- Skip to main content
- Keyboard shortcuts for audio player

This experimental drug could change the field of cancer research

Sacha Pfeiffer

Jonaki Mehta

The new treatment is categorized as immunotherapy. skaman306/Getty Images hide caption
The new treatment is categorized as immunotherapy.
A tiny group of people with rectal cancer just experienced something of a scientific miracle: their cancer simply vanished after an experimental treatment.
In a very small trial done by doctors at New York's Memorial Sloan Kettering Cancer Center, patients took a drug called dostarlimab for six months. The trial resulted in every single one of their tumors disappearing. The trial group included just 18 people, and there's still more to be learned about how the treatment worked. But some scientists say these kinds of results have never been seen in the history of cancer research.
Dr. Hanna Sanoff of the University of North Carolina's Lineberger Comprehensive Cancer Center joined NPR's All Things Considered to outline how this drug works and what it could mean for the future of cancer research. Although she was not involved with the study, Dr. Sanoff has written about the results.
This interview has been lightly edited
On her first reaction to the results: I mean, I am incredibly optimistic. Like you said in the introduction, we have never seen anything work in 100 percent of people in cancer medicine.
On how the drug works to treat cancer: This drug is one of a class of drugs called immune checkpoint inhibitors. These are immunotherapy medicines that work not by directly attacking the cancer itself, but actually getting a person's immune system to essentially do the work. These are drugs that have been around in melanoma and other cancers for quite a while, but really have not been part of the routine care of colorectal cancers until fairly recently.
On the kinds of side effects patients experienced: Very, very few in this study - in fact, surprisingly few. Most people had no severe adverse effects at all.
On how this study could be seen as 'practice-changing': Our hope would be that for this subgroup of people - which is only about five percent to 10 percent of people who have rectal cancer - if they can go on and just get six months of immunotherapy and not have any of the rest of this - I don't even know the word to use. Paradigm shift is often used, but this really absolutely is paradigm-shifting.
On why the idea of being able to skip surgery for cancer treatment is so revolutionary: In rectal cancer, this is part of the conversation we have with someone when they're diagnosed. We are very hopeful for being able to cure you, but unfortunately, we know our treatments are going to leave you with consequences that may, in fact, be life-changing. I have had patients who, after their rectal cancer, have barely left the house for years - and in a couple of cases, even decades - because of the consequences of incontinence and the shame that's associated with this.
On next steps for the drug: What I'd really like us to do is get a bigger trial where this drug is used in a much more diverse setting to understand what the real, true response rate is going to be. It's not going to end up being 100 percent. I hope I bite my tongue on that in the future, but I can't imagine it will be 100 percent. And so when we see what the true response rate is, that's when I think we can really do this all the time.
This piece was reported by Sacha Pfeiffer, produced by Jonaki Mehta and edited by Kathryn Fox. It was adapted for the web by Manuela Lopez Restrepo.
- cancer treatment
FDA approves groundbreaking treatment for advanced melanoma
The Food and Drug Administration on Friday approved a new cancer therapy that could one day transform the way a majority of aggressive and advanced tumors are treated.
The treatment, called Amtagvi, from Iovance Biotherapeutics , is for metastatic melanoma patients who have already tried and failed other drugs. It’s known as TIL therapy and involves boosting the number of immune cells inside tumors, harnessing their power to fight the cancer.
It’s the first time a cellular therapy has been approved to treat solid tumors. The drug was given a fast-track approval based on the results of a phase 2 clinical trial. The company is conducting a larger phase 3 trial to confirm the treatment’s benefits. The therapy’s list price — the price before insurance and other potential discounts — is $515,000 per patient.
“This is going to be huge,” said Dr. Elizabeth Buchbinder, a senior physician at Dana-Farber Cancer Institute in Boston. Melanoma is “not one of those cancers where there’s like 20 different” possible treatments, she said. “You start running out of options fast.”

Friday’s approval is only for melanoma, the deadliest form of skin cancer , but experts say it holds promise for treating other solid tumors, which account for 90% of all cancers.
“It is our hope that future iterations of TIL therapy will be important for lung cancer, colon cancer , head and neck cancer, bladder cancer and many other cancer types,” said Dr. Patrick Hwu, chief executive of the Moffitt Cancer Center in Tampa, Florida. Moffitt has been involved with Iovance’s clinical trials of TIL therapy.
TIL stands for tumor-infiltrating lymphocytes, which are immune cells that exist within tumors . But there are nowhere nearly enough of those cells to effectively fight off cancer cells. TIL therapy involves, in part, extracting some of those immune cells from the patient’s tumor and replicating them billions of times in a lab, then reinfusing them back into the patient.
It’s similar to CAR-T cell therapy, where healthy cells are taken out of a person’s body and then modified in a lab to fight cancers. That’s usually used for hard-to-treat blood cancers such as leukemia and lymphoma. With TIL therapy, the cells used are already programmed to recognize cancer — no lab modifications needed — they just need a boost in numbers to fight it.
Like CAR-T, TIL therapy is a one-time treatment, though the entire process can take up to eight weeks. The TIL cells are first harvested from the tumor through a minimally invasive procedure and then grown and multiplied in the lab, a process that takes 22 days, according to Iovance.
While that’s happening, patients are given chemotherapy to clear out their immune cells to make room for the billions of new melanoma-fighting TIL cells. Once the TIL cells are reinfused back into the body, patients get a drug called interleukin-2 to further stimulate those cells.
Hwu said that most side effects in patients undergoing TIL therapy are not from the reinfusion of cells, but from the chemotherapy and the interleukin-2. These can include nausea and extreme fatigue, and patients are also vulnerable to other illnesses because the body is depleted of disease-fighting white blood cells.
Putting billions of cells back into the body is not entirely risk-free, however, said Dr. William Dahut, chief scientific officer of the American Cancer Society. It’s possible that the body’s immune system could overreact in what’s known as a cytokine storm, which can cause flu-like symptoms, low blood pressure and organ damage. “There are risks for immune-related side effects, which could be serious,” he said.
Common side effects associated with Amtagvi can include abnormally fast heart rate, fluid buildup, rash, hair loss and feeling short of breath, the FDA said.
Those side effects can be managed, said Dr. Steven Rosenberg, chief of the surgery branch at the National Cancer Institute. “They’re a small price to pay for a growing cancer that would otherwise be lethal.”
Overall, Dahut said the approval of TIL therapy is “meaningful.”
“What’s nice about this is that patients will receive a wide variety of tumor fighting lymphocytes that will be able to have the capacity to overcome resistance and actually be a living therapy over time, too, to target additional cancer cells should they develop,” Dahut said.
In addition to melanoma, Dahut said that TIL therapy is most likely to be useful in cancers that respond to drugs that “take the brakes off the immune system,” called checkpoint inhibitors .
“Those would be things like non-small cell lung cancer, kidney cancer, maybe bladder cancer, that we know are responsive to immune-based therapies to begin with,” he said. “Many of those patients relapse, so another immune-based therapy that works in a different way, seems to me, the most likely way for this to be effective.”
Much more research is needed, and it may be years before TIL therapy is approved for other types of cancer.
One of Iovance’s clinical trials investigating TIL therapy for non-small cell lung cancer was forced to pause when a participant died. While the death is under investigation, the company said it may have been the result of either chemotherapy or interleukin 2 — therapies meant to knock down each patients’ immune system before they can get the reinfusion of their TIL cells.
The therapy is not expected to work for every metastatic melanoma patient. Clinical trial data that Iovance submitted to the FDA showed that tumors shrank in about a third of patients who received TIL therapy.
Of those patients, about half saw their tumors shrink for at least one year, Dr. Friedrich Graf Finckenstein, chief medical officer of Iovance Biotherapeutics. “Some of these patients even had their tumor completely disappear,” he said.
Another study, conducted in the Netherlands , did a head-to-head analysis of TIL therapy and another form of immunotherapy, called ipilimumab. Twenty percent of the patients who received TIL had complete remissions, compared with 7% of patients who got ipilimumab. Iovance was not involved with the Dutch trial.
The goal of the therapy, Hwu said, “is to get rid of the cancer and have it stay away. These immune cells stay in the body and live in the body for decades.”
The technology has been in development and studied for nearly 40 years. It was Rosenberg who pioneered TIL therapy — first describing how it could shrink melanoma tumors in the New England Journal of Medicine in 1988 .
“I’ve been waiting for a very long time to see this given to patients, because I know that it can cure some patients that have metastatic melanoma that cannot be affected by any other treatment,” Rosenberg said.
It’s worked so far for Dan Bennett, 59, of Clermont, Florida. Bennett was diagnosed with melanoma in 2011 after his daughter noticed a suspicious mole on his neck that had changed color.
Despite surgery, chemotherapy and radiation, his cancer kept returning. In 2014, his doctors at Moffitt recommended he try TIL therapy.
“At first, we were pretty leery about it because it was unproven,” Bennett said. Ten years later, Bennett is convinced the TIL therapy is the reason he has survived so long with stage 4 melanoma, which usually has a five-year survival rate of 22.5% .
“I would recommend any experimental drug if it’s your last opportunity,” he said. “You owe it to yourself and your family to do whatever you can to stay alive and to be a productive member of society.”
Buchbinder, the Dana-Faber doctor, was not involved with Iovance’s TIL therapy trial for melanoma, but she is scheduled to begin similar trials with other drugmakers.
“We literally have patients right now waiting for approval because they are hoping they’ll be able to go on it,” Buchbinder said. “It is definitely a practice-changing therapy.”
Erika Edwards is a health and medical news writer and reporter for NBC News and "TODAY."
Anne Thompson is NBC News’ chief environmental affairs correspondent.
Marina Kopf is an associate producer with the NBC News Health and Medical Unit.
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Sustainability
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
New cancer treatment may reawaken the immune system
Press contact :, media download.
*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."
Previous image Next image
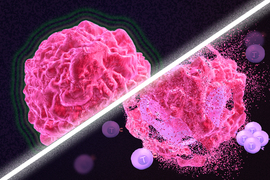
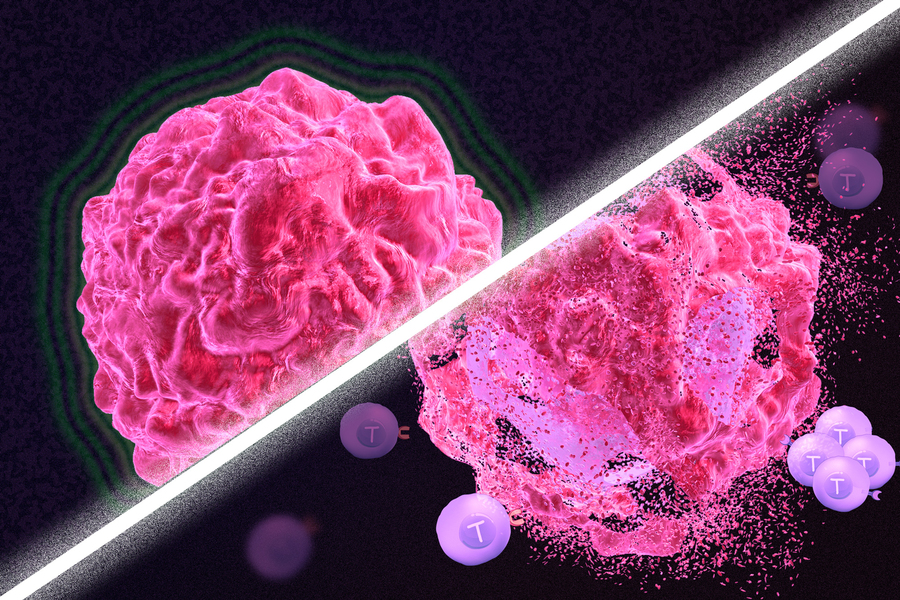
Immunotherapy is a promising strategy to treat cancer by stimulating the body’s own immune system to destroy tumor cells, but it only works for a handful of cancers. MIT researchers have now discovered a new way to jump-start the immune system to attack tumors, which they hope could allow immunotherapy to be used against more types of cancer.
Their novel approach involves removing tumor cells from the body, treating them with chemotherapy drugs, and then placing them back in the tumor. When delivered along with drugs that activate T cells, these injured cancer cells appear to act as a distress signal that spurs the T cells into action.
“When you create cells that have DNA damage but are not killed, under certain conditions those live, injured cells can send a signal that awakens the immune system,” says Michael Yaffe, who is a David H. Koch Professor of Science, the director of the MIT Center for Precision Cancer Medicine, and a member of MIT’s Koch Institute for Integrative Cancer Research.
In mouse studies, the researchers found that this treatment could completely eliminate tumors in nearly half of the mice.
Yaffe and Darrell Irvine, who is the Underwood-Prescott Professor with appointments in MIT’s departments of Biological Engineering and Materials Science and Engineering, and an associate director of the Koch Institute, are the senior authors of the study, which appears today in Science Signaling . MIT postdoc Ganapathy Sriram and Lauren Milling PhD ’21 are the lead authors of the paper.
T cell activation
One class of drugs currently used for cancer immunotherapy is checkpoint blockade inhibitors, which take the brakes off of T cells that have become “exhausted” and unable to attack tumors. These drugs have shown success in treating a few types of cancer but do not work against many others.
Yaffe and his colleagues set out to try to improve the performance of these drugs by combining them with cytotoxic chemotherapy drugs, in hopes that the chemotherapy could help stimulate the immune system to kill tumor cells. This approach is based on a phenomenon known as immunogenic cell death, in which dead or dying tumor cells send signals that attract the immune system’s attention.
Several clinical trials combining chemotherapy and immunotherapy drugs are underway, but little is known so far about the best way to combine these two types of treatment.
The MIT team began by treating cancer cells with several different chemotherapy drugs, at different doses. Twenty-four hours after the treatment, the researchers added dendritic cells to each dish, followed 24 hours later by T cells. Then, they measured how well the T cells were able to kill the cancer cells. To their surprise, they found that most of the chemotherapy drugs didn’t help very much. And those that did help appeared to work best at low doses that didn’t kill many cells.
The researchers later realized why this was so: It wasn’t dead tumor cells that were stimulating the immune system; instead, the critical factor was cells that were injured by chemotherapy but still alive.
“This describes a new concept of immunogenic cell injury rather than immunogenic cell death for cancer treatment,” Yaffe says. “We showed that if you treated tumor cells in a dish, when you injected them back directly into the tumor and gave checkpoint blockade inhibitors, the live, injured cells were the ones that reawaken the immune system.”
The drugs that appear to work best with this approach are drugs that cause DNA damage. The researchers found that when DNA damage occurs in tumor cells, it activates cellular pathways that respond to stress. These pathways send out distress signals that provoke T cells to leap into action and destroy not only those injured cells but any tumor cells nearby.
“Our findings fit perfectly with the concept that ‘danger signals’ within cells can talk to the immune system, a theory pioneered by Polly Matzinger at NIH in the 1990s, though still not universally accepted,” Yaffe says.
Tumor elimination
In studies of mice with melanoma and breast tumors, the researchers showed that this treatment eliminated tumors completely in 40 percent of the mice. Furthermore, when the researchers injected cancer cells into these same mice several months later, their T cells recognized them and destroyed them before they could form new tumors.
The researchers also tried injecting DNA-damaging drugs directly into the tumors, instead of treating cells outside the body, but they found this was not effective because the chemotherapy drugs also harmed T cells and other immune cells near the tumor. Also, injecting the injured cells without checkpoint blockade inhibitors had little effect.
“You have to present something that can act as an immunostimulant, but then you also have to release the preexisting block on the immune cells,” Yaffe says.
Yaffe hopes to test this approach in patients whose tumors have not responded to immunotherapy, but more study is needed first to determine which drugs, and at which doses, would be most beneficial for different types of tumors. The researchers are also further investigating the details of exactly how the injured tumor cells stimulate such a strong T cell response.
The research was funded, in part, by the National Institutes of Health, the Mazumdar-Shaw International Oncology Fellowship, the MIT Center for Precision Cancer Medicine, and the Charles and Marjorie Holloway Foundation.
Share this news article on:
Related links.
- Department of Biology
- Department of Biological Engineering
- Department of Materials Science and Engineering
- Koch Institute
- Ragon Institute
Related Topics
- Biological engineering
Related Articles
Cancer biologists identify new drug combo
A boost for cancer immunotherapy
Fighting cancer with the power of immunity
Previous item Next item
More MIT News

New security protocol shields data from attackers during cloud-based computation
Read full story →

How social structure influences the way people share money

Mars’ missing atmosphere could be hiding in plain sight

Startup helps people fall asleep by aligning audio signals with brainwaves

Study evaluates impacts of summer heat in U.S. prison environments
Fifteen Lincoln Laboratory technologies receive 2024 R&D 100 Awards
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
March 1, 2024
11 min read
These Cancers Were Beyond Treatment—But Might Not Be Anymore
New drugs called antibody-drug conjugates help patients with cancers that used to be beyond treatment
By Jyoti Madhusoodanan
Keith Negley
I n the long and often dispiriting quest to cure cancer, the 1998 approval of the drug Herceptin was a tremendously hopeful moment. This drug for breast cancer was the first to use a tumor-specific protein as a homing beacon to find and kill cancer cells. And it worked. Herceptin has benefited nearly three million people since that time, dramatically increasing the 10-year survival rate—and the cancer-free rate—for what was once one of the worst medical diagnoses. “Honestly, it was sort of earth-shattering,” says oncologist Sara M. Tolaney of the Dana-Farber Cancer Institute in Boston.
But the drug has a major limitation. Herceptin's beacon is a protein called HER2, and it works best for people whose tumors are spurred to grow by the HER2 signal—yet that's only about one fifth of breast cancer patients. For the other 80 percent of the approximately 250,000 people diagnosed with the disease every year in the U.S., Herceptin offers no benefits.
The hunt for better treatments led researchers to reimagine targeted therapies. By 2022 they had developed one that linked Herceptin to another cancer-killing drug. This therapy, for the first time, could damage tumors that had vanishingly low levels of HER2. The drug, named Enhertu, extended the lives of people with breast cancer by several months, sometimes longer. And it did so with fewer severe side effects than standard chemotherapies. The U.S. Food and Drug Administration approved its use in that year.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The news got even better in 2023. Researchers reported that Enhertu appeared to work even on tumors with seemingly no HER2 at all. (It's possible the cancers did have the protein but at very low levels that escaped standard detection methods.) “Exciting!” says oncologist Shanu Modi of Memorial Sloan Kettering (MSK) Cancer Center in New York City, who helped to run the study that led to Enhertu's approval. “They did this provocative test and saw this almost 30 percent response rate” in tumors apparently lacking the cancer protein, she notes.
Enhertu belongs to an ingenious and growing class of targeted cancer drugs called antibody-drug conjugates, or ADCs. The compounds are built around a particular antibody, an immune system protein that homes in on molecules that are abundant on cancer cells. The antibody is linked to a toxic payload, a drug that kills those cells. An ADC's affinity for cancer means it spares healthy cells, avoiding many of the side effects of traditional chemotherapy. And each antibody can be paired with several different drugs. This Lego-like assembly opens up a world of mix-and-match possibilities. Researchers can use the same drug to treat many cancers by switching up the antibody, or they can attack one type of tumor with many different ADCs that target several cancer biomarkers on the cells. This ability “changes the way we think about drug development,” Tolaney says.
The idea for ADCs is not entirely new—the first one was cleared for patient use in 2000—but recently scientists have learned intricate chemical construction techniques that make the compounds much more effective, and they have identified new cancer-specific targets. These advances have driven a wave of new development. Fourteen ADCs have been approved for breast, bladder, ovarian, blood, and other cancers. Approximately 100 others are in the preclinical pipeline. One ADC for breast cancer, known as T-DM1, proved much more effective than Herceptin and has now become the standard of care for early stages of disease. “It is pretty cool to see how things have changed so quickly,” Tolaney says. Buoyed by the successes, researchers and pharmaceutical companies are pouring resources into developing more powerful ADCs—perhaps even ones that can work across a wide range of cancer types. Pharma giants such as Gilead, Roche and BioNTech have invested heavily in their ADC programs; in October 2023, for example, Merck put $4 billion into a partnership with Daiichi Sankyo, the biotechnology firm that partnered with AstraZeneca to produce Enhertu.
But the new drugs are still beset by some mysterious problems. Some ADCs have side effects similar to those caused by traditional chemotherapies—which shouldn't happen, because the drugs are supposed to target cancer cells alone. On patient forums, people describe needing to reduce their doses because of intolerable nausea or fatigue. These drawbacks limit ADCs' use, so scientists and pharma companies are urgently trying to figure out what is causing them.
In the clinical trial that led to Enhertu's approval, patients typically had already received different kinds of chemotherapy drugs, such as medications that stop cells from multiplying. But these drugs—and other forms of chemotherapy—do not distinguish between a cancer cell and a healthy one. Any cell trying to make DNA or multiply is vulnerable, and normal tissue as well as tumors can be attacked. Fully 64 percent of people on standard chemotherapy experience nausea, diarrhea, fatigue, and other negative side effects. For many, these can be as debilitating as cancer itself. Such effects limit the dose people can take and the length of treatment, leaving windows of opportunity for tumors to grow resistant and rebound.
For many years researchers have sought less toxic alternatives, envisioning precision drugs that target cancers and spare healthy cells. The idea of ADCs sprang from the exquisite specificity of antibodies. If highly toxic forms of chemotherapy could be strapped onto antibodies, the toxins would reach only the cancer cells and no others. Although the concept was straightforward, attempts at making ADCs faltered for decades.
Some of the earliest attempts used drugs that just weren't strong enough. In the 1950s, for instance, researchers linked a drug named methotrexate to an antibody that targets carcinoembryonic antigen, a common tumor marker, and tested whether the construct could treat advanced colorectal and ovarian cancers in people. The drug bound to its target but had little therapeutic effect. Researchers then swung too far to the other end of the spectrum and tried using much more toxic drugs instead. But these drugs triggered serious side effects.
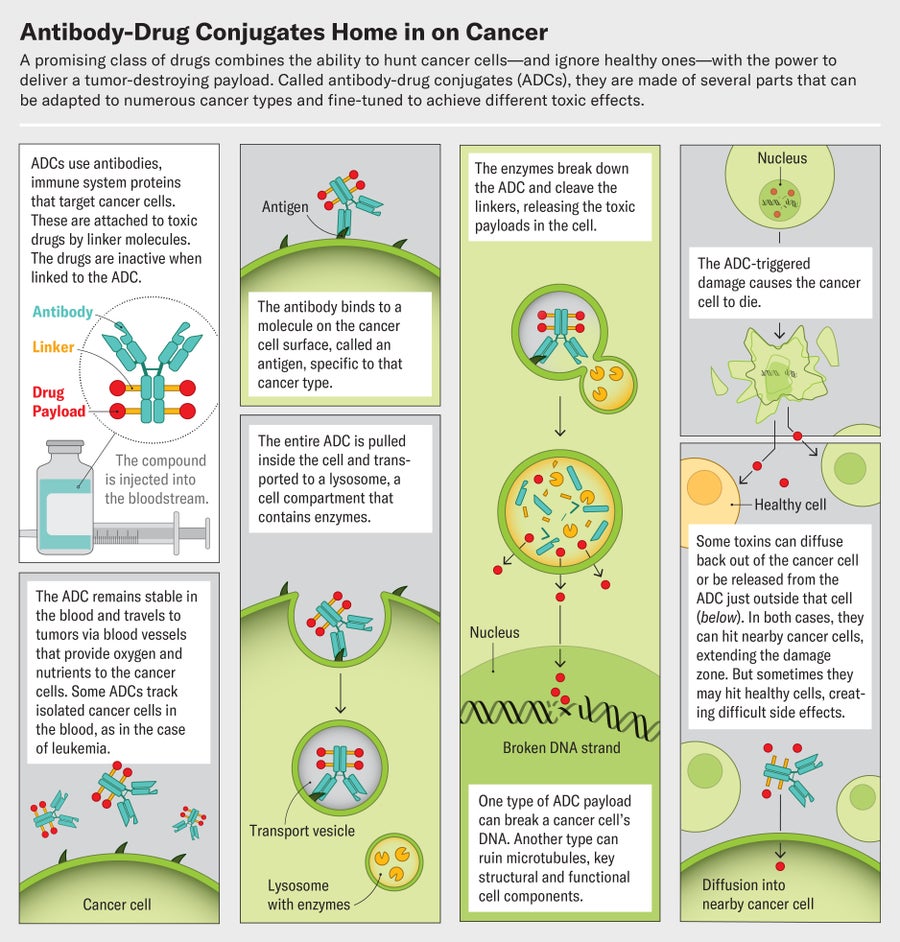
Credit: Jen Christiansen; Graphics consultant: Greg Thurber/University of Michigan
Greg Thurber, a chemical engineer at the University of Michigan, looked into this conundrum. He began working on ADCs when studying how antibodies spread through the body to bind to their targets. After ADCs infiltrate a tumor through its network of blood vessels, the compounds slip out of these vessels and into cancer cells to kill them, Thurber says. But the ADCs that existed at the time never got past the cells just outside the blood vessels. They bound too tightly. The key to improved effects, it turned out, was tailoring the antibody parts so they zeroed in on cancer cells but had a loose enough grip for some to slip into the interior of the tumor. “A lot of people in the field had a very simple concept—we put a chemotherapy drug on an antibody, it targets it to the cancer cell, and it will avoid healthy tissue,” Thurber says. “That's not at all how they work in reality.”
Tinkering with the drug component of ADCs, as well as the antibody, eventually led to a cancer-killing sweet spot. In 2013 the fda greenlit T-DM1 for breast cancer. Its antibody is trastuzumab (the “T” in T-DM1), the same antibody used in Herceptin. The drug attached to this antibody is notable because it's too dangerous to be used on its own. Known as emtansine, it was initially discovered in the 1970s but shelved because it was too toxic to too many cells. Tethered together as T-DM1, however, the drug and antibody generally stayed away from healthy cells and proved to be a potent and precise combination.
In the early 2000s Modi helped to conduct a trial of T-DM1—branded Kadcyla by its maker, Genentech—in people who had an especially difficult disease: advanced HER2-positive breast cancer that had spread throughout the body. Only those who had run out of other treatment options were enrolled. “We were taking people who in some cases were really looking to go to hospice,” Modi says. Yet “almost every patient who was enrolled on that drug had benefits. It was really so satisfying.”
In another trial of about 1,500 people with early breast cancer, an interim data analysis, published in 2019, estimated that 88 percent of those who received T-DM1 would be cancer-free three years later, compared with just 77 percent of those who received Herceptin alone. The drug has proved “more active than most of the therapies we were giving to patients, and it was associated with a better safety profile,” Modi says.
Kadcyla's success against difficult-to-treat cancers didn't just transform some patients' lives. It pumped enthusiasm—and, perhaps more important, pharmaceutical industry dollars—into the idea of ADCs. Researchers now knew that when pieced together correctly, it was possible to load an antibody with drugs too toxic to be used otherwise and still produce a medicine that worked better than traditional chemotherapy.
Several similarly designed ADCs have been approved for a range of different cancer types. Many of these carry drugs that inhibit the enzyme topoisomerase 1, which is essential for DNA replication. Like emtansine, the drug used in Kadcyla, newer topoisomerase inhibitors are too toxic to be used as freestanding drugs but are much less harmful when they're largely restricted to tumor cells. And Kadcyla itself, after being shown to slow or stall late-stage breast cancer, is being tested on patients with very early-stage disease to see whether treatment at that point can not only slow cancer down but actually cure it. Its success “was sort of the catalyst for continued exploration,” Modi says. “Can we build on this? Can we do even better?”
D oing better, it turns out, involves designing good linker molecules that tie the antibody to the drug. These tiny structures act like chemical triggers. They must remain perfectly stable until they reach their target, then unclip from the antibody to discharge their payload at the tumor. Some of the earliest attempts at making ADCs failed not because of their antibodies or drugs but as a result of unstable linkers.
Modern ADCs rely on two types of linkers. One kind remains unbroken even when the ADC reaches its target. The other kind, known as cleavable linkers, are chemicals that break in response to very specific cues, such as enzymes that are abundant in tumors, in the spaces between individual cancer cells. Once an ADC is within the tumor's boundaries, these enzymes cleave the linker and release the drug payload.
Cleavable linkers are showing impressive advantages, and more than 80 percent of currently approved ADCs now use them. An ADC with a noncleavable linker will kill only the cell it attaches to, but one that splits up could place drug molecules near neighboring tumor cells and destroy them as well. This so-called bystander effect can make the drugs much more effective, Thurber says.
Enhertu, for instance, uses the same antibody as Kadcyla but with a cleavable linker (Kadcyla uses a noncleavable version) and a different drug. Each Enhertu antibody carries approximately eight drug molecules, compared with about three per antibody in Kadcyla. In one recent study, researchers compared the effects of these two drugs in people with HER2-positive breast cancers. Enhertu was the clear winner. It stopped tumor growth for more than two years on average, whereas Kadcyla did so for just six months. “It was a landslide in terms of how much better it was,” Tolaney says. “It's a really nice example of how ADC technology leads to dramatic differences in outcomes.”
The bystander effect also explains, in part, why Enhertu is effective against tumors that have barely any HER2: once the ADC enters a tumor and the drug molecules detach, they can kill neighboring tumor cells even if those bystanders don't carry much HER2 on their surface. This action, along with the use of a diagnostic test that can miss extremely low HER2 levels, could explain the results from the trial where the drug seemed to work on tumors with no HER2. That trial employed an assay known as an IHC test. It is generally used to categorize cancers as HER2 positive or negative, not to measure the amount of the protein present. A negative result typically means 10 percent or fewer of the tumor's cells have HER2 on their surfaces. Yet 10 percent may be enough to attract a few Enhertu particles, and the bystander effect might be sufficient to destroy tumor cells, Modi says.
Enhertu is not the only ADC that appears to work this way. In a 2022 study, researchers found that Trodelvy, an ADC that targets a surface protein known as TROP2, seemed to be more effective than standard chemotherapy for people with metastatic triple-negative breast cancer, a particularly hard-to-treat disease. Trodelvy was better irrespective of how much or how little TROP2 was detected on tumors. “That, to me, is wild,” Tolaney says. “We're excited about it because these cancers are having benefits [apparently] without the target.”
This new generation of ADCs is making a difference in other types of cancers previously thought to be intractable, such as metastatic bladder cancer. In 2021 the fda approved Trodelvy and another ADC named Padcev to treat this illness. For 30 years the standard of care for this type of bladder cancer was chemotherapy alone, says oncologist David J. Benjamin, who treats genitourinary cancers at Hoag Family Cancer Institute in southern California. “Now we have multiple new treatments, and two of them happen to be antibody-drug conjugates,” Benjamin says. In clinical trials for patients with advanced bladder cancer, Padcev combined with a drug that stimulates the immune system shrank tumors or stalled their growth in more than 60 percent of people. In a whopping 30 percent of those who received the two-drug combination, their cancer completely disappeared—an unprecedented success.
But even newer ADCs aren't without problems. The bystander effect, which makes them so effective, can spread far enough from the tumor to affect healthy cells, causing hair loss, nausea, diarrhea, fatigue, and other side effects that are disturbingly similar to the fallout of old-school chemo. ADCs also have been linked to a variety of eye problems ranging from conjunctivitis to severe vision loss.
Another explanation for these nasty effects is that there are no protein targets that are exclusive to cancer cells. These proteins, also known as antigens, are more abundant in cancers but may appear in normal cells. That makes some binding of ADCs to healthy cells unavoidable. “I can't think of any examples of true tumor-specific antigens,” says Matthew Vander Heiden, a molecular biologist at the Koch Institute at the Massachusetts Institute of Techonology. Further, ADCs, like any other medicine or antibody, are eventually ingested and metabolized by noncancerous cells. This process fragments them into smaller pieces, releasing payload drugs from their linkers and triggering reactions.
Still, the ability to take ADCs apart and tweak their components—something that isn't possible with traditional treatments—offers researchers the chance to find versions with fewer side effects and more advantages. At present, most ADCs are used at the maximum dose a person can tolerate. That might not be true with future versions. When developing a medication, whether it's a simple painkiller, a chemotherapy or an ADC, researchers begin by figuring out the lowest dose at which the drug is effective. Then they work out the highest dose that people can receive safely. The space between those two doses, known as a therapeutic window, is usually small. But the ability to swap components offers ADC researchers many routes to widening it. Eventually drugmakers might create ADCs so effective that patients never need to take the highest tolerable dose—a much lower one would eliminate tumors without creating unintended consequences such as nausea or hair loss.
Shifting away from toxic chemotherapy-based drugs as payloads could also reduce side effects. Some recently approved ADCs, for instance, link antibodies to drugs that can activate the body's own immune system to attack cancer cells rather than relying on cell-poisoning chemicals. In addition, scientists are exploring ways to deliver radiation therapy directly to tumors by tethering antibodies to radioisotopes. Joshua Z. Drago, an oncologist at MSK Cancer Center, says that with the right kind of linkers, ADCs “could theoretically deliver any kind of small-molecule medication.”
Ultimately, recombined and improved components could lead to the type of swap that cancer patients really care about: exchanging their disease for a cure.
Jyoti Madhusoodanan is a science journalist based in Portland, Ore. She covers health, medicine and the life sciences.

New insights into serine hydroxymethyltransferase aid cancer drug design
- Download PDF Copy
In just two neutron experiments, scientists discovered remarkable details about the function of an enzyme that can aid drug design for aggressive cancers.
The scientists, working at the Department of Energy's Oak Ridge National Laboratory, used neutrons at the Spallation Neutron Source and the High Flux Isotope Reactor to identify exact atomic-scale chemistry in serine hydroxymethyltransferase, or SHMT, a metabolic enzyme necessary for cell division.
Cancer hijacks chemical reactions in the metabolic pathway that involves SHMT and other critical enzymes and turns the entire process into a runaway train, rapidly reproducing cancer cells. Designing an inhibitor to block the enzyme's function, which falls early in the metabolic pathway, could derail cancer's attempts to overtake it. The Royal Society of Chemistry published the team's findings in C hemical Science.
"I think neutrons will be highly sought after in future structure-based drug design," said ORNL's Victoria Drago, the lead author and a biochemist working in collaboration with Andrey Kovalevsky, a distinguished R&D scientist at ORNL, who uses neutron diffraction to illuminate protein structures. "This paper is a good example of how quickly neutrons can produce information that has been the subject of debate for a very long time. Studies on SHMT function and its catalytic mechanism date back to the early 1980s."
The exact catalytic mechanism and the roles of various amino acid residues in the enzyme's active site have been debated for decades. In the current study, the researchers observed that just one amino acid residue, a glutamate, regulates chemical reactions for this enzyme.
The neutron data clearly show that the glutamate, which is an acid, has the proton on it. You might expect it to already have given up its proton. But because it's able to carry that proton around, it can transfer it back and forth. So, it acts as an acid and a base." Robert Phillips, co-author, professor of chemistry, University of Georgia
In a pathway known as one-carbon metabolism, this enzyme works inside a cell's mitochondria, or energy producer. It converts the amino acid serine into another amino acid called glycine by transferring a carbon atom to tetrahydrofolate, a reduced form of folic acid. This reaction produces building blocks for the synthesis of nucleic acids, such as DNA and RNA, and other biological molecules critical to cell division. The glutamate controls this process.
In a prior experiment, the team combined two techniques, neutron and X-ray crystallography at physiologically relevant room temperature, to understand SHMT and to map its protein structure before its interaction with tetrahydrofolate. In the current experiment, the researchers captured the enzyme at the next step, establishing certainty about how the enzyme's reaction mechanism actually works.
Painting the picture with neutrons
Neutrons see light elements, such as hydrogen, and X-rays see heavier elements, such as carbon, nitrogen and oxygen. Neutron diffraction at SNS and HFIR, in-house X-ray diffraction at ORNL and synchrotron X-ray diffraction at Argonne National Laboratory's Advanced Photon Source provided insights the team needed to definitively characterize the enzyme's chemical reaction.
"Neutrons allow us to see hydrogen atoms, and hydrogen drives chemistry," Drago said. "Enzymes are about 50% made up of hydrogen atoms. In terms of electrostatics, hydrogen also carries a positive charge, which dictates the environment of the enzyme. Once you have a crystal that will diffract neutrons, you have everything you need. You see the positions where hydrogens are located and, equally as important, the positions lacking hydrogens. You get the whole picture."
Related Stories
- How nutrition and telomere dynamics shape beauty and aging in women
- COVID-19 reduces male fertility by affecting semen quality and hormone levels
- Natural antioxidants could delay age-related decline in male testosterone production
As shown in the animation, cancer cell mitochondria overproduce the SHMT enzyme, a tetramer constructed from four identical peptide chains, or protomers, shown in gray. SHMT functions by using pyridoxal-5′-phosphate, covalently bound to SHMT, and tetrahydrofolate, shown in gold and purple, respectively. Tetrahydrofolate acts as a substrate that binds to the active sites of all four protomers. The hydrogen atoms, shown flashing in green, revealed the exact catalytic mechanism and the roles of various amino acid residues in the enzyme's active sites. Once the enzyme releases tetrahydrofolate, an inhibitor, shown in blue, could be designed to block further chemical reactions at these sites, arresting the one-carbon metabolic pathway in cancer cells.
"The locations of the hydrogen atoms determine protonation states of specific chemical groups inside the enzyme's active sites," Kovalevsky said. "Thus, they provide information on the electric charge distribution, or electrostatics. This knowledge is crucial to designing small-molecule inhibitors that would bind to SHMT, replacing tetrahydrofolate and halting the enzyme function."
Cells contain thousands of enzymes functioning as catalysts that speed up biochemical reactions needed for bodily functions -; from breathing to producing hormones to nerve function. Enzymes also provide a place to tuck chemicals that target specific processes. Other enzymes in the one-carbon metabolic pathway are already well-known targets for cancer drugs such as methotrexate and fluorouracil. However, SHMT comes earlier in this pathway, presenting an opportunity to stop cancer earlier.
But the difficulties with treating cancer relate in part to its stealthy attacks on metabolic processes. Unlike drug resistance in infectious diseases, if one path does not work well, cancer recalibrates other metabolic processes to overproduce cancer cells.
"Now that we know the atomic details for SHMT, we can inform the design of an inhibitor to target this specific protein as part of a combination therapy," Kovalevsky said. "If you compare it to treating infectious diseases, this is much more difficult because in cancer chemotherapy, you usually target your own proteins, which is why patients experience side effects. In infectious diseases, the proteins you target belong to the viruses or the bacteria. But with cancer, you have to kill your own cells. The idea here is to kill the cancer sooner and have less of an effect on the patient."
Speeding the pace of discovery
The team used neutrons at the MaNDi instrument at SNS and the IMAGINE instrument at HFIR for its research. ORNL's recent Proton Power Upgrade project added stronger beams for all the instruments at SNS. Stronger proton beams mean more neutrons. More neutrons mean shorter data collection times with smaller samples, speeding answers that help the scientists design smarter drugs to treat diseases.
"Discovery research is absolutely essential," said William Nelson, director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. "We're moving ever closer to the space where, with the help of AI, we will be able to sequence a gene in somebody's cancer, predict what the protein structure would look like and make a drug to tuck in; it will work great, and we'll do it in an hour and a half. But we're not there yet. So, the more we know about the actual protein structure, chemical structure and the way things interact, the better we're going to be able train AI models to predict things we don't know right away."
Nelson was not an author of either ORNL-led study. As director of the Sidney Kimmel Comprehensive Cancer Center and professor at the Johns Hopkins School of Medicine, he teaches urology, medicine, pathology, and radiation oncology and molecular radiation sciences.
SNS and HFIR are DOE Office of Science user facilities at ORNL.
Oak Ridge National Laboratory
Drago, V. N., et al . (2024). Universality of critical active site glutamate as an acid–base catalyst in serine hydroxymethyltransferase function. Chemical Science . doi.org/10.1039/d4sc03187c .
Posted in: Medical Research News | Biochemistry | Medical Condition News
Tags: Amino Acid , Bacteria , Breathing , Cancer , Cell , Cell Division , chemical reaction , Chemicals , Chemotherapy , Crystallography , Diffraction , DNA , Drugs , Enzyme , Fluorouracil , Folic Acid , Gene , Glycine , Infectious Diseases , Laboratory , Medicine , Metabolism , Methotrexate , Mitochondria , Molecule , Nerve , Oncology , Oxygen , Pathology , Protein , Research , RNA , Serine , Urology , X-Ray
Suggested Reading

Cancel reply to comment
- Trending Stories
- Latest Interviews
- Top Health Articles

Leveraging the power of automation to boost research
Lukasz Wulnikowski
In this interview, NewsMedical talks to Cerba's Lukasz Wulnikowski about the power of automation and how it has drastically changed the way the company approaches kit building lines.

Automating research - solutions and best practices
Coen Stalpers
In this interview, NewsMedical speaks with Cerba's Coen Staplers about the benefits of lab automation, including the integration of AI technologies.

How can microdialysis benefit drug development
Ilona Vuist
In this interview, discover how Charles River uses the power of microdialysis for drug development as well as CNS therapeutics.

Latest News

Newsletters you may be interested in
Your AI Powered Scientific Assistant
Hi, I'm Azthena, you can trust me to find commercial scientific answers from News-Medical.net.
A few things you need to know before we start. Please read and accept to continue.
- Use of “Azthena” is subject to the terms and conditions of use as set out by OpenAI .
- Content provided on any AZoNetwork sites are subject to the site Terms & Conditions and Privacy Policy .
- Large Language Models can make mistakes. Consider checking important information.
Great. Ask your question.
Azthena may occasionally provide inaccurate responses. Read the full terms .
While we only use edited and approved content for Azthena answers, it may on occasions provide incorrect responses. Please confirm any data provided with the related suppliers or authors. We do not provide medical advice, if you search for medical information you must always consult a medical professional before acting on any information provided.
Your questions, but not your email details will be shared with OpenAI and retained for 30 days in accordance with their privacy principles.
Please do not ask questions that use sensitive or confidential information.
Read the full Terms & Conditions .
Provide Feedback

Login to your account
If you don't remember your password, you can reset it by entering your email address and clicking the Reset Password button. You will then receive an email that contains a secure link for resetting your password
If the address matches a valid account an email will be sent to __email__ with instructions for resetting your password
| Property | Value |
|---|---|
| Status | |
| Version | |
| Ad File | |
| Disable Ads Flag | |
| Environment | |
| Moat Init | |
| Moat Ready | |
| Contextual Ready | |
| Contextual URL | |
| Contextual Initial Segments | |
| Contextual Used Segments | |
| AdUnit | |
| SubAdUnit | |
| Custom Targeting | |
| Ad Events | |
| Invalid Ad Sizes |
- Submit Article
Access provided by
Risks and benefits of anticancer drugs in advanced cancer patients: A systematic review and meta-analysis
Download started
- Download PDF Download PDF
- Randomized trial
- Best supportive care
- Meta-analysis
Evidence before this study
Added value of this study, implications of all the available evidence, 1 introduction, 2.1 search strategy and selection criteria, 2.2 data analysis, 2.3 ethic statement, 2.4 role of the funding source.

| Trials versus placebo | Trials versus BSC | All trials | |
|---|---|---|---|
| No. of trials | 102 | 26 | 128 |
| No. of patients: | 42,037 | 5395 | 47,432 |
| - Experimental arm | 26,047 (62·0%) | 2981 (55·3%) | 29,028 (61·2%) |
| - No active treatment arm | 15,990 (38·0%) | 2414 (44·7%) | 18,404 (38·8%) |
| Sponsor: | |||
| - Academic | 12 (11·8%) | 10 (38·5%) | 22 (17·2%) |
| - Industrial | 90 (88·2%) | 16 (61·5%) | 106 (82·8%) |
| Year of publication: | |||
| - 2000–2009 | 26 (25·5%) | 12 (46·2%) | 38 (29·7%) |
| - 2010–2020 | 76 (74·5%) | 14 (53·8%) | 90 (70·3%) |
| Cross-over allowed | 21 (20·6%) | 3 (11·5%) | 24 (18·8%) |
| Line of treatment: | |||
| - 1st line | 23 (22·5%) | 4 (15·4%) | 27 (21·1%) |
| - ≥ 2nd line | 79 (77·5%) | 22 (84·6%) | 101 (78·9%) |
| Clinical phase of the trial: | |||
| - Phase 2 | 30 (29·4%) | 9 (34·6%) | 39 (30·5%) |
| - Phase 3 | 72 (70·6%) | 17 (65·4%) | 89 (69·5%) |
| Criteria used for efficacy assessment: | |||
| - RECIST | 86 (84·3%) | 15 (57·7%) | 101 (78·9%) |
| - WHO criteria | 8 (7·8%) | 7 (26·9%) | 15 (11·7%) |
| - Other | 2 (2·0%) | 1 (3·8%) | 3 (2·3%) |
| - Not specified | 6 (5·9%) | 3 (11·5%) | 9 (7·0%) |
| Experimental treatment type: | |||
| - Chemotherapy | 6 (5·9%) | 13 (50·0%) | 19 (14·8%) |
| - Hormone therapy | 13 (12·7%) | 1 (3·8%) | 14 (10·9%) |
| - Molecularly targeted agent | 68 (66·7%) | 7 (26·9%) | 75 (58·6%) |
| - Immunotherapy | 15 (14·7%) | 5 (19·2%) | 20 (15·6%) |
| Mode of administration: | |||
| - Intravenous | 12 (11·8%) | 19 (73·1%) | 31 (24·2%) |
| - Oral | 80 (78·4%) | 3 (11·5%) | 83 (64·8%) |
| - Intramuscular | 6 (5·9%) | 1 (3·8%) | 7 (5·5%) |
| - Subcutaneous | 4 (3·9%) | 3 (11·5%) | 7 (5·5%) |
| Tumor type: | |||
| - Hepatocellular carcinoma | 22 (21·6%) | 2 (7·7%) | 24 (18·8%) |
| - Non-small cell lung cancer | 15 (14·7%) | 5 (19·2%) | 20 (15·6%) |
| - Prostate adenocarcinoma | 17 (16·7%) | 0 | 17 (13·3%) |
| - Colorectal cancer | 10 (9·8%) | 4 (15·4%) | 14 (10·9%) |
| - Gastric cancer | 6 (5·9%) | 3 (11·5%) | 9 (7·0%) |
| - Neuroendocrine tumor | 7 (6·9%) | 0 | 7 (5·5%) |
| - Pancreatic adenocarcinoma | 2 (2·0%) | 3 (11·5%) | 5 (3·9%) |
| - Renal cell carcinoma | 5 (4·9%) | 0 | 5 (3·9%) |
| - Thyroid cancer | 5 (4·9%) | 0 | 5 (3·9%) |
| - Mesothelioma | 2 (2·0%) | 3 (11·5%) | 5 (3·9%) |
| - Gastro-intestinal stromal carcinoma | 4 (3·9%) | 1 (3·8%) | 5 (3·9%) |
| - Sarcoma | 3 (2·9%) | 1 (3·8%) | 4 (3·1%) |
| - Urothelial cancer | 0 | 2 (7·7%) | 2 (1·6%) |
| - Biliary cancer | 2 (2·0%) | 0 | 2 (1·6%) |
| - Head and neck squamous cell carcinoma | 0 | 1 (3·8%) | 1 (0·8%) |
| - Small cell lung cancer | 0 | 1 (3·8%) | 1 (0·8%) |
| - Melanoma | 1 (1·0%) | 0 | 1 (0·8%) |
| - Glioblastoma | 1 (1·0%) | 0 | 1 (0·8%) |
| Overall response rate: | |||
| - Experimental arm | 6·7% (1087/16,260) | 9·7% (214/2200) | 7·0% (1301/18,460) |
| - No active treatment arm | 1·2% (117/9367) | 0·7% (13/1810) | 1·2% (130/11,177) |
- Open table in a new tab

| Experimental arm | No active treatment arm | |||||
|---|---|---|---|---|---|---|
| Grade III, IV | Grade V | All | Grade III, IV | Grade V | All | |
| All trials | 33·5% (117 trials) | 7·8% (67 trials) | 77·0% (113 trials) | 23·3% (113 trials) | 7·3% (58 trials) | 63·1% (111 trials) |
| Type of control arm: | ||||||
| - Placebo | 32·8% | 8·0% | 76·7% | 23·0% | 7·6% | 64·6% |
| - Best supportive care | 40·8% | 4·5% | 80·4% | 26·2% | 3·9% | 51·1% |
| Experimental treatment type: | ||||||
| - Chemotherapy | 41·4% | 2·1% | 85·4% | 21·9% | 0·4% | 61·8% |
| - Hormone therapy | 33·5% | 5·9% | 70·4% | 32·5% | 5·3% | 69·6% |
| - Molecular targeted agent | 30·5% | 6·6% | 73·5% | 19·5% | 6·7% | 57·5% |
| - Immunotherapy | 40·0% | 16·4% | 91·5% | 31·1% | 15·2% | 83·9% |
| Year of publication: | ||||||
| - 2000–2009 | 23·0% | 3·5% | 68·0% | 17·6% | 3·9% | 52·4% |
| - 2010–2020 | 37·5% | 8·5% | 80·3% | 25·9% | 8·0% | 67·8% |
4 Discussion
Contributors section, data sharing statement, declaration of competing interest, appendix supplementary materials (2), article metrics.
- Download Hi-res image
- Download .PPT
- Institutional Access: Log in to ScienceDirect
- New Subscriber: Claim access with activation code. New subscribers select Claim to enter your activation code.
The Lancet Choice
Your Account
Manage your account, subscriptions and profile.
MyKomen Health
ShareForCures
In Your Community
In Your Community
View resources and events in your local community.
Change your location:

Susan G. Komen®
One moment can change everything.
What’s New in Breast Cancer
This section gives an overview of new breast cancer treatment breakthroughs and recent developments in research that are fueling new ways to assess risk, and prevent, detect, diagnose and treat breast cancer. Advances in breast cancer care are evaluated through a rigorous process that includes clinical trials and regulatory approvals before being considered standards of care and included in breast cancer care guidelines. Komen’s research team monitors the rapidly evolving breast cancer landscape, and here we will highlight new breast cancer treatment breakthroughs, innovations in technology or key advances that may be added or are new to guidelines. We will share these research advancements to empower patients with knowledge to help them make informed decisions with their doctors.
Use these links to jump to the topics below.
- Emerging Areas in Metastatic Breast Cancer Treatment
- Clinical Trials
Treatments and Drugs
For patients, new treatments can mean more options and more hope. Researchers are working to develop new breast cancer treatment breakthroughs, such as more effective drugs that will specifically target breast cancer cells, minimize side effects and prevent breast cancer cells from coming back. While some treatments increase the effectiveness of existing drugs, others may offer new, innovative strategies for attacking tumor cells.
As of August 2023, the following new treatments and drugs are currently in clinical trials and have not yet received FDA approval:
- A new antibody-drug conjugate called datopotamab deruxtecan (Dato-DXd) is currently being evaluated in three Phase 3 clinical trials for advanced estrogen receptor-positive (ER+) [1] breast cancer, metastatic triple negative [ 2 ] breast cancer and early triple negative [ 3 ] breast cancer (TNBC). Dato-DXd specifically targets a protein called TROP2, a biomarker that can be used to target cancer cells instead of healthy cells. Another TROP2-targeting therapy called sacituzumab govitecan has already been approved for TNBC and estrogen-receptor-positive breast cancer. Dato-DXd uses a different chemotherapy drug and delivery system compared to sacituzumab govitecan.
- People with metastatic estrogen receptor-positive breast cancer that progresses after their initial treatment are prone to developing mutations in the estrogen receptor (ER) gene (ESR1)[ 4 ]. ESR1 mutations cause the ER protein to be constantly active, driving tumor growth even in the presence of drugs designed to stop the ER from working. Lasofoxifene is a new type of hormone therapy being studied that stops the ER even when it’s mutated. Recent findings from the phase 2 ELAINE 2 clinical trial showed lasofoxifene plus the CDK4/6 inhibitor abemeciclib resulted in participants’ cancer remaining stable for a median of 13 months. Based on these results, the new phase 3 ELAINE 3 trial will compare lasofoxifene with the current standard of care fulvestrant (hormone therapy) in combination with a CDK4/6 inhibitor. If successful, patients may have a new hormone therapy option.
- Pembrolizumab is currently the only immunotherapy drug available for people with TNBC . The phase 2 BEGONIA clinical trial recently reported promising results for an immunotherapy drug called durvalumab (Imfinizi) in combination with a TROP-2 targeting antibody-drug conjugate being studied called Dato-DXd [ 5 ]. In this clinical trial, 62 people with metastatic triple-negative breast cancer were treated with this novel drug combination, and their cancer remained stable for a median of 13.8 months. Based on these exciting results, there are now three phase 3 clinical trials testing this drug combination in different breast cancer settings (TROPION-Breast03, TROPION-Breast04, TROPION-Breast05).
New and improved technologies may be able to increase the speed and accuracy of detecting, diagnosing or monitoring breast cancer for progression and response to treatment.
- Doctors may use PET scans, or positron emission tomography, to scan for evidence that breast cancer has spread or metastasized. Once breast cancer has spread, the metastases may have evolved to a different type of breast cancer than the original tumor. These differences mean the metastases and the original tumor may not respond to the same treatments. A diagnostic imaging agent called Cerianna (fluoroestradiol F-18 or FES PET) allows doctors to use PET scans to learn if estrogen receptors are present in metastatic lesions. If a person has metastatic lesions that are estrogen receptor-positive, they may respond well to hormone therapy. This agent was recently incorporated in the National Comprehensive Cancer Network (NCCN) guidelines [ 6 ] as an option for some people with metastatic or recurrent estrogen receptor-positive breast cancer to consider [ 7 ].
- Dormant cancer cells are cells that did not die from a person’s initial treatment. These cells can “hibernate” undetected for unknown reasons until they begin to grow again. The bone is a common place for dormant breast cancer cells to hide and possibly grow. In the phase 2 CLEVER clinical trial [ 8 ], presented at the European Society of Clinical Oncology in October 2023 by Komen Scholar Dr. Angela DeMichele, researchers tested whether they could find dormant cancer cells in participants’ bone marrow and eliminate them. Study results showed researchers were able to find and remove dormant cancer cells from about 80% of the participants. While larger studies will be needed to confirm these results, the CLEVER study shows this promising approach may prevent breast cancer recurrence .
- Doctors are getting closer to identifying which patients with early HER2-positive breast cancer can safely avoid chemotherapy by using the HER2DX genomic test. HER2DX is the first test specifically designed to identify HER2-positive patients at high and low risk for recurrence . For some people, being able to avoid chemotherapy without compromising long-term outcomes will lead to a better quality of life.

Research can take decades to reach the bedside, but what discoveries are just around the corner for patients? Susan G. Komen shares all of this and more through Breast Cancer Breakthroughs, a virtual education series focusing on the new science and technology advancements that are poised to make a difference for patients in the near future. Sign up for Breast Cancer Breakthroughs to never miss an episode.

Kimberly’s Story: Finding Joy in the Midst of a Metastatic Breast Cancer Diagnosis
After Kimberly Reinika’s mother passed away in 2019 from ovarian cancer, she worried that it would ultimately take her life, too. “That was the cancer I was checking for,” she said.
Approaches to Care
With knowledge gained from clinical trials, researchers are seeking new ways to improve patient outcomes while using existing drugs. Some new breast cancer treatment breakthroughs are the result of combining certain drugs, finding which patients can skip certain elements of treatment or changing the order of their treatments to maximize effectiveness or minimize side effects.
- All people with early TNBC are currently treated with immunotherapy and chemotherapy prior to surgery , then they receive immunotherapy for up to 27 weeks after their surgery. A new phase 3 clinical trial called OptimICE-PCR led by Komen Scholar Dr. Sara Tolaney, will test a new approach. The study will determine whether simply observing patients is as effective as getting immunotherapy after surgery in preventing breast cancer from coming back, if their initial treatment successfully got rid of all the breast cancer [ 9 ]. While effective, immunotherapy comes with side effects and may not be needed in some treatment plans. This study will help identify who can safely de-escalate their immunotherapy treatment while maintaining good outcomes.
- Results from the phase 3 DESTINY-Breast06 clinical trial [ 10 ], presented at the American Society of Clinical Oncology (ASCO) meeting in June 2024, showed patients with metastatic estrogen receptor-positive (ER-positive), HER2-low and HER2-ultralow breast cancer had about a 5-month progression-free survival benefit with trastuzumab deruxtecan compared to chemotherapy . HER2-ultralow is a new designation and means that there is a very small amount of detectable HER2 in a tumor. Trastuzumab deruxtecan has already been shown to be effective in HER2-low metastatic breast cancer, but this is the first study showing that people with HER2-ultralow metastatic breast cancer may benefit as well. With these new findings, about 85% of patients with metastatic ER-positive breast cancer may become eligible for this treatment.
- New data from the Young Women’s Breast Cancer Study, led by Komen Chief Scientific Advisor Dr. Ann Partridge, found 73% of women with stage I-III breast cancer who attempted to get pregnant after completing their breast cancer treatment were successful. [ 11 ] This study, presented at the 2024 Annual ASCO meeting, is one of the most comprehensive studies attempting to answer this question to date. The results highlight the importance of making sure women have access to fertility preservation when they begin their breast cancer treatment.
- Komen Scholar Dr. Bryan Schneider conducted the phase 2 EAZ171 clinical trial, which tested whether certain gene mutations could predict the likelihood of developing a side effect from some types of chemotherapy called taxane-induced peripheral neuropathy (TIPN), which is more common among Black women. This side effect causes pain, numbness and tingling in the extremities and can also lead to the treatment being stopped. Komen grantee Dr. Tarah Ballinger presented the results of the EAZ171 study at the 2024 Annual ASCO meeting. [ 12 ] The study found that while the gene mutations were unable to predict the likelihood of developing TIPN, researchers did identify a chemotherapy regimen that resulted in fewer instances of TIPN. These results provide some of the best evidence available to date to personalize chemotherapy treatment for Black women .
Komen will be closely monitoring the results of these studies and more at upcoming scientific conferences and hopes to see more promising data regarding new ways to prevent, detect, diagnose and treat breast cancer.

It Looks Promising: Uncovering New Possibilities in Breast Cancer Prevention
Is breast cancer prevention possible? Komen Scientific Advisory Board Member Dr. Kornelia Polyak is exploring a new strategy to identify and eliminate cell precursors from which tumors can grow.

Help discover cures to breast cancer, faster. New treatment breakthroughs for breast cancer come from researchers learning from people who have breast cancer, but our current data sources only represent a small portion of the breast cancer community. Help us discover the cures to breast cancer, faster, by joining ShareForCures.
What’s New in Breast Cancer References
- https://classic.clinicaltrials.gov/ct2/show/NCT05104866
- https://clinicaltrials.gov/study/NCT05374512
- https://classic.clinicaltrials.gov/ct2/show/NCT05629585
- https://breast-cancer-research.biomedcentral.com/articles/10.1186/s13058-021-01462-3
- https://oncologypro.esmo.org/meeting-resources/esmo-congress/datopotamab-deruxtecan-dato-dxd-durvalumab-d-as-first-line-1l-treatment-for-unresectable-locally-advanced-metastatic-triple-negative-breast
- https://www.gehealthcare.com/about/newsroom/press-releases/ge-healthcare-announces-fes-pet-imaging-recommendation-in-nccn-clinical-practice-guidelines-in-oncology-nccn-guidelines
- https://www.nccn.org/patients/guidelines/content/PDF/breast-invasive-patient.pdf (page 16)
- https://ascopost.com/news/october-2023/novel-strategies-for-eliminating-dormant-tumor-cells-in-breast-cancer-survivors/
- https://www.cancer.gov/research/participate/clinical-trials-search/v?id=NCI-2022-07859&r=1
- https://ascopost.com/news/june-2024/t-dxd-improves-progression-free-survival-in-patients-with-breast-cancer-previously-treated-with-endocrine-therapy/
- https://www.dana-farber.org/newsroom/news-releases/2024/most-young-women-treated-for-breast-cancer-can-have-children-study-shows#:~:text=Most%20young%20women%20treated%20for%20breast%20cancer%20can%20have%20children%2C%20study%20shows,-Posted%20date&text=New%20research%20by%20Dana%2DFarber,and%20want%20to%20have%20children.
- https://www.komen.org/blog/personalized-chemo/
TOOLS & RESOURCES
NEED HELP OR MORE INFORMATION?
1-877 GO KOMEN (1-877-465-6636)
Educational Resources
Komen Financial Assistance Program
Advances in Leukemia Research
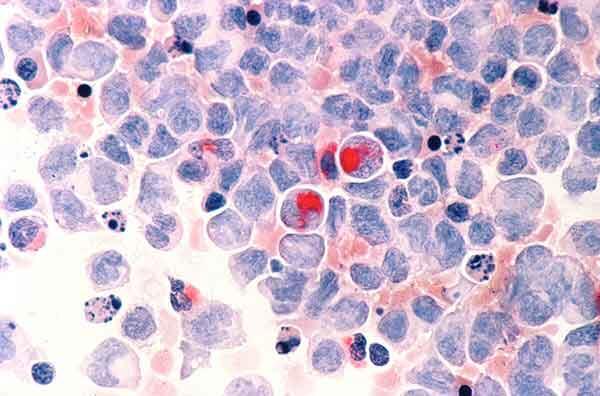
Human cells with acute myelocytic leukemia.
NCI-funded researchers are working to advance our understanding of how to treat leukemia. With progress in both targeted therapies and immunotherapies, leukemia treatment has the potential to become more effective and less toxic.
This page highlights some of the latest research in leukemia, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.
Leukemia Treatment for Adults
The mainstays of leukemia treatment for adults have been chemotherapy , radiation therapy , and stem cell transplantation . Over the last two decades, targeted therapies have also become part of the standard of care for some types of leukemia. These treatments target proteins that control how cancer cells grow, divide, and spread. Different types of leukemia require different combinations of therapies. For a complete list of all currently approved drugs, see Drugs Approved for Leukemia.
Although much progress has been made against some types of leukemia, others still have relatively poor rates of survival. And, as the population ages, there is a greater need for treatment regimens that are more effective and less toxic than standard chemotherapy.
Acute Lymphoblastic Leukemia (ALL) Treatment
Adult acute lymphoblastic leukemia (ALL) is a type of cancer in which the bone marrow makes too many lymphocytes (a type of white blood cell). It usually gets worse quickly and needs rapid treatment. Some recent research includes:
Combining less-toxic therapies
The intensive chemotherapy treatments used for ALL have serious side effects that many older patients cannot tolerate. Targeted therapies may have fewer side effects than chemotherapy. Clinical trials are now testing whether combinations of these types of therapies can be used instead of chemotherapy for older patients with a form of ALL called B-cell ALL.
Immunotherapy
Immunotherapies are treatments that help the body’s immune system fight cancer more effectively. Immunotherapy strategies being used or tested in ALL include:
CAR T-cell therapy
CAR T-cell therapy is a type of treatment in which a patient’s own immune cells are genetically modified to treat their cancer.
- Currently, one type of CAR T cell therapy is approved for the treatment of some children and young adults with B-cell precursor ALL . This CAR T cell therapy is now being explored for use in older adults with B-cell ALL.
- A second CAR T-cell therapy has also been approved for adults with B-cell precursor ALL that has not responded to treatment or has returned after previous treatment.
CAR T cell therapies are now being explored for other uses in ALL. For example, scientists hope that it will be possible to use CAR T-cell therapy to delay—or even replace—stem-cell transplantation in older, frailer patients.
Bispecific T-cell engagers
Another immunotherapy being tested in ALL is bispecific T-cell engagers (BiTEs). These drugs attach to immune cells and cancer cells, enabling the immune cells to easily find and destroy the cancer cell by bringing them closer together.
Once such BiTE, called blinatumomab (Blincyto) , was recently shown to improve survival for people with ALL who are in remission after chemotherapy , even when there is no trace of their disease. In 2024, FDA approved blinatumomab for adult and pediatric patients one month and older with a specific type of B-cell precursor ALL. The approval is for use as part of consolidation chemotherapy, which is treatment that is given after cancer has disappeared following initial therapy.
Improving treatment for adolescents and young adults (AYAs)
An intensive treatment regimen developed for children with ALL has been found to also improve outcomes for newly diagnosed AYA patients . The pediatric regimen more than doubled the median length of time people lived without their cancer returning compared with an adult treatment regimen. Further studies are now testing the addition of targeted therapies to the combination .
Acute Myeloid Leukemia (AML) Treatment
Acute myeloid leukemia (AML) is the most common type of acute leukemia in adults. It can cause a buildup of abnormal red blood cells, white blood cells, or platelets.
AML tends to be aggressive and is harder to treat than ALL. However, AML cells sometimes have gene changes that cause the tumors to grow but can be targeted with new drugs. Researchers are starting to look at whether genomic sequencing of tumor cells can help doctors choose the best treatment (such as chemotherapy, targeted therapy, stem-cell transplant, or a combination of therapies) for each patient. Scientists are also testing other ways to treat AML.
New Treatment Option for Some People with AML
Combining ivosidenib with chemo is effective for AML with an IDH1 gene mutation.
Targeted therapies
Targeted therapies recently approved to treat AML with certain gene changes include Enasidenib (Idhifa) , Olutasidenib (Rezlidhia) , Ivosidenib (Tibsovo) , Venetoclax (Venclexta) , Gemtuzumab ozogamicin (Mylotarg) , Midostaurin (Rydapt) , Gilteritinib (Xospata) , Glasdegib (Daurismo) , and Quizartinib (Vanflyta) .
An NCI-sponsored precision medicine study called MyeloMATCH is now enrolling people with newly diagnosed AML or a related but less aggressive cancer called myelodysplastic syndrome (MDS) . Participants will undergo genomic testing of blood and bone marrow samples to see if they have specific genetic alterations that can be matched to corresponding targeted therapies.
Other ways to treat AML
- Testing newer targeted therapies. Researchers continue to develop new drugs to shut down proteins that some leukemias need to grow. For example, new drugs called menin inhibitors stop cancer-promoting genes from being expressed.
- Studying ways to target AML cells indirectly. These include testing ways to make cancer cells more vulnerable to new and existing treatments.
- Targeting AML and related conditions. MDS can eventually progress to AML. Researchers are testing HDAC inhibitors and other drugs that alter how genes are switched on and off in both MDS and AML.
- Reducing side effects. Some older adults cannot tolerate the intensive treatments most commonly used for AML. Studies have recently found that several drug combinations can help older people with AML live longer while avoiding many serious side effects. New treatments to relieve symptoms of MDS have also been developed.
- Immunotherapy. CAR T-cells and BiTEs are being tested in people with AML.
Chronic Myelogenous Leukemia (CML) Treatment
Chronic myelogenous leukemia (CML) is a type of cancer in which the bone marrow makes too many granulocytes (a type of white blood cell). These granulocytes are abnormal and can build up in the blood and bone marrow so there is less room for healthy white blood cells, red blood cells, and platelets. CML usually gets worse slowly over time.
Blocking an abnormal protein
Most people with CML have a specific chromosome alteration called the Philadelphia chromosome , which produces an abnormal protein that drives the growth of leukemia cells. Targeted therapies that block this abnormal protein— imatinib (Gleevec) , nilotinib (Tasigna) , dasatinib (Sprycel) , and ponatinib (Iclusig) —have radically changed the outlook for people with CML, who now have close to a normal life expectancy.
Testing new combination therapies
Some people with CML continue to have detectable cancer cells in their body even after long-term treatment with drugs that target the protein produced by the Philadelphia chromosome. NCI-sponsored trials are testing whether the addition of immunotherapy or other targeted therapies to these drugs can reduce the number of CML cells in such patients.
Looking at whether patients can stop taking therapy
Researchers have found that some drugs that target the protein produced by the Philadelphia chromosome can be safely stopped in some CML patients rather than taken for life. These patients must undergo regular testing to ensure the disease has not come back.

Chronic Lymphocytic Leukemia (CLL) Treatment
Like ALL, chronic lymphocytic leukemia (CLL) is a type of cancer in which the bone marrow makes too many lymphocytes (a type of white blood cell). But unlike ALL, CLL is slow growing and worsens over time.
Targeted therapy
Ibrutinib (Imbruvica) . The targeted therapy ibrutinib (Imbruvica) was the first non-chemotherapy drug approved to treat CLL. It shuts down a signaling pathway called the B-cell receptor signaling pathway, which is commonly overactive in CLL cells. Depending on people’s age , ibrutinib may be given in combination with another targeted drug, rituximab (Rituxan) .
Clinical trials have shown that ibrutinib benefits both younger and older patients with CLL.
Venetoclax (Venclexta) and obinutuzumab (Gazyva) . In 2019, the Food and Drug Administration (FDA) approved the second chemotherapy-free initial treatment regimen for CLL , containing the targeted therapies venetoclax (Venclexta) and obinutuzumab (Gazyva) .
Other combinations of these drugs plus ibrutinib are now being used or tested for CLL, including • ibrutinib and venetoclax in people with newly diagnosed CLL • ibrutinib, obinutuzumab, and venetoclax in older adults with newly diagnosed CLL • ibrutinib and obinutuzumab with or without venetoclax in younger adults with newly diagnosed CLL
An ongoing trial at NCI is also testing whether giving the combination of venetoclax and obinutuzumab to some people with CLL before symptoms develop can help them live longer overall.
Zanubrutinib (Brukinsa) . In early 2023, the FDA approved a drug that works in a similar manner to ibrutinib, called zanubrutinib (Brukinsa) , for people with CLL. A large study showed that zanubrutinib alone has fewer side effects and is more effective than ibrutinib for people whose leukemia has returned after initial treatment. More research is now needed to understand how to best combine zanubrutinib with other newer therapies, such as venetoclax.
CAR T-cell therapy is also being tested in adults with CLL. Researchers would like to know if using this type of immunotherapy early in the course of treatment would be more effective than waiting until the cancer recurs.
Hairy Cell Leukemia (HCL) Treatment
Hairy cell leukemia (HCL) is a type of cancer in which the bone marrow makes too many lymphocytes (a type of white blood cell). The disease is called hairy cell leukemia because the abnormal lymphocytes look "hairy" when viewed under a microscope. This rare type of leukemia gets worse slowly, or sometimes does not get worse at all.
Combinations of drugs
Researchers are studying combinations of drugs to treat HCL. For example, in a recent small study, a combination of two targeted therapies— vemurafenib (Zelboraf) and rituximab (Rituxan) — led to long-lasting remissions for most participants with HCL that had come back after previous treatments. More drug combinations are currently being tested in clinical trials.
Leukemia Treatment for Children
For the two most common types of leukemia, AML and ALL, standard leukemia treatments for children have been chemotherapy, radiation therapy, and stem-cell transplant. Despite great improvements in survival for children with many types of leukemia, some treatments don't always work. Also, some children later experience a relapse of their disease. Others live with the side effects of chemotherapy and radiation therapy for the rest of their lives, highlighting the need for less toxic treatments.
Now researchers are focusing on targeted drugs and immunotherapies for the treatment of leukemia in children. Newer chemotherapy drugs are also being tested.
Targeted Therapies
Targeted therapies that have been approved or are being studied for children with leukemia include:
- imatinib (Gleevec) and dasatinib (Sprycel), which are approved for the treatment of children with CML as well as those with a specific type of ALL. The approvals are for children whose cancer cells have the Philadelphia chromosome.
- sorafenib (Nexavar) , which has been studied in combination with standard chemotherapy for children with AML whose leukemia has changes in a gene called FLT3. The addition of sorafenib to standard treatment was safe, and its addition may improve survival time free from leukemia. Other ongoing clinical trials are testing drugs that target FLT3 more specifically than sorafenib (such as gilteritinib).
- larotrectinib (Vitrakvi) , which is being tested in children with leukemia that has a specific change in a gene called NTRK .
More possible targets for the treatment of childhood cancers are discovered every year, and many new drugs that could potentially be used to treat cancers that have these targets are being tested through the Pediatric Preclinical In Vivo Testing Consortium (PIVOT) .
CAR T-cell therapy has recently generated great excitement for the treatment of children with relapsed ALL. One CAR T-cell therapy, tisagenlecleucel (Kymriah) , was approved in 2017 for some children with relapsed ALL.
Researchers continue to address remaining challenges about the use of CAR T-cell therapy in children with leukemia:
- Sometimes, leukemia can become resistant to tisagenlecleucel. Researchers in NCI’s Pediatric Oncology Branch have developed CAR T cells that target leukemia cells in a different way. An ongoing clinical trial is testing whether the combination of these two types of CAR T cells can provide longer-lasting remissions.
- CAR T cells are currently only approved for use in leukemia that has relapsed or proved resistant to standard treatment. A clinical trial from the Children's Oncology Group ( COG ) is now testing tisagenlecleucel as part of first-line therapy in children with ALL at high risk of relapse.
- More research is needed to understand which children who receive CAR T cells are at high risk of developing resistance to treatment. Researchers also plan to test whether strategies such as combining CAR T-cell therapy with other immunotherapies may help prevent resistance from developing.
- Other research, both in NCI’s Pediatric Oncology Branch and at other institutions, is focused on creating CAR T-cell therapies that work for children with other types of childhood leukemia, such as AML. Several clinical trials of these treatments, including one led by NCI researchers , are now under way.
Two other drugs that use the body’s immune system to fight cancer have shown promise for children with leukemia:
- In clinical trials, the drug was shown to be more effective than chemotherapy in treating ALL that has relapsed in children and young adults.
- An NCI-sponsored trial is now testing the drug as part of treatment for newly diagnosed ALL in children, adolescents, and young adults.
- A drug called inotuzumab ozogamicin (Besponsa) is being tested in children with relapsed B-cell ALL. This drug consists of an antibody that can bind to cancer cells linked to a drug that can kill those cells. An NCI-sponsored trial is also testing the drug as part of treatment for newly diagnosed ALL in children and adolescents at higher risk of relapse.
Chemotherapy
In addition to targeted therapies and immunotherapies, researchers are also working to develop new chemotherapy drugs for leukemia and find better ways to use existing drugs. In 2018, a large clinical trial showed that adding the drug nelarabine (Arranon) to standard chemotherapy improves survival for children and young adults newly diagnosed with T-cell ALL.
Other drugs are being tested that may make standard chemotherapy drugs more effective. These drugs include venetoclax , which has been approved for older adults with some types of leukemia and is now being tested in children .
Survivorship
Children’s developing brains and bodies can be particularly sensitive to the harmful effects of cancer treatment. Because many children treated for cancer go on to live long lives, they may be dealing with these late effects for decades to come.
The NCI-funded Childhood Cancer Survivor Study , ongoing since 1994, tracks the long-term harmful effects of treatments for childhood cancer and studies ways to minimize these effects. NCI also funds research into addressing ways to help cancer survivors cope with and manage health issues stemming from cancer treatment, as well into altering existing treatment regimens to make them less toxic in the long term.
For example, one study found that, in children with ALL, radiation therapy to prevent the cancer from returning in the brain is likely unnecessary . The study found that radiation can even be omitted for children at the highest risk of the cancer coming back, reducing the risk of future problems with thinking and memory, hormone dysfunction, and other side effects of radiation to the brain.
Preventing and Treating Graft Versus Host Disease
Many people with leukemia—both adults and children—have a stem-cell transplant as part of their treatment. If the new stem cells come from a donor, the immune cells they produce may be able to attack any cancer cells that remain in the body.
But sometimes, immune cells produced by donor stem cells attack healthy tissues of the body instead. This condition, called graft versus host disease ( GVHD ), can affect nearly every organ and can cause many painful and debilitating symptoms.
In recent years, several drugs have been approved by the FDA for the treatment of GVHD, including:
• ibrutinib, which is also used as a treatment for some types of leukemia • ruxolitinib (Jakafi) • belumosudil (Rezurock)
Researchers are also testing ways to prevent GVHD from developing in the first place. For example, a recent study found that removing certain immune cells from donated stem cells before they are transplanted may reduce the risk of chronic GVHD without any apparent increase in the likelihood of relapse.
NCI-Supported Research Programs
Many NCI-funded researchers working at the NIH campus and across the United States and the world are seeking ways to address leukemia more effectively. Some research is basic, exploring questions as diverse as the biological underpinnings of cancer. And some is more clinical, seeking to translate this basic information into improving patient outcomes. The programs listed below are a small sampling of NCI’s research efforts in leukemia.
NCI’s Leukemia Specialized Programs of Research Excellence (SPORE) promotes collaborative, interdisciplinary research. SPORE grants involve both basic and clinical/applied scientists working together. They support the efficient movement of basic scientific findings into clinical settings, as well as studies to determine the biological basis for observations made in individuals with cancer or in populations at risk for cancer.
The Targeting Fusion Oncoproteins in Childhood Cancers (TFCC) Network is forming a collaborative team of investigators to advance the understanding of how fusion proteins contribute to pediatric cancers, and how they might be targeted with new treatments. Fusion proteins, which can occur when parts of different chromosomal regions are joined, may drive the development of many cancers in children.
NCI has also formed partnerships with the pharmaceutical industry, academic institutions, and individual investigators for the early clinical evaluation of innovative cancer therapies. The Experimental Therapeutics Clinical Trials Network (ETCTN) was created to evaluate these therapies using a coordinated, collaborative approach to early-phase clinical trials.
The Pediatric Early-Phase Clinical Trials Network was established to help identify and develop effective new drugs for children and adolescents with cancer. The network’s focus is on phase I and early phase II trials, as well as pilot studies of novel drugs and treatment regimens to determine their tolerability.
NCI’s Pediatric Preclinical In Vivo Testing Consortium (PIVOT) develops mouse models to allow early, rapid testing of new drugs for pediatric cancers, including leukemia. The models are all derived from tissue samples taken from patients’ tumors. The consortium partners both with commercial drug companies and with drug development efforts at universities and cancer centers.
The NCI-supported Children’s Oncology Group develops and conducts both clinical trials of initial treatments and clinical trials for after cancer relapse for children and adolescents with ALL, AML, and CML.
Researchers in NCI’s Division of Cancer Epidemiology and Genetics (DCEG) investigate novel, molecular biomarkers for leukemia, as well as clarify relationships of established risk factors. Studies include those looking at environmental and workplace exposure, families with multiple leukemia cases, and inherited bone marrow failure syndromes to name a few.
Clinical Trials
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Search NCI-Supported Clinical Trials to find leukemia-related trials now accepting patients.
Leukemia Research Results
The following are some of our latest news articles on leukemia research:
- Quizartinib Approval Adds New Treatment Option for AML, Including in Older Patients
- Blinatumomab Increases Survival for Infants with an Aggressive Type of ALL
- Revumenib Shows Promise in Treating Advanced Acute Myeloid Leukemia
- Help Desk for Oncologists Treating People with a Rare Leukemia Pays Big Dividends
- Zanubrutinib’s Approval Improves Targeted Treatment for CLL
- Trial Suggests Expanded Role for Blinatumomab in Treating ALL
View the full list of Leukemia Research Results and Study Updates .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NEWS FEATURE
- 25 October 2022
Cancer drugs are closing in on some of the deadliest mutations
- Heidi Ledford
You can also search for this author in PubMed Google Scholar
Chemists have struggled to design drugs that interact with the KRAS protein’s relatively smooth surface. Credit: Alamy
When Terri Conneran, a mother-of-three and a former corporate accountant in Charlotte, North Carolina, was diagnosed with lung cancer in 2017, she sought support from people going through the same thing. A handful of groups had been springing up on social media based on the specific mutations found in people’s tumours.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 610 , 620-622 (2022)
doi: https://doi.org/10.1038/d41586-022-03392-2
Skoulidis, F. et al. N. Engl. J. Med. 384 , 2371–2381 (2021).
Article PubMed Google Scholar
Fakih, M. G. et al. Lancet Oncol. 23 , 115–124 (2022).
Ostrem, J. M., Peters, U., Sos, M. L., Wells, J. A. & Shokat, K. M. Nature 503 , 548–551 (2013).
Wang, X. et al. J. Med. Chem. 65 , 3123–3133 (2022).
Canon, J. et al. Nature 575 , 217–223 (2019).
Zhang, Z. et al. Cancer Cell 40 , 1060–1069 (2022).
Download references
Reprints and permissions
Related Articles
- Biotechnology
- Drug discovery
Childhood leukaemia in Down’s syndrome primed by blood-cell bias
News & Views 25 SEP 24
The type 2 cytokine Fc–IL-4 revitalizes exhausted CD8+ T cells against cancer
Article 25 SEP 24

Single-cell CAR T atlas reveals type 2 function in 8-year leukaemia remission

Pain: recognizing the power of non-pharmaceutical interventions
Outlook 25 SEP 24

Could biomarkers mean better pain treatment?
Jekyll and Hyde flip of the script when bacteria invert gene sequences

Revolutionary drug for schizophrenia wins US approval
News 27 SEP 24

Pain researchers must learn from the opioid crisis
Why do obesity drugs seem to treat so many other ailments?
News Feature 25 SEP 24
Independent Group Leader Positions in Computational and/or Experimental Medical Systems Biology
NIMSB is recruiting up to 4 Independent Group Leaders in Computational and/or Experimental Medical Systems Biology
Greater Lisbon - Portugal
NOVA - NIMSB
CZS Junior Group Leader for “Molecular Systems Engineering/DNA Nanoscience”
Johannes Gutenberg University Mainz (JGU) is one of the largest universities in Germany. Thanks to its location in the Rhine-Main science region, t...
Mainz, Rheinland-Pfalz (DE)
Johannes Gutenberg University Mainz (JGU)
Position Recruitment of Vice Dean
We are seeking talents for Vice Dean Positions.
Shenzhen, Guangdong, China
Shenzhen University of Advanced Technology
The recruitment for Earth Science High-talent in IDSSE, CAS
Seeking global talents in the field of Earth Science and Ocean Engineering.
Sanya, Hainan, China
Institute of Deep-sea Science and Engineering, Chinese Academy of Sciences
Locum Chief Editor, Nature Structural & Molecular Biology
Job Title: Locum Chief Editor, Nature Structural & Molecular Biology Location: New York or Milan Closing date: Friday, October 18th This job is of...
New York City, New York (US)
Springer Nature Ltd
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Men get breast cancer, too. But they can't always access new drugs
Breast cancer is widely researched, but some men suffering from the illness say they can’t benefit.

Social Sharing
This story is part of CBC Health's Second Opinion, a weekly analysis of health and medical science news emailed to subscribers on Saturday mornings. If you haven't subscribed yet, you can do that by clicking here .
Warren Kotler has outlived his prognosis.
Eight years ago, Kotler was told he had three to five years to live. The diagnosis: Stage 4 metastatic breast cancer. It's a common illness among women, but a rare one among men, who account for only one per cent of cases.
Kotler, 61, has received a mix of drug treatments and several courses of radiation therapy. His quality of life is excellent, he said: he got married two years ago. He travels often. He regularly goes on long bike rides.
Despite that, the Toronto man knows the cancer could eventually outsmart his treatments. The plan he and his medical team developed: "Stick around long enough. There's new drugs that are going to be coming on, and hopefully those will be of benefit," Kotler said.
This summer, his oncologist suggested a new drug: capivasertib, sold as Truqap.
Clinical studies suggest the drug, which was approved in Canada in January 2024, could hold off the cancer from progressing for several months for patients with a type of advanced breast cancer known as HR positive, HER2-negative. These cancers respond to hormone-therapy drugs and do not have abnormal levels of the protein HER2, which can accelerate tumour growth. The drug stops the cancer from growing by blocking AKT – one of the enzymes needed for cell growth.

But Truqap is expensive. Canada's Drug Agency says it costs around $10,000 for a 28-day supply. And while Kotler is able to get provincial help for some of his other pricey drugs through Ontario's Trillium Drug Program, Truqap is not covered by the program.
Kotler's medical team has asked the drug manufacturer to cover the cost of the drug on a compassionate basis through a patient support program it runs. AstraZeneca said it cannot.
The reason? Health Canada has only approved the drug for women.
Too few men in study, says Health Canada
Some other jurisdictions — the United States and the European Union — have approved the use of the drug for both men and women following a clinical trial.
When CBC News asked Health Canada about the decision, the department pointed to its regulatory decision summary for the drug, which says too few men were involved in the Phase 3 clinical study : seven out of around 700 participants.
For those men, the drug appeared to stop the cancer from getting worse for about two months — compared to around seven months for the entire study population. Health Canada raised concerns over the toxicity of the drug, including side effects like diarrhea, rash and nausea.
While side effects are a part of many treatments, there is a weighing of risk and benefit — and whether medications used at the end stages of cancer meaningfully improve survival and quality of life. A group of cancer doctors in North America say medications with marginal benefits are being overused for patients who are nearing the end of their lives. They say ultimately whether a patient decides if a drug is worth taking is a deeply personal choice, and one that should come after an honest conversation about the reality of what a drug can do.
But Canada's Drug Agency, an independent non-profit organization that provides objective evidence to healthcare decision-makers, came to a different conclusion than Health Canada in the case of Truqap.
In a reimbursement review for Truqap, its expert review committee said the drug should be reimbursed for all adult patients — with conditions. The proportion of men in the study, it said, reflects the rate of breast cancer among men, and because there were so few men involved in the study, it was impossible to say for sure the drug would be less effective in male patients.
For Kotler, who is no stranger to the side effects of cancer drugs, it's about having the option.
"In terms of quality of life, absolutely, I want to make an informed choice," he said.
"I don't have a choice with Truqap. It's not available to me."

Accessing new breast cancer treatments a challenge for some male patients
'it makes no sense' says cancer doctor.
Dr. Philippe Bedard, Kotler's oncologist at Toronto's Princess Margaret Cancer Centre, thinks the drug could be an effective option for some patients like Kotler.
"It's very frustrating," he said.
"Historically, men have been excluded from these types of clinical trials that test new drugs. And what we've learned is that the disease biology is really similar in men and women."
Breast cancer is a rare illness among male patients.The Canadian Cancer Society estimates 290 men will be diagnosed with the disease this year and 60 will die, compared to some 28,000 women diagnosed and 5,500 who die from breast cancer each year in Canada.
Because of how rare the disease is among men, it could take much longer to gather the same amount of data for male patients as for women. In the case of Ibrance, another drug used to treat hormone receptor-positive, HER2-negative breast cancer, Health Canada expanded the drug approval for men three years after the drug was approved for women — after examining data based on the real-world use of the drug among male patients.
Dr. Gerald Batist, the director of the Segal Cancer Centre at Montreal's Jewish General Hospital, says in situations like these, regulatory bodies need to use "some common sense and scientific reasoning."
"It's a very unusual cancer in men. But they do behave very much like breast cancers in women. We treat them very much like breast cancers in women. So it makes no sense," he said.
In this case, Batist thinks flexibility is warranted, even as Health Canada balances risk and benefit.
"There's a lack of data, but I think we have to recognize there's a little bit of a limitation because of the numbers of the incidence in this. We have to look around the world and see other expert panels, regulatory agencies that have approved this drug," he said.
- Is extending life by weeks worth the toll some cancer drugs take? Doctors push for 'common-sense oncology'
"They don't want to expose anyone to undue toxicity. On the other hand, we're at a point where we want more access to better drugs that will help people, and that's very urgent."
An urgency patients like Kotler know well.
"I need to continue. I have a lot to do. I have a big list. I have three kids," he said.
ABOUT THE AUTHOR

Senior Health Reporter
Jennifer Yoon covers the latest health news for CBC News on television, radio and digital. You can reach her at [email protected].
Related Stories
- Doctor calls for changes after patient's $700 ultrasound quote
- New Brunswick introduces liquid biopsy option for lung cancer patients
Add some “good” to your morning and evening.
A vital dose of the week's news in health and medicine, from CBC Health. Delivered to your inbox every Saturday morning.
This site is protected by reCAPTCHA and the Google Privacy Policy and Google Terms of Service apply.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pharmaceuticals (Basel)

Special Issue “Anticancer Drugs 2021”
Associated data.
Data is contained in the article.
This Special Issue of Pharmaceuticals is devoted to significant advances achieved in the field of Anticancer Drugs in 2021. Recent findings and trends in the design, synthesis and mechanism of action and therapeutic applications of anticancer drugs are presented. These research studies demonstrate the relevance of medicinal chemistry and the pharmaceutical sciences in cancer research. The research illustrates the exciting opportunities that contemporary drug design offers for the discovery of new therapies and diagnostics for cancer and offers perspectives on the future directions of anticancer therapeutics. Resistance to anticancer drugs has become a major threat to the success of chemotherapeutic agents, and therefore the discovery and development of new anticancer drugs for clinical use is extremely challenging. Investigations into anticancer drugs covers a vast area of research and includes natural products, design and synthesis of new molecular entities, molecular modelling, computational techniques and development of molecular and biochemical tests. Heterocycles are well represented in this collection with the inclusion of novel compounds targeting kinases, tubulin, thymidylate synthase, histone deacetylase, HER2 and apoptosis-related proteins. Original research into amelioration of toxicity associated with current chemotherapeutics and resistance is also featured.
In 2020, the FDA approved 18 new cancer drugs, including the HER2-directed margetuximab, sacituzumab govitecan [a TROP2-targeted antibody–drug conjugate (ADC) for triple-negative breast cancer] and the BCMA-targeted ADC belantamab mafodotin for multiple myeloma. Among the kinase inhibitors approved were the HER2 kinase inhibitor tucatinib, together with the RET kinase inhibitors selpercatinib and pralsetinib with indication for RET fusion-positive NSCLC. Lurbinectedin, approved for multiple myeloma, covalently binds to the DNA minor groove. Despite the continued impact of COVID-19, 15 new cancer drugs were approved by the FDA in 2021. The allosteric inhibitor sotorasib targets KRAS-G12C mutated NSCLC, while the novel allosteric HIF-2α inhibitor belzutivan targets von Hippel-Lindau tumours. Dostarlimab, a PD1/PDL1-targeted antibody for endometrial cancer was approved, together with the ADCs loncastuximab teserine, a CD19-targeted ADC for B-cell lymphomas, and tisotumab vedotin, a tissue targeted ADC approved for cervical cancer. The bispecific antibody amivantamab targeting EGFR and MET gained approval for small molecule-resistant NSCLC, the kinase inhibitor mobocertinib selectively inhibits EGFR in NSCLC and the kinase inhibitor asciminib was approved for Philadelphia chromosome-positive CML. Several BCMA-targeted CAR-T cell therapies were also approved in 2021.
Protein kinase inhibitors (PKIs) are clinically significant drugs in the treatment of cancer and inflammatory diseases, with over 535 reported PKs and over 70 PKIs approved by the FDA. Imran et al. have reviewed the USFDA PKI patent approvals for the period 2001 to 31 May 2021 and have provided a comprehensive timeline depicting the PKI approvals, molecular structures, primary targets and approved indications [ 1 ]. Availability of generic PKI drugs in the USA market is also discussed together with the development of PKIs with structurally varied scaffolds, chemotypes and pharmacophores. The development of larotrectinib and entrectinib as tissue-agnostic anti-cancer tropomycin receptor kinase (Trk) inhibitors is reviewed by Han [ 2 ]. In clinical trials with larotrectinib and entrectinib in patients with a wide range of tumour types with various types of Trk fusion, clinical benefits were observed indicating tumour-agnostic activity. It is concluded that the adoption of the tissue-agnostic approach has accelerated the clinical development of Trk inhibitors.
The simultaneous inhibition of multiple protein kinases targets involved in cancer progression is a possible route to increasing potency and overcoming resistance. Many multi-kinase inhibitors occupy only the hinge and hydrophobic region in the ATP binding site. Mashelkar et al. designed multi-kinase inhibitors that occupy the ribose pocket, along with the hinge and hydrophobic region [ 3 ] and identified a novel 4′-thionucleoside with potent anticancer activity and marked inhibition of TRKA, CK1δ, and DYRK1A/1B kinases, with potential for developing anti-cancer drugs. Previtali et al. reported the anti-cancer mechanism of a novel 3,4′-substituted diaryl guanidinium compound that inhibits BRAF through a hypothetical type-III allosteric mechanism [ 4 ]. Following a docking study using an active triphosphate-containing BRAF protein, a variety of structural modifications were evaluated in leukaemia, breast, cervical and colorectal carcinoma cell lines with pro-apoptotic effects. A divergent effect on inhibition of MAPK/ERK signalling pathway was demonstrated, confirming that diaryl guanidinium compounds are excellent hit molecules for new anticancer therapies. Elrayess et al. reported a series of thieno[2,3-d][1,2,3]triazine and acetamide derivatives as dual epidermal growth factor receptor (EGFR) and human EGFR-related receptor 2 (HER2) inhibitors targeting non-small cell lung cancer (NSCLC) [ 5 ]. The lead compound was cytotoxic at nanomolar levels in the H1299 cell line, with activity against EGFR and HER2 comparable to imatinib and was identified as a promising agent for NSCLC.
Ibrahim et al. investigated the design and synthesis of a series of dual targeting hybrid molecules by combining histone deacetylase (HDAC) inhibition with epidermal growth factor receptor (EGFR-TK) inhibition [ 6 ]. The novel hydroxamic acid hybrids were cytotoxic in cancer cell lines, proapoptotic, showed increased expression of caspases 3/8 and Bax and down-regulation in Bcl-2 and inhibition of both EGFR and HDAC1 enzymes. Balbuena-Rebolledo identified several FDA-approved drugs as potential inhibitors of the intracellular domain of epidermal growth factor receptor 1 (EGFR) and human epidermal receptor 2 (HER2) which are important targets for cancer drugs [ 7 ]. FDA-approved drugs with similar structures to lapatinib and gefitinib were identified in the DrugBank. Docking and molecular dynamics simulations on the selected compounds identified interactions with the ligand-binding sites of EGFR and HER2, without interaction with residues involved in drug resistance; cytotoxicity was confirmed in breast cancer cell lines. These repurposed compounds may offer possible new anticancer treatments by targeting HER2 and EGFR.
Sorafenib is an orally administered kinase inhibitor used to treat advanced hepatocellular and renal cell cancer. Inconsistencies in treatment efficacy and tolerability may be attributed to variability in sorafenib exposure over time. Ruanglertboon et al. developed a concentration-guided sorafenib dosing protocol to increase the proportion of patients that achieve a sorafenib C max within the required range by using a model to simulate sorafenib exposure [ 8 ].
Nagy et al. investigated the inhibition of Bcl-2 as a promising strategy for cancer treatment [ 9 ]. Benzimidazole and indole-containing analogues of the Bcl-2 inhibitor obatoclax were designed by introduction of alkylamine or carboxyhydrazine methylene linkers to facilitate improved hydrophobic Bcl-2 binding. Anti-cancer activity was confirmed in MDA-MB-231 (breast cancer) and A549 (lung adenocarcinoma) cells with significantly upregulated expression of pro-apoptotic Bax and caspase-3, -8 and -9, and downregulation of anti-apoptotic Bcl-2. Espadinha et al. reported indole-based tryptophanol-derived polycyclic compounds as activators of the tumour suppressor protein p53, a therapeutic target in many cancers [ 10 ]. A novel series of enantiomerically pure tryptophanol-derived small molecules was optimised, and absolute configuration established by X-ray analysis. These compounds target human gastric adenocarcinoma (AGS) cells while mediating apoptosis via increase in caspase 3/7 activity. In vitro stability and metabolic studies identified potent lead compounds for further studies. Stecoza et al. have developed a series of new 2,5-diaryl/heteroaryl-1,3,4-oxadiazoles as novel chemotherapeutic agents [ 11 ]. Following evaluation in human colon and breast cancer cell lines, STAT3 and miR-21 are suggested as the most probable targets for these compounds suggesting future studies to improve the anticancer profile and to reduce the toxicological risks.
The nuclear export receptor exportin-1 (XPO1, CRM1) is a relevant target in haematological malignancies. The XPO1 inhibitor leptomycin B interacts with XPO1 by covalent interaction with Cys528. Gargantilla et al. synthesised a series of chalcones designed to react with XPO1 thiol groups via hetero-Michael addition reactions [ 12 ]. Reactions of selected chalcones with GSH demonstrated potential reversible covalent interaction with XPO1 thiols. Good correlation was observed in antiproliferative assays with cancer cell lines and as XPO1 inhibitors. Thymidylate synthase (TS) is an established target in cancer treatment, as it is directly involved in DNA synthesis. Alam et al. developed potential chemotherapeutic hybrid compounds containing 1,2,3-triazole and 1,3,4-oxadiazole heterocycles [ 13 ]. Evaluation for inhibition of breast and human colorectal carcinoma demonstrated superior activity to tamoxifen and 5-fluorouracil, with inhibition of thymidylate synthase enzyme superior to pemetrexed. The DNA repair enzyme tyrosyl-DNA-phosphodiesterase 1 (TDP1) acts by removal of TOP1-DNA adducts stabilized by TOP1 inhibitors. The combination of a terpene resin acid backbone with an adamantane fragment as a DNA repair inhibitor was reported by Kolaleva et al. [ 14 ]. The linker type and length, diterpene and adamantane moieties were optimised. The synthesized compounds were effective inhibitors of TDP1 while molecular modelling indicated that the TDP1 intermediate (covalent complex of TDP1 with DNA) may be stabilised as observed for topoisomerase−DNA covalent complexes by camptothecins. The highly glycosylated transmembrane mucin (MUC) proteins are over-expressed in different types of cancers and are both promising cancer therapeutic targets and also biomarkers for human cancer. Current efforts to develop MUC1- and MUC16-targeted cancer therapies include antibody-based therapeutics, small molecule inhibitors, vaccines and cell therapies. Lee et al. have comprehensively reviewed the various therapeutic agents targeting mucins which are under different stages of clinical trial for several cancers [ 15 ].
The synthesis and biochemical evaluation of novel hybrids of the microtubule targeting benzophenone phenstatin and the aromatase triazole inhibitor letrozole are reported by Ana et al. [ 16 ]. The compounds demonstrated potency in MCF-7 and MDA-MB-231 breast cancer cells, together with significant G 2 /M phase cell cycle arrest, induction of apoptosis, inhibition of tubulin polymerisation and selective inhibition of aromatase. These hybrids are promising candidates for development as antiproliferative, aromatase inhibitory and microtubule-disrupting agents for breast cancer. The antitumour activity of hybrid compounds based on the structure of combretastatin A-4 and 2,3-diphenyl-2 H -indazole has been evaluated by Perez-Villanueva et al [ 17 ]. Selected hybrid compounds possess significant cytotoxic activity superior to cisplatin against HeLa and SK-LU-1 cells, with similar potency to imatinib against K562 cells, inhibited tubulin polymerisation and induced G 2 /M arrest. Balandis et al. synthesized a series of new imidazole derivatives incorporating a novel benzenesulfonamide moiety [ 18 ]. Evaluation against MDA-MB-231 breast cancer and human malignant melanoma (IGR39) cell identified the optimal aryl and imidazole substitution required. A core pharmacophore for the design of anticancer compounds against aggressive and invasive cancers such as malignant melanoma and triple-negative breast cancer was identified. 1,3-Disubstituted derivatives of urea and thiourea are reported to possess antiproliferative properties against various solid and leukaemia tumour cell lines. Strzyga-Lach et al. have developed a series of selective 3-(trifluoromethyl)phenylthiourea analogues with selective cytotoxic effects against human colon, prostate and leukaemia cell lines [ 19 ]. The most potent compounds showed pro-apoptotic activity and inhibited release of the cytokine IL-6 in the colon SW480 and SW620 cells lines. The anticancer effects of xanthones may be attributed to caspase activation, DNA cross-linking, inhibition of kinases and topoisomerase. Recent advances in the discovery of xanthone derivatives with anticancer activity, both isolated from natural sources and synthetic examples are reviewed by Kurniawan et al., together with potential further developments of active, selective and efficient anticancer drugs based on xanthone derivatives [ 20 ].
Toxicity and drug resistance remains a challenging issue in cancer drug development. The type II topoisomerase inhibitor mitoxantrone (MTX) is used in to treat several cancers and refractory multiple sclerosis. Reis-Mendes et al. investigated the cardiotoxicity of MTX to determine if inflammation or oxidative stress-related pathways are involved [ 21 ]. Histopathology results indicated that MTX caused cardiotoxicity while inflammation may be an important trigger to MTX-induced cardiotoxicity in adult mice, with increased expression of NF-κB p65, NF-κB p52 and TNF-α with decrease in IL-6. The widely used DNA alkylating agent cyclophosphamide (CPX) causes toxic effects in the urogenital system. Merwid-Ląd examined the effect of morin-5′-sulfonic acid sodium salt (NaMSA) on CPX-induced urogenital toxicity in rats by histological evaluation, morphological changes and relative decrease in sperm count [ 22 ]. Co-administration of NaMSA reversed most of the morphological changes and may attenuate CPX-induced histological changes in the urogenital tract. The nephroprotective effects of the beta-adrenergic antagonist carvedilol on CPX-induced urotoxicity was also examined [ 23 ]. When co-administered with mesna, carvedilol improved kidney function and reversed histological abnormalities in bladders, presumably via antioxidant and anti-inflammatory effects. Multidrug-Resistant (MDR) cancers modulate chemotherapeutic efficacy through drug efflux. Pulukuri et al. investigated P-glycoprotein 1 mediated efflux of Toll-Like Receptor (TLR 7/8) agonist immunotherapies [ 24 ]. The imidazoquinoline TLR agonists imiquimod, resiquimod and gardiquimod are P-gp substrates. Imidazoquinoline efflux occurs through P-gp and is enhanced for imiquimod due to acquired drug resistance. This enhancement could be beneficial for modulating the activity of tumour-infiltrating immune cells in local proximity to cancer cells
The research presented in this Special Issue contains contributions which are focussed on cancer drug discovery together with important reviews in specific areas of cancer therapeutics. This Special Issue highlights both the challenges and opportunities in the discovery and development of both novel small-molecule cancer drugs and applications of innovative cancer therapies and demonstrates the direction and potential for future research in these areas.
Acknowledgments
We thank the authors for their hard work to produce an up-to-date and comprehensive Issue on anticancer drugs.
Author Contributions
M.J.M. and N.M.O. compiled the editorial manuscript and M.J.M. submitted the manuscript. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

- Artificial intelligence, automation and robotics

Elnur - stock.adobe.com
Medtech startup brings Oracle AI to bear on cancer drug research
Learn how oracle cloud infrastructure and its cutting-edge ai features are bringing new benefits to cancer care by helping doctors identify better treatment options.

- Alex Scroxton, Security Editor
In recent years, human ingenuity and scientific know-how has developed advanced drugs promising relief and even recovery to millions of people worldwide diagnosed and living with previously incurable forms of cancer.
Historically, identifying the active ingredient for candidate drugs is not an easy process. Drug discovery often drew on old knowledge of traditional folk medicine – drugs such as aspirin began in this way, or came about by happy accident, such as in the case of penicillin, the first identified antibiotic, which was spotted by accident on a mouldy petri dish in 1928.
A century after Alexander Fleming’s chance encounter with penicillium chrysogenum, modern-day drug discovery is big business, with a large and ever-growing pharmaceutical industry pouring billions of dollars into the process, often backed by governments – as was the case with the first Covid-19 vaccines – but the process is still hard and dogged by inefficiencies.
What if there was a better way? With the technology being developed and used at Imagene AI , an Israel-based startup, we may be on the verge of big change. Imagene and its founder and CEO Dean Bitan are hoping to kickstart the process of drug discovery and find new ways to help oncologists provide the best care possible by bringing artificial intelligence (AI) capabilities courtesy of Oracle to bear on the scourge of cancer.
Bitan is a lifelong technologist who earned his degree in computer science at the tender age of 15. But it was some years later when he became interested in the field of cancer research. Cancer is a disease that will touch the lives of almost everybody on the planet in some way during their lifetimes, and in Bitan’s case, he sadly lost a close relative to it.
Sitting down with Computer Weekly on the fringes of Oracle Cloud World in Las Vegas , Bitan says: “I was interested in technology and entrepreneurship and that was my field. Initially, I had no relationship to cancer.
“But when it happened, I learned a lot about the disease, and I learned about the gaps and what might we do better…There are not always enough opportunities in terms of drugs – we see many cancer types where we don’t have enough drugs to offer patients.
“So that’s the story of Imagene, I decided to get into that field and I really wanted to assist physicians to better navigate the diagnostics and treatment decisions,” says Bitan.
Is AI the answer?
Did Bitan always have an inkling that AI might offer a path forward? He says he started considering the possibility very early on in the research that Imagene grew from.
“The assumption was that we might be able to leverage technology into that area. [But] I’m an engineer. And talking with physicians, there’s a different mindset,” says Bitan.
“What we did was to sit together and think – we know what the challenge is, how can we do it better? Some physicians had told me, before we established the company, that they have a strong sense of intuition when they look at a biopsy image.
“They say they can identify patterns that are probably related to the presence of biomarkers indicating whether a patient will respond, or not, to a specific drug. They build that up over many years of practice and observing their patients, but that is something that can be enhanced…Intuition and AI go very well together. So, we understood that we would try to see if we could discover more information out of those biopsy images.”
Bitan and his team leveraged 630,000 anonymised biopsy images taken from multiple cancers at multiple sites in the body, and from there developed a foundation model to deliver what he now describes as “oncology intelligence”.
This 1.1 billion parameter foundation model is called CanvOI. At its heart, it captures the complex features and patterns in biopsy images that a human might never see unaided to enhance a researcher’s understanding of various pathological features and derive new understanding from that.
The ultimate idea is to deliver a “robust vision data backbone” for the development of downstream applications in oncology research. This doesn’t just have to apply to identifying new drugs, it can also predict how people with various different biomarkers might respond to them, eventually enabling frontline physicians to deliver bespoke cancer care based on their patients’ unique physiologies.
OCI powers CanvOI
Imagene’s model runs on Oracle Cloud Infrastructure (OCI), taking advantage of OCI AI Infrastructure and OCI Supercluster, which can scale to tens of thousands of GPUs right now for AI inference , and will be able to tick over 130,000 in the very near future.
Bitan says that by applying Oracle’s compute capabilities and Imagene’s new approach to digital pathology foundation models, CanvOI is already achieving industry-leading performance in its various tasks, even when using minimally labelled data.
“Oracle is in a unique place to support AI companies in those challenges. So, when we talk about computing power, the fact that they had that strategic agreement with Nvidia allows us to get more availability of GPUs. And more availability of GPUs means more computing power and it’s better for us,” he says.
“We need companies like Oracle to go with us on this long journey because the challenges are real, and we want to continue and show more milestones related to this. With ChatGPT and LLMs we saw how the technology, in less than two years, moved from elementary school level to high school level, and now they are talking about PhD expert level. We want to see similar stuff in the world of oncology, and we’ve done it with biopsy images, but as we move forward, we will add more models.”
More widely, CanvOI forms the cornerstone of the firm’s new OISuite, a platform designed to support researchers and diagnostics developers and enable them to explore answers to a wide range of questions needed to conduct their research. Bitan says this alleviates the need for AI expertise and data acquisition, enabling new breakthroughs while still adhering to the highest possible standards of data privacy and security. On which note, says Bitan, all the data used by Imagene’s systems is de-identified in advance.
“And of course,” he continues, “we work based on the highest standards of GDPR, HIPAA compliance, etcetera. That much is obvious, but besides that, we are also working on zero-trust approaches, we run vulnerability scans, we encrypt data at rest and in transit, despite the fact it is de-identified.”
Future goals
Imagene is already working with medical institutions in multiple countries, including world-renowned US research facilities such as Johns Hopkins in Baltimore, and Northwestern University in Chicago, as well as leading cancer centres in Brazil and Israel. Bitan wants to go further, to bring in not only academic medical centres, but private and reference labs as well.
“In the field of cancer research, we don’t have the privilege not to do what we can because every day is important,” he says.
Looking further ahead, Bitan says he sees opportunities to apply the technology developed at Imagene to other areas of medical research as well, such as Covid-19 or HIV/AIDS.
“We will go towards those areas as we move forward. You will see more and more models that aggregate different modalities – so not only biopsy images, we could maybe add radiology, MRIs and X-rays. Or microbiomes or maybe even genome sequencing. Then we will be able to answer much more complex questions related to different aspects of healthcare,” he concludes hopefully.
Read more from Oracle OpenWorld 2024
- Oracle opens up its annual CloudWorld event with a plethora of announcements, launching a partnership with cloud giant AWS and expanding previously announced tie-ups with Google Cloud and Microsoft Azure .
- Oracle’s leadership was in celebratory mood opening its annual CloudWorld jamboree, and with its announcement of a new cloud partnership with AWS, multicloud futures were high on everyone’s agenda .
- Oracle delivered more AI features at its conference, including over 50 AI agents and a new generative AI RAG Agent in OCI .
Read more on Artificial intelligence, automation and robotics

MRI-based machine learning approach predicts breast cancer metastasis

NIH-supported researchers enhance urine tests for prostate cancers

Deep learning tool predicts brain metastasis in lung cancer patients

Machine Learning, ‘Liquid Biopsy’ to Bolster Early Cancer Detection
The U.S. Commerce Department's proposal to ban software and hardware for connected vehicles imported from China or Russia might ...
Apple joined a list of big tech companies voluntarily committing to responsible AI. But without regulation, there is no legal ...
Since the U.S. lacks an overarching AI policy, insiders worry that existing AI harms aren't being addressed and that artificial ...
The Institute for Security and Technology's Ransomware Task Force says a shift to big game hunting tactics led to a significant ...
Three vulnerabilities in Ivanti products have come under attack by unknown threat actors in recent weeks, including two flaws in ...
CrowdStrike changed the way it rolls out content updates as a result of the global IT outage caused by a faulty update in July.
CI/CD processes help deploy code changes to networks. Integrating a CI/CD pipeline into automation makes networks more reliable, ...
Predictive analytics can project network traffic flows, predict future trends and reduce latency. However, tools continue to ...
Test scripts are the heart of any job in pyATS. Best practices for test scripts include proper structure, API integration and the...
Hock Tan unscored the importance of customers at the Boston VMware User Group UserCon, but users remain uneasy about the recent ...
Extreme heat and inadequate cooling systems can lead to power failures in data centers. Calculate the duration of your UPS ...
Intel's turnaround efforts are progressing, but the chipmaker will need more than government funding and a deal that expands its ...
The vendor's latest set of capabilities includes prebuilt applications and automated features aimed at making it easier to use ...
Metadata management tools can range from comprehensive packages of many features to masters of a specific niche. Consider 9 of ...
The vendor's new feature aims to aid organizations that deal with sensitive data by enabling them to manage pipelines from ...
- MTS Biological Sciences Developments in Oncology Over the Last Year Showing Promise for Cancer Patients and Others
- Laboratory Technology
- Natural & Clinical Science
- Medical IT & Administrative
- Patient-Facing Technology
- Cytologist (Cytotechnologist)
- Dental Lab Technician
- Histotechnologist
- Medical Lab Assistant
- Medical Lab Technician
- Biological Sciences
- Biomedical Science
- Biotechnology
- Health Sciences
- Infection Preventionist
- Medical Laboratory Scientist
- Nutritionist & Dietitian
- Pathologists' Assistant (PathA)
- Pre-Vet (Veterinarian)
- Biomedical Equipment Technician
- Biomedical Informatics
- Health Informatics
- Health Information Management
- Health Information Technology
- Healthcare Administration
- Medical Billing & Coding
- Nursing Informatics
- Sterile Processing Technician
- Anesthesia Technician & Technologist
- Audiologist & SLP
- Cardiovascular Technologist
- Dental Assistant
- Dental Hygienist
- Diagnostic Medical Sonographer
- Dialysis Technician
- EKG Technician
- EMT & Paramedic
- Kinesiologist
- Mammography Technologist
- Medical Assistant
- MRI Technologist
- Neurodiagnostic Technologist
- Nuclear Medicine Technologist
- Ophthalmic Technician
- Pharmacy Technician
- Phlebotomist
- Physical Therapist Assistant & Aide
- Psychiatric & Mental Health Technician
- Radiation Therapist
- Radiologic Technologist
- Rehabilitation Technician
- Respiratory Therapist
- Surgical Technologist
Certification Guides
Career guides, interviews & features, developments in oncology over the last year showing promise for cancer patients and others, search for schools.
When you click on a sponsoring school or program advertised on our site, or fill out a form to request information from a sponsoring school, we may earn a commission. View our advertising disclosure for more details.
Leaps forward in tech and science this past year promise greater accessibility and quality of care for Americans suffering from nearly all forms of cancer.
MedicalTechnologySchools.com analyzed academic studies and resources from leading cancer research institutions, including the Mayo Clinic and the Cancer Research Institute , to round up the latest advancements in oncology—the science of diagnosing, preventing, and treating various forms of cancer.
Evidence of cancer dates back to at least 1600 BC when medical records in Egyptian hieroglyphics described surgical procedures and an understanding of benign and malignant tumors. The modern science of oncology, which we now appreciate for its ability to greatly extend life expectancies for those living with cancer, began in earnest a little more than 100 years ago.
Around the turn of the 20th century, Marie Curie’s work in chemistry and radiology, or the science of X-rays and radiation, was fundamental to the evolution of cancer research. Dedicated medical personnel such as medical laboratory scientists continued this work, with the assistance of support staff such as radiation therapists and radiologic technologists . In the post-World War II years, advancements in computer technology allowed truly modern techniques for diagnosis and treatment to flourish, including robot-assisted, noninvasive surgery and targeted therapies that don’t harm healthy parts of the body.
An estimated 650,000 people receive chemotherapy or radiation therapy every year. New developments are underway by medical technology firms and researchers that have the potential to improve the effects of radiation therapy or bypass its need altogether for the millions of people diagnosed with cancer every year.
Artificial Intelligence Applications in Cancer Detection and Treatment
Aspects of computer science algorithms have been put to work detecting cancer and pinpointing more effective forms of treatment for about the last 20 years, though research in the area is becoming more popular surrounding the launch of powerful and accessible generative AI tools in late 2022. Mentions of AI in cancer research have appeared in more than 1,000 research journal publications each year since 2022.
One of the more recent research findings is the potential for AI to do a better job than humans in spotting hard-to-identify signs of emerging colon and rectal cancer, the second most common cause of death from cancer and the number one cause for men under 50 . The study adds to a growing body of research suggesting AI algorithms can improve early detection, one of the best-known ways to beat colorectal cancer .
Genome Sequencing Opens up the Potential for More Targeted Treatments
Genome sequencing , or the mapping of the genetic tissues within the human body, has evolved since the 1980s as a tool for personalizing health care treatments rather than applying potentially less-effective blanket treatments.
Cancers involve mutations of the cell that can vary greatly from case to case. In January, researchers published the largest whole-genome sequencing study of its kind. The study included data on nearly 14,000 tumors, which the authors believe will impact how doctors decide on treatments and therapies for cancer patients.
The Promise of Pre-chemotherapy Surgery
Pancreatic cancer is among the most aggressive forms of cancer with one of the lowest survival rates. Even when caught early, the average patient survives just three-and-a-half years , according to Johns Hopkins.
A minimally invasive surgery performed before recently diagnosed pancreatic cancer patients begin chemotherapy has shown signs of helping doctors better treat it before it spreads, according to a study published last July in the Journal of the American College of Surgeons.
Reducing the Need for Mastectomy Procedures
Another study published in the Journal of Clinical Oncology could provide hope for those diagnosed with breast cancer who have multiple tumors in one or more of their breasts.
Typically, doctors might advise a person with multiple breast tumors to pursue a mastectomy, whereby the breast tissue is removed through surgery. The study demonstrated that tumor removal combined with radiation treatment can be effective at removing the cancer and preventing any recurrence while avoiding a full mastectomy.
“Some patients may still prefer or require a mastectomy, and that is a perfectly fine approach,” Mayo Clinic surgeon and lead author of the study Dr. Judy Boughey said in a statement last year. “But being able to provide more patients diagnosed with breast cancer with a choice is a great step forward.”
Less Invasive Treatments for Head, Neck Cancers
Advancements in proton beam therapy promise to provide a more targeted approach to treatment for people suffering from head and neck cancers located near vital body parts, like the brain and spinal cord.
While not typically a painful procedure, few centers offer proton therapy treatment, according to Johns Hopkins. This past year, regulators approved using a new proton therapy medical device made by Israeli firm P-Cure, which produces a system compact enough to fit into existing radiation therapy rooms .
Precise Biology-guided Radiation for Bone and Lung Cancer Therapy
Lung cancer causes one in five deaths among all forms of cancer and is the leading cause of all cancer deaths. This past year, top cancer centers, including the University of Texas Southwestern Medical Center, rolled out new biology-guided radiation technology to help target multiple cancerous areas within the affected body parts while not damaging healthy surrounding tissue. Using radioactivity, the treatment causes cancer cells to produce a signal that they can target with radiation beams.

Dom DiFurio is a writer and researcher whose work has been published in The Washington Post, USA Today, and ESPN Magazine . Additionally, he’s been featured in local and regional newsrooms across the country. Notably, he has been recognized by the Society for Advancing Business Editing and Writing, the Texas Associated Press Managing Editors, and Columbia University for his work.
Related Articles
- Online Biochemistry Degree Programs
- Online Degrees in Neuroscience - Bachelor’s, Master’s
- Online Degree Programs in Genetics
- Online Master’s Programs in Molecular Biology & Diagnostic Science
- Online Biology Degree Programs
Related Programs
New drug shows promise in slowing growth of bowel cancer
- 20 September 2021
A new drug has shown promise in slowing the regrowth of tumours among some bowel cancer patients, according to new findings of a major trial funded by an NIHR and MRC partnership and run by researchers at UCL in collaboration with Oxford, Leeds and Cardiff universities.
The results of the FOCUS4-C trial, which was funded by the EME Programme - an NIHR and MRC partnership, Cancer Research UK and AstraZeneca, was presented on Saturday (18 September) at the European Society of Medical Oncology and published in the Journal of Clinical Oncology .
The trial looked at whether a drug called adavosertib, taken in the form of a daily pill, could delay tumour regrowth among patients with an aggressive sub-type of inoperable bowel cancer who have limited treatment options.
Comparing 44 patients who took adavosertib with 25 patients who did not, the researchers found that the drug delayed tumour growth by about two months on average and had relatively few side effects. The drug had more effect in the 31 patients with left-sided/rectal tumours, increasing overall survival – that is, patients lived longer.
The researchers caution that these are early results and that larger trials are needed to establish whether the drug improves survival compared to standard treatment.
The trial tested adavosertib among patients who were on a treatment break following chemotherapy but the drug could potentially benefit patients with other types of bowel cancer or alongside standard treatments in other lines of therapy.
The subset of patients who took part in the trial had tumours with two common mutations, RAS and TP53, that the researchers hypothesised would make the tumours more sensitive to the effects of the drug. About a third of all colorectal cancer patients have tumours with these two mutations.
Bowel cancer is the fourth most common cancer in the UK and the second biggest cancer killer. Over 42,000 people are diagnosed with bowel cancer every year in the UK.
Lead author Dr Jenny Seligmann, of the University of Leeds, said: “These results show promising signs that adavosertib may be effective in delaying re-growth of bowel cancer in some patients and is well tolerated. The findings are particularly encouraging as the subset of patients involved represent a third of all bowel cancer patients and, while other patients have treatments developed specifically for their tumour types, this group currently has very limited treatment options.”
The findings come from one part of a large collaborative UK trial called FOCUS4 which aimed to investigate the best ways to help people with inoperable bowel cancer who have already received some chemotherapy.
More than 1,400 bowel cancer patients took part in the FOCUS4 trial programme. Blood samples and tumours were analysed and some of those enrolled took part in additional randomised controlled trials that tested new drugs in people whose cancer had particular chemical changes that suggested those drugs might be effective.
Professor Nick Lemoine, Medical Director of the NIHR Clinical Research Network said: "Defining the key molecular changes that drive a particular individual's cancer will allow the right patient to receive the right drug at the right time. The early results of this trial for bowel cancer patients suggest that detecting changes in two genes could allow selection for active treatment with a well-tolerated drug given by mouth. Clinical trials such as FOCUS4 allow multiple drugs to be tested in parallel arms of the study, speeding up the time to build convincing evidence for their use in routine NHS practice in the future."
Adavosertib kills cancer cells by inhibiting WEE1, a protein that helps to regulate the process of cell division in the tumour by ensuring that any DNA damage is repaired before cells divide.
Researchers believed that tumours with the mutations RAS and TP53 would be particularly sensitive to this form of attack, as these mutations have already placed the process of cell replication under stress.
FOCUS4 chief investigator, Professor Tim Maughan, of the University of Oxford, said: “WEE1 inhibitors target the DNA repair process in tumour cells. A similar strategy is used to treat some ovarian and breast cancers with drugs called PARP inhibitors. However, this is the first time this strategy has been successfully used to treat bowel cancer.”
Side effects of the drug included fatigue, diarrhoea, neutropenia (involving low levels of white blood cells called neutrophils), and nausea, but none of these occurred in more than 11% of patients.
A second new study from a separate part of the FOCUS4 trial called FOCUS4-N , also published in the Journal of Clinical Oncology , looked at outcomes among patients who had a complete break from treatment following chemotherapy, comparing them to outcomes among those who continued chemotherapy using a simpler tablet called capecitabine.
The researchers found that, among those who had a complete break, the cancer started to grow somewhat sooner than in those on continued maintenance therapy, but that maintenance therapy did not lead to an increase in how long people lived.
Lead author Professor Richard Adams, of Cardiff University, said: “The findings will help to inform discussions between patients and clinicians about treatment options at the end of four months of therapy - that is, whether to stay on oral chemotherapy long-term or have a complete break in treatment – giving patients better control of their cancer management.”
Read more about this trial on our project page .
Share this page
Latest news.

New findings on the use of molnupiravir to treat COVID-19

New funding opportunities for novel brain tumour research launched

ADHD digital test approved for use by the NHS
Explore UCD
- (opens in a new window) University Strategy
- University Governance
- President's Office
- Equality, Diversity & Inclusion
- Campus Development
- Course Catalogue
- Study at UCD
- Current Students
- Campus Accommodation
- International Student Experience
- Access & Lifelong Learning
- Careers Network
- Sports Clubs
- (opens in a new window) Student Societies
Research & Innovation
- Innovation at NovaUCD
- Graduate Studies
- Support for Researchers
- (opens in a new window) Find a UCD Researcher
- UCD College of Arts and Humanities
- UCD College of Business
- UCD College of Engineering and Architecture
- UCD College of Health and Agricultural Sciences
- UCD College of Science
- UCD College of Social Sciences and Law
- All Colleges and Schools
- News & Opinion
- Work at UCD
- UCD in the Community
- Global Partnerships
- (opens in a new window) UCD Foundation
- University Relations
Key Services
- Staff Directory
- Sport & Fitness
- IT Services
- (opens in a new window) Commuting
- (opens in a new window) UCD Map
- (opens in a new window)
- UCD Ulysses Medal for US oncologist Dr Dennis Slamon who developed breast cancer drug Herceptin
Friday, 27 September, 2024
Posted 27 September, 2024
Dr Dennis Slamon with his UCD Ulysses, received for his decades of pioneering breast cancer research Credit: Peter Houlihan / Fennell Photography
An American oncologist credited with saving the lives of hundreds of thousands of women worldwide by transforming the treatment of breast cancer, Dr Dennis Slamon has received the UCD Ulysses Medal from University College Dublin .
In presenting Dr Slamon with the Ulysses Medal, UCD recognises that his pursuit of novel therapies for breast cancer for over 30 years have shaped the field of precision medicine and paved the way for other targeted therapies in oncology.
“Dr Slamon is one of the most inspirational medical research scientists at work today,” said UCD President Professor Orla Feely .
“His pioneering research resulted in the groundbreaking development of the breast cancer drug Herceptin which has proven to be a lifesaving therapy for women with HER2-positive breast cancer.
“This therapy has saved the lives of hundreds of thousands of breast cancer patients worldwide, including an estimated 1,000 women in Ireland,” added President Feely.
“Each and every day, several thousand women around the world begin potentially life-saving and life-prolonging treatments for breast cancer directly linked to Dr Slamon’s scientific discoveries - highlighting the incredible difference that scientific endeavour can make to our world.”
“Dr Slamon is a most worthy recipient of the UCD Ulysses Medal, the highest honour that University College Dublin can bestow,” she continued.

Dr Slamon with his UCD Ulysses alongside UCD President Professor Orla Feely Credit: Peter Houlihan / Fennell Photography
The breast cancer drug Herceptin developed from the work of Dr Salmon is the first gene-based drug approved by the US Food and Drug Administration to fight cancer. It is now a cornerstone in the treatment of HER2-positive breast cancer.
In spearheading its lengthy development, Dr Slamon forged a close relationship with Professor John Crown, a Consultant Medical Oncologist at St Vincent's Hospital in Dublin, who encouraged Ireland's participation in the early Herceptin trials.
Since then, hundreds of Irish women have taken part in clinical breast cancer trials led by Slamon and the All-Ireland Cooperative Oncology Research group. According to Professor Crown, who delivered the citation at the award ceremony, Dr Slamon’s “contribution to cancer research is unparalleled.”
“His early research which identified the critical importance of the HER2 gene in patients with the most aggressive type of Breast cancer was initially greeted with widespread scepticism.
“However, the brilliance of his work and the doggedness of his determination succeeded in proving the importance of his findings. Drugs which have been developed as a result of this research have revolutionised the treatment of many patients with Breast cancer, and more recently other cancers.”

Dr Slamon forged a close relationship with Professor John Crown, who delivered the citation at the award ceremony, during Ireland's participation in the early Herceptin trials Credit: Peter Houlihan / Fennell Photography
“In addition, he has done pioneering research in the area of oestrogen receptor positive Breast cancer; research which led to the development of an entirely new class of anti-cancer drugs, the CDK46 inhibitors,” he said. “Dr Slamon has been a great friend to Ireland. He has a long collaboration with St. Vincent’s Hospital Oncology Unit, UCD and DCU, mentoring some of our brightest young Irish cancer researchers. “This Ulysses Award, the latest of many international recognitions of his contribution, is richly deserved.” Dr Slamon serves as director of the Revlon/UCLA Women's Cancer Research Program at the UCLA Jonsson Comprehensive Cancer Centre. He is also chief of the Division of Hematology/Oncology and executive vice chair for research for UCLA's Department of Medicine. He is now co-ordinating the worldwide NATALEE study in which ribociclib is being tested as a component of the treatment for earlier stage oestrogen receptor positive breast cancer. Between this and HER2 altered breast cancer, approximately 85% of all metastatic breast cancer patients now will receive treatments which derived originally from his work. A 1975 honours graduate of the University of Chicago's Pritzker School of Medicine, Dr Slamon has received nearly two dozen research awards including Lasker-DeBakey Clinical Medical Research Award and the Szent-Györgyi Prize for Progress in Cancer Research. He also won the Sjöberg Prize from the Royal Swedish Academy of Sciences and Sweden's Sjöberg Foundation, two awards that have historically been significantly predictive of subsequently winning the Nobel prize.

Numerous faculty and staff from the UCD School of Medicine, and elsewhere, came to the Ulysses Medal ceremony in recognition of Dr Slamon's work Credit: Peter Houlihan / Fennell Photography The UCD Ulysses Medal is the highest honour that the university can bestow, and was inaugurated in 2005, as part of the university’s 150th anniversary celebrations, to highlight the ‘creative brilliance’ of its most famous alumnus, James Joyce.
It is awarded to individuals whose work has made an outstanding global contribution.
Previous recipients of the Ulysses Medal include Godfather of AI, Professor Geoffrey Hinton (2024); Booker Prize winning Canadian novelist, Margaret Atwood (2018); and world leading philosopher and social theorist, Professor Jürgen Habermas (2010).
By: David Kearns , Digital Journalist / Media Officer, UCD University Relations
To contact the UCD News & Content Team, email: [email protected]
- New eLetter in Science debunks Meta-funded study suggesting its news-feed algorithms are not major drivers of misinformation
- Study shows that ocean waves can grow beyond known limits
Most shared
- UCD creative writing graduate wins Publishing Ireland’s inaugural Breakthrough Award
- UCD honorary degrees for Oscar nominated Irish language filmmakers
- UCD alum named FBD’s Young Farmer of the Year
UCD academics on The Conversation
- Opinion: Complaints are different when customers think a company cares
- Opinion: The leap year is February 29, not December 32 due to a Roman calendar quirk – and fastidious medieval monks
- Opinion: Nigeria’s ban on alcohol sold in small sachets will help tackle underage drinking


IMAGES
VIDEO
COMMENTS
In a very small trial done by doctors at New York's Memorial Sloan Kettering Cancer Center, patients took a drug called dostarlimab for six months. The trial resulted in every single one of their ...
Medical advances are accelerating the battle against cancer. Here are 11 recent developments. 1. Personalized cancer vaccines. Thousands of NHS cancer patients in England could soon access trials of a new vaccine treatment. It's designed to prime the immune system to target cancer cells and reduce recurrence risk.
The Food and Drug Administration on Friday approved a new cancer therapy that could one day transform the way a majority of aggressive and advanced tumors are treated. The treatment, called ...
The research was funded, in part, by the National Institutes of Health, the Mazumdar-Shaw International Oncology Fellowship, the MIT Center for Precision Cancer Medicine, and the Charles and Marjorie Holloway Foundation. ... Cancer biologists identify new drug combo. A boost for cancer immunotherapy. Fighting cancer with the power of immunity ...
People with cancer who take immunotherapy drugs often develop skin side effects, including itching and painful rashes. New research in mice suggests these side effects may be caused by the immune system attacking new bacterial colonies on the skin. Implanted "Drug Factories" Deliver Cancer Treatment Directly to Tumors.
A new, systematic analysis of cancer cells identifies 370 candidate priority drug targets across 27 cancer types, including breast, lung and ovarian cancers. By looking at multiple layers of ...
Targeted therapies have swiftly taken a prominent position in cancer research and clinical oncology in recent decades, thanks to the molecular insights into oncogenic processes and mechanisms ...
The drug, named Enhertu, extended the lives of people with breast cancer by several months, sometimes longer. And it did so with fewer severe side effects than standard chemotherapies. The U.S ...
By Levi Gadye. UC San Francisco researchers have designed a candidate drug that could help make pancreatic cancer, which is almost always fatal, a treatable, perhaps even curable, condition. The new drug candidate permanently modifies a wily cancer-causing mutation, called K-Ras G12D, that is responsible for nearly half of all pancreatic cancer ...
Cancer remains one of the greatest health threats globally. In 2020, there were an estimated 19.3 million new diagnoses of cancer and 10 million deaths. 1 More than 50% of incident cases occurred in low- and middle-income countries (LMICs), where patients face disproportionately high incidence to mortality ratios. By 2040, it is estimated that the number of patients with an indication for ...
Nanomedicine is a new multidisciplinary field that is garnering a lot of interest and investigation. Nanomedicine shows great potential for cancer diagnosis and treatment.
Researchers should focus on a new approach to cancer treatment involving the pathophysiology of the diseases, the discovery of human genome sequence, and novel molecular targets that kill cancer cells. 3. The management of cancer requires pharmacologic and non-pharmacologic treatment algorism like surgery, radiation, and chemotherapy, depending ...
Among the array of new cancer therapeutics approved by the US Food and Drug Administration (FDA) in 2023, patients stood to benefit substantially, notably with momelotinib (Ojjaara), a JAK ...
Organoid biology will further develop with a goal of translating the research into personalized therapy. These research areas may result in the creation of new cancer treatments in the future. Keywords: exosomes, immunotherapy, microbiome, organoid. Cancer research has made remarkable progress and new discoveries are beginning to be made.
New trends are evolving in the drug discovery paradigm that includes designing new derivatives of old drugs with high efficacy and lower toxicities. 269, 270 Several natural compounds isolated from microbial, plants, or natural sources were suggested to hold enormous potential to target stress response pathways by mounting a hyperactive ...
The oral medication works by targeting PARP, an enzyme in the body that helps to repair damaged cells — including cancer cells. By inhibiting PARP, the drug stops the repair of cancerous cells to prevent them from growing. The drug's approvals in 2014, 2018 and 2022 were based on clinical trials led or co-led by Susan Domchek, MD, executive ...
More neutrons mean shorter data collection times with smaller samples, speeding answers that help the scientists design smarter drugs to treat diseases. "Discovery research is absolutely essential ...
Background: Randomized clinical trials (RCTs) of anticancer drugs without active comparators in patients who have exhausted standard of care treatment options are debated. We aimed to quantify the safety and the efficacy of anticancer drugs in advanced cancer patients who have exhausted standard of care treatments from RCTs without active comparators.Methods: This systematic review and meta ...
The results could pave the way for Pfizer's drug ponsegromab to become the first-ever approved treatment specifically for cancer cachexia.
Learn about new breast cancer treatment breakthroughs and recent developments in research that are fueling new ways to assess risk, prevent, detect and diagnose breast cancer. ... researchers are seeking new ways to improve patient outcomes while using existing drugs. Some new breast cancer treatment breakthroughs are the result of combining ...
Persistent symptoms led to a shocking diagnosis of stage 4 colorectal cancer that had already spread to her liver. She had cancer treatment and eventually a liver transplant thanks to a living organ donation from her brother in 2020. The cancer subsided, but then returned — this time, to her lungs.
NCI-funded researchers are working to advance our understanding of how to treat leukemia. With progress in both targeted therapies and immunotherapies, leukemia treatment has the potential to become more effective and less toxic. This page highlights some of the latest research in leukemia, including clinical advances that may soon translate ...
WHIPPANY, N.J., September 26, 2024 - Bayer today announced the submission of a supplemental new drug application (sNDA) to the U.S. Food and Drug Administration (FDA) for the oral androgen receptor inhibitor (ARi) NUBEQA® (darolutamide) in combination with androgen deprivation therapy (ADT) for the treatment of patients with metastatic hormone-sensitive prostate cancer (mHSPC).
Such mutations are particularly prevalent in some of the deadliest cancers: more than 80% of pancreatic cancers carry a KRAS mutation, for example, as do roughly 30% of lung adenocarcinomas and ...
Breast cancer is one of the most-researched illnesses in the world. But some men suffering from the disease say they're not able to benefit from new research and treatments.
Data Availability Statement. This Special Issue of Pharmaceuticals is devoted to significant advances achieved in the field of Anticancer Drugs in 2021. Recent findings and trends in the design, synthesis and mechanism of action and therapeutic applications of anticancer drugs are presented. These research studies demonstrate the relevance of ...
In recent years, human ingenuity and scientific know-how has developed advanced drugs promising relief and even recovery to millions of people worldwide diagnosed and living with previously ...
Mentions of AI in cancer research have appeared in more than 1,000 research journal publications each year since 2022. One of the more recent research findings is the potential for AI to do a better job than humans in spotting hard-to-identify signs of emerging colon and rectal cancer, the second most common cause of death from cancer and the ...
More than 1,400 bowel cancer patients took part in the FOCUS4 trial programme. Blood samples and tumours were analysed and some of those enrolled took part in additional randomised controlled trials that tested new drugs in people whose cancer had particular chemical changes that suggested those drugs might be effective.
"His pioneering research resulted in the groundbreaking development of the breast cancer drug Herceptin which has proven to be a lifesaving therapy for women with HER2-positive breast cancer. "This therapy has saved the lives of hundreds of thousands of breast cancer patients worldwide, including an estimated 1,000 women in Ireland ...