- Open access
- Published: 17 January 2024

Nutrient patterns and risk of diabetes mellitus type 2: a case-control study
- Morteza haramshahi 1 ,
- Thoraya Mohamed Elhassan A-Elgadir 2 ,
- Hamid Mahmood Abdullah Daabo 3 ,
- Yahya Altinkaynak 4 ,
- Ahmed Hjazi 5 ,
- Archana Saxena 6 ,
- Mazin A.A. Najm 7 ,
- Abbas F. Almulla 8 ,
- Ali Alsaalamy 9 &
- Mohammad Amin Kashani 10
BMC Endocrine Disorders volume 24 , Article number: 10 ( 2024 ) Cite this article
2419 Accesses
1 Altmetric
Metrics details
Backgrounds
Although the significance of diet in preventing or managing diabetes complications is highlighted in current literature, there is insufficient evidence regarding the correlation between nutrient patterns and these complications. The objective of this case-control study is to investigate this relationship by analyzing the dietary intake of nutrients in participants with and without type 2 diabetes (T2D).
A case-control study was conducted at the Tabriz Center of Metabolism and Endocrinology to investigate the relationship between nutrient patterns and type 2 diabetes (T2D). The study enrolled 225 newly diagnosed cases of T2D and 225 controls. The dietary intake of nutrients was assessed using a validated semi-quantitative food frequency questionnaire (FFQ). Principal component analysis using Varimax rotation was used to obtain nutrient patterns. Logistic regression analysis was performed to estimate the risk of T2D.
The participants’ mean (SD) age and BMI were 39.8 (8.8) years and 27.8 (3.6) kg/m2, respectively. The results identified three major nutrient patterns. The first nutrient pattern was characterized by high consumption of sucrose, animal protein, vitamin E, vitamin B1, vitamin B12, calcium, phosphorus, zinc, and potassium. The second nutrient pattern included fiber, plant protein, vitamin D, Riboflavin, Vitamin B5, copper, and Magnesium. The third nutrient pattern was characterized by fiber, plant protein, vitamin A, riboflavin, vitamin C, calcium, and potassium. Individuals in the highest tertile of nutrient pattern 3 (NP3) had a lower risk of T2D compared to those in the lowest tertile after adjusting for confounders. The odds ratio was 0.52 with a 95% confidence interval of 0.30–0.89 and a P_trend of 0.039.
This study found that conforming to a nutrient pattern consisting of plant protein, vitamin C, vitamin A, vitamin B2, potassium, and calcium is linked to a lower likelihood of developing T2D.The initial results suggest that following a nutrient pattern that includes these nutrients may reduce the risk of T2D. However, further research is required to confirm the relationship between nutrient patterns and T2D.
Peer Review reports
Type 2 diabetes is a significant concern for public health in developed nations. It leads to high rates of illness and death and places a significant financial burden on healthcare systems [ 1 , 2 ]. In the past few decades, there has been a sharp increase in the occurrence of diabetes, and is expected to continue increasing, with an estimated 693 million people living with the disease by 2045 [ 1 ]. Complications associated with type 2 diabetes can also contribute to premature death. A concerning aspect of the disease is that a significant proportion of cases (40%) go undetected [ 3 ], and there is also an increasing prevalence of prediabetes, which raises the risk of developing type 2 diabetes and other chronic diseases [ 1 ].
The connection between diet and type 2 diabetes has been extensively studied, including the examination of dietary patterns and individual foods or nutrient patterns [ 4 , 5 , 6 , 7 ]. Various sources have suggested that chronic diseases may be influenced by a combination of nutrients [ 8 ]. In the field of nutritional epidemiology, the examination of dietary patterns has emerged as a viable approach to investigate the correlation between diet and disease. This method involves using statistical techniques to combine multiple foods or nutrients into dietary or nutrient patterns, which are believed to provide a more detailed understanding of the connection between diet and disease. It has been suggested that the impact of individual nutrients or foods on chronic disease may be too subtle to detect, but their collective effect within a pattern may be more indicative [ 9 ].
There have been some recent studies examining the effect of nutrient patterns on chronic disease such as, non-alcoholic fatty liver, breast and gastric cancer, Polycystic Ovary Syndrome (PCOs) and metabolic syndrome [ 10 , 11 , 12 , 13 , 14 ]. For example, it was found that a nutrient pattern consisting mainly of protein, carbohydrates, and various sugars was linked to a higher risk of Metabolic Syndrome (MetS) in both men and women, whereas a pattern characterized by copper, selenium, and several vitamins was linked to greater odds of MetS [ 14 ]. A prospective study conducted among participants of the Tehran Lipid and Glucose Study indicates that a nutrient pattern rich in vitamin A, vitamin C, vitamin B6, potassium, and fructose is associated with a reduced risk of insulin-related disorders [ 15 ]. Although there have been limited investigations on the connection between nutrient patterns and the likelihood of developing diabetes, the present study seeks to explore this relationship by analyzing the adherence to different nutrient patterns and its effect on the risk of type 2 diabetes.
Study population
This study utilized a case-control design and involved participants between the ages of 18 and 60 who had been diagnosed with type 2 diabetes within the previous six months based on specific glucose level criteria (FBS levels of ≥ 126 mg/dl and 2 h-PG levels of ≥ 200 mg/dl [ 17 ]). Healthy individuals within the same age range were also included, with specific glucose level criteria (FBS levels of < 100 mg/dl and 2 h-PG levels of < 200 mg/dl [ 17 ]). The study excluded individuals with certain chronic diseases, Type 1 Diabetes, gestational diabetes, those following specific dietary patterns or taking certain medications, pregnant and breastfeeding women, those with a family history of diabetes or hypertension, and those who did not complete the food frequency questionnaire (more than 35 items) or whose reported energy intake was outside of a specific range (range of 800–4200 kcal [ 18 ]).
This study enrolled 450 adult participants, with 225 individuals in the case group and 225 in the control group. The case group was selected using a simple sampling method from patients diagnosed with diabetes at the Tabriz Center of Metabolism and Endocrinology as a referral center affiliated to tabriz University of Medical Sciences from January 2021 to March 2022, as well as through a two-stage cluster sampling method among patients referred to private endocrinologists to enhance the sample’s external validity. Participants in the control group were also selected through a two-stage cluster sampling method from individuals who had undergone blood glucose checkups at the Tabriz Center of Metabolism and Endocrinology, a referral center affiliated with Tabriz University of Medical Sciences, within the past six months. All participants provided informed consent at the beginning of the study. The study was financially supported by Tabriz University of Medical Sciences and is related to project NO. 1400/63,145.
Dietary assessment
To collect dietary intake information, personal interviews and a semi-quantitative food frequency questionnaire (FFQ) consisting of 168 food items were used [ 16 ]. The FFQ asked about the frequency of consumption for each item over the course of one year, with the year before diagnosis for the case group and the year before the interview for the control group. Participants were also asked about the frequency of consumption (per day, week, month, or year) for each type of food. to ensure consistency in measurements, a nutritionist provided instructions on converting the size of reported food items from household measures to grams using four scales. The quantity of food consumed by each individual was calculated based on their intake in grams and reported on a daily basis. The nutrient composition of all foods was derived by using modified nutritionist IV software.
Nutrient pattern assessment
We conducted factor analyses using a comprehensive set of 34 nutrients, encompassing various macronutrients, micronutrients, and other dietary components. These included sucrose, lactose, fructose, fiber, animal protein, plant protein, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, cholesterol, as well as an array of vitamins and minerals such as A, D, E, K, C, thiamine (B1), riboflavin (B2), niacin (B3), pantothenic acid (B5), pyridoxine (B6), folate (B9), B12, calcium, phosphorus, iron, zinc, copper, magnesium, manganese, chromium, selenium, sodium, potassium, and caffeine. The dietary intake of these 34 nutrients per 1,000 Kcal of energy intake was computed and utilized as input variables. Subsequently, nutrient patterns (NPs) were derived through principal component analysis (PCA) with varimax rotation, based on the correlation matrix. Factor scores for each participant were then calculated by aggregating the frequency of consumption and multiplying it by the factor loadings across all 34 nutrients. To assess the statistical correlation between variables and evaluate the adequacy of the sample size, we employed the Bartlett test of sphericity ( P < 0.001) and the Kaiser-Mayer-Olkin test (0.71), respectively.
Assessment of other variables
To obtain the participants’ anthropometric measurements, weight and height were measured using a seca scale, and the participants’ BMI was determined by dividing their weight in kilograms by the square of their height in meters. Waist circumference was measured using a metal anthropometric tape, and the participants’ hip circumference was measured using a metal anthropometric tape while standing [ 17 ]. Daily physical activity was measured using a physical activity questionnaire [ 18 ], and personal questioning was employed to gather information on population and socioeconomic characteristics, including marital status, academic degree, and smoking.
Statistical analysis
Statistical analysis was performed using the Statistical Package Software for Social Science, version 21. The normality of the data was assessed using Kolmogorov-Smirnov’s test and histogram chart. The characteristics and dietary intakes of the case and control groups were presented as mean ± SD or median and frequency (percentages). Independent sample t-tests and chi-square tests were used to compare continuous and categorical variables, respectively, between the case and control groups.
The participants’ mean (SD) age and BMI were 39.8 (8.8) years and 27.8 (3.6) kg/m2, respectively. The mean (SD) BMI in the case group was 30.5 ± 4.1, and in the control group, it was 25.2 ± 3.2 kg/m2. The mean (SD) physical activity in the case group was 1121 ± 611 MET/min/week, and in the control group, it was 1598 ± 940 MET/min/week. There were significant differences in BMI and physical activity between the two groups. The mean (SD) waist circumference in the case group was 109.32 ± 10.28 cm, and in the control group, it was 87.25 ± 9.35 cm. The mean (SD) hip circumference in the case group was 107.25 ± 8.61 cm, and in the control group, it was 91.44 ± 6.17 cm. The study identified three primary nutrient patterns (NPs) with eigenvalues greater than 2. Table 1 displays the factor loadings for nutrient patterns, which accounted for 56.11% of the total nutrient variation. The high intake of sucrose, animal protein, phosphorus, zinc, potassium, calcium, vitamin E, vitamin B1 and vitamin B12 were the distinguishing features of the first pattern. The second nutrient pattern was positively associated with copper, magnesium, fiber, vitamin D, B2, B5 and plant protein but had a negative correlation with lactose and saturated fatty acids. On the other hand, the high intake of fiber, vitamin A, B2, vitamin C, plant protein and potassium were the distinguishing features of the third pattern.
The following are the characteristics of T2D patients compared to the control group, as shown in Table 2 : Higher BMI, More likely to be smokers, Lower physical activity levels, higher FBS, HbA1C, Insulin ( p < 0.05). Other variables did not differ significantly between the two groups ( p > 0.05). Additionally, T2D patients had a greater intake of energy and vitamin B3 but consumed less plant protein, vitamin A, vitamin E, vitamin B2, and zinc ( p < 0.05).
Table 3 summarizes the partial correlation coefficient between NPs and food sources, with NP1 showing a strong positive correlation with low-fat dairy, NP2 with refined grains, and NP3 with fruits and vegetables.
Table 4 demonstrates the relationships between NPs and T2D. After adjusting for age and sex, there was no significant link between each nutrient pattern (NP) and T2D. However, when adjusting for other factors such as BMI, physical activity, smoking, and energy intake, individuals in the highest tertile of NP1 and NP2 did not show a significant association with T2D compared to those in the lowest tertile. On the other hand, those in the highest tertile of NP3 had a lower probability of developing T2D than those in the lowest tertile (OR: 0.52, 95%CI: 0.30–0.89, P_trend = 0.039).
In this study, three major NPs were identified. After adjusting for potential confounders, we observed a significant inverse association between the Third NP and the odds of T2D. The high intake of fiber, vitamin A, B2, vitamin C, plant protein and potassium were the distinguishing features of the third pattern.
Dietary patterns, such as healthy, Mediterranean, traditional, and Western dietary patterns, have recently received significant attention in studying the connection between diet and health. When looking at the relationship between nutrients and disease incidence, it is more challenging to evaluate when considering individual foods and the metabolism of all nutrients together [ 19 ]. It is therefore more effective to take a broader view and consider diet as a whole. Dietary and nutrient patterns can have a greater impact on health than specific nutrients or nutritional groups. There is supporting evidence that links high calorie or high glycemic index foods with an increased risk of T2D. The quality of one’s diet is also associated with the risk, progression, and side effects of T2D [ 20 ]. Establishing a desirable food pattern has become a priority in public health efforts to prevent T2D. By studying dietary and nutrient patterns, we can gain a comprehensive understanding of an individual’s overall diet beyond just the consumption of specific nutrients and food groups. Moreover, it is easier for people to understand health recommendations when presented as dietary patterns rather than focusing solely on individual nutrients [ 19 ].
A previous cross-sectional study investigated the relationship between NPs and fasting glucose and glycated hemoglobin levels among apparently healthy black South Africans. The study stratified 2,010 participants by gender and urban/rural status and identified three nutrient patterns per stratum. In rural women, a nutrient pattern driven by starch, dietary fiber, and B vitamins was significantly associated with lower fasting glucose and glycated hemoglobin levels. A nutrient pattern that included vitamin B1, zinc, and plant protein was linked to notable decreases in glycated hemoglobin and fasting glucose levels in rural men. These findings suggest that nutrient patterns that are plant-based are linked to lower levels of fasting glucose and glycated hemoglobin [ 21 ].
Iwasaki et al. found that specific nutrient patterns were associated with lower risks of MetS. One nutrient pattern high in potassium, fiber, and vitamins, while another pattern high in vitamin B2, saturated fatty acids and calcium [ 22 ]. A recent study found that a nutrient pattern characterized by high intake of calcium, potassium, fats, cholesterol, vitamins B2, B12, A, D, K and C was positively linked to MetS [ 23 ]. Salehi-Sahlabadi et al. found that adhering to a nutrient pattern rich in potassium, vitamin A, fructose, vitamin C and vitamin B6 was negatively associated with the likelihood of NAFLD [ 11 ]. A nutrient pattern high in potassium, vitamin A, vitamin B6, vitamin C and fructose was associated with a reduced risk of hyperinsulinemia, IR, and dyslipidemia among participants in Tehran, according to a prospective study [ 11 , 24 , 25 ].
Due to several variations among studies exploring NPs linked to chronic diseases, including differences in the number of nutrients, populations, study designs and outcomes there has been a considerable diversity in the identified NPs, with only a few NPs being replicated across studies. Our study is the first of its kind to explore the correlation between nutrient patterns and T2D in this context.
In our study, there was no association between NPs 1 and 2 and T2D. This lack of correlation may be attributed to the absence of harmful nutrients or food categories linked to diabetes in these NPs. NP3 in this study, unlike other NPs, is positively associated with beneficial food groups such as nuts, fruits, plant oil and vegetables, and negatively associated with unhealthy food groups like red-processed meat, snacks, high-fat dairy and refined grains. A recent systematic review and meta-analysis found that individuals who consumed higher amounts of fruits and vegetables had a lower risk of developing type 2 diabetes [ 26 ]. Moreover, the consumption of vegetables was found to have an inverse relationship with ALT, TC and LDL levels among adults, while fruit consumption was associated with a positive reduction in visceral fat [ 27 , 28 ]. Another study suggested that an increased intake of vegetables and fruits could potentially lower the risk of MetS [ 29 ]. According to a study, greater nut consumption was significantly linked to a reduced prevalence of T2D [ 30 ]. Consuming fruits and vegetables is a crucial component of a healthful dietary pattern that can lower the risk of type 2 diabetes [ 31 ]. On the other hand, Consuming a Western dietary pattern, which primarily consists of fast foods, high-fat dairy, refined grains, soft drinks and processed meat has been found to be correlated with an increased risk of type 2 diabetes [ 31 ].
Several mechanisms have been identified that explain the positive associations between the components of NP 3 and T2D or its risk factors. Vitamin intake has been shown to play a role in the development of T2D through various pathways. Consuming vitamin C has been found to have beneficial effects in reducing the risk of type 2 diabetes mellitus. These effects can be attributed to the following actions of vitamin C: vasodilator, cytoprotective, platelet anti-aggregator and anti-mutagenic. To achieve this, the body increases the production of several substances including prostaglandin E1, PGI2, endothelial nitric oxide, and lipoxin A4. Additionally, the body restores the Arachidonic Acid content to normal levels [ 32 ]. Vitamin A has a multifaceted role in cell regulation beyond its antioxidant function. It contributes to gene regulation, epithelial cell integrity, and resistance to infection. Research suggests that vitamin A also enhances antioxidant enzyme function in the body. Research has indicated a link between vitamin A deficiency and type 2 diabetes mellitus (T2DM), which suggests that vitamin A may have a role in the biology of T2DM [ 33 ]. Moreover, a meta-analysis has found that replacing animal protein with plant protein can lead to minor improvements in glycemic control for individuals with diabetes [ 34 ]. According to a recent meta-analysis, increasing the consumption of fruits, especially berries, yellow vegetables, cruciferous vegetables, green leafy vegetables is associated with a lower risk of developing type 2 diabetes. These results support the recommendation to incorporate more fruits and vegetables into the diet as a way to prevent various chronic diseases, including type 2 diabetes [ 35 ]. A study showed that maintaining adequate potassium intake could regulate insulin secretion and carbohydrate metabolism, leading to the prevention of obesity and metabolic syndrome (MetS) [ 36 ].
A number of research studies conducted in the Western societies have shown that Western dietary pattern including higher intake of red meat, processed meat, and refined grains is significantly associated with increased risk of T2D [ 37 , 38 ]. For example, in the 12-years cohort prospective study, van Dam et al. investigated dietary pattern of 42,504 American white men at the age range of 40–75 years old using the FFQ. After controlling the confounders, the risk of T2D increased 60% in people adherent to the western-like dietary pattern [ 38 ]. The rapid process of change in lifestyle, diets, and physical activity that have been occurred as a result of extended urbanization, improved economic status, change of work pattern toward jobs, and change in the processes of producing and distributing nutrients during the recent years in developing countries have led people to more consumption of fast food and processed foods [ 20 ].
Significant research has been conducted on the impact of nutrient type and sequence on glucose tolerance. Multiple studies have shown that manipulating the sequence of food intake can enhance glycemic control in individuals with type 2 diabetes in real-life situations. The glucose-lowering effect of preload-based nutritional strategies has been found to be more pronounced in type 2 diabetes patients compared to healthy individuals. Moreover, consuming carbohydrates last, as part of meal patterns, has been proven to improve glucose tolerance and reduce the risk of weight gain [ 39 ]. Recent findings on meal sequence further emphasize the potential of this dietary approach in preventing and managing type 2 diabetes [ 40 ].
Several studies have shown that food from a short supply chain has a significant impact on metabolic syndrome. The length of the food supply chain is important in determining the risk of metabolic syndrome in a population [ 41 ]. Research indicates that people who consume food from short supply chains have a lower prevalence of metabolic syndrome compared to those who consume food from long supply chains. Specifically, food from short supply chains is associated with lower levels of triglycerides and glucose, which leads to a reduced occurrence of metabolic syndrome [ 42 ]. Adhering to the Mediterranean diet with a short supply chain is also found to significantly reduce the prevalence of metabolic syndrome. Therefore, these studies provide evidence that food from short supply chains positively affects metabolic parameters and the occurrence of metabolic syndrome [ 41 ].
The study we conducted presented several advantages. It was the first case-control research to investigate the correlation between nutrient patterns and the likelihood of developing type 2 diabetes (T2D). While numerous studies have explored the relationship between dietary patterns and diabetes, there is a scarcity of research specifically focusing on nutrient patterns in individuals with type 2 diabetes. Furthermore, the collection of dietary intake data was carried out through face-to-face interviews conducted by trained dieticians to minimize measurement errors. However, this study also had some limitations. Case-control studies are susceptible to selection and recall biases. Additionally, the use of factor analysis to identify patterns, and the potential influence of research decisions on the number of factors and nutrient factor loadings in each pattern, should be considered. Lastly, despite the use of a validated semi-quantitative FFQ (food frequency questionnaire), there remains a possibility of measurement error due to dietary recall. The study’s findings and limitations contribute to the ongoing discourse on the role of nutrient patterns in the development of T2D and the importance of considering these factors in future research and preventive strategies.
Conclusions
The results of this study indicate that conforming to a nutrient pattern consisting of plant protein, vitamin C, vitamin A, vitamin B2, potassium, and calcium is linked to a lower likelihood of developing T2D. Our investigation did not reveal any significant correlation between other nutrient patterns and T2D risk. However, additional research is necessary to authenticate these initial findings and establish the correlation between nutrient patterns and T2D.
Data availability
Upon reasonable request, the corresponding author can provide the datasets that were produced and analyzed during the current study.
Ogurtsova K, Guariguata L, Barengo NC, Ruiz PL-D, Sacre JW, Karuranga S, et al. IDF Diabetes Atlas: global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. 2022;183:109118.
Article PubMed Google Scholar
Teo ZL, Tham Y-C, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–91.
Sugiyama T, Yanagisawa-Sugita A, Tanaka H, Ihana-Sugiyama N, Imai K, Ohsugi M, et al. Different incidences of diabetic retinopathy requiring treatment since diagnosis according to the course of diabetes diagnosis: a retrospective cohort study. Sci Rep. 2023;13(1):10527.
Article CAS PubMed PubMed Central Google Scholar
Hodge AM, English DR, O’Dea K, Giles GG. Dietary patterns and diabetes incidence in the Melbourne Collaborative Cohort Study. Am J Epidemiol. 2007;165(6):603–10.
Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr. 2017;147(6):1174–82.
Article CAS PubMed Google Scholar
Sami W, Ansari T, Butt NS, Ab Hamid MR. Effect of diet on type 2 diabetes mellitus: a review. Int J Health Sci. 2017;11(2):65.
Google Scholar
Miller V, Micha R, Choi E, Karageorgou D, Webb P, Mozaffarian D. Evaluation of the quality of evidence of the association of foods and nutrients with cardiovascular disease and diabetes: a systematic review. JAMA Netw open. 2022;5(2):e2146705–e.
Article PubMed PubMed Central Google Scholar
Salehi-Sahlabadi A, Teymoori F, Jabbari M, Momeni A, Mokari-Yamchi A, Sohouli M, et al. Dietary polyphenols and the odds of non-alcoholic fatty liver disease: a case-control study. Clin Nutr ESPEN. 2021;41:429–35.
Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L, Keshteli AH, Feizi A, Feinle-Bisset C, et al. Nutrient patterns and their relation to general and abdominal obesity in Iranian adults: findings from the SEPAHAN study. Eur J Nutr. 2016;55:505–18.
Panjeshahin A, Salehi-Abargouei A, Ghadiri-Anari A, Rasouli A, Hosseinzadeh M. The Association between nutrient patterns and polycystic ovary syndrome: a case-control study. J Nutr Food Secur. 2022.
Salehi-Sahlabadi A, Teymoori F, Ahmadirad H, Mokhtari E, Azadi M, Seraj SS, et al. Nutrient patterns and non-alcoholic fatty liver disease in Iranian Adul: a case-control study. Front Nutr. 2022;9:977403.
Fereidani SS, Eini-Zinab H, Heidari Z, Jalali S, Sedaghat F, Rashidkhani B. Nutrient patterns and risk of breast cancer among Iranian women: a case-control study. Asian Pac J cancer Prevention: APJCP. 2018;19(9):2619.
CAS Google Scholar
Narmcheshm S, Sasanfar B, Hadji M, Zendehdel K, Toorang F, Azadbakht L. Patterns of nutrient intake in relation to gastric cancer: a case control study. Nutr Cancer. 2022;74(3):830–9.
Khayyatzadeh SS, Moohebati M, Mazidi M, Avan A, Tayefi M, Parizadeh SMR, et al. Nutrient patterns and their relationship to metabolic syndrome in Iranian adults. Eur J Clin Invest. 2016;46(10):840–52.
Teymoori F, Mokhtari E, Salehi P, Hosseini-Esfahani F, Mirmiran P, Azizi F. A nutrient pattern characterized by vitamin A, C, B6, potassium, and fructose is associated with reduced risk of insulin-related disorders: a prospective study among participants of Tehran lipid and glucose study. Diabetol Metab Syndr. 2021;13(1):12.
Esmaillzadeh A, Azadbakht L. Major dietary patterns in relation to general obesity and central adiposity among Iranian women. J Nutr. 2008;138(2):358–63.
Wang J, Thornton JC, Bari S, Williamson B, Gallagher D, Heymsfield SB, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutr. 2003;77(2):379–84.
Committee IR. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)-short and long forms. http://www.ipaq.ki.se/scoring.pdf ; 2005.
Mandalazi E, Drake I, Wirfält E, Orho-Melander M, Sonestedt E. A high diet quality based on dietary recommendations is not associated with lower incidence of type 2 diabetes in the Malmö Diet and Cancer Cohort. Int J Mol Sci. 2016;17(6):901.
Beigrezaei S, Ghiasvand R, Feizi A, Iraj B. Relationship between dietary patterns and incidence of type 2 diabetes. Int J Prev Med. 2019;10:122.
Chikowore T, Pisa PT, Van Zyl T, Feskens EJ, Wentzel-Viljoen E, Conradie KR. Nutrient patterns associated with fasting glucose and glycated haemoglobin levels in a black South African population. Nutrients. 2017;9(1):9.
Iwasaki Y, Arisawa K, Katsuura-Kamano S, Uemura H, Tsukamoto M, Kadomatsu Y, et al. Associations of nutrient patterns with the prevalence of metabolic syndrome: results from the baseline data of the Japan multi-institutional collaborative cohort study. Nutrients. 2019;11(5):990.
Sadeghi O, Sadeghi A, Mozaffari-Khosravi H, Shokri A. The association between nutrient patterns and metabolic syndrome among Iranian adults: cross-sectional analysis of Shahedieh cohort study. Public Health Nutr. 2021;24(11):3379–88.
Mottaghian M, Salehi P, Teymoori F, Mirmiran P, Hosseini-Esfahani F, Azizi F. Nutrient patterns and cardiometabolic risk factors among Iranian adults: Tehran lipid and glucose study. BMC Public Health. 2020;20:1–12.
Article Google Scholar
Teymoori F, Mokhtari E, Salehi P, Hosseini-Esfahani F, Mirmiran P, Azizi F. A nutrient pattern characterized by vitamin A, C, B6, potassium, and fructose is associated with reduced risk of insulin-related disorders: a prospective study among participants of Tehran lipid and glucose study. Diabetol Metab Syndr. 2021;13(1):1–13.
Halvorsen RE, Elvestad M, Molin M, Aune D. Fruit and vegetable consumption and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis of prospective studies. BMJ Nutr Prev Health. 2021;4(2):519.
Mollahosseini M, Daneshzad E, Rahimi MH, Yekaninejad MS, Maghbooli Z, Mirzaei K. The association between fruit and vegetable intake and liver enzymes (aspartate and alanine transaminases) in Tehran, Iran. Ethiop J Health Sci. 2017;27(4):401–10.
Plaz Torres MC, Bodini G, Furnari M, Marabotto E, Zentilin P, Giannini EG. Nuts and non-alcoholic fatty liver disease: are nuts safe for patients with fatty liver disease? Nutrients. 2020;12(11):3363.
Lee M, Lim M, Kim J. Fruit and vegetable consumption and the metabolic syndrome: a systematic review and dose–response meta-analysis. Br J Nutr. 2019;122(7):723–33.
Muley A, Fernandez R, Ellwood L, Muley P, Shah M. Effect of tree nuts on glycemic outcomes in adults with type 2 diabetes mellitus: a systematic review. JBI Evid Synthesis. 2021;19(5):966–1002.
Beigrezaei S, Ghiasvand R, Feizi A, Iraj B. Relationship between dietary patterns and incidence of type 2 diabetes. Int J Prev Med. 2019;10.
Das UN. Vitamin C for type 2 diabetes mellitus and hypertension. Arch Med Res. 2019;50(2):11–4.
Iqbal S, Naseem I. Role of vitamin A in type 2 diabetes mellitus biology: effects of intervention therapy in a deficient state. Nutrition. 2015;31(7–8):901–7.
Viguiliouk E, Stewart SE, Jayalath VH, Ng AP, Mirrahimi A, De Souza RJ, et al. Effect of replacing animal protein with plant protein on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2015;7(12):9804–24.
Wang PY, Fang JC, Gao ZH, Zhang C, Xie SY. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: a meta-analysis. J Diabetes Invest. 2016;7(1):56–69.
Cai X, Li X, Fan W, Yu W, Wang S, Li Z, et al. Potassium and obesity/metabolic syndrome: a systematic review and meta-analysis of the epidemiological evidence. Nutrients. 2016;8(4):183.
van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary patterns and risk for type 2 diabetes mellitus in US men. Ann Intern Med. 2002;136(3):201–9.
Fung TT, Schulze M, Manson JE, Willett WC, Hu FB. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med. 2004;164(20):2235–40.
Wheeler ML, Dunbar SA, Jaacks LM, Karmally W, Mayer-Davis EJ, Wylie-Rosett J, et al. Macronutrients, Food groups, and eating patterns in the management of diabetes: a systematic review of the literature, 2010. Diabetes Care. 2012;35(2):434–45.
Dwibedi C, Mellergård E, Gyllensten AC, Nilsson K, Axelsson AS, Bäckman M, et al. Effect of self-managed lifestyle treatment on glycemic control in patients with type 2 diabetes. Npj Digit Med. 2022;5(1):60.
Santulli G, Pascale V, Finelli R, Visco V, Giannotti R, Massari A, et al. We are what we eat: impact of food from short supply chain on metabolic syndrome. J Clin Med. 2019;8(12):2061.
De Rosa M, Giannotti R, Pascale A, Finelli R, Ilario M, Ciccarelli M, et al. P6280 food of short supply chain impacts metabolism and cardiovascular risk. A survey in Southern Italy. Eur Heart J. 2018;39(suppl1):ehy566. P6280.
Download references
Acknowledgements
The researchers express their gratitude towards all the individuals who volunteered to take part in the study.
This research received no external funding.
Author information
Authors and affiliations.
Faculty of medicine, Tabriz University of medical sciences, Tabriz, Iran
Morteza haramshahi
Department of clinical biochemistry, College of medicine, King Khalid University, Abha, Saudi Arabia
Thoraya Mohamed Elhassan A-Elgadir
Fharmacy Department, Duhok polytechnic, University Duhok, Kurdistan, Iraq
Hamid Mahmood Abdullah Daabo
Department of Medical Services and Techniques, Ardahan University, Ardahan, Turkey
Yahya Altinkaynak
Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Prince Sattam bin Abdulaziz University, Jeddah, Saudi Arabia
Ahmed Hjazi
Department of Management, Uttaranchal Institute of Management, Uttaranchal University, Dehradun, Uttarakhand, India
Archana Saxena
Pharmaceutical Chemistry Department, College of Pharmacy, Al-Ayen University, Thi-Qar, Iraq
Mazin A.A. Najm
College of technical engineering, The Islamic University, Najaf, Iraq
Abbas F. Almulla
College of technical engineering, Imam Ja’afar Al-Sadiq University, Al‐Muthanna, 66002, Iraq
Ali Alsaalamy
Department of Medicinal Chemistry, Faculty of Pharmacy, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
Mohammad Amin Kashani
You can also search for this author in PubMed Google Scholar
Contributions
The study’s protocol was designed by M.K., M.H., and T.E., while H.A., Y.A., and A.H. carried out the research. A.S. analyzed the data and prepared the initial draft of the manuscript. M.N., A.FA., and A.A. interpreted the data and provided critical feedback on the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Correspondence to Mohammad Amin Kashani .
Ethics declarations
Ethics approval and consent to participate.
This study was performed in line with the principles of the Declaration of Helsinki. Informed consent was obtained from all participants or their legal guardians. Approval was granted by the Research Ethics Committee of Islamic Azad University of Medical Sciences (Approval number: IR.AUI.MEDICINE. REC.1401.147).
Consent for publication
Not applicable.
Competing interests
The authors declared no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
haramshahi, M., A-Elgadir, T.M.E., Daabo, H.M.A. et al. Nutrient patterns and risk of diabetes mellitus type 2: a case-control study. BMC Endocr Disord 24 , 10 (2024). https://doi.org/10.1186/s12902-024-01540-5
Download citation
Received : 04 November 2023
Accepted : 09 January 2024
Published : 17 January 2024
DOI : https://doi.org/10.1186/s12902-024-01540-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Nutrient pattern
BMC Endocrine Disorders
ISSN: 1472-6823
- General enquiries: [email protected]
- Cancer Nursing Practice
- Emergency Nurse
- Evidence-Based Nursing
- Learning Disability Practice
- Mental Health Practice
- Nurse Researcher
- Nursing Children and Young People
- Nursing Management
- Nursing Older People
- Nursing Standard
- Primary Health Care
- RCN Nursing Awards
- Nursing Live
- Nursing Careers and Job Fairs
- CPD webinars on-demand
- --> Advanced -->
| | | |

- Clinical articles
- CPD articles
- CPD Quizzes
- Expert advice
- Clinical placements
- Study skills
- Clinical skills
- University life
- Person-centred care
- Career advice
- Revalidation
Art & Science Previous Next
Type 2 diabetes: a case study, priscilla cunningham nursing student, queen’s university belfast, belfast, northern ireland, helen noble lecturer, health services research, school of nursing and midwifery, queen’s university belfast, belfast, northern ireland.
Increased prevalence of diabetes in the community has been accompanied by an increase in diabetes in hospitalised patients. About a quarter of these patients experience a hypoglycaemic episode during their admission, which is associated with increased risk of mortality and length of stay. This article examines the aetiology, pathophysiology, diagnosis and treatment of type 2 diabetes using a case study approach. The psychosocial implications for the patient are also discussed. The case study is based on a patient with diabetes who was admitted to hospital following a hypoglycaemic episode and cared for during a practice placement. The importance of early diagnosis of diabetes and the adverse effects of delayed diagnosis are discussed.
Nursing Standard . 29, 5, 37-43. doi: 10.7748/ns.29.5.37.e9142
This article has been subject to double blind peer review
Received: 20 May 2014
Accepted: 15 July 2014
Blood glucose - case study - diabetes - glucose testing - hyperglycaemia - hypoglycaemia - insulin resistance - sulfonylureas - type 2 diabetes
User not found
Want to read more?
Already have access log in, 3-month trial offer for £5.25/month.
- Unlimited access to all 10 RCNi Journals
- RCNi Learning featuring over 175 modules to easily earn CPD time
- NMC-compliant RCNi Revalidation Portfolio to stay on track with your progress
- Personalised newsletters tailored to your interests
- A customisable dashboard with over 200 topics
Alternatively, you can purchase access to this article for the next seven days. Buy now
Are you a student? Our student subscription has content especially for you. Find out more

07 October 2014 / Vol 29 issue 5
TABLE OF CONTENTS
DIGITAL EDITION
- LATEST ISSUE
- SIGN UP FOR E-ALERT
- WRITE FOR US
- PERMISSIONS
Share article: Type 2 diabetes: a case study
We use cookies on this site to enhance your user experience.
By clicking any link on this page you are giving your consent for us to set cookies.
- DOI: 10.2337/DIASPECT.16.1.32
- Corpus ID: 73083750
Case Study: A Patient With Uncontrolled Type 2 Diabetes and Complex Comorbidities Whose Diabetes Care Is Managed by an Advanced Practice Nurse
- G. Spollett
- Published 2003
- Diabetes Spectrum
5 Citations
Management of ketosis-prone type 2 diabetes mellitus., integrating a pico clinical questioning to the ql4pomr framework for building evidence-based clinical case reports, nursing practice guideline for foot care for patients with diabetes in thailand, goal-driven structured argumentation for patient management in a multimorbidity setting, logic and argumentation: third international conference, clar 2020, hangzhou, china, april 6–9, 2020, proceedings, 18 references, using a primary nurse manager to implement dcct recommendations in a large pediatric program, diabetes in urban african americans. iii. management of type ii diabetes in a municipal hospital setting., primary care outcomes in patients treated by nurse practitioners or physicians: a randomized trial., caring for a child with diabetes: the effect of specialist nurse care on parents' needs and concerns., standards of medical care for patients with diabetes mellitus, management of patients with diabetes by nurses with support of subspecialists., a practical approach to type 2 diabetes., the diabetes control and complications trial (dcct): the trial coordinator perspective, oral antihyperglycemic therapy for type 2 diabetes: scientific review., caring for feet: patients and nurse practitioners working together., related papers.
Showing 1 through 3 of 0 Related Papers
- Diabetes & Primary Care
- Vol:24 | No:06
Interactive case study: The elderly and type 2 diabetes
Share this article + Add to reading list – Remove from reading list ↓ Download pdf

Diabetes & Primary Care ’s series of interactive case studies is aimed at all healthcare professionals in primary and community care who would like to broaden their understanding of diabetes.
The care of older people with type 2 diabetes is complicated, as the prognosis and appropriate treatment goals vary greatly between individuals. The three mini-case studies developed for this issue of the journal take us through the basic considerations of managing type 2 diabetes in the elderly.
The format uses typical clinical scenarios as tools for learning. Information is provided in short sections, with most ending in a question to answer before moving on to the next section.
Working through the case studies will improve our knowledge and problem-solving skills in diabetes care by encouraging us to make evidence-based decisions in the context of individual cases.
Readers are invited to respond to the questions by typing in your answers. In this way, we are actively involved in the learning process, which is hopefully a much more effective way to learn.
By actively engaging with these case histories, I hope you will feel more confident and empowered to manage such presentations effectively in the future.
Marianne , who is 71 years old, has type 2 diabetes but lives a very active life, with little in the way of comorbidities. However, despite treatment with metformin 1000 mg twice daily, her glycaemic control has deteriorated in recent years.
Mike is 78 years old and has long-standing type 2 diabetes. Six years ago he suffered a myocardial infarction. He takes a range of medication to address his hyperglycaemia, hypertension and low mood. He lives alone, but uses a stick to walk and receives practical help from his daughter. Recently, he has been experiencing shakiness and sweating after gardening, and dizziness on standing. His BP is 117/58 mmHg and HbA 1c is 51 mmol/mol.
Claire is an 81-year-old who lives in a care-home. She has Alzheimer’s disease and long-standing type 2 diabetes. A stroke 4 years ago left her with unilateral weakness, and she has frequent lower urinary tract infections and episodes of urinary incontinence. For her hyperglycaemia, hypertension and various other health concerns, she is taking over a dozen medications. A review of her diabetes is due.
The health and care needs of each of these people differ greatly. By working through their case studies, we will consider the following issues, and more:
- Agreeing glycaemic targets in the elderly.
- Assessment of frailty and the importance of a holistic approach to managing diabetes in the elderly.
- Choice of medications and concerns over hypoglycaemia.
- Deintensification and simplification of medication regimens.
Click here to see the case study.
Diabetes Distilled: Diabetes-related foot ulcers – detailed advice for primary care
Conference over coffee: diabetes and obesity within multiple long-term condidions, lada – assessing diabetes in a non-overweight younger person, challenges and opportunities in reducing risk of diabetes-related cardiovascular disease: making every contact count, diabetes distilled: pneumonia hospitalisation associated with long- and short-term risk of cardiovascular mortality, editorial: a tribute to dr michael mosley, pcds news: obesity survey results.
Review and guidelines highlight opportunities for primary care to really make a difference.
25 Jul 2024

The interactions between diabetes, obesity and long-term conditions, including cardiovascular disease, chronic kidney disease and cancer.
23 Jul 2024

The characteristics and clinical implications of LADA, its differential diagnosis and its possible management strategies.
18 Jul 2024

Exploring the unique opportunities general practice nurses have to promote primary and secondary prevention of CVD.
17 Jul 2024
Sign up to all DiabetesontheNet journals
- CPD Learning
- Journal of Diabetes Nursing
- Diabetes Care for Children & Young People
- The Diabetic Foot Journal
- Diabetes Digest
Useful information
- Terms and conditions
- Privacy policy
- Editorial policies and ethics

By clicking ‘Subscribe’, you are agreeing that DiabetesontheNet.com are able to email you periodic newsletters. You may unsubscribe from these at any time. Your info is safe with us and we will never sell or trade your details. For information please review our Privacy Policy .
Are you a healthcare professional? This website is for healthcare professionals only. To continue, please confirm that you are a healthcare professional below.
We use cookies responsibly to ensure that we give you the best experience on our website. If you continue without changing your browser settings, we’ll assume that you are happy to receive all cookies on this website. Read about how we use cookies .
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Predictors of diabetic retinopathy in type 2 diabetes: a cross-sectional study.

1. Introduction
2. materials and methods, 2.1. study design and population, 2.2. data collection and medical assessment, 2.3. statistical analysis, 4. discussion, 5. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022 , 183 , 109119. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ting, D.S.W.; Cheung, G.C.M.; Wong, T.Y. Diabetic Retinopathy: Global Prevalence, Major Risk Factors, Screening Practices and Public Health Challenges: A Review. Clin. Exper. Ophthalmol. 2016 , 44 , 260–277. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045. Ophthalmology 2021 , 128 , 1580–1591. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vujosevic, S.; Aldington, S.J.; Silva, P.; Hernández, C.; Scanlon, P.; Peto, T.; Simó, R. Screening for Diabetic Retinopathy: New Perspectives and Challenges. Lancet Diabetes Endocrinol. 2020 , 8 , 337–347. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- DCCT Research Group. Diabetes Control and Complications Trial (DCCT): Results of Feasibility Study. The DCCT Research Group. Diabetes Care 1987 , 10 , 1–19. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive Blood-Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes (UKPDS 33). Lancet 1998 , 352 , 837–853. [ Google Scholar ] [ CrossRef ]
- Ruta, L.M.; Magliano, D.J.; Lemesurier, R.; Taylor, H.R.; Zimmet, P.Z.; Shaw, J.E. Prevalence of Diabetic Retinopathy in Type 2 Diabetes in Developing and Developed Countries. Diabet. Med. 2013 , 30 , 387–398. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rein, D.B.; Wittenborn, J.S. Prevalence of Diabetic Retinopathy in Health Care Settings—An Early Warning Sign? JAMA Ophthalmol. 2024 , 142 , 607–608. [ Google Scholar ] [ CrossRef ]
- Guseh, J.S. Aging of the World’s Population. In Encyclopedia of Family Studies ; Shehan, C.L., Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 1–5. ISBN 978-1-119-08562-1. [ Google Scholar ]
- Nicholson, K.; Almirall, J.; Fortin, M. The Measurement of Multimorbidity. Health Psychol. 2019 , 38 , 783–790. [ Google Scholar ] [ CrossRef ]
- Cicek, M.; Buckley, J.; Pearson-Stuttard, J.; Gregg, E.W. Characterizing Multimorbidity from Type 2 Diabetes: Insights from Clustering Approaches. Endocrinol. Metab. Clin. N. Am. 2021 , 50 , 531–558. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Graue-Hernandez, E.O.; Rivera-De-La-Parra, D.; Hernandez-Jimenez, S.; Aguilar-Salinas, C.A.; Kershenobich-Stalnikowitz, D.; Jimenez-Corona, A. Prevalence and Associated Risk Factors of Diabetic Retinopathy and Macular Oedema in Patients Recently Diagnosed with Type 2 Diabetes. BMJ Open Ophthalmol. 2020 , 5 , e000304. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhang, M.; Wu, J.; Wang, Y.; Wu, J.; Hu, W.; Jia, H.; Sun, X. Associations between Blood Pressure Levels and Diabetic Retinopathy in Patients with Diabetes Mellitus: A Population-Based Study. Heliyon 2023 , 9 , e16830. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, L.; Gao, J.; Rao, X.; Liu, X. Relationship between Atherosclerotic Cardiovascular Disease and Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus. Medicine 2024 , 103 , e38051. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hafeez, M.; Achar, P.; Neeralagi, M.; Naik, G.T. Correlation between Diabetic Retinopathy and Diabetic Peripheral Neuropathy in Patients with Type II Diabetes Mellitus. J. Pharm. Bioallied Sci. 2022 , 14 , S658–S661. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chatziralli, I.P. The Role of Dyslipidemia Control in the Progression of Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus. Diabetes Ther. 2017 , 8 , 209–212. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kiziltoprak, H.; Tekin, K.; Inanc, M.; Goker, Y.S. Cataract in Diabetes Mellitus. World J. Diabetes 2019 , 10 , 140–153. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Klein, B.E.; Klein, R.; Moss, S.E. Incidence of Cataract Surgery in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Am. J. Ophthalmol. 1995 , 119 , 295–300. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ojha, S.; Kukreja, P.; Verma, S. Association of Intraocular Pressure with Blood Sugar Levels in Patients of Type 2 Diabetes Mellitus and Control Group. TNOA J. Ophthalmic Sci. Res. 2022 , 60 , 294. [ Google Scholar ] [ CrossRef ]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.K.; et al. Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology 2018 , 125 , 1608–1622. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Acan, D.; Calan, M.; Er, D.; Arkan, T.; Kocak, N.; Bayraktar, F.; Kaynak, S. The Prevalence and Systemic Risk Factors of Diabetic Macular Edema: A Cross-Sectional Study from Turkey. BMC Ophthalmol. 2018 , 18 , 91. [ Google Scholar ] [ CrossRef ]
- Mugharbel, K.M.; Al-Mansouri, M.A. Prevalence of Obesity among Type 2 Diabetic Patients in Al-Khobar Primary Health Care Centers. J. Family Community Med. 2003 , 10 , 49–53. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, W.; Gong, X.; Wang, W.; Xiong, K.; Meng, J.; Li, Y.; Wang, L.; Liang, X.; Jin, L.; Huang, W. Association of Different Kinds of Obesity with Diabetic Retinopathy in Patients with Type 2 Diabetes. BMJ Open 2022 , 12 , e056332. [ Google Scholar ] [ CrossRef ]
- Zhou, Q.; Sun, J.; Wu, Z.; Wu, W.; Zhang, X.; Pan, Q.; Qi, H.; Yuan, H.; Shi, H.; Cao, S.; et al. The Older, the Less Potential Benefit for Type 2 Diabetes from Weight Control. BMC Geriatr. 2022 , 22 , 346. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Galaviz, K.I.; Narayan, K.M.V.; Lobelo, F.; Weber, M.B. Lifestyle and the Prevention of Type 2 Diabetes: A Status Report. Am. J. Lifestyle Med. 2018 , 12 , 4–20. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Durlach, V.; Vergès, B.; Al-Salameh, A.; Bahougne, T.; Benzerouk, F.; Berlin, I.; Clair, C.; Mansourati, J.; Rouland, A.; Thomas, D.; et al. Smoking and Diabetes Interplay: A Comprehensive Review and Joint Statement. Diabetes Metab. 2022 , 48 , 101370. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cai, X.; Chen, Y.; Yang, W.; Gao, X.; Han, X.; Ji, L. The Association of Smoking and Risk of Diabetic Retinopathy in Patients with Type 1 and Type 2 Diabetes: A Meta-Analysis. Endocrine 2018 , 62 , 299–306. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hillier, T.A.; Pedula, K.L. Complications in Young Adults with Early-Onset Type 2 Diabetes: Losing the Relative Protection of Youth. Diabetes Care 2003 , 26 , 2999–3005. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Aljefri, S.; Al Adel, F. The Validity of Diabetic Retinopathy Screening Using Nonmydriatic Fundus Camera and Optical Coherence Tomography in Comparison to Clinical Examination. Saudi J. Ophthalmol. 2020 , 34 , 266–272. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zeppieri, M.; Marsili, S.; Enaholo, E.S.; Shuaibu, A.O.; Uwagboe, N.; Salati, C.; Spadea, L.; Musa, M. Optical Coherence Tomography (OCT): A Brief Look at the Uses and Technological Evolution of Ophthalmology. Medicina 2023 , 59 , 2114. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Agarwal, D.; Gelman, R.; Prospero Ponce, C.; Stevenson, W.; Christoforidis, J.B. The Vitreomacular Interface in Diabetic Retinopathy. J. Ophthalmol. 2015 , 2015 , 392983. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hagag, A.; Gao, S.; Jia, Y.; Huang, D. Optical Coherence Tomography Angiography: Technical Principles and Clinical Applications in Ophthalmology. Taiwan. J. Ophthalmol. 2017 , 7 , 115. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tol, A.; Baghbanian, A.; Mohebbi, B.; Shojaeizadeh, D.; Azam, K.; Shahmirzadi, S.E.; Asfia, A. Empowerment Assessment and Influential Factors among Patients with Type 2 Diabetes. J. Diabetes Metab. Disord. 2013 , 12 , 6. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pourhabibi, N.; Mohebbi, B.; Sadeghi, R.; Shakibazadeh, E.; Sanjari, M.; Tol, A.; Yaseri, M. Determinants of Poor Treatment Adherence among Patients with Type 2 Diabetes and Limited Health Literacy: A Scoping Review. J. Diabetes Res. 2022 , 2022 , 2980250. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| Variable | Value |
|---|---|
| Number of patients | 302 |
| Women Nr (%) | 145 (48.0%) |
| Age (years) | 64 [57; 70] |
| DM duration (years) | 12 [6; 17] |
| BMI (Kg/m ) | 27 [25; 30.8] |
| HbA1c (%) | 7.5 [7; 8] |
| Mean IOP (mmHg) | 15 [13; 17] |
| IOP right eye (mmHg) | 14 [12; 16] |
| IOP left eye (mmHg) | 16 [14; 18] |
| Cataract or IOL implant Nr (%) | 61 (20.2%) |
| DR Nr (%) | 105 (34.8%) |
| Hypertension Nr (%) | 212 (69.5%) |
| CVD Nr (%) | 236 (78.2%) |
| Diabetic polyneuropathy Nr (%) | 191 (63.3%) |
| CKD Nr (%) | 84 (27.8%) |
| Dyslipidaemia Nr (%) | 150 (49.7%) |
| Smoker status Nr (%) | 59 (19.5%) |
| Variable | Value |
|---|---|
| Number of patients | 105 |
| Women Nr (%) | 50 (47.6%) |
| Age (years) | 66 [59; 73] |
| DM duration (years) | 15 [7.9; 20] |
| BMI (Kg/m ) | 28.3 [25.7; 31] |
| HbA1c (%) | 8 [7.5; 8.8] |
| Mean IOP (mmHg) | 15.5 [14; 17.5] |
| IOP right eye (mmHg) | 15 [14; 17] |
| IOP left eye (mmHg) | 16 [14; 18] |
| Cataract or IOL implant Nr (%) | 22 (21%) |
| DME Nr (%) | 19 (18.1%) |
| Hypertension Nr (%) | 82 (78.1%) |
| CVD Nr (%) | 91 (86.7%) |
| Diabetic polyneuropathy Nr (%) | 75 (71.4%) |
| CKD Nr (%) | 41 (39.1%) |
| Dyslipidaemia | 62 (59.0%) |
| Smoker status Nr (%) | 27 (25.7%) |
| Argon laser photocoagulation Nr (%) | 56 (53.3%) |
| Parameter | Mild NPDR | Moderate NPDR | Severe NPDR | PDR | p * |
|---|---|---|---|---|---|
| HbA1c (%) | 7.1 [7; 7.45] | 8 [7.5; 8.6] | 8 [7.45; 8.2] | 8.9 [8; 9.1] | <0.0001 |
| DM duration (years) | 8 [5; 10] | 5 [3; 11.5] | 15.5 [12.5; 20] | 20 [15; 23] | <0.0001 |
| Age (years) | 68 [60.5; 72] | 69 [58.2; 75.7] | 64.5 [56; 70.5] | 64.5 [60; 73] | 0.51 |
| Variable | With DR | Without DR | |||||
|---|---|---|---|---|---|---|---|
| n | Median | Average Rank | n | Median | Average Rank | p | |
| DM duration | 105 | 15.0000 | 169.8524 | 197 | 12.0000 | 141.7183 | 0.0076 |
| HbA1c | 105 | 8.0000 | 199.0190 | 197 | 7.2000 | 126.1726 | <0.0001 |
| BMI | 105 | 28.2828 | 170.2476 | 197 | 27.0000 | 0.0064 | |
| IOP OD | 105 | 14.0000 | 121.7476 | 197 | 13.5000 | 109.2863 | 0.1530 |
| IOP OS | 105 | 16.0000 | 156.3095 | 197 | 16.0000 | 148.9365 | 0.4821 |
| Age | 105 | 66.0000 | 171.4619 | 197 | 63.0000 | 140.8604 | 0.0037 |
| DR | Without DR | p * | Chi-Squared | |
|---|---|---|---|---|
| Cataract Nr (%) | 22 (21%) | 39 (19.8%) | 0.8120 | 0.0565 |
| Variable | Odds Ratio | 95% CI |
|---|---|---|
| HbA1c | 2.6624 | 1.9309 to 3.6710 |
| Age | 1.0500 | 1.0184 to 1.0826 |
| DM duration | 1.0182 | 0.9859 to 1.0515 |
| Comorbidity | OR | 95% CI | Z Statistic | Significance Level |
|---|---|---|---|---|
| HTA | 1.8375 | 1.0618 to 3.1797 | 2.174 | p = 0.0297 |
| CVD | 2.3310 | 1.2221 to 4.4462 | 2.569 | p = 0.0102 |
| Polyneuropathy | 1.7026 | 1.0215 to 2.8379 | 2.042 | p = 0.0412 |
| CKD | 2.2943 | 1.3672 to 3.8502 | 3.144 | p = 0.0017 |
| Dyslipidaemia | 1.8275 | 1.1299 to 2.9558 | 2.458 | p = 0.0140 |
| Smoker status | 1.7740 | 0.9945 to 3.1647 | 1.941 | p = 0.0522 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Ivanescu, A.; Popescu, S.; Ivanescu, R.; Potra, M.; Timar, R. Predictors of Diabetic Retinopathy in Type 2 Diabetes: A Cross-Sectional Study. Biomedicines 2024 , 12 , 1889. https://doi.org/10.3390/biomedicines12081889
Ivanescu A, Popescu S, Ivanescu R, Potra M, Timar R. Predictors of Diabetic Retinopathy in Type 2 Diabetes: A Cross-Sectional Study. Biomedicines . 2024; 12(8):1889. https://doi.org/10.3390/biomedicines12081889
Ivanescu, Adriana, Simona Popescu, Radu Ivanescu, Monica Potra, and Romulus Timar. 2024. "Predictors of Diabetic Retinopathy in Type 2 Diabetes: A Cross-Sectional Study" Biomedicines 12, no. 8: 1889. https://doi.org/10.3390/biomedicines12081889
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

- Previous Article
Presentation
Clinical pearls, article information, case study: diabetic ketoacidosis in type 2 diabetes: “look under the sheets”.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Brian J. Welch , Ivana Zib; Case Study: Diabetic Ketoacidosis in Type 2 Diabetes: “Look Under the Sheets”. Clin Diabetes 1 October 2004; 22 (4): 198–200. https://doi.org/10.2337/diaclin.22.4.198
Download citation file:
- Ris (Zotero)
- Reference Manager
Diabetic ketoacidosis (DKA) is a cardinal feature of type 1 diabetes. However, there is a strong, almost dogmatic, errant perception by physicians that DKA is a complication that only occurs in patients with type 1 diabetes. This is not true. DKA does occur in type 2 diabetes; however, it rarely occurs in the absence of a precipitating event.
R.T., a 25-year-old African-American man with type 2 diabetes presented with a 5-day history of nausea and vomiting. He also reported a 2-week history of polyuria and polydipsia and a 10-lb weight loss. A review of symptoms was pertinent for a 5-day history of persistent lower back pain.
The patient was diagnosed with type 2 diabetes 5 years ago when he presented to a different hospital with symptoms of polyuria, polydipsia, and weight loss. He was given a prescription for a sulfonylurea, which he says he took until his initial prescription ran out 1 month later. He had not taken any other medication since that time.
Physical examination revealed an afebrile, obese man (BMI 40 kg/m 2 ) with prominent acanthosis nigricans, no retinopathy by direct funduscopic exam, and a normal neurological exam, including motor function and sensation. The patient had no tenderness to palpation over the lumbrosacral spine or paraspinous muscles despite his complaint of lower back pain.
The laboratory data showed an anion gap, metabolic acidosis, and hyperglycemia (pH of 7.14, anion gap of 24, bicarbonate 6 mmol/l, urinary ketones 150 mg/dl, glucose 314 mg/dl) consistent with the diagnosis of DKA. His white blood count was 20,400/μl. Urinalysis demonstrated no evidence of infection. The patient's hemoglobin A 1c (A1C) was 13.5%.
The patient was admitted and treated aggressively with intravenous fluid and an insulin-glucose infusion. A non-contrast magnetic resonance imaging(MRI) of the lumbosacral spine (L-spine) was obtained because of the patient's persistent complaint of lower back pain. The L-spine MRI results were negative for pathology. However, R.T. reported increasing discomfort and now noted weakness and numbness in his bilateral lower extremities.
Neurology was consulted, and during their assessment, the patient became incontinent and was found to have 0/5 strength in the lower extremities,severely compromised sensation, and decreased rectal tone. A contrast MRI of both the thoracic and lumbar spine was ordered, and the patient was found to have a T10-T12 epidural abscess ( Figure 1 ).

Epidural abscess precipitating DKA in a type 2 diabetic patient.
The patient's antibiotic coverage was broadly expanded, high-dose intravenous steroids were initiated, and neurosurgery was urgently consulted. Emergent evacuation of the epidural abscess with laminectomies of T10-T12 was performed without complication.
R.T.'s neurogenic bladder resolved without further intervention. After intensive inpatient rehabilitation, he had 3/5 strength in bilateral lower extremities and was still unable to ambulate.
S.D., a 39-year-old white man with type 2 diabetes and mild mental retardation, presented with a 3-week history of polyuria and polydipsia, as well as dysuria, left hip pain, and a feeling of incomplete bladder emptying. Because of the severity of his left hip discomfort, the patient required a cane to ambulate.
The patient was diagnosed with type 2 diabetes 4 years ago on the basis of an elevated fasting blood glucose level during a routine medical examination. He was started on oral hypoglycemic agents, but he discontinued them after 1 month because he was unable to pay for them.
On physical exam, S.D. was afebrile but tachycardic (heart rate 131 bpm)and hypertensive (blood pressure 192/118 mmHg). General examination revealed a wasted, severely volume-depleted man. Thrush was observed on oropharyngeal exam. Cardiopulmonary and abdominal examinations were unremarkable. The patient had point tenderness on the anterior aspect of his left hip. Rectal examination revealed a non-tender prostate.
The laboratory data showed an anion gap, metabolic acidosis, and hyperglycemia (pH 7.24, bicarbonate 9 mmol/l, anion gap 24, urinary ketones 150 mg/dl, and glucose 322 mg/dl) consistent with the diagnosis of DKA. Urinalysis was remarkable for large blood, 4+ bacteria, and > 400 white blood cells. S.D.'s serum white blood count was 22,200, and his erythrocyte sedimentation rate was 109 mm/hour. His A1C result was 12.6%.
The patient was admitted and treated with intravenous fluids and an insulin-glucose infusion. Cultures were obtained. S.D. was started empirically on ticarcillin/clavulanic acid because of concern for left hip osteomyelitis and complicated urinary tract infection. An MRI of the left hip was ordered to evaluate for suspected osteomyelitis. Unexpectedly, it revealed left hip myonecrosis and a large loculated prostatic abscess( Figure 2 ).

Prostatic abscess precipitating DKA in a type 2 diabetic patient.
Urology was consulted, and the patient underwent transurethral drainage of the prostatic abscess. Methicillin-sensitive Staphylococcus aureus grew from both blood and urine cultures. S.D. was treated with intravenous antibiotics per culture sensitivities. The myonecrosis was treated conservatively.
The patient recovered well. He was started on subcutaneous insulin and discharged home to complete a 2-week course of intravenous antibiotics.
What is the mechanism of DKA?
Why does DKA occur in type 2 diabetes?
DKA is a cardinal feature of type 1 diabetes, which has led to the widespread errant perception that it is a complication unique to type 1 diabetes. However, it has been repeatedly reported that DKA does occur in patients with type 2 diabetes. 1 - 5 Moreover, as the cases presented here illustrate, it can occur even in patients who were previously insulinindependent.
A recent study evaluating 138 consecutive admissions for DKA at a large academic center observed that 21.7% had type 2 diabetes. 6 Nearly 70% of the admissions involved discontinuation of medications, and almost half had an identifiable infection when an intensive search was undertaken.
A review of the mechanism of DKA is important. Ketoacidosis occurs as a function not only of severe insulin deficiency, but also of elevated glucagon levels. Insulin is an anabolic hormone. Severe insulin deficiency results in decreased glucose utilization by muscle and an unregulated increase in lipolysis. This leads to an enhanced delivery of gluconeogenetic precursors(glycerol and alanine) to the liver. Furthermore, removal of the normal suppressive effect of insulin causes glucagon elevation. 7 , 8 Glucagon is a catabolic hormone. Glucagon promotes gluconeogenesis, decreases oxidation of free fatty acids to triglycerides, and promotes hepatic ketogenesis. 9
Importantly, the concentration of insulin required to suppress lipolysis is only one-tenth of that required to promote glucose utilization. 10 Typically, moderate insulin deficiency (as observed in patients with type 2 diabetes) is associated with sufficient insulin to block lipolysis (and therefore ketoacid formation), but not enough to promote glucose utilization. This leads to hyperglycemia without formation of the ketoacids.
When DKA occurs in patients with type 2 diabetes, the presumed mechanism of ketoacidosis is the combination of relative insulin deficiency and increased secretion of glucagon (as well as other counteregulatory hormones such as cortisol, catecholamines, and growth hormone) in response to stress from 1 ) overwhelming infection, 2 ) infarction of tissue, or 3 ) other severe illness. The elevated catecholamines further suppress insulin secretion to perpetuate a downward spiral. The increased glucagons-to-insulin ratio causes a mismatch that promotes unregulated lipolysis and proteolysis with subsequent uninterrupted formation of ketoacids.
To summarize, DKA is not a unique feature of type 1 diabetes. Though much more common in type 1 diabetes, it does occur in patients with type 2 diabetes, as illustrated by these case reports. However, it is rare for DKA to occur in type 2 diabetes in the absence of some precipitating event. When DKA occurs in an individual with type 2 diabetes, the clinician should “look under the sheets” and initiate an intensive search for the precipitating factor. Once identified, the trigger should be treated promptly and appropriately.
DKA does occur in type 2 diabetes.
DKA in type 2 diabetes rarely occurs without a trigger.
When it does, an intensive search for the precipitating factor should be undertaken.
Brian J. Welch, MD, and Ivana Zib, MD, are fellows in the Division of Endocrinology and Metabolism at the University of Texas Southwestern Medical Center in Dallas.
The authors thank Philip Raskin, MD, for his support and guidance.
Email alerts
- Online ISSN 1945-4953
- Print ISSN 0891-8929
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 15 August 2024
Evaluation of a specialist nurse-led structured self-management training for peer supporters with type 2 diabetes mellitus with or without comorbid hypertension in Slovenia
- Tina Virtič Potočnik 1 , 2 ,
- Matic Mihevc 1 , 3 ,
- Črt Zavrnik 1 , 3 ,
- Majda Mori Lukančič 1 ,
- Nina Ružić Gorenjec 1 , 4 ,
- Antonija Poplas Susič 1 , 3 &
- Zalika Klemenc-Ketiš 1 , 2 , 3
BMC Nursing volume 23 , Article number: 567 ( 2024 ) Cite this article
234 Accesses
1 Altmetric
Metrics details
The training of peer supporters is critical because the success of the entire peer support intervention depends on the knowledge and experience that peer supporters can share with other patients. The objective of this study was to evaluate the pilot implementation of a specialist nurse-led self-management training programme for peer supporters with type 2 diabetes mellitus (T2DM) with or without comorbid hypertension (HTN) at the primary healthcare level in Slovenia, in terms of feasibility, acceptability, and effectiveness.
A prospective pre-post interventional pilot study was conducted in two Community Health Centres (CHC) in Slovenia from May 2021 to August 2022. Purposive sampling was employed to recruit approximately 40 eligible volunteers to become trained peer supporters. A specialist nurse-led structured training lasting 15 h over a 2-month period was delivered, comprising four group and two individual sessions. The comprehensive curriculum was based on interactive verbal and visual learning experience, utilising the Diabetes Conversation Maps™. Data were collected from medical records, by clinical measurements, and using questionnaires on sociodemographic and clinical data, the Theoretical Framework of Acceptability, knowledge of T2DM and HTN, and the Appraisal of Diabetes Scale, and evaluation forms.
Of the 36 participants, 31 became trained peer supporters (retention rate of 86.1%). Among them, 21 (67.7%) were women, with a mean age of 63.9 years (SD 8.9). The training was evaluated as satisfactory and highly acceptable. There was a significant improvement in knowledge of T2DM ( p < 0.001) and HTN ( p = 0.024) among peer supporters compared to baseline. Six months post-training, there was no significant improvement in the quality of life ( p = 0.066), but there was a significant decrease in body mass index (BMI) ( p = 0.020) from 30.4 (SD 6.2) at baseline to 29.8 (SD 6.2).
The pilot implementation of a specialist nurse-led self-management training for peer supporters was found to be feasible, acceptable, and effective (in the study group). It led to improvements in knowledge, maintained disease control, and promoted positive self-management behaviours among peer supporters, as evidenced by a decrease in their BMI over six months. The study emphasises the need for effective recruitment, training, and retention strategies.
Trial registration
The research is part of the international research project SCUBY: Scale up diabetes and hypertension care for vulnerable people in Cambodia, Slovenia and Belgium, which is registered in ISRCTN registry ( https://www.isrctn.com/ISRCTN41932064 ).
Peer Review reports
New models for comprehensive, patient-centred, integrated care have been introduced in Slovenian primary care to improve the quality of care for people with type 2 diabetes mellitus (T2DM) and hypertension (HTN) [ 1 , 2 , 3 , 4 ]. One example of an evidence-based model of such care is the Integrated Care Package [ 5 ], which encompasses elements of early detection and diagnosis, treatment in primary care, health education, self-management support by patients and caregivers, and collaboration among caregivers [ 5 , 6 ]. The integrated care provided for patients with T2DM and HTN in Slovenia is generally of high quality. However, the implementation of self-management support is only weakly developed [ 7 ]. The provision of self-management support for T2DM and HTN requires the ongoing engagement and motivation of patients, which cannot be adequately addressed by the healthcare system alone [ 8 , 9 ]. Consequently, the focus of patient-centered care should shift from healthcare institutions to the patient’s local and home environment [ 10 ]. One potential solution is the introduction of peer support by appropriately trained lay people, which would empower patients, family members and other informal caregivers in the local community [ 7 ]. This form of collaboration between peer supporters, patients, healthcare providers, and the local community is not yet established in Slovenia. Therefore, there is a necessity to investigate and implement this approach to scale-up integrated care for individuals with T2DM and HTN.
Patients are well-suited for the role of volunteer peer supporters because they can share first-hand knowledge, similar experiences and lifestyle issues with others who have the same chronic disease. As they operate within the local community, there are no demographic, language or cultural barriers between them. Peer supporters do not possess medical qualifications; rather, their role is to complement health services by providing practical assistance to individuals living with the same chronic disease. This assistance encompasses a range of activities, including offering guidance on coping with daily life, creating a supportive emotional and social environment, and providing ongoing support to assist with the lifelong needs of disease self-management [ 11 , 12 , 13 ]. Several systematic reviews have demonstrated that peer support interventions significantly improve glycaemic outcomes in adults with T2DM who receive such support [ 14 , 15 , 16 ]. A systematic review and meta-analysis on the effects of peer support interventions on other cardiovascular disease risk factors in adults with T2DM found a positive effect only on recipients’ systolic blood pressure (SBP) but not on diastolic blood pressure (DBP), cholesterol, body mass index (BMI), diet, or physical activity [ 17 ].
Training and coordinating peer supporters is crucial for the success of the peer support intervention, as it is essential that peer supporters have the knowledge and experience to effectively assist others [ 11 , 12 ]. The main problem is the lack of studies describing training models that provide comprehensive knowledge and enhance the ability of peer supporters to support self-management. The literature predominantly focuses on the peer support intervention itself and only a handful on peer supporter’s training, changes in knowledge, skills acquired [ 19 , 20 , 21 ] or impact on health outcomes [ 22 ]. There is a lack of guidelines in the methodology of training programme, including recruitment strategies, materials used, individuals delivering the training and duration of the training [ 11 , 12 , 18 , 21 , 23 , 24 ].
The primary objective of this study was to assess the feasibility and acceptability of a specialist nurse-led structured self-management training programme for peer supporters with T2DM, with or without comorbid HTN, at the primary healthcare level in Slovenia. Additionally, the study aimed to determine the improvement in peer supporters in terms of changes in their acquired knowledge about T2DM and HTN, quality of life and clinical outcomes.
Study design and settings
This was a prospective pre-post interventional pilot study conducted in two Community Health Centres (CHCs) in Slovenia. The initial criteria for the selection of the CHCs was based on the objective of ensuring both urban and rural settings. The CHC Ljubljana is situated in the largest municipality and capital city of Slovenia. It serves approximately 300,000 residents and is representative of an urban setting, contributing 38.4% of Slovenia's total GDP in 2022. In contrast, CHC Slovenj Gradec, located in the smallest municipality in Slovenia, serves an estimated population of 17,000 residents, representing a rural region. This CHC contributed 6.4% of Slovenia's total GDP in 2022 [ 25 ]. This approach considered the different cultural and social environments in urban and rural areas, and acknowledged that distinct forms of peer support are acceptable in each setting [ 26 ].
The study was nested within a larger parent study, which spanned from May 2021 to December 2023. Its objective was to develop an evidence-based model of peer support for people with T2DM, with or without comorbid HTN, at the primary healthcare level in Slovenia. The peer support intervention was a prospective, mixed-methods pilot study that commenced with the recruitment of eligible individuals with T2DM and HTN through purposive sampling, with the objective of training them as peer supporters via specialist nurse-led structured self-management training. Each trained peer supporter voluntarily shared their knowledge and experience at monthly group meetings with up to 10 people with T2DM and HTN over a three-month period in the local community. Data was collected through series of interviews, focus groups, and questionnaires to evaluate the role of peer support. This involved introducing trained peer supporters, determining the relationships between peer support and patient-reported quality of life and level of empowerment, and assessing the acceptability and feasibility of the peer support intervention [ 27 ].
The study was approved by the National Medical Ethics Committee (reference number 0120–219/2019/4, approved on 24 May 2019).
Participants and recruitment
Purposive sampling was employed to recruit eligible patients with T2DM, with or without comorbid HTN, from two CHCs by registered nurses and family medicine physicians. These patients were interested in serving as volunteer peer supporters. The purposive sampling method ensured that the recruited participants were suitable for the peer supporter role based on their responsibility, confidence, communication skills and willingness to collaborate with an educator from the CHC. It is important to note that peer supporters should be aware that they are not medical professionals and should not attempt to provide medical treatment or diagnosis. In the event that a situation arises that is beyond the scope of their knowledge and experience, it is recommended that they refer the recipient of peer support to a healthcare professional for appropriate care [ 27 ].
Inclusion criteria were as follows: i) a confirmed diagnosis of T2DM with fasting blood glucose (BG) value ≥ 7.0 mmol/l or venous plasma glucose ≥ 11.1 mmol/l two hours after glucose tolerance test or at any random opportunity, or glycated haemoglobin (HbA1c) ≥ 6.5% [ 28 ], ii) with or without comorbid HTN with a 7-day mean home BP values ≥ 135/85 mmHg or with 24-h blood pressure monitoring mean ≥ 130/80 mmHg [ 29 ], iii) for a duration of at least one year. This was deemed necessary in order to ensure that participants have had sufficient time to adapt to their diagnosis, understand their treatment regimen, and develop a baseline level of disease management.
Exclusion criteria included: type 1 diabetes or gestational diabetes, < 18 years of age and a documented diagnosis of cognitive decline obtained from the participant’s medical records. This diagnosis was based on comprehensive assessments of the individual’s clinical presentation, medical history, and relevant test results conducted by family physicians and other healthcare professionals.
Participation in the study was voluntary. All participants received an explanation of the study objectives and a participant information sheet that provided additional information. To participate in the study, it was obligatory to sign the informed consent form.
Structured self-management educational training
The self-management training was designed to empower peer supporters and equip them with comprehensive knowledge of T2DM and HTN and communication skills to provide effective peer support to other patients with T2DM, with or without comorbid HTN. The training was led by an educator with the expertise of a registered nurse with specialised knowledge in the field of health education of people with T2DM—a specialist nurse. There was ongoing consultation with the mentor-educator throughout the training, who remained their mentor while providing peer support, either in person, by telephone or by email. In addition, a specialist nurse actively promoted the awareness and value of peer support, thereby reducing the spread of misinformation and concerns about recommending it [ 11 , 17 ].
The training lasted a total of 15 h over a period of 2 months and consisted of four group sessions and two individual sessions. The training was organised in small groups of 6–10 candidates and conducted in accordance with the T2DM education [ 30 ] and treatment [ 28 ] guidelines. To ensure a consistent programme, each educator led the training based on the comprehensive curriculum (Table 1 ). To provide a comprehensive and interactive verbal and visual learning experience and to facilitate T2DM self-management through a patient-centred approach, the educators used Diabetes Conversation Maps™. Several well-established models of health behavior, such as the Biopsychosocial Model of health and illness, were considered in the development of this effective health education tool [ 31 ].
After the group sessions, participants had two individual sessions with the educator, a specialist nurse. The focus was on analysing the themes from the group session (Table 1 ), reviewing the self-monitoring diary of BG and BP, assessing the knowledge gained and discussing the aims of voluntary peer support, the role of a trained peer supporter and opportunities of organising peer group meetings, and ways of further collaboration with healthcare professionals, patients, and the local community. Throughout the training, the educator taught participants how to communicate assertively and used motivational and coaching techniques to approach volunteering and working with people. At the end of the 15-h training, each participant was given four different Conversation Maps™ and a honorary certificate of the acquired title of “trained peer supporter” and CHC ambassador at the award ceremony to ackowledge the completion of the training, and to acknowledge the participants’ efforts [ 27 ]. The study flow chart is presented in Fig. 1 .
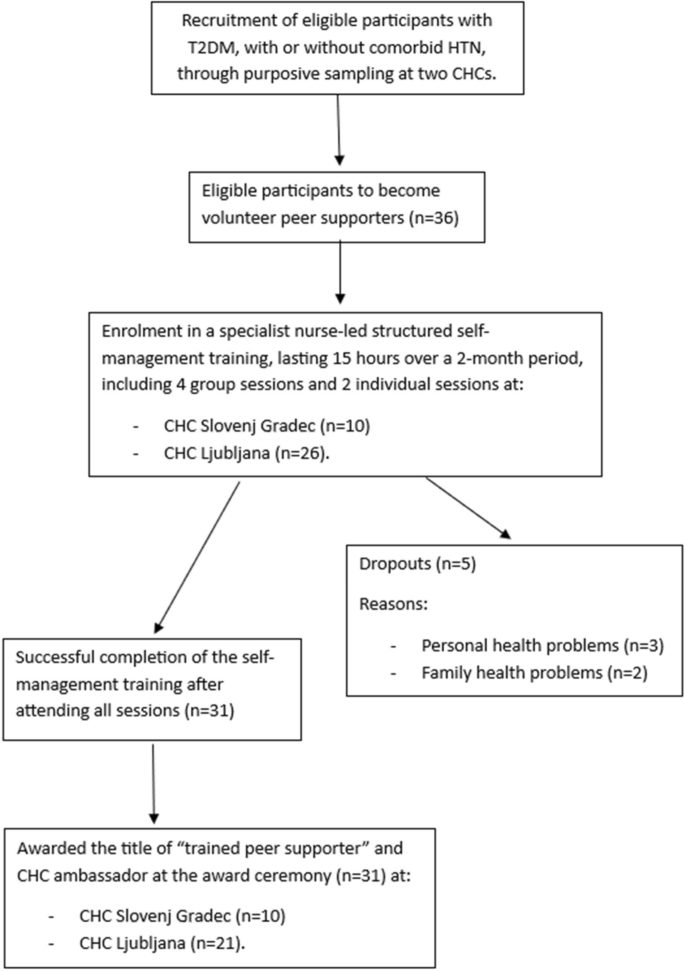
Study flow chart (n, number; T2DM, type 2 diabetes mellitus; HTN, hypertension; CHC, Community Health Centre)
Theoretical intervention model
The theory of change underlying the intervention was based on the hypothesis that training peer supporters would influence their knowledge, perceptions, and intentions, which in turn would lead to changes in self-management behavior and ultimately improved health outcomes. This would also enable effective delivery of peer support, resulting in behavior change and health benefits among people with T2DM, with or without comorbid HTN, receiving peer support. The theory of planned behavior [ 32 ] was used to predict and explain behavior change. Our pilot study protocol is schematically presented in Fig. 2 , outlining its objectives in terms of feasibility, acceptability, and effectiveness (in the study group). The ongoing collaboration between trained peer supporters, people with T2DM, with or without comorbid HTN, caregivers in the local community, and healthcare professionals aims to make them partners in health and care.

Schematic presentation of the pilot study and the theory of change framework (HTN –hypertension; T2DM – type 2 diabetes mellitus)
Instruments and data collection
The study lasted from May 2021 to August 2022. Data were collected from medical records, clinical measurements were conducted by a registered nurse at both the pre- and post-intervention stages, and structured questionnaires were completed by the peer supporters at entry into the study (baseline) and after completing the training. At the conclusion of the training, peer supporters were invited to complete an evaluation form as the sole method to provide qualitative feedback with quotations on their overall satisfaction with the training. Variables were observed across several categories (Table 2 ).
Participants underwent anthropometric and biochemical measurements at baseline and 6 months after completing the training. Measurements were performed by a registered nurse at CHC using a validated scale and blood pressure monitor. SBP and DBP were measured as recommended in the guidelines [ 29 ]. HbA1c level and fasting BG value were determined using peripheral venous blood sampling. To assess the acceptability of the healthcare intervention Sekhon et al. developed the TFA tool (Table 3 ) [ 33 ]. Specifically, we used a 19-items TFA questionnaire (Appendix 1) developed by Timm et al. [ 34 ], which covers all 7 domains of acceptability based on the TFA tool: affective attitude, burden, ethicality, intervention coherence, opportunity costs, perceived effectiveness and self-efficacy [ 33 ]. Each item is rated on a 5-point Likert scale, the score for each of the 7 domains and the total score range between 1 and 5. To assess knowledge about HTN and T2DM, we used validated Slovenian versions of the Hypertension Knowledge Test (HKT) [ 35 ] with 11 true/false questions and the first 14-item questionnaire of the Diabetes Knowledge Test (DKT) [ 36 ], the result of both is between 0 and 100%. The Appraisal of Diabetes Scale (ADS) [ 37 ] was used to assess the individual’s appraisal of T2D, which is diabetes-specific indicator of quality of life [ 38 ], consists of 28 items on a 5-point Likert scale yielding the final score between 7 and 35 where lower score is better.
Sample size elaboration
We employed purposive sampling method to recruit approximately 40 eligible individuals (30 from CHC Ljubljana and 10 from CHC Slovenj Gradec) with T2DM, with or without comorbid HTN, to become volunteer peer supporters. Each peer supporter was expected to share their knowledge and experience with around 10 patients with the same chronic condition in their local community, potentially providing support to up to 400 patients. Considering an estimated dropout rate of 20%, we anticipated that 32 peer supporters would remain, each supporting a group of 8 patients, resulting in 256 patients receiving peer support. The power analysis was done for the sample size of patients receiving peer support for the two outcomes in that larger parent study. Specifically, for the ADS score, a planned sample size of 256 patients achieves 80% power to detect a mean difference (between pre- and post-intervention) of 1.6 using two-tailed paired samples t-test, assuming the SD of differences of 9.3 (this represents the largest possible SD if the differences in ADS scores are normally distributed, given their range is at most [-28,28]) [ 27 ].
Statistical analysis
We summarised categorical variables with frequencies and percentages, and numerical variables with means and standard deviations (SD) or medians and interquartile ranges (IQR) in the case of asymmetric distributions (determined by Shapiro–Wilk normality test and visual inspection of graphs). To compare numerical variables between pre- and post-intervention, we used paired-samples t -test (together with 95% confidence interval (CI) for the mean difference) or Wilcoxon signed-rank test in the case of asymmetric distributions. A p -value of < 0.05 was considered statistically significant.
Of 36 patients (10 from CHC Slovenj Gradec and 26 from CHC Ljubljana) with T2DM, with or without comorbid HTN, recruited for the study, 31 (86.1%) attended all meetings, successfully completed the specialist nurse-led training, and became trained peer supporters. All the results are for the sample of 31 trained peer supporters.
Sociodemographic data and clinical history
The basic socio-demographic characteristics of the 31 trained peer supporters are shown in Table 4 . Among them, 21 (67.7%) were women, with a mean age of 63.9 (SD 8.9) years. They had all been treated for T2DM for a median duration of 15.0 years (IQR 5.0 – 20.5). As a comorbidity, 24 (77.4%) peer supporters had HTN. The median duration of treatment was 8.5 years (IQR 2.8 – 18.2). Of the 31 trained peer supporters, 7 (22.6%) were treated non-pharmacologically with diet and exercise, 13 (41.9%) with hypoglycaemic agents, 5 (16.1%) with a combination of hypoglycaemics and insulin, and 6 (19.3%) with insulin alone.
Acceptability of the self-management educational training
Participants rated the training as highly acceptable in all 7 domains, with median scores ranging from 4.0 to 5.0 and the lowest first quartile being 4.0 (Table 5 ). The median total score was 4.5 with IQR (4.1 – 4.7).
Peer supporters’ satisfaction with educational training
Some of the quotations from the evaluation forms highlight the satisfaction with the training: “It is fascinating how much I have learned about both diseases, even though I have been living with T2DM and HTN for years;” “I can always contact my educator by mail or phone if I have a problem;” “The training encouraged me to continue with a healthy lifestyle and to take greater control of my health;” “This programme gave me additional motivation to maintain my health and to share my experiences with others;” “I believe that the Conversation Maps are great; when I showed them at home, the words about T2DM just rolled out of my tongue.”
Knowledge about T2DM and HTN
After completing the training, knowledge of T2DM and HTN increased significantly ( p < 0.001 and p = 0.024, respectively). The mean knowledge of T2DM at baseline was 72.9% (SD 15.6%, median 79.0%, IQR (64.0% – 86.0%)), the mean difference in knowledge of T2DM was 9.4% (SD 12.9%, median 8.0%, IQR (0.0% – 14.5%)) with 95% CI for the mean difference (4.7%, 14.1%). The median knowledge of HTN at baseline was 91.0% with IQR (77.5% – 91.0%), the median difference in knowledge of HTN was 0.0% but with IQR (0.0% – 9.0%).
Quality of life
Quality of life with T2DM was not significantly better after the completed training ( p = 0.066). Participants' perceived burden of T2DM decreased from a mean score of 16.1 (SD 3.5) to 14.8 (SD 4.2) after the training (lower ADS score is better), the 95% CI for the mean difference was (-0.1, 2.7).
Clinical outcomes
The mean anthropometric and biochemical measurements at baseline and 6 months after completion of the training are shown in Table 6 . Peer supporters' weight decreased significantly ( p = 0.022) from 85.8 (SD 19.5) kg at baseline to 84.2 (SD 20.0) kg 6 months after training, and BMI decreased from 30.4 (SD 6.2) to 29.8 (SD 6.2) ( p = 0.020). Changes in fasting BG, HbA1c, SBP and DBP were not significant.
Our pilot study indicates that specialist nurse-led self-management training for peer supporters is feasible, acceptable, effective (in the study group), and highly valued by participants. The training enabled peer supporters to acquire knowledge about T2DM and HTN and equipped them with self-management skills to effectively support other people with the same chronic condition by sharing first-hand knowledge, similar experiences and lifestyle issues. Our study was unique in measuring changes in clinical measures of peer supporters in primary care settings. Peer supporters were successful in maintaining disease control and making positive changes in their self-management behaviours, as reflected in the reduction in their BMI over the six-months following the training.
The literature has not used rigorous approaches to recruit appropriate peer supporters [ 19 , 21 ]. Recruitment has mainly been done through referrals from healthcare professionals based on candidate interest in volunteering and diagnosis of T2DM as inclusion criteria [ 21 , 39 ]. In contrast to our study, some listed inclusion criteria of acceptable glycemic control (HbA1c ≤ 8.5%) [ 21 , 23 , 39 , 40 ], which could increase the retention rate and improve the chances of success [ 21 ]. We used the purposeful sampling method to ensure that recruited participants were suitable for the peer supporter role. Recruitment of peer supporters should emphasize the importance of their personal experience with the same chronic condition as people they will be supporting. This unique perspective allows them to better understand and empathize with the challenges that their support recipients are facing [ 12 ]. We believe it is important to promote this uniqueness when recruiting peer supporters, as it can help to build trust and confidence in the support programme.
There is limited data on the socio-demographic characteristics of peer supporters; most were female and had at least a high school education [ 21 , 39 , 41 , 42 ], which is consistent with the findings of our study. Most of our trained peer supporters were retired, had a longer duration of T2DM and were older than in other studies [ 21 , 39 , 43 ]. In one study, 90% of peer supporters were unemployed [ 43 ]. The Slovenian peer supporters were mainly older, disease-experienced individuals who were no longer involved in the daily stress of work. They rated the training as very acceptable. Participating in the training was effortless for them, it fitted well with their life beliefs and values, and they understood the process of the whole intervention. They felt empowered and confident in their ability to transfer the knowledge and skills they had acquired to other patients.
There are no clear recommendations on who should lead the training of peer supporters (nurse educator, multidisciplinary team, research expert, etc.) and how long the training should last (from a few hours to several months) [ 12 , 18 , 19 , 20 , 24 , 39 , 42 ]. Training programmes were mostly based on a structured curriculum [ 12 , 18 , 20 , 21 , 23 , 40 ]. Teaching methods included role-playing [ 12 , 20 , 21 , 43 ], brainstorming, group facilitation simulations [ 20 ], PowerPoint presentations [ 12 ], training booklets [ 19 , 21 ], and Conversation Maps™ [ 19 ]. We used four different Diabetes Conversation Maps™ as teaching tools, and trained peer supporters were given the same collection of four Maps™ to bring to peer support meetings after completing the training. These maps are designed to be interactive and engaging, encouraging participants to talk about the challenges of living with T2DM and HTN, to share their stories, knowledge and experiences, and to emphasise the importance of medication adherence, healthy lifestyles and regular check-ups with healthcare professionals. The maps help to create a structured and supportive environment where participants can learn from each other and feel empowered to take control of their disease management [ 31 , 44 ]. Our detailed self-management training programme (Table 1 ) makes the lesson preparation transparent and allows for replication when designing future interventions.
Consistent with the findings of our pilot study, other studies have also shown that the development of self-management educational training leads to improved knowledge of T2DM among peer supporters [ 19 , 43 ]. Six months after the training, peer supporters' weight and BMI decreased significantly compared with baseline measurements. There were no significant differences in the measurements of fasting BG, HbA1c, SBP and DBP after six months, nor were the changes that occurred clinically significant. We did not expect clinically significant changes in such a short period of time, as we believe that a longer study period is needed to detect significant changes. In addition, the peer supporters already had well-controlled clinical parameters at baseline. The results are still relevant as they show that patients were able to maintain their disease control and even improve some clinical parameters over the six-month period. Peer supporters who can model healthy behaviours and share their own experiences of disease management may be more effective in helping others to make positive changes in their own lives. To our knowledge, only Yin et al. have investigated the effects of peer support on the health of peer supporters. However, their study was conducted in hospital-based diabetes clinics and involved a multidisciplinary team to train the peer supporters, unlike our primary care setting. They found improvements in peer supporters self-care behaviours and maintenance of their glycaemic control over 4 years [ 22 ].
The actual implementation of our research depends on the willingness and motivation of individuals to provide peer support voluntarily, so a gradual decline in motivation and in some cases withdrawal can be expected [ 11 ]. We recognised the importance of acceptability in the evaluation of the healthcare interventions [ 33 ]. Participants assessed our training as highly acceptable and satisfactory. Consequently, we found that participation in the training was high and consistent, with 86.1% of patients successfully completing the training and becoming trained peer supporters. The reasons for dropping out were all external, such as changes in personal or family health status, rather than dissatisfaction with the programme or its content. The demographic and clinical characteristics of the non-completers were diverse, supporting the assertion of external reasons for dropping out (they were aged 57–77 years, with a gender split of 3 women and 2 men, 4 were retired and 1 was still working, 4 had completed secondary school and 1 university, had been managing T2DM for a range of 5–30 years, with only 2 having HTN as a comorbidity). In the study by Chan et al. 74.7% completed the training and 41.8% agreed to continue providing peer support [ 39 ]. In a study by Afshar et al., the retention rate among peer leaders ranged from 56 to 88% [ 21 ]. To overcome this problem, it is important to focus on engagement and recognition strategies, such as good communication, collaboration among stakeholders and a clear presentation of the benefits of peer support [ 11 ]. The future connection and collaboration between trained peer supporters, patients, family members, caregivers in the local community and health professionals could make them partners in health and care. Together they could achieve the ultimate goal of a comprehensive, patient-centred approach: empowering individuals to take an active role in managing their illness and achieving their health goals [ 45 ].
Strengths and limitations
Peer supporters are becoming an integral part of diabetes management. This study addresses an important gap in person-centred diabetes care by providing new insights into the feasibility and acceptability of a training programme for peer supporters. To ensure that the intervention is well organised, effective and sustained, emphasis needs to be placed on recruiting, training and retaining peer supporters for ongoing effective self-management and support of others with the same chronic condition. This can be achieved through several key strategies, including purposive sampling to select suitable candidates for the peer supporter role, the involvement of a mentor-educator to provide ongoing support and supervision, regular evaluation and monitoring of the training to identify challenges and areas for improvement, and the acknowledgement of peer supporters with honorary titles and certificates. The study provided valuable insights that could contribute to the successful implementation of peer support training interventions in diabetes care.
Our study has several limitations. Firstly, the lack of a control group of potential peer supporters who did not attend the training makes it impossible to estimate the real effectiveness of the training programme, and further research with a control group is needed. We decided not to use a control group due to our limited sources and our goal to train as many peer supporters as possible in a short period of time. Secondly, the use of the same DKT and HKT questionnaires at the beginning and the end of the two-month training means that participants already knew the questions, which could influence their actual knowledge. However, previous studies showing improved knowledge of T2DM after training [ 19 , 43 ], also repeated the same test, suggesting that question familiarity is not predictive of the second test results. Thirdly, it is not possible to measure the long-term effects as the questionnaires were only measured after the training was compiled, and clinical outcomes were only measured 6 months after the training. Fourthly, we cannot say that 15 h of training is sufficient. Therefore, a follow-up evaluation is needed to examine retention and acquisition of skills and knowledge for ongoing peer support intervention. Fifthly, in anticipation of a small sample size and difficulty in recruiting a large enough sample of participants with both T2DM and HTN who were willing to become peer supporters, we included in the pilot study all individuals with a confirmed diagnosis of T2DM, regardless of whether they had comorbid HTN. In addition, the use of purposive sampling introduces potential bias and limits the generalisability of the findings. Finally, we did not formally evaluate the teaching effectiveness or information transfer skills of the peer supporters. However, to the best of our knowledge, no studies [ 11 , 12 , 18 , 21 , 23 , 24 ] have included teaching skills in peer support training programmes, as the focus has been on practical and experiential skills that are crucial for managing their condition.
Conclusions
The structured self-management training for peer supporters, led by a specialist nurse, was found to be highly acceptable, effective (in the study group), and feasible, indicating significant potential for scaling-up integrated care for people with T2DM, with or without comorbid HTN, at the primary healthcare level in Slovenia. Trained peer supporters improved their knowledge and gained self-management skills, leading to positive changes in their behaviour, as evidenced by a decrease in their BMI over six months. The training programme enabled them to effectively support others with the same chronic condition by sharing first-hand knowledge, similar experiences, and lifestyle advice. However, further research is needed to confirm the true effectiveness of the training programme with a control group and to improve the quality of the peer support provided.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available because the data is part of an unpublished dissertation but are available from the corresponding author upon reasonable request.
Abbreviations
Type 2 diabetes mellitus
- Hypertension
Community Health Centres
Body mass index
Systolic blood pressure
Diastolic blood pressure
Blood glucose
Glycated haemoglobin
Appraisal of Diabetes Scale
Theoretical Framework of Acceptability
Diabetes Knowledge Test
Hypertension Knowledge Test
Standard deviation
Interquartile range
Confidence interval
Klemenc-Ketiš Z, Švab I, Poplas SA. Implementing quality indicators for diabetes and hypertension in family medicine in Slovenia. Slov J Public Health. 2017;56(4):211–9. https://doi.org/10.1515/sjph-2017-0029 .
Article Google Scholar
Poplas Susic A, Svab I, Klemenc KZ. Upgrading the model of care in family medicine: a Slovenian example. Public Health Panor. 2018;4(4):550–5.
Google Scholar
Tušek-Bunc K, Petek Ster M, Petek D. Correlation of coronary heart disease patient assessments of chronic illness care and quality of care procedures. Acta Medico-Biotech. 2018;11(1):45–53.
Mihevc M, Virtič Potočnik T, Zavrnik Č, Šter MP, Klemenc-Ketiš Z, Poplas Susič A. Beyond diagnosis: Investigating factors influencing health-related quality of life in older people with type 2 diabetes in Slovenia. Prim Care Diabetes. 2024;18(2):157–62. https://doi.org/10.1016/j.pcd.2024.01.010 .
Article PubMed Google Scholar
Beaglehole R, Epping-Jordan J, Patel V, Chopra M, Ebrahim S, Kidd M, et al. Improving the prevention and management of chronic disease in low-income and middle-income countries: a priority for primary health care. Lancet Lond Engl. 2008;372(9642):940–9. https://doi.org/10.1016/S0140-6736(08)61404-X .
van Olmen J, Menon S, Poplas Susič A, Ir P, Klipstein-Grobusch K, Wouters E, et al. Scale-up integrated care for diabetes and hypertension in Cambodia, Slovenia and Belgium (SCUBY): a study design for a quasi-experimental multiple case study. Glob Health Action. 2020;13(1):1824382.
Article PubMed PubMed Central Google Scholar
Klemenc-Ketis Z, Stojnić N, Zavrnik Č, Ružić Gorenjec N, Danhieux K, Lukančič MM, et al. Implementation of integrated primary care for patients with diabetes and hypertension: a case from Slovenia. Int J Integr Care. 2021;21(3):15. https://doi.org/10.5334/ijic.5637 .
American Diabetes Association Professional Practice Committee. Improving care and promoting health in populations: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Supplement_1):S8-16. https://doi.org/10.2337/dc22-S001 .
Virtič Potočnik T, Ružić Gorenjec N, Mihevc M, Zavrnik Č, Mori Lukančič M, Poplas Susič A, et al. Person-centred diabetes care: examining patient empowerment and diabetes-specific quality of life in Slovenian adults with type 2 diabetes. Healthcare. 2024;12(9):899.
Zavrnik Č, Danhieux K, Monarres MH, Stojnić N, Lukančič MM, Martens M, et al. Scaling-up an integrated care for patients with non-communicable diseases: an analysis of healthcare barriers and facilitators in Slovenia and Belgium. Zdr Varst. 2021;60(3):158–66. https://doi.org/10.2478/sjph-2021-0023 .
Aziz Z, Riddell MA, Absetz P, Brand M, Oldenburg B, Australasian Peers for Progress Diabetes Project Investigators. Peer support to improve diabetes care: an implementation evaluation of the Australasian peers for progress diabetes program. BMC Public Health. 2018;18(1):262. https://doi.org/10.1186/s12889-018-5148-8 .
Garner NJ, Pascale M, France K, Ferns C, Clark A, Auckland S, et al. Recruitment, retention, and training of people with type 2 diabetes as diabetes prevention mentors (DPM) to support a healthcare professional-delivered diabetes prevention program: the Norfolk Diabetes Prevention Study (NDPS). BMJ Open Diabetes Res Care. 2019;7(1):e000619.
Evans M, Daaleman T, Fisher EB. Peer support for chronic medical conditions. In: Avery JD. Peer Support in Medicine. Springer International Publishing; 2021;49–69. Available from: http://link.springer.com/10.1007/978-3-030-58660-7_3 . Accessed 31 May 2024.
Patil SJ, Ruppar T, Koopman RJ, Lindbloom EJ, Elliott SG, Mehr DR, et al. Peer support interventions for adults with diabetes: a meta-analysis of hemoglobin A1c outcomes. Ann Fam Med. 2016;14(6):540–51. https://doi.org/10.1370/afm.1982 .
Zhang X, Yang S, Sun K, Fisher EB, Sun X. How to achieve better effect of peer support among adults with type 2 diabetes: a meta-analysis of randomized clinical trials. Patient Educ Couns. 2016;99(2):186–97. https://doi.org/10.1016/j.pec.2015.09.006 .
Qi L, Liu Q, Qi X, Wu N, Tang W, Xiong H. Effectiveness of peer support for improving glycaemic control in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. BMC Public Health. 2015;15:471. https://doi.org/10.1186/s12889-015-1798-y .
Article CAS PubMed PubMed Central Google Scholar
Patil SJ, Ruppar T, Koopman RJ, Lindbloom EJ, Elliott SG, Mehr DR, et al. Effect of peer support interventions on cardiovascular disease risk factors in adults with diabetes: a systematic review and meta-analysis. BMC Public Health. 2018;18(1):398. https://doi.org/10.1186/s12889-018-5326-8 .
Egbujie BA, Delobelle PA, Levitt N, Puoane T, Sanders D, van Wyk B. Role of community health workers in type 2 diabetes mellitus self-management: a scoping review. Nkomazana O, editor. PLOS ONE. 2018;13(6):e0198424. https://doi.org/10.1371/journal.pone.0198424 .
Aponte J. Diabetes training for community health workers. J Community Med Health Educ. 2015;05(06):378. https://doi.org/10.4172/2161-0711.1000378 .
Tang TS, Funnell MM, Gillard M, Nwankwo R, Heisler M. The development of a pilot training program for peer leaders in diabetes. Diabetes Educ. 2011;37(1):67–77. https://doi.org/10.1177/0145721710387308 .
Afshar R, Tang TS, Askari AS, Sidhu R, Brown H, Sherifali D. Peer support interventions in type 2 diabetes: review of components and process outcomes. J Diabetes. 2020;12(4):315–38. https://doi.org/10.1111/1753-0407.12999 .
Yin J, Wong R, Au S, Chung H, Lau M, Lin L, et al. Effects of providing peer support on diabetes management in people with type 2 diabetes. Ann Fam Med. 2015;13(Suppl_1):S42-9. https://doi.org/10.1370/afm.1853 .
Riddell MA, Dunbar JA, Absetz P, Wolfe R, Li H, Brand M, et al. Cardiovascular risk outcome and program evaluation of a cluster randomised controlled trial of a community-based, lay peer led program for people with diabetes. BMC Public Health. 2016;16(1):864. https://doi.org/10.1186/s12889-016-3538-3 .
Lu S, Leduc N, Moullec G. Type 2 diabetes peer support interventions as a complement to primary care settings in high-income nations: A scoping review. Patient Educ Couns. 2022;105(11):3267–78. https://doi.org/10.1016/j.pec.2022.08.010 .
Statistical Office of the Republic of Slovenia. Gross domestic product by region in Slovenia, 2022. Available from: https://www.stat.si/StatWeb/en/News/Index/11537 . Accessed 31 May 2024.
Warshaw H, Edelman D. Building bridges through collaboration and consensus: expanding awareness and use of peer support and peer support communities among people with diabetes, caregivers, and health care providers. J Diabetes Sci Technol. 2019;13(2):206–12. https://doi.org/10.1177/1932296818807689 .
Virtič T, Mihevc M, Zavrnik Č, Mori Lukančič M, Poplas Susič A, Klemenc-Ketiš Z. Peer support as part of scaling-up integrated care in patients with type 2 diabetes and arterial hypertension at the primary healthcare level: a study protocol. Slov J Public Health. 2023;62(2):93–100. https://doi.org/10.2478/sjph-2023-0013 .
Committee American Diabetes Association Professional Practice. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(Supplement_1):S17-38. https://doi.org/10.2337/dc22-S002 .
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104. https://doi.org/10.1093/eurheartj/ehy339 .
Janjoš T, Poljanec Bohnec M, Tomažin-Šporar M. Kurikulum za edukacijo o oskrbi odraslih bolnikov s sladkorno boleznijo. Ljubljana: Zbornica zdravstvene in babiške nege Slovenije - Zveza strokovnih društev medicinskih sester, babic in zdravstvenih tehnikov Slovenije, Sekcija medicinskih sester in zdravstvenih tehnikov v endokrinologiji. 2012.
Reaney M, Eichorst B, Gorman P. From acorns to oak trees: the development and theoretical underpinnings of diabetes conversation map education tools. Diabetes Spectr. 2012;25(2):111–6. https://doi.org/10.2337/diaspect.25.2.111 .
Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50(2):179–211. https://doi.org/10.1016/0749-5978(91)90020-T .
Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17(1):88. https://doi.org/10.1186/s12913-017-2031-8 .
Timm L, Annerstedt KS, Ahlgren JÁ, Absetz P, Alvesson HM, Forsberg BC, et al. Application of the theoretical framework of acceptability to assess a telephone-facilitated health coaching intervention for the prevention and management of type 2 diabetes. PLoS ONE. 2022;17(10):e0275576. https://doi.org/10.1371/journal.pone.0275576 .
Prevolnik Rupel V, Ogorevc M, Poplas-Susič A. Knowledge of disease among patients with type 2 diabetes and hypertension in slovenia. Value Health. 2020;23:S620. https://doi.org/10.1016/j.jval.2020.08.1311 .
Eva Turk, Palfy M, Prevolnik Rupel V. General knowledge about diabetes in the elderly diabetic population in Slovenia. Zdr Vest. 2012;81:517–25.
Carey MP, Jorgensen RS, Weinstock RS, Sprafkin RP, Lantinga LJ, Carnrike CL, et al. Reliability and validity of the appraisal of diabetes scale. J Behav Med. 1991;14(1):43–51. https://doi.org/10.1007/BF00844767 .
Article CAS PubMed Google Scholar
Oluchi SE, Manaf RA, Ismail S, Kadir Shahar H, Mahmud A, Udeani TK. Health related quality of life measurements for diabetes: a systematic review. Int J Environ Res Public Health. 2021;18(17):9245. https://doi.org/10.3390/ijerph18179245 .
Chan JCN, Sui Y, Oldenburg B, Zhang Y, Chung HHY, Goggins W, et al. Effects of telephone-based peer support in patients with type 2 diabetes mellitus receiving integrated care: a randomized clinical trial. JAMA Intern Med. 2014;174(6):972. https://doi.org/10.1001/jamainternmed.2014.655 .
Thom DH, Ghorob A, Hessler D, De Vore D, Chen E, Bodenheimer TA. Impact of peer health coaching on glycemic control in low-income patients with diabetes: a randomized controlled trial. Ann Fam Med. 2013;11(2):137–44. https://doi.org/10.1370/afm.1443 .
Spencer MS, Kieffer EC, Sinco B, Piatt G, Palmisano G, Hawkins J, et al. Outcomes at 18 months from a community health worker and peer leader diabetes self-management program for latino adults. Diabetes Care. 2018;41(7):1414–22. https://doi.org/10.2337/dc17-0978 .
Debussche X, Besançon S, Balcou-Debussche M, Ferdynus C, Delisle H, Huiart L, et al. Structured peer-led diabetes self-management and support in a low-income country: The ST2EP randomised controlled trial in Mali. PLoS ONE. 2018;13(1):e0191262.
Gyawali B, Mishra SR, Neupane D, Vaidya A, Sandbæk A, Kallestrup P. Diabetes management training for female community health volunteers in Western Nepal: an implementation experience. BMC Public Health. 2018;18(1):641. https://doi.org/10.1186/s12889-018-5562-y .
Ghafoor E, Riaz M, Eichorst B, Fawwad A, Basit A. Evaluation of diabetes conversation map™ education tools for diabetes self-management education. Diabetes Spectr. 2015;28(4):230–5. https://doi.org/10.2337/diaspect.28.4.230 .
Poitras ME, Maltais ME, Bestard-Denommé L, Stewart M, Fortin M. What are the effective elements in patient-centered and multimorbidity care? A scoping review. BMC Health Serv Res. 2018;18(1):446. https://doi.org/10.1186/s12913-018-3213-8 .
Download references
Acknowledgements
We want to thank all peer supporters who participated in this study.
The research is part of the international research project SCUBY, funded from the European Union’s Horizon 2020 programme under grant agreement number 825432. The funding is not involved in study design, data collection, analysis and interpretation of data, writing of the paper or decision to submit the article for publication.
Author information
Authors and affiliations.
Primary Healthcare Research and Development Institute, Community Health Centre Ljubljana, Metelkova 9, 1000, Ljubljana, Slovenia
Tina Virtič Potočnik, Matic Mihevc, Črt Zavrnik, Majda Mori Lukančič, Nina Ružić Gorenjec, Antonija Poplas Susič & Zalika Klemenc-Ketiš
Faculty of Medicine, Department of Family Medicine, University of Maribor, Taborska 8, 2000, Maribor, Slovenia
Tina Virtič Potočnik & Zalika Klemenc-Ketiš
Faculty of Medicine, Department of Family Medicine, University of Ljubljana, Poljanski Nasip 58, 1000, Ljubljana, Slovenia
Matic Mihevc, Črt Zavrnik, Antonija Poplas Susič & Zalika Klemenc-Ketiš
Faculty of Medicine, Institute for Biostatistics and Medical Informatics, University of Ljubljana, Vrazov Trg 2, 1000, Ljubljana, Slovenia
Nina Ružić Gorenjec
You can also search for this author in PubMed Google Scholar
Contributions
TVP, MML, TPS, ZKK, MM and ČZ were responsible for study conception and design. MML and TVP performed the data collection. TVP and NRG contributed to the data analysis and interpretation. TV drafted the manuscript under the supervision of ZKK. To ensure the quality of the study MM, ČZ, NRG, TPS, MML and ZZK made critical revisions to the paper. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Tina Virtič Potočnik .
Ethics declarations
Ethics approval and consent to participate.
The research was approved on 24 May 2019 by the Slovenian National Medical Ethics Committee (reference number 0120–219/2019/4), which is exclusively responsible for making determinations on ethical issues that are relevant to the unification of ethical practices in the Republic of Slovenia. The study followed the Declaration of Helsinki on ethical standards. Written informed consent was obtained from all the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Virtič Potočnik, T., Mihevc, M., Zavrnik, Č. et al. Evaluation of a specialist nurse-led structured self-management training for peer supporters with type 2 diabetes mellitus with or without comorbid hypertension in Slovenia. BMC Nurs 23 , 567 (2024). https://doi.org/10.1186/s12912-024-02239-7
Download citation
Received : 24 March 2023
Accepted : 07 August 2024
Published : 15 August 2024
DOI : https://doi.org/10.1186/s12912-024-02239-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Peer support
- Type 2 Diabetes Mellitus
- Self-Management
- Training Program
- Empowerment
- Primary Healthcare
BMC Nursing
ISSN: 1472-6955
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Biomed Eng Online

A case study of type 2 diabetes self-management
1 Department of Biomedical Engineering, Texas A&M University, College Station, Texas, 77843-3120 USA
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Associated Data
It has been established that careful diabetes self-management is essential in avoiding chronic complications that compromise health. Disciplined diet control and regular exercise are the keys for the type 2 diabetes self-management. An ability to maintain one's blood glucose at a relatively flat level, not fluctuating wildly with meals and hypoglycemic medical intervention, would be the goal for self-management. Hemoglobin A1c (HbA1c or simply A1c) is a measure of a long-term blood plasma glucose average, a reliable index to reflect one's diabetic condition. A simple regimen that could reduce the elevated A1c levels without altering much of type 2 diabetic patients' daily routine denotes a successful self-management strategy.
A relatively simple model that relates the food impact on blood glucose excursions for type 2 diabetes was studied. Meal is treated as a bolus injection of glucose. Medical intervention of hypoglycaemic drug or injection, if any, is lumped with secreted insulin as a damping factor. Lunch was used for test meals. The recovery period of a blood glucose excursion returning to the pre-prandial level, the maximal reach, and the area under the excursion curve were used to characterize one's ability to regulate glucose metabolism. A case study is presented here to illustrate the possibility of devising an individual-based self-management regimen.
Results of the lunch study for a type 2 diabetic subject indicate that the recovery time of the post-prandial blood glucose level can be adjusted to 4 hours, which is comparable to the typical time interval for non-diabetics: 3 to 4 hours. A moderate lifestyle adjustment of light supper coupled with morning swimming of 20 laps in a 25 m pool for 40 minutes enabled the subject to reduce his A1c level from 6.7 to 6.0 in six months and to maintain this level for the subsequent six months.
Conclusions
The preliminary result of this case study is encouraging. An individual life-style adjustment can be structured from the extracted characteristics of the post-prandial blood glucose excursions. Additional studies are certainly required to draw general applicable guidelines for lifestyle adjustments of type 2 diabetic patients.
It is well established that diabetes can lead to acute and chronic complications, compromising the health and quality of life. Results from various studies [ 1 ] have demonstrated that improved control of blood glucose in type 2 diabetes reduces related complications. Type 2 diabetes results from the metabolic problem that is related to certain tissue resistance to insulin action and to the inability of the pancreas to appropriately regulate the quantity of insulin for glucose metabolism. These metabolic abnormalities lead to the many complications of diabetes. Type 2 diabetes historically occurs predominantly in adults aged 40 and over. A recent trend, however, indicates that children and adolescents of minority ethnic groups, especially in African Americans and American Indians, are increasingly susceptible to type 2 diabetes [ 2 ]. With the prevalence of type 2 diabetes and its associated risk for serious complications, issues related to proactive self-management become an urgent concern.
Dietary management is frequently referred as the cornerstone, or the initial step, in treating of type 2 diabetes mellitus. Foods containing carbohydrates play an important role in the diet. The glycemic Index (GI) ranks foods according to their post-prandial glycemic responses. The GI was introduced more than twenty years ago and has been widely adopted in diabetes management in Australia, New Zealand, Canada, the United Kingdoms, and France [ 3 ]. The World Health Organization states that it is important to consider the GI in constructing a healthful diet because low GI foods help control blood sugar levels by producing minimal fluctuations in blood glucose [ 4 ]. For diabetic patients, choosing low GI foods is particularly important because consumption of high GI foods often results in far more exaggerated glycemic responses, creating a need for drug or insulin therapy [ 3 , 5 ].
Most published GI lists are for single food items only. A GI is a numerical measure of how a carbohydrate would increase one's blood glucose level over a period of two (for normal) or three hours (for diabetic patients) after eating [ 6 , 7 ]. The area of elevated blood glucose level from the baseline (the pre-prandial measure) is expressed as a percent of the area for the same amount of a reference carbohydrate such as a pure glucose or a white bread (usually 50 g) [ 8 , 9 ]. To plan a complete meal using the weighted mean [ 6 ] for various food items is not only tedious, but also impractical.
Diet exchange lists are usually recommended for diabetic patients to use in formulating a sensible meal plan. However, an exchange list is not always convenient to use. Moreover, there is a lack of ethnic diet exchange lists. For a member of an ethnic minority to follow a diet exchange list, he or she must prepare his or her own meal away from the rest of the family. Nutall and Chasuk [ 10 ] have stressed that dietary recommendations for type 2 diabetes should be flexible and highly individualized, yet most of the prepared meal programs and exchange-list diets for diabetes have not had individualization in mind nor are they designed for ethnic minorities.
When diet alone cannot effectively control the type 2 diabetic conditions, medical interventions, such as insulin injections or dispensing hypoglycaemic pills, are usually the next step of managing type 2 diabetes mellitus. Medical interventions notoriously exacerbate the fluctuation of blood glucose excursions. Even with the smallest dosage of hypoglycaemic drug (5 mg glucotrol or glyburide) once in the morning, the subject of this study still experienced frequent acute hypoglycaemias. Besides, his A1c levels hovered around 6.5 levels for many years following his physician's advice of taking 5 mg glucotrol per day. It became obvious that a properly designed drug dispensing regimen was needed to avoid hypoglycaemic bouts and effectively reduce A1c levels.
Fasting blood glucose measurements are not consistent indicators, fluctuating widely from a low of 70 mg/dL to a high of 200 mg/dL (with most frequent range lay between 90 to 150 mg/dL) that were experienced by this type 2 diabetic subject prior to the model-based lifestyle adjustment. Initially, the subject tried to adjust lifestyle based on fasting glucose measurements, but it was not successful. His A1c measurements crept from 6.3 to 6.7 in a year. As glucose binds irreversibly to haemoglobin molecules within red blood cells, the amount of glucose that is bound to haemoglobin is directly tied to the concentration of glucose in the blood. The average life span of erythrocytes is about 120 days [ 11 ], measuring the amount of glucose bound to haemoglobin – by the A1c measurement – can provide an estimate of average blood sugar level during the 3 to 4 months period. It is obvious that A1c is a more reliable indicator than fasting glucose measurements for an effective blood glucose control self-management.
It has been established that exercise can effectively alleviate diabetic conditions. Although no rigorous investigation has been performed here, nor is the focus of this current study, a forty-minute exercise of swimming, or weight lifting, or jogging, or any combination of these, prior to a meal or 3 to 4 hours after a meal, can significantly depress the volunteer's post-prandial blood glucose levels. However, it is impractical to substitute hypoglycemic pills with a multiple daily exercise schedule. A sensible lifestyle adjustment is required to manage the diabetic conditions without altering much of daily routines.
Post-prandial blood glucose excursions (time series) for type 2 diabetes vary widely depending on the variety and the amount of food consumed. It also depends on long and short term physical conditions (exercise routines and stress levels such as insomnia) to a lesser scale. The recovery periods of blood glucose excursions returning to the pre-prandial level (or baseline) for diabetics are generally longer than those for non-diabetics. Although a simple glucose-insulin interaction compartmental model exists [ 12 ], not all the model parameters are readily interpretable. In addition, no case study is given to illustrate its potential applications. Compartmental models can provide first-order approximations that may be sufficient for specific goals. Simple models may not duplicate real phenomena but may reveal enough clues for which alternative approaches or experimental designs may come to light.
A biophysically-based model of impulse-force-generated heavily damped oscillatory system is used here to capture the post-prandial blood glucose characteristics of type 2 diabetes. The model follows the general approach of glucose-insulin interaction model (bolus injection of glucose) with a few modifications, for which parameters can readily be interpreted and a case study is presented for exploring its potential applications. Rather than using single food items for their published GI values, or its cumbersome weighted mean of multiple ingredients in a meal, normally consumed lunch for the subject was used for the test meal. Based on the preliminary results obtained from the model, a moderate lifestyle adjustment was devised for the subject: swimming 20 laps for 40 minutes in a 25 m pool in the morning and dispensing 1/4 of 5 mg glyburide 1/2 to 1 hour before lunch and dinner – that enables him to reduce 10% of his A1c level in six months and maintain the desirable lower level for the subsequent six months.
The subject is a mid-sixty healthy male of 180 lbs with 5'10" frame, leading a productive professional life. He has been diagnosed with type 2 diabetes for more than 30 years. Initially, he was on diet regimen for nearly twenty years and then was instructed by his physician to dispense 5 mg glucotrol once every morning. He experienced frequent acute hypoglycemia that led him to discuss a possible self-managed regimen with his family physician.
Lunch was chosen as the test meal for having sufficient time to take post-prandial measurements. The test meals were 15 sets of lunches that consisted either (1) 10 to 12 oz of steamed rice, stir-fried vegetables with 4 oz canned tuna (or steamed cod), or (2) 10 to 12 oz spaghetti with 6 medium sized meat balls (from Sam's family package). Five sets of data each were collected from: (i) without taking hypoglycemic pills before test meals; (ii) 1/4 size of 5 mg glyburide pills were dispensed pre-prandially right before the meal and (iii) 1/4 size of 5 mg glyburide pills were dispensed pre-prandially an hour before the test meals. One pre- and 8 to 12 post-prandial blood glucose measurements were taken at 30-minute intervals starting at the beginning of a meal (meal is usually consumed in 15 minutes): (i) for 6 hours, (ii) for 5 hours, and (iii) for 4 hours. In addition, for case (iii) two reference measurements were taken with one right before dispensing the pill and one an hour after completion of the 8 post prandial measurements, i.e ., at hour 5, for a total of 11 readings.
The purpose of the first set of measurements was to establish the baseline for this diabetic subject: the recovery period of post-prandial blood glucose excursion without medication. The second and the third sets of the trials were designed to quantitatively measure the hypoglycemic drug effects and the most optimal time frame to administer the pills. Raw data were averaged and the corresponding standard deviations were also calculated for 5 replicates at given times. The averaged data were then used for modeling analysis.
Model formulation
The post-prandial blood glucose excursion can be considered as a hormone regulated resilient system. The food intake is treated as a bolus injection of glucose, and thus the impulse force f ( t ); effects of exercises and hypoglycemic medication are lumped as the damping factor, β . The differential equation of such an oscillatory system, that is used to describe post-prandial blood glucose excursions, can be found in many physics texts:
where x represents blood glucose level over the baseline at time t , ω 0 is the system natural frequency [ 12 ]. The pre-prandial blood glucose levels are generally fluctuating with relatively insignificant magnitudes thus can be approximated as a flat level. If the impulse force f ( t ) takes the form of the Dirac delta function, F δ ( t -0) with F being a food intake dependent parameter, the solution of Eq. (1) is
Parametric estimation
For a given blood glucose excursion, data was taken every 30 minute interval from the time a meal was initially consumed, from which the excursion peak ( MR ), x max , and the corresponding time τ to reach MR can both be estimated. Setting dx / dt = 0 in Eq. (2), the time τ can be expressed as:
Substituting Eq. (3) into Eq.(2), we have
The area under an excursion curve, AUC , can also be obtained:
where T = 2 π / ω is the period of oscillation. The reason for setting the upper integral limit to T /2 is because the damping factor β effectively depresses the glucose excursion levels x near zero for t > T /2, i.e ., it ripples about pre-prandial level. The time T /2 is therefore defined as the recovery period ( RP ). For type 2 diabetic patients who are not in a properly structured regimen, the recovery periods are often longer than 5 hours, by which time the next meal arrives and induces another blood glucose upswing.
Equations (3) – (5) can be used to estimate the three parameters, F , ω and β , from the measurable quantities of τ , x max , and AUC . The procedure is briefly described below:
1. Assign T as twice the roughly estimated recovery period in hours, which can be obtained from the raw data and thus ω = 2 π / T .
4. Fine tune these three parameters by using MATLAB function fminsearch to minimize [ AUC data - AUC ( F , β , ω )] 2 , where AUC data is calculated from the averaged data points by the trapezoidal rule and AUC ( F , β , ω ) is calculated from Eq. (5).
5. These three parameters can further be fine-tuned by fminsearch (sum of squared errors between the averaged data points and the model predicted values).
Two MATLAB user defined functions: GlucoseModel (for No pill and Pill at meal) and GlucoseModel1 (for Pill one hour prior) to estimate these model parameters and calculating the relevant diabetic characteristic measures: τ , x max , AUC are listed in the Additional files 1 and 2 , respectively.
Table Table1 1 lists the fine-tuned values of model parameters: F , ω , β , and those characteristic parameters: RP , τ , x max , and AUC , the latter three are calculated from Eqs. (3) to (5). Also included in Table Table1 1 are the fitting statistics R 2 values that indicate how well model curves fit the data.
Model and characteristic parameters for the post-prandial blood glucose excursion
| Parameters | No pill | 1/4 pill at time 0 | 1/4 pill at time -1 |
| (mg/dL/hr) | 47.1 | 73.8 | 59.3 |
| (hr ) | 0.46 | 0.67 | 0.84 |
| (hr ) | 0.35 | 0.56 | 0.44 |
| (hr) | 2.60 | 1.76 | 1.56 |
| (hr): / | 6.77 | 4.71 | 3.72 |
| (mg/dL) | 59.8 | 62.5 | 49.4 |
| (mg-hr/dL) | 248 | 179 | 118 |
| R | 0.92 | 0.99 | 0.97 |
The parametric value of F is the result of food impact, or the rate of glucose being absorbed into the blood stream. The interpretation of F is rather difficult as the liver acts as a storage compartment for glucose [ 12 ]. Liver regulates blood plasma glucose levels; if it is too high, the excess will be stored in the liver, and the reverse process will take place if the plasma glucose is too low. Although all three model parameters: F , ω , and β are more or less influenced by the liver function, the impact on F deems more pronounced as it has a direct impact on the glucose levels in the blood stream. As the function of the liver is not included in the current model, the estimated F values can only be loosely inferred as a function of insulin level, F increases as hypoglycemic drug depresses the blood glucose levels that in turn increases the absorption rate of glucose into the blood stream as in the case of 1/4 pill taken right before the meal. When the drug is taken an hour before the meal, the liver may have sufficient time to regulate blood glucose levels that additional glucose absorption becomes less intensive.
Ratio of characteristic parameters for the post-prandial blood glucose excursion
| Characteristic ratio | No pill | 1/4 pill at time 0 | 1/4 pill at time -1 |
| / | 0.627 | 0.614 | 0.653 |
| 2.97 | 2.96 | 2.95 | |
| / | 0.384 | 0.374 | 0.419 |
| / | 36.6 | 38.0 | 31.7 |
| /( ) | 0.612 | 0.608 | 0.642 |
No pill trial
Parametric values for no-pill trial reveal that glucose absorption rate is generally slower (low F value) in comparison with the other two cases. The exceedingly long RP of nearly 7 hours is undesirable: as it implies that the next meal time arrives before the blood glucose level could return to the baseline, i.e ., an elevated blood glucose level would be sustained for a prolonged period of time. The high RP and AUC are unmistakably the characteristics for type 2 diabetes. Figure Figure1 1 compares the model and the data with the corresponding standard deviation bars. Model curves are extended for an additional hour beyond the last data point (and in all the figures herewith) to denote the trend of blood glucose excursion.

Post-prandial glucose excursion: no pill trial
1/4 of 5 mg glyburide taken right before the meal
The blood glucose characteristics are significantly improved with a 1/4 size of 5 mg glyburide taken right before lunch. Increased ω and β values translate to significantly lower RP and AUC with virtually unchanged x max . Although the mean RP is less than 5 hours, it is still a bit too long in comparison with the non-diabetics [ 12 ] (~ 4 hours). A higher F value than the one for no-pill trial may partly due to the liver intervention. Figure Figure2 2 compares the model and the data. From the figure one can tell that hypoglycemic drug has an effective delayed effect of about two hours as the rising portion of the model is almost identical to the one for no-pill trial with both x max are about 60, which may be the result of liver function that with initial stimulation of hypoglycemic drug, liver may also release glucose. As the hypoglycemic drug effect persists, the liver ceases to interfere.

Post-prandial glucose excursion: 1/4 pill right before the meal
1/4 of 5 mg glyburide taken an hour before the meal
From the personal experience of the participating subject, the hypoglycemia usually occurs 3 to 4 hours after taking the pill. The trial described in the previous section also reveals that no significant hypoglycemic drug effect is detected in the initial two hours. In order to learn the drug impact on an empty stomach, an additional glucose measurement was made prior to taking the hypoglycemic pill at -1 hour. Another measurement was also taken an hour after the blood glucose excursion returned to the baseline ( i.e ., at hour 5). This is meant to check if the blood glucose would remain near the baseline level. The drop of blood glucose levels between -1 and 0 hours are roughly 10 mg/dL, which can be contributed to the mild liver intervention. No net hypoglycemic drug effect is taking place before the meal as evidenced from the initial rise of the blood excursion curve as shown in Fig. Fig.3 3 (in comparison with Fig. Fig.2), 2 ), where only data between hour 0 and hour 4 were used to generate the model curve. Indeed, all parametric values are improved significantly: both PR and x max are decreased by 20% and their combination that reflected in AUC dropped nearly 35% in comparison to those for pill taken at meal trial as shown in Table Table1. 1 . The food impact parameter F decreased a little from the one for pill at meal trial, which may indicate an hour after dispensing the pill, a quasi-equilibrium state has been reached among the liver function, hypoglycemic drug effects, and the bolus injection of glucose. The system frequency ω increased for more than 25%, which gives a shorter RP that compares favorably with non-diabetics. The drop of damping factor β may be the result of low F , as both τ and x max are already significantly reduced that further strengthening of β becomes unnecessary. The hour 5 measurements confirm that although the model curve shows a decreasing trend, upon returning to the base level the blood glucose excursions practically stabilizes. In addition, the volunteer patient did not experience any hypoglycemia even two to three hours after the final post-prandial measurement.

Post-prandial glucose excursion: 1/4 pill an hour before the meal
This simple impulse-forced model provides a means to shape a self-management regimen for the type 2 diabetic subject: a moderate meal coupled with minimal amount of medical intervention has effectively modulated the blood glucose excursion by reducing its recovery periods and fluctuation amplitudes. Based on the model, the type 2 diabetic subject was able to adjust a lifestyle that include (a) 40 minute swimming in a 25 m pool in the morning, (b) a fruit of mid-size apple or its equivalent and a cup of coffee with cream for breakfast without taking hypoglycaemic pill, (c) moderate lunch with 1/4 size of 5 mg glyburide taken 1/2 to 1 hour before the meal, (d) moderate early dinner, 4 hours prior to bed time, with 1/4 size of 5 mg glyburide taken 1/2 to 1 hour before the meal, (e) snack a mid-size banana, or a small bag (3.5 oz) of peanuts, or 6 crackers when needed in between meals. With this regimen, he was able to reduce his A1c level from 6.7 to 6.0 in 6 months and maintained at this level for the subsequent 6 months. Moreover, he has not had any hypoglycaemic bouts ever since he particitipated in this study more than two years ago.
Elevated blood glucose excursions during the night would boost the A1c levels. To keep a low average fluctuation of blood glucose excursion amplitudes, the evening meal is crucial. In order to avoid hypoglycaemia during the sleep, an early dinner is advised. The subject has been able to keep post-prandial blood glucose levels within 200 mg/dL with the mean fasting reading of 90 ± 20 mg/dL. Occasionally he consumes a can of beer or sugar free deserts. Although no rigorous study has been performed, a forty-minute exercise of swimming, or weight lifting, or jogging, or any combination of these is roughly equivalent to the effect of 1/4 size of 5 mg glyburide. Nonetheless, it is impractical to exercise more than once a day, thus the subject takes 2.5 mg of hypoglycemic pill a day instead. His physician originally prescribed him to take one 5 mg hypoglycemic pill daily. That was more that 10 years ago. The regimen did not work very well as he experienced hypoglycaemic bouts often. This model-based regimen not only reduced A1c level but entirely eliminated hypoglycaemic symptoms. In addition, one fasting blood glucose measurement in the morning is sufficient for him to maintain a healthy daily routine of exercise, consuming meals/snacks and leading a productive life with mental and physical activities.
Lifestyle adjustments are the best regimens for many chronicle ailments such as diabetes, hypertension, high cholesterol levels, etc . Although this model-based self-management regimen for the type 2 diabetic subject is only a case study, it certainly provides a general guideline for an applicable life-style adjustment. Currently not all the model parameters are entirely clear, additional data are required to draw a meaningful general conclusion. A pilot project of testing this regimen on six type 2 diabetic patients in a regional nursing home is proposed for the next phase of study.
Although derived characteristic parameters: RP and AUC (to a lesser degree, τ and x max ), carry clear meaning that can be used to characterize type 2 diabetic subjects from non-diabetics, the implications of model parameters, F , ω and β are not as translucent. With additional data, one may be able to draw plausible conclusions about (a) how F is influenced by food intakes, drug (delaying) effects, and liver (regulatory) functions; and (b) how ω and β behave, whether they are independent of F and of each other, or all three somewhat mutually dependent. Better understanding of these parameters would definitely enhance the self-management for type 2 diabetes.
This model-based lifestyle adjustment has another advantage: it can be used to manage each individual needs. Nutall and Chasuk [ 10 ] have stressed that dietary recommendation for type 2 diabetes should be flexible and highly individualized; most of prepared meal programs and exchange-list diets for diabetes have not had individualization in mind nor are they designed for ethnic minorities. Once we have a comprehensive understanding of these parameters, it is possible to tailor individual lifestyle adjustment accordingly.
For those individuals who are interested in self-managing the type 2 diabetes, the general advice is: avoiding big meals, may snack moderately between meals, eat an early dinner – about 4 hours before bedtime, and exercise regularly. If one is interested in "normal" meal effects on one's post-prandial blood glucose excursion, taking a pre-prandial blood glucose measurement prior to a typical lunch and 8 to 10 post-prandial measurements at half-hour intervals for 5 or more replicates and follow the procedure described here to obtain these characteristic parameters RP , τ , x max , and AUC . Applying a small dosage of medical intervention prior to a meal can keep the blood glucose at a relatively flat level and depress the overnight blood glucose excursion; however, this practice needs the approval from one's family physician and is not recommended here.
Authors' contributions
Sole authorship: data collection/analysis, model building, parameter estimation/interpretation, and the design of life-style adjustment regimen for the participating subject.
Supplementary Material
MATLAB user defined function: GlucoseModel (for No pill and Pill at meal) to estimate model parameters: F , β , ω and to calculate the relevant diabetic characteristic measures: τ , x max , AUC .
MATLAB user defined function: GlucoseModel1 (for Pill one-hour prior) to estimate model parameters: F , β , ω and to calculate the relevant diabetic characteristic measures: τ , x max , AUC .
Acknowledgements
The author wishes to express his appreciation to Ms. Katherine Jakubik for her editing efforts, to Professor Jame B. Bassingthwaighte and two other anonymous reviewers for their critical comments to an earlier version of this manuscript.
- Ratner RE. Type 2 diabetes mellitus: the grand overview. Diabet Med. 1998; 15 :S4–7. doi: 10.1002/(SICI)1096-9136(1998120)15:4+<S4::AID-DIA735>3.3.CO;2-T. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jiwa F. Diabetes in the 1990s – an overview. Stat Bull Metrop Co. 1997; 78 :2–8. [ PubMed ] [ Google Scholar ]
- Brand-Miller J. The Glucose Revolution. Marlowe & Company; 1999. [ Google Scholar ]
- Linder L. What's your number, sweetie? The Washington Post, May 1, HE08. 2001.
- Franz M. In defence of the American Diabetes Association's recommendations on the glycemic index. Nutrition Today. 1999; 34 :78–81. [ Google Scholar ]
- Wolever TMS, Jenkins D, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implication. American Journal of Clinical Nutrition. 1991; 54 :846–854. [ PubMed ] [ Google Scholar ]
- Brand-Miller J. Diets with a low glycemic index: From theory to practice. Nutrition Today. 1999; 34 :64–72. [ Google Scholar ]
- Gannon MC, Nuttall FQ. Factors Affecting Interpretation of Postprandial Glucose and Insulin Areas. Diabetes Care. 1987; 10 :759–763. [ PubMed ] [ Google Scholar ]
- Truswell AS. Glycemic index of foods. Eur J Clin Nutr. 1992; 46 :S91–S101. [ PubMed ] [ Google Scholar ]
- Nuttall FQ, Chasuk RM. Nutrition and the management of type 2 diabetes. Journal of Family Practice. 1998; 47 :S45–53. [ PubMed ] [ Google Scholar ]
- Fournier RL. Basic Transport Phenomena in Biomedical Engineering. Taylor & Francis; 1998. [ Google Scholar ]
- Fisher RJ. Compartmental analysis. In: Enderle J, Blanchard S, Bronzino J, editor. Introduction to Biomedical Engineering. London: Academic Press; 2000. pp. 369–410. [ Google Scholar ]
Comparison of diabetic retinopathy screening between hospital-based multidisciplinary and general practice-based settings: insights from a regional study in Italy
- Original Article
- Open access
- Published: 19 August 2024

Cite this article
You have full access to this open access article

- Chiara Olivieri 1 , 2 ,
- Mattia Salato 3 ,
- Alessandra Campanella 4 ,
- Paola Marolo 1 , 2 ,
- Guglielmo Parisi 1 , 2 ,
- Giovanni Neri 1 , 2 ,
- Mario Damiano Toro 5 ,
- Antonio Scarmozzino 6 ,
- Fabio Broglio 7 ,
- Enrico Borrelli ORCID: orcid.org/0000-0003-2815-5031 1 , 2 na1 &
- Michele Reibaldi 1 , 2 na1
To compare diabetic retinopathy screening among patients with type 1 or type 2 diabetes under care in two distinct setups: hospital-based multidisciplinary and general practice-based.
Materials and methods
In this retrospective observational case series, we collected data from a total of 133 diabetic patients: subjects from the hospital-based multidisciplinary setting were referred by the diabetologist and screened by an ophthalmologist using the Optomed Aurora IQ fundus camera. These patients were compared with those who underwent DR screening arranged through a general practice-based setting.
The proportion of patients treated with insulin was higher in the hospital-based multidisciplinary group, both considering the totality patients and those affected by type 2 diabetes (71.6% vs. 32.2%; p < 0.001, and 58.8% vs. 31.0%; p = 0.004 respectively). Patients from the hospital-based multidisciplinary group had a longer mean diabetes duration (19.6 vs 14.9 years, p < 0.001), underwent DR screening more frequently in the previous three years (2.9 vs 1.4, p < 0.001), the mean time between two DR screenings was shorter (14.6 vs 77.9 weeks, p < 0.001), and DR was detected more frequently (32,4% vs 13.5%; p = 0.011).
We were able to demonstrate that patients screened in the multidisciplinary center, which had characteristics predisposing to a higher risk of DR, were more likely to be diagnosed with DR on time, with a higher mean number of DR screenings and a shorted interval between diabetic and ophthalmological assessments.
Explore related subjects
- Medical Imaging
Avoid common mistakes on your manuscript.
Introduction
According to the 10th edition of the IDF Diabetes Atlas, the estimated global prevalence of diabetes was 10.5% in 2021, impacting 537 million adults aged between 20 and 79 years [ 1 ]. Approximately 45% of diabetes cases remain undiagnosed, with a disproportionately higher proportion in low-income countries, where the number of diabetic patients is expected to increase, this partly due to their aging populations [ 2 ]. Diabetes accounts for 12.2% of global deaths across all causes and significantly influences total healthcare expenses globally [ 3 ].
Individuals with diabetes should access medical care through an integrated team-based approach, fostering a collaborative partnership between patients and physicians [ 4 ]. This approach aims to prevent or postpone complications including nephropathy, retinopathy, neuropathy, and cardiovascular issues. Diabetic retinopathy (DR) is a microvascular complication which represents the leading cause of blindness among working-age adults in high income countries. Its occurrence is associated with the duration of diabetes, chronic hyperglycemia, the presence of nephropathy, and hypertension [ 5 , 6 , 7 ].
Additionally, in a recent study has shown the useful role of serum levels of different types of miRNA as an useful biomarker for the early detection of DR since some miRNAs regulate the insulin secretion and play an important role in the pathophysiology of DR [ 8 ].
Given that DR represents an important public health concern, and considering the availability of a straightforward, safe, and validated screening test (i.e., retinal photography) along with effective treatments, this complication meets all criteria for screening [ 9 ].
The Professional Practice Committee (PPC) of the American Diabetes Association (ADA) advises eye examinations for retinopathy screening within five years following a diagnosis of type 1 diabetes and promptly after the diagnosis of type 2 diabetes, since these patients generally have had years of undiagnosed disease. Follow-up examinations should be conducted annually thereafter [ 4 , 10 ]. Screening for DR focuses on identifying microvascular retinal alterations. Detecting these changes is highly significant as it could prompt adjustments in systemic treatment or the initiation of ocular therapies. Screening for DR can be performed through fundus examination using either direct or indirect ophthalmoscopy or slit lamp biomicroscopic examination or fundus photography. It must be emphasized that novel imaging techniques, such as structural optical coherence tomography (OCT) and OCT angiography (OCTA), exhibit high sensitivity in detecting early vascular changes [ 11 , 12 , 13 ]. However, their use is still not included in DR screening.
In Western countries, despite the widespread occurrence of DR, only 60 to 65% of diabetes patients undergo annual screening examinations [ 14 , 15 ]. Multiple studies have found that lower educational attainment, lower income, belonging to a minority racial group, recent immigration, living in rural areas, and lacking health insurance are associated with significantly lower rates of DR screening [ 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 ]. Importantly, there are notable challenges involved in organizing screenings for DR. Firstly, diabetic patients and their caregivers must attend multiple appointments to assess the presence of diabetes-related systemic complications. This significant commitment may limit their ability to participate in all scheduled screening examinations within the designated timeframe. Secondly, there is a shortage of experienced healthcare professionals capable of accurately grading the severity of DR. Thirdly, achieving effective collaboration among various specialists is not always accomplished, potentially delaying the timely referral for each specialized assessment.
The Italian healthcare system is a complex mix of public and private healthcare. The Italian Government allocates funding that encompasses a universal public health insurance scheme aimed at offering complimentary or subsidized healthcare services. These services cover a range of treatments provided by healthcare professionals including general practitioners and specialists. Most DR screenings in Italy are conducted by ophthalmologists, both in private clinics and public healthcare facilities. Typically, patients are referred to these screenings by general practitioners and diabetologists. Nevertheless, there are hospital-based multidisciplinary setups where patients have the option to undergo both diabetologist assessment and DR screening within the same facility, either on the same day or with only a few days between appointments.
The objective of this study is to provide a basic overview of the distinctions in DR screening between a hospital-based multidisciplinary setup and general practice-based setting in a region located in Northern Italy.
The Institutional Review Board (IRB) of University of Turin was notified about this retrospective observational case series.
For inclusion in the study, patients were required to have either type 1 or type 2 diabetes. Those included in the analysis had undergone at least two DR screening visits, with the most recent one being considered for analysis. Exclusion criteria consisted of: (i) diagnosis of gestational diabetes; (ii) a history of prior ocular treatment for DR; (iii) presence of DR prior to the latest DR screening; and (iv) the presence of concomitant ocular diseases.
The subjects analyzed in this study were drawn from two distinct DR screening setups: (i) hospital-based multidisciplinary, and (ii) general practice-based.
In the hospital-based multidisciplinary setting (located at the San Giovanni Antica Sede (SGAS) Hospital, part of the "City of Health and Science" Hospital in Turin, Italy), patients with a diabetes diagnosis are referred and regularly monitored by diabetologists. Within this setup, an ophthalmologist is available for DR screening twice a week, exclusively for patients referred by diabetologists within the same center. This arrangement allows for DR screening to be conducted on the same day as the diabetology assessment or shortly thereafter. During the screening process, the ophthalmologist utilizes the Optomed Aurora IQ fundus camera after administering tropicamide for dilation. Subsequently, the ophthalmologist reviews the captured images to identify any signs of DR. If DR signs are detected, the patient is then referred to the Medical Retina Service of the Ophthalmology Department at the “City of Health and Science” Hospital in Turin, Italy. All consecutive diabetic patients who met the inclusion and exclusion criteria who were screened between November 2023 and December 2023 were retrospectively analyzed (i.e., 74 patients).
Patients from the hospital-based multidisciplinary setting were compared with diabetic patients who underwent DR screening arranged through a general practice-based setting within the same geographical area. Out of approximately 1,500 patients receiving care in this setting, 98 patients had a diagnosis of diabetes mellitus, and 59 of them met the inclusion and exclusion criteria, ultimately being included in the analysis.
The following variables were collected and incorporated into the analysis: age, gender, type and duration of diabetes, therapy for diabetes, time elapsed since the last DR screening assessment, and time between the last diabetes assessment and DR screening.
Data analysis and statistics
To assess for deviations from normal distribution, the Shapiro–Wilk test was applied to all variables. Mean values and standard deviations (SD) were calculated for each quantitative variable. Comparisons between groups were made using student T-test. Fischer’s exact test was employed to compare categorical variables. A multivariate regression analysis was conducted to explore the factors that primarily influenced a positive screening, with the presence of DR at the screening as the dependent variable.
All statistical analyses were carried out with the Jamovi software (version 2.4.12.0), setting the threshold for statistical significance at p < 0.05.
Patients’ characteristics
A total of 133 diabetic patients (i.e., 74 from the hospital-based multidisciplinary setup and 59 from the general practice-based setting) were included in this analysis. Mean ± SD age of participants was 62.7 ± 15.6 years and 70.7 ± 11.6 in the two groups, respectively ( p < 0.001). Males and females were similarly represented in the two groups ( p = 0.467) (Table 1 ).
Twenty-three out of 74 patients in the hospital-based multidisciplinary group were affected by type 1 diabetes, while only 1 patient in the general practice-based setting had a diagnosis of type 1 diabetes. The percentage of patients treated with insulin was higher in the hospital-based multidisciplinary group (71.6% vs. 32.2%; p < 0.001), even considering only patients with type 2 diabetes (58.8% vs. 31.0%; p = 0.004) (Table 1 , Fig. 1 ). Duration of diabetes was 19.6 ± 12.4 years in the hospital-based multidisciplinary group and 14.9 ± 9.6 years in the general practice-based setting group ( p < 0.001) (Table 1 , Fig. 2 ).
Grouped column chart showing the relative frequencies of qualitative clinical characteristics in the study cohort. Each chart shows the relative frequencies of patients with a specific clinical characteristic. The relative frequencies are given as a percentage of patients with a specific qualitative finding in a distinct group (patients from a hospital-based multidisciplinary setup vs. general practice-based setting). P values for each comparison are reported in the figure and details are presented in Table 1

Box and whisker plots showing quantitative variables in diabetic patients. Each box displays mean (cross within the box), median (central horizontal line) and interquartile range (horizontal extremes of the box) values for each metric. The ends of the whiskers illustrate the minimum and maximum values. Outliers are visualized as dots not included in whiskers. Each graph reports comparisons for a specific quantitative characteristic. Details on comparisons are presented in Table 1
Diabetic retinopathy screening’s characteristics
Mean ± SD number of DR screening in the last 3 years was 2.9 ± 0.8 and 1.4 ± 1.1 in the hospital-based multidisciplinary and general practice-based settings, respectively ( p < 0.001). The time between last diabetic assessment and DR screening was 14.6 ± 14.5 weeks for patients under care in the hospital-based multidisciplinary setting and 77.9 ± 97.0 weeks for patients under care in the general practice-based setting ( p < 0.001). Diabetic patients with a first diagnosis of DR during the latest screening were more prevalent in the hospital-based multidisciplinary group, as compared with those under care in the general practice-based setting (32.4% vs. 13.5%, p = 0.011) (Table 1 , Fig. 2 ).
Among the 59 patients under care in the general practice-based setting, 5 had their most recent diabetic retinopathy screening conducted at a private practice.
The multivariate regression analysis revealed that disease duration ( p = 0.006) and insulin therapy ( p = 0.005) were the primary factors associated with the detection of DR during the screening (Table 2 ).
In this study, we provided a basic overview of the distinctions in DR screening features between a hospital-based multidisciplinary setup and a general practice-based setup within a region situated in Northern Italy. Overall, our findings revealed differences in the populations served by these specific settings, particularly in terms of the type and duration of diabetes. Importantly, individuals receiving care in the hospital-based multidisciplinary setup demonstrated a greater likelihood of undergoing timely and suitable DR screening compared to those referred from general practice. Moreover, patients undergoing DR screening in a multidisciplinary setting were more inclined to receive a diagnosis of DR during the ophthalmology screening, underscoring the importance of timely screening in this cohort.
As mentioned above, as per the guidelines established by the PPC AND ADA, individuals diagnosed with type 1 diabetes should undergo eye examinations for DR screening within five years of diagnosis, while those with type 2 diabetes should undergo screening promptly after diagnosis. Subsequent follow-up examinations should be conducted annually thereafter [ 4 , 10 ]. The purpose of screening for DR is to identify cases requiring timely full ophthalmic examination and treatment to prevent permanent visual impairment. In our study cohort of patients undergoing DR screening through a hospital-based multidisciplinary setup, we found that the average number of DR screenings conducted in the last three years was 2.88. The latter results underscore the appropriateness of DR screening in accordance with the recommended guidelines, indicating a commendable adherence to the screening protocols.
The most relevant risk factors for the development of DR are the duration of diabetes, a diagnosis of type 1 diabetes and poor glycemic control [ 15 , 24 , 25 , 26 , 27 ]. In our study, we observed that diabetic individuals receiving care in the hospital-based multidisciplinary setup were more inclined to have type 1 diabetes and had a longer duration of diabetes compared to those patients for whom DR screening was organized through a general practice-based setup. Moreover, within the hospital-based multidisciplinary setup, diabetic patients were more commonly undergoing treatment with insulin, even considering only individuals with type 2 diabetes. This underscores the observation that even in cases of type 2 diabetes, the proportion of our study cohort’s patients undergoing insulin treatment is higher compared to those in the general practice-based setup. The aforementioned observations collectively suggest that individuals within the study cohort who are at higher risk of developing diabetes-related complications, such as type 1 diabetic patients with a longer disease duration or type 2 diabetic patients undergoing insulin treatment, are the ones undergoing DR screening organized through a hospital-based multidisciplinary setup.
Consistently with the above mentioned findings, a higher percentage of patients undergoing DR screening through a hospital-based multidisciplinary setup exhibited signs of DR (i.e., 32.4% compared to 13.5% in our study cohort). In cases where DR is present, a prompt ophthalmologic assessment is recommended to prevent further deterioration of vision. Within the hospital-based multidisciplinary setup, the average time between the last diabetic assessment and DR screening is approximately 14 weeks, a duration significantly shorter than that observed in the general practice-based setup. This aspect is crucial, underscoring the importance of promptly referring individuals to ophthalmologists in cases where DR is detected.
While a multidisciplinary setup proves effective in conducting DR screening, it does come with limitations. First, these setups are often centralized, requiring patients and caregivers to travel long distances to access them. However, optimal outcomes are achieved when screening is offered at locations and times that match the needs of the patient, not the provider [ 28 , 29 ]. In this regard, a general practice-based setup offers advantages, as it is more widespread and does not necessitate significant travel for patients. Second, implementing annual screening for all individuals with diabetes, regardless of their risk of DR, is evidently challenging to deliver and sustain within a multidisciplinary setup due to the restricted number of healthcare providers available in this environment [ 15 ]. Hence, conducting DR screening via a general practice-based setup is indispensable. However, the latter setting has limitations. For instance, there may be insufficient infrastructure to conduct screening efficiently, and patients might have to visit private ophthalmologists for screening, incurring personal expenses, or experiencing longer wait times compared to a multidisciplinary setup.
Several European studies have reported that extending the screening interval from annually to every 2 or 3 years in patients with diabetes who initially show no evidence of retinopathy can be cost-effective [ 30 , 31 ]. However, in these cases it is extremely important to differentiate patients into low-risk and high-risk groups, as doing so has the potential to further enhance cost-effectivenes [ 32 , 33 ]. In our study cohort, it appears that this risk stratification is observed, as patients undergoing DR screening through a general practice-based setup tend to have a lower risk of diabetes complications. This is evident as most of these patients have short-duration type 2 diabetes and are not receiving insulin treatment. Furthermore, the low incidence of signs of DR observed during screening further supports this observation.
A more effective use of appropriate digital retinal imaging coupled with telemedicine to transmit images is expected to substantially transform DR screening and enhance its effectiveness. Incorporating telemedicine into a general practice-based setup for DR screening would offer several advantages, including the ability to provide screening promptly and conveniently at locations and times that align with patient needs. This approach could be particularly beneficial for patients at low risk of DR, potentially improving DR screening outcomes in this population.
This study has certain limitations that need to be acknowledged. Firstly, while representative, the study only included two examples of multidisciplinary and general practice-based setups, which might limit the generalizability of the findings. Additionally, the study relied on DR screening results documented in patient’s records to determine screening frequency and other variables. Consequently, there is a possibility that some patients may have undergone screening, but if it was not recorded, it could not be accounted for or included in the analysis. Importantly, the diabetes care model examined in the Piedmont region is labeled as “integrated management,” dividing diabetic patients between hospital-based multidisciplinary and general practice-based setups. The latter setup mainly manages more complex cases, with general practitioners responsible for conducting retinography every two years on less complex cases. This undoubtedly influenced certain findings, potentially leading to notable variations in other regions across Italy.
In conclusion, the present study provided a basic overview of the distinctions in DR screening features between a hospital-based multidisciplinary setup and a general practice-based setup within a region situated in Northern Italy. Our findings indicate an effective DR risk stratification between these two settings, with patients at elevated risk of DR more commonly seen in the hospital-based multidisciplinary setting. However, the general practice-based setup presents advantages that could be enhanced with the implementation of telemedicine.
Guariguata L, Whiting D, Weil C et al (2011) The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Res Clin Pract 94:322–332
Article PubMed Google Scholar
Sun H et al (2022) IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119
IDF Diabetes Atlas 10th Edition . www.diabetesatlas.org .
Standards of medical care in diabetes-2010. Diabetes Care vol. 33 Preprint at https://doi.org/10.2337/dc10-S011 (2010).
Klein, R. Ttyperglycemia and Microvascular and Macrovascular Disease Diabetes . http://diabetesjournals.org/care/article-pdf/18/2/258/443442/18-2-258.pdf .
Estacio RO et al (1998) Overt albuminuria predicts diabetic retinopathy in hispanics with NIDDM. Am j kidney dis 31(947):953
Google Scholar
Leske MC et al (2005) Hyperglycemia, blood pressure, and the 9-year incidence of diabetic retinopathy: the Barbados eye studies. Ophthalmology 112:799–805
Mastropasqua R et al (2021) Serum microRNA Levels in Diabetes Mellitus. Diagnostics 11:248
Article Google Scholar
Querques G (2019) Eye complications of diabetes. Acta Diabetol 56:971. https://doi.org/10.1007/s00592-019-01377-8
Harris, M. I., Klein, R., Welborn, T. A. & Knuiman, M. W. O R I G I N A L Onset of NIDDM Occurs at Least 4–7 Yr Before Clinical Diagnosis . http://diabetesjournals.org/care/article-pdf/15/7/815/441555/15-7-815.pdf .
Viggiano P et al (2023) In vivo assessment of associations between photoreceptors structure and macular perfusion in type 1 diabetes. Br J Ophthalmol 107:1672–1679
Borrelli E et al (2021) Volume rendered 3D OCTA assessment of macular ischemia in patients with type 1 diabetes and without diabetic retinopathy. Sci Rep. https://doi.org/10.1038/s41598-021-99297-7
Article PubMed PubMed Central Google Scholar
Tombolini B, Borrelli E, Sacconi R et al (2022) Diabetic macular ischemia. Acta Diabetol. https://doi.org/10.1007/s00592-021-01844-1
Gibson DM (2019) Estimates of the percentage of us adults with diabetes who could be screened for diabetic retinopathy in primary care settings. JAMA Ophthalmol 137:440–444
Vujosevic S et al (2020) Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol 8:337–347
Shi Q, Zhao Y, Fonseca V et al (2014) Racial disparity of eye examinations among the U.S. working-age population with diabetes 2002–2009. Diabetes Care 37:1321–1328
Lim A et al (2008) Prevalence and risk factors of diabetic retinopathy in a multi-racial underserved population. Ophthalmic Epidemiol 15:402–409
French DD et al (2017) Payment reform needed to address health disparities of undiagnosed diabetic retinopathy in the city of Chicago. Ophthalmol Ther 6:123–131
Lovshin JA, Shah BR (2017) Inadequate screening for retinopathy among recent immigrants with type 2 diabetes despite universal health care: a population-based study. J Diabetes Complications 31:664–668
Gibson DM (2014) Eye care availability and access among individuals with diabetes, diabetic retinopathy, or age-related macular degeneration. JAMA Ophthalmol 132:471
Fathy C, Patel S, Sternberg P et al (2016) Disparities in adherence to screening guidelines for diabetic retinopathy in the united states: a comprehensive review and guide for future directions. Semin Ophthalmol 31:364–377
Eppley SE, Mansberger SL, Ramanathan S et al (2019) Characteristics associated with adherence to annual dilated eye examinations among us patients with diagnosed diabetes. Ophthalmology 126:1492–1499
Tran EMT, Bhattacharya J, Pershing S (2017) Self-reported receipt of dilated fundus examinations among patients with diabetes: medicare expenditure panel survey, 2002–2013. Am J Ophthalmol 179:18–24
Lu J et al (2018) Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 41:2370–2376
Article CAS PubMed Google Scholar
Chew EY et al (2014) The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes. Ophthalmology 121:2443–2451
UK Prospective Diabetes Study (UKPDS) Group*. (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). The Lancet 352 837–853
Lachin JM, Genuth S, Nathan DM et al (2008) Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—revisited. Diabetes 57:995–1001
Orton E, Forbes-Haley A, Tunbridge L et al (2013) Equity of uptake of a diabetic retinopathy screening programme in a geographically and socio-economically diverse population. Public Health 127:814–821
Pasquel FJ et al (2015) Cost-effectiveness of different diabetic retinopathy screening modalities. J Diabetes Sci Technol 10:301–307
Looker HC et al (2013) Predicted impact of extending the screening interval for diabetic retinopathy: the Scottish Diabetic Retinopathy Screening programme. Diabetologia 56:1716–1725
Article CAS PubMed PubMed Central Google Scholar
Agardh E, Tababat-Khani P (2011) Adopting 3-year screening intervals for sight-threatening retinal vascular lesions in type 2 diabetic subjects without retinopathy. Diabetes Care 34:1318–1319
Lund SH et al (2016) Individualised risk assessment for diabetic retinopathy and optimisation of screening intervals: a scientific approach to reducing healthcare costs. Br J Ophthalmol 100:683–687
Stratton IM, Aldington SJ, Taylor DJ et al (2013) A simple risk stratification for time to development of sight-threatening diabetic retinopathy. Diabetes Care 36:580–585
Download references
Acknowledgements
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement.
Author information
Enrico Borrelli and Michele Reibaldi contributed equally to the work presented here and should therefore be regarded as equivalent senior authors.
Authors and Affiliations
Department of Surgical Sciences, University of Turin, Turin, Italy
Chiara Olivieri, Paola Marolo, Guglielmo Parisi, Giovanni Neri, Enrico Borrelli & Michele Reibaldi
Department of Ophthalmology, “City of Health and Science” Hospital, Turin, Italy
Medical School, University of Turin, Turin, Italy
Mattia Salato
General Practice Clinic, Ciriè, Turin, Italy
Alessandra Campanella
Public Health Department, Eye Clinic, University of Naples Federico II, 80138, Naples, Italy
Mario Damiano Toro
Department of Quality and Cure Safety, Città Della Salute E Della Scienza, Turin, Italy
Antonio Scarmozzino
Division of Endocrinology, Diabetes and Metabolism, Department of Medical Sciences, University of Turin, Corso Dogliotti 14, 10126, Turin, Italy
Fabio Broglio
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Enrico Borrelli .
Ethics declarations
Conflict of interest.
The authors have no proprietary, funding, or conflicts of interest to disclose.
Ethical Standard Statement
This was a retrospective observational case series that adhered to the tenets of the Declaration of Helsinki.
Informed consent
Informed consent was obtained for retrospective analysis of data.
Additional information
Managed by Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Olivieri, C., Salato, M., Campanella, A. et al. Comparison of diabetic retinopathy screening between hospital-based multidisciplinary and general practice-based settings: insights from a regional study in Italy. Acta Diabetol (2024). https://doi.org/10.1007/s00592-024-02354-6
Download citation
Received : 25 April 2024
Accepted : 04 August 2024
Published : 19 August 2024
DOI : https://doi.org/10.1007/s00592-024-02354-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diabetic retinopathy
- Find a journal
- Publish with us
- Track your research
This paper is in the following e-collection/theme issue:
Published on 16.8.2024 in Vol 26 (2024)
Short-Term Effects of an eHealth Care Experiential Learning Program Among Patients With Type 2 Diabetes: Randomized Controlled Trial
Authors of this article:

Original Paper
- Yu-Shan Cheng 1 , MSN ;
- Cheng-Pei Lin 2, 3 , PhD ;
- Lu-Yen Anny Chen 4 , PhD ;
- Wei-Ren Hwang 5 , MHA, MD ;
- Yi-Chun Lin 5, 6 , MD ;
- Yu-Chi Chen 4 , PhD
1 School of Nursing, The University of Texas at Austin, Austin, TX, United States
2 Institute of Community Health Care, College of Nursing, National Yang Ming Chiao Tung University, Taipei, Taiwan
3 Cicely Saunders Institute of Palliative Care, Policy and Rehabilitation, King's College London, London, United Kingdom
4 Institute of Clinical Nursing, College of Nursing, National Yang Ming Chiao Tung University, Taipei, Taiwan
5 Rong-Yang Clinic, Taipei, Taiwan
6 Division of Endocrinology and Metabolism, Department of Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
Corresponding Author:
Yu-Chi Chen, PhD
Institute of Clinical Nursing
College of Nursing
National Yang Ming Chiao Tung University
No155, Section 2
Li-Nong Street, Beitou District
Taipei, 112
Phone: 886 228267093
Email: [email protected]
Background: Type 2 diabetes is a chronic disease with a significant medical burden. eHealth care integrates medicine and technology to enhance the outcomes of such patients; however, adequate eHealth literacy (eHL) is necessary for that to happen. Fostering eHL is crucial for patients with diabetes to engage with eHealth care and receive quality care and timely support. Experiential learning theory can enhance patients’ eHL and skills to use eHealth care technology in their daily care.
Objective: This study explored the effectiveness of an eHealth care experiential learning program in improving eHL, patient health engagement, and eHealth care use status among patients with type 2 diabetes in 3 months.
Methods: In this randomized controlled trial, patients under case management services from various clinics in Taiwan were randomly assigned to either the intervention group receiving the 6-session eHealth care experiential learning program or the control group receiving the usual care. Data were collected using structured questionnaires at 3 time points: pretest, postintervention, and 3 months after the intervention. Descriptive data were presented using frequency distribution, percentage, mean, and SD. The outcomes were analyzed using a generalized estimating equation method by intention-to-treat analysis.
Results: A total of 92 participants (46 in each group) were recruited in this study. Of these, 86 completed the course and follow-up evaluations with a mean age of 62.38 (SD 12.91) years. After completing the intervention, the intervention group had significantly higher posttest scores in eHL (β=19.94, SE 3.52; P <.001), patient health engagement (β=.28, SE 0.13; P =.04), and eHealth use (β=3.96, SE 0.42; P <.001) than the control group. Furthermore, the intervention group maintained these significant improvements in eHL (β=18.19, SE 3.82; P <.001) and eHealth use (β=3.87, SE 0.49; P <.001) after 3 months.
Conclusions: Participating in the eHealth care experiential learning program resulted in significant improvements in eHL, patient health engagement, and eHealth use among patients with type 2 diabetes. Our interventional program can inform future clinical practice and policies to strengthen self-management skills and facilitate the use of health technology in caring for patients with chronic diseases.
Trial Registration: ClinicalTrials.gov NCT05180604; https://clinicaltrials.gov/ct2/show/NCT05180604
Introduction
Diabetes is a chronic disease, and its prevalence is rapidly increasing globally. It is currently ranked among the top 10 causes of death worldwide. According to the World Health Organization, over 460 million people have diabetes, with this number expected to reach 700 million by 2045 [ 1 , 2 ]. Diabetes is often accompanied by several complications, leading to soaring medical expenses that impose a heavy burden on patients and their families. In the United States, the cost of direct and indirect care for diabetes is US $327 billion. Moreover, from 2012 to 2017, the economic costs of diabetes increased by 26% in the United States [ 3 ]. Therefore, preventing the progression of diabetes is a crucial public health issue.
Diabetes carries significant risks, including pathological changes, irreversible complications, and even death. To effectively manage diabetes, maintain a stable condition, and mitigate these risks, attentive, self-managed daily care by patients is necessary [ 4 ]. Patients need to engage in various self-management tasks such as monitoring their blood sugar regularly, managing medication, exercising regularly, eating a healthy diet, and monitoring their condition periodically [ 5 , 6 ]. These interventions can slow disease progression, prevent complications, reduce medical expenses, and enable patients to coexist with the disease [ 7 ].
With the rapid development of eHealth technology, using eHealth care systems for chronic disease management has become a growing trend. This innovative strategy allows individuals to efficiently engage in disease self-management in their daily lives and meet continuous care demands. Available applications include those offering physiological monitoring, recordkeeping for self-management at home, health consultation guidance, location and access to emergency services, and communication through social media [ 8 , 9 ]. However, while eHealth applications offer multiple health care solutions, they also experience potential problems, especially if patients are unfamiliar with mobile health or do not know how to use eHealth care systems. In that case, they may not be able to access the service, be apprehensive, or refuse to use such systems altogether. This can result in decreased willingness and motivation to use eHealth care for self-management, leading to deteriorating health outcomes [ 10 , 11 ]. Therefore, improving the engagement of patients with diabetes in the eHealth care system is crucial for continuing to provide them with high-quality care.
eHealth literacy (eHL) refers to an individual’s ability to seek, find, understand, and evaluate health information obtained from electronic sources such as websites, mobile apps, and other digital platforms [ 12 , 13 ]. It involves the skills, knowledge, and capabilities needed to navigate, comprehend, and use digital health resources effectively to make informed decisions on health-related matters. Patients with insufficient eHL find it difficult to use eHealth care applications and respond to their requirements such as completing web-based registration and electronic card check-in, which can result in increased passive resistance to self-management. Conversely, those with higher eHL levels can navigate and operate eHealth care effectively [ 14 - 16 ]. Moreover, enhancing patients’ engagement in self-management of their health is key to promoting their further use of smart health care systems in the eHealth era. Such patient health engagement is a continuous, dynamic process through which patients’ cognition, emotions, and behaviors drive psychological change across the following 4 stages: blackout, arousal, adhesion, and eudaimonic project [ 17 , 18 ]. As a result, patient health engagement affects the health outcomes of chronic disease management.
Introducing smart health care applications could be a beneficial strategy for patients; however, if they cannot engage in smart health care or face serious challenges while using eHealth in their daily care, they may feel reluctant, or even refuse, to use eHealth. Thus, teaching patients to use eHealth care systems and improving their eHL based on their pace may help them engage in eHealth care. In addition, encouraging patients to engage in practical work, discuss with each other, and reflect on their health issues during the learning process can increase their knowledge, attitudes, and behaviors [ 19 , 20 ]. Experiential learning theory (ELT) emphasizes the role of a patient’s experience in developing the skills they need to function in their daily life. This theory has been widely used in education and shown to improve learners’ overall knowledge, attitudes, and behaviors [ 21 - 26 ]. In the eHealth era, it is crucial to improve patients’ eHL to enable them to engage in self-management behaviors. Patients with higher levels of eHL are better able to effectively implement eHealth systems in their daily self-management [ 27 , 28 ], corresponding with the expected achievement indicators of this research. Therefore, we propose that adapting ELT as an intervention for patients’ eHealth care education would enhance their eHL, allow them to engage in smart technology health care, and increase their use of health technology, thus enabling eHealth care to become a part of the lives of patients with diabetes. Ultimately, not only does it enhance self-management behavior, but it can also achieve disease control in the long run, preventing deterioration and complications.
Thus, this study aimed to explore the effectiveness of an eHealth care experiential learning program in improving eHL, patient health engagement, and eHealth care use status among patients with type 2 diabetes.
This randomized controlled trial reports on the results of a 3-month study that included 92 patients with type 2 diabetes that examined the short-term effects on eHL, patient health engagement, and eHealth care use status. The experimental group received an eHealth care experiential learning program, while the control group received the usual standard care. CONSORT (Consolidated Standards of Reporting Trials) guidelines were used to report the findings ( Multimedia Appendix 1 ).
Study Design
This was a single-blind, randomized controlled trial, in which the research team performed random allocation by drawing slots. The study process flowchart is presented in Figure 1 .

Participants
The study included participants who met the following criteria: (1) diagnosis of type 2 diabetes and receiving case management services for at least 3 months, (2) aged 20 years or older, (3) able to communicate in Mandarin or Taiwanese, and (4) possess a mobile phone or tablet with an internet connection. Patients with serious diseases such as general paralysis, mental disorders, and cognitive function abnormalities were excluded. Enrollment took place from July to September 2020, with the intervention period from September to December 2020, and the posttest completed in December 2020 and March 2021.
To detect a clinically significant effect, defined by the within or between-group interaction and follow-up time, on the outcome indicators, a sample size of 82 was calculated using repeated measures ANOVA between the factors based on an effect size of 0.25, as per Cohen guidelines [ 29 ]. This used an intercluster correlation coefficient of 0.5, a power of 0.8, and an α of .05. To account for a potential percentage dropout rate of 20%, the total sample size was increased to 92 participants. Ultimately, the study recruited 92 patients with type 2 diabetes.
We recruited patients with type 2 diabetes from various metabolic clinics throughout northern Taiwan. These clinics are integrated into Taiwan’s nationwide health insurance system to ensure all citizens have access to medical services. They are also part of the Diabetes Shared Care Program in Taiwan, which provides specialized care for individuals with metabolic disorders. In these clinics, patients receive comprehensive care from a multidisciplinary team comprising metabolic doctors, nurses, case managers, dieticians, and pharmacists. The services provided include the essential components of effective diabetes management such as regular laboratory tests, health examinations, and health education programs.
Before starting the study, our research team collaborated with the clinic management to establish a clear and mutually agreed-upon research process. To recruit participants, the clinics displayed posters providing information about the study. Research assistants were available to thoroughly explain the research procedures to interested individuals and obtain informed consent. Participants were asked to complete a pretest only after completing the explanation process and obtaining informed consent. This ensured a thorough and ethical approach to participant recruitment and data collection.
Next, after the preassessment, those willing participants were added with a case number. The research team used a computer program to draw slots, assigning the cases to the intervention or control group using a lottery system. The intervention group participated in a 3-month, 6-session eHealth care experiential learning program, while the control group received the usual care and an eHealth care manual. In Taiwan, the usual care refers to the standard treatment and follow-up in outpatient units. Typically, physicians schedule appointments every 3 months for patients with type 2 diabetes to conduct blood tests and prescribe medications. This care often includes health consultations by case managers or nutritional counseling. Both groups underwent posttesting twice: immediately after the 6-session course and 3 months later. Patients with chronic diseases usually have regular medical appointments every 3 months in Taiwan, as mandated by the national health insurance. As such, the control group completed their regular medical appointments during the same period.
Intervention
The 6-session eHealth care experiential learning program ( Multimedia Appendix 2 ), which focused on self-management of diabetes, was based on the eHL framework (eHLF) [ 30 , 31 ] and ELT cycle [ 20 ]. Over 3 years, our research team developed a program specifically designed for patients with type 2 diabetes and the challenges health care providers encounter in managing this condition. In the initial year, we began a qualitative study to gain in-depth insights into the needs and challenges associated with diabetes care. This study revealed several critical aspects for enhancing self-management among patients with diabetes. One of the pivotal insights from the study was the vital role of eHealth care for enhancing home care for patients, particularly those with chronic diseases such as diabetes, for which consistent health monitoring at home is essential. Patients’ 3-monthly clinical visits further highlighted the need for effective home-based health monitoring and guidance. These insights underscored their struggle to engage in meaningful health care discussions, often due to their unfamiliarity with eHealth technologies and a lack of knowledge on self-management practices.
In the program’s second year, we initially developed 8 sessions to address these identified gaps. However, after conducting a Delphi study and a pilot study to test the program’s feasibility and usability, we streamlined it to 6 sessions. This refinement process intended to provide a more effective and concentrated participant learning experience focused on the program content. The program’s overall development cost, including expenses for wearable devices and Bluetooth-enabled health monitors (eg, blood pressure monitors, glucose monitors, and scales), amounted to approximately US $66.
Therefore, the program followed the learning cycle, with the following activities and strategies, divided into 4 stages: concrete experience, reflective observation, abstract conceptualization, and active experimentation. First, the concrete experience stage introduced the importance of health care management and eHealth care for patients with diabetes. The program provided real-life examples and scenarios to help patients understand the significance of eHealth care in managing their condition.
Second, various methods were used in the reflective observation stage to encourage patients to reflect on their learning experiences. Videos were played to show the practical implementation of eHealth care. Group discussions were held to facilitate dialogue and share new insights among patients participating in the eHealth care program. Additionally, each session began with a review to help increase patients’ familiarity with eHealth care devices. This approach ensured a blend of visual learning and active participation, which enhanced the program’s overall effectiveness.
Third, the abstract conceptualization stage aimed to clarify the concepts of eHealth care using social media platforms such as LINE and Facebook and internet groups. Patients engaged in web-based discussions to deepen their understanding of eHealth care practices. Moreover, group discussions fostered dialogue among participants around their eHealth care practice experiences.
Finally, the active experimentation stage allowed patients to learn by actively engaging in related health situations. In this stage, they had the opportunity to choose suitable health technology software, hardware, and wearable devices for their specific diseases in a simulation environment.
Then, patients participated in group and individual competitions that involved using eHealth care tools effectively. They were assisted in setting practice and self-management goals and encouraged to use eHealth activities at home. Overall, this experiential learning program followed a systematic learning cycle that involved introducing concepts, facilitating reflection, deepening understanding, and encouraging active application and experimentation with eHealth care tools and techniques.
Our eHealth care experiential learning program comprised 6 biweekly sessions over 3 months, each lasting around 90 minutes. These sessions, conducted by the project investigator (YCC), combined theoretical knowledge with extensive hands-on training and at-home practice using various eHealth tools and applications commonly used in Taiwanese clinical settings. This included wearable devices such as Xiaomi, health care applications such as Health2Sync, LINE, My Health Bank, and blood pressure and glucose monitors. These hardware and software tools are designed to track various health records and statuses such as heart rates, sleep patterns, blood sugar levels, blood pressure, diet, and medication adherence. With Bluetooth connectivity, they seamlessly integrate into everyday health management and have been widely adopted in clinical settings due to their effectiveness and ease of use.
The six sessions were as follows:
- “A new realm of health technology care”: Introduced smart health care functions, eHealth applications, and motivated patients to engage with eHealth technologies while reflecting on their health care experiences.
- “Fighting against sugar”: Emphasized the importance of regular health monitoring (blood sugar, blood pressure, and diet) in diabetes care, focusing on self-management, demonstrating diabetes health care technology, and providing patients with practice tools.
- “Trick of the trade for chronic kidney disease prevention”: Focused on diabetes complications, particularly chronic kidney disease, discussing self-management and relevant health care technologies that could be used for diabetic nephropathy.
- “eHealth care by your side”: Used competitions and hands-on activities to foster patient engagement with health care technology. Patients were encouraged to operate the technology independently and discuss any challenges they faced. This approach not only motivated active participation but also facilitated learning from real-life experiences, enabling patients to better manage their health conditions using these technologies.
- “My smart eHealth in daily care”: Focused on how health care technology can enhance patients’ lives. The participants were taught to correctly operate various eHealth components and integrate smart health care into their daily self-management. This approach emphasized practical use, encouraging patients to apply smart health care technologies to manage their health and benefit from their functionality in everyday life.
- “My eHealth care practice journey”: Synthesized the key elements from the previous sessions. This final session focused on ensuring that patients could accurately operate smart health care applications, reinforcing their ability and willingness to engage in self-care management using health care technology. This comprehensive review and hands-on practice session aimed to solidify patients’ understanding and proficiency in applying these technologies for their ongoing health management.
Overall, these sessions aimed to provide patients with the knowledge and skills to effectively use eHealth tools for managing their health, enhancing their outcomes through technology-driven self-management. During each session, the project investigator (YCC) began by encouraging participants to ask questions and provide feedback on the previous session for 15 minutes. Following this, the project investigator introduced the current session’s content, and research assistants assisted participants in the practical operation of health care technology and tools for the remaining 15-20 minutes. After the session, participants could practice one-on-one and discuss their learning challenges with the research team. Additionally, between the 2 sessions, the research team used a social media platform to coach participants and provide feedback on any questions. Throughout the sessions, various teaching aids and equipment were used, including learning manuals, social media platforms, health applications, and wearable health devices. The participants in each session simultaneously experienced 4 ELT learning cycles. This approach enabled them to experience intelligent care and learn by practicing daily self-management using eHealth and engaging in the smart health care system.
Data Collection
The data collection was blind to grouping. A single-blind method was applied. The clinics’ case managers collected the posttest data. The participants filled out the questionnaires independently during their medical appointments every 3 months. The case managers assisted participants if they required help with completing these questionnaires. All participants completed the questionnaires during admission (T0). The participants in the intervention group completed the posttest 1 (T1) questionnaires after completing the 3-month intervention program (T1) and again after a 3-month follow-up (T2) during their regular medical appointments. The participants in the control group completed the 2 posttest questionnaires during their regular medical appointments scheduled at 3 monthly intervals, 3 (T1) and 6 months (T2) after enrolling in this study.
Measurement
This study used questionnaires to collect data, which included sociodemographic data, the eHealth Literacy Questionnaire (eHLQ), the Patient Health Engagement Scale, and the eHealth Care Use Scale ( Multimedia Appendix 3 [ 13 , 32 - 34 ]).
Sociodemographic Data
Sociodemographic data included age, sex, education, economic status, number of comorbidities, perceived severity of illness, and health status.
eHLQ Instrument
The 35-item eHLQ is based on the eHLF, which refers to the ability to seek, find, understand, and appraise health information from electronic sources and apply the knowledge gained to address or solve a health problem. The eHLQ is typically structured to cover various dimensions of eHL, including technical skills, ability to understand health information, critical evaluation of web-based sources, and ability to actively engage with digital health technologies [ 13 ]. It comprises the following seven subscales: (1) using technology to process health information, (2) understanding the concept and language of health, (3) actively participating in technology service abilities, (4) feeling safe and self-controllable about personal health information, (5) motivation to participate in information technology services, (6) obtaining useful information technology services, and (7) information technology services that meet personal needs. Each item was scored on a 4-point scale (1=strongly disagree, 2=disagree, 3=agree, and 4=strongly agree). The higher the score, the better the patients’ eHL. The total score ranged from 35 to 140. The original scale’s reliability was 0.8, and it had good construct and discriminant validity [ 13 ]. The Chinese version of the eHLQ has been found to have a content validity of 0.97, and the Cronbach α for the entire scale was 0.98, with subscales ranging from 0.74 to 0.97 [ 32 ].
Patient Health Engagement Scale
Graffigna and Barello [ 33 , 35 ] developed the Patient Health Engagement Scale using the patient health engagement model. The scale investigates if patients are constantly engaging in their health [ 33 , 35 ]. It includes the following four positions along a continuum of engagement: (1) blackout, (2) arousal, (3) adhesion, and (4) eudaimonic project. The scale can conceptualize the psychological aspects of patient health engagement and includes 5 ordinal items. To avoid social desirability bias, 4 positions of raw patient health engagement were categorized into a 7-point scale: 1 and 2=blackout, 3 and 4=arousal, 5 and 6=adhesion, and 7=eudaimonic project. To acquire the final engagement position, the 4 positions of patient health engagement were arranged from lowest to highest. The median score corresponds to the third position, which represents the patient health engagement position. These 4 positions arise from conjoint cognitive (thinking), emotional (feeling), and conative (acting) enactment. The Cronbach α for this scale was 0.85, and its retest reliability was 0.95, indicating good reliability and validity [ 33 ]. The Chinese version of the scale translated by Zhang et al [ 34 ] had an internal consistency of 0.89 and a retest reliability between 0.52 and 0.79.
eHealth Care Use Scale
We used a self-developed scale to investigate various types of eHealth care use and monitoring items in daily disease management. The scale had high internal consistency (Cronbach α=0.92) and good content validity (content validity=0.95). The 4 types of eHealth care included were computer or internet systems, mobile apps, health monitoring systems, and wearable devices (eg, pedometers, smart bracelets, heart rate monitors, blood pressure monitors, blood glucose meters, and weight scales). The scoring method was as follows: 0=no use of any eHealth types, 1=use of 1 type, 2=use of 2 types, 3= use of 3 types, and 4=use of 4 types. Higher scores indicated a greater variety of eHealth types used.
Furthermore, the monitoring items encompassed blood pressure, blood sugar, weight, diet, sleep, heart rate, steps, and other health data. The scoring method for the monitoring items was as follows: 0=not monitoring any health data, 1=monitoring 1 type, 2=monitoring 2 types, 3=monitoring 3 types, 4=monitoring 4 types, 5=monitoring 5 types, 6=monitoring 6 types, and 7=monitoring 7 types. A higher score indicated a greater number of health data items being monitored. The total score reflected overall eHealth care use with a higher score indicating more comprehensive use.
Statistical Analysis
The data were analyzed using SPSS (version 24.0; IBM Corp). Descriptive statistics were used to report continuous data using scores, means, and SDs. Categorical data were presented as numbers and percentages. Independent sample t tests (2-tailed) and chi-square tests (2-tailed) were used to compare the homogeneity of the pretest data between the 2 groups. To avoid overestimating the effect of the intervention while not ruling out loss to follow-up, intention-to-treat analysis was used.
The generalized estimating equation (GEE) was used to examine the difference in effectiveness, with statistical significance set at P <.05. This statistical analysis method can accommodate repeated responses from each participant. The autoregressive (1) model of the GEE using a linear regression model was used to estimate whether there was a significant difference in the improvement of the outcome indicators between the 2 groups over time. To reduce interference factors, sex and economic status—both of which showed significant differences in the initial test after randomization—were included as covariates in the corrected GEE model. This adjustment addresses the potential impact of sex and economic status imbalances and the interaction between time and group. Consequently, the reliability of our findings is enhanced despite the imbalance issue following randomization.
Ethical Considerations
This study was approved by the institutional review board of the university with which the research team is affiliated (YM106120E-3). Before data collection, we provided the participants with an explanation of the research objectives, process, and questionnaire content, both verbally and in writing. Once participants completed a questionnaire at each time point—pretest, postintervention, and 3 months after the intervention—they were able to receive an NT $100 voucher (a currency exchange rate of NT $1=US $0.33 is applicable). We also assured them that their participation would be confidential and would affect neither their treatment rights nor their health care. Data were collected after the participants had signed the consent form. The participants were free to withdraw from the study at any point, even after providing consent.
Participant Characteristics
A total of 86 participants completed the 2 posttests, including 41 (48%) in the intervention group and 45 (52%) in the control group. The total participant attrition rate was 7% (n=6). The rates of subject loss in the intervention and control groups were 11% (5/46) and 2% (1/46), respectively. The initial 92 participants were included in the final analysis ( Table 1 ).
| Variable | Total (N 92) | Intervention group (n 46) | Control group (n 46) | test ( ) or chi-square test ( ) | ||
| Age (years), mean (SD) | 62.38 (13) | 64.96 (10) | 59.80 (15) | 1.9 (90) | ||
| 8.0 (1) | ||||||
| Male | 33 (36) | 10 (22) | 23 (50) | |||
| Female | 59 (64) | 36 (78) | 23 (50) | |||
| 6.1 (4) | ||||||
| Elementary school and below | 18 (20) | 6 (13) | 12 (26) | |||
| Junior high school | 16 (17) | 11 (24) | 5 (11) | |||
| High or vocational school | 32 (35) | 14 (30) | 18 (39) | |||
| College or university | 21 (23) | 13 (28) | 8 (17) | |||
| Graduate school | 5 (5) | 2 (4) | 3 (7) | |||
| ), n (%) | 9.7 (4) | |||||
| <10,000 | 29 (32) | 16 (35) | 13 (28) | |||
| 10,001-20,000 | 24 (26) | 6 (13) | 18 (39) | |||
| >20,001-30,000 | 19 (21) | 13 (28) | 6 (13) | |||
| >30,001-40,000 | 15 (16) | 9 (20) | 6 (13) | |||
| >40,001 | 5 (5) | 2 (4) | 3 (7) | |||
| 1.98 (1) | 1.83 (1) | 2.13 (1) | –1.4 (90) | |||
| 1 item, n (%) | 38 (41) | 20 (44) | 18 (39) | |||
| ≧2 items, n (%) | 54 (59) | 26 (57) | 28 (61) | |||
| 0.8 (2) | ||||||
| Not serious | 28 (30) | 12 (26) | 16 (35) | |||
| Normal | 62 (67) | 33 (72) | 29 (63) | |||
| Serious | 2 (2) | 1 (2) | 1 (2) | |||
| 4.3 (2) | ||||||
| Bad | 18 (20) | 7 (15) | 11 (24) | |||
| Normal | 54 (59) | 26 (57) | 28 (61) | |||
| Good | 20 (22) | 13 (28) | 7 (15) | |||
| eHealth literacy, mean (SD) | 93.82 (19) | 90.96 (19) | 96.67 (18) | –1.5 (90) | ||
| Patient health engagement, mean (SD) | 2.92 (1) | 2.91 (1) | 2.96 (1) | –0.5 (90) | ||
| eHealth care use, mean (SD) | 2.27 (2) | 2.17 (2) | 2.37 (2) | –0.4 (90) | ||
a Independent sample t test (2-tailed).
b Chi-square test (2-tailed).
c The difference between the 2 groups at a significance level of .01 (2-tailed).
d A currency exchange rate of NT $1=US $0.33 is applicable.
The mean age of the 92 participants was 62.38 (SD 12.9) years, and 59 (64%) were female participants, while 33 (36%) were male participants. Regarding education level, the highest proportion had a high school or higher vocational education, accounting for 32 (35%) participants. The intervention group consisted mostly of female participants, whereas the control group had an equal distribution of male and female participants. There was a significant difference in sex between the 2 groups ( χ 2 1 =8.0, P =.005). Regarding economic status, the intervention group had more participants with an income of NT $20,000-30,000, while the control group had more participants earning NT $10,000-20,000. There was a significant difference in economic status between the 2 groups ( χ 2 4 =9.7, P =.046). In terms of comorbidities, 54 (59%) individuals had at least 2 types of chronic diseases. In contrast, 38 (41%) individuals had 1 type. Moreover, 62 (67%) participants indicated a general level of subjective disease severity. Similarly, regarding self-rated health status, 54 (59%) participants considered their health status general ( Table 1 ).
There were no significant differences in most characteristics between the 2 groups, except for sex and economic status. Furthermore, the baseline assessment of the outcome indicators, including eHL, patient health engagement, and eHealth care use, showed no significant differences.
Effectiveness of the eHealth Care Experiential Learning Program
The results revealed significant differences in the patients’ eHL, patient health engagement, and eHealth use after participating in the eHealth care experiential learning program ( Table 2 , Multimedia Appendix 4 , and Figure 2 ). Moreover, there were also significant differences in the eHL subscales ( Multimedia Appendix 5 ).

| β (SE) | value | ||||||
| Intercept | 91.12 (4.06) | <.001 | |||||
| Group (intervention vs control) | –6.11 (3.58) | .09 | |||||
| T2 vs T0 | 2.11 (2.03) | .30 | |||||
| T1 vs T0 | .09 (1.39) | .95 | |||||
| Female vs male | –1.19 (3.08) | .70 | |||||
| ) | |||||||
| >40,001 versus <10,000 | 13.95 (4.63) | .003 | |||||
| >30,001-40,000 versus <10,000 | 8.41 (4.08) | .04 | |||||
| >20,001-30,000 versus <10,000 | 13.55 (3.78) | <.001 | |||||
| 10,001-20,000 versus <10,000 | 6.08 (4.21) | .15 | |||||
| Intervention*T2 versus control*T2 | 18.19 (3.82) | <.001 | |||||
| Intervention*T1 versus control*T1 | 19.94 (3.52) | <.001 | |||||
| Intercept | 3.05 (0.11) | <.001 | |||||
| Group (intervention vs control) | –.07 (0.14) | .59 | |||||
| T2 versus T0 | .05 (0.08) | .52 | |||||
| T1 versus T0 | –.09 (0.08) | .28 | |||||
| Female versus male | –.12 (0.12) | .32 | |||||
| ) | |||||||
| >40,001 versus <10,000 | –.43 (0.28) | .12 | |||||
| >30,001-40,000 versus <10,000 | –.06 (0.17) | .73 | |||||
| >20,001-30,000 versus <10,000 | .15 (0.13) | .25 | |||||
| 10,001-20,000 versus <10,000 | –.05 (0.13) | .70 | |||||
| Intervention T2 versus control T2 | .24 (0.14) | .07 | |||||
| Intervention T1 versus control T1 | .28 (0.13) | .04 | |||||
| Intercept | 1.54 (0.47) | .001 | |||||
| Group (intervention vs control) | –.21 (0.48) | .66 | |||||
| T2 versus T0 | .35 (0.30) | .23 | |||||
| T1 versus T0 | .07 (0.25) | .79 | |||||
| Female versus male | .30 (0.44) | .49 | |||||
| >40,001 versus <10,000 | 2.16 (0.60) | <.001 | |||||
| >30,001-40,000 versus <10,000 | .70 (0.57) | .22 | |||||
| >20,001-30,000 versus <10,000 | .97 (0.55) | .08 | |||||
| 10,001-20,000 versus <10,000 | .82 (0.55) | .14 | |||||
| Intervention T2 versus control T2 | 3.87 (0.49) | <.001 | |||||
| Intervention T1 versus control T1 | 3.96 (0.42) | <.001 | |||||
a The difference between the 2 groups at a significance level of .001 (2-tailed).
b A currency exchange rate of NT $1=US $0.33 is applicable.
c The difference between the 2 groups at a significance level of .05 (2-tailed).
The intervention group showed a significantly higher increase in eHL scores than the control group at both posttest time points. Specifically, the improvement in the intervention group was greater than that in the control group (β=19.94, SE 3.52; P <.001 and β=18.19, SE 3.82; P <.001) at T1 and T2. These findings indicate that the intervention positively improved eHL, which persisted for up to 3 months afterward.
Similarly, patient health engagement increased following the intervention, with a higher mean score change in the intervention group than in the control group. Specifically, the improvement in the intervention group was significantly higher than in the control group (β=.28, SE 0.13; P =.03) at the initial posttest (T1). However, at T2 (3 months after the intervention), there was no significant difference between the 2 groups in the extent of the change (β=.25, SE 0.14; P =.06). The effect of the learning program on the patients’ eHealth use was also examined. We compared the test scores of the intervention group at the 2 posttest time points and found that the mean score increase in the intervention group was higher than that in the control group. Specifically, the improvement in the intervention group was significantly greater than in the control group at T1 and T2 (β=3.96, SE 0.42; P <.001 and β=3.87, SE 0.49; P <.001).
Principal Findings
This study examined the impact of an experiential learning program focused on eHealth care among individuals with type 2 diabetes. We found significant improvements in the intervention group’s eHL, patient health engagement, and eHealth use compared with the control group following a series of educational sessions. The experiential learning program immersed participants in daily care activities and provided real-life experiences, resulting in a low attrition rate and positive acceptance of health care technology. These findings indicate that the program effectively enhanced participants’ skills, attitudes, and behaviors. Enhancing eHL is crucial in the rapidly evolving eHealth era, in which an increasing array of eHealth devices is becoming available. When equipped with adequate eHL, patients with chronic diseases are better positioned to engage in self-management and address health inequities [ 36 ].
While many previous studies have conducted eHealth interventions, their findings on the effectiveness of eHealth care vary [ 37 ]. This theory-based eHealth intervention focuses on practical, experiential learning and the learning cycle, thus bridging this gap in the research because of its broad generalizability. After participants joined the program, we also found that increasing eHL is crucial to improving their health-related behavior. Patients became more actively involved in self-management when using the eHealth system. Consequently, this program demonstrates the potential for future application in clinical care, enabling patients to actively engage in a smart eHealth care system, implement self-management skills, and maintain disease control to improve quality of life. Moreover, validating our program with a clinical population of patients with diabetes underscores its clinical relevance and applicability, thereby demonstrating its value in real-world health care settings. The successful implementation and positive outcomes in this context indicate that the program can also be used to manage other chronic conditions.
The study found that participation in experiential learning courses effectively improved individuals’ eHL, enabling them to use technology for daily self-management tasks. The course curriculum was based on the eHLF, encompassing all stages of the learning process. By engaging in experiential learning activities, participants applied learning cycles and integrated teaching strategies mirroring real-world situations in chronic disease management. This approach aligns with Kolb’s theory, emphasizing learning through everyday life experiences, where knowledge is generated and transformed through practical engagement [ 19 , 20 ]. Learners fully engage in learning by incorporating active participation and hands-on experiences, fostering interest, encouraging questioning, and promoting reflective practices [ 20 ]. Our program comprised activities that closely resembled real-life situations, adhering to the principles of the eHLF to promote active participation among the learners. For example, during the concrete experience stage, we introduced eHealth care for the self-management of chronic diseases through lectures and videos to enhance patients’ understanding of eHealth care. This approach enhances their interest in digital services, prompting them to take a more active role in managing their health, as indicated by the increased patient health engagement shown in Figure 2 . Moreover, in the active experimentation stage, we assisted patients in setting personal goals and facilitated hands-on learning by using various eHealth care devices. These activities provided them with access to appropriate digital services tailored to their health care needs and were designed to meet the unique challenges and requirements of the learners. As depicted in Figure 2 , the intervention group recorded higher scores in eHealth care use than the control group. Through these experiential learning opportunities, participants gain knowledge and insights that evolve into personal skills they can integrate into their daily lives. This process empowers them to make informed decisions about their health and well-being, ultimately enhancing their eHL. By engaging in experiential learning courses, participants develop the necessary skills and confidence in the digital health domain [ 22 , 38 ].
Participants demonstrated enhanced overall eHL and significant progress in all 7 subscales. Specifically, notable improvements were observed in subscale 1 (using technology to process health information) and subscale 2 (understanding health concepts and language). To improve the ability to process information, the program effectively incorporated sessions that elucidated the importance of disease management, demonstrated appropriate health care technology applications, and introduced the concept of smart health care. This comprehensive learning program enabled participants to internalize these concepts and cultivate their abilities while actively engaging them in health care. Previous research has shown that explaining the principles that necessitate improvement not only enhances knowledge and skills but also diminishes barriers to implementation [ 20 , 26 ]. We also observed evident improvements in subscales 3, 5, 6, and 7.
To enhance their understanding of eHealth care, we introduced the patients in this study to various eHealth applications such as smart health care applications, wearable devices, and social media through case studies, lectures, and videos. We also used visual aids, questions, and case studies to enhance their knowledge about health care technology. Thus, patients could select suitable health care technologies that meet their individual care needs. Previous studies have indicated that higher health awareness makes it easier for individuals to comprehend health-related information and actively seek to improve their self-management abilities [ 6 , 39 ]. People can integrate knowledge through practical experience, case studies, and handouts [ 22 , 24 ].
In addition, the issue of information security often affects willingness to use eHealth technologies; however, subscale 4 showed clear improvements in this study. Each session provided information about health-related security, ways to preserve health information, and strategies for fraud prevention. Moreover, we encouraged patients to ask questions and express their concerns, which were clarified by the research team. Patients were informed about security measures and policies and how health care professionals use them. As certain software and tools can determine authenticity, patients’ trust in technological information can be promoted through our intervention. Previous studies have suggested that asking and answering questions [ 40 ], assisting in the operation [ 26 ], and clarifying concerns [ 41 ] can help build people’s trust in their abilities.
We also observed an improvement in patient health engagement. Although there was no significant difference in patient health engagement after 3 months, the level of engagement reached the arousal stage. Patient health engagement is a continuous, dynamic psychological process through which patients’ cognition, emotions, and behaviors interact. This indicates that participants gradually accepted their illness and actively started to engage in disease management [ 35 ]. Previous research has highlighted the lack of knowledge on self-management of diseases and how to effectively use tools for disease management, leading to low engagement [ 6 , 42 ]. In this study, implementing the eHLF helped systematically organize participants’ care needs and connect them to relevant eHealth applications. This enabled patients to understand, take action, and gradually internalize these practices while passing through the learning cycle. Faiola et al [ 42 ] also emphasized providing systematic mobile-based health frameworks based on patients’ needs, empowering lifestyle changes, and promoting sustainable healthy behaviors.
Additionally, patient health engagement was measured on a 5-point scale, making it difficult to observe significant changes. Nevertheless, Figure 2 demonstrates the rise in patient health engagement, which can be attributed to patients learning to use health care technology daily and setting goals to track their health status. Through this program, we helped patients accumulate concrete, real-life self-care experiences, transformed abstract care concepts into usable knowledge, and assisted in operating health technology applications. Using their life experiences in case studies and addressing their questions reduced anxiety about technology use, promoted patient identification, and facilitated learning and engagement [ 6 , 43 ]. Furthermore, patients also participated in group discussions, which previous studies have shown to increase their sense of reality and engagement with eHealth care services [ 22 , 23 ]. This further supported patients’ engagement by fostering a sense of community and shared experiences. As a result, patients are actively engaged with health care technology in their daily lives.
Finally, the intervention significantly improved participants’ eHealth use. Our experiential learning program encouraged patients to participate in 6 sessions of the experience cycle. During these sessions, we demonstrated the operation of eHealth care devices and assisted each patient in selecting suitable devices according to their health conditions and abilities. Additionally, we provided simulation scenarios related to eHealth care, allowing patients to gradually familiarize themselves with operating these health care devices. Patients were encouraged to ask questions, and immediate answers were provided. Once taught, patients could use health technology for self-management independently. Educating individuals based on ELT can enhance their learning and skills [ 4 , 22 , 26 ]. Moreover, patients who engage in hands-on practice and receive information from simulation scenarios experience reduced fear and increased acceptance of new things [ 38 ]. Furthermore, we used LINE, a communication app, to remotely accompany patients in their daily lives. A web-based patient support group was established that allowed patients to ask questions at any time and receive emotional support. We also addressed patients’ questions individually whenever they encountered difficulties in using eHealth care. As a result, patients gained problem-solving skills and overcame any fear or hesitation in using health technology. These findings align with previous studies showing that when patients recognize the need to use such devices, they become more willing to share their needs and engage in discussions [ 9 , 43 , 44 ]. Additionally, when patients truly experience the benefits of using health technology, they integrate it as a helpful and effective part of their lives [ 45 , 46 ]. Therefore, our program has the potential to sustain patients’ eHealth use.
Limitations
This study has several limitations. The 3-month follow-up period may not fully capture the long-term effectiveness of the intervention. Recognizing the importance of longer follow-ups for assessing the sustainability of health behaviors and disease management outcomes, future research should consider extended follow-up periods to more accurately assess health behavior and health outcome changes. Moreover, several strategies can be implemented to sustain the long-term effects of eHealth care experiential learning program. Empowering patients to participate actively in their health management is crucial. Continuous support and counseling through regular refresher sessions can help reinforce learning and maintain patient engagement. Therefore, integrating the program into routine clinical practice is essential, involving health care providers in the ongoing monitoring and supporting patients’ eHealth activities to maintain the program’s benefits. Providing peer support and fostering a shared responsibility for health management can also contribute to sustaining the program’s impact. The extended follow-up includes collecting data on motivation (empowerment), health behaviors (such as patient health engagement, self-management, and eHealth use), and disease control indicators such as blood sugar levels, hemoglobin A 1c %, and lipid profiles. We will share these long-term findings in future publications. Additionally, a sex disparity was observed, with female participants being more active in health-related activities than male participants, partly due to the higher employment rates among male participants limiting their availability. Future studies should explore strategies to increase male’s participation to improve external validity. Furthermore, this study’s reliance on quantitative data may overlook insights that qualitative research can provide. Incorporating qualitative approaches in future research could yield a deeper understanding of user perspectives and the effectiveness of the ELT-based intervention. Addressing these limitations would enhance our understanding of the intervention’s long-term impact and its applicability to a broader population of patients with chronic illnesses.
Conclusions
This study provided compelling evidence of theory-based interventions in eHealth care, displaying their effectiveness in improving eHL, patient health engagement, and eHealth use. By applying ELT, patients with type 2 diabetes demonstrated notable enhancements in their abilities and skills to use eHealth care technology. This approach clarifies the generalizability of the intervention and its components and contributes significantly to bridging the research gap in understanding the impact of eHL on patient behavior. A key finding from this study, after participants engaged with the program, was the crucial role of increasing eHL in improving patients’ health-related behaviors. The study further underscored the significance of the eHLF as a fundamental knowledge base, acquired through immersive, real-world, self-management tasks using eHealth care tools within the learning cycle by demonstrating the tangible benefits of a theory-driven eHealth intervention in a clinical setting. The interventions designed to mimic patients’ daily life situations closely facilitated the optimal learning outcomes and consequent changes in their health behavior. As such, integrating patient-centered care becomes imperative in the health care system to actively involve patients in eHealth care programs and promote improved disease management in the future.
Acknowledgments
This research was funded by the Ministry of Science and Technology, Taiwan (MOST-107-2314-B-010-014-MY3) and the Yen Tjing Ling Medical Foundation, Taiwan (CI-111-38). The authors thank the research assistants, case managers, and all the participants for their time and effort.
Conflicts of Interest
None declared.
CONSORT (Consolidated Standards of Reporting Trials) checklist.
Content of the eHealth care experiential learning program.
The study questionnaire.
Mean changes in the measures of eHealth literacy, patient health engagement, and eHealth use over time (N=92).
Mean changes in the 7 eHealth literacy subscales over time (N=92).
- The top 10 causes of death. World Health Organization. URL: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death [accessed 2022-08-24]
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [ FREE Full text ] [ CrossRef ] [ Medline ]
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Helou N, Talhouedec D, Zumstein-Shaha M, Zanchi A. A multidisciplinary approach for improving quality of life and self-management in diabetic kidney disease: a crossover study. J Clin Med. 2020;9(7):2160. [ CrossRef ] [ Medline ]
- Saghaee A, Ghahari S, Nasli-Esfahani E, Sharifi F, Alizadeh-Khoei M, Rezaee M. Evaluation of the effectiveness of persian diabetes self-management education in older adults with type 2 diabetes at a diabetes outpatient clinic in Tehran: a pilot randomized control trial. J Diabetes Metab Disord. 2020;19(2):1491-1504. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Davis J, Fischl AH, Beck J, Browning L, Carter A, Condon JE, et al. 2022 National standards for diabetes self-management education and support. Sci Diabetes Self Manag Care. 2022;48(1):44-59. [ CrossRef ] [ Medline ]
- Carpenter R, DiChiacchio T, Barker K. Interventions for self-management of type 2 diabetes: an integrative review. Int J Nurs Sci. 2019;6(1):70-91. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chen Y, Chang P. Using eHealth in the continuity care of chronic kidney disease: opportunities and considerations. Hu Li Za Zhi. 2016;63(2):12-18. [ CrossRef ] [ Medline ]
- Bodie GD, Dutta MJ. Understanding health literacy for strategic health marketing: eHealth literacy, health disparities, and the digital divide. Health Mark Q. 2008;25(1-2):175-203. [ CrossRef ] [ Medline ]
- Powell J, Inglis N, Ronnie J, Large S. The characteristics and motivations of online health information seekers: cross-sectional survey and qualitative interview study. J Med Internet Res. 2011;13(1):e20. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kayser L, Kushniruk A, Osborne RH, Norgaard O, Turner P. Enhancing the effectiveness of consumer-focused health information technology systems through eHealth literacy: a framework for understanding users' needs. JMIR Hum Factors. 2015;2(1):e9. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kayser L, Karnoe A, Furstrand D, Batterham R, Christensen KB, Elsworth G, et al. A multidimensional tool based on the eHealth literacy framework: development and initial validity testing of the eHealth Literacy Questionnaire (eHLQ). J Med Internet Res. 2018;20(2):e36. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gilstad H. Toward a comprehensive model of eHealth literacy. 2014. Presented at: Proceedings of the 2nd European Workshop on Practical Aspects of Health Informatics (PAHI 2014); May 19, 2014:63-72; Trondheim, Norway. URL: https://ceur-ws.org/Vol-1251/paper7.pdf [ CrossRef ]
- Kaufman DR, Mirkovic J, Chan C. eHealth literacy as a mediator of health behaviors. In: Patel VL, Arocha JF, Ancker JS, editors. Cognitive Informatics in Health and Biomedicine. Health Informatics. Cham. Springer; 2017:271-297.
- Schulz PJ, Fitzpatrick MA, Hess A, Sudbury-Riley L, Hartung U. Effects of eHealth literacy on general practitioner consultations: a mediation analysis. J Med Internet Res. 2017;19(5):e166. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Barello S, Graffigna G, Vegni E. Patient engagement as an emerging challenge for healthcare services: mapping the literature. Nurs Res Pract. 2012;2012:905934. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Menichetti J, Libreri C, Lozza E, Graffigna G. Giving patients a starring role in their own care: a bibliometric analysis of the on-going literature debate. Health Expect. 2016;19(3):516-526. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kolb D. Experiential Learning: Experience as the Source of Learning and Development. Englewood Cliffs, NJ. Prentice Hall, Inc; 1984.
- Kolb D. Experiential Learning: Experience as the Source of Learning and Development. 2nd Edition. Upper Saddle River, NJ. Pearson FT Press; 2015.
- Kao CC. Applications of the Kolb Experiential Learning Cycle to improve the attitude of nursing students toward older people. Macau J Nur. 2018;16(1-2):1-6. [ FREE Full text ] [ CrossRef ]
- Hsu H, Hsiao J, Yeh C, Huang C. Effects of experiential learning theory in the simulation experience course for nursing students. VGH Nur. 2019;36(4):425-432. [ FREE Full text ] [ CrossRef ]
- Dewi EU, Nursalam, Mahmudah, Yunitasari E. The effect of peer support psychoeducation based on experiential learning on self-care demands among breast cancer patients with post-chemotherapy. J Public Health Res. 2023;12(1):22799036221146901. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Falloon G. Using simulations to teach young students science concepts: an experiential learning theoretical analysis. Comput Educ. 2019;135:138-159. [ CrossRef ]
- Li C, Yang Y, Jing Y. Formulation of teaching strategies for graduation internship based on the experiential learning styles of nursing undergraduates: a non-randomized controlled trial. BMC Med Educ. 2022;22(1):153. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Rasouli S, Namnabati M. Nurses' learning of infants' venipuncture based on Kolb's learning theory. J Neonatal Nurs. 2019;25(5):245-248. [ CrossRef ]
- Hennemann S, Witthöft M, Bethge M, Spanier K, Beutel ME, Zwerenz R. Acceptance and barriers to access of occupational e-mental health: cross-sectional findings from a health-risk population of employees. Int Arch Occup Environ Health. 2018;91(3):305-316. [ CrossRef ] [ Medline ]
- Guo SH, Hsing H, Lin J, Lee C. Relationships between mobile eHealth literacy, diabetes self-care, and glycemic outcomes in Taiwanese patients with type 2 diabetes: cross-sectional study. JMIR Mhealth Uhealth. 2021;9(2):e18404. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cohen S. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of hHealth. Thousand Oaks, CA. Sage Publications, Inc; 1988:31-67.
- Norgaard O, Furstrand D, Klokker L, Karnoe A, Batterham R, Kayser L, et al. The e-health literacy framework: a conceptual framework for characterizing e-health users and their interaction with e-health systems. KM&EL. 2015;7(4):522-540. [ FREE Full text ] [ CrossRef ]
- Norman CD, Skinner HA. eHealth literacy: essential skills for consumer health in a networked world. J Med Internet Res. 2006;8(2):e9. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chen Y, Cheng C, Osborne RH, Kayser L, Liu C, Chang L. Validity testing and cultural adaptation of the eHealth Literacy Questionnaire (eHLQ) among people with chronic diseases in Taiwan: mixed methods study. J Med Internet Res. 2022;24(1):e32855. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Graffigna G, Barello S, Bonanomi A, Lozza E. Measuring patient engagement: development and psychometric properties of the Patient Health Engagement (PHE) Scale. Front Psychol. 2015;6:274. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhang Y, Graffigna G, Bonanomi A, Choi K, Barello S, Mao P, et al. Adaptation and validation of a Chinese version of Patient Health Engagement Scale for patients with chronic disease. Front Psychol. 2017;8:104. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Graffigna G, Barello S. Modelling patient engagement in healthcare: insight for research and practice. In: Patient Engagement. Latin America. Sciendo Migration; 2015:27-43.
- Refahi H, Klein M, Feigerlova E. e-Health literacy skills in people with chronic diseases and what do the measurements tell us: a scoping review. Telemed J E Health. 2023;29(2):198-208. [ CrossRef ] [ Medline ]
- Wang Y, Min J, Khuri J, Xue H, Xie B, Kaminsky LA, et al. Effectiveness of mobile health interventions on diabetes and obesity treatment and management: systematic review of systematic reviews. JMIR Mhealth Uhealth. 2020;8(4):e15400. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhang L, Liu J, Yan C, Zhao Z, Tang J, Ma X. The effect of experiential learning on attitude and willingness to insulin therapy in patients with type 2 diabetes. Int J Clin Exp Med. 2016;9(9):18104-18113. [ FREE Full text ]
- Chen X, Hay JL, Waters EA, Kiviniemi MT, Biddle C, Schofield E, et al. Health literacy and use and trust in health information. J Health Commun. 2018;23(8):724-734. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chmil JV, Turk M, Adamson K, Larew C. Effects of an experiential learning simulation design on clinical nursing judgment development. Nurse Educ. 2015;40(5):228-232. [ CrossRef ] [ Medline ]
- Bonner GJ, Freels S, Ferrans C, Steffen A, Suarez ML, Dancy BL, et al. Advance care planning for African American caregivers of relatives with dementias: cluster randomized controlled trial. Am J Hosp Palliat Care. 2021;38(6):547-556. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Faiola A, Papautsky EL, Isola M. Empowering the aging with mobile health: a mHealth framework for supporting sustainable healthy lifestyle behavior. Curr Probl Cardiol. 2019;44(8):232-266. [ CrossRef ] [ Medline ]
- Shen H, van der Kleij R, van der Boog PJM, Wang W, Song X, Li Z, et al. Digital tools/eHealth to support CKD self-management: a qualitative study of perceptions, attitudes and needs of patients and health care professionals in China. Int J Med Inform. 2022;165:104811. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Knowles M, Holton IE, Swanson R. The Adult Learner: The Definitive Classic in Adult Education and Human Resource Development. 8th Edition. London and New York. Routledge; 2014.
- Burner ER, Menchine MD, Kubicek K, Robles M, Arora S. Perceptions of successful cues to action and opportunities to augment behavioral triggers in diabetes self-management: qualitative analysis of a mobile intervention for low-income Latinos with diabetes. J Med Internet Res. 2014;16(1):e25. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Öberg U, Isaksson U, Jutterström L, Orre CJ, Hörnsten Å. Perceptions of persons with type 2 diabetes treated in Swedish primary health care: qualitative study on using eHealth services for self-management support. JMIR Diabetes. Mar 12, 2018;3(1):e7. [ FREE Full text ] [ CrossRef ] [ Medline ]
Abbreviations
| Consolidated Standards of Reporting Trials |
| eHealth literacy |
| eHealth literacy framework |
| eHealth Literacy Questionnaire |
| experiential learning theory |
| generalized estimating equation |
Edited by S Ma; submitted 09.10.23; peer-reviewed by W Wei, M Savolainen, M Shen; comments to author 14.12.23; revised version received 07.02.24; accepted 21.06.24; published 16.08.24.
©Yu-Shan Cheng, Cheng-Pei Lin, Lu-Yen Anny Chen, Wei-Ren Hwang, Yi-Chun Lin, Yu-Chi Chen. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 16.08.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research (ISSN 1438-8871), is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
PPARγ2 P12A polymorphism and albuminuria in patients with type 2 diabetes: a meta-analysis of case-control studies
Affiliation.
- 1 Department of Cardiovascular, Endocrine and Metabolic Diseases, IRCCS Casa Sollievo della Sofferenza,San Giovanni Rotondo, Italy. [email protected]
- PMID: 21493814
- DOI: 10.1093/ndt/gfr187
Background: Insulin resistance has a role in diabetic nephropathy. The A12 variant of the PPARγ2 P121A polymorphism has been firmly associated with reduced risk of insulin resistance, while its role on the risk of albuminuria in patients with type 2 diabetes is uncertain. This study investigated whether the PPARγ2 P12A polymorphism modulates the risk of albuminuria in these patients.
Methods: We tested the association between the A12 variant and albuminuria in three new case-control studies in diabetic patients from Italy (n = 841, n = 623 and n = 714 patients, respectively) and then performed a meta-analysis of all studies available to date. The nine studies we meta-analysed (six previously published and three presented here) comprised a total of 2376 cases and 4188 controls.
Results: In none of the three new studies was a significant association observed with odds ratio (OR) [95% confidence intervals (95% CI)] being 1.115, 0.799 and 0.849 (P = 0.603, 0.358 and 0.518, respectively). At meta-analysis, the overall OR (95% CI) for association between A12 and albuminuria was 0.694 (0.528-0.912). A significant heterogeneity of the genetic effect was observed (P = 0.026), which was totally explained by the different method of urine collection and albuminuria definition utilized across the studies. In fact, most of the effect was observed in the four studies determining albumin excretion rate rather than in those using albumin concentration in a single spot (OR, 95% CI: 0.529, 0.397-0.706, P = 0.0000164 and 0.919, 0.733-1.153, P = 0.47, respectively).
Conclusion: The present study shows that the PPARγ2 Ala12 variant is significantly associated with a reduced risk of albuminuria among patients with type 2 diabetes.
PubMed Disclaimer
Similar articles
- The PPARγ2 P12A polymorphism is not associated with all-cause mortality in patients with type 2 diabetes mellitus. Pacilli A, Prudente S, Copetti M, Fontana A, Mercuri L, Bacci S, Marucci A, Alberico F, Viti R, Palena A, Lamacchia O, Cignarelli M, De Cosmo S, Trischitta V. Pacilli A, et al. Endocrine. 2016 Oct;54(1):38-46. doi: 10.1007/s12020-016-0906-9. Epub 2016 Mar 8. Endocrine. 2016. PMID: 26956846
- The association between the PPARγ2 Pro12Ala polymorphism and nephropathy susceptibility in type 2 diabetes: a meta-analysis based on 9,176 subjects. Wang L, Teng Z, Cai S, Wang D, Zhao X, Yu K. Wang L, et al. Diagn Pathol. 2013 Jul 15;8:118. doi: 10.1186/1746-1596-8-118. Diagn Pathol. 2013. PMID: 23856170 Free PMC article. Review.
- The PPARγ2 Pro12Ala variant is protective against progression of nephropathy in people with type 2 diabetes. Lapice E, Monticelli A, Cocozza S, Pinelli M, Cocozza S, Bruzzese D, Riccardi G, Vaccaro O. Lapice E, et al. J Transl Med. 2015 Mar 12;13:85. doi: 10.1186/s12967-015-0448-6. J Transl Med. 2015. PMID: 25889595 Free PMC article.
- Peroxisome proliferator activated receptor γ2 gene Pro12Ala gene polymorphism in type 2 diabetes and its relationship with diabetic nephropathy. Azab MM, Abdel-Azeez HA, Zanaty MF, El Alawi SM. Azab MM, et al. Clin Lab. 2014;60(5):743-9. doi: 10.7754/clin.lab.2013.130443. Clin Lab. 2014. PMID: 24839816
- Effects of Pro12Ala polymorphism in peroxisome proliferator-activated receptor-γ2 gene on metabolic syndrome risk: a meta-analysis. Zhang R, Wang J, Yang R, Sun J, Chen R, Luo H, Liu D, Cai D. Zhang R, et al. Gene. 2014 Feb 1;535(1):79-87. doi: 10.1016/j.gene.2013.07.087. Epub 2013 Sep 5. Gene. 2014. PMID: 24012868
- Type 2 diabetes linked FTO gene variant rs8050136 is significantly associated with gravidity in gestational diabetes in a sample of Bangladeshi women: Meta-analysis and case-control study. Amin USM, Rahman TA, Hasan M, Tofail T, Hasanat MA, Seraj ZI, Salimullah M. Amin USM, et al. PLoS One. 2023 Nov 30;18(11):e0288318. doi: 10.1371/journal.pone.0288318. eCollection 2023. PLoS One. 2023. PMID: 38033012 Free PMC article.
- Decisive evidence corroborates a null relationship between MTHFR C677T and chronic kidney disease: A case-control study and a meta-analysis. Chang HL, Chen GR, Hsiao PJ, Chiu CC, Tai MC, Kao CC, Tsai DJ, Su H, Chen YH, Chen WT, Su SL. Chang HL, et al. Medicine (Baltimore). 2020 Jul 17;99(29):e21045. doi: 10.1097/MD.0000000000021045. Medicine (Baltimore). 2020. PMID: 32702845 Free PMC article.
- PPARG Pro12Ala Polymorphism with CKD in Asians: A Meta-Analysis Combined with a Case-Control Study-A Key for Reaching Null Association. Chen HC, Chen WT, Sung TL, Tsai DJ, Lin C, Su H, Lin YF, Chiu HY, Su SL. Chen HC, et al. Genes (Basel). 2020 Jun 26;11(6):705. doi: 10.3390/genes11060705. Genes (Basel). 2020. PMID: 32604723 Free PMC article.
- ISN Forefronts Symposium 2015: Nuclear Receptors and Diabetic Nephropathy. Zheng B, Chen L, Gonzalez FJ. Zheng B, et al. Kidney Int Rep. 2016 Sep;1(3):177-188. doi: 10.1016/j.ekir.2016.07.007. Epub 2016 Aug 5. Kidney Int Rep. 2016. PMID: 28932823 Free PMC article.
- Effects of glycaemic management on diabetic kidney disease. MacIsaac RJ, Jerums G, Ekinci EI. MacIsaac RJ, et al. World J Diabetes. 2017 May 15;8(5):172-186. doi: 10.4239/wjd.v8.i5.172. World J Diabetes. 2017. PMID: 28572879 Free PMC article. Review.
Publication types
- Search in MeSH
Related information
- Gene (GeneRIF)
- Nucleotide (RefSeq)
- Protein (RefSeq)
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
- Silverchair Information Systems
- Genetic Alliance
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
How to Lower Fasting Blood Sugar Naturally
Dietary and Lifestyle Changes That Lower Morning Blood Sugar
- Why It Occurs
Change Your Exercise Routine
Take apple cider vinegar.
- Limit Your Carbs
- Avoid "Bad" Fat
- Prevent Hypoglycemia
Get Quality Sleep
Follow your diabetes plan.
Your fasting blood sugar, sometimes called your morning blood sugar, is the amount of glucose (sugar) in your blood after not eating for eight to 10 hours. It offers a more accurate picture of how well you are managing your blood sugar in the absence of food.
There are several ways to naturally control your fasting blood sugar If you have diabetes . These can be especially useful if you find that your morning blood sugar levels are suddenly high and don't know why.
With the right dietary and lifestyle changes, your fasting blood sugar should be well within the optimal range when you test in the morning. These include exercising regularly, managing your carb and fat intake, getting plenty of sleep, and keeping to your treatment plan.
The article explains why morning blood sugar levels may be high and natural ways to lower them if you have diabetes.
Verywell / Julie Bang
Why Blood Sugar Is High in the Morning
Ideally, when testing your blood glucose (sugar) in the morning, it should be between 70 and 99 milligrams per deciliter (mg/dL) if your diabetes is well-controlled.
But oftentimes, a person will test themselves and find that their blood sugar is high despite doing everything their healthcare provider told them to do. This may be due to a relatively common event called the dawn phenomenon that affects roughly 50% people of living with type 1 or type 2 diabetes .
The dawn phenomenon is caused by a chain of events that occurs while you are sleeping:
- In people with diabetes, the body will often produce less insulin at night. Insulin is the hormone that tells the liver when to stop producing glucose.
- As the morning nears, hormones like cortisol and glucagon are released as part of the body's sleep-wake cycle to "fuel" cells with glucose in preparation for the day ahead.
- The combination of low insulin and high hormones contributes to a spike in blood sugar (known as hyperglycemia ) first thing in the morning.
As common as the dawn phenomenon is, there are seven things you can do to better avoid these early-morning blood sugar spikes.
Exercise lowers blood sugar by increasing insulin sensitivity. This means that your body uses insulin and glucose more effectively.
Studies have shown that exercising in the afternoon or just after dinner helps stabilize insulin levels at night. By keeping your insulin levels at a steadier state, the body can counter the natural surge in glucose in the morning.
You don't need a hardcore workout to achieve this. Instead, aim for low-intensity exercises like:
If morning levels are still high, doing moderate-intensity exercise before breakfast can help bring down your blood sugar levels fast while improving glucose control throughout the day.
Some alternative practitioners endorse the use of apple cider vinegar to counter the effects of morning blood sugar spikes. Apple cider vinegar does not "treat" diabetes but may provide short-term blood sugar control.
According to a study published in the Journal of Evidence-Based Integrative Medicine , taking 2 tablespoons (1,400 milligrams) of apple cider vinegar can significantly lower fasting blood sugar levels 30 minutes after consumption. After 60 minutes, no benefit is seen.
Side effects include stomach upset and sore throat. Over time, the risks may outweigh the benefits as the long-term use of apple cider vinegar can lead to tooth enamel loss, throat burns, and bone mineral loss. Drug interactions are also common.
Limit Evening Carbs
Diet plays a major role in managing diabetes and maintaining healthy blood sugar levels. This is particularly true when it comes to eating carbohydrates .
While carbs are a critical part of any diet, they need to be consumed in moderation if you have diabetes. This is because the body converts 100% of carbs into glucose. So, if you eat carbs late at night, the level of glucose in your blood will rise as insulin levels start to decrease.
If you are hungry before bedtime, opt for a high-fiber or high-protein, low-fat snack that can satisfy your hunger without significantly affecting your blood sugar.
Examples include:
- Fresh fruit and vegetables
- Fat-free or low-fat yogurt
- Fat-free popcorn
- Low-fat granola
- Hard-boiled egg
- Small apple and reduced-fat cheese
Limiting your evening carb intake is one way to avoid morning spikes. But you also need to be mindful of how many carbs you eat at dinner, counting carbs so that you don't exceed the recommended per-meal intake.
The American Diabetes Association recommends between 45 and 60 grams (g) of carbs per meal and between 15 and 20 g of carbs per snack.
Watch Dinnertime Fat
Healthy fats are an essential part of a balanced diet. However, fat slows down digestion. By doing so, high-fat dinners can delay the normal post-meal rise in glucose until the following morning.
Fatty foods also contribute to obesity , a leading risk factor for diabetes as well as a leading risk factor for poor blood sugar control.
Rather than eating "bad" saturated fats derived from animals that are hard to digest, opt for "good" monosaturated and polyunsaturated fats that are derived from plants and are easier to digest. This is one measure that can help naturally lower blood sugar in people with diabetes.
Tree nuts, including almonds, cashews, pecans, and walnuts
Olives and olive oil
Oily fish (salmon, sardines, herring, mackerel, tuna)
Flaxseeds and flaxseed oil
Peanuts and peanut butter
Nut butters
Fatty red meat, including ground beef
Processed meats, like bologna, hot dogs, sausage, bacon
High-fat dairy, including milk, cream, cheese, and ice cream
Butter, margarine, or shortening
Cream and gravy sauces
Fried foods
Baked goods, like muffins, cookies, and cakes
Prevent Nighttime Hypoglycemia
Nighttime low blood sugar ( hypoglycemia ) can cause a rebound in blood sugar levels in the morning. This is referred to as the Somogyi effect .
In people without diabetes, glucose and insulin levels tend to stay flat and constant throughout the night, with a slight increase in insulin just before dawn. In people with diabetes, insulin levels typically decrease at night.
So, if blood sugar levels are low, the body will sense this and release excess cortisone and glucagon in the early morning hours to compensate. Without enough insulin to "put the brakes" on glucose production, hyperglycemia will occur.
Unlike the dawn phenomenon that can affect people with otherwise well-controlled diabetes, the Somogyi effect often occurs in people whose diabetes is poorly controlled.
To avoid nighttime hypoglycemia:
- Never skip dinners.
- Recognize the early signs of hypoglycemia so that you can act accordingly.
- Check your blood sugar before bedtime, adjusting your medications as needed.
- Avoid heavy exercise before bedtime which can contribute to hypoglycemia.
- Limit alcohol which is a leading risk factor for hypoglycemia.
If you have diabetes, getting too little sleep can reduce your ability to control your blood sugar by disrupting your normal sleep-wake cycle.
When you are sleep-deprived, hormones aren't released during the early morning hours as they should and blood sugar levels tend to rise precipitously. At the same time, for reasons that are not entirely understood, cells don't respond as well to insulin as well as they are meant to (a condition known as insulin resistance) . This almost invariably leads to high blood sugar.
Studies have shown that poor sleepers with diabetes have 23% higher glucose levels in the morning and 48% higher insulin levels than good sleepers with diabetes. High blood sugar and high insulin are characteristic of insulin resistance.
By contrast, getting a solid seven hours of sleep per night is associated with a decrease in insulin resistance.
There are several key ways to improve your sleep:
- Keep your bedroom dark, quiet, and relaxing.
- Keep the bedroom temperature cool, ideally around 65 degrees.
- Remove electronic devices from the bedroom.
- Take time to mentally unwind and relax before bedtime.
- Have a nightly bedtime routine, like taking a shower or reading.
- Get into bed only when you are tired.
The best way to control your diabetes at nighttime or daytime is to work with your healthcare provider and follow the prescribed treatment plan.
This includes:
- Taking your medication every day as prescribed
- Monitoring your blood sugar as directed
- Eating regularly and with the correct balance of carbs, proteins, and healthy fat
- Exercising routinely, combining resistance training to build lean muscle with aerobics to improve cardiovascular health
- Achieving and maintaining your ideal weight
- Keeping your regular healthcare appointments
- Refilling your drugs on time so you don't miss a dose
- Advising your healthcare provider about any problems you may be experiencing, including high blood sugar in the morning
High fasting blood sugar in the morning is not uncommon, even among people with well-controlled diabetes. You can better avoid this by making some healthy lifestyle changes, like exercising routinely, limiting your nighttime carbs, avoiding saturated fats, getting plenty of sleep, and keeping to your treatment plan.
Apple cider vinegar has also been proposed as a complementary way to control blood sugar.
Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern . J Educ Health Promot . 2014;3:1. doi:10.4103/2277-9531.127541
Zheng X, Qi Y, Bi L, et al. Effects of Exercise on blood glucose and glycemic variability in type 2 diabetic patients with dawn phenomenon . Biomed Res Int . 2020;2020:6408724. doi:10.1155/2020/6408724
Sampath Kumar A, Maiya AG, Shastry BA, et al. Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta-analysis . Ann Phys Rehabil Med . 2019;62(2):98-103. doi:10.1016/j.rehab.2018.11.001
Siddiqui FJ, Assam PN, de Souza NN, Sultana R, Dalan R, Chan ES. Diabetes control: is vinegar a promising candidate to help achieve targets? . J Evid Based Integr Med . 2018;23:2156587217753004. doi:10.1177/2156587217753004
American Diabetes Association. What can I eat? The diabetes guide to healthy food choices .
American Diabetes Association. What can I eat? Smart snacks .
Wolpert HA, Atakov-Castillo A, Smith SA, Steil GM. Dietary fat acutely increases glucose concentrations and insulin requirements in patients with type 1 diabetes: implications for carbohydrate-based bolus dose calculation and intensive diabetes management . Diabetes Care . 2013;36(4):810-6. doi:10.2337/dc12-0092
American Diabetes Association. Fats .
Grandner MA, Seixas A, Shetty S, Shenoy S. Sleep duration and diabetes risk: population trends and potential mechanisms . Curr Diab Rep. 2016;16(11):106. doi:10.1007/s11892-016-0805-8
Singh T, Ahmed TH, Mohamed N, et al. Does insufficient sleep increase the risk of developing insulin resistance: a systematic review . Cureus . 2022;14(3):e23501. doi:10.7759/cureus.23501
By Kimberly Charleson Kimberly is a health and wellness content writer crafting well-researched content that answers your health questions.
- Open access
- Published: 19 August 2024
The prognostic value of triglyceride-glucose index to adverse renal outcomes in patients with type 2 diabetes mellitus: results from the cohort study of ACCORD
- Pan Yu 1 na1 ,
- Jiaxi Pu 1 , 2 , 3 na1 ,
- Qiongjing Yuan 1 , 2 , 3 ,
- Ling Huang 1 , 2 , 3 ,
- Lijian Tao 1 , 2 , 3 &
- Zhangzhe Peng 1 , 2 , 3
Diabetology & Metabolic Syndrome volume 16 , Article number: 201 ( 2024 ) Cite this article
Metrics details
The triglyceride-glucose (TyG) index is a new and good biomarker of insulin resistance (IR). The prognostic utility of the TyG index for patients with type 2 diabetes mellitus (T2DM) remains uncertain. Our study seeks to elucidate the connection between the TyG index and adverse renal outcomes within a T2DM population, while also examining if these relationships are influenced by subgroup variations.
We analyzed data from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, involving 10,196 T2DM participants, to assess the link between triglyceride-glucose levels and adverse renal outcomes. This evaluation included Restricted Cubic Spline (RCS) analysis, Kaplan–Meier survival analysis, and Multivariate Cox proportional regression. Additionally, we examined the interaction between subgroups concerning adverse renal outcomes.
During a 7-year follow-up, 5824 patients (57.1%) experienced worsening renal function, 2309 patients (23.2%) developed albuminuria, and 280 patients (2.7%) advanced to renal failure. After adjusting for a range of confounding variables, triglyceride-glucose levels were significantly linked to both worsening renal function ( p < 0.001) and the onset of albuminuria ( p = 0.020). Nonetheless, no significant association was observed between triglyceride-glucose levels and renal failure ( p = 0.247). Furthermore, there was no significant subgroups interaction to the associations between TyG levels and adverse renal outcomes.
Our study underscores the significant relationship between the triglyceride-glucose index and the risk of adverse renal outcomes in patients with T2DM. The TyG index, as a readily calculable measure, offers clinicians a valuable tool for anticipating the risk of adverse renal outcomes in this patient population.
Introduction
The global prevalence of diabetes among adults aged 20 to 79 years is projected to rise from 6.4%, affecting 285 million individuals in 2010, to 7.7%, impacting 439 million adults by 2030. This period is expected to witness a 69% surge in the number of adults with diabetes in developing countries and a 20% increase in developed countries [ 1 ]. The kidney represents a critical site of microvascular damage in diabetes, with approximately 50% of individuals with type 2 diabetes mellitus (T2DM) developing diabetic kidney disease (DKD), characterized by impaired renal function, elevated urinary albumin excretion, or both [ 2 ]. DKD has emerged as the leading cause of end-stage renal disease (ESRD) in the United States and many developed nations, accounting for 30–50% of new ESRD cases [ 3 ]. Among the long-term complications of diabetes, DKD places the most substantial burden on patients, manifesting in significant financial costs and adverse impacts on daily life. Notably, individuals with DKD face a heightened risk of adverse health outcomes, such as frailty, reduced quality of life, ESRD, progressive damage to other organs, and premature death. The majority of excess mortality associated with T2DM is notably concentrated among those suffering from DKD [ 4 ], underscoring the imperative to mitigate the incidence of adverse renal outcomes and alleviate the associated prognosis through early diagnosis and intervention.
Insulin resistance (IR), a state wherein physiological concentrations of insulin elicit a diminished biological response, has been implicated in the pathogenesis of various metabolic disorders [ 5 ]. The triglyceride-glucose (TyG) index has been developed as a biochemical surrogate for the identification of IR in both diabetic and nondiabetic individuals [ 6 ]. While the hyper-insulinemic-euglycemic clamp test remains the gold standard for assessing IR, this method is time-consuming and laborious which renders it impractical for widespread clinical application [ 7 ]. Consequently, the TyG index, derived from fasting triglyceride and glucose levels, has gained recognition as a straightforward, accessible, and cost-effective surrogate marker for IR [ 8 ]. Previous research has established the TyG index as an independent predictor of future stroke, myocardial infarction, cardiovascular mortality, and all-cause/non-cardiovascular mortality in the general population, highlighting its role in forecasting cardiovascular and metabolic diseases [ 9 , 10 , 11 ]. Moreover, the TyG index has demonstrated clinical utility in predicting adverse cardiovascular events in patients with or without diabetes who have pre-existing cardiovascular disease [ 12 , 13 , 14 ]. However, the association between TyG and the risk of adverse renal outcomes remains less well-defined. While several studies have reported a correlation between elevated TyG levels and an increased risk of chronic kidney disease (CKD) [ 15 , 16 , 17 , 18 ], others, such as Pan et al., have not identified a significant association between TyG levels and CKD in patients with T2DM [ 19 ]. The inconsistency in these findings can be attributed to limitations in sample sizes, differences in study populations, and varying degrees of adjustment for confounding factors. As such, further research with specific populations, larger sample sizes and adjusting for a range of confounding variables is warranted to clarify the relationship between TyG and CKD.
Therefore, this study aims to assess the associations between the TyG index and adverse renal outcomes in patients with T2DM utilizing data from the ACCORD trial and to explore potential modifications of these associations within subgroups.
Study design and participants
Our study engaged in a retrospective analysis utilizing data derived from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, funded by the National Heart, Lung, and Blood Institute. The ACCORD trial was characterized by a multicenter, randomized, double 2 × 2 factorial design, aimed at exploring the effects of three distinct medical treatment strategies on the morbidity and mortality among individuals with T2DM. These strategies included the glycemia, lipid, and blood pressure trials, each designed to evaluate their respective impacts on cardiovascular disease (CVD) outcomes. The trial enrolled 10,251 middle-aged and elderly participants, diagnosed with T2DM, featuring an average glycated hemoglobin level of 8.3% and a median diabetes duration of 10 years. Recruitment spanned from June 2001 to October 2005, across 77 research sites in the United States and Canada. Inclusion criteria targeted individuals at high risk for CVD events, attributable to either existing clinical CVD, a pronounced likelihood of CVD, or the presence of two or more CVD high-risk factors. The specific criteria for inclusion and exclusion were detailed in the foundational ACCORD study documentation [ 20 ]. Our analysis excluded participants who lacked baseline TyG values. Notably, the employment of the ACCORD dataset in our investigation received approval from the National Heart, Lung, and Blood Institute, thus upholding the required ethical and regulatory standards.
Data collection and outcomes
The dataset for this analysis comprised a comprehensive array of variables, including demographic details (age, sex, race, educational attainment, body mass index, smoking status, and alcohol use). Clinical indicators common to the cohort were also meticulously recorded, encompassing blood pressure, glycated hemoglobin (HbA1c) levels, the duration of diabetes, cardiovascular disease history, lipid profiles, heart rate, and details of treatment regimens. Additionally, established kidney risk factors, such as estimated glomerular filtration rate (eGFR), serum creatinine (SCr) levels, and urinary albumin levels, were evaluated. The TyG index, serving as a key variable, was calculated using the formula: TyG index = Ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL) / 2].
Initially, the cohort consisted of 10,251 individuals diagnosed with type 2 diabetes mellitus, from which those lacking baseline TyG values were excluded, narrowing the focus to 10,196 patients for our study.
The study specifically aimed to explore three adverse renal outcomes based on ACCORD trial definitions: the doubling of serum creatinine levels or a significant decrease in estimated glomerular filtration rate by more than 20 mL/min corresponding to worsening renal function, the onset of albuminuria, and the occurrence of renal failure including end-stage renal disease or serum creatinine levels exceeding 3.3 mg/dL corresponding.
Statistical analysis
In our analysis, continuous variables were summarized using either the mean and standard deviation or the median and interquartile range, while categorical variables were expressed as proportions. To compare groups, we applied the unpaired Student’s t test or the Mann–Whitney U test for continuous variables and the Chi-square or Fisher’s exact test for categorical variables. The cutoff point for TyG levels was determined through maximally selected log-rank statistics. Kaplan–Meier Survival analysis and Cox proportional hazards models, adjusted for potential confounders, were utilized to calculate the prevalence of events and estimate survival times across TyG categories, and to analyze the time-to-event outcomes, respectively. To explore the relationship between TyG levels and various adverse renal outcomes, we employed restricted cubic spline analysis, allowing for the investigation of both linear and nonlinear associations. Model adjustments were made in three stages, based on established potential confounders of TyG’s association with renal outcomes. The model 1 adjusted for demographics and clinical measures including age, sex, education attainment, race, smoking status, alcohol use, blood pressures, and body mass index (BMI). The model 2 added metabolic factors such as glycated hemoglobin (HbA1c), duration of diabetes, serum creatinine, and urinary albumin to the adjustments. Finally, model 3 further adjusted for the use of angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB) treatment, glycemia trial, blood pressure trial, and lipid trial. All analyses were conducted using R version 4.3.0. A two-sided p value<0.05 was considered statistically significant in our analysis.
Baseline characteristics of the patients
The baseline characteristics of the study cohort were comprehensively outlined in Table 1 . With an average age of 62.77 years, the demographic distribution included 61.5% males and 62.5% identifying as White. The median TyG index was established at 9.10. Notably, a critical threshold for serum TyG levels linked to adverse renal outcomes was identified as 10.27, determined through maximally selected log-rank statistics, depicted in Fig. 1 . A detailed analysis of baseline demographic and biochemical measurements, categorized by TyG levels, was presented in Table 1 . This analysis underscored the association of elevated baseline TyG levels with several demographic and clinical parameters, including age, sex, race, educational attainment, BMI, duration of diabetes, SBP, DBP, heart rate, the use of insulin, HbA1c, FPG, and lipid profiles (TC, TG, HDL-C, VLDL-C), as well as serum potassium, estimated glomerular filtration rate, serum creatinine, urinary creatinine, urinary albumin and urinary albumin to creatinine ratio (UACR) levels. Higher TyG levels correlated with increased triglycerides, BMI, HbA1c, and fasting plasma glucose, indicating more severe insulin resistance. Moreover, patients with elevated TyG levels demonstrated a significantly higher risk of developing adverse renal outcomes, as statistically substantiated by the p value for worsening renal function ( p < 0.001), albuminuria ( p = 0.003) and renal failure ( p = 0.045), as shown in Table 1 .
Maximally selected log-rank statistics for cutoff point of TyG
The linear or nonlinear relationship between TyG and adverse renal outcomes
In our investigation, we explored the nature of the relationship—whether linear or nonlinear—between the TyG index and various adverse renal outcomes. Through the application of restricted cubic spline analysis, we sought to thoroughly examine this association. The analysis yielded evidence of a linear relationship between TyG levels and the adverse renal outcomes, namely worsening renal function ( p for nonlinearity = 0.1239), albuminuria ( p for nonlinearity = 0.5465), and renal failure ( p for nonlinearity = 0.7337). Consequently, these results suggested collectively that the risk associated with adverse renal outcomes increases in a linear manner in relation to TyG levels.
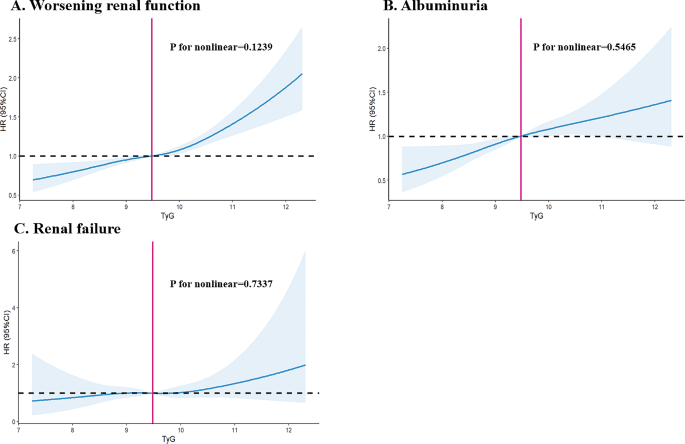
The linear relationship between TyG and adverse renal outcomes by performing Restricted cubic spline analysis
The risk of adverse renal outcomes were related with TyG levels
The association between TyG levels and the risks of adverse renal outcomes was elucidated through Kaplan-Meier survival analysis, with the findings presented in Fig. 3 . This analysis revealed that individuals with lower TyG levels exhibited a higher survival probability, implying a more favorable renal prognosis. The log-rank test was utilized to evaluate the statistical significance of the observed differences, indicating that higher TyG levels were significantly associated with an increased risk of adverse renal outcomes. Specifically, the disparities for worsening renal function, albuminuria, and renal failure were statistically significant, with p value of less than 0.0001 for worsening renal function, 0.00032 for albuminuria, and 0.025 for renal failure, underscoring the critical impact of TyG levels on renal health.
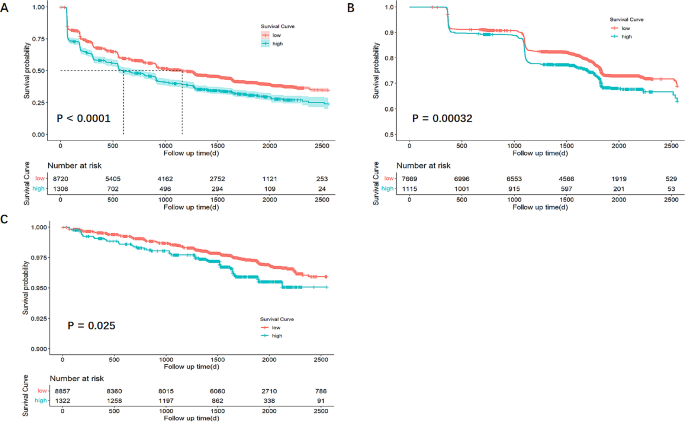
Kaplan - Meier survival analysis for adverse renal outcomes according to binary of TyG levels. ( A ) Worsening renal function; ( B ) Albuminuria; ( C ) Renal failure
Baseline TyG levels and adverse renal outcomes
In evaluating the relationship between baseline TyG levels and adverse renal outcomes, our study implemented Cox proportional regression analysis across three progressively adjusted models. Model 1 accounted for age, sex, education attainment, race, BMI, smoking status, alcohol use, DBP, and SBP. Model 2 expanded upon Model 1 by including HbA1c, diabetes duration, SCr and UACR. Model 3 further incorporated treatments with ACEI, ARB, glycemia trial, blood pressure trial, and lipid trial, building on the covariates of Model 2. The analyses demonstrated that, following adjustments for both demographic and conventional renal risk factors in Model 3, baseline TyG levels were independently associated with the incidence of adverse renal outcomes in patients with T2DM. Specifically, the hazard ratios (HRs) for the adverse renal outcomes in comparison to lower TyG levels were as follows: For worsening renal function, HR was 1.24 (95% CI 1.14–1.35, p < 0.001); for albuminuria, HR was 1.19 (95% CI 1.03–1.37, p = 0.020); and for renal failure, HR was 1.24 (95% CI 0.86–1.79, p = 0.247), as detailed in Table 2 .
Interaction between subgroups to adverse renal outcomes
To explore the relationship between the TyG levels and adverse renal outcomes across various subgroups, interaction between subgroups to adverse renal outcomes was also evaluated. Analysis indicated that the p value for interaction among subgroups concerning worsening renal function was not statistically significant ( p for interaction > 0.05), as depicted in Fig. 4 . Further examination of the interactions relating to albuminuria and renal failure can be found in Supplemental Fig. 1 and Fig. 2 , respectively. These findings suggested that the relationship between TyG levels and adverse renal outcomes remains consistent and significant across different subgroups of patients with T2DM, indicating a uniform impact of TyG levels on renal health irrespective of subgroup distinctions.
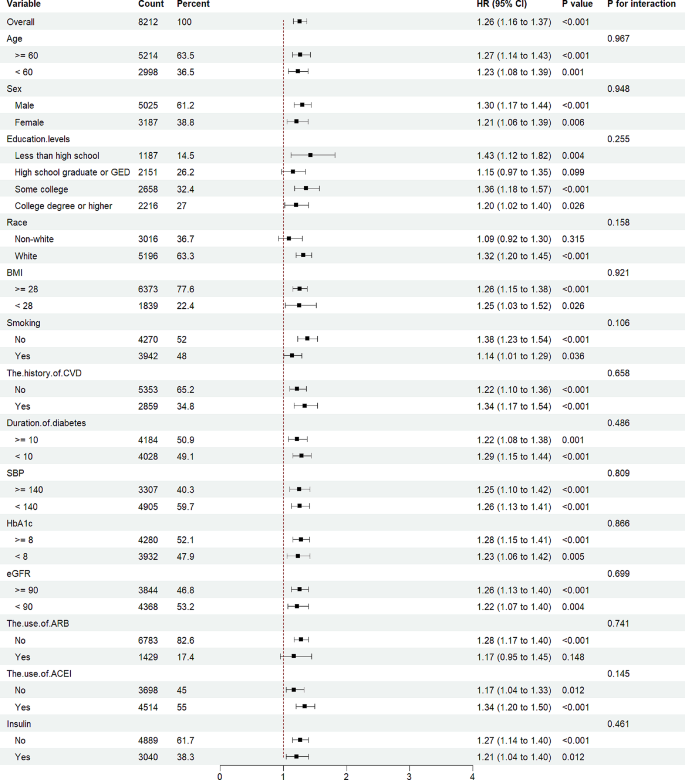
Interaction between subgroups to worsening renal function
Our investigation focused on the relationship between TyG index levels and adverse renal outcomes within a cohort of individuals with T2DM from the ACCORD study, also examining the consistency of these associations across different subgroups. We discovered a significant link between baseline TyG levels and the incidence of adverse renal outcomes, a relationship that persisted across various subgroups. Remarkably, this association remained evident even when accounting for established risk factors like serum creatinine levels, urinary protein content, and the use of ARB or ACEI. These findings highlighted the TyG index as a reliable predictor for early adverse renal outcomes among T2DM patients. Nonetheless, it’s critical to emphasize that the effectiveness of TyG in predicting late-stage renal adverse outcomes warrants further exploration. This study firstly utilized the ACCORD trial and underscored the TyG index’s potential as a tool in the early identification of T2DM patients in the United States at risk for renal complications, independent of traditional risk factors, suggesting its utility in clinical practice and future research directions.
Our study delved into the TyG index, it is very clear that the TyG index is an indicator composed of two risk factors, namely lipid-related and glucose-related factors, which reflect insulin resistance in the human body [ 21 ]. Previous research has described that fasting plasma glucose primarily reflects insulin resistance in the liver, while fasting triglycerides mainly reflect insulin resistance in adipocytes [ 9 ]. In fact, it was documented that the TyG index was the best index to identify individuals with insulin resistance, even superior to visceral adiposity indicators and other lipid parameters [ 7 , 8 , 22 ]. This index, first proposed in 2008, was shown to outperform the homeostasis model assessment-insulin resistance (HOMA-IR) index in identifying IR, demonstrating a sensitivity of 84.0% and specificity of 45.0% in a significant cross-sectional study of apparently healthy individuals [ 23 ]. Further research by Guerrero-Romero et al. in 2010 involving 99 participants with varying body weights and glucose tolerance highlighted the TyG index’s optimal performance for IR assessment, exhibiting high specificity (85.0%) and sensitivity (96.5%) compared to the gold standard, the Euglycemic-Hyperinsulinemia Clamp Test [ 22 ]. Since its inception, the TyG index has been established as a reliable and accessible tool for evaluating IR in individuals at high risk through extensive clinical studies. Previous research primarily focused on cardiovascular diseases (CVDs) such as coronary artery calcification (CAC), acute coronary syndrome (ACS), heart failure (HF), arterial stiffness (AS), stent restenosis, and stable coronary artery disease (CAD) [ 21 ]. Laura et al. utilized a large sample from the Vascular Metabolic CUN cohort (VMCUN cohort) over a median follow-up of 10 years to first suggest a positive association between the TyG index and CVD events, including, peripheral arterial disease (PAD), cerebrovascular disease and coronary heart disease (CHD), independent of confounding factors [ 24 ]. Despite the extensive application of the TyG index in cardiovascular research, limited studies have explored its relationship with adverse renal outcomes. Our current investigation assesses the TyG index as a potential risk factor for incident adverse renal outcomes in the T2DM population, with a focus on evaluating whether this significant association is consistent across different subgroups.
The relationship between triglyceride-glucose (TyG) index levels and chronic kidney disease (CKD) has been explored in some studies, revealing inconsistent results. Despite these discrepancies, a growing body of evidence suggested a strong link between TyG levels and adverse renal outcomes. A community-based cross-sectional study identified a significant association between higher TyG index levels and increased micro-albuminuria [ 25 ], while a cohort study in China reported that elevated TyG index levels were significantly correlated with a higher risk of developing albuminuria, particularly among individuals with metabolic dysfunction [ 26 ]. Further research has shown associations of TyG levels with acute kidney injury (AKI) [ 27 ], end-stage renal disease [ 28 ], hyperuricemia [ 29 ] and worsening renal function [ 30 , 31 ]. These findings underscore the necessity for additional studies to clarify the relationship between the TyG index and CKD, especially in patients with T2DM who are at risk of insulin resistance. This area remains pivotal for ongoing research and clinical investigation.
This investigation aimed to assess the TyG index as a predictor for adverse renal outcomes among individuals with T2DM. Our findings robustly supported the TyG index as a valuable predictor for early renal adverse outcomes. After adjusting for confounding factors, the study revealed a significant association between TyG levels and key adverse renal outcomes, including worsening renal function and the onset of albuminuria. The UACR and eGFR, well-established markers for chronic kidney disease risk assessment, underscore the importance of regular monitoring to manage renal function in diabetic patients effectively. The potential lag in these markers becoming abnormal, indicating already present advanced kidney damage, highlights the urgent need for innovative biomarkers for early detection. Our results demonstrated TyG’s capability to effectively predict early renal adverse outcomes, addressing a critical gap in current biomarkers and facilitating timely interventions for individuals with elevated TyG levels. Notably, our study did not observe a significant link between TyG levels and the progression to renal failure, likely due to the constrained follow-up period which limited observing these specific outcome events. This absence of statistical significance underlines the necessity for extended research to further elucidate TyG’s relationship with late-stage renal adverse outcomes. Despite these considerations, our research offers compelling evidence of the TyG index as a reliable early indicator of adverse renal outcomes, presenting significant clinical utility.
The TyG index’s predictive capacity for cardiovascular diseases has been linked to several molecular mechanisms, including smooth muscle cell dysfunction, coagulation, endothelial dysfunction, metabolic flexibility [ 21 ]. However, the specific mechanisms connecting the TyG index to incident adverse renal outcomes are less defined, though several plausible explanations exist. Firstly, insulin resistance is known to correlate with elevated levels of inflammatory markers [ 32 ] and inflammation has been recognized as an independent risk factor for incident adverse renal outcomes. Secondly, IR may activate the mitochondrial electron transport chain, leading to the production of reactive oxidative stress (ROS), which in turn can cause kidney tissue fibrosis [ 15 ]. Thirdly, hyperinsulinemia, often associated with IR, can detrimentally impact renal function by promoting glomerular hyperfiltration, endothelial dysfunction, and increased vascular permeability [ 33 ]. Therefore, individuals with higher TyG index values are likely to experience more severe kidney function impairment and are at a higher risk for adverse renal outcomes.
The utilization of a large sample size, access to high-quality subject information, and the application of various statistical methods enable our study to identify independent associations between the TyG index and adverse renal outcomes, leading to robust conclusions. Nonetheless, it’s essential to recognize our study’s limitations. Even though we incorporate most recognized risk factors for adverse renal outcomes into our multivariable regression models, we cannot completely exclude the possibility of residual confounding factors. Moreover, it’s important to highlight that our findings are observational. Although they strongly indicate a correlation between TyG levels and adverse renal outcomes, future prospective intervention studies are crucial to definitively determine TyG levels’ causal effects on these outcomes. Additionally, due to a lack of fasting insulin levels in the ACCORD trial, we cannot compare whether TyG is better than HOMA-IR in predicting the occurrence of adverse renal outcomes. Finally, the population of the ACCORD trial included only high-risk patients with type 2 diabetes and additional studies are necessary to increase the generalizability of the results.
Our investigation focused on elucidating the relationship between the TyG index and adverse renal outcomes in individuals with T2DM. The outcomes of this study are significant, demonstrating a clear association between baseline TyG levels and adverse renal outcomes. Furthermore, our findings indicate that the TyG index could be a useful tool for risk stratification in predicting adverse renal outcomes among patients with T2DM. However, to enhance our understanding and validate these associations, further research is necessary. Future studies should aim to corroborate our results and explore whether interventions aimed at reducing TyG levels could offer benefits to patients with T2DM exhibiting elevated TyG levels. Such research has the potential to inform clinical practices, thereby improving the management and care of diabetic individuals at risk of developing adverse renal outcomes.
Data availability
No datasets were generated or analysed during the current study.
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14.
Article PubMed CAS Google Scholar
Dwyer JP, Parving HH, Hunsicker LG, Ravid M, Remuzzi G, Lewis JB. Renal dysfunction in the Presence of Normoalbuminuria in Type 2 diabetes: results from the DEMAND Study. Cardiorenal Med. 2012;2:1–10.
Umanath K, Lewis JB. Update on Diabetic Nephropathy: Core Curriculum 2018. Am J Kidney Dis. 2018;71:884–95.
Article PubMed Google Scholar
Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–8.
Article PubMed PubMed Central CAS Google Scholar
Caro JF. Clinical review 26: insulin resistance in obese and nonobese man. J Clin Endocrinol Metab. 1991;73:691–5.
Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10:74.
Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL. Assessment of pancreatic beta-cell function: review of methods and clinical applications. Curr Diabetes Rev. 2014;10:2–42.
Sanchez-Garcia A, Rodriguez-Gutierrez R, Mancillas-Adame L, Gonzalez-Nava V, Diaz Gonzalez-Colmenero A, Solis RC, Alvarez-Villalobos NA, Gonzalez-Gonzalez JG. Diagnostic Accuracy of the Triglyceride and Glucose Index for Insulin Resistance: A Systematic Review. Int J Endocrinol 2020, 2020:4678526.
Lopez-Jaramillo P, Gomez-Arbelaez D, Martinez-Bello D, Abat MEM, Alhabib KF, Avezum A, Barbarash O, Chifamba J, Diaz ML, Gulec S, et al. Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Healthy Longev. 2023;4:e23–33.
Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18:361.
Yao Y, Wang B, Geng T, Chen J, Chen W, Li L. The association between TyG and all-cause/non-cardiovascular mortality in general patients with type 2 diabetes mellitus is modified by age: results from the cohort study of NHANES 1999–2018. Cardiovasc Diabetol. 2024;23:43.
Chiu H, Tsai HJ, Huang JC, Wu PY, Hsu WH, Lee MY, Chen SC. Associations between Triglyceride-Glucose Index and Micro- and Macro-angiopathies in Type 2 Diabetes Mellitus. Nutrients 2020, 12.
Yu Y, Meng Y, Liu J. Association between the triglyceride-glucose index and stroke in middle-aged and older non-diabetic population: a prospective cohort study. Nutr Metab Cardiovasc Dis. 2023;33:1684–92.
Xiong S, Chen Q, Zhang Z, Chen Y, Hou J, Cui C, Cheng L, Su H, Long Y, Yang S, et al. A synergistic effect of the triglyceride-glucose index and the residual SYNTAX score on the prediction of intermediate-term major adverse cardiac events in patients with type 2 diabetes mellitus undergoing percutaneous coronary intervention. Cardiovasc Diabetol. 2022;21:115.
Ren X, Jiang M, Han L, Zheng X. Association between triglyceride-glucose index and chronic kidney disease: a cohort study and meta-analysis. Nutr Metab Cardiovasc Dis. 2023;33:1121–8.
Chen N, Ma LL, Zhang Y, Chu X, Dong J, Yan YX. Association of long-term triglyceride-glucose index patterns with the incidence of chronic kidney disease among non-diabetic population: evidence from a functional community cohort. Cardiovasc Diabetol. 2024;23:7.
Kunutsor SK, Seidu S, Kurl S, Laukkanen JA. Baseline and usual triglyceride-glucose index and the risk of chronic kidney disease: a prospective cohort study. Geroscience; 2024.
Yu C, Shi Y, Wang T, Zhu L, Zhou W, Bao H, Cheng X. Triglyceride-glucose index change and chronic kidney disease progression in a Chinese hypertensive population. Front Endocrinol (Lausanne). 2024;15:1342408.
Pan Y, Zhong S, Zhou K, Tian Z, Chen F, Liu Z, Geng Z, Li S, Huang R, Wang H et al. Association between Diabetes Complications and the Triglyceride-Glucose Index in Hospitalized Patients with Type 2 Diabetes. J Diabetes Res : 2021, 2021:8757996.
Group AS, Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:i21–33.
Article Google Scholar
Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21:68.
Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, Jacques-Camarena O, Rodriguez-Moran M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–51.
Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304.
Sanchez-Inigo L, Navarro-Gonzalez D, Fernandez-Montero A, Pastrana-Delgado J, Martinez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46:189–97.
Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, Teliewubai J, Zhang Y, Xu Y. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. 2019;18:95.
Article PubMed PubMed Central Google Scholar
Gao W, Wang J, Chen Y, Qiao H, Qian X, Xin Z, Zhao Z, Wang T, Xu Y, Xu M, et al. Discordance between the triglyceride glucose index and HOMA-IR in incident albuminuria: a cohort study from China. Lipids Health Dis. 2021;20:176.
Yang Z, Gong H, Kan F, Ji N. Association between the triglyceride glucose (TyG) index and the risk of acute kidney injury in critically ill patients with heart failure: analysis of the MIMIC-IV database. Cardiovasc Diabetol. 2023;22:232.
Fritz J, Brozek W, Concin H, Nagel G, Kerschbaum J, Lhotta K, Ulmer H, Zitt E. The triglyceride-glucose index and obesity-related risk of end-stage kidney disease in Austrian adults. JAMA Netw Open. 2021;4:e212612.
Li Q, Shao X, Zhou S, Cui Z, Liu H, Wang T, Fan X, Yu P. Triglyceride-glucose index is significantly associated with the risk of hyperuricemia in patients with diabetic kidney disease. Sci Rep. 2022;12:19988.
Lei L, Liang H, Qu Y, Zhong Q, Zhang Q, Dai L, Lu J, Xiao M, Zhao Z, Zhou F, et al. Association between triglyceride-glucose index and worsening renal function in the elderly. Front Nutr. 2022;9:951564.
Cui C, Liu L, Zhang T, Fang L, Mo Z, Qi Y, Zheng J, Wang Z, Xu H, Yan H, et al. Triglyceride-glucose index, renal function and cardiovascular disease: a national cohort study. Cardiovasc Diabetol. 2023;22:325.
Chen J, Wildman RP, Hamm LL, Muntner P, Reynolds K, Whelton PK, He J, Third National H, Nutrition Examination S. Association between inflammation and insulin resistance in U.S. nondiabetic adults: results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2960–5.
Schrauben SJ, Jepson C, Hsu JY, Wilson FP, Zhang X, Lash JP, Robinson BM, Townsend RR, Chen J, Fogelfeld L, et al. Insulin resistance and chronic kidney disease progression, cardiovascular events, and death: findings from the chronic renal insufficiency cohort study. BMC Nephrol. 2019;20:60.
Download references
Acknowledgements
We are thankful for the contributions the ACCORD group made in data collection and sharing.
This project was supported by the Major Program of the National Natural Science Foundation of China (82090024).
Author information
Pan Yu and Jiaxi Pu contributed equally to this work.
Authors and Affiliations
Department of Nephrology, Xiangya Hospital, Central South University, No. 87 Xiangya Road, Kaifu District, Changsha, Hunan Province, China
Pan Yu, Jiaxi Pu, Qiongjing Yuan, Ling Huang, Lijian Tao & Zhangzhe Peng
Hunan Key Lab of Organ Fibrosis, Changsha, China
Jiaxi Pu, Qiongjing Yuan, Ling Huang, Lijian Tao & Zhangzhe Peng
National International Collaborative Research Center for Medical Metabolomics, Central South University, Xiangya Hospital, Changsha, China
You can also search for this author in PubMed Google Scholar
Contributions
ZZP, JXP and PY contributed to study design. JXP, ZZP and PY contributed to data acquisition. PY, JXP and ZZP contributed to data analysis. PY, LH, JXP and ZZP contributed to drafting of the manuscript. LJT contributed to supervision and mentorship. The final version of the manuscript was read and approved by all authors.
Corresponding author
Correspondence to Zhangzhe Peng .
Ethics declarations
Ethics approval.
The use of the ACCORD dataset in this study has been approved by the National Heart, Lung, and Blood Institute.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Figure S1:
Interaction between subgroups to albuminuria
Supplementary Material 2: Figure S2:
Interaction between subgroups to renal failure
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Yu, P., Pu, J., Yuan, Q. et al. The prognostic value of triglyceride-glucose index to adverse renal outcomes in patients with type 2 diabetes mellitus: results from the cohort study of ACCORD. Diabetol Metab Syndr 16 , 201 (2024). https://doi.org/10.1186/s13098-024-01439-0
Download citation
Received : 01 June 2024
Accepted : 05 August 2024
Published : 19 August 2024
DOI : https://doi.org/10.1186/s13098-024-01439-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Type 2 diabetes Mellitus
- Triglyceride-glucose
- Insulin resistance
- Adverse renal outcomes
Diabetology & Metabolic Syndrome
ISSN: 1758-5996
- Submission enquiries: [email protected]

IMAGES
COMMENTS
The following case study illustrates the clinical role of advanced practice nurses in the management of a patient with type 2 diabetes. Case Presentation A.B. is a retired 69-year-old man with a 5-year history of type 2 diabetes.
It is possible for a patient to have type 1 or type 2 diabetes in addition to MODY, so this patient should be screened for diabetes according to recommendations for the general population (e.g., in the event that she has a risk factor for diabetes, such as obesity). 1 Since the mild hyperglycemia associated with GCK-MODY is asymptomatic (and ...
B.L. is a 58-year-old white woman who has been referred to the pharmacist clinician for pharmacotherapy assessment and diabetes management. Her multiple medical conditions include type 2 diabetes diagnosed in 1995, hypertension, hyperlipidemia, asthma, coronary artery disease, persistent peripheral edema, and longstanding musculoskeletal pain secondary to a motor vehicle accident.
CASE. A 47-year-old woman was found to have hyperglycemia at a health fair when a random blood glucose level was 227 mg/dL (12.6 mmol/L). Several days later, a fasting blood glucose value was 147 mg/dL (8.2 mmol/L). She has no previous history of diabetes, is alarmed by the possibility of having this disorder, and seeks your advice.
Diabetes mellitus currently affects 6.4% or 285 million adults worldwide, and that number is expected to increase to 7.7% or 439 million by 2030. 1 In the United States, the prevalence of diabetes in adults increased from 11.3% in 2010 to 12.3% in 2012. 2 The current type 2 diabetes mellitus (T2DM) epidemic is closely associated with a parallel obesity epidemic, with more than 85% of patients ...
The most common pattern of dyslipidemia in patients with type 2 diabetes is elevated triglycerides and decreased HDL levels.1 Although coexistent increases in small, dense LDL cholesterol particles—not the triglycerides themselves—may be responsible for the increase in cardiovascular risk, hypertriglyceridemia poses a significant burden on ...
A recent study reported that oral medication was eventually needed to control hyperglycemia in patients with diabetes refractory to management with proper lifestyle modification.12 However, lifestyle modification is important, and is a cornerstone in the treatment of diabetes, and it should be a mandatory treatment for type 2 diabetes.
The present case-control study was designed to primarily test whether the prevalence of cardiovascular disease, and secondarily of other underlying conditions, differ between patients with type 2 diabetes hospitalized for Covid-19 (cases), compared to patients with type 2 diabetes without signs or symptoms of SARS-CoV-2 infection (controls).
Background To be diagnosed with type 2 diabetes is a challenge for every patient. There are previous studies on patients' experience in general but not addressing the increased cardiovascular risk and multifactorial treatment. The aim of this study was to explore the thoughts, experiences and reactions of newly diagnosed patients with diabetes to this diagnosis and to the risk of developing ...
Study population. This study utilized a case-control design and involved participants between the ages of 18 and 60 who had been diagnosed with type 2 diabetes within the previous six months based on specific glucose level criteria (FBS levels of ≥ 126 mg/dl and 2 h-PG levels of ≥ 200 mg/dl []).Healthy individuals within the same age range were also included, with specific glucose level ...
This article examines the aetiology, pathophysiology, diagnosis and treatment of type 2 diabetes using a case study approach. The psychosocial implications for the patient are also discussed. The case study is based on a patient with diabetes who was admitted to hospital following a hypoglycaemic episode and cared for during a practice ...
The three mini-case studies presented with this issue of the journal take you through what to consider in making an accurate diagnosis of type 2 diabetes. The format uses typical clinical scenarios as tools for learning. Information is provided in short sections, with most ending in a question to answer before moving on to the next section.
A case study is presented here to illustrate the possibility of devising an individual-based self-management regimen. Results: Results of the lunch study for a type 2 diabetic subject indicate that the recovery time of the post-prandial blood glucose level can be adjusted to 4 hours, which is comparable to the typical time interval for non ...
This article examines the aetiology, pathophysiology, diagnosis and treatment of type 2 diabetes using a case study approach. The psychosocial implications for the patient are also discussed. The case study is based on a patient with diabetes who was admitted to hospital following a hypoglycaemic episode and cared for during a practice ...
The specialized role of nursing in the care and education of people with diabetes has been in existence for more than 30 years and the emergence and subsequent growth of advanced practice in nursing during the past 20 years has expanded the direct care component, incorporating aspects of both nursing and medical care while maintaining the teaching and counseling roles. The specialized role of ...
Diabetes patients can draw on an increasing number of eHealth apps to support them in the self-management of their disease. While studies so far have focused on patients with type 1 diabetes, we explored how patients with type 2 diabetes mellitus (T2DM) integrate eHealth apps into their practices aimed at managing and coping with the disease, which aspects were considered particularly valuable ...
The three mini-case studies developed for this issue of the journal take us through the basic considerations of managing type 2 diabetes in the elderly. The format uses typical clinical scenarios as tools for learning. Information is provided in short sections, with most ending in a question to answer before moving on to the next section.
Type 2 diabetes mellitus is a state when pancreas unable to make sufficient insulin and results in lack of insulin production. It is diagnosed after the 40 years of age [8].Diabetes mellitus is a ...
It is possible for a patient to have type 1 or type 2 diabetes in addition to MODY, ... observational case control studies. PLoS One 2013;8(6):e65326-e65326. ...
If you are interested in taking the full course for CME credit, check it out here: https://med.stanford.edu/cme/courses/online/diabetes.htmlFaculty or studen...
Background: Type 2 diabetes mellitus (T2DM) represents one of the most impacting health issues of the modern era, as it is associated with an extensive range of comorbidities. Diabetic retinopathy (DR) is one the utmost severe diabetes complications as it is one of the major causes of vision loss among these patients. Our present research aims to evaluate the most frequent risk factors related ...
To summarize, DKA is not a unique feature of type 1 diabetes. Though much more common in type 1 diabetes, it does occur in patients with type 2 diabetes, as illustrated by these case reports. However, it is rare for DKA to occur in type 2 diabetes in the absence of some precipitating event.
The training of peer supporters is critical because the success of the entire peer support intervention depends on the knowledge and experience that peer supporters can share with other patients. The objective of this study was to evaluate the pilot implementation of a specialist nurse-led self-management training programme for peer supporters with type 2 diabetes mellitus (T2DM) with or ...
Results. Results of the lunch study for a type 2 diabetic subject indicate that the recovery time of the post-prandial blood glucose level can be adjusted to 4 hours, which is comparable to the typical time interval for non-diabetics: 3 to 4 hours. A moderate lifestyle adjustment of light supper coupled with morning swimming of 20 laps in a 25 ...
Purpose To compare diabetic retinopathy screening among patients with type 1 or type 2 diabetes under care in two distinct setups: hospital-based multidisciplinary and general practice-based. Materials and methods In this retrospective observational case series, we collected data from a total of 133 diabetic patients: subjects from the hospital-based multidisciplinary setting were referred by ...
Background: Type 2 diabetes is a chronic disease with a significant medical burden. eHealth care integrates medicine and technology to enhance the outcomes of such patients; however, adequate eHealth literacy (eHL) is necessary for that to happen. Fostering eHL is crucial for patients with diabetes to engage with eHealth care and receive quality care and timely support.
This study investigated whether the PPARγ2 P12A polymorphism modulates the risk of albuminuria in these patients. Methods: We tested the association between the A12 variant and albuminuria in three new case-control studies in diabetic patients from Italy (n = 841, n = 623 and n = 714 patients, respectively) and then performed a meta-analysis ...
Studies have shown that exercising in the afternoon or just after dinner helps stabilize insulin levels at night. ... The prevention and control the type-2 diabetes by changing lifestyle and ... Qi Y, Bi L, et al. Effects of Exercise on blood glucose and glycemic variability in type 2 diabetic patients with dawn phenomenon. Biomed Res Int. 2020 ...
Recent studies and national cohort data show alarming rise over the past 5 years in the number of people younger than 40 years being diagnosed with type 2 diabetes. This increase is a concern because life years lost,1 comorbidity risks, and suffering are substantially greater when type 2 diabetes develops at younger than when it develops at older ages. Several aspects of this worrying trend ...
The triglyceride-glucose (TyG) index is a new and good biomarker of insulin resistance (IR). The prognostic utility of the TyG index for patients with type 2 diabetes mellitus (T2DM) remains uncertain. Our study seeks to elucidate the connection between the TyG index and adverse renal outcomes within a T2DM population, while also examining if these relationships are influenced by subgroup ...