
- My presentations

Auth with social network:
Download presentation
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you!
Presentation is loading. Please wait.
CHAPTER 1 Physical Quantities, Units and Measurement.
Published by Andrea Moore Modified over 8 years ago
Similar presentations
Presentation on theme: "CHAPTER 1 Physical Quantities, Units and Measurement."— Presentation transcript:

The Système International SI Units GEL2006 More free powerpoints at

Mefemetics! By Murphski, Papa, and Mogi. What are the 7 base SI units? Base quantityNameSymbol lengthmeterm masskilogramkg timeseconds electric currentampereA.

CHEMISTRY 161 Chapter 1. Chemistry arabic: al-kimya greek: khymeia latin: chimica ‘FUSION’ ~ 3,500 BC.

Unit Conversion Review
Quantitative information (qualitative data would be descriptions of your observations). Measurements represent quantities (something that has magnitude,

Chapter 1 Legacy On Units of Measure. The Metric System In the past, many different units were used to measure things (ex. Foot, cubit, furlong, fathom)

Unit Conversions and Dimensional Analysis. Measurements in physics - SI Standards (fundamental units) Fundamental units: length – meter (m) time – second.

MEASUREMENT. TYPES QUALITATIVE –RESULTS ARE DESCRIPTIVE (NONNUMERIC: BIG, SMALL, PINCH, ETC.)

Introduction to Chemistry. No eating or drinking! Wear goggles at all times! Use common sense!

Phys141 Principles of Physical Science Chapter 1 Measurement Instructor: Li Ma Office: NBC 126 Phone: (713) Webpage:

Length Volume Mass Temperature Time Pressure Energy Amount of a Substance Luminous Intensity Electric Current Meters (m) Liters (L) Kilograms (kg) Kelvin.

Derived Units Foreign Language? Units We discussed standard units like the meter for distance, seconds for time, and gram for mass Today we.

Units of Measurement.
Measurements Integrated Science Chapter 2 Section 2 Pages

Originally developed during the French Revolution Refined by the French Academy of Sciences in 1791 in a group that included Lavoisier and.

Unit 2 Chapters 3 & 4. Review Qualitative measurement Qualitative measurement Uses descriptive wordsUses descriptive words Quantitative measurement Quantitative.

October 7, 2009 IOT POLY ENGINEERING I1-25 DRILL: A.LIST 5 BASE UNITS AND DESCRIBE WHAT THEY ARE USED TO MEASURE. B.LIST 5 DERIVED UNITS AND DESCRIBE WHAT.

AGENDA Unit 1 Measurement and Matter Test 1/24/12 Notes/handouts Elements Quiz #1-20 on Friday.

Chapter 2 Measurements and Calculations. Sect. 2-1: Scientific Method Scientific Method Scientific Method ▫ Observing and collecting Data ▫ Qualitative.

Scientific Method and M Measurement
About project
© 2024 SlidePlayer.com Inc. All rights reserved.
The Science of Physics
Chapter Overview
- Nature of physics and its related fields
- Scientific method of inquiry
- Role of models
- Basic SI units
- Precision vs. accuracy
- Scientific notation
- Significant digits
- Various ways of summarizing data
- Dimensional analysis
- Estimation procedures
Section 1.1 What is Physics?
- Identify activities and fields that involve the major areas within physics
- Describe the processes of the scientific method
- Describe the role of models and diagrams in physics
1.1 What is Physics?
- The study of the physical world
- Use a small number of basic concepts, equations, and assumptions to describe the physical world
- Can be used to make predictions about a broad range of phenomena
- Appliances, tools, buildings, inventions are all basic physics principles put to test
Thermodynamics – Efficient engines, use of coolants
Electromagnetism – Battery, starter, headlights
Optics – Headlights, rearview mirrors
Vibrations and mechanical waves – Shock absorbers, radio speakers, sound insulation
Mechanics – spinning motion of the wheels, tires that provide enough friction or traction
Physics is Everywhere
- When you buy ice cream, why do you put it in the freezer when you get home?
- **Any problem that deals with temperature, size, motion, position, shape, or color involves physics**
- There are major areas of physics that deal with each of these
- Design, build, and operate
- Best shape so that is remains stable and floating, yet quick and maneuverable
- Knowledge of fluids
- Efficient shape for sails and how to arrange them
- Understanding motion and its causes
- Balancing loads
- So port isn't heavier than starboard
- Knowledge on how the keel keeps the boat moving in one direction
- Even though the wind i s blowing in another
The Scientific Method
- No single procedure is always taken in an experiment
- Certain common steps in all good scientific investigations
- There was a car accident and the police were investigating… use the scientific method:
- Observations/Data:
- Hypothesis:
- Experiments/Tests:
- Interpret/Revise Hypothesis:
- Conclusion:
- Simple models are often used to explain the most fundamental features of various phenomena
- Common technique
- Break an event down into different parts
- Use a model for each section
WE WILL ALWAYS DRAW
MODELS!!!!!!
- Observations
- Ball’s size, spin, weight, color, surroundings, time in the air, speed, and sound when hitting ground
- Identify the system
- A single object and the items immediately affecting it
- Ball and its motion
- Disregard any characteristics that don't matter
- Color, sound when hitting the ground
- In some studies of motion, even size and spin are disregarded
Models Help Build Hypothesis
- A hypothesis is a reasonable explanation for observations
- Can be tested with additional experiments
- Modeling a situation can help identify variables as well
- Galileo’s ‘thought experiment’
Models Help Guide Experiments
- Galileo performed many experiments
- Observing weight only
- Used same size objects, just different weight
- No way to eliminate air resistance
- Used rolling ball down smooth ramps as a model
- The steeper the ramp, the closer the representation
Experiments
- Must deal with variables
- Majority of the time a controlled experiment
- Only one variable changed at a time
- Used same set of different weight balls
- Just down a steeper ramp each time
Hypothesis to Prediction
- Until the invention of the air pump, it was impossible to perform direct tests in the absence of air resistance
- Reasonably accurate predictions were still made
- Experiments are run until results match each other and are in agreement with the hypothesis
- If not there could be error
- Then the hypothesis must be revised
- Conclusions
- Are only valid if they can be duplicated and verified by other people under the same conditions
- Not only so scientists conduct experiments to test hypothesis
- They also RESEARCH!!!
- Steps to doing scientific research
- Identifying reliable resources
- Searching the sources to find references
- Checking carefully for opposing views
- Documenting sources
- Presenting findings to other scientists for review and discussion
Section 1.2 Measurements In Experiments
- List basic SI units and the quantities they describe
- Convert measurements into scientific notation
- Distinguish between accuracy and precision
- Use significant figures in measurements and calculations
Numbers as Measurements
- When in physics numbers will never stand alone
- Means absolutely nothing
- Must have units following the number
- (anything labeled without units will be wrong) ☺
- Length, mass, time, or something else?
- If length: inches, centimeters, kilometers, l ight-years?
- The units helps tell us what kind of physical quantity being measured
- Basic dimensions – length, mass, time
- There are many other dimensions as well
- Force, velocity , energy, volume, and acceleration
- All combinations of length, mass, and time
- SI is the standard measurement system for science
- Scientists like to use the same system of units for measurement
- If not that would be a lot of converting ☹
- 7 base units that each describe a single dimension
- Length – meters (m)
- Mass – grams (g)
- Time – seconds (s)
- Other units derived from the 3 bases
SI Prefixes
- A very wide range of measurements will be used
- 100,000,000,000,000,000 m for distances between stars
- .000 000 001 m distances between atoms in a solid
- Can deal with powers of ten
- Prefixes to go with the powers
Conversions
- Using SI, with the prefixes and same base
- Conversion factors will always = 1
- Any measurement multiplied by a fraction will be multiplied by 1
- The number and unit will change but the quantity will stay the same
Dimensional Analysis
- Mathematical techniques that uses conversion factors to convert from one unit to another
- A typical bacterium has a mass of about 2.0μg. Express this in terms of grams and kilograms.
- The mass of an average person is 60,000,000 mg. Express this in grams and kilograms.
Dimension and Units Must Agree
- Can’t measure a length then label in kilograms (kg)
- Must make sure use correct unit
- We will ALWAYS use metric!!
- No inches, feet, miles, lbs, tons
Accuracy and Precision
- The closeness of measurements to the correct or accepted value
- Closeness of a set of measurements of the same quantity made in the same way
Accepted Value = 55 km/h
Problems with Accuracy are Due to Error
- Experimental work is never free of error
- Important to minimize as much as possible
- Should never have human error
- Mistake in reading measurement
- Mistake in recording results
- Method should always be the same
- Same instrument
- Check calculations
Precision of Instrument
- Poor accuracy can be corrected
- Precision based on the instrument
- Instruments can only be so precise
Precise to the .1
Estimate the last place
Significant Figures
- Measurement that consists of all known digits with an uncertain digit at the end
- Uncertain digit
- The digit that you as the experimenter must estimate
- All digits are significant, but not necessarily certain
- Insignificant digits are never reported
- YOU WILL ALWAYS NEED TO USE SIGNIFICANT FIGURES!!!!!
Sig Fig Rules
Sample Problems
- How many significant figures?
- Always round to significant figures
- If adding 2 numbers with 3 significant figures each
- Answer will have 3 significant figures
- Use normal rounding
- 5 and up – round up
- 4 and down – stay the same
Sig Fig Math
- Adding and Subtracting
- Answer must have the same number of digits to the right of the decimal point as there are in the measurement having the fewest digits to the right of the decimal point.
- 2.59 + 6.8974 = 9.49
- Multiplying and Dividing
- Answer can have no more significant figures than are in the measurement with the fewest number of significant figures.
- 3.05/8.47 = .360
Practice Problems
- 5.44m – 2.6103m =
- 2.4g/mL x 15.82 mL =
Conversion Factors and Sig Figs
- Because a measurement is considered exact, after conversion there is no rounding
Section 1.3 The Language of Physics
- Interpret data in tables and graphs, and recognize equations that summarize data
- Distinguish between conventions for abbreviating units and quantities
- Use dimensional analysis to check the validity of expressions
- Perform order of magnitude calculations
Mathematics and Physics
- Tools are used to summarize and analyze data and observations
- Often times mathematical relationships
- In forms of charts and graphs
- Provides a visual of time versus distance
- Can determine distance traveled at any time
- Through this equation
(change in position m) = 4.9 x (time of fall s) 2
- How far would the ball have fallen at .500 s?
Equations Indicate Relationships
- Equations show how two or more variables are related
- Many equations do not have numbers
- But symbols representing physical constants
- Δ means difference or change in
- Usually final minus initial
- Units should help with equations
- Units must cancel correctly
- Want the units that match your answer
- If finding velocity should end with units of m/s
Units or Variables?
- Variables are usually boldface
- Stand for a measurement with specific units
- Always check the context of the problem
- Find the mass of something
- Mass is variable m, units would be g or kg
- Examples of Variables
- Δx, Δy, Δt, c, m, a, v
- Examples of Units
- m, kg, m/s, m/s 2 , s
- Use to check validity of equations
- A car is moving at a speed of 88 km/h and has traveled 725 km, how long did this trip take?
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

62 templates

pink flowers
255 templates

15 templates

64 templates

22 templates

greek mythology
42 templates
Math Subject for Middle School - 6th Grade: Measurement
It seems that you like this template, math subject for middle school - 6th grade: measurement presentation, free google slides theme, powerpoint template, and canva presentation template.
If this ruler is 30 centimeters long, what's its length in meters? This can be the math lesson you could be giving to your students right now, so why don't you download this template and use it as an extra material for your class? The backgrounds are textured and some of them contain resources that are very thematic. It's like giving your lesson a more formal touch, while making it accessible for everyone.
Features of this template
- 100% editable and easy to modify
- 35 different slides to impress your audience
- Contains easy-to-edit graphics such as graphs, maps, tables, timelines and mockups
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides, Canva, and Microsoft PowerPoint
- 16:9 widescreen format suitable for all types of screens
- Includes information about fonts, colors, and credits of the free resources used
How can I use the template?
Am I free to use the templates?
How to attribute?
Attribution required If you are a free user, you must attribute Slidesgo by keeping the slide where the credits appear. How to attribute?

Register for free and start downloading now
Related posts on our blog.

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.

Premium template
Unlock this template and gain unlimited access

- Preferences

THE SI system and units of measurement - PowerPoint PPT Presentation

THE SI system and units of measurement
Units of measurement measurement is used to measure quantities. quantity is something that has magnitude, size, or amount (volume). a quantity is not the same as a ... – powerpoint ppt presentation.
- Units of Measurement
- Measurement is used to measure quantities.
- Quantity is something that has magnitude, size, or amount (volume).
- A quantity is not the same as a measurement.
- In the late 18th century, scientists used the metric system. The metric system is a forerunner to the SI System.
- Scientists all over the world use a single measurement system called Le Systeme International dUnits, abbreviated SI.
- It was adopted in 1960 and has both base units and derived units.
- The most common base units that we will study include
- The SI standard unit for length is the meter.
- A distance of 1m is about the width of an average doorway.
- To express longer distances, the kilometer (km) is used. One kilometer is equal to 1000 m.
- To express shorter distances, the centimeter (cm) is used. One centimeter is equal to 1/100 of a meter.
- Length can be measured using a meter stick or rulers.
- Mass is a measure of the quantity of matter. The standard unit for mass is the kilogram (kg).
- The gram (g), which is 1/1000 of a kg is used for measuring masses of small objects such as flasks and beakers. Mass is typically measured using a balance.
- Mass is different from weight. Weight is a measure of the gravitational pull on matter. The weight of an object increases as gravity acts on it. The weight of an object on the moon is about 1/6th of its weight on Earth. So, a human on Earth who weighed 126 lbs. would weight how much on the moon?
- The standard unit of measurement for time is the second (s).
- Time can be measured using stop watches, clocks, count down timers, and other time pieces.
- Larger amounts of time are measured in minutes and hours.
- There are 60 seconds in one minute. There are 60 minutes in one hour. Given that there are 24 hours in one day, how many seconds are there in one day?
- The standard unit of measurement for temperature is degrees Kelvin (K).
- Temperature can also be measured in degrees Celsius (C) and degrees Fahrenheit (F).
- To convert degrees Celsius (C) to degrees Fahrenheit (F) multiply by 1.8 and then add 32. To convert degrees Fahrenheit to degrees Celsius, subtract 32 and then divide by 1.8
- Temperature is measured using a thermometer. (measures the degree of heat or coolness)
- Derived units are combinations of base units. They are produced by multiplying or dividing standard units. The derived units we will study include
- Area is length times the width. It is expressed as square meters.
- Area can also be expressed as cubic centimeters.
- What is the area of a rectangle that has an a length of 6 cm and a width of 16 cm?
- Volume is the amount of space occupied by an object. The derived SI unit for volume is cubic meters. The cubic meter is rather large, so a more common unit of cubic centimeters is more commonly used.
- Non-SI units are also used to measure volume such as the milliliter (mL) and the liter (L), which is 1000 cm3. There are 1000 mL in 1 L.
- Beakers, flasks, and graduated cylinders are often used to determine the volume of liquids.
- Density is the ratio of mass to volume, or mass divided by volume. It can be written
- densitymass/volume or Dm/V
- Density is a characteristic physical property of a substance that does not depend on the size of the sample. As the mass of an object increases, its volume increases.
- Question Are raisins, more or less dense than soda water? Lets see!
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics , the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.

Understanding your lab values and other CKD health numbers
Last Updated: September 05, 2023
Medically reviewed by NKF Patient Education Team
Table of Contents
About your lab values and other ckd health numbers, measuring your general health, measuring your kidney health, measuring your balance of important minerals and acidity, measuring ckd complications: nutrition & malnourishment, measuring ckd complications: anemia, measuring ckd complications: mineral and bone disorder (ckd-mbd), measuring ckd complications: cardiovascular disease (cvd), measuring ckd risk factors: diabetes, questions for your healthcare team, more resources.
It is normal to feel like living with chronic kidney disease (CKD) sometimes means you need to learn a new language. CKD is a complex condition that worsens over time. Early on, only a small number of tests may be needed. As CKD gets worse, your kidneys have a harder time doing all their jobs like helping make red blood cells, balancing important minerals, and keeping your bones healthy. So, you may notice more tests being checked and/or checked more often as your CKD gets worse. You may also need extra tests to monitor for other health conditions that are related to kidney disease.
Having regular visits with your healthcare professionals and getting your recommended lab work done can help you stay up to date with your health. But you may not be familiar with why some of these measures of your kidney-related health and wellbeing are used. So, the information below serves as a quick guide to the different types of health information that you may see in your medical record that is important for people living with CKD. Some of these tests require a blood or urine sample (also called “lab tests” or “labs”) – these are usually sent to a laboratory for measurement. Other measures, like weight or blood pressure, are usually done in an exam room.
If you have questions about your results, always talk with your healthcare professional first before taking any action.
Everybody's situation is different - some of these tests may not apply to you. Similarly, your situation may need a test that is not included in this list. Also, having test results that are not in the "normal" range (as provided on your lab sheet) doesn't always mean there is a problem or concern.
Blood pressure
A healthy blood pressure is very important for your kidneys and overall health.
- High blood pressure puts extra stress on your kidneys, heart, and blood vessels - increasing your risk for heart attack, stroke, and worsening kidney disease.
- Low blood pressure makes it hard for your blood to deliver oxygen and nutrients to all the different parts of your body. This increases your risk for acute kidney injury . It can also increase your risk for dizziness and falling.
Your blood pressure is reported as 2 separate numbers – for example “120/80” or “120 over 80”. The first/top number (called “systolic pressure”) is the pressure in your blood vessels during each heartbeat - when blood is actively pumped out of your heart to the rest of your body. The second/bottom number (called “diastolic pressure”) is the pressure in your blood vessels when your heart is resting between each beat.
The recommended blood pressure target may vary depending on factors like your age, other health conditions, risk of falling, and whether you are on dialysis . Ask your healthcare professional what your goal blood pressure should be.
Maintaining a healthy weight is important for your overall health. The definition of healthy weight depends on many other factors like your height, age, and other health conditions. So, ask your healthcare professional what a healthy body weight is for you.
- If you are underweight or losing weight without trying, you may not be getting the right nutrition to stay healthy.
- If you are overweight or slowly gaining weight, you may be getting too many calories and/or not enough physical activity.
In either of these situations, working with a dietitian can help you find ways to safely add or remove extra calories to your diet and make sure you get the right nutrition.
- Sudden weight gain can also be a serious problem, especially if you are on dialysis and/or have heart failure. It may be a sign of too much fluid in your body, especially if it also comes with symptoms like swelling, shortness of breath, and/or a rise in blood pressure.
If you are on dialysis and/or living with heart failure, it is important to ask your healthcare professional what your dry weight is – your “normal” weight without any extra fluid in your body. As part of your treatment plan, your healthcare professional may recommend weighing yourself at a certain time every day. After you check your weight, compare the number to your dry weight number. If your weight has gone up or down by too much (as defined by your healthcare professional’s directions), contact your dialysis center or clinic for further instructions.
Serum (blood) creatinine
Creatinine is a waste product in your blood that comes from the digestion of protein in your food and the normal breakdown of muscle tissue. It is removed from your body through the kidneys. If you have kidney disease, the kidneys can have trouble removing creatinine from your blood. So, the level of creatinine in your blood starts to go up. High creatinine levels can be a sign of acute kidney injury and/or chronic kidney disease . A “normal” creatinine level in the blood is hard to define because it can change depending on your age, sex, body size, and other factors.
Cystatin C is a protein that is produced by the cells in your body. Like creatinine, it is also removed from the body through the kidneys. If you have kidney disease, the kidneys can have trouble removing cystatin C from your blood. So, the level of cystatin C in your blood starts to go up. For some people, this blood test can be helpful to measure instead of (or in addition to) your serum creatinine to check your kidney health. This test is not as common as the creatinine test and can be more expensive.
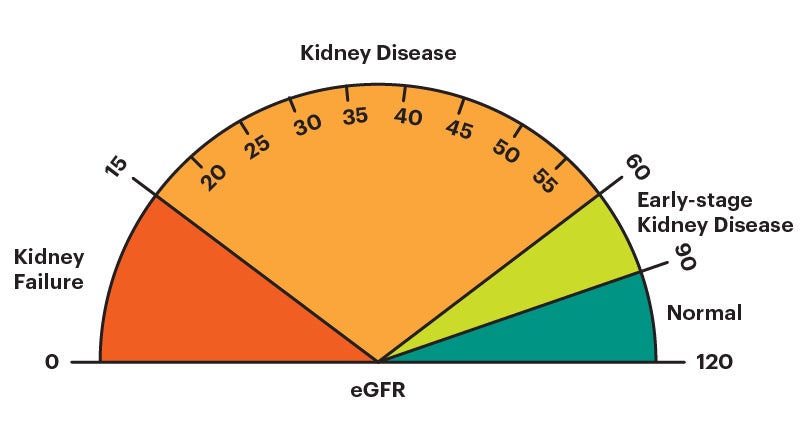
Estimated glomerular filtration rate (eGFR)
The estimated glomerular filtration rate (eGFR) is an estimate of how well your kidneys are removing waste products from the blood. It is calculated using your serum (blood) creatinine level, age, and sex. It can also be calculated using your cystatin C level instead of, or in addition to, your serum (blood) creatinine level. A “normal” eGFR varies according to age – it naturally decreases as you get older. For this test, a higher number is better .
If you have chronic kidney disease (CKD) , the eGFR is used to determine your CKD stage . In general, an eGFR value lower than 60 is a sign that the kidneys may not be working properly. An eGFR lower than 15 is a marker of kidney failure .
Measured glomerular filtration rate (mGFR)
In less common situations where a more accurate measure of your kidney function is needed, your healthcare provider may order a measured glomerular filtration rate (mGFR) . The mGFR is a direct measure of how well your kidneys are removing waste products from the blood. It can be a complicated and lengthy process. So, it is not used as often as the estimated GFR (eGFR).
Your healthcare professional may recommend this test if a more accurate measure of your kidney function is needed. There are many ways to complete this test – some involve collecting all the urine you make in 24 hours; others involve multiple blood samples taken from your arm over several hours. The mGFR is sometimes called a different name - measured creatinine clearance (mCrCl).
Blood urea nitrogen (BUN)
Urea nitrogen is a waste product in your blood that comes from the breakdown of protein in the foods you eat. It is removed from the body through the kidneys. A “normal” BUN level varies, and usually increases as you get older. Checking your BUN level is usually not very helpful by itself. So, your healthcare provider will likely compare your BUN level to your creatinine and eGFR levels when evaluating your kidney health.
Urine albumin-creatinine ratio (uACR)
The urine albumin-creatinine ratio (uACR) test measures the amount of two different substances in your urine (pee) – albumin (protein) and creatinine. Healthy kidneys keep the albumin in your blood while filtering the creatinine out into the urine. So, this test checks to see how well your kidneys are keeping albumin in your body and sending creatinine out.
The uACR is calculated by comparing the amount of albumin in your urine with the amount of creatinine in your urine to find the ratio. A “normal” uACR level is less than 30 mg/g. For this test, a lower number is better . A uACR level of 30 mg/g or more can be a sign of albuminuria .
When you check the results from this test on your lab report, you may see many different numbers. Focus on the result that has the word ratio in the name. For example, the name on your report may be “alb/creat ratio”, “albumin/creat ratio”, or “albumin/creat ratio, random urine”.
Urine protein-creatinine ratio (uPCR)
This test is very similar to the uACR test described above. But instead of measuring only the amount of albumin in your urine (pee), it measures all the different proteins that may be present. In some forms of kidney disease (like IgA nephropathy , lupus nephritis , or glomerulonephritis ) or when testing children for protein in their urine , your healthcare professional may choose to measure your uPCR instead of uACR. A “normal” uPCR level is less than 150 mg/g. For this test, a lower number is better . A uPCR level of 150 mg/g or more can be a sign of proteinuria .
Potassium is an important mineral found throughout your body. It is needed for many of your body’s functions – like keeping your heart beating regularly and muscles working properly. Your kidneys help keep the right amount of potassium in the blood.
In more advanced stages of chronic kidney disease , your kidneys may have a hard time removing extra potassium from the blood, especially if you are on dialysis. People living with CKD can also be at risk for low potassium, especially during earlier stages of CKD. The recommended goal potassium level for most people is between 3.5 and 5.
Sodium is an important mineral that helps balance the amount of fluid in your body. It also helps your nerves and muscles to work properly. Your kidneys play an active role in keeping your fluid levels balanced, partly by helping get rid of any extra sodium in your body through your urine.
In more advanced stages of chronic kidney disease , your kidneys may have a hard time balancing your fluid and blood sodium levels. This can increase your risk of high blood pressure, edema (swelling), and/or heart failure.
Having a sodium level higher or lower than the goal range can be a result of many things. So, your healthcare professional will likely compare your results from this test with your other test results (such as serum creatinine, glucose, potassium, carbon dioxide, and/or urine tests). When looked at together, your healthcare professional can provide you with custom guidance for resolving the issue (if applicable).
However, it is also possible to have a normal sodium level while still consuming too much sodium (salt). When your blood sodium level goes up, your body tries to balance it out by holding on to extra water. This is what causes symptoms like thirst, swelling, high blood pressure, and/or shortness of breath. It is important to limit your sodium (salt) intake to less than 2300 mg per day. Your healthcare professional may advise an even lower target depending on your other health conditions.
Serum (blood) bicarbonate / carbon dioxide (CO2)
Bicarbonate is needed in your blood to stop it from getting too acidic. Most of the bicarbonate in your body is in the form of carbon dioxide (CO2), a waste product from when your body turns food into energy. So, another name for this blood test is your “serum carbon dioxide (CO2)” level.
The kidneys work together with the lungs to keep your bicarbonate (carbon dioxide) level in your blood in the goal range. In more advanced stages of chronic kidney disease , your kidneys may have a hard time removing extra acidic waste products from the blood. This is also known as metabolic acidosis . A bicarbonate/CO2 level less than 22 mEq/L can be a sign your blood has too much acid – talk with your healthcare professional about a treatment plan if your labs show a CO2 (bicarbonate) level less than 22.
Serum albumin
Good nutrition is important for providing your body with the resources to fight infections, repair body tissue, and build new muscle. Having a more advanced stage of chronic kidney disease increases your risk of problems with your nutritional health and malnourishment, especially for people who are on dialysis . It is not possible to measure your nutritional health directly, so a variety of tests can be used to check the highest risk areas for health problems.
Albumin is an important protein normally found in the blood that serves many roles in the body. These roles include building muscle, repairing tissue, and fighting infection. A low level of albumin in your blood may be caused by not getting enough protein or calories in your diet, especially if you are on hemodialysis . Since albumin is made in the liver, low albumin levels can also be a sign of liver problems. A low albumin level may lead to health problems such as difficulty fighting off infections. When compared against your other health information and test results, your serum albumin level can help your healthcare professional see if you are getting enough nutrients in your diet.
Normalized protein nitrogen appearance (nPNA)
If you are on dialysis , the nPNA can be a helpful tool that your healthcare professional may use to see if you are eating enough protein. This number is calculated using your blood urea nitrogen (BUN) level just before your dialysis session, your weight, and the amount of protein you ate (including food, drink, and supplements) during a specific period of time. The amount of urea nitrogen in your urine may also be used.
When compared against your other health information and test results, your nPNA results can help your healthcare professional see if you are getting enough protein in your diet.
Another name for this test is the normalized protein catabolic rate (nPCR).
Subjective global assessment (SGA)
If you are on dialysis , your dietitian may use the SGA to help check for signs of nutrition problems. The dietitian will ask you a few questions about your daily diet and any symptoms you may have, measure your weight, and then check your fat and muscle stores in your face, hands, arms, shoulders, and legs. All this information can help your dietitian see if you are getting enough nutrition in your diet. If there are any concerns, your dietitian will work with you to create a plan to help you get the nutrition you need.
Anemia happens when you have low levels of red blood cells in your blood. Red blood cells carry oxygen from your lungs to the rest of your body. The kidneys play a very active role in helping your body make these red blood cells. Also, people living with advanced CKD can have problems absorbing iron from food. They are also at high risk for repeated blood loss from frequent blood tests and during dialysis . This makes the kidneys try to make even more red blood cells when they are having trouble keeping up in the first place. So, having a more advanced stage of chronic kidney disease increases your risk of anemia, especially for people who are on dialysis .
Keep in mind that anemia is not always caused by CKD and the descriptions below are general guides to the most common tests. If you have anemia, talk with your healthcare professional about what the primary cause may be and how you can treat it.
Hemoglobin (Hgb)
Hemoglobin is the protein in your red blood cells that carries oxygen. For adults and children over 15 years living with CKD, anemia is suspected when the hemoglobin level is under 13 g/dL (in males) or under 12 g/dL (in females).
Hematocrit (Hct)
Hematocrit is very similar to hemoglobin. It is a measure of how many red blood cells your body is making. The number is the actual percentage of your blood sample that is made up of red blood cells.
Ferritin (pronounced FAIR-ritt-in) is the stored form of iron found in your body. So, your ferritin level is a measure of how much iron your body has available to use at any given moment. Iron is an important ingredient for making hemoglobin. Having enough iron (ferritin) available is important for being able to make more red blood cells. Having a low level of ferritin means you may need an iron supplement to help treat your anemia.
Transferrin saturation (TSAT)
Transferrin (pronounced trans-FAIR-rin) is a protein that helps move iron throughout your body. So, your transferrin saturation (TSAT, pronounced TEE-sat) number helps show what percent of the transferrin in your blood is currently attached to iron. Your healthcare professional will likely look at your TSAT number in combination with your ferritin level to decide the best way to treat your anemia. In general, a TSAT number of 20% or more is considered “normal”.
Mean corpuscular volume (MCV)
The mean corpuscular volume (MCV) is a blood test that measures the average size of your blood cells. When combined with your other test results and medical history, it can help your healthcare professionals identify the best treatment for your anemia.
For example, an MCV below the reference range suggests the anemia may be caused by low iron. Similarly, an MCV above the reference range suggests the anemia may be caused by low vitamin B12 and/or folic acid.
Your kidneys play a very active role in balancing the ingredients needed for healthy bones – calcium, phosphorus, and vitamin D. In more advanced stages of chronic kidney disease , your kidneys may have a hard time activating vitamin D (which is needed to absorb calcium from your food) and removing extra phosphorus from the blood. This increases your risk of having calcium and phosphorus levels that are out of balance (also known as secondary hyperparathyroidism ). Without close monitoring and treatment, this can cause CKD-related bone disease (also known as CKD-mineral and bone disorder or CKD-MBD).
Parathyroid hormone (PTH)
Parathyroid hormone (PTH), also known as intact parathyroid hormone (iPTH), helps balance the levels of calcium and phosphorus in your blood. When your blood level of calcium goes down, your body makes more PTH to raise it, usually by releasing calcium (and phosphorus) from your bones. PTH also helps remove extra phosphorus from your blood through the kidneys. So, the kidneys play a very active role in this complex process.
Even though this test has the word “thyroid” in the name, it is entirely separate from anything related to your thyroid function. The name “parathyroid” comes from the place where the hormone comes from - very small glands in your neck that sit very close to your thyroid.
A “normal” PTH level in the blood is hard to define because it depends on many other factors (including your stage of CKD, phosphorus level, and calcium level). Ask your healthcare professional what your custom target PTH level should be.
Serum calcium
Calcium is an important mineral your body needs for strong bones, and for your nerves, muscles, and heart to work properly. Your kidneys play a very active role in balancing your calcium levels to make sure the level is just right. If your body needs more calcium, your kidneys activate vitamin D to help absorb more calcium from your food and drink. Your body is not able to absorb the calcium without it. In more advanced stages of chronic kidney disease , your kidneys may have a hard time activating vitamin D. This makes it very hard for your body to absorb enough calcium from your food. So, your body starts breaking down bones to supply the calcium. This increases your risk of CKD-related bone disease .
For people living with advanced CKD, your healthcare professional will likely compare your results from this test with your phosphorus and PTH levels. Looking at these three tests together will help them provide you with custom guidance about your target for each item.
Serum Phosphorus
Phosphorus (sometimes called phosphate) is an important mineral that your body needs to make strong bones, store energy, and maintain your tissues and cells. Your kidneys play a very active role in keeping your phosphorus levels in the goal range. In more advanced stages of chronic kidney disease , your kidneys may have a hard time removing extra phosphorus from the blood, especially if you are on dialysis. This increases your risk of having phosphorus levels that are too high, and can also lead to CKD-related bone disease .
For people living with advanced CKD, your healthcare professional will likely compare your results from this test with your calcium and PTH levels. Looking at these three tests together will help them provide you with custom guidance about your target for each item.
Vitamin D (25-hydroxyvitamin D; 25(OH)D; calcidiol)
Vitamin D is something your body needs to absorb calcium from your food. Vitamin D is needed for strong teeth and bones. It also helps keep your muscles, nerves, and immune system working well. Your body gets vitamin D from sun exposure and the food that you eat. Once absorbed, your liver converts the vitamin D into its storage form to save for later, also known as 25-hydroxyvitamin D or calcidiol. Your kidneys are responsible for activating the stored vitamin D when your body needs it.
In more advanced stages of chronic kidney disease , your kidneys may have a hard time activating vitamin D. This increases your risk of not absorbing enough calcium from your food. So, your body can start breaking down bones to get the calcium it needs. This increases your risk of CKD-related bone disease . Having a low 25-hydroxyvitamin D (calcidiol) level suggests you may not have enough stored vitamin D ready to use when your body needs it.
People living with chronic kidney disease are at an increased risk of having cardiovascular disease (heart attack or stroke). This is especially true if you have an advanced stage of CKD and/or if you have albuminuria . Your risk of CVD (heart attack or stroke) is increased even more if you also have high cholesterol.
Cholesterol is a fat-like substance found throughout your body and in your blood. It is important for keeping your cells and organs healthy. Your body gets cholesterol from two places – it is absorbed from food and made in your liver. Too much cholesterol in your blood can lead to it attaching to the walls of your blood vessels, making them narrow or blocking them altogether.
A typical cholesterol test usually checks your blood for four different things:
Total cholesterol (TC)
Total cholesterol is the total level of cholesterol in your blood. This number includes all the major types of cholesterol that exist in your blood (LDL cholesterol, HDL cholesterol, and triglycerides). For most people, a level below 200 mg/dL is considered the goal. However, there may be situations where a higher TC number is okay – for example, people who have very high HDL (“good”) cholesterol levels. So, your healthcare professional may advise you to focus more attention on the numbers for the different types of cholesterol listed below.
HDL cholesterol (HDL-C)
HDL cholesterol is the level of “good cholesterol” in your blood. It has this name because it helps remove extra fats from your blood. This lowers the risk of having blocked arteries and lowers your risk of heart disease. For this test, a higher number is better , especially if it is 40 mg/dL or higher.
LDL cholesterol (LDL-C)
LDL cholesterol is the level of “bad cholesterol” in your blood. It has this name because it increases the amount of fat that attaches to the walls of your blood vessels. This increases the risk of blockages in your arteries and raises your risk of heart disease. For this test, a lower number is better . A number less than 100 mg/dL is generally considered to be at goal. People who already have heart disease or are at very high risk for developing heart disease may have an even lower LDL-C goal.
Triglycerides (TG or “trigs”)
Triglycerides are a mixture of fats and carbohydrates (sugars) that your body uses as an energy source. A high triglyceride level can increase the risk of heart disease and pancreatitis. For this test, a lower number is better , especially if it is less than 150 mg/dL.
High blood glucose (sugar) levels over a long period of time can damage the kidneys. So, diabetes is a very strong risk factor for developing chronic kidney disease. This is especially true if your blood sugar levels are higher than your goal range for long periods of time. Two of the most common tests used to diagnose and monitor diabetes are the hemoglobin A1C and serum (blood) glucose level.
Hemoglobin A1C
Your hemoglobin A1C, often just called “A1C”, is a blood test that describes your average blood sugar over the past 2-3 months.
- If you have not been diagnosed with diabetes before , this test can be used to check for it. An A1C of 5.7% or more can be a sign that you are at high risk for developing diabetes. An A1C of 6.5% or more can be a sign that you have diabetes. A repeat test is usually recommended to confirm the results are accurate before a diagnosis can be made.
- If you have been diagnosed with diabetes before , this test is used to see how well you are managing it. The goal for most adults living with diabetes is an A1C of 7% or lower. Some people may need a higher or lower goal depending on their clinical situation. Ask your healthcare professional what your goal A1C level should be.
The A1C test may not be as accurate for people on dialysis or receiving erythropoietin stimulating agents (ESAs, a medication for anemia caused by CKD).
Serum (blood) glucose (sugar)
Glucose (sugar) is an important source of energy for your body, including your brain and red blood cells. This test describes the amount of glucose that is in your blood at the time of testing.
- A fasting blood glucose level of 100 mg/dL or higher is a sign that you may be at high risk for developing diabetes.
- A fasting level of 126 mg/dL or more is a sign that you may have diabetes.
- In both cases, “fasting” means you have not had anything to eat or drink (except water) during the 8-12 hours before the test.
- A blood sugar level of 200 mg/dL or more at any time is also a sign that you may have diabetes.
- In all of these cases, a repeat test is usually recommended to confirm the results are accurate before a diagnosis can be made.
- If you have been diagnosed with diabetes before , this test is used to see how well you are managing it. Keeping your blood glucose (sugar) level within your goal range is important. A blood sugar level that is too low (less than 70 mg/dL) can starve your brain and other parts of the body of energy. A blood sugar level that is too high can cause damage to your kidneys, heart, and other organs in your body. Everyone’s goal range is different – ask your healthcare professional what your goal range is.
- Am I at a healthy weight?
- What is my recommended goal blood pressure?
- What is my goal A1C and/or blood glucose level?
- What is my “dry weight”? What should I do if my weight at home is much higher or lower than my “dry weight”?
- Are there any test results in my lab work that you are especially concerned about or that I should pay special attention to?
- Which of my results have a different goal level or range than the “normal” range that is shared with my lab results?
- Kidney Failure and the Kidney Failure Risk Equations (KFRE)
- Dining out with confidence
- Nutrition and Chronic Kidney Disease
What’s your story?
We want to hear about your unique experience with a kidney transplant, living donation, or kidney disease. Your story may be the one that gives someone hope.
How helpful was this content?
Related kidney topics, estimated glomerular filtration rate (egfr), contrast dye and the kidneys, know your kidney numbers: two simple tests, can my gfr get better, related news and stories.

March 15, 2023
Pushing for Kidney Disease Screenings to Help the Patients that Need Them Most

December 20, 2022
5 People with Kidney Disease Share Their COVID-19 Experiences

January 13, 2023
Early Diagnosis of Kidney Disease: Incentivizing Comprehensive Testing

November 22, 2022
Flu Season is Back. How People with Kidney Disease Can Prepare

October 27, 2022
What Your Urine Says About Your Kidney Health

October 04, 2022
What the New eGFR Calculation Means for Your Kidney Disease Diagnosis and Treatment
Physical Quantities, Units and Measurement
Nov 13, 2014
770 likes | 1.96k Views
Physical Quantities, Units and Measurement. Name: ________________ Class: _________________ Index: ________________. Physics is the study of the natural world around us – from the very large, such as the solar system, to the very small, such as the atom.

Share Presentation
- physical quantities
- object figure 3
- measuring cylinder figure 15

Presentation Transcript
Physical Quantities, Units and Measurement Name: ________________ Class: _________________ Index: ________________
Physics is the study of the natural world around us – from the very large, such as the solar system, to the very small, such as the atom. The study of Physics is commonly divided into major topics such as General Physics, Thermal Physics, Light, Waves and Sound, Electricity and Magnetism. All these topics are related to two main ideas: Matter and Energy.
What is Physics?
A physical quantity is a quantity that can be measured. It consists of a numerical magnitude and a unit. There are altogether seven basic physical quantities.
All other common physical quantities such as area, volume and speed are derived from these seven quantities. They are called derived quantities. For example, speed is derived from length (distance travelled) and time.
Prefixes for SI Units The use of prefixes will make it more convenient to express physical quantities that are either very big or very small.
Apparatus for Measuring Length. • Metre Rule – A metre rule is used to measure length. The smallest division on a meter rule is 1 mm (0.1 cm or 0.001 m). We attribute an uncertainty of 0.1 cm in the measured length. In conducting experiments in the school laboratory, we frequently use other types of ruler such as the half-metre rule and the 30-cm plastic rule. Figure 1
A B E F D C Table 1
Measurements in Table 1 are called raw data. They are obtained from a • measuring instrument and consists of two parts: • a numerical value, and • a unit . • All raw data are expressed to a fixed number of decimal places in a • specified unit. This usually represents the precision to which the instrument • can supply. Therefore, the length obtained with a meter rule should be • given consistently to: • three decimal places in metres (m), or • one decimal place in centimetres (cm), or • to the nearest millimetre (mm). • Length of wooden plank = 1.260 m • Numerical ValueUnit • Width of wooden plank = 9.8 cm
When using a metre rule, care must be taken to avoid parallax error (Figure 2). This arises when the eye is not positioned vertically above the mark to be measured. At the incorrect position marked I, the mark seems to correspond to the 5.1 cm line on the scale. When viewed vertically at C, it is 5.0 cm. Figure 2
Check the ruler for end errors before making a measurement. This could arise when the end of the ruler is worn or the zero mark is not at the edge of the object (Figure 3). Figure 3
To measure the extension of a loaded spring, a needle can be attached on the base of the load and its tip protruding onto a scale along the line of sight of the person taking the reading. A vertical plane mirror fixed behind the ruler can help to eliminate parallax error (Figure 4). Figure 4
Set squares are used to align objects to the metre-rule scale to improve accuracy of reading. This is illustrated in Figure 5 where the diameter of the sphere can be obtained by subtracting R2 with R1.Measurements should be repeated by rotating the sphere. Figure 5
(b) Vernier Calipers A vernier caliper is used to measure short lengths such as internal diameter of a test tube. The smallest length that the vernier caliper (Figure 6) can measure is 0.1 mm. We attribute an uncertainty of 0.1 mm to the measured length when we use a vernier caliper. Figure 6
When the jaws of the vernier caliper are closed, the zeros of the main scale and vernier scale should coincide. If it doesn’t, the discrepancy is known as zero-error (Figure 7) and has to be subtracted from the value obtained through measurement. The zero error is an example of systematic error that will cause the measured value to be either too large or too small when compared to the true value. Correct measurement = Actual measurement – zero error Figure 7
Treatment of zero error is shown in Figure 8. Figure 8
(c) Micrometer Screw Gauge A micrometer screw gauge is used to measure lengths of small objects such as the diameter of human hair with a precision better than that of the vernier calipers. Figure 9 shows an actual micrometer screw gauge. The smallest length that the micrometer can measure is 0.01 mm. This is the precision of the micrometer. The spindle will move 0.01 mm horizontally away from the anvil when the thimble moves vertically downwards by one small division. Figure 9
When the anvil and spindle are closed, the zero mark on the circular scale coincides with the horizontal line on the main scale. If it doesn’t, the discrepancy is known as zero-error (Figure 10) and has to be subtracted from the value obtained by measurement. Zero error is an example of a systematic error that will cause the measured value to be either too large or too small when compared to the true value. Correct measurement = Actual measurement – zero error Figure 10
Treatment of zero-error is shown in Figure 11. Figure 11
Table 2 summarizes the precision of the metre rule, vernier calipers and the micrometer screw gauge. Table 2
To find the average diameter of a uniform rod (Figure 12), we take readings at several positions (at least 3). Average Diameter = (X1 + X2 +X3 ) / 3 Reading for X1 Reading for X2 Reading for X3 Figure 12
Techniques in measuring small quantities without using special instruments. • Thickness of 1 piece of paper • - Use a metre rule to measure the thickness of 100 sheets of paper. • - Thickness of 1 sheet of paper = Total Thickness / 100. • 2) Diameter of a piece of wire • - Wind the wire around a thin rod or a pencil to form a tight coil, say 18 turns (Figure 13). Use a metre rule to measure the length of the coil, d cm. • - Diameter of the wire = d / 18 Figure 13
Orders of Magnitude
Apparatus for measuring volume and mass • Measuring cylinder • The SI unit for the measurement of volume is m3. As volumes measured in the laboratory are small, alternative units such as dm3, cm3 and mm3 are used. • The conversion factors are given below: • 1cm3 = 1 ml • 1 dm3 = 1000 cm3 = 1 litre • 1 m3 = 1000 dm3 Figure 14
The precision of some apparatus used to measure volume is summarized in Table 3. Table 3
When the volume of a small irregular solid is required, it could be lowered directly into a cylinder containing a known initial volume of water. The volume of the solid can be obtained by subtracting the final volume of water to the initial volume. If the solid is too large and irregular, the solid is immersed in a displacement can filled to the brim with water. The volume of water can be read directly from the measuring cylinder (Figure 15). Figure 15
(b) Measuring mass • Mass is commonly measured by one • of the following measuring • instruments. • Spring Balance (or Newton-meter) • Spring balance makes use of the force of gravity acting on an object to extend or compress a spring. It measures weight when an object is attached to the hook. Modern spring balances are calibrated in both kilogram and newton (Figure 16). Figure 16
2) Electronic Balance (or top pan balance) The mass to be measured is placed on a top pan. The force of gravity deforms a substance within the balance. This alters the resistance and the current flowing through. The variation of the current with mass is then calibrated in grams. See Figure 17. Figure 17
The SI unit used in the spring balance is the Newton (N). The unit displayed on the electronic balance is the gram (g). The precision of some commonly used electronic balances are summarized in Table 4. Table 4
(c) Techniques in measuring small volume and small mass • Volume of 1 ball bearing • (i) Use a measuring cylinder which is partially filled with water. The volume of water in the measuring cylinder V1 is read. 100 ball bearings are then placed inside the measuring cylinder and the new reading V2 is taken. See Figure 18. • (ii) The volume of each ball bearing = (V2 – V1) / 100 Figure 18 2) Mass of 1 sheet of paper (i) Use a balance to measure the mass of 100 sheets of paper. (ii) Mass of 1 sheet of paper = Total mass / 100
Using a pendulum to tell time A simple pendulum can be used to measure time more accurately. It consists of a heavy object called a bob, like a small ball, attached to a string. The string is fixed at one end. If we swing the pendulum, it will move back and forth at regular intervals. Each complete to-and-fro motion is one oscillation. The period of the simple pendulum is the time taken for one complete oscillation.
Apparatus for Measuring Time (a) Analogue and digital stopwatches Time can be measured with an analogue stopwatch or a digital stopwatch (Figure 19). The oscillations of natural vibrations of crystals are used in clocks and watches as they are small, accurate and require very little electrical energy. Analogue stopwatch Digital stopwatch Figure 19 The SI unit for time is the second (s).
The precision of commonly used analogue stopwatch and digital stopwatch are summarized in Table 4. Table 5
(b) Techniques in measuring time in oscillations A simple pendulum consists of a bob suspended from a fixed support by a string (Figure 20). One period of a simple pendulum is the time taken for a complete oscillation. The period increases as the length of the pendulum increases. Figure 20
Apparatus for measuring temperature • Liquid-in-glass thermometer • An example of a liquid-in-glass thermometer is the mercury-in-glass • thermometer. It has a range of -10°C to 110°C. • The SI unit for temperature is the kelvin (K). • The precision of the thermometer is summarized in Table 5. Table 6
(b) Techniques in measuring temperature When taking a reading, it is essential that the eye be at the same level as the mercury meniscus (Figure 21). This is to prevent parallax error. Figure 21 Figure 22 Temperature should be measured to half a degree (i.e. +/- 0.5°C). However an uncertainty of +/- 0.2°C could be obtained if a hand lens is used (Figure 22).
(c) Digital thermometer The digital thermometer shown in Figure 23 makes use of a thermocouple as its temperature sensor. Temperature within the range of -50°C to 1300°C can be read on the digital liquid crystal display. An accuracy of 0.1°C can be obtained from the instrument. Figure 23
Apparatus for measuring electrical quantities • Ammeter • An ammeter is used to measure electric current. The SI unit for current is the ampere (A). An ammeter is always connected in series with other electrical components in the circuit (Figure 24). Before switching the circuit on, the ammeter should be checked for zero error. If the needle is not at zero graduation, the error can be eliminated by adjusting the screw at the needle pivot with a five cents coin or a screw driver. When taking the reading, the image of the needle in the mirror can be aligned with the needle to remove parallax error. Figure 24
(2) Voltmeter The voltmeter is an instrument that measures potential difference and its SI unit is the volt (V). The voltmeter is always connected in parallel across a resistor to determine the effective potential difference (Figure 25). In setting up an electrical circuit, it is advisable to connect all the components in series first. The voltmeter should be the last instrument to be connected. This is to reduce confusion and to prevent the voltmeter from being connected in series with resistances in the circuit. Figure 25
(3) Galvanometer The galvanometer is an electrical meter tat measures small current or the presence of a current. Its distinguishable feature is a small zero mark at the center of the scale (Figure 26). Figure 26
(4) Digital multimeter A digital multimeter is a versatile electronic instrument that can be used as an ammeter, voltmeter or an ohmmeter. By incorporating shunts, multipliers, variable resistors and an in-build battery, it is capable of measuring alternating current (a.c.), direct current (d.c.), voltages as well as resistances in an electric circuit (Figure 27). Figure 27
Precision of electrical instruments. Table 7
Apparatus and techniques in alignment • To ensure that a ruler is set up vertically • Set up a plumb line and placed the ruler parallel to it. If the ruler appears to be parallel to the plumb line from the front and the side, the ruler must be vertical (Figure 28). Figure 28
(b) To ensure that a ruler is set up horizontally (1) Set up a plumb line and place the ruler perpendicular to the plumb line as shown in Figure 29. Use a set square to ensure that the angle between the plumb line and the ruler is 90°. Figure 29
(2) Place a spirit level on the meter rule and adjust the bubble to the centre of the spirit level (Figure 30). Figure 30
(3) Another method is to measure both ends of the meter rule from the top of the bench (Figure 31). Figure 31
(c) Measurement of angles The most common instrument used to measure angles is the protractor. Readings will be inaccurate if there is parallax error or poor alignment of the protractor. Figure 32 illustrates the incorrect and correct methods of alignment when measuring the angle Ɵ of the slope and is obtained by reading the outer scale of the protractor. Figure 32
References: • http://geology.com/nasa/nasa-universe-pictures.shtml • http://www.measuringgauges.net/product.asp?page=6&classid= • http://www.phy.uct.ac.za/courses/c1lab/vernier1.html • Physics Insight “O” Level 2nd Edition. Loo Wan Yong, Loo Kwok Wai.
- More by User
Physical Quantities
Physical Quantities. Definition, Types, Symbols and Units. Definitions of Physical Quantities. A physical quantity is a physical property that can be quantified by measurement. Types of physical quantities. Scalar quantities
7.11k views • 41 slides

Components, Quantities, and Units
Introduction. This chapter will give you a preview of the types of things you will study throughout this book. Objectives. Recognize common electrical components and measuring instrumentsState basic electrical and magnetic quantities and their unitsUse Scientific notation to express quantitiesUse
580 views • 25 slides

Units and Measurement
Units and Measurement. Physics Mrs. Coyle. International Space Station http://apod.nasa.gov/apod/image/0706/iss_sts117_big.jpg. It All Starts with a Ruler!!!. Math and Units. Math- the language of Physics SI Units – International System MKS Meter m Mass kg Time s
671 views • 29 slides
Chapter 1: Units, Physical Quantities and Vectors
Chapter 1: Units, Physical Quantities and Vectors. About Physics. What is Physics?. Phys ’ ics [ Gr. Physika, physical or natural things ] Originally, natural sciences or natural philosophy The science of dealing with properties, changes, interaction, etc., of matter and energy
1.61k views • 59 slides
SI Base Quantities and Units
SI Base Quantities and Units. Metric(SI) Prefixes. Chapter1:Length. 1m 10 -7 of the distance from the equator to the pole 1m = length of the path traveled by light in vacuum during the time interval of 1/299,792,458 of a second. Mass.
501 views • 11 slides

Physical quantities, units and measurements
Physical quantities, units and measurements. Base quantities and units. Multiplying or dividing by base quantities produces derived quantities. Some quantities are dimensionless, eg., relative density. Examples of derived quantity . Common multiples and submultiples of units. submultiples.
1.33k views • 21 slides

Quantities factor units
Quantities and Units. Quantities factor units. Quantity and unit. Quantity. Subject / measureable First two columns from binas table 6. Grootheid en eenheid. Quantity examples. Speed v Volume V Time t Temperature T Force F Frequency f.
295 views • 20 slides

MEASUREMENT, QUANTITIES and UNITS
MEASUREMENT, QUANTITIES and UNITS. MEASUREMENT IS THE PROSES OF COMPARING A QUANTITY WITH ANOTHER QUANTITY WHICH IS USED AS UNIT. Measuring is to Compare a physical quantity of an object with a unit of measurement to get the value.
651 views • 11 slides
2. Measurement of physical quantities
LECTURE 2. Contents. 2. Measurement of physical quantities 2.1. Acquisition of information: active and passive information 2.2. Units, systems of units, standards 2.2.1. Units 2.2.1. Systems of units 2.2.1. Standards 2.3. Primary standards
671 views • 32 slides

Radiation Quantities and Units
Radiation Quantities and Units. Chapter 3 Sherer Chapter 33 Bushong. Somatic effects Long term somatic Genetic effects Box 3-1. Quality Factor, pg 56 Sherer "rate in which energy is deposited in the form of a charged particle or ion pair as it travels through matter" LET pg 57 Sherer
358 views • 17 slides

Quantities and Units
Quantities and Units. Afit Sutiyawan , S.Pd. Environmental.
251 views • 8 slides

Fundamental Quantities and Units
Fundamental Quantities and Units. Fundamental Quantity MKS Unit * Length (Meters) * Mass (Kilograms) * Time (Seconds). Motion. Motion. The symbol “ Δ ” means “Change in”. Motion. The symbol “ Δ ” means “Change in”. The speed changes by 4 m/sec every second.
527 views • 23 slides

Radiation Quantities and Units. Chapter 3 Sherer. Review-Pages 53-56. Somatic effects Long term somatic Genetic effects Box 3-1. Page 56-58- specifically. Tolerance dose was replaced with Maximum Permissible dose (MPD)-dose limits-effective dose
273 views • 10 slides
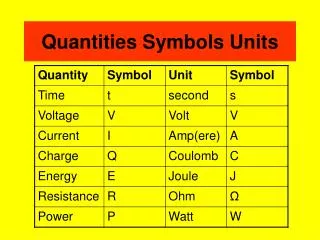
Quantities Symbols Units
Quantities Symbols Units. Electrical formulas VIR. V. volts. I. R. amps. ohms. Electrical formulas PIV. P. Watts. I. V. Amps. Volts. Electrical formulas EPt. E. Joules. P. t. Watts. seconds. Electrical formulas EQV. E. Joules. V. Q. Volts. Coulombs.
1.14k views • 16 slides

Physical Quantities. There are 2 main parts to a physical quantity. 5.01 H 10 2 km. {. {. Number Unit. 320 mL. {. {. Number Unit. The number contains 2 pieces of information. value and an indication of precision. 320 - value is 320 (obvious?).
323 views • 5 slides
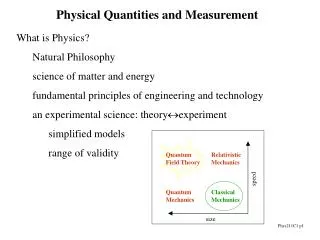
Physical Quantities and Measurement
Quantum Field Theory. Relativistic Mechanics. speed. Quantum Mechanics. Classical Mechanics. size. Physical Quantities and Measurement. What is Physics? Natural Philosophy science of matter and energy fundamental principles of engineering and technology
445 views • 11 slides

Quantities , Unit and Measurement
Quantities , Unit and Measurement. Sumber Gambar http://theworldoffii.blogspot.com/2008/07/alat-ukur-massa.html. Sumber Gambar : site: gurumuda.files.wordpress.com. Quantities and Unit. The kinds of quantities.
557 views • 28 slides

Physical Quantities, Units & Measurement (QUM)
Physical Quantities, Units & Measurement (QUM). MC textbook, Chp 1 & 4 GLM textbook, Chp 1 & 4. Mars Climate Orbiter. This is the Mars Climate Orbiter. It was launched by NASA to study the atmosphere on Mars in 1998 It costs US$125 million It took over 9 months to travel from Earth to Mars
1.13k views • 60 slides

UNITS AND MEASUREMENT
UNITS AND MEASUREMENT. Physical Quantity – Fundamental & Derived Quantities Unit – Fundamental & Derived Units Characteristics of Standard Unit fps, cgs, mks & SI System of Units Definition of Fundamental SI units Measurement of Length – Large Distances and Small Distances
2.5k views • 73 slides

Units and Measurement. Science Tools. A) SI Units, Scientific Notation, Measurement, Accuracy, Precision, Error. Math and Units. Math- the language of Science SI Units – International System MKS Meter m Mass kg Time s National Bureau of Standards Prefixes. SI Unit Prefixes.
498 views • 44 slides
Units and Measurement. Kinds of Units. Base Derived. Math and Units. SI Units – International System MKS (Base Units) Meter m Mass kg Time s. Derived SI Units (examples). Units. Derived Units Velocity = Distance /Time => Unit of Velocity is _____
322 views • 28 slides
Electrical Quantities and Units
Electrical Quantities and Units. Electrical Charges (Q). All matters made of atoms which consist of electrons, protons and neutrons. Protons and neutrons are in the nucleas and electrons are moving around them.
834 views • 31 slides
IEEE Account
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
Age estimation on post-mortem CT based on pelvic bone mineral density measurement and the state of putrefaction: a multivariate method
- Original Article
- Published: 20 August 2024
Cite this article

- Eulalie Pefferkorn 1 ,
- Ophélie Guillerme 1 ,
- Pauline Saint-Martin 1 ,
- Frédéric Savall 2 ,
- Fabrice Dedouit 2 &
- Norbert Telmon 2
Age-at-death estimation is an important issue in forensic medicine and anthropology. Initially, methods relied on morphological criteria, but with the advancement of radiology, new techniques such as morphological studies on multi-slice computed tomography (CT) reconstructions have emerged. Recent studies have shown promising results by investigating the correlation between age and bone mineral density (BMD). However, there is currently a lack of data on post-mortem CTs (PMCT) involving decomposed bodies, and limited information exists regarding changes in Hounsfield Units measurement in a post-mortem context. In light of these gaps, our study aimed to examine the relationship between age at death and pubic and ilium BMD using a sample of forensic bodies. We also aimed to determine whether post-mortem processes, such as putrefaction, could interfere with this correlation. Our retrospective analysis encompassed 637 PMCTs conducted before medicolegal autopsies at Tours University Hospital. Utilizing simple and multiple linear regressions, we investigated the correlation between age and pubic and ilium BMD, as well as the relationship between BMD and the radiologic alteration index (RAI), a scale employed to quantify the degree of putrefaction. Our findings indicate promising outcomes in age-at-death estimation using pubic and/or ilium BMD for bodies exhibiting no or moderate decomposition (RAI < 80), particularly among individuals under 40 years old. However, for highly decomposed corpses (RAI ≥ 80), the presence of gas infiltration significantly influences the BMD of both the ilium and pubis. Consequently, we advocate for the incorporation of the RAI score into the age estimation equation to enhance the accuracy of our results in such cases. Further investigation involving a larger cohort of decomposed bodies could facilitate refinement and validation of our method within this specific population.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Explore related subjects
- Medical Imaging
Data availability
Not applicable.
Code availability
Cunha E, Baccino E, Martrille L, Ramsthaler F, Prieto J, Schuliar Y et al (2009) The problem of aging human remains and living individuals: a review. Forensic Sci Int 193(1–3):1–13
Article PubMed Google Scholar
Konigsberg LW, Herrmann NP, Wescott DJ, Kimmerle EH (2008) Estimation and Evidence in Forensic Anthropology: Age-at-Death. J Forensic Sci 53(3):541–557
Baccino E, Ubelaker DH, Hayek LA, Zerilli A (1999) Evaluation of seven methods of estimating age at death from mature human skeletal remains. J Forensic Sci 44(5):931–936
Fanton L, Gustin MP, Paultre U, Schrag B, Malicier D (2010) Critical study of observation of the sternal end of the right 4th rib. J Forensic Sci 55(2):467–472
Brooks S, Suchey J (1990) Skeletal age determination based on the os pubis : a comparison of the Acsadi-Nemeskeri and Suchey-Brooks methods. Hum Evol 5(3):5227–238
Article Google Scholar
McKern T, Stewart T. Skeletal age changes in young American males : analysed from the standpoint of age identification. (Report NM: QR and DCT, ed.)
Barrier P, Dedouit F, Braga J, Joffre F, Rougé D, Rousseau H et al (2009) Age at death estimation using multislice computed tomography reconstructions of the posterior pelvis. J Forensic Sci 54(4):773–778
Dedouit F, Telmon N, Costagliola R, Otal P, Florence LL, Joffre F et al (2007) New identification possibilities with postmortem multislice computed tomography. Int J Legal Med 121(6):507–510
Krämer JA, Schmidt S, Jürgens KU, Lentschig M, Schmeling A, Vieth V (2014) Forensic age estimation in living individuals using 30T MRI of the distal femur. Int J Legal Med. 128(3):509–14
Štern D, Payer C, Urschler M (2019) Automated age estimation from MRI volumes of the hand. Med Image Anal 58:101538
Secco L, Padalino P, Franceschetto L, Viero A, Pizzi M, De Conti G et al (2024) Micro-CT evaluation of morphological degenerative features of sterno-clavicular joint for age-at-death estimation in forensic anthropology – A qualitative analysis. Leg Med 1:67
Google Scholar
Wade A, Nelson A, Garvin G, Holdsworth DW (2011) Preliminary radiological assessment of age-related change in the trabecular structure of the human os pubis. J Forensic Sci 56(2):312–319
Pasquier E, De Saint Martin Pernot L, Burdin V, Mounayer C, Le Rest C, Colin D et al (1999) Determination of age at death : Assessment of an algorithm of age prediction using numerical three-dimensional CT data from pubic bones. Am J Phys Anthropol. 108(3):261–8
Mays S (2015) The effect of factors other than age upon skeletal age indicators in the adult. Ann Hum Biol 42(4):332–341. https://doi.org/10.3109/03014460.2015.1044470
Virtama P (1969) Radiographic measurements of cortical bone ; variations in a normal population between 1 and 90 years of age. Acta radio. (Stockholm : [s.n.] 1969., ed.)
Schreiber JJ, Anderson PA, Rosas HG et al (2011) Hounsfield Units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Jt Surg Am 93:1057–1063. https://doi.org/10.2106/JBJS.J.00160
Castillo RF, Ruiz MD (2011) Assessment of age and sex by means of DXA bone densitometry: Application in forensic anthropology. Forensic Sci Int 209(1–3):53–8. https://doi.org/10.1016/j.forsciint.2010.12.008
Dubourg O, Faruch-Bilfeld M, Telmon N, Maupoint E, Saint-Martin P, Savall F (2019) Correlation between pubic bone mineral density and age from a computed tomography sample. Forensic Sci Int 298:345–350
Coelho O, Navega D (2017) DXAGE : A new method for age at death estimation based on femoral bone mineral density and artificial neural networks. p 1–7
Bascou A, Dubourg O, Telmon N, Dedouit F, Saint-Martin P, Savall F (2021) Age estimation based on computed tomography exploration: a combined method. Int J Legal Med [Internet]. 2:10–4. https://doi.org/10.1007/s00414-021-02666-0
Ford JM, Kumm TR, Decker SJ (2020) An analysis of hounsfield unit values and volumetrics from computerized tomography of the proximal femur for sex and age estimation. J Forensic Sci 65(2):591–596
Villa C, Hansen MN, Buckberry J, Cattaneo C, Lynnerup N (2013) Forensic age estimation based on the trabecular bone changes of the pelvic bone using post-mortem CT. Forensic Sci Int [Internet]. 233(1–3):393–402. https://doi.org/10.1016/j.forsciint.2013.10.020
Beauthier JP (2011) De l’ouverture des corps aux racines de la médecine légale. In: De boeck (ed) Traité de médecine légale. 2e édition. p 21–4
Lee S, Chung CK, Oh SH, Park SB (2013) Correlation between bone mineral density measured by dual-energy X-ray absorptiometry and hounsfield units measured by diagnostic CT in lumbar spine. J Korean Neurosurg Soc 54(5):384–389
Article PubMed PubMed Central Google Scholar
Egger C, Vaucher P, Doenz F, Palmiere C, Mangin P, Grabherr S (2012) Development and validation of a postmortem radiological alteration index: the RA-Index. Int J Legal Med 126(4):559–566
Wikistat (2016) Introduction au bootstrap, pp 1–3. Available from: http://wikistat.fr/pdf/st-m-app-bootstrap . Accessed 15 May 2024
Muggeo VMR (2003) Estimating regression models with unknown break-points. Stat Med 22(19):3055–3071
Lo C, Ferna R (2011) Assessment of age and sex by means of DXA bone densitometry : application in forensic anthropology. 209:53–8
Curate F, Albuquerque A, Cunha EM (2013) Age at death estimation using bone densitometry: testing the Fernández Castillo and López Ruiz method in two documented skeletal samples from Portugal. Forensic Sci Int 226(1–3):296.e1-296.e6. https://doi.org/10.1016/j.forscii
Schreiber JJ, Anderson PA, Hsu WK (2014) Use of computed tomography for assessing bone mineral density. Neurosurg Focus 37(1):E4
Consonni V, Ballabio D, Todeschini R (2010) Evaluation of model predictive ability by external validation techniques. J Chemom 24(3–4):194–201
Savall F, Rérolle C, Hérin F, Dédouit F, Rougé D, Telmon N et al (2016) Reliability of the Suchey-Brooks method for a French contemporary population. Forensic Sci Int 266:586.e1-586.e5
San Millán M, Rissech C, Turbón D (2013) A test of Suchey-Brooks (pubic symphysis) and Buckberry-Chamberlain (auricular surface) methods on an identified Spanish sample: Paleodemographic implications. J Archaeol Sci 40(4):1743–1751
Hoppa RD (2000) Population variation in osteological aging criteria: an example from the pubic symphysis. Am J Phys Anthropol 111:185–191. https://doi.org/10.1002/(SICI)1096-8644(200002)111:2%3c185::AID-AJPA5%3e3.0.CO;2-4
Warming L, Hassager C, Christiansen C (2002) Changes in bone mineral density with age in men and women: A longitudinal study. Osteoporos Int 13(2):105–112
Hedlund LR, Gallagher JC (1989) The effect of age and menopause on bone mineral density of the proximal femur. J Bone Miner Res 4(4):639–642
Download references
Author information
Authors and affiliations.
Institute of Legal Medicine, CHRU TOURS, Trousseau Hospital, Avenue de La République, 37170, Chambray-Lès-Tours, France
Eulalie Pefferkorn, Ophélie Guillerme & Pauline Saint-Martin
Center of Anthropobiology and Genomics of Toulouse, UMR 5288 (CNRS/UT3) - Faculté de Médecine de Purpan, 37 allées J. Guesde, 31000, Toulouse, France
Frédéric Savall, Fabrice Dedouit & Norbert Telmon
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Eulalie Pefferkorn .
Ethics declarations
Declarations.
Not applicable considering the Jardé law concerning interventional reseach on humans and Helsinki declaration.
Consent to participate and publish
Competing interests, additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Pefferkorn, E., Guillerme, O., Saint-Martin, P. et al. Age estimation on post-mortem CT based on pelvic bone mineral density measurement and the state of putrefaction: a multivariate method. Int J Legal Med (2024). https://doi.org/10.1007/s00414-024-03316-x
Download citation
Received : 17 June 2024
Accepted : 13 August 2024
Published : 20 August 2024
DOI : https://doi.org/10.1007/s00414-024-03316-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Age estimation
- Postmortem CTs
- Bone mineral density
- Radiological alteration index
- Anthropology
- Find a journal
- Publish with us
- Track your research

IMAGES
COMMENTS
Contains easy-to-edit graphics such as graphs, maps, tables, timelines and mockups. Includes 500+ icons and Flaticon's extension for customizing your slides. Designed to be used in Google Slides, Canva, and Microsoft PowerPoint. 16:9 widescreen format suitable for all types of screens. Includes information about fonts, colors, and credits of ...
Units of Energy. Energy is the capacity to do work, or to produce heat. Energy can also be measured, and two common units are: Joule (J) = the SI unit of energy, named after James Prescott Joule. calorie (cal) = the heat needed to raise 1 gram of water by 1 oC.
SI units are common today Measuring length 70 km/h 4.5 m Vehicles Not Exceeding 1500 kg In Unladen Weight Are classified into two types: Base quantities Derived quantities 1.1 Physical Quantities Base quantity is like the brick - the basic building block of a house Derived quantity is like the house that was build up from a collection of ...
Download ppt "CHAPTER 1 Physical Quantities, Units and Measurement." Physical Quantities A property that can be measured and is presented with magnitude and unit. Look into your textbook contents page, what do you think are the quantities that is measured in each chapter? Examples - mass, weight, speed, density, time, volume, area, length etc.
1.1 What is Physics? The study of the physical world. Use a small number of basic concepts, equations, and assumptions to describe the physical world. Can be used to make predictions about a broad range of phenomena. Appliances, tools, buildings, inventions are all basic physics principles put to test.
Length. A. 1 meter or 105 centimeters B. 4 kilometers or 4400 meters C. 12 centimeters or 102 millimeters D. 1200 millimeters or 1 meter km m cm mm Metric Units The basic unit of length in the metric system in the meter and is represented by a lowercase m. Standard: The distance traveled by light in absolute vacuum in 1⁄299.792.458 of a second.
MEASUREMENT IS THE PROSES OF COMPARING A QUANTITY WITH ANOTHER QUANTITY WHICH IS USED AS UNIT. Measuring is to Compare a physical quantity of an object with a unit of measurement to get the value. QUANTITIES ARE EVERYTHING THAT CAN BE MEASURED,HAS VALUE AND HAS THE CERTAIN UNITS. UNITS ARE A STATEMENT THAT EXPLAINS THE VALUE OF A QUANTITY.
Features of this template. Contains easy-to-edit graphics such as graphs, maps, tables, timelines and mockups. Includes 500+ icons and Flaticon's extension for customizing your slides. Designed to be used in Google Slides, Canva, and Microsoft PowerPoint. 16:9 widescreen format suitable for all types of screens.
Presentation Transcript. Physics and Physical Measurement Topic 1.2 Measurement and Uncertainties. The S.I. system of fundamental and derived units. Standards of Measurement • SI units are those of the Système International d'Unités adopted in 1960 • Used for general measurement in most countries.
Description: Units and Measurement is the first topic studied in Physics initially. So, one must know about the basic terms of unit and dimensions. This presentation is in demand to provide knowledge of basic terms of unit and measurements to the students. - PowerPoint PPT presentation. Number of Views: 12464. Slides: 9. Provided by: matrixsikar.
CHAPTER 1 UNIT & MEASUREMENT.ppt - Free download as Powerpoint Presentation (.ppt), PDF File (.pdf), Text File (.txt) or view presentation slides online. This document provides an overview of units and measurement in technician science. It discusses base and derived quantities, scalar and vector quantities, measurement prefixes, and standard scientific notation.
It's time to start teaching measurement to your young learners. There are so many things to be learnt that we have to take a step back and look at where we need to start from. That's easy. You can start off using this Units of Measurement Warm-Up PowerPoint. In it, you can find many types of measurement, including length. This weight, volume, and Length PowerPoint is great to use for ...
the vocabulary we use to discuss the measurement. There are then a selection of slides at the end that create a quiz, asking the children to choose the correct way of measuring in different scenarios. Perfect for introducing measures in Kindergarten, this PowerPoint is perfect for the beginning of units on height, length, weight, capacity and time.
Units and Measurement - Units and Measurement is the first topic studied in Physics initially. So, one must know about the basic terms of unit and dimensions. This presentation is in demand to provide knowledge of basic terms of unit and measurements to the students. | PowerPoint PPT presentation | free to view
Unit Measurement of any physical quantity involves comparison with a certain basic, arbitrarily chosen, internationally accepted reference standard called unit. Fundamental Units The units of the fundamental or base quantities are called fundamental or base units. Examples:metre, kilogramme, second, etc. Derived Units The units of the derived ...
Physical Quantities, Units and Measurements - Free download as Powerpoint Presentation (.ppt), PDF File (.pdf), Text File (.txt) or view presentation slides online. Lesson slides for Physics chapter on Physical Quantities, Units and Measurements. Includes quantities and SI units, conversion of units, vernier calipers, micrometer, vectors and scalars, ticker tape timer and pendulum.
The mGFR is a direct measure of how well your kidneys are removing waste products from the blood. It can be a complicated and lengthy process. So, it is not used as often as the estimated GFR (eGFR). Your healthcare professional may recommend this test if a more accurate measure of your kidney function is needed. There are many ways to complete ...
Physical Quantities, Units and Measurement Name: _____ Class: _____ Index: _____. Physics is the study of the natural world around us - from the very large, such as the solar system, to the very small, such as the atom. The study of Physics is commonly divided into major topics such as General Physics, Thermal Physics, Light, Waves and Sound, Electricity and Magnetism.
This transformation necessitates convenient measurement sensor and gesture recognition algorithms with shorter recognition delays and more reliable recognition results. In pursuit of this objective, we propose a novel pipeline for continuous and rapid gesture recognition employing a portable inertial measurement unit (IMU) device.
Age-at-death estimation is an important issue in forensic medicine and anthropology. Initially, methods relied on morphological criteria, but with the advancement of radiology, new techniques such as morphological studies on multi-slice computed tomography (CT) reconstructions have emerged. Recent studies have shown promising results by investigating the correlation between age and bone ...