- Privacy Policy

Home » Evaluating Research – Process, Examples and Methods

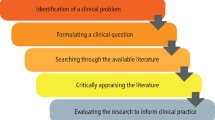
Evaluating Research – Process, Examples and Methods
Table of Contents

Evaluating Research
Definition:
Evaluating Research refers to the process of assessing the quality, credibility, and relevance of a research study or project. This involves examining the methods, data, and results of the research in order to determine its validity, reliability, and usefulness. Evaluating research can be done by both experts and non-experts in the field, and involves critical thinking, analysis, and interpretation of the research findings.
Research Evaluating Process
The process of evaluating research typically involves the following steps:
Identify the Research Question
The first step in evaluating research is to identify the research question or problem that the study is addressing. This will help you to determine whether the study is relevant to your needs.
Assess the Study Design
The study design refers to the methodology used to conduct the research. You should assess whether the study design is appropriate for the research question and whether it is likely to produce reliable and valid results.
Evaluate the Sample
The sample refers to the group of participants or subjects who are included in the study. You should evaluate whether the sample size is adequate and whether the participants are representative of the population under study.
Review the Data Collection Methods
You should review the data collection methods used in the study to ensure that they are valid and reliable. This includes assessing the measures used to collect data and the procedures used to collect data.
Examine the Statistical Analysis
Statistical analysis refers to the methods used to analyze the data. You should examine whether the statistical analysis is appropriate for the research question and whether it is likely to produce valid and reliable results.
Assess the Conclusions
You should evaluate whether the data support the conclusions drawn from the study and whether they are relevant to the research question.
Consider the Limitations
Finally, you should consider the limitations of the study, including any potential biases or confounding factors that may have influenced the results.
Evaluating Research Methods
Evaluating Research Methods are as follows:
- Peer review: Peer review is a process where experts in the field review a study before it is published. This helps ensure that the study is accurate, valid, and relevant to the field.
- Critical appraisal : Critical appraisal involves systematically evaluating a study based on specific criteria. This helps assess the quality of the study and the reliability of the findings.
- Replication : Replication involves repeating a study to test the validity and reliability of the findings. This can help identify any errors or biases in the original study.
- Meta-analysis : Meta-analysis is a statistical method that combines the results of multiple studies to provide a more comprehensive understanding of a particular topic. This can help identify patterns or inconsistencies across studies.
- Consultation with experts : Consulting with experts in the field can provide valuable insights into the quality and relevance of a study. Experts can also help identify potential limitations or biases in the study.
- Review of funding sources: Examining the funding sources of a study can help identify any potential conflicts of interest or biases that may have influenced the study design or interpretation of results.
Example of Evaluating Research
Example of Evaluating Research sample for students:
Title of the Study: The Effects of Social Media Use on Mental Health among College Students
Sample Size: 500 college students
Sampling Technique : Convenience sampling
- Sample Size: The sample size of 500 college students is a moderate sample size, which could be considered representative of the college student population. However, it would be more representative if the sample size was larger, or if a random sampling technique was used.
- Sampling Technique : Convenience sampling is a non-probability sampling technique, which means that the sample may not be representative of the population. This technique may introduce bias into the study since the participants are self-selected and may not be representative of the entire college student population. Therefore, the results of this study may not be generalizable to other populations.
- Participant Characteristics: The study does not provide any information about the demographic characteristics of the participants, such as age, gender, race, or socioeconomic status. This information is important because social media use and mental health may vary among different demographic groups.
- Data Collection Method: The study used a self-administered survey to collect data. Self-administered surveys may be subject to response bias and may not accurately reflect participants’ actual behaviors and experiences.
- Data Analysis: The study used descriptive statistics and regression analysis to analyze the data. Descriptive statistics provide a summary of the data, while regression analysis is used to examine the relationship between two or more variables. However, the study did not provide information about the statistical significance of the results or the effect sizes.
Overall, while the study provides some insights into the relationship between social media use and mental health among college students, the use of a convenience sampling technique and the lack of information about participant characteristics limit the generalizability of the findings. In addition, the use of self-administered surveys may introduce bias into the study, and the lack of information about the statistical significance of the results limits the interpretation of the findings.
Note*: Above mentioned example is just a sample for students. Do not copy and paste directly into your assignment. Kindly do your own research for academic purposes.
Applications of Evaluating Research
Here are some of the applications of evaluating research:
- Identifying reliable sources : By evaluating research, researchers, students, and other professionals can identify the most reliable sources of information to use in their work. They can determine the quality of research studies, including the methodology, sample size, data analysis, and conclusions.
- Validating findings: Evaluating research can help to validate findings from previous studies. By examining the methodology and results of a study, researchers can determine if the findings are reliable and if they can be used to inform future research.
- Identifying knowledge gaps: Evaluating research can also help to identify gaps in current knowledge. By examining the existing literature on a topic, researchers can determine areas where more research is needed, and they can design studies to address these gaps.
- Improving research quality : Evaluating research can help to improve the quality of future research. By examining the strengths and weaknesses of previous studies, researchers can design better studies and avoid common pitfalls.
- Informing policy and decision-making : Evaluating research is crucial in informing policy and decision-making in many fields. By examining the evidence base for a particular issue, policymakers can make informed decisions that are supported by the best available evidence.
- Enhancing education : Evaluating research is essential in enhancing education. Educators can use research findings to improve teaching methods, curriculum development, and student outcomes.
Purpose of Evaluating Research
Here are some of the key purposes of evaluating research:
- Determine the reliability and validity of research findings : By evaluating research, researchers can determine the quality of the study design, data collection, and analysis. They can determine whether the findings are reliable, valid, and generalizable to other populations.
- Identify the strengths and weaknesses of research studies: Evaluating research helps to identify the strengths and weaknesses of research studies, including potential biases, confounding factors, and limitations. This information can help researchers to design better studies in the future.
- Inform evidence-based decision-making: Evaluating research is crucial in informing evidence-based decision-making in many fields, including healthcare, education, and public policy. Policymakers, educators, and clinicians rely on research evidence to make informed decisions.
- Identify research gaps : By evaluating research, researchers can identify gaps in the existing literature and design studies to address these gaps. This process can help to advance knowledge and improve the quality of research in a particular field.
- Ensure research ethics and integrity : Evaluating research helps to ensure that research studies are conducted ethically and with integrity. Researchers must adhere to ethical guidelines to protect the welfare and rights of study participants and to maintain the trust of the public.
Characteristics Evaluating Research
Characteristics Evaluating Research are as follows:
- Research question/hypothesis: A good research question or hypothesis should be clear, concise, and well-defined. It should address a significant problem or issue in the field and be grounded in relevant theory or prior research.
- Study design: The research design should be appropriate for answering the research question and be clearly described in the study. The study design should also minimize bias and confounding variables.
- Sampling : The sample should be representative of the population of interest and the sampling method should be appropriate for the research question and study design.
- Data collection : The data collection methods should be reliable and valid, and the data should be accurately recorded and analyzed.
- Results : The results should be presented clearly and accurately, and the statistical analysis should be appropriate for the research question and study design.
- Interpretation of results : The interpretation of the results should be based on the data and not influenced by personal biases or preconceptions.
- Generalizability: The study findings should be generalizable to the population of interest and relevant to other settings or contexts.
- Contribution to the field : The study should make a significant contribution to the field and advance our understanding of the research question or issue.
Advantages of Evaluating Research
Evaluating research has several advantages, including:
- Ensuring accuracy and validity : By evaluating research, we can ensure that the research is accurate, valid, and reliable. This ensures that the findings are trustworthy and can be used to inform decision-making.
- Identifying gaps in knowledge : Evaluating research can help identify gaps in knowledge and areas where further research is needed. This can guide future research and help build a stronger evidence base.
- Promoting critical thinking: Evaluating research requires critical thinking skills, which can be applied in other areas of life. By evaluating research, individuals can develop their critical thinking skills and become more discerning consumers of information.
- Improving the quality of research : Evaluating research can help improve the quality of research by identifying areas where improvements can be made. This can lead to more rigorous research methods and better-quality research.
- Informing decision-making: By evaluating research, we can make informed decisions based on the evidence. This is particularly important in fields such as medicine and public health, where decisions can have significant consequences.
- Advancing the field : Evaluating research can help advance the field by identifying new research questions and areas of inquiry. This can lead to the development of new theories and the refinement of existing ones.
Limitations of Evaluating Research
Limitations of Evaluating Research are as follows:
- Time-consuming: Evaluating research can be time-consuming, particularly if the study is complex or requires specialized knowledge. This can be a barrier for individuals who are not experts in the field or who have limited time.
- Subjectivity : Evaluating research can be subjective, as different individuals may have different interpretations of the same study. This can lead to inconsistencies in the evaluation process and make it difficult to compare studies.
- Limited generalizability: The findings of a study may not be generalizable to other populations or contexts. This limits the usefulness of the study and may make it difficult to apply the findings to other settings.
- Publication bias: Research that does not find significant results may be less likely to be published, which can create a bias in the published literature. This can limit the amount of information available for evaluation.
- Lack of transparency: Some studies may not provide enough detail about their methods or results, making it difficult to evaluate their quality or validity.
- Funding bias : Research funded by particular organizations or industries may be biased towards the interests of the funder. This can influence the study design, methods, and interpretation of results.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Research Objectives – Types, Examples and...

Research Project – Definition, Writing Guide and...

Research Questions – Types, Examples and Writing...

Significance of the Study – Examples and Writing...

Purpose of Research – Objectives and Applications

Literature Review – Types Writing Guide and...
- The Open University
- Accessibility hub
- Guest user / Sign out
- Study with The Open University
My OpenLearn Profile
Personalise your OpenLearn profile, save your favourite content and get recognition for your learning
About this free course
Become an ou student, download this course, share this free course.

Start this free course now. Just create an account and sign in. Enrol and complete the course for a free statement of participation or digital badge if available.
1 Important points to consider when critically evaluating published research papers
Simple review articles (also referred to as ‘narrative’ or ‘selective’ reviews), systematic reviews and meta-analyses provide rapid overviews and ‘snapshots’ of progress made within a field, summarising a given topic or research area. They can serve as useful guides, or as current and comprehensive ‘sources’ of information, and can act as a point of reference to relevant primary research studies within a given scientific area. Narrative or systematic reviews are often used as a first step towards a more detailed investigation of a topic or a specific enquiry (a hypothesis or research question), or to establish critical awareness of a rapidly-moving field (you will be required to demonstrate this as part of an assignment, an essay or a dissertation at postgraduate level).
The majority of primary ‘empirical’ research papers essentially follow the same structure (abbreviated here as IMRAD). There is a section on Introduction, followed by the Methods, then the Results, which includes figures and tables showing data described in the paper, and a Discussion. The paper typically ends with a Conclusion, and References and Acknowledgements sections.
The Title of the paper provides a concise first impression. The Abstract follows the basic structure of the extended article. It provides an ‘accessible’ and concise summary of the aims, methods, results and conclusions. The Introduction provides useful background information and context, and typically outlines the aims and objectives of the study. The Abstract can serve as a useful summary of the paper, presenting the purpose, scope and major findings. However, simply reading the abstract alone is not a substitute for critically reading the whole article. To really get a good understanding and to be able to critically evaluate a research study, it is necessary to read on.
While most research papers follow the above format, variations do exist. For example, the results and discussion sections may be combined. In some journals the materials and methods may follow the discussion, and in two of the most widely read journals, Science and Nature, the format does vary from the above due to restrictions on the length of articles. In addition, there may be supporting documents that accompany a paper, including supplementary materials such as supporting data, tables, figures, videos and so on. There may also be commentaries or editorials associated with a topical research paper, which provide an overview or critique of the study being presented.
Box 1 Key questions to ask when appraising a research paper
- Is the study’s research question relevant?
- Does the study add anything new to current knowledge and understanding?
- Does the study test a stated hypothesis?
- Is the design of the study appropriate to the research question?
- Do the study methods address key potential sources of bias?
- Were suitable ‘controls’ included in the study?
- Were the statistical analyses appropriate and applied correctly?
- Is there a clear statement of findings?
- Does the data support the authors’ conclusions?
- Are there any conflicts of interest or ethical concerns?
There are various strategies used in reading a scientific research paper, and one of these is to start with the title and the abstract, then look at the figures and tables, and move on to the introduction, before turning to the results and discussion, and finally, interrogating the methods.
Another strategy (outlined below) is to begin with the abstract and then the discussion, take a look at the methods, and then the results section (including any relevant tables and figures), before moving on to look more closely at the discussion and, finally, the conclusion. You should choose a strategy that works best for you. However, asking the ‘right’ questions is a central feature of critical appraisal, as with any enquiry, so where should you begin? Here are some critical questions to consider when evaluating a research paper.
Look at the Abstract and then the Discussion : Are these accessible and of general relevance or are they detailed, with far-reaching conclusions? Is it clear why the study was undertaken? Why are the conclusions important? Does the study add anything new to current knowledge and understanding? The reasons why a particular study design or statistical method were chosen should also be clear from reading a research paper. What is the research question being asked? Does the study test a stated hypothesis? Is the design of the study appropriate to the research question? Have the authors considered the limitations of their study and have they discussed these in context?
Take a look at the Methods : Were there any practical difficulties that could have compromised the study or its implementation? Were these considered in the protocol? Were there any missing values and, if so, was the number of missing values too large to permit meaningful analysis? Was the number of samples (cases or participants) too small to establish meaningful significance? Do the study methods address key potential sources of bias? Were suitable ‘controls’ included in the study? If controls are missing or not appropriate to the study design, we cannot be confident that the results really show what is happening in an experiment. Were the statistical analyses appropriate and applied correctly? Do the authors point out the limitations of methods or tests used? Were the methods referenced and described in sufficient detail for others to repeat or extend the study?
Take a look at the Results section and relevant tables and figures : Is there a clear statement of findings? Were the results expected? Do they make sense? What data supports them? Do the tables and figures clearly describe the data (highlighting trends etc.)? Try to distinguish between what the data show and what the authors say they show (i.e. their interpretation).
Moving on to look in greater depth at the Discussion and Conclusion : Are the results discussed in relation to similar (previous) studies? Do the authors indulge in excessive speculation? Are limitations of the study adequately addressed? Were the objectives of the study met and the hypothesis supported or refuted (and is a clear explanation provided)? Does the data support the authors’ conclusions? Maybe there is only one experiment to support a point. More often, several different experiments or approaches combine to support a particular conclusion. A rule of thumb here is that if multiple approaches and multiple lines of evidence from different directions are presented, and all point to the same conclusion, then the conclusions are more credible. But do question all assumptions. Identify any implicit or hidden assumptions that the authors may have used when interpreting their data. Be wary of data that is mixed up with interpretation and speculation! Remember, just because it is published, does not mean that it is right.
O ther points you should consider when evaluating a research paper : Are there any financial, ethical or other conflicts of interest associated with the study, its authors and sponsors? Are there ethical concerns with the study itself? Looking at the references, consider if the authors have preferentially cited their own previous publications (i.e. needlessly), and whether the list of references are recent (ensuring that the analysis is up-to-date). Finally, from a practical perspective, you should move beyond the text of a research paper, talk to your peers about it, consult available commentaries, online links to references and other external sources to help clarify any aspects you don’t understand.
The above can be taken as a general guide to help you begin to critically evaluate a scientific research paper, but only in the broadest sense. Do bear in mind that the way that research evidence is critiqued will also differ slightly according to the type of study being appraised, whether observational or experimental, and each study will have additional aspects that would need to be evaluated separately. For criteria recommended for the evaluation of qualitative research papers, see the article by Mildred Blaxter (1996), available online. Details are in the References.
Activity 1 Critical appraisal of a scientific research paper
A critical appraisal checklist, which you can download via the link below, can act as a useful tool to help you to interrogate research papers. The checklist is divided into four sections, broadly covering:
- some general aspects
- research design and methodology
- the results
- discussion, conclusion and references.
Science perspective – critical appraisal checklist [ Tip: hold Ctrl and click a link to open it in a new tab. ( Hide tip ) ]
- Identify and obtain a research article based on a topic of your own choosing, using a search engine such as Google Scholar or PubMed (for example).
- The selection criteria for your target paper are as follows: the article must be an open access primary research paper (not a review) containing empirical data, published in the last 2–3 years, and preferably no more than 5–6 pages in length.
- Critically evaluate the research paper using the checklist provided, making notes on the key points and your overall impression.
Critical appraisal checklists are useful tools to help assess the quality of a study. Assessment of various factors, including the importance of the research question, the design and methodology of a study, the validity of the results and their usefulness (application or relevance), the legitimacy of the conclusions, and any potential conflicts of interest, are an important part of the critical appraisal process. Limitations and further improvements can then be considered.

Evaluating Research Articles
Understanding research statistics, critical appraisal, help us improve the libguide.

Imagine for a moment that you are trying to answer a clinical (PICO) question regarding one of your patients/clients. Do you know how to determine if a research study is of high quality? Can you tell if it is applicable to your question? In evidence based practice, there are many things to look for in an article that will reveal its quality and relevance. This guide is a collection of resources and activities that will help you learn how to evaluate articles efficiently and accurately.
Is health research new to you? Or perhaps you're a little out of practice with reading it? The following questions will help illuminate an article's strengths or shortcomings. Ask them of yourself as you are reading an article:
- Is the article peer reviewed?
- Are there any conflicts of interest based on the author's affiliation or the funding source of the research?
- Are the research questions or objectives clearly defined?
- Is the study a systematic review or meta analysis?
- Is the study design appropriate for the research question?
- Is the sample size justified? Do the authors explain how it is representative of the wider population?
- Do the researchers describe the setting of data collection?
- Does the paper clearly describe the measurements used?
- Did the researchers use appropriate statistical measures?
- Are the research questions or objectives answered?
- Did the researchers account for confounding factors?
- Have the researchers only drawn conclusions about the groups represented in the research?
- Have the authors declared any conflicts of interest?
If the answer to these questions about an article you are reading are mostly YESes , then it's likely that the article is of decent quality. If the answers are most NOs , then it may be a good idea to move on to another article. If the YESes and NOs are roughly even, you'll have to decide for yourself if the article is good enough quality for you. Some factors, like a poor literature review, are not as important as the researchers neglecting to describe the measurements they used. As you read more research, you'll be able to more easily identify research that is well done vs. that which is not well done.
Determining if a research study has used appropriate statistical measures is one of the most critical and difficult steps in evaluating an article. The following links are great, quick resources for helping to better understand how to use statistics in health research.

- How to read a paper: Statistics for the non-statistician. II: “Significant” relations and their pitfalls This article continues the checklist of questions that will help you to appraise the statistical validity of a paper. Greenhalgh Trisha. How to read a paper: Statistics for the non-statistician. II: “Significant” relations and their pitfalls BMJ 1997; 315 :422 *On the PMC PDF, you need to scroll past the first article to get to this one.*
- A consumer's guide to subgroup analysis The extent to which a clinician should believe and act on the results of subgroup analyses of data from randomized trials or meta-analyses is controversial. Guidelines are provided in this paper for making these decisions.
Statistical Versus Clinical Significance
When appraising studies, it's important to consider both the clinical and statistical significance of the research. This video offers a quick explanation of why.
If you have a little more time, this video explores statistical and clinical significance in more detail, including examples of how to calculate an effect size.
- Statistical vs. Clinical Significance Transcript Transcript document for the Statistical vs. Clinical Significance video.
- Effect Size Transcript Transcript document for the Effect Size video.
- P Values, Statistical Significance & Clinical Significance This handout also explains clinical and statistical significance.
- Absolute versus relative risk – making sense of media stories Understanding the difference between relative and absolute risk is essential to understanding statistical tests commonly found in research articles.
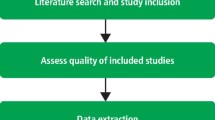
Critical appraisal is the process of systematically evaluating research using established and transparent methods. In critical appraisal, health professionals use validated checklists/worksheets as tools to guide their assessment of the research. It is a more advanced way of evaluating research than the more basic method explained above. To learn more about critical appraisal or to access critical appraisal tools, visit the websites below.

- Last Updated: Jun 11, 2024 10:26 AM
- URL: https://libguides.massgeneral.org/evaluatingarticles

- University Libraries
- Research Guides
- Critical Evaluation
Authority: Critical Evaluation
- World Views and Voices
- Understanding Peer Review
Critical Evaluation of Information Sources
After initial evaluation of a source, the next step is to go deeper. This includes a wide variety of techniques and may depend on the type of source. In the case of research, it will include evaluating the methodology used in the study and requires you to have knowledge of those discipline-specific methods. If you are just beginning your academic career or just entered a new field, you will likely need to learn more about the methodologies used in order to fully understand and evaluate this part of a study.
Lateral reading is a technique that can, and should, be applied to any source type. In the case of a research study, looking for the older articles that influenced the one you selected can give you a better understanding of the issues and context. Reading articles that were published after can give you an idea of how scholars are pushing that research to the next step. This can also help with understanding how scholars engage with each other in conversation through research and even how the academic system privileges certain voices and established authorities in the conversation. You might find articles that respond directly to studies that provide insight into evaluation and critique within that discipline.
Evaluation at this level is central to developing a better understanding of your own research question by learning from these scholarly conversations and how authority is tested.
Check out the resources below to help you with this stage of evaluation.
Scientific Method/Methodologies
Here is a general overview of how the scientific method works and how scholars evaluate their work using critical thinking. This same process is used when scholars write up their scholarly work.
The Steps of the Scientific Method
Question something that was observed, do background research to better understand, formulate a hypothesis (research question), create an experiment or method for studying the question, run the experiment and record the results, think critically about what the results mean, suggest conclusions and report back, lateral reading.
Critical Thinking
Thinking critically about the information you encounter is central to how you develop your own conclusions, judgement, and position. This analysis is what will allow you to make a valuable contribution of your own to the scholarly conversation.
- TEDEd: Dig Deeper on the 5 Tips to Improve Your Critical Thinking
- The Foundation for Critical Thinking: College and University Students
- Stanford Encyclopedia of Philosophy: Critical Thinking
Scholarship as Conversation
It sounds pretty bad if you say an article was retracted, but is it always? As with most things, it depends on the context. Someone retracting a statement made based on false information or misinformation is one thing. It happens fairly often in the case of social media--removed tweets or Instagram posts for example.
In scholarship, there are a number of reasons an article might be retracted. These range from errors in the methods used, experiment structure, data, etc. to issues of fraud or misrepresentation. Central to scholarship is the community of scholars actively participating in the scholarly conversation even after the peer review process. Careful analysis of published research by other scholars is vital to course correction.
In science research, it's a central part of the process ! An inherent part of discovery is basing conclusions on the information at hand and repeating the process to gather more information. If further research is done that provides new information and insight, that might mean an older conclusion gets corrected. Uncertainty is unsettling, but trust in the process means understanding the important role of retraction.
- << Previous: Evaluation
- Next: Resources >>
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 20 January 2009
How to critically appraise an article
- Jane M Young 1 &
- Michael J Solomon 2
Nature Clinical Practice Gastroenterology & Hepatology volume 6 , pages 82–91 ( 2009 ) Cite this article
52k Accesses
99 Citations
448 Altmetric
Metrics details
Critical appraisal is a systematic process used to identify the strengths and weaknesses of a research article in order to assess the usefulness and validity of research findings. The most important components of a critical appraisal are an evaluation of the appropriateness of the study design for the research question and a careful assessment of the key methodological features of this design. Other factors that also should be considered include the suitability of the statistical methods used and their subsequent interpretation, potential conflicts of interest and the relevance of the research to one's own practice. This Review presents a 10-step guide to critical appraisal that aims to assist clinicians to identify the most relevant high-quality studies available to guide their clinical practice.
Critical appraisal is a systematic process used to identify the strengths and weaknesses of a research article
Critical appraisal provides a basis for decisions on whether to use the results of a study in clinical practice
Different study designs are prone to various sources of systematic bias
Design-specific, critical-appraisal checklists are useful tools to help assess study quality
Assessments of other factors, including the importance of the research question, the appropriateness of statistical analysis, the legitimacy of conclusions and potential conflicts of interest are an important part of the critical appraisal process
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Making sense of the literature: an introduction to critical appraisal for the primary care practitioner
How to appraise the literature: basic principles for the busy clinician - part 2: systematic reviews and meta-analyses

How to appraise the literature: basic principles for the busy clinician - part 1: randomised controlled trials
Druss BG and Marcus SC (2005) Growth and decentralisation of the medical literature: implications for evidence-based medicine. J Med Libr Assoc 93 : 499–501
PubMed PubMed Central Google Scholar
Glasziou PP (2008) Information overload: what's behind it, what's beyond it? Med J Aust 189 : 84–85
PubMed Google Scholar
Last JE (Ed.; 2001) A Dictionary of Epidemiology (4th Edn). New York: Oxford University Press
Google Scholar
Sackett DL et al . (2000). Evidence-based Medicine. How to Practice and Teach EBM . London: Churchill Livingstone
Guyatt G and Rennie D (Eds; 2002). Users' Guides to the Medical Literature: a Manual for Evidence-based Clinical Practice . Chicago: American Medical Association
Greenhalgh T (2000) How to Read a Paper: the Basics of Evidence-based Medicine . London: Blackwell Medicine Books
MacAuley D (1994) READER: an acronym to aid critical reading by general practitioners. Br J Gen Pract 44 : 83–85
CAS PubMed PubMed Central Google Scholar
Hill A and Spittlehouse C (2001) What is critical appraisal. Evidence-based Medicine 3 : 1–8 [ http://www.evidence-based-medicine.co.uk ] (accessed 25 November 2008)
Public Health Resource Unit (2008) Critical Appraisal Skills Programme (CASP) . [ http://www.phru.nhs.uk/Pages/PHD/CASP.htm ] (accessed 8 August 2008)
National Health and Medical Research Council (2000) How to Review the Evidence: Systematic Identification and Review of the Scientific Literature . Canberra: NHMRC
Elwood JM (1998) Critical Appraisal of Epidemiological Studies and Clinical Trials (2nd Edn). Oxford: Oxford University Press
Agency for Healthcare Research and Quality (2002) Systems to rate the strength of scientific evidence? Evidence Report/Technology Assessment No 47, Publication No 02-E019 Rockville: Agency for Healthcare Research and Quality
Crombie IK (1996) The Pocket Guide to Critical Appraisal: a Handbook for Health Care Professionals . London: Blackwell Medicine Publishing Group
Heller RF et al . (2008) Critical appraisal for public health: a new checklist. Public Health 122 : 92–98
Article Google Scholar
MacAuley D et al . (1998) Randomised controlled trial of the READER method of critical appraisal in general practice. BMJ 316 : 1134–37
Article CAS Google Scholar
Parkes J et al . Teaching critical appraisal skills in health care settings (Review). Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No.: cd001270. 10.1002/14651858.cd001270
Mays N and Pope C (2000) Assessing quality in qualitative research. BMJ 320 : 50–52
Hawking SW (2003) On the Shoulders of Giants: the Great Works of Physics and Astronomy . Philadelphia, PN: Penguin
National Health and Medical Research Council (1999) A Guide to the Development, Implementation and Evaluation of Clinical Practice Guidelines . Canberra: National Health and Medical Research Council
US Preventive Services Taskforce (1996) Guide to clinical preventive services (2nd Edn). Baltimore, MD: Williams & Wilkins
Solomon MJ and McLeod RS (1995) Should we be performing more randomized controlled trials evaluating surgical operations? Surgery 118 : 456–467
Rothman KJ (2002) Epidemiology: an Introduction . Oxford: Oxford University Press
Young JM and Solomon MJ (2003) Improving the evidence-base in surgery: sources of bias in surgical studies. ANZ J Surg 73 : 504–506
Margitic SE et al . (1995) Lessons learned from a prospective meta-analysis. J Am Geriatr Soc 43 : 435–439
Shea B et al . (2001) Assessing the quality of reports of systematic reviews: the QUORUM statement compared to other tools. In Systematic Reviews in Health Care: Meta-analysis in Context 2nd Edition, 122–139 (Eds Egger M. et al .) London: BMJ Books
Chapter Google Scholar
Easterbrook PH et al . (1991) Publication bias in clinical research. Lancet 337 : 867–872
Begg CB and Berlin JA (1989) Publication bias and dissemination of clinical research. J Natl Cancer Inst 81 : 107–115
Moher D et al . (2000) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUORUM statement. Br J Surg 87 : 1448–1454
Shea BJ et al . (2007) Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 7 : 10 [10.1186/1471-2288-7-10]
Stroup DF et al . (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283 : 2008–2012
Young JM and Solomon MJ (2003) Improving the evidence-base in surgery: evaluating surgical effectiveness. ANZ J Surg 73 : 507–510
Schulz KF (1995) Subverting randomization in controlled trials. JAMA 274 : 1456–1458
Schulz KF et al . (1995) Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273 : 408–412
Moher D et al . (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Medical Research Methodology 1 : 2 [ http://www.biomedcentral.com/ 1471-2288/1/2 ] (accessed 25 November 2008)
Rochon PA et al . (2005) Reader's guide to critical appraisal of cohort studies: 1. Role and design. BMJ 330 : 895–897
Mamdani M et al . (2005) Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 330 : 960–962
Normand S et al . (2005) Reader's guide to critical appraisal of cohort studies: 3. Analytical strategies to reduce confounding. BMJ 330 : 1021–1023
von Elm E et al . (2007) Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335 : 806–808
Sutton-Tyrrell K (1991) Assessing bias in case-control studies: proper selection of cases and controls. Stroke 22 : 938–942
Knottnerus J (2003) Assessment of the accuracy of diagnostic tests: the cross-sectional study. J Clin Epidemiol 56 : 1118–1128
Furukawa TA and Guyatt GH (2006) Sources of bias in diagnostic accuracy studies and the diagnostic process. CMAJ 174 : 481–482
Bossyut PM et al . (2003)The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 138 : W1–W12
STARD statement (Standards for the Reporting of Diagnostic Accuracy Studies). [ http://www.stard-statement.org/ ] (accessed 10 September 2008)
Raftery J (1998) Economic evaluation: an introduction. BMJ 316 : 1013–1014
Palmer S et al . (1999) Economics notes: types of economic evaluation. BMJ 318 : 1349
Russ S et al . (1999) Barriers to participation in randomized controlled trials: a systematic review. J Clin Epidemiol 52 : 1143–1156
Tinmouth JM et al . (2004) Are claims of equivalency in digestive diseases trials supported by the evidence? Gastroentrology 126 : 1700–1710
Kaul S and Diamond GA (2006) Good enough: a primer on the analysis and interpretation of noninferiority trials. Ann Intern Med 145 : 62–69
Piaggio G et al . (2006) Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 295 : 1152–1160
Heritier SR et al . (2007) Inclusion of patients in clinical trial analysis: the intention to treat principle. In Interpreting and Reporting Clinical Trials: a Guide to the CONSORT Statement and the Principles of Randomized Controlled Trials , 92–98 (Eds Keech A. et al .) Strawberry Hills, NSW: Australian Medical Publishing Company
National Health and Medical Research Council (2007) National Statement on Ethical Conduct in Human Research 89–90 Canberra: NHMRC
Lo B et al . (2000) Conflict-of-interest policies for investigators in clinical trials. N Engl J Med 343 : 1616–1620
Kim SYH et al . (2004) Potential research participants' views regarding researcher and institutional financial conflicts of interests. J Med Ethics 30 : 73–79
Komesaroff PA and Kerridge IH (2002) Ethical issues concerning the relationships between medical practitioners and the pharmaceutical industry. Med J Aust 176 : 118–121
Little M (1999) Research, ethics and conflicts of interest. J Med Ethics 25 : 259–262
Lemmens T and Singer PA (1998) Bioethics for clinicians: 17. Conflict of interest in research, education and patient care. CMAJ 159 : 960–965
Download references
Author information
Authors and affiliations.
JM Young is an Associate Professor of Public Health and the Executive Director of the Surgical Outcomes Research Centre at the University of Sydney and Sydney South-West Area Health Service, Sydney,
Jane M Young
MJ Solomon is Head of the Surgical Outcomes Research Centre and Director of Colorectal Research at the University of Sydney and Sydney South-West Area Health Service, Sydney, Australia.,
Michael J Solomon
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jane M Young .
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Young, J., Solomon, M. How to critically appraise an article. Nat Rev Gastroenterol Hepatol 6 , 82–91 (2009). https://doi.org/10.1038/ncpgasthep1331
Download citation
Received : 10 August 2008
Accepted : 03 November 2008
Published : 20 January 2009
Issue Date : February 2009
DOI : https://doi.org/10.1038/ncpgasthep1331
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Emergency physicians’ perceptions of critical appraisal skills: a qualitative study.
- Sumintra Wood
- Jacqueline Paulis
- Angela Chen
BMC Medical Education (2022)
An integrative review on individual determinants of enrolment in National Health Insurance Scheme among older adults in Ghana
- Anthony Kwame Morgan
- Anthony Acquah Mensah
BMC Primary Care (2022)
Autopsy findings of COVID-19 in children: a systematic review and meta-analysis
- Anju Khairwa
- Kana Ram Jat
Forensic Science, Medicine and Pathology (2022)
The use of a modified Delphi technique to develop a critical appraisal tool for clinical pharmacokinetic studies
- Alaa Bahaa Eldeen Soliman
- Shane Ashley Pawluk
- Ousama Rachid
International Journal of Clinical Pharmacy (2022)
Critical Appraisal: Analysis of a Prospective Comparative Study Published in IJS
- Ramakrishna Ramakrishna HK
- Swarnalatha MC
Indian Journal of Surgery (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- University of Oregon Libraries
- Research Guides
How to Write a Literature Review
- 5. Critically Analyze and Evaluate
- Literature Reviews: A Recap
- Reading Journal Articles
- Does it Describe a Literature Review?
- 1. Identify the Question
- 2. Review Discipline Styles
- Searching Article Databases
- Finding Full-Text of an Article
- Citation Chaining
- When to Stop Searching
- 4. Manage Your References
Critically analyze and evaluate
Tip: read and annotate pdfs.
- 6. Synthesize
- 7. Write a Literature Review
Ask yourself questions like these about each book or article you include:
- What is the research question?
- What is the primary methodology used?
- How was the data gathered?
- How is the data presented?
- What are the main conclusions?
- Are these conclusions reasonable?
- What theories are used to support the researcher's conclusions?
Take notes on the articles as you read them and identify any themes or concepts that may apply to your research question.
This sample template (below) may also be useful for critically reading and organizing your articles. Or you can use this online form and email yourself a copy .
- Sample Template for Critical Analysis of the Literature
Opening an article in PDF format in Acrobat Reader will allow you to use "sticky notes" and "highlighting" to make notes on the article without printing it out. Make sure to save the edited file so you don't lose your notes!
Some Citation Managers like Mendeley also have highlighting and annotation features.Here's a screen capture of a pdf in Mendeley with highlighting, notes, and various colors:

Screen capture from a UO Librarian's Mendeley Desktop app
- Learn more about citation management software in the previous step: 4. Manage Your References
- << Previous: 4. Manage Your References
- Next: 6. Synthesize >>
- Last Updated: Aug 12, 2024 11:48 AM
- URL: https://researchguides.uoregon.edu/litreview
Contact Us Library Accessibility UO Libraries Privacy Notices and Procedures

1501 Kincaid Street Eugene, OR 97403 P: 541-346-3053 F: 541-346-3485
- Visit us on Facebook
- Visit us on Twitter
- Visit us on Youtube
- Visit us on Instagram
- Report a Concern
- Nondiscrimination and Title IX
- Accessibility
- Privacy Policy
- Find People
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Critically appraising...
Critically appraising qualitative research
- Related content
- Peer review
- Ayelet Kuper , assistant professor 1 ,
- Lorelei Lingard , associate professor 2 ,
- Wendy Levinson , Sir John and Lady Eaton professor and chair 3
- 1 Department of Medicine, Sunnybrook Health Sciences Centre, and Wilson Centre for Research in Education, University of Toronto, 2075 Bayview Avenue, Room HG 08, Toronto, ON, Canada M4N 3M5
- 2 Department of Paediatrics and Wilson Centre for Research in Education, University of Toronto and SickKids Learning Institute; BMO Financial Group Professor in Health Professions Education Research, University Health Network, 200 Elizabeth Street, Eaton South 1-565, Toronto
- 3 Department of Medicine, Sunnybrook Health Sciences Centre
- Correspondence to: A Kuper ayelet94{at}post.harvard.edu
Six key questions will help readers to assess qualitative research
Summary points
Appraising qualitative research is different from appraising quantitative research
Qualitative research papers should show appropriate sampling, data collection, and data analysis
Transferability of qualitative research depends on context and may be enhanced by using theory
Ethics in qualitative research goes beyond review boards’ requirements to involve complex issues of confidentiality, reflexivity, and power
Over the past decade, readers of medical journals have gained skills in critically appraising studies to determine whether the results can be trusted and applied to their own practice settings. Criteria have been designed to assess studies that use quantitative methods, and these are now in common use.
In this article we offer guidance for readers on how to assess a study that uses qualitative research methods by providing six key questions to ask when reading qualitative research (box 1). However, the thorough assessment of qualitative research is an interpretive act and requires informed reflective thought rather than the simple application of a scoring system.

Box 1 Key questions to ask when reading qualitative research studies
Was the sample used in the study appropriate to its research question.
Were the data collected appropriately?
Were the data analysed appropriately?
Can I transfer the results of this study to my own setting?
Does the study adequately address potential ethical issues, including reflexivity?
Overall: is what the researchers did clear?
One of the critical decisions in a qualitative study is whom or what to include in the sample—whom to interview, whom to observe, what texts to analyse. An understanding that qualitative research is based in experience and in the construction of meaning, combined with the specific research question, should guide the sampling process. For example, a study of the experience of survivors of domestic violence that examined their reasons for not seeking help from healthcare providers might focus on interviewing a …
Log in using your username and password
BMA Member Log In
If you have a subscription to The BMJ, log in:
- Need to activate
- Log in via institution
- Log in via OpenAthens
Log in through your institution
Subscribe from £184 *.
Subscribe and get access to all BMJ articles, and much more.
* For online subscription
Access this article for 1 day for: £50 / $60/ €56 ( excludes VAT )
You can download a PDF version for your personal record.
Buy this article
Critically Analyzing Information Sources: Critical Appraisal and Analysis
- Critical Appraisal and Analysis
Initial Appraisal : Reviewing the source
- What are the author's credentials--institutional affiliation (where he or she works), educational background, past writings, or experience? Is the book or article written on a topic in the author's area of expertise? You can use the various Who's Who publications for the U.S. and other countries and for specific subjects and the biographical information located in the publication itself to help determine the author's affiliation and credentials.
- Has your instructor mentioned this author? Have you seen the author's name cited in other sources or bibliographies? Respected authors are cited frequently by other scholars. For this reason, always note those names that appear in many different sources.
- Is the author associated with a reputable institution or organization? What are the basic values or goals of the organization or institution?
B. Date of Publication
- When was the source published? This date is often located on the face of the title page below the name of the publisher. If it is not there, look for the copyright date on the reverse of the title page. On Web pages, the date of the last revision is usually at the bottom of the home page, sometimes every page.
- Is the source current or out-of-date for your topic? Topic areas of continuing and rapid development, such as the sciences, demand more current information. On the other hand, topics in the humanities often require material that was written many years ago. At the other extreme, some news sources on the Web now note the hour and minute that articles are posted on their site.
C. Edition or Revision
Is this a first edition of this publication or not? Further editions indicate a source has been revised and updated to reflect changes in knowledge, include omissions, and harmonize with its intended reader's needs. Also, many printings or editions may indicate that the work has become a standard source in the area and is reliable. If you are using a Web source, do the pages indicate revision dates?
D. Publisher
Note the publisher. If the source is published by a university press, it is likely to be scholarly. Although the fact that the publisher is reputable does not necessarily guarantee quality, it does show that the publisher may have high regard for the source being published.
E. Title of Journal
Is this a scholarly or a popular journal? This distinction is important because it indicates different levels of complexity in conveying ideas. If you need help in determining the type of journal, see Distinguishing Scholarly from Non-Scholarly Periodicals . Or you may wish to check your journal title in the latest edition of Katz's Magazines for Libraries (Olin Reference Z 6941 .K21, shelved at the reference desk) for a brief evaluative description.
Critical Analysis of the Content
Having made an initial appraisal, you should now examine the body of the source. Read the preface to determine the author's intentions for the book. Scan the table of contents and the index to get a broad overview of the material it covers. Note whether bibliographies are included. Read the chapters that specifically address your topic. Reading the article abstract and scanning the table of contents of a journal or magazine issue is also useful. As with books, the presence and quality of a bibliography at the end of the article may reflect the care with which the authors have prepared their work.
A. Intended Audience
What type of audience is the author addressing? Is the publication aimed at a specialized or a general audience? Is this source too elementary, too technical, too advanced, or just right for your needs?
B. Objective Reasoning
- Is the information covered fact, opinion, or propaganda? It is not always easy to separate fact from opinion. Facts can usually be verified; opinions, though they may be based on factual information, evolve from the interpretation of facts. Skilled writers can make you think their interpretations are facts.
- Does the information appear to be valid and well-researched, or is it questionable and unsupported by evidence? Assumptions should be reasonable. Note errors or omissions.
- Are the ideas and arguments advanced more or less in line with other works you have read on the same topic? The more radically an author departs from the views of others in the same field, the more carefully and critically you should scrutinize his or her ideas.
- Is the author's point of view objective and impartial? Is the language free of emotion-arousing words and bias?
C. Coverage
- Does the work update other sources, substantiate other materials you have read, or add new information? Does it extensively or marginally cover your topic? You should explore enough sources to obtain a variety of viewpoints.
- Is the material primary or secondary in nature? Primary sources are the raw material of the research process. Secondary sources are based on primary sources. For example, if you were researching Konrad Adenauer's role in rebuilding West Germany after World War II, Adenauer's own writings would be one of many primary sources available on this topic. Others might include relevant government documents and contemporary German newspaper articles. Scholars use this primary material to help generate historical interpretations--a secondary source. Books, encyclopedia articles, and scholarly journal articles about Adenauer's role are considered secondary sources. In the sciences, journal articles and conference proceedings written by experimenters reporting the results of their research are primary documents. Choose both primary and secondary sources when you have the opportunity.
D. Writing Style
Is the publication organized logically? Are the main points clearly presented? Do you find the text easy to read, or is it stilted or choppy? Is the author's argument repetitive?
E. Evaluative Reviews
- Locate critical reviews of books in a reviewing source , such as the Articles & Full Text , Book Review Index , Book Review Digest, and ProQuest Research Library . Is the review positive? Is the book under review considered a valuable contribution to the field? Does the reviewer mention other books that might be better? If so, locate these sources for more information on your topic.
- Do the various reviewers agree on the value or attributes of the book or has it aroused controversy among the critics?
- For Web sites, consider consulting this evaluation source from UC Berkeley .
Permissions Information
If you wish to use or adapt any or all of the content of this Guide go to Cornell Library's Research Guides Use Conditions to review our use permissions and our Creative Commons license.
- Next: Tips >>
- Last Updated: Jun 21, 2024 3:08 PM
- URL: https://guides.library.cornell.edu/critically_analyzing
JEPS Bulletin
The Official Blog of the Journal of European Psychology Students
How to critically evaluate the quality of a research article?

So what’s the criteria to determine whether a result can be trusted? As it is taught in the first classes in psychology, errors may emerge from any phase of the research process. Therefore, it all boils down to how the research has been conducted and the results presented.
Meltzoff (2007) emphasizes the key issues that can produce flawed results and interpretations and should therefore be carefully considered when reading articles. Here is a reminder on what to bear in mind when reading a research article:
Research question The research must be clear in informing the reader of its aims. Terms should be clearly defined, even more so if they’re new or used in specific non-spread ways. You as a reader should pay particular attention should to errors in logic, especially those regarding causation, relationship or association.
Sample To provide trustworthy conclusions, a sample needs to be representative and adequate. Representativeness depends on the method of selection as well as the assignment. For example, random assignment has its advantages in front of systematic assignment in establishing group equivalence. The sample can be biased when researchers used volunteers or selective attrition. The adequate sample size can be determined by employing power analysis.
Control of confounding variables Extraneous variation can influence research findings, therefore methods to control relevant confounding variables should be applied.
Research designs The research design should be suitable to answer the research question. Readers should distinguish true experimental designs with random assignment from pre-experimental research designs.
Criteria and criteria measures The criteria measures must demonstrate reliability and validity for both, the independent and dependent variable.
Data analysis Appropriate statistical tests should be applied for the type of data obtained, and assumptions for their use met. Post hoc tests should be applied when multiple comparisons are performed. Tables and figures should be clearly labelled. Ideally, effect sizes shou
ld be included throughout giving a clear indication of the variables’ impact.
Discussion and conclusions Does the study allow generalization? Also, limitations of the study should be mentioned. The discussion and conclusions should be consistent with the study’s results. It’s a common mistake to emphasizing the results that are in accordance with the researcher’s expectations while not focusing on the ones that are not. Do the authors of the article you hold in hand do the same?
Ethics Last but not least, ere the ethical standards met? For more information, refer to the APA’s Ethical Principles of Psychologists and Code of Conduct (2010).
References American Psychological Association (2010, June 1). American Psychological Association Ethical Principles of Psychologists and Code of Conduct . Retrieved July 28, 2011 from http://www.apa.org/ethics/code/index.aspx
Meltzoff, J. (2007). Critical Thinking About Research. Washington, DC: American Psychological Association.
Edited by: Maris Vainre
Zorana Zupan
Share this:
Related posts:.
- How to critically evaluate internet-based sources?
- How to write a good literature review article?
- How to Read and Get the Most Out of a Journal Article
- Can you find an article in 5 sec? The world of DOIs
How to do library research: Critically Evaluate Information
- Refine your Topic
- Background Information & Facts
- Develop Keywords
- Find Books & eBooks
- Quick Article How-To
- Detailed Article How-To
- Evaluating Web Sources
- How to Create an Annotated Bibliography
Critically Evaluate Information Links
Primary and secondary sources, common information source formats.
Critically evaluating sources using the CRAP Method
Uses of different types of sources
Evaluating web sources
What is a primary source?
A primary source is original information about an event, person, object, product, or work of art in which there has been no comment or analysis about it; a primary source presents information in its original form, neither interpreted nor condensed nor evaluated by other writers.
Think about it in your own context. What primary sources have you created to tell the story of you? (For example, maybe a tweet, a picture of something you enjoy doing, or your student ID card).
What is a secondary source?
A secondary source is an interpretation of a primary source. For example, if your professor asks you to view a film and then write a paragraph about it's meaning to you, the film is the primary source, while your analysis of the film is the secondary source.
What do we mean by “sources”?
|
|
|
|
|
Available in print or as ebooks from your academic library; these are often "monographs" or books that focus closely on a research topic. |
SU Libraries’ Quick Search: See: | Books contain background or historical facts and can be used to frame an analysis or argument. Locate only the information you need in books by skimming chapter titles or by finding keywords in the Index located at the end. |
|
Articles are published within newspapers, magazines, and scholarly journals on a daily, weekly, monthly, or quarterly basis. | Go to SU Libraries home page, Find See: | Because they are published more frequently than books, articles can contain the most up-to-date information on a topic. Information found in scholarly journal articles can be used to support specific aspects of your analysis or argument. |
|
Any information made public on the open web. | Search engines on the open web such as Google See Critically Evaluating Sources above. | Although you should generally begin research using the SU Libraries’ resources, you may need to find additional sources using popular search engines like Google. Information from the open web can be created by anyone, so it’s important to any sources you might consider for use in academic work. |
Critically Evaluate Information
Critically Evaluating Sources ( what do we mean by "sources"? )
For college-level research, you'll want to consider using only the highest-quality information sources that you can find. Between the internet and SU’s library, the “ best ” information can depend on the assignment. Here are some ways to determine the best information sources to lend support to your own research.
Use the C.R.A.P. method to evaluate information that you may consider using: (Currency, Relevance, Author expertise, and Purpose)
|
|
|
|
|
|
| Check your research assignment directions. Some majors/disciplines require students to use only the most current scholarship in the field, while current scholarship is not important for others. In the sciences, the most up-to-date research is often considered the most valuable. For example, a research study about new technology from ten years ago might be less important than a study conducted last year. |
|
|
| Every single source that shows up in your work should be there for a reason and your reader should not have to guess what that reason is. Every source that you include in your research work should be there to strengthen your idea or argument. |
|
| Consider an author’s credentials before you commit to using the information that they have made public. Experts often have advanced academic degrees, institutional affiliations, and long track records of publishing articles and books containing earlier research. Find author information: – About the Author page – the article citation or the article itself. – See Evaluating Web Sources below | |
|
| An author’s bias can affect the validity or even the truthfulness of the information they have made public, treating one side of an issue more favorably than another. For example, an Apple website will claim its iPhone is the best in the world. Motorola will claim its Droid is the best. They both make this claim because they want to sell their phones. Consumer Reports, an un-biased group who rates products, will study both phones and determine superiority based on what consumers desire and they make that viewpoint known to readers. Using biased sources to support your ideas makes it easy for your audience to challenge the validity of your argument or analysis.
|

Bean, J. C. (2011). Designing and sequencing assignments to teach undergraduate research. In Engaging ideas: The professor's guide to integrating writing, critical thinking, and active learning in the classroom (2nd ed., p. 239). San Francisco: Jossey-Bass.
This information is an adaptation of Evaluating Information – Applying the CRAAP Test created by Meriam Library, CSU, Chico
- << Previous: Detailed Article How-To
- Next: Evaluating Web Sources >>
- Last Updated: Jul 8, 2021 12:52 PM
- URL: https://libraryguides.salisbury.edu/howtodolibrary

- Asbury University
- Research Help
- Critical Evaluation of Sources
- Why Evaluate?
Critical Evaluation of Sources: Why Evaluate?
- Introduction and Criteria
- Web and Articles
While most of us realize that we can’t trust all the information we see or read, we don’t always spend a lot of time considering how we actually make decisions about what to trust. Whether we’re watching the news, reading a friend’s blog, researching a health condition, or using information in some other way, we generally draw on our own values and life experiences to make relatively quick judgments about the validity of the information we are exposed to or seek out. Sometimes we don’t even consider the fact that we made a judgment about what to trust in the first place. We’re simply on autopilot.
Although the amount of deep thinking we need to put into evaluating the validity of an information source can vary depending on the significance of the situation, we ultimately make better decisions and construct more convincing arguments when we have a strong understanding of the quality of the information we’re using (or not using). This is especially true in an academic context, where our ability to create knowledge and meaning depends on our ability to analyze and interpret information with precision.
To evaluate information, then, is to analyze information from a critical perspective. The evaluative process requires us to step back and carefully consider the sources we use and how we use them, to not rush to judgment but to think through the content of the articles we’re reading or the online search results we’re browsing. We also need to consider the relationships among different sources and how they work together to form “conversations” around certain topics or issues. A “conversation” in this sense refers to the diverse perspectives and arguments surrounding a particular research question (or set of questions).
The questions in this guide can help you think through the evaluation of information sources. Keep in mind that evaluation is not simply about determining whether a source is “reliable” or “not reliable.” It’s rarely that easy or straightforward. Instead, it’s more useful to consider the degree to which a source is reliable for a given purpose. The primary goal of evaluation is to understand the significance and value of a source in relation to other sources and your own thinking on a topic.
Note that some evaluative questions will be more important than others depending on your needs as a researcher. Figuring out which questions are important to ask in a given situation is part of the research process. Also note that your evaluation of a source may evolve over time. For instance, a source that seems very useful early on may prove less useful as your project develops. Likewise, a source that seems insignificant at the beginning of a project may turn out to be your most significant source later in the research process.
From: http://louisville.libguides.com/evaluation
This evaluation process is really no different than the process people use everyday as they acquire all types of information from a neighbor, a friend, a newspaper, a television broadcast, or a bulletin board flyer.
All of this happens so automatically, you don't even realize you're doing it. While you should evaluate all of information sources (books, periodical articles, etc.) before using them in your research, it is most vital that you evaluate the information you find on the Internet. Every book and article published (even those available in Internet-based databases) goes through some sort of evaluation process, but Web pages go through no such pre-publication evaluation.
The number of resources available via the Internet is immense. Companies, organizations, educational institutions, communities and individual people all serve as information providers for the Internet community. Savvy members of the Internet community are aware that there are few, if any, quality controls for the information that is made available. Accurate and reliable data may share the computer screen with data that is inaccurate, unreliable, or even purposely false. In addition, the differences between the two types of data may be imperceptible, especially for someone who is not an expert in the topic area. Because the Internet is not the responsibility of any one organization or institution, it seems unlikely that any universal quality control will be established in the near future. In view of this, members of the Internet community must prepare themselves to be critically skilled consumers of the information they find.
Hoaxes, Fallacies, Propaganda - OH MY!
Types of hoaxes with examples - http://virtualchase.justia.com/hoaxes-and-other-bad-information
Listing of Types of Fallacies - http://www.nizkor.org/features/fallacies/
Propaganda - http://guides.library.jhu.edu/content.php?pid=198142&sid=1657614
Critical Thinkers
Characteristics of Critical Thinkers: http://www.mhhe.com/socscience/philosophy/reichenbach/m1_chap02studyguide.html
Defining Critical Thinking
A Source: http://www.criticalthinking.org/pages/defining-critical-thinking/766
Which states:
The Problem Everyone thinks; it is our nature to do so. But much of our thinking, left to itself, is biased, distorted, partial, uninformed or down-right prejudiced. Yet the quality of our life and that of what we produce, make, or build depends precisely on the quality of our thought. Shoddy thinking is costly, both in money and in quality of life. Excellence in thought, however, must be systematically cultivated.
A Definition Critical thinking is that mode of thinking - about any subject, content, or problem - in which the thinker improves the quality of his or her thinking by skillfully taking charge of the structures inherent in thinking and imposing intellectual standards upon them.
The Result A well cultivated critical thinker:
- raises vital questions and problems, formulating them clearly and precisely;
- gathers and assesses relevant information, using abstract ideas to interpret it effectively comes to well-reasoned conclusions and solutions, testing them against relevant criteria and standards;
- thinks openmindedly within alternative systems of thought, recognizing and assessing, as need be, their assumptions, implications, and practical consequences; and
- communicates effectively with others in figuring out solutions to complex problems.
Critical thinking is, in short, self-directed, self-disciplined, self-monitored, and self-corrective thinking. It presupposes assent to rigorous standards of excellence and mindful command of their use. It entails effective communication and problem solving abilities and a commitment to overcome our native egocentrism and sociocentrism.
Critical Thinking
Critical Thinking and introduction to the basic skills by William Hughes 1992 Broadview Press Ltd. Lewiston, NY Isbn 1-921149-73-2 The primary focus of critical thinking skills is on determining whether arguments are sound, i.e. whether they have true premises and logical strength.But determining the soundness of arguments is not a simple matter, for three reasons.First, before we can assess an argument we must determine its precise meaning. Second, determining the truth or falsity of statements is often a difficult task. Third, assessing argument is complex because there are several different types of inferences and each type requires a different kind of assessment. There three types of skills—
interpretive skills, verification skills, and reason skills—constitutes what are usually referred to as critical thinking skills. … mastering critical thinking skills is also a matter of intellectual self-respect. We all have the capacity to learn how to distinguish good arguments from bad ones and to work out for ourselves what we ought and ought not to believe, and it diminishes us as persons if we let others do our thinking for us. If we are not prepared to think for ourselves, and to make the effort to learn how do this well, we will always remain slaves to the ideas and values of others and to our own ignorance. P. 11 Argumentation and Debate Critical thinking for reasoned decision Making Austin J. Freeley and David L. Steinberg 10th edition 2000 Wadsworth/Thomson Learning Belmont, CA Isbn 0-534-46115-2
Critical thinking: the ability to analyze, criticize, and advocate ideas; to reason inductively and deductively; and to reach factual or judgmental conclusions based on sound inferences drawn from unambiguous statements of knowledge or belief. P. 458 Author: Theresa Rienzo, Reference Librarian,James Edward Tobin Library, Molloy College 1000 Hempstead Ave. Rockville Centre, NY 11571
Copied from: http://molloy.libguides.com/criticalthinking
A critical thinking model with elements: http://www.criticalthinking.org/ctmodel/logic-model1.htm
From: http://www.edpsycinteractive.org/topics/cogsys/critthnk.html
- << Previous: Introduction and Criteria
- Next: Analysis >>
- Last Updated: Feb 26, 2019 2:33 PM
- URL: https://asbury.libguides.com/criteval
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Oxidative stress and cataract formation: evaluating the efficacy of antioxidant therapies.

1. Introduction
2. the structure and physiology of the lens, physiology of the lens and its redox regulatory mechanisms, 3. ocular damages induced by reactive oxygen species: cataracto-genesis, 3.1. antioxidative systems, 3.2. protein aggregation, cross-linking, and light-scattering, 3.3. lipid peroxidation and loss of membrane integrity, 4. antioxidant strategies for the prevention and management of cataracts, 4.1. dietary nutrients and supplements, 4.1.1. vitamins c and e, 4.1.2. lutein and zeaxanthin, 4.2. potential pharmacological agents with antioxidative properties for cataract prevention and treatment, 4.2.1. n-acetyl-carnosine, 4.2.2. n-acetylcysteine amide, 4.2.3. resveratrol, 4.2.4. baicalein, 4.2.5. metformin, 4.3. nanotechnology-based drug delivery systems for cataract prevention and treatment, 4.3.1. n-acetylcarnosine nanoparticles, 4.3.2. resveratrol nanoparticles and nanovesicles, 4.3.3. baicalin, 4.3.4. cerium oxide, 4.4. gene therapy for cataract prevention and treatment, 4.4.1. suicide gene therapy, 4.4.2. rna interference, 4.4.3. crispr-cas9, 5. challenges and limitations of antioxidants and novel therapeutic approaches, 6. recommendations and future directions, 7. conclusions, author contributions, conflicts of interest.
- Pesudovs, K.; Lansingh, V.C.; Kempen, J.H.; Tapply, I.; Fernandes, A.G.; Cicinelli, M.V.; Arrigo, A.; Leveziel, N.; Briant, P.S.; Vos, T.; et al. Global estimates on the number of people blind or visually impaired by cataract: A meta-analysis from 2000 to 2020. Eye 2024 , 38 , 2156–2172. [ Google Scholar ] [ CrossRef ]
- Fang, R.; Yu, Y.F.; Li, E.J.; Lv, N.X.; Liu, Z.C.; Zhou, H.G.; Song, X.D. Global, regional, national burden and gender disparity of cataract: Findings from the global burden of disease study 2019. BMC Public Health 2022 , 22 , 2068. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Marques, A.P.; Ramke, J.; Cairns, J.; Butt, T.; Zhang, J.H.; Jones, I.; Jovic, M.; Nandakumar, A.; Faal, H.; Taylor, H.; et al. The economics of vision impairment and its leading causes: A systematic review. eClinicalMedicine 2022 , 46 , 101354. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Buscho, S.E.; Sharifi, A.; Cayenne, S.; Zhang, Y.; Merkley, K.H.; Gupta, P.K. Racial Disparities in Cataract Surgery Timeline and Intraocular Lens Selection: A Retrospective Study. Transl. Vis. Sci. Technol. 2023 , 12 , 20. [ Google Scholar ] [ CrossRef ]
- Lee, C.S.; Su, G.L.; Baughman, D.M.; Wu, Y.; Lee, A.Y. Disparities in delivery of ophthalmic care; An exploration of public Medicare data. PLoS ONE 2017 , 12 , e0182598. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fang, Z.; Chen, X.Y.; Lou, L.X.; Yao, K. Socio-economic disparity in visual impairment from cataract. Int. J. Ophthalmol. 2021 , 14 , 1310–1314. [ Google Scholar ] [ CrossRef ]
- Nizami, A.A.; Gulani, A.C. Cataract. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2024. [ Google Scholar ]
- Ho, M.C.; Peng, Y.J.; Chen, S.J.; Chiou, S.H. Senile cataracts and oxidative stress. J. Clin. Gerontol. Geriatr. 2010 , 1 , 17–21. [ Google Scholar ] [ CrossRef ]
- Shui, Y.-B.; Fu, J.-J.; Garcia, C.; Dattilo, L.K.; Rajagopal, R.; McMillan, S.; Mak, G.; Holekamp, N.M.; Lewis, A.; Beebe, D.C. Oxygen Distribution in the Rabbit Eye and Oxygen Consumption by the Lens. Investig. Opthalmology Vis. Sci. 2006 , 47 , 1571–1580. [ Google Scholar ] [ CrossRef ]
- Wu, S.Y.; Leske, M.C. Antioxidants and cataract formation: A summary review. Int. Ophthalmol. Clin. 2000 , 40 , 71–81. [ Google Scholar ] [ CrossRef ]
- Danysh, B.P.; Duncan, M.K. The lens capsule. Exp. Eye Res. 2009 , 88 , 151–164. [ Google Scholar ] [ CrossRef ]
- Griep, A.E. Cell cycle regulation in the developing lens. Semin. Cell Dev. Biol. 2006 , 17 , 686–697. [ Google Scholar ] [ CrossRef ]
- Pan, Y.; Liu, Z.; Zhang, H. Research progress of lens zonules. Adv. Ophthalmol. Pract. Res. 2023 , 3 , 80–85. [ Google Scholar ] [ CrossRef ]
- Zhang, X.; Liu, B.; Lal, K.; Liu, H.; Tran, M.; Zhou, M.; Ezugwu, C.; Gao, X.; Dang, T.; Au, M.-L.; et al. Antioxidant System and Endoplasmic Reticulum Stress in Cataracts. Cell. Mol. Neurobiol. 2023 , 43 , 4041–4058. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nandi, S.K.; Nahomi, R.B.; Rankenberg, J.; Glomb, M.A.; Nagaraj, R.H. Glycation-mediated inter-protein cross-linking is promoted by chaperone-client complexes of α-crystallin: Implications for lens aging and presbyopia. J. Biol. Chem. 2020 , 295 , 5701–5716. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Reddy, G.B.; Kumar, P.A.; Kumar, M.S. Chaperone-like activity and hydrophobicity of alpha-crystallin. IUBMB Life 2006 , 58 , 632–641. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Horwitz, J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA 1992 , 89 , 10449–10453. [ Google Scholar ] [ CrossRef ]
- Shang, F.; Zetterberg, M.; Zhang, X.; Dudek, E.J.; Taylor, A. Ubiquitin–Proteasome Pathway Is an Important Protein Quality Control Mechanism in the Lens. Investig. Ophthalmol. Vis. Sci. 2006 , 47 , 1520. [ Google Scholar ]
- Li, M.; Liu, S.; Huang, W.; Zhang, J. Physiological and pathological functions of βB2-crystallins in multiple organs: A systematic review. Aging 2021 , 13 , 15674–15687. [ Google Scholar ] [ CrossRef ]
- Myers, J.B.; Haddad, B.G.; O’neill, S.E.; Chorev, D.S.; Yoshioka, C.C.; Robinson, C.V.; Zuckerman, D.M.; Reichow, S.L. Structure of native lens connexin 46/50 intercellular channels by cryo-EM. Nature 2018 , 564 , 372–377. [ Google Scholar ] [ CrossRef ]
- Berthoud, V.M.; Beyer, E.C. Oxidative Stress, Lens Gap Junctions, and Cataracts. Antioxid. Redox Signal 2009 , 11 , 339–353. [ Google Scholar ] [ CrossRef ]
- Mathias, R.T.; White, T.W.; Gong, X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol. Rev. 2010 , 90 , 179–206. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhu, Y.; Xu, S.; Eisenberg, R.S.; Huang, H. A Bidomain Model for Lens Microcirculation. Biophys. J. 2019 , 116 , 1171–1184. [ Google Scholar ] [ CrossRef ]
- Liu, J.; Riquelme, M.A.; Li, Z.; Li, Y.; Tong, Y.; Quan, Y.; Pei, C.; Gu, S.; Jiang, J.X. Mechanosensitive collaboration between integrins and connexins allows nutrient and antioxidant transport into the lens. J. Cell Biol. 2020 , 219 , e202002154. [ Google Scholar ] [ CrossRef ]
- Liu, X.; Hao, J.; Xie, T.; Malik, T.H.; Liu, C.; Shu, C.; Lu, C.; Zhou, D. Nrf2 as a target for prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell. 2017 , 16 , 934–942. [ Google Scholar ] [ CrossRef ]
- Periyasamy, P.; Shinohara, T. Age-related cataracts: Role of unfolded protein response, Ca2+ mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection. Prog. Retin. Eye Res. 2017 , 60 , 1–19. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Guo, Y.; Yu, S.; Zhang, C.; Kong, A.N.T. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic. Biol. Med. 2015 , 88 (Pt B) , 337–349. [ Google Scholar ] [ CrossRef ]
- Ma, T.J.; Lan, D.H.; He, S.Z.; Ye, Z.; Li, P.; Zhai, W.; Chen, W.Q.; Huang, Y.; Fu, Y.; Sun, A.; et al. Nrf2 protects human lens epithelial cells against H2O2-induced oxidative and ER stress: The ATF4 may be involved. Exp. Eye Res. 2018 , 169 , 28–37. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020 , 21 , 421–438. [ Google Scholar ] [ CrossRef ]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011 , 15 , 1583–1606. [ Google Scholar ] [ CrossRef ]
- Reddy, V.N.; Kasahara, E.; Hiraoka, M.; Lin, L.R.; Ho, Y.S. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Exp. Eye Res. 2004 , 79 , 859–868. [ Google Scholar ] [ CrossRef ]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019 , 2019 , 9613090. [ Google Scholar ] [ CrossRef ]
- Chamberlain, C.G.; Mansfield, K.J.; Cerra, A. Glutathione and catalase suppress TGFbeta-induced cataract-related changes in cultured rat lenses and lens epithelial explants. Mol. Vis. 2009 , 15 , 895–905. [ Google Scholar ] [ PubMed ]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011 , 15 , 1957–1997. [ Google Scholar ] [ CrossRef ]
- Varadaraj, K.; Gao, J.; Mathias, R.T.; Kumari, S.S. GPX1 knockout, not catalase knockout, causes accelerated abnormal optical aberrations and cataract in the aging lens. Mol. Vis. 2022 , 28 , 11–20. [ Google Scholar ] [ PubMed ]
- Huynh, T.P.N.; Bowater, R.P.; Bernuzzi, F.; Saha, S.; Wormstone, I.M. GSH Levels Serve as a Biological Redox Switch Regulating Sulforaphane-Induced Cell Fate in Human Lens Cells. Investig. Opthalmology Vis. Sci. 2021 , 62 , 2. [ Google Scholar ] [ CrossRef ]
- Li, B.; Kim, J.Y.; Martis, R.M.; Donaldson, P.J.; Lim, J.C. Characterisation of Glutathione Export from Human Donor Lenses. Transl. Vis. Sci. Technol. 2020 , 9 , 37. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Reddy, V.N. Glutathione and its function in the lens—An overview. Exp. Eye Res. 1990 , 50 , 771–778. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Giblin, F.J. Glutathione: A vital lens antioxidant. J. Ocul. Pharmacol. Ther. 2000 , 16 , 121–135. [ Google Scholar ] [ CrossRef ]
- Pfaff, A.; Chernatynskaya, A.; Vineyard, H.; Ercal, N. Thiol antioxidants protect human lens epithelial (HLE B-3) cells against tert-butyl hydroperoxide-induced oxidative damage and cytotoxicity. Biochem. Biophys. Rep. 2022 , 29 , 101213. [ Google Scholar ] [ CrossRef ]
- Goyal, M.M.; Gajjar, D.U.; Patel, D.B.; Sune, P.; Vasavda, A.R. Effect of vitamin C and E activity on surgically removed cataractous human lens epithelium cells. Indian J. Clin. Biochem. IJCB 2009 , 24 , 375–380. [ Google Scholar ] [ CrossRef ]
- Hammond Christopher, J.; Snieder Harold Spector Tim, D.; Gilbert Clare, E. Genetic and Environmental Factors in Age-Related Nuclear Cataracts in Monozygotic and Dizygotic Twins. N. Engl. J. Med. 2000 , 342 , 1786–1790. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhu, X.; Li, D.; Du, Y.; He, W.; Lu, Y. DNA hypermethylation-mediated downregulation of antioxidant genes contributes to the early onset of cataracts in highly myopic eyes. Redox Biol. 2018 , 19 , 179–189. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lv, J.; Xing, Y. Effects of UV on apoptotic factors in lens epithelial cells of an animal model. Exp. Ther. Med. 2018 , 16 , 2309–2312. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Katoh, N.; Jonasson, F.; Sasaki, H.; Kojima, M.; Ono, M.; Takahashi, N.; Sasaki, K.; Reykjavik Eye Study Group Cortical lens opacification in Iceland. Cortical lens opacification in Iceland. Risk factor analysis—Reykjavik Eye Study. Acta Ophthalmol. Scand. 2001 , 79 , 154–159. [ Google Scholar ] [ CrossRef ]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal Diseases Associated with Oxidative Stress and the Effects of a Free Radical Scavenger (Edaravone). Oxid. Med. Cell Longev. 2017 , 2017 , 9208489. [ Google Scholar ] [ CrossRef ]
- Ahmad, A.; Ahsan, H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J. Immunoass. Immunochem. 2020 , 41 , 257–271. [ Google Scholar ] [ CrossRef ]
- Zhang, M.; Zhang, R.; Zhao, X.; Ma, Z.; Xin, J.; Xu, S.; Guo, D. The role of oxidative stress in the pathogenesis of ocular diseases: An overview. Mol. Biol. Rep. 2024 , 51 , 454. [ Google Scholar ] [ CrossRef ]
- Atalay, E.; Oğurel, T.; Derici, M.K. The role of oxidative damage in cataract etiopathogenesis. Ther. Adv. Ophthalmol. 2023 , 15 , 25158414231168813. [ Google Scholar ] [ CrossRef ]
- Quan, Y.; Du, Y.; Wu, C.; Gu, S.; Jiang, J.X. Connexin hemichannels regulate redox potential via metabolite exchange and protect lens against cellular oxidative damage. Redox Biol. 2021 , 46 , 102102. [ Google Scholar ] [ CrossRef ]
- Wang, G.; Quan, Y.; Ma, B.; Gu, S.; Jiang, J.X. Functional Interplay of Connexin Hemichannels in Glutathione Export from Lens Epithelial Cells and in Fiber Cell protection against Oxidative Stress. Investig. Ophthalmol. Vis. Sci. 2023 , 64 , 5118. [ Google Scholar ]
- Lasmaini, O.; Hidayat, M.; Wati, R. The Effect of Topical Glutathione on Malondialdehyde Levels in Rat with Cataract-Induced Sodium Selenite. Biosci. Med. J. Biomed. Transl. Res. 2023 , 7 , 3356–3361. [ Google Scholar ] [ CrossRef ]
- Schmid, P.W.N.; Lim, N.C.H.; Peters, C.; Back, K.C.; Bourgeois, B.; Pirolt, F.; Richter, B.; Peschek, J.; Puk, O.; Amarie, O.V.; et al. Imbalances in the eye lens proteome are linked to cataract formation. Nat. Struct. Mol. Biol. 2021 , 28 , 143–151. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Miller, A.P.; O’Neill, S.E.; Lampi, K.J.; Reichow, S.L. The α-crystallin Chaperones Undergo a Quasi-ordered Co-aggregation Process in Response to Saturating Client Interaction. J. Mol. Biol. 2024 , 436 , 168499. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Moosavi-Movahedi, F.; Saboury, A.A.; Ghasemi, A.; Pirhaghi, M.; Mamashli, F.; Mohammad-Zaheri, M.; Arghavani, P.; Yousefi, R.; Moosavi-Movahedi, A.A. Exploring the significance of potassium homeostasis in copper ion binding to human αB-Crystallin. Int. J. Biol. Macromol. 2024 , 263 , 130261. [ Google Scholar ] [ CrossRef ]
- Michiel, M.; Duprat, E.; Skouri-Panet, F.; Lampi, J.A.; Tardieu, A.; Lampi, K.J.; Finet, S. Aggregation of deamidated human βB2-crystallin and incomplete rescue by α-crystallin chaperone. Exp. Eye Res. 2010 , 90 , 688–698. [ Google Scholar ] [ CrossRef ]
- Ren, L.; Hu, L.; Zhang, Y.; Liu, J.; Xu, W.; Wu, W.; Xu, J.; Chen, X.; Yao, K.; Yu, Y. Cataract-causing S93R mutant destabilized structural conformation of βB1 crystallin linking with aggregates formation and cellular viability. Front. Mol. Biosci. 2022 , 9 , 844719. [ Google Scholar ] [ CrossRef ]
- Hill, J.A.; Nyathi, Y.; Horrell, S.; von Stetten, D.; Axford, D.; Owen, R.L.; Beddard, G.S.; Pearson, A.R.; Ginn, H.M.; Yorke, B.A. An ultraviolet-driven rescue pathway for oxidative stress to eye lens protein human gamma-D crystallin. Commun. Chem. 2024 , 7 , 81. [ Google Scholar ] [ CrossRef ]
- Budnar, P.; Tangirala, R.; Bakthisaran, R.; Rao, C.M. Protein Aggregation and Cataract: Role of Age-Related Modifications and Mutations in α-Crystallins. Biochem. Mosc. 2022 , 87 , 225–241. [ Google Scholar ] [ CrossRef ]
- Bellmaine, S.; Schnellbaecher, A.; Zimmer, A. Reactivity and degradation products of tryptophan in solution and proteins. Free Radic. Biol. Med. 2020 , 160 , 696–718. [ Google Scholar ] [ CrossRef ]
- Truscott, R.J.W.; Friedrich, M.G. Molecular Processes Implicated in Human Age-Related Nuclear Cataract. Investig. Ophthalmol. Vis. Sci. 2019 , 60 , 5007–5021. [ Google Scholar ] [ CrossRef ]
- Taylor, A.; Davies, K.J.A. Protein oxidation and loss of protease activity may lead to cataract formation in the aged lens. Free Radic. Biol. Med. 1987 , 3 , 371–377. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rocha, M.A.; Sprague-Piercy, M.A.; Kwok, A.O.; Roskamp, K.W.; Martin, R.W. Chemical Properties Determine Solubility and Stability in βγ-Crystallins of the Eye Lens. ChemBioChem 2021 , 22 , 1329–1346. [ Google Scholar ] [ CrossRef ]
- Nandwani, N.; Surana, P.; Negi, H.; Mascarenhas, N.M.; Udgaonkar, J.B.; Das, R.; Gosavi, S. A five-residue motif for the design of domain swapping in proteins. Nat. Commun. 2019 , 10 , 452. [ Google Scholar ] [ CrossRef ]
- Anbaraki, A.; Ghahramani, M.; Muranov, K.O.; Kurganov, B.I.; Yousefi, R. Structural and functional alteration of human αA-crystallin after exposure to full spectrum solar radiation and preventive role of lens antioxidants. Int. J. Biol. Macromol. 2018 , 118 (Pt A) , 1120–1130. [ Google Scholar ] [ CrossRef ]
- Paviani, V.; Junqueira de Melo, P.; Avakin, A.; Di Mascio, P.; Ronsein, G.E.; Augusto, O. Human cataractous lenses contain cross-links produced by crystallin-derived tryptophanyl and tyrosyl radicals. Free Radic. Biol. Med. 2020 , 160 , 356–367. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wilmarth, P.A.; Tanner, S.; Dasari, S.; Nagalla, S.R.; Riviere, M.A.; Bafna, V.; Pevzner, P.A.; David, L.L. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: Does deamidation contribute to crystallin insolubility? J. Proteome Res. 2006 , 5 , 2554–2566. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vetter, C.J.; Thorn, D.C.; Wheeler, S.G.; Mundorff, C.C.; Halverson, K.A.; Wales, T.E.; Shinde, U.P.; Engen, J.R.; David, L.L.; Carver, J.A.; et al. Cumulative deamidations of the major lens protein γS-crystallin increase its aggregation during unfolding and oxidation. Protein Sci. 2020 , 29 , 1945–1963. [ Google Scholar ] [ CrossRef ]
- He, Y.; Kang, J.; Song, J. ATP antagonizes the crowding-induced destabilization of the human eye-lens protein γS-crystallin. Biochem. Biophys. Res. Commun. 2020 , 526 , 1112–1117. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Timsina, R.; Khadka, N.K.; Maldonado, D.; Mainali, L. Interaction of alpha-crystallin with four major phospholipids of eye lens membranes. Exp. Eye Res. 2021 , 202 , 108337. [ Google Scholar ] [ CrossRef ]
- Timsina, R.; Hazen, P.; Trossi-Torres, G.; Khadka, N.K.; Kalkat, N.; Mainali, L. Cholesterol Content Regulates the Interaction of αA-, αB-, and α-Crystallin with the Model of Human Lens-Lipid Membranes. Int. J. Mol. Sci. 2024 , 25 , 1923. [ Google Scholar ] [ CrossRef ]
- Yang, X.; Xu, J.; Fu, C.; Jia, Z.; Yao, K.; Chen, X. The cataract-related S39C variant increases γS-crystallin sensitivity to environmental stress by destroying the intermolecular disulfide cross-links. Biochem. Biophys. Res. Commun. 2020 , 526 , 459–465. [ Google Scholar ] [ CrossRef ]
- Chen, S.; Guo, J.; Xu, W.; Song, H.; Xu, J.; Luo, C.; Yao, K.; Hu, L.; Chen, X.; Yu, Y. Cataract-related variant R114C increases βA3-crystallin susceptibility to environmental stresses by disrupting the protein senior structure. Int. J. Biol. Macromol. 2024 , 262 Pt 2 , 130191. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ghahramani, M.; Yousefi, R.; Krivandin, A.; Muranov, K.; Kurganov, B.; Moosavi-Movahedi, A.A. Structural and functional characterization of D109H and R69C mutant versions of human αB-crystallin: The biochemical pathomechanism underlying cataract and myopathy development. Int. J. Biol. Macromol. 2020 , 146 , 1142–1160. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Simonelli, F.; Nesti, A.; Pensa, M.; Romano, L.; Savastano, S.; Rinaldi, E.; Auricchio, G. Lipid peroxidation and human cataractogenesis in diabetes and severe myopia. Exp. Eye Res. 1989 , 49 , 181–187. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nam, T.G. Lipid peroxidation and its toxicological implications. Toxicol. Res. 2011 , 27 , 1–6. [ Google Scholar ] [ CrossRef ]
- Babizhayev, M.A. Analysis of Lipid Peroxidation and Electron Microscopic Survey of Maturation Stages during Human Cataractogenesis. Drugs R&D 2005 , 6 , 345–369. [ Google Scholar ] [ CrossRef ]
- Kreuzer, M.; Dučić, T.; Hawlina, M.; Andjelic, S. Synchrotron-based FTIR microspectroscopy of protein aggregation and lipids peroxidation changes in human cataractous lens epithelial cells. Sci. Rep. 2020 , 10 , 15489. [ Google Scholar ] [ CrossRef ]
- Wei, Z.; Hao, C.; Huangfu, J.; Srinivasagan, R.; Zhang, X.; Fan, X. Aging lens epithelium is susceptible to ferroptosis. Free Radic. Biol. Med. 2021 , 167 , 94–108. [ Google Scholar ] [ CrossRef ]
- Fan, X.; Wei, Z.; Hao, C.; Huangfu, J.; Srinivasagan, R.; Zhang, X. Lipid peroxidation induces ferroptosis in the lens epithelium. Investig. Ophthalmol. Vis. Sci. 2022 , 63 , 1146. [ Google Scholar ]
- Sardelli, G.; Scali, V.; Signore, G.; Balestri, F.; Cappiello, M.; Mura, U.; Del Corso, A.; Moschini, R. Response of a Human Lens Epithelial Cell Line to Hyperglycemic and Oxidative Stress: The Role of Aldose Reductase. Antioxidants 2023 , 12 , 829. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Liu, S.; Jin, Z.; Xia, R.; Zheng, Z.; Zha, Y.; Wang, Q.; Wan, X.; Yang, H.; Cai, J. Protection of Human Lens Epithelial Cells from Oxidative Stress Damage and Cell Apoptosis by KGF-2 through the Akt/Nrf2/HO-1 Pathway. Oxidative Med. Cell. Longev. 2022 , 2022 , 6933812. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Khoo, H.E.; Ng, H.S.; Yap, W.S.; Goh, H.J.H.; Yim, H.S. Nutrients for Prevention of Macular Degeneration and Eye-Related Diseases. Antioxidants 2019 , 8 , 85. [ Google Scholar ] [ CrossRef ]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021 , 13 , 615. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ringvold, A. The Significance of Ascorbate in the Aqueous Humour Protection Against UV-A and UV-B. Exp. Eye Res. 1996 , 62 , 261–264. [ Google Scholar ] [ CrossRef ]
- Shahidi, F.; Pinaffi-Langley, A.C.C.; Fuentes, J.; Speisky, H.; de Camargo, A.C. Vitamin E as an essential micronutrient for human health: Common, novel, and unexplored dietary sources. Free Radic. Biol. Med. 2021 , 176 , 312–321. [ Google Scholar ] [ CrossRef ]
- Ishikawa, Y.; Hashizume, K.; Kishimoto, S.; Tezuka, Y.; Nishigori, H.; Yamamoto, N.; Kondo, Y.; Maruyama, N.; Ishigami, A.; Kurosaka, D. Effect of vitamin C depletion on UVR-B induced cataract in SMP30/GNL knockout mice. Exp. Eye Res. 2012 , 94 , 85–89. [ Google Scholar ] [ CrossRef ]
- Haque, S.E.; Gilani, K.M.A. Effect of ambroxol, spirulina and vitamin-E in naphthalene induced cataract in female rats. Indian. J. Physiol. Pharmacol. 2005 , 49 , 57–64. [ Google Scholar ] [ PubMed ]
- Mares-Perlman, J.A.; Lyle, B.J.; Klein, R.; Fisher, A.I.; Brady, W.E.; VandenLangenberg, G.M.; Trabulsi, J.N.; Palta, M. Vitamin supplement use and incident cataracts in a population-based study. Arch. Ophthalmol. 2000 , 118 , 1556–1563. [ Google Scholar ] [ CrossRef ]
- Ghazala; Siddiqui, J.A.; Ali, N.; Ali, S.L.; Shaikh, G.S.; Qureshi, A. Serum vitamin A, E & C in cortical & nuclear cataract patients. Prof. Med. J. 2021 , 28 , 1137–1141. [ Google Scholar ] [ CrossRef ]
- Lim, J.C.; Caballero Arredondo, M.; Braakhuis, A.J.; Donaldson, P.J. Vitamin C and the Lens: New Insights into Delaying the Onset of Cataract. Nutrients 2020 , 12 , 3142. [ Google Scholar ] [ CrossRef ]
- Christen, W.G.; Glynn, R.J.; Sesso, H.D.; Kurth, T.; MacFadyen, J.; Bubes, V.; Buring, J.E.; Manson, J.E.; Gaziano, J.M. Age-related cataract in a randomized trial of vitamins E and C in men. Arch. Ophthalmol. 2010 , 128 , 1397–1405. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gritz, D.C.; Srinivasan, M.; Smith, S.D.; Kim, U.; Lietman, T.M.; Wilkins, J.H.; Priyadharshini, B.; John, R.K.; Aravind, S.; Prajna, N.V.; et al. The Antioxidants in Prevention of Cataracts Study: Effects of antioxidant supplements on cataract progression in South India. Br. J. Ophthalmol. 2006 , 90 , 847–851. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Srinivasan, M.; Ravindran, R.D.; O’Brien, K.S.; Kim, U.R.; Wilkins, J.H.; Whitcher, J.P.; Lietman, T.M.; Gritz, D.C.; Keenan, J.D. Antioxidant Vitamins for Cataracts: 15-Year Follow-up of a Randomized Trial. Ophthalmology 2020 , 127 , 986–987. [ Google Scholar ] [ CrossRef ]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch. Ophthalmol. 2001 , 119 , 1439–1452. [ Google Scholar ] [ CrossRef ]
- Zheng Selin, J.; Rautiainen, S.; Lindblad, B.E.; Morgenstern, R.; Wolk, A. High-Dose Supplements of Vitamins C and E, Low-Dose Multivitamins, and the Risk of Age-related Cataract: A Population-based Prospective Cohort Study of Men. Am. J. Epidemiol. 2013 , 177 , 548–555. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Barker, F.M., 2nd; Snodderly, D.M.; Johnson, E.J.; Schalch, W.; Koepcke, W.; Gerss, J.; Neuringer, M. Nutritional manipulation of primate retinas, V: Effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Investig. Ophthalmol. Vis. Sci. 2011 , 52 , 3934–3942. [ Google Scholar ] [ CrossRef ]
- Varma, S.D.; Kovtun, S.; Hegde, K.R. Role of ultraviolet irradiation and oxidative stress in cataract formation-medical prevention by nutritional antioxidants and metabolic agonists. Eye Contact Lens. 2011 , 37 , 233–245. [ Google Scholar ] [ CrossRef ]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016 , 50 , 34–66. [ Google Scholar ] [ CrossRef ]
- Walchuk, C.; Suh, M. Nutrition and the aging retina: A comprehensive review of the relationship between nutrients and their role in age-related macular degeneration and retina disease prevention. Adv. Food Nutr. Res. 2020 , 93 , 293–332. [ Google Scholar ] [ CrossRef ]
- Vu, H.T.V.; Robman, L.; Hodge, A.; McCarty, C.A.; Taylor, H.R. Lutein and Zeaxanthin and the Risk of Cataract: The Melbourne Visual Impairment Project. Investig. Opthalmology Vis. Sci. 2006 , 47 , 3783. [ Google Scholar ] [ CrossRef ]
- Moeller, S.M. Associations Between Age-Related Nuclear Cataract and Lutein and Zeaxanthin in the Diet and Serum in the Carotenoids in the Age-Related Eye Disease Study (CAREDS), an Ancillary Study of the Women’s Health Initiative. Arch. Ophthalmol. 2008 , 126 , 354. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Liu, X.H.; Yu, R.B.; Liu, R.; Hao, Z.X.; Han, C.C.; Zhu, Z.H.; Ma, L. Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis. Nutrients 2014 , 6 , 452–465. [ Google Scholar ] [ CrossRef ]
- Chew, E.Y.; SanGiovanni, J.P.; Ferris, F.L.; Wong, W.T.; Agron, E.; Clemons, T.E.; Sperduto, R.; Danis, R.; Chandra, S.R.; Blodi, B.A.; et al. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013 , 131 , 843–850. [ Google Scholar ] [ CrossRef ]
- Zetterberg, M.; Montan, P.; Kugelberg, M.; Nilsson, I.; Lundström, M.; Behndig, A. Cataract Surgery Volumes and Complications per Surgeon and Clinical Unit: Data from the Swedish National Cataract Register 2007 to 2016. Ophthalmology 2020 , 127 , 305–314. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lu, A.; Duan, P.; Xie, J.; Gao, H.; Chen, M.; Gong, Y.; Li, J.; Xu, H. Recent progress and research trend of anti-cataract pharmacology therapy: A bibliometric analysis and literature review. Eur. J. Pharmacol. 2022 , 934 , 175299. [ Google Scholar ] [ CrossRef ]
- Babizhayev, M.A.; Yermakova, V.N.; Semiletov, Y.A.; Deyev, A.I. The natural histidine-containing dipeptide Nalpha-acetylcarnosine as an antioxidant for ophthalmic use. Biochem. Biokhimiia 2000 , 65 , 588–598. [ Google Scholar ]
- Williams, D.L.; Munday, P. The effect of a topical antioxidant formulation including N-acetyl carnosine on canine cataract: A preliminary study. Vet. Ophthalmol. 2006 , 9 , 311–316. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Babizhayev, M.A. Rejuvenation of visual functions in older adult drivers and drivers with cataract during a short-term administration of N-acetylcarnosine lubricant eye drops. Rejuvenation Res. 2004 , 7 , 186–198. [ Google Scholar ] [ CrossRef ]
- Martis, R.M.; Grey, A.C.; Wu, H.; Wall, G.M.; Donaldson, P.J.; Lim, J.C. N-Acetylcysteine amide (NACA) and diNACA inhibit H(2)O(2)-induced cataract formation ex vivo in pig and rat lenses. Exp. Eye Res. 2023 , 234 , 109610. [ Google Scholar ] [ CrossRef ]
- Maddirala, Y.; Tobwala, S.; Karacal, H.; Ercal, N. Prevention and reversal of selenite-induced cataracts by N-acetylcysteine amide in Wistar rats. BMC Ophthalmol. 2017 , 17 , 54. [ Google Scholar ] [ CrossRef ]
- Higashi, Y.; Higashi, K.; Mori, A.; Sakamoto, K.; Ishii, K.; Nakahara, T. Anti-cataract Effect of Resveratrol in High-Glucose-Treated Streptozotocin-Induced Diabetic Rats. Biol. Pharm. Bull. 2018 , 41 , 1586–1592. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Singh, A.; Bodakhe, S.H. Resveratrol delay the cataract formation against naphthalene-induced experimental cataract in the albino rats. J. Biochem. Mol. Toxicol. 2020 , 34 , e22420. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, P.; Yao, Z.; He, Z. Resveratrol protects against high glucose-induced oxidative damage in human lens epithelial cells by activating autophagy. Exp. Ther. Med. 2021 , 21 , 440. [ Google Scholar ] [ CrossRef ]
- Machado, N.D.; Gutiérrez, G.; Matos, M.; Fernández, M.A. Preservation of the Antioxidant Capacity of Resveratrol via Encapsulation in Niosomes. Foods 2021 , 10 , 988. [ Google Scholar ] [ CrossRef ]
- Hu, N.; Jin, F.Y.; Gao, M.M.; Liu, L.J.; Wang, J.H.; Yang, B.F.; Li, C.L. Baicalein improves Na 2 SeO 3 induced cataract by enhancing the antioxidant capacity of juvenile Sprague Dawley Rat. J. Ethnopharmacol. 2024 , 320 , 117433. [ Google Scholar ] [ CrossRef ]
- Chen, M.; Fu, Y.; Wang, X.; Wu, R.; Su, D.; Zhou, N.; Qi, Y. Metformin protects lens epithelial cells against senescence in a naturally aged mouse model. Cell Death Discov. 2022 , 8 , 8. [ Google Scholar ] [ CrossRef ]
- March, M.E.; Gutierrez-Uzquiza, A.; Snorradottir, A.O.; Matsuoka, L.S.; Balvis, N.F.; Gestsson, T.; Nguyen, K.; Sleiman, P.M.A.; Kao, C.; Isaksson, H.J.; et al. NAC blocks Cystatin C amyloid complex aggregation in a cell system and in skin of HCCAA patients. Nat. Commun. 2021 , 12 , 1827. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zheng, Y.; Liu, Y.; Ge, J.; Wang, X.; Liu, L.; Bu, Z.; Liu, P. Resveratrol protects human lens epithelial cells against H 2 O 2 -induced oxidative stress by increasing catalase, SOD-1, and HO-1 expression. Mol. Vis. 2010 , 16 , 1467–1474. [ Google Scholar ]
- Hu, Z.; Guan, Y.; Hu, W.; Xu, Z.; Ishfaq, M. An overview of pharmacological activities of baicalin and its aglycone baicalein: New insights into molecular mechanisms and signaling pathways. Iran. J. Basic. Med. Sci. 2022 , 25 , 14–26. [ Google Scholar ] [ CrossRef ]
- Chen, M.; Zhang, C.; Zhou, N.; Wang, X.; Su, D.; Qi, Y. Metformin alleviates oxidative stress-induced senescence of human lens epithelial cells via AMPK activation and autophagic flux restoration. J. Cell Mol. Med. 2021 , 25 , 8376–8389. [ Google Scholar ] [ CrossRef ]
- Goutham, G.; Manikandan, R.; Beulaja, M.; Thiagarajan, R.; Arulvasu, C.; Arumugam, M.; Setzer, W.N.; Daglia, M.; Nabavi, S.M. A focus on resveratrol and ocular problems, especially cataract: From chemistry to medical uses and clinical relevance. Biomed. Pharmacother. 2017 , 86 , 232–241. [ Google Scholar ] [ CrossRef ]
- Wang, Y.; Xia, R.; Hu, H.; Peng, T. Biosynthesis, characterization and cytotoxicity of gold nanoparticles and their loading with N-acetylcarnosine for cataract treatment. J. Photochem. Photobiol. B. 2018 , 187 , 180–183. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vora, D.; Heruye, S.; Kumari, D.; Opere, C.; Chauhan, H. Preparation, Characterization and Antioxidant Evaluation of Poorly Soluble Polyphenol-Loaded Nanoparticles for Cataract Treatment. AAPS PharmSciTech 2019 , 20 , 163. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, N.; Zhao, Z.; Ma, H.; Liu, Y.; Nwafor, E.-O.; Zhu, S.; Jia, L.; Pang, X.; Han, Z.; Tian, B.; et al. Optimization and Characterization of Low-Molecular-Weight Chitosan-Coated Baicalin mPEG-PLGA Nanoparticles for the Treatment of Cataract. Mol. Pharm. 2022 , 19 , 3831–3845. [ Google Scholar ] [ CrossRef ]
- Zhou, Y.; Li, L.; Li, S.; Li, S.; Zhao, M.; Zhou, Q.; Gong, X.; Yang, J.; Chang, J. Autoregenerative redox nanoparticles as an antioxidant and glycation inhibitor for palliation of diabetic cataracts. Nanoscale 2019 , 11 , 13126–13138. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kwon, H.J.; Cha, M.-Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s Disease. ACS Nano. 2016 , 10 , 2860–2870. [ Google Scholar ] [ CrossRef ]
- Maccarone, R.; Tisi, A.; Passacantando, M.; Ciancaglini, M. Ophthalmic Applications of Cerium Oxide Nanoparticles. J. Ocul. Pharmacol. Ther. 2020 , 36 , 376–383. [ Google Scholar ] [ CrossRef ]
- Milazzo, S.; Grenot, M.; Benzerroug, M. La cataracte secondaire. J. Fr. Ophtalmol. 2014 , 37 , 825–830. [ Google Scholar ] [ CrossRef ]
- Da, W.J.; Shang, Z.J.; Xia, L.X.; Jie, W.K.; Meng, L.; Yan, M.Y.; Hua, W.X. Knockout of TGF-β receptor II by CRISPR/Cas9 delays mesenchymal transition of Lens epithelium and posterior capsule opacification. Int. J. Biol. Macromol. 2024 , 259 , 129290. [ Google Scholar ] [ CrossRef ]
- Kubo, E.; Shibata, T.; Singh, D.P.; Sasaki, H. Roles of TGF β and FGF Signals in the Lens: Tropomyosin Regulation for Posterior Capsule Opacity. Int. J. Mol. Sci. 2018 , 19 , 3093. [ Google Scholar ] [ CrossRef ]
- Thompson, B.; Davidson, E.A.; Chen, Y.; Orlicky, D.J.; Thompson, D.C.; Vasiliou, V. Oxidative stress induces inflammation of lens cells and triggers immune surveillance of ocular tissues. Chem. Biol. Interact. 2022 , 355 , 109804. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Malecaze, F.; Decha, A.; Serre, B.; Penary, M.; Duboue, M.; Berg, D.; Levade, T.; Lubsen, N.H.; Kremer, E.J.; Couderc, B. Prevention of posterior capsule opacification by the induction of therapeutic apoptosis of residual lens cells. Gene Ther. 2006 , 13 , 440–448. [ Google Scholar ] [ CrossRef ]
- Malecaze, F.; Lubsen, N.H.; Serre, B.; Decha, A.; Duboue, M.; Penary, M.; Berg, D.; Arnaud, J.-D.; Titeux, M.; Kremer, E.J.; et al. Lens cell targetting for gene therapy of prevention of posterior capsule opacification. Gene Ther. 2006 , 13 , 1422–1429. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jiang, Y.X.; Lu, Y.; Liu, T.J.; Yang, J.; Chen, Y.; Fang, Y.W. Using HSV-TK/GCV suicide gene therapy to inhibit lens epithelial cell proliferation for treatment of posterior capsular opacification. Mol. Vis. 2011 , 17 , 291–299. [ Google Scholar ] [ PubMed ]
- Huang, W.R.; Fan, X.X.; Tang, X. SiRNA targeting EGFR effectively prevents posterior capsular opacification after cataract surgery. Mol. Vis. 2011 , 17 , 2349–2355. [ Google Scholar ]
- Zhang, R.; Wei, Y.-H.; Zhao, C.-Y.; Song, H.-Y.; Shen, N.; Cui, X.; Gao, X.; Qi, Z.-T.; Zhong, M.; Shen, W. EDIL3 depletion suppress epithelial-mesenchymal transition of lens epithelial cells via transforming growth factor β pathway. Int. J. Ophthalmol. 2018 , 11 , 18–24. [ Google Scholar ] [ CrossRef ]
- Zheng, D.; Song, T.; Zhongliu, X.; Wu, M.; Liang, J.; Liu, Y. Downregulation of transforming growth factor-β type II receptor prohibit epithelial-to-mesenchymal transition in lens epithelium. Mol. Vis. 2012 , 18 , 1238–1246. [ Google Scholar ]
- Li, P.; Jing, J.; Hu, J.; Li, T.; Sun, Y.; Guan, H. RNA Interference Targeting Snail Inhibits the Transforming Growth Factor β 2-Induced Epithelial-Mesenchymal Transition in Human Lens Epithelial Cells. J. Ophthalmol. 2013 , 2013 , 869101. [ Google Scholar ] [ CrossRef ]
- Sun, J.; Xie, L.; Wang, Y.; Liu, T. Inhibition of human lens epithelial B-3 cell proliferation by adenovirus-mediated transfer of antisense c-myc construct. Graefes Arch. Clin. Exp. Ophthalmol. 2005 , 243 , 601–606. [ Google Scholar ] [ CrossRef ]
- Couderc, B.C.; de Neuville, S.; Douin-Echinard, V.; Serres, B.; Manenti, S.; Darbon, J.-M.; Malecaze, F. Retrovirus-mediated transfer of a suicide gene into lens epithelial cells in vitro and in an experimental model of posterior capsule opacification. Curr. Eye Res. 1999 , 19 , 472–482. [ Google Scholar ] [ CrossRef ]
- Malecaze, F.; Couderc, B.; De Neuville, S.; Serres, B.; Mallet, J.; Douin-Echinard, V.; Manenti, S.; Revah, F.; Darbon, J.-M. Adenovirus-Mediated Suicide Gene Transduction: Feasibility in Lens Epithelium and in Prevention of Posterior Capsule Opacification in Rabbits. Hum. Gene Ther. 1999 , 10 , 2365–2372. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zheng, Y.-P.; Zhang, S.-B.; Wang, F.; Liu, H.; Zhang, W.; Song, B.; Liu, Z.-Y.; Xiong, L.; Fan, Y.-Z.; Liao, D.-Y. Effects of lentiviral RNA interference-mediated downregulation of integrin-linked kinase on biological behaviors of human lens epithelial cells. Int. J. Ophthalmol. 2016 , 9 , 21–28. [ Google Scholar ] [ CrossRef ]
- Drag, S.; Dotiwala, F.; Upadhyay, A.K. Gene Therapy for Retinal Degenerative Diseases: Progress, Challenges, and Future Directions. Investig. Opthalmology Vis. Sci. 2023 , 64 , 39. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gao, X.; Cheng, J.; Lu, C.; Li, X.; Li, F.; Liu, C.; Zhang, M.; Zhu, S.; Ma, X. A Novel Mutation in the Connexin 50 Gene ( GJA8 ) Associated with Autosomal Dominant Congenital Nuclear Cataract in a Chinese Family. Curr. Eye Res. 2010 , 35 , 597–604. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shi, Y.; Li, X.; Yang, J. Mutations of CX46/CX50 and Cataract Development. Front. Mol. Biosci. 2022 , 9 , 842399. [ Google Scholar ] [ CrossRef ]
- Ma, B.; Jing, R.; Liu, J.; Yang, L.; Li, J.; Qin, L.; Cui, L.; Pei, C. CTGF Contributes to the Development of Posterior Capsule Opacification: An in vitro and in vivo study. Int. J. Biol. Sci. 2018 , 14 , 437–448. [ Google Scholar ] [ CrossRef ]
- Guo, M.; Su, F.; Chen, Y.; Su, B. Interfering Hsa_circRNA_0060640 Suppresses TGF-β2-Induced Proliferation, Motility and EMT in Human Lens Epithelium Cells by Targeting miR-214-3p and Collagen Type I alpha2 Chain. Curr. Eye Res. 2022 , 47 , 735–746. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Treatment | Structure and Description | Implications for Cataract Treatment | References |
|---|---|---|---|
| N-acetylcarnosine | Prodrug of l-carnosine. | Reduces lens opacification in canine cataracts, NACS eyedrops improve visual acuity and glare sensitivity in humans with cataracts. | [ , , ] |
| N-acetylcysteine amide | Analog of NAC, a glutathione prodrug. | NACA intraperitoneal injection prevents sodium selenite-induced cataract formation in rats, NACA eye drops reverse sodium selenite-induced cataract grade in rats, NACA and diNACA reduce H O -induced lens opacity in pig and rat lenses, with NACA increasing antioxidant levels as well. | [ , ] |
| diNACA | Analog of NACA. | ||
| Resveratrol | Polyphenolic phytoalexin produced in plants, trans isomer is more bioactive. | Delays diabetic cataract formation in rats, mitigates oxygen-mediated protein oxidation in diabetic rats, protects human lens epithelial cells against oxidative damage, increases antioxidant levels and delays lenticular opacity in rats with naphthalene-induced cataracts. | [ , , , ] |
| Baicalein | Antioxidant flavonoid. | In rats with sodium-selenite induced cataracts, it decreases dense opacity of the lens, increases soluble protein content, reduces oxidative stress, and prevents damage of lens epithelial cells. | [ ] |
| Metformin | Chronic low dose of metformin in mice significantly decreased lens opacity and lens epithelial cell senescence, which increasing autophagy. | [ ] |
| Drug | Nanotechnology Used | Outcomes | Reference |
|---|---|---|---|
| NACS | Encapsulated NACS into gold nanoparticles. | Attenuated NACS toxicity at high concentrations, increased biocompatibility and bioavailability. | [ ] |
| Resveratrol | Encapsulated resveratrol into lipid cyclodextrin-based nanoparticles. | Increased levels of antioxidant markers in bovine lens cultures to a higher degree than resveratrol alone. | [ ] |
| Resveratrol | Encapsulated resveratrol into niosomes. | Maintained antioxidant capacity of resveratrol, prevented light irradiation-induced isomer conversion of resveratrol to its less bioactive cis isomer. | [ ] |
| Baicalin | Encapsulated baicalin into chitosan-coated mPEG-PLGA nanoparticles. | Increased cellular uptake of baicalin, increased corneal retention of baicalin in rabbits, increased antioxidant levels and decreased oxidative stress markers in rabbits with selenite-induced cataract to a greater degree than baicalin alone. | [ ] |
| CeO | Encapsulated CeO in PEG-PLGA coated nanoparticles. | Allowed for water soluble formation of CeO suitable for biological use. Decreased peroxide and superoxide concentrations in lens epithelial cell cultures. Reduced oxidative stress markers, increased antioxidant levels, and attenuated cataract development in rats with diabetic cataracts. | [ ] |
| Gene(s) of Interest | Outcomes | Reference |
|---|---|---|
| Suicide Gene Therapy | ||
| Procaspase 3 or Bax | Overexpression of pro-apoptotic molecules was successfully targeted to rabbit residual lens epithelial cells, and sufficiently prevented PCO in rabbits. | [ , ] |
| HSV-tk (plus treatment with GNV) | HSV-tk was successfully expressed in HLECs and, when treated with GNV, was able to cause cell death. | [ ] |
| RNA Interference | ||
| EGF | siRNA successfully inhibited cell proliferation of HLECs and significantly reduced PCO in a rat model. | [ ] |
| EDIL3 | Knockdown significantly reduced HLEC proliferation and migration in vitro. | [ ] |
| TGF-βRII | RNAi significantly reduced LEC migration. | [ ] |
| Snail | siRNA successfully inhibited TGF-βII-mediated EMT of human epithelial cells. | [ ] |
| ILK | shRNA significantly decreased migration, increased apoptosis, and caused arresting of cells at G1/S transition. | [ ] |
| CRISPR-Cas9 | ||
| TGF-βRII | TGF-βRII knockout caused significant decrease in PCO incidence for rabbit PCO model, as well as significant decreased in in vitro HLEC proliferation. | [ ] |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Kulbay, M.; Wu, K.Y.; Nirwal, G.K.; Bélanger, P.; Tran, S.D. Oxidative Stress and Cataract Formation: Evaluating the Efficacy of Antioxidant Therapies. Biomolecules 2024 , 14 , 1055. https://doi.org/10.3390/biom14091055
Kulbay M, Wu KY, Nirwal GK, Bélanger P, Tran SD. Oxidative Stress and Cataract Formation: Evaluating the Efficacy of Antioxidant Therapies. Biomolecules . 2024; 14(9):1055. https://doi.org/10.3390/biom14091055
Kulbay, Merve, Kevin Y. Wu, Gurleen K. Nirwal, Paul Bélanger, and Simon D. Tran. 2024. "Oxidative Stress and Cataract Formation: Evaluating the Efficacy of Antioxidant Therapies" Biomolecules 14, no. 9: 1055. https://doi.org/10.3390/biom14091055
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Vet Sci
Levels of Evidence, Quality Assessment, and Risk of Bias: Evaluating the Internal Validity of Primary Research
Jan m. sargeant.
1 Department of Population Medicine, Ontario Veterinary College, University of Guelph, Guelph, ON, Canada
Marnie L. Brennan
2 Centre for Evidence-Based Veterinary Medicine, School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington Campus, Loughborough, United Kingdom
Annette M. O'Connor
3 Department of Large Animal Clinical Sciences, College of Veterinary Medicine, Michigan State University, East Lansing, MI, United States
Clinical decisions in human and veterinary medicine should be based on the best available evidence. The results of primary research are an important component of that evidence base. Regardless of whether assessing studies for clinical case management, developing clinical practice guidelines, or performing systematic reviews, evidence from primary research should be evaluated for internal validity i.e., whether the results are free from bias (reflect the truth). Three broad approaches to evaluating internal validity are available: evaluating the potential for bias in a body of literature based on the study designs employed (levels of evidence), evaluating whether key study design features associated with the potential for bias were employed (quality assessment), and applying a judgement as to whether design elements of a study were likely to result in biased results given the specific context of the study (risk of bias assessment). The level of evidence framework for assessing internal validity assumes that internal validity can be determined based on the study design alone, and thus makes the strongest assumptions. Risk of bias assessments involve an evaluation of the potential for bias in the context of a specific study, and thus involve the least assumptions about internal validity. Quality assessment sits somewhere between the assumptions of these two. Because risk of bias assessment involves the least assumptions, this approach should be used to assess internal validity where possible. However, risk of bias instruments are not available for all study designs, some clinical questions may be addressed using multiple study designs, and some instruments that include an evaluation of internal validity also include additional components (e.g., evaluation of comprehensiveness of reporting, assessments of feasibility or an evaluation of external validity). Therefore, it may be necessary to embed questions related to risk of bias within existing quality assessment instruments. In this article, we overview the approaches to evaluating internal validity, highlight the current complexities, and propose ideas for approaching assessments of internal validity.
Introduction
Every day in clinical practice, veterinary professionals need to make decisions ranging from a decision as to whether (or not) to use an intervention or to apply a diagnostic test, to decisions about the overall management of complex clinical conditions. Increasingly, it is expected that clinical decisions are evidence-based. Evidence-based veterinary medicine incorporates clinician experience, client preferences, animal needs, and scientific evidence when making clinical decisions ( 1 ). In this approach, scientific evidence is obtained from relevant research. When research-based evidence does not exist, other sources of evidence, such as expert opinion may need to be used. Traditional narrative reviews provide an overview of a topic, and thus may be an attractive way of quickly acquiring knowledge for making clinical decisions. However, narrative reviews generally do not provide information on the identification and selection of the primary research being summarized (if any), the methodological quality of the studies, or the magnitude of the expected effect ( 2 , 3 ).
Formal methods have been developed to systematically identify, select, and synthesize the available evidence to assist veterinary professionals in evidence-based decision-making. These include critically appraised topics (CATs) ( 4 ), systematic review and meta-analysis (SR-MA) ( 5 – 7 ), and clinical practice guidelines ( 8 ) (see Box 1 for a short overview of these methods). These evidence synthesis approaches have different purposes which results in different processes and endpoints, but each includes an assessment of the internal validity of the research used. Critical appraisal of an individual study also includes an evaluation of internal validity, in addition to an evaluation of feasibility and generalizability ( 10 ). The evaluation of internal validity is the focus of this article. Understanding the different ways internal validity can be assessed, and the assumptions associated with these approaches, is necessary for researchers evaluating internal validity, and for veterinary professionals to assess studies for integration of evidence into practice.
Overview of synthesis methods used in veterinary practice and research.
Systematic review, meta-analysis, and network meta-analysis: Systematic review is a structured methodology for identifying, selecting and evaluating all relevant research to address a structured question, which may relate to descriptive characteristics such as prevalence, etiology, efficacy of interventions, or diagnostic test accuracy ( 5 ). Meta-analysis is the statistical combination of results from multiple studies. For addressing questions on intervention efficacy, meta-analysis provides an overall effect size for pairwise comparisons between two intervention groups. Network meta-analysis allows an estimation of the comparative efficacy across all available intervention options ( 6 ), which may provide more relevant information for veterinary professionals when there are multiple intervention options available. However, systematic reviews with pairwise meta-analysis or network meta-analysis require that a body of research exists that can be synthesized to address a clinical question and can also be resource and time intensive to conduct. Therefore, there are many clinical questions for which formally synthesized research summaries do not exist.
Critically appraised topics: Critically appraised topics (CATs) use the same principles as systematic reviews to address clinical questions but employ a more rapid approach, particularly in relation to the screening and summation of the evidence. They were designed to be employed by clinicians as a way of rapidly gathering and interpreting evidence on clinical questions relating to specific cases ( 4 ). Therefore, there is a greater risk that research addressing the question may be missed. However, in the absence of a well conducted systematic review or meta-analysis, CATs can provide a faster evaluation of research addressing a clinical question and can be undertaken by veterinary professionals who may have fewer resources and potentially less methodological or statistical expertise, particularly if they are freely available and accessible.
Clinical practice guidelines: Veterinary professionals often are involved in the management of complex clinical conditions, where an array of questions need to be addressed, including those related to etiology, prognosis, diagnostic test accuracy, and intervention efficacy. Clinical practice guidelines are intended to assist healthcare professionals in assessing more than one aspect of case approach, including appropriate prevention, diagnosis, treatment, or clinical management of diseases, disorders, and other health conditions ( 9 ). Although there are differences in the methods among authors and institutions, the key elements of guideline development include the establishment of a multidisciplinary working group to develop the guidelines, the involvement of appropriate stakeholders, identification of the topic area, systematic searches for research evidence, assessment of the internal validity of studies comprising the evidence base, a process for drafting recommendations, and ongoing review and updating of the guidelines as new evidence becomes available ( 8 ).
Internal validity refers to the extent to which the study results reflect the true state of nature (i.e., whether the effect size estimated in a study is free from systematic error, also called bias) ( 11 ). Although there are a large number of named biases ( 12 ), for studies that assess interventions or risk factors, the biases can be categorized into three broad types of bias: selection bias, information bias, and confounding ( 13 ). Selection bias impacts the effect size if, compared to the source population, the exposure or intervention groups differ in the distribution of factors associated with the outcome at the time the study population is selected, or if differential loss to follow up between groups occurs during the study. In case-control studies, selection bias occurs if cases or controls are selected based on criteria that are related to the exposure of interest. Information bias occurs when there are errors in measuring the exposure or intervention, or the outcome, or both. Finally, confounding is a mixing of effects that occurs when a variable (the confounder) that is independently associated with both the exposure and the outcome is not properly controlled. When confounding is not controlled, the estimate of the relationship between the exposure and the outcome will be biased.
There are several terms used to describe the approaches to assessing internal validity of primary research studies, including evidence hierarchies and levels of evidence, quality assessment, and risk of bias assessment. The use of these terms may be confusing, and it is not uncommon for some of these terms to be used interchangeably ( 14 , 15 ). Also, authors may mislabel the approaches and some evaluation tools (instruments) available for assessing internal validity may include additional components, such as those related to comprehensiveness (quality) of reporting, feasibility of applying an intervention, or external validity. Finally, some instruments may use the approach as a label for the instrument [e.g., Cochrane's risk of bias tool ( 16 ), which is an instrument that employs a risk of bias approach] and other instruments may not include the approach in the instrument name [e.g., the Jadad scale ( 17 ), which employs a quality assessment approach]. In an evaluation of the comprehensiveness of reporting in animal health systematic reviews (SRs), Sargeant et al., ( 18 )found that a range of instruments involving all three approaches had been used for assessing the internal validity of primary research studies. Although a large number of instruments are available, the approaches within each instrument used to assess internal validity can be grouped into three broad categories: based on study design, based on the presence or absence of design features, or based on a judgement about bias in the context of the study. These categories generally correspond to levels of evidence, quality assessment, and risk of bias, respectively. Therefore, our objective was to review these approaches to assessing internal validity as distinct entities and to describe the assumptions associated with each approach. Although we provide examples of specific instruments that include an evaluation of internal validity, our focus is on the approaches, rather than the tools. We discuss advances in the use of these approaches to assessing internal validity in human healthcare and propose a process for veterinary medicine for selecting the approach with the least assumptions as appropriate to the clinical question, the purpose of the assessment, and the research found that addresses the question of interest. The target audience for this article is individuals who assess internal validity of studies, individuals who develop instruments that include items related to the assessment of internal validity, and those who use evidence synthesis products created by others, such as systematic reviews or clinical practice guidelines.
Evaluating Internal Validity by Study Design: Levels of Evidence
Levels of evidence is an approach to evaluating the internal validity of a body of evidence, based on the potential for bias which is inherent to the employed study designs that were used to address the clinical question. The concept behind levels of evidence is that there is a hierarchy of study designs, with different study designs having different potential for bias. The way evidence hierarchies are used is based on either the name of the design or the description of the design. Readers of a study look for this information, then determine the design and assign a level of evidence. No further differentiation of methodological features or judgment is conducted.
Evidence hierarchies were initially introduced in 1979 by the Canadian Task Force on the Periodic Health Examination ( 19 ), with further development into an evidence pyramid by David Sackett in 1989 ( 20 ). A pyramid shaped figure commonly is used to illustrate the hierarchy of study designs for evaluating the efficacy of an intervention under realistic-use conditions (owned animals, as opposed to experimental settings), with the potential for bias decreasing from the base to the top of the pyramid ( Figure 1 ). Thus, study designs on the top of the pyramid represent those with inherently lower risk of bias compared to study designs lower on the hierarchy. The pyramid shape acknowledges that the quantity of research tends to decrease in the higher levels of evidence (for instance, there will be a larger volume of randomized controlled trials (RCTs) compared to SR-MA). Suggested modifications to the evidence pyramid for veterinary intervention studies include dividing RCTs into those conducted under realistic-use conditions vs. those conducted in nonrealistic-use conditions (e.g., research facility) ( 21 ), the inclusion of challenge trials (where disease outcomes are deliberately induced) below RCTs in the pyramid ( 21 , 22 ), and increasing the interpretability of the concept for students by displaying the hierarchy as a staircase rather than a pyramid ( 23 ).

Illustration of an evidence pyramid hierarchy for addressing intervention studies in veterinary medicine. SR, systematic review; MA, meta-analysis; RCT, randomized controlled trial.
The concept of evaluating the potential for bias in an individual study based on the study design can be extended to an evaluation of the potential for bias in a body of literature. This approach for evaluating the internal validity of a body of literature is referred to as “levels of evidence”. The approach is applied by identifying research (or other evidence) that pertains to the clinical question, determining the study design used for each of the studies, and then assigning each study to a level of evidence based on that design. For instance, a framework for levels of evidence in veterinary clinical nutrition has been proposed by Roudebush et al. ( 24 ). In this framework, level 1 evidence corresponds to at least 1 appropriately designed RCT in the target species with natural disease development, level 2 evidence would correspond to RCTs in laboratory settings with natural disease development, level 3 evidence would be obtained from non-randomized trials, deliberate disease induction trials, analytical observational studies or case series, and level 4 evidence would correspond to expert opinion, descriptive studies, studies in other species, or pathophysiological justification. Therefore, if the clinical question involves interventions, and the evidence found to address the question consists of 2 RCTs, 3 case-control studies, and 3 case series, the evidence would be designated as “level 1 evidence” because study designs with the highest evidentiary level in the available research consisted of RCTs. If all available evidence was from expert opinion, the body of research would comprise “level 4” evidence. This evidence would represent the best available evidence to inform decision-making at the time the assessment was made, although the overall level assigned would change as higher evidentiary level information becomes available.
The levels of evidence approach may be perceived as a quick and easy approach to assessing internal validity because it requires only a knowledge of the study design employed and not the individual features of a study that may or may not be associated with the potential for bias. However, that ease of use is based on very strong assumptions: 1) that study design maps directly to bias, 2) that authors always correctly label study designs, and 3) that authors execute and report study designs appropriately. The approach also pertains to a body of evidence, implying that there are multiple comparable studies available to address the question of interest.
An important critique of levels of evidence is that the approach focuses on the study design, rather than the actual design features that were used or the context of the study. Thus, although this framework illustrates the inherent potential for bias of the different study designs, it does not provide a consideration of the methodological rigor with which any specific individual study was conducted ( 25 ). For instance, although a well-conducted cohort study may be less biased than a poorly executed RCT, this nuance is not captured by a levels of evidence approach. Additionally, levels of evidence are based on the potential for confounding and selection biases, but there is no mechanism to evaluate the potential for information bias because this is linked to the outcome and the levels of evidence approach is based on features at the study, rather than outcome, level. For instance, RCTs provide a higher level of evidence compared to observational studies because random allocation to intervention groups minimizes the potential for confounding, and case-control studies provide a lower level of evidence than cohort studies because they are more prone to selection bias. However, a RCT that used a subjectively measured outcome would be assigned a higher level of evidence than a cohort study with an objective outcome, although the observational study may have a lower risk of information bias. Finally, studies may be mislabeled in terms of their study design; there is empirical evidence that this occurs in the veterinary literature ( 26 – 28 ). For example, studies labeled as case series in veterinary medicine frequently include a component corresponding to a cohort study design ( 27 ); these studies may be assigned an inappropriately low level of evidence if individuals classifying these studies rely on authors terminology rather than the complete design description to determine the design employed.
An additional consideration is that for questions related to aspects of clinical care other than selection of interventions, the framework and positioning of study designs included in Figure 1 may not be appropriate. Levels of evidence schema are available for other clinical questions, such as prognosis, diagnostic test accuracy, disease screening, and etiology ( 29 , 30 ).
Evaluating Internal Validity Based on Inclusion of Study Features Associated With Bias: Quality Assessment
As the name implies, quality assessment represents an evaluation of the quality of a primary research article. However, the term “quality” is difficult to specifically define in the context of evidence-based medicine, in that it does not appear to have been used consistently in the literature. The Merriam-Webster dictionary defines quality as “how good or bad something is” or “a high level of value or excellence” ( https://www.merriam-webster.com/dictionary/quality ). Quality generally is understood to be a multi-dimensional concept. While clear definitions are difficult to find in the research literature, the lay literature includes numerous treaties on the dimensions of quality. One example is the eight dimensions of quality delineated by David Gavin, which include performance, features, reliability, conformance, durability, serviceability, aesthetics, and perceived quality ( https://en.wikipedia.org/wiki/Eight_dimensions_of_quality ).
The findings from a review ( 31 ) identified that available instruments labeled as quality assessment tools varied in clarity and often involved more than just assessing internal validity. In addition to including an assessment of internal validity, quality assessment instruments also generally contain elements related to quality of reporting or an assessment of the inclusion of study features not directly related to bias, such as whether ethical approval was sought or whether the study participants were similar to those animals in the care of the individual doing the critique ( 14 , 31 – 33 ).
Quality assessment as an approach to evaluating internal validity involves an evaluation of the presence or absence of design features, i.e., a methodological checklist ( 14 , 15 ). For example, the Jadad scale ( 17 ) involves completing a checklist of whether the study was described as randomized, whether the study was described as double blind, and whether there was a description of withdrawals and dropouts, with points assigned for each category. Therefore, the Jadad scale uses a quality assessment approach to evaluating internal validity. In terms of assumptions, the quality assessment approach also makes strong assumptions, although these are less than those used in levels of evidence assessments. Instead of mapping bias to the study design, quality assessment maps bias to a design feature i.e., if a trial was randomized, it is assumed to be “good quality” and if the trial was not randomized the assumption is that it is “poor quality”. The same process is followed for additional study aspects, such a blinding or losses to follow-up, and an overall assessment of quality is then based on how the study 'performs' against these questions.
Quality assessment also considers more than just confounding and selection bias as components of internal validity. The inclusion of blinding as a design feature of interest illustrates this. Blinding as a design feature is intended to reduce the potential for differential care as a source of confounding bias (blinding of caregivers) or may be intended to reduce the potential for information bias (blinding of outcome assessors). Conducting a quality assessment is more complicated and time-consuming than evaluating levels of evidence because the presence or absence of the specific design features needs to be identified and validated within the study report. However, the approach requires only that the person evaluating internal validity can identify whether (or not) a design feature was used. Therefore, this approach requires more technical expertise that the levels of evidence approach, but less than the risk of bias approach.
Evaluating Internal Validity by Making Contextualized Judgements on Potential Occurrence of Bias: Risk of Bias Assessment
Risk of bias assessments have been developed specifically for evaluating the potential for elements of the design or conduct employed within a study to lead to a biased effect size ( 34 , 35 ). The components of risk of bias assessments are selected based on empirical evidence of their association with estimates of effect sizes ( 24 , 32 ). The way risk of bias assessments work is that individuals evaluating a study for internal validity answer a series of signaling questions about the presence or absence of design features followed by a judgment about the potential for the use of the design feature to lead to a biased estimate in the context of the specific study. A conclusion is then reached about potential for bias based on all evaluated design features in the context of the study. Thus, a risk of bias assessment makes fewer assumptions about the link between study design and design features compared to quality assessment. For instance, a quality assessment for an RCT would include an evaluation as to whether blinding of outcome assessors occurred, whereas a risk of bias assessment would involve an evaluation not only as to whether blinding was used, but also a judgement as to whether a lack of blinding of outcome assessors would be likely to lead to a biased estimate given the context of the study and the outcome measures used. Thus, a RCT that did not include blinding of outcome assessors might be rated as poor on a quality assessment but might not be a concern in a risk of bias assessment if the outcomes were measured objectively, precluding the likelihood that the estimate would be biased by a knowledge of the intervention group when classifying the outcomes. Because of the necessity of making a judgement about the potential that bias is associated with design features in the context of a specific topic area, this approach requires the highest level of knowledge of study design and bias. The risk of bias approach also generally is conducted at the outcome level, rather than at the study level. For instance, an unblinded RCT of interventions to treat lameness might be considered to have a high risk of bias if the outcome was assessed by owners (a subjective outcome) but not if the outcome was assessed by force plate measurement (an objective outcome). For a level of evidence assessment, the assessment of internal validity would be high quality because the trial was an RCT. For quality assessment, the study may be considered poor quality because it was unblinded, but the overall judgement would be dependent on a number of other study design flaws identified. Finally, in a risk of bias assessment, the study would likely be low risk of bias for the objective outcome and high risk of bias for the subjective outcome if blinding was not used.
Some components of a risk of bias assessment are the same as those included in a quality assessment approach (e.g., an assessment of randomization, allocation concealment, and blinding could be included in both). However, the way the assessment is done differs, with quality assessments generally involving present/absent judgements as opposed to assessments as to whether the risk of bias is likely or not. Hartling et al. ( 14 ) applied two instruments using a quality assessment approach and one instrument using a risk of bias approach to a sample of 163 trials and found that there was low correlation between quality assessment and risk of bias approaches when comparing the assessment of internal validity.
Although the critical elements for risk of bias are well described for RCTs in human healthcare and to a large extent in veterinary RCTs, these elements are not as well described for non-randomized trials and observational studies where allocation to groups is not under the control of the investigator. There are some risk of bias tools available for assessing risk of bias in non-randomized studies, such as ROBINS-I ( 36 ). However, ROBINS-I has been criticized for being challenging to use and for having low reliability, particularly amongst less experienced raters ( 37 , 38 ). A review and critique of approaches to risk of bias assessment for observational studies is available ( 39 ). It is anticipated that risk of bias tools for observational study designs, including studies related to questions of prognosis and causation, will continue to evolve as new instruments are developed and validated.
Historical Contexts and Comparisons of Internal Validity Assessment Approaches
Currently, the available approaches to assessing internal validity tend to be used for different applications. Levels of evidence have previously been used for creating evidence-based recommendations or clinical practices guidelines ( 30 , 40 , 41 ), where it is anticipated that multiple study designs may have been used to address the clinical question(s) of interest. Both quality assessment and risk of bias assessment approaches have been used as a component of systematic reviews with meta-analysis or network meta-analysis, as the intended product of these reviews is to summarize a single parameter (such as incidence or prevalence) or a summary effect size (such as a risk ratio, odds ratio, or hazard ratio) where it is desired that the estimate is unbiased. Often, that estimate is derived from studies with the same study design or a narrow range of study designs from high levels in the evidence hierarchy for the research question type. Therefore, the focus is on a specific parameter estimate based on multiple studies, rather than a descriptive summary of the evidentiary strength of those studies.
However, the different approaches are not necessarily mutually exclusive, but are nested within each other based on assumptions, and the methodology and use of the different approaches has evolved over time. As previously described, a criticism of the use of levels of evidence is that the potential for bias is based on the study design that was employed, rather than the methodological rigor of a specific study ( 42 ). For this reason, many frameworks for levels of evidence included wording such as “appropriately designed” ( 24 ) or “well designed” ( 41 )for the study designs, although the criteria for determining whether a study was designed and executed with rigor generally is not described. A lack of transparency for the criteria for evaluating internal validity of studies within an evidence level is problematic for individuals wishing to use the results. An example of the evolution toward more transparent considerations of internal validity of individual studies within a levels of evidence framework is seen in the progression of the Australian National Health and Medical Research Council (NHMRC) system for evaluating evidence in the development of clinical practice guidelines. The designation of levels of evidence in this framework originally was based on levels of evidence, with descriptors such as “properly-designed” or “well-designed” included for each type of study design ( 40 ). A concern with this approach was that the framework was not designed to address the strength of evidence from individual studies within each evidence level ( 43 ). Therefore, the framework was modified to include the use of risk of bias evaluations of individual studies within each evidence level. The combined use of levels of evidence and risk of bias assessment of studies within each level of evidence now forms the “evidence base” component of the NHMRC's FORM framework for the development of evidence-based clinical guidelines ( 44 ).
Another example of the evolution of approaches to assessing internal validity is from the Cochrane Back review group, who conduct systematic reviews of neck and back pain. The initial methods guidelines, published in 1997, recommended that a quality assessment be performed on each included study, with each item in the quality assessment tool scored based on whether the authors reported their use ( 45 ). Updated methods guidelines were published in 2003 ( 46 ). The framework for levels of evidence in this guidance was restricted to a consideration of randomized controlled trials and non-randomized controlled clinical trials, as these were considered the study designs that potentially were appropriate to address research questions in this content area. In the updated guidelines, the recommendations for the assessment of internal validity moved to a risk of bias approach, where judgements were made on whether the characteristics of each study were likely to lead to biased study results. In the 2003 methods guidelines, levels of evidence were recommended as an approach to qualitative analysis rather than the use of “vote counting” (summing the number of studies where a positive or negative outcome was reported). The guidelines were again updated in 2009 ( 47 ). In this version, the assessment of the internal validity of individual studies explicitly employed a risk of bias approach. It was further recommended that the use of evidence levels as a component of a qualitative synthesis be replaced with a formal rating of the quality of the evidence for each of the included outcomes. It was recommended that review authors use the GRADE approach for this component. The GRADE approach explicitly includes a consideration of the risk of bias across all studies included in the review, as well as an assessment of the consistency of results across studies, the directness of the evidence to the review question, the precision in the effect size estimate, and the potential for publication bias ( 48 ).
The examples from the human medical literature illustrate that assessment of internal validity need not be static, and that modifications to our approach to assessing internal validity can strengthen the evidence base for clinical decision making. When developing or using tools which include an evaluation of internal validity, the assessment of internal validity should use the approach with the least assumptions about bias. This implies that the risk of bias approach, where context specific judgements are made related to the potential for bias, is the preferred approach for assessing internal validity. The risk of bias approach is well developed for RCTs. Therefore, when RCTs are included in the evidence available to address a clinical question, a risk of bias assessment approach should be used. When evaluating internal validity as a component of a SR-MA, the Cochrane ROB2.0 tool ( 16 ) could be used for this purpose. Modifications to this tool have been proposed for evaluating trials in livestock trials ( 49 – 51 ). For critical appraisal instruments for RCTs, where additional components such as feasibility and external validity are a desired component, the questions or items within the instrument that are specific to assessing internal validity still could follow a risk of bias approach by specifically requiring a judgement on the potential for bias. Similarly, the use of questions or items requiring a judgement on the potential for bias also could be used for evaluation of RCTs included in clinical practice guidelines when RCTs are present in the evidence base.
However, there are circumstances where these recommendations may not be appropriate or sufficient, such as for observational studies where risk of bias assessment instruments do not formally exist, or where a variety of study designs have been identified that answer the clinical question (particularly non-intervention type questions). When observational studies are used as evidence, individuals assessing internal validity may wish to evaluate risks of bias for each study ad hoc by considering the specific risks of bias related to selection bias, information bias, and confounding in the context of the topic area. However, this approach requires considerable methodological expertise. Alternatively, a quality assessment approach could be used to evaluate internal validity for observational studies, recognizing that more assumptions related to the potential for bias are involved. As instruments for evaluating the risk of bias for observational studies are developed and validated, these could replace ad hoc or quality assessment approaches.
For situations where the evidence base includes multiple study types, such as clinical practice guidelines, the use of levels of evidence may be useful for framing the potential for bias inherent in the studies identified to address the clinical questions. However, within each evidence level, there still is a need to evaluate the internal validity of each study. The proposed approach for situations where RCTs and observational studies are included in the evidence base was described in the preceding paragraphs. For lower levels of evidence, such as case series, textbooks and narrative reviews, and expert opinion, levels of evidence could be used to emphasize that these types of evidence have high potential for bias based on their design.
Broader Considerations
It should be noted that although this article has focused on approaches to evaluating internal validity of studies, this is only one component of the assessment of evidence. Critical appraisal, CATs, SR-MA, and clinical practice guidelines explicitly incorporate other aspects of decision-making, including a consideration of the magnitude and precision of an intervention effect or the potential clinical impact, the consistency of the research results across studies, the applicability (external validity and feasibility) of the research results, and the directness of the evidence to a clinical situation (for instance, whether the study populations are similar to those in a practice setting). However, a discussion of these components for decision-making is beyond the scope of the current study. The interested reader is referred to further details on the components used in evaluating evidence for CATs ( 4 ), for SR-MA using the GRADE approach ( 52 ), for network meta-analysis ( 53 ) and for clinical practice guidelines ( 8 , 44 ).
Author Contributions
JS drafted the manuscript. All authors contributed equally to the conceptualization of this work. All authors read and approved the final contents.
Partial funding support was obtained from the University of Guelph Research Leadership Chair (Sargeant).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Research on the DCT vehicle starting process evaluation based on LSTM neural network with attention mechanism
- Original Article
- Published: 21 August 2024
Cite this article

- Zeyu Xu 1 &
- Haijiang Liu 1
24 Accesses
Explore all metrics
Currently, with the advancement of dual-clutch transmission (DCT) control systems and vehicle performance, it is necessary to develop better objective evaluation methods for DCT vehicles. The starting process is a critical element affecting the driving and riding experience of DCT vehicles. Therefore, it is crucial to establish and improve a starting process evaluation model for the objective evaluation to DCT vehicles and optimization to DCT control strategies. This paper proposes a new method to evaluate the DCT vehicle starting process objectively. The method analyzes and models the time-series signals of the driving data using the LSTM neural network and uses the attention mechanism to improve the evaluation performance and enhance the interpretability of the evaluation results. Taking the dynamic performance evaluation as an example, the evaluation results indicate that the proposed model is better than the conventional methods, showing notable efficacy and preponderance.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Model Predictive Adaptive Cruise Control of Intelligent Electric Vehicles Based on Deep Reinforcement Learning Algorithm FWOR Driver Characteristics
An intelligent energy management and traffic predictive model for autonomous vehicle systems
Dual-attention network with multitask learning for multistep short-term speed prediction on expressways
Explore related subjects.
- Artificial Intelligence
T. Ali Böke, E. İsa Albak and N. Kaya, Correlation between objective and subjective tests for vehicle ride comfort evaluations, Proceedings of the Institution of Mechanical Engineers, Part D: Journal of Automobile Engineering , 237 (4) (2022) 706–721.
Google Scholar
W. Zhou, X. Guo and X. Pei, Objective evaluation of drivability in passenger cars with dual-clutch transmission: a case study of static gearshift condition, Mathematical Problems in Engineering , 2020 (2020) 1–13.
W. Huang, J. Liu and Y. Ma, Drivability evaluation model of engine start based on principal component analysis and support vector regression, SAE Technical Paper (2019) 2019-01-0932.
Y. Lei, Y. Zhang, Y. Fu, L. Wang and L. Zhang, Objective evaluation model of automatic transmission shift quality based on multi-hierarchical grey relational analysis, SAE Technical Paper (2018) 2018-01-0405.
W. Huang and H. Liu, Application of fuzzy dynamic weights drivability evaluation model in tip-in condition, Journal of Vibration and Control , 25 (4) (2018) 739–747.
Article Google Scholar
P. Schoeggl and E. Ramschak, Vehicle driveability assessment using neural networks for development, calibration and quality tests, SAE 2000 World Congress , Detroit, USA (2000).
W. Huang, H. J. Liu and Y. F. Ma, Drivability evaluation model using principal component analysis and optimized extreme learning machine, Journal of Vibration and Control , 25 (16) (2019) 2274–2281.
M. Wang, W. Miao, Y. Tan, K. Wu, X. Li, Y. Gu and L. Chen, Evaluation and prediction method of automotive electronic accelerator pedal based on support vector regression, Proceedings of the Institution of Mechanical Engineers, Part D: Journal of Automobile Engineering , 238 (4) (2024).
W. Zhou, X. Guo and C. Zhang, A novel objective evaluation method of drivability for passenger cars considering subjective and objective consistency, Proceedings of the Institution of Mechanical Engineers, Part D: Journal of Automobile Engineering , 237 (4) (2022) 607–621.
Z. Xu and H. Liu, Research on the identification of DCT vehicle driver’s starting intention based on LSTM neural network and multi-sensor data fusion, Proceedings of the Institution of Mechanical Engineers, Part D: Journal of Automobile Engineering , 237 (12) (2022) 2928–2941.
J. Khan, E. Lee and K. Kim, A higher prediction accuracy-based alpha-beta filter algorithm using the feedforward artificial neural network, CAAI Transactions on Intelligence Technology , 8 (4) (2023) 1124–1139.
O. J. Awujoola, P. Odion, A. Evwiekpaefe and G. N. Obunadike, Multi-stream fast Fourier convolutional neural network for automatic target recognition of ground military vehicle, Artificial Intelligence and Applications , 2 (2) (2022) https://doi.org/10.47852/bonviewAIA2202412 .
M. W. Jiang, P. Y. Zeng and K. Wang, FECAM: frequency enhanced channel attention mechanism for time series forecasting, Adv Eng Inform , 58 (2023) 12.
C. L. Zhou, Q. Shi and D. He, Spectral-spatial sequence characteristics-based convolutional transformer for hyperspectral change detection, CAAI Transactions on Intelligence Technology , 8 (4) (2023) 1237–1257.
H. Cao, Y. Wu, Y. Bao, X. Feng, S. Wan and C. Qlan, UTrans-Net: A model for short-term precipitation prediction, Artificial Intelligence and Applications , 1 (2) (2022) 106–113.
S. C. Limeros, S. Majchrowska, J. Johnander, C. Petersson, M. A. Sotelo and D. F. Llorca, Towards trustworthy multi-modal motion prediction: holistic evaluation and interpretability of outputs, CAAI Transactions on Intelligence Technology , 9 (3) (2024) 557–572.
M. Asadzadehkaljahi, A. Halder, P. Shivakumara and U. Pal, Spatiotemporal FFT based approach for arbitrarily moving object classification in videos of protected and sensitive scenes, Artificial Intelligence and Applications (2023).
S. Tang, W. Su, Y. Yang, L. Chen and M. Ye, Model adaptation via credible local context representation, CAAI Transactions on Intelligence Technology (2023).
W. Sun, Y. L. Hua and G. Q. Liu, A test study of wet dual clutch transmission during vehicle launch, Advanced Materials Research , 490–495 (2012) 86–90.
D. Qin, Y. Liu and J. Hu, Control and simulation of launch with two clutches for dual clutch transmissions, Chinese Journal of Mechanical Engineering , 46 (18) (2010) 121–127.
L. Li, Z. Zhu and X. Wang, Identification of a driver’s starting intention based on an artificial neural network for vehicles equipped with an automated manual transmission, Proceedings of the Institution of Mechanical Engineers, Part D: Journal of Automobile Engineering , 230 (10) (2016) 1417–1429.
K. Chen, X. Zhang, Y. Liu and J. Ma, An improved denoise method based on EEMD and optimal wavelet threshold for model building of OPAX, Proceedings of the Institution of Mechanical Engineers, Part D: Journal of Automobile Engineering , 235 (14) (2021) 3530–3544.
Y. LeCun, Y. Bengio and G. Hinton, Deep learning, Nature , 521 (7553) (2015) 436–444.
Y. Zhao, P. Joshi and D. G. Shin, Recurrent neural network for gene regulation network construction on time series expression data, IEEE International Conference on Bioinformatics and Biomedicine (BIBM) , IEEE, San Diego, CA (2019) 610–615.
J. S. Zhang and X. C. Xiao, Predicting chaotic time series using recurrent neural network, Chin. Phys. Lett. , 17 (2) (2000) 88–90.
M. Husken and P. Stagge, Recurrent neural networks for time series classification, Neurocomputing , 50 (2003) 223–235.
Y. G. Liu, P. Zhao and D. T. Qin, Driving intention identification based on long short-term memory neural network, 16th IEEE Vehicle Power and Propulsion Conference (VPPC) , Hanoi, Vietnam (2019).
S. Hochreiter and J. Schmidhuber, Long short-term memory, Neural Comput. , 9 (8) (1997) 1735–1780.
Y. Wang, C. Yang and D. Xu, Evaluation and prediction method of rolling bearing performance degradation based on attention-LSTM, Shock and Vibration , 2021 (2021) 1–15.
X. Kun, T. Shan, T. Yi and C. Chao, Attention-based long short-term memory network temperature prediction model, 2021 7th International Conference on Condition Monitoring of Machinery in Non-Stationary Operations (CMMNO) , Guanzhou, China (2021) 278–281.
Chapter Google Scholar
N. Fatima, Enhancing performance of a deep neural network: a comparative analysis of optimization algorithms, ADCAIJ: Advances in Distributed Computing and Artificial Intelligence Journal , 9 (2) (2020) 79–90.
Article MathSciNet Google Scholar
D. P. Kingma and J. L. Ba, ADAM: A method for stochastic optimization, 3rd International Conference on Learning Representations , San Diego, USA (2015).
Download references
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. U1764259).
Author information
Authors and affiliations.
School of Mechanical Engineering, Tongji University, Shanghai, 201804, People’s Republic of China
Zeyu Xu & Haijiang Liu
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Zeyu Xu .
Additional information
Zeyu Xu is currently pursuing the Ph.D. degree in mechanical engineering with Tongji University. His research interests include vehicle evaluation, vehicle detection, data mining and condition identifycation.
Haijiang Liu was born in 1976. He received the B.S., M.S., and Ph.D. degrees in mechanical engineering from Chongqing University. He is currently a Professor and a Doctoral Supervisor with the School of Mechanical Engineering, Tongji University. His research interests include vehicle evaluation, vehicle detection and intelligent manufacturing technology.
Rights and permissions
Reprints and permissions
About this article
Xu, Z., Liu, H. Research on the DCT vehicle starting process evaluation based on LSTM neural network with attention mechanism. J Mech Sci Technol (2024). https://doi.org/10.1007/s12206-024-0811-8
Download citation
Received : 04 March 2024
Revised : 21 April 2024
Accepted : 14 May 2024
Published : 21 August 2024
DOI : https://doi.org/10.1007/s12206-024-0811-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Attention mechanism
- DCT vehicle
- Dual-clutch transmission
- Drivability
- Starting process
- Find a journal
- Publish with us
- Track your research

IMAGES
COMMENTS
By evaluating research, individuals can develop their critical thinking skills and become more discerning consumers of information. Improving the quality of research: Evaluating research can help improve the quality of research by identifying areas where improvements can be made. This can lead to more rigorous research methods and better ...
Critically evaluate the research paper using the checklist provided, making notes on the key points and your overall impression. Discussion. Critical appraisal checklists are useful tools to help assess the quality of a study. Assessment of various factors, including the importance of the research question, the design and methodology of a study ...
Abstract. Critically reviewing the literature is an indispensible skill which is used throughout a research career. This demystifies the processes involved in systematically and critically reviewing the literature to demonstrate knowledge, identify research ideas and questions, position research and develop theory.
Understanding and Evaluating Research: A Critical Guide shows students how to be critical consumers of research and to appreciate the power of methodology as it shapes the research question, the use of theory in the study, the methods used, and how the outcomes are reported. The book starts with what it means to be a critical and uncritical ...
INTRODUCTION. Critical appraisal of a research paper is defined as "The process of carefully and systematically examining research to judge its trustworthiness, value and relevance in a particular context."[] Since scientific literature is rapidly expanding with more than 12,000 articles being added to the MEDLINE database per week,[] critical appraisal is very important to distinguish ...
Critical Appraisal. Critical appraisal is the process of systematically evaluating research using established and transparent methods. In critical appraisal, health professionals use validated checklists/worksheets as tools to guide their assessment of the research. It is a more advanced way of evaluating research than the more basic method ...
Critical evaluation is the process of examining the research for the strength or weakness of the findings, validity, relevance, and usefulness of the research findings. [ 1] The availability of extensive information and the difficulty in differentiating the relevant information obligate the primary need of critical appraisal.
Critical appraisal. ' The process of carefully and systematically examining research to judge its trustworthiness, and its value and relevance in a particular context '. -Burls A [ 1] The objective of medical literature is to provide unbiased, accurate medical information, backed by robust scientific evidence that could aid and enhance ...
Critical Evaluation of Information Sources. After initial evaluation of a source, the next step is to go deeper. This includes a wide variety of techniques and may depend on the type of source. In the case of research, it will include evaluating the methodology used in the study and requires you to have knowledge of those discipline-specific ...
Key Points. Critical appraisal is a systematic process used to identify the strengths and weaknesses of a research article. Critical appraisal provides a basis for decisions on whether to use the ...
to your own research at all stages. This approach will help you when conducting a critical evaluation of the literature (moving towards a systematic review and evidence synthesis; see Chapter 2). When you come to design your own research, critical appraisal across multiple stud-
Take notes on the articles as you read them and identify any themes or concepts that may apply to your research question. This sample template (below) may also be useful for critically reading and organizing your articles. Or you can use this online form and email yourself a copy.
Critically reviewing the literature is an indispensible skill which is used throughout a research career. This article demystifies the processes involved in systematically and critically reviewing the literature to demonstrate knowledge, identify research ideas, position research and develop theory. Although aimed primarily at research students ...
Academic Journals. Evaluating Research in Academic Journals is a guide for students who are learning how to. evaluate reports of empirical research published in academic journals. It breaks down ...
What is needed when faced with such research are different criteria; a new list to work with and apply to critically evaluate the research. At other times guidelines may contain criteria that are now deemed problematic and perhaps outdated. Hence, it is vital to not only stay up-to-date with contemporary debates, but also to avoid thinking of ...
Six key questions will help readers to assess qualitative research #### Summary points Over the past decade, readers of medical journals have gained skills in critically appraising studies to determine whether the results can be trusted and applied to their own practice settings. Criteria have been designed to assess studies that use quantitative methods, and these are now in common use.
Primary sources are the raw material of the research process. Secondary sources are based on primary sources. For example, if you were researching Konrad Adenauer's role in rebuilding West Germany after World War II, Adenauer's own writings would be one of many primary sources available on this topic.
Critical appraisal is the course of action for watchfully and systematically examining research to assess its reliability, value and relevance in order to direct professionals in their vital clinical decision making [ 1 ]. Critical appraisal is essential to: Continuing Professional Development (CPD).
Criteria and criteria measures. The criteria measures must demonstrate reliability and validity for both, the independent and dependent variable. Data analysis. Appropriate statistical tests should be applied for the type of data obtained, and assumptions for their use met. Post hoc tests should be applied when multiple comparisons are performed.
Critically Evaluating Sources (what do we mean by "sources"?) For college-level research, you'll want to consider using only the highest-quality information sources that you can find. Between the internet and SU's library, the "best" information can depend on the assignment. Here are some ways to determine the best information sources to ...
critiquing the literature, critical analysis, reviewing the literature, evaluation and appraisal of the literature which are in essence the same thing (Bassett and Bassett, 2003). Terminology in research can be confusing for the novice research reader where a term like 'random' refers to an organized manner of selecting items or participants ...
The primary goal of evaluation is to understand the significance and value of a source in relation to other sources and your own thinking on a topic. Note that some evaluative questions will be more important than others depending on your needs as a researcher. Figuring out which questions are important to ask in a given situation is part of ...
This comprehensive review investigates the pivotal role of reactive oxygen species (ROS) in cataract formation and evaluates the potential of antioxidant therapies in mitigating this ocular condition. By elucidating the mechanisms of oxidative stress, the article examines how ROS contribute to the deterioration of lens proteins and lipids, leading to the characteristic aggregation, cross ...
Systematic review, meta-analysis, and network meta-analysis: Systematic review is a structured methodology for identifying, selecting and evaluating all relevant research to address a structured question, which may relate to descriptive characteristics such as prevalence, etiology, efficacy of interventions, or diagnostic test accuracy ().Meta-analysis is the statistical combination of results ...
Currently, with the advancement of dual-clutch transmission (DCT) control systems and vehicle performance, it is necessary to develop better objective evaluation methods for DCT vehicles. The starting process is a critical element affecting the driving and riding experience of DCT vehicles. Therefore, it is crucial to establish and improve a starting process evaluation model for the objective ...