- main menu Back One Level
- Discoveries
- The Philadelphia Chromosome
- History and Implications for the Future
- Related Information
- Nobel in Chemistry: Irwin A. Rose, PhD
- Nobel in Physiology or Medicine: Baruch S. Blumberg, MD, PhD
- The Two-Hit Theory: Alfred Knudson

Knudson's "Two-Hit" Theory of Cancer Causation
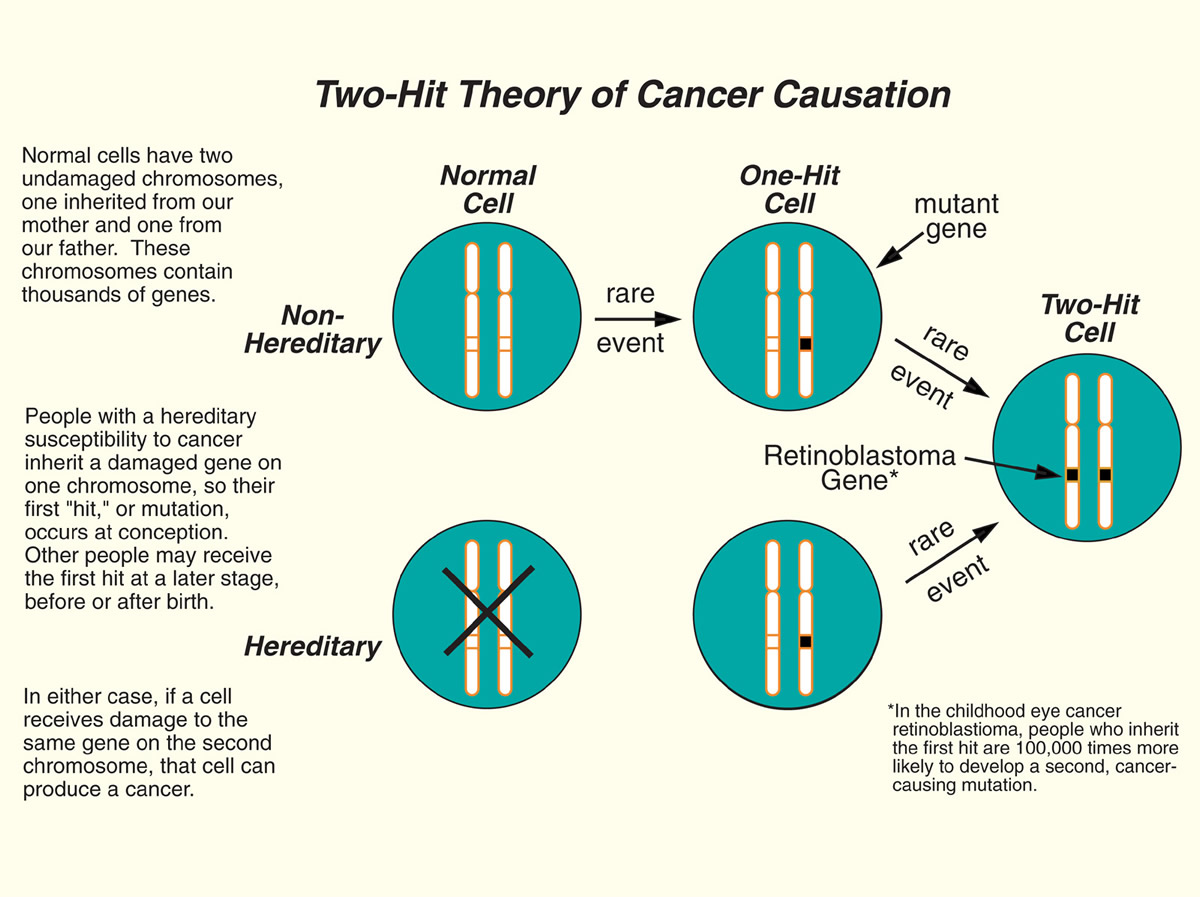
The "two-hit" hypothesis provided a unifying model for understanding cancer that occurs in individuals who carry a "susceptibility gene" and cancers that develop because of randomly induced mutations in otherwise normal genes. Tumor-suppressor genes, in particular, are important targets for cancer prevention research, since they normally function to apply the brakes to cellular growth.
Like many significant conceptual leaps in science, Knudson's "two-hit" hypothesis was met with skepticism when he first published it in 1971, yet Knudson's powerful insights into the development of cancer hold implications for both cancer treatment and prevention.
Defects in tumor-suppressor genes permit abnormal, cancerous growth, so devising ways to remedy such flaws or replace the gene's missing product through medication are of interest to researchers.
Alfred G. Knudson Jr., MD, PhD
A geneticist and physician, Dr. Knudson (August 9, 1922 – July 10, 2016) was internationally recognized for his "two-hit" theory of cancer causation, which explained the relationship between the hereditary and non-hereditary forms of a cancer and predicted the existence of tumor-suppressor genes that can suppress cancer cell growth. This now-confirmed theory has advanced understanding of errors in the genetic program that turn normal cells into cancer cells.
Distinguished Awards
Among Knudson's many professional distinctions, he received the 2004 Kyoto Prize , considered among the world's leading awards for lifetime achievement. He also earned the 1998 Albert Lasker Award for Clinical Medical Research , one of seven Lasker Awards presented that year. Considered "America's Nobels," Lasker Awards rank among the highest recognition for careers of distinguished work because of the extremely rigorous process of nomination and selection conducted by a jury of the world's top scientists.
In 1999, Knudson received the Distinguished Career Award of the American Society of Hematology/Oncology and the international John Scott Award from the City of Philadelphia. In 2000, the American Academy of Dermatology honored him with its Lila Gruber Memorial Cancer Research Award for researchers whose lifetime contributions have been outstanding in importance and distinction.
When Dr. Knudson became an AACR honorary member in 2011 he reviewed his distinguished career, and his most significant accomplishments that laid the groundwork for the tumor suppressor concept, in an interview with AACR News.
In September 2002, Knudson received Fox Chase Cancer Center's 14th annual Wick R. Williams Memorial Award. The American Society of Clinical Oncology also honored him with its 2002 Special Award in the form of a Pediatric Oncology Lectureship recognizing individuals who are accomplished in pediatric oncology.
In addition, Knudson has received the 1988 Charles S. Mott Prize of the General Motors Cancer Research Foundation; the American Cancer Society's 1989 Medal of Honor; the 1990 Founders' Award of the Chemical Industry Institute for Toxicology; the American Radium Society's 1990 Janeway Medal; Memorial Sloan-Kettering Cancer Center's 1990 Katharine Berkan Judd Award; the 1991 William Allan Memorial Award of the American Society of Human Genetics; M. D. Anderson Cancer Center's 1995 Bertner Award; Switzerland's 1995 Charles Rodolphe Brupbacher Foundation Award; the 1996 Robert J. and Claire Pasarow Foundation Award; the 1996 Durham City of Medicine Award; Canada's 1997 Gairdner Foundation International Award; and the American Society of Clinical Oncology's 1997 Karnofsky Memorial Lecture Award.
Knudson's Background and Education
Born in Los Angeles in 1922, Knudson received his BS from California Institute of Technology in 1944, his MD from Columbia University in 1947 and his PhD from California Institute of Technology in 1956. He held a Guggenheim fellowship from 1953 to 1954.
Knudson came to Fox Chase from the University of Texas Graduate School of Biomedical Sciences, where he was dean, and the M.D. Anderson Hospital and Tumor Institute in Houston, Texas, where he specialized in pediatrics and biology. Previously, he was associate dean for basic sciences at the State University of New York at Stony Brook from 1966 to 1969. He began his affiliation with Fox Chase in 1970 as a member of its scientific advisory committee before joining the Center staff in 1976.
Knudson and his wife, Anna T. Meadows, MD, a pediatric oncologist at Children's Hospital of Philadelphia, collaborated on the study of the genetics of childhood cancer.
He died July 10, 2016.
Career Highlights
Dr. Knudson became an honorary member of the American Association for Cancer Research (AACR) in 2011, and an inaugural Fellow of the AACR Academy in March 2013.
In honor of his contributions to biomedical science, Knudson has been elected to the National Academy of Sciences and was named a Fox Chase Distinguished Scientist and senior advisor to the president in 1992. He was instrumental as a leader of Fox Chase's molecular oncology program from 1989 to 1999. Previously, Knudson served as director of Fox Chase's Institute for Cancer Research from 1976 until 1982, Center president from 1980 to 1982 and scientific director from 1982 to 1983.
In 1995, Knudson was appointed as special advisor to Dr. Richard Klausner, then director of the National Cancer Institute. While continuing his work at Fox Chase, Knudson also worked closely with Dr. Joseph Fraumeni in NCI's Division of Cancer Epidemiology and Genetics. Knudson served as acting director of its human genetics program until September 1999, when he returned to Fox Chase full-time.
- Share with Facebook facebook-icon
- Share with twitter twitter-icon
- Share with email email-icon
- Print this print-icon
Related Articles

Remembering Alfred G. Knudson

Dr. Knudson and the Two-Hit Theory

Beatrice Mintz, PhD
American Association for Cancer Research to Inaugurate Two Fox Chase Scientists into the First Class of the Fellows of the AACR
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

The Two-Hit Hypothesis Meets Epigenetics
Jean-pierre issa.
Coriell Institute for Medical Research, Camden, New Jersey.
The landmark paper by Kane and colleagues was the first report of DNA methylation in the promoter of the human MLH1 gene in sporadic colon cancers with mismatch repair (MMR) deficiency. In both cell lines and primary tumors, promoter methylation was associated with loss of MLH1 protein expression and with a lack of mutations in the MLH1 coding region. Together with subsequent papers that showed that this methylation was directly responsible for loss of MLH1 expression and MMR deficiency, the observation expanded the two-hit hypothesis of tumor suppressor gene loss in cancer to include both genetic and epigenetic mechanisms of gene inactivation. More broadly, the paper contributed to normalization of the hypothesis of an epigenetic basis for cancer development.
Cancer: Genetic or Epigenetic Disease?
The idea of cancer as a disease of epigenetic regulation was proposed in the 1960’s based on unexpected reversal of the cancer phenotype when malignant cells undergo epigenetic reprogramming though embryogenesis ( 1 ). Epigenetic mechanisms were still mysterious at the time, and in the absence of a molecular explanation, the concept was rapidly forgotten. By the 1970’s, studies of familial cancer incidence had firmly established a genetic basis for inherited cancer, and it was hypothesized that sporadic cancers would follow the same path. Knudson formulated the two-hit hypothesis to explain familial versus sporadic cancer epidemiology, proposing that the same genes are involved in both cases ( 2 ). This was confirmed molecularly with studies on familial cancer genes such as TP53 ( 3 ). The dogma evolved to a model whereby the two hits are mutations or deletions in the same tumor suppressor gene (TSG; Fig. 1 ); familial cases would be explained by an inherited hit followed by a sporadic hit, while nonfamilial cases are caused by two rare sporadic hits, explaining the delayed age of onset when compared with familial cancers ( 2 ).

The two-hit hypothesis meets epigenetics. By the 1990’s, Knudson’s two-hit hypothesis had evolved to postulate that familial cancer predisposition is due to a germline mutation in a TSG (top left), while actual cancer development follows a sporadic mutation (or deletion) in the second allele (top right). It also postulated that sporadic cancers of the same tissue type were due to two acquired hits (mutation or deletion) in the same TSG (bottom). The paper by Kane and colleagues (and other papers in that period) revised that model for sporadic cancers to include epigenetic inactivation by promoter DNA hypermethylation as either the first hit, the second hit, or both (bottom right). The figure is a simplification; subsequent data showed that in very rare cases familial cancers can be caused by germline hypermethylation, that the second hit in familial cancers can also be DNA hypermethylation, and, in the case of MLH1 in colon cancer, the “hits” in sporadic cases are almost always hypermethylation caused by the CpG island methylator phenotype.
Inherited mutations in MLH1 cause the Lynch syndrome, a familiar colon cancer predisposition disease ( 3 ). Biallelic inactivation of MLH1 causes mismatch repair (MMR) deficiency, accumulation of mutations, and eventual cancer formation. The phenotype of MMR deficiency was known before the genotype was elucidated (it was termed microsatellite instability or MSI at the time), and it was clear early on that MMR defects were present in both familial and sporadic colon cancers ( 4 ). In accordance with Knudson’s hypotheses, it was expected that the genes that cause familial versus sporadic MMR-deficient cancers would be the same. Kane and colleagues tried to confirm this ( 4 ). Unexpectedly, while they found that sporadic MSI+ cases had loss of MLH1 protein, they could find no coding sequence mutations in this gene, challenging the prevailing notion at the time.
In the 1980’s, the unorthodox idea of an epigenetic basis for cancer resurfaced, powered by an actual proposed mechanism—permanent silencing of TSG expression by promoter DNA methylation ( 5 ). As besets many “out-of-the-box” hypotheses, this one was met with considerable early skepticism. Nevertheless, after Kane and colleagues observed unexplained loss of MLH1 expression in colon cancers, they went on to show that those sporadic cell lines and primary tumors that lacked MLH1 expression also had dense DNA methylation in a CpG rich area in the gene promoter ( 4 ). This observation was therefore consistent with Knudson’s tenets, but with a twist—the gene in sporadic and familial cancers was the same ( MLH1 ) but the mechanism of inactivation was different (DNA methylation vs. mutations; Fig. 1 ). The fact that MLH1 was a bona fide cancer predisposition gene (a “gatekeeper” in Vogelstein’s model; ref. 3 ) inactivated by DNA methylation was a turning point in the wide acceptance of the epigenetic basis of cancer hypothesis. It was further buttressed by the demonstration that the MMR phenotype can be reversed by removal of DNA methylation ( 6 ) and also by the identification of other TSGs with similar genetic/epigenetic mechanisms of inactivation ( 5 ). As can be seen in cancer biology textbooks 25 years later, the paper by Kane and colleagues contributed to rewriting the chapters on the mechanistic basis of cancer development—cancer is now seen as a genetic and an epigenetic disease.
The Two-Hit Hypothesis Revised
An important consequence of the paper by Kane and colleagues (and other papers at the time) was the modification of the two-hit hypothesis to include both genetic and epigenetic mechanisms of gene inactivation ( Fig. 1 ). Because epigenetic changes are reset during embryogenesis, it is not surprising that, with rare (and fascinating) exceptions, all familial cancers are caused by genetic changes. For reasons not yet fully understood, some TSGs are resistant to epigenetic inactivation. In those cases, the second hit in familial cancers as well as both hits in sporadic cancers are also genetic. For the other TSGs (such as MLH1 , VHL , CDKN2A , BRCA1 , and others), the second hit in familial cases can be either genetic or epigenetic, and both hits in sporadic cases can similarly be either genetic or epigenetic. Lynch syndrome provides an interesting study in this genetic/epigenetic duality. The MSH2 gene is a frequent cause of familial disease but is almost never epigenetically inactivated, and it is very rarely responsible for sporadic colon cancers. By contrast, the MLH1 gene is also a frequent cause of familial disease, but it is susceptible to epigenetic inactivation and is almost always responsible for MMR-deficient sporadic colon cancers.
Cycles of Instability
In addition to revising the two-hit hypothesis, the paper by Kane and colleagues suggested that epigenetic changes can trigger genetic changes by inducing DNA repair defects. In an interesting additional twist, MLH1 methylation in sporadic cancers was subsequently shown to be caused by a broader epigenetic deregulation, the CpG island methylator phenotype (CIMP; ref. 7 ). CIMP+ cancers inactivate multiple TSGs simultaneously, and this phenotype of epigenetic instability has been described in almost all cancer cell types ( 7 ). Intriguingly, MLH1 is a common CIMP target in colon and endometrial cancers, but not in other CIMP+ cases. In acute myelogenous leukemia (AML) and in glioblastoma multiforme (GBM), CIMP is caused by genetic defects in the TCA cycle, namely mutations in IDH1 or IDH2 ( 8 ). These mutations lead to accumulation of metabolites that inactivate the TET enzymes, which are responsible for protection against DNA methylation in CpG islands ( 8 ). Thus, mutations in metabolic regulation genes can lead to epigenetic instability. In turn, epigenetic instability can lead to genetic instability by inducing DNA repair defects ( 7 ). As one can imagine, these cycles of instability can be repeated many times in the lifetime of a cancer, contributing to progression, metastasis, and therapeutic resistance.
Unlike AML and GBM, mutations in TCA cycle genes have not been commonly seen in CIMP+ colon cancers ( 9 ) or gastric cancers. Even in AML, CIMP can only be explained by mutations in about half the cases. The etiology of CIMP in mutation-negative cases remains speculative. There is a strong association between CIMP and Epstein-Barr virus in gastric cancers ( 7 ), and an intriguing link between CIMP and fusobacterium in colon cancers ( 9 ), suggesting an environmental etiology to CIMP in some cases. It is possible for example that infections lead to metabolic deregulation, disrupting TET function and leading to DNA hypermethylation.
Clinical Implications
The diagnosis of a familial cancer syndrome can save lives through early screening and intervention. Lynch syndrome has an incomplete penetrance and can first manifest at an advanced age. While colon cancers are routinely screened for MMR defects, a positive result does not always trigger an investigation of germline defects because the frequency of sporadic MMR-deficient cancers is higher than that of familial MMR-deficient cancers after the age of 60. The paper by Kane and colleagues (and subsequent confirmation) suggests that MLH1 methylation can serve as a quick additional screen in this respect. MMR-deficient colon cancers that lack MLH1 expression but have no MLH1 promoter DNA methylation should trigger a very high index of suspicion for Lynch syndrome.
Finally, it is worth noting that epigenetic changes mediated by DNA methylation are potentially reversible through the use of drugs that target DNA methyltransferases ( 10 ). The paper by Kane and colleagues ( 4 ) and other papers showing the importance of DNA methylation in TSG regulation in cancer ( 5 ) triggered a revival in interest in these drugs, eventually leading to their FDA approval and broad usage in patients with myeloid malignancies ( 10 ). While their activity in solid tumors remains limited, there continues to be interest in developing DNA methylation–based treatment or prevention strategies that could be especially useful in cases with CIMP and MLH1 promoter DNA methylation.
Conclusions
The paper by Kane and colleagues had a straightforward message: MLH1 expression was missing in MMR-deficient sporadic colon cancers and this was associated with promoter DNA methylation rather than coding mutations in the gene ( 4 ). This deceptively simple message had a profound influence in that it lent legitimacy to the hypothesis of DNA methylation–mediated TSG inactivation in cancer. It helped rewrite textbooks—“Cancer is a genetic and an epigenetic disease.”
Acknowledgments
The author is funded by the NCI R01 CA158112, P50 CA100632, and P50 CA254897. The author would also like to acknowledge the many scientists who contributed to generating the concepts described in the commentary but whose primary papers could not be cited due to space constraints.
Authors’ Disclosures
J.J. Issa reports personal fees from Ascentage Pharma and personal fees from Daiichi Sankyo outside the submitted work.
- November 1999
'Two-Hit' Hypothesis
Much of what scientists know about the origins of cancer and the role of tumor suppressors can be traced back 28 years to the elegant theory of cancer researcher alfred g. knudson. widely thought to be one of the most significant theories in modern biology, knudson's "two-hit" hypothesis was recognized nov. 19 at the john scott awards in philadelphia, along with the revolutionary research of benoit mandelbrot, the discoverer of the powerful mathematical laws governing fractal geometry and self-s.
Much of what scientists know about the origins of cancer and the role of tumor suppressors can be traced back 28 years to the elegant theory of cancer researcher Alfred G. Knudson . Widely thought to be one of the most significant theories in modern biology, Knudson's "two-hit" hypothesis was recognized Nov. 19 at the John Scott Awards in Philadelphia, along with the revolutionary research of Benoit Mandelbrot , the discoverer of the powerful mathematical laws governing fractal geometry and self-similarity. 1
Even amid ongoing rumors...
Interested in reading more?
Become a member of.
- public health

Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals
You are here
- Volume 38, Issue 2
- Two hits revisited again
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- I P M Tomlinson a ,
- R Roylance a ,
- R S Houlston b
- a Molecular and Population Genetics Laboratory, Imperial Cancer Research Fund, 44 Lincoln's Inn Fields, London WC2A 3PX, UK, b Section of Cancer Genetics, Institute of Cancer Research, Cotswold Road, Sutton, Surrey SM2 5NG, UK
- Dr Tomlinson, i.tomlinson{at}icrf.icnet.uk
INTRODUCTION AND METHODS Since the concept of the “two hit hypothesis” was introduced over 20 years ago, a wealth of genetic data has accumulated on the mutations found at tumour suppressor loci. Perhaps surprisingly, these data conceal large gaps in our knowledge which genetic and functional studies are beginning to uncover. The “two hit hypothesis” must be updated to take account of this new information.
RESULTS AND DISCUSSION Here, we discuss both the results of recent studies and some of the questions that they highlight. In particular, how valid are conclusions from inherited Mendelian syndromes when applied to sporadic cancers? Why is allelic loss so common and how does it occur? Are the “two hits” random or interdependent? Is abolition of protein function always optimal for tumorigenesis? Can “third hits” occur and, if so, why? How can mismatch repair deficiency and the methylator phenotype be incorporated into the “two hit” hypothesis? We suggest that the “two hit hypothesis” is not fixed but is evolving as our knowledge expands.
- two hit model
- tumour suppressor
- carcinogenesis
https://doi.org/10.1136/jmg.38.2.81
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Read the full text or download the PDF:
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Hereditary cancer: two hits revisited
Affiliation.
- 1 Institute for Cancer Research, Fox Chase Cancer Center, Philadelphia, PA USA.
- PMID: 8601560
- DOI: 10.1007/BF01366952
According to a "two-hit" model, dominantly inherited predisposition to cancer entails a germline mutation, while tumorigenesis requires a second, somatic, mutation. Non-hereditary cancer of the same type requires the same two hits, but both are somatic. The original tumor used in this model, retinoblastoma, involves mutation or loss of both are somatic. The original tumor used in this model, retinoblastoma, involves mutation or loss of both copies of the RB1 tumor-suppressor gene in both hereditary and non-hereditary forms. In fact, most dominantly inherited cancers show this relationship. New dominantly inherited cancers show this relationship. New questions have arisen, however. When a tumor-suppressor gene is ubiquitously expressed, why is there any specificity of tumor predilection? In some instances, it is clear that two hits produce only a benign precursor lesion and that other genetic events are necessary. As the number of necessary events increase, the impact of the germline mutation diminishes. The number of events is least for embryonal tumors, and relatively small for certain sarcomas. Stem-cell proliferation evidently plays a key role early in carcinogenesis. In some tissues it is physiological, as in embryonic development and in certain tissues in adolescence. In adult renewal tissues, the sites of the common carcinomas, mutation may be necessary to impair the control of switching between renewal and replicative cell divisions; the APC gene may be the target of such a mutation.
PubMed Disclaimer
Similar articles
- Nakahara memorial lecture. Hereditary cancer, oncogenes, and anti-oncogenes. Knudson AG Jr. Knudson AG Jr. Princess Takamatsu Symp. 1989;20:15-29. Princess Takamatsu Symp. 1989. PMID: 2577335 Review.
- Hereditary predisposition to cancer. Knudson AG. Knudson AG. Ann N Y Acad Sci. 1997 Dec 29;833:58-67. doi: 10.1111/j.1749-6632.1997.tb48593.x. Ann N Y Acad Sci. 1997. PMID: 9616740 Review.
- Molecular biology of colorectal cancer. Gryfe R, Swallow C, Bapat B, Redston M, Gallinger S, Couture J. Gryfe R, et al. Curr Probl Cancer. 1997 Sep-Oct;21(5):233-300. doi: 10.1016/s0147-0272(97)80003-7. Curr Probl Cancer. 1997. PMID: 9438104 Review.
- Overview: genes that predispose to cancer. Knudson AG Jr. Knudson AG Jr. Mutat Res. 1991 Apr;247(2):185-90. doi: 10.1016/0027-5107(91)90013-e. Mutat Res. 1991. PMID: 2011135 Review.
- Hereditary cancer and its clinical implications: a view. Den Otter W, Koten JW, Van der Vegt BJ, Beemer FA, Boxma OJ, De Graaf PW, Derkinderen DJ, Hill FW, Huber J, Klein WR, et al. Den Otter W, et al. Anticancer Res. 1990 Mar-Apr;10(2B):489-95. Anticancer Res. 1990. PMID: 2190527 Review.
- Exome Sequencing Reveals Novel Germline Variants in Breast Cancer Patients in the Southernmost Region of Thailand. Sukpan P, Sangkhathat S, Sriplung H, Laochareonsuk W, Choochuen P, Auseng N, Khoonjan W, Salaeh R, Thangnaphadol K, Wanawanakorn K, Kanokwiroon K. Sukpan P, et al. J Pers Med. 2023 Nov 9;13(11):1587. doi: 10.3390/jpm13111587. J Pers Med. 2023. PMID: 38003901 Free PMC article.
- The clinical, genetic, and immune landscape of meningioma in patients with NF2-schwannomatosis. Gregory GE, Islim AI, Hannan CJ, Jones AP, Hammerbeck-Ward C, Rutherford SA, Freeman SR, Lloyd S, Kalamarides M, Smith MJ, Couper K, McBain CA, Jenkinson MD, Brough D, King AT, Evans DG, Pathmanaban ON. Gregory GE, et al. Neurooncol Adv. 2023 Jun 3;5(Suppl 1):i94-i104. doi: 10.1093/noajnl/vdac127. eCollection 2023 May. Neurooncol Adv. 2023. PMID: 37287576 Free PMC article.
- Multi-omic profiling reveals an RNA processing rheostat that predisposes to prostate cancer. Stentenbach M, Ermer JA, Rudler DL, Perks KL, Raven SA, Lee RG, McCubbin T, Marcellin E, Siira SJ, Rackham O, Filipovska A. Stentenbach M, et al. EMBO Mol Med. 2023 Jun 7;15(6):e17463. doi: 10.15252/emmm.202317463. Epub 2023 Apr 24. EMBO Mol Med. 2023. PMID: 37093546 Free PMC article.
- Interrogating the Human Diplome: Computational Methods, Emerging Applications, and Challenges. Chan AP, Choi Y, Rangan A, Zhang G, Podder A, Berens M, Sharma S, Pirrotte P, Byron S, Duggan D, Schork NJ. Chan AP, et al. Methods Mol Biol. 2023;2590:1-30. doi: 10.1007/978-1-0716-2819-5_1. Methods Mol Biol. 2023. PMID: 36335489
- Molecular and clinicopathological analysis of three cases of gastric juvenile polyposis. Yamashiro Y, Yanai Y, Takeda T, Hayashi T, Akazawa Y, Yatagai N, Ueyama H, Eguchi H, Nagahara A, Yao T, Saito T. Yamashiro Y, et al. JGH Open. 2022 Jun 28;6(8):531-538. doi: 10.1002/jgh3.12781. eCollection 2022 Aug. JGH Open. 2022. PMID: 35928693 Free PMC article.
- Science. 1990 Nov 30;250(4985):1233-8 - PubMed
- Nature. 1984 May 10-16;309(5964):174-6 - PubMed
- Mol Cell Biol. 1990 Nov;10(11):5772-81 - PubMed
- Cell. 1979 May;17(1):43-52 - PubMed
- Science. 1987 Mar 13;235(4794):1394-9 - PubMed
Publication types
- Search in MeSH

Related information
- Cited in Books
LinkOut - more resources
Other literature sources.
- The Lens - Patent Citations
- Genetic Alliance
Research Materials
- NCI CPTC Antibody Characterization Program
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Advertisement

- Previous Article
- Next Article
Cancer: Genetic or Epigenetic Disease?
The two-hit hypothesis revised, cycles of instability, clinical implications, conclusions, authors' disclosures, acknowledgments, the two-hit hypothesis meets epigenetics.
Cancer Res 2022;82:1167–9
- Funder(s): NCI
- Award Id(s): R01 CA158112 , P50 CA100632 , P50 CA254897
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
- Version of Record April 1 2022
Jean-Pierre Issa; The Two-Hit Hypothesis Meets Epigenetics. Cancer Res 1 April 2022; 82 (7): 1167–1169. https://doi.org/10.1158/0008-5472.CAN-22-0405
Download citation file:
- Ris (Zotero)
- Reference Manager
The landmark paper by Kane and colleagues was the first report of DNA methylation in the promoter of the human MLH1 gene in sporadic colon cancers with mismatch repair (MMR) deficiency. In both cell lines and primary tumors, promoter methylation was associated with loss of MLH1 protein expression and with a lack of mutations in the MLH1 coding region. Together with subsequent papers that showed that this methylation was directly responsible for loss of MLH1 expression and MMR deficiency, the observation expanded the two-hit hypothesis of tumor suppressor gene loss in cancer to include both genetic and epigenetic mechanisms of gene inactivation. More broadly, the paper contributed to normalization of the hypothesis of an epigenetic basis for cancer development.
See related article by Kane and colleagues, Cancer Res 1997;57:808–11
The idea of cancer as a disease of epigenetic regulation was proposed in the 1960's based on unexpected reversal of the cancer phenotype when malignant cells undergo epigenetic reprogramming though embryogenesis ( 1 ). Epigenetic mechanisms were still mysterious at the time, and in the absence of a molecular explanation, the concept was rapidly forgotten. By the 1970's, studies of familial cancer incidence had firmly established a genetic basis for inherited cancer, and it was hypothesized that sporadic cancers would follow the same path. Knudson formulated the two-hit hypothesis to explain familial versus sporadic cancer epidemiology, proposing that the same genes are involved in both cases ( 2 ). This was confirmed molecularly with studies on familial cancer genes such as TP53 ( 3 ). The dogma evolved to a model whereby the two hits are mutations or deletions in the same tumor suppressor gene (TSG; Fig. 1 ); familial cases would be explained by an inherited hit followed by a sporadic hit, while nonfamilial cases are caused by two rare sporadic hits, explaining the delayed age of onset when compared with familial cancers ( 2 ).

The two-hit hypothesis meets epigenetics. By the 1990's, Knudson's two-hit hypothesis had evolved to postulate that familial cancer predisposition is due to a germline mutation in a TSG (top left), while actual cancer development follows a sporadic mutation (or deletion) in the second allele (top right). It also postulated that sporadic cancers of the same tissue type were due to two acquired hits (mutation or deletion) in the same TSG (bottom). The paper by Kane and colleagues (and other papers in that period) revised that model for sporadic cancers to include epigenetic inactivation by promoter DNA hypermethylation as either the first hit, the second hit, or both (bottom right). The figure is a simplification; subsequent data showed that in very rare cases familial cancers can be caused by germline hypermethylation, that the second hit in familial cancers can also be DNA hypermethylation, and, in the case of MLH1 in colon cancer, the “hits” in sporadic cases are almost always hypermethylation caused by the CpG island methylator phenotype.
Inherited mutations in MLH1 cause the Lynch syndrome, a familiar colon cancer predisposition disease ( 3 ). Biallelic inactivation of MLH1 causes mismatch repair (MMR) deficiency, accumulation of mutations, and eventual cancer formation. The phenotype of MMR deficiency was known before the genotype was elucidated (it was termed microsatellite instability or MSI at the time), and it was clear early on that MMR defects were present in both familial and sporadic colon cancers ( 4 ). In accordance with Knudson's hypotheses, it was expected that the genes that cause familial versus sporadic MMR-deficient cancers would be the same. Kane and colleagues tried to confirm this ( 4 ). Unexpectedly, while they found that sporadic MSI+ cases had loss of MLH1 protein, they could find no coding sequence mutations in this gene, challenging the prevailing notion at the time.
In the 1980's, the unorthodox idea of an epigenetic basis for cancer resurfaced, powered by an actual proposed mechanism—permanent silencing of TSG expression by promoter DNA methylation ( 5 ). As besets many “out-of-the-box” hypotheses, this one was met with considerable early skepticism. Nevertheless, after Kane and colleagues observed unexplained loss of MLH1 expression in colon cancers, they went on to show that those sporadic cell lines and primary tumors that lacked MLH1 expression also had dense DNA methylation in a CpG rich area in the gene promoter ( 4 ). This observation was therefore consistent with Knudson's tenets, but with a twist—the gene in sporadic and familial cancers was the same ( MLH1 ) but the mechanism of inactivation was different (DNA methylation vs. mutations; Fig. 1 ). The fact that MLH1 was a bona fide cancer predisposition gene (a “gatekeeper” in Vogelstein's model; ref. 3 ) inactivated by DNA methylation was a turning point in the wide acceptance of the epigenetic basis of cancer hypothesis. It was further buttressed by the demonstration that the MMR phenotype can be reversed by removal of DNA methylation ( 6 ) and also by the identification of other TSGs with similar genetic/epigenetic mechanisms of inactivation ( 5 ). As can be seen in cancer biology textbooks 25 years later, the paper by Kane and colleagues contributed to rewriting the chapters on the mechanistic basis of cancer development—cancer is now seen as a genetic and an epigenetic disease.
An important consequence of the paper by Kane and colleagues (and other papers at the time) was the modification of the two-hit hypothesis to include both genetic and epigenetic mechanisms of gene inactivation ( Fig. 1 ). Because epigenetic changes are reset during embryogenesis, it is not surprising that, with rare (and fascinating) exceptions, all familial cancers are caused by genetic changes. For reasons not yet fully understood, some TSGs are resistant to epigenetic inactivation. In those cases, the second hit in familial cancers as well as both hits in sporadic cancers are also genetic. For the other TSGs (such as MLH1 , VHL , CDKN2A , BRCA1 , and others), the second hit in familial cases can be either genetic or epigenetic, and both hits in sporadic cases can similarly be either genetic or epigenetic. Lynch syndrome provides an interesting study in this genetic/epigenetic duality. The MSH2 gene is a frequent cause of familial disease but is almost never epigenetically inactivated, and it is very rarely responsible for sporadic colon cancers. By contrast, the MLH1 gene is also a frequent cause of familial disease, but it is susceptible to epigenetic inactivation and is almost always responsible for MMR-deficient sporadic colon cancers.
In addition to revising the two-hit hypothesis, the paper by Kane and colleagues suggested that epigenetic changes can trigger genetic changes by inducing DNA repair defects. In an interesting additional twist, MLH1 methylation in sporadic cancers was subsequently shown to be caused by a broader epigenetic deregulation, the CpG island methylator phenotype (CIMP; ref. 7 ). CIMP+ cancers inactivate multiple TSGs simultaneously, and this phenotype of epigenetic instability has been described in almost all cancer cell types ( 7 ). Intriguingly, MLH1 is a common CIMP target in colon and endometrial cancers, but not in other CIMP+ cases. In acute myelogenous leukemia (AML) and in glioblastoma multiforme (GBM), CIMP is caused by genetic defects in the TCA cycle, namely mutations in IDH1 or IDH2 ( 8 ). These mutations lead to accumulation of metabolites that inactivate the TET enzymes, which are responsible for protection against DNA methylation in CpG islands ( 8 ). Thus, mutations in metabolic regulation genes can lead to epigenetic instability. In turn, epigenetic instability can lead to genetic instability by inducing DNA repair defects ( 7 ). As one can imagine, these cycles of instability can be repeated many times in the lifetime of a cancer, contributing to progression, metastasis, and therapeutic resistance.
Unlike AML and GBM, mutations in TCA cycle genes have not been commonly seen in CIMP+ colon cancers ( 9 ) or gastric cancers. Even in AML, CIMP can only be explained by mutations in about half the cases. The etiology of CIMP in mutation-negative cases remains speculative. There is a strong association between CIMP and Epstein-Barr virus in gastric cancers ( 7 ), and an intriguing link between CIMP and fusobacterium in colon cancers ( 9 ), suggesting an environmental etiology to CIMP in some cases. It is possible for example that infections lead to metabolic deregulation, disrupting TET function and leading to DNA hypermethylation.
The diagnosis of a familial cancer syndrome can save lives through early screening and intervention. Lynch syndrome has an incomplete penetrance and can first manifest at an advanced age. While colon cancers are routinely screened for MMR defects, a positive result does not always trigger an investigation of germline defects because the frequency of sporadic MMR-deficient cancers is higher than that of familial MMR-deficient cancers after the age of 60. The paper by Kane and colleagues (and subsequent confirmation) suggests that MLH1 methylation can serve as a quick additional screen in this respect. MMR-deficient colon cancers that lack MLH1 expression but have no MLH1 promoter DNA methylation should trigger a very high index of suspicion for Lynch syndrome.
Finally, it is worth noting that epigenetic changes mediated by DNA methylation are potentially reversible through the use of drugs that target DNA methyltransferases ( 10 ). The paper by Kane and colleagues ( 4 ) and other papers showing the importance of DNA methylation in TSG regulation in cancer ( 5 ) triggered a revival in interest in these drugs, eventually leading to their FDA approval and broad usage in patients with myeloid malignancies ( 10 ). While their activity in solid tumors remains limited, there continues to be interest in developing DNA methylation–based treatment or prevention strategies that could be especially useful in cases with CIMP and MLH1 promoter DNA methylation.
The paper by Kane and colleagues had a straightforward message: MLH1 expression was missing in MMR-deficient sporadic colon cancers and this was associated with promoter DNA methylation rather than coding mutations in the gene ( 4 ). This deceptively simple message had a profound influence in that it lent legitimacy to the hypothesis of DNA methylation–mediated TSG inactivation in cancer. It helped rewrite textbooks—“Cancer is a genetic and an epigenetic disease.”
J.J. Issa reports personal fees from Ascentage Pharma and personal fees from Daiichi Sankyo outside the submitted work.
The author is funded by the NCI R01 CA158112, P50 CA100632, and P50 CA254897. The author would also like to acknowledge the many scientists who contributed to generating the concepts described in the commentary but whose primary papers could not be cited due to space constraints.
commentary-article
- Methylation of the hMLH1 Promoter Correlates with Lack of Expression of hMLH1 in Sporadic Colon Tumors and Mismatch Repair-defective Human Tumor Cell Lines 1
Citing articles via
Email alerts.
- Online First
- Collections
- Online ISSN 1538-7445
- Print ISSN 0008-5472
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Information on Advertising & Reprints
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Oncogenomics
- Published: 06 October 2008
APC and the three-hit hypothesis
- S Segditsas 1 na1 ,
- A J Rowan 1 na1 ,
- K Howarth 1 na1 ,
- A Jones 1 ,
- S Leedham 2 ,
- N A Wright 2 ,
- P Gorman 1 ,
- W Chambers 1 , 3 ,
- E Domingo 1 ,
- R R Roylance 1 ,
- E J Sawyer 1 ,
- O M Sieber 1 nAff5 &
- I P M Tomlinson 1
Oncogene volume 28 , pages 146–155 ( 2009 ) Cite this article
4852 Accesses
49 Citations
Metrics details
The seminal ‘two-hit hypothesis’ implicitly assumes that bi-allelic tumour suppressor gene (TSG) mutations cause loss of protein function. All subsequent events in that tumour therefore take place on an essentially null background for that TSG protein. We have shown that the two-hit model requires modification for the APC TSG, because mutant APC proteins probably retain some function and the two hits are co-selected to produce an optimal level of Wnt activation. We wondered whether the optimal Wnt level might change during tumour progression, leading to selection for more than two hits at the APC locus. Comprehensive screening of a panel of colorectal cancer (CRC) cell lines and primary CRCs showed that some had indeed acquired third hits at APC . These third hits were mostly copy number gains or deletions, but could be protein-truncating mutations. Third hits were significantly less common when the second hit at APC had arisen by copy-neutral loss of heterozygosity. Both polyploid and near-diploid CRCs had third hits, and the third hits did not simply arise as a result of acquiring a polyploid karyotype. The third hits affected mRNA and protein levels, with potential functional consequences for Wnt signalling and tumour growth. Although some third hits were probably secondary to genomic instability, others did appear specifically to target APC . Whilst it is generally believed that tumours develop and progress through stepwise accumulation of mutations in different functional pathways, it also seems that repeated targeting of the same pathway and/or gene is selected in some cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
251,40 € per year
only 5,03 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others

Mutational landscape of cancer-driver genes across human cancers
Landscape and function of multiple mutations within individual oncogenes

Source, co-occurrence, and prognostic value of PTEN mutations or loss in colorectal cancer
Albuquerque C, Breukel C, van der Luijt R, Fidalgo P, Lage P, Slors FJ et al . (2002). The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet 11 : 1549–1560.
Article CAS Google Scholar
Fiegler H, Carr P, Douglas EJ, Burford DC, Hunt S, Scott CE et al . (2003). DNA microarrays for comparative genomic hybridization based on DOP-PCR amplification of BAC and PAC clones. Genes Chromosomes Cancer 36 : 361–374.
Fodde R, Smits R . (2001). Disease model: familial adenomatous polyposis. Trends Mol Med 7 : 369–373.
Gaasenbeek M, Howarth K, Rowan AJ, Gorman PA, Jones A, Chaplin T et al . (2006). Combined array-comparative genomic hybridization and single-nucleotide polymorphism-loss of heterozygosity analysis reveals complex changes and multiple forms of chromosomal instability in colorectal cancers. Cancer Res 66 : 3471–3479.
Hanahan D, Weinberg RA . (2000). The hallmarks of cancer. Cell 100 : 57–70.
Jones AM, Thirlwell C, Howarth KM, Graham T, Chambers W, Segditsas S et al . (2007). Analysis of copy number changes suggests chromosomal instability in a minority of large colorectal adenomas. J Pathol 213 : 249–256.
Knudson AG . (2001). Two genetic hits (more or less) to cancer. Nat Rev Cancer 1 : 157–162.
Lamlum H, Ilyas M, Rowan A, Clark S, Johnson V, Bell J et al . (1999). The type of somatic mutation at APC in familial adenomatous polyposis is determined by the site of the germline mutation: a new facet to Knudson's ‘two-hit’ hypothesis. Nat Med 5 : 1071–1075.
Leslie A, Stewart A, Baty DU, Mechan D, McGreavey L, Smith G et al . (2006). Chromosomal changes in colorectal adenomas: relationship to gene mutations and potential for clinical utility. Genes Chromosomes Cancer 45 : 126–135.
Michor F, Iwasa Y, Vogelstein B, Lengauer C, Nowak MA . (2005). Can chromosomal instability initiate tumorigenesis? Semin Cancer Biol 15 : 43–49.
Schneikert J, Grohmann A, Behrens J . (2007). Truncated APC regulates the transcriptional activity of beta-catenin in a cell cycle dependent manner. Hum Mol Genet 16 : 199–209.
Sieber OM, Heinimann K, Gorman P, Lamlum H, Crabtree M, Simpson CA et al . (2002). Analysis of chromosomal instability in human colorectal adenomas with two mutational hits at APC. Proc Natl Acad Sci USA 99 : 16910–16915.
Sieber OM, Segditsas S, Knudsen AL, Zhang J, Luz J, Rowan AJ et al . (2006). Disease severity and genetic pathways in attenuated familial adenomatous polyposis vary greatly but depend on the site of the germline mutation. Gut 55 : 1440–1448.
Sieber OM, Tomlinson SR, Tomlinson IPM . (2005). Tissue, cell and stage specificity of (epi)mutations in cancers. Nat Rev Cancer 5 : 649–655.
Spirio LN, Samowitz W, Robertson J, Robertson M, Burt RW, Leppert M et al . (1998). Alleles of APC modulate the frequency and classes of mutations that lead to colon polyps. Nat Genet 20 : 385–388.
Su LK, Barnes CJ, Yao W, Qi Y, Lynch PM, Steinbach G . (2000). Inactivation of germline mutant APC alleles by attenuated somatic mutations: a molecular genetic mechanism for attenuated familial adenomatous polyposis. Am J Hum Genet 67 : 582–590.
Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ et al . (2007). The genomic landscapes of human breast and colorectal cancers. Science 318 : 1108–1113.
Download references
Acknowledgements
We are grateful to colleagues at St Mark's Hospital for tissue collection, to several kind providers of cell lines and to the Mutation Detection Facility, Cancer Research UK London Research Institute.
Author information
Present address: 5Current address: Ludwig Colon Cancer Initiative Laboratory, Ludwig Institute for Cancer Research, PO Box 2008, Royal Melbourne Hospital, VIC 3050, Australia,
S Segditsas, A J Rowan and K Howarth: These authors contributed equally to this work.
Authors and Affiliations
Molecular and Population Genetics Laboratory, London Research Institute, Cancer Research UK, London, UK
S Segditsas, A J Rowan, K Howarth, A Jones, P Gorman, W Chambers, E Domingo, R R Roylance, E J Sawyer, O M Sieber & I P M Tomlinson
Histopathology Laboratory, London Research Institute, Cancer Research UK, London, UK
S Leedham & N A Wright
Department of Colorectal Surgery and Genetics Knowledge Park, Oxford Radcliffe Hospitals, Oxford, UK
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to I P M Tomlinson .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Segditsas, S., Rowan, A., Howarth, K. et al. APC and the three-hit hypothesis. Oncogene 28 , 146–155 (2009). https://doi.org/10.1038/onc.2008.361
Download citation
Received : 20 February 2008
Revised : 07 July 2008
Accepted : 28 August 2008
Published : 06 October 2008
Issue Date : 08 January 2009
DOI : https://doi.org/10.1038/onc.2008.361
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- tumour suppressor
- copy number change
- ‘just right’
This article is cited by
Copy number of the adenomatous polyposis coli gene is not always neutral in sporadic colorectal cancers with loss of heterozygosity for the gene.
- Peter Zauber
- Stephen Marotta
- Marlene Sabbath-Solitare
BMC Cancer (2016)
DDX3 promotes tumor invasion in colorectal cancer via the CK1ε/Dvl2 axis
- Tsung-Ying He
Scientific Reports (2016)
The mini-driver model of polygenic cancer evolution
- Francesc Castro-Giner
- Peter Ratcliffe
- Ian Tomlinson
Nature Reviews Cancer (2015)
Insertional mutagenesis identifies multiple networks of cooperating genes driving intestinal tumorigenesis
- H Nikki March
- Alistair G Rust
- David J Adams
Nature Genetics (2011)
β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation
- Felipe C Geyer
- Magali Lacroix-Triki
- Jorge S Reis-Filho
Modern Pathology (2011)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
COMMENTS
Two-hit hypothesis. The Knudson hypothesis, also known as the two-hit hypothesis, is the hypothesis that most tumor suppressor genes require both alleles to be inactivated, either through mutations or through epigenetic silencing, to cause a phenotypic change. [ 1] It was first formulated by Alfred G. Knudson in 1971 [ 2] and led indirectly to ...
Today, this hypothesis serves as the basis for researchers' understanding of how mutations in tumor suppressor genes drive cancer. The two-hit hypothesis arose of out Knudson's interest in the ...
The plot from which Knudson proposed the two-hit hypothesis (Knudson, 1971, with the permission of the National Academy of Sciences, USA). Interestingly, Knudson's statistical approach anticipated much of today's cancer research literature; that is to say, the work consisted entirely of dataset analysis and mathematical modeling.
The "two-hit" hypothesis provided a unifying model for understanding cancer that occurs in individuals who carry a "susceptibility gene" and cancers that develop because of randomly induced mutations in otherwise normal genes. Tumor-suppressor genes, in particular, are important targets for cancer prevention research, since they normally ...
It takes two events in both hereditary and non-hereditary cases; the only difference is the timing of the first event - before or after conception. My hypothesis explained why a child born with the first hit inherited in all of the cells (including both retinas) would be more likely to get cancer in both eyes, and at an early age.
The Two‐hit Hypothesis, a "Driver" in the Development of the Field of CANCER GENETICS. The dominant nature of hereditary cancer had been recognized as a trait long before the basic techniques of molecular biology were established. Although there were reports describing the loss of specific regions of certain chromosomes in particular ...
The 'two-hit' hypothesis of tumorigenesis, originally proposed in 1971 by Alfred Knudson using retinoblastoma as a model, explained the role of recessive tumour suppressor genes (TSGs) in ...
The "two-hit" hypothesis 15 suggests that two successive hits are needed to turn a normal cell into a tumour cell. This concept was proposed by Alfred Knudson in 1971 and is also known as the Knudson hypothesis. In familial cancers one hit is inherited and all that is needed is one additional "hit" on the other allele for tumours to form.
conception. My hypothesis explained why a child born with the first hit inherited in all of the cells (including both retinas) would be more likely to get cancer in both eyes, and at an early age. I suggested that a child born without the first hit would develop cancers at a later age, and only in one eye, as the likelihood of having two events ...
The Two-Hit Hypothesis Revised. An important consequence of the paper by Kane and colleagues (and other papers at the time) was the modification of the two-hit hypothesis to include both genetic and epigenetic mechanisms of gene inactivation (Fig. 1). Because epigenetic changes are reset during embryogenesis, it is not surprising that, with ...
Few ideas in cancer genetics have been as influential as the "two-hit" theory of tumor suppressors. This idea was introduced in 1971 by Al Knudson in a paper in the Proceedings of the National Academy of Science and forms the basis for our current understanding of the role of mutations in cancer. In …
Knudson's two-hit cancer hypothesis had a huge impact. Until this point, cancer was thought to be caused by the activation of oncogenes. Now the search was on for tumour-suppressor genes — whose ...
FIGURE 1: The plot from which Knudson proposed the two-hit hypothesis (Knudson, 1971, with the permission of the National Academy of Sciences, USA). Interestingly, Knudson's statistical approach anticipated much of today's cancer research literature; that is to say, the work consisted entirely of dataset analysis and mathematical modeling.
Much of what scientists know about the origins of cancer and the role of tumor suppressors can be traced back 28 years to the elegant theory of cancer researcher Alfred G. Knudson. Widely thought to be one of the most significant theories in modern biology, Knudson's "two-hit" hypothesis was recognized Nov. 19 at the John Scott Awards in Philadelphia, along with the revolutionary research of ...
INTRODUCTION AND METHODS Since the concept of the "two hit hypothesis" was introduced over 20 years ago, a wealth of genetic data has accumulated on the mutations found at tumour suppressor loci. Perhaps surprisingly, these data conceal large gaps in our knowledge which genetic and functional studies are beginning to uncover. The "two hit hypothesis" must be updated to take account of ...
Unlike oncogenes, tumor suppressor genes generally follow the two-hit hypothesis, which states both alleles that code for a particular protein must be affected before an effect is manifested. [7] If only one allele for the gene is damaged, the other can still produce enough of the correct protein to retain the appropriate function.
The classic two-hit model posits that both alleles of a tumor suppressor gene (TSG) must be inactivated to cause cancer. ... a Tested hypothesis. b Test for whether interactions change between ...
The Two-hit Hypothesis, a "Driver" in the Development of the Field of CANCER GENETICS. The dominant nature of hereditary cancer had been recognized as a trait long before the basic techniques of molecular biology were established. Although there were reports describing the loss of specific regions of certain chromosomes in particular types ...
Abstract. According to a "two-hit" model, dominantly inherited predisposition to cancer entails a germline mutation, while tumorigenesis requires a second, somatic, mutation. Non-hereditary cancer of the same type requires the same two hits, but both are somatic. The original tumor used in this model, retinoblastoma, involves mutation or loss ...
The two-hit hypothesis meets epigenetics. By the 1990's, Knudson's two-hit hypothesis had evolved to postulate that familial cancer predisposition is due to a germline mutation in a TSG (top left), while actual cancer development follows a sporadic mutation (or deletion) in the second allele (top right).
The 'two-hit' hypothesis (Knudson, 2001) has dominated cancer genetics for over 20 years. In this model, one copy of a tumour suppressor gene (TSG) is inactivated by a nonsense or frameshift ...
The two-hit hypothesis is a promising avenue for research into the development of schizophrenia. Basically, this hypothesis suggests that some people might be susceptible to schizophrenia because ...
The Two-hit Hypothesis, a "Driver" in the Development of the Field of CANCER GENETICS. The dominant nature of hereditary cancer had been recognized as a trait long before the basic techniques of molecular biology were established. Although there were reports describing the loss of specific regions of certain chromosomes in particular types ...