Understand the principles of Human Research Ethics
Are you supporting or planning to engage in research with or about people, their data or their tissue? A new, self-paced learning module now available on Canvas entitled Human Research Ethics introduces its values, principles and review.

The module provides information to help you design a human research project and understand how ethics reviewers will consider your design against the guidance provided in the National Statement on Ethical Conduct in Human Research .
Based on, and directly cross referencing with the National Statement, the module outlines why research ethics review was introduced internationally. It introduces the key agencies and documents associated with Australian ethics review and points out important changes in 2023 National Statement. These include a refined risk matrix approach and the requirements of granting an exemption from ethics review.
Merrilee Kessler, UTS Research Ethics Coordinator said that UTS commissioned this module for its research community.
“We wanted to provide a course that introduced the key research ethics concepts such as risk and benefit, consent, recruitment and data management, in a way that helps researchers understand the language and intent of ethics review,” she said.

The module highlights the kinds of responses needed in an ethics application to show that the research design meets the expectations of the National Statement. It looks at current issues like AI, social media in research and Big Data.
“It also includes research that UTS doesn’t come across as often, but which provides great scope for ethical investigation, such as Genomic research and Animal-to-human xenotransplantation,” Merrilee explained.
We wanted to provide a course that introduced the key research ethics concepts such as risk and benefit, consent, recruitment and data management
Keith Heggart, UTS researcher and ethics reviewer, was one of the first to complete the module.
“I think it’s important for early career researchers to understand that going through the ethics process is not just a matter of ticking the right boxes and filling in the right forms. Instead, it’s a thoughtful, nuanced engagement with ideas like justice, beneficence and risk,” he said.
“Done well, careful completion of the ethics approval process improves research projects. This course does a great job of carefully explaining this point, and as such, I recommend it for all early career researchers - and experienced ones too!”
Done well, careful completion of the ethics approval process improves research projects.

Contributing to research excellence
Importantly, the Human Research Ethics module covers key aspects of the UTS Research Outcomes Capability Framework | RES Hub (uts.edu.au) including those relating to:
- Research life cycle: Best practice in research project management from research ethics and integrity, policy and procedures, data management policy and systems to IP management and security.
- Research leadership: Confidence to champion research integrity and best practice in research project management, mentor successfully and deliver ethical and robust research with integrity.
- Creativity and innovation: Knowledge of ‘human-centred’ research methods and practices.
- Indigenous led knowledges and research: Knowledge and understanding of Indigenous Research Ethics (e.g. AIATSIS, NHMRC, community protocols) and the ability to ensure that research processes and outputs will not harm Indigenous peoples and communities.
“We also look at the relevant UTS policies, procedures and systems,” added Merrilee.
“At the end of the course, participants will have the ability to identify and manage risk and appropriately manage data – including by planning for re-use and reproducibility, archiving and sharing with appropriate audiences.”
About the Human Research Ethics Module
Human Research Ethics takes approximately two hours to complete and can be accessed as many times as needed once an account has been opened.
A Certificate of Completion is available for download.
Access the training at https://canvas.uts.edu.au/enroll/GEKRK8
Find ethics training and support
- Visit the Ethics Sharepoint site - Research Ethics and Integrity - Home (sharepoint.com)
- Make an appointment to attend clinics for Animal Ethics, General Research Ethics or Health Related Research - Ethics clinics (sharepoint.com)
- Register for Good Clinical Practice (GCP) training- GCP training page
Need more information? Get in touch with the Research Ethics team by email [email protected]
RES Hub acknowledges and pays respect to the Gadigal people of the Eora Nation, the Boorooberongal people of the Dharug Nation, the Bidiagal people and the Gamaygal people, upon whose ancestral lands where UTS now stands.
We pay respect to their Elders; past, present, emerging and future as the traditional custodians of Country and knowledge for this land. We recognise their continued connection to the land and waters and the continuation of their cultural, spiritual, and educational practices. We extend this respect to all Aboriginal and/or Torres Strait Islander peoples who visit RES Hub.
As a place for the UTS community to connect and collaborate, RES Hub acknowledges the long-standing traditional practices of these communities in gathering to share experience, knowledge, and history. RES Hub acknowledges that sovereignty was never ceded. This is and always will be Aboriginal Land.
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.

Standards and Operational Guidance for Ethics Review of Health-Related Research with Human Participants
- Copyright and Permissions
The new WHO publication “Standards and operational guidance for ethics review of health-related research with human participants”, is a compilation of 10 standards that are applicable to the ethics review of health related research with human participants. This document is intended to provide guidance on the research ethics review process, not to take a substantive position on how particular ethical dilemmas in health-related research should be resolved. It is designed to serve as a basis upon which Research Ethics Committees (RECs) can develop their own specific practices and written procedures, and benchmark their achievements.
The term “standards” is used to delineate general principles and norms that all research ethics systems are expected to follow. This publication highlights the importance of a systems approach to research ethics, and includes and delineates the role of national governments and relevant legal and regulatory authorities.
- Collapse All
- Acknowledgements
- Standard 1 Responsibility for establishing the research ethics review system
- Standard 2 Composition of research ethics committees
- Standard 3 Research ethics committee resources
- Standard 4 Independence of research ethics committees
- Standard 5 Training the research ethics committee
- Standard 6 Transparency, accountability, and quality of the research ethics committee
- Standard 7 Ethical basis for decision-making in research ethics committees
- Standard 8 Decision-making procedures for research ethics committees
- Standard 9 Written policies and procedures
- Standard 10 Researchers' responsibilities
- Annex 1 Guidelines and codes of best practice and statutes and regulations
- Annex 2 Guidance for developing terms of reference for the Secretariat of the research ethics committee
- Annex 3 Guidance for developing written procedures for the research ethics committee
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers' products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use.
All rights reserved. Publications of the World Health Organization are available on the WHO web site ( www.who.int ) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: tni.ohw@sredrokoob ).
Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press through the WHO web site ( http://www.who.int/about/licensing/copyright_form/en/index.html ).
- Cite this Page Standards and Operational Guidance for Ethics Review of Health-Related Research with Human Participants. Geneva: World Health Organization; 2011.
- PDF version of this title (1.2M)
Other titles in this collection
- WHO Guidelines Approved by the Guidelines Review Committee
Related information
- NLM Catalog Related NLM Catalog Entries
Similar articles in PubMed
- Law, ethics and research ethics committees. [Med Law. 2002] Law, ethics and research ethics committees. Beyleveld D. Med Law. 2002; 21(1):57-75.
- American Society of Clinical Oncology policy statement: oversight of clinical research. [J Clin Oncol. 2003] American Society of Clinical Oncology policy statement: oversight of clinical research. American Society of Clinical Oncology. J Clin Oncol. 2003 Jun 15; 21(12):2377-86. Epub 2003 Apr 29.
- Payment of research participants: current practice and policies of Irish research ethics committees. [J Med Ethics. 2013] Payment of research participants: current practice and policies of Irish research ethics committees. Roche E, King R, Mohan HM, Gavin B, McNicholas F. J Med Ethics. 2013 Sep; 39(9):591-3. Epub 2012 Dec 1.
- Review Research ethics committees in developing countries and informed consent: with special reference to Turkey. [J Lab Clin Med. 2003] Review Research ethics committees in developing countries and informed consent: with special reference to Turkey. Oguz NY. J Lab Clin Med. 2003 May; 141(5):292-6.
- Review Biomedical Research Ethics Committees in sub-Saharan Africa: a collective review of their structure, functioning, and outcomes. [J Empir Res Hum Res Ethics. 2015] Review Biomedical Research Ethics Committees in sub-Saharan Africa: a collective review of their structure, functioning, and outcomes. Silaigwana B, Wassenaar D. J Empir Res Hum Res Ethics. 2015 Apr; 10(2):169-84. Epub 2015 Mar 6.
Recent Activity
- Standards and Operational Guidance for Ethics Review of Health-Related Research ... Standards and Operational Guidance for Ethics Review of Health-Related Research with Human Participants
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
National Institute of Environmental Health Sciences
Your environment. your health., what is ethics in research & why is it important, by david b. resnik, j.d., ph.d..
December 23, 2020
The ideas and opinions expressed in this essay are the author’s own and do not necessarily represent those of the NIH, NIEHS, or US government.

When most people think of ethics (or morals), they think of rules for distinguishing between right and wrong, such as the Golden Rule ("Do unto others as you would have them do unto you"), a code of professional conduct like the Hippocratic Oath ("First of all, do no harm"), a religious creed like the Ten Commandments ("Thou Shalt not kill..."), or a wise aphorisms like the sayings of Confucius. This is the most common way of defining "ethics": norms for conduct that distinguish between acceptable and unacceptable behavior.
Most people learn ethical norms at home, at school, in church, or in other social settings. Although most people acquire their sense of right and wrong during childhood, moral development occurs throughout life and human beings pass through different stages of growth as they mature. Ethical norms are so ubiquitous that one might be tempted to regard them as simple commonsense. On the other hand, if morality were nothing more than commonsense, then why are there so many ethical disputes and issues in our society?
Alternatives to Animal Testing
Alternative test methods are methods that replace, reduce, or refine animal use in research and testing
Learn more about Environmental science Basics
One plausible explanation of these disagreements is that all people recognize some common ethical norms but interpret, apply, and balance them in different ways in light of their own values and life experiences. For example, two people could agree that murder is wrong but disagree about the morality of abortion because they have different understandings of what it means to be a human being.
Most societies also have legal rules that govern behavior, but ethical norms tend to be broader and more informal than laws. Although most societies use laws to enforce widely accepted moral standards and ethical and legal rules use similar concepts, ethics and law are not the same. An action may be legal but unethical or illegal but ethical. We can also use ethical concepts and principles to criticize, evaluate, propose, or interpret laws. Indeed, in the last century, many social reformers have urged citizens to disobey laws they regarded as immoral or unjust laws. Peaceful civil disobedience is an ethical way of protesting laws or expressing political viewpoints.
Another way of defining 'ethics' focuses on the disciplines that study standards of conduct, such as philosophy, theology, law, psychology, or sociology. For example, a "medical ethicist" is someone who studies ethical standards in medicine. One may also define ethics as a method, procedure, or perspective for deciding how to act and for analyzing complex problems and issues. For instance, in considering a complex issue like global warming , one may take an economic, ecological, political, or ethical perspective on the problem. While an economist might examine the cost and benefits of various policies related to global warming, an environmental ethicist could examine the ethical values and principles at stake.
See ethics in practice at NIEHS
Read latest updates in our monthly Global Environmental Health Newsletter

Many different disciplines, institutions , and professions have standards for behavior that suit their particular aims and goals. These standards also help members of the discipline to coordinate their actions or activities and to establish the public's trust of the discipline. For instance, ethical standards govern conduct in medicine, law, engineering, and business. Ethical norms also serve the aims or goals of research and apply to people who conduct scientific research or other scholarly or creative activities. There is even a specialized discipline, research ethics, which studies these norms. See Glossary of Commonly Used Terms in Research Ethics and Research Ethics Timeline .
There are several reasons why it is important to adhere to ethical norms in research. First, norms promote the aims of research , such as knowledge, truth, and avoidance of error. For example, prohibitions against fabricating , falsifying, or misrepresenting research data promote the truth and minimize error.
Join an NIEHS Study
See how we put research Ethics to practice.
Visit Joinastudy.niehs.nih.gov to see the various studies NIEHS perform.

Second, since research often involves a great deal of cooperation and coordination among many different people in different disciplines and institutions, ethical standards promote the values that are essential to collaborative work , such as trust, accountability, mutual respect, and fairness. For example, many ethical norms in research, such as guidelines for authorship , copyright and patenting policies , data sharing policies, and confidentiality rules in peer review, are designed to protect intellectual property interests while encouraging collaboration. Most researchers want to receive credit for their contributions and do not want to have their ideas stolen or disclosed prematurely.
Third, many of the ethical norms help to ensure that researchers can be held accountable to the public . For instance, federal policies on research misconduct, conflicts of interest, the human subjects protections, and animal care and use are necessary in order to make sure that researchers who are funded by public money can be held accountable to the public.
Fourth, ethical norms in research also help to build public support for research. People are more likely to fund a research project if they can trust the quality and integrity of research.
Finally, many of the norms of research promote a variety of other important moral and social values , such as social responsibility, human rights, animal welfare, compliance with the law, and public health and safety. Ethical lapses in research can significantly harm human and animal subjects, students, and the public. For example, a researcher who fabricates data in a clinical trial may harm or even kill patients, and a researcher who fails to abide by regulations and guidelines relating to radiation or biological safety may jeopardize his health and safety or the health and safety of staff and students.
Codes and Policies for Research Ethics
Given the importance of ethics for the conduct of research, it should come as no surprise that many different professional associations, government agencies, and universities have adopted specific codes, rules, and policies relating to research ethics. Many government agencies have ethics rules for funded researchers.
- National Institutes of Health (NIH)
- National Science Foundation (NSF)
- Food and Drug Administration (FDA)
- Environmental Protection Agency (EPA)
- US Department of Agriculture (USDA)
- Singapore Statement on Research Integrity
- American Chemical Society, The Chemist Professional’s Code of Conduct
- Code of Ethics (American Society for Clinical Laboratory Science)
- American Psychological Association, Ethical Principles of Psychologists and Code of Conduct
- Statement on Professional Ethics (American Association of University Professors)
- Nuremberg Code
- World Medical Association's Declaration of Helsinki
Ethical Principles
The following is a rough and general summary of some ethical principles that various codes address*:

Strive for honesty in all scientific communications. Honestly report data, results, methods and procedures, and publication status. Do not fabricate, falsify, or misrepresent data. Do not deceive colleagues, research sponsors, or the public.

Objectivity
Strive to avoid bias in experimental design, data analysis, data interpretation, peer review, personnel decisions, grant writing, expert testimony, and other aspects of research where objectivity is expected or required. Avoid or minimize bias or self-deception. Disclose personal or financial interests that may affect research.

Keep your promises and agreements; act with sincerity; strive for consistency of thought and action.

Carefulness
Avoid careless errors and negligence; carefully and critically examine your own work and the work of your peers. Keep good records of research activities, such as data collection, research design, and correspondence with agencies or journals.

Share data, results, ideas, tools, resources. Be open to criticism and new ideas.
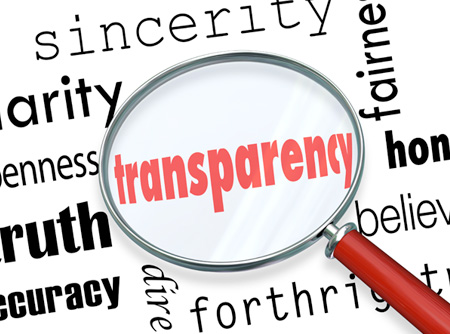
Transparency
Disclose methods, materials, assumptions, analyses, and other information needed to evaluate your research.

Accountability
Take responsibility for your part in research and be prepared to give an account (i.e. an explanation or justification) of what you did on a research project and why.

Intellectual Property
Honor patents, copyrights, and other forms of intellectual property. Do not use unpublished data, methods, or results without permission. Give proper acknowledgement or credit for all contributions to research. Never plagiarize.

Confidentiality
Protect confidential communications, such as papers or grants submitted for publication, personnel records, trade or military secrets, and patient records.

Responsible Publication
Publish in order to advance research and scholarship, not to advance just your own career. Avoid wasteful and duplicative publication.

Responsible Mentoring
Help to educate, mentor, and advise students. Promote their welfare and allow them to make their own decisions.

Respect for Colleagues
Respect your colleagues and treat them fairly.

Social Responsibility
Strive to promote social good and prevent or mitigate social harms through research, public education, and advocacy.

Non-Discrimination
Avoid discrimination against colleagues or students on the basis of sex, race, ethnicity, or other factors not related to scientific competence and integrity.

Maintain and improve your own professional competence and expertise through lifelong education and learning; take steps to promote competence in science as a whole.

Know and obey relevant laws and institutional and governmental policies.

Animal Care
Show proper respect and care for animals when using them in research. Do not conduct unnecessary or poorly designed animal experiments.

Human Subjects protection
When conducting research on human subjects, minimize harms and risks and maximize benefits; respect human dignity, privacy, and autonomy; take special precautions with vulnerable populations; and strive to distribute the benefits and burdens of research fairly.
* Adapted from Shamoo A and Resnik D. 2015. Responsible Conduct of Research, 3rd ed. (New York: Oxford University Press).
Ethical Decision Making in Research
Although codes, policies, and principles are very important and useful, like any set of rules, they do not cover every situation, they often conflict, and they require interpretation. It is therefore important for researchers to learn how to interpret, assess, and apply various research rules and how to make decisions and act ethically in various situations. The vast majority of decisions involve the straightforward application of ethical rules. For example, consider the following case:
The research protocol for a study of a drug on hypertension requires the administration of the drug at different doses to 50 laboratory mice, with chemical and behavioral tests to determine toxic effects. Tom has almost finished the experiment for Dr. Q. He has only 5 mice left to test. However, he really wants to finish his work in time to go to Florida on spring break with his friends, who are leaving tonight. He has injected the drug in all 50 mice but has not completed all of the tests. He therefore decides to extrapolate from the 45 completed results to produce the 5 additional results.
Many different research ethics policies would hold that Tom has acted unethically by fabricating data. If this study were sponsored by a federal agency, such as the NIH, his actions would constitute a form of research misconduct , which the government defines as "fabrication, falsification, or plagiarism" (or FFP). Actions that nearly all researchers classify as unethical are viewed as misconduct. It is important to remember, however, that misconduct occurs only when researchers intend to deceive : honest errors related to sloppiness, poor record keeping, miscalculations, bias, self-deception, and even negligence do not constitute misconduct. Also, reasonable disagreements about research methods, procedures, and interpretations do not constitute research misconduct. Consider the following case:
Dr. T has just discovered a mathematical error in his paper that has been accepted for publication in a journal. The error does not affect the overall results of his research, but it is potentially misleading. The journal has just gone to press, so it is too late to catch the error before it appears in print. In order to avoid embarrassment, Dr. T decides to ignore the error.
Dr. T's error is not misconduct nor is his decision to take no action to correct the error. Most researchers, as well as many different policies and codes would say that Dr. T should tell the journal (and any coauthors) about the error and consider publishing a correction or errata. Failing to publish a correction would be unethical because it would violate norms relating to honesty and objectivity in research.
There are many other activities that the government does not define as "misconduct" but which are still regarded by most researchers as unethical. These are sometimes referred to as " other deviations " from acceptable research practices and include:
- Publishing the same paper in two different journals without telling the editors
- Submitting the same paper to different journals without telling the editors
- Not informing a collaborator of your intent to file a patent in order to make sure that you are the sole inventor
- Including a colleague as an author on a paper in return for a favor even though the colleague did not make a serious contribution to the paper
- Discussing with your colleagues confidential data from a paper that you are reviewing for a journal
- Using data, ideas, or methods you learn about while reviewing a grant or a papers without permission
- Trimming outliers from a data set without discussing your reasons in paper
- Using an inappropriate statistical technique in order to enhance the significance of your research
- Bypassing the peer review process and announcing your results through a press conference without giving peers adequate information to review your work
- Conducting a review of the literature that fails to acknowledge the contributions of other people in the field or relevant prior work
- Stretching the truth on a grant application in order to convince reviewers that your project will make a significant contribution to the field
- Stretching the truth on a job application or curriculum vita
- Giving the same research project to two graduate students in order to see who can do it the fastest
- Overworking, neglecting, or exploiting graduate or post-doctoral students
- Failing to keep good research records
- Failing to maintain research data for a reasonable period of time
- Making derogatory comments and personal attacks in your review of author's submission
- Promising a student a better grade for sexual favors
- Using a racist epithet in the laboratory
- Making significant deviations from the research protocol approved by your institution's Animal Care and Use Committee or Institutional Review Board for Human Subjects Research without telling the committee or the board
- Not reporting an adverse event in a human research experiment
- Wasting animals in research
- Exposing students and staff to biological risks in violation of your institution's biosafety rules
- Sabotaging someone's work
- Stealing supplies, books, or data
- Rigging an experiment so you know how it will turn out
- Making unauthorized copies of data, papers, or computer programs
- Owning over $10,000 in stock in a company that sponsors your research and not disclosing this financial interest
- Deliberately overestimating the clinical significance of a new drug in order to obtain economic benefits
These actions would be regarded as unethical by most scientists and some might even be illegal in some cases. Most of these would also violate different professional ethics codes or institutional policies. However, they do not fall into the narrow category of actions that the government classifies as research misconduct. Indeed, there has been considerable debate about the definition of "research misconduct" and many researchers and policy makers are not satisfied with the government's narrow definition that focuses on FFP. However, given the huge list of potential offenses that might fall into the category "other serious deviations," and the practical problems with defining and policing these other deviations, it is understandable why government officials have chosen to limit their focus.
Finally, situations frequently arise in research in which different people disagree about the proper course of action and there is no broad consensus about what should be done. In these situations, there may be good arguments on both sides of the issue and different ethical principles may conflict. These situations create difficult decisions for research known as ethical or moral dilemmas . Consider the following case:
Dr. Wexford is the principal investigator of a large, epidemiological study on the health of 10,000 agricultural workers. She has an impressive dataset that includes information on demographics, environmental exposures, diet, genetics, and various disease outcomes such as cancer, Parkinson’s disease (PD), and ALS. She has just published a paper on the relationship between pesticide exposure and PD in a prestigious journal. She is planning to publish many other papers from her dataset. She receives a request from another research team that wants access to her complete dataset. They are interested in examining the relationship between pesticide exposures and skin cancer. Dr. Wexford was planning to conduct a study on this topic.
Dr. Wexford faces a difficult choice. On the one hand, the ethical norm of openness obliges her to share data with the other research team. Her funding agency may also have rules that obligate her to share data. On the other hand, if she shares data with the other team, they may publish results that she was planning to publish, thus depriving her (and her team) of recognition and priority. It seems that there are good arguments on both sides of this issue and Dr. Wexford needs to take some time to think about what she should do. One possible option is to share data, provided that the investigators sign a data use agreement. The agreement could define allowable uses of the data, publication plans, authorship, etc. Another option would be to offer to collaborate with the researchers.
The following are some step that researchers, such as Dr. Wexford, can take to deal with ethical dilemmas in research:
What is the problem or issue?
It is always important to get a clear statement of the problem. In this case, the issue is whether to share information with the other research team.
What is the relevant information?
Many bad decisions are made as a result of poor information. To know what to do, Dr. Wexford needs to have more information concerning such matters as university or funding agency or journal policies that may apply to this situation, the team's intellectual property interests, the possibility of negotiating some kind of agreement with the other team, whether the other team also has some information it is willing to share, the impact of the potential publications, etc.
What are the different options?
People may fail to see different options due to a limited imagination, bias, ignorance, or fear. In this case, there may be other choices besides 'share' or 'don't share,' such as 'negotiate an agreement' or 'offer to collaborate with the researchers.'
How do ethical codes or policies as well as legal rules apply to these different options?
The university or funding agency may have policies on data management that apply to this case. Broader ethical rules, such as openness and respect for credit and intellectual property, may also apply to this case. Laws relating to intellectual property may be relevant.
Are there any people who can offer ethical advice?
It may be useful to seek advice from a colleague, a senior researcher, your department chair, an ethics or compliance officer, or anyone else you can trust. In the case, Dr. Wexford might want to talk to her supervisor and research team before making a decision.
After considering these questions, a person facing an ethical dilemma may decide to ask more questions, gather more information, explore different options, or consider other ethical rules. However, at some point he or she will have to make a decision and then take action. Ideally, a person who makes a decision in an ethical dilemma should be able to justify his or her decision to himself or herself, as well as colleagues, administrators, and other people who might be affected by the decision. He or she should be able to articulate reasons for his or her conduct and should consider the following questions in order to explain how he or she arrived at his or her decision:
- Which choice will probably have the best overall consequences for science and society?
- Which choice could stand up to further publicity and scrutiny?
- Which choice could you not live with?
- Think of the wisest person you know. What would he or she do in this situation?
- Which choice would be the most just, fair, or responsible?
After considering all of these questions, one still might find it difficult to decide what to do. If this is the case, then it may be appropriate to consider others ways of making the decision, such as going with a gut feeling or intuition, seeking guidance through prayer or meditation, or even flipping a coin. Endorsing these methods in this context need not imply that ethical decisions are irrational, however. The main point is that human reasoning plays a pivotal role in ethical decision-making but there are limits to its ability to solve all ethical dilemmas in a finite amount of time.
Promoting Ethical Conduct in Science

Do U.S. research institutions meet or exceed federal mandates for instruction in responsible conduct of research? A national survey

Read about U.S. research instutuins follow federal manadates for ethics in research
Learn more about NIEHS Research
Most academic institutions in the US require undergraduate, graduate, or postgraduate students to have some education in the responsible conduct of research (RCR) . The NIH and NSF have both mandated training in research ethics for students and trainees. Many academic institutions outside of the US have also developed educational curricula in research ethics
Those of you who are taking or have taken courses in research ethics may be wondering why you are required to have education in research ethics. You may believe that you are highly ethical and know the difference between right and wrong. You would never fabricate or falsify data or plagiarize. Indeed, you also may believe that most of your colleagues are highly ethical and that there is no ethics problem in research..
If you feel this way, relax. No one is accusing you of acting unethically. Indeed, the evidence produced so far shows that misconduct is a very rare occurrence in research, although there is considerable variation among various estimates. The rate of misconduct has been estimated to be as low as 0.01% of researchers per year (based on confirmed cases of misconduct in federally funded research) to as high as 1% of researchers per year (based on self-reports of misconduct on anonymous surveys). See Shamoo and Resnik (2015), cited above.
Clearly, it would be useful to have more data on this topic, but so far there is no evidence that science has become ethically corrupt, despite some highly publicized scandals. Even if misconduct is only a rare occurrence, it can still have a tremendous impact on science and society because it can compromise the integrity of research, erode the public’s trust in science, and waste time and resources. Will education in research ethics help reduce the rate of misconduct in science? It is too early to tell. The answer to this question depends, in part, on how one understands the causes of misconduct. There are two main theories about why researchers commit misconduct. According to the "bad apple" theory, most scientists are highly ethical. Only researchers who are morally corrupt, economically desperate, or psychologically disturbed commit misconduct. Moreover, only a fool would commit misconduct because science's peer review system and self-correcting mechanisms will eventually catch those who try to cheat the system. In any case, a course in research ethics will have little impact on "bad apples," one might argue.
According to the "stressful" or "imperfect" environment theory, misconduct occurs because various institutional pressures, incentives, and constraints encourage people to commit misconduct, such as pressures to publish or obtain grants or contracts, career ambitions, the pursuit of profit or fame, poor supervision of students and trainees, and poor oversight of researchers (see Shamoo and Resnik 2015). Moreover, defenders of the stressful environment theory point out that science's peer review system is far from perfect and that it is relatively easy to cheat the system. Erroneous or fraudulent research often enters the public record without being detected for years. Misconduct probably results from environmental and individual causes, i.e. when people who are morally weak, ignorant, or insensitive are placed in stressful or imperfect environments. In any case, a course in research ethics can be useful in helping to prevent deviations from norms even if it does not prevent misconduct. Education in research ethics is can help people get a better understanding of ethical standards, policies, and issues and improve ethical judgment and decision making. Many of the deviations that occur in research may occur because researchers simply do not know or have never thought seriously about some of the ethical norms of research. For example, some unethical authorship practices probably reflect traditions and practices that have not been questioned seriously until recently. If the director of a lab is named as an author on every paper that comes from his lab, even if he does not make a significant contribution, what could be wrong with that? That's just the way it's done, one might argue. Another example where there may be some ignorance or mistaken traditions is conflicts of interest in research. A researcher may think that a "normal" or "traditional" financial relationship, such as accepting stock or a consulting fee from a drug company that sponsors her research, raises no serious ethical issues. Or perhaps a university administrator sees no ethical problem in taking a large gift with strings attached from a pharmaceutical company. Maybe a physician thinks that it is perfectly appropriate to receive a $300 finder’s fee for referring patients into a clinical trial.
If "deviations" from ethical conduct occur in research as a result of ignorance or a failure to reflect critically on problematic traditions, then a course in research ethics may help reduce the rate of serious deviations by improving the researcher's understanding of ethics and by sensitizing him or her to the issues.
Finally, education in research ethics should be able to help researchers grapple with the ethical dilemmas they are likely to encounter by introducing them to important concepts, tools, principles, and methods that can be useful in resolving these dilemmas. Scientists must deal with a number of different controversial topics, such as human embryonic stem cell research, cloning, genetic engineering, and research involving animal or human subjects, which require ethical reflection and deliberation.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Chapter 4: Research Ethics

2014, Doing Research in the Real World, 3rd edn

Related Papers
Journal of Emergency Nursing
Anne Manton
Bangladesh Journal of Physiology and Pharmacology
Mohammad Uzire Azam Khan
Ethics in research involving humans were first codified in 1946 as Nuremberg code. Subsequently other ethical declarations and guide lines were developed to protect the research participants as well as the researchers. The basic research bioethics includes three principles-respects for person, beneficence, and justice. To make a research with human subjects ethically sound the research protocol should have social and scientific values, fair subject selection, favorable risk benefit ratio, independent review, and informed consent of and respect for the participants. Above all the researcher should be honest and responsible enough to safeguard the rights and welfare of the research subjects. DOI: 10.3329/bjpp.v24i1.5734Bangladesh J Physiol Pharmacol 2008; 24(1&2) : 24-26
Reaz Mazumdar
Research involving human subjects are important to develop new therapeutics for the betterment of the human race. To take part in such research as volunteers is moral duty of any human. But such experiments should be justifiable and minimal risky for the participants. History of unethical research involving humans led to the development of many guidelines to make such research ethical as well as to gain maximum possible output. Several guidelines have been formulated to ensure research with human participants ethical. All the guidelines emphasize on one thing in particular- informed consent of the human subjects. Other considerations include rational benefit-harm ration, beneficence, justice, adequate research design and approval from proper authorities. All these guidelines aim to prevent any unethical research involving humans against their will.
Journal of Oral Health and Community Dentistry
sripriya nagarajan
Ghada Al Tajir
The twentieth century witnessed a succession of heinous experiments on human subjects in the name of science. The deplorable nature of these experiments led to the development of several guidelines that laid down the principles of research ethics. Respect, bene cence, and justice are the principles that form the foundation of research ethics today. These principles should be implemented through the channels of the informed consent process, privacy and con dentiality, risk bene t analysis, and fair recruitment. Proper implementation of research ethics ensures the protection of the rights and well-being of the participants. Some individuals are considered to be “vulnerable” in the research context because their autonomy is either diminished or lacking. Examples include children, some elderly persons, those with temporary or permanent cognitive impairment, prisoners, and refugees. Vulnerable groups require additional protection measures if they are involved in research. Public health research differs from general health research that necessitates additional ethical considerations. Research involving public health interventions or research conducted during public emergencies, such as natural disasters and disease outbreaks, has unique ethical challenges. Furthermore, in public health research, an understanding or familiarity with the community in which the research will be done is essential to ethical conduct of research. research ethics committees [otherwise known as institutional review boards (IRB)] play a central role in research involving human participants. The proposed research must be reviewed and approved prior to initiation and monitored thereafter with ongoing reviews of safety reports, progress reports, and emerging information or circumstances that may impact the study. A substantial number of conditions need to be met to ensure research starts and then remains ethical. Researchers should be quali ed by education, training, and experience to take on the role of investigators. The scienti c aspects of the research should be robust and valid and the research itself should be purposeful. It is important that ethical considerations be a constant, integrated into the research undertaking, from inception right through to the dissemination and or sharing of the results.
Amanda Hunn
Bodija Journal
Titilola H . Olojede
Ethical abuse of human subjects is still prevalent in researches. Biomedical research as the use of modern day medical technology to test hypothesis so as to deduce conclusions that are generalisable as theories and principles involve human subjects. If such research is not morally guided, it runs the danger of abusing the sacredness of human life and dehumanising the human person. Therefore, ethics as the philosophical science, which establishes the moral order of human acts would necessarily be required to check and balance the procedural systems of such research. It is within this context that this paper interrogates the role of ethics in biomedical research. This paper carefully and critically analyses how and why researchers abuse the human subject. Appealing to the Kantian moral imperatives, it opines that human subjects should never be treated as means to an end but as end in themselves. It succinctly points out the relevance of ethics in researches involving human subjects, particularly, biomedical research.
udo schuklenk
ABSTRACT This module will introduce you to the ethical concepts underlying applied ethical decision-making in the area of research involving human participants. We will also learn what the issues are that people involved in research on research ethics are concerned with. Ethics without an understanding of historical and legal context makes arguably little sense. It is for this reason that this module will begin with a brief history of research ethics and ends with a brief overview of the relevant national and international guidelines pertaining to ethical issues in research involving human participants.
Journal of the American Dietetic Association
Maureen Moran
Australasian Journal of Dermatology
David Wendler
RELATED PAPERS
The Southern Journal of Philosophy
Alexander Hadded
Lwandle Dumo
Samia Hurst
Journal of Microscopy and Ultrastructure
Shaista Guraya
Olutoye Olutayo
Emilee Moore
Brazilian Journal of Otorhinolaryngology (Impresso)
Marystella Takahashi , Henrique Ramos
Developing World Bioethics
GSC Advanced Research and Reviews
Nagla Hussien Mohamed Khalid
The International Journal of Tuberculosis and Lung Disease
Dirceu greco
European Textbook on Ethics in Research
Stephen Wilkinson
The American Journal of Bioethics
Alexander Kon
Annie Atkinson
The Journal of Law, Medicine & Ethics
Donald Chalmers
A Case-Based Textbook
Bernice S Elger
Ciênc. saúde coletiva
Silvia Koller
Michael Kalichman
Air Medical Journal
Edward Panacek
Tshidi M Wyllie
Tshidi M Wyllie,Ph.D.
American Journal of Bioethics
Mark Yarborough
Mwirigi Kiula
Journal of Medical Ethics
Lisbeth Franco
Michael O.S. Afolabi, PhD
Current Topics in Public Health
Charles Fokunang
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Fact sheets
- Facts in pictures
Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Health topics /
- Ethics and health
Global health ethics
Ethical questions related to health, health care, and public health cover topics as diverse as moral issues around reproduction, state obligations in the provision of health care services, and appropriate measures to control infectious disease. Scholars and health care professionals have debated ethical questions related to health and health care since the earliest days of medicine. Recent formal efforts to articulate international standards of ethics applicable to health and health care can be traced to the Nuremberg trials of 1947, during which the horrors of Nazi medical experiments came to light.
The principles that emerged from those trials, known as the Nuremberg Code, are broadly applicable to many types of health-related research involving human participants, including clinical trials. The growing breadth and complexity of contemporary health challenges have produced a range of difficult questions that cannot always be adequately addressed by relying exclusively on existing policies, guidelines or codes of conduct. Debates over access to new and expensive pharmaceuticals and medical technologies, as well as increasing awareness of the gross health disparities that exist both within and between countries, have called attention to the need for an ethics of health policy and practice.
Research ethics govern the standards of conduct for scientific researchers. It is important to adhere to ethical principles in order to protect the dignity, rights and welfare of research participants.
The WHO Manual (Section XV.2) defines research with human subjects as 'any social science, biomedical, behavioural, or epidemiological activity that entails systematic collection or analysis of data with the intent to generate new knowledge, in which human beings:
- are exposed to manipulation, intervention, observation, or other interaction with investigators either directly or through alteration of their environment; or
- become individually identifiable through investigator's collection, preparation, or use of biological material or medical or other records.
- Immunization raises a host of challenging ethical questions that researchers, governments, funders, pharmaceutical companies, and communities must confront.
- TB : Ethical issues include questions about the equitable distribution of resources, protection of vulnerable groups, respect for patient choice of treatment options and solidarity between communities during outbreaks.
- Zika has raised many specific ethical issues, in particular regarding pregnancy. At the same time, it has highlighted ethical issues that arise in vector-borne diseases more generally.
- The HIV epidemic has raised many ethical challenges for public health officials, researchers and clinicians, reaching from macro-level policy to micro-level clinical decisions.
- Questions and answers about ethics
- Ethics and COVID-19: resource allocation and priority setting
- Human Genome Editing (HGE) Registry
- Expert Group on Ethics and Governance of Artificial Intelligence for Health
- Health Ethics & Governance
- Ethics and COVID-19
WHO releases AI ethics and governance guidance for large multi-modal models
New Technical Advisory Group on Embedding Ethics in Health and Climate Change Policy (TAG-Ethics & Climate Health)
Launch of WHO tool for benchmarking ethics oversight of health-related research involving human participants
WHO kicks off deliberations on ethical framework and tools for social listening and infodemic management

WHO guidelines on ethical issues in public health surveillance

Ethics and governance of artificial intelligence for health: Guidance on large multi-modal models
Artificial Intelligence (AI) refers to the capability of algorithms integrated into systems and tools to learn from data so that they can perform automated...

Ethics and adaptive platform trial design in public health emergencies: meeting report,18-19 July 2022,...
Platform trials using adaptive methods have played a key role in the research response to COVID-19. They raise important ethical issues, however,...

WHO tool for benchmarking ethics oversight of health-related research involving human participants: user...
Jointly developed by WHO’s Regulatory System Strengthening, Regulation and Safety Unit and the Health Ethics & Governance Unit, it is intended...

WHO tool for benchmarking ethics oversight of health-related research involving human participants
Ensuring ethical standards and procedures for research with human beings
Developing normative guidance to address ethical challenges in global health
Supporting countries to manage ethical issues during outbreaks and emergencies
Engaging the global community in health ethics
Building ethics capacity
Framing the ethics of public health surveillance

Has the decolonizing global health agenda been colonized?

WHO Pandemic Ethics & Policy Summit: Prof David Archer, Chairperson of Nuffield Council on Bioethics

WHO Pandemic Ethics & Policy Summit - Charu Kauschic Chair of the Glopid-R Network

WHO Pandemic Ethics & Policy Summit: Laurence Lwoff
Related health topics.
Human genome editing
Vaccines and immunization

- My presentations
Auth with social network:
Download presentation
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you!
Presentation is loading. Please wait.
INTRODUCTION TO MEDICAL ETHICS
Published by Ira Rose Modified over 6 years ago
Similar presentations
Presentation on theme: "INTRODUCTION TO MEDICAL ETHICS"— Presentation transcript:

PHARMACIST CODE OF ETHICS

Lecture 3 Values & principles of professional ethics By Dr. Hala Yehia.

Ethics in HealthCare. Treating Patients With Dignity Sometimes health professionals get so wrapped up in the scientific principles of healthcare that.

Medical Ethics Lecturer :Noha Alaggad

The principles In Medical Ethics Lecturer :Noha Alaggad

Ethical and Moral Issues in Counseling

Mr. Caputo Unit #1 Lesson #5

Ethical and Legal Implications of Practice Chapter 5.

Ethical Issues.

Medical Ethics Dr. Raid Jastania. Right and Wrong.

Chapter 9 Ethical Issues.

What Would You Do? A Case Study in Ethics

2 Define the term “medical ethics” Differentiate between ethics and morality Differentiate between ethics and low.

Principles of medical ethics Lecture (4) Dr. rawhia Dogham.

Oviedo Convention and Its Protocols – Impact on Polish Law International Bioethics Conference Oviedo Convention in Central and Eastern European Countries.

Prepared by : Dr. Reem A.Jarra d. Introduction In their daily work nurses deal with events of : birth, death, & suffering. So they will be faced by many.

Ethics/Legal 6.03 Evaluate ethical and professional standards in a health care setting.

1 SCIENCES, TECHNOLOGY and MEDICINE SCIENCES, TECHNOLOGY and MEDICINE.

6.03 Ethics, Patient Rights, and Advance Directives for Healthcare

Legal Aspects of Health Information and Health Care Statistics Week 1 Robyn Korn, MBA, RHIA, CPHQ.
About project
© 2024 SlidePlayer.com Inc. All rights reserved.

Ethics in Research
Jul 23, 2014
120 likes | 429 Views
Ethics in Research. Chapter 2.1. What are ethics?. Ethics – the standards set for proper and responsible behavior. Ethical dilemmas . situations in which there is a choice to be made between two options, neither of which resolves the situation in an ethically acceptable fashion.
Share Presentation
- apa ethical guidelines
- varsity basketball

Presentation Transcript
Ethics in Research Chapter 2.1
What are ethics? • Ethics – the standards set for proper and responsible behavior
Ethical dilemmas • situations in which there is a choice to be made between two options, neither of which resolves the situation in an ethically acceptable fashion http://examples.yourdictionary.com/ethical-dilemma-examples.html
Personal Friendships Michael had several friends including Roger and Daniel. Roger has recently met and started dating a wonderful lady named Phyllis. He is convinced this is a long term relationship. Unknown to Roger, Michael observed them at a restaurant several days ago and realized Phyllis is the wife of his other friend Daniel. Michael is deciding whether to tell Roger that Phyllis is married when he receives a call from Daniel. Daniel suspects his wife is having an affair and since they and Michael share many friends and contacts, he asks if Michael has heard anything regarding an affair. http://examples.yourdictionary.com/ethical-dilemma-examples.html
Who lives? • A pregnant woman leading a group of people out of a cave on a coast is stuck in the mouth of that cave. In a short time high tide will be upon them, and unless she is unstuck, they will all be drowned except the woman, whose head is out of the cave. Fortunately, (or unfortunately,) someone has with him a stick of dynamite. There seems no way to get the pregnant woman loose without using the dynamite which will inevitably kill her; but if they do not use it everyone will drown. What should they do? http://examples.yourdictionary.com/ethical-dilemma-examples.html
Keep your word or not? The mood at Baileyville High School is tense with anticipation. For the first time in many, many years, the varsity basketball team has made it to the state semifinals. The community is excited too, and everyone is making plans to attend the big event next Saturday night. Jeff, the varsity coach, has been waiting for years to field such a team. Speed, teamwork, balance: they've got it all. Only one more week to practice, he tells his team, and not a rule can be broken. Everyone must be at practice each night at the regularly scheduled time: No Exceptions. Brad and Mike are two of the team's starters. From their perspective, they're indispensable to the team, the guys who will bring victory to Baileyville. They decide—why, no one will ever know—to show up an hour late to the next day's practice. Jeff is furious. They have deliberately disobeyed his orders. The rule says they should be suspended for one full week. If he follows the rule, Brad and Mike will not play in the semifinals. But the whole team is depending on them. What should he do? http://examples.yourdictionary.com/ethical-dilemma-examples.html
Ethics and Psychology • What ethical dilemmas might come up in your studies?
Ethical Guidelines • What ethical guidelines/rules should we follow?
APA Ethical Guidelines • Minimize misleading results • Protect the health, dignity & privacy of people involved • Obey all laws • Create rights and responsibilities for all involved before starting a study • Get signed permission • Do not lie or give false information to participants about the study • Use data and results appropriately
Ethics & Animal Rights • Do animals have rights? • Is it ok to hurt an animal if it helps save lives?
- More by User

Research Ethics in Undergraduate Research
Research Ethics in Undergraduate Research . Timothy Sparklin Administrator, Human and Animal Research Protections Office University of Maryland, Baltimore County September 28, 2009. Research ethics.
497 views • 10 slides

Ethics in Management Research
Ethics in Management Research. What are ethics? What are ethical principles Ethical business behaviour Brief history of evolution of ethics in research Ethical principles. Ethics in research Qualitative vs quantitative data. Introduction. What are ethics?.
1.01k views • 30 slides

Ethics in Psychological Research
Ethics in Psychological Research. Ethics – __________________ Research ethics – responsibility of researchers to be honest and respectful of all individuals affected by their research. Obligations as researchers
1.14k views • 15 slides

Ethics in Experimental Research
Ethics in Experimental Research. Showing concern for the welfare of human subjects. Horror Stories. Tuskegee syphilis study of 1932 Stanley Milgram’s conformity research of 1963 commercially funded, “for profit” research (2001): conflicts of interest and the “file drawer” problem
426 views • 20 slides

ETHICS in research
ETHICS in research . Eman raouf Faculty of science biochemistry2012. The content. important. Ethical issues. From school –to university !!!. why other countries successes in scientific research?. http://www.ru.nl/masters/programme/medical-sciences/bioethics /. The story.
636 views • 33 slides

Ethics in research
Ethics in research. Biomedical research ethics came about as a result of abuse to research participants in the past Nazis concentration camp experiments The Tuskegee Syphilis Study
1.14k views • 14 slides

Ethics in Research. Historical Context. Past German Experimentation Tuskegee Syphilis Study Present Cancer Research AIDS (AZT) Research. Historical Context. Ethical system designed to prevent people from being used as scientific guinea pigs
446 views • 25 slides

Ethics in Health Research
Ethics in Health Research. Prof. Ashry Gad Mohamed & Dr. Amna R. Siddiqui Department of Family and Community Medicine. OBJECTIVES OF THE LECTURE. At the end of the lecture students should: 1-define ethics in health research. 2-recognize the need for ethics in health research.
783 views • 40 slides

Ethics in Research. Based out of texts by Neuman and Babbie. Ethics of Omission – the parts of science done wrongly. Nobody starts out intending to violate ethical codes. Research as Pressure Cooker. Research Publish Prestige ???? Profit!. Scientific Misconduct.
520 views • 29 slides

Ethics in Psychological Research. Connections with last day. Psychologists strive for research which: Gathers empirical data and results Empirical – relying on or derived from experiment or observation Uses the scientific method
985 views • 11 slides

Ethics in Research . Qualitative Research Methods. Ethics in Research. Why Important? With ethics there is often no clear-cut answer. “It’s the right thing to do”. Ethics in Research. What are Ethics?
657 views • 25 slides

Ethics in Research. Little Albert. Little Albert. Why was this experiment performed? What was the result(s) of the experiment? What was the ethical dilemma in this experiment? Should this experiment be performed today?. The Monster Study. In 1939- orphans in state care –
440 views • 12 slides

Ethics in Educational Research
Ethics in Educational Research. EDDC 811 Summer 2012 6/7/12. Issues around ethics and research. Protection of participants Honesty in reporting Cheating and Plagiarism. Protecting Research Participants. Why do we have research protections? Past abuses
447 views • 10 slides

Ethics in Epidemiologic Research
Ethics in Epidemiologic Research. Javier Lopez-Zetina, PhD, MA Associate Professor Health Science Department California State University, Long Beach. Module Outline. 1. Overview of the IRB Principles of Beneficence, Justice and Respect for Persons
884 views • 27 slides

Ethics in Research. The Ethical Standards of the American Psychological Association http://www.apa.org/ethics/ (2002 Ethics code, to be effective June 1, 2003) http://www.apastyle.org
1.48k views • 15 slides

ETHICS IN SOCIAL RESEARCH
ETHICS IN SOCIAL RESEARCH. SKW 320. Research and Human as Subjects. Advances in human health and welfare Privileges by society, institutions, research subjects More benefits than risks. Considerations…. Critically evaluate the decision to conduct research. Comply with regulations.
550 views • 20 slides

Ethics in international Research
Ethics in international Research. Justice across national boundaries. Clinical Trials Group (ACTG) 076: Vertical Perinatal HIV Transmission.
463 views • 29 slides

Ethics in Research. Participating in Research. How would you have felt if you participated in Dr. Venkman’s study? Should there be guidelines for the appropriate behavior of psychologists?. Ethical Guidelines. Ethics – people should be treated as ends not means
356 views • 21 slides

Ethics in Research. Chapter 4. Ethics. Research ethics concerns the responsibility of researchers to be honest and respectful to all individuals who are affected by their research studies or their reports of the studies’ results. 2 ethical responsibilities.
446 views • 29 slides

Ethics in Research. Ethical researchers do not plagiarize or claim credit for the results of others; They do not misrepresent sources or invent results; They do not submit data whose accuracy they have reason to question, unless they raise the question;
248 views • 9 slides

256 views • 21 slides

3. Ethics in Research
3. Ethics in Research. What are some of the concern guiding ethical research?. What are the potential psychological threat to participants in behavioral science research projects?. What factors may interfere with participants’ freedom to choose when or not to participate in research?.
391 views • 22 slides
- Preferences

ETHICS IN GLOBAL HEALTH: BEYOND HIPPOCRATES - PowerPoint PPT Presentation

ETHICS IN GLOBAL HEALTH: BEYOND HIPPOCRATES
Ethics in global health: beyond hippocrates scott loeliger, md, ms mark stinson fellowship in global and underserved health contra costa family medicine residency – powerpoint ppt presentation.
- Review the historical context of ethics within medical training and practice.
- Understand the place of ethics within the new medical professionalism.
- Incorporate the concepts of ethical behavior and practice into service learning activities.
- Encourage open discussion about current controversies and new generation focus on global health work.
- Hippocratic Oath
- (4th Century BC)
- Universal Declaration of Human Rights (1948)
- The New Hippocratic Oath (1966)
- The Declaration of Alma Ata (1978)
- Declaration of Helsinki (1964-2004)
- Millennium Development Goals (2000)
- Primum non Nocerum to Primum non Tacere.
- Physician Charter on Medical Professionalism.
- Healing in the context of Social Justice.
- Residents with Skills Helpful or Dangerous?
- The Physician Charter
- Physician Charter Construct for Medical Professionalism
- Formal learning and didactics.
- Pre-experience preparation.
- Consideration of ethics in underserved local communities.
- Understanding complexities of global realities, institutions, processes and programs.
- Self- study and self-reflection.
- Short term clinical work combined with tourism.
- Attachment to clinical research project.
- Longer term work with NGOs or Universities.
- Advisor/Teacher or Medical Corps?
- All done in the name of Hippocrates is not right.
- Physician centered paradigm can distort true health improvements.
- Resource poor areas require careful attention to appropriate strategies.
- Attention to the Immediate versus the Sustainable.
- We are going there, whos coming here?
- Raised expectations without means to correct health manpower deficiencies.
- True professional exchanges, joining the growing global debate (Global Health Workforce Alliance).
- Translating Ours to Theirs.
- While working in health care, how to attend to social injustice and underlying factors of poor health.
- Training leaders, followers, co-conspirators or colleagues?
- Respect for emerging literature and research from abroad.
- Recently developed (2007-08).
- Included input from APHA, Partners in Health, Physicians for Human Rights, Save the Children, AMREF, GHETS, WHO, World Bank and others.
- Most recent consultation in Kampala during March global forum on human resourses for health.
- Next consultation at APHA meeting in October, 2008
- I. NGOs will engage in hiring practices that ensure long-term health system sustainability.
- II. NGOs will enact employee compensation practices that strengthen the public sector.
- III. NGOs will pledge to create and maintain human resources training and support systems that are good for the countries where they work.
- IV. NGOs will minimize the NGO management burden for ministries.
- V. NGOs will support Ministries of Health as they engage with communities.
- VI. NGOs will advocate for policies that promote and support the public sector.
- Global health research may have some ethical flaws.
- Interventions determined by narrow research goals may not be sustainable.
- Article 25 of Universal Declaration of Human Rights.
- WMA and Declaration of Helsinki.
- Think Global, Consider Local.
- Incorporate the Experience into Your Future Practice
- Work in Your Milieu to Integrate Service Learning into the Medical School and Residency Experience.
- In Your Medical Bag
- Stethoscope Check Ophthalmoscope-Check Sansome Guide Check Language Dictionary Check Ethical Guidelines-Check?
- 1 Filling In A Little Knowledge is a
- 2 Vertical Projects We Only Do
- 3 Ignoring Bureaucratic Barriers
- 4 NGO/Institutional Short Time Work
- Markle, W, et al. editors. Understanding Global Health. McGrawHill Medical, 2007, 362pp.
- Evert, J., et al. Developing Residency Training in Global Health A Guidebook. San Francisco Global Health Education Consortium, 2008. 119pp.
- ONeil, E. Awakening Hippocrates A primer on health, poverty and global service. AMA, 2006. 502 pp.
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics , the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.


IMAGES
VIDEO
COMMENTS
The module provides information to help you design a human research project and understand how ethics reviewers will consider your design against the guidance provided in the National Statement on Ethical Conduct in Human Research.. Based on, and directly cross referencing with the National Statement, the module outlines why research ethics review was introduced internationally.
Ethics in Healthcare: Current Ethics, Ethical vs Unethical Research through 1970. If you have any questions about the program you have just watched, you may call us at: (800) 424-4888 or fax (806) 743-2233. Direct your inquiries to Customer Service. Be sure to include the program number, title and speaker.
NSF. (1) Fabrication means making up data or results and recording or reporting them. (2) Falsification means manipulating research materials, equipment, or processes, or changing or omitting data or results such that the research is not accurately represented in the research record. (3) Plagiarism means the appropriation of another person's ...
This document aims to assist policy‑makers, health care providers and researchers to understand key concepts in health ethics and to identify basic ethical questions surrounding health and health care. It illustrates the challenges of applying ethical principles to global public health and outlines practical strategies for dealing with those challenges.
The Working Group decided to broaden the scope of the 2002 Guidelines from "biomedical research" to "health-related research". The Working Group considered biomedical research too narrow since that term would not cover research with health-related data, for example. At the same time, the Working
Writing Strategies and Ethical Considerations. Pages Sage Publishers 2003. Download ppt "ETHICS IN BIOMEDICAL RESEARCH". LEARNING OBJECTIVES Define the term "ethics" in health research Recognize the need for adherence to ethical principles in health research Describe the general ethical principles in health research Justify the role of ...
Measure 11.1.2.A "The purpose of this measure is to assess the health department's policies and process for the identification and resolution of ethical issues that arise from the department's program, policies, interventions, or employee/employer relations (emphasis added).". Dakota County Public Health Ethics Committee (#2102)
Ethics for health research is the enterprise that determines norms and values to guide the systematic reflection and scientific evaluation or assessment of clinical knowledge and any form of experimentation or survey, with the prime objective of promoting health care. Its sole intent is to benefit patients, to alleviate pain and to prevent ...
The new WHO publication "Standards and operational guidance for ethics review of health-related research with human participants", is a compilation of 10 standards that are applicable to the ethics review of health related research with human participants. This document is intended to provide guidance on the research ethics review process, not to take a substantive position on how ...
Ethics in Health Research. Prof. Ashry Gad Mohamed Dr. Aman /Dr. Salwa Tayel Department of Family and Community Medicine September 9, 2013. OBJECTIVES OF THE LECTURE. At the end of the lecture students should: 1-Define ethics in health research.
In any case, a course in research ethics can be useful in helping to prevent deviations from norms even if it does not prevent misconduct. Education in research ethics is can help people get a better understanding of ethical standards, policies, and issues and improve ethical judgment and decision making.
Chapter 4: Research Ethics. 2014, Doing Research in the Real World, 3rd edn. Ethics in research involving humans were first codified in 1946 as Nuremberg code. Subsequently other ethical declarations and guide lines were developed to protect the research participants as well as the researchers. The basic research bioethics includes three ...
The Global Health Ethics Unit provides a focal point for the examination of ethical issues raised by activities throughout the Organization. The unit also supports Member States in addressing ethical issues that arise in their own countries. This includes a range of global bioethics topics; from public health surveillance to developments in ...
7 ACTION It is the object of the ethics Action consists of "evaluation". "related life" "execution" Ethics deal with the intention, form, aim and result of the action. 8 VIRTUE Honesty, justice, respect, love, trust. Wisdom, continence, courage Moral perfection It is the basis of the ethical behaviors. 9 Medicine and the Ethics.
RESEARCH ETHICS. Dr. Don Vicente Carballo Real. Director for Research Development &. Community Extension Services. Delivered Lecture on September 14, 2018, SDCA. • 18th WMA General Assembly ...
Ethics in Health Research. Ethics in Health Research. Prof. Ashry Gad Mohamed & Dr. Amna R. Siddiqui Department of Family and Community Medicine. OBJECTIVES OF THE LECTURE. At the end of the lecture students should: 1-define ethics in health research. 2-recognize the need for ethics in health research. 783 views • 40 slides
Ethics and Research. Global health research may have some ethical flaws. Interventions determined by narrow research goals may not be sustainable. Article 25 of Universal Declaration of Human Rights. WMA and Declaration of Helsinki. 19 UNDERSERVED HEALTH CARE. Think Global, Consider Local. Incorporate the Experience into Your Future Practice