- Open access
- Published: 18 November 2022

What influences the implementation of kangaroo mother care? An umbrella review
- Qian Cai 1 , 2 ,
- Dan-Qi Chen 1 , 2 ,
- Hua Wang 2 ,
- Yue Zhang 2 ,
- Rui Yang 1 , 2 ,
- Wen-Li Xu 3 &
- Xin-Fen Xu 2 , 3
BMC Pregnancy and Childbirth volume 22 , Article number: 851 ( 2022 ) Cite this article
4704 Accesses
8 Citations
4 Altmetric
Metrics details
Kangaroo mother care (KMC) is an evidence-based intervention that reduces morbidity and mortality in preterm infants. However, it has not yet been fully integrated into health systems around the world. The aim of this study is to provide a cogent summary of the evidence base of the key barriers and facilitators to implementing KMC.
An umbrella review of existing reviews on KMC was adopted to identify systematic and scoping reviews that analysed data from primary studies. Electronic English databases, including PubMed, Embase, CINAHL and Cochrane Library, and three Chinese databases were searched from inception to 1 July 2022. Studies were included if they performed a review of barriers and facilitators to KMC. Quality assessment of the retrieved reviews was performed by at least two reviewers independently using the Joanna Briggs Institute (JBI) critical appraisal checklist and risk of bias was assessed with the Risk of Bias Assessment Tool for Systematic Reviews (ROBIS) tool. This umbrella review protocol was documented in the PROSPERO registry (CRD42022327994).
We generated 531 studies, and after the removal of duplicates and ineligible studies, six eligible reviews were included in the analysis. The five themes identified were environmental factors, professional factors, parent/family factors, access factors, and cultural factors, and the factors under each theme were divided into barriers or facilitators depending on the specific features of a given scenario.
Conclusions
Support from facility management and leadership and well-trained medical staff are of great significance to the successful integration of KMC into daily medical practice, while the parents of preterm infants and other family members should be educated and encouraged in KMC practice. Further research is needed to propose strategies and develop models for implementing KMC.
Peer Review reports
According to reports from the World Health Organization (WHO), with the development of assisted reproductive technology and the improvement of emergency and critical care technology, the incidence of premature birth is rising, and premature birth has become a global problem [ 1 ]. Nearly fifteen million preterm infants are born each year, and more than one million of them unfortunately die each year [ 2 ]. According to statistics, complications of preterm birth directly account for more than 35% of all neonatal deaths, while the proportion of deaths indirectly caused by preterm birth is even higher because preterm birth increases the risk of infant death from infection [ 3 ]. Many surviving preterm infants encounter plenty of problems due to premature birth, such as sensory impairment and cognitive and language impairment [ 4 , 5 , 6 ]. In addition, the birth of preterm infants may cause a substantial emotional crisis and economic cost to the family, as well as have an impact on public sector services such as education and other social support systems [ 7 , 8 ]. For mothers, preterm birth may also cause a range of perinatal diseases [ 5 , 9 ]. Therefore, effective evidence-based interventions that can be implemented at scale are urgently needed to reduce the incidence of preterm birth complications and neonatal mortality.
Kangaroo mother care (KMC) is one such evidence-based life-saving intervention for preterm infants [ 10 ]. In KMC, the mother (or father) puts her (his) naked preterm infant on her (his) chest in the same way as kangaroo parenting so that the preterm infant is capable of having early, continuous and long-term skin-to-skin contact with his or her mother (father); in addition, measures such as exclusive breastfeeding or breastfeeding, early discharge, and follow-up after discharge are taken for the preterm infants [ 11 , 12 ]. Compared with the conventional nursing mode, KMC is not only able to maintain the body temperature of preterm infants but also significantly reduces the risk of death in low-birth-weight infants by 36% while significantly reducing the risk of sepsis, hypoglycaemia, and hypothermia [ 13 ]. Numerous studies have shown that KMC is a safe, effective, and multifaceted intervention with many short-term and long-term positive effects for preterm infants, such as stabilizing the neonatal physiological state, enhancing immunity, increasing exclusive breastfeeding rates, and promoting mother-infant bonding [ 14 , 15 , 16 , 17 ].
Despite the clear benefits of KMC, this intervention has not yet been fully integrated into health systems around the world [ 18 , 19 ]. There are many barriers impeding the implementation of the KMC, including but not limited to lack of support from family members, lack of parental information, and lack of tools and resources [ 20 , 21 , 22 , 23 ]. Several studies have identified facilitators that may contribute to the implementation of KMC, such as providing KMC training programmes for parents and encouraging physicians to recommend KMC to parents [ 24 , 25 , 26 ]. Undoubtedly, a better understanding of these barriers and facilitators can optimize the implementation of KMC.
Studies on the subject of KMC have developed over many years, with extensive studies from around the world and several systematic reviews on KMC published. These studies spanned different clinical settings, and there are studies that have explored the influencing factors of KMC from different perspectives, such as caregivers (e.g., parents and families) and healthcare workers [ 27 , 28 , 29 ]. A certain number of barriers and facilitators have been identified in these studies. However, the complexity and diversity of conventional studies make KMC difficult to describe and understand and impose challenges for health professionals and administrators who try to apply KMC in health systems [ 22 , 30 ]. Therefore, it is necessary to robustly summarize the evidence base to identify and elucidate key barriers and facilitators to the implementation of KMC.
One available approach is the umbrella review, which involves the synthesis of existing reviews, enabling researchers to collect evidence from multiple healthcare facilities instead of conducting systematic reviews at each facility. Essentially, an umbrella review is a review of existing reviews to provide an overview of the available evidence on a specific topic and allow comparisons of published reviews [ 31 ]. Furthermore, an umbrella review is capable of compiling evidence bases related to specific issues in a relatively short time frame [ 32 ]. We adopted this comprehensive assessment approach to outline factors that may facilitate or inhibit KMC implementation and expansion.
Protocol and registration
A protocol was prospectively developed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines [ 33 ]. Following current recommendations, the protocol was made openly available through registration with the PROSPERO International Prospective Register of Systematic Reviews platform (registration number CRD42022327994).
Study design
This review was conducted according to the rules for conducting umbrella reviews and published approach [ 32 , 34 ], and was reported following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA 2020) statement [ 35 ]. The PRISMA checklist is shown in Additional file 1 .
Search strategy
Electronic databases, including PubMed, the Cochrane Database of Systematic Reviews, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the China National Knowledge Infrastructure (CNKI, for Chinese literature), SinoMed (for Chinese literature), and WAN FANG DATA (for Chinese literature), were searched to identify systematic reviews and meta-analyses (published from database inception to 1 July 2022.) of the factors influencing the implementation of KMC in preterm infants. Additionally, we manually searched reference lists from the screened articles to avoid the omission of any related articles. Also, we searched Google Scholar and OpenGrey for grey literature.
The search terms were constructed by combining subject terms and free words, while the language was limited to Chinese or English. The English search terms used were “prematur*/preterm*/premie*/neonat*/infant*/newborn*/low birth weight/LBW/ NICU”, “kangaroo mother care/kangaroo mother method/kangaroo care/kangaroo attachment/kangaroo contact/KMC/KC/skin-to-skin care/skin-to-skin contact/SSC/mother-infant contact”, and “systematic review/meta-analys”, and “早产儿/新生儿/低出生体重儿”“袋鼠护理/袋鼠式护理/皮肤接触”“系统评价/Meta分析/荟萃分析” were adopted as the Chinese search terms. More details of the search strategies are shown in Additional file 2 .
Inclusion criteria
This umbrella review included studies published in peer-reviewed journals and grey literature that addressed the research question. Articles were included if they were published in Chinese, English or in other language with the English version; identified factors impacting KMC implementation, including barriers and facilitators as primary or secondary objectives; and were a systematic review or meta-analysis. Moreover, to retrieve valuable information about the subject under study, we also decided to include scoping reviews, a type of review study that uses a systematic method of searching for information with the aim of accumulating as much evidence as possible and mapping the results. Screening of the searched articles and their subsequent full-text review were carried out based on the following inclusion criteria: (a) studies that used a systematic/scoping review and/or meta-analysis design, (b) studies focused on preterm infants with KMC, and (c) studies that aimed to identify factors associated with KMC implementation. In addition, articles fulfilling the following criteria were excluded: (a) reviews written in any language other than English or Chinese, (b) duplicate publications, and (c) articles or conference abstracts for which the full text was not available.
Study selection
Two researchers independently screened the literature according to the inclusion and exclusion criteria. In case of disagreement, the two researchers first discussed and attempted to resolve the disagreement. If the disagreement could not be resolved, a third researcher was invited to adjudicate. The literature screening process was as follows: (1) Endnote (a literature management software) was used to remove duplicate records; (2) the title and abstract of the articles were read in Endnote, and those that were not related to the subject, population and literature type were removed; (3) the full text of the remaining articles was downloaded, excluding those for which the full text could not be obtained; and (4) the full texts of the articles were read to further exclude literature according to the standard cited in the second step. The study selection process is summarized in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Quality assessment
The quality of the included reviews was assessed using the Joanna Briggs Institute (JBI) critical appraisal checklist for systematic reviews and research syntheses [ 36 ]. This assessment tool comprises 11 items, and the evaluation criteria for each item are “yes”, “no”, “unclear” or “not applicable”. Two members independently assessed the retrieved articles. Any disagreement between them was resolved by a third investigator.
Risk of bias assessment
Risk of bias of the included studies was evaluated by two reviewers using the Risk of Bias Assessment Tool for Systematic Reviews (ROBIS) [ 37 ]. In case of disagreement, a third reviewer was consulted until a final decision was made. ROBIS assesses four domains: 1) study eligibility criteria; 2) identification and selection of studies; 3) data collection and study appraisal; and 4) synthesis and findings. Each domain consists of five to six questions with six possible options: Yes, Probably yes, Probably No, No, Not indicated or Not applicable.
Data extraction
Two researchers independently used a unified Excel form that served as a data extraction sheet used to extract variables that were relevant to the scope of the current review, and another researcher verified the accuracy of the data extraction and quality assessment of all the included reviews. The extracted variables included the type of review, years covered, the total number of studies included in the review, country of origin, settings, aims/objectives and participants. As the aim was to provide a broad overview, all barriers and facilitators in all of the reviews were extracted except for those that were infrequently reported (i.e., those reported by only a few studies).
Data synthesis
After the data were extracted, a qualitative content analysis of the factors impacting KMC implementation was undertaken by the researcher. Each review article was read carefully to identify and extract the reported barriers and facilitators, and the researcher prepared the tables to summarize the data of all articles (see Additional file 3 ). The main key factors extracted from the articles were grouped and classified into themes to enhance the comprehension of the results outcomes. This classification of findings was performed based on the identified factors from the studies included in this review. Any uncertainties regarding the thematic categorizations were resolved through discussion and consensus by the reviewers.
Five hundred and thirty one hits retrieved in the initial search were exported into the reference management software Endnote, and 300 of them was left after duplicate records were excluded. A total of 285 references whose subject and theme were not matched were removed after title and abstract screening. Six eligible reviews were included after further full-text screening of the remaining 15 articles, as shown in Fig. 1 .
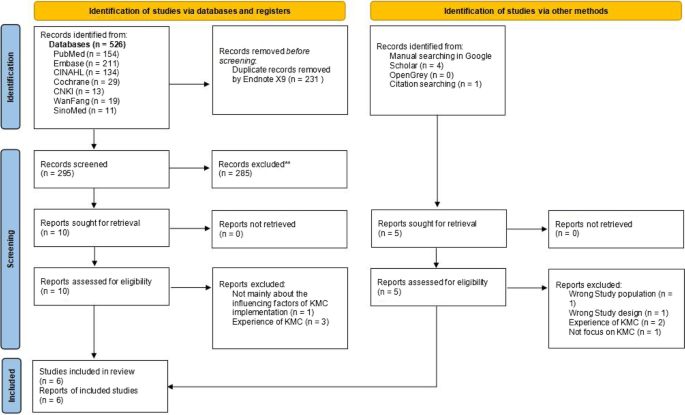
PRISMA flow diagram of barriers and facilitators to implementing KMC
Study characteristics
Table 1 provides an overview of five systematic reviews and one scope review related to KMC implementation as of July 1, 2022, all of which were published in 2015 and later, indicating this topic is relatively fresh. Two of the six articles described barriers and facilitators of KMC implementation from the perspective of caregivers of preterm infants [ 27 , 39 ]; one article explored these influencing factors from the the perspective of healthcare workers [ 28 ]; and the remaining articles discussed the factors affecting KMC implementation from both the perspectives of healthcare workers and parents of preterm infants [ 29 , 38 , 40 ].
The number of studies included in each review varied significantly, which often depended on the inclusion scope of the review [ 27 , 28 , 29 , 38 , 39 , 40 ]. For instance, two most recently published reviews included a smaller number of studies as it defined a specific study area [ 29 , 39 ]. Most of the studies included in the reviews were carried out in low-and middle-income counties and were conducted in health facility.
The methodological quality of the included 6 articles was evaluated by the JBI critical appraisal checklist. The ninth item “Was the likelihood of publication bias assessed” for all the included articles was “No” because publication bias are not assessed in all the included reviews. As the tools for evaluating the quality of the included studies and how to evaluate the quality of the included studies were not described in the two studies conducted by Seidman et al. [ 38 ] and Mathias et al. [ 39 ], so the fifth item “Were the criteria for appraising studies appropriate” and the sixth item “Was critical appraisal conducted by two or more reviewers independently” for these two studied was “No”, and the evaluation results of the remaining items were all “Yes”. The results of the quality appraisal of all the included studies are displayed in Additional file 4 .
After applying the ROBIS tool for risk of bias evaluation, of the six included systematic reviews, four were evaluated to have a high bias risk [ 27 , 28 , 38 , 40 ], and two present an unclear bias risk [ 29 , 39 ] (see Additional file 5 ). Main concerns regarding this aspect were related to (a) limiting searches with language restrictions; (b) lack of risk of bias evaluation; and (c) selection and data extraction not done in duplicate.
Barriers and facilitators of KMC
The five themes identified were environmental factors, professional factors, parent/family factors, access factors, and cultural factors. The subfactors under each theme were divided into barriers or facilitators according to the descriptions provided in the included reviews. A brief summary of the barriers and facilitators identified under each theme is presented in Table 2 . These are described in more detail below.
Environmental factors
This theme comprised facility conditions, resources and materials, and the healthcare system. Facility conditions mainly refer to hardware support in medical institutions, the most common factors being space and privacy. Lack of privacy and insufficient space and supplies directly hinder the implementation of KMC [ 27 , 28 , 29 , 38 , 39 , 40 ], while access to private space/privacy screens and sufficient space and supplies are key facilitators for the implementation of KMC [ 27 , 28 , 29 , 40 ]. In addition, factors such as temperature stability and a quiet and relaxed atmosphere in clinical facilities are conducive to the implementation of KMC [ 27 , 28 , 40 ]. Resources and materials refer to the environmental software support mainly related to resource management and material access. The most common barrier is a lack of KMC guidelines or protocols in the clinical unit [ 27 , 28 , 29 , 38 ], while the implementation of KMC would be enhanced if the clinical unit adopted KMC guidelines or protocols and displayed KMC pictures/posters, etc. [ 28 , 29 , 39 ]. The healthcare system mainly involves educational and policy factors. Inadequate/inconsistent training and unsupportive staffing policies are barriers to KMC implementation [ 28 , 29 , 39 , 40 ], while the integration of KMC into the healthcare curriculum and KMC-related policies are important facilitators for KMC implementation [ 29 , 40 ].
Professional factors
This theme encompassed three subthemes: professional perception, professional characteristics, and professional management. The main barriers under this theme included medical staff’s lack of belief in the efficacy or importance of the KMC [ 38 , 40 ] and their perceptions that KMC is unsafe [ 28 , 39 ] and imposes extra workload on them [ 38 ], the limited level of experience and knowledge of health care workers [ 28 , 29 , 38 ] and lack of communication with each other [ 28 ], high staff and leadership turnover [ 28 , 40 ] and lack of leadership and management support [ 28 , 38 , 40 ]. The main facilitators under this theme included medical staff’s belief in KMC benefits [ 28 , 29 , 40 ] and their sufficient experience, passion, and willingness to implement KMC [ 28 , 29 , 39 ]; leadership and management support [ 29 , 40 ]; and multiple health worker support [ 28 , 39 ].
Parent/family factors
This theme involved parental perception and motivation, parenting capacity, and parental support and empowerment. Experienced and perceived discomfort [ 29 , 39 ], a lack of awareness of the benefits of KMC [ 27 , 29 , 38 , 39 ], and fear/anxiety of hurting the infant [ 38 ] were the most frequently identified barriers to the implementation of KMC. Parenting capacity mainly refers to the health state of the parents of preterm infants. Medical issues such as pain/fatigue [ 27 , 38 , 40 ] and postpartum depression [ 27 , 29 , 38 ] and lack of confidence and knowledge on KMC [ 39 ] were the most common barriers. Support and empowerment refer to the availability of support from family members [ 27 , 29 , 38 , 39 , 40 ], medical staff [ 28 , 29 , 38 , 39 , 40 ], community [ 39 , 40 ], and peers [ 28 , 29 ], which facilitates the implementation of KMC and hinders implementation otherwise.
Access factors
This theme involved time, location, and financing. For medical staff, time was a key barrier; staff perceived that the implementation of KMC would increase their workload [ 28 , 29 , 38 , 40 ] and reduce time with other critical patients [ 28 , 40 ], and they had difficulty finding time for training [ 40 ]. For the parents of preterm infants, commuting from home and the medical unit was another barrier that caregivers were unable to devote sufficient time in KMC practice due to long commutes [ 27 ] or dealing with heavy household chores [ 39 ]. The costs of transportation, accommodation renting, and KMC implementation in the clinical ward were the immediate challenges [ 27 , 38 , 40 ]. Lower hospital costs to family [ 27 , 29 , 40 ], lower cost for health system [ 29 ] and unlimited visitation hours [ 27 , 28 , 40 ] were conducive to the implementation of KMC.
Cultural factors
This theme comprised traditional newborn care, traditional mindset, and gender roles. Traditional newborn care approaches, such as traditional bathing, carrying and breastfeeding practices [ 27 , 28 , 40 ], and the type of wrap [ 39 ] were identified as barriers to the implementation of KMC. However, some aspects of newborn care facilitated the implementation of KMC, i.e., advising mothers to delay bathing [ 28 ]. Some mindsets such as feeling ashamed of having a preterm infant [ 27 , 38 , 40 ], believing that skin-to-skin contact between the preterm infants and their caregivers was inappropriate [ 29 , 38 , 39 ] and considering KMC to be taboo [ 39 ] were identified barriers to the KMC implementation. Additionally, gender inequality existing in the division of labour between fathers and mothers [ 27 , 38 ] was not conducive to the implementation of KMC that KMC was regarded as a role responsibility of the mother, and the father was not allowed to participate in KMC [ 38 , 39 , 40 ] .
Our umbrella review highlighted different factors, each factor comprising barriers and facilitators, that influence the implementation of KMC, provide decision-makers in healthcare with an overview of the field and provide information for the implementation of KMC. All of the included reviews were published in 2015 or later, which confirms the growth and interest in the field of KMC. However, there is considerable heterogeneity in the evidence base on KMC, which makes translation into practice challenging.
Factors related to facility conditions, mainly including lack of privacy and insufficient space and supplies, were mentioned in all six included reviews, which might be related to the operation characteristics of KMC. Skin-to-skin contact is the most important part of the KMC procedure, which requires parents to undress their upper bodies and put their preterm infants on their chests, which is why a suitable physical environment is of great significance [ 11 , 12 ]. Studies have reported that mothers felt uncomfortable and exposed due to the continuous coming and going of medical staff during KMC when insufficient KMC private space was provided, which has proved to be a serious barrier affecting the implementation of KMC in many countries around the world [ 41 , 42 , 43 ]. Therefore, medical units should strive to provide enough quiet, comfortable, and private space for NICUs to implement KMC. Apart from physical facility conditions, resources and materials were another factor. Limited by facility space and human resources, some hospitals in China had to perform intermittent KMC instead of continuous KMC [ 44 ]. A multicountry analysis of health system bottlenecks from 12 African and Asian countries reported that insufficient essential supplies in facilities to support KMC was a barrier to the implementation of KMC [ 21 ].
KMC should be systematically implemented within a facility in accordance with relevant rules and regulations, for example, by adopting standard checklists for mothers and infants to ensure orderly and standardized KMC implementation. In a majority of the hospitals, nurses were required to commit to KMC-related tasks such as KMC recording, assessment, and data monitoring due to the lack of relevant rules and regulations, which meant an extra workload for the nurses [ 45 , 46 ]. Studies have shown that human resource challenges, record keeping, and data collection are barriers to KMC implementation in countries such as Malawi and Indonesia [ 28 , 47 ]. Documentation and annotation of KMC implementation were still not common practices in NICUs, while KMC-related information was imported through electronic medical records in most cases [ 28 , 48 ]. Chan et al. noted that the implementation of KMC was promoted when medical units improved their electronic medical records to allow nurses to record the onset and duration of KMC [ 28 ]. Therefore, the Ministry of Health and government agencies should formulate practical KMC implementation guidelines based on local conditions, and medical units should also formulate and standardize KMC implementation guidelines and programs to promote the implementation of KMC.
Lack of proper leadership, insufficient professionalism of personnel, and insufficient training were also obstacles to KMC implementation. A study on the introduction of KMC in Indonesian hospitals found that government support, hospital management, staff acceptance, and training were identified as key facilitators of KMC implementation [ 47 ]. In some regions, KMC-specific training programs were provisioned for medical staff by the government [ 49 ]. However, the number of staff participating in the training is very limited due to the long distance between the training site and the medical unit and the shortage of personnel in the hospitals, although many medical personnel were willing to participate in the training [ 42 , 50 ]. In other words, although policymakers and decision-makers tried to provide assistance and intervention programs for healthcare workers, they did not anticipate these barriers to attendance. Of course, the support from hospital administrators and leadership could provide more space and human resources to provision KMC, optimize or update the staffing configuration of neonatal care nurses, strengthen the professionalization of neonatal care by healthcare workers, and improve healthcare staff’s attitudes towards and perceptions of KMC [ 43 , 51 ].
The attitudes of the health caregivers towards KMC were also a factor influencing the adoption of KMC for parents. If there were staff in the hospital who were familiar with KMC and willing to educate parents on KMC knowledge, it would help parents of preterm infants to acquire KMC-related knowledge, which would promote KMC preferences and the early initiation of KMC [ 52 , 53 ]. Correspondingly, insufficient awareness of KMC and infant health among parents/family members was a barrier to the practice of KMC [ 22 ]. Despite the generally low awareness of KMC, the reviews reported that it was relatively easier to train mothers on KMC practices and that they were more adherent to KMC practices after understanding and accepting KMC [ 54 ]. Perceived, observed, and experienced effects of KMC could provide comfort and satisfaction to the parents of preterm infants, which promotes KMC use, whereas KMC is inhibited if parents and/or preterm infants experience KMC-related discomfort.
Lack of assistance is a barrier to KMC practice, whereas support from family, friends, and other mothers is a facilitator to the implementation of KMC. There were many different forms of support. For example, family members took turns embracing the preterm infants to free the mother from this practice [ 55 , 56 ]. Evidence from the literature has suggested that emotional support, as well as support and help with household chores, is also a facilitator for mothers [ 57 , 58 ]. Kangaroo nursing can be implemented not only by mothers but also by fathers, grandfathers, grandmothers, and other family members of preterm infants [ 43 , 59 ], and if family members do not understand this point, preterm infants might lose the opportunity to receive kangaroo care [ 60 ]. Therefore, different educational approaches should be adopted to educate families of preterm infants about their roles in KMC, with additional health promotions and activities targeting grandparents and other family members about the benefits of KMC and the significance of supporting mothers, which may increase the number of people receiving KMC.
However, KMC is not suitable for all situations. In some clinical scenarios where mothers of preterm infants have special health conditions, it could be very challenging to train mothers and facilitate KMC implementation. These challenges include the infant being too difficult to embrace, the infant being too heavy, and the mother experiencing chest or back discomfort or pain/fatigue [ 38 ]. The reviews showed that mothers’ medical conditions, including postepisiotomy pain repair [ 61 ], postcesarean recovery [ 62 ], postpartum depression and general maternal illness [ 48 ], were another challenge for KMC practice. Additionally, mothers may mentally struggle with KMC practices, including positioning problems (difficulty sleeping on the chest with infants), breast milk expression, and other breastfeeding-related issues [ 57 , 63 ]. In this case, family support and father involvement make a great difference [ 64 ]. Postpartum depression is a barrier to the implementation of KMC, but interestingly, mothers who practised KMC experienced reduced symptoms of postpartum depression [ 65 , 66 ].
Inviting parents to the NICU to perform KMC could result in extra costs. Studies performed in low-income countries have shown that commuting between home and KMC wards was a barrier to the implementation of KMC, and fees for mothers and babies staying in KMC wards were also considered a barrier [ 39 , 67 ]. Studies have shown that higher economic status is more conducive to the implementation of KMC [ 40 , 43 ]. Therefore, accessing financial resources from hospital administration and/or parental health insurance to facilitate KMC would be a necessary part of KMC expansion. Meanwhile, it is necessary to consider how to reduce hospital charges or provide certain transportation subsidies for families with infants whose hospitalization time exceeds the average length of stay. Limited visiting time in the NICU is another obstacle to the implementation of KMC, especially in the case of closed management such as the NICU in China. Extending the visit time could increase the adoption of KMC to some extent [ 68 , 69 ].
Different cultures, religions, and traditional beliefs in different countries influence perceptions of preterm infants and KMC. In many countries, carrying infants on the chest rather than on the back is considered inappropriate [ 41 ], and some cultures believe that skin-to-skin contact between an infant and his or her caregiver is not appropriate [ 27 ]. Understanding these culturally specific barriers, it is of great importance to adapt KMC promotion programmes to the needs of the population. In some countries, mothers are ridiculed for giving birth to preterm infants, which results in stigma [ 55 , 70 ]. Studies have reported that stigma about preterm infants creates anxiety and guilt in mothers, causing them to abandon their infants, which is a factor hindering the implementation of KMC [ 27 , 38 ]. Muddu et al. [ 71 ] found that fondness was an enabler for parents to accept their preterm infants and utilize KMC to support the improvement of their preterm infants’ health. Cultural barriers also encompass the practice of postpartum confinement and traditional resistance to confinement from grandparents and community members. Most mothers are advised to stay home after delivery in China and India [ 72 , 73 ], which has potential health benefits for mothers and newborns, but it also causes mothers and families to be hesitant to adopt KMC.
Traditional gender role factors were identified as barriers to male participation in neonatal care. KMC was regarded as a breach of social duty or responsibility by mothers in some countries where it is believed that mothers should take care of the family, and when mothers comply with this social duty and gender responsibility, the implementation of KMC becomes a challenge [ 74 ]; meanwhile, fathers are not encouraged to participate in KMC implementation in such cultures. Therefore, it is of great significance to develop interventions on how to encourage fathers to participate in KMC and reduce the stigma surrounding this infant care strategy [ 75 ]. As Dumbaugh et al. [ 76 ] pointed out, the inclusion of males in neonatal care must be done in a way that empowers women. Fathers who are successfully involved in KMC might become peer mentors or examples for others to address the problem of fathers’ reluctance to participate in neonatal care. The name of the intervention, “kangaroo mother care”, could also be modified, e.g., to “kangaroo care”, so that it does not directly imply that the practice is performed only by mothers.
Limitations
The findings in this manuscript are subject to some limitations. First, due to resource constraints, we only searched for English and Chinese reviews, and there was a possibility of missing some relevant studies. Another limitation of the umbrella review approach was that it could only report on what researchers have investigated and published [ 32 ]. For example, some factors might be highly influential, but if they were not adequately investigated in the included studies, they might be reported as less important, or they might not even be included in the review. To mitigate this issue, other key literature not identified in this review was actively referenced. Finally, a potential limitation to the umbrella review approach could be the risk that bias is transmitted upwards from primary studies to the reviews and then to the umbrella review.
Recommendations for future research
KMC implementation issues are likely to differ among different regions, so there remains a need for further research into sustainable development mechanisms in varied settings to promote the adoption of KMC. The generalizability of the findings worldwide and their translation into practice is uncertain. Most of the studies focused at the facility level, such as the NICU, which highlights the lack of community-level studies. Therefore, further research is needed to explore the factors influencing KMC implementation at home and in the community. Male involvement was identified as a facilitator to KMC implementation, but there was no study discussing hindrance factors of father involvement in care specifically. Therefore, further research is also needed to explore the hindrance and/or facilitating factors of male involvement in KMC care from the perspective of fathers. In addition, further research is also needed to test models for addressing barriers and supporting facilitators to promote and implement context-specific health system changes for greater uptake of KMC.
KMC is a complicated intervention that encounters unique barriers and facilitators in different aspects of healthcare systems. Our umbrella review prioritizes the main factors influencing KMC implementation and highlights some key areas that implementers and implementation researchers may need to focus on. KMC should be implemented more systematically and continuously to strengthen and expand its adoption.
The parents of preterm infants and other family members, the medical unit, and the medical staff contribute to a dynamic whole as a triangle, that are closely linked with one another. Support from facility management and leadership and well-trained medical staff are of great significance to the successful integration of KMC into daily medical practice, while the parents of preterm infants and other family members should be educated and encouraged to adopt KMC practice. Effectively integrating KMC into current health systems by addressing barriers and building trust will greatly improve neonatal survival rates.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request. All data were extracted from published systematic reviews and meta-analyses.
World Health Organization. Preterm birth. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (Accessed 13 May 2022).
Google Scholar
Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–46. https://doi.org/10.1016/S2214-109X(18)30451-0 .
Article PubMed Google Scholar
United Nations Inter-agency Group for Child Mortality Estimation (UN IGME), WHO, World Bank Group and United Nations. Levels & Trends in Child Mortality: Report 2019, Estimates developed by the United Nations Inter-agency Group for Child Mortality Estimation: United Nations Children’s Fund; 2019. Available online: https://www.unicef.org/reports/levels-and-trends-child-mortality-report-2019 (Accessed 16 May 2022)
Allotey J, Zamora J, Cheong-See F, Kalidindi M, Arroyo-Manzano D, Asztalos E, et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64 061 children. BJOG. 2018;125(1):16–25. https://doi.org/10.1111/1471-0528.14832 .
Article CAS PubMed Google Scholar
Ream MA, Lehwald L. Neurologic Consequences of Preterm Birth. Curr Neurol Neurosci Rep. 2018;18(8):48. https://doi.org/10.1007/s11910-018-0862-2 .
Dean B, Ginnell L, Ledsham V, Tsanas A, Telford E, Sparrow S, et al. Eye-tracking for longitudinal assessment of social cognition in children born preterm. J Child Psychol Psychiatry. 2021;62(4):470–80. https://doi.org/10.1111/jcpp.13304 .
Hodek JM, von der Schulenburg JM, Mittendorf T. Measuring economic consequences of preterm birth - Methodological recommendations for the evaluation of personal burden on children and their caregivers. Health Econ Rev. 2011;1(1):6. https://doi.org/10.1186/2191-1991-1-6 .
Article PubMed PubMed Central Google Scholar
Behrman RE, Butler AS, Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press (US). 2007.
Trumello C, Candelori C, Cofini M, Cimino S, Cerniglia L, Paciello M, et al. Mothers’ Depression, Anxiety, and Mental Representations After Preterm Birth: A Study During the Infant's Hospitalization in a Neonatal Intensive Care Unit. Front Public Health. 2018;6:359. https://doi.org/10.3389/fpubh.2018.00359 .
World Health Organization. WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. Geneva: World Health Organization; 2015. Available online: https://www.who.int/publications/i/item/9789241508988 (Accessed 16 May 2022)
World Health Organization. Kangaroo mother care: a practical guide. Geneva: World Health Organization; 2003. Available online: https://www.who.int/publications/i/item/9241590351 (Accessed 17 May 2022).
Chan GJ, Valsangkar B, Kajeepeta S, Boundy EO, Wall S. What is kangaroo mother care? Systematic review of the literature. J Glob Health. 2016;6(1):010701. https://doi.org/10.7189/jogh.06.010701 .
Boundy EO, Dastjerdi R, Spiegelman D, Fawzi WW, Missmer SA, Lieberman E, et al. Kangaroo Mother Care and Neonatal Outcomes: A Meta-analysis. Pediatrics. 2016;137(1):e20152238. https://doi.org/10.1542/peds.2015-2238 .
Article PubMed Central Google Scholar
Conde-Agudelo A, Díaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2016;2016(8):CD002771. https://doi.org/10.1002/14651858.CD002771 .
Sharma D, Farahbakhsh N, Sharma S, Sharma P, Sharma A. Role of kangaroo mother care in growth and breast feeding rates in very low birth weight (VLBW) neonates: a systematic review. J Matern Fetal Neonatal Med. 2019;32(1):129–42. https://doi.org/10.1080/14767058.2017.1304535 .
Cho ES, Kim SJ, Kwon MS, Cho H, Kim EH, Jun EM, et al. The Effects of Kangaroo Care in the Neonatal Intensive Care Unit on the Physiological Functions of Preterm Infants, Maternal-Infant Attachment, and Maternal Stress. J Pediatr Nurs. 2016;31(4):430–8. https://doi.org/10.1016/j.pedn.2016.02.007 .
Furman L. Kangaroo mother care 20 years later: connecting infants and families. Pediatrics. 2017;139(1):e20163332. https://doi.org/10.1542/peds.2016-3332 .
Stefani G, Skopec M, Battersby C, Matthew H. Why is Kangaroo Mother Care not yet scaled in the UK? A systematic review and realist synthesis of a frugal innovation for newborn care. BMJ Innov. 2022;8:9–20. https://doi.org/10.1136/bmjinnov-2021-000828 .
Article Google Scholar
Salim N, Shabani J, Peven K, Rahman QS, Kc A, Shamba D, et al. Kangaroo mother care: EN-BIRTH multi-country validation study. BMC Pregnancy Childbirth. 2021;21(Suppl 1):231. https://doi.org/10.1186/s12884-020-03423-8 .
Bergh AM, Kerber K, Abwao S, de Graft Johnson J, Aliganyira P, Davy K, et al. Implementing facility-based kangaroo mother care services: lessons from a multi-country study in Africa. BMC Health Serv Res. 2014;14:293. https://doi.org/10.1186/1472-6963-14-293 .
Vesel L, Bergh AM, Kerber KJ, Valsangkar B, Mazia G, Moxon SG, et al. Kangaroo mother care: a multi-country analysis of health system bottlenecks and potential solutions. BMC Pregnancy Childbirth. 2015;15(Suppl 2):S5. https://doi.org/10.1186/1471-2393-15-S2-S5 .
Bilal SM, Tadele H, Abebo TA, Tadesse BT, Muleta M, W/Gebriel F, et al. Barriers for kangaroo mother care (KMC) acceptance, and practices in southern Ethiopia: a model for scaling up uptake and adherence using qualitative study. BMC Pregnancy Childbirth. 2021;21(1):25. https://doi.org/10.1186/s12884-020-03409-6 .
Saltzmann AM, Sigurdson K, Scala M. Barriers to Kangaroo Care in the NICU: A Qualitative Study Analyzing Parent Survey Responses. Adv Neonatal Care. 2022;22(3):261–9. https://doi.org/10.1097/ANC.0000000000000907 .
Mathias CT, Mianda S, Ginindza TG. Facilitating factors and barriers to accessibility and utilization of kangaroo mother care service among parents of low birth weight infants in Mangochi District, Malawi: a qualitative study. BMC Pediatr. 2020;20(1):355. https://doi.org/10.1186/s12887-020-02251-1 .
Maniago JD, Almazan JU, Albougami AS. Nurses’ Kangaroo Mother Care practice implementation and future challenges: an integrative review. Scand J Caring Sci. 2020;34(2):293–304. https://doi.org/10.1111/scs.12755 .
Cattaneo A, Amani A, Charpak N, De Leon-Mendoza S, Moxon S, Nimbalkar S, et al. Report on an international workshop on kangaroo mother care: lessons learned and a vision for the future. BMC Pregnancy Childbirth. 2018;18(1):170. https://doi.org/10.1186/s12884-018-1819-9 .
Smith ER, Bergelson I, Constantian S, Valsangkar B, Chan GJ. Barriers and enablers of health system adoption of kangaroo mother care: a systematic review of caregiver perspectives. BMC Pediatr. 2017;17(1):35. https://doi.org/10.1186/s12887-016-0769-5 .
Chan G, Bergelson I, Smith ER, Skotnes T, Wall S. Barriers and enablers of kangaroo mother care implementation from a health systems perspective: a systematic review. Health Policy Plan. 2017;32(10):1466–75. https://doi.org/10.1093/heapol/czx098 .
Kinshella MW, Hiwa T, Pickerill K, Vidler M, Dube Q, Goldfarb D, et al. Barriers and facilitators of facility-based kangaroo mother care in sub-Saharan Africa: a systematic review. BMC Pregnancy Childbirth. 2021;21(1):176. https://doi.org/10.1186/s12884-021-03646-3 .
Bergh AM, de Graft-Johnson J, Khadka N, Om'Iniabohs A, Udani R, Pratomo H, et al. The three waves in implementation of facility-based kangaroo mother care: a multi-country case study from Asia. BMC Int Health Hum Rights. 2016;16:4. https://doi.org/10.1186/s12914-016-0080-4 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–40. https://doi.org/10.1097/XEB.0000000000000055 .
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/10.1186/2046-4053-4-1 .
Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Mental Health. 2018;21(3):95–100. https://doi.org/10.1136/ebmental-2018-300014 .
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71 .
Joanna Briggs Institute. Joanna Briggs Institute reviewers’ manual: 2017 edition. Australia: The Joanna Briggs Institute; 2017.
Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. https://doi.org/10.1016/j.jclinepi.2015.06.005 .
Seidman G, Unnikrishnan S, Kenny E, Myslinski S, Cairns-Smith S, Mulligan B, et al. Barriers and enablers of kangaroo mother care practice: a systematic review. PLoS One. 2015;10(5):e0125643. https://doi.org/10.1371/journal.pone.0125643 .
Article CAS PubMed PubMed Central Google Scholar
Mathias CT, Mianda S, Ohdihambo JN, Hlongwa M, Singo-Chipofya A, Ginindza TG. Facilitating factors and barriers to kangaroo mother care utilisation in low- and middle-income countries: A scoping review. Afr J Prim Health Care Fam Med. 2021;13(1):e1–e15. https://doi.org/10.4102/phcfm.v13i1.2856 .
Chan GJ, Labar AS, Wall S, Atun R. Kangaroo mother care: a systematic review of barriers and enablers. Bull World Health Organ. 2016;94(2):130–141J. https://doi.org/10.2471/BLT.15.157818 .
Charpak N, Ruiz-Peláez JG. Resistance to implementing kangaroo mother care in developing countries, and proposed solutions. Acta Paediatr. 2006;95(5):529–34. https://doi.org/10.1080/08035250600599735 .
Ferrarello D, Hatfield L. Barriers to skin-to-skin care during the postpartum stay. MCN Am J Matern Child Nurs. 2014;39(1):56–61. https://doi.org/10.1097/01.NMC.0000437464.31628.3d .
Yue J, Liu J, Williams S, Zhang B, Zhao Y, Zhang Q, et al. Barriers and facilitators of kangaroo mother care adoption in five Chinese hospitals: a qualitative study. BMC Public Health. 2020;20(1):1234. https://doi.org/10.1186/s12889-020-09337-6 .
Zhang B, Duan Z, Zhao Y, Williams S, Wall S, Huang L, et al. Intermittent kangaroo mother care and the practice of breastfeeding late preterm infants: results from four hospitals in different provinces of China. Int Breastfeed J. 2020;15(1):64. https://doi.org/10.1186/s13006-020-00309-5 .
Shah RK, Sainju NK, Joshi SK. Knowledge, Attitude and Practice towards Kangaroo Mother Care. J Nepal Health Res Counc. 2018;15(3):275–81. https://doi.org/10.3126/jnhrc.v15i3.18855 .
Adisasmita A, Izati Y, Choirunisa S, Pratomo H, Adriyanti L. Kangaroo mother care knowledge, attitude, and practice among nursing staff in a hospital in Jakarta, Indonesia. PLoS One. 2021;16(6):e0252704. https://doi.org/10.1371/journal.pone.0252704 .
Pratomo H, Uhudiyah U, Sidi IP, Rustina Y, Suradi R, Bergh AM, et al. Supporting factors and barriers in implementing kangaroo mother care in Indonesia. Paediatrica Indonesiana. 2012;52(1):43–50. https://doi.org/10.14238/pi52.1.2012.43-50 .
Lee HC, Martin-Anderson S, Dudley RA. Clinician perspectives on barriers to and opportunities for skin-to-skin contact for premature infants in neonatal intensive care units. Breastfeed Med. 2012;7(2):79–84. https://doi.org/10.1089/bfm.2011.0004 .
Sacks E, Bailey JM, Robles C, Low LK. Neonatal care in the home in northern rural Honduras: a qualitative study of the role of traditional birth attendants. J Perinat Neonatal Nurs. 2013;27(1):62–71. https://doi.org/10.1097/JPN.0b013e31827fb3fd .
Bergh AM, Rogers-Bloch Q, Pratomo H, Uhudiyah U, Sidi IP, Rustina Y, et al. Progress in the implementation of kangaroo mother care in 10 hospitals in Indonesia. J Trop Pediatr. 2012;58(5):402–5. https://doi.org/10.1093/tropej/fmr114 .
Yue J, Liu J, Zhao Y, Williams S, Zhang B, Zhang L, et al. Evaluating factors that influenced the successful implementation of an evidence-based neonatal care intervention in Chinese hospitals using the PARIHS framework. BMC Health Serv Res. 2022;22(1):104. https://doi.org/10.1186/s12913-022-07493-6 .
Deng Q, Zhang Y, Li Q, Wang H, Xu X. Factors that have an impact on knowledge, attitude and practice related to kangaroo care: National survey study among neonatal nurses. J Clin Nurs. 2018;27(21–22):4100–11. https://doi.org/10.1111/jocn.14556 .
Kinshella MW, Salimu S, Chiwaya B, Chikoti F, Chirambo L, Mwaungulu E, et al. “So sometimes, it looks like it's a neglected ward”: Health worker perspectives on implementing kangaroo mother care in southern Malawi. PLoS One. 2020;15(12):e0243770. https://doi.org/10.1371/journal.pone.0243770 .
Gill VR, Liley HG, Erdei C, Sen S, Davidge R, Wright AL, et al. Improving the uptake of Kangaroo Mother Care in neonatal units: A narrative review and conceptual framework. Acta Paediatr. 2021;110(5):1407–16. https://doi.org/10.1111/apa.15705 .
Nguah SB, Wobil PN, Obeng R, Yakubu A, Kerber KJ, Lawn JE, et al. Perception and practice of Kangaroo Mother Care after discharge from hospital in Kumasi, Ghana: a longitudinal study. BMC Pregnancy Childbirth. 2011;11:99. https://doi.org/10.1186/1471-2393-11-99 .
Kymre IG, Bondas T. Skin-to-skin care for dying preterm newborns and their parents--a phenomenological study from the perspective of NICU nurses. Scand J Caring Sci. 2013;27(3):669–76. https://doi.org/10.1111/j.1471-6712.2012.01076.x .
Chugh Sachdeva R, Mondkar J, Shanbhag S, Manuhar M, Khan A, Dasgupta R, et al. A Qualitative Analysis of the Barriers and Facilitators for Breastfeeding and Kangaroo Mother Care Among Service Providers, Mothers and Influencers of Neonates Admitted in Two Urban Hospitals in India. Breastfeed Med. 2019;14(2):108–14. https://doi.org/10.1089/bfm.2018.0177 .
Ariff S, Maznani I, Bhura M, Memon Z, Arshad T, Samejo TA, et al. Understanding Perceptions and Practices for Designing an Appropriate Community-Based Kangaroo Mother Care Implementation Package: Qualitative Exploratory Study. JMIR Form Res. 2022;6(1):e30663. https://doi.org/10.2196/30663 .
Kuo SF, Chen IH, Chen SR, Chen KH, Fernandez RS. The Effect of Paternal Skin-to-Skin Care: A Systematic Review and Meta-analysis of Randomized Control Trials. Adv Neonatal Care. 2022;22(1):E22–32. https://doi.org/10.1097/ANC.0000000000000890 .
Mu PF, Lee MY, Chen YC, Yang HC, Yang SH. Experiences of parents providing kangaroo care to a premature infant: A qualitative systematic review. Nurs Health Sci. 2020;22(2):149–61. https://doi.org/10.1111/nhs.12631 .
Brimdyr K, Widström AM, Cadwell K, Svensson K, Turner-Maffei C. A Realistic Evaluation of Two Training Programs on Implementing Skin-to-Skin as a Standard of Care. J Perinat Educ. 2012;21(3):149–57. https://doi.org/10.1891/1058-1243.21.3.149 .
Kymre IG, Bondas T. Balancing preterm infants’ developmental needs with parents’ readiness for skin-to-skin care: a phenomenological study. Int J Qual Stud Health Well-Being. 2013;8:21370. https://doi.org/10.3402/qhw.v8i0.21370 .
Blomqvist YT, Frölund L, Rubertsson C, Nyqvist KH. Provision of Kangaroo Mother Care: supportive factors and barriers perceived by parents. Scand J Caring Sci. 2013;27(2):345–53. https://doi.org/10.1111/j.1471-6712.2012.01040.x .
Filippa M, Saliba S, Esseily R, Gratier M, Grandjean D, Kuhn P. Systematic review shows the benefits of involving the fathers of preterm infants in early interventions in neonatal intensive care units. Acta Paediatr. 2021;110(9):2509–20. https://doi.org/10.1111/apa.15961 .
Kirca N, Adibelli D. Effects of mother-infant skin-to-skin contact on postpartum depression: A systematic review. Perspect Psychiatr Care. 2021;57(4):2014–23. https://doi.org/10.1111/ppc.12727 .
Scime NV, Gavarkovs AG, Chaput KH. The effect of skin-to-skin care on postpartum depression among mothers of preterm or low birthweight infants: A systematic review and meta-analysis. J Affect Disord. 2019;253:376–84. https://doi.org/10.1016/j.jad.2019.04.101 .
Blencowe H, Kerac M, Molyneux E. Safety, effectiveness and barriers to follow-up using an ‘early discharge’ Kangaroo Care policy in a resource poor setting. J Trop Pediatr. 2009;55(4):244–8. https://doi.org/10.1093/tropej/fmn116 .
Craig JW, Glick C, Phillips R, Hall SL, Smith J, Browne J. Recommendations for involving the family in developmental care of the NICU baby. J Perinatol. 2015;35(Suppl 1):S5–8. https://doi.org/10.1038/jp.2015.142 PMID: 26597804.
Calibo AP, De Leon MS, Silvestre MA, Murray JCS, Li Z, Mannava P, et al. Scaling up kangaroo mother care in the Philippines using policy, regulatory and systems reform to drive changes in birth practices. BMJ Glob Health. 2021;6(8):e006492. https://doi.org/10.1136/bmjgh-2021-006492 .
Hunter EC, Callaghan-Koru JA, Al Mahmud A, Shah R, Farzin A, Cristofalo EA, et al. Newborn care practices in rural Bangladesh: Implications for the adaptation of kangaroo mother care for community-based interventions. Soc Sci Med. 2014;122:21–30. https://doi.org/10.1016/j.socscimed.2014.10.006 .
Muddu GK, Boju SL, Chodavarapu R. Knowledge and awareness about benefits of Kangaroo Mother Care. Indian J Pediatr. 2013;80(10):799–803. https://doi.org/10.1007/s12098-013-1073-0 .
Wong J, Fisher J. The role of traditional confinement practices in determining postpartum depression in women in Chinese cultures: a systematic review of the English language evidence. J Affect Disord. 2009;116(3):161–9. https://doi.org/10.1016/j.jad.2008.11.002 .
Rao CR, Dhanya SM, Ashok K, Niroop SB. Assesment of cultural beliefs and practices during the postnatal period in a costal town of South India - A mixed method research study. Global J Med Public Health. 2014;3(5):1–8.
Opara PI, Okorie EMC. Kangaroo mother care: Mothers experiences post discharge from hospital. J Preg Neonatal Med. 2017;1(1):16–20. https://doi.org/10.35841/neonatal-medicine.1.1.16-20 .
Brotherton H, Daly M, Johm P, Jarju B, Schellenberg J, Penn-Kekana L, et al. “We All Join Hands”: Perceptions of the Kangaroo Method Among Female Relatives of Newborns in The Gambia. Qual Health Res. 2021;31(4):665–76. https://doi.org/10.1177/1049732320976365 .
Dumbaugh M, Tawiah-Agyemang C, Manu A, ten Asbroek GH, Kirkwood B, Hill Z. Perceptions of, attitudes towards and barriers to male involvement in newborn care in rural Ghana, West Africa: a qualitative analysis. BMC Pregnancy Childbirth. 2014;14:269. https://doi.org/10.1186/1471-2393-14-269 .
Download references
Acknowledgments
This research received no external funding.
Author information
Authors and affiliations.
Nursing Department, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
Qian Cai, Dan-Qi Chen & Rui Yang
Zhejiang University School of Medicine Women’s Hospital, No. 1 Xueshi Road, Shangcheng District, Hangzhou, 310006, Zhejiang Province, China
Qian Cai, Dan-Qi Chen, Hua Wang, Yue Zhang, Rui Yang & Xin-Fen Xu
Women’s Hospital School of Medicine Zhejiang University Haining Branch/Haining Maternity and Child Health Care Hospital, No. 6 Qinjian Road, Haizhou Street, Haining, 314400, Zhejiang Province, China
Wen-Li Xu & Xin-Fen Xu
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization, QC, XFX and HW; Methodology, QC, DQC and YZ; Validation, QC, DQC and RY and YZ; Formal Analysis, QC; Resources, QC, YZ and WLX; Writing – Original Draft Preparation, QC; Writing – Review & Editing, QC, DQC, RY and XFX; Supervision, XFX and HW. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Xin-Fen Xu .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
PRISMA 2020 Checklist.
Additional file 2.
Search strategies for English and Chinese databases.
Additional file 3.
1. Articles presenting barriers to implementing KMC. 2. Articles presenting facilitators to implementing KMC.
Additional file 4.
Result of the quality appraisal of included studies.
Additional file 5.
Risk of Bias analysis using ROBIS tool.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Cai, Q., Chen, DQ., Wang, H. et al. What influences the implementation of kangaroo mother care? An umbrella review. BMC Pregnancy Childbirth 22 , 851 (2022). https://doi.org/10.1186/s12884-022-05163-3
Download citation
Received : 07 July 2022
Accepted : 27 October 2022
Published : 18 November 2022
DOI : https://doi.org/10.1186/s12884-022-05163-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Kangaroo mother care
- Preterm birth
- Umbrella review
- Implementation
- Facilitators
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Barriers and Enablers of Kangaroo Mother Care Practice: A Systematic Review
* E-mail: [email protected]
Affiliation Harvard T. H. Chan School of Public Health, Boston, Massachusetts, United States of America
Affiliation Boston Consulting Group, Boston, Massachusetts, United States of America
Affiliation Boston Consulting Group, New York City, New York, United States of America
Affiliation Bill & Melinda Gates Foundation, Seattle, Washington, United States of America
- Gabriel Seidman,
- Shalini Unnikrishnan,
- Emma Kenny,
- Scott Myslinski,
- Sarah Cairns-Smith,
- Brian Mulligan,
- Cyril Engmann

- Published: May 20, 2015
- https://doi.org/10.1371/journal.pone.0125643
- Reader Comments
Kangaroo mother care (KMC) is an evidence-based approach to reducing mortality and morbidity in preterm infants. Although KMC is a key intervention package in newborn health initiatives, there is limited systematic information available on the barriers to KMC practice that mothers and other stakeholders face while practicing KMC. This systematic review sought to identify the most frequently reported barriers to KMC practice for mothers, fathers, and health practitioners, as well as the most frequently reported enablers to practice for mothers. We searched nine electronic databases and relevant reference lists for publications reporting barriers or enablers to KMC practice. We identified 1,264 unique publications, of which 103 were included based on pre-specified criteria. Publications were scanned for all barriers / enablers. Each publication was also categorized based on its approach to identification of barriers / enablers, and more weight was assigned to publications which had systematically sought to understand factors influencing KMC practice. Four of the top five ranked barriers to KMC practice for mothers were resource-related: “Issues with the facility environment / resources,” “negative impressions of staff attitudes or interactions with staff,” “lack of help with KMC practice or other obligations,” and “low awareness of KMC / infant health.” Considering only publications from low- and middle-income countries, “pain / fatigue” was ranked higher than when considering all publications. Top enablers to practice were included “mother-infant attachment” and “support from family, friends, and other mentors.” Our findings suggest that mother can understand and enjoy KMC, and it has benefits for mothers, infants, and families. However, continuous KMC may be physically and emotionally difficult, and often requires support from family members, health practitioners, or other mothers. These findings can serve as a starting point for researchers and program implementers looking to improve KMC programs.
Citation: Seidman G, Unnikrishnan S, Kenny E, Myslinski S, Cairns-Smith S, Mulligan B, et al. (2015) Barriers and Enablers of Kangaroo Mother Care Practice: A Systematic Review. PLoS ONE 10(5): e0125643. https://doi.org/10.1371/journal.pone.0125643
Academic Editor: Zulfiqar A. Bhutta, The Hospital for Sick Children, PAKISTAN
Received: August 22, 2014; Accepted: March 24, 2015; Published: May 20, 2015
Copyright: © 2015 Seidman et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: The Bill & Melinda Gates Foundation provided funding for this review. Two authors (BM, CE) were employees of the foundation at the time of writing. They were not involved in collection or analysis of data, but did provide input into revisions / edits of the manuscript.
Competing interests: The authors declare that no competing interests exist.
Introduction
Preterm birth is a major global health issue, with 15 million preterm births occurring each year, and over 1 million of these preterm infants dying each year [ 1 ]. Preterm birth complications directly account for greater than 35% of all neonatal deaths each year, and preterm birth indirectly contributes to an even greater percentage because it increases the risk that an infant will die from infection. Preterm births are on the rise globally, both in high-income and low-income settings [ 1 ]. The 10 countries with highest rates of preterm births include those that are high-income, such as the USA, middle-income such as India, China, the Philippines, Indonesia and Brazil, and low-income such as Nigeria, Pakistan, Bangladesh, Democratic Republic of Congo [ 1 ]. Thus interventions that are feasible and applicable in both high- and low-income settings are highly desired.
Kangaroo mother care (KMC) is an evidence-based approach to reducing mortality and morbidity in preterm infants which was first developed in Bogotá Colombia. According to the World Health Organization's definition, KMC consists of prolonged skin-to-skin (STS) contact between mother and infant, exclusive breastfeeding whenever possible, early discharge with adequate follow-up and support, and initiation of the practice in the facility and continuation at home [ 2 ]. In a meta-analysis, KMC was shown to significantly reduce preterm mortality at 40–41 weeks' corrected gestational age by 40% and to improve other outcomes including severe infection / sepsis, emotional attachment in mothers, and weight gain versus conventional neonatal care in preterm infants [ 3 ]. Another meta-analysis showed a similar mortality benefit, although it included fewer studies in its analysis [ 4 ]. Research from various countries also suggests that KMC is a cost-effective method for treating preterm infants [ 5 , 6 ], that mothers who have practiced KMC may find it acceptable [ 6 – 8 ], and that KMC can have a positive impact on the health of mothers in certain cases [ 9 , 10 ]. Therefore, KMC is a highly relevant intervention that should be considered for scaling across geographies. Although the WHO definition of KMC specifies that the practice should be initiated in a facility setting, several studies and trials have explored whether KMC can be effective in a community-initiated setting, and the effectiveness of KMC in this context has not yet been conclusively determined [ 11 , 12 ].
In spite of these benefits, mothers may face barriers to practice, some of which may prevent them from achieving the continuous STS contact with their infants (a defining feature of KMC). For example, a survey of 46 mothers of preterm infants who were trained on KMC in a facility in Andhra Pradesh, India found that only 6.5% of mothers felt that providing KMC for 12 hours / day or greater was feasible, whereas 52% of mothers felt that only 1 hour / day was practical[ 8 ]. Similarly, in a trial of community-initiated KMC with 1,565 mother-infant pairs, only 23.8% practiced STS for more than 7 hours / day in the first 48 hours of life, and the average number of hours of STS during days 3–7 of life was 2.7 ± 3.4 hours [ 11 ]. Barriers to the other components of KMC, including breastfeeding [ 12 , 13 ], and adequate follow-up after discharge [ 14 , 15 ], have also been noted.
KMC has emerged as a key intervention package for a number of newborn health initiatives, and this is epitomized by the Every Newborn Action Plan (ENAP) [ 16 ]. Additionally a recent convening of ideas from 600 key programmers, policymakers, researchers and stakeholders in newborn health, using the Child Health and Nutrition Research Initiative [CHNRI] method, highlighted KMC as a top preterm intervention agenda [ 17 ]. Many agencies, such as Save the Children's Saving Newborn Lives III (SNL), USAID, WHO and the Bill & Melinda Gates Foundation, and some countries, such as Malawi and South Africa, have also made KMC a priority [ 18 – 22 ].
Therefore, to adequately implement and effectively scale-up this intervention, it is critical to understand the key factors that contribute to a mother's (in)ability to practice KMC. However, there is a dearth of synthesized information on all of the sociocultural, resourcing, and experiential factors that influence a mother's practice of KMC. Accordingly, this review sets out to synthesize existing literature on the factors which influence a mother's ability to practice KMC by answering two questions. First, what are the most frequently cited barriers that could prevent a mother from successfully practicing KMC? These barriers can exist at multiple levels, including barriers to implementation of a KMC program, deficiencies in the program itself, or specific challenges associated with the practice of KMC which the mother has to perform. Second, are there any key positive factors, cited in the relevant literature, that can enable a mother to practice KMC? We believe that it is of utmost importance to consider these different types of barriers together (along with key enablers to practice), even though the solutions for solving each barrier might be different. Even though the specific barriers most relevant for mothers may vary based on context, a comprehensive list of this type will give program implementers, policymakers, and researchers a synthesized set of factors to consider as they attempt to implement new or improve existing KMC programs.
Methodology
Search strategy and selection criteria.
We undertook a systematic review according to PRISMA 2009 guidelines to answer these two questions [ 23 ]. (See S1 Appendix for complete PRISMA checklist). We developed a review protocol with methods and eligibility criteria that were specified in advance. We included any publication in our study that met the following criteria: 1) the aim of the study was to document experiences implementing KMC, STS, or other interventions related to Reproductive, Maternal, Newborn, & Child Health and Nutrition (RMNCH&N) that may have included KMC / STS, or the publication had relevant information on specific barriers to implementation listed in the abstract; 2) the study was published in a peer-reviewed journal; 3) the study included data on the sample population, sample size, and location of implementation; 4) the study was original research; and 5) the study was published in English. Studies testing the efficacy of KMC or STS practice (e.g. randomized controlled trials) were included if issues of acceptability, feasibility, or barriers to practice for parents or practitioners were documented in the abstract. Any publication published before August 13, 2013 (the date of the final database search) was eligible for inclusion. We excluded literature reviews, conference proceedings, letters to the editor, and abstracts in order to prevent double counting of data and to ensure that all barriers were understood in the context of the entire study.
We searched nine electronic databases: PubMed, EMBASE, Scopus, Web of Science, and the WHO Regional Databases (AIM, LILACS, IMEMR, IMSEAR, and WPRIM). We searched all databases using the following search terms: "Kangaroo Mother Care" OR "Kangaroo Care" OR "Skin to skin care". In addition, because at least one relevant article identified from a list of references in a literature review included the terms Kangaroo Mother Care in quotations and the term Skin to skin, we also searched PubMed for "'Kangaroo Mother Care'" and "Skin to skin". We used broad search criteria to ensure that relevant articles were not missed, and we then filtered and excluded many articles based on the eligibility criteria mentioned above. Reference lists from literature reviews identified in the database search were also scanned for relevant titles, and articles were also identified in consultation with the authors on this study. Recommendations for studies to be included in the review were also received from participants at the KMC Acceleration Meeting in Istanbul, October 2013[ 24 ] and in consultation with leaders in the fields of KMC and newborn health.
Data collection
After our initial database search and identification of additional studies through recommendations and scans of reference lists, study titles and abstracts were screened by two reviewers (GS and EK) for inclusion. In situations when a study's eligibility was disputed, a third reviewer (SU) provided an independent assessment until consensus was reached.
96 articles were reviewed to identify a comprehensive list of barriers to KMC practice in advance of the KMC Acceleration Convening [ 24 ]. A data extraction sheet was piloted and tested using these 96 articles. This piloting allowed for preliminary identification of relevant barriers and enablers to be included in the final review as well as final determination of stakeholders to be included in the review: mothers, fathers, community health workers, nurses, physicians, and program managers. The final tool included fields for collecting publication details, relevant study characteristics (sample size, location, and a short description of each study), barriers for each stakeholder group, and enablers to practice for mothers. Results from the preliminary analysis were shared at the KMC Acceleration Convening, ensuring that key stakeholders in the KMC community generally supported the methodology (described in further detail in the next section) and found the preliminary results to be consistent with their experiences [ 24 ]. This convening included researchers and practitioners from many different low- and middle-income countries (LMIC) across Latin America, Sub-Saharan Africa, and Asia, as well as major foundations and civil society organizations involved in RMNCH&N
Once the tool and list of studies was finalized, data was captured from each article into the tool independently by two reviewers (GS and EK) and a third reviewer (SU) provided independent assessment in case of disputes. The main outcome of interest was the frequency with which a barrier / enabler was mentioned across publications. Using frequency of mention allowed for a synthesized view of the barriers / enablers to practice listed in the relevant literature. The data collection process involved identifying barriers and enablers of KMC practice listed in each study (either through qualitative or quantitative findings) and categorizing them into one of the pre-determined categories of barriers / enablers in the tool. There was no limit to the number of barriers / enablers that could be found in a single study, but each study could only count toward a given barrier / enabler once. For example, if a study mentioned several statistics all indicating that mothers' low awareness of KMC was a barrier to practice, this would be coded as a single instance of low awareness among mothers in the tool. In cases where a barrier or enabler was listed for parents in general and did not distinguish between mothers and fathers, this barrier was listed as a barrier for mothers. In cases where a barrier was listed for both nurses and physicians but did not distinguish between the two, this barrier was listed as a barrier for nurses. Barriers / enablers were grouped into three different categories—resourcing, experiential, and sociocultural—based on consensus among all authors. Definitions for these three categories are included in S2 Appendix .
Risk of bias and publication weighting methodology
The goal of this study was to synthesize existing literature on barriers to and enablers of KMC practice. As noted, there is limited systematically organized information on this topic. Therefore, in order to ensure that our review captured as many relevant qualitative and quantitative findings as possible, we chose to include any study identified through our search strategy which had information on barriers and enablers to KMC practice, even if studying this topic was not the primary purpose of the publication.
As one might expect based on this search strategy, our findings included many studies which had observational information on barriers to / enablers of KMC practice. Given the limited amount of synthesized information on barriers to KMC practice, we felt it was important to include these observational findings so that relevant programmatic experience informed this review. At the same time, however, we also sought to ensure that our analysis was weighted toward data from publications which had explicitly studied barriers to KMC practice (rather than giving those data equal weighting to observational findings).
Therefore, we developed a methodology to weight findings from each publication based on the way in which the data was identified and captured. Other public health literature reviews have used similar methods to quantify qualitative data drawn from multiple sources of varying quality and relevance [ 25 – 28 ]. We categorized each publication into one of four types: Indirect study, Exploratory study, Systematic study, and Prioritized study. Indirect studies were defined as those which did not set out to study barriers to / enablers of KMC practice, but which identified and documented these factors (ie, through observational findings). Exploratory studies were defined as those which set out to identify barriers / enablers to KMC practice but which did not pre-specify factors under consideration (ie, were not explicitly testing hypotheses about which barriers / enablers would influence practice). Systematic studies were defined as those which set out to identify barriers / enablers of KMC practice and which did pre-specify the factors under consideration but which did not prioritize among these barriers. Prioritized studies were defined in the same way as systematic studies with the exception that these studies also prioritized the barriers to KMC practice. Our indexed ranking methodology gave the most weight to Prioritized studies, the second-most weight to Systematic studies, the third-most weight to Exploratory studies, and the least weight to Indirect studies. ( S2 Appendix provides more detail on full methodology describing indexed ranking process.) Note that in our findings and discussion, we refer to "top-ranked" barriers to practice for mothers and other groups. Top-ranked barriers are those that received the highest score based on this indexed ranking, which accounts for both frequency of mention across publications and weighting of each piece of evidence based on the publication type.
Each study was placed into one of these categories independently by two reviewers (GS and EK), and in cases of a discrepancy, a third reviewer provided an independent assessment (SU). Of the 103 publications included in this review, there were only 12 discrepancies (11.65%) in categorization between the first two readers, suggesting that this method is reliable for categorizing publications. Our data capture tool included a field to categorize each publication into one of these four categories.
Study selection
From our database search, a total of 1,260 unique publications were identified, and four others were identified through snowballing. Of these 1,264, 168 met preliminary eligibility criteria based on a scan of the title and abstract; all others were excluded because they did not meet at all eligibility criteria discussed in the Methodology section. Of these 168, 51 were eliminated after full-text screening because they did not have relevant data (i.e. barriers to newborn health intervention rollout were listed, but no barriers specific to KMC / STS were listed) or because only an abstract was available, and 14 did not have full text available in English. This resulted in 103 articles deemed relevant for inclusion in the review. Fig 1 represents the study selection for inclusion in the systematic review. A full list of publications included in this review can be found in S3 Appendix .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0125643.g001
Of these 103 articles, 49 were from high-income countries HIC [ 29 ], 22 were from Sub-Saharan Africa, 15 were from South Asia, five were from North Africa / the Middle East, five were from Latin America / Caribbean, three were from Eastern Europe, two were from East Asia / Southeast Asia / Pacific, and two were from LMIC in multiple regions.
Nine of the publications were classified as Prioritized, 48 were classified as Systematic, 31 were classified as Exploratory, and 15 were classified as Indirect. Indirect studies included randomized controlled trials that discussed barriers to implementation and practice, two case studies of individuals' experiences with KMC, and studies on practices throughout the NICU which included information on KMC or STS practice.
A complete dataset used for analyses can be found in S1 Dataset .
Barriers and enablers of KMC practice for mothers
Of the top five barriers to KMC practice identified for mothers, four were resource-related. The top two barriers to practice identified—"Issues with facility environment / resources" and "Negative impressions of staff attitudes or interactions"—were specific to the facility setting. "Fear / anxiety of hurting the infant," an experiential barrier to practice, was ranked third. Resource-related barriers that are relevant both inside and outside the facility—"Lack of help with KMC practice and other obligations" and "Low awareness of KMC / infant health"—were ranked fourth and fifth. When considering publications from LMIC only, four of the five top barriers were the same as when all publications were considered. The only difference is that "Negative impressions of staff attitudes or interactions" dropped significantly (to 11th), and "Pain / fatigue" emerged as the fourth-highest-ranked barrier, just after "Fear / anxiety of hurting the infant." The full rankings of barriers identified for mothers can be found in Fig 2A , and the full ranking of barriers identified for mothers from LMIC only can be found in Fig 2B .
a) Indexed ranking of barriers to adoption of KMC for mothers in all countries, and b) indexed ranking of barriers to adoption of KMC for mothers in LMIC only.
https://doi.org/10.1371/journal.pone.0125643.g002
Experiential factors emerged as the top enablers to KMC practice for mothers. "Mother-infant attachment," "Feelings of confidence / empowerment," and "Ease of practice / preference over traditional care" emerged as three of the top five enablers both when considering all publications and just those from LMIC. "Support from family, friends, and other mothers," a resourcing enabler, was also in the top five enablers when considering all publications, and it was the top-ranked enabler when considering publications only from LMIC. "Support from staff or community health worker (CHW)" was the fourth-highest-ranked enabler when considering all publications, but was ranked seventh when considering LMIC only. "Understanding of efficacy" was also ranked among the top five enablers to practice when considering LMIC only. The full ranking of enablers for mothers across all publications and in LMIC only can be found in Fig 3A and Fig 3B , respectively.
https://doi.org/10.1371/journal.pone.0125643.g003
Barriers of KMC for nurses
Resourcing and sociocultural factors emerged as the top barriers to KMC adoption for nurses. The resourcing barriers "Actual increased workload / staff shortages" and "Lack of clear guidelines / training" were in the top five barriers for nurses when considering publications from all geographies and just those from LMIC. The sociocultural barriers "General lack of buy-in / belief in efficacy" and "Concerns about other medical conditions / care" were also in the top five barriers for nurses when considering publications from all geographies and just those from LMIC. (Note that a data point was counted in the "Concerns about other medical conditions / care" category when the publication indicated that nurses' beliefs countered guidelines for KMC practice or when there was lack of consensus among nurses about whether KMC was safe to practice when an infant had a certain condition). The full ranking of barriers to adoption for nurses across all publications and in LMIC only can be found in Fig 4A and Fig 4B , respectively.
a) Indexed ranking of barriers to adoption of KMC for nurses in all countries, and b) indexed ranking of barriers to adoption of KMC for nurses in LMIC.
https://doi.org/10.1371/journal.pone.0125643.g004
Barriers for fathers, CHW's, physicians, and program managers
Much less data was available for fathers, physicians, and program managers than was for mothers and nurses. Full rankings of barriers for these stakeholders across all publications can be found in Figs 5 – 7 .
https://doi.org/10.1371/journal.pone.0125643.g005
https://doi.org/10.1371/journal.pone.0125643.g006
https://doi.org/10.1371/journal.pone.0125643.g007
The top-ranked barrier for fathers was "Lack of opportunity to practice." The top-ranked barrier for physicians was "General lack of buy-in / belief in efficacy." The top-ranked barrier for program managers was "Need for high-touch support from staff."
The aim of this systematic review was to identify the most frequently cited barriers to KMC adoption, as well as enablers to practice. Given the increasing importance of KMC in addressing the global health challenge of preterm birth and death, synthesizing the experiential, resourcing, and sociocultural barriers that could prevent a mother from effectively practicing KMC is critical to effectively implementing this intervention. Although much has been written on this topic, nearly half (44.6%) of the publications identified for inclusion in this review were categorized as either Exploratory or Indirect, suggesting that there is lots of data relevant to the promotion of KMC that is not organized in a systematic way which can readily guide program implementation.
Based on the list of barriers and enablers found in the publications identified, we have identified five key insights which we believe are relevant for program implementers and researchers. Each of these insights is detailed below.
Mothers are generally able to understand and accept KMC
Low awareness of KMC and infant health more broadly was the fourth-highest-ranked barrier to KMC practice across all publications, and the highest barrier to KMC practice when considering only publications from LMIC. However, this barrier may be over-represented in the literature on KMC because it is easily testable and many publications that implemented KMC in a new setting surveyed pre-existing levels of awareness to establish a baseline. Lack of information about KMC, hypothermia, or newborn health was identified across HIC (Sweden [ 30 , 31 ], Unite[ 32 ]d States[ 33 ]) and LMIC (Bangladesh [ 11 ], Egypt [ 34 , 35 ], Ghana [ 36 , 37 ], India [ 8 , 32 , 38 ], South Africa [ 22 , 39 ], and Zimbabwe [ 40 ]).
In spite of low general awareness of KMC, however, the literature from LMIC suggests that it is easy to train mothers on KMC practices and that they can understand the practice. For example, a training program in India found that 88% of mothers were able to understand KMC with a single training session [ 10 ]. Similarly, during site visits to facilities practicing KMC in Ghana, researchers found that mothers practicing KMC were able name its benefits [ 41 ]. Mothers were also able to understand the KMC messages delivered by community health workers in a community setting in Bangladesh [ 12 ].
Mothers' understanding of the practice also seems to enhance their adherence to practice. In South Africa, for example, mothers' "main motivation for embracing [KMC] was the well-being of their infants" [ 22 ]. Similarly, studies in Ghana found, "all mothers recognised that their babies' small weights put them at risk of illness and death and appreciated that [STS] could improve their health and survival,"[ 37 ] and, "as a motivational factor, mothers and health workers also mentioned various success stories of infants who had survived having been nursed in KMC." [ 41 ] Belief in the efficacy of KMC as an enabling factor for practice was also mentioned in HIC. One case study from the United States describes how the mother used research articles demonstrating KMC's benefits to convince facility staff to let her practice KMC [ 42 ].
Mothers can enjoy practicing KMC, and the practice has benefits for mothers and families
Mothers not only are able to understand and accept KMC, but also they may enjoy the practice. Mother-infant attachment was the top-ranked enabler for KMC practice, and evidence for this enabler came from across HIC and LMIC. In Colombia, for example, sensitivity to infants was significantly higher among mothers practicing KMC compared to control (p<.05), and cognitive fostering was significantly higher among KMC mothers compared to control after 14 days (p<.05) [ 43 ]. Similarly, in India, KMC mothers were more likely to spend time with their baby "beyond the usual care taking" (p<.05), derive pleasure from their baby (p<.05), and only go out for "totally unavoidable" reasons (p<.05) compared to controls [ 44 ]. Qualitative findings from HIC also support these findings [ 13 , 30 , 45 ].
Several studies have shown that KMC has positive impact on the mother. Although postpartum depression can be a barrier to practicing KMC [ 46 ], those mothers who do practice KMC may experience a reduction in postpartum depression symptoms [ 9 , 47 ]. They may also experience an increased sense of competence [ 43 ]. Evidence from HIC also suggests that KMC has a beneficial impact on overall family dynamics. For example, one study from Israel found family cohesiveness was higher among KMC families as compared to controls [ 48 ]. Similarly, qualitative findings from Sweden indicate that KMC "strengthened the mother-father-child unit" [ 49 ]. Although further research may be needed to replicate these findings in low- and middle-income countries, it is clear that KMC can be a beneficial intervention not only for the infant, but also for the mother and the family.
Practicing KMC is often difficult
"Pain / fatigue" emerged as one of the top five barriers to KMC practice when considering all publications and only publications from LMIC. This set of barriers included finding the baby too difficult or heavy to hold [ 12 ], discomfort on the chest or back [ 46 ], and exhaustion [ 50 ], among others. Further, one should note that we identified other barriers that, taken together with the "Pain / fatigue" barrier, indicate that mothers may struggle with the practice. These barriers include "Positioning issues," including difficulty sleeping with the infant on the chest [ 40 ], "Breastmilk expression and other breastfeeding-related issues,"[ 8 ] discomfort related to temperature [ 50 ], and "Issues with clothing / infants' medical devices"[ 30 , 51 ]. Of course, mothers' medical issues also pose a major barrier to practice. These medical issues included pain from episiotomy repair [ 52 ], recovery from caesarean section[ 46 ], postpartum depression[ 46 ], and general maternal illness [ 12 , 53 ].
These barriers suggest that practicing continuous KMC is likely very challenging for mothers, especially those who have low motivation and medical issues.
Support for mothers can make KMC practice easier
In addition to being physically taxing for mothers, KMC also limits the mother's ability to take care of other tasks and obligations. "Lack of help with KMC practice and other obligations" was ranked among the top five barriers to KMC practice across all publications and when looking only at LMIC. Obligations related to mothers' daily routine came up in publications from countries such as Zimbabwe [ 40 ], Uganda [ 54 ], Ghana [ 36 ], and Sweden [ 30 ].
Conversely, "Support from family, friends, and other mothers" emerged as the third-highest-ranked enabler to practice across publications and the top enabler of practice in LMIC. This support took many different forms. Family members would often take turns holding the infant in KMC to give the mother a break from the practice [ 7 , 10 , 55 ]. They would also take care of other tasks that the mother otherwise would have had to deal with, including childcare and housekeeping [ 56 , 57 ]. Qualitative evidence also indicates that emotional support provides an important, and sometimes crucial, enabler to practice. For example, in Malawi, when looking to overcome issues of fear or embarrassment for the mothers, implementers found, "the most effective way to ensure KMC continues at home is to involve the grandma during the admission" [ 58 ]. Similarly critical roles of family members providing emotional support were documented in Ghana [ 36 ] and South Africa [ 39 ].
Several studies also documented the role that other mothers could play in training or supporting mothers in KMC practice. For example, in a study investigating a community-based application of KMC in Bangladesh, one third of mothers who had been trained on community-initiated KMC reported teaching the practice to others [ 11 ]. There is quantitative evidence from Ghana that this phenomenon has an impact on practice; infants in a region where some women had been trained on STS but whose mothers had not been taught STS were more likely to receive STS than infants born in regions where no mothers had been taught STS (RR Any [STS care] : 1.28; 95% CI: 0.92–1.79; RR > 2 h [STS care] : 1.64; 95% CI: 0.80–3.39), thereby suggesting that mothers discussed their STS practice with each other [ 37 ]. Qualitative findings also indicate that KMC mothers support other mothers starting the practice on the ward. In South Africa, for example, KMC mothers supported each other on the ward in various ways: "they reminded each other about the importance of KMC for their babies; discussed how to comfort their babies, and how to kangaroo the infants properly, as demonstrated; and exchanged ideas on how to minimise discomfort" [ 22 ]. Similar experiences were found in Mozambique [ 59 ] and Mexico, Indonesia, and Ethiopia.
Interestingly, "Support from staff or community health workers" was the fourth-highest-ranked enabler for practice across publications but fell to seventh when looking only at publications from LMIC. Although further research is needed, this finding, combined with the finding that support from family, friends, and other mothers is a top enabler to practice, indicates that the community may play a critical role in promoting KMC practice in low-resource settings. Going forward, it will be important for researchers and implementers to understand how the community can complement a facility-based approach to scale-up with community engagement activities, drive demand for the practice, and ensure infants receive quality KMC care.
Physical environment and resourcing factors can be barriers to practice, but these are under-studied in the community setting
"Issues with facility environment / resources" emerged as the top barrier to practice for mothers, and this factor includes an array of different issues. These issues included crowdedness and noisiness [ 22 , 50 , 60 ], lack of privacy [ 61 , 62 ], lack of food and supplies [ 40 , 54 ], and uncomfortable beds [ 13 , 22 ]. It is important to remember that, due to the nature of KMC guidelines, facility-related issues may be over-represented in these findings. Data regarding nurses' barriers to adoption also suggests that resource-related factors, such as workload, play an important role in the implementation of KMC.
It is also important to note that there is a paucity of information available on physical and resourcing barriers to practice for mothers practicing KMC in the community. Of the 103 articles included in this review, only 16 focused on community-initiated KMC or had a substantial focus on community-based practice and perspectives. Thus, although a lack of resources in the community, such as comfortable beds and readily available food, may be an equally common barrier, the data on this topic is currently limited by the focus of existing literature. Of course, institution-initiated KMC is more commonly accepted as an evidence-based practice [ 3 ], which may account for some of the lack of research on practice outside the facility. However, because facility and community practice of KMC actually represent a continuum, with infants moving back and forth between the two, there is still opportunity to study community barriers to practice, even within a facility-initiated KMC program [ 24 ].
Directions for Future Research and Practice
This systematic review prioritizes the main factors that influence KMC practice, and, in doing so, highlights some key areas that implementers and implementation researchers may need to focus on when promoting KMC. Given that local circumstances, including cultural attitudes and support for the mother, have an impact on KMC practice, it is critical to understand the context-specific factors that might impact a KMC program. Qualitative and ethnographic research, including interviews with mothers who have practiced KMC and healthcare providers, as well focus groups with community members, can achieve this goal. Implementers should also study the effectiveness of various user-centric designs for promoting KMC, including different mechanisms to ensure the mother has support for practice.
In addition, this review points out the difficulty that mothers have practicing continuous KMC (at least 20 hours of STS / day). Accordingly, more research and analysis is needed to understand the dose-response effect of KMC. If mothers could practice for shorter periods of time without reducing the mortality impact of the practice, KMC might be more feasible and easier to scale. Researchers should re-examine existing data on the number of hours of STS that infants received and the associated mortality impact, as well as track actual STS hours in forthcoming continuous KMC programs in order to compare infants who received at least 20 hours of STS with those who received fewer (ie, infants whose mothers deviated from the protocol).
Limitations of this Study
This review is limited by definitional challenges related to the practice and implementation of KMC. Since WHO guidelines currently do not recommend community-initiated KMC, there is likely significant bias in the literature toward institution-related barriers to KMC practice [ 2 ]. Therefore, it is likely that more research will focus on issues related to providing KMC in the facility than on issues related to the community, such as cultural perceptions of KMC. However, because mothers and newborns require a continuum of care that extends into both the facility and community, there are likely important barriers to the practice of KMC that relate to community beliefs about newborn care which may be underrepresented in this review.
There also exists some inconsistency in the definition of KMC practice. Even studies included in the Cochrane Review's meta-analysis of KMC , which used rigorous publication inclusion criteria and which helped establish KMC as an evidence-based practice for reducing preterm mortality and morbidity, had widely varying applications of KMC [ 3 ]. For example, Worku et al. did not require infants to be stabilized before beginning KMC, even though most other studies included in the meta-analysis did [ 63 ]. Similarly, the studies included in this meta-analysis had a wide range in the number of hours of STS care actually practiced by mothers and guardians: while some studies reported continuous contact for approximately 20 hours / day [ 64 ], others reported an average of only 1–2 hours of STS care / day [ 65 , 66 ]. Unfortunately, dose-response data for KMC is not available. Given that variations in the application of KMC exist and do not always follow WHO guidelines, our review necessarily includes publications that reflect this variation. By incorporating findings from the broadest range of publications which report barriers to KMC practice, including those publications which only sought to implement STS care (the hallmark component of KMC) and not its other components, we believe we have captured the full range of barriers that one could face when implementing a KMC program.
In addition, the majority of papers identified focuses on mothers and excludes fathers' and other family members' perspectives, and they focus on nurses and exclude physicians' perspectives. Although this likely reflects the reality of the situation that mothers practice KMC more often than fathers and nurses train parents on KMC more often than physicians, future research may need to focus on barriers to practice for fathers and physicians.
There is also a risk that the barriers identified across studies are not the most important barriers to practice, but rather the most easily observable barriers. As mentioned, this review is designed to synthesize the literature on barriers to practice in order to serve as a starting point for future research, rather than to determine which barriers are most critical to overcome in order to ensure the maximum number of hours of STS contact. Because this study included qualitative and observational information from many sources, including publications which did not explicitly set out to address the topic of barriers to KMC practice, it would be impossible to determine which of these barriers are most important (ie, in order to increase the number of hours that a mother can practice STS).
Finally, this review is limited by the fact that only studies published in English were included; in particular, there may be data from non-English-speaking LMIC that have relevant information on barriers / enablers to KMC practice which are not included in this review.
As KMC gains momentum with the rollout of various other Reproductive, Maternal, Newborn, & Child Health and Nutrition programs, including ENAP, it is critical to understand the barriers to practice for the end-users, often the mother, of this life-saving practice, which has many additional benefits for infants and mothers. This systematic review sought to synthesize the most frequently cited barriers to practice for mothers, fathers, CHW's, nurses, physicians, and program managers, as well as the most commonly cited enablers to practice for mothers. The findings from this review can be used to guide future programmatic research efforts aiming to understand how to effectively implement KMC at scale, as well as the design or update of implementation efforts across geographies.
Supporting Information
S1 appendix. prisma checklist..
https://doi.org/10.1371/journal.pone.0125643.s001
S2 Appendix. Detailed methodology for indexed ranking of barriers / enablers.
https://doi.org/10.1371/journal.pone.0125643.s002
S3 Appendix. Full list of publications included in analysis for systematic review.
https://doi.org/10.1371/journal.pone.0125643.s003
S1 Dataset. Complete dataset used for analysis in systematic review.
https://doi.org/10.1371/journal.pone.0125643.s004
Acknowledgments
Funding for this study was provided by the Bill & Melinda Gates Foundation. Funders were not involved in collection, analysis, or interpretation of data. Funders did review drafts of this manuscript. Employees of a for-profit company (Boston Consulting Group) were involved in writing this review, but the outcome of the engagement was not contingent upon the findings or analysis in this paper or any other part of the engagement with the foundation.
Author Contributions
Conceived and designed the experiments: GS SU SCS BM CE. Analyzed the data: GS SU EK SM. Wrote the paper: GS SU CE.
- 1. March of Dimes, PMNCH, Save the Children, WHO (2012) Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization. https://doi.org/10.1186/1742-4755-10-S1-S1 pmid:24625113
- 2. Department of Reproductive Health and Research W (2003) Kangaroo mother care: a practical guide. Geneva: World Health Organization.
- 3. Conde-Agudelo A, Díaz-Rossello J (2014) Kangaroo mother care to reduce morbidity and mortality in low birthweight infants (Review).
- View Article
- PubMed/NCBI
- Google Scholar
- 5. Broughton E, Gomez I, N S, Vindell C (2013) The cost-savings of implementing kangaroo mother care in Nicaragua.
- 8. Muddu GK, Boju SL, Chodavarapu R (2013) Knowledge and Awareness about Benefits of Kangaroo Mother Care. Indian J Pediatr.
- 16. PMNCH. "Every Newborn." Available: www.everynewborn.org . Accessed 2013 Dec 1.
- 17. Yoshida S, Rudan I, Lawn JE, Wall S, Souza JP, Bahl R, et al. (2014) Defining newborn health research agenda beyond 2015. Submitted for publication.
- 18. Save the Children (2013) Saving Newborn Lives: Bending the Curve; Accelerating Progress in Newborn Survival and Health. The Bill & Melinda Gates Foundation. [Personal correspondance with Becky Ferguson: August 2013].
- 19. MCHIP (2012). "MCHIP and USAID Host First LAC Conference on Kangaroo Mother Care." Available: http://www.mchip.net/node/699 . Accessed 2014 Jan 16.
- 20. World Health Organization (2014). "Kangaroo mother care to reduce morbidity and mortality and improve growth in low-birth-weight infants." Available: http://www.who.int/elena/titles/kangaroo_care_infants/en/ . Accessed: 2014 Jan 16.
- 21. Bergh A-M, Banda L, Lipato T, Ngwira G, Luhanga R, Ligowe R (2012) Evaluation of kangaroo mother care services in Malawi. MCHIP.
- 29. Central Intelligence Agency (2013) The World Factbook 2013–2014. In: Agency CI, editor. Washington, DC.
- 33. Bramson L, Lee JW, Moore E, Montgomery S, Neish C, Bahjri K, et al. (2010) Effect of early skin-to-skin mother–infant contact during the first 3 hours following birth on exclusive breastfeeding during the maternity hospital stay. Journal of Human Lactation.
Journal Article
Jun 15, 2016
Kangaroo Mother Care (KMC)
What is kangaroo mother care? Systematic review of the literature

Additional Authors
Grace J Chan, Bina Valsangkar, Sandhya Kajeepeta , Ellen O Boundy, Stephen Wall
Related Topics
Subscribe to our newsletter.
Kangaroo mother care (KMC), often defined as skin– to–skin contact between a mother and her newborn, frequent or exclusive breastfeeding, and early discharge from the hospital has been effective in reducing the risk of mortality among preterm and low birth weight infants. Research studies and program implementation of KMC have used various definitions.
To describe the current definitions of KMC in various settings, analyze the presence or absence of KMC components in each definition, and present a core definition of KMC based on common components that are present in KMC literature.
We conducted a systematic review and searched PubMed, Embase, Scopus, Web of Science, and the World Health Organization Regional Databases for studies with key words “kangaroo mother care”, “kangaroo care” or “skin to skin care” from 1 January 1960 to 24 April 2014. Two independent reviewers screened articles and abstracted data.
We screened 1035 articles and reports; 299 contained data on KMC and neonatal outcomes or qualitative information on KMC implementation. Eighty–eight of the studies (29%) did not define KMC. Two hundred and eleven studies (71%) included skin–to–skin contact (SSC) in their KMC definition, 49 (16%) included exclusive or nearly exclusive breastfeeding, 22 (7%) included early discharge criteria, and 36 (12%) included follow–up after discharge. One hundred and sixty–seven studies (56%) described the hours per day of SSC.
Conclusions
There exists significant heterogeneity in the definition of KMC. A large number of studies did not report definitions of KMC. Skin–to–skin contact is the core component of KMC, whereas components such as breastfeeding, early discharge, and follow–up care are context specific. To implement KMC effectively development of a global standardized definition of KMC is needed.
Related Resources
May 19, 2023
Kangaroo Mother Care (KMC) | Newborn
Follow-up of Kangaroo Mother Care programmes in the last 28 years: results from a cohort of 57 154 low-birth-weight infants in Colombia

May 18, 2023
Infection | Kangaroo Mother Care (KMC)
Effect on neonatal sepsis following immediate kangaroo mother care in a newborn intensive care unit: a post-hoc analysis of a multicentre, open-label, randomised controlled trial

May 16, 2023
Newborn | Kangaroo Mother Care (KMC)
Global Position Paper on Kangaroo Mother Care: A transformative innovation in healthcare


Kangaroo Mother Care: Implementation strategy for scale-up adaptable to different country contexts

- kangaroo-mother-care-kmc
We have a new look and feel!
We may look different, but we have not lost the essence of who we are; a learning platform that provides evidence-based knowledge to those working to ensure that newborns survive and thrive. We’d love to hear what you think of the new HNN!
Contact us!
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Supplements
- BMJ Journals
You are here
- Volume 8, Issue 6
- Kangaroo mother care for preterm or low birth weight infants: a systematic review and meta-analysis
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-2259-5693 Sindhu Sivanandan 1 ,
- http://orcid.org/0000-0003-1474-1451 Mari Jeeva Sankar 2
- 1 Neonatology , Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) , Puducherry , India
- 2 Pediatrics , All India Institute of Medical Sciences , New Delhi , India
- Correspondence to Dr Mari Jeeva Sankar; jeevasankar{at}gmail.com
Importance The Cochrane review (2016) on kangaroo mother care (KMC) demonstrated a significant reduction in the risk of mortality in low birth weight infants. New evidence from large multi-centre randomised trials has been available since its publication.
Objective Our systematic review compared the effects of KMC vs conventional care and early (ie, within 24 hours of birth) vs late initiation of KMC on critical outcomes such as neonatal mortality.
Methods Eight electronic databases, including PubMed ® , Embase, and Cochrane CENTRAL, from inception until March 2022, were searched. All randomised trials comparing KMC vs conventional care or early vs late initiation of KMC in low birth weight or preterm infants were included.
Data extraction and synthesis The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered with PROSPERO.
Main outcomes and measures The primary outcome was mortality during birth hospitalization or 28 days of life. Other outcomes included severe infection, hypothermia, exclusive breastfeeding rates, and neurodevelopmental impairment. Results were pooled using fixed-effect and random-effects meta-analyses in RevMan 5.4 and Stata 15.1 (StataCorp, College Station, TX).
Results In total, 31 trials with 15 559 infants were included in the review; 27 studies compared KMC with conventional care, while four compared early vs late initiation of KMC. Compared with conventional care, KMC reduces the risks of mortality (relative risk (RR) 0.68; 95% confidence interval (CI) 0.53 to 0.86; 11 trials, 10 505 infants; high certainty evidence) during birth hospitalisation or 28 days of age and probably reduces severe infection until the latest follow-up (RR 0.85, 95% CI 0.79 to 0.92; nine trials; moderate certainty evidence). On subgroup analysis, the reduction in mortality was noted irrespective of gestational age or weight at enrolment, time of initiation, and place of initiation of KMC (hospital or community); the mortality benefits were greater when the daily duration of KMC was at least 8 hours per day than with shorter-duration KMC. Studies comparing early vs late-initiated KMC demonstrated a reduction in neonatal mortality (RR 0.77, 95% CI 0.66 to 0.91; three trials, 3693 infants; high certainty evidence) and a probable decrease in clinical sepsis until 28-days (RR 0.85, 95% CI 0.76 to 0.96; two trials; low certainty evidence) following early initiation of KMC.
Conclusions and relevance The review provides updated evidence on the effects of KMC on mortality and other critical outcomes in preterm and low birth weight infants. The findings suggest that KMC should preferably be initiated within 24 hours of birth and provided for at least 8 hours daily.
- public health
- systematic review
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Data are available upon reasonable request from the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjgh-2022-010728
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Kangaroo mother care (KMC) is a simple and cost-effective intervention that decreases neonatal mortality and the risk of infection in low birth weight infants.
The WHO recommends the initiation of KMC among low birth weight infants after clinical stabilisation.
WHAT THIS STUDY ADDS
Compared with conventional care, KMC initiated either in the hospital or at home reduces mortality during birth hospitalisation or 28 days of age and probably reduces severe infection until the latest follow-up among preterm and low birth weight infants.
KMC provided for at least 8 hours a day probably results in greater benefits than a shorter duration of KMC.
KMC initiated within 24 hours of birth reduces neonatal mortality and may reduce clinical sepsis until 28 days compared with later initiation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The results of this updated review will likely influence health providers to initiate KMC in all low birth weight and preterm infants managed in health facilities and at home. Efforts might be undertaken to initiate KMC within 24 hours of birth and to provide it for at least 8 hours a day.
Introduction
Prematurity (gestational age <37 weeks) and low birth weight (defined as <2500 g) are important causes of neonatal and infant mortality and long-term neurodevelopmental disability. 1 Low- and middle-income countries (LMIC) have the highest burden of preterm and low birth weight infants. Kangaroo mother care (KMC) is a simple and cost-effective intervention that has been shown to reduce neonatal mortality and the risk of infection in low birth weight infants. 2 The Cochrane review on KMC, published in 2016, included 21 studies involving 3042 infants and demonstrated a significant reduction in the risks of mortality and severe infection in low birth weight infants. 3
New evidence from large multi-country and community-based randomised trials became available after the publication of the Cochrane review. 4 5 A few of these trials examined the effect of early KMC, that is, KMC initiated within the first 24 hours of delivery. 5 6 The timing of initiation of KMC is critical because KMC is usually commenced after the infant is stabilised. The WHO guidelines also recommend the initiation of KMC after clinical stabilisation. However, stabilisation of preterm/low birth weight neonates may take anything from hours to days, depending on the gestation, birth weight, and general condition at birth. The median age at initiation of KMC in the facility-based studies included in the Cochrane review varied from 3 to 24 days. KMC initiated after 3 days of life would not naturally reduce the risk of deaths occurring in the first 3 days, which account for about 62% of total neonatal deaths. 7 The efficacy and safety of early initiation of KMC – within 24 hours of life – are unknown.
This systematic review aimed to compare the effects of (a) KMC with conventional care and (b) early initiation, that is, KMC within 24 hours of age, with late initiation of KMC on neonatal and infant mortality and severe morbidities among low birth weight and preterm infants. This review would provide critical evidence for policymakers and other stakeholders and may help to formulate clinical practice guidelines.
Inclusion and exclusion criteria
Our review included individually-randomised and cluster-randomised trials that compared KMC with conventional care or early initiation (ie, in the first 24 hours after birth) of KMC with late-initiated KMC among low birth weight and preterm infants, irrespective of the duration of KMC, infant stability at enrolment, study setting, and breastfeeding patterns. Trials reported as only abstracts were included if sufficient information on study methods was available to assess the eligibility and the risk of bias. We excluded quasi-randomised and crossover trials, studies evaluating KMC among term infants or those with birthweight >2500 g, and studies assessing KMC on only physiological parameters, pain scores, maternal mental health, infant colic, or during neonatal transport or as a part of a package of interventions.
Search strategy
We systematically reviewed the relevant publications by searching the electronic databases of MEDLINE (1966 to March 2022) via PubMed ® and OVID, Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1 to March 2022), EMBASE (1988 to March 2022), CINAHL (1981 to March 2022), and the databases PsycINFO, AMED, EMCARE, BNI from inception until March 2022. We used the search terms “kangaroo care,” “kangaroo mother care,” “skin-to-skin care,” and “neonates or infants” in the search strategy. The search was initially conducted until March 2021 (for the presentation of review findings to the WHO Guideline Development Group of the guidelines on the care of low birth weight infants); the search was then updated to March 2022. The search strategy, search results, and the definitions used in the review are provided in online supplemental file 1 . We also searched the databases of clinical trials and reference lists of retrieved articles for eligible studies.
Supplemental material
The primary outcome was mortality during birth hospitalisation or by day 28 of life. Other outcomes were mortality by 6–12 months of age, severe infections, infant growth, neurodevelopment, hypothermia, length of hospital stay, readmission to hospital, and exclusive breastfeeding at discharge and at one and 6 months of age.
Data extraction
The two review authors (SS and MJS) extracted data using a standardised and pre-tested data abstraction form. The data included study characteristics, sample size, details of KMC initiation, duration, breastfeeding, time of hospital discharge, study setting (hospital or community), outcomes including neonatal mortality, hypothermia, sepsis, rates of exclusive breastfeeding, and weight gain. Discrepancies, if any, were resolved by mutual discussion between the reviewers.
Quality assessment and statistical analysis
The review authors independently evaluated the quality of studies using Cochrane’s Risk of Bias-1 tool, extracted data, and synthesised the effect estimates – relative risks (RR) or mean difference (MD) – using RevMan version 5.4 (The Cochrane Collaboration, 2020) or Stata 15.1 (StataCorp, College Station, TX, USA). The RR and 95% confidence intervals (CI) were calculated based on the extracted frequencies and denominators. Results were pooled using fixed-effect meta-analyses using the Mantel-Haenszel method. The heterogeneity of the pooled studies was assessed using the test of homogeneity of study-specific effect sizes and the I 2 statistic, in addition to visual confirmation from forest plots. If substantial heterogeneity was detected, the reasons for heterogeneity were explored. If there was no critical clinical or methodological heterogeneity among the studies, we pooled their results using the random-effects model. We evaluated the likelihood of potential publication bias using funnel plots.
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach 8 to assess the quality of evidence for critical outcomes such as mortality at discharge, severe infection/sepsis at the latest follow-up, weight gain, exclusive breastfeeding, and neurodevelopmental outcomes. Evidence from randomised controlled trials was considered high quality; still, it could be downgraded by one or two levels for serious and very serious limitations, respectively, based on the risk of bias, imprecision, inconsistency, indirectness of study results, and publication bias. The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered in PROSPERO (CRD42021240336).
Planned subgroup analyses
For the comparison of KMC vs conventional care, we performed subgroup analyses according to different gestational and birth weight categories and by median duration KMC in hours (<8 hours, 8–16 hours, and >16 hours); time of initiation of KMC – early (≤24 hours of life) vs late initiation; stable vs unstable neonates; health facility vs community settings; and countries (high income vs LMIC settings).
Patient and public involvement
The study is a systematic review of the existing literature on the efficacy of KMC in preterm and low birth weight infants. No subjects were enrolled in the review. Therefore, parents, parent advisors, or the public were not involved in developing the research question and outcome measures.
Role of the funding source
The WHO, Geneva, funded the review. The WHO staff helped finalise the protocol and the manuscript; they had no role in the literature search, data extraction, or data analysis. The corresponding author had the final responsibility for the decision to submit for publication.
Of the 3458 records identified from the database and bibliographic searches, 31 4–6 9–35 studies enrolling 15 559 infants were included in the review ( figure 1 ); 25 studies were conducted in LMIC (two from multiple countries 5 14 while seven were conducted in high-income countries 12 20 24 26 29 30 34 (Appendix). Twenty-seven studies compared KMC with conventional care, while four compared early with late initiation of KMC. 5 6 24 25 KMC was initiated in the health facility in 29 studies and at home (community) in two trials. 4 11 While the sample sizes of earlier hospital-based studies ranged from 28 to 777, the most recent facility-based study – WHO iKMC study 5 – had a sample size of 3211. Of the two community-based studies, one trial had enrolled around 8400 infants. 4 Only six studies included infants with birthweight <1500 g. 12 13 19 28 30 34 Figure 2 depicts the risk of bias in the included studies in specific domains. Many studies had an unclear or high risk of selection bias (due to a lack of information on allocation concealment) and detection bias (because the outcomes assessors were not masked to the intervention group).
- Download figure
- Open in new tab
- Download powerpoint
Flow chart of search results (adapted from PRISMA 2009 flow diagram).
Risk of bias in included studies. Green circle indicates low-risk, red indicates high-risk and yellow, unclear-risk of bias.
KMC versus conventional newborn care
The comparison included 27 studies that enrolled 11 956 infants. The characteristics of included studies are provided in table 1 . All but one study enrolled infants after stabilisation (variably defined in different studies as cardiorespiratory stability, off oxygen or any form of respiratory support, or off intravenous fluids). KMC was started within 24 hours after birth in two studies, between 1 and 7 days in 10 studies, and after 7 days in 12 studies (3 studies did not report the time of initiation). The duration of KMC was <8 hours in 9 studies, 8–16 hours in 9 studies, and >16 hours in 4 studies (5 studies did not report the duration).
- View inline
KMC vs conventional newborn care – characteristics of included studies
Pooled analysis revealed a 32% reduction in mortality during birth hospitalisation or by 28 days after birth or 40 weeks of postmenstrual age (risk ratio (RR) 0.68; 95% CI (CI) 0.53 to 0.86; I 2 =0%; 12 studies; 10 505 infants; fixed-effect model; high certainty evidence; figure 3 ). The funnel plot did not show any evidence of a potential publication bias ( online supplemental efigure 1 ). The benefits of KMC in the primary outcome of mortality during birth hospitalisation or by 28 days of age were observed in all subgroup analyses: gestational age category (≤34 weeks vs. >34 weeks), weight at birth/enrolment (≤2000 g vs. >2000 g), setting (health facility vs. community) and time of initiation of KMC (within 24 hours after birth vs later); the benefits were greater when the daily duration of KMC was at least 8 hours per day than with shorter duration ( online supplemental efigure 2 ). Pooled analysis of 4 studies that had reported mortality by 6 months of age showed a 25% reduction in mortality (RR 0.75; 95% CI 0.62 to 0.92; fixed-effect model; high certainty of evidence).
Kangaroo mother care (KMC) vs. conventional care –Risk ratio of mortality during birth hospitalisation or 28 days of life.
KMC probably results in a 15% reduction in severe infection/sepsis at the latest follow-up (RR 0.85, 95% CI 0.79 to 0.92; 9 trials, 9847 infants; moderate certainty evidence) and 68% reduction in the risk of hypothermia (RR 0.32, 95% CI 0.19 to 0.53; 11 trials, 1169 infants; moderate-certainty evidence). Infants in the KMC arm had a higher gain in anthropometric parameters, namely weight gain per day and length and head circumference gain per week ( table 2 ). The exclusive breastfeeding rates were higher at discharge/28 days of life (RR 1.48, 95% CI 1.44 to 1.52; 9 trials, 9983 infants, very low certainty evidence), but the evidence was uncertain; also, there was no difference in breastfeeding rates at 1–3 months of age. KMC may result in little to no difference in the Griffith Quotients or the risk of cerebral palsy at 12 months of corrected age 36 or IQ scores at 20 years of age.
KMC vs conventional newborn care: key outcomes
Early-initiated versus late-initiated KMC
The evidence was derived from 4 trials that enrolled 3603 infants. One study was done in a high-come country (Sweden), 2 studies were done in low-income countries (Madagascar and The Gambia), and 1 study was multi-country conducted in LMICs (Ghana, India, Malawi, Nigeria, and Tanzania). All trials were conducted in health facilities. Infant stability at enrolment, duration of KMC achieved, and time of initiation of KMC in the included studies are provided in table 3 . In two studies (Mörelius et al 24 and WHO iKMC) 5 KMC was initiated in the delivery room. Brotherton et al 6 enrolled moderately unstable infants in the early KMC arm and stable infants after >24 hour of admission in the control arm. Nagai et al began KMC within 24 hours of birth in the early arm and after 24 hours in the late arm.
Early vs late-initiated KMC – characteristics of included studies
Early-initiated KMC showed a reduction in the risks of mortality by 28 days of age (RR 0.78, 95% CI 0.66 to 0.92; 3 trials, 3533 infants, high certainty evidence; online supplemental efigure 3 ) and hypothermia by discharge or at 28 days (RR 0.74, 95% CI 0.61 to 0.90; high certainty evidence). It probably reduces the risk of clinical sepsis until 28-day follow-up (RR 0.85, 95% CI 0.76 to 0.96; table 4 ; low certainty evidence) and improves exclusive breastfeeding at discharge (RR 1.1.2, 95% CI 1.10 to 1.19; moderate certainty evidence). There was also a decrease in the length of hospital stay ( table 4 ).
Early vs late-initiated KMC – critical outcomes
On subgroup analysis, there was evidence of a reduction in 28-day mortality for infants with GA ≤34 weeks and BW ≤2000, but there was little data for infants >34 weeks and weighing >2000 g at birth. The mortality reduced with a duration of KMC of at least >16 hours per day, with little data for daily KMC duration of <8 hours or 8–16 hours per day.
Quality of the evidence
For the comparison of KMC vs conventional newborn care, the certainty of the evidence was assessed as high for neonatal mortality and moderate for sepsis/severe infection and hypothermia ( table 5 ). For early vs late-initiated KMC, the certainty of the evidence was high for neonatal mortality and hypothermia, moderate for exclusive breastfeeding at discharge, and low for nosocomial clinical sepsis ( table 6 ). A few outcomes, such as weight gain, breastfeeding, and length of hospital stay, showed a high degree of heterogeneity, partly due to clinical and methodological heterogeneity among the studies (varied definitions of hypothermia and time points of assessment; different methods of breastfeeding assessment, etc.).
Summary of findings – KMC vs conventional newborn care
Summary of findings – early initiated KMC vs late-initiated KMC in preterm or low-birth weight infants
The systematic review showed that KMC reduces mortality during birth hospitalisation or by 28 days of age and probably reduces severe infection at the latest follow-up in preterm and low birth weight infants in health facilities and at home. KMC may result in a slight increment in growth parameters (weight and length) and exclusive breastfeeding rates at discharge. KMC may result in little to no difference in neurodevelopmental outcomes at 12 months compared with conventional care. Compared with delayed initiation (>24 hours) of KMC, early-initiated KMC (<24 hours) results in a 33% reduction in mortality by 28 days and a slight reduction in clinical sepsis by 28 days.
Three recent systematic reviews examined the effect of KMC compared with conventional care on infant clinical outcomes. 3 37 38 The Cochrane review in 2016 found 21 studies enrolling 3042 low birth weight infants. 3 Our systematic review used a similar search strategy and inclusion criteria and included studies until 2022. We found 10 newer studies that provided data on 12 517 additional infants with similar gestation and birth weight range. The Cochrane review reported a similar decrease in mortality at discharge or 40 weeks of postmenstrual age (RR 0.60, 95% CI 0.39 to 0.92; 8 trials, 1736 infants) and similar effects on infection, hypothermia, and anthropometry. However, the certainty of the evidence was graded as moderate to very low in the Cochrane review. The addition of information from 12,000-odd infants has improved the precision and certainty of the evidence of the critical outcomes in the current review. In 2020, a systematic review of 416 preterm neonates reported that KMC significantly reduced apneic events in preterm neonates. 38 Another review in 2019 concluded that KMC had a significant positive impact on growth and breastfeeding rates in very low birth weight (VLBW) neonates. 37
We investigated the effect of mean duration KMC in hours and prespecified three categories (<8 hours, 8–16 hours, and >16 hours). The effects on mortality were comparable in the >16 hour and 8–16 hour groups, but there was insufficient data in the <8 hours group. The Cochrane review (2016) explored the effects of the duration of KMC in three different categories; <2 hours and 6–15 hours, and >20 hours per day, and found benefits only when KMC was done for 20 hours or more. We found beneficial effects of KMC in prespecified subgroups of ≤2.0 kg and >2.0 kg and infants with gestational age ≤34 and >34 weeks at birth. T he two community- based studies that enrolled infants at home also showed significant benefits on mortality. We found no additional trials – other than the study by Worku et al included in the Cochrane review – that compared KMC with conventional care in unstable infants.
Only one systematic review – the Cochrane review published in 2016 – has evaluated the effects of early vs late initiation of KMC in low birth weight infants. It also used a cut-off of 24 hours to define early initiation but found only one study of 73 relatively stable low birth weight infants. 25 Our review included three additional studies that recruited 3530 preterm/low birth weight infants and found significant beneficial effects with early initiation of KMC. 5 6 24
The results of our review have substantial implications for policymaking, particularly in LMIC. First, KMC should be provided to all low birth weight and preterm infants irrespective of the settings – both health facilities and at home. Second, given the probable dose-effect response, KMC should preferably be practiced for at least 8 hours a day for optimal benefits. Third, KMC should be initiated within the first 24 hours of life. Indeed, our findings have helped to make recommendations on KMC in the new WHO guidelines on the care of preterm and low birth weight neonates. 39
The strengths of the current review include a comprehensive and systematic search of the literature with updated evidence to March 2022. Compared with the existing Cochrane reviews on KMC, our review identified additional studies that had enrolled almost 13 000 low birth weight infants, which resulted in high precision of estimates and improved the certainty of the evidence. The review also had some limitations. The included studies were not blinded, although outcome assessors were blinded in many studies. However, the risk of bias in the included studies was generally low, and the certainty of the evidence for the primary outcomes was moderate to high. Very low birth weight, extremely preterm neonates, and severely unstable neonates were often excluded from studies. More evidence is needed before extrapolating the study results in these high-risk groups.
To conclude, our findings support the practice of KMC for preterm and low birth weight infants as soon as possible after birth and for at least 8 hours a day. Future research should focus on overcoming barriers and facilitators to large-scale implementation of KMC in facility and community settings. Data on long-term neurodevelopmental outcomes are also needed.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
Acknowledgments.
We acknowledge the support and guidance provided by Dr. Rajiv Bahl, Dr. Karen Edmond, and Dr. Shuchita Gupta from the WHO, Geneva, in finalising the protocol and interpreting the results.
- Milner KM ,
- Roberts G , et al
- Boundy EO ,
- Dastjerdi R ,
- Spiegelman D , et al
- Conde-Agudelo A ,
- Díaz-Rossello JL
- Mazumder S ,
- Dube B , et al
- WHO Immediate KMC Study Group ,
- Naburi H , et al
- Brotherton H ,
- Kebbeh B , et al
- Sankar MJ ,
- Natarajan CK ,
- Das RR , et al
- Balshem H ,
- Helfand M ,
- Schünemann HJ , et al
- Acharya N ,
- Bhatta NK , et al
- Sharma R , et al
- Alisjahbana A ,
- Lrawaty S , et al
- Ferguson AE ,
- Morales Y , et al
- Cattaneo A ,
- Davanzo R ,
- Worku B , et al
- Charpak N ,
- Ruiz-Peláez JG ,
- Figueroa de CZ , et al
- Bhavana DrD ,
- Lakshmi DBV ,
- Sruthi DT , et al
- Pratiwi I ,
- Soetjiningsih S ,
- Gathwala G ,
- Ghavane S ,
- Subramanian S , et al
- Hake-Brooks SJ ,
- Anderson GC
- Kanbur W , et al
- Kumbhojkar S ,
- Md Mahbubul H ,
- Md Maksudur R
- Mörelius E ,
- Örtenstrand A ,
- Theodorsson E , et al
- Andrianarimanana D ,
- Rabesandratana N , et al
- Nimbalkar SM ,
- Patel DV , et al
- Ramanathan K ,
- Deorari AK , et al
- Roberts KL ,
- Paynter C ,
- Quevedo M , et al
- Camacho LW ,
- Rojas EP , et al
- Suman RPN ,
- Zhang Y , et al
- Whitelaw A ,
- Heisterkamp G ,
- Sleath K , et al
- Ruiz-Pelaez JG ,
- Figueroa de C Z , et al
- Farahbakhsh N ,
- Sharma S , et al
- Montealegre-Pomar A ,
- Bohorquez A ,
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Data supplement 2
- Press release
Handling editor Seema Biswas
Contributors Both authors, MJS and SS, contributed equally to protocol development, literature search, data extraction and analysis and interpretation. SS drafted the manuscript with inputs from MJS. Both authors reviewed and approved the final manuscript. MJS acts as the guarantor of the paper.
Funding The World Health Organization. Grant number- not applicable.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
- Submit your manuscript
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Mothers’ perceptions of the practice of kangaroo mother care for preterm neonates in sub-Saharan Africa: a systematic review of qualitative evidence
Bayo, Pontius 1 ; Alobo, Gasthony 1 ; Sauvé, Caroline 2 ; Feyissa, Garumma Tolu 3,4
1 Department of Obstetrics and Gynecology, St. Mary's Hospital Lacor, Gulu, Uganda
2 Centre Hospitalier de l’Université de Montréal, Quebec, QC, Canada
3 Department of Health, Behavior and Society, Jimma University Institute of Health, Jimma, Ethiopia
4 Ethiopian Evidence Based Healthcare and Development Centre: A JBI Centre of Excellence, Jimma, Ethiopia
Correspondence: Pontius Bayo, [email protected]
The authors declare no conflict of interest.
Objective:
The objective of this review was to explore the experiences of mothers with the practice of kangaroo mother care for preterm neonates at home in sub-Saharan Africa.
Introduction:
Newborn deaths globally have remained high despite the significant reductions in deaths among under-fives over the past few decades. More than 7000 deaths occur daily around the globe, but mostly in sub-Saharan Africa. Of these deaths, 60% to 80% are due to preterm birth and low birth weight. Kangaroo mother care is known to offer a cheap and effective way to care for low birth weight, preterm neonates; however, its practice is still low. There is limited evidence on the factors that hinder or facilitate the practice of kangaroo mother care at the community level.
Inclusion criteria:
The review considered studies conducted in sub-Saharan Africa on the perceptions and experiences of mothers who had given birth to preterm babies and had practiced kangaroo mother care wholly or in part at home. Qualitative studies in English and French conducted from January 1979 to March 2019 were considered for inclusion if they exclusively used qualitative research methods including, but not limited to, phenomenology, grounded theory, ethnography, action research, or feminist research.
Methods:
PubMed, Embase, Web of Science, Scopus, African Index Medicus (AIM), Academic Search Complete, CINAHL Complete, Education Source, and Health Source: Nursing/Academic Edition were searched in March 2019. Eligible studies were critically appraised using the standardized JBI tool. Findings were pooled using the meta-aggregative approach, and confidence was assessed according to the ConQual approach.
Results:
Following the systematic search and critical appraisal process, six studies were included in the review for data extraction and synthesis of findings. Three of the six studies were based on in-depth individual interviews, while two employed both individual interviews and focus group discussions, and one study used only focus group discussions. Twenty-six primary findings were generated from the review process that were aggregated into 10 categories, which generated four meta-synthesized findings:
- i) Cultural and contextual factors: The traditional way of carrying babies on the back and providing them warmth through lighting lamps or charcoal make kangaroo mother care appear odd and shameful (level of confidence: low).
- ii) The technical content of the intervention: The practice of kangaroo mother care is perceived to be technically cumbersome, especially because it has to be continuous; there is fear of making the baby's cord bleed; it creates difficulty in positioning for breastfeeding; and there is difficulty in maintaining the position while sleeping and doing other household chores (level of confidence: moderate).
- iii) Health system factors: The health care systems have no clear strategies to promote kangaroo mother care at the community level. Most mothers learned about the practice for the first time from health care workers only after birthing; however, peer-to-peer information sharing was noted to be a powerful source of trusted information about kangaroo mother care. Community leaders and religious leaders could be used to promote use of kangaroo mother care (level of confidence: moderate).
- iv) Individual and family factors: Although mothers realize the importance of kangaroo mother care for their infants’ recovery, their individual and family conditions affect their decision to practice the intervention (level of confidence: moderate).
Conclusions:
There is a link between the perceptions and experiences of kangaroo mother care that influences its practice in sub-Saharan Africa. The health care systems have failed to create awareness among communities before the birth of a preterm neonate. The traditional practices make kangaroo mother care stigmatizing at the community level, and the practice is perceived to be difficult and cumbersome, requiring substantial social support. Strategies to make the practice less cumbersome need to be devised, focusing on the comfort of mothers. Further qualitative studies are needed to explore community-level experiences of kangaroo mother care in sub-Saharan Africa.
ConQual Summary of Findings

Introduction
Although the morality rate of children younger than five years has declined significantly in the past few decades, the neonatal mortality rate has remained high, with about 7000 deaths occurring around the globe daily. 1 Approximately 46% of the global deaths among under-fives are in the neonatal period and mostly in sub-Saharan Africa and southern Asia. 2 The neonatal mortality rate in sub-Saharan Africa and southern Asia are 34 and 32 per 1000 live births, respectively, compared with 1 to 4 per 1000 live births in the world's wealthiest countries. 2,3 Preterm births with the accompanying low birth weight (LBW) account for 60% to 80% of these neonatal deaths. 4 Those preterm newborns who survive early neonatal death have a high risk of infections, developmental delays, and death at infancy or during childhood. 5 Low-income countries bear the highest burden of LBW infants, with 18 million LBW infants born annually in these countries. 6 Out of the 11 countries with preterm birth rates greater than 15%, nine are in sub-Saharan Africa. 7
Kangaroo mother care (KMC) offers an alternative care method for LBW babies compared with conventional care that is highly technical, involving the use of an incubator, cardiopulmonary monitors, and continuous pressure ventilators. 8,9 The key components of KMC include skin-to-skin contact between the neonate and the mother's chest between the breasts, exclusive breastfeeding, emotional and physical maternal support, and early health-facility discharge for continued home practice of the intervention. 10 Kangaroo mother care has been shown to promote bonding between parent and child, 11 facilitate breastfeeding, 12 and stabilize the neonatal body temperature as well as the heart and respiratory rates. 13-15 Kangaroo mother care, therefore, does not only reduce the need for expensive equipment for neonatal care in low-resourced settings, but it also offers increased opportunities for health education and parental involvement in care provision even in high-income countries. 16
However, despite the knowledge of the benefits of KMC, the level of practice has not achieved scale. 17,18 Many health systems disregard KMC and prefer to invest in incubator care, 19 or little time is spent by health care workers to promote its implementation, 20 while others lack the required skills to be able to support the mothers. 18 Only a limited number of health facilities in low-income countries have managed to provide a conducive environment for effective implementation of KMC. 17 There is, therefore, limited opportunity for interaction with mothers and communities to explain and understand the benefits of KMC. 21 There is also no effective follow-up of the practice in the communities, even if there is a successful initiation at the health facility. 10 For example, in Ghana, only 58% of mothers are able to practice KMC outdoors while at home after a successful initiation from the health facility. 22 Other parents are not able to offer continuous KMC, only practicing intermittently when it is convenient. 17
There are also several barriers at the community level that have been identified by mothers and their families and associated with sociocultural backgrounds. In India, mothers feel uncomfortable while breastfeeding or while being taught KMC in groups and are unwilling to wear clothing with open fronts. 23 Some mothers have perceived medical fears, such as causing the neonate to vomit, transmitting infection to the neonate, and causing bleeding at the umbilical cord. 23 The breastfeeding component of KMC is considered a barrier by some mothers as it interferes with the skin-to-skin contact, especially if breast milk needs to be expressed. 24 The posture assumed during KMC is uncomfortable for some caregivers and results in inadequate sleep. 24 However, despite these barriers, studies have also demonstrated factors that facilitate the practice of KMC at the community level. 24,25 Access to ready family support, compassionate health care providers who guide parents through the process of KMC, and a conducive government policy environment that favors parents, such as allowing time off work, are factors that can promote KMC practice.
Unlike many other child health interventions, the success of KMC in reducing neonatal morbidity and mortality depends on adequate participation of the parents and other family members. 26 The existing systematic reviews on the practice of KMC focused on the perspectives of health care workers and the practice of KMC in health facilities. 27-30 These reviews have also combined studies from high- and low-income countries, making it challenging to explore the barriers to and facilitators of KMC practice at the community level in the context of sub-Saharan Africa.
This review aimd to close this gap and synthesize the evidence about the experiences of mothers regarding the practice of KMC at the community level in sub-Saharan Africa, providing an understanding of the facilitators and barriers. The findings presented in this review can act as a guide to inform development of KMC programs in sub-Saharan Africa.
Review question
What are the experiences of mothers with the practice of KMC for preterm neonates at home in sub-Saharan Africa? The secondary questions are:
- i) How do women perceive the benefits of practicing KMC?
- ii) What are the facilitators and barriers perceived by women?
Inclusion criteria
Participants.
The review considered studies that included mothers who have given birth to preterm babies before 37 completed weeks of gestation and are practicing or have practiced KMC either fully or partially at home.
Phenomena of interest
This review considered studies that explored the perceptions, views, experiences, attitudes, and beliefs of mothers regarding the practice of KMC at home.
This review only considered studies that were conducted in sub-Saharan Africa.
Types of studies
This review considered studies that exclusively used qualitative research methods including, but not limited to, designs such as phenomenology, grounded theory, ethnography, action research, and feminist research. Only studies written in English and French were included. We also considered studies in other languages if an English translation was available. Studies published from January 1979 to the search date of March 2019 were included, as KMC was first developed in 1978. 31
This review was conducted in accordance with the JBI methodology for systematic reviews of qualitative evidence, 32 and follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 33 The review also follows an a priori protocol. 34
Search strategy
The search strategy aimed to locate both peer-reviewed publications and gray literature. An initial limited search of PubMed was undertaken, followed by analysis of the text words contained in the title and abstract, and of the index terms used to describe the articles. This informed the development of a search strategy, which was tailored for each information source. The full search strategies are presented in Appendix I. The reference lists of all studies selected for critical appraisal were screened for additional studies.
The databases searched included MEDLINE (Ovid), MEDLINE (PubMed), Embase (Ovid), Evidence-Based Medicine Reviews (Ovid), Web of Science (Clarivate Analytics), Scopus (Elsevier), African Index Medicus, Academic Search Complete (EBSCO), CINAHL Complete (EBSCO), Education Source (EBSCO), and Health Source: Nursing/Academic Edition (EBSCO). Sources of gray literature searched included JSTOR, OpenGrey, Google Scholar, and reference lists of identified articles.
Study selection
Following the search, all identified citations were collated and uploaded into EndNote v.X9.2 (Clarivate Analytics, PA, USA) and duplicates removed. Titles and abstracts were screened by two independent reviewers (PB, GA) for assessment against the inclusion criteria for the review. Studies that met or could potentially meet the inclusion criteria were retrieved in full and their details imported into the JBI System for the Unified Management, Assessment and Review of Information (JBI SUMARI; JBI, Adelaide, Australia). The full texts of selected studies were retrieved and assessed in detail against the inclusion criteria. Full-text studies that did not meet the inclusion criteria were excluded, and reasons for exclusion are provided in Appendix II. Included studies were critically appraised. The results of the search are presented in a PRISMA flow diagram 33 (see Figure 1 ). Any disagreements that arose between the reviewers were resolved through discussion, and there was no need for a third reviewer.

Assessment of methodological quality
Selected studies were critically appraised by two independent reviewers (PB, GA) for methodological quality using the JBI qualitative assessment and review checklist. 32 Any disagreements that arose between the reviewers were resolved through discussion, and there was no need for a third reviewer. The results of critical appraisal are reported in narrative form.
All studies, regardless of the results of their methodological quality, underwent data extraction and synthesis where possible. This was to avoid missing any evidence.
Data extraction
Data was extracted from studies included in the review by two independent reviewers (PB, GA) using the standardized JBI data extraction tool. 32 The data extracted included specific details about the populations, context, culture, geographical location, study methods, and the phenomena of interest relevant to the review question. Findings and their illustrations were extracted and assigned a level of credibility: unequivocal, credible, or not supported.
Data synthesis
Qualitative research findings were pooled using JBI SUMARI with the meta-aggregation approach. 32 This involved the aggregation or synthesis of findings to generate a set of statements that represent that aggregation through assembling the findings and categorizing them on the basis of similarity in meaning. Findings were verbatim extracts from the author's analytic interpretations of their data accompanied by illustrations. Two or more similar findings were grouped together into a category, and an explanatory statement was constructed to convey an inclusive meaning to that group of findings. Similar categories were then synthesized into a single comprehensive synthesized finding and an overarching explanatory statement constructed to convey the whole, inclusive meaning that can be used as a basis for evidence-based practice.
Assessing confidence in the findings
The final synthesized findings were graded according to the ConQual approach for establishing confidence in the output of qualitative research synthesis and presented in a Summary of Findings. 35 The Summary of Findings includes the major elements of the review and details how the ConQual score is developed. Each synthesized finding from the review is presented along with the type of research informing it, a score for dependability, a score for credibility, and the overall ConQual score.
Study inclusion
From our database search, 434 publications were identified. After removing duplicates, 98 articles were left for screening of titles and abstracts. Of these, 90 articles were excluded: 47 used quantitative methods, 17 did not use a primary data analysis, 12 had a different phenomenon of interest, 11 had different participants, and three were facility-based studies. Of the eight studies remaining, two were eliminated after full-text screening (Appendix II) because they both included ineligible participants (ie, health workers). Finally, there were six articles eligible for inclusion in the review. Figure 1 presents the study selection process for inclusion in the review.
Methodological quality
Overall, the methodological quality of all the included studies was rated as moderate using the JBI-QARI critical appraisal checklist for interpretive and critical research. 32 None of the included studies stated a clear alignment between the methodology and philosophical perspective; however, all the studies demonstrated congruity between methodology and research questions, data collection methods, representation and analysis of data, and interpretation of results. None of the studies adequately located the researcher either culturally or theoretically, nor did they state the influence of the researcher on the research and/or vice versa. All the studies presented the participants and their voices adequately, and the conclusions drawn in the research reports originated from the analysis and/or interpretation of the data.
As stated previously, all studies, regardless of the results of their methodological quality, underwent data extraction and synthesis. We could not take our ratings as concrete proxies for quality of research as they were based entirely on our interpretation of the included data or lacking information. Moreover, journal length and editorial requirements could have impacted the depth of methodological information provided.
Characteristics of included studies
The characteristics of included studies are summarized in Appendix III. All the included studies were published after 2000 with 50% published between 2010 and 2018. Three of the six studies were based on in-depth individual interviews, 36-38 while two used both individual interviews and focus group discussions, 25,39 and one study used only focus group discussions. 40 Two of the studies were from South Africa, 37,38 and one each from Uganda, 39 Malawi, 25 Zimbabwe, 40 and Ghana. 36 Three of the studies were conducted in rural settings 25,36,39 while others 37,38,40 were in an urban setting. The phenomena of interest in all the studies were experiences and/or views and/or perceptions of KMC.
In three studies, 37,38,40 participants were recruited from a health facility setting while in the others, 25,36,39 recruitment was done from the community. All studies included mothers who had either practiced KMC previously or were currently practicing it for their preterm neonates; however, in 50% of the studies, 25,38,39 other participants were also involved, including fathers to preterm neonates, health workers, and community health workers whose views/perceptions are not included in further analysis of this review.
Review findings
Synthesized finding 1: cultural and contextual factors.
The traditional way of carrying babies on the back and providing them warmth through lighting lamps or charcoal make KMC appear odd and shameful.
This finding was synthesized from two categories that were derived from four primary findings (all credible), which were supported by illustrations taken directly from the papers 36,37,39,40 that represented the voices of the participants on the practice of KMC (see Table 1 ).
| Findings | Categories | Synthesized finding |
| The mothers considered the position of the neonate in KMC as odd and shameful (C) | KMC technique creates a stigma among the mothers in the community | The traditional way of carrying babies on the back and providing them warmth through lighting lamps or charcoal make KMC appear odd and shameful |
| Friends’, neighbours’, and communities’ reactions to kangaroo care: Mothers feared stigmatizing comments from these groups as some of them thought mothers were hiding stolen property in their chests (C) | ||
| Care for preterm babies at community level: Although the need for warm care for a preterm baby was well known among the respondents, community members had little knowledge on STS care or KMC. The generation of warmth was improvised through covering and wrapping babies in many clothes, lighting lamps and charcoal stoves placed under the baby's bed, and hot water jerry cans or plastic bottles put in close proximity to the baby (C) | Existence of traditional methods to keep neonates warm | |
| Husbands’ reactions to kangaroo care at home: Many mothers said their husbands were very supportive of the kangaroo care method; however, some said their husbands were not keen on the method (C) |
“ We don’t do it like that! The people outside would even laugh if I go out in that state [with the baby in STSC position]. I would be staying indoors for one month after the baby is born, so someone else should explain that [practice] to them .” 36 (p.45-6)
“ At home I didn’t have spectators … I felt at peace and I could hold her and put her on me and it was beautiful .” 37 (p.23)
Although relatives were generally supportive and helped the mothers with household chores, they were said to be very inquisitive and subjected the mothers to a lot of questions. 40
“The midwife told us to cover the baby in a clean place, not to bathe it, to get cooking oil and a clean cloth and smear it always. She also told us to put a lamp or a charcoal stove where it sleeps so that it gets some warmth and never to remove it from its cover or bed till after one month.” 39 (p.1144)
“ Preterm infants have always been there and so what's new, why change the method of care .” 40 (p.132)
Synthesized finding 2: The technical content of the intervention
The practice of KMC is perceived to be technically cumbersome, especially because it has to be continuous; there is fear of making the baby's cord to bleed; it creates difficulty in positioning for breastfeeding; and there is difficulty in maintaining the position while sleeping and doing other household chores.
This finding was synthesized from three categories that were derived from seven primary findings (two unequivocal and five credible), which were supported by illustrations taken directly from the papers 36,37 that represented the voices of the participants on the practice of KMC (see Table 2 ).
| Findings | Categories | Synthesized finding |
| Adjustments, roles, and responsibilities: Provision of 24-hour KMC created extra work, roles, and responsibilities (U) | KMC is labor-intensive and time-consuming | The practice of KMC is perceived to be technically cumbersome, especially because it has to be provided continuously; there is fear of making the baby's cord bleed; it creates difficulty in positioning for breastfeeding; and there is difficulty in maintaining the position while sleeping and while doing other household chores |
| The challenges for caring for preterm babies included: increased workload for women; labor-intensive, time-consuming, and tiring care; limited male involvement other than financial support; expenses because of the need to buy fuel (charcoal and paraffin) and oil to smear on the baby; accessing care at a facility when the infant is sick; and the nature of rural homes (small, congested, and dusty) (C) | ||
| Some mothers feared that their babies would fall when they stood up or moved during KMC or that they might hurt the umbilicus of the neonate (U) | Fears about injury to the neonate and self | |
| Possible problems to the promotion of KMC practice at the community level that were mentioned in FGDs included fear of hurting the baby because “the cord is still fresh”; women need to work yet “the baby has to be in the chest all the time"; and the perception that KMC is tiring (C) | ||
| Women observed in the study always used the cradle hold position for breastfeeding their baby and were not very receptive to trying out other breastfeeding positions (C) | Technical difficulty | |
| They thought that the practice hooked them in one position for a long time and this was unhealthy for them (C) | ||
| Some mothers did say that it was difficult to sleep with an infant on their chest at first, but they had persevered for the sake of the infant. A few others said it took time to get used to cooking and doing other household chores with an infant on their chest (C) |
The mothers perceived the practice to be cumbersome to implement, labor-intensive, technically difficult to get a resting position for the mother and a position to breastfeed adequately, and they feared for the safety of the neonate and self. The mothers are used to the cradle hold position for breastfeeding and are not willing to adopt new positions. They also thought that the practice restricted them to one position for a long time and this was unhealthy for them.
“ All the time you have your baby with you … sleep with you, eat with you, walk with you .” 37 (p.24)
“ I would not want to sit for long because it will cause waist pains, but if I had to use something to sit against then I will try it .” 36 (p.46)
“I could not do that until the cord has fallen off after the first week [of the baby's life], it could cause pain and bleeding to the cord.” 36 (p.45)
“I thought I would feel some pains but when I tried it I had no pains in the chest, breast or stomach. I was surprised after I tried it but it seemed the baby liked it because he kept quiet when placed there.” 36 (p.45)
“Well obviously I’ve got a husband and another child at home, and obviously have to cook … you have to clean and do a lot of other things, besides looking after yourself and the baby.” 37 (p.22)
“It is tiresome because day, night, day, night, you can even become sick. You can even start bleeding again.” 39 (p.1144)
Synthesized finding 3: Health system factors
The health care systems have no clear strategies to promote KMC at the community level; most mothers learned about the practice for the first time from health care workers only after birthing; however, peer-to-peer information sharing was noted to be a powerful source of trusted information about KMC and the community leaders and religious leaders could be used to promote use of KMC.
This finding was synthesized from two categories that were constructed from six primary findings (two unequivocal and four credible) supported by illustrations taken directly from the papers 25,40 that represented the voices of the participants on the practice of KMC (see Table 3 ).
| Findings | Categories | Synthesized finding |
| Sources of information on preterm birth and KMC: Despite health facilities and health workers being the primary trusted sources of health information in general, peer-to-peer information sharing was the major source of information on KMC for pregnant women (C) | Sources and timing of information to mothers on KMC | The health care systems have no clear strategies to promote the practice at the community level. Most mothers learned about the practice for the first time from health care workers only after birthing; however, peer-to-peer information sharing is a powerful source of trusted information about KMC. Community leaders and religious leaders could also promote use of KMC |
| Timing of education on KMC: It was only following delivery that they learned the specifics of this practice and were trained on how to do it (U) | ||
| Men's involvement in KMC: Men were often left out of the conversation about preterm babies and KMC (C) | Promotion of KMC | |
| Role of community leaders: Enforcing penalties against those engaging in child marriage, partnering with health workers to promote family planning and counseling on preterm birth, holding community meetings on preterm births and KMC, and creating a network of leaders to better address the issue (U) | ||
| Role of religious leaders: Can encourage the family to take care of the preterm baby and assist them with their needs, both spiritual and monetary (C) | ||
| Mothers’ recommendations about care of preterm infants: Mothers suggested the method be promoted through drama, radio, and television (C) |
“I didn’t know anything until the time my baby was born.” 25 (p.5)
“We had never learnt about it [KMC], but when the babies were born, the doctors taught us about it.” 25 (p.5)
“My friend's sister was the one who was helping me and advising me on how to take care of my child since she also has the child who was born before the expected time of delivery.” 25 (p.4)
“Mothers who practiced kangaroo care before should be used in drama sessions, for example for a production on ‘parents day’ at schools.” 40 (p.132)
“Mass campaigns on kangaroo care for communities and the public were urgently needed.” 40 (p.133)
“What is important for the leaders is to come together, to work together and sensitize each other and the people in the community about different programs.” 25 (p.5)
“They must convene meetings to tell the people about this so that if it happens to anyone, they must attach the baby to their stomach.” 25 (p.6)
“If the chief mobilizes the community, everyone will be present and able to listen and get the information through community drama, songs, poems and the like.” 25 (p.6)
“They have the power to advise people even in churches because a lot of […] women gather there, so the church can offer advice.” 25 (p.6)
“They should also encourage their flocks to take care of the preterm babies, they should advise them the advantages of taking care of the children and the good thing about skin to skin care practice.” 25 (p.6)
Synthesized finding 4: Individual and family factors
Although mothers realize the importance of KMC for their infant's recovery, their individual and family conditions affect their decision to practice the intervention (extreme anxiety and fear of harming the preterm neonate and hurting themselves negatively affect the decision, while the availability of social support from the family, prior knowledge, and experience of the advantages of KMC positively influence the decision ).
This finding was synthesized from three categories that were derived from nine primary findings (five unequivocal and four credible) supported by illustrations taken directly from the articles 25,36-38 that represented the voices of the participants on the practice of KMC (see Table 4 ).
| Findings | Categories | Synthesized finding |
| Unforeseen, unprepared, and uncertain – the experience of birth: Parents have to deal with loss and grief related to the early and abrupt termination of pregnancy, feelings of emptiness, uncertainty regarding the infant's prognosis, and fear related to touch (C) | Extreme fear and anxiety about the preterm baby and self | Although mothers realize the importance of KMC for their infants’ recovery, their individual and family conditions affect their decision to practice the intervention (extreme anxiety and fear of harming the preterm neonate and hurting themselves negatively affect the decision while the availability of social support from the family, prior knowledge, and experience of the advantages of KMC positively influence the decision) |
| Feelings before KMC: Mothers described their feelings before starting KMC as being afraid, anxious, or confused (C) | ||
| Living-in challenges: The KC ward confines the mother's world to a bed permanently raised at a 45-degree angle in a room shared with seven other mother-infant couples. The living-in dominated the mothers’ lives (C) | Social support perceived by mothers | |
| A network of encouragement and support: Relatives were said to be very inquisitive, asking a lot of questions, but were usually supportive and helped with household chores so that the mother could attend to the infant (U) | ||
| Shifting perceptions: Through the process of learning about KMC and gaining these skills, many parents involved in the practice noted a shift in their perceptions (U) | ||
| Management of the mothers: Mothers felt that the nursing staff in the health facilities had been a pillar of strength to them. Mothers acknowledged and described the encouragement that they had received from other mothers (C) | ||
| Prior knowledge of advantages of kangaroo care: Women understood that the method was useful for keeping the infant warm, enhancing mother–baby bonding, and for closely monitoring the condition of the baby and early detection of “colour” changes, breathing difficulties, vomiting, and choking (U) | Knowledge of the advantages of KMC, and especially the observation of weight gain by their preterm neonates, is a motivating factor to mothers | |
| An intimate connection: Despite the initial anxiety and environmental barriers, KMC facilitated a special connection between parent and infant. In addition, to the feelings of excitement generated by the weight gain, feelings of emotional calmness and tranquility were also expressed (U) | ||
| During KMC: Despite some apprehension by mothers prior to commencing KMC, mothers acknowledged feelings of elation and excitement whilst practicing KMC. It was usually the baby's weight gain that triggered this response. In addition to the feelings of excitement generated by the weight gain, feelings of emotional calmness and tranquility were also expressed (U) |
“I was so afraid of KMC as it was the first time, I was seeing it being done.” 38 (p.64)
“ -- yes, I did have a doubt, I doubted whether KMC would work.” 38 (p.64)
“My first experience with kangaroo caring [for] her… when I took her out and put her against my skin, it was just … a great sense of relief for the first time really I felt bonded with her, that it was my daughter. So I think that kangaroo care helps to bridge that initial … gap that is between a mother and her preterm baby.” 37 (p.21)
“Ah! The bond, the feeling. I can’t explain how special it was.” 38 (p.65)
“[My family] also saw that after being cared for and weighed, the baby was improving. This made everyone encourage me to keep the baby attached to the chest.” 25 (p.8)
“I wouldn’t have gone through with it if it wasn’t for the Sisters (registered midwives).” 38 (p.65)
“ Don’t be frightened, just be positive, it is very good. You will find that it works .” 38 (p.65)
“This is new! We have not seen this before, but if it is good for the baby we will do it.” 36 (p.47)
“The problem with men is that they don’t go inside the room or ward when their wives or relatives are in the [KMC] ward but instead they stay outside and call their relatives to see them whilst there.” 25 (p.5)
“Kangaroo care has become so much part of me now. I am now owning this thing. I was thinking what was kangaroo care? It was nothing up in the sky, it was nothing other than what I was doing, all the other ideas, the breathing and the posture were part of it, and I developed it for myself so that it became more meaningful.” 37 (p.22)
This review collates the available qualitative data from sub-Saharan Africa on mothers’ perceptions of KMC practice for preterm neonates at home, and presents a set of related factors that enhance and/or hinder the practice. Figure 2 shows a summary of these factors to illustrate the links.

In this review, mothers reported being told about KMC for the first time only after birthing a preterm baby, 25 and that they perceived the practice to be cumbersome and time-consuming compared with other traditional and cultural alternatives of looking after neonates. 40 In a quantitative study in eastern Ethiopia, although 100% of the mothers had heard about KMC, up to 30% did not know the main benefit of KMC. 41 In this same study, 69% of mothers did not know how long KMC was meant to be practiced and most of the respondents (58%) mentioned a lack of knowledge regarding KMC as the main barrier to the practice of KMC. 41
This lack of knowledge about KMC among mothers may reflect a general health system gap. The women acknowledged the health system as the trusted source of information, for example, but they gained much more encouragement and confidence from their peers, as also demonstrated in randomized controlled trials elsewhere. 42,43 The presence of clear policy guidelines that promote community involvement and engagement to understand the benefits of KMC are likely to increase awareness of the practice. It has already been determined, for example, that where health workers had adequate skills and believed in the benefits of KMC, mothers received more opportunities to learn and practice the intervention. 26 In a multi-country study, the absence of a national KMC policy and/or dissemination of KMC service guidelines – even in health facilities – was identified as a major bottleneck to the increased implementation of KMC practice. 17 This study states that, overall, KMC is not prioritized by authorities and institutionalization of the practice has failed in most countries.
The findings in this review showed that mothers were anxious about the survival of their preterm babies, no matter what the interventions were; had lost hope; and felt inadequate. As shown in other literature, many cultures ascribe to women the role of “giving life” and mothers feel guilty, sad, and anxious if this life comes in the form of a preterm baby who may not survive; some consider it a form of supernatural punishment. 44 Preterm birth is associated with significant mental health challenges, especially depression, anxiety, and maternal distress, that affect the concentration of the mothers to practice interventions such as KMC, including exclusive breastfeeding. 45,46 It has also been shown that some mothers who are highly anxious and distressed show little emotional warmth in their interaction with their neonates. 47
Practicing KMC as recommended is perceived to be difficult, cumbersome, and could potentially cause injury to the neonate and pose breastfeeding challenges. Similar findings have been reported in another review that included studies from both high- and low-income countries. 28 Despite these expressed difficulties, KMC programs in low-income countries have failed to devise means to make it easier for mothers to practice the intervention. A study in Uganda showed that mothers maintained a skin-to-skin position for an average of only three hours per day compared with 20 hours per day recommended by the World Health Organization. 48 A recent study in Malawi has shown how women found a customized KMC wrap very comfortable, which increased the duration mothers kept their babies in skin-to-skin positions. 49
Quantitative studies have already proved that peer support improves the confidence of mothers with preterm babies, and thus increases implementation of the intervention. 42 This is important especially in many lower-income countries where many births still take place outside health institutions and where LBW infants are common, and clinical assessments of weight and breathing cannot be done easily. 50,51 Even for those who give birth in a health institution and receive counseling from health workers, the motivation to continue practicing from home is sometimes low. 52 In Bangladesh, neonatal health was significantly improved only when skin-to-skin contact was for seven or more hours per day and this, in turn, was associated with having made contacts with community workers during pregnancy. 53 These community workers were specially trained on KMC and made contacts with the pregnant women in their communities to educate and encourage them to practice KMC.
The birth of a preterm neonate and the need to practice KMC brings unique challenges to the family. In this review, the women expressed the need for support from their spouses and other family members to participate in the skin-to-skin care, take care of other children in the household, and perform other household chores. 25 Another study in a similar setting showed that the availability of help with other household responsibilities and other family members offering skin-to-skin care were important in increasing the duration of KMC practice. 54 The husband's will and that of other close relatives to support the mothers in practicing KMC depended on the belief in or experience of the benefits of the intervention. After an initial sense of anxiety, fear, and self-blame, 37 the husbands were cooperative and willing to support their wives – even in providing the skin-to-skin care – when they noticed the neonates gaining weight. 25 So even if social norms exist that delineate roles and do not allow husbands to actively participate in skin-to-skin care or directly help with household chores, 27,30 they are likely to overcome these norms and offer social support and motivate the mothers to practice KMC for longer periods of time.
Although the main source of information for mothers about how to practice KMC is the professional health care system, they rely on members of the community close to them, especially their peers who have experienced the benefits of KMC. The studies in this current review also discussed the benefit of involving influential community leaders, such as the traditional chiefs and religious leaders to create awareness of KMC. 25 The women have argued that these leaders are capable of mobilizing the communities and are more likely to be listened to, so involving them in mass campaigns during public gatherings or on radio talk shows, for example, could lead to behavior change.
This argument recognizes that KMC practice can be influenced by the behavior of the mother, partner, or other family members. However, behavior change can result from the conscious, rational decision of an individual under internal constraints that pushes them towards behaviors that facilitate their participation in KMC. It can also be a result of external pressure and supervision to observe social norms for collective social capital. 55 The conscious, rational, individual decisions also depend on access to knowledge, which the community leaders can be trained to provide, and the establishment of social norms. In a clinical trial in Bangladesh, community workers were trained to create awareness of KMC among pregnant women and those who were in immediate puerperium. 43 About 94% of women who had made contact with community workers during pregnancy practiced continuous KMC for more than seven hours a day.
Limitations
This review had significant limitations. First, there was a dearth of qualitative evidence in sub-Saharan Africa about women practicing KMC solely at home. There is a substantial number of quantitative studies, especially in the health care systems, that did not necessarily provide evidence on mothers practicing KMC at home. Secondly, this review was limited to studies in English and French, which meant that studies published in other languages were ineligible. Additionally, the included studies underwent data extraction regardless of their methodological quality. This may have affected the accuracy or the completeness of the findings reported in those studies; however, this ensured that no experiences expressed by women were ignored in this review.
Conclusions
This review identified a link between mothers’ perceptions and experiences of KMC and the influence on its practice in sub-Saharan Africa. The health care systems have failed to create awareness of KMC among communities before the birth of a preterm neonate; the traditional practices make KMC stigmatizing at a community level; and KMC is perceived to be difficult and cumbersome, requiring substantial social support. Strategies to make KMC less cumbersome need to be devised, and more qualitative studies are needed to explore community-level experiences of KMC in sub-Saharan Africa.
Recommendations for practice
This review has generated recommendations based on the illustrations, findings, categories, and the synthesized findings from the available qualitative evidence. The intention is to provide evidence to inform policy and practice to improve the uptake of KMC by the mothers of preterm neonates in sub-Saharan Africa.
Health care systems need to develop and implement deliberate KMC information, education, and communication strategies to be delivered to the communities much earlier during and/or before pregnancy. Mothers who have benefited previously from KMC intervention can be champions to communicate the information on KMC, such as on radio talk shows or dramas. The community leaders, including religious and traditional leaders, can be key in mobilizing communities to practice KMC, especially advocating for male and other family members’ involvement, dispelling the associated stigma attached to the practice. The health care systems should seek to understand the components of the intervention that make it cumbersome and focus on developing strategies to improve the comfort of the mother, as well as increase the availability and accessibility of devices for safely holding preterm neonates while performing other household chores, and educating mothers on their use. The policy environment should outline processes and communication strategies to address the feelings of anxiety about KMC.
Recommendations for research
This review focused on the perceptions and experiences of mothers practicing KMC at home and highlights key issues that are interlinked: the practice of the KMC is stigmatizing in the communities, and is also considered to be technically cumbersome, so the mothers resort to traditional and cultural alternatives that have not been tested. However, there is a paucity of evidence on KMC practice at home. Further research is needed to have a deeper understanding of mothers’ varying acceptance of KMC and the behaviors, assumptions, and cultures that may influence the practice. The research should focus on the cultural factors that may influence the practice of KMC. There should also be focus on strategies to make KMC less cumbersome and improve the mother's comfort while practicing KMC.
Acknowledgments
The Innovating for Maternal and Child Health in Africa initiative, African Population and Health Research Centre, and JBI for encouraging and providing the technical support for developing this review.
Appendix I: Search strategy
Cinahl (ebsco).
Search date: March 22, 2019

Embase (Ovid)
1 low birth weight/ (33,140)
2 very low birth weight/ (11,430)
3 extremely low birth weight/ (2949)
4 prematurity/ (92,592)
5 premature labor/ (41,902)
6 low birth weight.kw,tw. (33,000)
7 preterm.kw,tw. (92,829)
8 premature.kw,tw. (144,036)
9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (280,274)
10 developing countr ∗ .kw,tw. (72,092)
11 developing nation ∗ .kw,tw. (3447)
12 developing population ∗ .kw,tw. (348)
13 developing world ∗ .kw,tw. (10,331)
14 less developed countr ∗ .kw,tw. (1373)
15 less developed nation ∗ .kw,tw. (69)
16 less developed population ∗ .kw,tw. (3)
17 less developed world ∗ .kw,tw. (67)
18 lesser developed countr ∗ .kw,tw. (53)
19 lesser developed nation ∗ .kw,tw. (9)
20 lesser developed population ∗ .kw,tw. (0)
21 lesser developed world ∗ .kw,tw. (1)
22 under developed countr ∗ .kw,tw. (176)
23 under developed nation ∗ .kw,tw. (7)
24 under developed population ∗ .kw,tw. (3)
25 under developed world ∗ .kw,tw. (6)
26 underdeveloped countr ∗ .kw,tw. (1161)
27 underdeveloped nation ∗ .kw,tw. (90)
28 underdeveloped population ∗ .kw,tw. (11)
29 underdeveloped world ∗ .kw,tw. (55)
30 low income countr ∗ .kw,tw. (7114)
31 low income nation ∗ .kw,tw. (95)
32 low income population ∗ .kw,tw. (1626)
33 lower income countr ∗ .kw,tw. (518)
34 lower income nation ∗ .kw,tw. (8)
35 lower income population ∗ .kw,tw. (85)
36 underserved countr ∗ .kw,tw. (42)
37 underserved nation ∗ .kw,tw. (23)
38 underserved population ∗ .kw,tw. (3793)
39 underserved world ∗ .kw,tw. (5)
40 under served countr ∗ .kw,tw. (7)
41 under served nation ∗ .kw,tw. (1)
42 under served population ∗ .kw,tw. (126)
43 under served world ∗ .kw,tw. (0)
44 deprived countr ∗ .kw,tw. (31)
45 deprived nation ∗ .kw,tw. (11)
46 deprived population ∗ .kw,tw. (358)
47 deprived world ∗ .kw,tw. (0)
48 poor countr ∗ .kw,tw. (2707)
49 poor nation ∗ .kw,tw. (252)
50 poor population ∗ .kw,tw. (559)
51 poor world ∗ .kw,tw. (72)
52 poorer countr ∗ .kw,tw. (342)
53 poorer nation ∗ .kw,tw. (51)
54 poorer population ∗ .kw,tw. (66)
55 poorer world ∗ .kw,tw. (3)
56 developing econom ∗ .kw,tw. (551)
57 less developed econom ∗ .kw,tw. (18)
58 lesser developed econom ∗ .kw,tw. (1)
59 under developed econom ∗ .kw,tw. (2)
60 underdeveloped econom ∗ .kw,tw. (18)
61 middle income econom ∗ .kw,tw. (69)
62 low income econom ∗ .kw,tw. (46)
63 lower income econom ∗ .kw,tw. (2)
64 low gdp.kw,tw. (191)
65 low gnp.kw,tw. (7)
66 low gross domestic.kw,tw. (27)
67 low gross national.kw,tw. (10)
68 lower gdp.kw,tw. (62)
69 lower gnp.kw,tw. (1)
70 lower gross domestic.kw,tw. (12)
71 lower gross national.kw,tw. (5)
72 lmic.kw,tw. (1920)
73 lmics.kw,tw. (2843)
74 third world.kw,tw. (3510)
75 lami countr ∗ .kw,tw. (48)
76 transitional countr ∗ .kw,tw. (222)
77 cote d’ivoire.kw,tw. (2307)
78 cabo verde.kw,tw. (64)
79 eastern africa.kw,tw. (984)
80 western africa.kw,tw. (751)
81 east africa.kw,tw. (4082)
82 west africa.kw,tw. (9185)
83 central africa.kw,tw. (3138)
84 sub-saharan africa.kw,tw. (22,730)
85 subsaharan africa.kw,tw. (242)
86 zimbabwe.kw,tw. (5419)
87 zambia.kw,tw. (5307)
88 uganda.kw,tw. (14,443)
89 togo.kw,tw. (1539)
90 gambia.kw,tw. (2319)
91 tanzania.kw,tw. (12,865)
92 swaziland.kw,tw. (958)
93 sudan.kw,tw. (8283)
94 south sudan.kw,tw. (521)
95 south africa.kw,tw. (35,703)
96 somalia.kw,tw. (1342)
97 sierra leone.kw,tw. (2135)
98 seychelles.kw,tw. (673)
99 senegal.kw,tw. (6076)
100 rwanda.kw,tw. (3019)
101 “republic of the congo”.kw,tw. (3290)
102 nigeria.kw,tw. (32114)
103 niger.kw,tw. (14,894)
104 namibia.kw,tw. (1485)
105 “sao tome and principe”.kw,tw. (143)
106 mozambique.kw,tw. (3511)
107 mauritius.kw,tw. (932)
108 mali.kw,tw. (3889)
109 malawi.kw,tw. (6884)
110 madagascar.kw,tw. (4691)
111 liberia.kw,tw. (1488)
112 lesotho.kw,tw. (688)
113 kenya.kw,tw. (18,198)
114 ivory coast.kw,tw. (1836)
115 guinea-bissau.kw,tw. (1034)
116 guinea.kw,tw. (100,706)
117 ghana.kw,tw. (10,149)
118 gabon.kw,tw. (1816)
119 ethiopia.kw,tw. (13,263)
120 eritrea.kw,tw. (532)
121 equatorial guinea.kw,tw. (494)
122 djibouti.kw,tw. (389)
123 “democratic republic of the congo”.kw,tw. (2826)
124 comoros.kw,tw. (316)
125 chad.kw,tw. (1154)
126 central african republic.kw,tw. (997)
127 cameroon.kw,tw. (7101)
128 burundi.kw,tw. (801)
129 burkina faso.kw,tw. (4326)
130 botswana.kw,tw. (2348)
131 benin.kw,tw. (4354)
132 angola.kw,tw. (1360)
133 “Africa south of the Sahara”/ (12,710)
134 Central Africa/ (1287)
135 Cameroon/ (6213)
136 Central African Republic/ (797)
137 Chad/ (739)
138 Congo/ (2984)
139 Democratic Republic Congo/ (3143)
140 Equatorial Guinea/ (407)
141 Gabon/ (1559)
142 Africa/ (52,806)
143 “Sao Tome and Principe”/ (67)
144 Burundi/ (746)
145 Djibouti/ (290)
146 Eritrea/ (438)
147 Ethiopia/ (13,738)
148 Kenya/ (18,437)
149 Rwanda/ (3130)
150 Somalia/ (1795)
151 South Sudan/ (212)
152 Sudan/ (5528)
153 Tanzania/ (13,088)
154 Uganda/ (14,217)
155 Angola/ (1236)
156 Botswana/ (2302)
157 Lesotho/ (614)
158 Malawi/ (6463)
159 Namibia/ (1416)
160 Mozambique/ (3190)
161 South Africa/ (44,862)
162 Swaziland/ (845)
163 Zambia/ (5439)
164 Zimbabwe/ (5861)
165 Benin/ (2192)
166 Burkina Faso/ (3770)
167 Cabo Verde/ (307)
168 Cote d’Ivoire/ (2922)
169 Gambia/ (2530)
170 Ghana/ (9801)
171 Guinea/ (1651)
172 Guinea-Bissau/ (920)
173 Liberia/ (1428)
174 Mali/ (3061)
175 Mauritania/ (570)
176 Niger/ (1921)
177 Nigeria/ (33724)
178 Senegal/ (5719)
179 Sierra Leone/ (1997)
180 Togo/ (1251)
181 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101 or 102 or 103 or 104 or 105 or 106 or 107 or 108 or 109 or 110 or 111 or 112 or 113 or 114 or 115 or 116 or 117 or 118 or 119 or 120 or 121 or 122 or 123 or 124 or 125 or 126 or 127 or 128 or 129 or 130 or 131 or 132 or 133 or 134 or 135 or 136 or 137 or 138 or 139 or 140 or 141 or 142 or 143 or 144 or 145 or 146 or 147 or 148 or 149 or 150 or 151 or 152 or 153 or 154 or 155 or 156 or 157 or 158 or 159 or 160 or 161 or 162 or 163 or 164 or 165 or 166 or 167 or 168 or 169 or 170 or 171 or 172 or 173 or 174 or 175 or 176 or 177 or 178 or 179 or 180 (497,640)
182 kangaroo care/ (928)
183 kangaroo care.kw,tw. (392)
184 skin to skin.kw,tw. (7637)
185 kangaroo mother care.kw,tw. (453)
186 kangaroo mother method.kw,tw. (21)
187 kangaroo mother.kw,tw. (478)
188 182 or 183 or 184 or 185 or 186 or 187 (8428)
189 9 and 181 and 188 (152)
MEDLINE (Ovid)
Search date: March 20, 2019
1 Infant, Low Birth Weight/ (17,646)
2 Infant, Very Low Birth Weight/ (7919)
3 Infant, Extremely Low Birth Weight/ (1683)
4 Premature Birth/ (11,873)
5 Obstetric Labor, Premature/ (13,030)
6 low birth weight.kw,tw. (22,957)
7 preterm.kw,tw. (57,796)
8 premature.kw,tw. (100,135)
9 Infant, Extremely Premature/ (1832)
10 Premature Birth/ (11,873)
11 Infant, Premature/ (50,622)
12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (187,921)
13 developing countr ∗ .kw,tw. (74,593)
14 developing nation ∗ .kw,tw. (2184)
15 developing population ∗ .kw,tw. (261)
16 developing world ∗ .kw,tw. (7038)
17 less developed countr ∗ .kw,tw. (1105)
18 less developed nation ∗ .kw,tw. (53)
19 less developed population ∗ .kw,tw. (1)
20 less developed world ∗ .kw,tw. (60)
21 lesser developed countr ∗ .kw,tw. (43)
22 lesser developed nation ∗ .kw,tw. (6)
23 lesser developed population ∗ .kw,tw. (0)
24 lesser developed world ∗ .kw,tw. (0)
25 under developed countr ∗ .kw,tw. (86)
26 under developed nation ∗ .kw,tw. (4)
27 under developed population ∗ .kw,tw. (1)
28 under developed world ∗ .kw,tw. (3)
29 underdeveloped countr ∗ .kw,tw. (776)
30 underdeveloped nation ∗ .kw,tw. (67)
31 underdeveloped population ∗ .kw,tw. (9)
32 underdeveloped world ∗ .kw,tw. (39)
33 low income countr ∗ .kw,tw. (4568)
34 low income nation ∗ .kw,tw. (50)
35 low income population ∗ .kw,tw. (1881)
36 lower income countr ∗ .kw,tw. (322)
37 lower income nation ∗ .kw,tw. (5)
38 lower income population ∗ .kw,tw. (54)
39 underserved countr ∗ .kw,tw. (24)
40 underserved nation ∗ .kw,tw. (13)
41 underserved population ∗ .kw,tw. (2083)
42 underserved world ∗ .kw,tw. (3)
43 under served countr ∗ .kw,tw. (2)
44 under served nation ∗ .kw,tw. (1)
45 under served population ∗ .kw,tw. (57)
46 under served world ∗ .kw,tw. (0)
47 deprived countr ∗ .kw,tw. (18)
48 deprived nation ∗ .kw,tw. (6)
49 deprived population ∗ .kw,tw. (237)
50 deprived world ∗ .kw,tw. (0)
51 poor countr ∗ .kw,tw. (1913)
52 poor nation ∗ .kw,tw. (160)
53 poor population ∗ .kw,tw. (403)
54 poor world ∗ .kw,tw. (49)
55 poorer countr ∗ .kw,tw. (249)
56 poorer nation ∗ .kw,tw. (49)
57 poorer population ∗ .kw,tw. (52)
58 poorer world ∗ .kw,tw. (1)
59 developing econom ∗ .kw,tw. (326)
60 less developed econom ∗ .kw,tw. (14)
61 lesser developed econom ∗ .kw,tw. (1)
62 under developed econom ∗ .kw,tw. (3)
63 underdeveloped econom ∗ .kw,tw. (16)
64 middle income econom ∗ .kw,tw. (37)
65 low income econom ∗ .kw,tw. (30)
66 lower income econom ∗ .kw,tw. (2)
67 low gdp.kw,tw. (117)
68 low gnp.kw,tw. (6)
69 low gross domestic.kw,tw. (19)
70 low gross national.kw,tw. (8)
71 lower gdp.kw,tw. (34)
72 lower gnp.kw,tw. (0)
73 lower gross domestic.kw,tw. (7)
74 lower gross national.kw,tw. (1)
75 lmic.kw,tw. (901)
76 lmics.kw,tw. (1597)
77 third world.kw,tw. (2730)
78 lami countr ∗ .kw,tw. (34)
79 transitional countr ∗ .kw,tw. (132)
80 cote d’ivoire.kw,tw. (1749)
81 cabo verde.kw,tw. (54)
82 eastern africa.kw,tw. (3802)
83 western africa.kw,tw. (2943)
84 east africa.kw,tw. (3329)
85 west africa.kw,tw. (7328)
86 central africa.kw,tw. (2598)
87 sub-saharan africa.kw,tw. (15,239)
88 subsaharan africa.kw,tw. (135)
89 zimbabwe.kw,tw. (4467)
90 zambia.kw,tw. (4036)
91 uganda.kw,tw. (10635)
92 togo.kw,tw. (1200)
93 gambia.kw,tw. (2022)
94 tanzania.kw,tw. (9702)
95 swaziland.kw,tw. (668)
96 sudan.kw,tw. (6325)
97 south sudan.kw,tw. (345)
98 south africa.kw,tw. (25,155)
99 somalia.kw,tw. (1102)
100 sierra leone.kw,tw. (1612)
101 seychelles.kw,tw. (570)
102 senegal.kw,tw. (4810)
103 rwanda.kw,tw. (2134)
104 “republic of the congo”.kw,tw. (2241)
105 nigeria.kw,tw. (20,945)
106 niger.kw,tw. (9827)
107 namibia.kw,tw. (1116)
108 “sao tome and principe”.kw,tw. (120)
109 mozambique.kw,tw. (2646)
110 mauritius.kw,tw. (759)
111 mali.kw,tw. (2796)
112 malawi.kw,tw. (5003)
113 madagascar.kw,tw. (3822)
114 liberia.kw,tw. (1213)
115 lesotho.kw,tw. (530)
116 kenya.kw,tw. (13,826)
117 ivory coast.kw,tw. (1601)
118 guinea-bissau.kw,tw. (832)
119 guinea.kw,tw. (101,126)
120 ghana.kw,tw. (7065)
121 gabon.kw,tw. (1460)
122 ethiopia.kw,tw. (9726)
123 eritrea.kw,tw. (405)
124 equatorial guinea.kw,tw. (349)
125 djibouti.kw,tw. (315)
126 “democratic republic of the congo”.kw,tw. (1886)
127 comoros.kw,tw. (263)
128 chad.kw,tw. (945)
129 central african republic.kw,tw. (853)
130 cameroon.kw,tw. (5261)
131 burundi.kw,tw. (669)
132 burkina faso.kw,tw. (3118)
133 botswana.kw,tw. (1733)
134 benin.kw,tw. (2626)
135 angola.kw,tw. (1121)
136 Africa South of the Sahara/ (10,160)
137 Africa, Central/ (1232)
138 Cameroon/ (4983)
139 Central African Republic/ (737)
140 Chad/ (678)
141 Congo/ (1714)
142 Democratic Republic of the Congo/ (3822)
143 Equatorial Guinea/ (243)
144 Gabon/ (1389)
145 Africa, Eastern/ (3930)
146 “Sao Tome and Principe”/ (9)
147 Burundi/ (618)
148 Djibouti/ (211)
149 Eritrea/ (300)
150 Ethiopia/ (10,689)
151 Kenya/ (14,516)
152 Rwanda/ (2133)
153 Somalia/ (1467)
154 South Sudan/ (105)
155 Sudan/ (4473)
156 Tanzania/ (10,256)
157 Uganda/ (10812)
158 Africa, Southern/ (2265)
159 Angola/ (920)
160 Botswana/ (1583)
161 Lesotho/ (386)
162 Malawi/ (4650)
163 Namibia/ (967)
164 Mozambique/ (2128)
165 South Africa/ (38,717)
166 Swaziland/ (502)
167 Zambia/ (4113)
168 Zimbabwe/ (5462)
169 Africa, Western/ (5563)
170 Benin/ (1414)
171 Burkina Faso/ (2949)
172 Cabo Verde/ (170)
173 Cote d’Ivoire/ (2943)
174 Gambia/ (2318)
175 Ghana/ (7084)
176 Guinea/ (954)
177 Guinea-Bissau/ (874)
178 Liberia/ (1097)
179 Mali/ (2190)
180 Mauritania/ (413)
181 Niger/ (1104)
182 Nigeria/ (26403)
183 Senegal/ (5427)
184 Sierra Leone/ (1363)
185 Togo/ (1053)
186 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101 or 102 or 103 or 104 or 105 or 106 or 107 or 108 or 109 or 110 or 111 or 112 or 113 or 114 or 115 or 116 or 117 or 118 or 119 or 120 or 121 or 122 or 123 or 124 or 125 or 126 or 127 or 128 or 129 or 130 or 131 or 132 or 133 or 134 or 135 or 136 or 137 or 138 or 139 or 140 or 141 or 142 or 143 or 144 or 145 or 146 or 147 or 148 or 149 or 150 or 151 or 152 or 153 or 154 or 155 or 156 or 157 or 158 or 159 or 160 or 161 or 162 or 163 or 164 or 165 or 166 or 167 or 168 or 169 or 170 or 171 or 172 or 173 or 174 or 175 or 176 or 177 or 178 or 179 or 180 or 181 or 182 or 183 or 184 or 185 (406,419)
187 Kangaroo-Mother Care Method/ (322)
188 kangaroo care.kw,tw. (275)
189 skin to skin.kw,tw. (4551)
190 kangaroo mother care.kw,tw. (276)
191 kangaroo mother method.kw,tw. (18)
192 kangaroo mother.kw,tw. (279)
193 187 or 188 or 189 or 190 or 191 or 192 (4983)
194 12 and 186 and 193 (89)
MEDLINE (PubMed)
“Infant, Low Birth Weight”[Mesh] OR “Infant, Very Low Birth Weight”[Mesh] OR “Infant, Extremely Low Birth Weight”[Mesh] OR “Premature Birth”[Mesh] OR “Obstetric Labor, Premature”[Mesh] OR low birth weight[tiab] OR low birth weight[ot] OR preterm[tiab] OR preterm[ot] OR premature[tiab] OR premature[ot] OR “Infant, Extremely Premature”[Mesh] OR “Premature Birth”[Mesh] OR “Infant, Premature”[Mesh]
“developing country”[ot] OR “developing countries”[ot] OR “developing nation”[ot] OR “developing nations”[ot] OR “developing population”[ot] OR “developing populations”[ot] OR “developing world”[ot] OR “less developed country”[ot] OR “less developed countries”[ot] OR “less developed nation”[ot] OR “less developed nations”[ot] OR “less developed population”[ot] OR “less developed populations”[ot] OR “less developed world”[ot] OR “lesser developed country”[ot] OR “lesser developed countries”[ot] OR “lesser developed nation”[ot] OR “lesser developed nations”[ot] OR “lesser developed population”[ot] OR “lesser developed populations”[ot] OR “lesser developed world”[ot] OR “under developed country”[ot] OR “under developed countries”[ot] OR “under developed nation”[ot] OR “under developed nations”[ot] OR “under developed population”[ot] OR “under developed populations”[ot] OR “under developed world”[ot] OR “underdeveloped country”[ot] OR “underdeveloped countries”[ot] OR “underdeveloped nation”[ot] OR “underdeveloped nations”[ot] OR “underdeveloped population”[ot] OR “underdeveloped populations”[ot] OR “underdeveloped world”[ot] OR “low income country”[ot] OR “low income countries”[ot] OR “low income nation”[ot] OR “low income nations”[ot] OR “low income population”[ot] OR “low income populations”[ot] OR “lower income country”[ot] OR “lower income countries”[ot] OR “lower income nation”[ot] OR “lower income nations”[ot] OR “lower income population”[ot] OR “lower income populations”[ot] OR “underserved country”[ot] OR “underserved countries”[ot] OR “underserved nation”[ot] OR “underserved nations”[ot] OR “underserved population”[ot] OR “underserved populations”[ot] OR “underserved world”[ot] OR “under served country”[ot] OR “under served countries”[ot] OR “under served nation”[ot] OR “under served nations”[ot] OR “under served population”[ot] OR “under served populations”[ot] OR “under served world”[ot] OR “deprived country”[ot] OR “deprived countries”[ot] OR “deprived nation”[ot] OR “deprived nations”[ot] OR “deprived population”[ot] OR “deprived populations”[ot] OR “deprived world”[ot] OR “poor country”[ot] OR “poor countries”[ot] OR “poor nation”[ot] OR “poor nations”[ot] OR “poor population”[ot] OR “poor populations”[ot] OR “poor world”[ot] OR “poorer country”[ot] OR “poorer countries”[ot] OR “poorer nation”[ot] OR “poorer nations”[ot] OR “poorer population”[ot] OR “poorer populations”[ot] OR “poorer world”[ot] OR “developing economy”[ot] OR “developing economies”[ot] OR “less developed economy”[ot] OR “less developed economies”[ot] OR “lesser developed economy”[ot] OR “lesser developed economies”[ot] OR “under developed economy”[ot] OR “under developed economies”[ot] OR “underdeveloped economy”[ot] OR “underdeveloped economies”[ot] OR “middle income economy”[ot] OR “middle income economies”[ot] OR “low income economy”[ot] OR “low income economies”[ot] OR “lower income economy”[ot] OR “lower income economies”[ot] OR “low gdp”[ot] OR “low gnp”[ot] OR “low gross domestic”[ot] OR “low gross national”[ot] OR “lower gdp”[ot] OR “lower gnp”[ot] OR “lower gross domestic”[ot] OR “lower gross national”[ot] OR lmic[ot] OR lmics[ot] OR “third world”[ot] OR “lami country”[ot] OR “lami countries”[ot] OR “transitional country”[ot] OR “transitional countries”[ot] OR cote d’ivoire[tiab] OR cote d’ivoire[ot] OR cabo verde[tiab] OR cabo verde[ot] OR eastern africa[tiab] OR eastern africa[ot] OR western africa[tiab] OR western africa[ot] OR east africa[tiab] OR east africa[ot] OR west africa[tiab] OR west africa[ot] OR central africa[tiab] OR central africa[ot] OR sub-saharan africa[tiab] OR sub-saharan africa[ot] OR subsaharan africa[tiab] OR subsaharan africa[ot] OR zimbabwe[tiab] OR zimbabwe[ot] OR zambia[tiab] OR zambia[ot] OR uganda[tiab] OR uganda[ot] OR togo[tiab] OR togo[ot] OR gambia[tiab] OR gambia[ot] OR tanzania[tiab] OR tanzania[ot] OR swaziland[tiab] OR swaziland[ot] OR sudan[tiab] OR sudan[ot] OR south sudan[tiab] OR south sudan[ot] OR south africa[tiab] OR south africa[ot] OR somalia[tiab] OR somalia[ot] OR sierra leone[tiab] OR sierra leone[ot] OR seychelles[tiab] OR seychelles[ot] OR senegal[tiab] OR senegal[ot] OR sao tome and principe[tiab] OR sao tome and principe[ot] OR rwanda[tiab] OR rwanda[ot] OR republic of the congo[tiab] OR republic of the congo[ot] OR nigeria[tiab] OR nigeria[ot] OR niger[tiab] OR niger[ot] OR namibia[tiab] OR namibia[ot] OR mozambique[tiab] OR mozambique[ot] OR mauritius[tiab] OR mauritius[ot] OR mauritania[tiab] OR mauritania[ot] OR mali[tiab] OR mali[ot] OR malawi[tiab] OR malawi[ot] OR madagascar[tiab] OR madagascar[ot] OR liberia[tiab] OR liberia[ot] OR lesotho[tiab] OR lesotho[ot] OR kenya[tiab] OR kenya[ot] OR ivory coast[tiab] OR ivory coast[ot] OR guinea-bissau[tiab] OR guinea-bissau[ot] OR guinea[tiab] OR guinea[ot] OR ghana[tiab] OR ghana[ot] OR gabon[tiab] OR gabon[ot] OR ethiopia[tiab] OR ethiopia[ot] OR eritrea[tiab] OR eritrea[ot] OR equatorial guinea[tiab] OR equatorial guinea[ot] OR djibouti[tiab] OR djibouti[ot] OR democratic republic of the congo[tiab] OR democratic republic of the congo[ot] OR comoros[tiab] OR comoros[ot] OR chad[tiab] OR chad[ot] OR central african republic[tiab] OR central african republic[ot] OR cameroon[tiab] OR cameroon[ot] OR burundi[tiab] OR burundi[ot] OR burkina faso[tiab] OR burkina faso[ot] OR botswana[tiab] OR botswana[ot] OR benin[tiab] OR benin[ot] OR angola[tiab] OR angola[ot] OR “Africa South of the Sahara”[Mesh] OR “Africa, Central”[Mesh] OR “Cameroon”[Mesh] OR “Central African Republic”[Mesh] OR “Chad”[Mesh] OR “Congo”[Mesh] OR “Democratic Republic of the Congo”[Mesh] OR “Equatorial Guinea”[Mesh] OR “Gabon”[Mesh] OR “Sao Tome and Principe”[Mesh] OR “Africa, Eastern”[Mesh] OR “Burundi”[Mesh] OR “Djibouti”[Mesh] OR “Eritrea”[Mesh] OR “Ethiopia”[Mesh] OR “Kenya”[Mesh] OR “Rwanda”[Mesh] OR “Somalia”[Mesh] OR “South Sudan”[Mesh] OR “Sudan”[Mesh] OR “Tanzania”[Mesh] OR “Uganda”[Mesh] OR “Africa, Southern”[Mesh] OR “Angola”[Mesh] OR “Botswana”[Mesh] OR “Lesotho”[Mesh] OR “Malawi”[Mesh] OR “Namibia”[Mesh] OR “Mozambique”[Mesh] OR “South Africa”[Mesh] OR “Swaziland”[Mesh] OR “Zambia”[Mesh] OR “Zimbabwe”[Mesh] OR “Africa, Western”[Mesh] OR “Benin”[Mesh] OR “Burkina Faso”[Mesh] OR “Cabo Verde”[Mesh] OR “Cote d’Ivoire”[Mesh] OR “Gambia”[Mesh] OR “Ghana”[Mesh] OR “Guinea”[Mesh] OR “Guinea-Bissau”[Mesh] OR “Liberia”[Mesh] OR “Mali”[Mesh] OR “Mauritania”[Mesh] OR “Niger”[Mesh] OR “Nigeria”[Mesh] OR “Senegal”[Mesh] OR “Sierra Leone”[Mesh] OR “Togo”[Mesh]
“Kangaroo-Mother Care Method”[Mesh] OR kangaroo care[tiab] OR kangaroo care[ot] OR skin to skin[tiab] OR kangaroo mother care[tiab] OR kangaroo mother care[ot] OR kangaroo mother method[tiab] OR kangaroo mother method[ot] OR kangaroo mother[tiab] OR kangaroo mother[ot]
Evidence-Based Medicine Reviews (Ovid)
1 low birth weight.kw,tw. (3972)
2 preterm.kw,tw. (10,843)
3 premature.kw,tw. (10,979)
4 premature labo?r.kw,tw. (978)
5 1 or 2 or 3 or 4 (20,935)
6 developing countr ∗ .kw,tw. (3813)
7 developing nation ∗ .kw,tw. (112)
8 developing population ∗ .kw,tw. (11)
9 developing world ∗ .kw,tw. (463)
10 less developed countr ∗ .kw,tw. (111)
11 less developed nation ∗ .kw,tw. (5)
12 less developed population ∗ .kw,tw. (0)
13 less developed world ∗ .kw,tw. (3)
14 lesser developed countr ∗ .kw,tw. (2)
15 lesser developed nation ∗ .kw,tw. (0)
16 lesser developed population ∗ .kw,tw. (0)
17 lesser developed world ∗ .kw,tw. (0)
18 under developed countr ∗ .kw,tw. (870)
19 under developed nation ∗ .kw,tw. (107)
20 under developed population ∗ .kw,tw. (61)
21 under developed world ∗ .kw,tw. (169)
22 underdeveloped countr ∗ .kw,tw. (34)
23 underdeveloped nation ∗ .kw,tw. (3)
24 underdeveloped population ∗ .kw,tw. (0)
25 underdeveloped world ∗ .kw,tw. (1)
26 low income countr ∗ .kw,tw. (1000)
27 low income nation ∗ .kw,tw. (11)
28 low income population ∗ .kw,tw. (207)
29 lower income countr ∗ .kw,tw. (78)
30 lower income nation ∗ .kw,tw. (0)
31 lower income population ∗ .kw,tw. (7)
32 underserved countr ∗ .kw,tw. (2)
33 underserved nation ∗ .kw,tw. (4)
34 underserved population ∗ .kw,tw. (315)
35 underserved world ∗ .kw,tw. (0)
36 under served countr ∗ .kw,tw. (3)
37 under served nation ∗ .kw,tw. (3)
38 under served population ∗ .kw,tw. (25)
39 under served world ∗ .kw,tw. (0)
40 deprived countr ∗ .kw,tw. (3)
41 deprived nation ∗ .kw,tw. (3)
42 deprived population ∗ .kw,tw. (46)
43 deprived world ∗ .kw,tw. (0)
44 poor countr ∗ .kw,tw. (135)
45 poor nation ∗ .kw,tw. (11)
46 poor population ∗ .kw,tw. (45)
47 poor world ∗ .kw,tw. (4)
48 poorer countr ∗ .kw,tw. (30)
49 poorer nation ∗ .kw,tw. (0)
50 poorer population ∗ .kw,tw. (4)
51 poorer world ∗ .kw,tw. (0)
52 developing econom ∗ .kw,tw. (25)
53 less developed econom ∗ .kw,tw. (1)
54 lesser developed econom ∗ .kw,tw. (0)
55 under developed econom ∗ .kw,tw. (11)
56 underdeveloped econom ∗ .kw,tw. (1)
57 middle income econom ∗ .kw,tw. (22)
58 low income econom ∗ .kw,tw. (9)
59 lower income econom ∗ .kw,tw. (0)
60 low gdp.kw,tw. (41)
61 low gnp.kw,tw. (1)
62 low gross domestic.kw,tw. (1)
63 low gross national.kw,tw. (2)
64 lower gdp.kw,tw. (2)
65 lower gnp.kw,tw. (0)
66 lower gross domestic.kw,tw. (0)
67 lower gross national.kw,tw. (0)
68 lmic.kw,tw. (153)
69 lmics.kw,tw. (214)
70 third world.kw,tw. (90)
71 lami countr ∗ .kw,tw. (11)
72 transitional countr ∗ .kw,tw. (21)
73 cote d’ivoire.kw,tw. (213)
74 cabo verde.kw,tw. (1)
75 eastern africa.kw,tw. (28)
76 western africa.kw,tw. (9)
77 east africa.kw,tw. (136)
78 west africa.kw,tw. (299)
79 central africa.kw,tw. (48)
80 sub-saharan africa.kw,tw. (1459)
81 subsaharan africa.kw,tw. (2)
82 zimbabwe.kw,tw. (524)
83 zambia.kw,tw. (622)
84 uganda.kw,tw. (1560)
85 togo.kw,tw. (60)
86 gambia.kw,tw. (375)
87 tanzania.kw,tw. (1219)
88 swaziland.kw,tw. (69)
89 sudan.kw,tw. (229)
90 south sudan.kw,tw. (17)
91 south africa.kw,tw. (2735)
92 somalia.kw,tw. (45)
93 sierra leone.kw,tw. (143)
94 seychelles.kw,tw. (29)
95 senegal.kw,tw. (294)
96 rwanda.kw,tw. (221)
97 “republic of the congo”.kw,tw. (210)
98 nigeria.kw,tw. (1170)
99 niger.kw,tw. (190)
100 namibia.kw,tw. (57)
101 “sao tome and principe”.kw,tw. (5)
102 mozambique.kw,tw. (240)
103 mauritius.kw,tw. (36)
104 mali.kw,tw. (329)
105 malawi.kw,tw. (822)
106 madagascar.kw,tw. (121)
107 liberia.kw,tw. (78)
108 lesotho.kw,tw. (64)
109 kenya.kw,tw. (1634)
110 ivory coast.kw,tw. (57)
111 guinea-bissau.kw,tw. (199)
112 guinea.kw,tw. (720)
113 ghana.kw,tw. (711)
114 gabon.kw,tw. (117)
115 ethiopia.kw,tw. (633)
116 eritrea.kw,tw. (38)
117 equatorial guinea.kw,tw. (25)
118 djibouti.kw,tw. (23)
119 “democratic republic of the congo”.kw,tw. (202)
120 comoros.kw,tw. (23)
121 chad.kw,tw. (64)
122 central african republic.kw,tw. (50)
123 cameroon.kw,tw. (317)
124 burundi.kw,tw. (61)
125 burkina faso.kw,tw. (437)
126 botswana.kw,tw. (205)
127 benin.kw,tw. (206)
128 angola.kw,tw. (50)
129 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101 or 102 or 103 or 104 or 105 or 106 or 107 or 108 or 109 or 110 or 111 or 112 or 113 or 114 or 115 or 116 or 117 or 118 or 119 or 120 or 121 or 122 or 123 or 124 or 125 or 126 or 127 or 128 (19,495)
130 kangaroo care.kw,tw. (194)
131 skin to skin.kw,tw. (870)
132 kangaroo mother care.kw,tw. (166)
133 kangaroo mother method.kw,tw. (5)
134 kangaroo mother.kw,tw. (175)
135 130 or 131 or 132 or 133 or 134 (1083)
136 5 and 129 and 135 (44)
Appendix II: Studies ineligible following full-text review
Mazumder S, Upadhyay RP, Hill Z, Taneja S, Dube B, Kaur J, et al. Kangaroo mother care: using formative research to design an acceptable community intervention. BMC Public Health. 2018;18(1):307.
Reason for exclusion: Ineligible participants
Rasaily R, Ganguly KK, Roy M, Vani SN, Kharood N, Kulkarni R, et al. Community based kangaroo mother care for low birth weight babies: a pilot study. Indian J Med Res. 2017;145(1):51-7.
Appendix III: Characteristics of included studies

Appendix IV: Study findings with illustrations

kangaroo mother care; mothers; perceptions; skin to skin care; sub-Saharan Africa
- + Favorites
- View in Gallery
Readers Of this Article Also Read
New parents\' experiences of postpartum depression - a systematic review of qualitative evidence</strong>', 'holopainen arja phd; hakulinen-viitanen, tuovi phd', 'jbi evidence synthesis', '2012', '10', '56' , 'p 1-10');" onmouseout="javascript:tooltip_mouseout()" class="ejp-uc__article-title-link"> new parents' experiences of postpartum depression - a systematic review of..., experiences of transition to motherhood among pregnant women following assisted ..., the hospitalised patients’ experience of being in protective/source isolation: a systematic review of qualitative evidence</strong>', 'vottero beth rn phd cne; rittenmeyer, leslie rn, psy.d, cne', 'jbi evidence synthesis', '2012', '10', '16' , 'p 935-976');" onmouseout="javascript:tooltip_mouseout()" class="ejp-uc__article-title-link"> the hospitalised patients’ experience of being in protective/source isolation:..., women\'s experiences of menopause: a systematic review protocol of qualitative evidence</strong>', 'hoga luiza ak rn phd; rodolpho, juliana rc rn, msn; gon\u00e7alves, bruna g rn, msn; quirino, bruna', 'jbi evidence synthesis', 'july 2014', '12', '7' , 'p 72-81');" onmouseout="javascript:tooltip_mouseout()" class="ejp-uc__article-title-link"> women's experiences of menopause: a systematic review protocol of qualitative..., the effects of induction of labor prior to post-term in low risk pregnanices: a systematic review protocol</strong>', 'rydahl eva mhsc; eriksen, lena mhsc', 'jbi evidence synthesis', 'june 2014', '12', '6' , 'p 36-48');" onmouseout="javascript:tooltip_mouseout()" class="ejp-uc__article-title-link"> the effects of induction of labor prior to post-term in low risk pregnanices: a ....
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Observatory
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
Kangaroo mother care started immediately after birth critical for saving lives, new research shows
Immediate kangaroo mother care for preterm and low birthweight babies requires dedicated Mother-Newborn Intensive Care Units
GENEVA, 27 May 2021
- Kangaroo mother care, which involves skin-to-skin contact and exclusive breastfeeding , significantly improves a premature or low birthweight baby’s chances of survival
- Starting kangaroo mother care immediately after birth has the potential to save up to 150,000 more lives each year, compared with the current recommendation of starting it only once a baby is stable
- Mother-Newborn Intensive Care Units (ICUs) will be critical to support the mother, or a surrogate, in providing this immediate, ongoing skin-to-skin contact from birth.
The results of a new clinical trial published today in the New England Journal of Medicine , show that immediate kangaroo mother care, which involves skin-to-skin contact with the mother and exclusive breastfeeding, started as soon as a preterm or low birthweight baby is born, dramatically improves survival.
Current World Health Organization (WHO) recommendations indicate starting kangaroo mother care only after the baby is stabilized in an incubator or warmer, which can take on average 3-7 days. This new study suggests that, when compared with the existing practice, starting kangaroo mother care immediately after birth can save up to 150,000 more lives each year .
“Keeping the mother and baby together right from birth with zero separation will revolutionize the way neonatal intensive care is practiced for babies born early or small,” said Dr Rajiv Bahl, Head of the Newborn Unit at WHO , and the coordinator of the study. “When started at the soonest possible time, kangaroo mother care can save more lives, improve health outcomes for babies and ensures the constant presence of the mother with her sick baby.”
The results of the immediate kangaroo mother care study indicate the need for a global paradigm shift in the care of small babies with zero separation of babies from their mothers by having dedicated Mother-Newborn ICUs . “The best way to nurture the newly born low birthweight baby, including in high-income countries, is through ongoing skin-to-skin contact with the mother, in a mother-newborn couplet care unit that provides care and medical treatment for both,” said Dr Bjorn Westrup, of the Karolinska Institute, Sweden, and a technical expert for the study.
Kangaroo mother care is already known to be effective, reducing mortality by 40% among hospitalized infants with a birth weight less than 2.0 kg when started once they are clinically stable. However, this important new study provides new evidence to show a further 25% reduction when it is initiated immediately after birth, either with the mother or a surrogate.
Dr Queen Dube , one of the study investigators , and Director of Health Services in Malawi said , “Separating mothers from small and sick newborns adds stress for both mum and baby, at a time when they often both need close contact - immediate Kangaroo Mother Care overcomes this barrier. Keeping the mother and the baby together helps the baby to survive and thrive.
Mother-Newborn ICUs have been established in some countries so that mothers can always be with their babies to provide continuous kangaroo care. Mothers receive their own post-birth care in these wards without being separated from their baby. If a mother is unwell, the selection of a surrogate ensures that the provision of kangaroo care continues until the mother recovers.
During the clinical trial, which was conducted across five countries in Africa and Asia, mothers or surrogates provided approximately 17 hours of skin-to-skin contact per day while in a Mother-Newborn ICU. Delivery of the intervention required close collaboration between obstetric and neonatal departments. It is crucial to note that quality care for all newborns and mothers was provided in the trial which included provision of respiratory support if required, thermal care, breastfeeding support and prevention and management of infections.
Immediate kangaroo mother care had several other benefits in addition to improved survival. It reduced infections and hypothermia, which are two big killers of small babies. The babies also had more opportunity to breastfeed.
Dr Harish Chellani , one of the study investigators, from Vardhman Mahavir Medical College and Safdarjung Hospital, India, observed, “Health care providers have been separating small and sick babies from their mothers for decades believing that was best for them. The new evidence from this study means we must establish the practice of immediate kangaroo mother care globally”.
WHO is in the process of reviewing its current recommendations on kangaroo mother care, published in 2015, in light of the new evidence that has become available .
About the study
This study was a two arm, randomised controlled trial set in high volume, public tertiary care units in Ghana, India, Malawi, Nigeria and Tanzania. The babies in the immediate kangaroo mother care group started the intervention as soon as possible after birth and got an average of 17 hours per day in the Mother-Newborn ICU in the first three days. In the control group, kangaroo mother care was started only after the baby was stable and the babies got about 1.5 hours per day in the neonatal ICU; both the study groups got kangaroo mother care thereafter (about 19 hours / day). The study planned to include 4200 infants but was stopped early due to clear evidence of benefit on survival.
Kangaroo mother care and COVID-19:
The COVID-19 pandemic is affecting the quality of care provided to babies in all regions of the world and threatening implementation of life-saving interventions like breastfeeding and kangaroo mother care. A recent analysis showed that there is an increased risk of death among preterm or low birth weight babies if kangaroo mother care is not practiced, and this risk is 65-fold higher than the risk of death due to COVID-19 infection among newborns.
Dr Suman P N Rao, St. John's Medical College, Bangalore, India , co-author of both papers said , “Kangaroo mother care is one of our most cost-effective ways to protect small and sick newborns. Now it is more critical than ever to ensure mothers are supported to do kangaroo mother care and that healthcare professionals feel safe and comfortable to support this lifesaving intervention.”
Study sites
- Vardhman Mahavir Medical College & Safdarjung Hospital, New Delhi, India
- Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
- University of Malawi, College of Medicine, Blantyre, Malawi
- Obafemi Awolowo University, Ilfe-Ife, Nigeria
- Kwame Nkrumah University of Science and Technology and Komfo ANokye Teaching Hospital, Kumasi, Ghana
Media Contacts
Laura Keenan
Communications officer World Health Organization

IMAGES
VIDEO
COMMENTS
Kangaroo mother care (KMC), often defined as skin-to-skin contact between a mother and her newborn, frequent or exclusive breastfeeding, and early discharge from the hospital has been effective in reducing the risk of mortality among preterm and low birth weight infants. ... We conducted a systematic review of the KMC literature to 1 ...
Develop and disseminate kangaroo mother care protocols, policies, and guidelines. An absence of protocols, policies, and guidelines was a common barrier discovered in the literature. Without these critical documents, it results in the "default" procedure of separating infants from their mothers, which has been the norm for HCPs.
Percentages of correct answers on knowledge and attitude toward kangaroo mother care. General knowledge, knowledge on benefits, and attitude scores were grouped into four categories (scores 0 to 25, 25 to below 50, 50 to below 75, and 75 to 100).
Among infants with a birth weight between 1.0 and 1.799 kg, those who received immediate kangaroo mother care had lower mortality at 28 days than those who received conventional care with kangaroo ...
Of the 2875 papers identified, we included 112 studies with qualitative data on barriers to and enablers of kangaroo mother care ( Fig. 1 ). Most of the studies were published between 2010 and 2015 (66; 59%) and had less than 50 participants (67; 60%). Nearly half of the studies were surveys or interviews (50; 45%).
Kangaroo mother care literature review screening form based on our inclusion criteria. Using stan-dardized data abstraction forms, two reviewers abstracted data independently from all included articles and reports. At each stage, reviewers compared results to ensure agree-ment. In the case of disagreement between the two review-
In conclusion, the study underscores the potential benefits and positive impact of Kangaroo Mother Care on mother's experience. The findings advocate for the broad implementation of Kangaroo Mother Care as a valuable strategy in neonatal care, offering a holistic approach to improving the well-being of both mothers and newborns.
Introduction. Kangaroo Mother Care (KMC) is the treatment of preterm infants by their mothers, who make skin-to-skin contact with the infants. 1 KMC improves growth, reduces morbidity, and decreases the duration of hospitalization. 2, 3 It also increases the compatibility between the mothers' and infants' saliva levels 4 and results in a significant improvement in vital physiological ...
Background Kangaroo mother care (KMC) is an evidence-based intervention that reduces morbidity and mortality in preterm infants. However, it has not yet been fully integrated into health systems around the world. The aim of this study is to provide a cogent summary of the evidence base of the key barriers and facilitators to implementing KMC. Methods An umbrella review of existing reviews on ...
Kangaroo mother care (KMC) is an evidence-based approach to reducing mortality and morbidity in preterm infants. Although KMC is a key intervention package in newborn health initiatives, there is limited systematic information available on the barriers to KMC practice that mothers and other stakeholders face while practicing KMC. This systematic review sought to identify the most frequently ...
Complications of preterm birth are the leading cause of death among newborns.1 Kangaroo mother care can include early and continuous skin-to-skin contact, breastfeeding, early discharge from the health-care facility and supportive care.2 The clinical eficacy and health benefits of kangaroo mother care have been demonstrated in multiple settings.
Abstract. Background: Kangaroo mother care (KMC), often defined as skin-to-skin contact between a mother and her newborn, frequent or exclusive breastfeeding, and early discharge from the hospital has been effective in reducing the risk of mortality among preterm and low birth weight infants. Research studies and program implementation of KMC ...
WHO extends sincere appreciation to the Kangaroo Mother Care (KMC) Working Group convened by the WHO Strategic and Technical Advisory Group of Experts (STAGE) for Maternal, Newborn, Child and Adolescent Health and Nutrition (MNCAH&N), listed below. All the participating organizations endorsed the position paper. STAGE MNCAH&N KMC Working Group
We conducted a systematic review and searched PubMed, Embase, Scopus, Web of Science, and the World Health Organization Regional Databases for studies with key words "kangaroo mother care", "kangaroo care" or "skin to skin care" from 1 January 1960 to 24 April 2014. Two independent reviewers screened articles and abstracted data.
A comprehensive literature review is needed to improve understanding of specific barriers that are most relevant to nurses and the improvement of this practice. This review investigates nurses' barriers in implementing Kangaroo Mother Care, in order to illustrate directions for future research. Methods: This study was based on integrative ...
Recent systematic reviews have collated the numerous studies that identify diverse barriers and enablers to the use of Kangaroo Mother Care. In this narrative review, we combine the findings of these systematic reviews with more recent studies to propose a conceptual framework, encompassing factors that may affect the initiation and maintenance ...
Kangaroo mother care (KMC) is an intervention aimed at improving outcomes among preterm and low birth weight newborns. ... Dr Boundy conceptualized and designed the study, conducted the literature review, collected the data, conducted the analyses, created the tables and figures, and drafted and revised the manuscript; Dr Dastjerdi conducted ...
In this multicenter trial, the initiation of con-tinuous kangaroo mother care soon after birth in infants with a birth weight between 1.0 and 1.799 kg improved neonatal survival by 25% as compared ...
Importance The Cochrane review (2016) on kangaroo mother care (KMC) demonstrated a significant reduction in the risk of mortality in low birth weight infants. New evidence from large multi-centre randomised trials has been available since its publication. Objective Our systematic review compared the effects of KMC vs conventional care and early (ie, within 24 hours of birth) vs late initiation ...
Kangaroo mother care (KMC) is an evidence-based approach to reducing mortality and morbidity in preterm infants. ... In addition, because at least one relevant article identified from a list of references in a literature review included the terms Kangaroo Mother Care in quotations and the term Skin to skin, we also searched PubMed for ...
Despite this, implementation of kangaroo care globally remains low. The objectives of this review were to: (a) synthesize evidence on parents' and healthcare practitioners' perceptions, experiences, knowledge of, and attitudes toward kangaroo care of preterm babies in hospital settings; and (b) establish parents' satisfaction with kangaroo care.
Develop and disseminate kangaroo mother care protocols, policies, and guidelines. An absence of protocols, policies, and guidelines was a common barrier discovered in the literature. Without these critical documents, it results in the "default" procedure of separating infants from their mothers, which has been the norm for HCPs. Key. Conclusion
-fives over the past few decades. More than 7000 deaths occur daily around the globe, but mostly in sub-Saharan Africa. Of these deaths, 60% to 80% are due to preterm birth and low birth weight. Kangaroo mother care is known to offer a cheap and effective way to care for low birth weight, preterm neonates; however, its practice is still low. There is limited evidence on the factors that hinder ...
Review. An online search was conducted using PubMed, Google Scholar, and Web of Science to find scholarly articles. Key Medical Subject Heading (MeSH) search terms such as "Kangaroo mother care benefits," "Low birth weight baby," "neonatal child," "preterm child," and "infant child development" were used.
Europe PMC is an archive of life sciences journal literature. Methods. We searched PubMed, Embase, Scopus, Web of Science and the World Health Organization's regional databases, for studies on "kangaroo mother care" or "kangaroo care" or "skin-to-skin care" from 1 January 1960 to 19 August 2015, without language restrictions.
Immediate kangaroo mother care for preterm and low birthweight babies requires dedicated Mother-Newborn Intensive Care Units GENEVA, 27 May 2021Kangaroo mother care, which involves skin-to-skin contact and exclusive breastfeeding, significantly improves a premature or low birthweight baby's chances of survival Starting kangaroo mother care immediately after birth has the potential to save up ...