
- Table of Contents
- Random Entry
- Chronological
- Editorial Information
- About the SEP
- Editorial Board
- How to Cite the SEP
- Special Characters
- Advanced Tools
- Support the SEP
- PDFs for SEP Friends
- Make a Donation
- SEPIA for Libraries
- Entry Contents

Bibliography
Academic tools.
- Friends PDF Preview
- Author and Citation Info
- Back to Top
Darwin: From the Origin of Species to the Descent of Man
This entry offers a broad historical review of the origin and development of Darwin’s theory of evolution by natural selection through the initial Darwinian phase of the “Darwinian Revolution” up to the publication of the Descent of Man in 1871. The development of evolutionary ideas before Darwin’s work has been treated in the separate entry evolutionary thought before Darwin . Several additional aspects of Darwin’s theory of evolution and his biographical development are dealt with in other entries in this encyclopedia (see the entries on Darwinism ; species ; natural selection ; creationism ). The remainder of this entry will focus on aspects of Darwin’s theory not developed in the other entries. It will also maintain a historical and textual approach. Other entries in this encyclopedia cited at the end of the article and the bibliography should be consulted for discussions beyond this point. The issues will be examined under the following headings:
1.1 Historiographical Issues
1.2 darwin’s early reflections, 2.1. the concept of natural selection.
- 2.2. The Argument of the Published Origin
3.1 The Popular Reception of Darwin’s Theory
3.2 the professional reception of darwin’s theory, 4.1 the genesis of darwin’s descent, 4.2 darwin on mental powers, 4.3 the ethical theory of the descent of man.
- 4.4 The Reception of the Descent
5. Summary and Conclusion
Other internet resources, related entries, acknowledgments, 1. the origins of darwin’s theory.
Charles Darwin’s version of transformism has been the subject of massive historical and philosophical scholarship almost unparalleled in any other area of the history of science. This includes the continued flow of monographic studies and collections of articles on particular aspects of Darwin’s theory (Prestes 2023; R. J. Richards and Ruse 2016; Ruse 2013a, 2009a,b,c; Ruse and Richards 2009; Hodge and Radick 2009; Hösle and Illies 2005; Gayon 1998; Bowler 1996; Depew and Weber 1995; Kohn 1985a). The continuous production of popular and professional biographical studies on Darwin provides ever new insights (Ruse et al. 2013a; Johnson 2012; Desmond and Moore 1991, 2009; Browne 1995, 2002; Bowlby 1990; Bowler 1990). In addition, major editing projects on Darwin’s manuscripts and the completion of the Correspondence , project through the entirety of Darwin’s life, continue to reveal details and new insights into the issues surrounding Darwin’s own thought (Keynes [ed.] 2000; Burkhardt et al. [eds] 1985–2023; Barrett et al. [eds.] 1987). The Cambridge Darwin Online website (see Other Internet Resources ) serves as an international clearinghouse for this worldwide Darwinian scholarship, functioning as a repository for electronic versions of all the original works of Darwin, including manuscripts and related secondary materials. It also supplies a continuously updated guide to current literature.
A long tradition of scholarship has interpreted Darwin’s theory to have originated from a framework defined by endemic British natural history, a British tradition of natural theology defined particularly by William Paley (1743–1805), the methodological precepts of John Herschel (1792–1871), developed in his A Preliminary Discourse on the Study of Natural Philosophy (1830 [1987]), and the geological theories of Charles Lyell (1797–1875). His conversion to the uniformitarian geology of Charles Lyell and to Lyell’s advocacy of “deep” geological time during the voyage of the HMS Beagle (December 1831–October 1836), has been seen as fundamental in his formation (Norman 2013; Herbert 2005; Hodge 1983). Complementing this predominantly anglophone historiography has been the social-constructivist analyses emphasizing the origins of Darwin’s theories in British Political Economy (Young 1985: chps. 2, 4, 5). It has also been argued that a primary generating source of Darwin’s inquiries was his involvement with the British anti-slavery movement, a concern reaching back to his revulsion against slavery developed during the Beagle years (Desmond and Moore 2009).
A body of recent historiography, on the other hand, drawing on the wealth of manuscripts and correspondence that have become available since the 1960s (online at Darwin online “Papers and Manuscripts” section, see Other Internet Resources ) has de-emphasized some of the novelty of Darwin’s views and questions have been raised regarding the validity of the standard biographical picture of the early Darwin. These materials have drawn attention to previously ignored aspects of Darwin’s biography. In particular, the importance of his Edinburgh period from 1825–27, largely discounted in importance by Darwin himself in his late Autobiography , has been seen as critical for his subsequent development (Desmond and Moore 1991; Hodge 1985). As a young medical student at the University of Edinburgh (1825–27), Darwin developed a close relationship with the comparative anatomist Robert Edmond Grant (1793–1874) through the student Plinian Society, and in many respects Grant served as Darwin’s first mentor in science in the pre- Beagle years (Desmond and Moore 1991, chp. 1). Through Grant he was exposed to the transformist theories of Jean Baptiste Lamarck and the Cuvier-Geoffroy debate centered on the Paris Muséum nationale d’histoire naturelle (see entry on evolutionary thought before Darwin , Section 4).
These differing interpretive frameworks make investigations into the origins of Darwin’s theory an active area of historical research. The following section will explore these origins.
In its historical origins, Darwin’s theory was different in kind from its main predecessors in important ways (Ruse 2013b; Sloan 2009a; see also the entry on evolutionary thought before Darwin ). Viewed against a longer historical scenario, Darwin’s theory does not deal with cosmology or the origins of the world and life through naturalistic means, and therefore was more restricted in its theoretical scope than its main predecessors influenced by the reflections of Georges Louis LeClerc de Buffon (1707–1788), Johann Herder (1744–1803, and German Naturphilosophen inspired by Friederich Schelling (1775–1854) . This restriction also distinguished Darwin’s work from the grand evolutionary cosmology put forth anonymously in 1844 by the Scottish publisher Robert Chambers (1802–1871) in his immensely popular Vestiges of the Natural History of Creation , a work which in many respects prepared Victorian society in England, and pre-Civil War America for the acceptance of a general evolutionary theory in some form (Secord 2000; MacPherson 2015). It also distinguishes Darwin’s formulations from the theories of his contemporary Herbert Spencer (1820–1903).
Darwin’s theory first took written form in reflections in a series of notebooks begun during the latter part of the Beagle voyage and continued after the return of the Beagle to England in October of 1836 (Barrett et al., 1987). His reflections on the possibility of species change are first entered in March of 1837 (“Red Notebook”) and are developed in the other notebooks (B–E) through July of 1839 (Barrett et al. 1987; Hodge 2013a, 2009). Beginning with the reflections of the third or “D” “transmutation” Notebook, composed between July and October of 1838, Darwin first worked out the rudiments of what was to become his theory of natural selection. In the parallel “M” and “N” Notebooks, dating between July of 1838 and July of 1839, and in a loose collection called “Old and Useless Notes”, dating from approximately 1838–40, he also developed many of his main ideas on human evolution that would only be made public in the Descent of Man of 1871 (below, Section 4).
To summarize a complex issue, these Notebook reflections show Darwin proceeding through a series of stages in which he first formulated a general theory of the transformation of species by historical descent from common ancestors. He then attempted to work out a causal theory of life that would explain the tendency of life to complexify and diversify (Hodge 2013a, 2009, 1985; Sloan 1986). This causal inquiry into the underlying nature of life, and with it the search for an explanation of life’s innate tendency to develop and complexify, was then replaced by a dramatic shift in focus away from these inquiries. This concern with a causal theory of life was then replaced by a new emphasis on external forces controlling population, a thesis developed from his reading of Thomas Malthus’s (1766–1834) Essay on the Principle of Population (6th ed. 1826). For Malthus, human populaton was assumed to expand geometrically, while food supply expanded arithmetically, leading to an inevitable struggle of humans for existence. The impact of Darwin’s reading of this edition of the Essay in August of 1838, was dramatic. It enabled him to theorize the existence of a constantly-acting dynamic force behind the transformation of species.
Darwin’s innovation was to universalize the Malthusian “principle of population” to apply to all of nature. In so doing, Darwin effectively introduced what may be termed an “inertial” principle into his theory, although such language is never used in his text. Newton’s first law of motion, set forth in his Mathematical Principles of Natural Philosophy (1st ed. 1687), established his physical system upon the tendency of all material bodies to persist eternally either at rest or in uniform motion in a straight line, requiring a causal force explanation for any deviations from this initial state. But Newton did not seek a deeper metaphysical explanation of this inertial state. Law One is simply an “axiom” in Newton’s Principia. Similarly, the principle of population supplied Darwin with the assumption of an initial dynamic state of affairs that was not itself explained within the theory—there is no attempt to account causally for this tendency of living beings universally to reproduce geometrically. Similarly for Darwin, the principle of population functions axiomatically, defining a set of initial conditions from which any deviance from this ideal state demands explanation.
This theoretical shift enabled Darwin to bracket his earlier efforts to develop a causal theory of life, and focus instead on the means by which the dynamic force of population was controlled. This allowed him to emphasize how controls on population worked in company with the phenomenon of slight individual variation between members of the same species, in company with changing conditions of life, to produce a gradual change of form and function over time, leading to new varieties and eventually to new species. This opened up the framework for Darwin’s most important innovation, the concept of “natural” selection.
2. Darwinian Evolution
The primary distinguishing feature of Darwin’s theory that separates it from previous explanations of species change centers on the causal explanation he offered for how this process occurred. Prior theories, such as the theory of Jean-Baptiste Lamarck (see entry on evolutionary thought before Darwin ), relied on the inherent dynamic properties of matter. The change of species was not, in these pre-Darwinian efforts, explained through an adaptive process. Darwin’s emphasis after the composition of Notebook D on the factors controlling population increase, rather than on a dynamic theory of life grounded in vital forces, accounts for many of the differences between Darwin’s theory and those of his predecessors and contemporaries.
These differences can be summarized in the concept of natural selection as the central theoretical component of Darwinian theory. However, the exact meaning of this concept, and the varying ways he stated the principle in the Origin over its six editions (1859–1872), has given rise to multiple interpretations of the meaning of this principle in the history of Darwinism, and the different understandings of his meaning deeply affected different national and cultural receptions of his theory (see below, Section 3 .1).
One way to see the complexity of Darwin’s own thinking on these issues is to follow the textual development of this concept from the close of the Notebook period (1839) to the publication of the Origin of Species in 1859. This period of approximately twenty years involved Darwin in a series of reflections that form successive strata in the final version of his theory of the evolution of species. Understanding the historical sequence of these developments also has significance for subsequent controversies over this concept and the different readings of the Origin as it went through its successive revisions. This historical development of the concept also has some bearing on assessing Darwin’s relevance for more general philosophical questions, such as those surrounding the relevance of his theory for such issues as the concept of a more general teleology of nature.
The earliest set of themes in the manuscript elaboration of natural selection theory can be characterized as those developed through a particular form of the argument from analogy. This took the form of a strong “proportional” form of the analogical argument that equated the relation of human selection to the development of domestic breeds as an argument of the basic form: human selection is to domestic variety formation as natural selection is to natural species formation (White, Hodge and Radick 2021, chps. 4–5). This makes a direct analogy between the actions of nature with those of humans in the process of selection. The specific expressions, and changes, in this analogy are important to follow closely. As this was expressed in the first coherent draft of the theory, a 39-page pencil manuscript written in 1842, this discussion analogized the concept of selection of forms by human agency in the creation of the varieties of domestic animals and plants, to the active selection in the natural world by an almost conscious agency, a “being infinitely more sagacious than man (not an omniscient creator)” who acts over “thousands and thousands of years” on “all the variations which tended towards certain ends” (Darwin 1842 in Glick and Kohn 1996, 91). This agency selects out those features most beneficial to organisms in relation to conditions of life, analogous in its action to the selection by man on domestic forms in the production of different breeds. Interwoven with these references to an almost Platonic demiurge are appeals to the selecting power of an active “Nature”:
Nature’s variation far less, but such selection far more rigid and scrutinizing […] Nature lets <<an>> animal live, till on actual proof it is found less able to do the required work to serve the desired end, man judges solely by his eye, and knows not whether nerves, muscles, arteries, are developed in proportion to the change of external form. (Ibid., 93)
These themes were continued in the 230 page draft of his theory of 1844. Again he referred to the selective action of a wise “Being with penetration sufficient to perceive differences in the outer and innermost organization quite imperceptible to man, and with forethought extending over future centuries to watch with unerring care and select for any object the offspring of an organism produced” (Darwin 1844 in ibid., 101). This selection was made with greater foresight and wisdom than human selection. As he envisions the working of this causal agency,
In accordance with the plan by which this universe seems governed by the Creator, let us consider whether there exist any secondary means in the economy of nature by which the process of selection could go on adapting, nicely and wonderfully, organisms, if in ever so small a degree plastic, to diverse ends. I believe such secondary means do exist. (Ibid., 103).
Darwin returned to these issues in 1856, following a twelve-year period in which he published his Geological Observations on the Volcanic Islands (1844), the second edition of his Journal of Researches (1845), Geological Observations on South America (1846), the four volumes on fossil and living barnacles ( Cirripedia ) (1851, 54, 55), and Geological Observations on Coral Reefs (1851). In addition, he published several smaller papers on invertebrate zoology and on geology, and reported on his experiments on the resistance of seeds to salt water, a topic that would be of importance in his explanation of the population of remote islands.
These intervening inquiries positioned Darwin to deal with the question of species permanence against an extensive empirical background. The initial major synthesis of these investigations takes place in his long manuscript, or “Big Species Book”, commenced in 1856, known in current scholarship as the “Natural Selection” manuscript. This formed the immediate background text behind the published Origin . Although incomplete, the “Natural Selection” manuscript provides insights into many critical issues in Darwin’s thinking. It was also prepared with an eye to the scholarly community. This distinguishes its content and presentation from that of the subsequent “abstract” which became the published Origin of Species . “Natural Selection” contained tables of data, references to scholarly literature, and other apparatus expected of a non-popular work, none of which appeared in the published Origin .
The “Natural Selection” manuscript also contained some new theoretical developments of relevance to the concept of natural selection that are not found in earlier manuscripts. Scholars have noted the introduction in this manuscript of the “principle of divergence”, the thesis that organisms under the action of natural selection will tend to radiate and diversify within their “conditions of life”—the contemporary name for the complex of environmental and species-interaction relationships (Kohn 1985b, 2009). Although the concept of group divergence under the action of natural selection might be seen as an implication of Darwin’s theory from his earliest formulations of the 1830s, nonetheless Darwin’s explicit definition of this as a “principle”, and its discussion in a long late insertion in the “Natural Selection” manuscript, suggests its importance for Darwin’s mature theory. The principle of divergence was now seen by Darwin to form an important link between natural variation and the conditions of existence under the action of the driving force of population increase.
Still evident in the “Natural Selection” manuscript is Darwin’s implicit appeal to some kind of teleological ordering of the process. The action of the masculine-gendered “wise being” of the earlier manuscripts, however, has now been given over entirely to the action of a selective “Nature”, now referred to in the traditional feminine gender. This Nature,
…cares not for mere external appearance; she may be said to scrutinise with a severe eye, every nerve, vessel & muscle; every habit, instinct, shade of constitution,—the whole machinery of the organisation. There will be here no caprice, no favouring: the good will be preserved & the bad rigidly destroyed.… Can we wonder then, that nature’s productions bear the stamp of a far higher perfection than man’s product by artificial selection. With nature the most gradual, steady, unerring, deep-sighted selection,—perfect adaption [sic] to the conditions of existence.… (Darwin 1856–58 [1974: 224–225])
The language of this passage, directly underlying statements about the action of “natural selection” in the first edition of the published Origin , indicates the complexity in the exegesis of Darwin’s meaning of “natural selection” when viewed in light of its historical genesis (Ospovat 1981). The parallels between art and nature, the intentionality implied in the term “selection”, the notion of “perfect” adaptation, and the substantive conception of “nature” as an agency working toward certain ends, all render Darwin’s views on teleological purpose more complex than they are typically interpreted from the standpoint of contemporary Neo-selectionist theory (Lennox 1993, 2013). As will be discussed below, the changes Darwin subsequently made in his formulations of this concept over the history of the Origin have led to different conceptions of what he meant by this principle.
The hurried preparation and publication of the Origin between the summer of 1858 and November of 1859 was prompted by the receipt on June 18 of 1858 of a letter and manuscript from Alfred Russel Wallace (1823–1913) that outlined his remarkably similar views on the possibility of continuous species change under the action of a selection upon natural variation (Wallace 1858 in Glick and Kohn 1996, 337–45). This event had important implications for the subsequent form of Darwin’s published argument. Rapidly condensing the detailed arguments of the unfinished “Natural Selection” manuscript into shorter chapters, Darwin also universalized several claims that he had only developed with reference to specific groups of organisms, or which he had applied only to more limited situations in the manuscript. This resulted in a presentation of his theory at the level of broad generalization. The absence of tables of data, detailed footnotes, and references to the secondary literature in the published version also resulted in predictable criticisms which will be discussed below in Section 3.2 .
2.2. The Central Argument of the Published Origin
The Origin of Species by Means of Natural Selection, or the Preservaton of Favoured Races in the Struggle for Life was issued in London by the publishing house of John Murray on November 24, 1859 (Darwin 1859 [1964]). The structure of the argument presented in the published Origin has been the topic of considerable literature and can only be summarized here. Although Darwin himself described his book as “one long argument”, the exact nature of this argument is not immediately transparent, and alternative interpretations have been made of his reasoning and rhetorical strategies in formulating his evolutionary theory. (Prestes 2023; White, Hodge and Radick 2021; Hodge 2013b, 1977; Hoquet 2013; Hull 2009; Waters 2009; Depew 2009; Ruse 2009; Lennox 2005; Hodge 1983b).
The scholarly reconstruction of Darwin’s methodology employed in the Origin has taken two primary forms. One approach has been to reconstruct it from the standpoint of currently accepted models of scientific explanation, sometimes presenting it as a formal deductive model (Sober 1984). Another, more historical, approach interprets his methodology in the context of accepted canons of scientific explanation found in Victorian discussions of the period (see the entry on Darwinism ; Prestes 2023; White, Hodge and Radick 2021; Hodge 2013b, 1983b, 1977; Hoquet 2013; Hull 2009; Waters 2009; Depew 2009; Lennox 2005). The degree to which Darwin did in fact draw from the available methodological discussions of his contemporaries—John Herschel, William Whewell, John Stuart Mill—is not fully clear from available documentary sources. The claim most readily documented, and defended particularly by White, Hodge and Radick (2021) and M. J. S. Hodge (1977, 1983a), has emphasized the importance of John Herschel’s A Preliminary Discourse on the Study of Natural Philosophy (1830 [1987]), which Darwin read as a young student at Cambridge prior to his departure on the HMS Beagle in December of 1831.
In Herschel’s text he would have encountered the claim that science seeks to determine “true causes”— vera causae— of phenomena through the satisfaction of explicit criteria of adequacy (Herschel, 1830 [1987], chp. 6). This concept Newton had specified in the Principia as the third of his “Rules of Reasoning in Philosophy” (see the entry on Newton’s philosophy , Section 4). Elucidation of such causes was to be the goal of scientific explanation. Vera causae , in Herschel’s formulation, were those necessary to produce the given effects; they were truly active in producing the effects; and they adequately explained these effects.
The other plausible methodological source for Darwin’s mature reasoning was the work of his older contemporary and former Cambridge mentor, the Rev. William Whewell (1794–1866), whose three-volume History of the Inductive Sciences (Whewell 1837) Darwin read with care after his return from his round-the-world voyage (Ruse 2013c, 1975). On this reading, a plausible argument has been made that the actual structure of Darwin’s text is more closely similar to a “Whewellian” model of argument. In Whewell’s accounts of his philosophy of scientific methodology (Whewell 1840, 1858), the emphasis of scientific inquiry is, as Herschel had also argued, to be placed on the discovery of “true causes”. But evidence for the determination of a vera causa was to be demonstrated by the ability of disparate phenomena to be drawn together under a single unifying “Conception of the Mind”, exemplified for Whewell by Newton’s universal law of gravitation. This “Consilience of Inductions”, as Whewell termed this process of theoretical unification under a few simple concepts, was achieved only by true scientific theories employing true causes (Whewell 1840: xxxix). It has therefore been argued that Darwin’s theory fundamentally produces this kind of consilience argument, and that his methodology is more properly aligned with that of Whewell.
A third account, related to the Whewellian reading, is that of David Depew. Building on Darwin’s claim that he was addressing “the general naturalist public,” Darwin is seen as developing what Depew has designated as “situated argumentation”, similar to the views developed by contemporary Oxford logician and rhetorical theorist Richard Whately (1787–1863) (Depew 2009). This rhetorical strategy proceeds by drawing the reader into Darwin’s world by personal narration as it presents a series of limited issues for acceptance in the first three chapters, none of which required of the reader a considerable leap of theoretical assent, and most of which, such as natural variation and Malthusian population increase, had already been recognized in some form in the literature of the period.
As Darwin presented his arguments to the public, he opens with a pair of chapters that draw upon the strong analogy developed in the manuscripts between the action of human art in the production of domestic forms, and the actions of selection “by nature.” The resultant forms are presumed to have arisen through the action of human selection on the slight variations existing between individuals within the same species. The interpretation of this process as implying directional, and even intentional, selection by a providential “Nature” that we have seen in the manuscripts was, however, downplayed in the published work through the importance given by Darwin to the role of “unconscious” selection, a concept not encountered in the Natural Selection manuscript. Such selection denotes the practice even carried out by aboriginal peoples who simply seek to maintain the integrity and survival of a breed or species by preserving the “best” forms.
The domestic breeding analogy is, however, more than a decorative rhetorical strategy. It repeatedly functions for Darwin as the principal empirical example to which he could appeal at several places in the text as a means of visualizing the working of natural selection in nature, and this appeal remains intact through the six editions of the Origin.
From this model of human selection working on small individual natural variations to produce the domestic forms, Darwin then developed in the second chapter the implications of “natural” variation, delaying discussion of the concept of natural selection until Chapter IV. The focus of the second chapter introduces another important issue. Here he extends the discussion of variation developed in Chapter I into a critical analysis of the common understanding of classification as grounded on the definition of species and higher groups based on the possession of essential defining properties. It is in this chapter that Darwin most explicitly develops his own position on the nature of organic species in relation to his theory of descent. It is also in this chapter that he sets forth the ingredients for his attack on one meaning of species “essentialism”.
Darwin’s analysis of the “species question” involves a complex argument that has many implications for how his work was read by his contemporaries and successors, and its interpretation has generated a considerable literature (see the entries on species and Darwinism ; Mallet 2013; R. A. Richards 2010; Wilkins 2009; Stamos 2007; Sloan 2009b, 2013; Beatty 1985).
Prior tradition had been heavily affected by eighteenth-century French naturalist Buffon’s novel conception of organic species in which he made a sharp distinction between “natural” species, defined primarily by fertile interbreeding, and “artificial” species and varieties defined by morphological traits and measurements upon these (see the entry on evolutionary thought before Darwin , Section 3.3). This distinction was utilized selectively by Darwin in an unusual blending of two traditions of discussion that are conflated in creative ways in Darwin’s analysis.
Particularly as the conception of species had been discussed by German natural historians of the early nineteenth-century affected by distinctions introduced by philosopher Immanuel Kant (1724–1804), “Buffonian” species were defined by the material unity of common descent and reproductive continuity. This distinguished them by their historical and material character from the taxonomic species of the “Linnean” tradition of natural history. This distinction between “natural” and “logical” species had maintained a distinction between problems presented in the practical classification of preserved specimens, distinguished by external characters, and those relating to the unity of natural species, which was grounded upon reproductive unity and the sterility criterion (Sloan 2009b).
Remarkable in Darwin’s argument is the way in which he draws selectively in his readings from these two preexistent traditions to undermine the different grounds of species “realism” assumed within both of these traditions of discourse. One framework—what can be considered in his immediate context the “Linnean” tradition—regarded species in the sense of universals of logic or class concepts, whose “reality” was often grounded on the concept of divine creation. The alternative “Buffonian” tradition viewed species more naturalistically as material lineages of descent whose continuity was determined by some kind of immanent principle, such as the possession of a conserving “internal mold” or specifying vital force (see evolutionary thought before Darwin 3.3). The result in Darwin’s hands is a complex terminological interweaving of concepts of Variety, Race, Sub-species, Tribe, and Family that can be shown to be a fusion of different traditions of discussion in the literature of the period. This creative conflation also led to many confusions among his contemporaries about how Darwin actually did conceive of species and species change in time.
Darwin addresses the species question by raising the problems caused by natural variation in the practical discrimination of taxa at the species and varietal levels, an issue with which he had become closely familiar in his taxonomic revision of the Sub-class Cirripedia (barnacles) in his eight-year study on this group. Although the difficulty of taxonomic distinctions at this level was a well-recognized problem in the literature of the time, Darwin subtly transforms this practical problem into a metaphysical ambiguity—the fuzziness of formal taxonomic distinctions created by variation in preserved specimens is seen to imply a similar ambiguity of “natural” species boundaries.
We follow this in reading how natural variation is employed by Darwin in Chapter Two of the Origin to break down the distinction between species and varieties as these concepts were commonly employed in the practical taxonomic literature. The arbitrariness apparent in making distinctions, particularly in plants and invertebrates, meant that such species were only what “naturalists having sound judgment and wide experience” defined them to be ( Origin 1859 [1964], 47). These arguments form the basis for claims by his contemporaries that Darwin was a species “nominalist”, who defined species only as conventional and convenient divisions of a continuum of individuals.
But this feature of Darwin’s discussion of species captures only in part the complexity of his argument. Drawing also on the tradition of species realism developed within the “Buffonian” tradition, Darwin also affirmed that species and varieties are defined by common descent and material relations of interbreeding. Darwin then employed the ambiguity of the distinction between species and varieties created by individual variation in practical taxonomy to undermine the ontological fixity of “natural” species. Varieties are not simply the formal taxonomic subdivisions of a natural species as conceived in the Linnaean tradition. They are, as he terms them, “incipient” species (ibid., 52). This subtly transformed the issue of local variation and adaptation to circumstances into a primary ingredient for historical evolutionary change. The full implications to be drawn from this argument were, however, only to be revealed in Chapter Four of the text.
Before assembling the ingredients of these first two chapters, Darwin then introduced in Chapter Three the concept of a “struggle for existence”. This concept is introduced in a “large and metaphorical sense” that included different levels of organic interactions, from direct struggle for food and space to the struggle for life of a plant in a desert. Although described as an application of Thomas Malthus’s parameter of geometrical increase of population in relation to the arithmetical increase of food supply, Darwin’s use of this concept in fact reinterprets Malthus’s principle, which was formulated only with reference to human population in relation to food supply. It now becomes a general principle governing all of organic life. Thus all organisms, including those comprising food for others, would be governed by the tendency to geometrical increase.
Through this universalization, the controls on population become only in the extreme case grounded directly on the traditional Malthusian limitations of food and space. Normal controls are instead exerted through a complex network of relationships of species acting one on another in predator-prey, parasite-host, and food-web relations. This profound revision of Malthus’s arguments rendered Darwin’s theory deeply “ecological” as this term would later be employed. We can cite two thought experiments employed by Darwin himself as illustrations (ibid., 72–74). The first concerns the explanation of the abundance of red clover in England. This Darwin sees as dependent on the numbers of pollinating humble bees, which are controlled in turn by the number of mice, and these are controlled by the number of cats, making cats the remote determinants of clover abundance. The second instance concerns the explanation of the abundance of Scotch Fir. In this example, the number of fir trees is limited indirectly by the number of cattle.
With the ingredients of the first three chapters in place, Darwin was positioned to assemble these together in his grand synthesis of Chapter Four on “natural” selection. In this long discussion, Darwin develops the main exposition of his central theoretical concept. For his contemporaries and for the subsequent tradition, however, the meaning of Darwin’s concept of “natural” selection was not unambiguously evident for reasons we have outlined above, and these unclarities were to be the source of several persistent lines of disagreement and controversy.
The complexities in Darwin’s presentation of his central principle over the six editions of the published Origin served historically to generate several different readings of his text. In the initial introduction of the principle of natural selection in the first edition of Darwin’s text, it is characterized as “preservation of favourable variations and the rejection of injurious variations” (ibid., 81). When Darwin elaborated on this concept in Chapter Four of the first edition, he continued to describe natural selection in language suggesting that it involved intentional selection, continuing the strong art-nature analogy found in the manuscripts. For example:
As man can produce and certainly has produced a great result by his methodical and unconscious means of selection, what may not nature effect? Man can act only on external and visible characters: nature cares nothing for appearances, except in so far as they may be useful to any being. She can act on every internal organ, on every shade of constitutional difference, on the whole machinery of life. Man selects only for his own good; Nature only for that of the being which she tends. Every selected character is fully exercised by her; and the being is placed under well-suited conditions of life. (Ibid., 83)
The manuscript history behind such passages prevents the simple discounting of these statements as mere rhetorical imagery. As we have seen, the parallel between intentional human selectivity and that of “nature” formed the proportional analogical model upon which the concept of natural selection was originally constructed.
Criticisms that quickly developed over the overt intentionality embedded in such passages, however, led Darwin to revise the argument in editions beginning with the third edition of 1861. From this point onward he explicitly downplayed the intentional and teleological language of the first two editions, denying that his appeals to the selective role of “nature” were anything more than a literary figure. Darwin then moved decisively in the direction of defining natural selection as the description of the action of natural laws working upon organisms rather than as an efficient or final cause of life. He also regrets in his Correspondence his mistake in not utilizing the designation “natural preservation” rather than “natural selection” to characterize his principle (letter to Lyell 28 September 1860, Burkhardt Correspondence 8, 397; also see Darwin Correspondence Project in Other Internet Resources ). In response to criticisms of Alfred Russel Wallace, Darwin then adopted in the fifth edition of 1869 his contemporary (1820–1903) Herbert Spencer’s designator, “survival of the fittest”, as a synonym for “natural selection” (Spencer 1864, 444–45; Darwin 1869, 72). This redefinition further shifted the meaning of natural selection away from the concept that can be extracted from the early texts and drafts. These final statements of the late 1860s and early 70s underlie the tradition of later “mechanistic” and non-teleological understandings of natural selection, a reading developed by his disciples who, in the words of David Depew, “had little use for either his natural theodicy or his image of a benignly scrutinizing selection” (Depew 2009, 253). The degree to which this change preserved the original strong analogy between art and nature can, however, be questioned. Critics of the use of this analogy had argued since the original formulations that the comparison of the two modes of selection actually worked against Darwin’s theory (Wallace 1858 in Glick and Kohn 1997, 343). This critique would also be leveled against Darwin in the critical review of 1867 by Henry Fleeming Jenkin discussed below.
The conceptual synthesis of Chapter Four also introduced discussions of such matters as the conditions under which natural selection most optimally worked, the role of isolation, the causes of the extinction of species, and the principle of divergence. Many of these points were made through the imaginative use of “thought experiments” in which Darwin constructed possible scenarios through which natural selection could bring about substantial change.
One prominent way Darwin captured for the reader the complexity of this process is reflected in the single diagram to appear in all the editions of the Origin . In this illustration, originally located as an Appendix to the first edition, but thereafter moved into Chapter Four, Darwin summarized his conception of how species were formed and diverged from common ancestral points. This image also served to depict the frequent extinction of most lineages, an issue developed in detail in Chapter Ten. It displayed pictorially the principle of divergence, illustrating the general tendency of populations to diverge and fragment under the pressure of population increase. It supplied a way of envisioning relations of taxonomic affinity to time, and illstrated the persistence of some forms unchanged over long geological periods in which stable conditions prevail.

Figure: Tree of life diagram from Origin of Species ( Origin 1859:“Appendix”.
Remarkable about Darwin’s diagram of the tree of life is the relativity of its coordinates. It is first presented as applying only to the divergences taking place in taxa at the species level, with varieties represented by the small lower-case letters within species A–L of a “wide ranging genus”, with the horizontal lines representing time segments measured in terms of a limited number of generations. However, the attentive reader could quickly see that Darwin’s destructive analysis of the distinction between “natural” and “artificial” species in Chapter Two, implied the relativity of the species-variety distinction, this diagram could represent eventually all organic relationships, from those at the non-controversial level of diverging varieties within fixed species, to those of the relations of Species within different genera. Letters A–L could also represent taxa at the level of genera, families or orders. The diagram can thus be applied to relationships between all levels of the Linnaean hierarchy with the time segments representing potentially vast expanses of time, and the horizontal spread of branches the degree of taxonomic divergence over time. In a very few pages of argument, the diagram was generalized to represent the most extensive group relations, encompassing the whole of geological time. Extension of the dotted lines at the bottom could even suggest, as Darwin argues in the last paragraph of the Origin , that all life was a result of “several powers, having been originally breathed into a few forms or into one” (Darwin 1859 [1964], 490). This could suggest a single naturalistic origin of all original forms either by material emergence, or through the action of a vitalistic power of life. Darwin’s use of Biblical language could also be read as allowing for the action of a supernatural cause.
In response to criticisms concerning this latter point, Darwin quickly added to the final paragraph in the second edition of 1860 the phrase “by the Creator” (1860: 484), which remained in all subsequent editions. as did the quotations on the frontispiece from familiar discussions in British natural theology concerning creation by secondary causation. Conceptual space was thereby created for the reading of the Origin by some contemporaries, notably by the Harvard botanist Asa Gray (1810–88), as compatible with traditional natural theology (Gray 1860).
The sweep of the theoretical generalization that closed the natural selection chapter, one restated even more generally in the final paragraph of the book, required Darwin to deal with several obvious objections to the theory that constitute the main “defensive” chapters of the Origin (Five–Nine), and occupy him through the numerous revisions of the text between 1859 and 1872. As suggested by David Depew, the rhetorical structure of the original text developed in an almost “objections and response” structure that resulted in a constant stream of revisions to various editions of the original text as Darwin engaged his opponents (Depew 2009; Peckham 2006). Anticipating at first publication several obvious lines of objection, Darwin devoted much of the text of the original Origin to offering a solution in advance to predictable difficulties. As Darwin outlined these main lines of objection, he discussed, first, the apparent absence of numerous slight gradations between species, both in the present and in the fossil record, of the kind that would seem to be predictable from the gradualist workings of the theory (Chps. Six, Nine). Second, the gradual development of organs and structures of extreme complexity, such as the vertebrate eye, an organ which had since Antiquity served as a mainstay of the argument for external teleological design (Chp. Six). Third, the evolution of the elaborate instincts of animals and the puzzling problem of the evolution of social insects that developed sterile neuter castes, proved to be a particularly difficult issue for Darwin in the manuscript phase of his work and needed some account (Chp. Seven). As a fourth major issue needing attention, the traditional distinction between natural species defined by interfertility, and artificial species defined by morphological differences, required an additional chapter of analysis in which he sought to undermine the absolute character of the interbreeding criterion as a sign of fixed natural species (Chp. Eight).
In Chapter Ten, Darwin developed his interpretation of the fossil record. At issue was the claim by Lamarckian and other transformists, as well as Cuvierian catastrophists such as William Buckland (1784–1856) (see the entry on evolutionary thought before Darwin , Section 4.1), that the fossil record displayed a historical sequence beginning with simpler plants and animals, arriving either by transformism or replacement, at the appearance of more complex forms in geological history. Opposition to this thesis of “geological progressionism” had been made by none other than Darwin’s great mentor in geology, Charles Lyell in his Principles of Geology (Lyell 1832 [1990], vol. 2, chp. xi; Desmond 1984; Bowler 1976). Darwin defended the progressionist view against Lyell’s arguments in this chapter.
To each of the lines of objection to his theory, Darwin offered his contemporaries plausible replies. Additional arguments were worked out through the insertion of numerous textual insertions over the five revisions of the Origin between 1860 and 1872, including the addition of a new chapter to the sixth edition dealing with “miscellaneous” objections, responding primarily to the criticisms of St. George Jackson Mivart (1827–1900) developed in his Genesis of Species (Mivart 1871).
For reasons related both to the condensed and summary form of public presentation, and also as a reflection of the bold conceptual sweep of the theory, the primary argument of the Origin could not gain its force from the data presented by the book itself. Instead, it presented an argument from unifying simplicity, gaining its force and achieving assent from the ability of Darwin’s theory to draw together in its final synthesizing chapters (Ten–Thirteen) a wide variety of issues in taxonomy, comparative anatomy, paleontology, biogeography, and embryology under the simple principles worked out in the first four chapters. This “consilience” argument might be seen as the best reflection of the impact of William Whewell’s methodology (see above).
As Darwin envisioned the issue, with the acceptance of his theory, “a grand untrodden field of inquiry will be opened” in natural history. The long-standing issues of species origins, if not the explanation of the ultimate origins of life, as well as the causes of their extinction, had been brought within the domain of naturalistic explanation. It is in this context that he makes the sole reference in the text to the claim that “light will be thrown on the origin of man and his history”. And in a statement that will foreshadow the important issues of the Descent of Man of 1871, he speaks of how “Psychology will be based on a new foundation, that of the necessary acquirement of each mental power and capacity by gradation” (ibid., 488)
3. The Reception of the Origin
The broad sweep of Darwin’s claims, the brevity of the empirical evidence actually supplied in the Origin , and the implications of his theory for several more general philosophical and theological issues, opened up a controversy over Darwinian evolution that has waxed and waned over more than 160 years. The theory was inserted into a complex set of different national and cultural receptions the study of which currently forms a scholarly industry in its own right. European, Latin American and Anglophone receptions have been most deeply studied (Bowler 2013a; Gayon 2013; Largent 2013; Glick 1988, 2013; Glick and Shaffer 2014; Engels and Glick 2008; Gliboff 2008; Numbers 1998; Pancaldi, 1991; Todes 1989; Kelly 1981; Hull 1973; Mullen 1964). To these have been added analyses of non-Western recptions (Jin 2020, 2019 a,b; Yang 2013; Shen 2016; Elshakry 2013; Pusey 1983). These analyses display common patterns in both Western and non-Western readings of Darwin’s theory, in which these receptions were conditioned, if not determined, by the pre-existing intellectual, scientific, religious, social, and political contexts into which his works were inserted.
In the anglophone world, Darwin’s theory fell into a complex social environment that in the United States meant into the pre-Civil War slavery debates (Largent 2013; Numbers 1998). In the United Kingdom it was issued against the massive industrial expansion of mid-Victorian society, and the development of professionalized science. To restrict focus to aspects of the British reading public context, the pre-existing popularity of the anonymous Vestiges of the Natural History of Creation of 1844, which had reached 11 editions and sold 23,350 copies by December of 1860 (Secord “Introduction” to Chambers 1844 [1994], xxvii]), with more editions to appear by the end of the century, certainly prepared the groundwork for the general notion of the evolutionary origins of species by the working of secondary natural laws. The Vestiges ’s grand schema of a teleological development of life, from the earliest beginnings of the solar system in a gaseous nebula to the emergence of humanity under the action of a great “law of development”, had also been popularized for Victorian readers by Alfred Lord Tennyson’s epic poem In Memoriam (1850). This Vestiges backdrop provided a context in which some could read Darwin as supplying additional support for the belief in an optimistic historical development of life under teleological guidance of secondary laws with the promise of ultimate historical redemption. Such readings also rendered the Origin seemingly compatible with the progressive evolutionism of Darwin’s contemporary Herbert Spencer (see the entry on Herbert Spencer ). Because of these similarities, Spencer’s writings served as an important vehicle by which Darwin’s views, modified to fit the progressivist views expounded by Spencer, were first introduced in non-Western contexts (Jin 2020, 2019 a,b; Lightman [ed.] 2015; Pusey 1983). Such popular receptions ignored or revised Darwin’s concept of evolution by natural selection to fit these progressivist alternatives.
Outside the United Kingdom, the receptions of Darwin’s work display the importance of local context and pre-existent intellectual and social conditions. Three examples—France, Germany, and China—can be elaborated upon. In France, Darwin’s theory was received against the background of the prior debates over transformism of the 1830s that pitted the theories of Lamarck and Etienne Geoffroy St. Hilaire against Cuvier (Gayon 2013; entry on evolutionary thought before Darwin , 4.1). At least within official French Academic science, these debates had been resolved generally in favor of Cuvier’s anti-transformism. The intellectual framework provided by the “positive philosophy” of Auguste Comte (1798–1857) also worked both for and against Darwin. On one hand, Comte’s emphasis on the historical progress of science over superstition and metaphysics allowed Darwin to be summoned in support of a theory of the progress of science. The Origin was so interpreted in the preface to the first French translation of the Origin made by Clémence Royer (Harvey 2008). On the other hand, the Comtean three stages view of history, with its claim of the historical transcendence of speculative and metaphysical periods of science by a final period of experimental science governed by determinate laws, placed Darwinism in a metaphysical phase of speculative nature philosophy. This view is captured by the assessment of the leading physiologist and methodologist of French Science, Claude Bernard (1813–78). As he stated this in his 1865 treatise on scientific methodology, Darwin’s theory was to be regarded with those of “a Goethe, an Oken, a Carus, a Geoffroy Saint Hilaire”, locating it within speculative philosophy of nature rather than granting it the status of “positive” science (Bernard 1865 [1957], 91–92]).
In the Germanies, Darwin’s work entered a complex social, intellectual and political situation in the wake of the failed efforts to establish a liberal democracy in 1848. It also entered an intellectual culture strongly influenced by the pre-existent philosophical traditions of Kant, Schelling’s Naturphilosophie , German Romanticism, and the Idealism of Fichte and Hegel (R. J. Richards 2002, 2008, 2013; Gliboff 2007, 2008; Mullen 1964). These factors formed a complex political and philosophical environment into which Darwin’s developmental view of nature and theory of the transformation of species was quickly assimilated, if also altered. Many readings of Darwin consequently interpreted his arguments against the background of Schelling’s philosophy of nature. The marshalling of Darwin’s authority in debates over scientific materialism were also brought to the fore by the enthusiastic advocacy of Darwinism in Germany by University of Jena professor of zoology Ernst Heinrich Haeckel (1834–1919). More than any other individual, Haeckel made Darwinismus a major player in the polarized political and religious disputes of Bismarckian Germany (R. J. Richards 2008). Through his polemical writings, such as the Natural History of Creation (1868), Anthropogeny (1874), and Riddle of the Universe (1895–99), Haeckel advocated a materialist monism in the name of Darwin, and used this as a stick with which to beat traditional religion. Much of the historical conflict between religious communities and evolutionary biology can be traced back to Haeckel’s polemical writings, which went through numerous editions and translations, including several English and American editions that appeared into the early decades of the twentieth century.
To turn to a very different context, that of China, Darwin’s works entered Chinese discussions by a curious route. The initial discussions of Darwinian theory were generated by the translation of Thomas Henry Huxley’s 1893 Romanes Lecture “Evolution and Ethics” by the naval science scholar Yan Fu (1854–1921), who had encountered Darwinism while being educated at the Royal Naval College in Greenwich from 1877 to 1879. This translation of Huxley’s lecture, published in 1898 under the name of Tianyan Lun , was accompanied with an extensive commentary by Yan Fu that drew heavily upon the writings of Herbert Spencer which Yan Fu placed in opposition to the arguments of Huxley. This work has been shown to have been the main vehicle by which the Chinese learned indirectly of Darwin’s theory (Jin 2020, 2019 a, b; Yang 2013; Pusey 1983). In the interpretation of Yan Fu and his allies, such as Kan Yuwei (1858–1927), Darwinism was given a progressivist interpretation in line with aspects of Confucianism.
Beginning in 1902, a second phase of Darwinian reception began with a partial translation of the first five chapters of the sixth edition of the Origin by the Chinese scientist, trained in chemistry and metallurgy in Japan and Germany, Ma Junwu (1881–1940). This partial translation, published between 1902 and 1906, again modified the text itself to agree with the progressive evolutionism of Spencer and with the progressivism already encountered in Yan Fu’s popular Tianyan Lun. Only in September of 1920 did the Chinese have Ma Junwu’s full translation of Darwin’s sixth edition. This late translation presented a more faithful rendering of Darwin’s text, including an accurate translation of Darwin’s final views on natural selection (Jin 2019 a, b). As a political reformer and close associate of democratic reformer Sun Yat-Sen (1866–1925), Ma Junwu’s interest in translating Darwin was also was involved with his interest in revolutionary Chinese politics (Jin 2019a, 2022).
The reception of the Origin by those who held positions of professional research and teaching positions in universities, leadership positions in scientific societies, and employment in museums, was complex. These individuals were typically familiar with the empirical evidence and the technical scientific issues under debate in the 1860s in geology, comparative anatomy, embryology, biogeography, and classification theory. This group can usually be distinguished from lay interpreters who may not have made distinctions between the views of Lamarck, Chambers, Schelling, Spencer, and Darwin on the historical development of life.
If we concentrate attention on the reception by these professionals, Darwin’s work received varied endorsement (Hull 1973). Many prominent members of Darwin’s immediate intellectual circle—Adam Sedgwick, William Whewell, Charles Lyell, Richard Owen, and Thomas Huxley—had previously been highly critical of Chambers’s Vestiges in the 1840s for its speculative character and its scientific incompetence (Secord 2000). Darwin himself feared a similar reception, and he recognized the substantial challenge facing him in convincing this group and the larger community of scientific specialists with which he interacted and corresponded widely. With this group he was only partially successful.
Historical studies have revealed that only rarely did members of the scientific elites accept and develop Darwin’s theories exactly as they were presented in his texts. Statistical studies on the reception by the scientific community in England in the first decade after the publication of the Origin have shown a complicated picture in which there was neither wide-spread conversion of the scientific community to Darwin’s views, nor a clear generational stratification between younger converts and older resisters, counter to Darwin’s own predictions in the final chapter of the Origin (Hull et al. 1978). These studies also reveal a distinct willingness within the scientific community to separate acceptance of Darwin’s more general claim of species descent with modification from common ancestors from the endorsement of his explanation of this descent through the action of natural selection on slight morphological variations.
Of central importance in analyzing this complex professional reception was the role assigned by Darwin to the importance of normal individual variation as the source of evolutionary novelty. As we have seen, Darwin had relied on the novel claim that small individual variations—the kind of differences considered by an earlier tradition as merely “accidental”—formed the raw material upon which, by cumulative directional change under the action of natural selection, major changes could be produced sufficient to explain the origin and subsequent differences in all the various forms of life over time. Darwin, however, left the specific causes of this variation unspecified beyond some effect of the environment on the sexual organs. Variation was presented in the Origin with the statement that “the laws governing inheritance are quite unknown” (Darwin 1859 [1964], 13). In keeping with his commitment to the gradualism of Lyellian geology, Darwin also rejected the role of major “sports” or other sources of discontinuous change in this process.
As critics focused their attacks on the claim that such micro-differences between individuals could be accumulated over time without natural limits, Darwin began a series of modifications and revisions of the theory through a back and forth dialogue with his critics that can be followed in his revisions to the text of the Origin . In the fourth edition of 1866, for example, Darwin inserted the claim that the continuous gradualism depicted by his branching diagram was misleading, and that transformative change does not necessarily go on continuously. “It is far more probable that each form remains for long periods unaltered, and then again undergoes modification” (Darwin 1866, 132; Peckham 2006, 213). This change-stasis-change model allowed variation to stabilize for a period of time around a mean value from which additional change could then resume. Such a model would, however, presumably require even more time for its working than the multi-millions of years assumed in the original presentation of the theory.
The difficulties in Darwin’s arguments that had emerged by 1866 were highlighted in a lengthy and telling critique in 1867 by the Scottish engineer Henry Fleeming Jenkin (1833–1885) (typically Fleeming Jenkin). Using an argument previously raised in the 1830s by Charles Lyell against Lamarck, Fleeming Jenkin cited empirical evidence from domestic breeding that suggested a distinct limitation on the degree to which normal variation could be added upon by selection (Fleeming Jenkin 1867 in Hull 1973). Using a loosely mathematical argument, Fleeming Jenkin argued that the effects of intercrossing would continuously swamp deviations from the mean values of characters and result in a tendency of the variation in a population to return to mean values over time. It is also argued that domestic evidence does not warrant an argument for species change. For Fleeming Jenkin, Darwin’s reliance on continuous additive deviation was presumed to be undermined by these arguments, and only more dramatic and discontinuous change—something Darwin explicitly rejected—could account for the origin of new species.
Fleeming Jenkin also argued that the time needed by Darwin’s theory to account for the history of life under the gradual working of natural selection was simply unavailable from scientific evidence, supporting this claim by an appeal to the physical calculations of the probable age of the solar system presented in publications by his mentor, the Glasgow physicist William Thompson (Lord Kelvin, 1824–1907) (Burchfield 1975). On the basis of Thompson’s quantitative physical arguments concerning the age of the sun and solar system, Fleeming Jenkin judged the time since the presumed first beginnings of life to be insufficient for the Darwinian gradualist theory of species transformation to have taken place.
Jenkin’s multi-pronged argument gave Darwin considerable difficulties and set the stage for more detailed empirical inquiries into variation and its causes by Darwin’s successors. The time difficulties were only resolved in the twentieth-century with the discovery of radioactivity that could explain why the sun did not lose heat in accord with Newtonian principles.
As a solution to the variation question, Darwin developed his “provisional hypothesis” of pangenesis, which he presented the year after the appearance of the Fleeming Jenkin review in his two-volume Variation of Plants and Animals Under Domestication (Darwin 1868; Olby 2013). Although this theory had been formulated independently of the Jenkin review (Olby 1963), in effect it functioned as Darwin’s reply to Jenkin’s critique. The pangenesis theory offered a causal theory of variation and inheritance through a return to a theory resembling Buffon’s theory of the organic molecules proposed in the previous century (see entry on evolutionary thought before Darwin section 3.2). Invisible material “gemmules” were presumed to exist within the cells. According to theory, these were subject to external alteration by the environment and other external causes. The gemmules were then shed continually into the blood stream (the “transport” hypothesis) and assembled by “mutual affinity for each other, leading to their aggregation either into buds or into the sexual elements” (Darwin 1868, vol. 2, 375). In this form they were then transmitted—the details were not explained—by sexual generation to the next generation to form the new organism out of “the modified physiological units of which the organism is built” (ibid., 377). In Darwin’s view, this hypothesis united together numerous issues into a coherent and causal theory of inheritance and explained the basis of variation. It also explained how use-disuse inheritance, a theory which Darwin never abandoned, could work.
The pangenesis theory, although not specifically referred to, seems to be behind an important distinction Darwin inserted into the fifth edition of the Origin of 1869 in his direct reply to the criticisms of Jenkin. In this textual revision, Darwin distinguished “certain variations, which no one would rank as mere individual differences”, from ordinary variations (Darwin1869, 105; Peckham 2006, 178–179). This revision shifted Darwin’s emphasis away from his early reliance on normal slight individual variation, and gave new status to what he now termed “strongly marked” variations. The latter were now the forms of variation to be given primary evolutionary significance. Presumably this strong variation was more likely to be transmitted to the offspring, although details are left unclear, and in this form major variation could presumably be maintained in a population against the tendency to swamping by intercrossing as Fleeming Jenkin had argued.
Darwin’s struggles over this issue defined a set of problems that British life scientists in particular were to deal with into the 1930s. These debates over the role of somatic variation in the evolutionary process placed Darwinism in a defensive posture that forced its supporters into major revisions in the Darwinian research program (Gayon 1998; Vorzimmer 1970). The consequence was a complex period of Darwinian history in which natural selection theory was rejected by many research, or defended in modified form by others (Bowler 1983, 2013a; Largent 2009).
4. Human Evolution and the Descent of Man
Darwin had retained his own conclusions on human evolution quietly in the background through the 1860’s while the defense of his general theory was conducted by advocates as diverse as Thomas Henry Huxley (1825–95) in England, Asa Gray (1810–88) in the United States, and Ernst Haeckel (1834–1919) in the emerging new Germany. Darwin’s own position on the “human question” remained unclear to the reading public, and his rhetorical situating of the Origin within a tradition of divine creation by secondary law, captured in the frontispiece quotations from William Whewell and Francis Bacon, allowed many before 1871 to see Darwin as more open to religious interpretations of human origins than those of some of his popularizers.
Darwin’s interest in developing his insights into the origins of human beings and the explanation of human properties through descent with modification was, however, evident in his correspondence as early as January of 1860 when he began collecting evidence on the expressions of the emotions in human beings (Browne 2002, chp. 9). He then developed a questionnaire specifically intended to gain such information from contacts in Patagonia and Tierra del Fuego (Radick 2018). Further engagement with these issues was then generated by the discussions of Lyell (1863) and A. R. Wallace (1864), both of whom suggested that natural selection could not account for the development of the “higher” rational faculties, language, and ethical motivation (R. J. Richards 1987, chp. 4). It was then in February of 1867 that Darwin decided to remove material from his massive manuscript of the Variation of Plants and Animals Under Domestication to create a “very small volume, ‘an essay on the origin of mankind’” (Darwin to Hooker, 8 February 1867 and CD to Turner, 11 February 1867, Burkhardt, Correspondence 15: 74, 80). At this time he also sent to several international correspondents a more detailed questionnaire asking for information on human emotional expression. Further impetus to develop his views was created by the arguments of William R. Greg (1809–1881) in an essay in Fraser’s Magazine (1868), with further support by arguments of A. R. Wallace in 1869, both of whom drew a sharp distinction between human properties and those of animals (R. J. Richards 1987, 172–184). These arguments denied that natural selection could explain the origins of these “higher powers”.
Darwin’s drafting of his views on human issues, begun in early 1868, expanded into a major enterprise in which he became deeply engaged with the issue of the implications of his theory for ethics. The result of this effort devoted to anthropological topics was two separate works: the Descent of Man and Selection in Relation to Sex , delivered to the publisher in June of 1870 with publication in 1871, and its companion, Expression of the Emotions in Man and Animals , which he commenced in early 1871 with publication in early 1872.
As commentators have noted, these two works differ markedly in their arguments, and reflect different relationships to Darwin’s causal theories of natural and sexual selection, with sexual selection predominting over natural selection for the major portion of the Descent , and both of these causal theories generally missing from the descriptive approach of the Expression (Radick 2018).
Sexual selection—the choosing of females by males or vice versa for breeding purposes—had received a general statement by Darwin in Chapter IV of the Origin , but this played only a minor role in the original argument, and its importance was denied by co-evolutionist A. R. Wallace. In the Descent this was now developed in extensive detail as a major factor in evolution that could even work against ordinary natural selection. Sexual selection could be marshaled to explain sexual dimorphism, and also the presence of unusual characters and properties of organisms—elaborate feeding organs, bright colors, and other seemingly maladaptive structures such as the antlers on the Irish Elk or the great horn on the Rhinoceros beetle—that would appear anomalous outcomes of ordinary natural selection working for the optimal survival of organisms in nature. In a dramatic extension of the principle to human beings, the combination of natural and sexual selection is used to explain the origins of human beings from simian ancestors. It also accounts for the sexual dimorphism in humans, and is a major factor accounting for the origin of human races (E. Richards 2017; R. A. Richards 2013).
Although the secondary causal role of sexual selection in the development of species generally was to be the main topic of the bulk of the Descent , this plays an ambiguous role initially in the “treatise on man” that occupies the initial chapters, and functions differently in his treatment of the origins of mental powers, the moral sense, and the origin of races in this opening discussion.
In constructing this presentation, Darwin reaches back to the early Notebooks that he had separated out from the “transformist” discussions to deal with his inquiries into ethics, psychology, and emotions (see Section 1.2 above). Of particular importance for the opening discussions of the Descent was the “M” notebook, commenced in July of 1838, and “N”, begun in October of that year. On occasion he also samples the collection of entries now entitled “Old and Useless Notes”, generally written between 1838 and 1840.
The initial topic of focus in the Descent deals with the far-reaching issues concerning the status and origin of human mental properties, faculties presumed traditionally to be possessed uniquely by human beings. These properties Darwin now places on an evolutionary continuum with those features of animal behavior long regarded as instinctual. In this he placed himself in opposition to the long tradition of discourse that had distinguished humans from animals due to the possession of a “rational principle” related to their possession of a rational soul. This tradition had been given a more radical foundation in the revolutionary reflections on the relation of mind and body initiated by René Descartes (1596–1650) in the middle of the seventeenth century. Descartes deepened this distinction with the separation of the two substances—thinking substance, or res cogitans , possessed only by humans, and extended material substance, res extensa that constituted the rest of the natural world, including animals and plants, rendering animals only lifeless machines without rational faculties.
Darwin’s collapse of this Cartesian barrier with his theory of human origins outlined in the Descent continued a discussion that had been a concern of his transformist predecessors, especially Jean Baptiste Lamarck (Sloan 1999). But Darwin took this issue to a new level as he interpreted the human-animal relationship in the context of his novel theory of divergent evolution from common ancestors. Darwin also broke with the view of humans as the summit of a natural teleological process. Darwin instead denies such teleological ordering, and effectively reduces human properties to those of animals—mental as well as physical—by tracing them to their origin in properties of lower organisms.
The warrant for the identification of human and animal mental properties, however, is not supported by substantial argumentation in the Descent. The opening discussions of the treatise summarize the anatomical evidence for “homologies” —true identities—between humans and animals due to descent from common ancestors, claims already set out in Chapter Thirteen of the Origin. But the transferal of this identity of structure to inner non-anatomical “mental” properties rested on premises that are not made explicit in this text, and were not identities drawn by Huxley, Wallace and Lyell, for example, in their treatments of humans in relation to evolutionary theory, although they acknowledged the anatomical continuities.
To understand Darwin’s arguments, it is useful to return to his Notebook discussions on which he was drawing for his reasoning (see above, Section 1.2). In his “C” Notebook, opened in February of 1838, Darwin has a remarkable entry that displays very early on his commitment to a metaphysical “monism”—the thesis that there is only one substance underlying both mind and body. With this goes the thesis of a parallelism of the complexity of mental properties with those of material structure. In this entry in “C” following on Darwin’s reflections on the issue of instinct, and also recording some of his observations on animals at the Regents Park zoological gardens, Darwin comments:
There is one living spirit, prevalent over this wor[l]d, (subject to certain contingencies of organic matter & chiefly heat), which assumes a multitude of forms <<each having acting principle>> according to subordinate laws.—There is one thinking […] principle (intimately allied to one kind of organic matter—brain. & which <prin> thinking principle. seems to be given or assumed according to a more extended relations [ sic ] of the individuals, whereby choice with memory, or reason ? is necessary.—) which is modified into endless forms, bearing a close relation in degree & kind to the endless forms of the living beings.— We see thus Unity in thinking and acting principle in the various shades of <dif> separation between those individuals thus endowed, & the community of mind, even in the tendency to delicate emotions between races, & recurrent habits in animals.— (Barrett 1987, 305)
As we follow these issues into the “M” Notebook, the assumption of a single “thinking principle,” allied to one kind of organic matter, seems then to underlie Darwin’s subsequent reflections on mind and matter. The “M” Notebook cites numerous “mental”properties common to humans and animals that generally parallel levels of material organization, similar to the identities expressed in the later Descent. The range of this universal extension of mental properties is far-reaching in these early discussions: consciousness and “free will” extends to all animals, including invertebrates:
With respect to free will, seeing a puppy playing cannot doubt that they have free will, if so all animals., then an oyster has & a polype (& a plant in some senses […]; now free will of oyster, one can fancy to be direct effect of organization, by the capacities its senses give it of pain or pleasure, if so free will is to mind, what chance is to matter […] (Barrett 1987, 536).
When these themes reappear in Chapter Two of the first edition of the Descent , Darwin seems to draw implicitly upon this matter-mind identity theory as an obvious consequence of his theory of descent from common ancestry. There he enumerates a long list of traditional human mental and emotional properties to claim that each of them are identities with the properties of simpler forms of life. The list is expansive: courage, deceit, play, kindness, maternal affection, self-complacency, pride, shame, sense of honor, wonder, dread, imitation, imagination, and dreaming. All are considered to be represented in a wide range of animals, with “play”and “recognition” found even in the ants.
When he addresses the more complex mental properties that specifically had been considered by a long tradition of discussion to be the distinctive human properties—possession of language, reason, abstract conceptual thinking, self-reflection—these again are treated as having their manifestations in other forms of life, with none of them unique to human beings. Language, the property that Descartes, for example, had considered to be the primary distinguishing character denoting the human possession of mind as distinct from matter, Darwin treats a developing in a gradual process from animal sounds that parallel the differentiation of species, illustrated by the fact that languages “like organic beings, can be classed in groups under groups” (Darwin 1871 [1981], 60). He closes his discussion of mental powers with an analysis of religious belief that derives it from imagination and belief in spirits found in aboriginal peoples. It can even be homologized with the “deep love of a dog for his master, associated with complete submissions, some fear, and perhaps other feelings” (ibid., 68). Darwin’s discussions of the relation of human and animal mental and emotional properties would set the agenda for a complex discussion that would carry into contemporary debates over animal cognition and the relations of human and animal properties (see the entries on animal cognition ; methods in comparative cognition ; and animal consciousness ).
The subsequent treatment of ethical issues in the third chapter of the Descent was for Darwin a topic to be approached “exclusively from the side of natural history” (ibid., 71). This issue also presented him with some of his most difficult conceptual problems (CD to Gray, 15 March 1870, Burkhardt, Correspondence 18, 68). In this discussion he also employs natural selection theory as an explanatory cause.
Under the heading of “Moral Sense”, Darwin offered some innovations in ethics that do not easily map on to standard ethical positions classified around the familiar categories of Rule or Act Utilitarianism, Kantian Deontology, Hedonism, and Emotivism. Darwin’s closest historical affinities are with the Scottish “Moral Sense” tradition of Frances Hutcheson (1694–1746), Adam Smith (1723?–1790), and David Hume (1711–1776). More immediately Darwin drew from the expositions of the moral sense theory by his distant relative, Sir James Macintosh (1765–1832) (R. J. Richards 1987, 114–122, 206–219).
Traditional moral sense theory linked ethical behavior to an innate property that was considered to be universal in human beings, although it required education and cultivation to reach its full expression (see the entry on moral sentimentalism ). This inherent property, or “moral” sense, presumably explained such phenomena as ethical conscience, the sense of moral duty, and it accounted for altruistic actions that could not be reduced to hedonic seeking of pleasure and avoiding pain. It also did not involve the rational calculation of advantage, or the maximization of greatest happiness by an individual prior to action, as implied by Utilitarianism. For this reason Darwin criticized John Stuart Mill’s version of Utilitarian theory because it relied on acquired habits and the calculation of advantage (Darwin 1871 [1981], 71n5).
Darwin’s reinterpretation of the moral sense tradition within his evolutionary framework also implied important transfomations of this theory of ethics. The moral sense was not to be distinguished from animal instinct but was instead derived historically from the social instincts and developed by natural selection. From this perspective, Darwin could claim a genuine identity of ethical foundations holding between humans and animals, with the precursors of human ethical behavior found in the behavior of other animals, particularly those with social organization. Natural selection then shaped these ethical instincts in ways that favored group survival over immediate individual benefit (ibid., 98). Human ethical behavior is therefore grounded in a natural property developed by natural selection, with the consequence that ethical actions can occur without moral calculus or rational deliberation.
When moral conflict occurs, this is generally attributed to a conflict of instincts, with the stronger of two conflicting instincts favored by natural selection insofar as it favors group benefit (ibid. 84). In human beings the “more enduring Social Instincts” thus come to override the less persistent “individual” instincts.
The adequacy of evolutionary ethical naturalism as a foundation for ethical realism proved to be a point of contention for Darwin’s contemporaries and successors following the publication of the Descent . For some moral philosophers, Darwin had simply reduced ethics to a property subject to the relativizing tendencies of natural selection (Farber 1994: chp. 5). It was, in the view of Darwin’s philosophical critics, to reduce ethics to biology and in doing so, to offer no way to distinguish ethical goods from survival advantages. Not even for some strong supporters of Darwinism, such as Thomas Huxley and Alfred Russel Wallace, was Darwin’s account adequate (ibid., chp. 4). Much of subsequent development of moral philosophy after Darwin would be grounded upon the canonical acceptance of the “is-ought” distinction, which emerged with new force from the critique of “evolutionary” ethical theory. This critique began with Thomas Huxley’s own break with Darwinian ethical theory in his Romanes Lecture, “Evolution and Ethics”of 1893 (Huxley 1893). This lecture, reflecting Huxley’s views eleven years after Darwin’s death, would play an important role in the Chinese reception of Darwinism (Huxley 1895; see above, section 3.1). This line of critique also received an influential academic expression in G. E. Moore’s (1873–1958) Principia Ethica —itself an attack on Spencer’s version of evolutionary ethics (Moore 1903). Debates over the adequacy of evolutionary ethics continue into the present (see the entries on biological altruism and morality and evolutionary biology ; see also, R. J. Richards 2015, 2009, 1999, 1987, Appendix 2; Charmetant 2013; Boniolo and DeAnna (eds.) 2006; Hauser 2006; Katz (ed.) 2000; Maienschein and Ruse (eds.) 1999).
4.4 Reception of the Descent
The international reception of the Descent of Man and Expression of the Emotions is a topic in need of the kind of detailed studies that surround the historical impact of the Origin. These works presented the reading public after 1871 with a more radical and controversial Darwin than had been associated with the author of the popular Journal of Researches or even the Origin itself, and his anthropological works created a watershed in the public reception of Darwin’s views (Radick 2013). The Descent finally made public Darwin’s more radical conclusions about human origins, and seemed to many of his readers, even those previously sympathetic to the Origin , to throw Darwin’s authority behind materialist and anti-religious forces. Public knowledge of Darwin’s own conclusions on human evolution before 1871 had rested on the one vague sentence on the issue in the Origin itself. The Descent made public his more radical conclusions. Even though the question of human evolution had already been dealt with in part by Thomas Huxley in his Man’s Place in Nature of 1863 (Huxley 1863), and by Charles Lyell in the same year in his Geological Evidences of the Antiquity of Man (Lyell 1863), followed by Alfred Russel Wallace’s articles in 1864 and 1870 (Wallace 1864 and online), these authors had either not dealt with the full range of questions presented by the inclusion of human beings in the evolutionary process, or they had emphasized the moral and mental discontinuity between humans and animals. Only Ernst Heinrich Haeckel had drawn out a more general reductive conception of humanity from evolutionary theory and he had not ventured into the specific issues of ethics, social organization, the origins of human races, and the relation of human mental properties to those of animals, all of which are dealt with in the Descent . Darwin’s treatise presented, as one commentator has put it, “a closer resemblance to Darwin’s early naturalistic vision than anything else he ever published” (Durant 1985, 294).
Darwin’s extension of his theory to a range of questions traditionally discussed within philosophy, theology, and social and political theory, has shaped the more general history of Darwinism since the 1870s. It set the agenda for much of the development of psychology of the late nineteenth century (R. J. Richards 1987). It also hardened the opposition of many religiously-based communities against evolutionary theory, although here again, distinctions must be made between different communities (Ellegård 1990, chp. 14). Such opposition was not simply based upon the denial of the literal scriptural account of the origins of humankind, an issue that played out differently within the main religious denominations (Haught 2013; Finnegan 2013; Swetlitz 2013; Artigas, Glick, & Martinez 2006; Moore 1979). The more fundamental opposition was due to the denial of distinctions, other than those of degree, between fundamental human properties and those of animals.
Furthermore, the apparent denial of some kind of divine guidance in the processes behind human evolution and the non-teleological character of Darwin’s final formulations of the natural selection theory in the fifth and sixth editions of the Origin , hardened this opposition. His adoption from Herbert Spencer of designator “survival of the fittest” as a synonym for “natural selection” in the fifth edition of 1869 added to this growing opposition. As a consequence, the favorable readings that many influential religious thinkers—John Henry Newman (1801–1890) is a good example—had given to the original Origin , disappeared. The rhetoric of the Descent , with its conclusion that “man is descended from a hairy quadruped, furnished with a tail and pointed ears” (Darwin 1871 [1981], 389), presented to the public a different Darwin than many had associated with the popular seagoing naturalist.
The new opposition to Darwin is reflected in the many hostile reviews of the Descent to appear in the periodical press (R. J. Richards 1987, 219–230). Particularly at issue were Darwin’s accounts of the origin of ethical principles and intelletual powers, including language, self-reflection, abstract thinking and religious belief as derivations from animal properties (Anon. 1871)
The profound revolution in thought that Darwin created, however, was eventually recognized even by his one-time harsh critics. The once leading British comparative anatomist Richard Owen (1804–1892), who had long been estranged from Darwin since his harsh review of the Origin in 1860, nonetheless could comment on the occasion of Darwin’s burial in Westminster Abbey in a letter to Horace Walpole:
The great value of Darwin’s series of works, summarizing all the evidence of Embryology, Paleontology, & Physiology experimentally applied in producing Varieties of Species, is exemplified in the general acceptance by Biologists of the Secondary Law, by Evolution, of the ‘Origin of Species’ […] In this respect Charles Darwin stands to Biology in the relation which Copernicus stood to Astronomy. […] [Copernicus] knew not how the planets revolved around the sun. To know that required the successive labours of a Galileo, a Kepler and finally a Newton […] Meanwhile our British Copernicus of Biology merits the honour and the gratitude of the Empire, which is manifest by a Statue in Westminster Abbey. (Richard Owen to Horace Walpole, 5 November, 1882, Royal College of Surgeons of England Archives, MS0025/1/5/4).
The subsequent history of the debates surrounding Darwin’s achievement forms a complex story that involves much of the history of life science, as well as ethical theory, psychology, philosophy, theology and social theory since 1870. For a general summary of recent scholarship see Ruse 2013a and articles from this encyclopedia listed below.
This article has intended to give a historical overview of the specific nature of Darwinian theory, and outline the ways in which it differed from the theories of predecessors in the nineteenth century (see the entry evolution before Darwin ). The eventual general consensus achieved by the middle of the twentieth century around the so-named “Synthetic” theory of evolution that would combine population genetics with a mathematical analysis of evolutionary change, has formed a successful research program for more than half a century (Smocovitis 1996; Mayr and Provine 1980; Provine 1971). This “synthesis” has been challenged in recent decades by the current movement known as evolutionary developmental theory, or “evo-devo”. This development represents in some important respects a return to presumably discarded traditions and lines of exploration of the nineteenth and early twentieth centuries which sought to link evolution with embryological development, and to a complex understanding of genetics, with re-examination of the effects of external conditions on inheritance (Gilbert 2015; Newman 2015; Laubichler and Maienschein 2007; Gissis and Jablonka 2011; Pigliucci and Müller 2010; Amundson 2005; Gilbert, Opitz and Raff 1996). Where these debates and revisions in evolutionary theory may lead in another fifty years is a matter of speculation (Gayon 2015 in Sloan, McKenny and Eggleson 2015).
More general philosophical issues associated with evolutionary theory—those surrounding natural teleology, ethics, the relation of evolutionary naturalism to the claims of religious traditions, the implications for the relation of human beings to the rest of the organic world—continue as issues of scholarly inquiry. The status of Darwin’s accounts of human mental powers and moral properties continue to be issues of philosophical debate. The adequacy of his reliance on sexual selection to explain sex and gender roles in human society form heated topics in some feminist scholarship. Such developments suggest that there are still substantial theoretical issues at stake that may alter the future understanding of evolutionary theory in important ways (Sloan, McKenny, & Eggleson [eds] 2015).
- Amundson, Ron, 2005, The Changing Role of the Embryo in Evolutionary Thought: Roots of Evo-Devo , Cambridge: Cambridge University Press. doi:10.1017/CBO9781139164856
- Anon., “Review of the Descent of Man and Selection in Relation to Sex” , Edinburgh Review 134 (July 1871), 195–235.
- Artigas, Mariano, Thomas F. Glick, and Rafael A. Martínez, 2006, Negotiating Darwin: The Vatican Confronts Evolution, 1877–1902 , Baltimore, MD: Johns Hopkins University Press.
- Barrett, Paul H., Peter J. Gautrey, Sandra Herbert, David Kohn, and Sydney Smith (eds.), 1987, Charles Darwin’s Notebooks: 1836–1844 , Cambridge: Cambridge University Press. [online manuscripts at Darwin’s notebooks and reading lists.]
- Beatty, John 1985, “Speaking of Species: Darwin’s Strategy”, in Kohn 1985a: 265–281. doi:10.1515/9781400854714.265
- Bernard, Claude, 1865 [1957], Introduction to the Study of Experimental Medicine , translated Henry Copley Greene, New York: Dover. Originally published in 1927, New York:Macmillan. [ Bernard 1865 available online ]
- Boniolo, Giovanni and Gabriele De Anna (eds.) 2006, Evolutionary Ethics an Contemporary Biology, Cambridge: Cambridge University Press.
- Bowlby, John, 1990, Charles Darwin: A New Life , New York: Norton.
- Bowler, Peter J., 1976, Fossils and Progress: Paleontology and the Idea of Progressive Evolution in the Nineteenth Century , New York: Science History.
- –––, 1983, The Eclipse of Darwinism: Anti-Darwinian Evolution Theories in the Decades Around 1900 , Baltimore, MD: Johns Hopkins University Press.
- –––, 1990, Charles Darwin: The Man and His Influence , Oxford: Blackwell.
- –––, 1996, Life’s Splendid Drama: Evolutionary Biology and the Reconstruction of Life’s Ancestry, 1860–1940 , Chicago: University of Chicago Press.
- –––, 2013a, “Darwinism in Britain”, in Ruse 2013a: 218–225. doi:10.1017/CBO9781139026895.028
- Browne, Janet, 1995, Charles Darwin: Voyaging , New York: Knopf.
- –––, 2002, Charles Darwin: The Power of Place , Princeton: Princeton University Press.
- Burchfield, Joe D., 1975, Lord Kelvin and the Age of the Earth , Chicago: University of Chicago Press.
- Burkhardt, Frederick et al., (eds.), 1985–2023, The Correspondence of Charles Darwin , 30 volumes. Cambridge: Cambridge University Press.
- Chambers, Robert, 1844 [1994], Vestiges of the Natural History of Creation , facsimile reprint of first edition, J. Secord (ed.), Chicago: University of Chicago Press. [ Chambers 1844 available online ]
- Charmetant, Erin. 2013,“Darwin and Ethics”, in Ruse 2013a, 188–194.
- Darwin, Charles Robert, 1836–1844 [1987], Charles Darwin’s Notebooks: 1836–1844 , Paul H. Barrett, Peter J. Gautrey, Sandra Herbert, David Kohn, and Sydney Smith (eds.), Cambridge: Cambridge University Press. See also the Darwin Online section on Darwin’s notebooks and reading lists .
- –––, 1856–1858 [1974], Charles Darwin’s “Natural Selection”, Being the Second Part of his Big Species Book Written from 1856 to 1858 , R.C. Stauffer (ed.), 1974, Cambridge: Cambridge University Press. [ Natural Selection 1974 available online ]
- –––, 1842 [1996], “1842 Sketch On Selection Under Domestication, Natural Selection, and Organic Beings in the Wild State”, selection in Glick and Kohn 1996, 89–99.
- –––, 1844a [1996], “1844 Essay: Variation of Organic Beings in the Wild State”, in Glick and Kohn 1996, 99–115.
- –––, 1859 [1964], On the Origin of Species By Means of Natural Selection , London: Murray. Facsimile reprint, ed. E. Mayr Cambridge, MA: Harvard University Press [ Origin first edition available online ]
- –––, 1860, second edition [ Origin second edition available online ]
- –––, 1861, third edition, [ Origin third edition available online ]
- –––, 1862, first French edition, De l’origine des espèces , Clémence Royer (trans.), Paris: Guillaumin. [ Origin French first edition available online ]
- –––, 1866, fourth edition, [ Origin fourth edition available online ]
- –––, 1869, fifth edition, [ Origin fifth edition available online ]
- –––, 1872, sixth edition, [ Origin sixth edition available online
- –––, 1868, The Variation of Animals and Plants Under Domestication , two volumes, London: John Murray. First edition available online
- –––, 1871 [1981], The Descent of Man, and Selection in Relation to Sex , two volumes, London: John Murray. Reprinted, ed John T. Bonner and Robert May, Princeton: Princeton University Press [ Descent 1871 available online ]
- –––, 1872, Expression of the Emotions in Man and the Animals , London: John Murray. [ Expression available online ]
- Depew, David J., 2009, “The Rhetoric of the Origin of Species”, in Ruse and Richards 2009: 237–255. doi:10.1017/CCOL9780521870795.015
- Depew, David J. and Bruce H. Weber, 1995, Darwinism Evolving: Systems Dynamics and the Genealogy of Natural Selection , Cambridge, MA: MIT Press.
- Desmond, Adrian J., 1984, Archetypes and Ancestors: Palaeontology in Victorian London, 1850–1875 , Chicago: University of Chicago Press.
- –––, and James R. Moore, 1991, Darwin , London: Michael Joseph.
- –––, and James R. Moore, 2009, Darwin’s Sacred Cause: How a Hatred of Slavery Shaped Darwin’s Views on Human Evolution , Boston: Houghton, Mifflin, Harcourt.
- Durant, John R., 1985, “The Ascent of Nature in Darwin’s Descent of Man ”, in Kohn 1985a: 283–306. doi:10.1515/9781400854714.283
- Ellegård, Alvar, 1990, Darwin and the General Reader; the Reception of Darwin’s Theory of Evolution in the British Periodical Press, 1859–1872 , reprint of 1958 edition, Chicago: University of Chicago Press.
- Elshakry, Marwa, 2013, Reading Darwin in Arabic, 1860–1950. Chicago: University of Chicago Press.
- Engels, Eve-Marie and Thomas F. Glick (eds.), 2008, The Reception of Charles Darwin in Europe , London/New York: Continuum.
- Farber, Paul Lawrence, 1994, The Temptations of Evolutionary Ethics , Berkeley: University of California Press.
- Finnegan, Diarmid A., 2013, “Darwin and Protestantism”, in Ruse 2013a: 468–475. doi:10.1017/CBO9781139026895.060
- Gayon, Jean, 1998, Darwinism’s Struggle for Survival: Heredity and the Hypothesis of Natural Selection , Cambridge: Cambridge University Press.
- –––, 2013, “Darwin and Darwinism in France before 1900”, in Ruse 2013a: 243–249. doi:10.1017/CBO9781139026895.031
- –––, 2015, “What Future for Darwinism?”, in Sloan, McKenny and Eggleson 2015: 404–423.
- Gilbert, Scott F., 2015, “Evolution Through Developmental Change”, in Sloan, McKenny, & Eggleson 2015: 35–60.
- –––, John M. Opitz, and Rudolf A. Raff, 1996, “Resynthesizing Evolutionary and Developmental Biology”, Developmental Biology , 173 (2): 357–372. doi:10.1006/dbio.1996.0032
- Gissis, Snait B. and Eva Jablonka (eds.), 2011, Transformations of Lamarckism: From Subtle Fluids to Molecular Biology , (Vienna Series in Theoretical Biology), Cambridge, MA: MIT Press.
- Gliboff, Sander, 2007, “H. G. Bronn and the History of Nature”, Journal of the History of Biology , 40 (2): 259–294. doi:10.1007/s10739-006-9114-4
- –––, 2008, H. G. Bronn, Ernst Haeckel, and the Origins of German Darwinism: A Study in Translation and Transformation , Cambridge, MA: MIT Press.
- Glick, Thomas F. (ed.), 1988, The Comparative Reception of Darwinism , New edition with new preface, Chicago: University of Chicago Press.
- –––, 2013, “Darwinism in Latin America”, in Ruse 2013a: 258–263. doi:10.1017/CBO9781139026895.033
- –––, and Elinor S. Shaffer (eds.), 2014, The Literary and Cultural Reception of Charles Darwin in Europe , London: Bloomsbury.
- –––, and David Kohn (eds.), 1996, On Evolution: The Development of the Theory of Natural Selection , Indianapolis, IN: Hackett.
- Gray, Asa, 1860, “Review: The Origin of Species by Means of Natural Selection”, American Journal of Science and Arts , series 2, 29: 153–184, written anonymously. doi:10.2475/ajs.s2-29.86.153 [ Gray 1860 available online ]
- Haeckel, Ernst, 1868 [1876], Natürliche Schöpfungsgeschichte , Berlin: G. Reimer. Translated as The History of Creation , two volumes, London: Henry S. King.
- –––, 1874 [1879], Anthropogenie oder Entwickelungsgeschichte des Menschen , translated as The Evolution of Man: A Popular Exposition of the Principal Points of Human Ontogeny and Phylogeny. New York: Appleton.
- –––, 1895–99 [1901], Die Welträthsel ( Riddle of the Universe ). Translated by Joseph McCabe, New York/London: Harper and Brothers.
- Harvey, Joy, 2008, “Darwin in French Dress: Translating, Publishing and Supporting Darwin in Nineteenth-Century France”, in Engels and Glick 2008: 354–374.
- Hauser, Marc D., 2006, Moral Minds: How Nature Designed Our Universal Sense of Right and Wrong , New York: Ecco.
- Haught, John F., 2013, “Darwin and Catholicism”, in Ruse 2013a: 485–492. doi:10.1017/CBO9781139026895.062
- Herbert, Sandra, 2005, Charles Darwin, Geologist , Ithaca, NY: Cornell University Press.
- Herschel, John F. W., 1830 [1987], A Preliminary Discourse on the Study of Natural Philosophy , reprint, Chicago: University of Chicago Press. [ Herschel 1830 available online ]
- Hodge, Jonathan [M.J.S.}, 1977, “The Structure and Strategy of Darwin’s ‘Long Argument’”, British Journal for the History of Science , 10(3): 237. doi:10.1017/S0007087400015685
- –––,1983a, “Darwin and the Laws of the Animate Part of the Terrestrial System (1835–1837): On the Lyellian Origins of His Zoonomical Explanatory Program”, Studies in the History of Biology , 6: 1–106.
- –––, 1983b, “The Development of Darwin’s General Biological Theorizing”, in D. S. Bendall, Evolution From Molecules to Men , Cambridge: Cambridge University Press, 43–62.
- –––, 1985, “Darwin as a Lifelong Generation Theorist”, in Kohn 1985a: 207–243. doi:10.1515/9781400854714.207
- –––, 2009, “The Notebook Programmes and Projects of Darwin’s London Years”, in Hodge and Radick 2009: 44–72, doi:10.1017/CCOL9780521884754.003
- –––, 2013a, “The Origins of the Origin ”, in Ruse 2013a: 64–71. doi:10.1017/CBO9781139026895.007
- –––, 2013b, “Darwin’s Book: On the Origin of Species ”, Science and Education , 22(9): 2267–2294. doi:10.1007/s11191-012-9544-7
- –––, and Gregory Radick (eds.), 2009, The Cambridge Companion to Darwin , Second edition, 2009, doi:10.1017/CCOL9780521884754.
- Hoquet, Thierry, 2013, “The Evolution of the Origin (1859–1872)”, in Ruse 2013a: 158–164. doi:10.1017/CBO9781139026895.020
- Hösle, Vittorio and Christian Illies (eds.), 2005, Darwinism and Philosophy , Notre Dame, IN: University of Notre Dame Press.
- Hull, David L. (ed.), 1973, Darwin and His Critics: The Reception of Darwin’s Theory of Evolution by the Scientific Community , Cambridge, MA: Harvard University Press.
- –––, 2009, “Darwin’s Science and Victorian Philosophy of Science”, in Hodge and Gregory Radick 2009: 173–196, doi:10.1017/CCOL9780521884754.008
- –––, Peter D. Tessner, and Arthur M. Diamond, 1978, “Planck’s Principle”, Science , 202(4369): 717–723. doi:10.1126/science.202.4369.717
- Huxley, Thomas Henry, 1863, Evidence as to Man’s Place in Nature , London: Williams and Norgate. [ Huxley 1863 available online ]
- –––, 1893, “Evolution and Ethics”, London: Macmillan. Romanes lecture.
- –––, 1895, Evolution and Ethics and Other Essays , London: Macmillan. Includes a “Prolegomena”. Parts translated into Chinese by Yan Fu as 天演論 ( Tianyan lun ), 1898. [ Huxley 1895 available online ] [ Yan Fu translation of Huxley 1895 available online ]
- Jenkin, H. Fleeming, 1867 [1973], “[Review] The Origin of Species”, The North British Review , 46 (June): 277–318. Reprinted in Hull 1973: 303–350. [ Jenkin 1867 available online ]
- Jin, Xiaoxing, 2019a, “Darwin in China, 1870–1935”, Unpublished Ph.D. Thesis, University of Notre Dame.
- –––, 2019b, “Translation and Transmutation: The Origin of Species in China”, The British Journal for the History of Science , 52(1): 117–141. doi:10.1017/S0007087418000808.
- –––, 2020, “The Evolution of Evolutionism in China, 1870–1930”, Isis 111(1): 46–66. doi: https://doi.org/10.1086/708367.
- –––, 2022, “The Evolution of Social Darwinism in China, 1895–1930”, Comparative Studies in Society and History 64(3): 690–721. doi: https://doi.org/10.1017/S0010417522000214.
- Johnson, Paul, 2012, Darwin: Portrait of a Genius , New York: Viking.
- Katz, Leonard D. (ed.), 2000, Evolutionary Origins of Morality: Cross-Disciplinary Perspectives , Exeter, UK: Imprint Academic.
- Kelly, Alfred, 1981, The Descent of Darwin: The Popularization of Darwinism in Germany, 1860–1914 , Chapel Hill, NC: University of North Carolina Press.
- Keynes, Richard (ed.), 2000, Charles Darwin’s Zoology Notes & Specimen Lists from H.M.S. Beagle , Cambridge: Cambridge University Press. [ Keynes 2000 available online ]
- Kohn, David (ed.), 1985a, The Darwinian Heritage , Princeton: Princeton University Press. doi:10.1515/9781400854714
- –––, 1985b, “Darwin’s Principle of Divergence as Internal Dialogue”, in Kohn 1985a: 245–257. doi:10.1515/9781400854714.245
- –––, 2009, “Darwin’s Keystone: The Principle of Divergence”, in Ruse and Richards 2009: 87–108, doi:10.1017/CCOL9780521870795.008.
- Laubichler, Manfred Dietrich and Jane Maienschein (eds.), 2007, From Embryology to Evo-Devo: A History of Developmental Evolution , (Dibner Institute Studies in the History of Science and Technology), Cambridge, MA: MIT Press.
- –––, 2013, “Developmental Evolution”, in Ruse 2013a: 375–382. doi:10.1017/CBO9781139026895.048
- Largent, Mark, 2013, “Darwinism in the United States, 1859–1930”, in Ruse 2013a: 226–234.
- –––, 2009. “The So-Called Eclipse of Darwinism”, Transactions of the American Philosophical Society , 99(4): 3–21.
- Lennox, James G., 1993, “Darwin Was a Teleologist”, Biology & Philosophy , 8(4): 409–421. doi:10.1007/BF00857687
- –––, 2005, “Darwin’s Methodological Evolution”, Journal of the History of Biology , 38(1): 85–99. doi:10.1007/s10739-004-6511-4
- –––, 2013, “Darwin and Teleology”, in Ruse 2013a: 152–157. doi:10.1017/CBO9781139026895.019
- Lightman, Bernard (ed.), 2015, Global Spencerism: The Communication and Appropriation of a British Evolutionist , Leiden: Brill. doi:10.1163/9789004264007
- Lyell, Charles, 1830–33 [1990], Principles of Geology , 3 vols. London: Murray, facsimile reprint, ed. Martin J. Rudwick, Chicago: University of Chicago Press.
- –––, 1863, The Geological Evidences of the Antiquity of Man , London: John Murray. [ Lyell 1863 available online ]
- MacPherson, Ryan, 2015, Debating Evolution Before Darwinism: An Exploration of Science and Religion in America, 1844–1859 , Mantako, MN: Into Your Hands Press.
- Maienschein, Jane and Michael Ruse (eds.), 1999, Biology and the Foundation of Ethics , Cambridge: Cambridge University Press. doi:10.1017/CBO9780511609077
- Mallet, James, 2013, “Darwin and Species”, in Ruse 2013a: 109–115. doi:10.1017/CBO9781139026895.013
- Malthus, Thomas, 1826, An Essay on the Principle of Population , 6th edition. London.[available online]
- Mayr, Ernst and William B. Provine (eds.), 1980, The Evolutionary Synthesis: Perspectives on the Unification of Biology , Cambridge, MA: Harvard University Press.
- Mivart, St. George Jackson, 1871, On the Genesis of Species , London: MacMillan [available online]
- Moore, George Edward, 1903, Principia Ethica , Cambridge: Cambridge University Press. [ G.E. Moore 1903 available online ]
- Moore, James R., 1979, The Post-Darwinian Controversies: A Study of the Protestant Struggle to Come to Terms with Darwin in Great Britain and America 1870–1900 , Cambridge: Cambridge University Press. doi:10.1017/CBO9780511622830
- Mullen, Pierce C., 1964, “The Preconditions and Reception of Darwinian Biology in Germany, 1800–1870”, Unpublished Ph.D Dissertation, University of California, Berkeley.
- Newman, Stuart A., 2015, “The Evolution of Evolutionary Mechanisms”, in Sloan, McKenny, and Eggleson 2015: 61–89.
- Norman, David, 2013, “Charles Darwin’s Geology”, in Ruse 2013a: 46–55. doi:10.1017/CBO9781139026895.005
- Numbers, Ronald L., 1998, Darwinism Comes to America , Cambridge, MA: Harvard University Press.
- Olby, Robert C., 1963, “Charles Darwin’s Manuscript of Pangenesis”, The British Journal for the History of Science , 1(3): 251–263. doi:10.1017/S0007087400001497
- –––, 2013, “Darwin and Heredity”, in Ruse 2013a: 116–123. doi:10.1017/CBO9781139026895.014
- Ospovat, Dov, 1981, The Development of Darwin’s Theory: Natural History, Natural Theology, and Natural Selection , Cambridge: Cambridge University Press.
- Pancaldi, Giuliano, 1983 [1991], Darwin in Italia: Impresa scientifica e frontiere culturali , (Saggi 248), Bologna: il Mulino. Translated as Darwin in Italy: Science across Cultural Frontiers , Ruey Brodine Morelli (trans.), Bloomington: Indiana University Press, 1991.
- Peckham, Morse (ed.), 2006, The Origin of Species: a Variorum Text , Philadelphia: University of Pennsylvania Press, reprint of 1959 edition.
- Pigliucci, Massimo and Gerd Müller (eds.), 2010, Evolution, the Extended Synthesis , Cambridge, MA: MIT Press.
- Prestes, Maria Elice Brzezinski (ed.), 2023, Understanding Evolution in Darwin ’s ‘Origin’: The Emerging Context of Evolutionary Thinking , Cham: Springer.
- Provine, William B., 1971, The Origins of Theoretical Population Genetics , Chicago: University of Chicago Press.
- Pusey, James Reeve, 1983, China and Charles Darwin , (Harvard East Asian Monographs 100), Cambridge, MA: Harvard University Press.
- Radick, Gregory, 2013, “Darwin and Humans”, in Ruse 2013a: 173–181. doi:10.1017/CBO9781139026895.022
- Richards, Evelleen, 2017, Darwin and the Making of Sexual Selection , Chicago: University of Chicago Press. doi:10.7208/chicago/9780226437064.001.0001
- Richards, Richard A., 2010, The Species Problem: A Philosophical Analysis , Cambridge: Cambridge University Press. doi:10.1017/CBO9780511762222
- –––, 2013, “Sexual Selection”, in Ruse 2013a: 103–108. doi:10.1017/CBO9781139026895.012
- Richards, Robert J., 1987, Darwin and the Emergence of Evolutionary Theories of Mind and Behavior , Chicago: University of Chicago Press.
- –––, 1999, “Darwin’s Romantic Biology: The Foundation of His Evolutionary Ethics”, in J. Maienschein and M. Ruse 1999 (eds.) 1999: 113–153. doi:10.1017/CBO9780511609077.007
- –––, 2002, The Romantic Conception of Life: Science and Philosophy in the Age of Goethe , Chicago: University of Chicago Press.
- –––, 2005, “Darwin’s Metaphysics of Mind”, in Hösle and Illies 2005, 166–180.
- –––, 2008, The Tragic Sense of Life: Ernst Haeckel and the Struggle Over Evolutionary Thought , Chicago: University of Chicago Press.
- –––, 2009, “Darwin on Mind, Morals and Emotions”, in Hodge and Radick 2009: 96–119, doi:10.1017/CCOL9780521884754.005
- –––, 2013, “The German Reception of Darwin’s Theory, 1860–1945”, in Ruse 2013a: 235–242. doi:10.1017/CBO9781139026895.030
- –––, 2015, “Darwin’s Evolutionary Ethics: the Empirical and Normative Justifications”, in Sloan, McKenny and Eggleson, 2015, 182–200.
- –––, and Michael Ruse, 2016, Debating Darwin , Chicago: University of Chicago Press.
- Ruse, Michael, 1975, “Darwin’s Debt to Philosophy: An Examination of the Influence of the Philosophical Ideas of John F. W. Herschel and William Whewell on the Development of Charles Darwin’s Theory of Evolution”, Studies in the History and Philosophy of Science , 6: 159–181.
- –––, 2009a, “The Origin of the Origin ”, in Ruse and R. J. Richards 2009: 14–14. doi:10.1017/CCOL9780521870795.003
- –––, 2009b, Defining Darwin: Essays on the History and Philosophy of Evolutionary Biology , Amherst, MA: Prometheus Books.
- –––, 2009c, Philosophy After Darwin: Classic and Contemporary Readings , Princeton: Princeton University Press.
- ––– (ed.), 2013a, The Cambridge Encyclopedia of Darwin and Evolutionary Thought , Cambridge: Cambridge University Press. doi:10.1017/CBO9781139026895
- –––, 2013b, “Evolution before Darwin”, in Ruse 2013a: 39–45. doi:10.1017/CBO9781139026895.004
- –––, 2013c, “The Origin of Species ”, in Ruse 2013a: 95–102. doi:10.1017/CBO9781139026895.011
- –––, and Robert J. Richards (eds.), 2009, The Cambridge Companion to the “Origin of Species” , Cambridge: Cambridge University Press. doi:10.1017/CCOL9780521870795
- Secord, James A., 2000, Victorian Sensation: The Extraordinary Publication, Reception, and Secret Authorship of Vestiges of the Natural History of Creation , Chicago: University of Chicago Press.
- Shen, Vincent, 2016, “Translation and Interpretation: the Case of Introducing Darwinian Evolutionism into China”, Universitas: Monthly Review of Philosophy and Culture , 43: 3–25.
- Sloan, Phillip R. 1986, “Darwin, Vital Matter, and the Transformism of Species”, Journal of the History of Biology , 19 (3): 369–445. doi:10.1007/BF00138286
- –––, 1999, “From Natural Law to Evolutionary Ethics in Enlightenment French Natural History”, in Maienschein and Ruse 1999: 52–83. doi:10.1017/CBO9780511609077.004
- –––, 2009a, “The Making of a Philosophical Naturalist”, in Hodge and Radick 2009: 21–43, doi:10.1017/CCOL9780521884754.002
- –––, 2009b, “Originating Species: Darwin on the Species Problem”, in Ruse and R. J. Richards 2009: 67–86. doi:10.1017/CCOL9780521870795.007
- –––, Gerald P McKenny, and Kathleen Eggleson (eds.), 2015, Darwin in the Twenty-First Century: Nature, Humanity, and God , Notre Dame, IN: University of Notre Dame Press.
- Smocovitis, Vassiliki Betty, 1996, Unifying Biology: The Evolutionary Synthesis and Evolutionary Biology , Princeton, NJ: Princeton University Press.
- Sober, Elliott, 1984, The Nature of Selection: Evolutionary Theory in Philosophical Focus , Cambridge, MA: MIT Press.
- Spencer, Herbert, 1864, Principles of Biology , London: Williams and Noegate. [ Spencer 1864 available online ]
- Stamos, David N., 2003, The Species Problem: Biological Species, Ontology, and the Metaphysics of Biology , Lanham, MA: Lexington Books.
- –––, 2007, Darwin and the Nature of Species , Albany, NY: State University of New York Press.
- Swetlitz, Marc, 2013, “Judaism, Jews, and Evolution”, in Ruse 2013a: 493–498. doi:10.1017/CBO9781139026895.063
- Theunissen, Bert, 2013, “The Analogy between Artificial and Natural Selection”, in Ruse 2013a: 88–94. doi:10.1017/CBO9781139026895.010
- Todes, Daniel Philip, 1989, Darwin Without Malthus: the Struggle for Existence in Russian Evolutionary Thought , New York and Oxford: Oxford University Press.
- Vorzimmer, Peter J., 1970, Charles Darwin, The Years of Controversy: The Origin of Species and Its Critics, 1859–1882 , Philadelphia: Temple University Press.
- Wallace, Alfred R., 1858, “On the Tendency of Varieties to Depart Indefinitely from the Original Type”, in Glick and Kohn 1996, 335–345.
- –––,“The Origin of Human Races and the Antiquity of Man Deduced from the Theory of ‘Natural Selection’”, Journal of the Anthropological Society of London , 2: clviii–clxxxvii. doi:10.2307/3025211
- Waters, C. Kenneth, 2009, “The Arguments in the Origin of Species ”, in Hodge and Radick 2009: 120–144, doi:10.1017/CCOL9780521884754.006
- Whewell, William, 1837, History of the Inductive Sciences, from the Earliest to the Present Times , three volumes, London: Parker.
- –––, 1840, The Philosophy of the Inductive Sciences , London: Parker. [ Whewell 1840 available online ]
- –––, 1858, Novum Organon Renovatum, Being the Second Part of the Philosophy of the Inductive Sciences , thirrd edition, London: Parker. [ Whewell 1858 available online ]
- White, Roger M., M. J. S. Hodge, and Gregory Radick, 2021, Darwin’s Argument by Analogy: From Artificial to Natural Selection , Cambridge: Cambridge University Press.
- Wilkins, John S., 2009, Species: a History of the Idea , Berkeley and Los Angeles: University of California Press.
- Yang Haiyan, 2013, “Encountering Darwin and Creating Darwinism in China”, in Ruse 2013a: 250–257. doi:10.1017/CBO9781139026895.032
- Yan Fu, 1898, Tianyan lun , translation of Huxley 1895, [available online ]
- Young, Robert M., 1985, Darwin’s Metaphor: Nature’s Place in Victorian Culture , Cambridge: Cambridge University Press.
How to cite this entry . Preview the PDF version of this entry at the Friends of the SEP Society . Look up topics and thinkers related to this entry at the Internet Philosophy Ontology Project (InPhO). Enhanced bibliography for this entry at PhilPapers , with links to its database.
- The Complete Works of Charles Darwin Online , maintained by John van Wyhe, Cambridge University Library. In particular note the Darwin Papers & Manuscripts section
- Darwin Manuscripts Project , maintained by David Kohn in cooperation with the American Museum of Natural History Research Library.
- Letter to Charles Lyell, 28 September 1860, DCP-LETT-2931
- Letter from J.D. Hooker, 8 February 1867, DCP-LETT-5395
- Letter to William Turner, 11 February 1867, DCP-LETT-5398
- Letter to Asa Gray, 15 March 1870, DCP-LETT-7132
- Ghiselin, Michael T., 2009, Darwin: A Reader’s Guide [PDF], Occasional Papers of the California Academy of Sciences 155.
- The Huxley File , maintained by Charles Blinderman and David Joyce (Clark University).
- Works by Ernst Heinrich Haeckel , Project Gutenberg.
- Wallace Online , maintained by John van Wyhe, Cambridge University Library.
adaptationism | altruism | altruism: biological | animal: cognition | animal: consciousness | biology: philosophy of | comparative cognition, methods in | creationism | Darwinism | evolution: concept before Darwin | evolution: cultural | fitness | genetics: ecological | life | morality: and evolutionary biology | moral sentimentalism | natural selection | natural selection: units and levels of | Newton, Isaac: philosophy | species | Spencer, Herbert | teleology: teleological notions in biology | Whewell, William
The author wishes to acknowledge the valuable comments on this version of the article by David Depew, Gregory Radick, M. J. S. Hodge, Alan Love, and Xiaoxing Jin. Additional comments were made on an earlier version by Michael Ruse, Robert J. Richards, Edward Zalta, M. Katherine Tillman, and the anonymous reviewers for the Stanford Encyclopedia of Philosophy. I am particularly indebted to Dr. Xiaoxing Jin for information contained in his substantial doctoral work and subsequent research on the reception of Darwinism into China. Responsibility for all interpretations is my own.
Copyright © 2024 by Phillip Sloan < sloan . 1 @ nd . edu >
- Accessibility
Support SEP
Mirror sites.
View this site from another server:
- Info about mirror sites
The Stanford Encyclopedia of Philosophy is copyright © 2024 by The Metaphysics Research Lab , Department of Philosophy, Stanford University
Library of Congress Catalog Data: ISSN 1095-5054
Sciencing_Icons_Science SCIENCE
Sciencing_icons_biology biology, sciencing_icons_cells cells, sciencing_icons_molecular molecular, sciencing_icons_microorganisms microorganisms, sciencing_icons_genetics genetics, sciencing_icons_human body human body, sciencing_icons_ecology ecology, sciencing_icons_chemistry chemistry, sciencing_icons_atomic & molecular structure atomic & molecular structure, sciencing_icons_bonds bonds, sciencing_icons_reactions reactions, sciencing_icons_stoichiometry stoichiometry, sciencing_icons_solutions solutions, sciencing_icons_acids & bases acids & bases, sciencing_icons_thermodynamics thermodynamics, sciencing_icons_organic chemistry organic chemistry, sciencing_icons_physics physics, sciencing_icons_fundamentals-physics fundamentals, sciencing_icons_electronics electronics, sciencing_icons_waves waves, sciencing_icons_energy energy, sciencing_icons_fluid fluid, sciencing_icons_astronomy astronomy, sciencing_icons_geology geology, sciencing_icons_fundamentals-geology fundamentals, sciencing_icons_minerals & rocks minerals & rocks, sciencing_icons_earth scructure earth structure, sciencing_icons_fossils fossils, sciencing_icons_natural disasters natural disasters, sciencing_icons_nature nature, sciencing_icons_ecosystems ecosystems, sciencing_icons_environment environment, sciencing_icons_insects insects, sciencing_icons_plants & mushrooms plants & mushrooms, sciencing_icons_animals animals, sciencing_icons_math math, sciencing_icons_arithmetic arithmetic, sciencing_icons_addition & subtraction addition & subtraction, sciencing_icons_multiplication & division multiplication & division, sciencing_icons_decimals decimals, sciencing_icons_fractions fractions, sciencing_icons_conversions conversions, sciencing_icons_algebra algebra, sciencing_icons_working with units working with units, sciencing_icons_equations & expressions equations & expressions, sciencing_icons_ratios & proportions ratios & proportions, sciencing_icons_inequalities inequalities, sciencing_icons_exponents & logarithms exponents & logarithms, sciencing_icons_factorization factorization, sciencing_icons_functions functions, sciencing_icons_linear equations linear equations, sciencing_icons_graphs graphs, sciencing_icons_quadratics quadratics, sciencing_icons_polynomials polynomials, sciencing_icons_geometry geometry, sciencing_icons_fundamentals-geometry fundamentals, sciencing_icons_cartesian cartesian, sciencing_icons_circles circles, sciencing_icons_solids solids, sciencing_icons_trigonometry trigonometry, sciencing_icons_probability-statistics probability & statistics, sciencing_icons_mean-median-mode mean/median/mode, sciencing_icons_independent-dependent variables independent/dependent variables, sciencing_icons_deviation deviation, sciencing_icons_correlation correlation, sciencing_icons_sampling sampling, sciencing_icons_distributions distributions, sciencing_icons_probability probability, sciencing_icons_calculus calculus, sciencing_icons_differentiation-integration differentiation/integration, sciencing_icons_application application, sciencing_icons_projects projects, sciencing_icons_news news.
- Share Tweet Email Print
Radiometric Dating
Evolutionary theory, evidence for evolution, evolution today, evolution and genetics.
- Home ⋅
- Science ⋅
Theory of Evolution: Definition, Charles Darwin, Evidence & Examples
In 1831, an inexperienced 22-year-old British naturalist named Charles Darwin jumped on the HMS Beagle and sailed the world on a five-year scientific voyage that earned him a place in science and history.
Known today as the “father of evolution,” Darwin amassed compelling evidence supporting the theory of evolution by natural selection. Earlier scholars, including his grandfather Erasmus Darwin, were mocked for presenting such unorthodox ideas as transmutation of species.
Darwin is credited with being the first scientist to persuasively argue a unifying theory of how species evolve and continue to change.
Brief Biography of Charles Darwin
Charles Darwin grew up on an idyllic English estate where he spent his days collecting rare beetles, moths and fossils. His love of nature persisted despite his father’s insistence that young Charles pursue a practical career in medicine at the renowned University of Edinburgh. Not to be deterred, Charles found a mentor in marine biologist Robert Grant and immersed himself in natural science.
Grant introduced Darwin to the idea that life sprung from a common ancestor by pointing out similarities between a human hand and a bird wing. Two years later, Darwin transferred to another school where he focused on botany.
His first professional job was working as a naturalist on the HMS Beagle, a survey boat that took him to exciting places like Brazil, Argentina, the Canary Islands, the Galapagos Islands and Sydney, Australia.
Darwin was influenced by the work of geologist Charles Lyell , who believed in the principle of uniformitarianism. Darwin and Lyell considered fossil records and striated layers in rock formations as evidence of slow and continual change. Darwin applied his knowledge of variation in plants, animals, fossils and rocks to the origin of the species by means of natural selection.
Pre-Darwinian Theories
Religious beliefs and science were closely intertwined in Victorian England. The Bible was the respected authority on how and when life on Earth was created by God. Many scientists acknowledged that species change over time but couldn’t comprehend how or why living organisms change once they appear.
French naturalist, Jean Baptiste Lamarck , was a pioneer in evolutionary theory who challenged the notion that species were immutable based on fossil records. He argued that traits could be acquired and passed along to the next generation.
For instance, Lamarck thought that so-called “nervous fluid” was secreted when giraffes reached for leaves, producing a longer neck that would be inherited by the next generation. Lamarck was ostracized for his suggestion that natural processes, rather than a divine design, determined the direction of life.
Influencer of Darwinian Theory
The 19th century was a turning point in how people viewed the history of life. Great minds from multiple disciplines influenced one another’s theories. Darwin followed the work of progressive thinkers of his time, such Thomas Malthus . A political economist, Malthus argued that people and animals overproduce and put a drain on resources. He advocated for regulation of family size as a means of population control.
Darwin saw some logic in Malthus’ arguments and applied the concept of overpopulation to the natural world. Darwin reasoned that animals compete for survival from the moment of birth.
When resources are scarce, competition is intense. Random, naturally occurring variations make some siblings more fit than others to successfully compete, mature and multiply.
Discovery of Natural Selection
In the 1850s, Alfred Russel Wallace collected thousands of exotic specimens and noticed regional differences in traits. He concluded that the best-suited organisms for a region were naturally more likely to survive and pass along their characteristics. Wallace shared his ideas with Darwin, who had been collecting evidence of natural selection for a much longer time.
Darwin had held off releasing his findings for fear of public ridicule. However, he did not want to see Wallace receive all the credit if the idea of national selection was favorably received. Soon after, Darwin and Wallace simultaneously presented their work to the Linnaean Society.
A year later, Darwin published his groundbreaking work On the Origin of Species .
Darwin’s Theory of Evolution: Definition
Darwin defined evolution as a process of “descent with modification.” He believed that some organisms within a species have trait variants that make them fitter and more likely to reproduce.
Over time, inherited modified traits become dominant in the population, and a new species may emerge. Taking the idea further, Darwin speculated that all life evolved from one common ancestor millions of years ago.
Descent from modification also explains extinction. Certain characteristics may be crucial to plant survival, such as thorns. In a heavily grazed area, plants without thorns could be consumed before they go to seed.
Traits acquired during the lifetimes of those eaten plants are not passed along to any offspring, with the exception of gene mutations in sex cells, such as exposure of germ cells to damaging radiation.
Theory of Evolution by Natural Selection
Darwin’s theory of evolution by natural selection solved the mystery of how evolution works. Darwin figured out that that certain traits and characteristics are better suited to the environment, which enables organisms with the adapted variant to better survive and multiply.
Slowly, over time, a once uncommon gene variant may eventually become the predominant gene in the population via natural selection.
Survival of the fittest is another premise of Darwinian evolutionary theory. However, this does not mean the biggest, fastest and toughest always win. Fitness is a fluid concept relative to the traits needed for survival at a particular time and place. Biodiversity makes a population stronger because change is ongoing, and the evolutionary process keeps pace.
Theory of Evolution: Evidence
Fossil records provide compelling evidence of the evolutionary history of living things. Gradual, incremental changes in land and marine fossils coincide with climate change or migration.
For example, the modern-day horse once looked more like a fox. Paleontologist can show how the ancient horse adapted by slowly acquiring hooves, height and flat teeth as an adaptive modification to living on open grasslands instead of the forest.
DNA extracted from the recovered bones and teeth of Neanderthals indicates that modern humans and Neanderthals descended from the same ancestral group, as supported by DNA sequence analysis . The Neanderthals moved out of Africa and hunted mammoths during the Ice Age.
Later, Homo sapiens and Neanderthals crossed paths again and had children together. Neanderthals died out, but many people today have Neanderthal gene variants in their human genome.
The now extinct Tiktaalik is an example of a missing link that shows when species evolved in very different directions. Tiktaalik was a large fish with characteristics of an amphibian, including a flat head and a neck. Around 375 million years ago, this “fishapod” adapted to living in shallow water and land. Tetrapods, or animals with four feet, descended from these primitive amphibians.
Reverse Evolution: Humans With Tails
Vestigial organs , like the human appendix, are remnants of a body part that once served a purpose. For instance, vestigial tails in humans are an unusual evolutionary throwback that occurs when the tail of the embryo fails to dissolve properly. Normally, the tail of the human embryo forms the coccyx (tailbone). On rare occasion, a baby will be born with a tail that may be fleshy or bony, and a few inches long.
According to the American Museum of Natural History , the small hind leg bones under the skin of boa constrictors and pythons reflect the snakes' evolutionary history. Boa constrictors and pythons descended from lizards that happened to be born with stubby legs. Short legs were better for survival than long legs in certain environments.
Genes for short legs became dominant in the population, and eventually legs disappeared except for unseen vestigial bones near the snakes' tails.
Theory of Evolution: Examples
While traveling the world on the HMS Beagle, Darwin was enthralled by the many different types of island finches. He noted that the finches had various adaptations to suit their environment, like changes in beak size and shape depending on the food they ate.
Darwin’s finches are a textbook example of adaptation and evolution on a small scale. Birds had migrated to the islands from the mainland, and species gradually evolved to fit their new environments. Natural selection happens because organisms in a population typically have randomly occurring gene variations and mutations that affect adaptation.
Evolution requires existing variation in the species. For example, giraffes with a random variation of an unusually long neck were better able to reach leaves in the canopy, making them fitter to survive and more likely to reproduce. Offspring with the same variation of a longer neck enjoyed the same evolutionary advantage at feeding time. The giraffe evolved over time to have the characteristic long neck seen today.
Divine Creation vs. Evolutionary Theory
Darwin’s ideas offended Christians who believed that God created the universe and made man in his image and likeness. The very suggestion that humans, worms and whales had a common ancestor seemed laughable at a time when DNA was not known or understood.
Although some questions remain, the theory of evolutionary change is widely accepted by scientists around the world now. The creationist view of human evolution is generally considered to represent a religious belief based on faith rather than a scientific theory.
Biological Evidence of Evolution
Darwin’s findings resulted from years of painstaking work classifying living organisms based on observed traits, behavior, vocalizations and overall appearance. He was able to develop his theory of evolution without knowing the exact mechanism behind it. The discovery of genes and alleles answered the question that Darwin could not solve.
Descent with modification is the result of gene recombination and mutations in germ cells that get handed down to the next generation. Genetic changes resulting from mutations can be harmless, helpful or detrimental. Genetic variations and modifications in populations often lead to the emergence of new species.
Molecular Biology and Evolutionary Evidence
A common ancestor is based on remarkable similarity in genetic material, genetic codes and gene expression. Cells of multicellular organisms grow, metabolize, divide and mutate much the same way. Molecular biology allows comparison of organisms and species at the cellular level.
Closely related organisms have similar sequences of amino acids in their genes. Certain genes may be almost identical in different species as the result of sharing a common ancestor. Humans and chimpanzees have an almost identical gene that encodes insulin.
Humans and chickens both code for insulin, but the genes have fewer similarities, revealing that humans are more closely related to monkeys than fowl.
Evolution Is Ongoing
Humans continue to evolve as a species. Blue eyes came about just 10,000 years ago when a gene mutation turned off the switch to produce brown eyes. Other relatively recent mutations include an ability to digest milk. The process of natural selection and survival of the fittest may have a more limited effect on modern human evolution, however.
Advances in modern medicine make it possible to survive diseases that would have once proved fatal. Many people are having babies when they are older, when the risks of genetic diseases may be greater. The theory of evolution holds that life will continue to diversify and adapt to changing conditions.
Related Articles
Genetic isolation and evolution.
- Smithsonian National Museum of Natural History: Introduction to Human Evolution
- Khan Academy: Evidence for Evolution
- UC Berkeley: Understanding Evolution: Early Concepts of Evolution: Jean Baptiste Lamarck
- Natural History Museum: Who Was Alfred Russel Wallace?
- University of California Museum of Paleontology: Thomas Malthus (1766-1834)
- American Museum of Natural History: Vestigial Organs
- Phys.org: We Are Still Evolving
- American Museum of Natural History: A Trip Around the World
About the Author
Dr. Mary Dowd studied biology in college where she worked as a lab assistant and tutored grateful students who didn't share her love of science. Her work history includes working as a naturalist in Minnesota and Wisconsin and presenting interactive science programs to groups of all ages. She enjoys writing online articles sharing information about science and education. Currently, Dr. Dowd is a dean of students at a mid-sized university.
Find Your Next Great Science Fair Project! GO
INFOGRAPHIC
Evolving theory of evolution.
Charles Darwin and Alfred Wallace developed the idea of evolution through natural selection. But this idea was not accepted by scientists until more evidence came along. Use this infographic to explore how Darwinism and genetics came together to explain what we know today about evolution.
Biology, Genetics
Loading ...
Idea for Use in the Classroom
Introduce and share the infographic with students. Define key ideas such as natural selection , inheritance, and DNA . These activities can be done as in-class assignments, small group work, or they can be completed outside of class time. Introduce students to Charles Darwin’s research, Gregor Mendel’s work with pea plants, and James Watson and Francis Crick’s determination of the structure of DNA .
Darwin Challenged
Have students work in pairs to research the scientists and ideas that were used to challenge Darwin’s theory about the role of natural selection . Ask the pairs to choose one scientist or idea and make a poster that explains why that scientist thinks Darwin is wrong. Then have students write a response from Charles Darwin explaining why his theory of natural selection is correct.
Have students search to find a tree of life diagram of human evolution . Ask students to identify where “Lucy” fits into the tree. Then ask students to research Lucy and write a short paragraph describing why she is an important find.
Darwin’s Finches
Have students work in groups to research the finches Darwin studied in the Galapagos. Have the groups select one type of finch and explain how its beak adapted through natural selection to gather its food. Encourage the groups to build models to demonstrate how the shape of the beak is best suited for obtaining certain types of food. Have the groups present their demonstration to the class.
Tweeting Science
Ask students to read through the timeline and select one of the entries. Then have them research that entry to learn more about how the discovery came about and what it adds to our understanding of evolution. After research, have students summarize how the entry supports the legacy of an idea by writing a 280-character tweet that announces the evidence.
Natural Selection
Tell students that Darwin, along with another scientist, Alfred Russel Wallace, were the first to propose the idea of evolution through natural selection. Today, this idea is widely accepted by scientists. Have students research and devise a demonstration of how natural selection works in plants or animals. Have students select a demonstration that they can present to the class.
Media Credits
The audio, illustrations, photos, and videos are credited beneath the media asset, except for promotional images, which generally link to another page that contains the media credit. The Rights Holder for media is the person or group credited.
Production Managers
Program specialists, last updated.
October 19, 2023
User Permissions
For information on user permissions, please read our Terms of Service. If you have questions about how to cite anything on our website in your project or classroom presentation, please contact your teacher. They will best know the preferred format. When you reach out to them, you will need the page title, URL, and the date you accessed the resource.
If a media asset is downloadable, a download button appears in the corner of the media viewer. If no button appears, you cannot download or save the media.
Text on this page is printable and can be used according to our Terms of Service .
Interactives
Any interactives on this page can only be played while you are visiting our website. You cannot download interactives.
Related Resources
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Special Collections
- Author Guidelines
- Submission Site
- Open Access
- Reasons to submit
- About BioScience
- Journals Career Network
- Editorial Board
- Advertising and Corporate Services
- Self-Archiving Policy
- Potentially Offensive Content
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
References cited.
- < Previous
Evolution: Evidence and Acceptance
Ross H. Nehm ( [email protected] ) is an associate professor of science education and evolution, ecology, and organismal biology at The Ohio State University, in Columbus. His research on evolution education was recently highlighted in Thinking Evolutionarily: Evolution Education Across the Life Sciences (National Academies Press, 2012).
- Article contents
- Figures & tables
- Supplementary Data
Ross H. Nehm, Evolution: Evidence and Acceptance, BioScience , Volume 62, Issue 9, September 2012, Pages 845–847, https://doi.org/10.1525/bio.2012.62.9.13
- Permissions Icon Permissions
The Evidence for Evolution. Alan R. Rogers. University of Chicago Press, 2011. 128 pp., illus. $18.00 (ISBN 9780226723822 paper).
A lthough scientists view evolution as an indisputable feature of the natural world, most Americans simply do not believe that it occurs, or they reject naturalistic explanations for biotic change. Empirical studies have revealed that students and teachers often know quite a bit about evolution but still do not accept it. This somewhat counterintuitive finding has been empirically corroborated and has led science educators to investigate this pattern in order to provide suggestions for effective evolution instruction (e.g., Rosengren et al. 2012 ). Within the lucid, compact, up-to-date, and highly readable pages of The Evidence for Evolution , author Alan R. Rogers takes an approach that most science educators have found inadequate: exclusively using logic, parsimony, and the force of evidence to precipitate conceptual change about evolutionary belief. Reactions from both supportive and dissenting readers to this nicely written text will depend on how much faith they place in the use of logic to challenge the worldviews of intelligent-design creationists.
Two premises appear to frame this short book: Biology courses and textbooks are focused on evolutionary mechanisms at the expense of the evidence for evolution, which most people are not aware of, and once disbelievers of evolution are exposed to the massive amount of evidence that exists, they will change their beliefs. I am not sure whether most biologists would agree with the first premise, given the increasingly elaborate coverage of evolution in textbooks. Indeed, having reviewed some of the best-selling introductory biology books ( Nehm et al. 2009 ), I know that many topics that Rogers discusses are, in fact, covered in these texts. I am also doubtful as to whether science educators would agree with the second premise: Empirical studies have shown that learning more about evolution often fails to precipitate a meaningful belief change.
Within the 10 chapters that form the structure of The Evidence for Evolution , the choice of topics is excellent. Also noteworthy are the use of fresh empirical examples, the integration of phylogenetic trees, and the inclusion of paleontological patterns, radiometric dating, and genomic data. The evidence for evolution is vast, and choosing appropriate examples for a short book is no small task.
Writing about evolution can be quite challenging, given that many students and teachers view teleological factors as sufficient explanations for evolutionary change. It is important, therefore, to clarify what we mean when we use such language ( Rector et al. 2012 ). At times, Rogers uses intentional or teleological language: “Every living thing must solve many engineering problems just to stay alive” (p. 34). Although biologists will understand what Rogers means, the same may not be true of novice readers. Individual organisms cannot willfully change the traits that they have (e.g., they cannot intentionally modify a phenotypic feature).
Language may also invoke ideas that are at odds with current scientific thinking, and although Rogers writes with precision and clarity, some exceptions are worth mentioning. Trait loss, for example, has been shown to be a particularly difficult concept for students and teachers to understand ( Nehm and Ha 2011 ). When describing the loss of whale limbs (“Over the next few million years, whales relied less and less on their legs,” p. 20, or “Hind limbs dwindled,” p. 22), his language may be in greater alignment with common misconceptions about use and disuse than with natural selection. When writing about evolution, scientists need to be more cognizant of readers' potential interpretations of the language that we use.

One literary device employed throughout the text is the contrast of supernatural explanations (e.g., “Perhaps we sprang from the hand of God,” p. 81) with naturalistic, evolutionary explanations. Although this approach makes the text engaging, it makes little sense from my perspective and has the potential to exacerbate readers' existing confusions about core ideas relating to the nature of science (NOS). Most students and teachers remain unaware of the ontological presuppositions that undergird the scientific process (e.g., methodological naturalism). By definition (e.g., from the National Academy of Sciences), science cannot speak to or evaluate the relative merits of supernatural explanations; no amount of evidence will ever be able to tip the scale in favor of a naturalistic explanation relative to a supernatural one or vice versa. It is not clear why Rogers takes this approach.
Students' and teachers' evolutionary acceptance levels are known to be related to their understanding of the NOS. Because many Americans are deeply confused about NOS concepts such as observation , inference , testability , theory , law , model , proof , experiment , and hypothesis ( Lederman 2007 ), addressing NOS misconceptions has become de rigueur in evolution education. I was surprised, therefore, to find that The Evidence for Evolution does not discuss what evidence is or how the term is used in evolutionary science. More problematic is the somewhat careless use of NOS terms (e.g., “this experiment proved that,” p. 12, emphasis added, and “we can also see new species forming ,” p. 16, emphasis added). In order to prevent the reinforcement of such NOS misconceptions (e.g., that scientific knowledge is certain because it is proven ; or the conflation of observation and inference ), the meanings of everyday and scientific terms must be carefully distinguished for readers.
To make the most of Rogers's important contribution, pairing The Evidence for Evolution with a textbook about the NOS (e.g., Espinoza 2012 ) is much more likely to achieve what the author admirably aspires to: an understanding, acceptance, and appreciation of evolutionary science. Facts, logic, and parsimony are unlikely, on their own, to affect most people's perceptions of the plausibility of evolution.
Espinoza F . 2012 . The Nature of Science: Integrating Historical, Philosophical, and Sociological Perspectives . Rowman and Littlefield .
Google Scholar
Google Preview
Lederman NG . 2007 . Nature of Science: Past, Present, and Future . Pages 831 – 880 in Abell SK Lederman NG , eds. Handbook of Research on Science Education . Erlbaum .
Nehm RH Ha M . 2011 . Item feature effects in evolution assessment . Journal of Research in Science Teaching 48 : 237 – 256 .
Nehm RH Poole TM Lyford ME Hoskins SG Carruth L Ewers BE Colberg PJS . 2009 . Does the segregation of evolution in biology textbooks and introductory courses reinforce students' faulty mental models of biology and evolution? Evolution: Education and Outreach 2 : 527 – 532 .
Rector MA Nehm RH Pearl D . 2012 . Learning the language of evolution: Lexical ambiguity and word meaning in student explanations . Research in Science Education . Forthcoming. (3 July 2012; www.springerlink.com/content/4117121q46082l30 ) doi:10.1007/s11165-012-9296-z
Rosengren KS Brem SK Evans EM Sinatra GM eds. 2012 . Evolution Challenges: Integrating Research and Practice in Teaching and Learning about Evolution . Oxford University Press .
Author notes
| Month: | Total Views: |
|---|---|
| December 2016 | 2 |
| January 2017 | 20 |
| February 2017 | 20 |
| March 2017 | 33 |
| April 2017 | 23 |
| May 2017 | 1 |
| June 2017 | 1 |
| August 2017 | 11 |
| September 2017 | 18 |
| October 2017 | 20 |
| November 2017 | 34 |
| December 2017 | 132 |
| January 2018 | 137 |
| February 2018 | 182 |
| March 2018 | 332 |
| April 2018 | 394 |
| May 2018 | 270 |
| June 2018 | 173 |
| July 2018 | 155 |
| August 2018 | 200 |
| September 2018 | 244 |
| October 2018 | 170 |
| November 2018 | 258 |
| December 2018 | 193 |
| January 2019 | 142 |
| February 2019 | 249 |
| March 2019 | 318 |
| April 2019 | 233 |
| May 2019 | 176 |
| June 2019 | 145 |
| July 2019 | 118 |
| August 2019 | 145 |
| September 2019 | 150 |
| October 2019 | 200 |
| November 2019 | 75 |
| December 2019 | 74 |
| January 2020 | 43 |
| February 2020 | 125 |
| March 2020 | 62 |
| April 2020 | 76 |
| May 2020 | 44 |
| June 2020 | 70 |
| July 2020 | 35 |
| August 2020 | 42 |
| September 2020 | 70 |
| October 2020 | 102 |
| November 2020 | 56 |
| December 2020 | 67 |
| January 2021 | 48 |
| February 2021 | 37 |
| March 2021 | 68 |
| April 2021 | 59 |
| May 2021 | 39 |
| June 2021 | 45 |
| July 2021 | 13 |
| August 2021 | 29 |
| September 2021 | 24 |
| October 2021 | 33 |
| November 2021 | 33 |
| December 2021 | 14 |
| January 2022 | 16 |
| February 2022 | 43 |
| March 2022 | 22 |
| April 2022 | 32 |
| May 2022 | 23 |
| June 2022 | 26 |
| July 2022 | 20 |
| August 2022 | 26 |
| September 2022 | 48 |
| October 2022 | 37 |
| November 2022 | 41 |
| December 2022 | 37 |
| January 2023 | 32 |
| February 2023 | 34 |
| March 2023 | 50 |
| April 2023 | 65 |
| May 2023 | 36 |
| June 2023 | 55 |
| July 2023 | 14 |
| August 2023 | 48 |
| September 2023 | 40 |
| October 2023 | 63 |
| November 2023 | 67 |
| December 2023 | 42 |
| January 2024 | 28 |
| February 2024 | 73 |
| March 2024 | 37 |
| April 2024 | 47 |
| May 2024 | 24 |
| June 2024 | 39 |
| July 2024 | 34 |
| August 2024 | 17 |
| September 2024 | 26 |
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1525-3244
- Copyright © 2024 American Institute of Biological Sciences
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Evotourism ®
A Smithsonian magazine special report
AT THE SMITHSONIAN
Seven new things we learned about human evolution in 2021.
Paleoanthropologists Briana Pobiner and Ryan McRae reveal some of the year’s best findings in human origins studies
Briana Pobiner and Ryan McRae
:focal(600x297:601x298)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/aa/12/aa12de6c-4859-4e5c-a2b3-3cccc3db95c3/teenagers-left-their-footprints-in-the-mud_1.jpeg)
This year—2021—has been a year of progress in overcoming the effects of the Covid-19 pandemic on human evolution research. With some research projects around the world back up and running, we wanted to highlight new and exciting discoveries from 13 different countries on five different continents. Human evolution is the study of what links us all together, and we hope you enjoy these stories we picked to show the geographic and cultural diversity of human evolution research, as well as the different types of evidence for human evolution, including fossils, archaeology, genetics, and even footprints!
New Paranthropus robustus fossils from South Africa show microevolution within a single species.
The human fossil record, like any fossil record, is full of gaps and incomplete specimens that make our understanding of complex evolutionary trends difficult. Identifying species and the process by which new species emerge from fossils falls in the realm of macroevolution , or evolution over broad time scales. These trends and changes tend to be more pronounced and easier to identify in the fossil record; think about how different a Tyrannosaurus rex and a saber-toothed cat are from each other. Human evolution only took place over the course of 5 to 8 million years, a much shorter span compared to the roughly 200 million years since dinosaurs and mammals shared a common ancestor. Because of this, smaller-scale evolutionary changes within a single species or lineage over time, called microevolution , is often difficult to detect.
Fossils of one early human species, Paranthropus robustus , are known from multiple cave sites in South Africa. Like other Paranthropus species, P. robustus is defined by large, broad cheeks, massive molars and premolars, and a skull highly adapted for intense chewing. Fossils of P. robustus from Swartkrans cave, just 20 miles west of Johannesburg, are dated to around 1.8 million years ago and show a distinct sagittal crest, or ridge of bone along the top of the skull, with their jaws indicating a more efficient bite force. Newly discovered fossils of P. robustus from Drimolen cave , about 25 miles north of Johannesburg, described by Jesse Martin from La Trobe University and colleagues in January, are at least 200,000 years older (2.04-1.95 million years old) and have a differently positioned sagittal crest and a less efficient bite force, among other small differences. Despite numerous disparities between fossils at the two sites, they much more closely resemble each other than any other known species of hominin. Because of this, researchers kept them as the same species from two different time points in a single lineage . The differences between fossils at the two sites highlight microevolution within this Paranthropus lineage .
Fossil children from Kenya, France, and South Africa tell us how ancient and modern human burial practices changed over time.
Most of the human fossil record includes the remains of adult individuals; that’s likely because larger and thicker adult bones, and bones of larger individuals, are more likely to survive the burial, fossilization, and discovery processes. The fossil record also gets much richer after the practice of intentional human burial began, starting at least 100,000 years ago .
In November, María Martinón-Torres from CENIEH (National Research Center on Human Evolution) in Spain, Nicole Boivin and Michael Petraglia from the Max Planck Institute for the Science of Human History in Germany, and other colleagues announced the oldest known human burial in Africa —a two-and-a-half to three-year-old child from the site of Panga ya Saidi in Kenya. The child, nicknamed “Mtoto” which means “child" in Kiswahili, was deliberately buried in a tightly flexed position about 78,000 years ago, according to luminescence dating. The way the child’s head was positioned indicates possible burial with a perishable support, like a pillow. In December, a team led by University of Colorado, Denver’s Jaime Hodgkins reported the oldest known burial of a female modern human infant in Europe . She was buried in Arma Veirana Cave in Italy 10,000 years ago with an eagle-owl talon, four shell pendants, and more than 60 shell beads with patterns of wear indicating that adults had clearly worn them for a long time beforehand. This evidence indicates her treatment as a full person by the Mesolithic hunter-gatherer group she belonged to. After extracted DNA determined that she was a girl, the team nicknamed her “Neve” which means “snow” in Italian. Aside from our own species, Neanderthals are also known to sometimes purposefully bury their dead . In December, a team led by Antoine Balzeau from the CNRS (the French National Centre for Scientific Research) and Muséum National d’Histoire Naturelle in France and Asier Gómez-Olivencia from the University of the Basque Country in Spain provided both new and re-studied information on the archaeological context of the La Ferrassie 8 Neanderthal skeleton, a two-year-old buried in France about 41,000 years ago. They conclude that this child, who is one of the most recently directly dated Neanderthals (by Carbon-14) and whose partial skeleton was originally excavated in 1970 and 1973, was purposefully buried . There have also been suggestions that a third species, Homo naledi , known from South Africa between about 335,000 and 236,000 years ago, purposefully buried their dead—though without any ritual context. In November, a team led by University of the Witwatersrand’s Lee Berger published two papers with details of skull and tooth fragments of a four to six-year-old Homo naledi child fossil , nicknamed “Leti” after the Setswana word “letimela” meaning “the lost one.” Given the location of the child’s skull found in a very narrow, remote and inaccessible part of the Rising Star cave system, about a half mile from Swartkrans, this first partial skull of a child of Homo naledi yet recovered might support the idea that this species also deliberately disposed of their dead.
The first Europeans had recent Neanderthal relatives, according to genetic evidence from Czechia and Bulgaria.
Modern humans, Homo sapiens , evolved in Africa and eventually made it to every corner of the world. That is not news. However, we are still understanding how and when the earliest human migrations occurred. We also know that our ancestors interacted with other species of humans at the time, including Neanderthals , based on genetic evidence of Neanderthal DNA in modern humans alive today—an average of 1.9 percent in Europeans.
Remains of some of the earliest humans in Europe were described this year by multiple teams, except they were not fully human. All three of the earliest Homo sapiens in Europe exhibit evidence of Neanderthal interbreeding (admixture) in their recent genealogical past. In April, Kay Prüfer and a team from the Max Planck Institute for the Science of Human History described a human skull from Zlatý kůň, Czechia, dating to around 45,000 years old . This skull contains roughly 3.2 percent Neanderthal DNA in the highly variable regions of the genome, comparable with other humans from around that time. Interestingly, some of these regions indicating Neanderthal admixture were not the same as modern humans, and this individual is not directly ancestral to any population of modern humans, meaning they belonged to a population that has no living descendants. Also in April, Mateja Hajdinjak and a team from the Max Planck Institute for Evolutionary Anthropology described three similar genomes from individuals found in Bacho Kiro Cave, Bulgaria, dating between 46,000 and 42,000 years old . These individuals carry 3.8, 3.4, and 3.0 percent Neanderthal DNA, more than the modern human average. Based on the distribution of these sequences, the team concluded that the three individuals each had a Neanderthal ancestor only six or seven generations back. This is roughly the equivalent length of time from the turn of the twentieth century to today. Interestingly, these three genomes represent two distinct populations of humans that occupied the Bulgarian cave—one of which is directly ancestral to east Asian populations and Indigenous Americans, the other of which is directly ancestral to later western Europeans. These findings suggest that there is continuity of human occupation of Eurasia from the earliest known individuals to present day and that mixing with Neanderthals was likely common, even among different Homo sapiens populations.
A warty pig from Indonesia, a kangaroo from Australia, and a conch shell instrument from France all represent different forms of ancient art.
Currently, the world’s oldest representational or figurative art is a cave painting of a Sulawesi warty pig found in Leang Tedongnge, Indonesia, that was dated to at least 45,500 years ago using Uranium series dating—and reported in January by a team led by Adam Brumm and Maxime Aubert from Griffith University. In February, a team led by Damien Finch from the University of Melbourne in Australia worked with the Balanggarra Aboriginal Corporation, which represents the Traditional Owners of the land in the Kimberly region of Australia, to radiocarbon date mud wasp nests from rock shelters in this area. While there is fossil evidence of modern humans in Australia dating back to at least 50,000 years ago , this team determined that the oldest known Australian Aboriginal figurative rock paintings date back to between around 17,000 and 13,000 years ago . The naturalistic rock paintings mainly depict animals and some plants; the oldest example is of a about 6.5 footlong kangaroo painting on the ceiling of a rock shelter dated to around 17,300 years ago. Right around that time, about 18,000 years ago, an ancient human in France cut off the top of a conch shell and trimmed its jagged outer lip smooth so it could be used as the world’s oldest wind instrument . A team led by Carole Fritz and Gilles Tostello from the Université de Toulouse in France reported in February that they re-examined this shell, discovered in Marsoulas Cave in 1931, using CT scanning. In addition to the modifications described above, they found red fingerprint-sized and shaped dots on the internal surface of the shell, made with ochre pigment also used to create art on the walls of the cave. They also found traces of a wax or resin around the broken opening, which they interpreted as traces of an adhesive used to attach a mouthpiece as found in other conch shell instruments.
Fossil finds from China and Israel complicate the landscape of human diversity in the late Pleistocene.
This year a new species was named from fossil material found in northeast China: Homo longi . A team from Hebei University in China including Qiang Ji, Xijun Ni, Qingfeng Shao and colleagues described this new species dating to at least 146,000 years old. The story behind the discovery of this cranium is fascinating! It was hidden in a well from the Japanese occupying forces in the town of Harbin for 80 years and only recently rediscovered. Due to this history, the dating and provenience of the cranium are difficult to ascertain, but the morphology suggests a mosaic of primitive-like features as seen in Homo heidelbergensis , and other more derived features as seen in Homo sapiens and Neanderthals . Although the cranium closely resembles some other east Asian finds such as the Dali cranium , the team named a new species based on the unique suite of features. This newly named species may represent a distinct new lineage, or may potentially be the first cranial evidence of an enigmatic group of recent human relatives—the Denisovans . Adding to the increasingly complex picture of late Pleistocene Homo are finds from Nesher Ramla in Israel dating to 120,000 to 130,000 years old , described in June by Tel Aviv University’s Israel Hershkovitz and colleagues. Like the Homo longi cranium, the parietal bone, mandible and teeth recovered from Nesher Ramla exhibit a mix of primitive and derived features. The parietal and mandible have stronger affiliations with archaic Homo , such as Homo erectus , while all three parts have features linking them to Neanderthals. Declining to name a new species , the team instead suggests that these finds may represent a link between earlier fossils with “Neanderthal-like features” from Qesem Cave and other sites around 400,000 years ago to later occupation by full Neanderthals closer to 70,000 years ago. Regardless of what these finds may come to represent in the form of new species, they tell us that modern-like traits did not evolve simultaneously, and that the landscape of human interaction in the late Pleistocene was more complex than we realize.
The ghosts of modern humans past were found in DNA in dirt from Denisova Cave in Russia.
Denisova Cave in Russia, which has yielded fossil evidence of Denisovans and Neanderthals (and even remains of a 13-year-old girl who was a hybrid with a Neanderthal mother and Denisovan father), is a paleoanthropological gift that keeps on giving! In June, a team led by Elena Zavala and Matthias Meyer from the Max Planck Institute for Evolutionary Anthropology in Germany and Zenobia Jacobs and Richard Roberts from the University of Wollongong in Australia analyzed DNA from 728 sediment samples from Denisova Cave —the largest analysis ever of sediment DNA from a single excavation site. They found ancient DNA from Denisovans and Neanderthals… and modern humans, whose fossils have not been found there, but who were suspected to have lived there based on Upper Paleolithic jewelry typically made by ancient modern humans found in 45,000-year-old layers there. The study also provided more details about the timing and environmental conditions of occupation of the cave by these three hominin species: first Denisovans were there, between 250,000 and 170,000 years ago; then Neanderthals arrived at the end of this time period (during a colder period) and joined the Denisovans, except between 130,000 and 100,000 years ago (during a warmer period) when only Neanderthal DNA was detected. The Denisovans who came back to the cave after 100,000 years ago have different mitochondrial DNA, suggesting they were from a different population. Finally, modern humans arrived at Denisova Cave by 45,000 years ago. Both fossil and genetic evidence point to a landscape of multiple interacting human species in the late Pleistocene, and it seems like Denisova Cave was the place to be!
Fossilized footprints bring to light new interpretations of behavior and migration in Tanzania, the United States and Spain.
Usually when we think of fossils, we think of the mineralized remnants of bone that represent the skeletons of long since passed organisms. Yet trace fossils, such as fossilized footprints, give us direct evidence of organisms at a specific place in a specific time. The Laetoli footprints , for example, represent the earliest undoubted bipedal hominin, Australopithecus afarensis (Lucy’s species) at 3.6 million years ago. In December, a team led by Ellison McNutt from Ohio University announced that their reanalysis of some of the footprints from Site A at Laetoli were not left by a bear, as had been hypothesized, but by a bipedal hominin. Furthermore, because they are so different from the well-known footprints from Site G, they represent a different bipedal species walking within 1 kilometer (0.6 miles) of each other within the span of a few days! Recently uncovered and dated footprints in White Sands National Park , New Mexico, described in September by a team led by Matthew Bennett of Bournemouth University, place modern humans in the area between 23,000 to 21,000 years ago. Hypotheses as to how Indigenous Americans migrated into North America vary in terms of method (ice-free land corridor versus coastal route) as well as timing. Regardless of the means by which people traveled to North America, migration was highly unlikely, if not impossible, during the last glacial maximum (LGM), roughly 26,000 to 20,000 years ago. These footprints place modern humans south of the ice sheet during this period, meaning that they most likely migrated prior to the LGM . This significantly expands the duration of human occupation past the 13,000 years ago supported by Clovis culture and the roughly 20,000 years ago supported by other evidence. Furthermore, it means that humans and megafauna, like giant ground sloths and wooly mammoths, coexisted for longer than previously thought, potentially lending credit to the theory that their extinction was not caused by humans. Also interesting is that most of these footprints were likely made by children and teenagers, potentially pointing to division of labor within a community. Speaking of footprints left by ancient children, a team led by Eduardo Mayoral from Universidad de Huelva reported 87 Neanderthal footprints from the seaside site of Matalascañas in southwestern Spain in March. Dated at about 106,000 years ago, these are now the oldest Neanderthal footprints in Europe, and possibly in the world. The researchers conclude that of the 36 Neanderthals that left these footprints, 11 were children; the group may have been hunting for birds and small animals, fishing, searching for shellfish… or just frolicking on the seashore. Aw.
A version of this article was originally published on the PLOS SciComm blog.
Get the latest on what's happening At the Smithsonian in your inbox.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/11-630x421.jpg)
Briana Pobiner | READ MORE
Briana Pobiner is a paleoanthropologist with the National Museum of Natural History’s Human Origins Program . She lead's the program's education and outreach efforts.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/Ryan_McCrae.jpg)
Ryan McRae | READ MORE
Dr. Ryan McRae is a paleoanthropologist studying the hominin fossil record on a macroscopic scale. He currently works for the National Museum of Natural History’s Human Origins Program as a contractor focusing on research, education, and outreach, and is an adjunct assistant professor of anatomy at the George Washington University School of Medicine and Health Sciences.
What is Darwin's Theory of Evolution?
Charles Darwin's Theory of Evolution is one of the most solid theories in science. But what exactly is it?

- Natural selection
- Origin of whales
- Rival theories of evolution
- Modern evolutionary synthesis
- Evidence for evolution
- Is evolution controversial?
Additional resources
The Theory of Evolution by natural selection was first formulated in Charles Darwin's book " On the Origin of Species " published in 1859. In his book, Darwin describes how organisms evolve over generations through the inheritance of physical or behavioral traits, as National Geographic explains. The theory starts with the premise that within a population, there is variation in traits, such as beak shape in one of the Galapagos finches Darwin studied.
According to the theory, individuals with traits that enable them to adapt to their environments will help them survive and have more offspring, which will inherit those traits. Individuals with less adaptive traits will less frequently survive to pass them on. Over time, the traits that enable species to survive and reproduce will become more frequent in the population and the population will change, or evolve, according to BioMed Central . Through natural selection, Darwin suggested, genetically diverse species could arise from a common ancestor .
Darwin did not know the mechanism by which traits were passed on, according to National Geographic. He did not know about genetics , the mechanism by which genes encode for certain traits and those traits are passed from one generation to the next. He also did not know about genetic mutation, which is the source of natural variation. But future research by geneticists provided the mechanism and additional evidence for evolution by natural selection
What is natural selection?
Darwin chose the term "natural selection" to be in contrast with "artificial selection," in which animal breeders select for particular traits that they deem desirable. In natural selection, it's the natural environment, rather than a human being, that does the selecting.
Put simply, the theory of evolution by means of natural selection can be described as "descent with modification," said Briana Pobiner , an anthropologist and educator at the Smithsonian National Museum of Natural History in Washington, D.C., who specializes in the study of human origins. The theory is sometimes described as "survival of the fittest," but that characterization can be misleading, Pobiner said. Here, "fitness" refers not to an organism's strength or athleticism but rather its ability to survive and reproduce.
Natural selection can alter a species in small ways, causing a population to change color or size over the course of several generations, according to The Natural History Museum . When this process happens over a relatively short period of time and in a species or small group of organisms, scientists call it " microevolution ."

But when given enough time and accumulated changes, natural selection can create entirely new species, a process known as "macroevolution," according to Derek Turner and Joyce C. havstad in " The Philosophy of Macroevolution ." This long-term process is what turned dinosaurs into birds , amphibious mammals (such as an animal called Indohyus ) into whales and a common ancestor of apes and humans into the people, chimps and gorillas we know today.
Darwin also described a form of natural selection that depends on an organism's success at attracting a mate — a process known as sexual selection, according to Nature Education . The colorful plumage of peacocks and the antlers of male deer are both examples of traits that evolved under this type of selection.
How did whales evolve?
One of the best examples scientists have of natural selection, is the evolution of whales . By using Darwin's theory as a guide, and understanding how natural selection works, biologists determined that the transition of early whales from land to water occurred in a series of predictable steps.
The evolution of the blowhole, for example, might have started with random genetic changes that resulted in at least one whale having its nostrils farther back on its head, according to Phys.org .
The whales with this adaptation would have been better suited to a marine lifestyle, since they would not have had to completely surface to breathe. Such individuals were more successful and had more offspring. In later generations, more genetic changes occurred, moving the nose farther back on the head.
Other body parts of early whales also changed. Front legs became flippers. Back legs disappeared. Their bodies became more streamlined, and they developed tail flukes to better propel themselves through water, according to the Natural History Museum .
Even though scientists could predict what early whales should look like, for a long time they lacked the fossil evidence to back up their claim. Creationists viewed this absence, not just with regard to whale evolution but more generally, as proof that evolution didn't occur, as pointed out in a Scientific American article .

However, since the early 1990s, scientists have found evidence from paleontology , developmental biology and genetics to support the idea that whales evolved from land mammals. These same lines of evidence support the theory of evolution as a whole.
In the first edition of "On the Origin of Species," Darwin speculated about how natural selection could cause a land mammal to turn into a whale. As a hypothetical example, Darwin used North American black bears ( Ursus americanus ), which were known to catch insects by swimming in the water with their mouths open, according to the Darwin Correspondence Project .
"I can see no difficulty in a race of bears being rendered, by natural selection, more aquatic in their structure and habits, with larger and larger mouths, till a creature was produced as monstrous as a whale," he speculated.
The idea didn't go over very well with the public or with other scientists. Darwin was so embarrassed by the ridicule he received that the swimming-bear passage was removed from later editions of the book. Scientists now know that Darwin had the right idea but the wrong animal. Instead of looking at bears, he should have been looking at cows and hippopotamuses .
Other theories of evolution
Darwin wasn't the first or only scientist to develop a theory of evolution. Around the same time as Darwin, British biologist Alfred Russel Wallace independently came up with the theory of evolution by natural selection, according to the Natural History Museum . However this had little impact.
"The concept of evolution as a historical event was a hot topic among biologists and geologists prior to Darwin’s book because there was so much evidence accumulating, but I suspect biological evolution hadn’t really impinged on people outside of the academic bunker," Dr. P John D. Lambshead, a retired science research leader in marine biodiversity, ecology, and evolution at The Natural History Museum, London, told All About History Magazine . "As long as science knew of no mechanism to explain how evolution happened it could be safely dismissed as a crank idea."
Meanwhile, French biologist Jean-Baptiste Lamarck proposed that an organism could pass on traits to its offspring, though he was wrong about some of the details, according to the University of California’s Museum of Paleontology .
Like Darwin, Lamarck believed that organisms adapted to their environments and passed on those adaptations. He thought organisms did this by changing their behavior and, therefore, their bodies — like an athlete working out and getting buff — and that those changes were passed on to offspring.

For example, Lamarck thought that giraffes originally had shorter necks but that, as trees around them grew taller, they stretched their necks to reach the tasty leaves and their offspring gradually evolved longer and longer necks. Lamarck also believed that life was somehow driven to evolve through the generations from simple to more complex forms, according to Understanding Evolution , an educational resource from the University of California Museum of Paleontology .
Though Darwin wasn't sure of the mechanism by which traits were passed on, he did not believe that evolution necessarily moved toward greater complexity, according to Understanding Evolution — rather, he believed that complexity arose through natural selection.
A Darwinian view of giraffe evolution, according to Quanta Magazine , would be that giraffes had natural variation in their neck lengths, and that those with longer necks were better able to survive and reproduce in environments full of tall trees, so that subsequent generations had more and more long-necked giraffes.
The main difference between the Lamarckian and Darwinian ideas of giraffe evolution is that there's nothing in the Darwinian explanation about giraffes stretching their necks and passing on an acquired characteristic.
What is modern evolutionary synthesis?
According to Pobiner, Darwin did not know anything about genetics. "He observed the pattern of evolution, but he didn't really know about the mechanism," she said. That came later, with the discovery of how genes encode different biological or behavioral traits, and how genes are passed down from parents to offspring. The incorporation of genetics into Darwin's theory is known as "modern evolutionary synthesis."
The physical and behavioral changes that make natural selection possible happen at the level of DNA and genes within the gametes, the sperm or egg cells through which parents pass on genetic material to their offspring. Such changes are called mutations . "Mutations are basically the raw material on which evolution acts," Pobiner said.
Mutations can be caused by random errors in DNA replication or repair, or by chemical or radiation damage, according to Nature Education . Usually, mutations are either harmful or neutral, but in rare instances, a mutation might prove beneficial to the organism. If so, it will become more prevalent in the next generation and spread throughout the population.
In this way, natural selection guides the evolutionary process, preserving and adding up the beneficial mutations and rejecting the bad ones. "Mutations are random, but selection for them is not random," Pobiner said.
But natural selection isn't the only mechanism by which organisms evolve, she said. For example, genes can be transferred from one population to another when organisms migrate or immigrate — a process known as gene flow. And the frequency of certain genes can also change at random, which is called genetic drift.
The reason Lamarck's theory of evolution is generally wrong is that acquired characteristics don't affect the DNA of sperm and eggs. A giraffe's gametes, for example, aren't affected by whether it stretches its neck; they simply reflect the genes the giraffe inherited from its parents. But as Quanta reported , some aspects of evolution are Lamarckian.
For example, a Swedish study published in 2002 in the European Journal of Human Genetics found that the grandchildren of men who starved as children during a famine passed on better cardiovascular health to their grandchildren. Researchers hypothesize that although experiences such as food deprivation don't change the DNA sequences in the gametes, they may result in external modifications to DNA that turn genes "on" or "off."
Such changes, called epigenetic changes, do not modify the actual DNA sequence itself. For instance, a chemical modification called methylation can affect which genes are turned on or off. Such epigenetic changes can be passed down to offspring. In this way, a person's experiences could affect the DNA he or she passes down, analogous to the way Lamarck thought a giraffe craning its neck would affect the neck length of its offspring.
What is the evidence for evolution?
The Theory of Evolution is one of the best-substantiated theories in the history of science. It is supported by evidence from a wide variety of scientific disciplines, including genetics, which shows that different species have similarities in their DNA .
There is also evidence supporting the Theory of Evolution in paleontology and geology. This is through the fossil record, which shows how that species that existed in the past are different from those present today, according to Bruce S. Lieberman and Roger L. Kaesler in " Prehistoric Life: Evolution and the Fossil Record " (Wiley, 2010).
There is also evidence for Darwin's theory found in developmental biology . It has been discovered that species that seem very different as adults pass through similar stages of embryological development, suggesting a shared evolutionary past, according to the open-access textbook " Concepts of Biology ."
Evidence for whale evolution from paleontology

The critical piece of evidence was discovered in 1994, when paleontologists found the fossilized remains of Ambulocetus natans , which means "swimming-walking whale," according to a 2009 review published in the journal Evolution: Education and Outreach . Its forelimbs had fingers and small hooves, but its hind feet were enormous relative to its size. The animal was clearly adapted for swimming, but it was also capable of moving clumsily on land, much like a seal.
When it swam, the ancient creature moved like an otter , pushing back with its hind feet and undulating its spine and tail.
Modern whales propel themselves through the water with powerful beats of their horizontal tail flukes, but A. natans still had a whip-like tail and had to use its legs to provide most of the propulsive force needed to move through water.
In recent years, more and more of these transitional species, or " missing links ," have been discovered, lending further support to Darwin's theory. For example, in 2007, a geologist discovered the fossil of an extinct aquatic mammal, called Indohyus , that was about the size of a cat and had hooves and a long tail.
Scientists think the animal belonged to a group related to cetaceans such as Ambulocetus natans . This creature is considered a "missing link" between artiodactyls — a group of hoofed mammals (even-toed ungulates) that includes hippos, pigs, and cows — and whales, according to the National Science Foundation .
Researchers knew that whales were related to artiodactyls, but until the discovery of this fossil, there were no known artiodactyls that shared physical characteristics with whales. After all, hippos, thought to be cetaceans' closest living relatives , are very different from whales. Indohyus , on the other hand, was an artiodactyl, indicated by the structure of its hooves and ankles, and it also had some similarities to whales, in the structure of its ears, for example.
Evidence for whale evolution from genetics & developmental biology

Genetic evidence also supports the idea that whales evolved from land mammals and provides information about the exact branching of the evolutionary tree. For instance, in 1999, researchers reported in the journal Proceedings of the National Academy of Sciences that according to genetic analysis of " jumping gene " sequences, which copy and paste themselves into genomes, hippos were whales' closest living relatives. Before 1985, researchers thought pigs were more closely related to whales, but this 1999 study overturned that idea, as the Associated Press reported.
In 2019, researchers reported in the journal Science Advances about which genes within the whale genome were inactivated during the process of the creature's evolution from land mammals, as Science Friday reported. The researchers could tell that certain genes, including one involved in making saliva, had been inactivated because there are remnants of them, which the researchers call genomic fossils, in whale genomes. This indicates that whales evolved from a salivating creature.
There's also evidence of cetacean evolution from developmental biology. Developmental biology illustrates the fact that animals that are very different as adults share similarities as embryos because they are evolutionarily related. For example, as embryos, cetaceans started to develop hind limbs, which disappear later in development, while the forelimbs remain and develop into flippers, according to the journal Evolution: Education and Outreach . This suggests that cetaceans evolved from a four-legged ancestor.
Is the theory of evolution controversial?
Despite the wealth of evidence from the fossil record, genetics and other fields of science, some people still question the theory of evolution 's validity. Some politicians and religious leaders denounce the theory, invoking a higher being as a designer to explain the complex world of living things, especially humans.
School boards debate whether the theory of evolution should be taught alongside other ideas, such as intelligent design or creationism.
Mainstream scientists see no controversy. "A lot of people have deep religious beliefs and also accept evolution," Pobiner said, adding, "there can be real reconciliation."
Evolution is well supported by many examples of changes in various species leading to the diversity of life seen today. "Natural selection, or to put it another way — variation, heredity, and differential fitness — is the core theory of modern biology," John Lambshead explains. "It is to biology what, say quantum mechanics and special relativity are to physics or the atomic model is to chemistry."
Additional reporting by contributors Alina Bradford, Ashley P. Taylor and Callum McKelvie
- The National Oceanic and Atmospheric Administration has a presentation on whale evolution.
- To read the theory in its original form, see Darwin's book, " On the Origin of Species ."
- Check out this article for an overview of natural selection.
- To understand the difference between a theory and fact, see this National Academy of Sciences website .
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Ashley P. Taylor is a writer based in Brooklyn, New York. As a science writer, she focuses on molecular biology and health, though she enjoys learning about experiments of all kinds. Ashley's work has appeared in Live Science, The New York Times blogs, The Scientist, Yale Medicine and PopularMechanics.com. Ashley studied biology at Oberlin College, worked in several labs and earned a master's degree in science journalism from New York University's Science, Health and Environmental Reporting Program.
Which animals are evolving fastest?
'I have never written of a stranger organ': The rise of the placenta and how it helped make us human
What is a quantum bit (qubit)?
Most Popular
- 2 80 million-year-old sea monster jaw filled with giant globular teeth for crushing prey discovered in Texas
- 3 Earth once wore a Saturn-like ring, study of ancient craters suggests
- 4 Watch mesmerizing video of weird waves that 'shape life itself' inside a fly embryo
- 5 A passing star may have kicked the solar system's weirdest moons into place
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Genet Mol Biol
- v.42(1); Jan-Mar 2019
Science and evolution
Claudia a.m. russo.
1 Universidade Federal do Rio de Janeiro, Universidade Federal do Rio de Janeiro, Departamento de Genética, Rio de Janeiro RJ , Brazil, Departamento de Genética, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Thiago André
2 Universidade Federal do Oeste do Pará, Universidade Federal do Oeste do Pará, Programa de Pós-Graduação em Biodiversidade, Santarém PA , Brazil, Programa de Pós-Graduação em Biodiversidade, Universidade Federal do Oeste do Pará, Santarém, PA, Brazil
Evolution is both a fact and a theory. Evolution is widely observable in laboratory and natural populations as they change over time. The fact that we need annual flu vaccines is one example of observable evolution. At the same time, evolutionary theory explains more than observations, as the succession on the fossil record. Hence, evolution is also the scientific theory that embodies biology, including all organisms and their characteristics. In this paper, we emphasize why evolution is the most important theory in biology. Evolution explains every biological detail, similar to how history explains many aspects of a current political situation. Only evolution explains the patterns observed in the fossil record. Examples include the succession in the fossil record; we cannot find the easily fossilized mammals before 300 million years ago; after the extinction of the dinosaurs, the fossil record indicates that mammals and birds radiated throughout the planet. Additionally, the fact that we are able to construct fairly consistent phylogenetic trees using distinct genetic markers in the genome is only explained by evolutionary theory. Finally, we show that the processes that drive evolution, both on short and long time scales, are observable facts.
In recent years, the teaching of creationism within science curricula has become a subject of public debate worldwide ( Miller et al. , 2006 ; Reiss 2011 ). Most of the attention has been given to cases in the United States of America ( Jackson et al. , 1995 ; Berkman and Plutzer, 2011 ; Baltzley, 2016 ; Ross, 2017 ), where many bills have been submitted to the Houses of Representatives encouraging teachers to express their criticism about evolution. In more serious cases, such as Turkey, evolution has recently been removed from the high school curriculum ( Kingsley, 2017 ), and in Brazil, intelligent design research has recently reached university level ( Silva, 2017 ). The rise of “anti-vaxxers” and “flat-earthers” openly demonstrates that the anti-science movement is not confined to biology, with devastating consequences such as the vaccine-preventable outbreaks ( Miller et al. , 2015 ). At the same time, the anti-science debates have been usually promoted by anti-scientists and have stayed marginal to scientific literature. This explains the rising trend and confirms the need for scientists to hastily step into the scene. With this in mind, we felt compelled to address basic aspects of science and of the scientific method in the evolution versus divine creation debate in a scientific journal.
Science can be defined as being both the criterion for gathering scientific data (scientific method), as well as the explanatory theories that were developed following its criteria (scientific knowledge) ( Project 2061 American Association for the Advancement of Science, 1993 ; Roberts 2007 ). A few centuries ago, scientists decided to select a small part of human knowledge to restrict the method used to assemble this knowledge. The use of the scientific method does not mean that this is more valuable than other types of knowledge; it is just more reliable in uncovering natural laws ( Atkins, 1995 ).
One should regard science as a process in which scientists formulate hypotheses to explain certain facts and to test their predictive models by confronting their predictions with new facts ( Gilbert, 1991 ). A fact is something that we observe. For instance, when we drop an object, it falls to the ground. This is a fact. The scientific theory that explains why objects fall is the theory of gravity . A valid scientific theory can never become a fact ( Gould, 1981 ), as there is always the possibility that a future explanation will better match newly discovered facts.
Evolution as a fact and theory
Evolution is a population concept. An individual does not evolve; only populations evolve in the face of the genetic changes accumulated from one generation to the next. The flu virus evolves. This explains why last years’ flu vaccine does not work on the current strain of the virus: only the resistant strains of the virus survived last year’s vaccine application. This is a textbook example of evolution by natural selection. Genetic modifications are encountered in the resistant strains; thus, evolution is a fact ( Gould, 1981 ). Mutation, migration, natural selection, and genetic drift are the evolutionary forces that drive genetic changes of natural populations from one generation to the next. This is known among biologists as microevolution.
On the other hand, evolutionary theory explains more than those facts that we can routinely observe. This makes it a theory, but is it just a theory? The word theory has distinct meanings in science and in lay language ( Ghose, 2013 ). A scientific theory is the utmost position an idea may reach in science. Outside of academia, however, a theory is equivalent to a hypothesis, an idea that explains facts but has never been tested ( Futuyama and Kirkpatrick, 2017 ). This occurs because there seems to be no need for a distinction between hypothesis and theory outside the scope of science. In science, however, this distinction is fundamental. An idea remains a hypothesis if it has never been confronted with new (independently collected) scientific data that would serve as a test for its predictions. If a hypothesis has endured further testing by subsequent scientific experiments, in time it becomes a valid scientific theory ( Figure 1 ).

For any given valid scientific theory there are three possibilities. The first possibility is that the true explanation for the facts is entirely different from the valid scientific theory. In this case, all scientific experiments aimed to test the theory were flawed in design or in the interpretation of the results. The second possibility is that the true explanation for the facts is more restricted than the current scientific theory claims. In this case, the predictions of the theory agreed with newly collected data because all tests focused on a single (and true ) aspect of the theory. Finally, the last alternative is that the true explanation for the facts is the scientific theory. Science has the tools to reject (first alternative) and to refine (second alternative) scientific theories when they are confronted with new data. However, even theories that endure many tests must still face these three possibilities, as, even in light of the true explanation, science does not furnish us the tools to perceive truthfulness.
A hallmark of natural sciences is that scientific hypotheses and scientific theories must make predictions about the natural world ( Paz-y-Miño and Spinosa, 2011 ). Often, the older the theory, the more reliable it is because it has survived many empirical tests. Furthermore, the more universal the theory, the more robust it becomes with time, as more tests would have been performed. According to Darwin, evolutionary theory is centred around two points ( Darwin, 1859 ). First, from one generation to the next, natural populations change over time by a process of natural selection. Second, all organisms have a common ancestor, and the time since this last common ancestor lived is inversely proportional to the similarities that the organisms will share today. Hence, evolutionary theory is universal because it includes all (living and fossil) biological diversity and has implications for all heritable characteristics of life. Since 1859, evolutionary theory has become the most universal and, hence, widely tested of the scientific theories in biology.
Today, Darwin’s original theory has been refined, as he himself anticipated that it would be ( Darwin, 1871 ). This occurred in many fronts because recent concepts, such as genetic drift and mutations, have provided more details on how natural populations evolve. One example is the understanding that, at the molecular level, random evolution, rather than natural selection, plays the most important role ( Kimura, 1991 ). This is known as the neutral theory, which completed its 50 th anniversary in 2018.
The substance of Darwin’s original theory, however, remains. Theodosius Dobzhansky (1973) shared his astonishment that Charles Darwin proposed the theory of evolution without many key biological concepts, such as that DNA is the molecule responsible for heredity. Half a century after Dobzhansky’s paper, it remains impressive that the theory of evolution still stands valid in light of the discoveries of the molecular biology revolution. Each newly sequenced genome tests some aspects of Darwin’s theory, and, on each case, the sequence has been consistent with Darwin’s prediction of the shared evolutionary history of life. The sharp increase in scope and universality of evolution has strengthened Darwin’s original proposal and made evolutionary theory one of the most reliable and tested theories in the natural sciences ( National Academy of Sciences, 2008 ).
Some creationists dispute this information, claiming that scientists discredit data that go against evolutionary theory. Nonetheless, there is no room for considering worldwide, long-lasting conspiracies in science, as scientific fame and recognition come from the demolition of old theories, not from adherence to them ( Atkins, 1995 ). Indeed, scientists themselves have challenged many aspects of the original Darwinian theory of evolution, such as the importance of neutral evolution, the discovery of epigenetics, the proposal of punctuated equilibrium, etc. When these challenges were first proposed, they were not ignored; they were published in top scientific journals and have been subject to meticulous research and have generated fruitful debates in the scientific arena.
Furthermore, if scientists were dishonestly accepting a false theory of evolution, Lamarck’s theory of inheritance of acquired characters would still be considered valid today. However, it is not. In the XIX century, August Weissman (1889) removed the tails of 20 generations of mice, but no significant decrease in length was found in the descendants’ tails. Scientists themselves devised the scientific experiment that bluntly rejected Lamarck’s proposal as a mechanism of evolution ( Dobzhansky, 1973 ). Scientists do not discredit data that goes against evolution; otherwise, Lamarck’s idea would still be accepted. They discredit scientific untestable theories and explanations that were not gathered using the scientific method.

The cornerstone of biology
Just as human history explains the geopolitical configurations of our world today, modern biological systems are a direct result of their evolutionary past. Hence, evolutionary theory is the cornerstone of the discipline of biology ( Rutledge and Warden, 2000 ). The discipline of biology today is an instantaneous portrayal of the dynamic evolutionary axis that arose with the origin of life and has been changing by evolution ever since ( Figure 2 ). With the first life, genetics, ecology, biochemistry and evolution began.

As a scientific theory, however, which facts does evolutionary theory explain? One pivotal example is the succession in the fossil record. This evolution, namely, macroevolution, explains the larger evolutionary picture that is the appearance of the greater groups, such as the evolution of mammals, insects, and plants. Fossilized mammals are easily recognized, as they have distinct types of teeth, such as molars, canines, and incisors. These vertebrates are also very likely to fossilize on account of their rigid teeth and hard cranium. If mammals are so easily fossilized, how can we explain a rich fossil record full of vertebrates and invertebrates with no mammalian fossil before 300 million years ago?
Similarly, if we dig deeper still, disclosing 500 million years old layers, we find no hard skeleton vertebrates but plenty of fossilized invertebrates in a boost of diversity that we call the Cambrian Explosion. There are no vertebrates in this explosion because vertebrates appear in a much later explosion. Digging even deeper, to 600 million years old records, we find strata with soft-bodied Ediacaran animals but no hard-shelled invertebrates and no vertebrates. In one billion years old strata, we find only single-celled organisms.
How can we find, in old strata, many single celled organisms but not a single mammalian tooth? The only reasonable explanation for these facts is that 400 million years ago, mammals had not yet evolved; 500 million years ago, vertebrates had not yet evolved; 600 million years ago, hard-shelled invertebrates had not yet evolved; and one billion years ago, multicellular life had not yet evolved. Smaller local successions are also observable in the fossil record; such as the beautiful strings of intermediate fossils that include amphibians ( Kustchera and Elliot, 2013 ), birds, whales ( Thewissen, 2009 ), horses, and humans. These successions in the fossil record are the most obvious evidence to macroevolution ( Figure 2 ). In fact, the entire fossil record is a set of millions of intermediate fossils that provide solid evidence of how macroevolution worked in the past billion years.
Evolutionary processes that drive micro and macroevolution are facts
To have a better understanding of evolution, we must discuss the processes that drive evolution. For this, we start by comparing processes that drive microevolution with those that drive macroevolution. Many of the same evolutionary processes that drive microevolution also drive macroevolution, namely natural selection, mutation, migration, and genetic drift. A lineage will tend to diversify if it has adaptations that increase survival and reproductive abilities compared to other species. This advantage will tend to increase population size and the geographical distribution of the ancestral species that will more likely speciate into two descendant species. Hence, according to this view, macroevolution is microevolution on a larger scale ( Zimmer, 2001 ), with biological speciation as the only additional process ( Russo et al. , 2016 ). Through speciation, one ancestral species gives rise to two descendant species that are reproductively incompatible with each other.
More than a million species have been described ( Mora et al. , 2011 ), and each biological species includes many interbreeding members. Also, most species are reproductively isolated from each other. The fact that we observe biological species with interbreeding members and reproductive isolation between species is compatible with both separate creation and macroevolution. So, which observable pattern would we expect if many speciation events generated the vast biological diversity from a single common ancestor? In this case, we would expect different degrees of similarity between reproductively isolated species. This is exactly what we observe. Some species are very similar, such as chimpanzees and gorillas, with most features shared between them. Other species, on the other hand, are morphologically so different that one must look into cytology, physiology, or comparative genomics to detect evidence of their common past. One example is a fern and a frog. For instance, the cellular respiration is a process shared by ferns and frogs and it is an evidence of their common ancestry. Only macroevolution explains well the distinct degrees of similarity between these four isolated species, as the age of their last common ancestor is inversely proportional to the similarity between any two species.
Furthermore, the existence of hybrids, such as the mule, the liger, the coywolf, is also only explained by the hierarchical common ancestry theory, not by separate creation. The hybrids are direct evidence of on-going processes of speciation. Thus, the presence of hybrids is what we would expect if all life had a common ancestry.
Other fossil record patterns are well explained by macroevolution. For instance, why do we find a major increase in mammalian fossil diversity only after the disappearance of non-avian dinosaurs approximately 65 million years ago? The same pattern is observed in the fossil record of birds. Macroevolution explains this well, as the extinction of dinosaurs eliminated competition, and the surviving ancestral mammals were able to increase in number and diversified through speciation, generating more species of their kind.
Final remarks
A single, very well designed experiment, performed in accordance with the utmost scientific standards, is what it takes to put any scientific theory to rest. Divine creation will never be part of science because science is not able to detect supernatural phenomena. Divine phenomena explain everything equally; hence, it provides no real explanatory (i.e., predictive) power. If we accept “God’s will” as an adequate explanation for a natural phenomenon, we eliminate the possibility of eventually being able to explain it naturally. Thus, the scientific revolution begun when we eliminated the divine as a scientific explanation.
Science, as a process, starts with the acceptance of our ignorance about a natural phenomenon and by seeking natural explanations for it. Hence, ignorance drives the engine of Science. Even if evolution were, hypothetically, rejected, contested by new data, scientists would have to study hard to find an alternative natural explanation that was able to explain everything that evolution explains today plus the new data that contested it.
Evolution is a fact and a well-supported scientific theory. It has endured daily and rigorous testing, and it stands as the unifying theory in biology ( Rutledge and Warden, 2000 ). This says nothing about whether God created or did not create the world, as science is unable to distinguish a divinely guided evolution from a materialistic evolution. God may well have created the biological world through natural selection, mutation, speciation, extinction, etc. Still, evolution and Science would remain unscathed as Science is not concerned with why or who , but only with how .
Some creationists say that we must bring the evolution versus creationist debate to the classroom and claim that the opposition to the debate is anti-scientific. However, science is not about blind criticism ( Meyer and El-Hani, 2013 ). Blind criticism is just as naïve as blind acceptance. Scientists must weigh the evidence before questioning a theory. The idea that all debates are equally scientific is misleading and it explains the sad emergence of flat-earthers and anti-vaxxers. A debate on what is the shape of our planet is not only pointless, but it is also dangerously harmful to the minds of the young students. A fruitful debate in a science class is restricted to those issues that lie within the scientific realm ( Baltzley, 2016 , Branch, 2016 ).
A recent study has suggested that science concepts, more than evolutionary basics, are critical to promoting evolution ( Dunk et al. , 2017 ). One way to reinforce these fundamentals would be the requirement of evolution and science fundaments in admission policies for biology professionals, particularly teachers ( Larkin and Perry-Ryder, 2015 ; see Rutledge and Warden, 2000 for statistics).
Associate Editor: Carlos F. M. Menck
- Atkins P. Science as truth. Hist Hum Sci. 1995; 8 :97–102. [ Google Scholar ] Atkins P (1995) Science as truth. Hist Hum Sci 8:97-102.
- Baltzley M. Institutionalizing creationism. Science. 2016; 35 :1285–1286. [ PubMed ] [ Google Scholar ] Baltzley M (2016) Institutionalizing creationism. Science 35:1285-1286. [ PubMed ]
- Berkman MB, Plutzer E. Defeating creationism in the courtroom, but not in the classroom. Science. 2011; 331 :404–405. [ PubMed ] [ Google Scholar ] Berkman MB and Plutzer E (2011) Defeating creationism in the courtroom, but not in the classroom. Science 331:404-405. [ PubMed ]
- Branch G. Keeping creationism out of classrooms. Science. 2016; 354 :715. [ PubMed ] [ Google Scholar ] Branch G (2016) Keeping creationism out of classrooms. Science 354:715. [ PubMed ]
- Darwin CR. The Origin of Species by means of natural selection or the preservation of favoured races in the struggle for life. John Murray; London: 1859. p. 502. [ PMC free article ] [ PubMed ] [ Google Scholar ] Darwin CR (1859) The Origin of Species by means of natural selection or the preservation of favoured races in the struggle for life. John Murray, London, 502 p. [ PMC free article ] [ PubMed ]
- Darwin CR. The descent of man and selection in relation to sex. John Murray; London: 1871. p. 450. [ Google Scholar ] Darwin CR (1871) The descent of man and selection in relation to sex. John Murray, London, 450 p.
- Dobzhansky T. Nothing in biology makes sense except in the light of evolution. Am Biol Teach. 1973; 75 :87–91. [ Google Scholar ] Dobzhansky T (1973) Nothing in biology makes sense except in the light of evolution. Am Biol Teach 75:87-91.
- Dunk RDP, Petto AJ, Wiles JR, Campbell BC. A multifactorial analysis of acceptance of evolution. Evol Edu Outreach. 2017; 10 :4. [ Google Scholar ] Dunk RDP, Petto AJ, Wiles JR and Campbell BC (2017) A multifactorial analysis of acceptance of evolution. Evol Edu Outreach 10:4.
- Futuyma DJ, Kirkpatrick M. Evolution. 4th edition. Sinauer; Sunderland: 2017. p. 594. [ Google Scholar ] Futuyma DJ and Kirkpatrick M (2017) Evolution. 4 th edition. Sinauer, Sunderland, 594 p.
- Ghose T. Just a theory: 7 Misused Science Words. 2013. [accessed 19 January 2019]. https://www.scientificamerican.com/article/just-a-theory-7-misused-science-words/ Ghose T (2013) Just a theory: 7 Misused Science Words, https://www.scientificamerican.com/article/just-a-theory-7-misused-science-words/ (accessed 19 January 2019)
- Gilbert SW. Model building and a definition of science. J Res Sci Teach. 1991; 28 :73–79. [ Google Scholar ] Gilbert SW (1991) Model building and a definition of science. J Res Sci Teach 28:73-79.
- Gould SJ. Evolution as fact and theory. Discover. 1981; 2 :34–37. [ Google Scholar ] Gould SJ (1981) Evolution as fact and theory. Discover 2:34-37.
- Jackson DF, Doster EC, Meadows L, Wood T. Hearts and minds in the science classroom: The education of a confirmed evolutionist. J Res Sci Teach. 1995; 32 :585–611. [ Google Scholar ] Jackson DF, Doster EC, Meadows L and Wood T (1995) Hearts and minds in the science classroom: The education of a confirmed evolutionist. J Res Sci Teach 32:585–611.
- Kimura M. The neutral theory of molecular evolution: A review of recent evidence. Jpn J Genet. 1991; 66 :367–386. [ PubMed ] [ Google Scholar ] Kimura M (1991) The neutral theory of molecular evolution: A review of recent evidence. Jpn J Genet 66:367-386. [ PubMed ]
- Kingsley P. Turkey drops evolution from curriculum, angering secularists. 2017. [accessed 19 January 2019]. https://www.nytimes.com/2017/06/23/world/europe/turkey-evolution-high-school-curriculum.html . Kingsley P (2017) Turkey drops evolution from curriculum, angering secularists, https://www.nytimes.com/2017/06/23/world/europe/turkey-evolution-high-school-curriculum.html (accessed 19 January 2019)
- Kutschera U, Elliot JM. Do mudskippers and lungfishes elucidate the early evolution of four-limbed vertebrates? Evol Edu Outreach. 2013; 6 :8. [ Google Scholar ] Kutschera U and Elliot JM (2013) Do mudskippers and lungfishes elucidate the early evolution of four-limbed vertebrates? Evol Edu Outreach 6:8.
- Larkin DB, Perry-Ryder GM. Without the light of evolution: A case study of resistance and avoidance in learning to teach high school biology. Sci Edu. 2015; 99 :549–576. [ Google Scholar ] Larkin DB and Perry-Ryder GM (2015) Without the light of evolution: A case study of resistance and avoidance in learning to teach high school biology. Sci Edu 99:549–576.
- Meyer D, El-Hani C. O que está em jogo no confronto entre criacionismo e evolução. Filos Hist Biol. 2013; 8 :211–222. [ Google Scholar ] Meyer D and El-Hani C (2013) O que está em jogo no confronto entre criacionismo e evolução. Filos Hist Biol 8:211-222.
- Miller JD, Scott EC, Okamoto S. Public acceptance of evolution. Sci. 2006; 313 :765–766. [ PubMed ] [ Google Scholar ] Miller JD, Scott EC and Okamoto S (2006) Public acceptance of evolution. Sci 313:765-766. [ PubMed ]
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: What does the evidence show? Vaccine. 2015; 33 :3288–3292. [ PMC free article ] [ PubMed ] [ Google Scholar ] Miller ER, Moro PL, Cano M and ShimabukuroTT (2015) Deaths following vaccination: What does the evidence show? Vaccine 33:3288-3292. [ PMC free article ] [ PubMed ]
- Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B. How many species are there on Earth and in the Ocean? PLoS Biology. 2011; 9 :e1001127. [ PMC free article ] [ PubMed ] [ Google Scholar ] Mora C, Tittensor DP, Adl S, Simpson, AGB and Worm B (2011) How many species are there on Earth and in the Ocean? PLoS Biology 9:e1001127. [ PMC free article ] [ PubMed ]
- National Academy of Sciences . Science, evolution and creationism. National academies; Washington: 2008. p. 88. [ Google Scholar ] National Academy of Sciences (2008) Science, evolution and creationism. National academies, Washington, 88 p. [ PMC free article ] [ PubMed ]
- Paz-y-Miño G, Spinosa A. On the theory of evolution versus the concept of evolution: Three observations. Evol Edu Outreach, 2011; 4 :308–312. [ PMC free article ] [ PubMed ] [ Google Scholar ] Paz-y-Miño G and Spinosa A (2011) On the theory of evolution versus the concept of evolution: Three observations. Evol Edu Outreach, 4:308-312. [ PMC free article ] [ PubMed ]
- Project 2061 American Association for the Advancement of Science . Benchmarks for science literacy. Oxford University Press; Oxford: 1993. p. 448. [ Google Scholar ] Project 2061 American Association for the Advancement of Science (1993) Benchmarks for science literacy. Oxford University Press, Oxford, 448 p.
- Reiss MJ. How should creationism and intelligent design be dealt with in the classroom? J Philos Edu. 2011; 45 :399–415. [ Google Scholar ] Reiss MJ (2011) How should creationism and intelligent design be dealt with in the classroom? J Philos Edu 45, 399–415.
- Roberts DA. Scientific Literacy/Science Literacy. In: Abell SK, Lederman NG, editors. Handbook of Research on Science Education. Routledge; New York: 2007. pp. 729–780. [ Google Scholar ] Roberts DA (2007) Scientific Literacy/Science Literacy. In: Abell SK and Lederman NG (eds) Handbook of Research on Science Education. Routledge, New York, pp 729-780.
- Ross E. Revamped ‘anti-science’ education bills in United States find success. 2017. [accessed 19 January 2019]. https://www.nature.com/news/revamped-anti-science-education-bills-in-united-states-find-success-1.21986 . Ross E (2017) Revamped ‘anti-science’ education bills in United States find success, https://www.nature.com/news/revamped-anti-science-education-bills-in-united-states-find-success-1.21986 (accessed 19 January 2019)
- Russo CAM, Aguiar B, Voloch CM, Selvatti AP. When Chinese masks meet phyologenetics. Am Biol Teacher. 2016; 78 :241–247. [ Google Scholar ] Russo CAM, Aguiar B, Voloch CM and Selvatti AP (2016) When Chinese masks meet phyologenetics. Am Biol Teacher 78:241-247.
- Rutledge ML, Warden MA. Evolutionary theory, the nature of science & High School Biology teachers: Critical relationships. Am Biol Teacher. 2000; 62 :23–31. [ Google Scholar ] Rutledge ML and Warden MA (2000) Evolutionary theory, the nature of science & High School Biology teachers: Critical relationships. Am Biol Teacher 62:23-31.
- Silva HM. Intelligent design endangers education. Science. 2017; 357 :880. [ PubMed ] [ Google Scholar ] Silva HM (2017) Intelligent design endangers education. Science 357:880. [ PubMed ]
- Thewissen JGM, Cooper LN, George JC, Bajpai S. From land to water: The origin of whales, dolphins and porpoises. Evol Edu Outreach. 2009; 2 :272–288. [ Google Scholar ] Thewissen JGM, Cooper LN, George JC and Bajpai S (2009) From land to water: The origin of whales, dolphins and porpoises. Evol Edu Outreach 2:272-288.
- Weismann A. Essays upon Heredity and Kindred Biological Problems. Clarendon; Oxford: 1889. [ Google Scholar ] Weismann A (1889) Essays upon Heredity and Kindred Biological Problems. Clarendon, Oxford.
- Zimmer C. Evolution, the triumph of an idea. Harper Collins; New York: 2001. p. 528. [ Google Scholar ] Zimmer C (2001) Evolution, the triumph of an idea. Harper Collins, New York, 528 p.

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction & Top Questions
Early life and education
- The Beagle voyage
- Evolution by natural selection: the London years, 1836–42
- The squire naturalist in Downe
- On the Origin of Species
- The patriarch in his home laboratory
- The private man and the public debate

What is Charles Darwin famous for?
What is evolution, as charles darwin understood it, what was charles darwin’s educational background, what was charles darwin’s family life like, what were the social impacts of charles darwin’s work.

Charles Darwin
Our editors will review what you’ve submitted and determine whether to revise the article.
- Stanford Encyclopedia of Philosophy - Darwinism
- National Center for Biotechnology Information - PubMed Central - Charles Darwin and the Origin of Life
- The Victorian Web - Charles Darwin
- Biology LibreTexts - Charles Darwin
- Internet Encyclopedia of Philosophy - Charles Darwin
- Academia - Charles Darwin
- Palomar College - Natural Selection
- Australian Dictionary of Biography - Biography of Charles Robert Darwin
- Charles Darwin - Children's Encyclopedia (Ages 8-11)
- Charles Darwin - Student Encyclopedia (Ages 11 and up)
- Table Of Contents
Charles Darwin’s theory of evolution by natural selection is the foundation upon which modern evolutionary theory is built. The theory was outlined in Darwin’s seminal work On the Origin of Species , published in 1859. Although Victorian England (and the rest of the world) was slow to embrace natural selection as the mechanism that drives evolution, the concept of evolution itself gained widespread traction by the end of Darwin’s life.
Charles Darwin’s theory of evolution had three main components: that variation occurred randomly among members of a species; that an individual’s traits could be inherited by its progeny; and that the struggle for existence would allow only those with favorable traits to survive. Although many of his ideas have been borne out by modern science, Darwin didn’t get everything right: traces of Jean-Baptiste Lamarck ’s outdated theory of evolution remained in Darwin’s own. He was also unable to correctly establish how traits were inherited, which wasn’t clarified until the rediscovery of Gregor Mendel ’s work with peas.
Growing up, Charles Darwin was always attracted to the sciences. In 1825 his father sent him to the University of Edinburgh to study medicine. There he was exposed to many of the dissenting ideas of the time, including those of Robert Edmond Grant, a former student of the French evolutionist Jean-Baptiste Lamarck . He transferred to Christ’s College, Cambridge, in 1828, where his mentors mostly endorsed the idea of providential design. A botany professor suggested he join a voyage on the HMS Beagle —a trip that would provide him with much of his evidence for the theory of evolution by natural selection .
Charles Darwin was born in England to a well-to-do family in 1809. His father was a doctor, and his mother—who died when he was only eight years old—was the daughter of a successful 18th-century industrialist. Darwin was not the first of his family to gravitate toward naturalism: his father’s father, Erasmus Darwin , was a physician, inventor, and poet who had developed his own theories on the evolution of species. Darwin later married his first cousin on his mother’s side, Emma Wedgwood. Together they had 10 children, 3 of whom died at a young age.
Charles Darwin’s theories hugely impacted scientific thought. But his ideas also affected the realms of politics, economics, and literature. More insidious were the ways that Darwin’s ideas were used to support theories such as social Darwinism and eugenics , which used biological determinism to advocate for the elimination of people deemed socially unfit. Although Darwin himself was an abolitionist, the social Darwinist ideas inspired by his work contributed to some of the most racist and classist social programs of the last 150 years.

Charles Darwin (born February 12, 1809, Shrewsbury, Shropshire , England—died April 19, 1882, Downe, Kent) was an English naturalist whose scientific theory of evolution by natural selection became the foundation of modern evolutionary studies. An affable country gentleman, Darwin at first shocked religious Victorian society by suggesting that animals and humans shared a common ancestry. However, his nonreligious biology appealed to the rising class of professional scientists, and by the time of his death evolutionary imagery had spread through all of science , literature, and politics. Darwin, himself an agnostic , was accorded the ultimate British accolade of burial in Westminster Abbey , London.

Darwin formulated his bold theory in private in 1837–39, after returning from a voyage around the world aboard HMS Beagle , but it was not until two decades later that he finally gave it full public expression in On the Origin of Species (1859), a book that has deeply influenced modern Western society and thought.
Darwin was the second son of society doctor Robert Waring Darwin and of Susannah Wedgwood, daughter of the Unitarian pottery industrialist Josiah Wedgwood . Darwin’s other grandfather, Erasmus Darwin , a freethinking physician and poet fashionable before the French Revolution , was author of Zoonomia; or the Laws of Organic Life (1794–96). Darwin’s mother died when he was eight, and he was cared for by his three elder sisters. The boy stood in awe of his overbearing father, whose astute medical observations taught him much about human psychology. But he hated the rote learning of Classics at the traditional Anglican Shrewsbury School, where he studied between 1818 and 1825. Science was then considered dehumanizing in English public schools, and for dabbling in chemistry Darwin was condemned by his headmaster (and nicknamed “Gas” by his schoolmates).
His father, considering the 16-year-old a wastrel interested only in game shooting, sent him to study medicine at Edinburgh University in 1825. Later in life, Darwin gave the impression that he had learned little during his two years at Edinburgh . In fact, it was a formative experience. There was no better science education in a British university. He was taught to understand the chemistry of cooling rocks on the primitive Earth and how to classify plants by the modern “natural system.” At the Edinburgh Museum he was taught to stuff birds by John Edmonstone, a freed South American slave , and to identify the rock strata and colonial flora and fauna.

More crucially, the university’s radical students exposed the teenager to the latest Continental sciences. Edinburgh attracted English Dissenters who were barred from graduating at the Anglican universities of Oxford and Cambridge , and at student societies Darwin heard freethinkers deny the Divine design of human facial anatomy and argue that animals shared all the human mental faculties. One talk, on the mind as the product of a material brain , was officially censored, for such materialism was considered subversive in the conservative decades after the French Revolution. Darwin was witnessing the social penalties of holding deviant views. As he collected sea slugs and sea pens on nearby shores, he was accompanied by Robert Edmond Grant, a radical evolutionist and disciple of the French biologist Jean-Baptiste Lamarck . An expert on sponges , Grant became Darwin’s mentor, teaching him about the growth and relationships of primitive marine invertebrates , which Grant believed held the key to unlocking the mysteries surrounding the origin of more-complex creatures. Darwin, encouraged to tackle the larger questions of life through a study of invertebrate zoology , made his own observations on the larval sea mat ( Flustra ) and announced his findings at the student societies.
The young Darwin learned much in Edinburgh’s rich intellectual environment , but not medicine: he loathed anatomy , and (pre- chloroform ) surgery sickened him. His freethinking father, shrewdly realizing that the church was a better calling for an aimless naturalist, switched him to Christ’s College, Cambridge, in 1828. In a complete change of environment, Darwin was now educated as an Anglican gentleman. He took his horse , indulged his drinking, shooting, and beetle-collecting passions with other squires’ sons, and managed 10th place in the Bachelor of Arts degree in 1831. Here he was shown the conservative side of botany by a young professor, the Reverend John Stevens Henslow , while that doyen of Providential design in the animal world, the Reverend Adam Sedgwick , took Darwin to Wales in 1831 on a geologic field trip.

Fired by Alexander von Humboldt ’s account of the South American jungles in his Personal Narrative of Travels , Darwin jumped at Henslow’s suggestion of a voyage to Tierra del Fuego , at the southern tip of South America , aboard a rebuilt brig , HMS Beagle . Darwin would not sail as a lowly surgeon-naturalist but as a self-financed gentleman companion to the 26-year-old captain, Robert Fitzroy , an aristocrat who feared the loneliness of command. Fitzroy’s was to be an imperial-evangelical voyage: he planned to survey coastal Patagonia to facilitate British trade and return three “savages” previously brought to England from Tierra del Fuego and Christianized. Darwin equipped himself with weapons, books (Fitzroy gave him the first volume of Principles of Geology , by Charles Lyell ), and advice on preserving carcasses from London Zoo ’s experts. The Beagle sailed from England on December 27, 1831.
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
5 facts about evolution and religion

Are faith and belief in evolution necessarily at odds? According to Pope Francis , the answer is no. Indeed, the pope recently reaffirmed the Roman Catholic Church’s view that “evolution in nature is not inconsistent” with church teaching on creation, pushing the debate on human origins back into the news .
Although most U.S. Catholics accept the idea of evolution in some form, a substantial percentage of American adults reject the scientific explanation for the origins of human life, and a number of religious groups in the U.S. maintain that Charles Darwin’s theory of evolution through natural selection is not correct because it conflicts with their views of creation.
Here are five facts about evolution and faith:
The Roman Catholic Church has long accepted – or at least not objected to – evolutionary theory. Pope Francis is not the first pontiff to publicly affirm that evolution is compatible with church teachings. In 1950, in the encyclical “ Humani Generis ,” Pope Pius XII said that Catholic teachings on creation could coexist with evolutionary theory. Pope John Paul II went a bit further in 1996 , calling evolution “more than a hypothesis.”
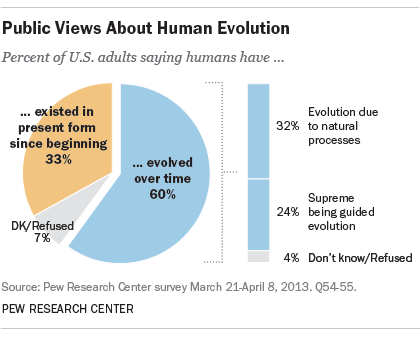
A minority of Americans fully accept the scientific explanation for the origins of human life. According to a 2013 Pew Research Center survey , 60% of Americans say humans have evolved over time, but only about half of that group (32% of U.S. adults overall) believes that humans and other living things evolved solely due to natural processes, the explanation accepted by the vast majority of scientists . About a quarter of U.S. adults (24%) say that humans and other life evolved, but that this evolution was guided by a supreme being. The same survey found that a third of Americans (33%) reject evolution entirely, saying humans and other living things have existed in their present form since the beginning of time.
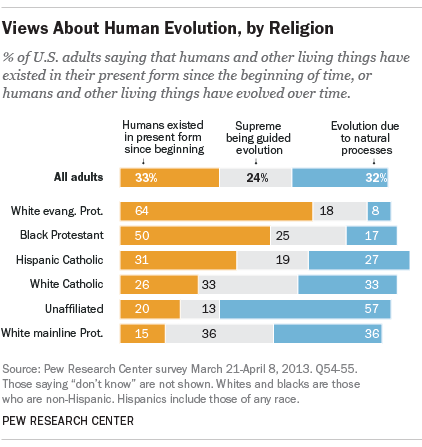
Of all the major religious groups in the U.S., white evangelical Protestants are the most likely to reject evolution. Nearly two-thirds (64%) of white evangelicals say that humans and other living things have always existed in their present form, while roughly one-in-ten white evangelicals (8%) say that humans evolved through natural processes. On the other end of the spectrum are the unaffiliated, a majority of whom (57%) said they believe that life evolved through natural processes.
The rejection of evolution by most evangelicals is largely mirrored by their churches, such as the Southern Baptist Convention and the Lutheran Church-Missouri Synod , which explicitly reject evolutionary theory as being in conflict with what they see as biblical truth.
About a quarter of white American Catholics (26%) say that they do not believe in evolution of any kind, despite the church’s acceptance of it . The share of Hispanic Catholics in the U.S. who reject evolution and say that humans have always existed in their present form is even higher (31%).
A series of court decisions prohibit the teaching of creationism or intelligent design in public schools. In spite of efforts in many American states and localities to ban the teaching of evolution in public schools or to teach alternatives to evolution, courts in recent decades have consistently rejected public school curricula that veer away from evolutionary theory. In Edwards v. Aguillard (1987), for instance, the U.S. Supreme Court ruled that a Louisiana law requiring public school students to learn both evolution and creation science violated the U.S. Constitution’s prohibition on the establishment of religion.
- Catholicism
- Religion & Politics
- Religion & Science

David Masci is a former senior writer/editor focusing on religion at Pew Research Center .
Majority of U.S. Catholics Express Favorable View of Pope Francis
9 facts about u.s. catholics, the pope is concerned about climate change. how do u.s. catholics feel about it, under pope francis, the college of cardinals has become less european, online religious services appeal to many americans, but going in person remains more popular, most popular.
901 E St. NW, Suite 300 Washington, DC 20004 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan, nonadvocacy fact tank that informs the public about the issues, attitudes and trends shaping the world. It does not take policy positions. The Center conducts public opinion polling, demographic research, computational social science research and other data-driven research. Pew Research Center is a subsidiary of The Pew Charitable Trusts , its primary funder.
© 2024 Pew Research Center
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 19 September 2024
9,000 years of genetic continuity in southernmost Africa demonstrated at Oakhurst rockshelter
- Joscha Gretzinger 1 ,
- Victoria E. Gibbon ORCID: orcid.org/0000-0001-7875-3297 2 ,
- Sandra E. Penske 1 ,
- Judith C. Sealy ORCID: orcid.org/0000-0001-5071-8211 3 ,
- Adam B. Rohrlach ORCID: orcid.org/0000-0002-4204-5018 1 , 4 ,
- Domingo C. Salazar-García 5 , 6 ,
- Johannes Krause ORCID: orcid.org/0000-0001-9144-3920 1 na1 &
- Stephan Schiffels ORCID: orcid.org/0000-0002-1017-9150 1 na1
Nature Ecology & Evolution ( 2024 ) Cite this article
74 Altmetric
Metrics details
- Archaeology
- Population dynamics
- Population genetics
Southern Africa has one of the longest records of fossil hominins and harbours the largest human genetic diversity in the world. Yet, despite its relevance for human origins and spread around the globe, the formation and processes of its gene pool in the past are still largely unknown. Here, we present a time transect of genome-wide sequences from nine individuals recovered from a single site in South Africa, Oakhurst Rockshelter. Spanning the whole Holocene, the ancient DNA of these individuals allows us to reconstruct the demographic trajectories of the indigenous San population and their ancestors during the last 10,000 years. We show that, in contrast to most regions around the world, the population history of southernmost Africa was not characterized by several waves of migration, replacement and admixture but by long-lasting genetic continuity from the early Holocene to the end of the Later Stone Age. Although the advent of pastoralism and farming substantially transformed the gene pool in most parts of southern Africa after 1,300 bp , we demonstrate using allele-frequency and identity-by-descent segment-based methods that the ‡Khomani San and Karretjiemense from South Africa still show direct signs of relatedness to the Oakhurst hunter-gatherers, a pattern obscured by recent, extensive non-Southern African admixture. Yet, some southern San in South Africa still preserve this ancient, Pleistocene-derived genetic signature, extending the period of genetic continuity until today.
Similar content being viewed by others

Population genomics of post-glacial western Eurasia

The genomic landscape of Mexican Indigenous populations brings insights into the peopling of the Americas

Genetic history from the Middle Neolithic to present on the Mediterranean island of Sardinia
Southern African populations today harbour genetic variation that traces deep human population history 1 , 2 , reflected also in the archaeological record with fossils of archaic Homo sapiens dating back to 260 thousand years ago (ka) before present ( bp ) (uncalibrated) and evidence for the presence of anatomically modern humans in South Africa from at least ~120 ka bp onwards 3 , 4 . While genetic investigations have extensively explored the significance of southern African population structure in human evolution, there is a noticeable gap in our understanding of the more recent demographic trajectories during the Holocene (the last 11,700 years), which remain relatively understudied genetically.
During the Holocene, major transformations in lithic industries and subsistence practices probably also reflect demographic shifts 5 , 6 . In the last 2,000 years, the spread of pastoralism and farming have resulted in repeated admixture events visible in genetic complexity in both ancient and contemporary populations 1 , 2 , 7 , 8 . First, the spread of herders contributed northeast African, Levantine-enriched ancestry to the genetic make-up of southern African hunter-gatherers 2 . Second, the influx of farmers closely related to present-day Bantu-language speakers introduced western African ancestry to San and Khoe populations 2 . Consequently, all contemporary San and Khoe groups exhibit at least 9% genetic admixture from non-San sources outside modern-day South Africa, Namibia and Botswana 1 , 2 , 7 , 8 , obscuring the population structures of the Later Stone Age (LSA) San population. To provide insights into early Holocene San population structure, we sampled and recovered genome-wide data from a series of individuals unearthed from the Oakhurst rockshelter in South Africa, offering a chronological spectrum spanning most of the Holocene.
Oakhurst rockshelter is located close to George in southernmost Africa, ~7 km from the coast (Fig. 1a ). Excavated in the first half of the twentieth century 9 , it is especially noteworthy for its substantial accumulation of deposit that spans 12,000 years. Its Early Holocene macrolithic stone artefact assemblage is characteristic of the period, similar to those found at many sites across South Africa and is today regarded as a distinctive technocomplex termed the ‘Oakhurst Complex’, named after the site 5 , 6 , 10 , 11 , 12 , 13 . At ~8,000 bp , the lithics change to microlithic ‘Wilton’ assemblages which persist through the Middle and Late Holocene, albeit with some temporal shifts, notably the addition of ceramics in the last 2,000 years (refs. 6 , 9 , 14 , 15 ). The site also preserves the complete and partial burials of 46 juvenile and adult individuals, spanning the complete period of occupation of the site 16 , 17 and providing a valuable resource for the study of LSA San population structure. Here, we present genome-wide data from 13 individuals, including the oldest DNA from South Africa, dating back to ~10,000 years (calibrated) cal bp .

a , Approximate locations of present-day populations and ancient individuals mentioned in the article. Present-day populations are coloured according to linguistic affiliation, as indicated in the legend. b , PCA on 212,000 SNPs with ancient individuals projected onto PC1 and PC2. Shown are the positions of each individual along the first and second axes of genetic variation, with symbols denoting the individual's population and linguistic affiliation using the same colour coding as in a .
We sampled skeletal remains from 13 individuals, each radiocarbon dated on bone collagen with dates ranging between approximately 10,000 and 1,300 cal bp (Supplementary Table 1 ). Nine of these 14 C dates were previously reported; for four samples we generated new 14 C dates. We prepared powder from skeletal material, extracted ancient DNA (aDNA) and converted it into double- or single-stranded libraries ( Methods; Supplementary Table 1 ). We selected 11 double-stranded and 15 single-stranded libraries for hybridization DNA capture to enrich for sequences that overlapped 1.24 million single-nucleotide polymorphisms (SNPs). For all 13 individuals, we determined the genetic sex and classified mitochondrial DNA and Y chromosome haplogroups for nine and five individuals, respectively, all of which are common in ancient and contemporary San and Khoe populations 1 , 2 , 18 , 19 , 20 , 21 , 22 , 23 , 24 (Supplementary Table 1 ). After quality filtering ( Methods ) and merging of duplicate libraries, we recovered genome-wide data sufficient for population genetic analysis for nine individuals, featuring on average 368,359 SNPs of the 1,240k panel, a mean mtDNA contamination of 1.7% and a mean X chromosome contamination of 1.5% (Supplementary Table 1 ).
Genetic affinities between Oakhurst and contemporary San and Khoe
To place the nine individuals from Oakhurst into a pan-African evolutionary context, we first constructed a population tree based on allele frequencies in ancient and present-day populations, using TreeMix 25 ( Methods ). Similar to contemporary San individuals 1 , 2 , 7 , the nine Oakhurst individuals diverge basal to all other human lineages (Extended Data Fig. 1a ). To investigate their genetic ancestry in detail, we performed principal component analysis (PCA) on a set of 24 contemporary San, Khoekhoe and Bantu-speaking populations from Namibia, Botswana and South Africa and projected new and published ancient genomes onto the first two principal components (Fig. 1 and Supplementary Tables 26 and 27 ). In line with previous analysis of San and Khoekhoe fine-scale population structure, we observed marked genetic differentiation between San and Khoe populations along PC2, reflecting the geographic separation between groups living north and south of the Kalahari Desert 7 , 8 , 26 , 27 , 28 , 29 . In total, we observe three principal clusters with the Kx`a-speaking Ju|’Hoan (genetic group label in figures and tables: Ju_hoan) and !Xuun (Xuun) representing the northern San ancestry component, the Khoe-Kwadi-speaking Nama as well as Tuu-speaking ‡Khomani (Khomani) and Karretjiemense (self-identification of these San descendants from the Karoo region of South Africa, the Afrikaans term translates to ‘the people of the cart’) (Karretjie) forming the southern San ancestry component and the Tuu-speaking Taa, Kx`a-speaking ǂHoan (Hoan) and Khoe-Kwadi-speaking Gǀui (Gui) and Gǁana (Gana) corresponding to the central San ancestry component. In this context, eight of the Oakhurst individuals cluster closely together with four previously published LSA hunter-gatherers from South Africa 1 , 2 within the diversity of the southern San and Khoekhoe cluster (Fig. 1b ). We also considered a slightly different PCA with a larger SNP overlap among fewer analysed populations (Extended Data Fig. 1b ), which proves useful for specific signals. Here, we see that the oldest individual in our dataset, OAK006, which dates between 9,900 and 10,500 cal BP (95% confidence interval (CI)), is slightly shifted in the direction of the northern San cluster (Extended Data Fig. 1b ). Unsupervised ancestry decomposition using DYSTRUCT 30 (Extended Data Fig. 2 ) shows overall a similar pattern as PCA (with K = 6 clusters): present-day southern San and Khoekhoe are assigned the same major ancestral component (shown in orange), which is maximized in the LSA individuals from Oakhurst, St. Helena, Faraoskop and Ballito Bay. In contrast, northern San exhibit a different component (shown in blue) that is maximized in northern Ju|’Hoan. Finally, a third component (shown in magenta) is maximized in Taa groups and also represents the largest San ancestry component in most remaining Khoe-Kwadi populations such as the ǂHoan, Gǀui, Gǁana and Tshwa (Extended Data Fig. 2 ).
To quantitatively test whether the observations from the PCA and ancestry clustering are consistent with patterns of shared genetic drift, we compute outgroup F 3 -statistics of the form F 3 (Archaic; Oakhurst, Test) between the Oakhurst individuals and present-day San and Khoe populations (Extended Data Fig. 3a and Supplementary Table 2 ). We also calculate the fixation index ( F ST ) by pairs and compare the two measures (Extended Data Fig. 3a and Supplementary Table 3 ). Although outgroup F 3 and F ST point estimates are significantly associated (Pearson’s correlation; t = −13.472, d.f. = 27, P = 1.683 × 10 −13 , r = −0.933) (Extended Data Fig. 3a ), the outgroup F 3 signal is mostly correlated with the proportion of indigenous San ancestry (measured using qpAdm; see Methods and analysis around Figs. 3 and 4 ) in the present-day populations (Pearson’s correlation; t = 16.67, d.f. = 9, P = 8.478 × 10 −13 , r = 0.968). F ST appears less affected by varying percentages of non-San ancestry (evidenced by a reduced correlation between F ST and San ancestry; Pearson’s correlation; t = −6.252, d.f. = 19, P = 5.278 × 10 −6 , r = −0.82) and is consequently able to detect subtle population structure that was obscured by later admixture events. We find the highest genetic affinity between Oakhurst and groups of the southern San cluster, namely the Karretjiemense, ‡Khomani and Nama (Extended Data Fig. 3b ). In general, F ST between the Oakhurst individuals and present-day San and Khoe-Kwadi-speaking groups is strongly correlated with latitude (Pearson’s correlation; t = 2.828, d.f. = 23, P = 0.009528, r = 0.508), demonstrating that San and Khoekhoe groups who live closer to Oakhurst rockshelter are still today more closely related to its LSA inhabitants than groups from further north. This is furthermore supported by the sharing of identity-by-descent (IBD) segments between the most recent individual, OAK007 (dating to ~1,344 cal bp ; 1,400-1,300 cal bp 95% CI) and present-day southern Africans. On average, OAK007 shares more and longer IBD segments with the Karretjiemense and ‡Khomani than with any other tested population, demonstrating direct genetic relatedness between the ancient hunter-gatherer and modern San and Khoe groups from South Africa (Extended Data Fig. 4 and Supplementary Table 24 ).
Genomic continuity since the early Holocene
We then proceeded to investigate individual changes in ancestry through time. First, we assessed the extent of genetic similarity between the Oakhurst individuals and previously published prehistoric genomes from South Africa, Cameroon 31 , Kenya 32 , 33 , Malawi 2 , 34 , Tanzania 34 and Zambia 34 by means of outgroup F 3 -statistics (Supplementary Table 4 ). All LSA genomes from South Africa are more like one another than any other tested prehistoric ancient African (Fig. 2 ). Yet, some fine-scale population stratification is evident, with the most recent sample OAK007 clustering together with two 2,000-year-old hunter-gatherers from St. Helena and Faraoskop. Together these samples form a sister clade with two contemporaneous samples from Ballito Bay, located in KwaZulu-Natal on the eastern coast of South Africa, within the diversity of the older Oakhurst samples. Visualizing the transformed pairwise-distance F 3 -matrix through multidimensional scaling, we find the Oakhurst individuals older than 1,300 cal bp shifted along coordinate 1 away from the younger LSA genomes and three historical San samples from Sutherland, Western Cape province (Extended Data Fig. 5a ). On the other hand, consistent with the affinities detected in PCA and DYSTRUCT analysis, the genomes of a 1,200-year-old pastoralist and four Iron Age farmers from South Africa cluster in the diversity of LSA genomes from Malawi and Cameroon, respectively, highlighting the impact of admixture events after 1,300 cal bp that contributed varying fractions of non-San ancestry to all populations in southern Africa 1 , 2 , 7 , 8 (Fig. 2 ).

Shown is a heat-map matrix of pairwise outgroup F 3 -statistics of the form F 3 (X, Y; Chimp). Hierarchical cluster analysis applying Ward’s minimum variance method to the rows is added as a dendrogram. Data can be found in Supplementary Table 4 .
To test whether the Oakhurst individuals already exhibit subtle excess affinity to non-San ancestries, we calculated F 4 -statistics 35 of the form F 4 (Archaic, Test; Ju_hoan_North, Tanzania_Luxmanda_3000BP) (Fig. 3a and Supplementary Table 6 ) and F 4 (Archaic, Test; Ju_hoan_North, Cameroon_SMA) (Extended Data Fig. 5b and Supplementary Table 5 ). We find that none of the Oakhurst individuals, including the most recent individual OAK007, shares significantly more genetic drift with Tanzania_Luxmanda_3000BP (associated with East African pastoralist ancestry) or Cameroon_SMA (representing Central and Western African ancestry) than the published LSA genomes, providing an early bound for the date of the arrival of non-San ancestry at the southern coast only after 1,300 cal bp . For all LSA samples from South Africa, we observe significantly higher affinity ( Z > 3) to present-day ‡Khomani than to Ju|’Hoan, confirming an old split age for the northern and southern San ancestries before 20,000 bp (refs. 7 , 8 , 27 , 36 ) and, thus, before the lake Makgadikgadi palaeo-wetland dried up 29 , 37 (Fig. 3b and Supplementary Table 7 ).

a , Individual F 4 -statistics of the form F 4 (Archaic, Test; Ju_hoan_North, Tanzania_Luxmanda_3000BP) through time for 21 ancient and 2 current-day Khomani genomes from South Africa. Error bars represent 2 s.e. Data can be found in Supplementary Tables 6 and 7 . b , Individual F 4 -statistics of the form F 4 (Archaic, Test; Ju_hoan_North, Khomani_San) through time for 21 ancient genomes from South Africa. c , Overview about population genetic changes in South Africa from 10,000 cal bp to the present-day. Arrows indicate P values from generalized-likelihood ratio tests implemented in qpWave testing for genetic continuity between temporally preceding and succeeding groups in the Northern Cape, Western Cape and KwaZulu-Natal, respectively. Discontinuities are explicitly marked as interrupted arrows. Pie charts depict the ancestry composition for each group derived from qpAdm modelling. Symbols and colours correspond to Fig. 1 . Data can be found in Supplementary Tables 8 and 9 .
To evaluate whether chronological groups in South Africa were consistent in sharing the same genetic make-up as the preceding and succeeding populations, we used qpWave 38 , 39 , 40 , a generalization of F 4 -statistics, testing for significant evidence of continuity (that is, we tested whether they were consistent with forming a clade at P > 0.01) (Supplementary Table 8 ). We find that groups of individuals from Oakhurst dating to 10,000 cal bp and 6,000 to 4,000 cal bp as well as groups from the western and eastern coast (St. Helena, Faraoskop, OAK007 and Ballito Bay, respectively; dating between 2,200 and 1,300 cal bp ) were all genetically indistinguishable (Fig. 3c ). On the other hand, we observe significant discontinuity between 1,300 and 1,200 cal bp , as well as between 1,200 and 400 cal bp , consistent with the independent arrivals of non-San East African pastoralist and West African farmer ancestry in South Africa (Fig. 3c ). To assess these demographic changes quantitatively, we used qpAdm 39 , 40 to successively model these groups as mixtures between local LSA, pastoralist and farmer ancestry components ( Methods ; Supplementary Table 9 ). We find no evidence of West African ancestry within the three Sutherland individuals 41 (dating to the second half of the nineteenth century), yet, we detect small amounts of Tanzania_Luxmanda_3000BP-related ancestry (11% ± 0.9%) comparable to the proportions measured in present-day ‡Khomani from the Northern Cape Province (9% ± 1%) (Fig. 3c ).
Overall, these observations indicate that between 10,000 and 1,300 cal bp , no ancestry from outside present-day South Africa arrived at Oakhurst rockshelter, demonstrating a remarkable degree of relative genetic continuity over a time range of nearly 9,000 years. Such a demographic pattern is exceptional in the global archaeogenetic record, yet, the Oakhurst samples do not exhibit signs of genetic isolation. While the conditional nucleotide diversity (CND; Methods ) of the Oakhurst individuals is lower than in LSA populations from Malawi, Kenya and Cameroon, it is comparable to the diversity measured in the published hunter-gatherers from the Western Cape and KwaZulu-Natal and higher than the CND in ancient hunter-gatherers from Serbia 42 , Japan 43 or Brazil 44 (Extended Data Fig. 6a ). Furthermore, we find the average heterozygosity levels within the three highest-coverage individuals (OAK007, OAK012 and OAK013) to be higher than in most present-day San and Khoe populations, disagreeing with a model of prolonged genetic isolation, yet supporting recent findings of a continuous, substantial reduction of effective population size in southern San and Khoe after 1,300 bp (ref. 45 ) (Extended Data Fig. 6b and Supplementary Table 25 ).
Demographic changes in the last 2,000 years
We use the increased availability of LSA data to quantify and characterize the demography of transitions in southern Africa during the last 2,000 years. Yet, these admixture events are challenging to reconstruct because of additional gene flow from at least two immigrant populations during prehistoric times 1 , 2 , 7 , 8 , 27 , 46 , 47 , 48 , 49 and additional inter- and intra-continental admixture following European settlement from the 1650s onwards 46 , 50 , 51 , 52 . This complex history hinders inferences about the timing and mode (for example, involving sex bias) of admixture events. To circumvent these issues, we focused on groups with only two of the various ancestries present in the region today and compared the resulting patterns to identify putative trajectories of non-LSA ancestries through Southern Africa. Specifically, we used qpAdm to test 1-source, 2-source or 3-source models (excluding individuals with European admixture for now) for present-day San, Khoe and Bantu-speaking populations. As sources, we used (1) Stone Age hunter-gatherers from South Africa (SA_LSA), (2) Tanzanzia_Luxmanda_3000BP and (3) present-day Mende, reflecting the local LSA, pastoralist and farming ancestries 2 , respectively ( Methods ; Extended Data Fig. 7 and Supplementary Tables 10 – 12 ).
On the basis of the estimated admixture proportions in the best-fitting model using the lowest number of sources, we grouped populations into primarily West African- or East African-admixed categories, excluding ambiguous cases with both non-San ancestries being present substantially (Supplementary Tables 13 – 16 ). Within these categories, we computed admixture dates for the West African component (Extended Data Fig. 8a and Supplementary Table 14 ) and the East African component (Extended Data Fig. 8b and Supplementary Table 13 ) in the relevant target groups. We find East African, Tanzania_Luxmanda-related ancestry gene flow into San and Khoe populations to be consistently older than the gene flow from West African-related groups. For the Tanzania_Luxmanda-related gene flow, we identify a mean admixture date of 1,068 bp among San and Khoe populations, agreeing with the observed East African ancestry in the 1,200-year-old pastoralist from Kasteelberg and the admixture date estimated for the nineteenth century Sutherland samples (1,228 ± 278 bp ). In contrast, West African admixture in San, Khoe and Bantu groups is dated consistently younger than the Tanzania_Luxmanda-related ancestry gene flow and also exhibits a difference between Bantu and San/Khoe. Specifically, the mean date for admixture of West African ancestry among Bantu-speaking populations in southern Africa (for example, Herero, Tswana and Kgalagadi) is estimated at 808 bp on average, remarkably agreeing with the dating of that admixture in the 400 cal bp Iron Age farmers from KwaZulu-Natal (832 ± 139 bp ). In contrast, the estimated date of West African ancestry in San and Khoe groups is more recent (578 bp ). This discrepancy might suggest successive waves of Bantu immigration 53 or continuing gene flow from Bantu-related groups into San and Khoe populations after the initial admixture event that contributed San and Khoe ancestry to Bantu-speaking groups (Extended Data Fig. 8 ).
For the mode of interaction between locals and newcomers, we find evidence for sex bias in most present-day San, Khoe and Bantu populations, with stronger signals in the San and Khoe populations compared to the Bantu-speaking groups (Fig. 4b and Supplementary Table 17 ). In general, both San and Khoe as well as Bantu groups exhibit significantly more SA_LSA ancestry on the X chromosome than the autosomes and (congruently) share more drift with SA_LSA on the X chromosome than on the autosomes (Fig. 4b ). This suggests that substantially more female than male San ancestors were involved in the admixture events following the spread of East African pastoralist and West African farmer ancestry, which is consistent with previous studies of uniparental and genome-wide markers 19 , 20 , 22 , 28 , 29 , 47 , 48 , 54 , 55 , 56 , 57 . Assuming a single admixture event (using the dates obtained from DATES analysis), we explicitly compared autosome to X chromosome ancestry to determine female ( sf ) and male contributions ( sm ) to the gene pools of selected Khoe and Bantu populations, using a previously described method 58 , 59 (Supplementary Table 20 ). For the Damara, we estimate that for each San man ∼ 1.4 San women contributed to the gene pool, for the ǂHoan ∼ 2.28 San women per San man, for the Shua ∼ 4, for the Haiǁom (Haiom) ∼ 5.2 and for South African Bantu ∼ 2.1 San women per San man.

a , Scatter plot of group-based jackknife point estimates for F 4 -statistics of the form F 4 (Archaic, X; Cameroon_LSA, SA_LSA) on loci of the X chromosome ( y axis) and the autosomes ( x axis), where X represents the ancient genomes ( n = 17) and two present-day San populations (Khomani_San and Ju_Hoan_North). Error bars, 1 s.e. Data can be found in Supplementary Table 18 . b , As a but for present-day San, Khoe and Bantu-speaking populations ( n = 25). Error bars, 1 s.e. Data can be found in Supplementary Table 17 . c , Summary of the inferred population history of the San and Khoe in southern Africa. Sex symbols indicate male- and female-biased reproduction. Note that pastoralism and farming both appeared in present-day South Africa at about the same time, 2,000 years ago. Symbols and colours correspond to Fig. 1 .
Although this signal of female-biased admixture is also evident in the historical Sutherland genomes and the 1,200-year-old pastoralist from Kasteelberg, we observe that the four Iron Age KwaZulu-Natal samples (SA_400BP) share more drift with SA_LSA on the autosomes than on the X chromosome, indicative of more male than female San ancestors (Fig. 4a and Supplementary Tables 18 and 19 ). This contradicts the pattern observed in most present-day Bantu or San and Khoe groups in South Africa and Botswana and might be related to changes in interaction between Bantu and San/Khoe groups after 400 bp . Overall, our results show that, despite an overarching trend of female-biased gene flow from San and Khoe populations into Bantu-speaking groups, the modes of interaction and reproduction were strongly influenced by locally and temporally defined factors after the initial arrival of the first farmers 47 .
Finally, we detect a comparatively recent admixture date corresponding to male-biased 28 , 46 , 50 , 51 , 52 gene flow from Europe into San/Khoe and mixed 60 groups from Colesberg and Wellington 7 (Supplementary Tables 21 – 23 ). This ancestry is best approximated by northwestern Europeans, as shown by admixture F 3 -statistics of the form F 3 (Test, Sutherland, Target) (Supplementary Table 23 ). For instance, for South Africans of mixed ancestry from Colesberg, we observed that F 3 values among 40 West Eurasian populations are minimized for Irish, Icelandic and Norwegian people, followed by English (all Z < −69). We date the arrival of northern European ancestry among these populations to 199 yr bp (Extended Data Fig. 8 and Supplementary Table 15 ), postdating the settlement of South Africa by Dutch and British immigrants from the mid-1600s onwards, a development that led ultimately to the demise of most San and Khoe genetic, linguistic and cultural diversity in the region 61 and lastingly affected the demographic trajectories in southern Africa 62 (Fig. 4c ). While population structure in South Africa partly collapsed, new extracontinent ancestries were introduced to the region, increasing the heterogeneity of the admixture landscape. To quantitatively estimate this influx from outside Africa and its impact on genetic diversity, we decomposed admixture sources using a supervised clustering approach implemented in the software ADMIXTURE 63 (Extended Data Fig. 9 and Supplementary Tables 21 and 22 ). For example, in South Africans of mixed ancestry from Colesberg, we observe on average 24.4% ± 3.2% South Asian ancestry and 2.8% ± 0.5% East Asian ancestry besides 8.2% ± 1.4% North European ancestry, yet only 35.5% ± 2.9% San-related ancestry. Besides South Africans of mixed ancestry, we furthermore detect European ancestry in the Karretjiemense (5.61%), ‡Khomani (9.45%) and Nama (6.83%), indicating that the southern San and Khoe were especially affected by admixture with European sources. Using the output from ADMIXTURE, we proceeded to measure the variability of ancestry components in the southern African groups via FSTruct 64 . We find that the relative levels of variation among southern San, namely the Karretjiemense, ‡Khomani and Nama are significantly higher than in any other present-day San or Khoe population because of the frequent presence of variable European ancestry components, comparable to the variability measured in South Africans of mixed ancestry from Colesberg and Wellington (Supplementary Tables 21 and 22 and Extended Data Fig. 9 ). This heterogeneity in non-Southern African admixture across individuals obscures the high genetic affinity to the ancient Oakhurst samples, as measured using F ST and IBD metrics and highlights the necessity of further sampling of local communities to adequately assess the effect of non-southern African admixture on the current genetic landscape of San populations in southernmost Africa. On the other hand, northern and central San feature significantly lower variability, which is similar to the diversity observed in neighbouring Bantu-speaking people, who also do not exhibit substantial proportions of non-African ancestry (Extended Data Fig. 9 ).
The question of population continuity or discontinuity during the LSA of southern Africa has been the focus of anthropological research for well over a century. Archaeogenetic research of the last two decades has revealed that the Holocene demographic histories of Stone Age Europe 39 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , Asia 43 , 72 , 73 , 74 , 75 , 76 , 77 and North Africa 78 , 79 , 80 were characterized by several episodes of large-scale migrations, either in the form of admixture with newcomers or by total replacement of the established inhabitants. While these biological transformations modified the genetic make-up of the local populations, they were also vectors for technological innovation, such as the introduction of new technologies, raw material uses or subsistence strategies. In contrast, for South Africa, we demonstrate that the local gene pool was characterized by a prolonged period of genetic continuity with no (detectable) gene flow from outside southern Africa. The earliest individual in our study that yielded aDNA showed a genetic make-up indistinguishable from the later inhabitants of Oakhurst rockshelter, suggesting that this local ‘southern’ San gene pool was formed more than 10,000 years ago and remained isolated from admixture with neighbouring ‘central’ and ‘northern’ San populations 7 , 8 , 26 , 27 , 28 , 29 or with more distant sources to the northeast, which admixed with San groups in Malawi and Tanzania 2 .
Consequently, the sequence of cultural change at Oakhurst, for example from the Oakhurst to Wilton technocomplexes 6 , 9 , 14 , 15 , appears to result from local development initiated by the indigenous inhabitants 14 , highlighting the role of in situ innovations followed by acculturation. Our data also demonstrate that subtle fluctuations in the craniofacial size of South African LSA coastal inhabitants 81 , 82 (for example, between 4,000 and 3,000 bp ) were not the product of genetic discontinuity but probably related to changes in environmental factors or population size 81 , 82 , 83 . Yet, we highlight that the inhabitants of Oakhurst were not a small, bottlenecked population. Genomic measurements of diversity indicate a degree of genetic variation comparable to other African hunter-gatherer populations and higher than Stone Age foragers from Europe or America. Furthermore, the current resolution of our methods and limited reference dataset in sub-Saharan Africa restricts our ability to detect subtle changes in group size or small-scale immigration of people from within southern Africa. However, our data are congruent with a population in reproductive isolation from other San (and non-San) populations over the whole period of occupation of the site.
This period of ~9,000 years of genetic continuity ends rather abruptly in the migration events which introduced East and West African-related ancestry to South Africa, accompanied by the spread of herding and farming 1 , 2 , 7 , 8 . On the basis of present available data, it appears that non-southern African ancestry reached the southernmost parts of South Africa only after 1,300 cal bp . There is, however, abundant archaeological evidence of marked changes in subsistence and settlement patterns among coastal and near-coastal communities in this region from ~2,000 cal bp . These changes have previously been interpreted as resulting from the disruption of hunter-gatherer communities by the emergence of herding 84 , 85 , 86 , 87 . Notably, a similar temporal discrepancy was observed during the Mesolithic–Neolithic translation in Europe, where the admixture between hunter-gatherers and incoming farmers postdates the emergence of agriculture by almost 2,000 years (ref. 88 ). This indicates that hunter-gatherers and farmers resided in close geographic proximity for a considerable time before mixing 88 and demonstrates that migration can precede any subsequent population admixture substantially. Alternatively, the practice of pastoralism may have spread to Southern Africa through a process of cultural diffusion in advance of substantial population expansion 89 , 90 , explaining the absence of any East African-related ancestry in South Africa before 1,300 cal bp .
Yet, the events of the last 1,300 years had a substantial impact on the local gene pool of South Africa. Today, all San and Khoe populations are admixed with one or both of East African Pastoralist and West African Farmer ancestry 1 , 2 , 7 , 8 . The collapse of the LSA population structure was accelerated by the arrival of European settlers in the mid-seventeenth century 62 . Together with the continuous loss of oral traditions, these issues contribute to our poor understanding of the prehistoric southern African population structure. Using allele-frequency and IBD segment-based analyses, we were able to show that the present-day San and Khoe inhabitants of South Africa are, despite recent periods of disruption under Dutch and British rule, still directly related to the ancient Oakhurst individuals of the last 10,000 years. Especially among the ‡Khomani, Karretjiemense and Nama, who belong to the most admixed San/Khoe groups in southern Africa, some individuals still trace most of their ancestry back to these LSA hunter-gatherers. This also applies to the three historic San individuals from Sutherland dating to the late nineteenth century, who show only minor ancestry contribution from outside southern Africa and otherwise close autosomal and mitochondrial similarity to the LSA Oakhurst population 41 , demonstrating that the early Holocene gene pool of the Western Cape persisted in some regions throughout the last 2,000 years without major changes and that in some parts of southern Africa the long-lasting population continuity was not completely disrupted.
Study design and ethics
The human remains from the Oakhurst rockshelter site are housed in the University of Cape Town (UCT) human skeletal repository. The approach for permission to use these samples for aDNA was followed according to ref. 91 , which included consultation with representatives from the San community in accordance with the South African Heritage Resources Agency and permission from the repository research committee. The Oakhurst samples were approved by the UCT human research ethics committee under ethics no. 715/2017 and Heritage Western Cape permit no. 17071302AS0718E.
The sampling strategy used was twofold: to be minimally invasive and serial sample through time in the occupation of the site. We selected only individuals with loose, broken or previously glued petrous bones so that upon return the samples could be re-glued back to their original state (only featuring a small, unnoticeable sampling hole). Additionally, a single tooth per individual was sampled. For the DNA libraries analysed in this study, 13 individuals were sampled (including 11 petrous bones and 12 teeth). OAK003.B and OAK003.C were initially thought to belong to the same individuals but in fact represent two distinct individuals. These small bone and tooth elements were shipped to Germany for sampling and (after processing) returned to South Africa. Collection of bone powder for aDNA extraction was performed as described in the section on ‘Ancient DNA work’ below.
New radiocarbon dates for this study were measured on the bone and tooth fragments sampled for DNA. These dates were obtained at the Curt-Engelhorn-Center Archaeometry gGmbH, Mannheim, using MICADAS-AMS. Collagen was extracted from the previously sampled bones, purified by ultrafiltration (fraction >30 kDa) and freeze-dried. The 14 C ages were normalized to δ 13 C = −25‰. The calibration was done using the SHCal20 calibration curve for the Southern Hemisphere 92 .
Site background
Oakhurst rockshelter (33° 59′ 00″ S–34° 00′ 00″ S and 22° 35′ 00″ E–22° 43′ 00″ E) is important in the history of LSA studies in southern Africa. Excavations by John Goodwin from 1932 to 1935 produced many artefacts and some human skeletons. The site has substantial deposits (>2 m deep) extending over the last 10,000 years. It was one of the first in South Africa to be excavated in accordance with professional standards, with Goodwin and his team carrying out meticulous excavation and detailed recording. In the 1930s, a main goal was a better understanding of the stratigraphic (and thus temporal) relationships between different stone artefact assemblages, seen at that time as different ‘cultures’.
Now that more sites have been excavated, we recognize that the large, relatively unstandardized stone artefacts from the lower part of the Oakhurst sequence form part of a widespread artefact-making tradition in the terminal Pleistocene/early Holocene, extending across South Africa and into Zimbabwe and southern Namibia. Although there are regional variations, these assemblages are sufficiently similar that they are generally grouped as the ‘Oakhurst technocomplex’, acknowledging their early recognition at this site. At Oakhurst, this technocomplex extends through approximately the lower half of the deposits and dates to 9,000–8,000 bp . At about 8,000 bp there was a shift to microlithic (Wilton) assemblages, also very widely distributed across the subcontinent (and into East Africa). Goodwin distinguished ‘Smithfield C’, ‘Wilton’, ‘Developed Wilton’ and ‘Wilton with pottery’ but today we see these as an evolving microlithic tradition. Selection of fine-grained stone raw materials facilitated the making of tiny artefacts, with materials probably sourced over considerable distances. We note that the inhabitants of Oakhurst continued to make microlithic artefacts into the last 2,000 years (when they also made pottery), as seen at the sites of Boomplaas, further inland and Die Kelders, on the coast further to the west. Along most of the southern Cape coast, however, preferences in the last ~3,500 years shifted back to macrolithic artefacts with very little retouch, often made on locally available quartzite. A greater degree of spatial heterogeneity in the last few millennia is consistent with higher population densities and more territorial settlement patterns, as seen amongst hunter-gatherers in coastal and riverine areas elsewhere in the world.
Excavations at Oakhurst were made considerably more challenging by the many burials, with some grave shafts and even graves intersecting others. Some individuals could be recovered in their entirety, sometimes with rich grave goods, for example grave VIa (UCT 204). Others had been dispersed by disturbances in antiquity or by the roots of plants or burrowing animals, making it difficult to assess how many individuals are represented in the remains recovered.
Ancient DNA work
Collection of bone powder.
Sampling of 23 bone and teeth samples took place in clean-room facilities dedicated to aDNA work at the Max Planck Institute for Science of Human History in Jena (MPI-SHH). The sampling workflow included documenting and photographing the provided samples. For teeth, we either cut along the cementum/enamel junction and collected powder by drilling into the pulp chamber or accessed the pulp chamber by drilling the tooth transversally. For the petrous bones, we cut the petrous pyramid longitudinally to drill the dense part directly from either side 93 . We collected 28–178 mg of bone or tooth powder per sample for DNA extractions.
For four bone samples, more bone powder was obtained for 14 C dating at the Curt-Engelhorn-Center Archaeometry gGmbH.
DNA extraction
The aDNA was extracted following a modified protocol of ref. 94 , as described in ref. 95 , where we replaced the extended-MinElute-column assembly for manual extractions with columns from the Roche High Pure Viral Nucleic Acid Large Volume Kit 96 and for automated extraction with a protocol that replaced spin columns with silica beads in the purification step 97 .
Library construction
We generated 23 double-indexed 98 , double-stranded libraries using 25 µl of DNA extract and following established protocols 99 . We applied the partial UDG (UDG half) 100 protocol to remove most of the aDNA damage while preserving the characteristic damage pattern in the terminal nucleotides. Additionally, we generated 15 double-indexed single-stranded libraries 101 using 20 µl of DNA extract and applied no UDG treatment.
Shotgun screening, capture and sequencing
Libraries were sequenced inhouse on an Illumina HiSeq 4000 platform to an average depth of 5 million reads and after demultiplexing processed through EAGER 102 . After an initial quality check based on the presence of aDNA damage and endogenous DNA >0.1%, we subsequently selected and enriched 11 double-stranded and 15 single-stranded libraries using in-solution capture probes synthesized by Agilent Technologies for ~1,240k SNPs along the nuclear genome 103 . The captured libraries were sequenced for ~34 million reads on average (minimum, 17 million; maximum, 52 million) using a single end (1 × 75 base pair (bp) reads) configuration. Taking all double- and single-stranded libraries together, we generated 40–139 million reads for the 13 individuals (on average 62 million reads).
aDNA data processing
Read processing and adna damage.
After demultiplexing based on a unique pair of indexes, raw sequence data were processed using EAGER 102 . This included clipping sequencing adaptors from reads with AdapterRemoval (v.2.3.1) 104 and mapping of reads with BWA (Burrows–Wheeler Aligner) v.0.7.12 (ref. 105 ) against the human reference genome hg19, with seed length (-l) disabled, maximum number of differences (-n) of 0.01 and a quality filter (-q) of 30. We removed duplicate reads with the same orientation and start and end positions using DeDup v.0.12.2 (ref. 102 ). Terminal base deamination damage calculation was done using mapDamage v.2.0.6 (ref. 106 ), specifying a length (-l) of 100 bp. For the ten libraries that underwent UDG half treatment, we used BamUtil v.1.0.14 ( https://genome.sph.umich.edu/wiki/BamUtil:_trimBam ) to clip two bases at the start and end of all reads for each sample to remove residual deaminations, thus removing genotyping errors that could arise as a result of aDNA damage.
Sex determination
To determine the genetic sex of the ancient individuals, we calculated the coverage on the autosomes as well as on each sex chromosome and subsequently normalized the X and Y reads by the autosomal coverage 107 . For that, we used a custom script ( https://github.com/TCLamnidis/Sex.DetERRmine ) for the calculation of each relative coverage as well as their associated error bars 108 . Females are expected to have an X rate of 1 and a Y rate of 0, whereas males are expected to have a rate of 0.5 for both X and Y chromosomes.
Contamination estimation
We used the ANGSD (analysis of next generation sequencing data) package 109 (v.0.923) to test for heterozygosity of polymorphic sites on the X chromosome in male individuals, applying a contamination threshold of 5% at the results of method 1. For male and female samples, we estimated contamination levels on the mtDNA using Schmutzi 110 (v.1.5.4) by comparing the consensus mitogenome of the ancient sample to a panel of 197 worldwide mitogenomes as a potential contamination source, applying a contamination threshold of 5%.
We used the program pileupCaller (v.1.4.0.2) ( https://github.com/stschiff/sequenceTools.git ) to genotype the trimmed BAM files of ten UDG half libraries. A pileup file was generated using samtools mpileup with parameters -q 30 -Q 30 -B containing only sites overlapping with our capture panel. From this file, for each individual and each SNP on the 1,240k panel 39 , 40 , 111 , one read covering the SNP was drawn at random and a pseudohaploid call was made; that is, the ancient individual was assumed homozygous for the allele on the randomly drawn read for the SNP in question. For the 15 single-stranded libraries that underwent no UDG treatment, we used the parameter -SingleStrandMode, which causes pileupCaller to ignore reads aligning to the forward strand at C/T polymorphisms and at G/A polymorphisms to ignore reads aligning to the reverse strand, which should remove postmortem damage in aDNA libraries prepared with the non-UDG single-stranded protocol. To maximize our resolution, we filled missing data in the single-stranded libraries with additional genotypes present in the trimmed, double-stranded libraries but not in the single-stranded libraries.
Mitochondrial and Y chromosome haplogroup assignment
To process the mtDNA data, we extracted reads from 1,240k data using samtools (v.1.3.1) 112 and mapped these to the revised Cambridge reference sequence. We subsequently called consensus sequences using Geneious R9.8.1 (ref. 113 ) and used HaploGrep 2 (v.2.4.0) 114 ( https://haplogrep.uibk.ac.at/ ; with PhyloTree v.17-FU1) to determine mitochondrial haplotypes. For the male individuals, we used pileup from the Rsamtools package to call the Y chromosome SNPs of the 1,240k SNP panel (mapping quality ≥30 and base quality ≥30). We then manually assigned Y chromosome haplogroups using pileups of Y-SNPs included in the 1,240k panel that overlap with SNPs included on the ISOGG SNP index v.15.73 (Y-DNA Haplogroup Tree 2019-2020; 2020.07.11).
Identity-by-descent
We imputed and phased individuals with >500,000 SNPs (OAK003B, OAK007, OAK012 and OAK013) using GLIMPSE 115 (v.2.0.0) ( https://github.com/odelaneau/GLIMPSE ), applying the default parameters and using the 1,000 genomes reference panel. Samples with >600,000 SNPs exhibiting a genotype posterior of ⩾ 0.99 after imputation were included in downstream IBD analysis. Subsequently, we used BEAGLE 116 , 117 (v.5.2) to phase the newly imputed genotypes. Following ref. 118 , the window and overlap lengths were set as wider than any chromosome (window length 380 cM and overlap length 190 cM) to maximize the information used for phasing the genomes. The 1,000 genomes phase 3 dataset ( https://bochet.gcc.biostat.washington.edu/beagle/1000_Genomes_phase3_v5a ) and GRCh37 genomic maps ( https://bochet.gcc.biostat.washington.edu/beagle/genetic_maps/ ) provided by BEAGLE were used for phasing. The identification of IBD segments was done using RefinedIBD 119 . The window size was set to 3 cM. The minimal size for a segment to be considered shared by IBD is 1 cM, the same threshold used in refs. 118 , 120 . Finally, we removed gaps between IBD segments that have at most one discordant homozygote and that are <0.6 cM in length and aggregated the sum and number of IBD segments between each pair of ancient and present-day individuals.
Kinship estimation
We calculated the pairwise mismatch rate 121 in all pairs of individuals from our pseudohaploid dataset to double-check for potential duplicate individuals and to determine first-, second- and third-degree relatives. However, no relatives were identified.
Diversity estimation
CND was estimated by counting the differences between the ascertained pseudohaploid genotype calls present in one pair of individuals from the same population as described in the section on ‘Kinship estimation’ above. For these comparisons we grouped the individuals per geographic origin and time period. Results are reported as boxplots, where each dot corresponds to the CND value for a unique pair of individuals. We also estimate average heterozygosity levels for the imputed genomes of OAK007, OAK012 and OAK013 as well as for two published Iron Age individuals from Botswana (XAR001 and XAR002) by taking the fraction of the number of heterozygous sites over the total number of sites across 22 autosomes 122 . Subsequently, we compared these estimates with the heterozygosity levels observed in 562 individuals belonging to 49 present-day sub-Saharan African populations.
Population genetic analysis
We merged our aDNA data with previously published datasets of ancient individuals reported by the Reich Lab in the Allen Ancient DNA Resource v.54.1 ( https://reich.hms.harvard.edu/allen-ancient-dna-resource-aadr-downloadable-genotypes-present-day-and-ancient-dna-data ) (1,240k SNP panel) 123 (Supplementary Table 26 ). Present-day data from primarily sub-Saharan Africans were assembled from refs. 7 , 8 , 36 , 111 , 124 (human origins SNP panel and human origins-Schlebusch SNP panel) (Supplementary Table 27 ). We excluded recently admixed individuals for PCA, DYSTRUCT and qpAdm analysis from ref. 7 (see Data Availability).
Within tables and figures, we refer to populations by the names given in the Allen Ancient DNA resource v.54.1 (ref. 123 ). Additionally, we refer to individuals from Oakhurst, St. Helena 2 , Faraoskop 2 and Ballito Bay 1 grouped together as South Africa LSA or SA_LSA. We follow the San Council recommendations in using population-specific terms whenever possible and alternatively use the terms San for Tuu and K’xaa language-speaking hunter-gatherer groups and Khoe for Khoe-Kwadi speakers. Within the text, we spell names of San and Khoe groups using click consonant symbols. Within figures and tables we refer to populations using the labels from the original publications of the genotype data. When necessary we collectively refer to groups with indigenous southern Africa-specific ancestry as having San-related ancestry. We used the label ‘coloured’ for some groups in the figures and supplementary tables following the labelling in the original publications of these genomes 1 , 7 . This bureaucratic denotation refers to South Africans of mixed ancestry, who represent a biologically heterogeneous group with variable and complex admixture from indigenous San and Khoe, European, Bantu-speaking African, Asian and Madagascan Cape slaves or migrants 60 . In the text, we use the label South Africans of mixed ancestry for these individuals, acknowledging that many are genetically homogenous to one origin yet were classified during apartheid under a single racial label, which is still used today 60 .
Principal components analysis
We carried out PCA using the smartpca software v.16000 from the EIGENSOFT package (v.6.0.1) 125 . We computed principal components on two different sets of southern African populations and projected ancient individuals using lsqproject: YES and shrinkmode: YES. Dataset (1) includes 24 San, Khoe and Bantu-speaking populations from three sources (refs. 7 , 8 , 111 ) as well as 212,000 SNPs (Fig. 1b ); dataset (2) includes only 22 San, Khoe and Bantu-speakining populations from two sources (refs. 25 , 111 ) but 597,000 SNPs (Extended Data Fig. 1b ). We highlight that the PCA computed on dataset (1) better reflects the genetic diversity within southern San (which is under-represented in dataset (2) because of the lack of samples from South Africa).
F -statistics
F 3 - and F 4 -statistics were computed with ADMIXTOOLS v.3.0 (ref. 35 ) ( https://github.com/DReichLab ). F 3 -statistics were calculated using qp3Pop (v.435). For F 4 -statistics, we used the qpDstat (v.755) and with the activated F 4 -mode. Significant deviation from zero can be interpreted as rejection of the tree population typology ((Outgroup, X);(Pop1, Pop2)). Under the assumption that no gene flow occurred between Pop1 and Pop2 and the Outgroup, a positive F -statistic suggests affinity between X and Pop2, whilst a negative value indicates affinity between X and Pop1. Standard errors were calculated with the default block jackknife 5 cM in size. As outgroup for F 3 - and F 4 -statistics, we used either diploid genotypes from two archaic human genomes (a Neanderthal 126 and a Denisovan 127 ) or haploid genotypes from one chimpanzee genome (the chimpanzee genome is required for technical reasons as an outgroup to all humans).
Fixation index
We calculated F ST using smartpca software v.16000 from the EIGENSOFT package (v.6.0.1) 125 with the fstonly, inbreed and fsthiprecision options set to YES.
Inference of mixture proportions
We estimated ancestry proportions using qpWave 39 , 128 (v.410) and qpAdm 39 (v.810) from ADMIXTOOLS v.3.0 (ref. 35 ) with the allsnps: YES and inbreeding: YES options and one basic set of 11 outgroups modified from ref. 33 : Mbuti, Dinka, Ju_hoan_North, Turkey_N 129 , Iran_GanjDareh_N 40 , French, Sardinian, Punjabi, Ami, Papuan and Karitiana.
For group-based qpAdm analysis, we tested for each ancient and present-day population 1-, 2- and 3-way admixtures scenarios between SA_LSA (consisting of Oakhurst, without OAK006, St. Helena, Faraoskop and Ballito Bay), Tanzania_Luxmanda_3000BP and Mende.DG 2 . We selected for each population the admixture model with P > 0.01 featuring the lowest number of sources.
To analyse potential sex bias in the admixture process, we used qpAdm to estimate SA_LSA admixture proportions on the autosomes (default option) and on the X chromosome (option “chrom: 23”) using the abovementioned outgroups. Following the approach established by ref. 42 , Z -scores were calculated for the difference between the autosomes and the X chromosome using the formula Z = \(\frac{{{\mathrm{pA}}}-{{\mathrm{pX}}}}{\sqrt{{\mathrm{\sigma {A}}}^{2}+{{\mathrm{\sigma X}}}^{2}}}\) where pA and pX are the SA_LSA admixture proportions on the autosomes and the X chromosome and σ A and σX are the corresponding jackknife standard deviations 42 . Thus, a negative Z -score means that there is more SA_LSA admixture on the X chromosome than on the autosomes, indicating that the SA_LSA admixture was female-biased.
Ancestry decomposition
We performed model-based clustering analysis using two different approaches: (1) We applied DYSTRUCT 30 , with a cluster number ( K ) ranging between 2 and 10. Mean radiocarbon ages for the ancient individuals included were converted to generation ages (assuming a generation time of 30 yr; refs. 130 , 131 ) and provided for the analysis. (2)We applied ADMIXTURE 63 in supervised mode using modern reference populations at K = 7. This analysis was run on haploid data with the parameter –haploid set to all (="*"). To obtain point estimates for populations, we averaged individual point estimates and calculated the s.e.m. As modern references we used the following groupings: San (Ju_hoan_North.DG, Khomani_San.DG), West Africa (YRI.SG, ESN.SG), East Africa (Somali, Masai, Sandawe), South Europe (TSI.SG, IBS.SG), North Europe (CEU.SG, GBR.SG), South Asia (PJL.SG, GIH.SG), East Asia (CHB.SG, JPT.SG) 111 , 132 , 133 . The Q matrix of this ADMIXTURE analysis was also used as input for FSTruct as described by the authors 64 .
Potential ascertainment bias
Recent analyses have shown that co-modelling more than one sub-Saharan African and/or archaic human groups (Neanderthals and Denisovans) using F -statistics in a non-outgroup-ascertained SNP panel can lead to false rejection of true demographic histories and failure to reject incorrect models in F 4 -derived methods like qpAdm 134 . However, most F 4 -statistics themselves remain minimally affected by ascertainment 134 . Regarding qpAdm analysis, we compared our results calculated on the complete 1,240k or human origins SNP panels with the published results by ref. 2 . That study 2 obtained an outgroup-ascertained set of 814,242 transversion SNPs polymorphic between the Denisovan and Neanderthal genomes to minimize the bias on F -statistics 2 . We find our qpAdm estimates to be highly correlated with the ones reported by Skoglund et al. 2 (Extended Data Fig. 7a ) (Pearson’s product-moment correlation; t = 22.244, d.f. = 17, P = 5.23 × 10 −14 , cor = 0.983), suggesting a negligible effect of ascertainment bias on our results. The qpWave analysis was restricted to A/T and G/C sites in the 1,240k SNP panels as recommended by ref. 134 .
Admixture dating
Admixture dates between SA_LSA and Tanzania_Luxmanda, Mende or English as sources were calculated using DATES (distribution of ancestry tracts of evolutionary signals) (v.4010) 88 using standard settings. A default bin size of 0.001 M is applied in our estimates (flag “binsize: 0.001” added). We used a standard of 29 years per generation to convert the generation times in years since admixture.
Maximum likelihood tree
We constructed maximum likelihood trees using TreeMix (v.1.12) 25 . For each tree, we performed a round of global rearrangements after adding all populations (-global) and calculated 100 bootstrap replicates to assess the uncertainty of the fitted model (-bootstrap). Sample size correction was disabled.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Raw sequence data (fastq files) and mapped data (bam files) from the 13 newly reported ancient individuals will be available before publication from the European Nucleotide Archive under accession no. PRJEB77188 . A poseidon package of the genotype data analysed in this paper is available on the Poseidon Community Archive ( https://www.poseidon-adna.org/#/archive_explorer ). Owing to ethical prescriptions of this research under UCT human research ethics no. 715/2017, DNA sequencing libraries, both before and after SNP capture, are available for replication upon request to the corresponding authors and the UCT Human Skeletal Repository Committee at [email protected] as aliquots, pending consultation, approval and permission by the UCT Skeletal Repository Committee and consulted San communities who granted the original sample access. Previously published genotype data for ancient and present-day individuals were reported by the Reich Lab in the Allen Ancient DNA Resource v.54.1 ( https://reich.hms.harvard.edu/allen-ancient-dna-resource-aadr-downloadable-genotypes-present-day-and-ancient-dna-data ). Additional previously published genotype data for the present-day San and Khoe samples from ref. 7 are available at the Arrayexpress database ( https://www.ebi.ac.uk/arrayexpress/ ) under accession no. E-MTAB-1259. The Genome Reference Consortium Human Build 37 (GRCh37/hg19) is available via the National Center for Biotechnology Information under accession no. PRJNA31257 . The revised Cambridge reference sequence is available via the National Center for Biotechnology Information under reference sequence NC_012920.1 .
Code availability
All software used in this work is publicly available. List of software and respective versions: AdapterRemoval (v.2.3.1), Burrows–Wheeler Aligner (v.0.7.12), DeDup (v.0.12.2), mapDamage (v.2.0.6), BamUtil (v.1.0.14), EAGER (v.1), Sex.DetERRmine (v.1.1.2) ( https://github.com/TCLamnidis/Sex.DetERRmine ), ANGSD (v.0.915), Schmutzi (v.1.5.4), PMDtools (v.0.50), pileupCaller (v.1.4.0.2), samtools (v.1.3.1), Geneious (R9.8.1), HaploGrep 2 (v.2.4.0), READ ( https://bitbucket.org/tguenther/read ) (v.f541d55), PLINK (v.1.90b3.29), Picard tools (v.2.27.3), smartpca (v.16000; EIGENSOFT v.6.0.1), qp3Pop (v.435; ADMIXTOOLS v.3.0), qpDstat (v.755; ADMIXTOOLS v.3.0), qpWave (v.410), qpAdm (v.810), DATES (v.4010), ADMIXTURE (v.1.3), GLIMPSE ( https://github.com/odelaneau/GLIMPSE ) (v.2.0.0), DyStruct ( https://github.com/tyjo/dystruct ) (v.2.0.0), BEAGLE (v.5.4), RefinedIBD (v.17Jan20.102), FSTruct ( https://github.com/MaikeMorrison/FSTruct ) (d39827e) and TreeMix (v.1.12). Data visualization and descriptive statistical tests were performed in R (v.4.1.1). The following R packages were used: Rsamtools (v.2.12.0), vegan (v.2.6-2), factoextra (v.1.0.7), ggplot2 (v.3.3.6), ggExtra (v.0.10.0), ggforce (v.0.3.3), rnaturalearth (v.0.1.0), sf (v.1.0.-8), raster (v.3.5-21), rgdal (v.1.5-32), spatstat (v.2.3-4), maptools (v.1.1-4), gstat (v.2.0-9), sp (v.1.5-0), labdsv (v.2.0-1), rcarbon (v.1.5.1), magrittr (v.2.0.3), dplyr (v.1.0.9), reshape 2 (v.1.4.4) and tidyverse (v.1.3.2). Y chromosome and mtDNA haplogroups were determined using the ISOGG SNP index (v.15.73) and PhyloTree (v.17-FU1) reference databases, respectively.
Schlebusch, C. M. et al. Southern African ancient genomes estimate modern human divergence to 350,000 to 260,000 years ago. Science 358 , 652–655 (2017).
Article CAS PubMed Google Scholar
Skoglund, P. et al. Reconstructing prehistoric African population structure. Cell 171 , 59–71 (2017).
Article CAS PubMed PubMed Central Google Scholar
Dusseldorp, G., Lombard, M. & Wurz, S. Pleistocene Homo and the updated Stone Age sequence of South Africa. S. Afr. J. Sci. 109 , 7 (2013).
Article Google Scholar
Mounier, A. & Mirazón Lahr, M. Deciphering African late middle Pleistocene hominin diversity and the origin of our species. Nat. Commun. 10 , 3406 (2019).
Article PubMed PubMed Central Google Scholar
Wadley, L. Legacies from the Later Stone Age. Goodwin Ser. 6 , 42–53 (1989).
Sealy, J. in Africa from MIS 6-2. Population Dynamics and Paleoenvironments (eds Jones, S. C. & Stewart, B. A.) 65–75 (Springer Nature, 2016).
Schlebusch, C. M. et al. Genomic variation in seven Khoe-San groups reveals adaptation and complex African history. Science 338 , 374–379 (2012).
Pickrell, J. K. et al. The genetic prehistory of southern Africa. Nat. Commun. 3 , 1143 (2012).
Article PubMed Google Scholar
Goodwin, A. J. H. Archaeology of the Oakhurst Shelter George, Part I: Course of the Excavation. Trans. R. Soc. S. Afr. 25 , 229–245 (1937).
Wadley, L. & Laue, G. Adullam Cave, eastern Free State, South Africa: test excavations at a multiple-occupation Oakhurst Industry site. South Afr. Humanit. 12 , 1–13 (2000).
Google Scholar
Low, M. Continuity, variability and the nature of technological change during the Late Pleistocene at Klipfonteinrand Rockshelter in the Western Cape, South Africa. Afr. Archaeol. Rev. 36 , 67–88 (2019).
Forssman, T. A review of hunter-gatherers in Later Stone Age research in Southern Africa. Goodwin Ser. 12 , 56–68 (2019).
Lombard, M. et al. The South African stone age sequence updated (II). South Afr. Archaeol. Bull. 77 , 172–212 (2022).
Fagan, B. M. The Glentyre Shelter and Oakhurst re-examined. South Afr. Archaeol. Bull. 15 , 80–94 (1960).
Schrire, C. Oakhurst: a re-examination and vindication. South Afr. Archaeol. Bull. 17 , 181–195 (1962).
Drennan, M. R. Archaeology of the Oakhurst Shelter, George. Part III: The Cave-Dwellers. Trans. R. Soc. S. Afr. 25 , 259–280 (1937).
Patrick, M. K. An Archaeological, Anthropological Study of the Human Skeletal Remains from the Oakhurst Rockshelter, George, Cape Province, Southern Africa (Univ. of Cape Town, 1989).
Underhill, P. A. et al. Y chromosome sequence variation and the history of human populations. Nat. Genet. 26 , 358–361 (2000).
Wood, E. T. et al. Contrasting patterns of Y chromosome and mtDNA variation in Africa: evidence for sex-biased demographic processes. Eur. J. Hum. Genet. 13 , 867–876 (2005).
Schlebusch, C. M., de Jongh, M. & Soodyall, H. Different contributions of ancient mitochondrial and Y-chromosomal lineages in ‘Karretjie people’ of the Great Karoo in South Africa. J. Hum. Genet. 56 , 623–630 (2011).
Barbieri, C. et al. Ancient substructure in early mtDNA lineages of southern Africa. Am. J. Hum. Genet. 92 , 285–292 (2013).
Schlebusch, C. M., Lombard, M. & Soodyall, H. MtDNA control region variation affirms diversity and deep sub-structure in populations from southern Africa. BMC Evol. Biol. 13 , 56 (2013).
Rito, T. et al. The first modern human dispersals across Africa. PLoS ONE 8 , e80031 (2013).
Naidoo, T. et al. Y-chromosome variation in Southern African Khoe-San populations based on whole-genome sequences. Genome Biol. Evol. 12 , 1031–1039 (2020).
Pickrell, J. K. & Pritchard, J. K. Inference of population splits and mixtures from genome-wide allele frequency data. PLoS Genet. 8 , e1002967 (2012).
Uren, C. et al. Fine-scale human population structure in Southern Africa reflects ecogeographic boundaries. Genetics 204 , 303–314 (2016).
Montinaro, F. et al. Complex ancient genetic structure and cultural transitions in Southern African populations. Genetics 205 , 303–316 (2017).
Vicente, M. et al. Male-biased migration from East Africa introduced pastoralism into southern Africa. BMC Biol. 19 , 259 (2021).
Pfennig, A., Petersen, L. N., Kachambwa, P. & Lachance, J. Evolutionary genetics and admixture in African populations. Genome Biol. Evol. 15 , evad054 (2023).
Joseph, T. A. & Pe’er, I. Inference of population structure from time-series genotype data. Am. J. Hum. Genet. 105 , 317–333 (2019).
Lipson, M. et al. Ancient West African foragers in the context of African population history. Nature 577 , 665–670 (2020).
Prendergast, M. E. et al. Ancient DNA reveals a multistep spread of the first herders into sub-Saharan Africa. Science 365 , eaaw6275 (2019).
Wang, K. et al. Ancient genomes reveal complex patterns of population movement, interaction, and replacement in sub-Saharan Africa. Sci. Adv. 6 , eaaz0183 (2020).
Lipson, M. et al. Ancient DNA and deep population structure in sub-Saharan African foragers. Nature 603 , 290–296 (2022).
Patterson, N. et al. Ancient admixture in human history. Genetics 192 , 1065–1093 (2012).
Mallick, S. et al. The Simons Genome Diversity Project: 300 genomes from 142 diverse populations. Nature 538 , 201–206 (2016).
Barbieri, C. et al. Unraveling the complex maternal history of Southern African Khoisan populations. Am. J. Phys. Anthropol. 153 , 435–448 (2014).
Reich, D., Thangaraj, K., Patterson, N., Price, A. L. & Singh, L. Reconstructing Indian population history. Nature 461 , 489–494 (2009).
Haak, W. et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature 522 , 207–211 (2015).
Lazaridis, I. et al. Genomic insights into the origin of farming in the ancient Near East. Nature 536 , 419–424 (2016).
Gibbon, V. E. et al. Confronting historical legacies of biological anthropology in South Africa-restitution, redress and community-centered science: the Sutherland Nine. PLoS ONE 18 , e0284785 (2023).
Mathieson, I. et al. The genomic history of southeastern Europe. Nature 555 , 197–203 (2018).
Wang, C.-C. et al. Genomic insights into the formation of human populations in East Asia. Nature 591 , 413–419 (2021).
Posth, C. et al. Reconstructing the deep population history of Central and South America. Cell 175 , 1185–1197 (2018).
van Eeden, G. et al. The recombination landscape of the Khoe-San likely represents the upper limits of recombination divergence in humans. Genome Biol. 23 , 172 (2022).
Choudhury, A. et al. Whole-genome sequencing for an enhanced understanding of genetic variation among South Africans. Nat. Commun. 8 , 2062 (2017).
Choudhury, A., Sengupta, D., Ramsay, M. & Schlebusch, C. Bantu-speaker migration and admixture in southern Africa. Hum. Mol. Genet. 30 , R56–R63 (2021).
Sengupta, D. et al. Genetic substructure and complex demographic history of South African Bantu speakers. Nat. Commun. 12 , 2080 (2021).
Fortes-Lima, C. A. et al. The genetic legacy of the expansion of Bantu-speaking peoples in Africa. Nature 625 , 540–547 (2024).
Patterson, N. et al. Genetic structure of a unique admixed population: implications for medical research. Hum. Mol. Genet. 19 , 411–419 (2010).
Petersen, D. C. et al. Complex patterns of genomic admixture within southern Africa. PLoS Genet. 9 , e1003309 (2013).
Hollfelder, N. et al. Patterns of African and Asian admixture in the Afrikaner population of South Africa. BMC Biol. 18 , 16 (2020).
Huffman, T. N. Handbook to the Iron Age: The Archaeology of Pre-Colonial Farming Societies in Southern Africa (Univ. KwaZulu-Natal Press, 2007).
Coelho, M., Sequeira, F., Luiselli, D., Beleza, S. & Rocha, J. On the edge of Bantu expansions: mtDNA, Y chromosome and lactase persistence genetic variation in southwestern Angola. BMC Evol. Biol. 9 , 80 (2009).
Quintana-Murci, L. et al. Strong maternal Khoisan contribution to the South African coloured population: a case of gender-biased admixture. Am. J. Hum. Genet. 86 , 611–620 (2010).
Barbieri, C., Butthof, A., Bostoen, K. & Pakendorf, B. Genetic perspectives on the origin of clicks in Bantu languages from southwestern Zambia. Eur. J. Hum. Genet. 21 , 430–436 (2012).
Bajić, V. et al. Genetic structure and sex-biased gene flow in the history of southern African populations. Am. J. Phys. Anthropol. 167 , 656–671 (2018).
Goldberg, A., Verdu, P. & Rosenberg, N. A. Autosomal admixture levels are informative about sex bias in admixed populations. Genetics 198 , 1209–1229 (2014).
Goldberg, A. & Rosenberg, N. A. Beyond 2/3 and 1/3: the complex signatures of sex-biased admixture on the X chromosome. Genetics 201 , 263–279 (2015).
Tawha, T., Dinkele, E., Mole, C. & Gibbon, V. E. Assessing zygomatic shape and size for estimating sex and ancestry in a South African sample. Sci. Justice 60 , 284–292 (2020).
Sinclair-Thomson, B. & Challis, S. Runaway slaves, rock art and resistance in the Cape Colony, South Africa. Azania Archaeol. Res. Afr. 55 , 475–491 (2020).
Schlebusch, C. M., Prins, F., Lombard, M., Jakobsson, M. & Soodyall, H. The disappearing San of southeastern Africa and their genetic affinities. Hum. Genet. 135 , 1365–1373 (2016).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19 , 1655–1664 (2009).
Morrison, M. L., Alcala, N. & Rosenberg, N. A. FSTruct: an FST-based tool for measuring ancestry variation in inference of population structure. Mol. Ecol. Resour. 22 , 2614–2626 (2022).
Raghavan, M. et al. Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature 505 , 87–91 (2014).
Allentoft, M. E. et al. Population genomics of Bronze Age Eurasia. Nature 522 , 167–172 (2015).
Jones, E. R. et al. Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nat. Commun. 6 , 8912 (2015).
Fu, Q. et al. The genetic history of Ice Age Europe. Nature 534 , 200–205 (2016).
Villalba-Mouco, V. et al. Survival of Late Pleistocene hunter-gatherer ancestry in the Iberian Peninsula. Curr. Biol. 29 , 1169–1177 (2019).
Posth, C. et al. Palaeogenomics of Upper Palaeolithic to Neolithic European hunter-gatherers. Nature 615 , 117–126 (2023).
Villalba-Mouco, V. et al. A 23,000-year-old southern Iberian individual links human groups that lived in Western Europe before and after the Last Glacial Maximum. Nat. Ecol. Evol. 7 , 597–609 (2023).
PubMed PubMed Central Google Scholar
Siska, V. et al. Genome-wide data from two early Neolithic East Asian individuals dating to 7700 years ago. Sci. Adv. 3 , e1601877 (2017).
Lipson, M. et al. Ancient genomes document multiple waves of migration in Southeast Asian prehistory. Science 361 , 92–95 (2018).
Yang, M. A. et al. Ancient DNA indicates human population shifts and admixture in northern and southern China. Science 369 , 282–288 (2020).
Ning, C. et al. Ancient genomes from northern China suggest links between subsistence changes and human migration. Nat. Commun. 11 , 2700 (2020).
Carlhoff, S. et al. Genome of a middle Holocene hunter-gatherer from Wallacea. Nature 596 , 543–547 (2021).
Wang, K. et al. Middle Holocene Siberian genomes reveal highly connected gene pools throughout North Asia. Curr. Biol. 33 , 423–433 (2023).
Fregel, R. et al. Ancient genomes from North Africa evidence prehistoric migrations to the Maghreb from both the Levant and Europe. Proc. Natl Acad. Sci. USA 115 , 6774–6779 (2018).
van de Loosdrecht, M. et al. Pleistocene North African genomes link Near Eastern and sub-Saharan African human populations. Science 360 , 548–552 (2018).
Simões, L. G. et al. Northwest African Neolithic initiated by migrants from Iberia and Levant. Nature 618 , 550–556 (2023).
Stynder, D. D., Ackermann, R. R. & Sealy, J. C. Craniofacial variation and population continuity during the South African Holocene. Am. J. Phys. Anthropol. 134 , 489–500 (2007).
Irish, J. D., Black, W., Sealy, J. & Ackermann, R. R. Questions of Khoesan continuity: dental affinities among the indigenous Holocene peoples of South Africa. Am. J. Phys. Anthropol. 155 , 33–44 (2014).
Pfeiffer, S. & Harrington, L. Growth, mortality, and small stature. Curr. Anthropol. 52 , 449–461 (2011).
Sealy, J. C. & Van der Merwe, N. J. Social, spatial and chronological patterning in marine food use as determined by delta 13 C measurements of Holocene human skeletons from the south-western Cape, South Africa. World Archaeol. 20 , 87–102 (1988).
Parkington, J. et al. in The Archaeology of Prehistoric Coastlines (eds Bailey, G. & Parkington, J. E.) 22–41 (Cambridge Univ. Press, 1988).
Sealy, J. Diet, mobility, and settlement pattern among Holocene hunter‐gatherers in Southernmost Africa. Curr. Anthropol. 47 , 569–595 (2006).
Jerardino, A. Large shell middens and hunter-gatherer resource intensification along the west coast of South Africa: the Elands Bay case study. J. Isl. Coast. Archaeol. 7 , 76–101 (2012).
Chintalapati, M., Patterson, N. & Moorjani, P. The spatiotemporal patterns of major human admixture events during the European Holocene. eLife 11 , e77625 (2022).
Jerardino, A., Fort, J., Isern, N. & Rondelli, B. Cultural diffusion was the main driving mechanism of the Neolithic transition in southern Africa. PLoS ONE 9 , e113672 (2014).
Sadr, K. Livestock first reached southern Africa in two separate events. PLoS ONE 10 , e0134215 (2015).
Gibbon, V. E. African ancient DNA research requires robust ethics and permission protocols. Nat. Rev. Genet. 21 , 645–647 (2020).
Hogg, A. G. et al. SHCal20 Southern Hemisphere calibration, 0–55,000 years cal BP. Radiocarbon 62 , 759–778 (2020).
Article CAS Google Scholar
Pinhasi, R. et al. Optimal ancient DNA yields from the inner ear part of the human petrous bone. PLoS ONE 10 , e0129102 (2015).
Dabney, J. et al. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc. Natl Acad. Sci. USA 110 , 15758–15763 (2013).
Velsko, I., Skourtanioti, E. & Brandt, G. Ancient DNA Extraction from Skeletal Material. protocols.io www.protocols.io/view/ancient-dna-extraction-from-skeletal-material-baksicwe (2020).
Korlević, P. et al. Reducing microbial and human contamination in DNA extractions from ancient bones and teeth. Biotechniques 59 , 87–93 (2015).
Rohland, N., Glocke, I., Aximu-Petri, A. & Meyer, M. Extraction of highly degraded DNA from ancient bones, teeth and sediments for high-throughput sequencing. Nat. Protoc. 13 , 2447–2461 (2018).
Kircher, M., Sawyer, S. & Meyer, M. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 40 , e3 (2012).
Meyer, M. & Kircher, M. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harb. Protoc. 2010 , db.prot5448 (2010).
Rohland, N., Harney, E., Mallick, S., Nordenfelt, S. & Reich, D. Partial uracil-DNA-glycosylase treatment for screening of ancient DNA. Philos. Trans. R. Soc. Lond. B 370 , 20130624 (2015).
Gansauge, M.-T. & Meyer, M. Single-stranded DNA library preparation for the sequencing of ancient or damaged DNA. Nat. Protoc. 8 , 737–748 (2013).
Peltzer, A. et al. EAGER: efficient ancient genome reconstruction. Genome Biol. 17 , 60 (2016).
Fu, Q. et al. An early modern human from Romania with a recent Neanderthal ancestor. Nature 524 , 216–219 (2015).
Schubert, M., Lindgreen, S. & Orlando, L. AdapterRemoval v2: rapid adapter trimming, identification, and read merging. BMC Res. Notes 9 , 88 (2016).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25 , 1754–1760 (2009).
Jónsson, H., Ginolhac, A., Schubert, M., Johnson, P. L. F. & Orlando, L. mapDamage2.0: fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics 29 , 1682–1684 (2013).
Mittnik, A., Wang, C.-C., Svoboda, J. & Krause, J. A molecular approach to the xexing of the triple burial at the Upper Paleolithic site of Dolní Věstonice. PLoS ONE 11 , e0163019 (2016).
Lamnidis, T. C. et al. Ancient Fennoscandian genomes reveal origin and spread of Siberian ancestry in Europe. Nat. Commun. 9 , 5018 (2018).
Korneliussen, T. S., Albrechtsen, A. & Nielsen, R. ANGSD: analysis of next generation sequencing data. BMC Bioinf. 15 , 356 (2014).
Renaud, G., Slon, V., Duggan, A. T. & Kelso, J. Schmutzi: estimation of contamination and endogenous mitochondrial consensus calling for ancient DNA. Genome Biol. 16 , 224 (2015).
Lazaridis, I. et al. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature 513 , 409–413 (2014).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25 , 2078–2079 (2009).
Kearse, M. et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28 , 1647–1649 (2012).
Weissensteiner, H. et al. HaploGrep 2: mitochondrial haplogroup classification in the era of high-throughput sequencing. Nucleic Acids Res. 44 , W58–W63 (2016).
Rubinacci, S., Ribeiro, D. M., Hofmeister, R. J. & Delaneau, O. Efficient phasing and imputation of low-coverage sequencing data using large reference panels. Nat. Genet. 53 , 120–126 (2021).
Browning, S. R. & Browning, B. L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 81 , 1084–1097 (2007).
Browning, B. L., Tian, X., Zhou, Y. & Browning, S. R. Fast two-stage phasing of large-scale sequence data. Am. J. Hum. Genet. 108 , 1880–1890 (2021).
Morez, A. et al. Imputed genomes and haplotype-based analyses of the Picts of early medieval Scotland reveal fine-scale relatedness between Iron Age, early medieval and the modern people of the UK. PLoS Genet. 19 , e1010360 (2023).
Browning, B. L. & Browning, S. R. Improving the accuracy and efficiency of identity-by-descent detection in population data. Genetics 194 , 459–471 (2013).
Margaryan, A. et al. Population genomics of the Viking world. Nature 585 , 390–396 (2020).
Kennett, D. J. et al. Archaeogenomic evidence reveals prehistoric matrilineal dynasty. Nat. Commun. 8 , 14115 (2017).
Wang, K. et al. High-coverage genome of the Tyrolean Iceman reveals unusually high Anatolian farmer ancestry. Cell Genomics 3 , 100377 (2023).
Mallick, S. et al. The Allen Ancient DNA Resource (AADR) a curated compendium of ancient human genomes. Sci. Data 11 , 182 (2024).
Skoglund, P. et al. Genetic evidence for two founding populations of the Americas. Nature 525 , 104–108 (2015).
Patterson, N., Price, A. L. & Reich, D. Population structure and eigenanalysis. PLoS Genet. 2 , e190 (2006).
Prüfer, K. et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 505 , 43–49 (2014).
Meyer, M. et al. A high-coverage genome sequence from an archaic Denisovan individual. Science 338 , 222–226 (2012).
Reich, D. et al. Reconstructing Native American population history. Nature 488 , 370–374 (2012).
Mathieson, I. et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature 528 , 499–503 (2015).
Moorjani, P. et al. A genetic method for dating ancient genomes provides a direct estimate of human generation interval in the last 45,000 years. Proc. Natl Acad. Sci. USA 113 , 5652–5657 (2016).
Wang, R. J., Al-Saffar, S. I., Rogers, J. & Hahn, M. W. Human generation times across the past 250,000 years. Sci. Adv. 9 , eabm7047 (2023).
Sudmant, P. H. et al. An integrated map of structural variation in 2,504 human genomes. Nature 526 , 75–81 (2015).
1000 Genomes Project Consortiumet al. A global reference for human genetic variation. Nature 526 , 68–74 (2015).
Flegontov, P. et al. Modeling of African population history using f -statistics is biased when applying all previously proposed SNP ascertainment schemes. PLoS Genet. 19 , e1010931 (2023).
Download references
Acknowledgements
We acknowledge and thank the descendants of Oakhurst and all those who have contributed to the global aDNA data. A special note of thanks to the individuals whose remains rest in the repositories assessed, without whom this research would not be possible. We thank the consulted communities, UCT human research ethics committee and Heritage Western Cape for permitting this research. We thank the curators and staff of the UCT human skeletal repository. V.E.G. acknowledges financial support for this research by the South African National Research Foundation (grant nos. 115357 and 120816) and J.C.S. by the South African Research Chairs Initiative (grant no. 84407). Opinions expressed and conclusions arrived at are those of the authors and not necessarily to be attributed to the South African National Research Foundation. We thank T. C. Lamnidis for his help in uploading the aDNA data to the European Nucleotide Archive.
Open access funding provided by Max Planck Society.
Author information
These authors contributed equally: Johannes Krause, Stephan Schiffels.
Authors and Affiliations
Max Planck Institute for Evolutionary Anthropology, Department of Archaeogenetics, Leipzig, Germany
Joscha Gretzinger, Sandra E. Penske, Adam B. Rohrlach, Johannes Krause & Stephan Schiffels
Division of Clinical Anatomy and Biological Anthropology, Department of Human Biology, University of Cape Town, Cape Town, South Africa
Victoria E. Gibbon
Department of Archaeology, University of Cape Town, Cape Town, South Africa
Judith C. Sealy
School of Computer and Mathematical Sciences, University of Adelaide, Adelaide, South Australia, Australia
Adam B. Rohrlach
Department of Geological Sciences, University of Cape Town, Cape Town, South Africa
Domingo C. Salazar-García
Departament de Prehistòria, Arqueologia i Història Antiga, Universitat de València, València, Spain
You can also search for this author in PubMed Google Scholar
Contributions
V.E.G., J.C.S., D.C.S.-G., S.S. and J.K. conceived the study. V.E.G., J.C.S. and D.C.S.-G. provided archaeological samples and context. S.E.P. performed laboratory analyses. J.G., S.E.P. and A.B.R. analysed data. V.E.G., J.C.S., S.S. and J.K. supervised the project. J.G., V.E.G., J.C.S. and S.S. wrote the paper with input from all coauthors.
Corresponding authors
Correspondence to Victoria E. Gibbon or Stephan Schiffels .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Ecology & Evolution thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 genetic affinities between ancient and present-day southern africans..
a ) Maximum likelihood tree, generated using TreeMix (see Methods ) of genome sequences from present-day and ancient populations, excluding populations with evidence of asymmetrical allele sharing with non-Africans indicative of recent gene flow. Branches of ancient individuals/groups are truncated for better readability. b ) Alternative PCA computed using 22 present-day populations from southern Africa and 597,000 SNPs. This PCA drops two populations from the 24 shown in Fig. 1b , for the benefit of using a larger number of SNPs. The arrows indicate the three distinct clines of genetic variation between Bantu groups and Northern or Southern San, respectively. Khoekhoe populations cluster in between the three poles.
Extended Data Fig. 2 Sample age-informed ancestry decomposition.
a ) Clustering of 162 ancient and present-day sub-Saharan African individuals (~212,000 SNPs) at K = 6 using DYSTRUCT. Major groups are indicated on top of the bar plots based on chronological, geographical and linguistic classifications. b ) Kriging interpolated distributions of southern (orange component), central (magenta component) and northern (blue component) San ancestry in present-day populations. Proportions for ancient LSA individuals are indicated as dots. White outlines indicate the present-day distributions of the Tuu (left and centre) and Kx’a (right) language groups.
Extended Data Fig. 3 Genetic affinities of Oakhurst individuals to present-day southern Africans in F-statistics.
a) Scatter plot of group-based jackknife point estimates from F ST (Y axis) and outgroup F 3 -statistics of the form F 3 (Archaic; Oakhurst, X), where X represents the present-day populations ( n = 32). Error bars represent 2 standard errors. The error band indicates the 95% confidence interval of the linear regression. Symbols and colours correspond to Fig. 1 . Data can be found in Supplementary Table 2 & 3 . b ) F ST distances visualized on a map of southern Africa. The populations exhibiting the highest genetic affinity to Oakhurst are highlighted. The location of Oakhurst is plotted in black. The beige-coloured region is the Kalahari semi-desert. Data can be found in Supplementary Table 3 .
Extended Data Fig. 4 Identity-by-descent segment sharing between imputed Oakhurst genomes and present-day sub-Saharan Africans.
a) Length of IBD segments shared between OAK007 and present-day Africans. The bounds of the box represent the 25 th and 75 th percentile, the centre represents the median, and Whiskers represent the smallest value greater than the 25 th Percentile minus 1.5 times the interquartile range and largest value less than the 75 th Percentile plus 1.5 times the interquartile range of the data, respectively. Outliers present the minimum and maximum values in the data and are depicted as large dots. Each dot corresponds to the summed IBD for a unique pair of OAK007 and an individual from the respective population ( n = 31). The boxplots are ordered according to the averaged length of IBD segments shared between all individuals of a population and OAK007 (including pairs with no IBD sharing). Populations on the left feature the longest mean IBD segment sharing with OAK007, populations on the right the shortest. Lengths of segments are given in centiMorgan (cM). Data can be found in Supplementary Table 24 . b ) Median IBD sharing between OAK007 and present-day populations visualized on a map of southern Africa. The colour of each dot corresponds to the median length of summed IBD segments shared with OAK007 per individual, the size corresponds to the median number of IBD segments shared per individual.
Extended Data Fig. 5 Population structure in ancient Africa.
a) MDS plot of the pairwise F 3 -matrix of the form F 3 (Chimp; X, Y) (shown in Fig. 2 ) transformed into distances using the formula 1 − F 3 . Data can be found in Supplementary Table 4 . b ) Individual F 4 -statistics of the form F 4 (Archaic, Test; Ju_hoan_North, Cameroon_SMA) through time for 21 ancient and 2 current-day Khomani genomes from South Africa. Error bars represent 2 standard errors. Data can be found in Supplementary Table 5 .
Extended Data Fig. 6 Genetic diversity in ancient and present-day sub-Saharan Africans.
a ) Conditional nucleotide diversity (measured as the intra-population pairwise mismatch rate) in ancient pseudo-haploid populations ( n = 10). Each dot depicts the pairwise mismatch rate between a pair of individuals from the same group. The bounds of the box represent the 25th and 75th percentile, the centre represents the median, and Whiskers represent the smallest value greater than the 25th Percentile minus 1.5 times the interquartile range and largest value less than the 75th Percentile plus 1.5 times the interquartile range, respectively. Outliers present the minimum and maximum values in the data and are depicted as large dots. The geographical origin of each ancient group is indicated on the world map. b ) Average heterozygosity in 5 imputed ancient genomes from South Africa and Botswana and 561 individuals from 49 present-day African populations. Each dot corresponds to the fraction of heterozygous sites over the total number of genotyped sites across 22 autosomes. The bounds of the box represent the 25th and 75th percentile, the centre represents the median, and Whiskers represent the smallest value greater than the 25th Percentile minus 1.5 times the interquartile range and largest value less than the 75th Percentile plus 1.5 times the interquartile range, respectively. Outliers present the minimum and maximum values in the data and are depicted as large dots. Data can be found in Supplementary Table 25 .
Extended Data Fig. 7 Ancestry composition of present-day San, Khoe and Bantu populations.
a ) Scatter plot of the proportions of SA_LSA ancestry measured by Skoglund et al. 2017 (represented by the St. Helena and Faraoskop genomes) and this study (represented by the Oakhurst, St. Helena, Faraoskop and Ballito Bay genomes) in present-day San, Khoe and Bantu-spreaking groups ( n = 19) using qpAdm. The error band indicates the 95% confidence interval of the linear regression. Symbols and colours correspond to Fig. 1 . b ) Fractions of SA_LSA ancestry in San, Khoe and Bantu groups stratified by language affiliation. The bounds of the box represent the 25th and 75th percentile, the centre represents the median, and Whiskers represent the smallest value greater than the 25th Percentile minus 1.5 times the interquartile range and largest value less than the 75th Percentile plus 1.5 times the interquartile range, respectively. c ) Ancestry compositions retrieved from 2- and 3-way qpAdm admixture modeling of present-day San, Khoe and Bantu groups ( n = 23) visualized on a map of southern Africa. Mean admixture dates from DATES analysis are indicated for selected populations. On the left for populations admixed between SA_LSA and Tanzania_Luxmanda_3000BP and on the right for populations admixed between SA_LSA and Mende. Arrows on maps indicate a general direction of influences rather than discrete routes of migration. Data can be found in Supplementary Table 10 - 12 .
Extended Data Fig. 8 Admixture dates in ancient and present-day San, Khoe and Bantu populations.
a ) Admixture date point estimates for the admixture (squares) between SA_LSA and Mende in groups with primarily West African-related admixture (based on qpAdm) ( n = 9). For ancient individuals, the mean radiocarbon date is indicated (circles). Error bars represent 1 standard error. b ) Admixture date point estimates for the admixture (squares) between SA_LSA and Tanzania_Luxmanda_3000BP in groups with primarily East African-related admixture (based on qpAdm) ( n = 14). Error bars represent 1 standard error. c ) Admixture date point estimates for the admixture (squares) between SA_LSA and English in groups with European-related ancestry ( n = 5). Error bars represent 1 standard error. d ) Schematic representation of the geographic origin and potential admixture date of non-Southern African ancestry found in present-day San and Khoe populations. Data can be found in Supplementary Table 13 - 16 .
Extended Data Fig. 9 Variability in admixture proportions among ancient and present-day San, Khoe, Bantu and Coloured populations.
a ) Pie charts depict the averaged ancestry composition for each group derived from supervised ADMIXTURE modeling at K = 7. Box- and violin plots depict the Bootstrap ( n = 1000) distributions of the ancestry variability measure, F ST /F ST max , obtained from FStruct ( methods ) calculated on the Q matrix from supervised ADMIXTURE for each population. The bounds of the box represent the 25th and 75th percentile, the centre represents the median, and Whiskers represent the smallest value greater than the 25th Percentile minus 1.5 times the interquartile range and largest value less than the 75th Percentile plus 1.5 times the interquartile range, respectively. b ) Scatter plot of the proportions of SA_LSA ancestry measured using qpAdm and the San proportion measured using supervised ADMIXTURE in present-day San, Khoe and Bantu-spreaking groups. Both estimates are significantly correlated (Pearson's correlation; t = 49.826, df = 20, p = 2.2e-16, cor = 0.996). The error band indicates the 95% confidence interval of the linear regression. Symbols and colours correspond to Fig. 1 . Data can be found in Supplementary Table 21 & 22 .
Supplementary information
Reporting summary, supplementary tables.
Supplementary Tables 1–27.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Gretzinger, J., Gibbon, V.E., Penske, S.E. et al. 9,000 years of genetic continuity in southernmost Africa demonstrated at Oakhurst rockshelter. Nat Ecol Evol (2024). https://doi.org/10.1038/s41559-024-02532-3
Download citation
Received : 31 January 2024
Accepted : 02 August 2024
Published : 19 September 2024
DOI : https://doi.org/10.1038/s41559-024-02532-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
A theory is an idea about how something in nature works that has gone through rigorous testing through observations and experiments designed to prove the idea right or wrong. When it comes to the evolution of life, various philosophers and scientists, including an eighteenth-century English doctor named Erasmus Darwin, proposed different ...
evolution, theory in biology postulating that the various types of plants, animals, and other living things on Earth have their origin in other preexisting types and that the distinguishable differences are due to modifications in successive generations. The theory of evolution is one of the fundamental keystones of modern biological theory.
evolution, Biological theory that animals and plants have their origin in other preexisting types and that the distinguishable differences are due to modifications in successive generations.It is one of the keystones of modern biological theory. In 1858 Charles Darwin and Alfred Russel Wallace jointly published a paper on evolution. The next year Darwin presented his major treatise On the ...
Evolutionary theory is the area that focuses on further development and refinement of the modern synthesis of evolution and genetics. Notable topics include the appropriate level of selection, the ...
This entry offers a broad historical review of the origin and development of Darwin's theory of evolution by natural selection through the initial Darwinian phase of the "Darwinian Revolution" up to the publication of the Descent of Man in 1871. The development of evolutionary ideas before Darwin's work has been treated in the separate entry evolutionary thought before Darwin.
Evolution articles from across Nature Portfolio. Evolution is the process of heritable change in populations of organisms over multiple generations. Evolutionary biology is the study of this ...
Evolution - Natural Selection, Adaptation, Genetics: The central argument of Darwin's theory of evolution starts with the existence of hereditary variation. Experience with animal and plant breeding had demonstrated to Darwin that variations can be developed that are "useful to man." So, he reasoned, variations must occur in nature that are favourable or useful in some way to the ...
Yes, urgently. Without an extended evolutionary framework, the theory neglects key processes, say Kevin Laland and colleagues. Charles Darwin conceived of evolution by natural selection without ...
Appendix A: About the Survey. The bulk of the analysis in this report stems from a Pew Research Center survey conducted by telephone with a national sample of adults (18 years of age or older) living in all 50 U.S. states and the District of Columbia. The results are based on 2,002 interviews (801 respondents were interviewed on a landline […]
The theory of evolution by natural selection is attributed to 19th century British naturalist Charles Darwin. The theory is widely accepted based on fossil records, DNA sequencing, embryology, comparative anatomy and molecular biology. Darwin's finches are examples of evolutionary adaptation.
Introduce students to Charles Darwin's research, Gregor Mendel's work with pea plants, and James Watson and Francis Crick's determination of the structure of . DNA.Darwin Challenged. Have students work in pairs to research the scientists and ideas that were used to challenge Darwin's theory about the role of . natural selection. Ask the ...
The Evidence for Evolution. Alan R. Rogers. University of Chicago Press, 2011. 128 pp., illus. $18.00 (ISBN 9780226723822 paper). Although scientists view evolution as an indisputable feature of the natural world, most Americans simply do not believe that it occurs, or they reject naturalistic explanations for biotic change.Empirical studies have revealed that students and teachers often know ...
Evolution is the change in the heritable characteristics of biological populations over successive generations. [1] [2] It occurs when evolutionary processes such as natural selection and genetic drift act on genetic variation, resulting in certain characteristics becoming more or less common within a population over successive generations. [3]The process of evolution has given rise to ...
Darwin and His Theory of Evolution. At first glance, Charles Darwin seems an unlikely revolutionary. Growing up a shy and unassuming member of a wealthy British family, he appeared, at least to his father, to be idle and directionless. But even as a child, Darwin expressed an interest in nature. Later, while studying botany at Cambridge ...
Biology - Evolution, Natural Selection, Adaptation: As knowledge of plant and animal forms accumulated during the 16th, 17th, and 18th centuries, a few biologists began to speculate about the ancestry of those organisms, though the prevailing view was that promulgated by Linnaeus—namely, the immutability of the species. Among the early speculations voiced during the 18th century, the British ...
In November, María Martinón-Torres from CENIEH (National Research Center on Human Evolution) in Spain, Nicole Boivin and Michael Petraglia from the Max Planck Institute for the Science of Human ...
The incorporation of genetics into Darwin's theory is known as "modern evolutionary synthesis." The physical and behavioral changes that make natural selection possible happen at the level of DNA ...
To mark the occasion, here are six facts about the public's views on evolution, as well as other aspects of the debate in the U.S. and elsewhere: Roughly eight-in-ten U.S. adults (81%) say humans have evolved over time, according to data from a new Pew Research Center study. This includes one-third of all Americans (33%) who say that humans ...
e. Many scientists and philosophers of science have described evolution as fact and theory, a phrase which was used as the title of an article by paleontologist Stephen Jay Gould in 1981. He describes fact in science as meaning data, not known with absolute certainty but "confirmed to such a degree that it would be perverse to withhold ...
Abstract. Evolution is both a fact and a theory. Evolution is widely observable in laboratory and natural populations as they change over time. The fact that we need annual flu vaccines is one example of observable evolution. At the same time, evolutionary theory explains more than observations, as the succession on the fossil record.
The great French naturalist Jean-Baptiste de Monet, chevalier de Lamarck, held the enlightened view of his age that living organisms represent a progression, with humans as the highest form. From this idea he proposed, in the early years of the 19th century, the first broad theory of evolution. Organisms evolve through eons of time from lower to higher forms, a process still going on, always ...
Charles Darwin's theory of evolution by natural selection is the foundation upon which modern evolutionary theory is built. The theory was outlined in Darwin's seminal work On the Origin of Species, published in 1859.Although Victorian England (and the rest of the world) was slow to embrace natural selection as the mechanism that drives evolution, the concept of evolution itself gained ...
Here are five facts about evolution and faith: The Roman Catholic Church has long accepted - or at least not objected to - evolutionary theory. Pope Francis is not the first pontiff to publicly affirm that evolution is compatible with church teachings. In 1950, in the encyclical " Humani Generis," Pope Pius XII said that Catholic ...
The theory that COVID-19 originated from a human interacting with an infected animal at a local market in late 2019 is backed by "very strong evidence." In January of 2020, 800 samples of stalls ...
V.E.G. acknowledges financial support for this research by the South African National Research Foundation (grant nos. 115357 and 120816) and J.C.S. by the South African Research Chairs Initiative ...