

Globalization, Biosecurity, and the Future of the Life Sciences (2006)
Chapter: 3 advances in technologies with relevance to biology: the future landscape, 3 advances in technologies with relevance to biology: the future landscape.
T his chapter provides an overview and a perspective on the breadth and types of technologies that may have an impact on the life sciences enterprise of the future, with the understanding that there are inherent difficulties in anticipating or predicting how any of these technologies alone or in combination will alter the nature of the future threat “landscape.”
Rather than attempt to cover the technology landscape in a comprehensive manner, this chapter (1) highlights technologies likely to have obvious or high-impact near-term consequences; (2) illustrates the general principles by which technological growth alters the nature of future biological threats; and, (3) highlights how and why some technologies are complementary or synergistic in bolstering defense against future threats while also enhancing or altering the nature of future threats.
There is immense diversity and rapid evolution of technologies with relevance to (or impact on) the life sciences enterprise. Their impact(s) may be beneficial or detrimental depending on how these tools and technologies are applied. Some may be seen as “coming out of left field”; that is, these technologies may have very different applications from those originally intended, or may be combined in unexpected, nontraditional configurations. The combination of nanotechnology and biotechnology is one such example of a synergistic combination.
Many of the technologies discussed in this chapter create novel opportunities for scientists (and others) to explore aspects of biological and chemical diversity that cannot be accessed through natural mechanisms
or processes. Given the unpredictable nature of technological change, it is difficult if not impossible to describe in definite terms what the global technology landscape will look like in 5 to 10 years, both with regard to the emergence of technologies with dual-use applications and the global geography of future breakthroughs. New, unexpected discoveries and technological applications in RNAi and synthetic biology arose even during the course of deliberations by this committee. If this report, with the same charge, were prepared even a year or two in the future, many of the details presented in this chapter would likely be different.
A CLASSIFICATION SCHEME FOR BIOLOGICAL TECHNOLOGIES
Despite the seemingly disparate and scattered goals of recent advances in life sciences technologies, the committee concluded that there are classes or categories of advances that share important features. These shared characteristics are based on common purposes, common conceptual underpinnings, and common technical enabling platforms. Thus, the technologies outlined in this chapter are categorized according to a classification scheme devised by the committee and organized around four groupings:
Acquisition of novel biological or molecular diversity. These are technologies driven by efforts to acquire or synthesize novel biological or molecular diversity, or a greater range of specificity, so that the user can then select what is useful from the large, newly-acquired diversity pool. The goal is to create collections of molecules with greater breadth of diversity than found so far in nature, as well as with types of diversity that may not exist in nature. The kinds of molecules that might be generated include, for example, enzymes with enhanced or altered activities, as well as molecules composed of “unnatural” amino acids. Technologies in this category include those dedicated toward DNA synthesis; the generation of new chemical diversity (i.e., through combinatorial chemistry); those that create novel DNA molecules (from genes to genomes) using directed in vitro molecular evolution (e.g., “DNA shuffling” 1 ); and those that amplify or simply collect previously uncharacterized sequences (genomes) directly from nature (i.e., bioprospecting). All of these technologies require a subsequent selection step, such that molecules, macromolecular complexes, or even microbes with the desired properties can be identified and isolated from a large and very diverse pool of possibilities. Toward this end, new high-throughput screening (including the use of robotics and advanced information management systems) have become critical enabling technologies.
Directed design. These are technologies that involve deliberate efforts to generate novel but predetermined and specific biological or molecular diversity. The use of these technologies begins with a more defined, preexisting understanding of the desired endproduct and its molecular features. One then synthesizes or re-engineers the desired product or its components. Examples include but are not limited to rational, structure-aided design of small-molecule ligands; the genetic engineering of viruses or microbes; and, the emerging field of “synthetic biology.”
Understanding and manipulation of biological systems. These are technologies driven by efforts to gain a more complete understanding of complex biological systems and an ability to manipulate such systems. Examples include “systems biology”; gene silencing (e.g., RNA interference); the generation of novel binding (affinity) reagents; technologies focused on developmental programs (e.g., embryonic stem cells); genomics and genomic medicine; the study of modulators of homeostatic systems; bioinformatics; and, advanced network theory.
Production, delivery, and “packaging.” These are technologies driven by efforts in the pharmaceutical, agriculture, and healthcare sectors to improve capabilities for producing, reengineering, or delivering biological or biology-derived products and miniaturizing these processes. Examples include the use of transgenic plants as production platforms, aerosol technology, microencapsulation, microfluidics/microfabrication; nanotechnology; and, gene therapy technology. [Some of these technologies are related to the manipulation of biological systems—e.g., nanotechnology—and may also be applied to the generation (category 1) or design (category 2) of novel biological diversity or to the analysis of complex biological systems (category 3).]
The classification scheme serves several important purposes. It:
highlights commonalities among technologies and, by so doing, draws attention to critical enabling features;
provides insight into some of the technical drivers behind biology-related technology;
facilitates predictions about future emerging technologies; and,
lends insight into the basis for complementarities or synergies among technologies and, as such, facilitates the analysis of interactions that lead to either beneficial or potentially malevolent ends.
Limitations of the classification scheme include the fact that it is based on a relatively small number of relevant technologies (i.e., the committee’s
list of technologies may be biased and is inevitably incomplete) and the acknowledgment that there are many ways to categorize these technologies. As a reflection of the latter dilemma, the committee found that some of the technologies discussed in this chapter could have been classified in more than one category. The category assignment in these cases was guided by the nature of the particular applications that the committee had in mind when considering each of the relevant technologies.
The examples below serve as a finite set of future technologies that represent and illustrate each of the four categories. For each example the following issues are addressed: the purpose of the technology, its current state of the art, and future applications. The coverage of these issues for each of the technologies is not intended to be exhaustive. The technologies covered in this chapter include not only those that open up new possibilities for the creation of novel or enhanced biological agents but also those that expose new vulnerabilities (i.e., targets for biological attack). Details are limited to those necessary for a clear explanation of the plausibility of use.
1. ACQUISITION OF NOVEL BIOLOGICAL OR MOLECULAR DIVERSITY
Given the clear capability of at least some microbes and viruses to evolve quickly, acquire new genes, and alter their behavior, it might seem reasonable that over hundreds of thousands of years all conceivable biological agents have been “built” and “tested” and that the agents seen today are the most “successful” of these. Thus, is there any reason to think that it might be possible to create a more successful biological agent? Possibly not, but it is important to understand that “successful” in this context means the most able to survive within, on, or near human populations over time. “Success” does not necessarily equate with virulence or pathogenicity, the ability to cause disease or injury.
The kinds of basic biological diversity found in nature today, or those that have potentially evolved in the natural world and been tested for fitness over time, may have been (and are still) limited by certain natural constraints, including available building blocks—nucleotides and amino acids; natural mechanisms for generating genetic diversity; and, the strength and nature of selective pressures over time. Nor has there been enough time over the history of the earth for nature to have explored more than a tiny fraction of the diversity that is possible. 2 The technologies described in this section are those that seek to create a much wider and deeper set of diverse biological molecules, many of which may never have been generated or given a fair chance for succeeding in nature (although success may be defined in different ways). 3
Techniques have been developed to expand both the diversity of nucleotide or amino acid sequences of nucleic acids or proteins, respectively (which in both cases ultimately hold the information specifying the folding and thus the conformation of biologically active molecules), or for creating a diversity of small molecules with different shapes, sizes, and charge characteristics. In addition, some investigators are creating unnatural nucleic acids and amino acids in order to test and explore possible structural constraints on molecules with biological function. All of these approaches result in novel types of genetic or molecular diversity that then require assessment of functional potential. This assessment typically takes the form of a screening process (i.e., deliberate examination of all molecules for a desired feature or function) or a selective process (i.e., one that imposes a selective advantage on those molecules that have a property of interest). While the technological processes of assessing and selecting molecules of interest—high-throughput screening and selection—have some features in common with the next category of technologies (i.e., directed design), they are included in this first category because of their critical enabling role in the exploration of molecular and biological diversity.
DNA Synthesis
Description.
DNA synthesis is a technology that enables the de novo generation of genetic sequences that specifically program cells for the expression of a given protein. It is not new, but technical enhancements continue to increase the speed, ease, and accuracy with which larger and larger sequences can be generated chemically. By the early 1970s, scientists had demonstrated that they could engineer synthetic genes. 4 However, it was the automation of de novo DNA synthesis and the development of the polymerase chain reaction (PCR) in the early 1980s that spawned the development of a series of cascading methodologies for the analysis of gene expression, structure, and function. Our ability to synthesize short oligonucleotides (typically 10 to 80 base pairs in length) rapidly and accurately has been an essential enabling technology for a myriad of advances, not the least of which has been the sequencing of the human genome.
The past few years have seen remarkable technological advances in this field, particularly with respect to the de novo synthesis of increasingly longer DNA constructs. The chemical synthesis and ligation of large segments of a DNA template, followed by enzymatic transcription of RNA led to the de novo creation of the poliovirus genome in 2002 (about 7,500 nucleotides in length), from which the infectious, virulent virus was res-
cued following its transfection into permissive cells. 5 The following year scientists announced the successful assembly of a bacterial virus genome. 6 Parallel efforts in industry and academia led to the synthesis and assembly of large segments of the hepatitis C virus genome, from which replication competent RNA molecules were rescued. These studies raised concerns in the media that larger, more complex organisms, such as the smallpox virus (which is approximately 186,000 base pairs long), might be within reach. 7
DNA synthesis technology is currently limited by the cost and time involved to create long DNA constructs of high fidelity as well as by its high error rate. Current estimates for generating even simple oligonucleotides are at least $0.10 per base (including synthesis of the oligonucleotides plus error correction). 8 See Figure 3-1 .

FIGURE 3-1 Cost per base of sequencing and synthesis.
SOURCE: Rob Carlson presentation to the committee, February 2004.
Current State of the Art
Several recent studies have demonstrated important steps toward making gene synthesis readily affordable and accessible to researchers with small budgets, by decreasing its cost and improving its error rate. 9 For example, in December 2004, as this committee deliberated its charge, scientists described a new microchip-based technology for the semiautomated multiplex synthesis of long oligonucleotides. 10 The researchers used the new technology to synthesize all 21 genes that encode proteins of the E. coli 30S ribosomal subunit. Almost simultaneously, another research group described a novel approach for reducing errors by more than 15-fold compared to conventional gene synthesis techniques, yielding DNA with one error per 10,000 base pairs. 11
Future Applications
Developments in DNA synthetic capacity have generated strong interest in the fabrication of increasingly larger constructs, including genetic circuitry, 12 the engineering of entire biochemical pathways, 13 and, as mentioned above, the construction of small genomes. 14 As a specific example of a potential future beneficial application of DNA synthesis, one research group has described a method for synthesizing terpenoid, a natural product used in commercial flavors, fragrances, and antimalarial and anticancer therapeutics, using recombinant DNA constructs. 15 Terpenoids are normally isolated from plant tissue and can only be recovered in small amounts. DNA synthesis technology could be used as an alternative method for producing high-value compounds.
DNA synthesis technology could allow for the efficient, rapid synthesis of viral and other pathogen genomes—either for vaccine or therapeutic research and development, or for malevolent purposes or with unintentional consequences. Given the latter risks, in 2004, George Church (Harvard Medical School, Cambridge, MA) drafted a proposal for decreasing biohazard risks (i.e., creating nearly extinct human viruses, such as polio, or novel pathogens, like IL-4 poxvirus) while minimizing the impact on legitimate research. The proposal focuses on instrument and reagent licensing (e.g., restricting the sale and maintenance of oligonucleotide synthesis machines to licensed entities); regulation for the screening of select agents; establishing a method for testing these newly implemented licensing and, screening systems; criteria for exemption from the whole process; and, strategies for keeping the cost down. 16 The proposal is mentioned here not to endorse it, but rather to highlight the need for a careful analysis and thoughtful discussion of the issues.
DNA Shuffling
Classical genetic breeding has proven itself over and over again throughout human history as a powerful means to improve plant and animal stocks to meet changing societal needs. The late 20th century discovery of restriction endonucleases, enzymes that cut DNA molecules at sites comprising specific short nucleotide sequences, and the subsequent emergence of recombinant DNA technology provided scientists with high-precision tools to insert (or remove) single genes into the genomes of a variety of viruses and organisms, leading, for example, to the introduction of production-enhancing traits into crop plants. 17 Most recently, a powerful mode of directed evolution known as “DNA shuffling”—also known as multigene shuffling, gene shuffling, and directed in vitro molecular evolution—has allowed scientists to greatly improve the efficiency with which a wide diversity of genetic sequences can be derived. A quantum leap in the ability to generate new DNA sequences, DNA shuffling can be used to produce large libraries of DNA that can then be subjected to screening or selection for a range of desired traits, such as improved protein function and/or greater protein production.
“Classical” single-gene breeding starts with a “parental” pool of related sequences (genes, etc.) and then breeds “offspring” molecules, which are subjected to screening and selection for the “best” offspring. The process is repeated for several generations. With DNA shuffling, sequence diversity is generated by fragmenting and then recombining related versions of the same sequence or gene from multiple sources (e.g., related species), resulting in “shuffling” of the DNA molecules. Basically, it allows for the simultaneous mating of many different species. The result is a collection of DNA mosaics. The reassortment that occurs during the shuffling process yields a higher diversity of functional progeny sequences than can be produced by a sequential single-gene approach.
In one of the earliest demonstrations of the technology, which involved shuffling four separately evolved genes (from four different microbial species), the shuffled “hybrids” encoded proteins with 270 to 540 times greater enzymatic activity than the best parental sequence. 18 Even if that same recombined enzyme could have been evolved through single-gene screening, the process would have been dramatically slower. But chances are it never would have evolved. Evidence from at least one study shows that the best parent is not necessarily the one closest in sequence to the best chimeric offspring and thus would probably not represent the best starting point for single-gene evolution (i.e., some other better-look-
ing parental sequence would have been chosen for single-gene directed evolution). 19
The technology has developed quickly, such that scientists are not just shuffling single genes, they are shuffling entire genomes. In 2002, biologists used whole-genome shuffling for the rapid improvement of tylosin production from the bacterium Streptomyces fradiae ; after only two rounds of shuffling, a bacterial strain was generated that could produce tylosin (an antibiotic) at a rate comparable to strains that had gone through 20 generations of sequential selection. 20 Also in 2002, a portion of the HIV genome was shuffled to create a new strain of HIV that was able to replicate in a monkey cell line that previously had been resistant to viral infection. 21 By 2003 the technique had advanced to the point where many mammalian DNA sequences could be shuffled together in a single bacterial cell line. In one study, scientists shuffled one gene of a cytokine from seven genetically similar mammalian species (including human) to generate an “evolved” cytokine that demonstrated a 10-fold increase in activity compared to the human cytokine alone. 22 It should be emphasized that the power of this technology (and any diversity generating procedure) is only fully realized if the molecules generated with the most enhanced, desired properties can be identified and isolated. Despite continual improvements in the throughput of current screening procedures, the use of conditions that impose strong selective pressures for emergence of molecules with the desired properties is far more efficient in finding the most potent molecule in the pool.
Ultimately, this rapid molecular method of directed evolution will allow biologists to generate novel proteins, viruses, bacteria, and other organisms with desired properties in a fraction of the time required with classical breeding and in a more cost-effective manner. For example, virologists are using DNA shuffling to optimize viruses for gene therapy and vaccine applications. 23 Synthetic biologists are using the technology to speed up their discovery process (see “ Synthetic Biology ” later in this chapter).
Bioprospecting
Description 24.
Bioprospecting is the search for previously unrecognized, naturally occurring, biological diversity that may serve as a source of material for use in medicine, agriculture, and industry. These materials include genetic blueprints (DNA and RNA sequences), proteins and complex biological compounds, and intact organisms themselves. Humans have been exploiting naturally-derived products for thousands of years. Even as high-throughput technologies like combinatorial chemistry, described above, have practically revolutionized drug discovery, modern therapeutics is still largely dependent on compounds derived from natural products. Excluding biologics (products made from living organisms), 60 percent of drugs approved by the Food and Drug Administration and pre-new drug application candidates between 1989 and 1995 were of natural origin. 25 Between 1983 and 1994, over 60 percent of all approved cancer drugs and cancer drugs at the pre-new drug application stage and 78 percent of all newly approved antibacterial agents were of natural origin. 26 Taxol, the world’s first billion-dollar anticancer drug, is derived from the yew tree. 27 Artemisinin, one of the most promising new drugs for the treatment of malaria, was discovered as a natural product of a fernlike weed in China called sweet wormwood. And aspirin—arguably one of the best known and most universally used medicines—is derived from salicin, a glycoside found in many species in the plant genera Salix and Populus .
Bioprospecting is not limited to plants, nor is drug discovery its only application. Most recently, with the use of molecular detection methods, scientists have uncovered a staggering number of previously unrecognized and uncharacterized microbial life forms. 28 Indeed, microbial genomes represent the largest source of genetic diversity on the planet—diversity that could be exploited for medical, agricultural, and industrial uses. Natural products discovered through bioprospecting microbial endophytes—microorganisms that reside in the tissues of living plants—include antibiotics, antiviral compounds, anticancer agents, antioxidants, antidiabetic agents, immunosuppressive compounds, and insectides. With respect to the last, bioinsecticides are a small but growing component of the insecticide market. Bioprospected compounds exhibiting potent insecticidal properties include nodulisporic compounds for use against blowfly larvae (isolated from a Nodulisporium spp . that inhabits the plant Bontia daphnoides ) 29 and benzofuran compounds for use against spruce budworm (isolated from an unidentified endophytic fungus from winter-green, Gaultheria procumbens ). 30 Of note, naphthalene, the ingredient in
mothballs, is a major product of an endophytic fungus, Muscodor vitigenus , which inhabits a liana, Paullina paullinioides . 31
Prospecting directly for DNA and RNA sequences that encode novel proteins with useful activities has become a potentially important scientific and business enterprise. This approach entails searches based on random expression of thousands or millions of sequences, followed by screening or selection for products with desired activities. 32 Sometimes the search focuses on families of related sequences that are predicted to encode products of interest, which are recovered directly from environments using sequence amplification technology. This kind of approach can synergize with the DNA shuffling technology described above. Recent, early forays into “community genomics,” or large-scale random sequencing of the DNA from complex environmental microbial communities, reflect the immense future potential of this approach for the discovery and harnessing of previously unimagined biological activities. 33
For example, Diversa Corporation (San Diego, CA) utilizes bioprospecting of microbial genomes to develop small molecules and enzymes for the pharmaceutical, agricultural, chemical, and industrial markets. 34 After collecting environmental samples of uncultured microorganisms and extracting the genetic material, researchers search for novel genes and gene pathways for potentially useful products, like enzymes with increased efficiencies and stabilities (e.g., high and low temperature stability, high or low pH tolerance, high or low salt tolerance). The samples are collected from environments ranging from thermal vents to contaminated industrial sites to supercooled sea ice.
Bioprospecting has also been applied to the discovery of microbial agents in efforts to better understand the diversity of microbes in the environment that might serve as human pathogens if provided the opportunity. It has been argued that by deliberately scrutinizing the kinds of vectors and reservoirs that exist in a local environment for previously unrecognized microbes, novel agents might be identified long before they are discovered to be human, animal or plant pathogens, thus providing early warning of potential disease-causing agents. 35 At the least, these surveys could expand our appreciation of microbial diversity and inferred microbial function. 36 For example, in 2002, using a broad-range PCR approach (i.e., using conserved priming sites for a group of related sequence targets, as opposed to specific primers for single unique targets), scientists discovered four novel Bartonella DNA sequences in 98 arthropod specimens (fleas, lice, and ticks) from Peru; three of the sequences were significantly different from previously characterized Bartonella species. 37 Bartonella s are vectorborne bacteria associated with numerous human and animal infections. 38 Rather than having any immediate known clinical
implications, this study illustrates the power of this generic approach as well as our incomplete understanding of Bartonella diversity.
Current methods include recovery of microbes using cultivation-based methods, serologic surveys of potential hosts, extraction/separation/purification of molecules with desired properties, amplification of families of related nucleic acid sequences using broad-range PCR (and similar techniques), shotgun cloning and sequencing of bulk DNA or cDNA from environments of interest, and the use of subtractive hybridization methods 39 to enrich for novel nucleic acid sequences in hosts or environments.
One might consider both molecular and traditional cultivation-based approaches for examining hosts, such as fruit bats and small rodents, which are already known to serve as reservoirs for important human microbial pathogens (Hendra and Nipah viruses, Borrelia spp. and other genera, respectively). As described above, the potential benefits associated with the discovery of novel products and microbial genetic diversity are innumerable.
Combinatorial Chemistry: Generating Chemical Diversity
Combinatorial chemistry refers to technologies and processes used for the rapid creation of large numbers of synthetic compounds (“libraries”), typically for the purposes of screening for activity against biological drug targets (see “High-throughput Screening”). Whereas DNA synthesis enables the acquisition of genetic sequence diversity, these techniques allow for the generation of libraries of chemical compounds having a diversity of shapes, sizes, and charge characteristics—all of which may be of interest for their varied abilities to interact with and bind to biologically active proteins or macromolecular complexes, thereby altering the biological properties of these proteins and complexes. Combinatorial chemistry techniques can be used to create a wide range of chemotypes or molecular motifs, ranging from large polycyclic compounds of a peptidic nature to smaller, presumably more druglike, compounds. Initially, it was believed that when used in combination with high-throughput screening technologies, combinatorial techniques would dramatically
accelerate the drug discovery process while reducing the associated up-front costs with the drug discovery effort. While this has not yet proven to be the case, most pharmaceutical companies are still heavily invested in combinatorial chemistry and are exploring the development and implementation of novel methods to create additional libraries of compounds. A recent trend noted in the pharmaceutical industry is the move from the development of large, unfocused, general screening libraries to smaller, less diverse libraries for screening against a particular target or family of related targets.
The origins of this new branch of chemistry can be traced back to the early 1960s, when methods were developed for the solid-phase synthesis of peptides. 40 This involved attaching an amino acid to a solid support (i.e., beads of plastic resin) and then adding amino acid residues, one by one in a stepwise fashion through the creation of covalent peptide chemical bonds, until the desired peptide product is created. The final polypeptide is released by chemically breaking its bond with the solid support and washing it free. 41 Subsequent modifications of the solid-phase synthesis process greatly enhanced the ability to generate a large number of peptides with specific amino acid sequences. 42 Individual peptides were synthesized on the ends of “pins” that were spatially oriented in a two-dimensional array designed to match up with the wells of a 96-well microtiter plate. This reduced the scale of the process and greatly facilitated the parallel synthesis of large numbers of peptides. A further modification of the technique enhanced the ability to create a diversity of peptide sequences by incorporating a combinatorial approach. 43 In this case, the solid-phase resin bearing the nascent synthetic peptide was enclosed in a mesh, or “tea bag.” Like the pin-based method, the tea-bag process facilitated the numerous washing and drying steps required for peptide synthesis and thus allowed for the parallel synthesis of many different peptides, each in its own tea bag. However, by mixing the resin from different tea bags after each individual stepwise addition of an amino acid residue, combinatorial peptide libraries involving a great diversity of amino acid sequences could be readily generated, in which each resin bead bears an individual peptide with a unique amino acid sequence. 44
After the compounds are synthesized and a library is constructed, a selection or screening strategy is needed to identify unique compounds of interest to the biological sciences. The most obvious method involves affinity isolation of the peptide of interest on an immobilized target molecule, followed by release of the peptide and analysis utilizing combinations of gas-phase chromatography, high-performance liquid chromatography (HPLC), mass spectrometry, and nuclear magnetic resonance (NMR). It is also possible to determine the structure of compounds still
attached to the resin, using “on-bead” analytical techniques such as infrared analysis, gel-phase NMR, matrix-assisted laser desorption ionization time-of-flight mass spectrometry, electrospray mass spectrometry, and HPLC chemiluminescence nitrogen detection. 45
While direct determination of structure, as described in the previous paragraph, works well for small libraries, these techniques are generally not applicable to large, mixture-based libraries. For libraries, various strategies have been developed that govern the reaction sequence by attaching a readable chemical “tag” to the bead while the molecule is being synthesized. One of the earliest tagging approaches employed the use of oligonucleotides. 46 In this approach, for every amino acid added to the peptide chain, a specific set of oligonucleotides was added to a separate chain that was attached to the same bead. PCR and DNA sequencing techniques were then used to decode the structure of the peptide. Numerous additional tagging techniques and agents have since been developed. 47
Solution-phase parallel synthesis is becoming the combinatorial chemistry technique of choice in the pharmaceutical industry, driven primarily by advances in laboratory automation, instrumentation, and informatics. Compounds can be synthesized either as single discrete compounds per reaction vessel or as mixtures of compounds in a single reaction vessel, so many of the same principles described above for solid-phase (resinbound) principles are applicable here as well. The primary advantage of solution-phase combinatorial chemistry lies in the increase in the number of chemical reactions/transformations that can be accessed, thereby greatly increasing the range of chemotypes (chemical scaffolds) that can be created.
The earliest reports of solution phase combinatorial chemistry techniques involved the use of a common multicomponent reaction, termed the Ugi reaction, in which an isocyanide, an aldehyde, an amine, and a carboxylic acid are combined in a single-reaction vessel to create a single major product. Using this synthetic approach coupled with advanced data analysis techniques, scientists were able to identify compounds with the desired biological effect after synthesizing only a 400-compound subset of the 160,000 possible products. This represents a 400-fold increase in discovery efficiency over conventional approaches.
The current trend in parallel solution-phase chemistry is leaning toward the development of smaller arrays (12 to 96 compounds) of simple to moderately complex chemical compositions. As the robotics and laboratory instrumentation required for parallel synthesis become more af-
fordable and readily accessible, the technology is being transferred into basic medicinal chemistry laboratories and becoming instrumental in the optimization of lead compounds (i.e., compounds that show potential to be developed into drugs). Such efforts are ideally carried out with knowledge of the structure of the target molecule, usually gained by application of either x-ray crystallography or NMR techniques. Structure-activity relationships are determined as lead compounds, identified initially through the screening of large libraries of compounds, are modified at specific sites, and the impact of the chemical modification on the desired biological properties of the compound is determined.
The purity and identity of combinatorially-produced compounds have been a source of recent great discussion and technological advance since, in order for any meaningful data to be produced from a biological assay, the purity of the compound of interest must be as high as possible. 48 The activity of the compound must also be confirmed by resynthesis of the specific molecule and repeat assays for biological activity.
Combinatorial chemistry techniques are not only useful for drug discovery and development, they are being used in the search for better superconductors, better phosphors for use in video monitors (phosphors are substances that emit light), better materials for use in computer magnetic and other storage devices, and better biosensors for the detection of medically-important molecules and environmental toxins. Combinatorial approaches have been used to develop a “nose chip” sensor capable of detecting and distinguishing among seven common solvents (toluene, chloroform, tetrahydrofuran, acetone, ethyl acetate, ethanol, and methanol). 49
Using combinatorial and high-throughput methods, the pharmaceutical industry synthesizes and screens several million new potential ligands annually. Although most companies have little use for the tens of thousands of these compounds identified each year as toxic, some might have potential as biochemical weapons ( Chapter 1 ). 50 Although most of the information derived from combinatorial and high-throughput technology is held in proprietary databases, a new public database recently proposed as part of the National Institutes of Health (NIH) Roadmap raises concerns about public access to dual-use information ( Chapter 1 , Box 1-1 ). The NIH Roadmap discovery effort is particularly worrisome in this regard, because of plans to optimize lead compounds shown to be capable of targeting specific cellular proteins. The goal is not to develop therapeutic agents but rather to provide a series of reagents, facilitating
further exploration of protein function and systems biology. 51 Such compounds may be relatively potent poisons.
While the technologies applied in combinatorial chemistry are not exceedingly complex, a wide variety of laboratory automation and instrumentation is needed to stage an effective combinatorial chemistry campaign.
High-Throughput Screening 52
High-throughput screening (HTS) refers to the process of examining large numbers of diverse biomolecular or chemical compounds in a rapid and efficient manner for properties of interest. Such technologies are essential to achieving any benefit from the construction of large and diverse libraries of compounds, as they are used to select a particular compound having the desired properties. These properties might include biochemical or enzymatic activities desired of a potential therapeutic agent or toxicity in such an agent that under usual circumstances one would wish to avoid. Advances in miniaturized screening technologies, bioinformatics, robotics, and a variety of other technologies have all contributed to the improved biological assay efficiency that characterizes HTS. In contrast to this paradigm, in which a large library of compounds (i.e., samples) is tested for one specific activity or set of activities, a variation on the HTS theme involves the testing of a single biological sample for a wide variety of activities. The best example of this is the use of DNA or oligonucleotide microarrays—also known as DNA chips. These are routinely used in both basic and applied research to facilitate the large-scale screening and monitoring of gene expression levels, gene function, and genetic variation in biological samples, and to identify novel drug targets.
The process of screening large numbers of compounds against potential disease targets is characterized by a collection of technologies that strive to increase biological assay efficiency through the application of miniaturized screening formats and advanced liquid handling, signal detection, robotics, informatics, and a variety of other technologies. Over the past several years, the industry has witnessed an evolution in screening capabilities, resulting in the ability of a user to screen more than 100,000 compounds per day for potential biological activity. Evaluating upward of 1 million compounds for biological (or various other) properties in a screening campaign is now commonplace in the pharmaceutical industry.
Effective HTS relies on robust assays that can detect and then translate biological or other activities into a format that can be readily interpreted. A wide variety of assays are currently in use, including:
cell-free colorimetric or chemiluminescence assays;
cell-free fluorescence resonance energy transfer assays;
cell-based reporter gene assays, usually with an enzymatic read-out;
cell-based fluorescence imaging assays;
NMR assays, which involve identifying small molecule ligands for macromolecular receptor targets;
affinity chromatography assays;
DNA microarrays (high density arrangements of double-stranded DNA clones (cDNA) or oligonucleotides that serve as identical or complementary probes, respectively, for specific genes, transcripts, or genome sequences); and
Other types of microarrays, including high-density arrangements of antibodies, nucleic acid or peptide aptamers, antigens (protein or lipid), MHC 53 -peptide antigen complexes, and intact cells.
Future Directions
Future advances in HTS—such as the development of one-step assays and increased miniaturization—will continue to increase the throughput and reduce the cost of HTS assays and may eventually allow the simultaneous monitoring of multiple endpoints (e.g., biological, toxicological) across a wide variety of targets. An analysis of the current HTS technology landscape reveals the following as potential opportunities and future directions:
further development of one-step (homogeneous) assays;
development of improved primary screening hardware;
miniaturization as a means to increase throughput and decrease cost;
improvements in the capabilities and efficiency of robotic systems in the life sciences;
application of HTS to lead compound optimization; and,
novel approaches for identification of biologically-relevant targets.
In short, HTS assays and technologies will permeate new sectors in the life sciences, affecting the productivity and speed of advances and discoveries in these varied sectors. The cost effectiveness of HTS assays and technologies will improve, such that tasks previously believed to be impractical will become quite tractable. Coupled with methods to generate enhanced sequence and structural diversity beyond that seen in nature, these assays and technologies will permit the identification and selection of novel molecules with important biological functions, with ramifications for all of the life sciences.
2. DIRECTED DESIGN
There are other technologies, besides those described in the previous category of technologies, that seek to generate new kinds of genetic or molecular diversity. However, in contrast to the technologies in the first category, these “directed design” approaches are more deliberate, and rely on preexisting knowledge with regard to what needs to be created.
Rational Drug Design
The methods described above, wherein a large library of diverse chemical compounds are screened using HTS methods to identify a smaller number of potential lead compounds with desired activities, are gradually being enhanced by less empirical approaches that are based on a greater understanding of biological systems (i.e., target: ligand interactions), identification of specific target molecules, and determination of the structure of a target molecule whose activity has been shown to be critical for the production of a particular disease or for maintenance of health. Such structural knowledge has grown rapidly over the past decade due to advances in x-ray crystallography, NMR technologies, and associated computational techniques that now allow for rapid determination of the structure of even large proteins or nucleic acid molecules at atomic-level resolution. A quick survey of the Protein Data Bank (PDB), 54 the global resource for all publicly available biological macromolecular structures, reveals that the number of structures deposited on an annual basis witnessed nearly a 10-fold increase between 1994 (3,091) and 2004 (28,992); see Figure 3-2 . With such structural knowledge of targets in hand, chemists can rationally pursue the design of novel chemical compounds that either bind to selected sites on the surface of these target molecules or mimic the structure of the target molecule and thereby compete for the binding to a receptor molecule.

FIGURE 3-2 Growth in the number of structures deposited per year (gray) and total holdings of the PDB (black) from the time the bank was founded.
SOURCE: Reprinted from Dutta, S. and H.M. Berman. 2005. Large macromolecular complexes in the protein data bank: A status report. Structure 13(3):382, with permission from Elsevier.
An excellent example of technological convergence exists with the field of in silico , or virtual, screening. This methodology capitalizes on the advances described above with respect to the determination of structures for target molecules as well as advances in computer hardware and specialized chemical informatics algorithms, so-called docking and scoring programs. Many thousands of virtual compounds can be rapidly and effectively assessed for potential target molecule complementarity, 55 as a prerequisite for biological activity, prior to any actual chemistry being carried out or biological assays being performed. The product of this computational effort is thus a rationally designed molecule that, once synthesized, can potentially serve as a lead compound in the drug discovery process.
Although rational drug design has received a great deal of attention from the pharmaceutical industry and is recognized as having great potential for the future, most efforts today by the drug discovery industry reflect a combination of structure-aided rational design of compounds and the HTS screening of libraries of diverse compounds. Thus, the use of structure, when known for a given molecular target, may come into play once a lead compound has been identified through an HTS process and efforts are made to optimize this lead and improve the biological activity or pharmacological properties of the compound. The field today is such that absence of knowledge of the structure of a targeted molecule is viewed as a critical impediment to the development of a new drug.
In contrast to the rational design of small-molecule therapeutics, the rational design of therapeutic nucleic-acid-based compounds is much easier in that such compounds are synthesized to be complementary to the targeted nucleic acid sequence. While nucleic acid therapeutics based on antisense oligonucleotides or ribozymes, enzymatically-active RNAs that cleave specific RNA target sequences, have been pursued for over a decade, their promise has not yet been realized due to difficulties in delivering stable compounds to desired sites. Significant advances are now occurring, however, in providing desired pharmacological properties to siRNA-based compounds and morpholino antisense oligonucleotides.
As the structure of greater numbers of potential target molecules are identified in the future and as both in silico screening and chemical synthesis methods continue to advance, it seems clear that a greater reliance
is likely to develop on these types of approaches. Greater application of rational, structure-based design approaches is likely to speed the discovery process significantly. While there are dual-use implications for such technologies, as there are for almost any advancing life sciences technology, the infrastructure required to pursue such structure-based design of novel biologically active compounds is likely to limit its use to the legitimate pharmaceutical industry for a number of years. It should be noted, however, that like the nucleotide sequence databases that are open to the public, rapidly growing numbers of protein structures are being placed in the public domain. This trend is likely to continue and even accelerate, and as the computer hardware and software requirements for viewing and interpreting such structures becomes increasingly simple, these approaches will become increasingly accessible to scientists outside the pharmaceutical industry.
Synthetic Biology
The fledgling 5-year-old-field of synthetic biology—which is attracting engineers and biologists in equal measure—means different things to different researchers. Engineers view it primarily as a way to fabricate useful microbes to do what no current technology can do (i.e., they view it as an engineering discipline). Biologists see it as a powerful new way to learn about underlying principles of cellular function.
Unlike systems biologists (see description later in this chapter), who adopt a big-picture approach to biology by analyzing troves of data on the simultaneous activity of thousands of genes and proteins, synthetic biologists reduce the very same systems to their simplest components. They create models of genetic circuits, build the circuits, see if they work, and adjust them if they do not, learning underlying principles of biology in the process. By examining simple patterns of gene expression and treating pieces of DNA as modules, which, like Legos™, can be spliced together, synthetic biologists construct what are effectively biochemical logic boards that control both intra- and extracellular activity.
Because the molecular nature of many cellular reactions is only partially understood, most synthetic genetic circuits require considerable further empirical refinement after the initial computational work. Some scientists use DNA shuffling to streamline the empirical process. After inserting mutated DNA circuits into cells and selecting for those cells (and the circuits therein) that performed the best, researchers can evolve an effective product in just a couple of generations. 56
One of the goals of the field is to transform bacteria into tiny programmable computers. Like electronic computers, the live bacterial circuits would use both analog and digital logic circuits to perform simple computations. For example, researchers are working to develop modular units, such as sensors and actuators, input and output devices, genetic circuits to control cells, and a microbial chassis in which to assemble these pieces. If they are successful, a “registry of biological parts” will allow researchers to go to the freezer, get a part, and hook it up. 57 The computing power of programmable cells will likely never rival that of their electronic counterparts. Rather, the beauty of synthetic biology lies in what living cells can do.
In 2000, a genetic “circuit” was created in E.coli that caused the cells to blink like a lighthouse. 58 The circuit, which was called “the repressilator,” was comprised of three repressor genes, one of which turned on a gene for green fluorescent protein (GFP), which, when activated, emits a green glow. Three years later another research group created a genetic circuit by crafting a “toggle switch” that could oscillate the circuit and alter its pattern depending on growth conditions. 59 Using this technique, investigators subsequently developed a procedure to re-engineer a bacterial protein that binds to TNT (an explosive) and that, when bound, activates a gene circuit that produces GFP. 60 This demonstrates an initial effort to engineer organisms that operate as biological sentinels, pinpointing explosives or detecting the presence of biological weapons.
In 2004, researchers in Israel designed a prototype “DNA computer” with the capacity to logically analyze mRNA disease indicators in vitro (i.e., in this case, early signs of prostate and lung cancer) and control the administration of biologically active ssDNA molecules, including drugs. 61 The procedure is relatively innocuous, requiring the injection of a very small amount of fluid containing billions of nanoparticles, each of which operates as a tiny computer by effectively interrogating the cell and detecting the presence of diagnostic DNA markers (e.g., mutated mRNA sequences or underexpressed or overexpressed mRNA). If the markers are present, the nanoparticle sends out a therapeutic short nucleic acid that can affect the level of gene expression.
Synthetic biology technology has many potential applications, including designing bacteria that can detect chemical or biological agent signatures, engineering bacteria that can clean up environmental pollutants, and engineering organisms or compounds that can diagnose disease or fix faulty genes. Although initial efforts are focused on microbial cells, some synthetic biologists imagine a day when they will be able to pro-
gram adult stem cells for therapeutic purposes (e.g., to patch up a damaged heart).
Engineering ethicist Aarne Vesilind (Bucknell University) is one of many scientists promoting the idea that synthetic biologists and ethicists hold an Asilomar-like conference on synthetic biology—much like that held at the dawn of genetic engineering research in the mid-1970s—to define bioengineers’ “responsibilities to society” should these engineered organisms survive outside the laboratory to cause harm to human health or the environment. 62 Several efforts have now been planned to examine the implications of this kind of work, including one foundation-funded study involving three institutions, two of which play a major role in synthetic genomics research. 63 In addition, the National Science Advisory Board for Biosecurity has identified synthetic genomics as a major area of interest. Many of the same issues are raised by the genetic engineering of viruses.
Genetic Engineering of Viruses
As described above, the development of recombinant DNA technology and the ability to manipulate DNA sequences in bacterial species such as E. coli has resulted over time in the capacity to insert almost any desired gene into almost any kind of prokaryotic or eukaryotic cell. Placing the DNA inserted under appropriate transcriptional controls, and the protein encoded by it under appropriate translational control, allows that gene to direct the expression of almost any kind of protein: a fluorescent marker (as in the GloFish described in Chapter 1 ), an enzyme that might function as a reporter, an antibiotic resistance marker, or even a toxin. Using very similar techniques, genes of interest (subject to size constraints) can be introduced into the genomes of many different types of DNA viruses, ranging from adenoviruses to herpesviruses. Such capabilities raise obvious and compelling dual-use concerns.
The introduction of heterologous gene sequences into the genomes of RNA viruses, or other types of modifications to the RNA genomes of these viruses, presents a special set of technical difficulties due to the fact that the genetic material is RNA, which is less stable than DNA and not as amenable to the genetic splicing techniques that have made recombinant DNA technology as versatile. However, this has been accomplished for a growing number of different types of RNA viruses. Moreover, given the small size of these RNA genomes, it has proven possible to synthesize completely de novo all the genetic material needed to recover fully infectious virus particles with near wild-type infectivity, virulence and replication potential.
RNA viruses come in several types, depending on the number of strands of RNA in each molecule of their genome (i.e., single-stranded or double-stranded RNA molecules) and the number of genomic segments (one or more). Genetic engineering of single-stranded RNA viruses in which the RNA is of positive polarity (i.e., the same sense as the messenger RNA that encodes the viral proteins) has proven most straightforward. It has been known for many years that genomic RNA isolated from positive-strand RNA viruses, such as poliovirus, is intrinsically infectious. When transfected (i.e., introduced) into a permissive cell in the absence of any accompanying proteins, such RNA will lead directly to the synthesis of the viral proteins, which will then begin to assemble the necessary replicative machinery to make additional copies of the RNA as well as more viral protein, leading ultimately to the assembly and “rescue” of fully infectious virus, which is then generally released from the cell.
To manipulate the viral RNA genome, scientists in the age of molecular biology have developed efficient enzymatic methods for creating complementary DNA (cDNA) copies of the viral genomic RNA using reverse transcriptase enzymes encoded by retroviruses. This cDNA can be engineered to have “sticky” ends, allowing it then to be molecularly cloned into E. coli , in which it can be manipulated by all the modern methods available. This can include the deletion of protein coding sequences, the creation of deletion or point mutations, or even the introduction of completely novel protein-coding sequences. The modified cDNA can then be placed downstream of an appropriate promoter sequence for a DNA-dependent RNA polymerase and a novel, molecularly engineered viral RNA genome efficiently transcribed in an in vitro transcription reaction. The transcribed RNA can then be transfected back into a permissive cell and, if the introduced mutations are compatible with continued viability of the virus, will give rise to novel infectious viruses.
The process by which virologists use this method, involving the conversion of the genetic sequence of the virus from RNA to DNA and back to RNA, generally in order to assess the impact of mutations on the viral life cycle or pathogenic properties, is known as “reverse genetic engineering.” This approach is widely used by positive-strand molecular virologists. First carried out in 1980 with poliovirus, 64 infectious cDNA clones have now been constructed for members of many positive-stranded RNA virus families, including brome mosaic virus, 65 yellow fever virus, 66 Sindbis virus, 67 citrus tristeza virus, 68 and equine arteritis virus. 69 In the case of hepatitis C virus, a positive-strand virus in the Flaviviridae , virus rescue has generally required injection of the synthetic RNA directly into the liver of a chimpanzee. On the other hand, fully infectious poliovirus, a member of the family Picornaviridae , has been recovered in a cell-free reaction carried out in vitro in an optimized cell extract system.
In the past, coronaviruses, which have the largest genomes of all positive-strand RNA viruses (around 30 kilobases long), were difficult to reverse engineer because of the sheer size and instability of their full-length cDNA clones in bacterial vectors. 70 However, recent technological advances have made it possible to reverse engineer even these largest of all known RNA viruses, 71 including the causative agent of severe acute respiratory syndrome (SARS), a previously undescribed coronavirus. 72
Similarly, the reverse genetic engineering of negative-strand RNA viruses 73 has proven much more difficult, given the fact that the RNA genomes of these viruses do not function directly as messenger RNAs and thus do not give rise to infectious virus progeny following their introduction into permissive cells. These RNAs require the expression of certain viral proteins, in order to make positive-strand copies of the negative-stranded RNA genome and to initiate the replicative cycle. The technology to accomplish this was first developed for influenza A virus in the late 1980s to early 1990s. Like the earlier efforts with positive-strand RNA viruses, these efforts not only have dramatically improved our understanding of how these viruses replicate, but have also created the means for genetically manipulating viral genomes in order to generate new viruses for use as live, attenuated vaccines or vectors. 74
Initially, reverse engineering of the influenza virus required the use of helper viruses, which provided proteins and RNA segments that the reconstituted in vitro RNPs (i.e., reconstituted ribonucleoprotein complexes containing RNA transcribed from the molecularly cloned cDNA) needed in order to be infectious following transfection into cells. Later, alternative methods for introducing influenza RNPs into cells were developed, including entirely plasmid-driven rescue that did not require the involvement of a helper virus. 75 The latter plasmid-based system allowed for easy engineering of viral genomes with multiple specific mutations. By 2001 at least one laboratory had generated a pathogenic H5N1 virus using reverse engineering. 76
In addition to influenza A virus, and as summarized in a paper that appeared in the Journal of Virology in 1999, 77 in its first decade the technology was used to reverse engineer, or “recover” many other negative-stranded RNA viruses including rabies virus, 78 vesicular stomatitis virus, 79 respiratory syncytial virus, 80 measles virus, 81 Sendai virus, 82 human parainfluenza type 3, 83 rinderpest virus, 84 simian virus, 85 bovine respiratory syncytial virus, 86 Newcastle disease virus, 87 and bunyavirus. 88
Most recently, as mentioned in Chapter 1 , reverse engineering has been used to produce infectious influenza A viruses containing the viral
haemagglutinin (HA) and neuraminidase (NA) genes of the strain that caused the devastating 1918-1919 “Spanish” influenza pandemic. Scientists demonstrated that the HA of the 1918 virus confers enhanced pathogenicity in mice to recent human viruses that are otherwise nonpathogenic in their murine host. HA is a major surface protein that stimulates the production of neutralizing antibodies in the host, and changes in the genome segment that encodes it may render the virus resistant to preexisting neutralizing antibodies, thus increasing the potential for epidemics or pandemics of disease. Moreover, the reverse engineered viruses expressing 1918 viral HA elicited hallmark symptoms of the illness produced during the original pandemic. 89
With the complete genetic sequencing of the H1N1 influenza A virus, referred to in Chapter 1 , some have questioned whether these studies should have been published 90 in the open literature given concerns that terrorists could, in theory, use the information to reconstruct the 1918 flu virus. 91 It should be noted that in addition to the “normal” scientific peer review, the editors of Science required the authors to demonstrate that they had obtained approval to publish their research from the director of the Centers for Disease Control and Prevention, and the director of the National Institute of Allergy and Infectious Diseases. 92 Furthermore, the National Science Advisory Board for Biosecurity (NSABB) was asked to consider these papers prior to publication and determined that the scientific benefit of the future use of this research far outweighed the potential risk of misuse. 93
Reverse engineering of the causative agent of SARS illustrates the many potential beneficial applications of the technology. In addition to opening up new opportunities for exploring the complexity of the SARS-coronavirus genome, the availability of a full-length cDNA provides a genetic template for manipulating the genome in ways that will allow for rapid and rational development and testing of candidate vaccines and therapeutics. 94 By mutating the many small proteins seemingly expressed by this unique coronavirus, scientists will learn their function in viral replication and/or pathogenesis and potentially identify useful targets for drug discovery efforts.
The influenza A reverse genetic engineering system serves as an excellent example of the potential for this technology to be used with the intent to do harm. As summarized in a 2003 article on the potential use of influenza virus as an agent for bioterrorism, with respect to advances that allowed for helper virus free production of a pathogenic H5N1 virus, virologist Robert M. Krug (University of Texas, Austin) has written:
There is every reason to believe that the same recombinant DNA techniques can be used to render this H5N1 virus transmissible from humans to humans. Furthermore, it should be possible to introduce mutations into such a recombinant virus so that it is resistant to currently available influenza virus antivirals (M2 inhibitors: amantadine and rimantadine; and NA inhibitors: zanamivir and oseltamivir), and so that it possesses an HA antigenic site that is unlike those in recently circulating human viruses. In fact, several viruses with different HA antigenic sites could be generated. The human population would lack immunological protection against such viruses, existing antiviral drugs would not afford any protection, and these viruses could be spread simply by release of an aerosol spray in several crowded areas. 95
3. UNDERSTANDING AND MANIPULATION OF BIOLOGICAL SYSTEMS
A more holistic understanding of complex biological systems (e.g., the workings of an intact cell, multicellular organism, or complex microbial community) is emerging through a set of technologies that allow for the collection of vast, comprehensive (highly parallel) sets of data for multiple kinds of biological processes, the integration of these data sets, and the identification of critical components or pathways. Critical components can then serve as targets for therapeutic and preventive intervention or manipulation; they can also serve as targets for malevolent manipulation and as the basis for novel kinds of biological attack. Concurrently, technologies that facilitate a better understanding of intracellular, organ, and whole-animal control “circuitry” will enhance the ability of scientists to manipulate these complex systems.
Examples of some technologies that are leading to this type of holistic overview include the emerging field/discipline of “systems biology” 96 and genomic medicine. Examples of the tools that could be used to manipulate complex biological systems include gene silencing, novel binding reagents (e.g., nucleic acid and peptide aptamers, engineered antibodies), and small-molecule modulators of physiological systems. In many ways this category of technologies opens up entirely novel aspects of the future biodefense and biothreat agent landscapes and changes the fundamental paradigm for future discussions on this topic.
RNA Interference
RNA interference—also known as RNAi and RNA silencing—was first observed in plants when it was noted that endogenous and “foreign” genes appeared to be turning each other off by a process initially termed
“co-suppression.” 97 What was initially thought to be peculiar to petunias was later found in other plants and also animals. The phenomenon is now known as RNA interference, and is recognized to be a common antiviral defense mechanism in plants and a common phenomenon in many other organisms, including mammals. It is also increasingly apparent that RNAi is intimately related to widespread regulation of gene expression by very small endogenously expressed RNA molecules, so-called micro-RNAs (miRNA). This field is exploding with new discoveries almost daily concerning the role of miRNAs in regulating gene expression during development and after. The interaction of endogenous miRNAs with cellular mRNAs encoding specific proteins leads to suppression of protein expression, either by impairing the stability of the mRNA or by suppressing its translation into protein. The fact that small, largely double-stranded RNAs of this type, about 21 nucleotides in length, could play such an apparently broad and fundamental role in development and in the control of cellular homeostasis was not at all appreciated just a few years ago and highlights the sudden, unpredictable paradigm shifts and sharp turns in the way scientists think that are possible in the advance of the life sciences ( Figure 3-3 ).
The basic molecular mechanism of RNAi is as follows. Long, double-stranded RNAs (dsRNAs; typically >200 nucleotides long) silence the expression of target genes upon entering a cellular pathway commonly referred to as the RNAi pathway. First, in the so-called initiation step, the dsRNAs are processed into 20 to 25 nucleotide small interfering RNAs (siRNAs) by an Rnase III-like enzyme called Dicer. The siRNAs then assemble into endoribonuclease-containing complexes known as RNA-induced silencing complexes (RISCs), unwinding in the process. The siRNA strands subsequently guide the RISCs to complementary RNA molecules, where RISC complex cleaves and destroys the cognate RNA (i.e., this is the effector step). miRNAs are generated in a similar fashion from endogenously expressed RNAs containing short hairpin structures, using a related Dicer-like protein. They are capable of similarly silencing gene expression but can also direct post-transcriptional silencing by blocking translation of a targeted host mRNA. This later effect typically depends on binding to a partially complementary target sequence near the 3’ end of the mRNA.
RNAi is highly specific and remarkably potent (only a few dsRNA molecules per cell are required for effective interference), and the interfering activity can occur in cells and tissues far removed from the site of introduction.
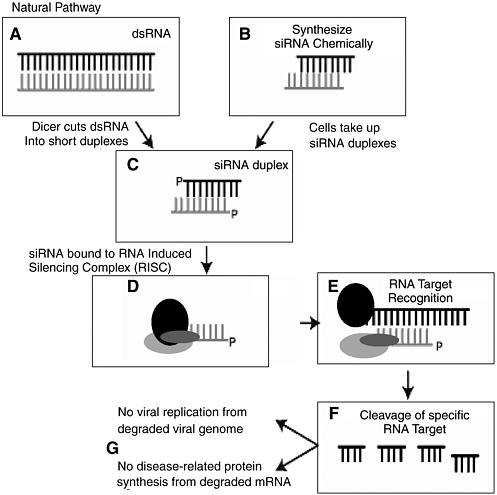
FIGURE 3-3 The process of RNA interference.
SOURCE: Steven Block, presentation to the committee, April 2004.
The technology is expected to prove particularly valuable in cases where the targeted RNA encodes genes and protein products inaccessible to conventional drugs (i.e., protein, small-molecule, and monoclonal antibody therapeutics). However, clinical delivery poses a significant challenge, as does the likelihood of undesirable silencing of nontargeted genes. 98 Yet several recent experiments indicate that investigators are well on their way to overcoming these challenges and creating an emerging dual-use risk in the form of bioengineered RNAi-based pathogens. In 2003, a German research team announced the successful lentivirus vector
delivery of in vivo gene silencing with RNAi. 99 Also in 2003, researchers announced the successful use of high-pressured, high-volume intravenous injection of synthetic siRNA. 100 Other studies have demonstrated the potential to deliver RNAi to specific organs, such as the eyes, 101 lungs, 102 and central nervous system. 103 Although human trials of RNAi have begun for the treatment of age-related macular degeneration, 104 a systemic mode of delivery would arguably have greater clinical utility. Substantial progress is being made toward this aim, however, using liposome and lipid nanoparticle formulations of chemically modified, and hence stabilized, siRNAs. Scientists at Sirna, a small biotech company working for well over a decade on nucleic-acid-based therapies, have recently described a 1,000-fold reduction in the amount of hepatitis B virus present in the blood of mice replicating this virus in the liver, following a series of three separate intravenous inoculations of a lipid nanoparticle formulated, chemically modified, siRNA.
In November 2004, researchers from Alnylam Pharmaceuticals used chemically modified siRNAs to silence genes encoding Apolipoprotein B (ApoB) in mice, resulting in decreased plasma levels of ApoB protein and reduced total cholesterol. 105 The study thus demonstrated systemic activity following a conventional clinical mode of delivery. Importantly, the delivery did not inadvertently impact nontargeted genes. Still, there are questions about the specificity of the siRNA, given that the investigators did not evaluate all proteins and given that they collected measurements over a relatively short period of time. 106 A longer, more comprehensive study would be necessary to evaluate more fully the specificity of the technique. However, while “off- target” effects of siRNAs are certainly of concern to regulators and industry proponents as well, it is likely they can be managed in much the same way that “off target” effects (i.e., unexpected toxic effects) of small-molecule therapeutics have been in the past.
Potential Applications
Observations that RNAi works in vivo in mammals has not only created opportunities for the development of new therapeutic tools but also spawned a new generation of genetic research in mammals. 107 For example, the vast majority of mammalian RNAi systems are driven by a polymerase III promoter, which can be manipulated such that the experimenter has the ability to turn the expression of a gene on and off at will, allowing for novel experimental designs. One could temporarily switch off a tumor suppressor gene suspected of providing genome protection (e.g., a checkpoint gene) and then turn it on again, allowing the experimenter to determine whether the gene is necessary for the initiation or
maintenance of tumorigenesis and whether it might be a good target for late-stage cancer treatments.
It is reasonable to expect significant additional advances in the formulation of siRNAs for use as pharmacological agents, particularly with contributions from the field of nanotechnology. As with so many of the technologies outlined in this chapter, just as RNAi promises new therapeutic options for cancer and other diseases, it could also be used to manipulate gene expression with the intent to do harm.
High-Affinity Binding Reagents (Aptamers and Tadpoles)
Aptamers are short, single-stranded nucleic acid or peptidic ligands that fold into well-defined three-dimensional shapes, allowing them to inhibit or modulate their protein targets with high affinity and specificity. Since their discovery in the early 1990s, 108 aptamers have been used in target validation, detection reagents, and functional proteomic tools. 109 Over the past decade, several studies have explored the potential of aptamers for therapeutic intervention, including the inhibition of targets associated with inflammatory processes, cancer, and other disorders. 110 Aptamers have been compared to monoclonal antibodies but with the added advantage that they are neither toxic nor immunogenic.
One of the first aptamers tested in an animal model was an antithrombin agent that blocks the proteolytic activity of thrombin, a protein involved in thrombosis (blood clot formation in a blood vessel). 111 In June 2004, Archemix Corp. (Cambridge, MA) and Nuvelo, Inc. (San Carlos, CA) announced that an Investigational New Drug application had been submitted to the FDA to begin a Phase I clinical trial with an antithrombin aptamer, ARC183, for potential use in coronary artery bypass graft surgery. 112 In another clinical trial, Eyetech Pharmaceuticals, Inc. (New York, NY) is testing Macugen, an aptamer that targets VEGF (vascular endothelial growth factor) as a treatment for age-related macular degeneration and diabetic macular edema. 113
In January 2005, scientists reported that they had created a new type of high-affinity binding reagent—“tadpoles”—that bind to specific targets, such as Bacillus anthracis protective antigen and the enzyme cofactor biotin, as examples. 114 Tadpoles are protein-DNA chimeras that contain a protein head coupled to an oligonucleotide tail. The head has an affinity for a specific target molecule; the tail, which contains a region for PCR
amplification, mediates detection. Tadpoles represent another type of high-affinity binding reagent with the power to not only detect but, with its DNA tail, “count” small numbers of proteins and other molecules in a precise fashion.
Their sensitivity, dynamic range, and, in the case of tadpoles, precise quantification make these high-affinity binding molecules potentially very useful tools for disease diagnosis and environmental detection, including pathogen and other biological agent detection in the event of a naturally occurring or deliberate biological attack.
Despite their promise as therapeutic agents, aptamers are very expensive to synthesize and are still a largely unknown entity (with respect to administration, formulation, adverse effects, etc.). So although several compounds have entered clinical trial, their future as biopharmaceuticals is unclear. 115 More certain is their role as valuable lead structures in small-molecule drug discovery (because they can be so readily modified and adapted to almost any kind of high-throughput readout format) and as molecular detection reagents (because of their high specificity).
Computational Biology and Bioinformatics 116
Life scientists have exploited computing for many years in some form or another. But what is different today—and will be increasingly so in the future—is that the knowledge of computing and mathematical theory needed to address many of the most challenging biological problems can no longer be easily acquired but requires instead a fusion of the disciplines of biology, computation, and informatics. A National Research Council (NRC) report entitled Catalyzing Inquiry at the Interface of Computing and Biology (December 2005) has pointed out that the kinds and levels of expertise needed to address the most challenging problems of contemporary biology stretch the current state of knowledge of the field. The report identifies four distinct but interrelated roles of computing for biology:
Computational tools are artifacts—usually implemented as software but sometimes hardware—that enable biologists to solve very specific and precisely defined problems. Such biologically oriented tools acquire, store, manage, query, and analyze biological data in a myriad of forms and in enormous volume for its complexity. These tools allow bi-
ologists to move from the study of individual phenomena to the study of phenomena in biological context, to move across vast scales of time, space, and organizational complexity and to utilize properties such as evolutionary conservation to ascertain functional details.
Computational models are abstractions of biological phenomena implemented as artifacts that can be used to test insight, to make quantitative predictions, and to help interpret experimental data. These models enable biological scientists to understand many types of biological data in context, and even at very large volumes, and to make model-based predictions that can then be tested empirically. Such models allow biological scientists to tackle harder problems that could not readily be posed without visualization, rich databases, and new methods for making quantitative predictions. Biological modeling itself has become possible because data are available in unprecedented richness and because computing itself has matured enough to support the analysis of such complexity.
A computational perspective or metaphor on biology applies the intellectual constructs of computer science and information technology as ways of coming to grips with the complexity of biological phenomena that can be regarded as performing information processing in different ways. This perspective is a source for information and computing abstractions that can be used to interpret and understand biological mechanisms and function. Because both computing and biology are concerned with function, information and computing abstractions can provide well-understood constructs that can be used to characterize the biological function of interest. Further, they may well provide an alternative and more appropriate language and set of abstractions for representing biological interactions, describing biological phenomena, or conceptualizing some characteristics of biological systems.
Cyberinfrastructure and data acquisition are enabling support technologies for 21st century biology. Cyberinfrastructure—high-end general-purpose computing centers that provide supercomputing capabilities to the community at large; well-curated data repositories that store and make available to all researchers large volumes and many types of biological data; digital libraries that contain the intellectual legacy of biological researchers and provide mechanisms for sharing, annotating, reviewing, and disseminating knowledge in a collaborative context; and high-speed networks that connect geographically distributed computing resources—will become an enabling mechanism for large-scale, data-intensive biological research that is distributed over multiple laboratories and investigators around the world. New data acquisition technologies such as genomic sequencers will enable researchers to obtain larger amounts of data of different types and at different scales, and advances in informa-
tion technology and computing will play key roles in the development of these technologies.
A new level of sophistication in computing and informatics is required for interpretation of much of the data generated today in the life sciences. These data are highly heterogenous in content and format, multimodal in collection, multidimensional, multidisciplinary in creation and analysis, multiscale in organization, and international in collaborations, sharing, and relevance. 117 Such data may consist of sequences, graphs, geometric information, scalar and vector fields, patterns of organization, constraints, images, scientific prose, and even biological hypotheses and evidence. These data may well be of very high dimension, since data points that might be associated with the behavior of an individual unit must be collected for thousands or tens of thousands of comparable units. The size and complexity of the data sets being generated require novel methods of analysis, which are being provided by computational biologists. For example, scientists at the U.S. Department of Energy’s Pacific Northwest National Laboratory have developed a new computational tool—called ScalaBLAST—that is a sophisticated “sequence alignment tool” and can divide the work of analyzing biological data into manageable fragments, so that large data sets can run on many processors simultaneously. The application of this technology means that large-scale problems—such as the analysis of an organism—can be solved in minutes rather than weeks. 118
The NRC report notes that these data are windows into structures of immense complexity. Biological entities (and systems consisting of multiple entities) are sufficiently complex that it may well be impossible for any human being to keep all of the essential elements in his or her head at once. Thus, advances in computational biology will be driven by the need to understand how complex biological systems operate and are controlled and will contribute fundamentally to the development of a systems view in biology.
The NRC report emphasizes that the life sciences of the future will be an information science and will “use computing and information technology as a language and a medium in which to manage the discrete, nonsymmetric, largely nonreducible, unique nature of biological systems and observations. In some ways, computing and information will have a relationship to the language of 21st century biology that is similar to the
relationship of calculus to the language of the physical sciences. Computing itself can provide biologists with an alternative and possibly more appropriate language and sets of intellectual abstractions for creating models and data representations of higher-order interactions, describing biological phenomena, and conceptualizing some characteristics of biological systems.” This potential is nowhere more evident than in the nascent field of systems biology.
Systems Biology
Systems biology—also known as integrative biology—uses high-throughput, genome-wide tools (e.g., microarrays) for the simultaneous study of complex interactions involving networks of molecules, including DNA, RNA, and proteins. It is, in a sense, classical physiology taken to a new level of complexity and detail. The term “systems” comes from systems theory or dynamic systems theory: Systems biology involves the application of systems- and signal-oriented approaches to understanding inter- and intracellular dynamic processes. 119 Systems-level problem solving in biology is based on the premise that cellular behavior is a complex system of dynamically interacting biomolecular entities. A systems biologist seeks to quantify all of the molecular elements that make up a biological system and then integrate that information into graphical network models that can serve as predictive hypotheses.
A growing number of researchers in the life sciences community are recognizing the usefulness of systems biology tools for analyzing complex regulatory networks (both inside the cell, and the regulatory networks that integrate and control the functions of distinctly different cell types in multicellular organisms like humans) and for making sense of the vast genomic and proteomic data sets that are so rapidly accumulating. 120 These efforts draw heavily on computational methods to model the biological systems, as described earlier. Systems biology is being seen as a valuable addition to the drug discovery toolbox. 121 In medicine, where disease is being viewed as a perturbation of the normal network structure of a system (i.e., disease-perturbed proteins and gene regulatory networks differ from their healthy counterparts, because of genetic or environmental influences), a systems biology approach can provide insights into how disease-related processes interact and are controlled, guide new diagnostic and therapeutic approaches, and enable a more predictive, preventive, personalized medicine. 122
This field is rapidly evolving, with the computational tools still in an immature state and inadequate for handling the reams of data derived from microarray assays and their functional correlates. Unconventional means of recording experimental results and conveying them rapidly to others in the field using an Internet-based approach are being pursued in an effort to manage the scale of data collection and analysis required for this effort. 123 Whereas scientists previously may have examined only a single facet of a signal transduction pathway involved, for example, in control of a cellular response to infection, they are now looking more broadly at the effect of a particular stimulus on multiple different pathways, including what happens at common nodes and the counter-regulatory pathways that are activated in response to a particular signal. They are coming to realize that many novel molecular mechanisms are involved in controlling these signaling pathways, not only phosphorylation and kinase activation as classically recognized in signal transduction but also specific protein conformational changes, the translocation of proteins to different cellular compartments, proteolytic cleavage of signaling partners and latent transcription factors, and the binding and release of modulatory proteins from key signaling intermediates. A similar multiplicity of mechanisms exists within the extracellular regulatory networks, that must ultimately take their cues from intracellular events. In all of these signaling networks, tremendous specificity of responses stems from the timing, duration, amplitude, and type of signal generated and the pathways from which it emanates. At present, perhaps it could be said that while the magnitude and nature of the challenge posed by systems biology are increasingly well recognized, it remains unclear exactly how these challenges will be met, or how successful such attempts to do so will be.
The rise of systems biology is expected to have profound implications for research, clinical practice, education, intellectual property, and industrial competitiveness. As computational technologies advance, simulation of complex biological systems will have more predictive accuracy, aspects of laboratory experimentation will replaced by more cost-effective computational approaches, and physicians will have new decision support tools to help them identify the best preventative and therapeutic approaches for individual genotypes and phenotypes.
Just as systems biology will profoundly alter the way scientists and physicians conduct their analyses, the same global problem-solving ap-
proach could serve as a tool for the identification of ways to deliberately manipulate biological systems with the intent to do harm.
Genomic Medicine
Genomic, or personalized, medicine refers to potential patient-tailored therapies made possible by improved molecular characterization of disease, technologies that allow for rapid genomic and proteomic analyses of individual patients, and advances in information technology that allow practitioners to access this information in meaningful ways. Scientists have known for a long time that human genetic variation is associated with many diseases and questions. With recent advances in technology that allow for quick, affordable genotypic assessments (i.e., from PCR to high-throughput sequencing), researchers have begun to understand the implications of human genetic variation for the treatment of disease. 124 Patient-tailored therapies hold forth great promise as a new way of treating, or preventing, disease and are an active area of research and investment.
Recent accomplishments in the field include the use of an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, gefitinib (i.e., Iressa), for use in the treatment of non-small cell lung cancer. Scientists have found that certain EGFR mutations may predict a patient’s sensitivity to the drug, meaning that some patients are more likely to benefit than others. Moreover, in one study the mutations (and benefits of treatment) were more prevalent among Japanese patients than U.S. patients, raising general questions about the ethnic or geographic specificity of this and other cancer drugs. 125
Herceptin provides another more publicized example of the potential for genomic medicine. In 1997, this drug became the first gene-based therapeutic licensed and marketed for use against breast cancer. Women with metastatic breast cancer whose cells produce the proteins HER2 and HER2/neu were given new hope in the form of this monoclonal antibody drug developed and manufactured by San Francisco-based Genentech. 126 Herceptin, an erbB2 monoclonal antibody, is now licensed for use in the 20 to 30 percent of breast cancer patients who overexpress this tyrosine kinase receptor. 127 Although mechanism-based cardiotoxicity has been observed, response rates of up to 60 to 70 percent have been reported for Herceptin in combination with paclitaxel or doxorubicin. 128 Similar
“proof of principle” is now emerging for the clinical activity of small-molecule inhibitors of oncogenic tyrosine kinases such as Glivec (imatinib) against chronic myeloid leukemia 129 and preclinical activity in tumor models driven by the tyrosine kinase activity of the platelet-derived growth factor and c- kit receptors. 130
Understanding and harnessing genomic variation are expected to contribute significantly to improving the health of people worldwide, including the developing world. 131 In recognition of this, Mexico is in the process of delivering one of the first genomic medicine platforms in Latin America, one that is expected to serve as a regional model for other countries in their efforts to ease health and financial burdens. The Mexican government and medical and biomedical research communities view the present time as a window of opportunity for investing in this emerging technological trend, so as to minimize the likelihood of needing to depend on foreign aid and sources in the future. 132 Likewise, genomic medicine activities in Singapore represent another national effort to gain leverage in this field. Already, high-tech manufacturing and financial services serve as the fulcrum of the Singaporean economy. Strengthening biotechnological capacity, including genomic medicine capacity, is viewed as the next high-tech step forward to accelerated economic growth. 133
Integrating personalized, or genomic, medicine into regular health care (in any country) will require overcoming two major challenges. First, it will be necessary to make the “$1,000 genome” a reality. The $1,000 genome refers to the cost of sequencing an individual’s entire genomic sequence and, although a somewhat arbitrary threshold, has come to represent the point at which the technology is finally affordable enough for widespread use. It is not clear how the $1,000 genome hurdle will be jumped, although biotech companies are trying. Some experts believe it will require a new technology. The second and arguably more significant challenge will be making the philosophical jump from the highly interventional, British-style school of medicine to a preventative, predictive health care paradigm. Genomic medicine is expected to revolutionize human medicine by altering the nature of diagnosis, treatment, and prevention. In traditional medicine, diagnosis is based on clinical criteria, treatment is population-based, and prevention is based on late-stage identification of disease. In genomic medicine, diagnosis is based on molecular criteria (e.g., the use of microarrays in cancer diagnosis), treatment is highly individualized (i.e., genomic based), and prevention is based on early-stage identification of who is at risk.
The same genomic sequences that will one day allow health care pro-
viders to identify and provide genotype-specific (and phenotype-specific) treatment may some day be exploited as targets for novel biological agents. Knowledge generated from genomic medicine could potentially be used to target specific ethnic, racial, or other population characteristics. Such weapons need not be hugely effective or even completely selective; proportional selectivity would be sufficient since, in addition to the direct effect of the weapon itself, the social tension, erosion, and (potential) fragmentation resulting from headlines of the “Mystery Virus Strikes Blacks, Spares Whites” variety would be likely to trigger effects far in excess of those from the disease itself.
While knowledge spreading from the various genome projects has fueled speculation in this area, two points should be kept in mind when considering this topic. First, the hugely large number of point mutations and other polymorphisms within the genome are not likely to lead to any selective targeting in the near future. Although techniques such as RNAi, as discussed previously, certainly have the capability to inhibit the expression of key genes with relevant single nucleotide polymorphisms (SNPs) within them, the proportion of such mutations lying in functionally important areas of the genome is small and the technical difficulties associated with exploiting them are real. Second, the idea is not new; South Africa’s Project Coast reportedly conducted experiments on vaccines designed to target fertility. 134
The technology to construct such weapons exists. For almost two decades, researchers have been using adenoviruses to target tumor cells in individuals and steadily refining their techniques for directing viral entry into cells. For example, it is now possible to modify through genetic approaches the fibers used by the virus for cellular attachment so that the virus attaches to particular cell types. 135 , 136 Studies have also shown that preferential attachment and infection of target cells can be markedly elevated. 137 , 138
Interestingly, while the availability of the complete human genome sequence has revealed numerous SNPs and other polymorphic elements—and has consequently raised greater concern about the possibility of using biological weapons to target specific racial or ethnic populations—the ability to identify 139 and exploit genetic differences among such populations does not require this new information. Adenoviruses could be used to deliver antibodies that target distinct ethnic groups with characteristic cell surface molecules, without needing to identify population-specific SNPs. 140 For example, human leukocyte antigens have distinctive distributions that vary with geographic origin (e.g., the common haplotype B8-DR3 is distributed almost uniquely among Northern European Caucasians; in the Mediterranean basin, this is replaced by B18-DR3).
Modulators of Homeostatic Systems
The stability and integrity (homeostasis) of the molecular circuits, pathways and networks responsible for diverse body functions are altered by disease and by exposure to noxious environmental pollutants and toxins (xenobiotics). The quest to identify the molecular circuits and control systems in each specialized cell type in the body, and to understand the perturbations that give rise to disease, is a dominant research theme in contemporary biology. Mapping the “molecular signatures” of the body’s biocircuits in health and disease is the primary technological catalyst in the development of new molecular diagnostic tests for detecting disease and the emergence of new disease classification schemes based on causal molecular pathologies rather than clinical symptoms. Analysis of the disease-induced perturbations in biocircuits also provides the intellectual foundation of modern drug discovery, which is based increasingly on rational design therapeutic agents directed against the specific molecular lesions responsible in disease etiology.
Burgeoning knowledge about the composition and regulation of homeostatic molecular circuits in the body’s cells, tissues, and organs, and their dysregulation in disease, epitomizes the dual-use dilemma created by rapid advances in systems biology. The life sciences are undergoing a profound transformation from their historical reliance on descriptive and phenomenological observations to now focus on the detailed underlying mechanisms of disease and identification of the “rule sets” that govern the assembly and function of biological systems in both health and disease. These insights hold great promise for future advances in medicine, agriculture, ecology, and the environmental sciences. But the very same knowledge about the homeostatic control of body biocircuits can be usurped for less beneficent intentions.
The rapid pace of research progress in revealing the detailed molecular circuit diagrams and control processes for every body function, dictates that the risk of evolution of new threats will escalate in parallel. In this context, the concept of a “biothreat agent” will expand beyond the current limited perspective of biothreats as being only “bugs” (i.e., pathogenic organisms) to include an entirely new category of threats—the biological circuit disruptors. 141
The commercial availability of large libraries of bioactive chemical compounds, together with automated high-throughput screening meth-
ods, allows the biological activities of thousands of chemical compounds to be assayed rapidly. Combinatorial chemistry and the directed evolution methods described earlier in this chapter are now used to routinely generate chemical libraries containing 10 4 to 10 7 compounds at relatively low cost (tens of thousands of dollars). In addition to “random” screening to identify compounds with the desired biological properties, robust knowledge about how the structure of a chemical compound correlates with causation of specific biological perturbations will permit increasingly accurate predictions about how the tertiary structure of bioactive molecules correlates with their affinity for, and reactivity with, specific molecular targets in the various cell lineages of the body.
The emerging field of toxicogenomics involves profiling the changes in gene and protein expression induced by chemicals found in the industrial workplace to assess potential risk from exposure to occupational and environmental hazards. The pharmaceutical industry and drug regulatory agencies such as the FDA (and their international counterparts) also have recognized the value of toxicogenomic profiling as a new tool to detect how investigational drugs might adversely affect genes important in drug metabolism or affect homeostatic genes that may lead to acute or chronic side effects. The current heightened public and legislative concern over drug safety will likely intensify pressures for the adoption of toxicogenomics as a routine part of the drug approval process. The benefits of toxicogenomics are self-evident. Once again, however, research that reveals structure-activity relationship (SAR) correlations between chemical structure(s) and specific toxicity events provide useful grist for the design of biological circuit disruptors in malevolent hands.
More robust correlations between chemical structure and therapeutic activity and absorption, distribution, metabolism, excretion, and toxicology (ADMET) properties will also come from research in the new field of chemical genomics (also referred to as chemogenomics or chemical biology). This emerging area of research seeks to establish the SAR rule of how chemical structure defines the selective interaction of different structural classes of molecules with various families of cellular proteins.
The Chemical Genomics Center, established in June 2004, by the Molecular Libraries and Imaging Implementation Group, as part of the NIH New Pathways to Discovery theme is but one example of an initiative that may eventually lead to potent new dual-use information. The center will be part of a consortium of chemical genomics screening centers to be located across the country whose purpose will be to identify small-molecule inhibitors of every important human cellular protein or signaling pathway. Part of the rationale for the chemical genomics initiative(s) is that, in contrast to researchers in the pharmaceutical industry, many academic and government scientists do not have easy access to large libraries
of small molecules (i.e., organic chemical compounds that are smaller than proteins and that can be used as tools to modulate gene function).
The database will give academic and government researchers an opportunity to identify useful biological targets and thereby contribute more vigorously to the early stages of drug development. With plans to screen more than 100,000 small-molecule compounds in its first year of operation, one of the goals of the Chemical Genomics Center network is to explore the areas of the human genome for which small-molecule chemical probes have yet to be identified. Data generated by the network will be deposited in a comprehensive database of chemical structures (and their biological activities). The database, known as PubChem, will be freely available to the entire scientific community. In addition to screening and probe data, it will list compound information from the scientific literature.
Should this come to pass, it will offer enormous opportunities for industry and academic scientists alike to pursue novel “drugable” targets in a search for small-molecule inhibitors of certain pathways that could offer substantial clinical benefit. However, the availability of information and reagents that enable one to disrupt critical human physiological systems has profound implications for the nature of the future biological and chemical threat spectrum. The difference between the NIH and industrial efforts resides in the fate of the information produced from these large-scale screening programs. Companies view their screening data and the accompanying SARs to be proprietary assets. Their data are viewed as a source of corporate competitive advantage and are not typically placed in the public domain. In contrast, the NIH data will be placed in the public domain, with the unavoidable accompanying complication of creating a rich source of SAR information that could potentially be exploited for malevolent use.
In the past, the dual-use risk of bioregulators was considered minimal because of their lack of suitability for aerosolization unless microencapsulated, their limited shelf life after atmospheric release, the fact that proteins denature at very high temperatures and lose activity at low temperatures, and high purchase costs. However, new knowledge and advancing technologies, particularly encapsulation technologies (as discussed elsewhere in this chapter), have raised concerns about the dual-use risk of bioregulators. 142
A greater understanding of how small molecules and naturally occurring bioregulatory peptides function in higher organisms will open up novel opportunities to design agents—for good or bad—that target par-
ticular physiological systems and processes, such as the brain and the immune system, in very precise ways. Scientists’ understanding of neuropeptides and their role in diverse physiological processes has advanced considerably over the last several decades. 143 This new knowledge, combined with the almost limitless size of the consumer market for pharmaceutical compounds that alleviate pain, depression, sleep disorders, and a wide range of other mental disorders, suggests that many new potentially dual-use psychoactive compounds will be discovered in the near future, including novel compounds that affect perception, sensation, cognition, emotion, mood, volition, bodily control, and alertness. 144 Several so-called “smart drugs”—brain-boosting medications that enhance memory or cognition—are already being sold or are in development. 145
4. PRODUCTION, DELIVERY, AND “PACKAGING”
The ability to manipulate “biological systems” in a defined, deliberate manner—for either beneficial or malevolent purposes—depends on the ability to produce and deliver such interventions. Technologies that allow for such production and delivery are evolving very quickly, driven by the goals and needs of the pharmaceutical, agricultural, and healthcare sectors. Some of these technologies, which clearly have immense potential future impact on biology, have not been traditionally viewed as biotechnologies or as having relevance to future biological threats. A prime example is the potential now offered by developments in nanoparticle science for the creation of novel and highly efficient delivery systems for previously difficult-to-deliver biologically-active compounds.
These technologies can be subdivided into those concerned with production, packaging, and delivery. Examples of production technologies with relevance to biology include microreactor technology (as used in the chemical engineering industrial sector), microfluidics and microfabrication technologies (e.g., currently being employed for next-generation detection tools), and transgenic plants. Examples of packaging technologies with relevance to biology include microencapsulation and nanotechnology. Examples of delivery technologies with relevance to biology include aerosol technology and gene therapy and gene vector technology.
Plants as Production Platforms—“Biopharming”
“Biopharming,” also called “molecular pharming,” is the harvest of bioactive molecules from mass-cultured organisms and crops for use as ingredients in industrial products and pharmaceuticals. Transgenic crop
plants, into which genes for bioactive compounds from other species have been inserted, serve as the basis of biopharming. (Biopharming differs from bioprospecting in that the latter is sourced in wild populations.) A novel advantage of biopharming is the crop-based production of vaccines and antibodies otherwise not possible or too expensive to produce using conventional methods. 146
As described in Chapter 1 , many different genetically engineered crop varieties with genes for therapeutic products have been developed: transgenic rice (beta carotene, human milk proteins, higher iron content, higher zinc content, low phytic acid, high phytase); transgenic potato (gene from grain amaranth for high protein content, antigens of cholera and diarrheal pathogens, and hepatitis B vaccine); transgenic maize (AIDS antigens, higher content of lysine and tryptophan, nutritive value equivalent to that of milk); transgenic fruits and vegetables (bananas, melons, brinjals [ Solanum melongena ], and tomatoes with subunit vaccines against rabies; AIDS antigens in tomatoes; and human glycoprotein in tomatoes to inhibit Helicobacter pylori against ulcers and stomach cancer); transgenic tobacco (human hemoglobin, human antibody against hepatitis B virus, and 50 percent lower nicotine), and genetically engineered coffee (decaffeinated by gene splicing). However, despite the existence of functional prototypes and evidence that the technology works, there are some technical, delivery, and regulatory challenges that are slowing progress in the field. 147
Plant manufacturing platforms may provide a cost-effective means to produce vaccines, offering the ability to address some of the problems associated with global vaccine manufacture and delivery. 148 They are also being used to experiment with plant-derived microbicides, with the goal of finding a cost-effective way to block HIV transmission, and they are being explored as a possible cost-effective way to produce antibodies for use against potential biowarfare agents. 149
However, transgenic plants could also be engineered to produce large quantities of bioregulatory or otherwise toxic proteins, which could either be purified from plant cells or used directly as biological agents. As with legitimate production, using transgenic plants as bioreactors would eliminate the need for mechanical equipment normally associated with the process. The technology would be limited to producing protein-based agents
(transgenic plants would be largely indistinguishable from nontransgenic crops), but it could potentially provide a covert means for producing large amounts of product. 150
Microfluidics and Microfabrication
Microfluidics and microfabrication are rapidly growing technologies in which a wide variety of processes and manipulations are carried out at miniaturized scales (e.g., nanoliter volumes) and in automated fashion. Microfluidic, or “lab-on-a-chip,” technology underlies many recent advances in point-of-care diagnostics, including DNA analysis, immunoassays, cell analysis, and enzyme-activity measurements. 151 Microfabrication involves building functional devices at the molecular size.
Fabricated on glass or plastic chips ranging in size from a microscope slide to a compact disk, microfluidic arrays require only very small (on the order of picoliters, 10 −12 liters) sample and reagent volumes. The most sophisticated systems are completely integrated, with sample introduction, preprocessing (e.g., cell lysis, dilution), reagent addition, and detection all conducted on the same chip. But most systems are bulkier and rely on external detector and other devices. Limitations of the current technology include reagent stability (or instability) and the need for liquid reagent reservoirs.
Microelectromechanical systems (MEMS) are a similar miniaturized technology. Unlike microfluidic systems, MEMS devices are self-contained and do not require reagents. Swallowed-capsule technology is a popular example of a MEMS: patients swallow a capsule containing all of the miniaturized equipment necessary for taking images in the gastrointestinal tract.
Nanotechnological advances are decreasing the size of microfluidic and other miniature diagnostic systems even further. For example, Biotrove, Inc. (Waltham, MA) has developed a nanoliter sample size real-time PCR machine that, when commercially available, will allow users to analyze thousands of samples simultaneously and for a much lower per-sample cost than with currently available high-throughput microarray systems. Other sampling problems come into play at smaller volumes (e.g., the small volume may not be representative of the whole sample or population). 152
As stated in a recent Science review on miniaturized diagnostic systems: “Farther down the road may be personalized health care with diagnosis and disease-monitoring occurring in the home with easy-to-use miniature devices.” Although this possibility may be farther into the future than the scope of this report covers, for regulatory as much as technical reasons, steps are being taken in this direction. For example, there have been several recent advances in convenient sampling methods, including breath and saliva sampling, that would be necessary before personalized diagnostic devices become a widely accepted component of personal health care.
Nanotechnology
Nanotechnology, which was defined in Chapter 2 , started off as little more than a clever means of making incredibly small things. In 1990, IBM scientists made headlines by painstakingly arranging 35 xenon atoms to spell out the company’s three-letter name, creating the world’s smallest corporate logo. Other scientists followed with an invisibly small “nanoguitar.” Its strings, each just a few atoms across, could be plucked by laser beams to play notes 17 octaves higher than those produced by a conventional guitar—well above the human hearing range. Novelties though they were, these feats proved that,with new tools in hand, scientists could arrange atoms as methodically as masons arrange bricks—and in doing so build materials never made in nature.
Last year alone, hundreds of tons of nanomaterials were made in U.S. labs and factories. Microscopically thin sheets of tightly woven carbon atoms are being wrapped around the cores of tennis balls to keep air from escaping; new fabrics have been endowed with nanofibers that keep stains from setting; some sunscreens have ultraviolet-absorbing nanoparticles so small they cannot reflect light, making them invisible; and tennis rackets and airplane bodies are being made with nanomaterials whose atoms have been carefully arranged to make them especially strong.
Current State of the Art 153
An intriguing feature of the nanoscale is that it is the scale on which biological systems build their structural components, like microtubules, microfilaments, and chromatin. 154 In other words, biochemistry is a nanoscale phenomenon. Even more intriguingly, a key property of these
biological structural components—including, of course, the DNA double helix—is self-assembly. In their quest to emulate these biological phenomena, scientists have created the field of DNA nanotechnology 155 and the closely related field of DNA-based computation by algorithm self-assembly. 156
Some of the most interesting nanotech research being conducted today falls within the realm of so-called DNA nanotechnology. DNA nanotechnology is the design and development of objects, lattices, and devices made of synthetic DNA. Since the DNA helix is naturally linear (i.e., unbranched), the assembly of structures or devices built with synthetic DNA requires constructing branched molecules that can then be connected to form structural networks, or motifs. The DNA motifs are combined by sticky-end cohesion, a high-specificity DNA reaction.
The DNA can be used as either “brick” and “mortar” in the construction of various kinds of nano-objects (so-called “high structural resolution DNA nanotech”) or as just mortar to join non-DNA particles (“compositional DNA nanotech”). The latter, which laboratories worldwide are involved with, can be used in many ways to organize large complexes. There are only about a dozen labs worldwide involved in high structural resolution DNA nanotech, the potential applications—which are many and varied—include architectural control and scaffolding (e.g., DNA-based computation), nanomechanical devices (e.g., nanorobotics and nanofabrication), and self-replicating nano-systems.
Self-assembling systems are completely autonomous devices that do not require the input of a person (or a robot) in order to function (i.e., as nanomechanical devices do). Last year an investigator at Purdue University made one of the first self-assembling nano-devices, in this case a DNAzyme, which can bind and cleave RNA molecules one by one. 157 Unimaginable just a couple of years ago, the creation of this device epitomizes the progress that the field of DNA nanotech has achieved in just a few years.
The future trajectory of the field, particularly the convergence of nanotechnology and molecular biology, is unclear, although it will almost certainly have multiple medical applications, including therapeutic delivery by nanoparticles. 158 In October 2004, scientists from the Institute of Bioengineering and Nanotechnology (Singapore) reported having invented a contact lens capable of releasing precise amounts of medication to treat glaucoma and other eye diseases. 159 Nanobiotechnology also promises multiple new approaches to molecular detection and diagnostics. 160
Just as nanotubes and other nanodevices promise novel advantageous means of drug delivery, there is considerable concern that the very same devices and particles could have inadvertent dangerous (i.e., toxic) consequences. Several recent studies have examined the possible toxicity of nanotechnology-derived products. 161 Likewise, the field opens up an entirely new means of potential deliberate misuse.
Aerosol Technology
Very broadly, aerosol science is an interdisciplinary field focused on the study of the presence and movement of biological particles in the earth’s atmosphere, including the impact of such particles of human populations, agriculture, and animals (including insect control). 162 The widespread aerial spraying of the Bacillus thuringiensis var. kurstaki (Btk), to protect forests from damage and defoliation caused by the spruce budworm, is a good example of how aerosol technology is being used and optimized. 163 Other examples of recent research in this field include a study on the use of animal models for understanding the threat to human health caused by inhalation of toxic airborne particulate matter; 164 a study on wind as a potential aerosolization mechanism for dispersing microorganisms at flooded wastewater irrigation sites (reuse of partially treated domestic wastewater is increasingly being done worldwide for agricultural irrigation purposes); 165 studies of plume characteristics of bioaerosols generated during the application of liquid biosolids to farmland, and the microbial risk to human health associated with this practice; 166 and studies on the aerial spraying of insecticides. 167
In biomedical research, aerosol science revolves around the study of the use of inhaled particulate matter as a means to treat human disease. Although its current widespread use is for local treatment of asthma and chronic obstructive pulmonary disease, direct administration of drugs to the respiratory tract has been effectively used or is being tested to treat bacterial lung infections, cystic fibrosis, and lung carcinoma. The effectiveness of aerosol delivery for systemic action is also being explored, as a novel, injection-free way to control pain and deliver various therapeutics for the treatment of diabetes, human growth hormone deficiency (in children), prostate cancer, and endometriosis. 168 Compared to oral delivery, advantages of aerosolized delivery 169 include its rapid speed of onset and even biodistribution. 170
In the drug delivery industry the three most common types of aerosol delivery devices currently in medical use are propellant metered-dose inhalers (pMDIs), dry powder inhalers (DPIs), and nebulizers. 171 Propellant MDIs are the most popular, since they are small, convenient, and self-powered (i.e., by the high-pressured contents of the “metering chamber”). The aerosol is drawn into a metering chamber, followed by propulsion of the solution as droplets into the lung. In the past, most pMDIs utilized suspensions or solutions of drugs in chlorofluorocarbons (CFCs). But CFC propellants are being phased out in favor of non-ozone-depleting hydrofluoroalkane inhalers. But the latter require device components and formulations that are different than those of CFC pMDIs, which has necessarily led to the creation of novel delivery means. 172
Many new DPI devices and technologies have been developed and patented since the first one was introduced in the 1960s. 173 DPIs deliver powdered dry particles into the lungs, relying on the energy produced by the forces of the inhaled airflow. Most powder products are mixtures of drug particles and large lactose carrier particles. The smaller particles are delivered to the lungs while the larger particles, which help with dispersion, are deposited on the mouthpiece. A variety of different technologies have been used in the development of DPIs, and performance varies widely among different types of inhalers. Attempts to improve the delivery of respirable dry products to the lower airways and lungs remains an active area of research. 174
Although air-jet nebulizers are inconvenient devices, due to their utilization of compressed gas (and thus requiring an air compressor) and their comparatively long aersolization time, their capability to deliver a high dose over an extended time period is widely considered an advantage over pMDIs and DPIs. 175 Nebulizers work by passing an air-jet stream (which is created by using compressed gas or piezoelectric ceramics) through a capillary tube that runs through a reservoir containing the drug; the drug solution is drawn out of the reservoir and deposited into the lungs in droplet form. In addition to their inconvenience, other limitations of the technology include the partial loss of drug dose during exhalation (since nebulizers generate aerosol continuously) and the large size of some of the devices. A variety of newer, “next-generation” nebulizers, which overcome some of these limitations, are being developed and produced.
In addition to the quality and features of the delivery device, critical to the delivery of the drugs to the lungs is the preparation of particles of correct size and shape for incorporation into aerosol products. Advances in powder technology and particle engineering play a significant role in
improving powder production and aerosol drug formulation (e.g., by improving particle dispersibility, control of particle morphology, physical and chemical stability). For example, supercritical fluid (SCF) processing has recently emerged as an alternative technology for designing particles to use in metered-dose and dry powder inhalers. 176 SCFs are substances that exist as a single phase but have the properties of both liquids and gases (at certain temperatures and pressures), and they can extract compounds from complex substrates much more quickly than liquid organic solvents can.
In addition to its pharmaceutical applications, SCF technology is being used in the food industry (for decaffeination of coffee and tea and extraction of edible oils); the flavor and fragrance industry (for extraction of aromas and flavors); the nutraceutical industry (for the extraction of active ingredients for nutraceuticals and purification of antioxidants for nutraceuticals), the paint/coating industry (for the production of small particles for paint coating applications); and for a variety of other industrial purposes (e.g., purification of natural and synthetic materials and polymers and production of small particles for explosives).
Biomedical advances in aerosol delivery technology are expected to improve drug delivery and patient adherence. Several companies are pursuing aerosolized insulin delivery as a non-invasive alternative to injectable insulin. It is widely believed that, once proven safe for prolonged use, aerosolized insulin delivery will stimulate further activity in this already very active field. Aerosol delivery is also being explored as a means of gene therapy.
Advances in drug delivery technology, including aerosol delivery, have raised concerns about the use of bioregulators for nefarious purposes. In the past, bioregulators have not generally been viewed as potential dual-use agents, largely because of the lack of effective delivery technology. 177
The dual-use risk of bioregulators was considered to be minimal due to their lack of suitability for aerosolization unless microencapsulated, their limited shelf life after atmospheric release, the fact that proteins denature at very high temperatures and lose activity at low temperatures, and high purchase costs. However, new knowledge and advancing technologies, particularly delivery technologies, have raised concerns about the dual-use risk of bioregulators. Potential delivery platforms include the use of bacterial plasmids or viral vectors for cloning the genes that encode bioregulators; use of transgenic insects (i.e., to secrete and inoculate the bioregulators); nanoscale delivery systems (e.g., engineered pro-
teins either within or bound to nanotubes); and microencapsulated delivery systems (i.e., incorporating vectors or the proteins themselves into biodegradable microspheres or liposomes for controlled release). 178 Given that anything less than 3 microns in diameter is respirable across what amounts to a 75-square-meter absorptive surface, miniaturization of respiratory delivery systems comes with considerable dual-use risk. Moreover, transgenic plants could be put to dual use as bioregulator-production factories.
Microencapsulation Technology
Microencapsulation is the envelopment of small solid particles, liquid droplets, or gas bubbles with a protective coating derived from any of a number of compounds (organic polymer, hydrocolloid, sugar, wax, fat, metal, or inorganic oxide). The capsules, which are basically miniature containers that protect their contents from evaporation, oxidation, and contamination and can be engineered with any of a variety of unique release mechanisms (e.g., from controlled, delayed, targeted release to biodegradable or salt-induced release), have countless applications.
Microencapsulation is not a new technology. Between the late 1940s and early 1960s, the concept of chemical microencapsulation generated interest in the pharmaceutical industry as an alternative mode of drug delivery that could offer sustained controlled release. Researchers and entrepreneurs continue to utilize and investigate advances in microencapsulation technology in efforts to make dosages more palatable, make active ingredients more stable and/or soluble, and otherwise improve drug delivery. 179 In the decades since the technology first emerged, many other life sciences industrial sectors have benefited tremendously from non-pharmaceutical applications of microencapsulation. In fact, it was partly in response to potential agrochemical applications of encapsulation technology that the Controlled Release Society, an international organization with 3,000 members from more than 50 countries, was formed in the mid-1970s (microencapsulation is the most common but not the only form of controlled release). As defined on its Web site, controlled release is “the field of scientific activity concerned with the control in time and space of the biological effects of therapeutic agents in human and animal health, and of other active agents in environmental, consumer and industrial applications.” 180
According to data provided by the Southwest Research Institute, the number of U.S. patents for encapsulation processes increased from about 1,250 during 1976-1980 to about 8,500 during 1996-2001. 181 U.S. patents
for nanoencapsulation grew from near zero to about 1,000 over the same time period.
There are two general categories of microencapsulation processes: physical (e.g., spray drying, fluid bed coating, co-extrusion, rotary disk atomization) and chemical (e.g., polymerization, phase separation, solvent evaporation, coacervation). Between 1996 and 2002, polymerization was the most commonly used process, based on U.S. patent data, followed by spray drying and rotary disk atomization.
Today, microencapsulation technology is being used in water treatment (to remove emulsified oils, heavy metals, phosphates, and suspended solids from wastewater), food and agriculture (to improve taste and mask odor; stabilize thermal, oxidative, and shelf-life properties of ingredients; and allow for more effective absorption of nutrients and vitamins), and in the cosmetics industry (to create “eye appeal” or a specific or special feel in a wide range of personal care products). It has also been used as a way to manage mercury-contaminated and other hazardous wastes. 182
Examples of recent use and exploration of this technology include an investigation by University of Saskatchewan researchers into the use of microencapsulated engineered cells as an alternative approach to cancer treatment. 183 The cells had been engineered to release a compound that kills tumor cells (i.e., functional necrosis factor-alpha). Implantation of encapsulated cells (into a mouse model system) led to tumor regression and slower tumor growth. In another study, researchers from the Netherlands tested the release, upon chewing, of flavored microencapsulates in Gouda cheese (the microencapsulates contained sunflower oil, lemon, and orange oil flavors). 184 Japanese researchers recently demonstrated the use of a novel nanoencapsulation drug delivery method for the external treatment of photo-damaged skin. 185 Advanced BioNutrition Corporation (Columbia, Maryland) was recently awarded a National Science Foundation grant to further develop its proprietary microencapsulation technology for the incorporation of functional ingredients—such as enzymes, fatty acids, probiotics, even vaccines—into its animal and human food products. 186 The company will use the money to scale up its microencapsulation technology production process.
An exciting future application is the transplantation of encapsulated live cells for therapeutic purposes. 187 Other future applications range from
teddy bears that release a scent to help children sleep to novel military applications. In January 2005, for example, Northrop Grumman (San Diego, CA) announced the development of new encapsulation technology that allows non-marinized 188 weapons and vehicles to be released by submarines.
Gene Therapy Technologies
Gene therapy is an experimental technique that uses “healthy” genes to treat or prevent disease. In most gene therapy studies, a “normal” gene is inserted into the genome to replace an “abnormal,” disease-causing gene. A carrier molecule—called a vector—must be used to deliver the “healthy” gene to a recipient’s target cells. Currently, the most commonly used vectors are viruses (including retroviruses, adenoviruses, adeno-associated viruses, and herpes simplex viruses) that have been genetically altered to carry normal human DNA. Nonviral options for gene delivery include the direct introduction of therapeutic DNA into target cells, although direct administration can only be used with certain tissues and requires large amounts of DNA (see Figure 3-4 ).

FIGURE 3-4 Viral vectors.
SOURCE: James Benjamin Petro, presentation to the committee, February 2004.
State of the Art
Gene therapy is still experimental, and most of the research performed to date has been conducted in animal trials (from rodents to primates). For example, in a study that appeared in Nature Medicine in March 2005, using a guinea pig model system, researchers from the University of Michigan and Kansai Medical University, Japan, reported that they had used gene therapy to restore hearing in mature deaf animals. 189 The evidence suggests that gene therapy can be used to regenerate functional hair cells, which are necessary to restore hearing, by using (in this case) an adenovector to deliver the “healthy” gene into nonsensory cells that reside in the deaf cochlea. The introduced gene, Atoh1 (also known as Math1), encodes a basic helix-loop-helix transcription factor and key regulator of hair cell development. Upon delivery, hearing is substantially improved.
The few human clinical trials that have been conducted have not been as successful as originally hoped. 190 Although substantial progress has been made, and some clinical successes seem to be on the horizon, further vector refinement and/or development is required before gene therapy will become standard care for any individual disorder.
When gene therapy does become a clinical reality, it will be used to correct faulty or defective disease-causing genes. But just as it will be used to delivery “healthy” genes into cells and tissues, gene therapy could potentially be used to deliver harmful genes.
Targeting Biologically-Active Materials to Specific Locations in the Body
The efficacy and safety of medical drugs, imaging agents, and vaccines depend on the ability to deliver these agents to the right location in the body and, ideally, with precision targeting only to the cells of interest. Selectivity in drug delivery reduces the exposure of nontarget tissues to the drug, thereby reducing the risk of unwanted drug actions and adverse events. However, this obvious therapeutic need is far from easy to achieve in practice. Selective targeting of bioactive molecules remains a largely unfulfilled objective in clinical therapeutics. The pharmaceutical and biotechnology industries, including companies that specialize only in the design of ways to optimize drug delivery, are investing substantial sums in research and development to achieve this attractive, yet elusive, goal.
Considerable ingenuity has been exhibited in designing “targeting”
vehicles and “homing” systems for precision delivery of drugs and imaging agents. These range from efforts to deliver materials to specific zones in the body (e.g., aerosol delivery to the lungs, selective drug delivery to different regions of the gastrointestinal tract) to the more challenging objectives of targeted delivery to specific cell types (e.g., cancer cells versus their normal counterparts) or delivery of a drug or other bioactive agents to a specific compartment inside the cell (e.g., nuclear uptake of genes into chromosomal DNA for gene therapy or targeted therapeutic ablation of deleterious genes).
A broad repertoire of targeting vehicles have been examined in this research effort. These include carrier particles containing encapsulated drugs (e.g., liposomes, nanoparticles, dendrimers); exploitation of the “homing” ability of microorganisms to bind selectively to specific cells (e.g., viruses or bacteria as vectors for targeted delivery of genes and proteins); and the coupling of drugs to cognate carrier molecules designed to recognize only the desired cell type and then release their therapeutic payload. A unifying theme linking these different approaches lies in engineering suitable “molecular recognition” systems whereby cognate molecules in/on the carrier system recognize and attach to molecules expressed exclusively on the desired target cell, tissue, or organ. Additional cognate molecular interaction systems can be designed to enhance the efficiency of drug uptake by cells once selective targeting has occurred and for directing the delivered drug or gene to the correct location inside the cell.
Two different technical approaches underpin technical strategies for targeted drug delivery. The first incorporates the targeting (homing) property into the drug itself so that it will interact only with target cells that bear a “receptor” molecule that recognizes a structural region (domain) on the drug molecule. In the second approach the cognate properties required for recognition and binding to target cells are engineered into a drug carrier rather than the drug itself. Drugs are associated with the carrier either via passive encapsulation (e.g., particulate carriers) or by chemical coupling to the carrier. Both approaches exploit cognate molecular interactions as a common design principle. Targeted delivery is achieved as a consequence of molecular recognition events that limit the interaction of the drug and/or the drug carrier to only those cells that express a specific molecular determinant that interacts with the drug or drug-carrier complex.
As emphasized in the earlier section on how knowledge of the body’s biocircuits can be used for both constructive and abusive purposes, the technical platforms for precision drug targeting pose a similar dual-use problem. Knowledge of how to target bioactive materials to specific cells can be usurped to disrupt or destroy vital functions in humans, animals,
or plants. However, the technical ease with which such assaults could be mounted will be influenced by the location of the target in the targeted host and the anatomic barriers that a targeting system must breach in order to reach its molecular locus of action.
One of the more concerning assaults, yet attainable even with today’s delivery technology, could arise from the use of targeted delivery systems to insert genes into chromosomal DNA. For example, viral delivery vectors developed for human gene therapy exploit the ability of viruses to bind selectively to specific cell types as a way to deliver genes encapsulated inside the viral particle into the target cells. The question of whether these viral delivery systems are applied to beneficent or malevolent goals is defined solely by the nature of the genetic payload incorporated into the vector. Although therapeutic gene therapy has yet to attain routine clinical utility, the extensive research literature on gene transfection technologies using viruses and various particulate carriers has demonstrated the feasibility of inserting exogenous genes in multiple cell types in the body. Future improvements in the efficiency of these delivery technologies can be confidently expected, with accompanying expansion in the horizons of both therapeutic and nefarious utility.
The delivery and expression of genes that code for the uncontrolled production of highly potent hormones and other natural bioactive mediators involved in homeostasis offer the simplest example of how this knowledge could be abused and used to expand the emerging threat spectrum. Alternatively, rather than using a transfected gene directly to produce a bioactive product to perturb body function, the transfected gene could act as a trigger for the abnormal expression or destruction of other genes vital to body homeostasis. In either of these settings the introduced gene is designed to integrate into the chromosomal DNA of the host. The disruptive effects could be manifest immediately as an acute event or the gene could lie silent in the genome for activation at a later time by a second external trigger.
An aphorism frequently cited in the design of drug delivery systems is that “the opportunities are limited only by the imagination of the inventor.” Theoretically, the ability to design drugs and carrier vehicles endowed with cognate molecular properties that enable them to home selectively to the desired target in the body is limited only by the availability of suitable molecular recognition molecules that can be incorporated into the delivery system to confer recognition and binding by molecules unique to the desired target cell. The availability of relevant molecular cognate pairs for the delivery system and for the target is an obligate prerequisite for targeting. However, this is but one component in the engineering of targeted delivery systems. For therapeutic applications, the tar-
FIGURE 3-5 Converging technologies. Biotechnology, nanotechnology, and information technology are converging in ways that will enable humans to do things never dreamt of until now.
SOURCE: Michael Morgan, presentation during the Cuernavaca workshop, September 2004.
geting system must also exhibit suitable absorption, distribution, metabolism, excretion, and toxicology (ADMET) properties.
Complementarity and Synergy of Technologies
Some futurists consider the convergence of bio-, nano-, and information technologies, along with the neuro- and cognitive sciences, a transformation that will prove as powerful as the Industrial Revolution ( Figure 3-5 ). However, the details and impact of possible convergent events are unclear at this time.
Enabling technologies are those that interact with each other to create novel products that would otherwise be impossible to achieve. Nanotechnology enables other technologies by providing a common hardware for molecular engineering and allowing for the realization of desirable architectures. Nanotechnology enables biotechnology by developing new imaging techniques, probes, and sensors; it also contributes to the miniatur-
ization demands of information technologies. Biotechnology enables other technologies by identifying chemical and physical processes and algorithmic structures in living systems that have a genetically based material organization. It enables nanotechnology by providing a paradigm that nanotechnologists use in developing systems; much of the work in nanotechnology involves mimicking biotechnological processes while simultaneously redesigning them to fit particular purposes. Biotechnology enables information technology by providing new systems of computing, some of which may be based on DNA. Information technology enables other technologies through its ability to represent physical states as information and model processes. It provides the computing power that is essential to all research; it enables nanotechnology through precision control of patterning and intervention; and it enables biotechnology by providing the means to model complex processes and thereby solve difficult research problems.
In addition to convergence, which leads to the emergence of entirely new disciplines such as DNA nanotechnology and bioinformatics, technologies combine and converge on a smaller, less dramatic scale all the time. In terms of future potential threats, one should note the importance of combinations or interactions involving technologies in any of the first three categories—the acquisition of biological or molecular diversity, directed design, and understanding and manipulation of biological systems—and technologies in the fourth category: production, delivery, and packaging. In other words, the impact, both beneficial and detrimental, of a small-molecule agent, synthetic agent, or an agent bred through “DNA shuffling” is enhanced by appropriate packaging and delivery. Indeed, growing concerns about the dual-use risk of bioregulators are partly in response to advances in microencapsulated delivery systems, which make the use of bioregulators for either beneficial or nefarious purposes more feasible.
Based on extensive deliberations on a wide range of advancing technologies with relevance to the life sciences, including many technologies and fields of knowledge not traditionally viewed within the rubric of bio technology, the committee was particularly struck by the extent to which various tools and technologies are interacting and converging 191 —both additively and synergistically—and creating unanticipated opportunities for these technologies to be used for either beneficial or malicious intent (or with beneficial intent but unintended consequences). As already mentioned, the convergence of nanotechnology and molecular biology serves as a prime example of how an entirely new discipline, DNA nanotechnology, can emerge unexpectedly and with profound consequences. Nanotechnology is also merging with encapsulation and micro-
fluidic technologies, providing the means for further miniaturization of already very low-volume biological sampling, detection, delivery, and other processes.
As one example, synthetic biologists are using their new tools in conjunction with nanotechniques to program cells with decision-making therapeutic power. For example, researchers have designed a prototype “DNA computer” with the capacity to logically analyze mRNA disease indicators in vitro (i.e., in this case, early signs of prostate and lung cancer) and control the administration of biologically active ssDNA molecules, including drugs. 192 The procedure is relatively innocuous, requiring the injection of a very small amount of fluid containing billions of nanoparticles, each of which operates as a tiny computer by effectively interrogating the cell and detecting the presence of diagnostic DNA markers (e.g., mutated mRNA sequences or underexpressed or overexpressed mRNA). If the markers are present, the nanoparticle sends out a therapeutic short nucleic acid that can affect the levels of gene expression.
The field of bioinformatics represents another key example of converging technologies—in this case biology, computer science, and information technologies—all of which have merged to form what is now a single discipline. Over the past 10 years, major advances in the field of molecular biology, coupled with advances in genomic technologies, have led to an explosive growth in biological information generated by the life sciences community. This deluge of genomic information, in turn, has led to an absolute requirement for computerized databases to store, organize, and index the data and for specialized tools to view and analyze the data. These databases and tools comprise the field of bioinformatics. Increasingly, biological studies begin with a scientist surveying databases to formulate specific hypotheses or design large-scale experiments, representing a dramatic shift in biology from a purely lab-based to an information-based science. Moreover, the growing availability of vast amounts of biological and other relevant information (e.g., small-molecule libraries) will also allow nonspecialists to tinker with or design constructs that, in the past, would have required years of education or training.
“During the century just begun, as our ability to modify fundamental life processes continues its rapid advance, we will be able not only to devise additional ways to destroy life but will also be able to manipulate it—including the processes of cognition, development, reproduction, and inheritance.”—Matthew Meselson 193
It is difficult to predict what the global technology landscape will look like in 20, 10, or even 5 years into the future. But it is not difficult to anticipate that as advances are made, so too will opportunities for misuse. This chapter summarizes information on emerging technologies that are expected to have significant economic, societal, and dual-use risk impacts in the near future. As highlighted during the committee’s international workshop in Cuernavaca, prominent among these are advances in knowledge and delivery technology that have increased the dual-use potential and risk of nonlethal bioregulators and the convergence of nano- and biotechnology in the form of DNA nanotechnology.
A major theme that emerged from the committee’s deliberations in Mexico was the notion that pathogens are not the only problematic agents of biological origin. Some argue that bioregulators, 194 which are nonpathogenic organic compounds, may pose a more serious dual-use risk than had previously been appreciated, particularly as improved targeted delivery technologies have made the potential dissemination of these compounds much more feasible than in the past. This shift in focus highlights the reality that the materials, equipment, and technology necessary for disseminating and delivering the agents to their intended recipient(s) are equally, if not more, important than the agents themselves in terms of their dual-use risk.
The immune and neuroendocrine systems 195 are particularly vulnerable to bioregulator modification. In fact, the capacity to develop bioweapons that can be aimed at the interaction of the immune and neuroendocrine systems again points to a shift in focus from the agents to, in this case, how a range of agents can be exploited (or created) to affect the human body in targeted, covert, and insidious ways.
A controversial issue that arose from these discussions is how all research on immune system evasion could be considered potentially dangerous, thus highlighting the very important need to uphold the norms of the Biological and Toxin Weapons Convention. Another important theme that emerged from discussions of the material presented here is the notion of time and how the advancing technology landscape has an uncertain future and unpredictable dual-use risk implications. This unpredictability poses a significant challenge for developing and implementing a strategy to manage these risks. These challenges—and potential solution sets—are discussed in the following chapter.
|
| Stemmer, W.P. 1994. Rapid evolution of a protein in vitro by DNA shuffling. 370(6488):389-391. |
|
| With approximately 10 microorganisms on earth today, even with a 10- |
|
| minute fission time, only about 10 have existed over the history of the earth, which is tiny compared to the number of possible 10 base pair DNA sequences. |
|
| See discussion of virulence and evolution of pathogens in . |
|
| Agarwal, K.L., et al. 1974. Total synthesis of the gene for an alanine transfer ribonucleic acid from yeast. 227(5253):27-34. |
|
| Cello, J., et al. 2002. Chemical synthesis of poliovirus cDNA: generation of infectious virus in the absence of natural template. 297(5583):1016-1018. |
|
| Smith, H.O., C.A. Hutchison, III, C. Pfannkoch, and J.C. Venter. 2003. Generating a synthetic genome by whole genome assembly: phiX174 bacteriophage from synthetic oligonucleotides. 100(26): 15440–15445. |
|
| Wade, N. 2005. “A DNA success raises bioterror concern. (January 12). Many experts in the field consider this view alarmist, since not only is the smallpox virus longer, but it cannot self-generate from its nucleotide sequence alone. |
|
| Carlson, R. 2003. The pace and proliferation of biological technologies. 1(3):203-214. |
|
| Carr, P.A., et al. 2004. Protein-mediated error correction for de novo DNA synthesis. 32(20); Richmond, K.E., et al. 2004. Amplification and assembly of chip-eluted DNA (AACED): a method for high-throughput gene synthesis. 32(17):5011–5018; Tian, J. et al. 2004. Accurate multiplex gene synthesis from programmable DNA microchips. 432(7020):1050-1054. |
|
| Tian, J. et al. 2004. Accurate multiplex gene synthesis from programmable DNA microchips. 432(7020):1050-1054. |
|
| Carr, P.A. et al. 2004. Protein-mediated error correction for DNA synthesis. 32(20):e162. |
|
| Elowitz, M.B. and S. Leibler. 2000. A synthetic oscillatory network of transcriptional regulators. 403(6767):335-338. |
|
| Martin, V.J., et al. 2003. Engineering a mevalonate pathway in for production of terpenoids. 21(7):796-802. |
|
| Hutchinson, C.A., et al. 1999. Global transposon mutagenesis and a minimal genome. 286(5447):2165-2169. |
|
| Martin, V.J. et al. 2003. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. 21(7):796-802. |
|
| Church, G. 2004. A synthetic biohazard non-proliferation proposal. Updated May 21, 2005. Available online at [accessed January 5, 2006]. The Alfred P. Sloan Foundation recently funded an joint activity by the Massachusetts Institute of Technology, the Venter Institute, and the Center for Strategic and International Studies to examine the benefits and risks of synthetic genomics and develop and analyze policy options for governance of the relevant technologies. A press release issued by the three institutions describing this study may be found online at . |
|
| Mann, C.C. 1999. Crop scientists seek a new revolution. 283(5400): 310-314. |
|
| Crameri, A. et al. 1998. DNA shuffling of a family of genes from diverse species accelerates directed evolution. 391(6664):288-291. |
|
| Ness, J.E. 1999. DNA shuffling of subgenomic sequences of subtilisin. 17(9):893-896. |
|
| Zhang, Y.Z. et al. 2002. Genome shuffling leads to rapid phenotypic improvement in bacteria. 415(6872):644-646. |
|
| Pekrun, K. et al. 2002. Evolution of a human immunodeficiency virus type 1 variant with enhanced replication in pig-tailed macaque cells by DNA shuffling. 76(6):2924-2935. |
|
| Leong, S.R. et al. 2003. Optimized expression and specific activity of IL-12 by directed molecular evolution. 100(3):1163-1168. |
|
| Soong, N.W., et al. 2000. Molecular breeding of viruses. 25 (4):436-439; Powell, S.K. et al. 2000. Breeding of retroviruses by DNA shuffling for improved stability and processing yields. 18(12):1279-1282. |
|
| Much of the information in this section is adapted from Strobel, G. and B. Daisy. 2003. Bioprospecting for microbial endophytes and their natural products. 67(4):491-502. Available online at , [accessed March 24, 2005]. |
|
| Grabley, S. and R. Thiericke, eds. 1999. Berlin: Springer-Verlag; 3-33. |
|
| Concepcion, G.P. et al. 2001. Screening for bioactive novel compounds. In Pointing, S.B. and K.D. Hyde, eds. 2001. Hong Kong: Fungal Diversity Press; 93-130 |
|
| Wani, M.C. et al. 1971. Plant antitumor agents, VI. The isolation and structure of taxol, anovel antileukemic and antitumor agent from Taxus brevifolia. 93:2325-2327. |
|
| Pace, N.R. 1997. A molecular view of microbial diversity and the biosphere. 276(5313):734-740; Venter, J.C. et al. 2004. Environmental genome shotgun sequencing of the Sargasso Sea. 304(5667):66-74. |
|
| Demain, A.L. 2000. Microbial natural products: a past with a future. In Wrigley, S.K., M.A. Hayes, R. Thomas, E.J.T. Chrystal, and N. Nicholson, eds. . The Royal Society of Chemistry, Cambridge, United Kingdom; 3-16. |
|
| Findlay, J.A. et al. 1997. Insect toxins from an endophyte fungus from wintergreen. 60:1214-1215. |
|
| Strobel, G. and B. Daisy. 2003. Bioprospecting for microbial endophytes and their natural products. 67(4):491-502. |
|
| Lorenz, P. and J. Eck. 2005. Metagenomics and industrial applications. 3(6):510-516. |
|
| Tyson, G.W. et al. 2004. Community structure and metabolism through reconstruction of microbial genomes from the environment. 428(6978):37-43; Venter, J.C. et al. 2004. Environmental genome shotgun sequencing of the Sargasso Sea. 304(5667):66-74; Tringe, S.G. et al. 2005. Comparative Metagenomics of Microbial Communities. 308(5721):554-557. |
|
| This search for novel microbial genomes to identify useful products is |
|
| achieved through the use of laboratory methods and queries of bioinformatics “libraries.” |
|
| Marshall, W.F. 3rd et al. 1994. Detection of DNA in museum specimines of 170:1027-1032; Mills, J.N. et al. 1999. Long-term studies of hantavirus reservoir populations in the southwestern United States: A synthesis. 5(1):135-142; Monroe, M.C. et al. 1999. Genetic diversity and distribution of borne hantaviruses in North America. 5(1):75-86. |
|
| Relman, D.A. 2002. Mining the natural world for new pathogens. 67(2):133-134. |
|
| Parola, P. et al. 2002. First molecular evidence of new s in fleas and a tick from Peru. 67(2):135-136. |
|
| Breitschwerdt, E.B. and Kordick, D.L. 2000. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. 13(3):428-438. |
|
| In higher eukaryotes, biological processes such as cellular growth and organogenesis are mediated by differential gene expression. To understand molecular regulation of these processes, differentially expressed genes of interest must be identified, cloned, and studied in detail. Subtractive cDNA hybridization has been a powerful tool in the identification and analysis of differentially expressed cDNAs. See . |
|
| Merrifield, R.B. 1963. Solid phase peptide synthesis: the synthesis of a tetrapeptide. 85:2149-2154. |
|
| A more detailed understanding of how the technology works requires understanding the basic chemistry of polypeptide formation: the general chemical formula for amino acids is H NCH(R)CO H. Amino acids can be linked together to form peptides by reacting the −NH group of one amino acid with the −CO H group of another, thus forming an amide bond. Solid-phase synthesis involves reacting the −CO H group with a CH Cl group on the resin, thereby leaving the −NH group free to form an amide bond with the second amino acid. The second amino acid is structurally modified, prior to mixing with first amino acid, in order to render its −NH group incapable of participating in an amide-forming reaction. The now protected second amino acid is added to the reaction mixture and a dipeptide, attached to the solid support, is created. The protecting group of the new dipeptide is removed, and a third protected amino acid is added to the mixture, resulting in a tripeptide. The process is continued until the desired product is created. |
|
| Geyson, M.H. et al. 1984. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. 81(13):3998-4002. |
|
| Houghton, R.A. 1985. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. 82(15): 5131-5135. |
|
| The general approach devised by Geyson and Houghten was modified further in the early 1990s, when Kit Lam developed a rapid method for producing and evaluating random libraries of millions of peptides. Initially applied to pep- |
|
| tides, solid phase synthesis was gradually extended to produce libraries of druglike small molecules, which were of greater interest to the drug discovery industry. In the early 1990s, Jonathan A. Ellman, University of California, Berkeley, used Geyson’s multi-pin approach to create a library of 192 structurally diverse benzodiazepines. Concurrently, Sheila H. DeWitt, then at Parke-Davis Pharmaceutical Research, Michigan, reported a technique and apparatus for the multiple, simultaneous synthesis of so-called “diversomers” (collections of organic compounds, including dipeptides, hydantoins, and benzodiazepines). These studies represented some of the earliest techniques for generating small molecule libraries. |
|
| Sanchez-Martin, R.M. et al. 2004. The impact of combinatorial methodologies on medicinal chemistry. 4(7): 653-669. |
|
| Needles, M.C. et al. 1993. Generation and screening of an oligonucleotide-encoded synthetic peptide library. 90(22):10700-10704. |
|
| Ohlmeyer, M.H.J. et al. 1993. Complex synthetic chemical libraries indexed with molecular tags. 90(23):10922-10926; Moran, E. J., et al. 1995. Radio frequency tag-encoded combinatorial library method for the discovery of tripeptide-substituted cinnamic acid inhibitors of the protein tyrosinase phosphatase PTP1B. 117(43):10787-10788; Nicolau, K.C. et al. 1995. Radiofrequency encoded combinatorial chemistry. 34(20): 2289-2291. |
|
| Reader, J. C. 2004. Automation in medicinal chemistry. 4(7):671-686. |
|
| Matzger, A.V. et al. 2000. Combinatorial approaches to the synthesis of vapor detector arrays for use in an electronic nose. 2(4):301-304. |
|
| Wheelis, M. 2002. Biotechnology and biochemical weapons. Spring:48-53. |
|
| Austin, C.P., L.S. Brady, T.R. Insel, and F.S. Collins. 2004. NIH Molecular Libraries Initiative. 306(5699):1138-1139. |
|
| See also, discussion of this issue in “The NIH Roadmap.” |
|
| Major Histocompatibility Complex (protein complexes that present antigens to lymphocytes). |
|
| Berman, H.M., J. Westbrook, Z. Feng, G. Gilliland, T.N. Bhat, H. Weissig, I.N. Shindyalov, and P.E. Bourne. 2004. The Protein Data Bank. 28(1):235-242. See [accessed January 5, 2006]. |
|
| The Smallpox Research Grid project distributed a screensaver to thousands of home computer owners to perform these calculations to identify drugs that might interfere with the enzyme that unwinds variola DNA to permit replication. The project is described at . (Altogether over 39,000 years of computer time were devoted to the project in less than six months, screening 35 million molecules against eight models of the target protein.) |
|
| Yokobayashi, Y. et al. 2002. Directed evolution of a genetic circuit. 99(26):16587-16591. |
|
| Registry of Standard Biological Parts. The Endy Lab, Massachusetts Institute of Technology. See [accessed January 5, 2006]. |
|
| Elowitz, M.B. and S. Liebler. 2000. A synthetic oscillatory network of transcriptional regulators. 403(6767):335-338. |
|
| Atkinson, M.R. et al. 2003. Development of genetic circuitry exhibiting toggle switch or oscillatory behavior in 113(5):597-607. |
|
| Looger, L.L. et al. 2003. Computational design of receptor and sensor proteins with novel functions. 423 (6936):185-90; DeGrado, W.F. 2003. Biosensor Design. 423(6936):132-133. |
|
| Benenson, Y. et al. 2004. An autonomous molecular computer for logical control of gene expression. 429(6990):423-429. |
|
| Ferber, D. 2004. Microbes made to order. 303(5655):158-161. |
|
| The Center for Strategic & International Studies (CSIS), the J. Craig Venter Institute (Venter Institute), and the Massachusetts Institute of Technology (MIT) have initiated a project, funded by the Alfred P. Sloan Foundation, to examine the societal implications of synthetic genomics, exploring risks and benefits as well as possible safeguards to prevent abuse, including bioterrorism. See further description online at . |
|
| Racaniello, V.R. and Baltimore, D. 1981. Cloned poliovirus complementary DNA is infectious in mammalian cells. 214(4523):916-919. |
|
| Ahlquist, P. et al. 1984. Multicomponent RNA plant virus infection derived from cloned viral cDNA. 81(22):7066–7070. |
|
| Rice, C.M., et al. 1989. Transcription of infectious yellow fever RNA from full-length cDNA templates produced by in vitro ligation. 1(3):285-296. |
|
| Rice, C.M. et al. 1987. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. 61(12): 3809–3819. |
|
| Satyanarayana, T. et al. 1999. An engineered closterovirus RNA replicon and analysis of heterologous terminal sequences for replication. 96(13):7433-7438. |
|
| Van Dinten, L.C. et al. 1997. An infectious arterivirus cDNA clone: Identification of a replicase point mutation that abolishes discontinuous mRNA transcription. 94(3):991–999. |
|
| Masters, P.S. 1999. Reverse genetics of the largest RNA viruses. 53:245-64. |
|
| Almazán, F., et al. 2000. Engineering the largest RNA virus genome as an infectious bacterial artificial chromosome. 97(10):5516–5521. |
|
| Yount, B., et al. 2003. Reverse genetics with a full-length infectious cDNA of severe acute respiratory syndrome coronavirus. 100(22):12995–13000. |
|
| Negative-stranded RNA viruses have a genome consisting of one or more molecules of single-stranded RNA that is of opposite polarity (i.e., complementary) to the positive-sense mRNA that encodes their proteins. |
|
| Enami, M. et al. 1990. Introduction of site-specific mutations into the genome of influenza virus. 87(10):3802–3805; Luytjes, M. et al. 1989. Amplification, expression, and packaging of foreign gene by influenza virus. 59(6):1107-1113. |
|
| Fodor, E. et al. 1999. Rescue of influenza A virus from recombinant DNA. 73(11):9679–9682; Neumann, G. et al. 1999. Generation of influenza A viruses entirely from cloned cDNAs. 96(16): 9345-9350. |
|
| Hatta, M. et al. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. 293(5536):1840-1842. |
|
| Fodor, E., et al. 1999. Rescue of influenza A virus from recombinant DNA. 73(11):9679-9682. |
|
| Schnell, M.J. et al. 1994. Infectious rabies viruses from cloned cDNA. 13(18):4195-4203. |
|
| Lawson, N.D. et al. 1995. Recombinant vesicular stomatitis virus from DNA. 92(10):4477–4481;Whelan, S.P. et al. 1995. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. 92(18):8388-8392. |
|
| Collins, P.L. et al. 1995. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5¢ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. 92(25):11563-11567; Jin H. et al. 1998. Recombinant human respiratory syncytial virus (RSV) from cDNA and construction of subgroup A and B chimeric RSV. 251(1):206-214. |
|
| Radecke, F. et al. 1995. Rescue of measles virus from cloned DNA. 14(23):5773-5784. |
|
| Garcin, D. et al. 1995. A highly recombinogenic system for the recovery of infectious Sendai paramyxovirus from cDNA: generation of a novel copy-back non-defective interfering virus. 14(24):6087; Kato, A. et al. 1996. Initiation of Sendai virus multiplication from transfected cDNA or RNA with negative or positive sense. 1(6):569-579. |
|
| Durbin, A.P. et al. 1997. Recovery of infectious human parainfluenza virus type 3 from cDNA. 235(2):323–332; Hoffman, M.A. and A.K. Banrjee. 1997. An infectious clone of human parainfluenza virus type 3. 71(6):4272-4277. |
|
| Baron, M.D. and T. Barrett. 1997. Rescue of rinderpest virus from cloned cDNA. 71(2):1265-1271. |
|
| He, B. et al. 1997. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. 237(2):249-260. |
|
| Buchholz, U.J. et al. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. 73(1):251-259. |
|
| Peeters, B.P.H. et al. 1999. Rescue of Newcastle disease virus from cloned cDNA: evidence that cleavability of the fusion protein is a major determinant for virulence. 73(6):5001-5009. |
|
| Bridgen, A. and R. Elliott. 1996. Rescue of a segmented negative-strand RNA virus entirely from cloned complementary DNAs. 93(26):15400-15404. |
|
| Kobasa, D. et al. 2004. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. 431(7009):703-707. |
|
| Tumpey, T.M., C.F. Basler, P.V. Aguilar, H. Zeng, A. Solórzano, D.E. Swayne, N.J. Cox, J.M. Katz, J.K. Taubenberger, P. Palese, and A. García-Sastre. 2005. Characterization of the Reconstructed 1918 Spanish Influenza Pandemic Virus. 310(5745):77-80; Taubenberger, J.K., A.H. Reid, R.M. Lourens, R. Wang, G. Jin and T.G. Fanning. 2005. Characterization of the 1918 influenza virus polymerase genes. 437(7060):889-893. |
|
| Kaiser, J. 2005. Resurrected influenza virus yields secrets of deadly 1918 pandemic. 310(5745):28-29. |
|
| Ibid. |
|
| Sharp, P.A. 2005. 1918 flu and responsible science. 310(5745):17. |
|
| Snijder, E.J. et al. 2003. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. 331(5):991-1004; Yount, B. et al. 2003. Reverse genetics with a full-length infectious cDNA of severe acute respiratory syndrome coronavirus. 100(22):12995–13000. |
|
| Krug, R.M. 2003. The potential use of influenza virus as an agent for bioterrorism. 57(1-2):147-150. |
|
| “Systems biology” is not a technology in the classic sense. It is an attempt to draw many disparate technologies together in the service of a new field, or perhaps in a new way of doing biology. |
|
| Napoli, C. et al. 1990. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. 2(4):279-289. |
|
| Jackson, A.L. et al. 2003. Expression profiling reveals off-target gene regulation by RNAi. 21(6):635-37; Scacheri, P.C. et al. 2004. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. 101(7):1892-1897. |
|
| Scherr, M. et al. 2003. Inhibition of GM-CSF receptor function by stable RNA interference in a NOD/SCID mouse hematopoietic stem cell transplantation model. 13(5):353-363. |
|
| Song, E. et al. 2003. RNA interference targeting Fas protects mice from fulminant hepatitis. 9(3):347-351. |
|
| Reich, S.J. et al. 2003. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. 9:210-216. |
|
| Zhang, X. et al. 2004. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. 279(11)10677-10684. |
|
| Dorn, G. et al. 2004. siRNA relieves chronic neuropathic pain. 32(5):e49. |
|
| See |
|
| Soutschek, J. et al. 2004. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. 432(7014):173-178. |
|
| Check, E. 2004. Hopes rise for RNA therapy as mouse study hits target. 432(7014):136. |
|
| Voorhoeve, P.M. and R. Agami. 2003. Knockdown stands up. 21(1):2-4. |
|
| Ellington, A.D. and J. W. Szostak. 1990. selection of RNA molecules that bind specific ligands. 346(6287):818-822; Tuerk, C. and L. Gold. 1990. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. 249(4968):505-510. |
|
| Block, C. et al. 2004. Photoaptamer arrays applied to multiplexed proteomic analysis. 4(3):609-618; Jayasena, S.D. 1999. Aptamers: an emerging class of molecules that rival antibodies in diagnostics. 45(9):1628-1650; Mayer, G. and A. Jenne. 2004. Aptamers in research and drug development. 18(6): 351-359. |
|
| Mayer, G. and A. Jenne. 2004. Aptamers in research and drug development. 18(6):351-359. |
|
| Wang, K.Y. et al. 1993. A DNA aptamer which binds to and inhibits thrombin exhibits a new structural motif for DNA. 32(8):1899-1904; Li, W.X., et al. 1994. A novel nucleotide-based thrombin inhibitor inhibits clot-bound thrombin and reduces arterial platelet thrombus formation. 83(3):6 -82. |
|
| See [accessed March 27, 2005]. |
|
| See [accessed March 27. 2005]. |
|
| Burbulis, I. et al. 2005. Using protein-DNA chimeras to detect and count small numbers of molecules. 2(1):31-37. |
|
| Mayer, G. and A. Jenne. 2004. Aptamers in research and drug development. 18(6):351-359. |
|
| The following section is taken from the Executive Summary of an NRC report entitled: (December 2005). |
|
| National Research Council. 2005. . Washington, DC: The National Academies Press. |
|
| Pacific Northwest National Laboratory. 2005. Genomic sequences processed in minutes, rather than weeks. , June 21. Available online at . |
|
| Wolkenhauer, O. et al. 2005. The dynamic systems approach to control and regulation of intracellular networks. 579(8):1846-1853. |
|
| Goldbeter, A. 2004. Computational biology: a propagating wave of interest. 14(15):601-602; Uetz, P. and R.L. Finley, Jr. 2005. From protein networks to biological systems. 579(8):1821-1827; Aloy, P. and R.B. Russell. 2005. Structure-based systems biology: a zoom lens for the cell. 579(8):1854-58; Rousseau, F. and J. Schymkowitz. 2005. A systems biology perspective on protein structural dynamics and signal transduction. 15(1):23-30. |
|
| Apic, G. et al. 2005. Illuminating drug discovery with biological pathways. |
|
| 579(8):1872-1877; Young, J.A. and E.A. Winzeler. 2005. Using expression information to discover new drug and vaccine targets in the malaria parasite Plasmodium falciparum. 6(1):17-26. |
|
| Hood, L. et al. 2004. Systems biology and new technologies enable predictive and preventative medicine. 306(5696):640-643. |
|
| AfCS Nature; The Signalling Gateway; See . |
|
| Balakrishnan, V.S. et al. 2005. Genomic medicine, gene polymorphisms, and human biological diversity. 18(1):37-40; Carr, K.M. et al. 2004. Genomic and proteomic approaches for studying human cancer: prospects for true patient-tailored therapy. 1(2):134-140. |
|
| Guillermo Paez, J. et al. 2004. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. 304(5676):1497-1500. |
|
| Marietti, C. 1999. Body Language; Health Care Informatics. [Online]. Available online at [accessed January 5, 2006]. |
|
| Workman, P. 2001. New drug targets for genomic cancer therapy: Successes, limitations, opportunities and future challenges. 1(1):33-47. |
|
| Ibid. |
|
| Schinder, T., W. Bornmann, P. Pellicena, W.T. Miller, B. Clarkson, J. Kuriyan. 2000. Structural mechanism for STI-571 inhibition of Abelson tyrosine kinase. 289(5486):1938-1941. |
|
| Workman, P. 2001. New drug targets for genomic cancer therapy: Successes, limitations, opportunities and future challenges. 1(1):33-47. |
|
| Daar, A.S. and P.A. Singer. 2005. Pharmacogenetics and geographical ancestry: implications for drug development and global health. 6(3):241-246. |
|
| National Research Council/Institute of Medicine. 2005. Washington, DC: The National Academies Press. |
|
| Ibid. |
|
| While the vaccine was not one that would specifically target black as opposed to white people, it was clearly intended to be used to limit fertility in black women. |
|
| Glasgow, J.N. et al. 2004. An adenovirus vector with a chimeric fiber derived from canine adenovirus type 2 displays novel tropism. 324(1): 103-16. |
|
| Nettelbeck, D.M. et al. 2004. Retargeting of adenoviral infection to melanoma: combining genetic ablation of native tropism with a recombinant bispecific single-chain diabody (scDb) adapter that binds to fiber knob and HMWMAA. 108(1):136-45. |
|
| Suzuki, T. et al. 2000. Adenovirus-mediated ribozyme targeting of HER-2/neu inhibits in vivo growth of breast cancer cells. 7(3):241-248. |
|
| Rein, D.T. et al. 2004. Gene transfer to cervical cancer with fiber-modified adenoviruses. 111(5):698-704. |
|
| A company called DNAprint Genomics has identified a number of genetic markers that correlate highly with racial or ethnic designations, many of them having to do with metabolizing toxins found in foods that are indigenous to certain areas. The markers identified by this firm are used to provide quantitative measures of an individual’s ancestry, according to four different “anthropological groups”—Native American; East Asian; Sub-Saharan Africa; and European. “European” can be broken down into Northern European; Southeastern European, Middle Eastern, and South Asian. For additional information on this company’s “products” see , and in particular a related site, . |
|
| The Sunshine Project. 2003. Emerging Technologies: Genetic Engineering and Biological Weapons. Background Paper #12. Available online at [accessed January 5, 2006]. |
|
| Kagan, E. 2001 Bioregulators as instruments of terror. 21(3): 607-618. See also, Wheelis, M. 2004. Will the new biology lead to new weapons? 34(July/August):6-13; and, Dando, M. 1999. British Medical Association. Amsterdam: Harwood Academic Publishers, especially Chapter 4 on “Genetic weapons.” See also Dando, M. 1996. Dulles, VA: Potomac Books, Inc., especially Chapters 5 and 8. |
|
| Wang, D. et al. 1999. Encapsulation of plasmid DNA in biodegradable poly(D, L-lactic-co-glycolic acid) microspheres as a novel approach for immunogene therapy. 57(1): 9-18; National Research Council/Institute of Medicine. 2005. Washington, DC: The National Academies Press. |
|
| Neuropeptides, a type of bioregulator found in nervous system tissue, have a powerful modulatory effect on the nervous and immune systems. |
|
| Wheelis, M. 2002. Biotechnology and biochemical weapons. 9(Spring):48-53. Available online at [accessed January 5, 2006]. |
|
| Healy, M. 2004. Sharper minds. (December 20): F1; Tully, T. et al. 2003. Targeting the CREB pathway for memory enhancers. 2(4):267-77. |
|
| National Research Council/Institute of Medicine. 2005. Washington, DC: The National Academies Press. |
|
| Ibid. |
|
| Arntzen, C. et al. 2005. Plant-derived vaccines and antibodies: potential and limitations. 23(15):1753-1756; Huang, Z. et al. 2005. Virus-like particle expression and assembly in plants: hepatitis B and Norwalk viruses. 23(15): 1851-1858; Thanavala, Y. et al. 2005. Immunogenicity in humans of an edible vaccine for hepatitis B. 102(9):3378-3382. |
|
| National Research Council/Institute of Medicine. 2005. Washington, DC: The National Academies Press. |
|
| Petro, J.B. et al. 2003. Biotechnology: Impact on biological warfare and |
|
| biodefense. 1(3):161-168. |
|
| Walt, D.R. 2005. Miniature analytical methods for medical diagnostics. 308(5719):217-219. |
|
| Ibid. |
|
| This section is based on the workshop presentation of N. Seeman, in National Research Council/Institute of Medicine. 2005. Washington, DC: The National Academies Press. |
|
| Seeman, N.C. and A.M. Belcher. 2002. Emulating biology: building nanostructures from the bottom up. 99(Suppl 2):6451-6455. |
|
| Seeman, N.C. 1982. Nucleic acid junctions and lattices. 99(2): 237-247; Seeman, N.C. 1999. DNA engineering and its application to nanotech-nology. 17(11):437-443. |
|
| Winfree, E. 1995. On the computational power of DNA annealing and ligation. In Lipton, R. and E. Baum, eds. 1995. . Am. Math. Society:199-215; and Adleman, L. 1994. Molecular computation of solutions to combinatorial problems. 266(5187): 1021-1024. |
|
| Chen, Y. and C. Mao. 2004. Putting a brake on autonomous DNA nanomotor. 126(28):8626-8627; Emerich, D.F. 2005. Nanomedicine—prospective therapeutic and diagnostic applications. 5(1):1-5. |
|
| Kohli, P. and Martin, C.R. 2005. Smart nanotubes for biotechnology. 6(1):35-47; Kubik, T. et al. 2005. Nanotechnology on duty in medical applications. 6(1):17-33. |
|
| . |
|
| Fortina, P. et al. 2005. Nanobiotechnology: the promise and reality of new approaches to molecular recognition. 23(4):168-173; Patolsky, F. et al. 2004. Electrical detection of single viruses. 101(39):14017-14022. |
|
| Warheit, D. et al. 2004. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. 77:117-125; Oberdorster, E. 2004. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. 112(10):1058-62. |
|
| Main, C.E. 2003. Aerobiological, ecological, and health linkages. 29(2-3):347-349. |
|
| Bauce, E. et al. 2004. subsp. aerial spray prescriptions for balsam fir stand protection against spruce budworm (Lepidoptera: Tortricidae). 97(5):1624-1634. |
|
| Martonen, T.B. and J.D. Schroeter. 2003. Risk assessment dosimetry model for inhaled particulate matter: I. human subjects. 138(1-2): 119-132. |
|
| Paez-Rubio, T. et al. 2005. Source bioaerosol concentration and rRNA gene- |
|
| based identification of microorganisms aerosolized at a flood irrigation wastewater reuse site. 71(2):804-810. |
|
| Tanner, B.D. et al. 2005. Bioaerosol emission rate and plume characteristics during land application of liquid class B biosolids. 39(6):1584-90; Brooks, J.B. et al. 2005. Estimation of bioaerosol risk of infection to residents adjacent to a land applied biosolids site using an empirically derived transport model. 98(2):397-405. |
|
| Brown, J.R. et al. 2005. Aerial optimization and canopy penetration study of Dibrom 14 Concentrate. 21(1): 106-113. |
|
| Chan, H.K. 2003. Inhalation drug delivery devices and emerging technologies. 13(9):1333-1343. |
|
| Edwards, D. 2002. Delivery of biological agents by aerosols. 48(1):2-6. |
|
| LiCalsi, C., M.l Maniaci, T. Christensen, E. Phillips, G.H. Ward, and C. Witham. 2001. A powder formulation of measles vaccine for aerosol delivery. 19(17-19):2629-2636. The authors describe a method to deliver live, attenuated, measles vaccine via the lungs. “In this study, live attenuated measles vaccine is micronized by jet milling to generate particle sizes appropriate for pulmonary delivery (1-5 µm). Milling does not induce detectable physical change and significant viral potency is maintained…. The measles vaccine formulation is dispersible …” |
|
| Clark, A.R. 1995. Medical aerosol inhalers: past, present, and future. 22:374-381. |
|
| Chan, H.K. 2003. Inhalation drug delivery devices and emerging technologies. 13(9):1333-1343. |
|
| Crowder, T.M. 2002. Fundamental effects of particle morphology on lung delivery: predictions of Stokes’ Law and the particular relevance to dry powder inhaler formulation and development. 19(3):239-245. |
|
| Garcia-Contreras, L. and H.D.C. Smyth. 2005. Liquid-spray or dry-powder systems for inhaled delivery of peptide and proteins? 3(1):29-45. |
|
| Chan, H.K. 2003. Inhalation drug delivery devices and emerging technologies. 13(9):1333-1343. |
|
| Tan, H.S. and S. Borsadia. 2001. Particle formation using supercritical fluids: pharmaceutical applications. 11(5):861-872. |
|
| Based on Elliott Kagan’s presentation at the Cuernavaca workshop. See National Research Council/Institute of Medicine. 2005. Washington, DC: The National Academies Press. |
|
| Wang, D. et al. 1999. Encapsulation of plasmid DNA in biodegradable poly(D,L-lactic-co-glycolic acid) microspheres as a novel approach for immunogene delivery. 57(1):19-18. |
|
| Dai, C. et al. 2005. Microencapsulation peptide and protein drugs delivery system. 41(2-3):117-20. |
|
| See [accessed May 12, 2005]. |
|
| See [accessed May 12, 2005]. |
|
| Randall, P. and S. Chattopadhyay. 2004. Advances in encapsulation technologies for the management of mercury-contaminated hazardous wastes. 114(1-3):211-223. |
|
| Hao, S. et al. 2005. A novel approach to tumor suppression using microencapsulated engineered J558/TNF-alpha cells. 27(1):56-60. |
|
| Weinbreck, F. et al. 2004. Microencapsulation of oils using whey protein/gum Arabic coacervates. 21(6):667-679. |
|
| Yamaguchi, Y. et al. 2005. Successful treatment of photo-damaged skin of nano-scale atRA particles using a novel transdermal delivery. 104(1):29-40. |
|
| See [accessed May 12, 2005]. |
|
| Chang, T.M. 2005. Therapeutic applications of polymeric artificial cells. 4(3):221-235; and Orive, G. et al. 2004. History, challenges and perspectives of cell microencapsulation. 22(2):87-92. |
|
| A new technology, which will allow weapons and vehicles to be released from submarines even if they were not originally designed for undersea use. |
|
| Izumikawa, M. et al. 2005. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. 11(3):271-276. |
|
| Parsons, D. 2005. Airway gene therapy and cystic fibrosis. 41(3):94-96. |
|
| Although “convergent technology” is a common term often used to refer to the convergence of specific types of technologies, we use it here loosely to refer to the convergence of technologies. |
|
| Benenson, Y. et al. 2004. An autonomous molecular computer for logical control of gene expression. 429(6990):423-429. |
|
| Kagan, E. 2001 Bioregulators as instruments of terror. 21(3): 07-618. See also, Wheelis, M. 2004. Will the new biology lead to new weapons? 34(July/August):6-13; and Dando, M. 1999. British Medical Association. Amsterdam: Harwood Academic Publishers, especially Chapter 4 on “Genetic weapons.” See also Dando, M. 1996. . Dullas, VA: Potomac Books, Inc., especially Chapter 8: “An assault on the brain?” and Chapter 5: “Lethal and non-lethal chemical agents.” |
|
| Ibid. |
|
| Nixdorff, K. and W. Bender. 2002. Ethics of university research, biotechnology and potential military spin-off. 40:15-35. See also Nixdorff, K., N. Davison, P. Millett, and S. Whitby. 2004. Technology and biological weapons: Future threats. , Number 2, University of Bradford, Department of Peace Studies. Available online at [accessed January 5, 2006]. |
This page intentionally left blank.
Biomedical advances have made it possible to identify and manipulate features of living organisms in useful ways—leading to improvements in public health, agriculture, and other areas. The globalization of scientific and technical expertise also means that many scientists and other individuals around the world are generating breakthroughs in the life sciences and related technologies. The risks posed by bioterrorism and the proliferation of biological weapons capabilities have increased concern about how the rapid advances in genetic engineering and biotechnology could enable the production of biological weapons with unique and unpredictable characteristics. Globalization, Biosecurity, and the Future of Life Sciences examines current trends and future objectives of research in public health, life sciences, and biomedical science that contain applications relevant to developments in biological weapons 5 to 10 years into the future and ways to anticipate, identify, and mitigate these dangers.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
Loading metrics
Open Access
The future is bright, the future is biotechnology
* E-mail: [email protected]
Affiliation Public Library of Science, San Francisco, California, United States of America and Cambridge, United Kingdom
- Richard Hodge,
- on behalf of the PLOS Biology staff editors

Published: April 28, 2023
- https://doi.org/10.1371/journal.pbio.3002135
- Reader Comments
As PLOS Biology celebrates its 20 th anniversary, our April issue focuses on biotechnology with articles covering different aspects of the field, from genome editing to synthetic biology. With them, we emphasize our interest in expanding our presence in biotechnology research.
Citation: Hodge R, on behalf of the PLOS Biology staff editors (2023) The future is bright, the future is biotechnology. PLoS Biol 21(4): e3002135. https://doi.org/10.1371/journal.pbio.3002135
Copyright: © 2023 Hodge, on behalf of the PLOS Biology staff editors. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: The authors received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
The PLOS Biology Staff Editors are Ines Alvarez-Garcia, Joanna Clarke, RichardHodge, Paula Jauregui, Nonia Pariente, Roland Roberts, and Lucas Smith.
This article is part of the PLOS Biology 20th Anniversary Collection.
Biotechnology is a revolutionary branch of science at the forefront of research and innovation that has advanced rapidly in recent years. It is a broad discipline, in which organisms or biological processes are exploited to develop new technologies that have the potential to transform the way we live and work, as well as to boost sustainability and industrial productivity. The new tools and products being generated have a wide range of applications across various sectors, including medicine, agriculture, energy, manufacturing and food.
PLOS Biology has traditionally published research reporting significant advances across a wide range of biological disciplines. However, our scope must continue to evolve as biology increasingly becomes more and more applied, generating technologies with potentially game-changing therapeutic and environmental impact. To that end, we recently published a collection of magazine articles focused on ideas for green biotechnologies that could have an important role in a sustainable future [ 1 ], including how to harness microbial photosynthesis to directly generate electricity [ 2 ] and using microbes to develop carbon “sinks” in the mining industry [ 3 ]. Moreover, throughout this anniversary year we are publishing Perspective articles that take stock of the past 20 years of biological research in a specific field and look forward to what is to come in the next 20 years [ 4 ]; in this issue, these Perspectives focus on different aspects of the broad biotechnology field—synthetic biology [ 5 ] and the use of lipid nanoparticles (LNPs) for the delivery of therapeutics [ 6 ].
One fast moving area within biotechnology is gene editing therapy, which involves the alteration of DNA to treat or prevent disease using techniques such as CRISPR-Cas9 and base editors that enable precise genetic modifications to be made. This approach shows great promise for treating a variety of genetic diseases. Excitingly, promising phase I results of the first in vivo genome editing clinical trial to treat several liver-related diseases were reported at the recent Keystone Symposium on Precision Genome Engineering. This issue of PLOS Biology includes an Essay from Porto and Komor that focuses on the clinical applications of base editor technology [ 7 ], which could enable chronic diseases to be treated with a ‘one-and-done’ therapy, and a Perspective from Hamilton and colleagues that outlines the advances in the development of LNPs for the delivery of nucleic acid-based therapeutics [ 6 ]. LNPs are commonly used as vehicles for the delivery of such therapeutics because they have a low immunogenicity and can be manufactured at scale. However, expanding the toolbox of delivery platforms for these novel therapeutics will be critical to realise their full clinical potential.
Synthetic biology is also a rapidly growing area, whereby artificial or existing biological systems are designed to produce products or enhance cellular function. By using CRISPR to edit genes involved in metabolic pathways, researchers can create organisms that produce valuable compounds such as biofuels, drugs, and industrial chemicals. In their Perspective, Kitano and colleagues take stock of the technological advances that have propelled the “design-build-test-learn” cycle methodology forward in synthetic biology, as well as focusing on how machine-learning approaches can remove the bottlenecks in these pipelines [ 5 ].
While the potential of these technologies is vast, there are also concerns about their safety and ethical implications. Gene editing, in particular, raises ethical concerns, as it could be used to create so-called “designer babies” with specific traits or to enhance physical or mental capabilities. There are also concerns about the unintended consequences of gene editing, such as off-target effects that could cause unintended harm. These technologies can be improved by better understanding the interplay between editing tools and DNA repair pathways, and it will be essential for scientists and policymakers to be cautious and work together to establish guidelines and regulations for their use, as outlined at the recent International Summit on Human Genome Editing .
Basic research has also benefitted from biotechnological developments. For instance, methodological developments in super-resolution microscopy offer researchers the ability to image cells at exquisite detail and answer previously inaccessible research questions. Sequencing technologies such as Nanopore sequencers are revolutionising the ability to sequence long DNA/RNA reads in real time and in the field. Great strides have also been made in the development of analysis software for structural biology purposes, such as sub-tomogram averaging for cryo-EM [ 8 ]. The rate of scientific discovery is now at an unprecedented level in this age of big data as a result of these huge technological leaps.
The past few years has also seen the launch of AI tools such as ChatGPT. While these tools are increasingly being used to help write students homework or to improve the text of scientific papers, generative AI tools hold the potential to transform research and development in the biotechnology industry. The recently developed language model ProGen can generate and then predict function in protein sequences [ 9 ], and these models can also be used to find therapeutically relevant compounds for drug discovery. Protein structure prediction programs, such as AlphaFold [ 10 ] and RosettaFold, have revolutionized structural biology and can be used for a myriad of purposes. We have recently published several papers that have utilized AlphaFold models to develop methods that determine the structural context of post-translational modifications [ 11 ] and predict autophagy-related motifs in proteins [ 12 ].
The future of biotechnology is clearly very promising and we look forward to being part of the dissemination of these important new developments. Open access science sits at the core of our mission and the publication of these novel technologies in PLOS Biology can help their widespread adoption and ensure global access. As we look forward during this year of celebration, we are excited that biotechnology research will continue to grow and become a central part of the journal. The future is bright and the future is very much biotechnology.
- View Article
- PubMed/NCBI
- Google Scholar
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Special Collections
- Author Guidelines
- Submission Site
- Open Access
- Reasons to submit
- About BioScience
- Journals Career Network
- Editorial Board
- Advertising and Corporate Services
- Self-Archiving Policy
- Potentially Offensive Content
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Conservation technology, sensor types, fixed and portable devices, large-scale monitoring systems, devices on vehicles: terrestrial and aquatic, airborne remote sensing, animal-borne devices: biologging and biotelemetry, wildlife tracking, conclusions, acknowledgments, references cited.
- < Previous
A Comprehensive Overview of Technologies for Species and Habitat Monitoring and Conservation
- Article contents
- Figures & tables
- Supplementary Data
José J Lahoz-Monfort, Michael J L Magrath, A Comprehensive Overview of Technologies for Species and Habitat Monitoring and Conservation, BioScience , Volume 71, Issue 10, October 2021, Pages 1038–1062, https://doi.org/10.1093/biosci/biab073
- Permissions Icon Permissions
The range of technologies currently used in biodiversity conservation is staggering, with innovative uses often adopted from other disciplines and being trialed in the field. We provide the first comprehensive overview of the current (2020) landscape of conservation technology, encompassing technologies for monitoring wildlife and habitats, as well as for on-the-ground conservation management (e.g., fighting illegal activities). We cover both established technologies (routinely deployed in conservation, backed by substantial field experience and scientific literature) and novel technologies or technology applications (typically at trial stage, only recently used in conservation), providing examples of conservation applications for both types. We describe technologies that deploy sensors that are fixed or portable, attached to vehicles (terrestrial, aquatic, or airborne) or to animals (biologging), complemented with a section on wildlife tracking. The last two sections cover actuators and computing (including web platforms, algorithms, and artificial intelligence).
For decades, technology has played an important role in how we study habitats and species of conservation concern, as well as helping us deal with threats to biodiversity. From humble beginnings of handcrafted devices, some technologies (e.g., camera trapping, radio tracking) have become standard tools in wildlife studies. Furthermore, the last couple of decades have seen an unprecedented explosion in technological development at all levels of society, including the digital revolution brought by computing and near-global connectivity, the rise of the DIY or maker communities, and more flexible manufacturing associated to the Fourth Industrial Revolution (Berger-Tal and Lahoz-Monfort 2018 ). With cheaper and faster than ever technology prototyping and manufacturing, novel ways of developing technology are emerging, including collaborative open source. All these developments have been slowly trickling into conservation, with a very broad range of established and emerging technologies developed, adopted from other disciplines, or used in novel ways and being trialed in the field (Pimm et al. 2015 ). Finally, there is a recent and growing international push for the conservation community to become innovation leaders rather than users of technologies developed for other purposes, progressively leading to the development and awareness of “conservation technology” as a discipline (Joppa 2015 , Berger-Tal and Lahoz-Monfort 2018 , Lahoz-Monfort et al. 2019 ). Despite all the exciting uses and developments, there has been no comprehensive overview of the use of this broad range of technologies for conservation purposes. We aim to provide the first snapshot of the current landscape of this emerging discipline as of 2020. It offers an up-to-date entry point for those coming into conservation (e.g., graduate students, new practitioners) and a broad but comprehensive overview of existing possibilities for those wanting to expand their use of technology or scout for new options. This overview will be equally useful for people working in other areas of applied ecology (e.g., wildlife monitoring for management of harvested populations) as most of these technologies will be useful beyond conservation.
Although the term technology can include practically any expression of human ingenuity applied to solving practical problems, in the present article, we follow a commonly used more restrictive definition as “machinery and equipment developed from the application of scientific knowledge” (Lexico 2021 ), including both physical tools (devices and machines, often relying on electronics) and more abstract methods associated with them (including computer programs and algorithms). Conservation technology naturally extends this definition to technology that is useful to achieving biodiversity conservation goals. We focus our overview on technologies that play a specific role (i.e., excluding generic devices such as laptops), whether they have been specifically created with biodiversity in mind (e.g., radio collar) or not (e.g., drone). We include two categories. First, established technologies are routinely deployed in conservation, normally backed by substantial field application and a solid body of scientific literature. This does not imply old fashioned or underperforming: Most have continued improving in functionality and performance over time. Second, novel technologies (or, often, novel uses of existing technologies) on the other hand would typically be at trial stage and often considered a risky investment in a critical conservation intervention. The conservation community at large would often view them with interest, and some programs and institutions would decide to invest in them. In covering novel applications of technology and giving them profile, we hope to hasten the understanding, adoption and acceptance of the possibilities brought by the latest options. Because the primary literature is necessarily limited, we have provided links to websites when possible. The line between established and novel is obviously fuzzy, and established technologies (e.g., acoustic loggers) can be used in novel ways (e.g., deployed from a drone over forest canopy). For well-documented established technologies, we provide references to more detailed discussions or specific reviews; we focus in the present article on their latest developments and mention future opportunities for improvement if relevant.
We have excluded from this overview a third category, emerging technologies, whose potential to contribute to conservation may have been claimed but not yet demonstrated in practice. These include both existing technologies with still largely untapped potential for application in conservation and technologies at conceptual or early stages of development. The former category includes some sensing technologies (the Internet of Things, mobile crowdsensing), 3D printing and additive manufacturing, and some computing technologies (virtual reality and augmented reality, gamification, blockchain, culturomics, and i-ecology). The latter includes advanced robotics, smart dust, synthetic biology (including gene drive), and biobatteries. We consider these beyond the scope of this already broad article because the purpose of covering emerging technologies would be different, focusing on opportunities rather than existing applications, therefore being somewhat speculative. Again, there is some degree of subjectivity in classifying a technology as emerging rather than novel .
Finally, we considered biotechnology (based on the processing of biological samples) outside the scope of this overview, focusing instead on technologies that are based on physical phenomena (e.g., sound or electromagnetic waves, including light and heat). We acknowledge the important contribution to conservation that some of these have, including conservation genomics, metagenomics (Russello et al. 2020 ) and genetic barcoding (Hebert et al. 2003 ), environmental DNA (Beng and Corlett 2020 ), and stable isotopes (Rubenstein and Hobson 2004 ). Beyond these specific references, the application of biotechnology to conservation was recently reviewed (Corlett 2017 ).
We acknowledge that the successful contribution of technology to conservation hinges on many important (often critical) considerations that are not purely technical. These include, for example, challenges associated with field deployment, ethical and social implications of the use of technology, hype or inflated expectations, overreliance on technological solutions. These deserve their own study and are not discussed in the present article, which is focused on the development and use of technology for conservation purposes. We have also purposely excluded any technology specific to animal husbandry and veterinary science (e.g., surgery or reproductive technologies).
An overview of a broad discipline such as conservation technology can be structured following different criteria (e.g., by conservation objective). We have chosen to follow what we believe is a natural description of the technology pipeline (figure 1 ), which goes from the physical (electronic components) to the abstract (algorithms): sensing and data acquisition (mostly hardware dependent); data transmission, handling, and storage (hardware but mostly computing and software); and data processing, analysis, and use (largely relying on software and algorithms).

Illustrative example of a technology pipeline (from sensing to analysis) and associated types of technologies, including (1) components, (2) devices, (3) systems, (4) computers and processing, (5) software and data management, (6) algorithms and methods. The example represents a simplified view of a network of fixed sensors that transmit data in real time to a back-end computing system.
Along this pipeline, we distinguish the following types of technology. (1) Components (e.g., electronic or mechanical) are the building blocks and will only be mentioned when relevant (e.g., sensors). (2) Devices (machines): Components and parts are assembled together to form devices, from the simple (e.g., camera trap) to the complex (e.g., satellite); this is typically, the lowest (most physical) level that is relevant for conservation. (3) Systems: Several devices must often work together to achieve a given functionality (e.g., a wireless sensor network includes several types of devices). (4) Computers and processing: the backbone of most conservation technology, including storing, retrieving, manipulating, processing and visualizing data and derived information. (5) Software and data management: Software (e.g., computer programs and apps) provides the brains that allow devices to fulfill their potential. Most modern electronic devices require some control software, either directly interacting with the user (e.g., in a laptop) or running independently (e.g., data processing software within a GPS unit). (6) Algorithms and methods: The highest level of abstraction, including signal processing and data analysis, these are typically implemented as software running on computing devices. (7) Services: Modern technology is inherently associated with some level of service provision. For example, cellular networks require dedicated large-scale infrastructure and complex computing and software systems; from the user's perspective, connectivity is a service that allows using devices such as mobile phones to communicate and access the Internet. Web and cloud services (data storage, processing and analysis of large data sets) are becoming increasingly popular. Figure 2 illustrates a selection of technologies deployed for conservation purposes.

Illustrative examples of conservation technologies. (a) Thermal and camera trap images (night infrared illumination) of Leadbeater's possums. Photograph: Zoos Victoria. (b) Low-cost open-source acoustic logger (AudioMoth) for passive acoustic monitoring. Photograph: Open Acoustic Devices. (c) Small vertical-looking pulse radar (BirdScan) used to study bird migration. Photograph: Swiss Ornithological Institute. (d) Small multirotor UAV (DJI Phantom3) with color camera and thermal sensor (FLIR Vue Pro R) used to detect wildlife (inset: two bridled nail-tail wallabies). Photographs: José Lahoz-Monfort. (e) Raspberry Pi–based video logger for sea turtles (Arribada PS-C). Photograph: Alasdair Davies. (f) Weddell seal with a satellite relay data logger on its head with CTD (conductivity, temperature, and depth) sensor, part of the IMOS system. Photograph: Rob Harcourt. (g) Traditional manual VHF radio tracking of orange-bellied parrots (inset, with antenna visible of tail-mounted transmitter). Photographs: Zoos Victoria. (h) Bespoke radio-tracking UAV (Wildlife Drones) deployed for automated VHF tracking of orange-bellied parrots. Photograph: Zoos Victoria. (i) Birds can carry small monitoring equipment attached to a tiny harness—in this image, a light-based geolocator (GDL2, 0.6g) and multisensor logger (GDL3-PAM, 1.4g; ambient light, atmospheric pressure, temperature, acceleration) Photographs: Swiss Ornithological Institute. (j) Automated monitoring of feeder visitation by the critically endangered helmeted honeyeaters using RFID microchips Photograph: Zoos Victoria. (k) Raspberry Pi–based low-cost time-lapse cameras to monitor penguin colonies in Antarctica, with pictures reviewed by citizen scientists on the Zooniverse platform. Photograph: Alasdair Davies. (l) Automated detection of Marsh deer from color photographs taken from a fixed-wing drone using a deep-learning model. Photograph: Ismael Brack.
We start with an overview of different relevant sensors types, often essential elements for many technologies. We then describe technologies that deploy sensors in devices that are fixed or portable, including large-scale networks; attached to vehicles (terrestrial or aquatic) or airborne, including satellites; attached to animals (biologging); or used to track moving wildlife. The last sections are dedicated to actuators and computing. Figure 3 shows a visual index of all the technologies covered by this article. We provide examples of conservation applications, both classic and novel whenever relevant, as well as a brief introduction to the principles behind a technology when deemed relevant for comprehension.

Index of technologies covered in this article, listed in the same order as in the article. The light and dark gray boxes correspond to sections and subsections.
One of the main purposes of using technology is to detect or gather data on wildlife, habitats, humans or their activities. This requires sensing abiotic, biotic and anthropic components of the environment. Most sensors are electronic components that convert a physical or chemical magnitude into an electric signal (either a current or voltage) whose value (or variation over time) can be measured, displayed, stored or further manipulated. We often use the term sensor to refer to a complete device (e.g., a camera trap as an optical sensor rather than the actual CMOS electronic component within it).
Environmental and atmospheric sensors
Sensors exist to measure different physical and chemical magnitudes that reflect relevant aspects of environments (e.g., soils, aquatic) and the atmosphere, from temperature to volatile compounds (for some examples, see table 1 ; see a list of sensors in agriculture in Aqeel-ur-Rehman et al. 2014 ). Often, several sensors are integrated into a single electronic component (e.g., temperature and humidity sensor) or device (e.g., pipe::scan water quality sensor; www.s-can.at/news/item/176-the-new-pipescan ). Most of these sensor types have been developed for industrial or agricultural applications, or atmospheric studies.
Examples of environmental and atmospheric sensors.
| Magnitude sensed or measured . | Example of sensor . |
|---|---|
| Temperature | Thermistors (resistance that varies with temperature) |
| Moisture content in soil or air (relative humidity), dew point | Electronic hygrometers (changes in capacitance or resistance) |
| Magnetic field (and its variation) | Magnetometers; the most common type are small solid-state Hall effect sensors (easily integrated in consumer electronics to sense the position of accessories) |
| Gas concentration (e.g., nitrogen dioxide, carbon monoxide, carbon dioxide, alcohol) | Gas detectors (e.g., based on electrochemical measurements or infrared) |
| Air quality, including particulate matter and volatile organic compounds, to monitor airborne pollutants | Air quality sensors (aka air pollution sensors) |
| Water quality, including physical properties (conductance, turbidity, color) and concentration of substances (e.g., salinity, pH, dissolved organic carbon, dissolved oxygen) | Water quality probes (often measure several magnitudes) |
| Volatile compounds | Electronic noses can recognize specific volatile compounds, or combinations of them (using sensor arrays). They require pattern-recognition algorithms trained to recognize target compounds. Some systems (e.g., Cyranose; ) have arrays of sensors that can be trained to detect different chemical profiles. |
| Magnitude sensed or measured . | Example of sensor . |
|---|---|
| Temperature | Thermistors (resistance that varies with temperature) |
| Moisture content in soil or air (relative humidity), dew point | Electronic hygrometers (changes in capacitance or resistance) |
| Magnetic field (and its variation) | Magnetometers; the most common type are small solid-state Hall effect sensors (easily integrated in consumer electronics to sense the position of accessories) |
| Gas concentration (e.g., nitrogen dioxide, carbon monoxide, carbon dioxide, alcohol) | Gas detectors (e.g., based on electrochemical measurements or infrared) |
| Air quality, including particulate matter and volatile organic compounds, to monitor airborne pollutants | Air quality sensors (aka air pollution sensors) |
| Water quality, including physical properties (conductance, turbidity, color) and concentration of substances (e.g., salinity, pH, dissolved organic carbon, dissolved oxygen) | Water quality probes (often measure several magnitudes) |
| Volatile compounds | Electronic noses can recognize specific volatile compounds, or combinations of them (using sensor arrays). They require pattern-recognition algorithms trained to recognize target compounds. Some systems (e.g., Cyranose; ) have arrays of sensors that can be trained to detect different chemical profiles. |
Optical sensors
Optical sensors react to light, usually referring to the visual (color) part of the spectrum (wavelengths of approximately 400–700 nanometers [nm]; figure 4 ) but often encompassing ultraviolet (approximately 10–400 nm) and infrared (approximately 700 nm–1000 micrometers [μm]) wavelengths.

Electromagnetic spectrum, with visible light expanded. Wavelength (in measures of distance) is the inverse of frequency (in hertz). Source: Adapted from Philip Ronan (license CC BY-SA 3.0).
Optical sensors range from simple electronic components that react to incident light (e.g., a light-dependent resistor) to more complex optical systems (e.g., digital camera CCD image sensor). Sensors measuring light intensity (radiance) in different frequencies are often called spectrometers (sometimes radiometers , when outside the visible spectrum). The most common application is in interpreting the images generated, but other applications exist (e.g., derive geographic latitude; see the “Wildlife tracking” section). Spectrometry can be used to study the chemical composition of a substance.
Dedicated ultraviolet (UV) sensors exist, but even normal digital camera sensors react to UV light. Although they are uncommon, there are imagers than can directly apply UV for conservation purposes (e.g., to detect white-nose syndrome in bats; Turner et al. 2014 ). The infrared (IR) band is broad and includes electromagnetic radiation of very different properties. Beyond imaging, IR light can be used to measure distances (e.g., rangefinders used in distance sampling surveys to estimate population density; Thomas et al. 2010 ) and in 3D scanning (e.g., mangrove roots; Kamal et al. 2014 ). The near infrared band (NIR; 700–1100 nm) typically conveys information uncorrelated with visible-spectrum images. NIR's characteristics are often important in remote sensing applications: Clear sky and water absorb NIR, whereas healthy vegetation reflects it, helping delineating habitats and assessing vegetation health. Some digital cameras can be modified to capture NIR. NIR is also used in night vision imagers (which are different from thermal imaging), which provide images even in low-light conditions, often assisted by emitted NIR light (e.g., “night mode” in some video cameras).
Thermal sensors
Thermal radiation (heat) is emitted by an object by the vibration of its molecules at a given temperature. A section of the infrared spectrum called thermal infrared (TIR) is particularly useful for monitoring wildlife, because animals and plants radiate in this band (and not in visible light) at their normal temperature; they are therefore detectable at night or in limited visibility conditions. Thermal imagers (or thermographic cameras) transform TIR radiation (3–14 μm) into an electrical signal that can form an image in a display or in a digital file. The most commonly used thermal sensors in consumer-grade devices are uncooled silicon-based microbolometers. TIR imagers and sensors are of much lower resolution than visible-light digital sensors (0.3 megapixels for a typical high-end commercial thermal sensor versus 80 megapixels for a high-end digital camera). Thermal sensors (including cameras, scopes, goggles, rifle scopes; handheld and airborne) have extensive industrial use (construction, electronics, firefighting). Low-cost, low-resolution (i.e., 160 × 120 pixels) imagers are now available as mobile phones accessories (e.g., FLIR ONE range; www.flir.com/flirone ) or for DIY electronics (e.g., FLIR Lepton Dev Kit; www.sparkfun.com/products/14654 ), opening the door for open-source options. Havens and Sharp ( 2016 ) provide a technical and practical introduction to thermal imagers (and night vision cameras).
Multispectral and hyperspectral sensors
Some spectrometers measure the energy in many different bands across a broad part of the electromagnetic spectrum. They are often deployed for remote sensing purposes onboard planes or satellites. Multispectral sensors (e.g., the Operational Land Imager onboard the Landsat 8 satellite) measure several (approximately 3–15) discrete spectral bands. Hyperspectral sensors measure many more (e.g., hundreds) of narrower contiguous spectral bands, often across broad parts of the electromagnetic spectrum (e.g., NASA's Hyperion spectrometer onboard the EO-1 satellite: 220 contiguous 10 nm bands in 0.4–2.5 µm). The higher spectral resolution can allow the detection of landscape features; for example, multispectral images could help locate forest areas, whereas hyperspectral images could map specific tree species (Miyoshi et al. 2020 ).
Lidar (short for light detection and ranging , also called laser altimetry ) is an active surveying method in which a laser is emitted toward an object (e.g., the ground, from an instrument onboard a plane) and its reflection measured by the same instrument. The time between emission and reception can be processed to obtain high-resolution information on the three-dimensional structure of the target. Different wavelengths (UV, visible, IR) can achieve different spatial resolutions (down to very small scales, such as centimeter scale), depending on the instrument and deployment. Airborne lidar can cover large spatial extents, but terrestrial (fixed) applications exist (e.g., to characterize forest structure). Bathymetric lidar to map underwater terrain uses different wavelengths to penetrate water. Melin and colleagues ( 2017 ) offer a good introduction to lidar focused on conservation applications.
The electromagnetic spectrum beyond TIR is typically referred to as radio waves (figure 4 ). Beyond radio communications, radio waves are the basis for radar (for radio detection and ranging ), an active sensing method for remote sensing. The two basic types are pulse Doppler radar (based on the frequency shift induced by moving objects to detect location and speed) and pulse echo radar (based on the time of flight to derive distance). Stationary radar systems are used to detect the location and speed of objects (e.g., maritime, aviation, or weather radars); they can also detect animals (e.g., birds; Gauthreaux and Belser 2003 ) or can be used for imaging purposes (deployed on a plane or satellite, for studying landscapes or deriving topography—e.g., digital elevation models). Portable low-power radars exist ( www.flir.com.au/surveillance/display/?id = 64731), including boards for DIY development (e.g., detection up to 10 meters [m]; BumbleBee, samraksh.com/index.php/products/all-products/32-product-pages/products-sensors/71-bumblebee-radar).
Passive acoustic sensors
Sound is a vibration that propagates through a medium (air, water, or a solid) as a pressure wave. Animal species are sensitive to different sound frequencies (hearing range), from very low (infrasound, below 20 Hz; e.g., elephants) to very high (ultrasound, above 20 kHz; e.g., bats). Sound can be sensed using microphones or hydrophones (underwater). Different sensing technologies are used depending on the desired characteristics (sound quality, sensitivity, directionality, size, cost). Sound recording devices have evolved from the traditional tape recorders to small (e.g., AudioMoth, www.openacousticdevices.info ; Song Meter Micro, www.wildlifeacoustics.com/products/song-meter-micro ) sound loggers. Passive acoustic sensors can also be used to locate wildlife (see the “Acoustic triangulation” section, under “Wildlife tracking”). Passive acoustic monitoring can also occur underwater; it is often called passive sonar when the objective is to detect acoustic signals.
Active sonar
Sonar (for sound navigation and ranging ) is a specific application of acoustics to communicate, navigate, detect or measure target objects, particularly underwater. Unlike in passive acoustics, active sonar systems listen to the reflection of an emitted sound pulse (ping) on a target object. A variety of technologies exist with different characteristics (e.g., spatial resolution, distance) and frequencies (from infra to ultrasounds). These include multibeam echosounders ( www.kongsberg.com/maritime/products/mapping-systems/mapping-systems/multibeam-echo-sounders ), higher-resolution, short-range “acoustic cameras” (e.g., DIDSON; www.soundmetrics.com/Products/DIDSON-Sonars ) and ocean acoustic waveguide remote sensing (for instantaneous imaging and continuous monitoring of fish populations over continental-shelf-scale areas; Jagannathan et al. 2009 ). Beyond classic military and civilian maritime applications, sonar is used to detect fish schools and can estimate biomass in fisheries studies, map depth (bathymetry), and help navigation by automated vehicles (e.g., robots).
Vibration sensors
Geophones convert ground movement (vibration, vertical or horizontal) into an electric signal, using different technologies. Their principal use is in seismology (at very large scales), but they can also be used to detect vibration created by smaller objects at more local scales, such as the vibrations emitted by elephants (Mortimer et al. 2018 ).
Position and motion sensors
Some sensors can measure locally a variety of magnitudes related to movement and position. An inexhaustive list includes accelerometers (acceleration), gyroscopes (rotation and tilt), rate gyroscopes (angular velocity), magnetometers (magnetic field, heading; can be used as compass). They have many applications, including robotics, gaming (gesture detection), image stabilization in cameras. Inertial measurement units (IMU) or inertial navigation systems (INS) integrate several position and motion sensors, facilitating stable flight (e.g., drone) and navigation by dead reckoning (continuous update of the position, orientation and velocity). Motion can also be detected on the basis of changes in the amount of heat received by a sensor (e.g., passive infrared [PIR] motion sensors in some security cameras and wildlife camera traps) or algorithmically by analysis of images (see the “Computing” section).
This section covers a range of devices that are either portable or can be deployed in fixed locations.
Optical cameras
Camera traps (or remotely triggered cameras) can capture still pictures or videos after being triggered by an animal. They have become essential tools for monitoring many (particularly terrestrial) species. This is a mature technology with a long history of development (more than100 years) and a rich literature, including dedicated books (e.g., O'Connell et al. 2011 ), reviews of devices, applications and associated statistical methods (e.g., Hamel et al. 2013 , Burton et al. 2015 ), and a recent comprehensive review providing best practices (Wearn and Glover-Kapfer 2017 ).
Modern camera traps typically consist of a digital image sensor, a PIR sensor (to detect animals in front of the camera), and auxiliary electronics (e.g., memory card) and protective casing. Price ($50–$1000) depends on many aspects (e.g., image quality, triggering delay, and capabilities). Camera traps have been used in conservation for different purposes, including (table 7-2 in Wearn and Glover-Kapfer 2017 ) monitoring wildlife population status (e.g., recording presence or absence, detection rate, or specific individuals with artificial or natural markings; searching for rare species; estimating biodiversity, including rapid assessments; studying habitat preferences and behavior; detecting poachers). They are often used for terrestrial animals of mid-size to large (e.g., cat to deer size) but can also detect smaller animals (e.g., rodents) at closer range. Although challenging, some studies have used them for arboreal animals. Ectotherms are also challenging to detect using a PIR sensor (body often close to ambient temperature).
More advanced camera trap features include infrared flash (night illumination without disturbing animals), wireless connectivity (pictures sent through cellular network or Wi-Fi), networked cameras (e.g., BuckEye Cam; www.buckeyecameras.com ), and remote checks of camera status. New intelligent camera traps (in-device image processing; see the “Computing” section) have important conservation applications (e.g., detecting humans for antipoaching, PoacherCam; www.panthera.org/conservation-technology/poachercam ) but are not yet widespread. A few open-source camera-trap systems have been developed, using off-the-shelf components (Williams et al. 2014 ), sometimes geared toward education (e.g., Naturebytes; naturebytes.org ). The future directions of camera-trapping technology were recently reviewed (Glover‐Kapfer et al. 2019 ).
Cameras are also used underwater. Baited remote underwater video stations (BRUVS) are becoming a popular tool to study demersal and nektonic communities, particularly fish (Langlois et al. 2020 provided a field and annotation guide). They most often work in a non-triggered way, with continuous or programmed recordings. Underwater triggered camera trapping is less common (Williams et al. 2014 ), with challenges including low light levels (particularly less than 40m), high pressure, and image triggering relying on image processing algorithms (infrared triggering does not work).
Managing, visualizing, and sorting massive amounts of images can become a resource-consuming task, even the limiting factor for a project. Software programs, applications, and algorithms (see the “Computing” section) have been developed recently to alleviate this burden, including automated sorting (e.g., images with no animals), automated species identification, online sharing to crowdsource human-based species identification.
Thermal imaging
The contrast between the heat emitted by animals and their immediate surroundings can help detect them efficiently and unobtrusively, particularly under some conditions (e.g., at night, with cryptic background or hidden by vegetation). TIR imaging has long been used to detect wildlife and monitor animal populations (Havens and Sharp 2016 ), particularly for large-bodied endotherms (e.g., ungulates); this is linked to hunting, one of the main uses of thermal imaging (handheld devices and rifle scopes). It has also been used for small mammals (e.g., rodents; Boonstra et al. 1994 ), birds (McCafferty 2013 ), nests, and cave roosts (Hristov et al. 2008 ). Infrared radiation attenuates very quickly in water, so thermal monitoring has been rather limited in aquatic environments (e.g., surfacing marine mammals and intertidal invertebrates; Lathlean and Seuront 2014 ). Some studies have shown thermal detection to be more efficient than spotlighting (Focardi et al. 2001 ), but this will depend on each case (species, habitat, environmental conditions). Thermal sensing is not always intuitive; many aspects influence the relationship between body temperature and how much TIR radiation hits a thermal sensor (e.g., ambient temperature, insulation by fur, surface temperature versus core body temperature, distance to target, field of view of the lens); pilot studies can help test whether the approach is sensible for a specific purpose.
Thermal imaging has been used in a variety of contexts beyond wildlife monitoring, including research on migrations (McCafferty 2013 ), behavior (e.g., flight patterns; Hristov et al. 2008 ), welfare and disease diagnosis (Cilulko et al. 2013 ), to avoid killing of animals (e.g., farmland bird nests, fawns) during mowing (Steen et al. 2012 ), to detect wind farm collisions of birds (Desholm et al. 2006 ).
The growing literature on the use of TIR for wildlife studies and monitoring includes a dedicated book (Havens and Sharp 2016 ). The use of this technology is likely to increase in the coming years driven by the decreasing cost of lightweight handheld devices.
Passive acoustics
Monitoring wildlife on the basis of the sounds they produce has a long tradition in ecology and conservation. Initially relying on human hearing and tape recorders, the discipline flourished with the development of modern passive acoustic monitoring (PAM) on the basis of acoustic loggers, electronic devices left unattended in the field for extended periods, recording sound at preprogrammed intervals or in response to acoustic triggers. Acoustic monitoring is a booming discipline and modern low-cost automated PAM could enable biodiversity monitoring at unprecedented spatial and temporal scales. Acoustic monitoring has a rich literature on applications (different species, locations) and technologies (e.g., automated species identification, statistical methods). A recent overview (Browning et al. 2017 ) covers from technology to survey design and data analysis; Sugai and colleagues ( 2019 ) offered a recent review and perspective.
Many animal taxa contain vocal species, notably many birds, frogs, insects, mammals (e.g., primates, rodents, cetaceans, and bats use ultrasonic vocalizations, and elephants use infrasonic) and even fish. Some illegal activities (e.g., chainsaws, gunshots) and invasive species (e.g., cane toads, Hu et al. 2009 ) can be detected acoustically. Sound propagates well underwater and PAM is used to monitor cetaceans and, increasingly, fish and invertebrates. PAM, particularly with recent cheaper devices, can be used to search for rare, elusive or sparse species (or their threats) in very large landscapes (Hill et al. 2018 ). Trends in species occupancy and abundance, and biodiversity indices (e.g., species richness) can be estimated from PAM data, often relying on robust statistical methods (e.g., spatially explicit capture–recapture, Royle et al. 2013 ; occupancy detection, Campos-Cerqueira and Aide 2016 ) to deal with imperfect detection (including false positives, particularly with automated identification). The recent discipline of soundscape ecology (Pijanowski et al. 2011 ) studies the relationship between ecological processes and the soundscape (spatiotemporal variation of sounds in a landscape), reflecting important ecosystem processes and human activities. Soundscape studies do not require identification of individual species but still generate large amounts of data. Methods have been developed to summarize soundscape properties (acoustic indices, Sueur et al. 2014 ) and facilitate interpretation (Towsey et al. 2014 ). Soundscape monitoring may provide affordable large-scale surveillance of ecosystem health (Farina 2014 ) and surrogates for monitoring biodiversity (Burivalova et al. 2018 ).
PAM traditionally relied on expensive commercial equipment ($800–$1200 per unit), labor-intensive retrievals of memory cards and manual checking of recordings for target sounds or training recognizer algorithms. The discipline is now reaching a new level of maturity, with cheaper and more intelligent equipment. Low-cost open-source devices have been proposed (e.g., DIY, Whytock and Christie 2016 ; integrated, Wijers et al. 2019 ). The promising AudioMoth (Hill et al. 2018 ), with its key characteristics (open-source design, bespoke programming, low energy consumption, a low-cost of approximately $80), represents a milestone in PAM technology. Automated acoustic species recognition has vastly improved, reducing labor and enabling real-time species detection (Aide et al. 2013 ). Modern electronics could allow in-device detection of species or events in real time, critical for some conservation applications (e.g., poacher gunshots; Wrege et al. 2017 ). Automating the data pipeline (handling, storing, processing) is essential to operationalize PAM at scale, given the massive amounts of data PAM typically generates, and some end-to-end solutions (e.g., ARBIMON includes acoustic stations wirelessly connected to a data repository; Aide et al. 2013 ) and backend support (e.g., Ecosounds online repository, www.ecosounds.org ; Wimmer et al. 2013 ) have been proposed.
Active sonar has a long-standing tradition in seafloor mapping and fisheries, with some applications to conservation including understanding the impact of fishing practices (Lucchetti and Sala 2012 ), surveying for threatened species (Flowers and Hightower 2013 ) and studying marine fauna (Giorli and Au 2017 ). These devices are often towed from a boat or deployed in buoys. Acoustic cameras (e.g., DIDSON and its successor ARIS) achieve sound-based real-time near-video imaging at short distances of tens of meters (Moursund et al. 2003 ). They are portable devices (including a handheld version for divers with a mask-mounted display; soundmetrics.com/Products/ARIS-Sonars/ARIS-Defender-3000) and can substitute optical cameras for detecting, identifying and counting fish species in turbid waters.
Environmental sensing
In the context of conservation, the most common use of many environmental and atmospheric sensors is to monitor environmental quality and detect changes in environmental conditions, mostly as background surveillance monitoring. For example, long-term ecosystem monitoring sites of NEON (the US National Ecological Observatory Network) deploy several automated environmental sensors (Thorpe et al. 2016 ), including water quality (e.g., dissolved oxygen, pH, nitrate), atmospheric (e.g., solar radiation, wind speed) or in the soil (e.g., temperature and moisture). iButton data loggers ( www.maximintegrated.com/en/products/ibutton/data-loggers.html ) are an increasingly popular small, low-cost, robust sensor that can log temperature or humidity in the field for long periods of time. Buoys and bottom-tethered devices are used to deploy environmental sensors at sea, often as multisensor platforms (e.g., the United Kingdom's autonomous SmartBuoys; www.cefas.co.uk/data-and-publications/smartbuoys ), providing long-term data to assess eutrophication, environmental variability and ground-truth satellite images. FieldKit ( www.fieldkit.org ), an open-source modular environmental sensing platform has just been released.
Vibration created by movement and low-frequency sounds can be recorded using geophones and processed using seismic signal processing techniques. It has been used to detect large mammals such as elephants (Mortimer et al. 2018 ), with potential for monitoring some species (Wood et al. 2005 ). Geophones have been used to study the behavior of small fossorial animals (Narins et al. 1997 ) and could potentially be used for monitoring. Early detection of large species that produce ground vibrations (e.g., elephants) can be used to reduce human–wildlife conflict (Anastácio et al. 2018 ). Some human activities could be tracked with geophones (e.g., mining blasts (Wrege et al. 2010 ) or vehicles associated to poaching or logging).
Subterranean activity can also be tracked using magneto-inductive localization by measuring magnetic field strength generated by antennas (e.g., badger tracking over 15 × 20-m area; Noonan et al. 2015 ).
Terrestrial radar
Ground-based stationary radars have been used to detect or track flying animals. The basic idea is not new (Vaughn 1985 ) and early radar ornithology provided the first sound evidence of nocturnal bird migration. The discipline has seen a revival, particularly following the expansion of wind turbines (to model collision risk with flying birds; Desholm et al. 2006 ), as well as access to cheaper equipment and online data from weather radar, and modern computing for dealing with large data volumes. Low-powered surveillance radars, which can detect bird movements within a few kilometers, have been used to study migrations for several decades. Long-range, powerful surveillance radars (including airport, weather station and military radars), which can detect birds at much larger ranges (100–240 kilometers [km]), have been used to study migration (Gauthreaux and Belser 2003 ) and estimate migration numbers (Dokter et al. 2018 , with weather radars) at continental scales.
With Doppler weather data (good coverage in the United States and Europe) now freely accessible online, international research collaboration is growing in this area (e.g., ENRAM; www.enram.eu ). Small purpose-built vertical-looking radar (e.g., BirdScan; swiss-birdradar.com ) can detect individual birds, bats, and insects flying over it and can be used for research on migrations (e.g., average flight direction and speed), including at high altitude and at night (Chapman et al. 2003 ), environmental consulting studies (e.g., planning infrastructure projects such as wind turbines), and conservation (e.g., temporary shutdown of wind turbines when large flocks detected). The taxon can sometimes be derived from radar signal analysis (Zaugg et al. 2008 ). A recent study compares the strengths and weaknesses of different radar types (from Doppler weather radar to dedicated bird radars) operated at the same location (Nilsson et al. 2018 ). Some conservation-focused applications exist (Gauthreaux and Belser 2003 ), including understanding bird migration patterns and stopover areas at continental scales. This technology is likely to see most conservation application for flying species, including insects (Drake and Reynolds 2012 ).
Terrestrial lidar
Portable terrestrial lidar (or terrestrial laser scanning, TLS) devices allow rapid collection of high-resolution (less than 1 centimeter [cm]) 3D spatial structure of natural habitats (Vierling et al. 2008 ). Portable devices include, for example, Echidna lidar (approximately 20 kilograms [kg]) as well as lighter options, optimized for rapid scanning and portability, such as the Compact Biomass Lidar (3.4 kg) or the handheld Zebedee (see links in Paynter et al. 2016 ). TLS data can help ground-truth areal lidar data, or conversely aerial data can scale up the more detailed parameters measured with ground-based lidar. Estimating tree structure can help monitor ecosystem condition and measure tropical forest carbon stocks (Tanago et al. 2018 ).
Other portable devices
Smartphones are currently widely available and usually carry several sensors beyond the obvious camera and microphone, including proximity (infrared or magnetic Hall effect), ambient light, atmospheric pressure, magnetometer (magnetic compass), accelerometer, gyroscope, temperature, and GPS. Moreover, they can send data remotely thanks to in-built connectivity (cellular and wifi). Some of these sensors are only available in higher-end phone models, but even a microphone paired with wireless connectivity can be an effective conservation tool (e.g., Rainforest Connection developed automated acoustic detection of illegal activities—machinery—on the basis of discarded phones and sound analysis in a cloud server; rfcx.org/our_work). Mobile phones as connected multisensor platforms may allow new ideas with great potential for conservation, such as crowdsourcing (see the “Computing” section).
Electronic noses have detected wildlife disease from carefully prepared lab samples (e.g., tuberculosis in badgers; Fend et al. 2005 ); applicability to field situations are currently being tested (Doty et al. 2020 ).
Integrated multisensor platforms
Sometimes several sensors are combined in the same device or station. This is typical for example of environmental sensing and can even scale up to very large networks (see the “Large-scale monitoring systems” section). There is an increasing interest in integrating wildlife monitoring sensors that would have typically been deployed independently. For example, the Instant Detect ( www.zsl.org/conservation/conservation-initiatives/conservation-technology/instant-detect ) platform expands the traditional camera trap to accommodate other sensors (e.g., acoustic), with real-time data communication via satellite. It has been trialed to detect small illegal fishing vessels ( www.zsl.org/conservation/conservation-initiatives/conservation-technology/detecting-illegal-fishing-vessels ). The AmiBio project ( www.evolving-science.com/research-grants/amibio-automatic-acoustic-monitoring-and-inventorying-biodiversity-00485 ) integrates acoustics with weather data. Automated biodiversity sampling stations are being designed (e.g., project AMMOD, www.zfmk.de/en/research/projects/ammod-a-weatherstation-counting-species-diversity , which includes DNA identification of insects, pollen and airborne spores; image recognition of birds, mammals, and nocturnal insects; acoustic detection of birds, bats, and grasshoppers; and analysis of biogenic scents). Other multisensor platforms include intensively monitored nest boxes (Zárybnická et al. 2016 ).
The last couple of decades have seen an increasing use of networks of advanced sensors in ecological studies (Porter et al. 2009 ), and the integration of several technologies forming complete monitoring or surveillance systems is possibly one of the most promising areas of conservation technology (Marvin et al. 2016 ), allowing greater spatial and temporal resolution. System in the present article refers to several devices interconnected into a single functional entity, most often with some degree of control software.
Networks of independent stations
A simple system may consist of a network of individual stations (see the multisensor examples in the previous section) that may be coordinated but do not directly communicate with each other wirelessly. Their strength resides in the mass of data gathered over time at many different locations, which can be jointly analyzed. Some of the largest such networks run at the continental (e.g., NEON in the United States; www.neonscience.org ) or planetary scale (e.g., ILTER; www.ilter.network ), with a strong emphasis on research collaboration and monitoring infrastructure.
Wireless sensor networks
Wireless sensor networks (WSN) can relay data back to a central facility wirelessly (e.g., satellite or cellular networks), and sometimes even let the sensor nodes talk to each other. The review by Porter and colleagues ( 2005 ) identified five reasons for using WSN: high observation frequency, wider area coverage, unobtrusive observation, real-time data acquisition, bidirectional communication, allowing control of sensor functions. Sensors can be deployed following different spatial configurations, depending on the hierarchy of nodes: Some are simple sensors, others can route data and commands toward a central control system. Some WSN nodes can be carried by animals (see the “Biologging” section). Sensor-to-sensor connectivity allows new functionalities (Collins et al. 2006 ), including mesh networks (nodes can relay data dynamically, making the system robust to failures in a single sensor), self-diagnosis (e.g., sensor failure detected by nearby sensors), in-device processing (e.g., remove outlier data by comparing with nearby sensors), automated adaptive sampling to react in real time to locally sensed events. Data relaying is the most common functionality in current WSNs; others are still under exploration. Optimal WSN configuration and control is an active area of research in engineering and computer science.
WSNs are a promising technology for environmental monitoring, with plenty of potential for ecological research (Porter et al. 2005 , 2009 , Collins et al. 2006 ), including in aquatic environments (for a review, see Xu et al. 2014 ). Although often the sensors are environmental or climatic, they can also include cameras or acoustic sensors (e.g., Cai et al. 2007 ). Conservation-specific WSNs are still rare, but examples exist, some of them experimental. These include biosecurity (for a review, see Jurdak et al. 2015 ), including invasive species detection (e.g., cane toads; Hu et al. 2009 ) and for monitoring remote locations (e.g., seabirds colonies; McKown et al. 2012 ).
The typical aim of having a sensor on a terrestrial or aquatic vehicle is to cover more ground than a walking human could, by relying on greater speed or autonomy. In contrast, airborne remote sensing has the added benefit of the sensor itself covering a greater area, thanks to a greater distance between sensor and sensed area.
Terrestrial vehicles
Some handheld sensors have been used for wildlife monitoring from cars (either fixed to the vehicle or held by a passenger). This is typically done to cover longer distances while maintaining a good chance to detect wildlife (e.g., handheld thermal imager from a slow car; Morelle et al. 2012 ). Cars increase coverage but not autonomy (a human driver is still needed). We consider autonomous terrestrial vehicles (cars and robots) to be an emerging technology with no current conservation applications; they must overcome the challenges of moving through natural environments.
Aquatic vehicles
Cameras and sensors deployed from vessels or attached to manned submersibles have long been used to study underwater environmental conditions and biodiversity, including for conservation purposes (e.g., imaging for marine conservation planning; Schlacher et al. 2010 ). Missions are expensive and limited in area coverage; we concentrate in the present article on discussing a relatively recent technology: unmanned aquatic vehicles, which add autonomy and can reach challenging locations. Beyond the generic term drone , the somehow overlapping terminology includes remotely operated vehicles (tethered and remotely controlled from the surface; Shepherd 2001 ), autonomous underwater vehicles (AUVs, which may have some autonomous behavior), unmanned underwater vehicles , autonomous surface vessels ( www.aims.gov.au/advanced-observation-technologies/autonomous-surface-vessels ), unmanned surface vehicles ( sailing drones for long deployments; www.saildrone.com ), and underwater gliders (which use small changes in buoyancy to move up and down; dx.doi.org/10.1051/matecconf/20141302020). The main differences include whether the vehicle operates above or below the surface, whether it is remotely controlled or autonomous, its type of movement (gliding, floating, rolling on the sea floor), and its propulsion system (wind, solar, fuel, or tethered for electricity supply over short distances). One or more sensors may be integrated within the body or on their surface, or attached to an extension arm. Many different sensors have been deployed, from cameras to sonar and environmental and chemical sensors (e.g., multisensor oceanic drone Saildrone; www.saildrone.com ), and include suction devices to capture specimens. Underwater drones have traditionally been large expensive experimental (or military) devices relying on important infrastructure for deployment. The recent emergence of smaller, autonomous, cheaper (even DIY; www.seaperch.org/index ) options might allow a resurgence in their use.
Aquatic drones can be deployed for a variety of tasks, depending on their autonomy and characteristics. A few examples related to conservation and biodiversity include biodiversity exploration (e.g., new species; Raskoff and Matsumoto 2004 ) and monitoring (e.g., video and sonar coral reef surveys; Singh et al. 2004 ), document threats (e.g., trawl fishing damage to deep-sea coral reefs; Hall-Spencer et al. 2002 ), invasive species management (e.g., automated detection of invasive starfish; www.araa.asn.au/acra/acra2005/papers/clement.pdf ), reducing the impact on marine mammals (e.g., hydrophone-based automated localization; www.navaldrones.com/ZRay.html ).
This section describes sensing devices that are used from airborne vehicles. The first part covers traditional remote sensing, from manned vehicles (like planes or helicopters) to satellites (including nanosatellites, a newer option), while we dedicate the second part to unmanned aerial vehicles (UAVs), a remote sensing technology that is rapidly becoming popular.
Traditional remote sensing
Although remote sensing literally refers to the study of objects without physical contact, the term is normally reserved for when observations are taken from a long distance, including monitoring from airborne or spaceborne sensors. Manned planes and helicopters have long tradition in wildlife monitoring, by humans or onboard sensors (e.g., distance sampling transects for cetaceans). Large scale (even global) ground coverage is obtained by sensors on board satellites; these programs are extremely expensive and traditionally handled by governmental organizations (e.g., NASA) or large private companies (but see the “Nanosatellites” section below). Images are then distributed as a product, often after substantial image processing. After acquiring images, advanced image processing skills are still often required for postprocessing and analysis. Satellite-based remote sensing is characterized by temporal (how often an area is covered), spatial (how much area covered) and spectral (what frequency bands) resolutions. Satellite-based remote sensing is a huge field, with a long history in environmental applications. There is a well-developed literature around environmental remote sensing (Wang et al. 2010 , Kuenzer et al. 2014 ), and ecological applications more specifically (reviewed in Pettorelli et al. 2014 ). In the present article, we give illustrative examples of biodiversity-related applications. Satellite-based remote sensing is probably one of the greatest technological leaps for studying biodiversity.
Color or NIR images: Traditionally, most biodiversity studies using satellite imagery relate remotely sensed habitat measures to species preferences (for a review, see Leyequien et al. 2007 ), including in marine environments (e.g., coral reefs, Xu and Zhao 2014 ). Different spectral bands quantify information that can be related to some aspect of biodiversity (e.g., geophysical variables such as sea-surface temperature), indices (e.g., normalized difference vegetation index), thematic variables (land or water cover), topographic variables (surface roughness), and image textures (patch size, habitat fragmentation, and connectivity). These can help map habitat boundaries, estimate habitat preferences and species distributions, assess vegetation and habitat status, locate human-induced pressures, and threats. Often the focus is on tracking their temporal variation (e.g., map deforestation). Detecting and counting animals is the most common application of aircraft-based monitoring; the coarser spatial resolution has traditionally limited the use of satellite imagery for this, although some examples exist, including through habitat modification by animals (e.g., bare ground around wombat warrens, Löffler and Margules 1980 ; fecal staining to count penguin colonies, Fretwell and Trathan 2009 ). Newer higher resolution sensors (e.g., 1.65 meters [m]; Geo-Eye satellite) allow direct individual counts but this is uncommon and relies on large size (e.g., large savannah mammals; Yang et al. 2014 ) or high contrast (e.g., albatrosses; Fretwell et al. 2017 ). High-resolution daily mapping of landscape based on large constellations of small satellites has great untapped potential for conservation ( www.sciencemag.org/news/2017/02/flotilla-tiny-satellites-will-photograph-entire-earth-every-day ).
Multispectral or hyperspectral images: Some well-known Earth-observing sensors are multispectral (e.g., satellite Landsat) or hyperspectral (Hyperion spectrometer in EO-1 satellite, NASA's aircraft-based AVIRIS). These provide more detail on vegetation, soils (geology, chemistry), and atmosphere than color or NIR sensors. Most applications relate directly to habitat, vegetation, and environmental conditions and only indirectly to assisting conservation. Hyperspectral imagery has been used for plant species identification, monitoring soil properties, mapping habitat, and assessing plant condition (Pettorelli et al. 2014 ). Plant communities can be mapped, even down to species level (Kuenzer et al. 2014 ), including invasives (Walsh et al. 2008 ).
Lidar: Airborne lidar has been used in ecology and conservation (for reviews, see Vierling et al. 2008 and Melin et al. 2017 ). It can characterize three-dimensional habitat structure in terrestrial and aquatic environments at high resolution over broad areas, with two benefits: replacing labor-intensive field measurements and measuring novel habitat characteristics. Habitat structure measurements (e.g., forest canopy, canopy cover, leaf area index) can generate predictors to model biodiversity, including species distributions (e.g., Goetz et al. 2007 ) and habitat quality. Airborne lidar can also assess land cover, topography, and hydrology. Mapping forest biomass can provide input into schemes for carbon emission reduction such as REDD+ (e.g., AToMS, several sensors; directory.eoportal.org/web/eoportal/airborne-sensors/atoms). Although not suited to monitor animals directly, lidar has been used indirectly (e.g., finding malleefowl mounds; www.nationalmalleefowl.com.au/uploads/pdfs/21_V%20Saffer_Use%20of%20LiDAR.pdf ).
Radar: Data from airborne radar can also be used to derive proxies of vegetation height and structure, bringing complementary information. An important radar-derived product is the global high-resolution digital elevation model, produced in 2000 by the Shuttle Radar Topography Mission. It has become a standard in spatial distribution studies, with many important topography-related suitability predictors (slope, aspect, ruggedness) derived from it.
Nanosatellites: Satellites have traditionally been expensive to design, manufacture and launch into orbit. Increasingly smaller (and cheaper) satellites have been developed in the last decades, from miniature (100–500 kg) to micro- (10–100 kg) and nanosatellites (1–10 kg). CubeSats (nanosats) are of particular interest for conservation. They have a modular design (10 × 10 × 10-cm units) and are often built from commercial components (even open-source development kits; www.cubesatkit.com ), and they offer more affordable access to space, and short project times of 9–24 months (Allan et al. 2018 ). CubeSats have started a revolution in Earth observation: large low-Earth orbit constellations allow covering a large part of the planet simultaneously, although at coarser resolution than the most advanced large satellites (Marvin et al. 2016 ). They promise near-real-time global monitoring at increasing resolution, allowing analysis of changes in ecosystems, land use, and threats (e.g., road construction, illegal fishing, oil spills), with great potential for conservation. For example, PlanetLabs ( www.planet.com ) images the entire Earth every day at 3–5 m resolution, with targeted monitoring at 72 cm (Boshuizen et al. 2014 ). Radar systems are also being deployed on nanosatellites experimentally (e.g., to detect and track on the ground objects irrespective of cloud cover; www.bbc.com/news/science-environment-43544211 ).
Unmanned aerial vehicles
Unmanned aerial vehicles (UAVs) or remotely piloted aircraft systems (RPAS), often referred to as drones , are aircrafts without an onboard human pilot. UAVs can have different degrees of autonomy ranging from full human manual control to preplanned missions followed autonomously (GPS navigation), all the way to onboard autonomous decision-making allowing obstacle navigation (usually military or experimental systems). Research is underway to develop swarming drones (group flight with coordinated actions through drone-to-drone communication).
Most UAVs belong to two main types: fixed-wing (unmanned planes, propelled by an engine or, less commonly, as a glider) and multirotor (unmanned multicopters, with several pairs of engine-powered rotor blades). Other more experimental options exist (e.g., flapping ornithopters) but have not been used for conservation yet. These two types have well-known characteristics (Anderson and Gaston 2013 ) that often sit at the opposite end of trade-offs. Compared with multirotor, fixed-wing drones typically fly longer and are less noisy, but are unable to hover or fly at very slow speeds, require open spaces for takeoff and landing, and more training. From their military origins, the last 5–10 years have seen the emergence of a consumer market for affordable (approximately A$2000) to inexpensive (approximately A$200) drones, particularly ready-to-go quadcopters with very stable flight. Despite lower capabilities compared with military-grade ones, they still bring great potential for conservation applications. Open-source DIY options (e.g., ArduPilot of the DIYDrones community; diydrones.com/notes/ArduPilot) have been nurtured by a community of enthusiasts.
Drones offer some clear benefits: access to remote, dangerous or difficult locations, efficient large-area coverage, better or new vantage points (e.g., above), safer than manned flights. Compared with satellites, drones offer controlled revisit periods, low-altitude flights, and much lower operational costs (Anderson and Gaston 2013 ).
The use of drones in natural environments still faces important challenges (Hardin et al. 2019 ), including limited flight times of most consumer-level options (less than 30 minutes) and strong legal restrictions (though sometimes easier in natural areas). Field trials often find this technology difficult or unsuitable; only recently, evidence is starting to unravel their true potential to study and protect biodiversity, with field testing and comparing their efficiency with traditional survey methods (Linchant et al. 2015 , Hodgson et al. 2018 ). UAVs won't be suitable for all species and locations, and some applications, particularly related to imagery (e.g., orthorectified images) require substantial expertise. Two other issues that require further consideration are the social and ethical issues of privacy (What is the impact of capturing video of humans living in the area? Sandbrook 2015 ) and animal welfare concerns (What is the impact of the drone on wildlife? Mulero-Pázmány et al. 2017 ).
Drones have been used in a variety of biodiversity-related applications, with a few general and review papers (Koh and Wich 2012 , Anderson and Gaston 2013 , Linchant et al. 2015 ) and a conservation-focused book (Wich and Koh 2018 ) offering insight. Some of these applications (reviewed in Christie et al. 2016 ) include monitoring marine and terrestrial wildlife, from orangutans to orcas, for population monitoring, detection of presence, or even individual identification and behavior studies; monitoring and mapping the status and changes of habitat, vegetation, and land use (e.g., to detect and measure illegal logging); managing threats on the ground (e.g., antipoaching surveillance). Organizations such as ConservationDrones (conservationdrones.org/mission) and the World Wildlife Fund ( www.worldwildlife.org/projects/wildlife-crime-technology-project ) have tested UAVs in a variety of applications. Reviews of nonconservation uses (e.g., environmental; Colomina and Molina 2014 , Pajares 2015 ) can offer inspiration for novel conservation applications—for example, monitoring forest fires (Merino et al. 2015 ) and invasive species (e.g., ResQu drone; research.csiro.au/robotics/project-resqu).
Other sensors beyond color cameras are increasingly used, including thermal (Christiansen et al. 2014 , Gonzalez et al. 2016 ), multispectral or hyperspectral, and even lidar or radar, although larger sensors require bigger UAVs. Advanced automated processing of images (see the “Computing” section) will greatly improve the usefulness of drone-based imagery (Dell et al. 2016 ). Drones could also help acoustic monitoring, with suspended microphones (Wilson et al. 2017 ) or deploying acoustic loggers in difficult places (e.g., forest canopy). Drones have been proposed as a data communication node (e.g., data retrieval while flying over a camera trap; Glover‐Kapfer et al. 2019 ). Many advanced applications are at research or early trial stages, including grasping objects in flight (Thomas et al. 2014 ) or perching (Doyle et al. 2013 ).
Biologging refers to the collection of data from sensors located on or inside an animal. Telemetry refers to the automated transmission of remotely gathered data. Biologging is often coupled with telemetry technology, sometimes termed biotelemetry (Cooke et al. 2004a ). Telemetry may also refer to the determination of an animal's position using data transmitted from the animal (e.g., a VHF—very high frequency—collar); such position telemetry is commonly called wildlife tracking, radio tracking , or wildlife telemetry . Biologging and tracking can lead to insights into animal health, ecology and behavior that may be critical for wildlife management and conservation. Overall, wildlife telemetry can be considered one of the areas of technology that has had a massive impact in conservation and ecology (Kays et al. 2015 ). Biotelemetry and tracking tags can be attached to wildlife externally (depending on the species, using collars and harnesses or glued to skin, scutes, fur, or feathers) or may be surgically implanted. To avoid the need for recapture, some tags can be programmed or remotely triggered for release, others can transmit data wirelessly to a receiver. Nevertheless, the capture, handling and carrying of tags may cause stress and changes in animal behavior, leading to ethical concerns about potential impacts of their (increasingly widespread) use (Cooke et al. 2017 ). Tags have been used with a variety of taxa, from large mammals to invertebrates, and sometimes provide insight extremely difficult to obtain through other observational approaches (e.g., diving sea mammals). Although used for decades, progress has been phenomenal in the last 10–15 years, including improved tools for data management, visualization, integration and analysis (Rutz and Hays 2009 ). Some telemetry tags can be expensive (well above $1000), which limits the number of individuals that can be tagged. Recently proposed open-source alternatives (e.g., Arribada's Horizon platform; blog.arribada.org/2019/12/08/new-horizons-open-access-argos-telemetry) could significantly reduce their price. Many limitations are still technological, including battery life, miniaturization, data transmission rates and sensor capabilities (Bograd et al. 2010 ).
This section deals with biologging and biotelemetry (i.e., data about the animal's body and environment), showcasing different types of animal-borne sensors; we treat location data separately in the next section (“Wildlife tracking”). Note these areas often overlap (some tags allow simultaneous tracking and biologging) and often better insight is obtained by merging biologging and position data.
Overview of biologging and biotelemetry
Biologging allows gathering data on physiology, behavior, energetics, basic ecology, and interactions of free-ranging, undisturbed animals, and even the environments in which they live. This is achieved by sensing and recording a staggering variety of physical and chemical magnitudes, from temperature to blood flow, from image to sound, from body position to proximity to other individuals (see table 2 in Cooke et al. 2004a ). Tags often include several sensors, alongside tracking technology (e.g., Milsar tags, include gyroscope, magnetometer, temperature, light and pressure sensors, and GPS; milsar.com/p/11-radiotag-telemetry). Some combinations are relatively standard for specific studies (e.g., time, temperature and depth recorders for fish; www.lotek.com/products/lat1000-series ). A well-developed literature exists around the more common types of biotelemetry, including general (e.g., Cooke et al. 2004a , Ropert-Coudert and Wilson 2005 ) and specific reviews (aquatic, Hussey et al. 2015 ; terrestrial, Kays et al. 2015 ); other types of biotelemetry are more experimental. We outline below the main types of biotelemetry technologies, with example applications.
Thanks to camera miniaturization, animal-borne videography can obtain information about the ecology (diet, habitat use) and behavior (mate selection, threat avoidance) that could be critical for conservation. For example, video loggers have been deployed to study ecology and behavior—for example, on crows (Rutz and Troscianko 2013 ) and Tasmanian devils (Andersen et al. 2020 ). So-called AVEDs (animal-borne video and environmental data collection systems) integrate environmental sensors (e.g., audio, location, temperature, acceleration). Moll and colleagues ( 2007 ) provided a review of the evolution, use and advantages or disadvantages of AVED technology. An important limitation is that video creates large amounts of data.
Microphones can be mounted on animals, independently or alongside a camera or other environmental sensors (often position, movement and orientation). They can record sounds produced by the animal carrying the microphone or tag, its conspecifics, but also the amount of sound an animal is exposed to. Acoustics also generates large amounts of data, and storage and manual reviewing are usually limiting factors. They have also been deployed on terrestrial and aquatic species. Johnson and colleagues ( 2009 ) reviewed the technology evolution and use for marine mammals (sound is key for their communication). Small acoustic devices have been developed recently (e.g., less than 1 gram [g] microphone backpack; Gill et al. 2016 ), including the recent open-source µMoth ( www.openacousticdevices.info/mmoth ).
Physiology and energetics
Many physiological parameters (e.g., body temperature, heart rate, metabolism, cellular respiration, blood flow, muscle activity, neural activity) can be measured with sensors attached externally or implanted within the animal's body (Laske et al. 2018 )—for example, tiny transmitters (less than 1 g) to monitor heart rate, wing beat rate, and respiration in free-flying songbirds and bats (Cooke et al. 2004a ) and electromyograms to identify stressors in fish to improve knowledge of salmon migration ecology (Cooke et al. 2004b ) and to measure heart rate, temperature, and activity in ruminants (Signer et al. 2010 ). Physiology and energetics studies are often complemented with fine-scale positional data (see the next section); this combination could help prevent poaching (Laske et al. 2018 ).
Body position and movement
Body position (e.g., head down could indicate grazing) or the amount of movement (e.g., acceleration achieved or speed profiles) is sometimes of interest when the focus is on behavior and interaction with the immediate environment, rather than absolute position or movement over the landscape (as in wildlife tracking). Accelerometers can provide high-resolution data to estimate energy expenditure and activity budgets (Brown et al. 2013 ), detect rare behavioral events such as predation (Rutz and Hays 2009 ) and derive behavioral states from body position (Martiskainen et al. 2009 ). Pressure sensors can monitor depth within the water column (e.g., cetacean diving profiles) or altitude of a bird during migration (Shipley et al. 2018 ). Magnetic contact switches can log events triggered by movement (e.g., prey ingestion by recording jaw opening; Plötz et al. 2002 ). Hall sensors can track the movement of body parts with small magnets attached to them (Wilson and Liebsch 2003 ).
Biologging tags can also collect high-quality data on the environment in which animals live, with two distinct purposes: studying how these animals relate to their environment, or using them as vehicles for environmental data collection. For example, marine animals are being used as oceanographers (Rutz and Hays 2009 ), with, for example, deep-diving elephant seals with conductivity, temperature, or depth sensors have become an essential source of temperature and salinity profiles in polar oceans (eurogoos.eu/download/IN-SITU-OBSERVATIONS-USING-TAGGED-ANIMALS-March-2017.pdf).
Individual identity: RFID and PIT tags
Identifying individual animals is important for many conservation studies and statistical methods (e.g., mark–recapture). Although low-tech marking options exist (e.g., visible and fluorescent tags; Catalano et al. 2001 ), technologies such as RFID and PIT tags can provide a robust long-term way to obtain unique identity for individuals, that in some cases can enable automated identification. Radio-frequency identification (RFID) uses an electromagnetic field to identify tags at (typically) short distances, and recover very simple information (e.g., individual identity code). RFID tags can be active (battery-supported constant transmission), battery-assisted passive (activated by a reader, but detection over longer distances thanks to a battery), or passive (no battery)—see Baratchi and colleagues ( 2013 , section 4.1) for more details. The transponders of passive RFID systems (PIT tags, for passive integrated transponders ) are the cheapest and smallest but require harvesting the electromagnetic field of the reader to transmit the information (e.g., identity) encoded in them, so they typically work at close or very close (touching) range. RFID has become extremely common in industry, commerce and agriculture. Commercial readers (approximately hundreds to thousands of dollars), much more expensive than tags (from a few cents), have historically limited the use of RFID in ecological studies. They have become increasingly accessible over the last decade, and even low-cost DIY alternatives have been designed for wildlife monitoring (e.g., open-source reader; Bridge and Bonter 2011 ).
PIT tags have long been used in wildlife studies, either injected into the animal's body or attached to it (Gibbons and Andrews 2004 ), and the tiny size of some tags even permits use on invertebrates (e.g., 9-microgram tags to study ant behavior; Robinson et al. 2009 ). PIT tags are often used for mark–recapture studies of large cohorts of fish (e.g., Zabel et al. 2005 marked more than 150,000 salmon). Demographic parameters and movement patterns can also be estimated with individual identification (Gibbons and Andrews 2004 ). Individual identity may be broadcasted, to locate specific individuals in large landscapes (e.g., for translocation) or read at close range (e.g., to confirm identity of illegally removed individuals; Gibbons and Andrews 2004 ).
Interactions and proximity
A special use of individual identity coupled with location data is to study proximity to another sensor using proximity loggers. In the present article, the interest is in the relative position of an animal with respect to another sensor-carrying animal or to a fixed sensor (e.g., on a fence or den entrance), rather than in absolute position. The proximity event is most often detected locally. For example, some proximity tags transmit an identifying signal and log duration of each proximity event—that is, when another tag comes closer than a predefined distance (Drewe et al. 2012 ). Proximity can also be derived externally using tracking data (e.g., GPS fixes; Böhm et al. 2008 ). Animal-borne proximity loggers tend to be expensive and often bulky, but smaller loggers have been developed recently (e.g., Encounternet system; Mennill et al. 2012 ). Proximity between two animals has been used to study intra- and interspecies interactions (see references in Drewe et al. 2012 ). Proximity to a fixed detector can also be ascertained with RFID (for reviews, see Gibbons and Andrews 2004 , Bonter and Bridge 2011 ). Potential conservation applications include tracking the use of specific features (e.g., caves, culverts under highways, bird feeders and nest boxes).
Wildlife tracking (position telemetry) is one of the most mature areas of conservation technology. The focus is on determining the absolute position (coordinates) of individuals and their movement over time (and, sometimes, associated quantities such as speed and acceleration). Real-time position knowledge can help locate a specific individual (e.g., for recapture) and assist wildlife management (see the “Virtual fencing” section). Movement data can inform about behavior and habitat preferences, supporting management (e.g., habitat protection or restoration priorities, the use of road underpasses). Tracking data, biotelemetry and remotely sensed habitat data together can provide a high-resolution picture of how and when animals move and interact with each other. There is a considerable literature around wildlife tracking, including general advice on deployment and data collection (e.g., manuals; Pride and Swift 1992 , Kenward 2000 ); statistical modeling (Patterson et al. 2008 ); review and future developments (Bridge et al. 2011 , Baratchi et al. 2013 , Hussey et al. 2015 , Kays et al. 2015 ); technology recommendations based on animal weight, environmental limitations, and technical requirements (Thomas et al. 2012 ). The basic trade-offs are between size, price and data collection capacity. There are two distinct flavors of studying movement ecology on the basis of spatiotemporal data (Baratchi et al. 2013 ): individual based (tracking some tagged individuals) versus place based (gathering detections at fixed detectors and fitting models that estimate probability of presence of an individual at a location); most technologies described in the present article belong to the first category. For example, bird migration can be studied by tracking a few individuals or by counting passing birds at fixed stations.
Technology overview
Most tracking systems involve at least one emitter and one receiver, one of which is animal borne. Tracking technologies can be divided in two main groups, depending on whether an animal-mounted device (tag) receives an external signal that allows it to calculate its coordinates locally (e.g., by triangulation, combining the estimated bearing to several sources) or transmits a signal that can be used externally to calculate its position, by triangulation (combining the estimated bearing to the source from several positions) or by proximity to a point of known position. Data gathered by tracking tags may be stored on board for manual retrieval, but some tags allow wireless data retrieval over relatively short distances (using VHF or UHF—ultrahigh frequency—receivers). Automated data downloading is possible, via satellite-based systems (e.g., Argos, Iridium, Globalstar), the cellular network (e.g., GSM) or, in some cases, WSNs. See (Bridge et al. 2011 , Thomas et al. 2012 ) for more details.
Many telemetry devices (e.g., radio collars, fish tags) are produced by a few companies specifically developing technology for wildlife management, research, hunting or agriculture or aquaculture. The recent OpenCollar initiative (opencollar.io) aims to develop open-source tracking collar hardware and software, potentially bringing down costs and providing higher design flexibility. Except in fisheries and a few terrestrial exceptions, tracking studies often involve a handful of individuals. Several initiatives pull data together from many individual studies. Movebank ( www.movebank.org ) is a free online database and web application for archiving and sharing wildlife tracking data (Kranstauber et al. 2011 ). Other national and international collaborations, often taxon specific, include the Ocean Tracking Network ( oceantrackingnetwork.org ), IMOS (the Integrated Marine Observing System; imos.org.au) and EUROMAMMALS (euromammals.org).
Radio-frequency triangulation
From its origins in the 1960s (Cochran and Lord 1963 ), radio-frequency-based tracking become a standard for wildlife tracking, only challenged by the emergence of satellite-based tracking in the 1990s, which massively improved area coverage. Radio-tracking collars emit a radio signal (commonly VHF, sometimes UHF) that can be tracked using a directional antenna. Each fix only provides the bearing to the tag, but a location estimate can be derived by triangulation by taking successive measures from different positions. Although radio-tracking collars were initially big (battery weight still often the limiting factor), technology miniaturization currently allows tagging small species (e.g., insects; Kissling et al. 2013 ). VHF tracking is labor intensive (it might take a day to locate an animal) and has limited coverage (a few hundred meters around smaller tags). Drone-based VHF triangulation has been trialed to avoid the difficulties of manual radio tracking (Cliff et al. 2018 , Shafer et al. 2019 ). Automated triangulation can be performed with fixed receivers (e.g., Kays et al. 2011 ).
Global navigation satellite system
Global navigation satellite systems (GNSS) are satellite-based navigation systems with global coverage. GPS (the US Global Positioning System) was the first (and most popular) system, but others have been developed (Russia's GLONASS, China's BeiDou Navigation Satellite System, EU's Galileo). A constellation of satellites orbiting the Earth continuously transmit radiofrequency signals that can be read by GPS tags; by calculating distances to several satellites of known position, the GPS tag can estimate its position. Location fixes can be stored on board or retrieved wirelessly. Cagnacci and colleagues ( 2010 ) and other above-mentioned reviews discuss the applications and potential of GPS-based wildlife telemetry. Although historically tag and battery size (more than 10 g; approximately 30 g with data transmission) limited the use of GPS to larger animals, modern lightweight GPS tags (2.5 g) have been deployed on medium-to-small size animals for extended periods (Recio et al. 2011 ). Fastloc-GPS (a proprietary improvement for rapid satellite signal acquisition; wildlifecomputers.com/data/technologies/fastloc) allows practical deployment of GPS tags for marine animals that only surface for a few seconds (Dujon et al. 2014 ). High tag cost (more than $2000) often limits the number of tracked individuals. Low-cost DIY GPS collars have been developed (e.g., with off-the-shelf GPS receivers, Allan et al. 2013 ; based on microcontrollers, Foley and Sillero‐Zubiri 2020) but are not widely used.
Satellite-based tracking
In contrast to the tags of GNSS systems, which calculate position locally from received signals, the tags of the long-standing Argos System( www.argos-system.org ) periodically transmit a radio signal to a suite of polar-orbiting satellites, which relay it to ground stations where location is calculated (on the basis of the Doppler effect due to the satellite's speed). It provides near-real-time global coverage, although with much coarser location accuracy (often more than 100 m) than GPS. It can also relay data from other tags, including GPS. Traditionally limited to medium or large species, recent developments (2 g solar-powered tags; www.microwavetelemetry.com/solar_2g_ptt ) allow studying movement in small birds (Kok et al. 2020 ).
A recent exciting development is the Icarus Initiative (for International Cooperation for Animal Research Using Space ; www.icarus.mpg.de/en ), which promises to track small animals and study migrations at a global scale (Wikelski et al. 2007 ) thanks to solar-powered tags weighing 5g (with plans to reach 1g, allowing deployment even on large insects). Current tags include GPS, 3D accelerometer, magnetometer, temperature and other sensors. Its central system was installed in 2019 onboard the International Space Station, flying overhead at least once a day over 90% of the Earth's surface. Hibernating tags wake up to transmit locally stored position and sensor data, which are relayed from the ISS to a ground station and stored in a central online database (Movebank) for user retrieval.
Inertial sensors and dead reckoning
Data from accelerometers, gyroscopes, magnetometers and pressure sensors can be used to derive (2D or 3D) speed and acceleration, providing a relative measure of movement or, if coupled with an initial absolute position, a high-resolution tracking method (aka dead reckoning or inertial measurement). As position error accumulates over time, it requires regular correction from an absolute measurement (e.g., GPS). This approach can augment other methods such as GPS tracking during periods when the main system may not work (e.g., surfacing marine mammals; Johnson et al. 2009 ).
Harmonic radar
Harmonic radar can track individual animals carrying small simple passive tags that reradiate an emitted radar signal (Kissling et al. 2013 ). Since the experimental trials in the 1990s (Riley et al. 1996 ), it is now an established approach to tracking insects (e.g., Cant et al. 2005 ) and frogs (Roznik and Alford 2015 ), with two main variants: Stationary scanning radar stations are more expensive but record direction and distance to the tag, up to 1 km, whereas cheaper, lightweight, handheld receivers (harmonic direction finders; recco.com ) only provide bearing, which can be used for triangulation at shorter ranges.
Acoustic triangulation
Triangulation of sound sources requires specific hardware—for example, time synchronized microphone arrays, with location estimated on the basis of differences in arrival times or amplitudes (Rhinehart et al. 2020 ). It can aid abundance estimation and help locate threats such as gunshots (Stevenson et al. 2015 ). Sound (audible or ultrasounds) is the most common form of underwater tracking because pressure waves propagate through water much better than radio waves. Sound-emitting acoustic tags have been deployed to track a variety of aquatic species, from fish (common in fisheries research) to marine mammals (Johnson et al. 2009 ). Acoustic triangulation is less common on land, often relying on naturally produced sounds rather than tags, which simplifies deployment but complicates analysis (sound events must be matched across receivers).
Light-derived position
Light-level tags ( geolocators or geologgers ) measure light levels at regular times. Locally stored data can be retrieved and used to estimate location by inferring solar position with respect to the horizon (estimating sunrise and sunset times). This system can have poor accuracy (errors up to 200 km, particularly in latitude) and suffers from different sources of error (e.g., shading of the tag), but with tag weights down to 0.6 g, it is often the only option to track small birds (study migration routes, phenology, overwintering areas) at global scale. Bridge and colleagues ( 2013 ) review the use of geolocators on small birds, and Lisovski and colleagues (2020) explain the concepts and provide a practical guide to the curation and analysis of geolocator data.
Fixed detection stations
The alternative to tracking the movement of tagged individuals is for fixed stations (sometimes organized in large networks) to detect the proximity of marked individuals. Traditional mark–recapture studies (e.g., banded birds detected at fixed stations) fall in this category, but in the last decades, technology has offered automated alternatives. Although some sensor types described in earlier sections (e.g., camera traps) can identify individuals on the basis of variation in their natural markings, some systems have been developed specifically for tracking. For example, the Australian IMOS ( imos.org.au ) includes small acoustic tags attached or implanted on marine species (crustaceans, fish, marine reptiles and mammals) by different research groups, which transmit unique identifier codes. Arrays of receivers around the country detect tags of passing animals and read their identity, automatically providing information about movement patterns. Similarly, the Motus Wildlife Tracking System ( motus.org ; Taylor et al. 2017 ) creates a network of land-based telemetry stations to track small flying animals, currently spanning 31 countries and more than 200 species tagged.
PIT tags (see the “Individual identity” section, under “Animal-borne devices”) can also be used in movement studies, on the basis of fixed stations with automated RFID scanning (Smyth and Nebel 2013 ). Given the short range of PIT tags, detectors are often located, for example, at large concentrations (e.g., colonies) or movement bottlenecks (e.g., road underpasses or culverts).
We end this section covering two types of wildlife tracking applications, which are likely to become increasingly used.
Virtual fencing
Virtual fencing is the use of nonphysical barriers or virtual boundaries, to control the location and movement of animals. Conservation applications are still relatively few but increasing (reviewed by Jachowski et al. 2014 ). It avoids blocking movement of nontarget wildlife species and people, and is cheaper than physical fences. Tracking technology allows two options. Animal-mounted training collars (livestock, www.agersens.com/eshepherd ; within wildlife, mainly wolves to date) act as proximity alarms, either triggering on-the-ground alarms (visual or auditory radio-activated guards) or delivering a cue to the animal (e.g., electric shock or sound) when they cross a virtual boundary. Real-time virtual fencing (geofencing) use animal-mounted real-time position tracking (e.g., GPS) to track position compared with some virtual fences (e.g., edge of wildlife reserve or farmland areas), with a notification sent to conservation staff or private landowners when an animal crosses a virtual fence, triggering an appropriate management response (e.g., translocation). Virtual fencing has been implemented to reduce human–wildlife conflict (box 1 in Jachowski et al. 2014 , Anastácio et al. 2018 ) and to protect critically endangered species (e.g., avoid Californian condors colliding with wind turbines; Sheppard et al. 2015 ). Animals can also be detected using fixed sensors (e.g., the Vertebrate Pest Detect-and-Deter; www.csiro.au/en/News/News-releases/2017/Keeping-pests-at-bay-the-hi-tech-way ).
Large-scale integrated surveillance systems
Data from different animal-borne sensors (including tracking) can be integrated in large-scale surveillance or monitoring systems. For example, Wall and colleagues ( 2014 ) developed a real-time monitoring system for elephants on the basis of GPS fixes transmitted to a cloud-based control system. Analysis of position data (e.g., for geofencing) and movement–behavior data (e.g., movement rate or immobility can indicate injury or death) allows rapid intervention. Similarly, the Domain Awareness System ( www.vulcan.com/News/2017/Domain-Awareness-System.aspx ) integrates data from several technologies in protected areas in Africa to provide real-time information on biodiversity, assets (e.g., patrols) and threats, to improve management and ranger deployment.
Actuators, the parts of a device that move and control a mechanism or system (e.g., gates, motors, pumps), are used to react on sensor information (Aqeel-ur-Rehman et al. 2014 ). Although not a new idea, conservation applications are still uncommon and limited to some specific situations that require acting on a sensed cue. We present in the present article a few examples and ideas, some at development and testing phases.
Wildlife traps may use electronics (e.g., motion sensors) to trigger the trap (Kittelson 2016 ); more advanced approaches could include individual (e.g., PIT tag reader) or species identification (e.g., from a camera) for targeted trapping. Some (e.g., for feral pigs) can also be activated remotely ( www.buckeyecam.com/site/assets/x80_activator_product_brief.pdf ). Bait stations could have actuators that deliver toxins for eradication (e.g., possums in New Zealand). Grooming traps are being trialed to spray a toxin on feral cats or foxes in Australia as they walk in front or inside a device, with infrared beams used as triggering mechanism (Read et al. 2014 ). Actuators can be used in remote feeders to release food in a programmed way (e.g., Parrott and Chasse 2005 ) or in response to local cues or remote monitoring through wireless camera traps (e.g., BuckEye Cams; www.buckeyecameras.com/products.html ). Actuators have been used as deterrents to reduce human–wildlife conflict and animal–vehicle collisions (see the “Virtual fencing” section above).
If the previous technologies represented the body (physical objects, or hardware in electronics lingo), we now turn to the brains, the more abstract functions of storing, manipulating and analyzing data, and making automated decisions. This includes algorithms (sets of rules that define a sequence of operations), signal processing and statistical methods (abstract ideas). These are implemented as software (programs or applications), which run on some piece of hardware with computing capabilities (e.g., a computer or microcontroller). Recent advances in and availability of computing and processing algorithms is expected to help realize the full potential of other technologies (e.g., data-gathering sensors). This section covers some selected computing technologies and applications that are having a substantial impact in conservation and biodiversity research. We do not discuss the most basic computing functionalities (e.g., spreadsheets, databases, GIS—geographic information system mapping) not specific to conservation.
Websites and online platforms
Databases or data repositories have become powerful tools thanks to online access through the Internet, from any connected device, including computers, mobile phones and tablets, but also increasingly automated devices without human supervision. Remote access is important both ways: Data can be retrieved at any time, but can also be submitted to online databases, manually (by humans) or automatically (by connected sensors). Classic sources of biodiversity records (museum or herbarium collections, dedicated sampling) are currently augmented with other sources, including DNA sampling, citizen science and remotely sensed data (Pimm et al. 2015 ). The front end (i.e., what the user sees) of online databases or platforms is often a website (a portal) or smartphone app, which facilitates manual data entry.
The power of these technologies resides in facilitating a massive accumulation of data over geographic and temporal scales that would be otherwise impossible. Some global online platforms such as the GBIF (Global Biodiversity Information Facility; www.gbif.org ) or eBird ( www.ebird.org ) gather millions of records. Many other international, national (e.g., Atlas of Living Australia; www.ala.org.au ) and even regional data portals exist, some of them with specific taxonomic focus (see examples in August et al. 2015 ). Some deal with specific data types (e.g., Xeno-canto for acoustics, www.xeno-canto.org ; Movebank for animal tracking data, www.movebank.org ); others have specific functionalities (e.g., iNaturalist, an online social network of citizen scientists and biologists that share observations; www.inaturalist.org ).
Mobile phones and apps
Mobile phones (including smartphones), ubiquitous across the world, represent one of the most powerful technology advancements for conservation. They can provide Internet access and allow easy deployment of applications (apps, small task-oriented programs) to facilitate a variety of objectives, including data entry by professionals, data gathering in citizen science or community monitoring projects, society and community engagement, and education. For example, the Forest Watcher app ( forestwatcher.globalforestwatch.org ) can be used to track and document deforestation (e.g., illegal logging events). Many repositories mentioned in the previous section (e.g., eBird) have associated mobile apps to facilitate data recording (with associated metadata; e.g., GPS coordinates). Bespoke data collection apps can be easily created or customized (e.g., Cybertracker for GPS and auxiliary data, www.cybertracker.org ; the highly customizable open-source community-driven app development platform Open Data Kit, opendatakit.org ). Low cost allows bottom-up citizen science to gather data and address local issues, avoiding the traditional reliance on institutions (August et al. 2015 ). Importantly, user interface and user experience must consider the target audience, which may include illiterate users or speakers of minority languages (e.g., pictograms in Cybertracker).
Crowdsourcing
Citizen science (the contribution of nonscientists to gathering data of scientific value) represents a form of crowdsourcing (outsourcing work to the crowd), where many individuals contribute to a common goal. Some of the largest citizen science communities include Zooniverse ( www.zooniverse.org ), iNaturalist and eBird. Through the Zooniverse web platform, individuals can help gather data for scientist-driven projects. Crowdsourcing can also harness the pattern recognition ability of the human brain, using volunteers to identify animal species in camera trap pictures accessed through an online portal or mobile app (e.g., Instant Wild; instantwild.zsl.org/intro). New technologies will bring opportunities and challenges to realize the potential of citizen science (Newman et al. 2012 ). Crowdfunding, another form of crowdsourcing, relies on online platforms such as Kickstarter ( www.kickstarter.com ) to allow people around the world to contribute financially to conservation projects (Gallo-Cajiao et al. 2018 ).
Artificial intelligence: Automation and autonomy
Artificial intelligence (AI) is one of the big technology promises for the coming decades, particularly coupled with big data. AI is a broad term that includes machine learning methods for data analysis (computational alternatives to classical statistics). We do not include in the present article classic forms of data analysis because they form a distinct body of knowledge not often associated with the term technology . Some of the algorithms required for AI fall within the discipline of signal processing. AI promises to bring automation (of tasks traditionally done by humans) and autonomy (facilitating or even taking over the process of decision-making).
Automation based on AI algorithms could strongly benefit conservation by accelerating the extraction of useful information from the increasing amounts of data being collected (Kwok 2019 ), particularly by sensors such as camera traps and acoustic loggers; such time-consuming tasks have traditionally been performed manually, often becoming the limiting factor of a project. Automation could even provide insights in near real time. The strong industry push for AI-based automation is also starting to benefit conservation (e.g., Microsoft's AI for Earth grant program; www.microsoft.com/en-us/ai/ai-for-earth-grants ). Deep learning, a family of artificial neural network approaches (LeCun et al. 2015 ), has recently become notorious for their success at achieving complex identification tasks. Their spread is being facilitated by open-source platforms supported by industry giants (e.g., Microsoft Cognitive Toolkit version 2.0, Google's Tensor Flow library). A recent review describes existing uses of deep learning in ecology (Christin et al. 2019 ).
A popular application is in automated identification of species (or sometimes even individuals) from content-rich data types such as images (including thermal; Corcoran et al. 2019 ), video and audio. It typically requires specialized skills to train models, but programs exist to facilitate this task. Recent examples with impressive performance include species identification from camera trap images (Norouzzadeh et al. 2018 ); the cloud-based Wildbook ( wildbook.org ), which can identify and track individuals of most striped, spotted, wrinkled or notched species; iNaturalist's SEEK app ( www.inaturalist.org/blog/23075-real-time-computer-vision-predictions-in-seek-by-inaturalist-version-2-0 ) for real-time species identification with smartphone cameras. Some applications target specific taxa (e.g., salamanders; Gamble et al. 2008 ) or may be broad (e.g., electronic surveillance of counting and sizing fish in fishing vessels to help protect fisheries; civileats.com/2018/05/10/the-future-of-fish-is-big-data-and-artificial-intelligence). Automated identification of humans in camera traps can help combat poaching (e.g., TrailGuard AI camera trap sends immediate wireless alerts to parks management when a person is detected, www.resolve.ngo/trailguard.htm ; trials in Tanzania led to 30 arrested poachers). Automated species identification within sound recordings has also advanced to the point where relatively high performance can be expected for many species (Stowell et al. 2019 ). Other automated tasks include automated counting of individuals of a target species from remotely sensed images, including from satellites (e.g., savannah mammals; Yang et al. 2014 ) and drones (e.g., seabirds in large colonies; Hodgson et al. 2018 ) and automated tracking of a moving object in video footage (Dell et al. 2016 ).
Autonomy is a growing area of application of AI algorithms. Real-time species identification is key to automating decision-making. For example, bait dispensers could release poison bait only when the target pest species is captured (grooming traps trialed for culling feral cats; www.ecologicalhorizons.com/assets/feral-cat-grooming-trap-jan2015.pdf ). AI algorithms were traditionally trained and applied on local powerful computers or in the cloud (large dedicated computing facilities accessed online) but the processing power of higher-end smartphones currently allow some AI applications to run locally ( www.inaturalist.org/blog/23075-real-time-computer-vision-predictions-in-seek-by-inaturalist-version-2-0 ). Furthermore, recently developed dedicated hardware (e.g., Google's Coral microcontroller, coral.withgoogle.com/docs/edgetpu/models-intro) for so-called edge AI or edge computing ( www.imagimob.com/blog/what-is-edge-ai ) allows algorithms (still trained on powerful computers) to be deployed and used in real time even on relatively simple devices (e.g., sensors, such as a camera trap or actuators), with potential to provide local decision-making capabilities. Autonomous navigation, underpinning (e.g., self-driving cars and autonomous drones) could have useful applications for conservation. Experimental examples already exist (e.g., RangerBot AUV, programmed to detect and kill invasive crown-of-thorns starfish in the Great Barrier Reef; www.wildlabs.net/resources/news/underwater-robot-trained-kill-coral-destorying-reef-starfish ).
Low-cost computing
The last 10 years or so have witnessed a revolution in low-cost computing, with several boards based on simple processors or microcontrollers (“dumber” cheaper version of the microprocessor that run the brains of a computer) have been developed commercially that are much simpler to use—even without specialized skills (Cressey 2017 ). Single-board processors such as Raspberry Pi ( www.raspberrypi.org ) and microcontroller-based boards such as Arduino ( www.arduino.cc ) have been heralded as opening the world of electronics to the general public. It has revolutionized the world of DIY electronics and the maker community with extremely low prices (e.g., approximately US$4 for Arduino), online communities (e.g., Instructables, www.instructables.com ) providing discussion and support and (often free) learning materials. Low-cost computing coupled with DIY electronics has been advocated as key to revolutionize wildlife data gathering, particularly in the context of the open-source movement (Greenville and Emery 2016 ). Recent examples include a Raspberry Pi–based open-source acoustic platform (Whytock and Christie 2016 ) and an Arduino-based radio-tracking collar (Foley and Sillero‐Zubiri 2020). Low-cost computing is not limited to these easy-to-use commercial boards. Others have integrated more efficient (e.g., better use of battery) microcontrollers into conservation-oriented products (e.g., AudioMoth acoustic device, Hill et al. 2018 ), but note this approach requires specialized engineering skills.
Our overview showcases the stunning breath of applications of technology in conservation. There has been a long tradition of using technology to aid studies of wildlife, reflected in some well-established tools, including camera trapping and radio tracking. But recent decades have seen a dramatic escalation in technology use and sophistication, particularly thanks to increased availability of remotely sensed products, computing power, and cheaper electronics. We conclude by synthesizing some key observations and trends.
From monitoring and research to conservation action
Many conservation technologies are used for wildlife and habitat monitoring. Data gathered using these devices and systems are essential to many conservation studies and, perhaps more importantly, underpin solid decision-making in conservation management. Often the purpose of monitoring is to ascertain the presence of a species or to track changes in species distribution and abundance (e.g., through capturing images or sounds). More complex data types (e.g., related to animal movement, behavior or physiology) are typically used in research to gain insight that can then be applied to aid conservation work. Fewer technologies are specifically created for on-the-ground conservation action (e.g., drones for antipoaching patrols, satellite imagery to detect deforestation in protected areas, virtual fencing, automated baiting stations).
From well-established to more recent applications of technologies
Conservation technologies can be classified on a continuous spectrum from well-established technologies that have become standard tools in conservation research and projects (e.g., radio tracking, camera traps), to novel applications of technology that are still not in widespread use (e.g., drone-based radio tracking, deep learning algorithms for automated detection of sounds or images). We can expect many of these novel applications to become established tools within the next decade. We note that well established should not be taken to mean outdated or underperforming; on the contrary, these technologies have often continued evolving following industry and research developments (e.g., intelligent camera traps, GPS-based wildlife tracking compared with earlier VHF tracking).
From generic to specific technologies
To date, conservation has mostly used technologies developed for other purposes—for example, military, consumer market or biomedical (Berger-Tal and Lahoz-Monfort 2018 ). Targeted development for conservation is increasingly more common as one progresses along the technology pipeline (figure 1 ): Very few (if any) sensors are created specifically for conservation; some devices are developed for biodiversity-related purposes (e.g., radio-tracking collars, acoustic loggers), but many others are not (e.g., thermal scopes, radar stations, AI methods); most systems established for conservation or ecology purposes are specifically built (even if using generic devices and sensors as elements); many if not most applications, web-based services, or algorithms (e.g., automatically identifying species in images, web portal for crowdsourcing) are specific to their conservation use (although run on computing resources and knowledge that are generic).
We believe this is likely to change in the near future, with calls to support the development of more targeted conservation technology (Lahoz-Monfort et al. 2019 ), especially at the device level. Whether generic or specific to conservation or ecology, most devices are commercial products; some open-source or DIY options exist, but they are currently the exception (we have highlighted some of these options above). This situation can be expected to change over the next decade (see below).
From labor intensive to increased automation
Early use of technologies in conservation has often been very manual and labor intensive (e.g., manual VHF radio tracking, walked transects with handheld thermal scopes, manual checking of images from camera traps or sounds from acoustic monitoring). However, changes over the last decade in many aspects of the technology pipeline have allowed an increasing volume of data being collected (cheaper technology), processed (cheaper computing), made widely available (Internet connectivity) and analyzed (statistical methods, automated analysis of images and sound, crowdsourcing tasks). Raw data are often only the first step, and can become a problem if they accumulate faster than they can be dealt with. Even now, there is often a substantial gap, with cheaper and more available devices creating volumes of data (particularly image and sound) that are difficult to handle and analyze. The bottleneck in biodiversity monitoring technology is moving from data acquisition to data handling. Advances in AI methods are enabling a much-needed increase in automation. This is a key development and we expect the situation to improve in the coming decade.
The next decade of conservation technology
We believe the following (in no particular order) are promising avenues for technology to aid conservation in the near future: AI-based automation and autonomy; increased integration of different technologies and associated data (Marvin et al. 2016 ); the Internet of Things providing increased capacity for sensors to talk to each other; open-source innovation providing new ways of designing, prototyping, and manufacturing technology specifically for conservation purposes (Lahoz-Monfort et al. 2019 ). Although outside the scope of our overview, we close this list mentioning another promising area (Corlett 2017 ): genetics or molecular technology providing increasing capacity to detect, identify, and manage populations and species and to modify organisms for conservation and environmental purposes (e.g., gene drive introducing disease resistance or sterility in invasive species).
We argue that the success of conservation technology as an emerging discipline will depend on both sides: developers and users. On one hand, learning how best to attract technologists to collaborations with the conservation community and ensure the technology prototyped can be converted into final products that can be scaled up for global impact and remain viable over the longer term (Lahoz-Monfort et al. 2019 ). On the other hand, we need to ensure that technology is fit-for-purpose (including withstanding harsh field conditions) and appropriate for the socioeconomic and cultural context in which it will be used (e.g., avoiding creating new forms of dependency of biodiversity-rich developing countries on developed nations). Open-source innovation may be a way forward to achieve these objectives.
The road ahead for conservation technology looks promising but challenging as the conservation community learns to better collaborate with technologists and avoid the pitfalls of misguided use. Our overview highlights that technology can make a great contribution to the conservation toolkit, but in the end, conservation primarily deals with human societies and human behavior, with all their complexities. Only by mastering all these aspects, and not simply developing new cool tech, will conservation technology achieve global impact to aid conservation in the twenty-first century.
We thank Craig Whiteford for comments and Michelle Cooper for early discussions, as well as Achaz von Hardenberg and two anonymous reviewers for constructive suggestions.

Author Biographical
José J. Lahoz-Monfort ( [email protected] ) is affiliated with the School of Ecosystem and Forest Sciences at the University of Melbourne, in Melbourne, Victoria, Australia. Michael J. L. Magrath ( [email protected] ) is affiliated with Wildlife Conservation and Science at Zoos Victoria and with the School of BioSciences at the University of Melbourne, in Melbourne, Victoria, Australia.
Aide TM , Corrada-Bravo C , Campos-Cerqueira M , Milan C , Vega G , Alvarez R . 2013 . Real-time bioacoustics monitoring and automated species identification . PeerJ 1 : e103 .
Google Scholar
Allan BM , Arnould JPY , Martin JK , Ritchie EG . 2013 . A cost-effective and informative method of GPS tracking wildlife . Wildlife Research 40 : 345 – 348 .
Allan BM , Nimmo DG , Ierodiaconou D , VanDerWal J , Koh LP , Ritchie EG . 2018 . Futurecasting ecological research: The rise of technoecology . Ecosphere 9 : e02163 .
Anastácio R , Cardoso S , Pereira MJ . 2018 . Spy out to protect: Sensing devices for wildlife virtual fencing . Open Journal of Ecology 08 : 192 .
Andersen GE , McGregor HW , Johnson CN , Jones ME . 2020 . Activity and social interactions in a wide-ranging specialist scavenger, the Tasmanian devil ( Sarcophilus harrisii ), revealed by animal-borne video collars . PLOS ONE 15 : e0230216 .
Anderson K , Gaston KJ. 2013 . Lightweight unmanned aerial vehicles will revolutionize spatial ecology . Frontiers in Ecology and the Environment 11 : 138 – 146 .
Aqeel-ur-Rehman Abbasi AZ , Islam N , Shaikh ZA . 2014 . A review of wireless sensors and networks’ applications in agriculture . Computer Standards and Interfaces 36 : 263 – 270 .
August T , Harvey M , Lightfoot P , Kilbey D , Papadopoulos T , Jepson P . 2015 . Emerging technologies for biological recording . Biological Journal of the Linnean Society 115 : 731 – 749 .
Baratchi M , Meratnia N , Havinga PJM , Skidmore AK , Toxopeus BAG . 2013 . Sensing solutions for collecting spatio-temporal data for wildlife monitoring applications: A review . Sensors 13 : 6054 – 6088 .
Beng KC , Corlett RT. 2020 . Applications of environmental DNA (eDNA) in ecology and conservation: Opportunities, challenges and prospects . Biodiversity and Conservation 29 : 2089 – 2121 .
Berger-Tal O , Lahoz-Monfort JJ. 2018 . Conservation technology: The next generation . Conservation Letters 11 : e12458 .
Bograd SJ , Block BA , Costa DP , Godley BJ . 2010 . Biologging technologies: New tools for conservation . Introduction Endangered Species Research 10 : 1 – 7 .
Böhm M , Palphramand KL , Newton-Cross G , Hutchings MR , White PCL . 2008 . Dynamic interactions among badgers: Implications for sociality and disease transmission . Journal of Animal Ecology 77 : 735 – 745 .
Bonter DN , Bridge ES. 2011 . Applications of radio frequency identification (RFID) in ornithological research: A review . Journal of Field Ornithology 82 : 1 – 10 .
Boonstra R , Krebs CJ , Boutin S , Eadie JM . 1994 . Finding mammals using far-infrared thermal imaging . Journal of Mammalogy 75 : 1063 – 1068 .
Boshuizen C , Mason J , Klupar P , Spanhake S . 2014 . Results from the Planet Labs Flock Constellation . AIAA/USU Conference on Small Satellites .
Google Preview
Bridge ES , Bonter DN. 2011 . A low-cost radio frequency identification device for ornithological research . Journal of Field Ornithology 82 : 52 – 59 .
Bridge ES , Kelly JF , Contina A , Gabrielson RM , MacCurdy RB , Winkler DW . 2013 . Advances in tracking small migratory birds: A technical review of light-level geolocation . Journal of Field Ornithology 84 : 121 – 137 .
Bridge ES et al. 2011 . Technology on the move: Recent and forthcoming innovations for tracking migratory birds . BioScience 61 : 689 – 698 .
Brown DD , Kays R , Wikelski M , Wilson R , Klimley AP . 2013 . Observing the unwatchable through acceleration logging of animal behavior . Animal Biotelemetry 1 : 20 .
Browning E , Gibb R , Glover-Kapfer P , Jones KE . 2017 . Passive Acoustic Monitoring in Ecology and Conservation . World Wildlife Fund. Conservation Technology Series no. 1 .
Burivalova Z , Towsey M , Boucher T , Truskinger A , Apelis C , Roe P , Game ET . 2018 . Using soundscapes to detect variable degrees of human influence on tropical forests in Papua New Guinea . Conservation Biology 32 : 205 – 215 .
Burton AC , Neilson E , Moreira D , Ladle A , Steenweg R , Fisher JT , Bayne E , Boutin S . 2015 . Wildlife camera trapping: A review and recommendations for linking surveys to ecological processes . Journal of Applied Ecology 52 : 675 – 685 .
Cagnacci F , Boitani L , Powell RA , Boyce MS . 2010 . Animal ecology meets GPS-based radiotelemetry: A perfect storm of opportunities and challenges . Philosophical Transactions of the Royal Society of London B 365 : 2157 – 2162 .
Cai J , Ee D , Pham B , Roe P , Zhang J . 2007 . Sensor Network for the monitoring of ecosystem: Bird species recognition . Paper presented at 3rd International Conference on Intelligent Sensors, Sensor Networks and Information Processing 2007, 3–6 December 2007 .
Campos-Cerqueira M , Aide TM. 2016 . Improving distribution data of threatened species by combining acoustic monitoring and occupancy modelling . Methods in Ecology and Evolution 7 : 1340 – 1348 .
Cant ET , Smith AD , Reynolds DR , Osborne JL . 2005 . Tracking butterfly flight paths across the landscape with harmonic radar . Proceedings of the Royal Society of London B 272 : 785 – 790 .
Catalano MJ , Chipps SR , Bouchard MA , Wahl DH . 2001 . Evaluation of injectable fluorescent tags for marking centrarchid fishes: Retention rate and effects on vulnerability to predation . North American Journal of Fisheries Management 21 : 911 – 917 .
Chapman JW , Reynolds DR , Smith AD . 2003 . Vertical-looking radar: A new tool for monitoring high-altitude insect migration . BioScience 53 : 503 – 511 .
Christiansen P , Steen KA , Jørgensen RN , Karstoft H . 2014 . Automated detection and recognition of wildlife using thermal cameras . Sensors 14 : 13778 – 13793 .
Christie KS , Gilbert SL , Brown CL , Hatfield M , Hanson L . 2016 . Unmanned aircraft systems in wildlife research: Current and future applications of a transformative technology . Frontiers in Ecology and the Environment 14 : 241 – 251 .
Christin S , É Hervet , Lecomte N . 2019 . Applications for deep learning in ecology . Methods in Ecology and Evolution 0 .
Cilulko J , Janiszewski P , Bogdaszewski M , Szczygielska E . 2013 . Infrared thermal imaging in studies of wild animals . European Journal of Wildlife Research 59 : 17 – 23 .
Cliff OM , Saunders DL , Fitch R . 2018 . Robotic ecology: Tracking small dynamic animals with an autonomous aerial vehicle . Science Robotics 3 : eaat8409 .
Cochran WW , Lord RD. 1963 . A radio-tracking system for wild animals . Journal of Wildlife Management 27 : 9 – 24 .
Collins SL et al. 2006 . New opportunities in ecological sensing using wireless sensor networks . Frontiers in Ecology and the Environment 4 : 402 – 407 .
Colomina I , Molina P. 2014 . Unmanned aerial systems for photogrammetry and remote sensing: A review . ISPRS Journal of Photogrammetry and Remote Sensing 92 : 79 – 97 .
Cooke SJ , Hinch SG , Wikelski M , Andrews RD , Kuchel LJ , Wolcott TG , Butler PJ . 2004a . Biotelemetry: A mechanistic approach to ecology . Trends in Ecology and Evolution 19 : 334 – 343 .
Cooke SJ , Nguyen VM , Kessel ST , Hussey NE , Young N , Ford AT . 2017 . Troubling issues at the frontier of animal tracking for conservation and management . Conservation Biology 31 : 1205 – 1207 .
Cooke SJ , Thorstad EB , Hinch SG . 2004b . Activity and energetics of free-swimming fish: Insights from electromyogram telemetry . Fish and Fisheries 5 : 21 – 52 .
Corcoran E , Denman S , Hanger J , Wilson B , Hamilton G . 2019 . Automated detection of koalas using low-level aerial surveillance and machine learning . Scientific Reports 9 : 3208 .
Corlett RT. 2017 . A bigger toolbox: Biotechnology in biodiversity conservation . Trends in Biotechnology 35 : 55 – 65 .
Cressey D. 2017 . The DIY electronics transforming research . Nature News 544 : 125 .
Dell AI et al. 2016 . Automated image-based tracking and its application in ecology . Trends in Ecology and Evolution 29 : 417 – 428 .
Desholm M , Fox AD , Beasley PDL , Kahlert J . 2006 . Remote techniques for counting and estimating the number of bird–wind turbine collisions at sea: A review . Ibis 148 : 76 – 89 .
Dokter AM , Farnsworth A , Fink D , Ruiz-Gutierrez V , Hochachka WM , Sorte FAL , Robinson OJ , Rosenberg KV , Kelling S . 2018 . Seasonal abundance and survival of North America's migratory avifauna determined by weather radar . Nature Ecology and Evolution 2 : 1603 – 1609 .
Doty AC , Wilson AD , Forse LB , Risch TS . 2020 . Assessment of the portable C-320 electronic nose for discrimination of nine insectivorous bat species: Implications for monitoring white-nose syndrome . Biosensors 10 : 12 .
Doyle CE , Bird JJ , Isom TA , Kallman JC , Bareiss DF , Dunlop DJ , King RJ , Abbott JJ , Minor MA . 2013 . An avian-inspired passive mechanism for quadrotor perching . IEEE/ASME Transactions on Mechatronics 18 : 506 – 517 .
Drake VA , Reynolds DR. 2012 . Radar Entomology: Observing Insect Flight and Migration . CABI .
Drewe JA , Weber N , Carter SP , Bearhop S , Harrison XA , Dall SRX , McDonald RA , Delahay RJ . 2012 . Performance of proximity loggers in recording intra- and inter-species interactions: A laboratory and field-based validation study . PLOS ONE 7 : e39068 .
Dujon AM , Lindstrom RT , Hays GC . 2014 . The accuracy of Fastloc-GPS locations and implications for animal tracking . Methods in Ecology and Evolution 5 : 1162 – 1169 .
Farina A. 2014 . Soundscape Ecology: Principles, Patterns, Methods, and Applications . Springer .
Fend R et al. 2005 . Use of an electronic nose to diagnose mycobacterium bovis infection in badgers and cattle . Journal of Clinical Microbiology 43 : 1745 – 1751 .
Flowers HJ , Hightower JE. 2013 . A novel approach to surveying sturgeon using side-scan sonar and occupancy modeling . Marine and Coastal Fisheries 5 : 211 – 223 .
Focardi S , De Marinis AM , Rizzotto M , Pucci A . 2001 . Comparative evaluation of thermal infrared imaging and spotlighting to survey wildlife . Wildlife Society Bulletin 29 : 133 – 139 .
Foley CJ , Sillero-Zubiri C. 2020 . Open-source, low-cost modular GPS collars for monitoring and tracking wildlife . Methods in Ecology and Evolution 11 : 553 – 558 .
Fretwell PT , Scofield P , Phillips RA . 2017 . Using super-high resolution satellite imagery to census threatened albatrosses . Ibis 159 : 481 – 490 .
Fretwell PT , Trathan PN. 2009 . Penguins from space: Faecal stains reveal the location of emperor penguin colonies . Global Ecology and Biogeography 18 : 543 – 552 .
Gallo-Cajiao E , Archibald C , Friedman R , Steven R , Fuller RA , Game ET , Morrison TH , Ritchie EG . 2018 . Crowdfunding biodiversity conservation . Conservation Biology 32 : 1426 – 1435 .
Gamble L , Ravela S , McGarigal K . 2008 . Multi-scale features for identifying individuals in large biological databases: An application of pattern recognition technology to the marbled salamander Ambystoma opacum . Journal of Applied Ecology 45 : 170 – 180 .
Gauthreaux SA , Belser CG. 2003 . Radar ornithology and biological conservation . Auk 120 : 266 – 277 .
Gibbons WJ , Andrews KM. 2004 . PIT tagging: Simple technology at its best . BioScience 54 : 447 – 454 .
Gill LF , D'Amelio PB , Adreani NM , Sagunsky H , Gahr MC , ter Maat A . 2016 . A minimum-impact, flexible tool to study vocal communication of small animals with precise individual-level resolution . Methods in Ecology and Evolution 7 : 1349 – 1358 .
Giorli G , Au WWL. 2017 . Combining passive acoustics and imaging sonar techniques to study sperm whales’ foraging strategies . The Journal of the Acoustical Society of America 142 : 1428 – 1431 .
Glover-Kapfer P , Soto-Navarro CA , Wearn OR . 2019 . Camera-trapping version 3.0: Current constraints and future priorities for development . Remote Sensing in Ecology and Conservation 5 : 209 – 223 .
Goetz S , Steinberg D , Dubayah R , Blair B . 2007 . Laser remote sensing of canopy habitat heterogeneity as a predictor of bird species richness in an eastern temperate forest, USA . Remote Sensing of Environment 108 : 254 – 263 .
Gonzalez LF , Montes GA , Puig E , Johnson S , Mengersen K , Gaston KJ . 2016 . Unmanned aerial vehicles (UAVs) and artificial intelligence revolutionizing wildlife monitoring and conservation . Sensors 16 : 97 .
Greenville AC , Emery NJ. 2016 . Gathering lots of data on a small budget . Science 353 : 1360 – 1361 .
Hall-Spencer J , Allain V , Fosså JH . 2002 . Trawling damage to Northeast Atlantic ancient coral reefs . Proceedings of the Royal Society of London B 269 : 507 – 511 .
Hamel S , Killengreen ST , Henden J-A , Eide NE , Roed-Eriksen L , Ims RA , Yoccoz NG . 2013 . Towards good practice guidance in using camera-traps in ecology: Influence of sampling design on validity of ecological inferences . Methods in Ecology and Evolution 4 : 105 – 113 .
Hardin PJ , Lulla V , Jensen RR , Jensen JR . 2019 . Small unmanned aerial systems (sUAS) for environmental remote sensing: Challenges and opportunities revisited . GIScience and Remote Sensing 56 : 309 – 322 .
Havens KJ , Sharp EJ. 2016 . Thermal Imaging Techniques to Survey and Monitor Animals in the Wild . A methodology . Academic Press .
Hebert PDN , Cywinska A , Ball SL , deWaard JR . 2003 . Biological identifications through DNA barcodes . Proceedings of the Royal Society of London B 270 : 313 – 321 .
Hill AP , Prince P , Covarrubias EP , Doncaster CP , Snaddon JL , Rogers A . 2018 . AudioMoth: Evaluation of a smart open acoustic device for monitoring biodiversity and the environment . Methods in Ecology and Evolution 9 : 1199 – 1211 . doi:10.1111/2041-210X.12955
Hodgson JC , Mott R , Baylis SM , Pham TT , Wotherspoon S , Kilpatrick AD , Raja Segaran R , Reid I , Terauds A , Koh LP . 2018 . Drones count wildlife more accurately and precisely than humans . Methods in Ecology and Evolution 9 : 1160 – 1167 .
Hristov NI , Betke M , Kunz TH . 2008 . Applications of thermal infrared imaging for research in aeroecology . Integrative and Comparative Biology 48 : 50 – 59 .
Hu W , Nirupama B , Chou CT , Jha S , Taylor A , Tran VN . 2009 . Design and evaluation of a hybrid sensor network for cane toad monitoring . ACM Transactions on Sensor Networks 5 : 2 – 28 .
Hussey NE et al. 2015 . Aquatic animal telemetry: A panoramic window into the underwater world . Science 348 : 1255642 .
Jachowski DS , Slotow R , Millspaugh JJ . 2014 . Good virtual fences make good neighbors: Opportunities for conservation . Animal Conservation 17 : 187 – 196 .
Jagannathan S et al. 2009 . Ocean Acoustic Waveguide Remote Sensing (OAWRS) of marine ecosystems . Marine Ecology Progress Series 395 : 137 – 160 .
Johnson M , de Soto NA , Madsen PT . 2009 . Studying the behaviour and sensory ecology of marine mammals using acoustic recording tags: A review . Marine Ecology Progress Series 395 : 55 – 73 .
Joppa LN. 2015 . Technology for nature conservation: An industry perspective . Ambio 44 : 522 – 526 .
Jurdak R , Elfes A , Kusy B , Tews A , Hu W , Hernandez E , Kottege N , Sikka P . 2015 . Autonomous surveillance for biosecurity . Trends in Biotechnology 33 : 201 – 207 .
Kamal S , Lee SY , Warnken J . 2014 . Investigating three-dimensional mesoscale habitat complexity and its ecological implications using low-cost RGB-D sensor technology . Methods in Ecology and Evolution 5 : 845 – 853 .
Kays R , Crofoot MC , Jetz W , Wikelski M . 2015 . Terrestrial animal tracking as an eye on life and planet . Science 348 : aaa2478 .
Kays R et al. 2011 . Tracking animal location and activity with an automated radio telemetry system in a tropical rainforest . Computer Journal 54 : 1931 – 1948 .
Kenward RE. 2000 . A Manual for Wildlife Radio Tagging . Academic Press .
Kissling WD , Pattemore DE , Hagen M . 2013 . Challenges and prospects in the telemetry of insects . Biological Reviews 89 : 511 – 530 .
Kittelson R. 2016 . Motion Activated Non-lethal Animal Trap . US patent no. US9439412B2 .
Koh LP , Wich SA. 2012 . Dawn of drone ecology: Low-cost autonomous aerial vehicles for conservation . Tropical Conservation Science 5 : 121 – 132 .
Kok EMA , Tibbitts TL , Douglas DC , Howey PW , Dekinga A , Gnep B , Piersma T . 2020 . A red knot as a black swan: How a single bird shows navigational abilities during repeat crossings of the Greenland Icecap . Journal of Avian Biology 51 : 02464 .
Kranstauber B , Cameron A , Weinzerl R , Fountain T , Tilak S , Wikelski M , Kays R . 2011 . The Movebank data model for animal tracking . Environmental Modelling and Software 26 : 834 – 835 .
Kuenzer C , Ottinger M , Wegmann M , Guo H , Wang C , Zhang J , Dech S , Wikelski M . 2014 . Earth observation satellite sensors for biodiversity monitoring: Potentials and bottlenecks . International Journal of Remote Sensing 35 : 6599 – 6647 .
Kwok R. 2019 . AI empowers conservation biology . Nature 567 : 133 .
Lahoz-Monfort JJ et al. 2019 . A call for international leadership and coordination to realize the potential of conservation technology . BioScience 69 : 823 – 832 .
Langlois T et al. 2020 . A field and video annotation guide for baited remote underwater stereo-video surveys of demersal fish assemblages . Methods in Ecology and Evolution 11 : 1401 – 1409 .
Laske TG , Evans AL , Arnemo JM , Iles TL , Ditmer MA , Fröbert O , Garshelis DL , Iaizzo PA . 2018 . Development and use of implantable cardiac monitors in free-ranging American black and Eurasian brown bears: System evolution and lessons learned . Animal Biotelemetry 6 : 13 .
Lathlean J , Seuront L. 2014 . Infrared thermography in marine ecology: Methods, previous applications and future challenges . Marine Ecology Progress Series 514 : 263 – 277 .
LeCun Y , Bengio Y , Hinton G . 2015 . Deep learning . Nature 521 : 436 – 444 .
Lexico . 2021 . “technology, n.” Oxford University Press . www.lexico.com/definition/technology [ accessed 26.4.2021 ].
Leyequien E , Verrelst J , Slot M , Schaepman-Strub G , Heitkönig IMA , Skidmore A . 2007 . Capturing the fugitive: Applying remote sensing to terrestrial animal distribution and diversity . International Journal of Applied Earth Observation and Geoinformation 9 : 1 – 20 .
Linchant J , Lisein J , Semeki J , Lejeune P , Vermeulen C . 2015 . Are unmanned aircraft systems (UASs) the future of wildlife monitoring? A review of accomplishments and challenges . Mammal Review 45 : 239 – 252 .
Lisovski S et al. 2020 . Light-level geolocator analyses: A user's guide . Journal of Animal Ecology 89 : 221 – 236 .
Löffler E , Margules C. 1980 . Wombats detected from space . Remote Sensing of Environment 9 : 47 – 56 .
Lucchetti A , Sala A. 2012 . Impact and performance of Mediterranean fishing gear by side-scan sonar technology . Canadian Journal of Fisheries and Aquatic Sciences 69 : 1806 – 1816 .
Martiskainen P , Järvinen M , Skön J-P , Tiirikainen J , Kolehmainen M , Mononen J . 2009 . Cow behaviour pattern recognition using a three-dimensional accelerometer and support vector machines . Applied Animal Behaviour Science 119 : 32 – 38 .
Marvin DC , Koh LP , Lynam AJ , Wich S , Davies AB , Krishnamurthy R , Stokes E , Starkey R , Asner GP . 2016 . Integrating technologies for scalable ecology and conservation . Global Ecology and Conservation 7 : 262 – 275 .
McCafferty DJ. 2013 . Applications of thermal imaging in avian science . Ibis 155 : 4 – 15 .
McKown MW , Lukac M , Borker B , Tershy B , Croll D . 2012 . A wireless acoustic sensor network for monitoring wildlife in remote locations . Journal of the Acoustical Society of America 132 : 2036 – 2036 .
Melin M , Shapiro AC , Glover-Kapfer P . 2017 . LIDAR for Ecology and Conservation . World Wildlife Fund. Conservation Technology Series no. 1 .
Mennill DJ , Doucet SM , Ward K-AA , Maynard DF , Otis B , Burt JM . 2012 . A novel digital telemetry system for tracking wild animals: A field test for studying mate choice in a lekking tropical bird . Methods in Ecology and Evolution 3 : 663 – 672 .
Merino L , Dios JRM , Ollero A . 2015 . Cooperative unmanned aerial systems for fire detection, monitoring, and extinguishing . Pages 2693 – 2722 in Valavanis K , Vachtsevanos GJ , eds. Handbook of Unmanned Aerial Vehicles . Springer .
Miyoshi GT , Arruda M dos S , Osco LP , Marcato Junior J , Gonçalves DN , Imai NN , Tommaselli AMG , Honkavaara E , Gonçalves WN . 2020 . A novel deep learning method to identify single tree species in UAV-based hyperspectral images . Remote Sensing 12 : 1294 .
Moll RJ , Millspaugh JJ , Beringer J , Sartwell J , He Z . 2007 . A new ‘view’ of ecology and conservation through animal-borne video systems . Trends in Ecology and Evolution 22 : 660 – 668 .
Morelle K , Bouche P , Lehaire F , Leeman V , Lejeune P . 2012 . Game species monitoring using road-based distance sampling in association with thermal imagers: A covariate analysis . Animal Biodiversity and Conservation 35 : 253 – 265 .
Mortimer B , Rees WL , Koelemeijer P , Nissen-Meyer T . 2018 . Classifying elephant behaviour through seismic vibrations . Current Biology 28 : R547 – R548 .
Moursund RA , Carlson TJ , Peters RD . 2003 . A fisheries application of a dual-frequency identification sonar acoustic camera . ICES Journal of Marine Science 60 : 678 – 683 .
Mulero-Pázmány M , Jenni-Eiermann S , Strebel N , Sattler T , Negro JJ , Tablado Z . 2017 . Unmanned aircraft systems as a new source of disturbance for wildlife: A systematic review . PLOS ONE 12 : e0178448 .
Narins PM , Lewis ER , Jarvis JJUM , O'Riain J . 1997 . The use of seismic signals by fossorial Southern African mammals: A neuroethological gold mine . Brain Research Bulletin 44 : 641 – 646 .
Newman G , Wiggins A , Crall A , Graham E , Newman S , Crowston K . 2012 . The future of citizen science: Emerging technologies and shifting paradigms . Frontiers in Ecology and the Environment 10 : 298 – 304 .
Nilsson C et al. 2018 . Field validation of radar systems for monitoring bird migration . Journal of Applied Ecology 55 : 2552 – 2564 .
Noonan MJ , Markham A , Newman C , Trigoni N , Buesching CD , Ellwood SA , Macdonald DW . 2015 . A new magneto-inductive tracking technique to uncover subterranean activity: What do animals do underground? Methods in Ecology and Evolution 6 : 510 – 520 .
Norouzzadeh MS , Nguyen A , Kosmala M , Swanson A , Palmer MS , Packer C , Clune J . 2018 . Automatically identifying, counting, and describing wild animals in camera-trap images with deep learning . Proceedings of the National Academy of Sciences 115 : E5716 – E5725 .
O'Connell AF , Nichols JD , Karanth KU . 2011 . Camera Traps in Animal Ecology: Methods and Analyses . Springer .
Pajares G. 2015 . Overview and current status of remote sensing applications based on unmanned aerial vehicles (UAVs) . Photogrammetric Engineering and Remote Sensing 81 : 281 – 329 .
Parrott J , Chasse M. 2005 . Intermittent Wildlife Feeder . US patent no. US20050241587A1 .
Patterson TA , Thomas L , Wilcox C , Ovaskainen O , Matthiopoulos J . 2008 . State–space models of individual animal movement . Trends in Ecology and Evolution 23 : 87 – 94 .
Paynter I et al. 2016 . Observing ecosystems with lightweight, rapid-scanning terrestrial lidar scanners . Remote Sensing in Ecology and Conservation 2 : 174 – 189 .
Pettorelli N , Laurance WF , O'Brien TG , Wegmann M , Nagendra H , Turner W . 2014 . Satellite remote sensing for applied ecologists: Opportunities and challenges . Journal of Applied Ecology 51 : 839 – 848 .
Pijanowski BC , Villanueva-Rivera LJ , Dumyahn SL , Farina A , Krause BL , Napoletano BM , Gage SH , Pieretti N . 2011 . Soundscape ecology: The science of sound in the landscape . BioScience 61 : 203 – 216 .
Pimm SL , Alibhai S , Bergl R , Dehgan A , Giri C , Jewell Z , Joppa L , Kays R , Loarie S . 2015 . Emerging technologies to conserve biodiversity . Trends in Ecology and Evolution 30 : 685 – 696 .
Plötz J , Bornemann H , Knust R , Schröder A , Bester M . 2002 . Foraging behaviour of Weddell seals, and its ecological implications . Pages 148 – 156 in Arntz WE , Clarke A , eds. Ecological Studies in the Antarctic Sea Ice Zone: Results of EASIZ Midterm Symposium . Springer .
Porter J et al. 2005 . Wireless sensor networks for ecology . BioScience 55 : 561 – 572 .
Porter JH , Nagy E , Kratz TK , Hanson P , Collins SL , Arzberger P . 2009 . New eyes on the world: Advanced sensors for ecology . BioScience 59 : 385 – 397 .
Pride IG , Swift SM. 1992 . Wildlife Telemetry: Remote Monitoring and Tracking of Animals . Ellis Horwood .
Raskoff KA , Matsumoto GI. 2004 . Stellamedusa ventana, a new mesopelagic scyphomedusa from the eastern Pacific representing a new subfamily, the Stellamedusinae . Journal of the Marine Biological Association of the United Kingdom 84 : 37 – 42 .
Read J , Gigliotti F , Darby S , Lapidge S . 2014 . Dying to be clean: Pen trials of novel cat and fox control devices . International Journal of Pest Management 60 : 166 – 172 .
Recio MR , Mathieu R , Denys P , Sirguey P , Seddon PJ . 2011 . Lightweight GPS-tags, one giant leap for wildlife tracking? An assessment approach . PLOS ONE 6 : e28225 .
Rhinehart TA , Chronister LM , Devlin T , Kitzes J . 2020 . Acoustic localization of terrestrial wildlife: Current practices and future opportunities . Ecology and Evolution 10 : 6794 – 6818 .
Riley JR , Smith AD , Reynolds DR , Edwards AS , Osborne JL , Williams IH , Carreck NL , Poppy GM . 1996 . Tracking bees with harmonic radar . Nature 379 : 29 – 30 .
Robinson EJH , Richardson TO , Sendova-Franks AB , Feinerman O , Franks NR . 2009 . Radio tagging reveals the roles of corpulence, experience and social information in ant decision making . Behavioral Ecology and Sociobiology 63 : 627 – 636 .
Ropert-Coudert Y , Wilson RP. 2005 . Trends and perspectives in animal-attached remote sensing . Frontiers in Ecology and the Environment 3 : 437 – 444 .
Royle JA , Chandler RB , Sollmann R , Gardner B . 2013 . Spatial Capture–Recapture . Academic Press .
Roznik EA , Alford RA. 2015 . Seasonal ecology and behavior of an endangered rainforest frog ( Litoria rheocola ) threatened by disease . PLOS ONE 10 : e0127851 .
Rubenstein DR , Hobson KA. 2004 . From birds to butterflies: Animal movement patterns and stable isotopes . Trends in Ecology and Evolution 19 : 256 – 263 .
Russello M , Amato G , DeSalle R , Knapp M . 2020 . Conservation genetics and genomics . Genes 11 : 318 .
Rutz C , Hays GC. 2009 . New frontiers in biologging science . Biology Letters 5 : 289 – 292 .
Rutz C , Troscianko J. 2013 . Programmable, miniature video-loggers for deployment on wild birds and other wildlife . Methods in Ecology and Evolution 4 : 114 – 122 .
Sandbrook C. 2015 . The social implications of using drones for biodiversity conservation . Ambio 44 : 636 – 647 .
Schlacher TA , Williams A , Althaus F , Schlacher-Hoenlinger MA . 2010 . High-resolution seabed imagery as a tool for biodiversity conservation planning on continental margins . Marine Ecology 31 : 200 – 221 .
Shafer MW , Vega G , Rothfus K , Flikkema P . 2019 . UAV wildlife radiotelemetry: System and methods of localization . Methods in Ecology and Evolution 10 : 1783 – 1795 .
Shepherd K. 2001 . Remotely Operated Vehicles (ROVs)* . Pages 742 – 747 in Steele JH , ed. Encyclopedia of Ocean Sciences , 2nd ed. Academic Press .
Sheppard JK , McGann A , Lanzone M , Swaisgood RR . 2015 . An autonomous GPS geofence alert system to curtail avian fatalities at wind farms . Animal Biotelemetry 3 : 1 – 8 .
Shipley JR , Kapoor J , Dreelin RA , Winkler DW . 2018 . An open-source sensor-logger for recording vertical movement in free-living organisms . Methods in Ecology and Evolution 9 : 465 – 471 .
Signer C , Ruf T , Schober F , Fluch G , Paumann T , Arnold W . 2010 . A versatile telemetry system for continuous measurement of heart rate, body temperature and locomotor activity in free-ranging ruminants . Methods in Ecology and Evolution 1 : 75 – 85 .
Singh H , Armstrong R , Gilbes F , Eustice R , Roman C , Pizarro O , Torres J . 2004 . Imaging coral I: Imaging coral habitats with the SeaBED AUV . Subsurface Sensing Technologies and Applications 5 : 25 – 42 .
Smyth B , Nebel S. 2013 . Passive Integrated Transponder (PIT) Tags in the Study of Animal Movement . Nature Education Knowledge 4 : 3 .
Steen KA , Villa-Henriksen A , Therkildsen OR , Green O . 2012 . Automatic detection of animals in mowing operations using thermal cameras . Sensors 12 : 7587 – 7597 .
Stevenson BC , Borchers DL , Altwegg R , Swift RJ , Gillespie DM , Measey GJ . 2015 . A general framework for animal density estimation from acoustic detections across a fixed microphone array . Methods in Ecology and Evolution 6 : 38 – 48 .
Stowell D , Wood MD , Pamuła H , Stylianou Y , Glotin H . 2019 . Automatic acoustic detection of birds through deep learning: The first Bird Audio Detection challenge . Methods in Ecology and Evolution 10 : 368 – 380 .
Sueur J , Farina A , Gasc A , Pieretti N , Pavoine S . 2014 . Acoustic indices for biodiversity assessment and landscape investigation . Acta Acustica united with Acustica 100 : 772 – 781 .
Sugai LSM , Silva TSF , Jr Ribeiro JW , Llusia D . 2019 . Terrestrial passive acoustic monitoring: Review and perspectives . BioScience 69 : 15 – 25 .
Tanago JG de et al. 2018 . Estimation of above-ground biomass of large tropical trees with terrestrial LiDAR . Methods in Ecology and Evolution 9 : 223 – 234 .
Taylor P et al. 2017 . The Motus Wildlife Tracking System: A collaborative research network to enhance the understanding of wildlife movement . Avian Conservation and Ecology 12 : 8 .
Thomas B , Holland JD , Minot EO . 2012 . Wildlife tracking technology options and cost considerations . Wildlife Research 38 : 653 – 663 .
Thomas J , Polin J , Sreenath K , Kumar V . 2014 . Avian-Inspired Grasping for Quadrotor Micro UAVs . Paper presented at ASME 2013 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference. 12 February 2014 .
Thomas L , Buckland ST , Rexstad EA , Laake JL , Strindberg S , Hedley SL , Bishop JRB , Marques TA , Burnham KP . 2010 . Distance software: Design and analysis of distance sampling surveys for estimating population size . Journal of Applied Ecology 47 : 5 – 14 .
Thorpe AS , Barnett DT , Elmendorf SC , Hinckley E-LS , Hoekman D , Jones KD , LeVan KE , Meier CL , Stanish LF , Thibault KM . 2016 . Introduction to the sampling designs of the National Ecological Observatory Network Terrestrial Observation System . Ecosphere 7 : e01627 .
Towsey M , Zhang L , Cottman-Fields M , Wimmer J , Zhang J , Roe P . 2014 . Visualization of long-duration acoustic recordings of the environment . Procedia Computer Science 29 : 703 – 712 .
Turner GG et al. 2014 . Nonlethal screening of bat-wing skin with the use of ultraviolet fluorescence to detect lesions indicative of white-nose syndrome . Journal of Wildlife Diseases 50 : 566 – 573 .
Vaughn CR. 1985 . Birds and insects as radar targets: A review . Proceedings of the IEEE 73 : 205 – 227 .
Vierling KT , Vierling LA , Gould WA , Martinuzzi S , Clawges RM . 2008 . Lidar: Shedding new light on habitat characterization and modeling . Frontiers in Ecology and the Environment 6 : 90 – 98 .
Wall J , Wittemyer G , Klinkenberg B , Douglas-Hamilton I . 2014 . Novel opportunities for wildlife conservation and research with real-time monitoring . Ecological Applications 24 : 593 – 601 .
Walsh SJ , McCleary AL , Mena CF , Shao Y , Tuttle JP , González A , Atkinson R . 2008 . QuickBird and Hyperion data analysis of an invasive plant species in the Galapagos Islands of Ecuador: Implications for control and land use management . Remote Sensing of Environment 112 : 1927 – 1941 .
Wang K , Franklin SE , Guo X , Cattet M , Wang K , Franklin SE , Guo X , Cattet M . 2010 . Remote sensing of ecology, biodiversity and conservation: A review from the perspective of remote sensing specialists . Sensors 10 : 9647 – 9667 .
Wearn OR , Glover-Kapfer P . 2017 . Camera-Trapping for Conservation: A Guide to Best-Practices . World Wildlife Fund. Conservation Technology Series no. 1 .
Whytock RC , Christie J. 2016 . Solo: An open source, customizable and inexpensive audio recorder for bioacoustic research . Methods in Ecology and Evolution 8 : 308 – 312 .
Wich SA , Koh LP. 2018 . Conservation Drones: Mapping and Monitoring Biodiversity . Oxford University Press .
Wijers M , Loveridge A , Macdonald DW , Markham A . 2019 . CARACAL: A versatile passive acoustic monitoring tool for wildlife research and conservation . Bioacoustics 30 : 41 – 57 .
Wikelski M , Kays RW , Kasdin NJ , Thorup K , Smith JA , Swenson GW . 2007 . Going wild: What a global small-animal tracking system could do for experimental biologists . Journal of Experimental Biology 210 : 181 – 186 .
Williams K , De Robertis A , Berkowitz Z , Rooper C , Towler R . 2014 . An underwater stereo-camera trap . Methods in Oceanography 11 : 1 – 12 .
Wilson AM , Barr J , Zagorski M . 2017 . The feasibility of counting songbirds using unmanned aerial vehicles . Auk 134 : 350 – 362 .
Wilson R , Liebsch N. 2003 . Up-beat motion in swinging limbs: New insights into assessing movement in free-living aquatic vertebrates . Marine Biology 142 : 537 – 547 .
Wimmer J , Towsey M , Planitz B , Williamson I , Roe P . 2013 . Analysing environmental acoustic data through collaboration and automation . Future Generation Computer Systems 29 : 560 – 568 .
Wood JD , O'Connell-Rodwell CE , Klemperer SL . 2005 . Using seismic sensors to detect elephants and other large mammals: A potential census technique . Journal of Applied Ecology 42 : 587 – 594 .
Wrege PH , Rowland ED , Keen S , Shiu Y . 2017 . Acoustic monitoring for conservation in tropical forests: Examples from forest elephants . Methods in Ecology and Evolution 8 : 1292 – 1301 .
Wrege PH , Rowland ED , Thompson BG , Batruch N . 2010 . Use of acoustic tools to reveal otherwise cryptic responses of forest elephants to oil exploration . Conservation Biology 24 : 1578 – 1585 .
Xu G , Shen W , Wang X , Xu G , Shen W , Wang X . 2014 . Applications of Wireless Sensor Networks in Marine environment monitoring: A survey . Sensors 14 : 16932 – 16954 .
Xu J , Zhao D. 2014 . Review of coral reef ecosystem remote sensing . Acta Ecologica Sinica 34 : 19 – 25 .
Yang Z , Wang T , Skidmore AK , de Leeuw J , Said MY , Freer J . 2014 . Spotting East African mammals in open savannah from space . PLOS ONE 9 : e115989 .
Zabel RW , Wagner T , Congleton JL , Smith SG , Williams JG . 2005 . Survival and selection of migrating salmon from capture–recapture models with individual traits . Ecological Applications 15 : 1427 – 1439 .
Zárybnická M , Kubizňák P , Šindelář J , Hlaváč V . 2016 . Smart nest box: A tool and methodology for monitoring of cavity-dwelling animals . Methods in Ecology and Evolution 7 : 483 – 492 .
Zaugg S , Saporta G , van Loon E , Schmaljohann H , Liechti F . 2008 . Automatic identification of bird targets with radar via patterns produced by wing flapping . Journal of the Royal Society Interface 5 : 1041 – 1053 .
| Month: | Total Views: |
|---|---|
| July 2021 | 1,155 |
| August 2021 | 1,083 |
| September 2021 | 397 |
| October 2021 | 916 |
| November 2021 | 740 |
| December 2021 | 457 |
| January 2022 | 428 |
| February 2022 | 419 |
| March 2022 | 467 |
| April 2022 | 466 |
| May 2022 | 384 |
| June 2022 | 361 |
| July 2022 | 324 |
| August 2022 | 345 |
| September 2022 | 525 |
| October 2022 | 600 |
| November 2022 | 587 |
| December 2022 | 534 |
| January 2023 | 341 |
| February 2023 | 365 |
| March 2023 | 525 |
| April 2023 | 587 |
| May 2023 | 346 |
| June 2023 | 291 |
| July 2023 | 326 |
| August 2023 | 316 |
| September 2023 | 475 |
| October 2023 | 511 |
| November 2023 | 619 |
| December 2023 | 512 |
| January 2024 | 666 |
| February 2024 | 771 |
| March 2024 | 929 |
| April 2024 | 815 |
| May 2024 | 868 |
| June 2024 | 566 |
| July 2024 | 577 |
| August 2024 | 710 |
| September 2024 | 555 |
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1525-3244
- Copyright © 2024 American Institute of Biological Sciences
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Volume 21 Supplement 9
Selected Articles from the 20th International Conference on Bioinformatics & Computational Biology (BIOCOMP 2019)
- Introduction
- Open access
- Published: 03 December 2020
Current trend and development in bioinformatics research
- Yuanyuan Fu 1 ,
- Zhougui Ling 1 , 2 ,
- Hamid Arabnia 3 &
- Youping Deng 1
BMC Bioinformatics volume 21 , Article number: 538 ( 2020 ) Cite this article
11k Accesses
21 Citations
4 Altmetric
Metrics details
This is an editorial report of the supplements to BMC Bioinformatics that includes 6 papers selected from the BIOCOMP’19—The 2019 International Conference on Bioinformatics and Computational Biology. These articles reflect current trend and development in bioinformatics research.
The supplement to BMC Bioinformatics was proposed to launch during the BIOCOMP’19—The 2019 International Conference on Bioinformatics and Computational Biology held from July 29 to August 01, 2019 in Las Vegas, Nevada. In this congress, a variety of research areas was discussed, including bioinformatics which was one of the major focuses due to the rapid development and requirement of using bioinformatics approaches in biological data analysis, especially for omics large datasets. Here, six manuscripts were selected after strict peer review, providing an overview of the bioinformatics research trend and its application for interdisciplinary collaboration.
Cancer is one of the leading causes of morbidity and mortality worldwide. There exists an urgent need to identify new biomarkers or signatures for early detection and prognosis. Mona et al. identified biomarker genes from functional network based on the 407 differential expressed genes between lung cancer and healthy populations from a public Gene Expression Omnibus dataset. The lower expression of sixteen gene signature is associated with favorable lung cancer survival, DNA repair, and cell regulation [ 1 ]. A new class of biomarkers such as alternative splicing variants (ASV) have been studied in recent years. Various platforms and methods, for example, Affymetrix Exon-Exon Junction Array, RNA-seq, and liquid chromatography tandem mass spectrometry (LC–MS/MS), have been developed to explore the role of ASV in human disease. Zhang et al. have developed a bioinformatics workflow to combine LC–MS/MS with RNA-seq which provide new opportunities in biomarker discovery. In their study, they identified twenty-six alternative splicing biomarker peptides with one single intron event and one exon skipping event; further pathways indicated the 26 peptides may be involved in cancer, signaling, metabolism, regulation, immune system and hemostasis pathways which validated by the RNA-seq analysis [ 2 ].
Proteins serve crucial functions in essentially all biological processes and the function directly depends on their three-dimensional structures. Traditional approaches to elucidation of protein structures by NMR spectroscopy are time consuming and expensive, however, the faster and more cost-effective methods are critical in the development of personalized medicine. Cole et al. improved the REDRAFT software package in the important areas of usability, accessibility, and the core methodology which resulted in the ability to fold proteins [ 3 ].
The human microbiome is the aggregation of microorganisms that reside on or within human bodies. Rebecca et al. discussed the tissue-associated microbial detection in cancer using next generation sequencing (NGS). Various computational frameworks could shed light on the role of microbiota in cancer pathogenesis [ 4 ]. How to analyze the human microbiome data efficiently is a huge challenge. Zhang et al. developed a nonparametric test based on inter-point distance to evaluate statistical significance from a Bayesian point of view. The proposed test is more efficient and sensitive to the compositional difference compared with the traditional mean-based method [ 5 ].
Human disease is also considered as the cause of the interaction between genetic and environmental factors. In the last decades, there was a growing interest in the effect of metal toxicity on human health. Evaluating the toxicity of chemical mixture and their possible mechanism of action is still a challenge for humans and other organisms, as traditional methods are very time consuming, inefficient, and expensive, so a limited number of chemicals can be tested. In order to develop efficient and accurate predictive models, Yu et al. compared the results among a classification algorithm and identified 15 gene biomarkers with 100% accuracy for metal toxicant using a microarray classifier analysis [ 6 ].
Currently, there is a growing need to convert biological data into knowledge through a bioinformatics approach. We hope these articles can provide up-to-date information of research development and trend in bioinformatics field.
Availability of data and materials
Not applicable.
Abbreviations
The 2019 International Conference on Bioinformatics and Computational Biology
Liquid chromatography tandem mass spectrometry
Alternative splicing variants
Nuclear Magnetic Resonance
Residual Dipolar Coupling based Residue Assembly and Filter Tool
Next generation sequencing
Mona Maharjan RBT, Chowdhury K, Duan W, Mondal AM. Computational identification of biomarker genes for lung cancer considering treatment and non-treatment studies. 2020. https://doi.org/10.1186/s12859-020-3524-8 .
Zhang F, Deng CK, Wang M, Deng B, Barber R, Huang G. Identification of novel alternative splicing biomarkers for breast cancer with LC/MS/MS and RNA-Seq. Mol Cell Proteomics. 2020;16:1850–63. https://doi.org/10.1186/s12859-020-03824-8 .
Article Google Scholar
Casey Cole CP, Rachele J, Valafar H. Increased usability, algorithmic improvements and incorporation of data mining for structure calculation of proteins with REDCRAFT software package. 2020. https://doi.org/10.1186/s12859-020-3522-x .
Rebecca M, Rodriguez VSK, Menor M, Hernandez BY, Deng Y. Tissue-associated microbial detection in cancer using human sequencing data. 2020. https://doi.org/10.1186/s12859-020-03831-9 .
Qingyang Zhang TD. A distance based multisample test for high-dimensional compositional data with applications to the human microbiome . 2020. https://doi.org/10.1186/s12859-020-3530-x .
Yu Z, Fu Y, Ai J, Zhang J, Huang G, Deng Y. Development of predicitve models to distinguish metals from non-metal toxicants, and individual metal from one another. 2020. https://doi.org/10.1186/s12859-020-3525-7 .
Download references
Acknowledgements
This supplement will not be possible without the support of the International Society of Intelligent Biological Medicine (ISIBM).
About this supplement
This article has been published as part of BMC Bioinformatics Volume 21 Supplement 9, 2020: Selected Articles from the 20th International Conference on Bioinformatics & Computational Biology (BIOCOMP 2019). The full contents of the supplement are available online at https://bmcbioinformatics.biomedcentral.com/articles/supplements/volume-21-supplement-9 .
Publication of this supplement has been supported by NIH grants R01CA223490 and R01 CA230514 to Youping Deng and 5P30GM114737, P20GM103466, 5U54MD007601 and 5P30CA071789.
Author information
Authors and affiliations.
Department of Quantitative Health Sciences, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu, HI, 96813, USA
Yuanyuan Fu, Zhougui Ling & Youping Deng
Department of Pulmonary and Critical Care Medicine, The Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou, 545005, China
Zhougui Ling
Department of Computer Science, University of Georgia, Athens, GA, 30602, USA
Hamid Arabnia
You can also search for this author in PubMed Google Scholar
Contributions
YF drafted the manuscript, ZL, HA, and YD revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Youping Deng .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Fu, Y., Ling, Z., Arabnia, H. et al. Current trend and development in bioinformatics research. BMC Bioinformatics 21 (Suppl 9), 538 (2020). https://doi.org/10.1186/s12859-020-03874-y
Download citation
Published : 03 December 2020
DOI : https://doi.org/10.1186/s12859-020-03874-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Bioinformatics
- Human disease
BMC Bioinformatics
ISSN: 1471-2105
- General enquiries: [email protected]
- Utility Menu
The Life Sciences Outreach Program
Enhancing biology education and connecting teachers from 2002 to 2021, modern tools in biology: cutting-edge techniques in context (2021), fall 2021 faculty speaker series for high school biology teachers.
The application for the Fall 2021 Faculty Speaker Series is closed. Thank you for your interest. Know any Chemistry or Physics teachers? Click here .
This year’s program will explore the symbiotic relationship between biology and technology. Advances in biological research rely on the development and constant improvement of technologies permitting the visualization, manipulation, and analysis of biological data. Harvard scientists will share how their research in fields such as genetics, cell biology, and microbiology both employ and inspire the development of exciting new tools like high-resolution microscopy, CRISPR, and game theory.
Program Format and Application Process
All participating teachers commit to attending 5 Thursday afternoon online sessions, beginning at 4PM. Each session will involve a short talk followed by a 15 minute Q&A period. After a quick (10 minute) break, we will reconvene to engage with an activity under the leadership of our presenters and LSO Fellows, ending around 6:15PM.
Because we will be utilizing breakout sessions within the Zoom platform, we may need to limit enrollment. We ask that teachers apply only if they can commit to full participation for each of the 5 afternoons.
Five Thursday afternoon sessions will take place from 4-6:15PM on:
- Week 1 October 7th
- Week 2 October 21st
- Week 3 November 4th
- Week 4 November 18th
- Week 5 December 2nd
This program is FREE of charge and funded by the Derek Bok Center for Teaching and Learning. All participants will receive a PDP certificate upon completion of the program.
Applications are due on Friday, September 17th. Applicants must be full-time high school life science teachers or curriculum directors at public, private, parochial or charter schools in New England. We will notify applicants of their status by September 24th and accepted applicants must respond to our acceptance email by October 1st. All program communication takes place via email.
Opportunity for Chemistry and Physics Educators
Do you have colleagues in Chemistry or Physics who would be interested in attending a talk as well?
Professor Suyang Xu from the Harvard University Department of Chemistry and Chemical Biology is offering a talk specifically for high school Chemistry and Physics teachers, “Material Sciences Research and the Development of Quantum Technologies,” on Thursday, October 28 at 4pm ET over Zoom. Please see below for the workshop description.
Please share the application link with colleagues who may be interested.
Quantum technologies have the power to change the world. By deepening our understanding of the motion of electrons, atoms and molecules, quantum mechanics will likely transform medicine, revolutionize communications, and reform artificial intelligence.
We will explore how quantum physics directly enables novel technologies such as quantum computers and quantum cryptography, and we will discuss current research on new materials which provide a platform for new quantum technologies. In particular, we will focus on a class of new materials called the topological quantum materials, which provide the necessary platform for new technologies.
Socially Engaged Learning at The Derek Bok Center: Life Sciences Outreach Partnership
The mission of the Derek Bok Center for Teaching and Learning is to elevate the quality of teaching at all levels. The Center offers avenues through which faculty and graduate students develop teaching and communication skills in their disciplines. The Socially Engaged Learning initiative at the Bok Center provides interested faculty and graduate students the unique opportunity to practice these skills in front of important external audiences. The Lifes Sciences Outreach Fall Faculty Speaker Series consists of faculty talks and activities led by faculty, graduate students and/or post-doctoral fellows. We encourage you to help us think about ways in which to leverage high school classroom experience and pedagogical expertise to help further graduate student development in the areas of teaching and communication.
If you have questions, please contact:
Susan Johnson Assistant Director of Socially Engaged Learning Derek Bok Center for Teaching and Learning [email protected]
- Lecture 1 (10/07/21) - Sean Eddy, "The Language of DNA"
- Lecture 2 (10/21/21) - Ali Zomorrodi, "Unraveling the Games that Microbes Play Using Mathematical and Systems Biology Modeling"
- Lecture 3 (11/04/21) - Brian Liau, "CRISPR/Cas9: Genetic Scissors - From Basic Research to Therapeutics"
- Lecture 4 (11/18/21) - Maxim Prigozhin, "Biological Microscopy in the 21st Century"; Doug Richardson, "Current Techniques in Microscopy"
- Lecture 5 (12/3/21) - Nick Hilgert and Emily Rivard, "Phase Separation in Biology"
- Open access
- Published: 26 February 2019
Stem cells: past, present, and future
- Wojciech Zakrzewski 1 ,
- Maciej Dobrzyński 2 ,
- Maria Szymonowicz 1 &
- Zbigniew Rybak 1
Stem Cell Research & Therapy volume 10 , Article number: 68 ( 2019 ) Cite this article
586k Accesses
958 Citations
53 Altmetric
Metrics details
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation. Quality control and teratoma formation assays are important procedures in assessing the properties of the stem cells tested. Derivation methods and the utilization of culturing media are crucial to set proper environmental conditions for controlled differentiation. Among many types of stem tissue applications, the use of graphene scaffolds and the potential of extracellular vesicle-based therapies require attention due to their versatility. The review is summarized by challenges that stem cell therapy must overcome to be accepted worldwide. A wide variety of possibilities makes this cutting edge therapy a turning point in modern medicine, providing hope for untreatable diseases.
Stem cell classification
Stem cells are unspecialized cells of the human body. They are able to differentiate into any cell of an organism and have the ability of self-renewal. Stem cells exist both in embryos and adult cells. There are several steps of specialization. Developmental potency is reduced with each step, which means that a unipotent stem cell is not able to differentiate into as many types of cells as a pluripotent one. This chapter will focus on stem cell classification to make it easier for the reader to comprehend the following chapters.
Totipotent stem cells are able to divide and differentiate into cells of the whole organism. Totipotency has the highest differentiation potential and allows cells to form both embryo and extra-embryonic structures. One example of a totipotent cell is a zygote, which is formed after a sperm fertilizes an egg. These cells can later develop either into any of the three germ layers or form a placenta. After approximately 4 days, the blastocyst’s inner cell mass becomes pluripotent. This structure is the source of pluripotent cells.
Pluripotent stem cells (PSCs) form cells of all germ layers but not extraembryonic structures, such as the placenta. Embryonic stem cells (ESCs) are an example. ESCs are derived from the inner cell mass of preimplantation embryos. Another example is induced pluripotent stem cells (iPSCs) derived from the epiblast layer of implanted embryos. Their pluripotency is a continuum, starting from completely pluripotent cells such as ESCs and iPSCs and ending on representatives with less potency—multi-, oligo- or unipotent cells. One of the methods to assess their activity and spectrum is the teratoma formation assay. iPSCs are artificially generated from somatic cells, and they function similarly to PSCs. Their culturing and utilization are very promising for present and future regenerative medicine.
Multipotent stem cells have a narrower spectrum of differentiation than PSCs, but they can specialize in discrete cells of specific cell lineages. One example is a haematopoietic stem cell, which can develop into several types of blood cells. After differentiation, a haematopoietic stem cell becomes an oligopotent cell. Its differentiation abilities are then restricted to cells of its lineage. However, some multipotent cells are capable of conversion into unrelated cell types, which suggests naming them pluripotent cells.
Oligopotent stem cells can differentiate into several cell types. A myeloid stem cell is an example that can divide into white blood cells but not red blood cells.
Unipotent stem cells are characterized by the narrowest differentiation capabilities and a special property of dividing repeatedly. Their latter feature makes them a promising candidate for therapeutic use in regenerative medicine. These cells are only able to form one cell type, e.g. dermatocytes.
Stem cell biology
A blastocyst is formed after the fusion of sperm and ovum fertilization. Its inner wall is lined with short-lived stem cells, namely, embryonic stem cells. Blastocysts are composed of two distinct cell types: the inner cell mass (ICM), which develops into epiblasts and induces the development of a foetus, and the trophectoderm (TE). Blastocysts are responsible for the regulation of the ICM microenvironment. The TE continues to develop and forms the extraembryonic support structures needed for the successful origin of the embryo, such as the placenta. As the TE begins to form a specialized support structure, the ICM cells remain undifferentiated, fully pluripotent and proliferative [ 1 ]. The pluripotency of stem cells allows them to form any cell of the organism. Human embryonic stem cells (hESCs) are derived from the ICM. During the process of embryogenesis, cells form aggregations called germ layers: endoderm, mesoderm and ectoderm (Fig. 1 ), each eventually giving rise to differentiated cells and tissues of the foetus and, later on, the adult organism [ 2 ]. After hESCs differentiate into one of the germ layers, they become multipotent stem cells, whose potency is limited to only the cells of the germ layer. This process is short in human development. After that, pluripotent stem cells occur all over the organism as undifferentiated cells, and their key abilities are proliferation by the formation of the next generation of stem cells and differentiation into specialized cells under certain physiological conditions.
Oocyte development and formation of stem cells: the blastocoel, which is formed from oocytes, consists of embryonic stem cells that later differentiate into mesodermal, ectodermal, or endodermal cells. Blastocoel develops into the gastrula
Signals that influence the stem cell specialization process can be divided into external, such as physical contact between cells or chemical secretion by surrounding tissue, and internal, which are signals controlled by genes in DNA.
Stem cells also act as internal repair systems of the body. The replenishment and formation of new cells are unlimited as long as an organism is alive. Stem cell activity depends on the organ in which they are in; for example, in bone marrow, their division is constant, although in organs such as the pancreas, division only occurs under special physiological conditions.
Stem cell functional division
Whole-body development.
During division, the presence of different stem cells depends on organism development. Somatic stem cell ESCs can be distinguished. Although the derivation of ESCs without separation from the TE is possible, such a combination has growth limits. Because proliferating actions are limited, co-culture of these is usually avoided.
ESCs are derived from the inner cell mass of the blastocyst, which is a stage of pre-implantation embryo ca. 4 days after fertilization. After that, these cells are placed in a culture dish filled with culture medium. Passage is an inefficient but popular process of sub-culturing cells to other dishes. These cells can be described as pluripotent because they are able to eventually differentiate into every cell type in the organism. Since the beginning of their studies, there have been ethical restrictions connected to the medical use of ESCs in therapies. Most embryonic stem cells are developed from eggs that have been fertilized in an in vitro clinic, not from eggs fertilized in vivo.
Somatic or adult stem cells are undifferentiated and found among differentiated cells in the whole body after development. The function of these cells is to enable the healing, growth, and replacement of cells that are lost each day. These cells have a restricted range of differentiation options. Among many types, there are the following:
Mesenchymal stem cells are present in many tissues. In bone marrow, these cells differentiate mainly into the bone, cartilage, and fat cells. As stem cells, they are an exception because they act pluripotently and can specialize in the cells of any germ layer.
Neural cells give rise to nerve cells and their supporting cells—oligodendrocytes and astrocytes.
Haematopoietic stem cells form all kinds of blood cells: red, white, and platelets.
Skin stem cells form, for example, keratinocytes, which form a protective layer of skin.
The proliferation time of somatic stem cells is longer than that of ESCs. It is possible to reprogram adult stem cells back to their pluripotent state. This can be performed by transferring the adult nucleus into the cytoplasm of an oocyte or by fusion with the pluripotent cell. The same technique was used during cloning of the famous Dolly sheep.
hESCs are involved in whole-body development. They can differentiate into pluripotent, totipotent, multipotent, and unipotent cells (Fig. 2 ) [ 2 ].
Changes in the potency of stem cells in human body development. Potency ranges from pluripotent cells of the blastocyst to unipotent cells of a specific tissue in a human body such as the skin, CNS, or bone marrow. Reversed pluripotency can be achieved by the formation of induced pluripotent stem cells using either octamer-binding transcription factor (Oct4), sex-determining region Y (Sox2), Kruppel-like factor 4 (Klf4), or the Myc gene
Pluripotent cells can be named totipotent if they can additionally form extraembryonic tissues of the embryo. Multipotent cells are restricted in differentiating to each cell type of given tissue. When tissue contains only one lineage of cells, stem cells that form them are called either called oligo- or unipotent.
iPSC quality control and recognition by morphological differences
The comparability of stem cell lines from different individuals is needed for iPSC lines to be used in therapeutics [ 3 ]. Among critical quality procedures, the following can be distinguished:
Short tandem repeat analysis—This is the comparison of specific loci on the DNA of the samples. It is used in measuring an exact number of repeating units. One unit consists of 2 to 13 nucleotides repeating many times on the DNA strand. A polymerase chain reaction is used to check the lengths of short tandem repeats. The genotyping procedure of source tissue, cells, and iPSC seed and master cell banks is recommended.
Identity analysis—The unintentional switching of lines, resulting in other stem cell line contamination, requires rigorous assay for cell line identification.
Residual vector testing—An appearance of reprogramming vectors integrated into the host genome is hazardous, and testing their presence is a mandatory procedure. It is a commonly used procedure for generating high-quality iPSC lines. An acceptable threshold in high-quality research-grade iPSC line collections is ≤ 1 plasmid copies per 100 cells. During the procedure, 2 different regions, common to all plasmids, should be used as specific targets, such as EBNA and CAG sequences [ 3 ]. To accurately represent the test reactions, a standard curve needs to be prepared in a carrier of gDNA from a well-characterized hPSC line. For calculations of plasmid copies per cell, it is crucial to incorporate internal reference gDNA sequences to allow the quantification of, for example, ribonuclease P (RNaseP) or human telomerase reverse transcriptase (hTERT).
Karyotype—A long-term culture of hESCs can accumulate culture-driven mutations [ 4 ]. Because of that, it is crucial to pay additional attention to genomic integrity. Karyotype tests can be performed by resuscitating representative aliquots and culturing them for 48–72 h before harvesting cells for karyotypic analysis. If abnormalities are found within the first 20 karyotypes, the analysis must be repeated on a fresh sample. When this situation is repeated, the line is evaluated as abnormal. Repeated abnormalities must be recorded. Although karyology is a crucial procedure in stem cell quality control, the single nucleotide polymorphism (SNP) array, discussed later, has approximately 50 times higher resolution.
Viral testing—When assessing the quality of stem cells, all tests for harmful human adventitious agents must be performed (e.g. hepatitis C or human immunodeficiency virus). This procedure must be performed in the case of non-xeno-free culture agents.
Bacteriology—Bacterial or fungal sterility tests can be divided into culture- or broth-based tests. All the procedures must be recommended by pharmacopoeia for the jurisdiction in which the work is performed.
Single nucleotide polymorphism arrays—This procedure is a type of DNA microarray that detects population polymorphisms by enabling the detection of subchromosomal changes and the copy-neutral loss of heterozygosity, as well as an indication of cellular transformation. The SNP assay consists of three components. The first is labelling fragmented nucleic acid sequences with fluorescent dyes. The second is an array that contains immobilized allele-specific oligonucleotide (ASO) probes. The last component detects, records, and eventually interprets the signal.
Flow cytometry—This is a technique that utilizes light to count and profile cells in a heterogeneous fluid mixture. It allows researchers to accurately and rapidly collect data from heterogeneous fluid mixtures with live cells. Cells are passed through a narrow channel one by one. During light illumination, sensors detect light emitted or refracted from the cells. The last step is data analysis, compilation and integration into a comprehensive picture of the sample.
Phenotypic pluripotency assays—Recognizing undifferentiated cells is crucial in successful stem cell therapy. Among other characteristics, stem cells appear to have a distinct morphology with a high nucleus to cytoplasm ratio and a prominent nucleolus. Cells appear to be flat with defined borders, in contrast to differentiating colonies, which appear as loosely located cells with rough borders [ 5 ]. It is important that images of ideal and poor quality colonies for each cell line are kept in laboratories, so whenever there is doubt about the quality of culture, it can always be checked according to the representative image. Embryoid body formation or directed differentiation of monolayer cultures to produce cell types representative of all three embryonic germ layers must be performed. It is important to note that colonies cultured under different conditions may have different morphologies [ 6 ].
Histone modification and DNA methylation—Quality control can be achieved by using epigenetic analysis tools such as histone modification or DNA methylation. When stem cells differentiate, the methylation process silences pluripotency genes, which reduces differentiation potential, although other genes may undergo demethylation to become expressed [ 7 ]. It is important to emphasize that stem cell identity, together with its morphological characteristics, is also related to its epigenetic profile [ 8 , 9 ]. According to Brindley [ 10 ], there is a relationship between epigenetic changes, pluripotency, and cell expansion conditions, which emphasizes that unmethylated regions appear to be serum-dependent.
hESC derivation and media
hESCs can be derived using a variety of methods, from classic culturing to laser-assisted methodologies or microsurgery [ 11 ]. hESC differentiation must be specified to avoid teratoma formation (see Fig. 3 ).
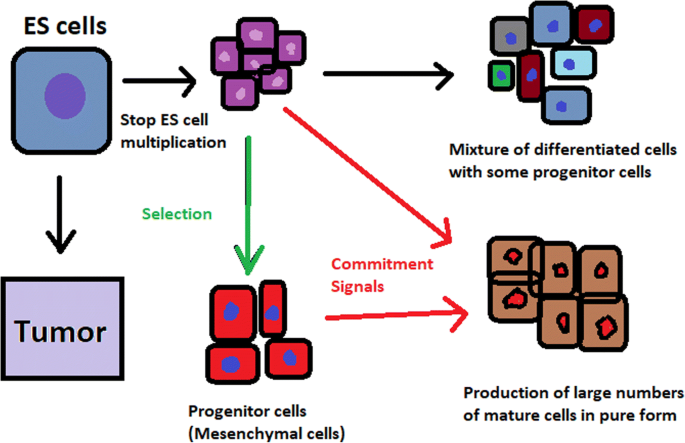
Spontaneous differentiation of hESCs causes the formation of a heterogeneous cell population. There is a different result, however, when commitment signals (in forms of soluble factors and culture conditions) are applied and enable the selection of progenitor cells
hESCs spontaneously differentiate into embryonic bodies (EBs) [ 12 ]. EBs can be studied instead of embryos or animals to predict their effects on early human development. There are many different methods for acquiring EBs, such as bioreactor culture [ 13 ], hanging drop culture [ 12 ], or microwell technology [ 14 , 15 ]. These methods allow specific precursors to form in vitro [ 16 ].
The essential part of these culturing procedures is a separation of inner cell mass to culture future hESCs (Fig. 4 ) [ 17 ]. Rosowski et al. [ 18 ] emphasizes that particular attention must be taken in controlling spontaneous differentiation. When the colony reaches the appropriate size, cells must be separated. The occurrence of pluripotent cells lasts for 1–2 days. Because the classical utilization of hESCs caused ethical concerns about gastrulas used during procedures, Chung et al. [ 19 ] found out that it is also possible to obtain hESCs from four cell embryos, leaving a higher probability of embryo survival. Additionally, Zhang et al. [ 20 ] used only in vitro fertilization growth-arrested cells.
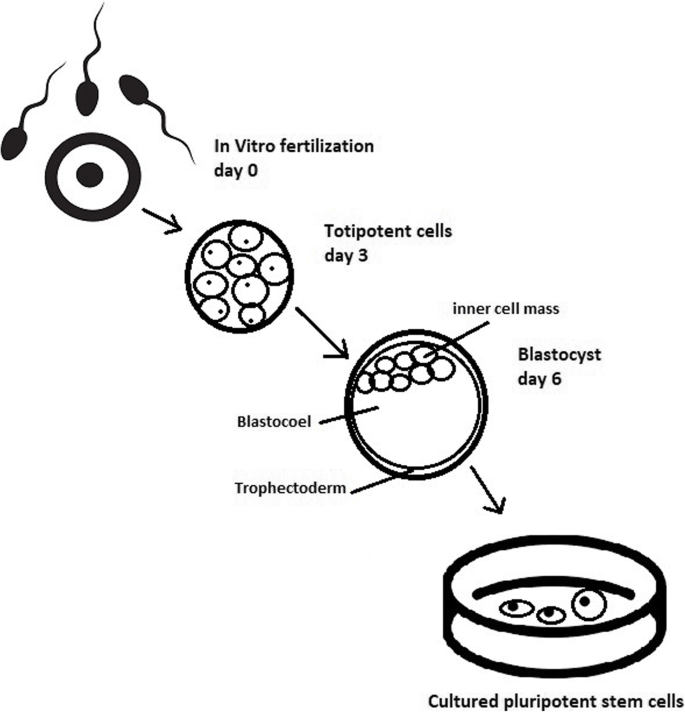
Culturing of pluripotent stem cells in vitro. Three days after fertilization, totipotent cells are formed. Blastocysts with ICM are formed on the sixth day after fertilization. Pluripotent stem cells from ICM can then be successfully transmitted on a dish
Cell passaging is used to form smaller clusters of cells on a new culture surface [ 21 ]. There are four important passaging procedures.
Enzymatic dissociation is a cutting action of enzymes on proteins and adhesion domains that bind the colony. It is a gentler method than the manual passage. It is crucial to not leave hESCs alone after passaging. Solitary cells are more sensitive and can easily undergo cell death; collagenase type IV is an example [ 22 , 23 ].
Manual passage , on the other hand, focuses on using cell scratchers. The selection of certain cells is not necessary. This should be done in the early stages of cell line derivation [ 24 ].
Trypsin utilization allows a healthy, automated hESC passage. Good Manufacturing Practice (GMP)-grade recombinant trypsin is widely available in this procedure [ 24 ]. However, there is a risk of decreasing the pluripotency and viability of stem cells [ 25 ]. Trypsin utilization can be halted with an inhibitor of the protein rho-associated protein kinase (ROCK) [ 26 ].
Ethylenediaminetetraacetic acid ( EDTA ) indirectly suppresses cell-to-cell connections by chelating divalent cations. Their suppression promotes cell dissociation [ 27 ].
Stem cells require a mixture of growth factors and nutrients to differentiate and develop. The medium should be changed each day.
Traditional culture methods used for hESCs are mouse embryonic fibroblasts (MEFs) as a feeder layer and bovine serum [ 28 ] as a medium. Martin et al. [ 29 ] demonstrated that hESCs cultured in the presence of animal products express the non-human sialic acid, N -glycolylneuraminic acid (NeuGc). Feeder layers prevent uncontrolled proliferation with factors such as leukaemia inhibitory factor (LIF) [ 30 ].
First feeder layer-free culture can be supplemented with serum replacement, combined with laminin [ 31 ]. This causes stable karyotypes of stem cells and pluripotency lasting for over a year.
Initial culturing media can be serum (e.g. foetal calf serum FCS), artificial replacement such as synthetic serum substitute (SSS), knockout serum replacement (KOSR), or StemPro [ 32 ]. The simplest culture medium contains only eight essential elements: DMEM/F12 medium, selenium, NaHCO 3, l -ascorbic acid, transferrin, insulin, TGFβ1, and FGF2 [ 33 ]. It is not yet fully known whether culture systems developed for hESCs can be allowed without adaptation in iPSC cultures.
Turning point in stem cell therapy
The turning point in stem cell therapy appeared in 2006, when scientists Shinya Yamanaka, together with Kazutoshi Takahashi, discovered that it is possible to reprogram multipotent adult stem cells to the pluripotent state. This process avoided endangering the foetus’ life in the process. Retrovirus-mediated transduction of mouse fibroblasts with four transcription factors (Oct-3/4, Sox2, KLF4, and c-Myc) [ 34 ] that are mainly expressed in embryonic stem cells could induce the fibroblasts to become pluripotent (Fig. 5 ) [ 35 ]. This new form of stem cells was named iPSCs. One year later, the experiment also succeeded with human cells [ 36 ]. After this success, the method opened a new field in stem cell research with a generation of iPSC lines that can be customized and biocompatible with the patient. Recently, studies have focused on reducing carcinogenesis and improving the conduction system.
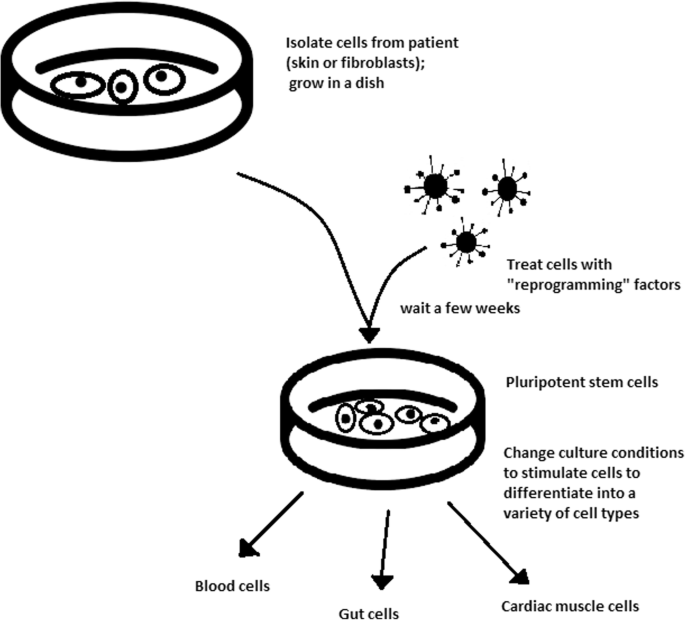
Retroviral-mediated transduction induces pluripotency in isolated patient somatic cells. Target cells lose their role as somatic cells and, once again, become pluripotent and can differentiate into any cell type of human body
The turning point was influenced by former discoveries that happened in 1962 and 1987.
The former discovery was about scientist John Gurdon successfully cloning frogs by transferring a nucleus from a frog’s somatic cells into an oocyte. This caused a complete reversion of somatic cell development [ 37 ]. The results of his experiment became an immense discovery since it was previously believed that cell differentiation is a one-way street only, but his experiment suggested the opposite and demonstrated that it is even possible for a somatic cell to again acquire pluripotency [ 38 ].
The latter was a discovery made by Davis R.L. that focused on fibroblast DNA subtraction. Three genes were found that originally appeared in myoblasts. The enforced expression of only one of the genes, named myogenic differentiation 1 (Myod1), caused the conversion of fibroblasts into myoblasts, showing that reprogramming cells is possible, and it can even be used to transform cells from one lineage to another [ 39 ].
Although pluripotency can occur naturally only in embryonic stem cells, it is possible to induce terminally differentiated cells to become pluripotent again. The process of direct reprogramming converts differentiated somatic cells into iPSC lines that can form all cell types of an organism. Reprogramming focuses on the expression of oncogenes such as Myc and Klf4 (Kruppel-like factor 4). This process is enhanced by a downregulation of genes promoting genome stability, such as p53. Additionally, cell reprogramming involves histone alteration. All these processes can cause potential mutagenic risk and later lead to an increased number of mutations. Quinlan et al. [ 40 ] checked fully pluripotent mouse iPSCs using whole genome DNA sequencing and structural variation (SV) detection algorithms. Based on those studies, it was confirmed that although there were single mutations in the non-genetic region, there were non-retrotransposon insertions. This led to the conclusion that current reprogramming methods can produce fully pluripotent iPSCs without severe genomic alterations.
During the course of development from pluripotent hESCs to differentiated somatic cells, crucial changes appear in the epigenetic structure of these cells. There is a restriction or permission of the transcription of genes relevant to each cell type. When somatic cells are being reprogrammed using transcription factors, all the epigenetic architecture has to be reconditioned to achieve iPSCs with pluripotency [ 41 ]. However, cells of each tissue undergo specific somatic genomic methylation. This influences transcription, which can further cause alterations in induced pluripotency [ 42 ].
Source of iPSCs
Because pluripotent cells can propagate indefinitely and differentiate into any kind of cell, they can be an unlimited source, either for replacing lost or diseased tissues. iPSCs bypass the need for embryos in stem cell therapy. Because they are made from the patient’s own cells, they are autologous and no longer generate any risk of immune rejection.
At first, fibroblasts were used as a source of iPSCs. Because a biopsy was needed to achieve these types of cells, the technique underwent further research. Researchers investigated whether more accessible cells could be used in the method. Further, other cells were used in the process: peripheral blood cells, keratinocytes, and renal epithelial cells found in urine. An alternative strategy to stem cell transplantation can be stimulating a patient’s endogenous stem cells to divide or differentiate, occurring naturally when skin wounds are healing. In 2008, pancreatic exocrine cells were shown to be reprogrammed to functional, insulin-producing beta cells [ 43 ].
The best stem cell source appears to be the fibroblasts, which is more tempting in the case of logistics since its stimulation can be fast and better controlled [ 44 ].
- Teratoma formation assay
The self-renewal and differentiation capabilities of iPSCs have gained significant interest and attention in regenerative medicine sciences. To study their abilities, a quality-control assay is needed, of which one of the most important is the teratoma formation assay. Teratomas are benign tumours. Teratomas are capable of rapid growth in vivo and are characteristic because of their ability to develop into tissues of all three germ layers simultaneously. Because of the high pluripotency of teratomas, this formation assay is considered an assessment of iPSC’s abilities [ 45 ].
Teratoma formation rate, for instance, was observed to be elevated in human iPSCs compared to that in hESCs [ 46 ]. This difference may be connected to different differentiation methods and cell origins. Most commonly, the teratoma assay involves an injection of examined iPSCs subcutaneously or under the testis or kidney capsule in mice, which are immune-deficient [ 47 ]. After injection, an immature but recognizable tissue can be observed, such as the kidney tubules, bone, cartilage, or neuroepithelium [ 30 ]. The injection site may have an impact on the efficiency of teratoma formation [ 48 ].
There are three groups of markers used in this assay to differentiate the cells of germ layers. For endodermal tissue, there is insulin/C-peptide and alpha-1 antitrypsin [ 49 ]. For the mesoderm, derivatives can be used, e.g. cartilage matrix protein for the bone and alcian blue for the cartilage. As ectodermal markers, class III B botulin or keratin can be used for keratinocytes.
Teratoma formation assays are considered the gold standard for demonstrating the pluripotency of human iPSCs, demonstrating their possibilities under physiological conditions. Due to their actual tissue formation, they could be used for the characterization of many cell lineages [ 50 ].
Directed differentiation
To be useful in therapy, stem cells must be converted into desired cell types as necessary or else the whole regenerative medicine process will be pointless. Differentiation of ESCs is crucial because undifferentiated ESCs can cause teratoma formation in vivo. Understanding and using signalling pathways for differentiation is an important method in successful regenerative medicine. In directed differentiation, it is likely to mimic signals that are received by cells when they undergo successive stages of development [ 51 ]. The extracellular microenvironment plays a significant role in controlling cell behaviour. By manipulating the culture conditions, it is possible to restrict specific differentiation pathways and generate cultures that are enriched in certain precursors in vitro. However, achieving a similar effect in vivo is challenging. It is crucial to develop culture conditions that will allow the promotion of homogenous and enhanced differentiation of ESCs into functional and desired tissues.
Regarding the self-renewal of embryonic stem cells, Hwang et al. [ 52 ] noted that the ideal culture method for hESC-based cell and tissue therapy would be a defined culture free of either the feeder layer or animal components. This is because cell and tissue therapy requires the maintenance of large quantities of undifferentiated hESCs, which does not make feeder cells suitable for such tasks.
Most directed differentiation protocols are formed to mimic the development of an inner cell mass during gastrulation. During this process, pluripotent stem cells differentiate into ectodermal, mesodermal, or endodermal progenitors. Mall molecules or growth factors induce the conversion of stem cells into appropriate progenitor cells, which will later give rise to the desired cell type. There is a variety of signal intensities and molecular families that may affect the establishment of germ layers in vivo, such as fibroblast growth factors (FGFs) [ 53 ]; the Wnt family [ 54 ] or superfamily of transforming growth factors—β(TGFβ); and bone morphogenic proteins (BMP) [ 55 ]. Each candidate factor must be tested on various concentrations and additionally applied to various durations because the precise concentrations and times during which developing cells in embryos are influenced during differentiation are unknown. For instance, molecular antagonists of endogenous BMP and Wnt signalling can be used for ESC formation of ectoderm [ 56 ]. However, transient Wnt and lower concentrations of the TGFβ family trigger mesodermal differentiation [ 57 ]. Regarding endoderm formation, a higher activin A concentration may be required [ 58 , 59 ].
There are numerous protocols about the methods of forming progenitors of cells of each of germ layers, such as cardiomyocytes [ 60 ], hepatocytes [ 61 ], renal cells [ 62 ], lung cells [ 63 , 64 ], motor neurons [ 65 ], intestinal cells [ 66 ], or chondrocytes [ 67 ].
Directed differentiation of either iPSCs or ESCs into, e.g. hepatocytes, could influence and develop the study of the molecular mechanisms in human liver development. In addition, it could also provide the possibility to form exogenous hepatocytes for drug toxicity testing [ 68 ].
Levels of concentration and duration of action with a specific signalling molecule can cause a variety of factors. Unfortunately, for now, a high cost of recombinant factors is likely to limit their use on a larger scale in medicine. The more promising technique focuses on the use of small molecules. These can be used for either activating or deactivating specific signalling pathways. They enhance reprogramming efficiency by creating cells that are compatible with the desired type of tissue. It is a cheaper and non-immunogenic method.
One of the successful examples of small-molecule cell therapies is antagonists and agonists of the Hedgehog pathway. They show to be very useful in motor neuron regeneration [ 69 ]. Endogenous small molecules with their function in embryonic development can also be used in in vitro methods to induce the differentiation of cells; for example, retinoic acid, which is responsible for patterning the nervous system in vivo [ 70 ], surprisingly induced retinal cell formation when the laboratory procedure involved hESCs [ 71 ].
The efficacy of differentiation factors depends on functional maturity, efficiency, and, finally, introducing produced cells to their in vivo equivalent. Topography, shear stress, and substrate rigidity are factors influencing the phenotype of future cells [ 72 ].
The control of biophysical and biochemical signals, the biophysical environment, and a proper guide of hESC differentiation are important factors in appropriately cultured stem cells.
Stem cell utilization and their manufacturing standards and culture systems
The European Medicines Agency and the Food and Drug Administration have set Good Manufacturing Practice (GMP) guidelines for safe and appropriate stem cell transplantation. In the past, protocols used for stem cell transplantation required animal-derived products [ 73 ].
The risk of introducing animal antigens or pathogens caused a restriction in their use. Due to such limitations, the technique required an obvious update [ 74 ]. Now, it is essential to use xeno-free equivalents when establishing cell lines that are derived from fresh embryos and cultured from human feeder cell lines [ 75 ]. In this method, it is crucial to replace any non-human materials with xeno-free equivalents [ 76 ].
NutriStem with LN-511, TeSR2 with human recombinant laminin (LN-511), and RegES with human foreskin fibroblasts (HFFs) are commonly used xeno-free culture systems [ 33 ]. There are many organizations and international initiatives, such as the National Stem Cell Bank, that provide stem cell lines for treatment or medical research [ 77 ].
Stem cell use in medicine
Stem cells have great potential to become one of the most important aspects of medicine. In addition to the fact that they play a large role in developing restorative medicine, their study reveals much information about the complex events that happen during human development.
The difference between a stem cell and a differentiated cell is reflected in the cells’ DNA. In the former cell, DNA is arranged loosely with working genes. When signals enter the cell and the differentiation process begins, genes that are no longer needed are shut down, but genes required for the specialized function will remain active. This process can be reversed, and it is known that such pluripotency can be achieved by interaction in gene sequences. Takahashi and Yamanaka [ 78 ] and Loh et al. [ 79 ] discovered that octamer-binding transcription factor 3 and 4 (Oct3/4), sex determining region Y (SRY)-box 2 and Nanog genes function as core transcription factors in maintaining pluripotency. Among them, Oct3/4 and Sox2 are essential for the generation of iPSCs.
Many serious medical conditions, such as birth defects or cancer, are caused by improper differentiation or cell division. Currently, several stem cell therapies are possible, among which are treatments for spinal cord injury, heart failure [ 80 ], retinal and macular degeneration [ 81 ], tendon ruptures, and diabetes type 1 [ 82 ]. Stem cell research can further help in better understanding stem cell physiology. This may result in finding new ways of treating currently incurable diseases.
Haematopoietic stem cell transplantation
Haematopoietic stem cells are important because they are by far the most thoroughly characterized tissue-specific stem cell; after all, they have been experimentally studied for more than 50 years. These stem cells appear to provide an accurate paradigm model system to study tissue-specific stem cells, and they have potential in regenerative medicine.
Multipotent haematopoietic stem cell (HSC) transplantation is currently the most popular stem cell therapy. Target cells are usually derived from the bone marrow, peripheral blood, or umbilical cord blood [ 83 ]. The procedure can be autologous (when the patient’s own cells are used), allogenic (when the stem cell comes from a donor), or syngeneic (from an identical twin). HSCs are responsible for the generation of all functional haematopoietic lineages in blood, including erythrocytes, leukocytes, and platelets. HSC transplantation solves problems that are caused by inappropriate functioning of the haematopoietic system, which includes diseases such as leukaemia and anaemia. However, when conventional sources of HSC are taken into consideration, there are some important limitations. First, there is a limited number of transplantable cells, and an efficient way of gathering them has not yet been found. There is also a problem with finding a fitting antigen-matched donor for transplantation, and viral contamination or any immunoreactions also cause a reduction in efficiency in conventional HSC transplantations. Haematopoietic transplantation should be reserved for patients with life-threatening diseases because it has a multifactorial character and can be a dangerous procedure. iPSC use is crucial in this procedure. The use of a patient’s own unspecialized somatic cells as stem cells provides the greatest immunological compatibility and significantly increases the success of the procedure.
Stem cells as a target for pharmacological testing
Stem cells can be used in new drug tests. Each experiment on living tissue can be performed safely on specific differentiated cells from pluripotent cells. If any undesirable effect appears, drug formulas can be changed until they reach a sufficient level of effectiveness. The drug can enter the pharmacological market without harming any live testers. However, to test the drugs properly, the conditions must be equal when comparing the effects of two drugs. To achieve this goal, researchers need to gain full control of the differentiation process to generate pure populations of differentiated cells.
Stem cells as an alternative for arthroplasty
One of the biggest fears of professional sportsmen is getting an injury, which most often signifies the end of their professional career. This applies especially to tendon injuries, which, due to current treatment options focusing either on conservative or surgical treatment, often do not provide acceptable outcomes. Problems with the tendons start with their regeneration capabilities. Instead of functionally regenerating after an injury, tendons merely heal by forming scar tissues that lack the functionality of healthy tissues. Factors that may cause this failed healing response include hypervascularization, deposition of calcific materials, pain, or swelling [ 84 ].
Additionally, in addition to problems with tendons, there is a high probability of acquiring a pathological condition of joints called osteoarthritis (OA) [ 85 ]. OA is common due to the avascular nature of articular cartilage and its low regenerative capabilities [ 86 ]. Although arthroplasty is currently a common procedure in treating OA, it is not ideal for younger patients because they can outlive the implant and will require several surgical procedures in the future. These are situations where stem cell therapy can help by stopping the onset of OA [ 87 ]. However, these procedures are not well developed, and the long-term maintenance of hyaline cartilage requires further research.
Osteonecrosis of the femoral hip (ONFH) is a refractory disease associated with the collapse of the femoral head and risk of hip arthroplasty in younger populations [ 88 ]. Although total hip arthroplasty (THA) is clinically successful, it is not ideal for young patients, mostly due to the limited lifetime of the prosthesis. An increasing number of clinical studies have evaluated the therapeutic effect of stem cells on ONFH. Most of the authors demonstrated positive outcomes, with reduced pain, improved function, or avoidance of THA [ 89 , 90 , 91 ].
Rejuvenation by cell programming
Ageing is a reversible epigenetic process. The first cell rejuvenation study was published in 2011 [ 92 ]. Cells from aged individuals have different transcriptional signatures, high levels of oxidative stress, dysfunctional mitochondria, and shorter telomeres than in young cells [ 93 ]. There is a hypothesis that when human or mouse adult somatic cells are reprogrammed to iPSCs, their epigenetic age is virtually reset to zero [ 94 ]. This was based on an epigenetic model, which explains that at the time of fertilization, all marks of parenteral ageing are erased from the zygote’s genome and its ageing clock is reset to zero [ 95 ].
In their study, Ocampo et al. [ 96 ] used Oct4, Sox2, Klf4, and C-myc genes (OSKM genes) and affected pancreas and skeletal muscle cells, which have poor regenerative capacity. Their procedure revealed that these genes can also be used for effective regenerative treatment [ 97 ]. The main challenge of their method was the need to employ an approach that does not use transgenic animals and does not require an indefinitely long application. The first clinical approach would be preventive, focused on stopping or slowing the ageing rate. Later, progressive rejuvenation of old individuals can be attempted. In the future, this method may raise some ethical issues, such as overpopulation, leading to lower availability of food and energy.
For now, it is important to learn how to implement cell reprogramming technology in non-transgenic elder animals and humans to erase marks of ageing without removing the epigenetic marks of cell identity.
Cell-based therapies
Stem cells can be induced to become a specific cell type that is required to repair damaged or destroyed tissues (Fig. 6 ). Currently, when the need for transplantable tissues and organs outweighs the possible supply, stem cells appear to be a perfect solution for the problem. The most common conditions that benefit from such therapy are macular degenerations [ 98 ], strokes [ 99 ], osteoarthritis [ 89 , 90 ], neurodegenerative diseases, and diabetes [ 100 ]. Due to this technique, it can become possible to generate healthy heart muscle cells and later transplant them to patients with heart disease.
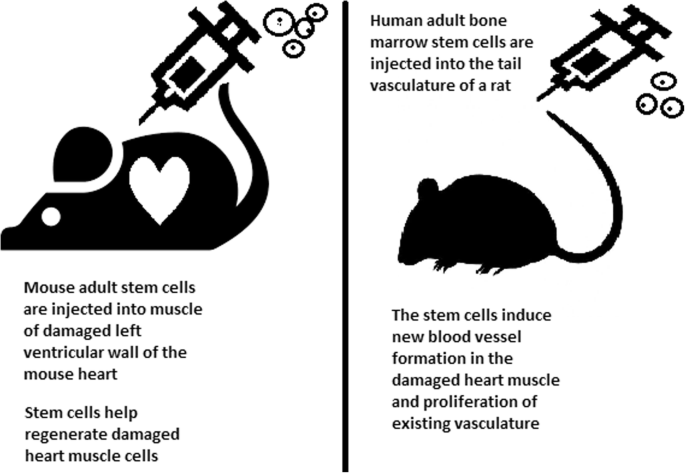
Stem cell experiments on animals. These experiments are one of the many procedures that proved stem cells to be a crucial factor in future regenerative medicine
In the case of type 1 diabetes, insulin-producing cells in the pancreas are destroyed due to an autoimmunological reaction. As an alternative to transplantation therapy, it can be possible to induce stem cells to differentiate into insulin-producing cells [ 101 ].
Stem cells and tissue banks
iPS cells with their theoretically unlimited propagation and differentiation abilities are attractive for the present and future sciences. They can be stored in a tissue bank to be an essential source of human tissue used for medical examination. The problem with conventional differentiated tissue cells held in the laboratory is that their propagation features diminish after time. This does not occur in iPSCs.
The umbilical cord is known to be rich in mesenchymal stem cells. Due to its cryopreservation immediately after birth, its stem cells can be successfully stored and used in therapies to prevent the future life-threatening diseases of a given patient.
Stem cells of human exfoliated deciduous teeth (SHED) found in exfoliated deciduous teeth has the ability to develop into more types of body tissues than other stem cells [ 102 ] (Table 1 ). Techniques of their collection, isolation, and storage are simple and non-invasive. Among the advantages of banking, SHED cells are:
Guaranteed donor-match autologous transplant that causes no immune reaction and rejection of cells [ 103 ]
Simple and painless for both child and parent
Less than one third of the cost of cord blood storage
Not subject to the same ethical concerns as embryonic stem cells [ 104 ]
In contrast to cord blood stem cells, SHED cells are able to regenerate into solid tissues such as connective, neural, dental, or bone tissue [ 105 , 106 ]
SHED can be useful for close relatives of the donor
Fertility diseases
In 2011, two researchers, Katsuhiko Hayashi et al. [ 107 ], showed in an experiment on mice that it is possible to form sperm from iPSCs. They succeeded in delivering healthy and fertile pups in infertile mice. The experiment was also successful for female mice, where iPSCs formed fully functional eggs .
Young adults at risk of losing their spermatogonial stem cells (SSC), mostly cancer patients, are the main target group that can benefit from testicular tissue cryopreservation and autotransplantation. Effective freezing methods for adult and pre-pubertal testicular tissue are available [ 108 ].
Qiuwan et al. [ 109 ] provided important evidence that human amniotic epithelial cell (hAEC) transplantation could effectively improve ovarian function by inhibiting cell apoptosis and reducing inflammation in injured ovarian tissue of mice, and it could be a promising strategy for the management of premature ovarian failure or insufficiency in female cancer survivors.
For now, reaching successful infertility treatments in humans appears to be only a matter of time, but there are several challenges to overcome. First, the process needs to have high efficiency; second, the chances of forming tumours instead of eggs or sperm must be maximally reduced. The last barrier is how to mature human sperm and eggs in the lab without transplanting them to in vivo conditions, which could cause either a tumour risk or an invasive procedure.
Therapy for incurable neurodegenerative diseases
Thanks to stem cell therapy, it is possible not only to delay the progression of incurable neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease (AD), and Huntington disease, but also, most importantly, to remove the source of the problem. In neuroscience, the discovery of neural stem cells (NSCs) has nullified the previous idea that adult CNS were not capable of neurogenesis [ 110 , 111 ]. Neural stem cells are capable of improving cognitive function in preclinical rodent models of AD [ 112 , 113 , 114 ]. Awe et al. [ 115 ] clinically derived relevant human iPSCs from skin punch biopsies to develop a neural stem cell-based approach for treating AD. Neuronal degeneration in Parkinson’s disease (PD) is focal, and dopaminergic neurons can be efficiently generated from hESCs. PD is an ideal disease for iPSC-based cell therapy [ 116 ]. However, this therapy is still in an experimental phase ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4539501 /). Brain tissue from aborted foetuses was used on patients with Parkinson’s disease [ 117 ]. Although the results were not uniform, they showed that therapies with pure stem cells are an important and achievable therapy.
Stem cell use in dentistry
Teeth represent a very challenging material for regenerative medicine. They are difficult to recreate because of their function in aspects such as articulation, mastication, or aesthetics due to their complicated structure. Currently, there is a chance for stem cells to become more widely used than synthetic materials. Teeth have a large advantage of being the most natural and non-invasive source of stem cells.
For now, without the use of stem cells, the most common periodontological treatments are either growth factors, grafts, or surgery. For example, there are stem cells in periodontal ligament [ 118 , 119 ], which are capable of differentiating into osteoblasts or cementoblasts, and their functions were also assessed in neural cells [ 120 ]. Tissue engineering is a successful method for treating periodontal diseases. Stem cells of the root apical areas are able to recreate periodontal ligament. One of the possible methods of tissue engineering in periodontology is gene therapy performed using adenoviruses-containing growth factors [ 121 ].
As a result of animal studies, dentin regeneration is an effective process that results in the formation of dentin bridges [ 122 ].
Enamel is more difficult to regenerate than dentin. After the differentiation of ameloblastoma cells into the enamel, the former is destroyed, and reparation is impossible. Medical studies have succeeded in differentiating bone marrow stem cells into ameloblastoma [ 123 ].
Healthy dental tissue has a high amount of regular stem cells, although this number is reduced when tissue is either traumatized or inflamed [ 124 ]. There are several dental stem cell groups that can be isolated (Fig. 7 ).
Localization of stem cells in dental tissues. Dental pulp stem cells (DPSCs) and human deciduous teeth stem cells (SHED) are located in the dental pulp. Periodontal ligaments stem cells are located in the periodontal ligament. Apical papilla consists of stem cells from the apical papilla (SCAP)
Dental pulp stem cell (DPSC)
These were the first dental stem cells isolated from the human dental pulp, which were [ 125 ] located inside dental pulp (Table 2 ). They have osteogenic and chondrogenic potential. Mesenchymal stem cells (MSCs) of the dental pulp, when isolated, appear highly clonogenic; they can be isolated from adult tissue (e.g. bone marrow, adipose tissue) and foetal (e.g. umbilical cord) [ 126 ] tissue, and they are able to differentiate densely [ 127 ]. MSCs differentiate into odontoblast-like cells and osteoblasts to form dentin and bone. Their best source locations are the third molars [ 125 ]. DPSCs are the most useful dental source of tissue engineering due to their easy surgical accessibility, cryopreservation possibility, increased production of dentin tissues compared to non-dental stem cells, and their anti-inflammatory abilities. These cells have the potential to be a source for maxillofacial and orthopaedic reconstructions or reconstructions even beyond the oral cavity. DPSCs are able to generate all structures of the developed tooth [ 128 ]. In particular, beneficial results in the use of DPSCs may be achieved when combined with other new therapies, such as periodontal tissue photobiomodulation (laser stimulation), which is an efficient technique in the stimulation of proliferation and differentiation into distinct cell types [ 129 ]. DPSCs can be induced to form neural cells to help treat neurological deficits.
Stem cells of human exfoliated deciduous teeth (SHED) have a faster rate of proliferation than DPSCs and differentiate into an even greater number of cells, e.g. other mesenchymal and non-mesenchymal stem cell derivatives, such as neural cells [ 130 ]. These cells possess one major disadvantage: they form a non-complete dentin/pulp-like complex in vivo. SHED do not undergo the same ethical concerns as embryonic stem cells. Both DPSCs and SHED are able to form bone-like tissues in vivo [ 131 ] and can be used for periodontal, dentin, or pulp regeneration. DPSCs and SHED can be used in treating, for example, neural deficits [ 132 ]. DPSCs alone were tested and successfully applied for alveolar bone and mandible reconstruction [ 133 ].
Periodontal ligament stem cells (PDLSCs)
These cells are used in periodontal ligament or cementum tissue regeneration. They can differentiate into mesenchymal cell lineages to produce collagen-forming cells, adipocytes, cementum tissue, Sharpey’s fibres, and osteoblast-like cells in vitro. PDLSCs exist both on the root and alveolar bone surfaces; however, on the latter, these cells have better differentiation abilities than on the former [ 134 ]. PDLSCs have become the first treatment for periodontal regeneration therapy because of their safety and efficiency [ 135 , 136 ].
Stem cells from apical papilla (SCAP)
These cells are mesenchymal structures located within immature roots. They are isolated from human immature permanent apical papilla. SCAP are the source of odontoblasts and cause apexogenesis. These stem cells can be induced in vitro to form odontoblast-like cells, neuron-like cells, or adipocytes. SCAP have a higher capacity of proliferation than DPSCs, which makes them a better choice for tissue regeneration [ 137 , 138 ].
Dental follicle stem cells (DFCs)
These cells are loose connective tissues surrounding the developing tooth germ. DFCs contain cells that can differentiate into cementoblasts, osteoblasts, and periodontal ligament cells [ 139 , 140 ]. Additionally, these cells proliferate after even more than 30 passages [ 141 ]. DFCs are most commonly extracted from the sac of a third molar. When DFCs are combined with a treated dentin matrix, they can form a root-like tissue with a pulp-dentin complex and eventually form tooth roots [ 141 ]. When DFC sheets are induced by Hertwig’s epithelial root sheath cells, they can produce periodontal tissue; thus, DFCs represent a very promising material for tooth regeneration [ 142 ].
Pulp regeneration in endodontics
Dental pulp stem cells can differentiate into odontoblasts. There are few methods that enable the regeneration of the pulp.
The first is an ex vivo method. Proper stem cells are grown on a scaffold before they are implanted into the root channel [ 143 ].
The second is an in vivo method. This method focuses on injecting stem cells into disinfected root channels after the opening of the in vivo apex. Additionally, the use of a scaffold is necessary to prevent the movement of cells towards other tissues. For now, only pulp-like structures have been created successfully.
Methods of placing stem cells into the root channel constitute are either soft scaffolding [ 144 ] or the application of stem cells in apexogenesis or apexification. Immature teeth are the best source [ 145 ]. Nerve and blood vessel network regeneration are extremely vital to keep pulp tissue healthy.
The potential of dental stem cells is mainly regarding the regeneration of damaged dentin and pulp or the repair of any perforations; in the future, it appears to be even possible to generate the whole tooth. Such an immense success would lead to the gradual replacement of implant treatments. Mandibulary and maxillary defects can be one of the most complicated dental problems for stem cells to address.
Acquiring non-dental tissue cells by dental stem cell differentiation
In 2013, it was reported that it is possible to grow teeth from stem cells obtained extra-orally, e.g. from urine [ 146 ]. Pluripotent stem cells derived from human urine were induced and generated tooth-like structures. The physical properties of the structures were similar to natural ones except for hardness [ 127 ]. Nonetheless, it appears to be a very promising technique because it is non-invasive and relatively low-cost, and somatic cells can be used instead of embryonic cells. More importantly, stem cells derived from urine did not form any tumours, and the use of autologous cells reduces the chances of rejection [ 147 ].
Use of graphene in stem cell therapy
Over recent years, graphene and its derivatives have been increasingly used as scaffold materials to mediate stem cell growth and differentiation [ 148 ]. Both graphene and graphene oxide (GO) represent high in-plane stiffness [ 149 ]. Because graphene has carbon and aromatic network, it works either covalently or non-covalently with biomolecules; in addition to its superior mechanical properties, graphene offers versatile chemistry. Graphene exhibits biocompatibility with cells and their proper adhesion. It also tested positively for enhancing the proliferation or differentiation of stem cells [ 148 ]. After positive experiments, graphene revealed great potential as a scaffold and guide for specific lineages of stem cell differentiation [ 150 ]. Graphene has been successfully used in the transplantation of hMSCs and their guided differentiation to specific cells. The acceleration skills of graphene differentiation and division were also investigated. It was discovered that graphene can serve as a platform with increased adhesion for both growth factors and differentiation chemicals. It was also discovered that π-π binding was responsible for increased adhesion and played a crucial role in inducing hMSC differentiation [ 150 ].
Therapeutic potential of extracellular vesicle-based therapies
Extracellular vesicles (EVs) can be released by virtually every cell of an organism, including stem cells [ 151 ], and are involved in intercellular communication through the delivery of their mRNAs, lipids, and proteins. As Oh et al. [ 152 ] prove, stem cells, together with their paracrine factors—exosomes—can become potential therapeutics in the treatment of, e.g. skin ageing. Exosomes are small membrane vesicles secreted by most cells (30–120 nm in diameter) [ 153 ]. When endosomes fuse with the plasma membrane, they become exosomes that have messenger RNAs (mRNAs) and microRNAs (miRNAs), some classes of non-coding RNAs (IncRNAs) and several proteins that originate from the host cell [ 154 ]. IncRNAs can bind to specific loci and create epigenetic regulators, which leads to the formation of epigenetic modifications in recipient cells. Because of this feature, exosomes are believed to be implicated in cell-to-cell communication and the progression of diseases such as cancer [ 155 ]. Recently, many studies have also shown the therapeutic use of exosomes derived from stem cells, e.g. skin damage and renal or lung injuries [ 156 ].
In skin ageing, the most important factor is exposure to UV light, called “photoageing” [ 157 ], which causes extrinsic skin damage, characterized by dryness, roughness, irregular pigmentation, lesions, and skin cancers. In intrinsic skin ageing, on the other hand, the loss of elasticity is a characteristic feature. The skin dermis consists of fibroblasts, which are responsible for the synthesis of crucial skin elements, such as procollagen or elastic fibres. These elements form either basic framework extracellular matrix constituents of the skin dermis or play a major role in tissue elasticity. Fibroblast efficiency and abundance decrease with ageing [ 158 ]. Stem cells can promote the proliferation of dermal fibroblasts by secreting cytokines such as platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), and basic fibroblast growth factor. Huh et al. [ 159 ] mentioned that a medium of human amniotic fluid-derived stem cells (hAFSC) positively affected skin regeneration after longwave UV-induced (UVA, 315–400 nm) photoageing by increasing the proliferation and migration of dermal fibroblasts. It was discovered that, in addition to the induction of fibroblast physiology, hAFSC transplantation also improved diseases in cases of renal pathology, various cancers, or stroke [ 160 , 161 ].
Oh [ 162 ] also presented another option for the treatment of skin wounds, either caused by physical damage or due to diabetic ulcers. Induced pluripotent stem cell-conditioned medium (iPSC-CM) without any animal-derived components induced dermal fibroblast proliferation and migration.
Natural cutaneous wound healing is divided into three steps: haemostasis/inflammation, proliferation, and remodelling. During the crucial step of proliferation, fibroblasts migrate and increase in number, indicating that it is a critical step in skin repair, and factors such as iPSC-CM that impact it can improve the whole cutaneous wound healing process. Paracrine actions performed by iPSCs are also important for this therapeutic effect [ 163 ]. These actions result in the secretion of cytokines such as TGF-β, interleukin (IL)-6, IL-8, monocyte chemotactic protein-1 (MCP-1), vascular endothelial growth factor (VEGF), platelet-derived growth factor-AA (PDGF-AA), and basic fibroblast growth factor (bFGF). Bae et al. [ 164 ] mentioned that TGF-β induced the migration of keratinocytes. It was also demonstrated that iPSC factors can enhance skin wound healing in vivo and in vitro when Zhou et al. [ 165 ] enhanced wound healing, even after carbon dioxide laser resurfacing in an in vivo study.
Peng et al. [ 166 ] investigated the effects of EVs derived from hESCs on in vitro cultured retinal glial, progenitor Müller cells, which are known to differentiate into retinal neurons. EVs appear heterogeneous in size and can be internalized by cultured Müller cells, and their proteins are involved in the induction and maintenance of stem cell pluripotency. These stem cell-derived vesicles were responsible for the neuronal trans-differentiation of cultured Müller cells exposed to them. However, the research article points out that the procedure was accomplished only on in vitro acquired retina.
Challenges concerning stem cell therapy
Although stem cells appear to be an ideal solution for medicine, there are still many obstacles that need to be overcome in the future. One of the first problems is ethical concern.
The most common pluripotent stem cells are ESCs. Therapies concerning their use at the beginning were, and still are, the source of ethical conflicts. The reason behind it started when, in 1998, scientists discovered the possibility of removing ESCs from human embryos. Stem cell therapy appeared to be very effective in treating many, even previously incurable, diseases. The problem was that when scientists isolated ESCs in the lab, the embryo, which had potential for becoming a human, was destroyed (Fig. 8 ). Because of this, scientists, seeing a large potential in this treatment method, focused their efforts on making it possible to isolate stem cells without endangering their source—the embryo.
Use of inner cell mass pluripotent stem cells and their stimulation to differentiate into desired cell types
For now, while hESCs still remain an ethically debatable source of cells, they are potentially powerful tools to be used for therapeutic applications of tissue regeneration. Because of the complexity of stem cell control systems, there is still much to be learned through observations in vitro. For stem cells to become a popular and widely accessible procedure, tumour risk must be assessed. The second problem is to achieve successful immunological tolerance between stem cells and the patient’s body. For now, one of the best ideas is to use the patient’s own cells and devolve them into their pluripotent stage of development.
New cells need to have the ability to fully replace lost or malfunctioning natural cells. Additionally, there is a concern about the possibility of obtaining stem cells without the risk of morbidity or pain for either the patient or the donor. Uncontrolled proliferation and differentiation of cells after implementation must also be assessed before its use in a wide variety of regenerative procedures on living patients [ 167 ].
One of the arguments that limit the use of iPSCs is their infamous role in tumourigenicity. There is a risk that the expression of oncogenes may increase when cells are being reprogrammed. In 2008, a technique was discovered that allowed scientists to remove oncogenes after a cell achieved pluripotency, although it is not efficient yet and takes a longer amount of time. The process of reprogramming may be enhanced by deletion of the tumour suppressor gene p53, but this gene also acts as a key regulator of cancer, which makes it impossible to remove in order to avoid more mutations in the reprogrammed cell. The low efficiency of the process is another problem, which is progressively becoming reduced with each year. At first, the rate of somatic cell reprogramming in Yamanaka’s study was up to 0.1%. The use of transcription factors creates a risk of genomic insertion and further mutation of the target cell genome. For now, the only ethically acceptable operation is an injection of hESCs into mouse embryos in the case of pluripotency evaluation [ 168 ].
Stem cell obstacles in the future
Pioneering scientific and medical advances always have to be carefully policed in order to make sure they are both ethical and safe. Because stem cell therapy already has a large impact on many aspects of life, it should not be treated differently.
Currently, there are several challenges concerning stem cells. First, the most important one is about fully understanding the mechanism by which stem cells function first in animal models. This step cannot be avoided. For the widespread, global acceptance of the procedure, fear of the unknown is the greatest challenge to overcome.
The efficiency of stem cell-directed differentiation must be improved to make stem cells more reliable and trustworthy for a regular patient. The scale of the procedure is another challenge. Future stem cell therapies may be a significant obstacle. Transplanting new, fully functional organs made by stem cell therapy would require the creation of millions of working and biologically accurate cooperating cells. Bringing such complicated procedures into general, widespread regenerative medicine will require interdisciplinary and international collaboration.
The identification and proper isolation of stem cells from a patient’s tissues is another challenge. Immunological rejection is a major barrier to successful stem cell transplantation. With certain types of stem cells and procedures, the immune system may recognize transplanted cells as foreign bodies, triggering an immune reaction resulting in transplant or cell rejection.
One of the ideas that can make stem cells a “failsafe” is about implementing a self-destruct option if they become dangerous. Further development and versatility of stem cells may cause reduction of treatment costs for people suffering from currently incurable diseases. When facing certain organ failure, instead of undergoing extraordinarily expensive drug treatment, the patient would be able to utilize stem cell therapy. The effect of a successful operation would be immediate, and the patient would avoid chronic pharmacological treatment and its inevitable side effects.
Although these challenges facing stem cell science can be overwhelming, the field is making great advances each day. Stem cell therapy is already available for treating several diseases and conditions. Their impact on future medicine appears to be significant.
After several decades of experiments, stem cell therapy is becoming a magnificent game changer for medicine. With each experiment, the capabilities of stem cells are growing, although there are still many obstacles to overcome. Regardless, the influence of stem cells in regenerative medicine and transplantology is immense. Currently, untreatable neurodegenerative diseases have the possibility of becoming treatable with stem cell therapy. Induced pluripotency enables the use of a patient’s own cells. Tissue banks are becoming increasingly popular, as they gather cells that are the source of regenerative medicine in a struggle against present and future diseases. With stem cell therapy and all its regenerative benefits, we are better able to prolong human life than at any time in history.
Abbreviations
Basic fibroblast growth factor
Bone morphogenic proteins
Dental follicle stem cells
Dental pulp stem cells
Embryonic bodies
Embryonic stem cells
Fibroblast growth factors
Good Manufacturing Practice
Graphene oxide
Human amniotic fluid-derived stem cells
Human embryonic stem cells
Human foreskin fibroblasts
Inner cell mass
Non-coding RNA
Induced pluripotent stem cells
In vitro fertilization
Knockout serum replacement
Leukaemia inhibitory factor
Monocyte chemotactic protein-1
Fibroblasts
Messenger RNA
Mesenchymal stem cells of dental pulp
Myogenic differentiation
Osteoarthritis
Octamer-binding transcription factor 3 and 4
Platelet-derived growth factor
Platelet-derived growth factor-AA
Periodontal ligament stem cells
Rho-associated protein kinase
Stem cells from apical papilla
Stem cells of human exfoliated deciduous teeth
Synthetic Serum Substitute
Trophectoderm
Vascular endothelial growth factor
Transforming growth factors
Sukoyan MA, Vatolin SY, et al. Embryonic stem cells derived from morulae, inner cell mass, and blastocysts of mink: comparisons of their pluripotencies. Embryo Dev. 1993;36(2):148–58
Larijani B, Esfahani EN, Amini P, Nikbin B, Alimoghaddam K, Amiri S, Malekzadeh R, Yazdi NM, Ghodsi M, Dowlati Y, Sahraian MA, Ghavamzadeh A. Stem cell therapy in treatment of different diseases. Acta Medica Iranica. 2012:79–96 https://www.ncbi.nlm.nih.gov/pubmed/22359076 .
Sullivan S, Stacey GN, Akazawa C, et al. Quality guidelines for clinical-grade human induced pluripotent stem cell lines. Regenerative Med. 2018; https://doi.org/10.2217/rme-2018-0095 .
Amps K, Andrews PW, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat. Biotechnol. 2011; 29 (12):1121–44.
Google Scholar
Amit M, Itskovitz-Eldor J. Atlas of human pluripotent stem cells: derivation and culturing. New York: Humana Press; 2012.
Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–46.
CAS PubMed Google Scholar
Kang MI. Transitional CpG methylation between promoters and retroelements of tissue-specific genes during human mesenchymal cell differentiation. J. Cell Biochem. 2007;102:224–39.
Vaes B, Craeye D, Pinxteren J. Quality control during manufacture of a stem cell therapeutic. BioProcess Int. 2012;10:50–5.
Bloushtain-Qimron N. Epigenetic patterns of embryonic and adult stem cells. Cell Cycle. 2009;8:809–17.
Brindley DA. Peak serum: implications of serum supply for cell therapy manufacturing. Regenerative Medicine. 2012;7:809–17.
Solter D, Knowles BB. Immunosurgery of mouse blastocyst. Proc Natl Acad Sci U S A. 1975;72:5099–102.
CAS PubMed PubMed Central Google Scholar
Hoepfl G, Gassmann M, Desbaillets I. Differentiating embryonic stem cells into embryoid bodies. Methods Mole Biol. 2004;254:79–98 https://doi.org/10.1385/1-59259-741-6:079 .
Lim WF, Inoue-Yokoo T, Tan KS, Lai MI, Sugiyama D. Hematopoietic cell differentiation from embryonic and induced pluripotent stem cells. Stem Cell Res Ther. 2013;4(3):71. https://doi.org/10.1186/scrt222 .
Article CAS PubMed PubMed Central Google Scholar
Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27(36):6032–42. https://doi.org/10.1016/j.biomaterials.2006.07.012 .
Article CAS PubMed Google Scholar
Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of the visceral yolk sac, blood islands, and myocardium. J Embryol Exp Morphol. 1985;87:27–45.
Kurosawa HY. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–98.
Heins N, Englund MC, Sjoblom C, Dahl U, Tonning A, Bergh C, Lindahl A, Hanson C, Semb H. Derivation, characterization, and differentiation of human embryonic stem cells. Stem Cells. 2004;22:367–76.
Rosowski KA, Mertz AF, Norcross S, Dufresne ER, Horsley V. Edges of human embryonic stem cell colonies display distinct mechanical properties and differentiation potential. Sci Rep. 2015;5:Article number:14218.
PubMed Google Scholar
Chung Y, Klimanskaya I, Becker S, Li T, Maserati M, Lu SJ, Zdravkovic T, Ilic D, Genbacev O, Fisher S, Krtolica A, Lanza R. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell. 2008;2:113–7.
Zhang X, Stojkovic P, Przyborski S, Cooke M, Armstrong L, Lako M, Stojkovic M. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006;24:2669–76.
Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protoc. 2012;7:2029–40.
Ellerström C, Hyllner J, Strehl R. single cell enzymatic dissociation of human embryonic stem cells: a straightforward, robust, and standardized culture method. In: Turksen K, editor. Human embryonic stem cell protocols. Methods in molecular biology: Humana Press; 2009. p. 584.
Heng BC, Liu H, Ge Z, Cao T. Mechanical dissociation of human embryonic stem cell colonies by manual scraping after collagenase treatment is much more detrimental to cellular viability than is trypsinization with gentle pipetting. Biotechnol Appl Biochem. 2010;47(1):33–7.
Ellerstrom C, Strehl R, Noaksson K, Hyllner J, Semb H. Facilitated expansion of human embryonic stem cells by single-cell enzymatic dissociation. Stem Cells. 2007;25:1690–6.
Brimble SN, Zeng X, Weiler DA, Luo Y, Liu Y, Lyons IG, Freed WJ, Robins AJ, Rao MS, Schulz TC. Karyotypic stability, genotyping, differentiation, feeder-free maintenance, and gene expression sampling in three human embryonic stem cell lines deri. Stem Cells Dev. 2004;13:585–97.
Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–6.
Nie Y, Walsh P, Clarke DL, Rowley JA, Fellner T. Scalable passaging of adherent human pluripotent stem cells. 2014. https://doi.org/10.1371/journal.pone.0088012 .
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7.
Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cellsexpress an immunogenic nonhuman sialic acid. Nat. Med. 2005;11:228–32.
Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336(6200):688–90. https://doi.org/10.1038/336688a0 .
Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnol. 2001;19:971–4. https://doi.org/10.1038/nbt1001-971 .
Article CAS Google Scholar
Weathersbee PS, Pool TB, Ord T. Synthetic serum substitute (SSS): a globulin-enriched protein supplement for human embryo culture. J. Assist Reprod Genet. 1995;12:354–60.
Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–9.
Sommer CA, Mostoslavsky G. Experimental approaches for the generation of induced pluripotent stem cells. Stem Cell Res Ther. 2010;1:26.
PubMed PubMed Central Google Scholar
Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140(12):2457–61 https://doi.org/10.1242/dev.092551 .
Shi D, Lu F, Wei Y, et al. Buffalos ( Bubalus bubalis ) cloned by nuclear transfer of somatic cells. Biol. Reprod. 2007;77:285–91. https://doi.org/10.1095/biolreprod.107.060210 .
Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Development. 1962;10:622–40 http://dev.biologists.org/content/10/4/622 .
CAS Google Scholar
Kain K. The birth of cloning: an interview with John Gurdon. Dis Model Mech. 2009;2(1–2):9–10. https://doi.org/10.1242/dmm.002014 .
Article PubMed Central Google Scholar
Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;24(51(6)):987–1000.
Quinlan AR, Boland MJ, Leibowitz ML, et al. Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming. Cell Stem Cell. 2011;9(4):366–73.
Maherali N, Sridharan R, Xie W, Utika LJ, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70.
Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, Yu J, Hebrok M, Hochedlinger K, Costello JF, Song JS, Ramalho-Santos M. Incomplete DNA methylation underlines a transcriptional memory of somatic cells in human IPS cells. Nat Cell Biol. 2011;13:541–9.
Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32 https://doi.org/10.1038/nature07314 .
Hilfiker A, Kasper C, Hass R, Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011;396:489–97.
Zhang Wendy, Y., de Almeida Patricia, E., and Wu Joseph, C. Teratoma formation: a tool for monitoring pluripotency in stem cell research. StemBook, ed. The Stem Cell Research Community . June 12, 2012. https://doi.org/10.3824/stembook.1.53.1 .
Narsinh KH, Sun N, Sanchez-Freire V, et al. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121(3):1217–21.
Gertow K, Przyborski S, Loring JF, Auerbach JM, Epifano O, Otonkoski T, Damjanov I, AhrlundRichter L. Isolation of human embryonic stem cell-derived teratomas for the assessment of pluripotency. Curr Protoc Stem Cell Biol . 2007, Chapter 1, Unit 1B 4. 3: 1B.4.1-1B.4.29.
Cooke MJ, Stojkovic M, Przyborski SA. Growth of teratomas derived from human pluripotent stem cells is influenced by the graft site. Stem Cells Dev. 2006;15(2):254–9.
Przyborski SA. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells. 2005;23:1242–50.
Tannenbaum SE, Turetsky TT, Singer O, Aizenman E, Kirshberg S, Ilouz N, Gil Y, Berman-Zaken Y, Perlman TS, Geva N, Levy O, Arbell D, Simon A, Ben-Meir A, Shufaro Y, Laufer N, Reubinoff BE. Derivation of xeno-free and GMP-grade human embryonic stem cells- platforms for future clinical applications. PLoS One. 2012;7:e35325.
Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12:243–52.
Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2007;60(2):199–214. https://doi.org/10.1016/j.addr.2007.08.036 .
Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–29.
Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–806.
Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–714.
Efthymiou AG, Chen G, Rao M, Chen G, Boehm M. Self-renewal and cell lineage differentiation strategies in human embryonic stem cells and induced pluripotent stem cells. Expert Opin Biol Ther. 2014;14:1333–44.
Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDRþembryonic-stem-cell-derived population. Nature. 2008;453:524–8.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52. https://doi.org/10.1038/nbt1393 .
Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–21.
Burridge PW, Zambidis ET. Highly efficient directed differentiation of human induced pluripotent stem cells into cardiomyocytes. Methods Mol Biol. 2013;997:149–61.
Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, Song X, Guo Y, Ding M, Deng H. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–39.
Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH. Directing human embryonic stem cell differentiation towards a renal lineage generates a selforganizing kidney. Nat Cell Biol. 2014;16:118–26.
Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, VunjakNovakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32:84–91.
Kadzik RS, Morrisey EE. Directing lung endoderm differentiation in pluripotent stem cells. Cell Stem Cell. 2012;10:355–61.
Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–97.
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9.
Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, Brison DR, Hardingham TE, Kimber SJ. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28:1187–94.
Jun Cai, Ann DeLaForest, Joseph Fisher, Amanda Urick, Thomas Wagner, Kirk Twaroski, Max Cayo, Masato Nagaoka, Stephen A Duncan. Protocol for directed differentiation of human pluripotent stem cells toward a hepatocyte fate. 2012. DOI: https://doi.org/10.3824/stembook.1.52.1 .
Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, Dudek H, Bumcrot D, Wang FY, Jones S, Shulok J, Rubin LL, Porter JA. Small-molecule modulators of hedgehog signaling: identification and characterization of smoothened agonists and antagonists. J Biol. 2002;1:10.
Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair celllike cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–16.
Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, Sasai Y, Takahashi M. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122:3169–79.
Kshitiz PJ, Kim P, Helen W, Engler AJ, Levchenko A, Kim DH. Control of stem cell fate and function by engineering physical microenvironments. Intergr Biol (Camb). 2012;4:1008–18.
Amps K, Andrews PW, Anyfantis G, Armstrong L, Avery S, Baharvand H, Baker J, Baker D, Munoz MB, Beil S, Benvenisty N, Ben-Yosef D, Biancotti JC, Bosman A, Brena RM, Brison D, Caisander G, Camarasa MV, Chen J, ChiaoE CYM, Choo AB, Collins D, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol. 2011;29:1132–44.
Nukaya D, Minami K, Hoshikawa R, Yokoi N, Seino S. Preferential gene expression and epigenetic memory of induced pluripotent stem cells derived from mouse pancreas. Genes Cells. 2015;20:367–81.
Murdoch A, Braude P, Courtney A, Brison D, Hunt C, Lawford-Davies J, Moore H, Stacey G, Sethe S, Procurement Working Group Of National Clinical H, E. S. C. F, National Clinical H, E. S. C. F. The procurement of cells for the derivation of human embryonic stem cell lines for therapeutic use: recommendations for good practice. Stem Cell Rev. 2012;8:91–9.
Hewitson H, Wood V, Kadeva N, Cornwell G, Codognotto S, Stephenson E, Ilic D. Generation of KCL035 research grade human embryonic stem cell line carrying a mutation in HBB gene. Stem Cell Res. 2016;16:210–2.
Daley GQ, Hyun I, Apperley JF, Barker RA, Benvenisty N, Bredenoord AL, Breuer CK, Caulfield T, Cedars MI, Frey-Vasconcells J, Heslop HE, Jin Y, Lee RT, Mccabe C, Munsie M, Murry CE, Piantadosi S, Rao M, Rooke HM, Sipp D, Studer L, Sugarman J, et al. Setting global standards for stem cell research and clinical translation: the 2016 ISSCR guidelines. Stem Cell Rep. 2016;6:787–97.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. https://doi.org/10.1016/j.cell.2006.07.024 .
Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–40.
Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, SuberbielleBoissel C, Tartour E, Desnos M, Larghero J. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36:2011–7.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–16.
Ilic D, Ogilvie C. Concise review: human embryonic stem cells-what have we done? What are we doing? Where are we going? Stem Cells. 2017;35:17–25.
Rocha V, et al. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12(1):34–4.
Longo UG, Ronga M, Maffulli N. Sports Med Arthrosc 17:112–126. Achilles tendinopathy. Sports Med Arthrosc. 2009;17:112–26.
Tempfer H, Lehner C, Grütz M, Gehwolf R, Traweger A. Biological augmentation for tendon repair: lessons to be learned from development, disease, and tendon stem cell research. In: Gimble J, Marolt D, Oreffo R, Redl H, Wolbank S, editors. Cell engineering and regeneration. Reference Series in Biomedical Engineering. Cham: Springer; 2017.
Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34.
Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177–82.
Li R, Lin Q-X, Liang X-Z, Liu G-B, et al. Stem cell therapy for treating osteonecrosis of the femoral head: from clinical applications to related basic research. Stem Cell Res Therapy. 2018;9:291 https://doi.org/10.1186/s13287-018-1018-7 .
Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49(5):1005–9.
Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325–30.
Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplast. 2012;27(5):679–86.
Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, Lehmann S, Lemaitre JM. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011;25:2248–53.
Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–8.
Petkovich DA, Podolskiy DI, Lobanov AV, Lee SG, Miller RA, Gladyshev VN. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metab. 2017;25:954–60 https://doi.org/10.1016/j.cmet.2017.03.016 .
Gerontology, Rejuvenation by cell reprogramming: a new horizon in. Rodolfo G. Goya, Marianne Lehmann, Priscila Chiavellini, Martina Canatelli-Mallat, Claudia B. Hereñú and Oscar A. Brown. Stem Cell Res Therapy . 2018, 9:349. https://doi.org/10.1186/s13287-018-1075-y .
Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E, Araoka T, Vazquez-Ferrer E, Donoso D, Roman JLXJ, Rodriguez-Esteban C, Nuñez G, Nuñez Delicado E, Campistol JM, Guillen I, Guillen P, Izpisua. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. 2016;167:1719–33.
de Lázaro I, Cossu G, Kostarelos K. Transient transcription factor (OSKM) expression is key towards clinical translation of in vivo cell reprogramming. EMBO Mol Med. 2017;9:733–6.
Sun S, Li ZQ, Glencer P, Cai BC, Zhang XM, Yang J, Li XR. Bringing the age-related macular degeneration high-risk allele age-related maculopathy susceptibility 2 into focus with stem cell technology. Stem Cell Res Ther. 2017;8:135 https://doi.org/10.1186/s13287-017-0584-4 .
Liu J. Induced pluripotent stem cell-derived neural stem cells: new hope for stroke? Stem Cell Res Ther. 2013;4:115 https://doi.org/10.1186/scrt326 .
Shahjalal HM, Dayem AA, Lim KM, Jeon TI, Cho SG. Generation of pancreatic β cells for treatment of diabetes: advances and challenges. Stem Cell ResTher. 2018;9:355 https://doi.org/10.1186/s13287-018-1099-3 .
Kroon E, Martinson LA, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26;443–52.
Arora V, Pooja A, Munshi AK. Banking stem cells from human exfoliated deciduous teeth. J Clin Pediatr Dent. 2009;33(4):289–94.
Mao JJ. Stem cells and the future of dental care. New York State Dental J. 2008;74(2):21–4.
Reznick, Jay B. Continuing education: stem cells: emerging medical and dental therapies for the dental Professional. Dentaltown Magazine . 2008, pp. 42–53.
Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26(7):1787–95.
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith A. Dental pulp tissue engineering with stem cells from exfoliated. J Endod. 2008;34(8):962–9.
Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146(4):519–32. https://doi.org/10.1016/j.cell.2011.06.052 .
Sadri-Ardekani H, Atala A. Testicular tissue cryopreservation and spermatogonial stem cell transplantation to restore fertility: from bench to bedside. Stem Cell ResTher. 2014;5:68 https://doi.org/10.1186/scrt457 .
Zhang Q, Xu M, Yao X, Li T, Wang Q, Lai D. Human amniotic epithelial cells inhibit granulosa cell apoptosis induced by chemotherapy and restore the fertility. Stem Cell Res Ther. 2015;6:152 https://doi.org/10.1186/s13287-015-0148-4 .
Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19:672–82. https://doi.org/10.1038/cr.2009.56 .
Dantuma E, Merchant S, Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell ResTher. 2010;1:37 https://doi.org/10.1186/scrt37 .
Wang Q, Matsumoto Y, Shindo T, Miyake K, Shindo A, Kawanishi M, Kawai N, Tamiya T, Nagao S. Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. J Med Invest. 2006;53:61–9. https://doi.org/10.2152/jmi.53.61 .
Article PubMed Google Scholar
Moghadam FH, Alaie H, Karbalaie K, Tanhaei S, Nasr Esfahani MH, Baharvand H. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation. 2009;78:59–68. https://doi.org/10.1016/j.diff.2009.06.005 .
Byrne JA. Developing neural stem cell-based treatments for neurodegenerative diseases. Stem Cell ResTher. 2014;5:72. https://doi.org/10.1186/scrt461 .
Awe JP, Lee PC, Ramathal C, Vega-Crespo A, Durruthy-Durruthy J, Cooper A, Karumbayaram S, Lowry WE, Clark AT, Zack JA, Sebastiano V, Kohn DB, Pyle AD, Martin MG, Lipshutz GS, Phelps PE, Pera RA, Byrne JA. Generation and characterization of transgene-free human induced pluripotent stem cells and conversion to putative clinical-grade status. Stem Cell Res Ther. 2013;4:87. https://doi.org/10.1186/scrt246 .
Peng J, Zeng X. The role of induced pluripotent stem cells in regenerative medicine: neurodegenerative diseases. Stem Cell ResTher. 2011;2:32. https://doi.org/10.1186/scrt73 .
Wright BL, Barker RA. Established and emerging therapies for Huntington’s disease. 2007;7(6):579–87 https://www.ncbi.nlm.nih.gov/pubmed/17896994/579-87 .
Lin NH, Gronthos S, Bartold PM. Stem cells and periodontal regeneration. Aust Dent J. 2008;53:108–21.
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55.
Ramseier CA, Abramson ZR, Jin Q, Giannobile WV. Gene therapeutics for periodontal regenerative medicine. Dent Clin North Am. 2006;50:245–63.
Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. OrthodCraniofac Res. 2005;8:191–9.
Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein. J Dent Res. 2004;83:590–5.
Hu B, Unda F, Bopp-Kuchler S, Jimenez L, Wang XJ, Haikel Y, et al. Bone marrow cells can give rise to ameloblast-like cells. J Dent Res. 2006;85:416–21.
Liu Y, Liu W, Hu C, Xue Z, Wang G, Ding B, Luo H, Tang L, Kong X, Chen X, Liu N, Ding Y, Jin Y. MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem Cells. 2011;29(11):1804–16. https://doi.org/10.1002/stem.728 .
Raspini G, Wolff J, Helminen M, Raspini G, Raspini M, Sándor GK. Dental stem cells harvested from third molars combined with bioactive glass can induce signs of bone formation in vitro. J Oral Maxillofac Res. 2018;9(1):e2. Published 2018 Mar 31. https://doi.org/10.5037/jomr.2018.9102 .
Christodoulou I, Goulielmaki M, Devetzi M, Panagiotidis M, Koliakos G, Zoumpourlis V. Mesenchymal stem cells in preclinical cancer cytotherapy: a systematic review. Stem Cell Res Ther. 2018;9(1;336). https://doi.org/10.1186/s13287-018-1078-8 .
Bansal R, Jain A. Current overview on dental stem cells applications in regenerative dentistry. J Nat Sci Biol Med. 2015;6(1):29–34. https://doi.org/10.4103/0976-9668.149074 .
Article PubMed PubMed Central Google Scholar
Edgar Ledesma-Martínez, Víctor Manuel Mendoza-Núñez, Edelmiro Santiago-Osorio. Mesenchymal stem cells derived from dental pulp: a review. Stem Cells Int . 2016, 4,709,572, p. doi: https://doi.org/10.1155/2016/4709572 ].
Grzech-Leśniak K. Making use of lasers in periodontal treatment: a new gold standard? Photomed Laser Surg. 2017;35(10):513–4.
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–12. https://doi.org/10.1073/pnas.0937635100 .
Yasui T, Mabuchi Y, Toriumi H, Ebine T, Niibe K, Houlihan DD, Morikawa S, Onizawa K, Kawana H, Akazawa C, Suzuki N, Nakagawa T, Okano H, Matsuzaki Y. Purified human dental pulp stem cells promote osteogenic regeneration. J Dent Res. 2016;95(2):206–14. https://doi.org/10.1177/0022034515610748 .
Yamamoto A, Sakai K, Matsubara K, Kano F, Ueda M. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res. 2014;78:16–20. https://doi.org/10.1016/j.neures.2013.10.010 .
d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;12, PMID: 19908196:75–83.
Wang L, Shen H, Zheng W, Tang L, Yang Z, Gao Y, Yang Q, Wang C, Duan Y, Jin Y. Characterization of stem cells from alveolar periodontal ligament. Tissue Eng. Part A. 2011;17(7–8):1015–26. https://doi.org/10.1089/ten.tea.2010.0140 .
Iwata T, Yamato M, Zhang Z, Mukobata S, Washio K, Ando T, Feijen J, Okano T, Ishikawa I. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol. 2010;37(12):1088–99. https://doi.org/10.1111/j.1600-051X.2010.01597.x .
Chen F-M, Gao L-N, Tian B-M, Zhang X-Y, Zhang Y-J, Dong G-Y, Lu H, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. https://doi.org/10.1186/s13287-016-0288-1 .
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol. 2011;56(7):709–21. https://doi.org/10.1016/j.archoralbio.2010.12.008 .
Han C, Yang Z, Zhou W, Jin F, Song Y, Wang Y, Huo N, Chen L, Qian H, Hou R, Duan Y, Jin Y. Periapical follicle stem cell: a promising candidate for cementum/periodontal ligament regeneration and bio-root engineering. Stem Cells Dev. 2010;19(9):1405–15. https://doi.org/10.1089/scd.2009.0277 .
Luan X, Ito Y, Dangaria S, Diekwisch TG. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006;15(4):595–608. https://doi.org/10.1089/scd.2006.15.595 .
Handa K, Saito M, Tsunoda A, Yamauchi M, Hattori S, Sato S, Toyoda M, Teranaka T, Narayanan AS. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect Tissue Res. 2002;43(2–3):406–8 PMID: 12489190.
Guo W, Chen L, Gong K, Ding B, Duan Y, Jin Y. Heterogeneous dental follicle cells and the regeneration of complex periodontal tissues. Tissue Engineering. Part A. 2012;18(5–6):459–70 https://doi.org/10.1089/ten.tea.2011.0261 .
Bai, Yudi et al. Cementum- and periodontal ligament-like tissue formation by dental follicle cell sheets co-cultured with Hertwig’s epithelial root sheath cells. Bone. 2011, 48, Issue 6, pp. 1417–1426, https://doi.org/10.1016/j.bone.2011.02.016 .
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. 2008, 34, pp. 962–969.
Dobie K, Smith G, Sloan AJ, Smith AJ. Effects of alginate, hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect Tissue Res 2. 2002;43:387–90.
Friedlander LT, Cullinan MP, Love RM. Dental stem cells and their potential role in apexogenesis and apexification. Int Endod J. 2009;42:955–62.
Cai J, Zhang Y, Liu P, Chen S, Wu X, Sun Y, Li A, Huang K, Luo R, Wang L, Liu Y, Zhou T, Wei S, Pan G, Pei D, Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen (Lond). July 30, 2013, 2(1), pp. 6, doi: https://doi.org/10.1186/2045-9769-2-6 .
Craig J. Taylor, Eleanor M. Bolton, and J. Andrew Bradley 2011 Aug 12 and https://doi.org/10.1098/rstb.2011.0030 ], 366(1575): 2312–2322. [doi:. Immunological considerations for embryonic and induced pluripotent stem cell banking,. Philos Trans R SocLond B Biol Sci. 2011, 366(1575), pp. 2312–2322, doi: https://doi.org/10.1098/rstb.2011.0030 .
T.R. Nayak, H. Andersen, V.S. Makam, C. Khaw, S. Bae, X.F. Xu, P.L.R. Ee, J.H. Ahn, B.H. Hong, G. Pastorin, B. Ozyilmaz, ACS Nano, 5 (6) (2011), pp. 4. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells,. ACS Nano. 2011, pp. 4670–4678.
Lee WC, Lim C, Shi H, Tang LAL, Wang Y, Lim CT, Loh KP. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5(9):7334–41.
Kenry LWC, Loh KP, Lim CT. When stem cells meet graphene: opportunities and challenges in regenerative medicine. Biomaterials. 2018;155:236–50.
Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, et al. Transfer of microRNAs by embryonic stem cell microvesicles. 2009. 2009, 4(3), p. https://doi.org/10.1371/journal.pone . 0004722.
Oh, Myeongsik, et al. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 2018, 19(6), p. 1715.
Ramirez MI. et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10:881–906.
Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–9.
Mateescu B, et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—an ISEV position paper. J. Extracell. Vesicles. 2017;6(1). https://doi.org/10.1080/20013078.2017.1286095 .
Nawaz M, et al. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016;2016:17. Article ID 1073140.
Helfrich, Y.R., Sachs, D.L. and Voorhees, J.J. Overview of skin aging and photoaging. Dermatol. Nurs. 20, pp. 177–183, https://www.ncbi.nlm.nih.gov/pubmed/18649702 .
Julia Tigges, Jean Krutmann, Ellen Fritsche, Judith Haendeler, Heiner Schaal, Jens W. Fischer, Faiza Kalfalah, Hans Reinke, Guido Reifenberger, Kai Stühler, Natascia Ventura, Sabrina Gundermann, Petra Boukamp, Fritz Boege. The hallmarks of fibroblast ageing, mechanisms of ageing and development, 138, 2014, Pages 26–44. 2014, 138, pp. 26–44, ISSN 0047–6374, https://doi.org/10.1016/j.mad.2014.03.004 .
Huh MI, Kim MS, Kim HK, et al. Effect of conditioned media collected from human amniotic fluid-derived stem cells (hAFSCs) on skin regeneration and photo-aging. Tissue Eng Regen Med. 2014;11:171 https://doi.org/10.1007/s13770-014-0412-1 .
Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31.
Liu J, Han G, Liu H, et al. Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: a possible role of Wnt and Akt signaling. PLoS One. 2013;8:e62844.
Oh M, et al. Promotive effects of human induced pluripotent stem cell-conditioned medium on the proliferation and migration of dermal fibroblasts. Biotechnol. Bioprocess Eng. 2017;22:561–8.
Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS One. 2008;3:e1886.
Bae J-S, Lee S-H, Kim J-E, Choi J-Y, Park R-W, Park JY, Park H-S, Sohn Y-S, Lee D-S, Lee EB. βig-h3 supports keratinocyte adhesion, migration, and proliferation through α3β1 integrin. Biochem. Biophys. Res. Commun. 2002;294:940–8.
Zhou B-R, Xu Y, Guo S-L, Xu Y, Wang Y, Zhu F, Permatasari F, Wu D, Yin Z-Q, Luo D. The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. BioMed Res. Int. 2013;519:126.
Peng Y, Baulier E, Ke Y, Young A, Ahmedli NB, Schwartz SD, et al. Human embryonic stem cells extracellular vesicles and their effects on immortalized human retinal Müller cells. PLoS ONE. 2018, 13(3), p. https://doi.org/10.1371/journal.pone.019400 .
Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003.
Mascetti VL, Pedersen RA. Human-mouse chimerism validates human stem cell pluripotency. Cell Stem Cell. 2016;18:67–72.
Gandia C, Armiñan A, García-Verdugo JM, Lledó E, Ruiz A, Miñana MD, Sanchez-Torrijos J, Payá R, Mirabet V, Carbonell-Uberos F, Llop M, Montero JA, Sepúlveda P. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2007;26(3):638–45.
Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ, Goebel WS. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008;14(2):149–56.
Garcia-Olmo D, Garcia-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–23.
de Mendonça CA, Bueno DF, Martins MT, Kerkis I, Kerkis A, Fanganiello RD, Cerruti H, Alonso N, Passos-Bueno MR. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J Craniofac Surg. 2008;19(1):204–10.
Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, Lee JS, Shi S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14(5):428–34.
Abbas, Diakonov I., Sharpe P. Neural crest origin of dental stem cells. Pan European Federation of the International Association for Dental Research (PEF IADR). 2008, Vols. Seq #96 - Oral Stem Cells.
Kerkis I, Ambrosio CE, Kerkis A, Martins DS, Gaiad TP, Morini AC, Vieira NM, Marina P, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs. J Transl Med. 2008;6:35.
Xianrui Yang, Li Li, Li Xiao, Donghui Zhang. Recycle the dental fairy’s package: overview of dental pulp stem cells. Stem Cell Res Ther . 2018, 9, 1, 1. https://doi.org/10.1186/s13287-018-1094-8 .
Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, Wang S. Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. 2010;19:1375–83.
Wang J, et al. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly (L-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010;6(10):3856–63.
Davies OG, Cooper PR, Shelton RM, Smith AJ, Scheven BA. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J Bone Miner Metab. 2015;33:371–82.
Huang GT-J, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066–73.
Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29(6):532–9.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30.
Nuti N, Corallo C, Chan BMF, Ferrari M, Gerami-Naini B. Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev Rep. 2016;12:511–23.
Ferro F, et al. Dental pulp stem cells differentiation reveals new insights in Oct4A dynamics. PloS One. 2012;7(7):e41774.
Conde MCM, Chisini LA, Grazioli G, Francia A, Carvalho RVd, Alcázar JCB, Tarquinio SBC, Demarco FF. Does cryopreservation affect the biological properties of stem cells from dental tissues? A systematic review. Braz Dent J. 2016;1210(6):633-40. https://doi.org/10.1590/0103-6440201600980 .
Papaccio G, Graziano A, d’Aquino R, Graziano MF, Pirozzi G, Menditti D, De Rosa A, Carinci F, Laino G. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006;208:319–25.
Alge DL, Zhou D, Adams LL, et. al. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4(1):73–81.
Jo Y-Y, Lee H-J, Kook S-Y, Choung H-W, Park J-Y, Chung J-H, Choung Y-H, Kim E-S, Yang H-C, Choung P-H. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–73.
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5.
Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005;20:1394–402.
Zainal A, Shahrul H, et al. In vitro chondrogenesis transformation study of mouse dental pulp stem cells. Sci World J. 2012;2012:827149.
Wei X, et al. Expression of mineralization markers in dental pulp cells. J Endod. 2007;33(6):703–8.
Dai J, et al. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials. 2012;33(31):7699–711.
Vasandan AB, et al. Functional differences in mesenchymal stromal cells from human dental pulp and periodontal ligament. J Cell Mol Med. 2014;18(2):344–54.
Werle SB, et al. Carious deciduous teeth are a potential source for dental pulp stem cells. Clin Oral Investig. 2015;20:75–81.
Nemeth CL, et al. Enhanced chondrogenic differentiation of dental pulp stem cells using nanopatterned PEG-GelMA-HA hydrogels. Tissue Eng A. 2014;20(21–22):2817–29.
Paino F, Ricci G, De Rosa A, D’Aquino R, Laino L, Pirozzi G, et al. Ecto-mesenchymal stem cells from dental pulp are committed to differentiate into active melanocytes. Eur. Cell Mater. 2010;20:295–305.
Ferro F, Spelat R, Baheney CS. Dental pulp stem cell (DPSC) isolation, characterization, and differentiation. In: Kioussi C, editor. Stem cells and tissue repair. Methods in molecular biology (methods and protocols): Humana Press. 2014;1210.
Ishkitiev N, Yaegaki K, Imai T, Tanaka T, Nakahara T, Ishikawa H, Mitev V, Haapasalo M. High-purity hepatic lineage differentiated from dental pulp stem cells in serum-free medium. J Endod. 2012;38:475–80.
Download references
Acknowledgements
Not applicable.
This work is supported by Wrocław Medical University in Poland.
Availability of data and materials
Please contact author for data requests.
Author information
Authors and affiliations.
Department of Experimental Surgery and Biomaterials Research, Wroclaw Medical University, Bujwida 44, Wrocław, 50-345, Poland
Wojciech Zakrzewski, Maria Szymonowicz & Zbigniew Rybak
Department of Conservative Dentistry and Pedodontics, Krakowska 26, Wrocław, 50-425, Poland
Maciej Dobrzyński
You can also search for this author in PubMed Google Scholar
Contributions
WZ is the principal author and was responsible for the first draft of the manuscript. WZ and ZR were responsible for the concept of the review. MS, MD, and ZR were responsible for revising the article and for data acquisition. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Wojciech Zakrzewski .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Zakrzewski, W., Dobrzyński, M., Szymonowicz, M. et al. Stem cells: past, present, and future. Stem Cell Res Ther 10 , 68 (2019). https://doi.org/10.1186/s13287-019-1165-5
Download citation
Published : 26 February 2019
DOI : https://doi.org/10.1186/s13287-019-1165-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Differentiation
- Pluripotency
- Induced pluripotent stem cell (iPSC)
- Stem cell derivation
- Growth media
- Tissue banks
- Tissue transplantation
Stem Cell Research & Therapy
ISSN: 1757-6512
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Advertisement
Tracing scientific and technological development in genetically modified crops
- Published: 18 September 2024
Cite this article

- Anurag Kanaujia 1 , 2 &
- Solanki Gupta 1 , 3
47 Accesses
1 Altmetric
Explore all metrics
Genetically Modified (GM) Organisms have been used in various domains since their introduction in the 1980s. According to ISAAA data, the use of GM crops in agriculture has also increased significantly in the past 30 years. However, even after 3 decades of commercialisation, GM crops are still surrounded with controversies with different countries adopting varying approaches to their introduction in the consumer markets, owing to different stances of various stakeholders. Motivated by this multitude of opinions, and absence of knowledge mapping, this study has undertaken scientometric analysis of the publication (Web of Science) and patent (Lens.org) data about genetically modified technology use in agriculture to explore the changing knowledge patterns and technological advancements in the area. It explores both scientific and technological perspectives regarding the use of Genetically Modified Crops, by using publication as well as patent data. The findings of this study highlight the major domains of research, technology development, and leading actors in the ecosystem. These findings can be helpful in taking effective policy decisions, and furthering the research activities. It presents a composite picture using both publications and patent data. Further, it will be of utility to explore the other technologies which are replacing GM technology in agriculture in future studies.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Socio-economic research on genetically modified crops: a study of the literature.
What are the socio-economic impacts of genetically modified crops worldwide? A systematic map protocol
Global networks of genetically modified crops technology: a patent citation network analysis
https://www.isaaa.org/gmapprovaldatabase/default.asp
https://www.statista.com/statistics/271897/leading-countries-by-acreage-of-genetically-modified-crops/
https://www.isaaa.org/gmapprovaldatabase/developerlist/default.asp
https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100
https://www.dutchgreenhouses.com/en/technology/hydroponics/
https://www.nal.usda.gov/farms-and-agricultural-production-systems/hydroponics
https://agriculture.canada.ca/en/news-agriculture-and-agri-food-canada/scientific-achievements-agriculture/what-hydroponics
https://www.fda.gov/food/agricultural-biotechnology/how-gmos-are-regulated-united-states
https://support.clarivate.com/ScientificandAcademicResearch/s/article/Web-of-Science-Core-Collection-Web-of-Science-Categories?language=en_US
https://www.bayer.com/media/en-us/bayer-closes-monsanto-acquisition/
Ahmad A, Jamil A, Munawar N (2023) GMOs or Non-GMOs? The CRISPR conundrum. Front Plant Sci 14:1232938. https://doi.org/10.3389/fpls.2023.1232938
Article PubMed PubMed Central Google Scholar
Arntzen CJ, Coghlan A, Johnson B, Peacock J, Rodemeyer M (2003) GM crops: science, politics and communication. Nat Rev Genet 4(10):839–843. https://doi.org/10.1038/nrg1185
Article CAS PubMed Google Scholar
Baudoin W, Nersisyan A, Shamilov A, Hodder A, Gutierrez D, De Pascale S, Qaryouti M (2013) Good agricultural practices for greenhouse vegetable crops. Food Agric Organ U N 217:1–449
Google Scholar
Brookes G (2022a) Genetically modified (GM) crop use 1996–2020: impacts on carbon emissions. GM Crops Food 13(1):242–261
Brookes G (2022b) Genetically modified (GM) crop use 1996–2020: environmental impacts associated with pesticide use change. GM Crops Food 13(1):262–289
Campanario JM (2011) Empirical study of journal impact factors obtained using the classical two-year citation window versus a five-year citation window. Scientometrics 87(1):189–204
Article Google Scholar
Carpenter JE (2011) Impact of GM crops on biodiversity. GM Crops 2(1):7–23
Article PubMed Google Scholar
Chen C (2017) Science mapping: a systematic review of the literature. J Data Inf Sci 2(2):1–40. https://doi.org/10.1515/jdis-2017-0006
Article CAS Google Scholar
Cobo MJ, López-Herrera AG, Herrera-Viedma E, Herrera F (2011) Science mapping software tools: review, analysis, and cooperative study among tools. J Am Soc Inf Sci Technol 62(7):1382–1402. https://doi.org/10.1002/asi.21525
Conner AJ, Glare TR, Nap JP (2003) The release of genetically modified crops into the environment: part II. Overview of ecological risk assessment. Plant J 33(1):19–46
Elisabetta R, Gabriele M, Massimo S, Sofia B, Giuseppe SM (2018) Structural trend and conceptual evolution of research on genetically modified organisms using a science mapping approach. J Clean Prod 205:329–338. https://doi.org/10.1016/j.jclepro.2018.09.118
FAO F (2018) The future of food and agriculture—alternative pathways to 2050. Food Agric Organ U N Rome 224:1–202
Fischer K, Ekener-Petersen E, Rydhmer L, Edvardsson Björnberg K (2015) Social impacts of GM crops in agriculture: a systematic literature review. Sustainability 7(7):8598–8620
Gazni A (2020) The growing number of patent citations to scientific papers: changes in the world, nations, and fields. Technol Soc 62:101276
Gerasimova K (2016) Debates on genetically modified crops in the context of sustainable development. Sci Eng Ethics 22:525–547
Gilbert N (2013) Case studies: a hard look at GM crops. Nature 497:24–26. https://doi.org/10.1038/497024a
Gonzalvo CM, Aala WJF, Maharjan KL (2022) Is implementing a biotech ban correct or not? Analysis of farmer perceptions and attitudes on the philippine supreme court’s ban on biotech crops. Sustainability 14(13):7919
Gupta S, Kanaujia A, Lathabai HH, Singh VK, Mayr P (2024) Patterns in the growth and thematic evolution of Artificial Intelligence research: a study using Bradford distribution of productivity and path analysis. Int J Intell Syst 2024(1):5511224
Herring RJ (2014) On risk and regulation: Bt crops in India. GM Crops Food 5(3):204–209
Hood WW, Wilson CS (2001) The literature of bibliometrics, scientometrics, and informetrics. Scientometrics 52:291–314. https://doi.org/10.1023/A:1017919924342
Hosseini MR, Martek I, Zavadskas EK, Aibinu AA, Arashpour M, Chileshe N (2018) Critical evaluation of off-site construction research: s scientometric analysis. Autom Constr 87:235–247. https://doi.org/10.1016/j.autcon.2017.12.002
Jayaraman K (2003) India debates results of its first transgenic cotton crop. Nature 421:681. https://doi.org/10.1038/421681a
Ji J, Barnett GA, Chu J (2019) Global networks of genetically modified crops technology: a patent citation network analysis. Scientometrics 118(3):737–762. https://doi.org/10.1007/s11192-019-03006-1
Jones MG, Fosu-Nyarko J, Iqbal S, Adeel M, Romero-Aldemita R, Arujanan M, Khoo K (2022) Enabling trade in gene-edited produce in Asia and Australasia: the developing regulatory landscape and future perspectives. Plants 11(19):2538
Kanaujia A, Bhattacharya S (2018) The GM crop debate in India: stakeholders’ interests, perceptions, trust and public policy. Asian Biotechnol Dev Rev 20(1/2):27–45
Kanaujia A, Bhattacharya S (2021) Genetically modified crops and Indian agriculture: issues relating to governance and regulation. Indian agriculture under the shadows of WTO and FTAs: issues and concerns. Springer, Singapore, pp 215–233. https://doi.org/10.1007/978-981-33-6854-5_11
Chapter Google Scholar
Klümper W, Qaim M (2014) A meta-analysis of the impacts of genetically modified crops. PLoS ONE 9(11):e111629
Liu Y (2016) Bibliometric review of international research on insect-resistant transgenic Bt rice. Chin J Appl Entomol 53(3):648–659
Lobato-Gómez M, Hewitt S, Capell T, Christou P, Dhingra A, Girón-Calva PS (2021) Transgenic and genome-edited fruits: background, constraints, benefits, and commercial opportunities. Hortic Res 8:166. https://doi.org/10.1038/s41438-021-00601-3
Nap JP, Metz PL, Escaler M, Conner AJ (2003) The release of genetically modified crops into the environment: part I. Overview of current status and regulations. Plant J 33(1):1–18
Parisi C, Tillie P, Rodríguez-Cerezo E (2016) The global pipeline of GM crops out to 2020. Nat Biotechnol 34(1):31–36. https://doi.org/10.1038/nbt.3449
Paull J (2015) GMOs and organic agriculture: Six lessons from Australia. Agric for 61(1):7–14
Perianes-Rodriguez A, Waltman L, Van Eck NJ (2016) Constructing bibliometric networks: a comparison between full and fractional counting. J Informet 10(4):1178–1195
Peschard K, Randeria S (2020) Taking Monsanto to court: legal activism around intellectual property in Brazil and India. J Peasant Stud 47(4):792–819
Pixley KV, Falck-Zepeda JB, Paarlberg RL, Phillips PW, Slamet-Loedin IH, Dhugga KS, Campos H, Gutterson N (2022) Genome-edited crops for improved food security of smallholder farmers. Nat Genet 54(4):364–367
Raman R (2017) The impact of genetically modified (GM) crops in modern agriculture: a review. GM Crops Food 8(4):195–208
Santillán-Fernández A, Salinas-Moreno Y, Valdez-Lazalde JR, Pereira-Lorenzo S (2021) Spatial-temporal evolution of scientific production about genetically modified maize. Agriculture 11(3):246
Scandizzo PL, and Savastano S (2010) The adoption and diffusion of GM crops in United States: A real option approach. CEIS Tor Vergata, Research Paper Series , 8 (4), CEIS Working Paper No. 169, Available at SSRN: https://ssrn.com/abstract=1653915 .
Sendhil R, Nyika J, Yadav S, Mackolil J, Workie E, Ragupathy R, Ramasundaram P (2022) Genetically modified foods: bibliometric analysis on consumer perception and preference. GM Crops Food 13(1):65–85. https://doi.org/10.1080/21645698.2022.2038525
Singh A, Kanaujia A, Singh VK, Vinuesa R (2024) Artificial intelligence for sustainable development goals: bibliometric patterns and concept evolution trajectories. Sustain Dev 32(1):724–754
Smyth SJ (2014) The state of genetically modified crop regulation in Canada. GM Crops Food 5(3):195–203
Su HN, Lee PC (2010) Mapping knowledge structure by keyword co-occurrence: a first look at journal papers in technology foresight. Scientometrics 85(1):65–79. https://doi.org/10.1007/s11192-010-0259
Zhang W, Xu X, Ming C, Mao Z, Shi J, Xiang Y (2016) Surviving in the dispute: a bibliometric analysis of global GMF-related research, 1995–2014. Scientometrics 109(1):359–375. https://doi.org/10.1007/s11192-016-1995-1
Zheng Y, Karimi-Maleh H, Fu L (2022) Advances in electrochemical techniques for the detection and analysis of genetically modified organisms: an analysis based on bibliometrics. Chemosensors 10(5):194. https://doi.org/10.3390/chemosensors10050194
Download references
Author information
Authors and affiliations.
Department of Computer Science, Banaras Hindu University, Varanasi, 221005, India
Anurag Kanaujia & Solanki Gupta
Delhi School of Analytics, University of Delhi, New Delhi, 110089, India
Anurag Kanaujia
School of Engineering and Technology, K. R. Mangalam University, Sohna Road, Gurgram, Haryana, 122103, India
Solanki Gupta
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization of Study, Data Collection, Analysis, Manuscript preparation and Manuscript Review—A.K. Operationalization, Data Processing analysis, Manuscript preparation and Manuscript Review—S.G.
Corresponding author
Correspondence to Anurag Kanaujia .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
(See Tables 7 and 8 ).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Kanaujia, A., Gupta, S. Tracing scientific and technological development in genetically modified crops. Transgenic Res (2024). https://doi.org/10.1007/s11248-024-00412-x
Download citation
Received : 11 October 2023
Accepted : 10 September 2024
Published : 18 September 2024
DOI : https://doi.org/10.1007/s11248-024-00412-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Genetically modified crops
- Scientometrics
- Patent analysis
- Agriculture
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 07 May 2021
Art as a tool for science
- David S. Goodsell ORCID: orcid.org/0000-0002-5932-2130 1 , 2
Nature Structural & Molecular Biology volume 28 , pages 402–403 ( 2021 ) Cite this article
23k Accesses
9 Citations
318 Altmetric
Metrics details
- Molecular modelling
- Research data
Artistic techniques are essential tools to visualize, understand and disseminate the results of scientific research. The field of structural biology has enjoyed a particularly productive marriage of art and science.
At a recent summer internship hosted by the Djerassi Resident Artists Program, I had the opportunity to watch several fine artists at work 1 . Once a year, this program tosses together six scientists and six artists for a month and provides them with the chance to create together. I was invited as one of the scientists. The experience was daunting, and in many ways, I came away with the impression that an artist’s task is infinitely more difficult than the work we face as scientists. Scientists work within very tight constraints: experiments must probe the nature of the world and must be reproducible; hypotheses must account for observations in a logical way; and most importantly, scientists must then devise new ways to test and possibly destroy these hypotheses with further experimentation. Fine artists have far fewer constraints. As they create work that speaks to their audiences, they are limited only by imagination and the technicalities and seductions of their chosen media. Consequently, fine artists need to create entire worlds from scratch. My time at this internship helped me to gain a better understanding of my own artwork, where the goal is more circumscribed: to create imagery as a tool for science.
Photographer Felice Frankel beautifully articulated this goal in relation to her own work: “I do not view myself as an artist because an artist has a personal agenda and a very particular point of view—that of communicating the part of herself she wants the world to perceive. One may view the images I take as artistic, but their primary purpose is to communicate scientific information” 2 . The idea of borrowing the techniques of fine art for scientific communication has proven useful throughout the history of science and is currently undergoing a renaissance with the SciArt movement. The SciArt community is a wonderfully heterogeneous mix of creative people: artists working on scientific themes, scientists using art in their science, and every combination in between.
The power of SciArt has perhaps its strongest manifestation in structural biology, where the things we study are particularly amenable to visual representation. Molecules have a size and shape, so synthetic imagery can trick us into thinking we can see them for ourselves. The early days of structural biology relied heavily on SciArt, better known at the time as “visualization.” Macromolecular X-ray crystallography was one of the early drivers of computer graphics hardware and software development, and as part of this, an entire visual language was invented to depict the structure and properties of proteins and nucleic acids 3 .
The impact of these visual tools is hard to measure, since they are so ingrained in every aspect of our work, both in research and in its dissemination. Today, we can head over to one of the worldwide Protein Data Bank sites ( https://wwpdb.org ) and instantly view more than 170,000 biomolecular structures using highly sophisticated graphics tools that are available, amazingly, directly in a web browser or on your phone. As structural biologists, we’re all intimately familiar with the uses of these methods. They allow us to pose structural questions on the fly and answer them interactively. We load a protein structure, measure distances and angles at coordination sites, look for neighboring amino acids and try to reconcile mutational data, color by surface charge or hydrophobicity to understand how this protein interacts with others, and so on. In my own research in computational biology and drug design, I use these tools every day without thinking twice. And when I want to present my work to other scientists or to a wider audience, I use these same tools, infused with a bit more artistic flair.
This type of SciArt—visualization—comes with strong constraints. Visualization is a tool for study, essentially extending the capabilities of our eyes, and must be treated like any of the other materials and methods that we employ in our research. The graphical approach needs to capture the salient properties of the molecule so that the insights we gain during the visualization will translate into insights about the biology. When used as figures in our papers, these images are documentary evidence of our discoveries and thus require a direct connection between the data and image, with no cherry-picked image processing or manual tweaking. To me, the constraints of scientific visualization are far more a joy than they are a curse. They invite me to focus on the goals of the image, and once these goals are set, I can leverage the creativity that we borrow from fine art to refine and simplify the visual method until it perfectly captures the desired properties of the molecule.
SciArt can also help us to see the larger context of our work. Artistic conceptions provide an easy way to explore speculative hypotheses about how our data fit together into a big picture. When constrained with a scientific sensibility, this is a powerful tool for synthesizing an increasingly comprehensive representation of the data to act as a touchstone for future thought and research. Speculative SciArtists continually ask difficult questions like this to explore unfamiliar worlds: Chesley Bonestell imagined what we would see if we stood on the surface of Titan; Isaac Asimov asked what it would be like to journey through the bloodstream. We can take this same approach as a scientific tool in structural biology.
In my postdoctoral work, I asked myself the question: “Can I paint an accurate picture of the molecular structure of a living cell?” After many hours in the library with the citation index and much enjoyable exploration of the Protein Data Bank (at the time, ~700-entries large!), my answer was “Almost.” With a liberal dose of artistic license and scientific intuition, I cobbled together as much information as I could find into an image of a portion of a bacterial cell 4 . This process was filled with hypotheses that needed answers: What direction do the peptidoglycan strands go? How bendy and supercoiled is the DNA? When RNA polymerase moves down the helical DNA strand, does the nascent mRNA end up wrapping around the DNA? In the years since then, as more and more structural, proteomics and ultrastructural data have become available, I have continued to update and refine this image (Fig. 1 ).
This watercolor painting integrates information from structural biology, microscopy and bioinformatics. I explored many hypotheses during creation of the painting, which required making decisions about, for example, sieving effects of the DNA (yellow) on the distribution of soluble molecules and details of the orientation and cross-linking of peptidoglycan chains (light turquoise) in the space between the membranes. This image is available under Creative Commons at the RCSB PDB ( https://doi.org/10.2210/rcsb_pdb/goodsell-gallery-028 ), along with more information on what is shown.
The process of creating this type of integrative image, rather than the final image itself, is arguably the most important aspect of the endeavor. This is when the fun begins, as it involves searching for information from multiple disciplines, fitting it together to build a larger picture, and filling the gaps with best guesses. I have since worked with many researchers to create similar integrative illustrations based on their work (see for example work on depicting autophagy with Daniel Klionsky 5 ). Invariably, the researchers learn as much as I do as we gather information on the parts of the painting related to their work, as well as information about the many other details that need to be included: the cellular context of their molecular work, or the molecular details of their cellular work.
In my laboratory, we are building software to help researchers create these types of integrative conceptions of their own work without the need for art classes and hours of painting. CellPAINT (Fig. 2 ) allows researchers to build up cellular illustrations that are similar to my paintings, using a set of molecular brushes that have molecule-like behaviors 6 . The goal is to put more tools into the hands of scientists, thereby reducing the barrier between their ideas and the manifestation of these ideas in images. In addition, by simplifying and streamlining the process of building these types of integrative illustrations, we can help in keeping up with the steady forward march of science. I always joke that my paintings go out-of-date the second I finish them. But that is the power of SciArt: it captures the current state of knowledge, warts and all, and hopefully spurs discussion and further exploration.
In the digital illustration program CellPAINT ( https://ccsb.scripps.edu/cellpaint ), molecules are chosen from a palette on the left and painted into the scene, and various options for painting, grouping, locking and erasing molecules are available on the right. Each of the molecular brushes is controlled by the behavior of the molecule, so the spike proteins will remain embedded in the viral membrane but antibodies will be free to diffuse around the virion.
Berrie, B. H. et al. Leonardo 52 , 220–229 (2019).
Article Google Scholar
Frankel, F. Science 280 , 1698–1700 (1998).
Article CAS Google Scholar
Olson, A. J. J. Mol. Biol. 430 , 3997–4012 (2018).
Goodsell, D. S. Trends Biochem. Sci. 16 , 203–206 (1991).
Goodsell, D. S. & Klionsky, D. J. Autophagy 6 , 3–6 (2010).
Gardner, A. et al. Front. Bioinform . https://doi.org/10.3389/fbinf.2021.660936 (2021).
Download references
Author information
Authors and affiliations.
Department of Integrative Structural and Computational Biology, the Scripps Research Institute, La Jolla, CA, USA
David S. Goodsell
Research Collaboratory for Structural Bioinformatics Protein Data Bank, Rutgers, the State University of New Jersey, Piscataway, NJ, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to David S. Goodsell .
Ethics declarations
Competing interests.
The author declares no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Goodsell, D.S. Art as a tool for science. Nat Struct Mol Biol 28 , 402–403 (2021). https://doi.org/10.1038/s41594-021-00587-5
Download citation
Published : 07 May 2021
Issue Date : May 2021
DOI : https://doi.org/10.1038/s41594-021-00587-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
A growing number of researchers in the life sciences community are recognizing the usefulness of systems biology tools for analyzing complex regulatory networks (both inside the cell, and the regulatory networks that integrate and control the functions of distinctly different cell types in multicellular organisms like humans) and for making ...
Biotechnology is a revolutionary branch of science at the forefront of research and innovation that has advanced rapidly in recent years. It is a broad discipline, in which organisms or biological processes are exploited to develop new technologies that have the potential to transform the way we live and work, as well as to boost sustainability ...
Plant biotechnology is a mature technology. Modern biotechnology was a scientifically obvious outcome of the striking advances in molecular biology that followed the discovery of the bacterial DNA restriction-modification system (Luria and Human, 1952; Luria, 1953; Dussoix and Arber, 1962; Nathans and Smith, 1975).Microorganisms and plants were the first organisms to be manipulated to serve ...
INTRODUCTION. Nearly a decade has passed since systems biology was introduced into the language of modern biology (Ideker et al. 2001, Kitano 2002).In that time it has expanded greatly in breadth; it now embraces much of the life sciences and is used to address many research problems across humans and diverse model species (Figure 1).Systems biology has also deepened considerably; many more ...
Abstract While we have a basic understanding of the functioning of the gene when coding sequences of specific proteins, we feel the lack of information on the role that DNA has on specific diseases or functions of thousands of proteins that are produced. Bioinformatics combines the methods used in the collection, storage, identification, analysis, and correlation of this huge and complex ...
In 1987, the New York Times Magazine characterized the Human Genome Project as the "biggest, costliest, most provocative biomedical research project in history." 2 But in the years between the ...
The term "biotechnology" refers to the application of techniques and the use of living organisms or biological processes to develop agricultural, industrial, or medical products. In particular, DNA technology is used in many aspects of modern biotechnology including DNA sequencing, analysis, cutting, and pasting.
Biotechnology is a broad discipline in which biological processes, organisms, cells or cellular components are exploited to develop new technologies. New tools and products developed by ...
Abstract. Integrating neural cultures developed through synthetic biology methods with digital computing has enabled the early development of Synthetic Biological Intelligence (SBI). Recently, key studies have emphasized the advantages of biological neural systems in some information processing tasks. However, neither the technology behind this ...
In modern science, cell biologists have access to an ever-growing armoury of microscopy tools, which can be used either alone or in combination and offer many improvements over the much older ...
Technologies and techniques. The development of a novel method, technique or technology can revolutionize the way in which we perform experiments and facilitate our understanding of fundamental ...
Optimal WSN configuration and control is an active area of research in engineering and computer science. WSNs are a promising technology for environmental monitoring, with plenty of potential for ecological research (Porter et al. 2005, 2009, Collins et al. 2006), including in aquatic environments (for a review, see Xu et al. 2014).
These articles reflect current trend and development in bioinformatics research. The supplement to BMC Bioinformatics was proposed to launch during the BIOCOMP'19—The 2019 International Conference on Bioinformatics and Computational Biology held from July 29 to August 01, 2019 in Las Vegas, Nevada. In this congress, a variety of research ...
The advent of recombinant DNA technology revolutionized the development in biology and led to a series of dramatic changes. It offered new opportunities for innovations to produce a wide range of therapeutic products with immediate effect in the medical genetics and biomedicine by modifying microorganisms, animals, and plants to yield medically ...
This year's program will explore the symbiotic relationship between biology and technology. Advances in biological research rely on the development and constant improvement of technologies permitting the visualization, manipulation, and analysis of biological data. ... Applicants must be full-time high school life science teachers or ...
First published: September 18, 2024. Genome design is the foundation of genome synthesis, which provides a new platform for deepening our understanding of biological systems by exploring the fundamental components and structure of the genome. Artificial genome designs can endow unnatural genomes with desired functions.
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and ...
Biological techniques are methods or procedures that are used to study living things. They include experimental and computational methods, approaches, protocols and tools for biological research ...
Using synthetic biology tools, microorganisms have been engineered to sense, quantify, and report the presence of specific environmental pollutants (Brutesco et al., 2017). These biosensors, reviewed in section two of this paper, provide simple and cost-effective techniques for monitoring water and soil quality.
Bioinformatics is defined as the application of tools of computation and analysis to the capture and interpretation of biological data. It is an interdisciplinary field, which harnesses computer science, mathematics, physics, and biology (fig 1). Bioinformatics is essential for management of data in modern biology and medicine.
2. The wider science and technology convergence. Science is about discovering, understanding, explaining and predicting patterns in natural phenomena, producing more accurate explanations of how the natural world works (Bertolaso, Citation 2013; Robson & McCartan, Citation 2016), regardless of potential applications.It is the result of deep curiosity and its goal is the pursuit of knowledge ...
Cognitive intelligence is a higher-level ability of induction, reasoning and acquisition of knowledge. It is inspired by cognitive science, brain science, and brain-like intelligence to endow machines with thinking logic and cognitive ability similar to human beings. Once a machine has the abilities of perception and cognition, it is often ...
Genetically Modified (GM) Organisms have been used in various domains since their introduction in the 1980s. According to ISAAA data, the use of GM crops in agriculture has also increased significantly in the past 30 years. However, even after 3 decades of commercialisation, GM crops are still surrounded with controversies with different countries adopting varying approaches to their ...
Artistic techniques are essential tools to visualize, understand and disseminate the results of scientific research. The field of structural biology has enjoyed a particularly productive marriage ...