

20 IB Chemistry IA ideas

Best IB Chemistry IA Ideas

Chemistry is a fundamental science that explores the composition, properties, and behavior of matter. It plays a crucial role in various fields, including medicine, agriculture, and environmental conservation. In this article, we will delve into an array of intriguing chemistry investigation ideas to inspire students' Chemistry Internal Assessment (IA) projects.
The experiments outlined in this article focus on exploring different aspects of chemical reactions and their applications. From studying the effects of catalysts on reaction rates to analyzing the efficiency of sunscreen brands in UV protection, these investigations provide opportunities for students to develop critical thinking and laboratory skills.
Moreover, safety is paramount when conducting any scientific experiment. Therefore, each project suggestion ensures adherence to safety guidelines and promotes responsible handling of chemicals. By following proper protocols and precautions, students can carry out these investigations with confidence while maintaining a safe learning environment.
Embark on a journey through the captivating world of chemistry as we present you with engaging IA ideas that combine scientific rigor with practical relevance.

Key Takeaways for IB Chemistry IA ideas
- Chemistry is a fundamental science that explores the composition, properties, and behavior of matter.
- Chemistry plays a crucial role in medicine, agriculture, and environmental conservation.
- Catalysts have a profound impact on reaction rates by providing an alternative reaction pathway with lower activation energy.
- Temperature significantly affects solubility, leading to an increase in solubility for most solid solutes in liquid solvents.
IB Chemistry IA Ideas - Investigating the Effects of Different Catalysts on Reaction Rates
The investigation delves into the intriguing realm of catalytic chemistry, exploring the profound impact that various catalysts have on the rates of chemical reactions. Catalysts play a crucial role in speeding up reactions by providing an alternative reaction pathway with lower activation energy. This allows for more successful collisions between reactant particles, leading to increased reaction rates. By investigating the effects of different catalysts on reaction rates, scientists can gain valuable insights into how to optimize chemical processes and improve efficiency.
When conducting experiments involving catalysts, it is important to prioritize safety precautions. Proper ventilation and protective equipment should be used to minimize exposure to potentially harmful substances. Additionally, care should be taken when handling chemicals and working with high temperatures or pressure conditions.
Transitioning into examining the relationship between temperature and solubility, it becomes evident that understanding the factors influencing solubility is essential in various applications such as pharmaceutical formulation and environmental engineering. Temperature significantly affects solute-solvent interactions and can have a profound impact on solubility. By investigating this relationship, researchers can uncover valuable information about how temperature influences the dissolution process and make informed decisions regarding solubility optimization in various industries. Read More About: 20 Ideas for your IB Chemistry — Peak Study Resources
IB Chemistry IA Ideas - Examining the Relationship Between Temperature and Solubility
Examining the relationship between temperature and solubility reveals a compelling interplay between these two factors. Solubility refers to the ability of a substance, known as the solute, to dissolve in another substance called the solvent. Temperature plays a crucial role in determining solubility as it affects the kinetic energy of particles. Generally, an increase in temperature leads to an increase in solubility for most solid solutes in liquid solvents.
Understanding this relationship is important for individuals who prioritize safety when handling chemicals. By knowing how temperature influences solubility, one can make informed decisions about mixing substances and avoid potential hazards. For example, if a particular solid substance is highly soluble at high temperatures but less soluble at lower temperatures, it would be wise to handle it with caution when dissolving it in a cold solvent.
Transitioning into exploring the effects of pH on enzyme activity, it is essential to consider other external factors that can impact chemical reactions. The pH level represents the acidity or alkalinity of a solution and has been found to influence enzyme activity significantly. Therefore, investigating how changes in pH affect enzyme function provides valuable insights for those concerned with maintaining optimal conditions for biological processes without explicitly stating 'step'.
IB Chemistry IA Ideas - Exploring the Effects of pH on Enzyme Activity
Exploring the effects of pH on enzyme activity provides valuable insights into the intricate relationship between chemical reactions and optimal conditions for biological processes. Enzymes are essential proteins that catalyze biochemical reactions in living organisms. They play a crucial role in maintaining homeostasis by regulating metabolic pathways. The activity of enzymes is highly dependent on their environment, including factors such as temperature, substrate concentration, and pH.
pH, or the measure of hydrogen ion concentration, can significantly impact enzyme activity. Each enzyme has an optimal pH range at which it functions most efficiently. Deviations from this range can lead to denaturation or inactivation of the enzyme. This phenomenon occurs because changes in pH disrupt the ionic and hydrogen bonding interactions that maintain the enzyme's three-dimensional structure.
Understanding the effects of pH on enzyme activity is important for several reasons. Firstly, it helps researchers design experiments that mimic physiological conditions accurately. Secondly, it aids in understanding how certain diseases or environmental factors can alter enzymatic function due to changes in pH levels within cells or tissues.
Transitioning into investigating the factors affecting the rate of rusting requires a shift from enzymology to material science principles. Therefore, studying how external factors influence corrosion rates provides further insight into chemical reactions occurring outside biological contexts while still exploring how various parameters affect reaction rates without compromising safety considerations. Read More About: IB Chemistry IA (Internal Assessment)
IB Chemistry IA Ideas - Investigating the Factors Affecting the Rate of Rusting
Investigating the factors affecting the rate of rusting provides valuable insights into the chemical reactions involved in corrosion processes and how external influences can influence their rates. Rusting, a common form of corrosion that occurs when iron or steel reacts with oxygen and moisture, can be influenced by various factors. These include:
- Humidity: High levels of humidity provide more moisture for the oxidation process to occur, accelerating rust formation.
- Oxygen concentration: The presence of oxygen is essential for rusting to take place. Higher levels of oxygen lead to faster rates of corrosion.
- Temperature: Elevated temperatures increase the kinetic energy of particles, enhancing reaction rates and promoting faster rusting.
- Presence of pollutants: Pollutants such as sulfur dioxide or chloride ions can accelerate metal corrosion by acting as catalysts or promoting electrochemical reactions.
- Surface area: A larger surface area exposed to air and moisture increases the likelihood of oxidation reactions occurring, facilitating rust formation.
Understanding these factors is crucial for industries and individuals seeking to mitigate corrosion risks and ensure safety. By analyzing the chemical composition of common household products, we can further explore effective strategies for preventing corrosion and protecting valuable assets from deterioration.
IB Chemistry IA Ideas - Analyzing the Chemical Composition of Common Household Products
Analyzing the chemical composition of common household products provides valuable insights into their potential for corrosion and the effectiveness of preventive measures. Understanding the chemicals present in these products allows us to identify potential corrosive agents and develop strategies to mitigate their damaging effects. For an audience concerned with safety, it is crucial to be aware of the chemical composition of household items that may come into contact with metals, as this knowledge can help prevent corrosion-related accidents.
Many common household products contain substances that can accelerate corrosion. For example, cleaning solutions often contain acidic compounds such as vinegar or lemon juice, which can react with metal surfaces and promote rusting. Similarly, certain detergents and soaps may contain salts or enzymes that corrode metal over time. By analyzing the chemical composition of these products, we can better understand how they interact with metals and devise appropriate preventive measures.
In the subsequent section about investigating the effects of different concentrations of acids on metal corrosion, we will explore how varying acid concentrations impact corrosion rates. This analysis builds upon our understanding of household product compositions by focusing specifically on acid-induced corrosion. By examining the relationship between acid concentration and metal degradation, we can further refine our knowledge on preventing and mitigating corrosion in various settings without compromising safety precautions.
IB Chemistry IA Ideas - Investigating the Effects of Different Concentrations of Acids on Metal Corrosion
In our previous discussion, we explored the chemical composition of common household products and its significance in our daily lives. Now, let's shift our focus to a different aspect of chemistry by investigating the effects of different concentrations of acids on metal corrosion. This topic holds great importance for industries that deal with metals, as understanding the corrosive properties of acids can help in developing effective preventive measures.
To delve deeper into this subject, we will examine it from various angles:
- Corrosion rates: We will analyze how different concentrations of acids affect the rate at which metals corrode. This will provide valuable insights into the reactivity of metals and their vulnerability to corrosion.
- Protective coatings: We will explore the effectiveness of different protective coatings in preventing metal corrosion when exposed to varying acid concentrations. This knowledge can guide industries in choosing appropriate coatings for their specific needs.
- Environmental impact: We will assess how different acid concentrations influence environmental degradation caused by metal corrosion. Understanding these effects is crucial for ensuring safety and sustainability in industrial practices.
By studying these aspects, we aim to contribute to a safer and more environmentally conscious industry that minimizes harmful impacts on both human health and nature. Transitioning seamlessly into our next section, let us now turn our attention towards examining the relationship between light intensity and photosynthesis rate, shedding light on yet another fascinating aspect of chemistry research.
IB Chemistry IA Ideas - Examining the Relationship Between Light Intensity and Photosynthesis Rate
Exploring the interplay between varying intensities of light and the rate of photosynthesis provides valuable insights into the fundamental processes involved in plant growth and energy conversion. Photosynthesis is a crucial process for plants, as it converts sunlight into chemical energy that can be used for growth and development. Light intensity plays a significant role in influencing the rate of photosynthesis, as it directly affects the amount of energy available for plants to convert.
When examining this relationship, it is important to consider safety precautions. It is essential to use appropriate equipment and ensure that the light sources are not too intense, as excessive light can cause damage to both plants and researchers. Additionally, protective measures such as wearing goggles should be taken when working with high-intensity lights.
Understanding how light intensity affects photosynthesis can have practical applications in optimizing plant growth conditions. By manipulating light intensity, growers can potentially enhance their crop yields or create specific environments suitable for different types of plants.
In the subsequent section about investigating the effects of different fertilizers on plant growth, we will delve into another aspect that contributes to overall plant health and productivity.
IB Chemistry IA Ideas - Investigating the Effects of Different Fertilizers on Plant Growth
Investigating the effects of different fertilizers on plant growth provides valuable insights into the role of nutrient availability in optimizing plant health and productivity. Understanding how various fertilizers affect plants can help farmers and gardeners make informed decisions about which fertilizer to use for their specific needs. Here are three key points to consider:
- Nutrient composition: Different fertilizers contain varying amounts of essential nutrients such as nitrogen, phosphorus, and potassium. These nutrients are crucial for plant growth and development. By comparing the growth rates of plants treated with different fertilizers, we can determine which nutrient compositions are most effective in promoting healthy plant growth.
- Environmental impact: It is important to consider the environmental impact of using different fertilizers. Some fertilizers may contain harmful chemicals that can leach into groundwater or contribute to air pollution. Choosing environmentally friendly fertilizers helps ensure the long-term sustainability of agricultural practices.
- Plant response: Plants respond differently to different types of fertilizers. Some plants may thrive with a particular fertilizer, while others may show signs of stress or exhibit stunted growth. By studying these responses, we can gain insights into the specific nutritional requirements of different plant species.
Investigating the effects of different fertilizers on plant growth not only helps optimize crop yields but also promotes sustainable farming practices that prioritize environmental safety and long-term productivity. Transitioning into the subsequent section about 'analyzing the efficiency of different sunscreen brands in UV protection,' understanding how various products perform under specific conditions is crucial for ensuring consumer safety and well-being without compromising sun protection efficacy.
IB Chemistry IA Ideas - Analyzing the Efficiency of Different Sunscreen Brands in UV Protection
Continuing our exploration of the effects of various substances on different processes, we now shift our focus to analyzing the efficiency of different sunscreen brands in UV protection. Sunscreen is an essential product for individuals who prioritize their safety under the sun. This research topic holds great significance as it enables us to evaluate and compare the effectiveness of various sunscreen formulations available in the market today.
The primary objective of this investigation is to determine which sunscreen brand provides optimal protection against harmful ultraviolet (UV) radiation. By subjecting multiple brands to rigorous testing procedures, we can obtain empirical data regarding their ability to block both UVA and UVB rays. The results obtained from this study will not only assist consumers in making informed choices about which sunscreen brand they should use but also have implications for public health.
Transitioning into the subsequent section, our inquiry into investigating the effects of different antacids on stomach acid neutralization will shed light on another significant aspect related to human health – digestion.
IB Chemistry IA Ideas - Investigating the Effects of Different Antacids on Stomach Acid Neutralization
Shifting our focus to the effects of different antacids on stomach acid neutralization, this investigation aims to examine the impact of various substances on the digestive process. With a growing concern for maintaining good health and well-being, it is imperative to understand how different antacids can contribute to regulating stomach acidity.
The study begins by selecting a range of commonly used antacid brands available in the market. These include calcium carbonate, magnesium hydroxide, aluminum hydroxide, and sodium bicarbonate. The experiment involves simulating stomach acid using hydrochloric acid and then adding measured amounts of each antacid. The resulting pH levels are then recorded at regular time intervals to determine the effectiveness of these substances in neutralizing stomach acid.
Understanding the effects of different antacids is crucial for individuals seeking relief from conditions such as heartburn or indigestion. By identifying which substances offer better neutralization properties, individuals can make informed decisions when choosing over-the-counter remedies.
Transitioning into the subsequent section about examining the relationship between concentration and reaction rate in a chemical reaction, further investigations will explore how varying concentrations can influence reaction rates without compromising safety precautions.
IB Chemistry IA Ideas - Examining the Relationship Between Concentration and Reaction Rate in a Chemical Reaction
An examination of the correlation between concentration and reaction rate in a chemical reaction reveals the significant impact that varying concentrations can have on the speed at which a reaction occurs. The concentration of reactants plays a crucial role in determining the rate of a chemical reaction. Here are three key points to consider when studying this relationship:
- Increased concentration leads to faster reaction rates: As the concentration of reactants increases, there are more particles available for collisions, increasing the frequency of successful collisions. This results in an increased rate of reaction.
- Collision theory explains the effect of concentration: According to collision theory, for a chemical reaction to occur, reactant particles must collide with sufficient energy and proper orientation. Higher concentrations increase collision frequency, making it more likely for successful collisions to take place.
- Safety considerations: When conducting experiments involving varying concentrations, it is essential to follow safety guidelines and wear appropriate protective equipment such as gloves and goggles. Additionally, ensure that all chemicals are handled carefully and stored properly to prevent accidents or unwanted reactions.
Transitioning into investigating the effects of different electrolytes on battery performance involves examining another aspect where understanding chemical reactions is crucial for ensuring safe and efficient technology development without compromising user safety.
IB Chemistry IA Ideas - Investigating the Effects of Different Electrolytes on Battery Performance
In the previous subtopic, we explored the relationship between concentration and reaction rate in a chemical reaction. Now, we shift our attention to another intriguing topic within the field of chemistry - investigating the effects of different electrolytes on battery performance.
Batteries play an essential role in powering various devices and technologies that are integral to our daily lives. Understanding how different electrolytes affect battery performance is crucial for optimizing their efficiency and safety. Electrolytes, which are substances capable of conducting electricity when dissolved or melted, are a vital component in batteries as they facilitate the movement of ions between electrodes.
By examining the impact of various electrolytes on battery performance, researchers can identify which combinations result in enhanced energy storage capacity, longer lifespan, and improved safety features. This investigation involves testing different types of electrolytes with varying chemical compositions and concentrations to determine their influence on factors such as charge retention, discharge rate, and overall stability.
The findings from this study have practical implications for designing more efficient batteries with improved performance characteristics. Additionally, understanding how different electrolyte compositions affect battery behavior contributes to enhancing device safety by minimizing the risk of overheating or leakage that could potentially lead to accidents or damage.
Frequently Asked Questions
How do different catalysts affect reaction rates in chemical reactions.
Different catalysts can alter the reaction rate in chemical reactions. By lowering the activation energy, they provide an alternative pathway for the reaction to occur, making it faster and more efficient. This allows reactions to proceed at lower temperatures, reducing potential safety hazards.
What is the relationship between temperature and solubility in different substances?
The relationship between temperature and solubility in different substances is that generally, as the temperature increases, the solubility of most solid solutes in water also increases. However, this relationship can vary for different substances.
How does pH affect the activity of enzymes?
The activity of enzymes is affected by pH levels. Changes in pH can alter the shape and structure of enzymes, affecting their ability to catalyze reactions. Maintaining optimal pH conditions is crucial for enzyme function and overall safety.
What factors contribute to the rate of rusting in metals?
Factors that contribute to the rate of rusting in metals include moisture, oxygen, and the presence of electrolytes. These factors accelerate the oxidation process, leading to the formation of rust on metal surfaces.
What is the chemical composition of common household products?
The chemical composition of common household products varies depending on the specific product. It is important to consult safety data sheets or labels for information regarding the chemicals present and their potential hazards.
In conclusion, this article provides a variety of interesting IB chemistry IA ideas that can be explored by students. The topics range from investigating the effects of catalysts and temperature on reaction rates to analyzing the chemical composition of household products and examining the efficiency of sunscreen brands. These experiments offer opportunities for students to deepen their understanding of key concepts in chemistry and develop their research skills. By selecting one of these IA ideas, students can engage in meaningful scientific investigations that contribute to their overall learning experience.

Hire a Tutor & Get Free Trial
25 IB Chemistry IA Topic Ideas

We all know that scoring superbly on internal assessments is a great way to boost our IB grades. But how do we get started on a lab report and, crucially, what should we write about? In general, keep in mind that for your Chemist ry IA we want to measuring how changing one variable has an effect on another variable.
If you’re struggling with a topic, consider some easily altered variables (temperature, surface area, concentration) and explore what effect changing these may have on your dependent variable, which should ideally be easy to measure (pH, gas produced, temperature, etc.). However, if you’re still feeling stuck then this week we’re going to take a look at 25 Chemistry IA topic ideas that we hope will spark some ideas for you!
NOTE: These topics are purely meant as inspiration and are not to be chosen blindly. Even though many of these topics led to high scores for some of our graduates in the past, it is important that you listen to the advice of your subject teacher before choosing any topic!
Get Support from A Top Tutor Today
At Lanterna we have hundreds of tutors who smashed Chemistry. They know exactly how to get an 7 in the exam and IA, and can give you tips and tricks on how you can do the same. What are you waiting for? Get your own tutor today !
1, Calculating absolute zero using gas volume
2, exploring the vitamin content of various healthy foodstuffs, 3, studying the dissolved oxygen content of a water body, 4, investigating the concentration of drugs within tablets (this could even be explored across different brands), 5, using calorimetry to determine enthalpy changes/the enthalpy of neutralisation, 6, determining the activation energy of a reaction, 7, exploring conditions under which lipase can be denatured, 8, exploring the speed of various chemical reactions using a spectrometer, 9, what is the activation energy needed to decompose a compound such as hydrogen peroxide, 10, find the calcium content of different milk brands, 11, explore the optimal conditions to electroplate metals by considering a variety of external factors, 12, using thermal decomposition try to identify the type of salt present in some compound, grab free chem resources , 13, explore and distinguish between methanol and ethanol using iodine and sodium hydroxide solutions, 14, use paper chromatography to separate pigments present in a tree leaf, 15, explore the effect of temperature on the strength of a ferromagnet, 16, describe the effect of varying temperature on the formation of rust on steel, 17, measure the amount of free caffeine in different coffee, tea, or other drink brands, 18, can different fruits be used in order to chelate heavy metals from polluted water sources, 19, an analysis into the different edta contents of a variety of shower cleaners, 20, speed of denaturation in various animal proteins using uv light, 21, synthesizing the sweetener dulcin from paracetamol, 22, considering and exploring the effectiveness of various brands of salts for snow removal, 23, using paper chromatography to analyse the various dyes present in different brands of jelly candy. , 24, measuring the change in iron levels of avocados as they go through different ripening stages, 25, measuring the energy content of a packet of cheetos.
For getting the ball rolling after you’ve grabbed a great Chemistry IA topic, there are numerous great websites with tips . But non-chemists scientists out there please don’t feel left out! Check out our previous blog about generating some inspiration for your reports! And finally, if you do require any additional assistance with tackling your IA’s then our online private tuition services might be perfect for you…
Check Out Our 30 Bio IA Topic Ideas !
Share article links
Related Articles

- Most Popular
30 IB Biology IA Topic Ideas!
Are you struggling with choosing your topic for your IB Biology IA? Don’t worry, we’ve all been there. Finding a topic is one of the – if not THE – most important part of writing your IA, so we want to make sure that you get it right! Luckily, there are so many great topics […]
20 IB Physics IA Topic Ideas!
Choosing where to start with an IA can be the hardest part, and this is definitely true for the Physics IA. We know that our topic has to be somewhat related to the syllabus, but where should we focus? Thankfully, we’ve asked some of our favourite IB graduates for some of the ideas they pursued! […]
25 History IA Topic Ideas!
Are you about to start your History internal assessment? We know the struggle. One of the most difficult parts about the task is finding a good History IA topic because it feels like you can just write about anything. The IB breaks it down into 7 main different types of topics that you can choose, […]
Best Chemistry IA Ideas in 2025 | Sorted by Major and Topic
Choosing the right Chemistry IA topic can feel like searching for a needle in a haystack, especially with the pressure to find something unique and impactful. Whether you're fascinated by the chemistry behind medicine, intrigued by the science of dentistry, or passionate about environmental challenges, this guide offers a treasure trove of IB Chemistry IA ideas.
We'll explore experiments that range from investigating the stability of over-the-counter medications to analyzing the impact of acid rain on soil health. These topics not only align with your syllabus but also give you the edge to create an IA that truly shines.
1. Medicine
- Sub-topic: Reactivity 3.1 (Proton transfer reactions)
- Syllabus Point: Reactivity 3.1.6 (Strong and weak acids and bases differ in the extent of ionization)
- Sub-topic: Structure 3.2 (Functional groups: Classification of organic compounds)
- Syllabus Point: Structure 3.2.2 (Functional groups and their reactions)
- Sub-topic: Reactivity 2.2 (How fast? The rate of chemical change)
- Syllabus Point: Reactivity 2.2.3 (Factors that influence the rate of a reaction)
- Syllabus Point: Structure 3.2.9 (Infrared spectra for bond identification)
- Syllabus Point: Structure 3.2.3 (Homologous series and their properties)
- Sub-topic: Structure 2.2 (The covalent model)
- Syllabus Point: Structure 2.2.8 (Intermolecular forces)
- Sub-topic: Reactivity 3.2 (Electron transfer reactions)
- Syllabus Point: Reactivity 3.2.3 (Reactivity of metals with aqueous metal ions)
2. Dentistry
- Syllabus Point: Reactivity 3.1.7 (Acids react with bases in neutralization reactions)
- Syllabus Point: Reactivity 3.1.4 (The pH scale and its relationship with [H+])
- Syllabus Point: Reactivity 3.2.1 (Oxidation and reduction can be described in terms of electron transfer)
- Syllabus Point: Structure 3.2.1 (Identification of functional groups)
- Sub-topic: Structure 2.4 (From models to materials)
- Syllabus Point: Structure 2.4.4 (Polymers: Natural and synthetic)
3. Engineering
- Syllabus Point: Reactivity 2.2.5 (Catalysts and reaction rates)
- Sub-topic: Reactivity 2.3 (How far? The extent of chemical change)
- Syllabus Point: Reactivity 2.3.4 (Le Châtelier’s principle)
- Sub-topic: Structure 2.3 (The metallic model)
- Syllabus Point: Structure 2.3.1 (Metallic bonding)
- Syllabus Point: Structure 2.3.3 (Properties of transition elements)
- Syllabus Point: Structure 2.3.2 (Strength of metallic bonds)
4. Environmental Science
- Syllabus Point: Structure 2.2.10 (Chromatography)
5. Pharmaceutical Sciences
6. biomedical engineering.
Once you've settled on an exciting Chemistry IA topic, the next step is ensuring your experiment is up to IB standards. You can do this by using coursework graders that not only reviews your work for alignment with the IB criteria but also offers personalized feedback to help you refine your investigation.
By using the coursework grader , you can identify areas for improvement, whether it's sharpening your research question or enhancing your data analysis. It's like having a virtual mentor guiding you towards a top-tier IA that impresses both your teachers and examiners.
Ace your exams with RevisionDojo
- Thousands of practice questions
- Study notes and flashcards for every topic and subject
- Free Jojo AI tutor

RevisionDojo, saved my exams. I went from a 4 to a 6 in math, a 5 to a 7 in econ and a 5 to a 7 in chem. ”
Joseph Chan
IBDP student
Rated Excellent
On Trustpilot

IB Chemistry IA: 60 Examples and Guidance
Charles Whitehouse
The IB Diploma programme offers a variety of assessments for students, including Internal Assessments (IAs), which are pieces of coursework marked by students’ teachers. The Chemistry IA is an assessment designed to test students' understanding of the material they have learned in their chemistry course and their ability to conduct independent research. The investigation should be a self-directed study that demonstrates the student's ability to design, execute, and evaluate a scientific investigation.
What is the IA?
The IA consists of a laboratory report that students must complete during their IB chemistry course. For assessments before May 2025, the report should be 6 to 12 pages in length and should include a research question, a methodology section, data analysis, and a conclusion. From May 2025 , the report should be a maximum of 3,000 words.
What should the IA be about?
When choosing a topic for their IA, expert IB tutors recommend that the students should keep in mind that the investigation should be related to the content of the IB Chemistry course. It should also be practical, feasible, and of sufficient complexity to demonstrate their understanding of the subject matter. Some examples of topics that have been used in the past include the determination of the concentration of a substance in a solution, the effect of temperature on a chemical reaction, and the rate of a chemical reaction.
What should the IA contain?
Once a topic has been chosen, students should write a research proposal outlining their investigation. The proposal should include a clear research question, a brief literature review, a detailed methodology, and a list of the equipment and materials that will be needed. The proposal should also include a risk assessment, outlining any hazards associated with the investigation and the measures that will be taken to minimize them.
After the proposal has been approved, students can begin their investigation. They should keep a detailed laboratory notebook, including all the data they collect, any observations they make, and any calculations they perform. They should also take photographs or videos of their experiment to document the process.
Once the investigation is complete, students should analyze their data and draw conclusions. They should process their data using appropriate techniques, such as statistical analysis or graphing, and present it in a clear and concise manner. They should also evaluate their methodology and results, highlighting any limitations or uncertainties.
Finally, students should write a report, summarizing their investigation. The report should include an introduction, a method section, a results section, a discussion section, and a conclusion. The report should also include a list of references, citing any sources that were used in the research proposal or during the investigation.
Have a look at our comprehensive set resources for IB Chemistry developed by expert IB teachers and examiners!
- IB Chemistry 2024 Study Notes
- IB Chemistry 2025 Study Notes
- IB Chemistry 2024 Questions
- IB Chemistry 2025 Questions
How can I do well in the IA?
To prepare for the IA, students should ensure that they understand the material covered in their chemistry course and should practice writing lab reports. They should also seek feedback from their teachers on their writing skills and their understanding of the research process, and can also enlist the help of an IB Chemistry tutor .
Before starting the IA, students should also familiarize themselves with the assessment criteria and the guidelines provided by the IB. This will allow them to show their full potential and achieve the highest mark possible. Students should also make sure that their report is well-written and properly formatted, and that it includes all the required sections.
The assessment criteria include the following:
Personal engagement : Students should engage with the exploration, which can be demonstrated through independent thinking and creativity. The research question or topic should be linked to something of personal significance or interest, and the student should show initiative in implementing the investigation. (2 marks)
Exploration : Students should identify a relevant and fully-focused research question, which is explored with appropriate background information and investigated with an appropriate methodology. The student should consider the safety, ethical, or environmental issues that are relevant to the methodology. (6 marks)
Analysis : Students should demonstrate the ability to analyze data and draw conclusions. They should show that they have used appropriate techniques to process and present data, and that they have identified patterns and trends in the data. The report should include quantitative and qualitative data, which supports a detailed and valid conclusion, following appropriate data processing. (6 marks)
Evaluation : Students should demonstrate an understanding of the limitations and uncertainties of their investigation. They should critically evaluate their methodology and results, and suggest ways in which the investigation could be improved or extended. (6 marks)
Communication : The investigation should be clearly presented, with an effective structure, concise writing, and appropriate use of subject-specific terminology. (4 marks)
What are some example research questions?
Here are a few examples of potential research questions compiled by expert IB Chemistry tutors that could inspire your Chemistry IA:
1 - How does the concentration of a solution affect the rate of reaction between hydrochloric acid and magnesium?
Conduct a series of experiments in which hydrochloric acid is added to different concentrations of magnesium in solution. The rate of reaction could be measured by tracking the production of hydrogen gas over time. The concentration of the solution could be varied by diluting the hydrochloric acid with water. The results could be plotted on a graph to show the relationship between concentration and rate of reaction. Control variables such as temperature and stirring rate would need to be kept constant throughout the experiments.
2 - Can the purity of a sample of aspirin be determined using thin-layer chromatography?
A sample of the aspirin would be dissolved in a suitable solvent and spotted onto a thin-layer chromatography plate. The plate would then be placed in a developing chamber containing a suitable solvent. As the solvent moves up the plate, it will separate the different components of the sample based on their polarity. The purity of the aspirin can be determined by comparing the distance traveled by the aspirin spot to the distance traveled by any impurities or other components present in the sample. This can be done by measuring the Rf value (the ratio of the distance traveled by the spot to the distance traveled by the solvent) for each component. A pure sample of aspirin would have an Rf value of 1, while impurities or other components would have lower Rf values.
3 - Investigating the effect of temperature on the solubility of a salt in water.
Prepare a saturated solution of the salt at room temperature. Then, heat the solution to a higher temperature and add more of the salt until it reaches saturation again. The amount of salt that can dissolve in the water at each temperature can be measured by weighing the solution before and after adding the salt. This process can be repeated at different temperatures to create a solubility curve. The curve can then be used to determine the effect of temperature on the solubility of the salt in water.
4 - How does the concentration of hydrochloric acid affect the reaction rate with sodium thiosulfate?
Conduct a series of experiments in which different concentrations of hydrochloric acid are mixed with a fixed amount of sodium thiosulfate. The reaction rate can be measured by observing the time it takes for the solution to turn cloudy, indicating that the reaction has occurred. The concentration of hydrochloric acid that produces the fastest reaction rate can be determined, and a graph can be created to show the relationship between concentration and reaction rate. Control variables such as temperature and stirring should be kept constant throughout the experiments.
5 - Can the enthalpy change of a chemical reaction be determined using Hess's law and calorimetry?
Use calorimetry to measure the enthalpy change of the individual reactions involved in the chemical reaction of interest. Then, use Hess's law to calculate the enthalpy change of the overall reaction. This would involve setting up a calorimeter, measuring the initial and final temperatures of the reactants and products, and calculating the heat absorbed or released during the reaction. The enthalpy change of the individual reactions could be determined by conducting them separately and measuring the heat change.
6 - Investigating the effect of different types of catalysts on the rate of decomposition of hydrogen peroxide.
Set up an experiment in which hydrogen peroxide is mixed with different types of catalysts, such as manganese dioxide, copper oxide, or iron oxide. The rate of decomposition of the hydrogen peroxide can be measured by monitoring the release of oxygen gas using a gas syringe or pressure sensor. The experiment would need to be repeated with each type of catalyst, and the results can be compared to determine which catalyst is most effective at increasing the rate of decomposition. Control variables such as temperature, concentration of hydrogen peroxide, and volume of catalyst would need to be kept constant.
7 - How does the pH of a solution affect the solubility of a sparingly soluble salt?
Prepare a solution of the sparingly soluble salt in water at a known concentration. Vary the pH of the solution using acidic or basic solutions. The solubility of the salt can be determined by measuring the concentration of the salt in the solution using techniques such as spectrophotometry or gravimetry. The solubility of the salt can then be plotted against the pH of the solution to determine the effect of pH on solubility. This process would need to be repeated for different concentrations of the salt to determine the impact of concentration on solubility.
8 - Can the concentration of a solution be determined using acid-base titration?
To determine the concentration of a solution using acid-base titration, a known volume of the solution would be added to a flask and an indicator would be added. A standardized solution of a strong acid or base would then be slowly added to the flask until the endpoint is reached, indicating that all the acid or base has reacted with the solution. The concentration of the solution can then be calculated based on the volume and concentration of the standardized solution used in the titration. This process would need to be repeated for each solution being tested.
9 - Investigating the effect of different types of surfactants on the surface tension of water.
Prepare solutions of different concentrations of the surfactants being tested. A drop of each solution would be placed on the surface of water and the surface tension of the water would be measured using a tensiometer. The process would be repeated for each concentration of surfactant being tested. The results would be plotted on a graph to determine the relationship between the concentration of surfactant and the surface tension of water.
10 - How does the concentration of a solution affect the rate of reaction between sodium thiosulfate and hydrochloric acid?
Conduct a series of experiments in which different concentrations of sodium thiosulfate and hydrochloric acid are mixed together in a controlled environment. The rate of reaction can be measured by observing the time it takes for the solution to turn cloudy due to the formation of sulfur. The concentration of the solution can be varied by diluting or concentrating the solutions before mixing them together. By comparing the rate of reaction at different concentrations, the relationship between concentration and rate of reaction can be determined.
11 - Can the concentration of copper in a brass alloy be determined using atomic absorption spectroscopy?
Prepare a series of standard solutions of known copper concentrations using a pure copper sample. The brass alloy would then be dissolved in acid and the resulting solution would be analyzed using atomic absorption spectroscopy. The absorption of light by the copper atoms in the solution would be measured and compared to the absorption of the standard solutions to determine the concentration of copper in the brass alloy. This process would need to be repeated for each brass alloy being tested.
12 - Investigating the effect of the length of an alkane chain on its boiling point.
Prepare a series of alkane samples with varying chain lengths. Each sample would be heated and the temperature at which it boils would be recorded. The boiling point of each alkane sample would be plotted against its chain length to determine the relationship between the two variables. This experiment would need to be repeated multiple times to ensure accuracy and consistency of results.
13 - How does the pH of a solution affect the color of an indicator?
Select an appropriate indicator that changes color within the pH range being tested. Prepare solutions with different pH values by adding acids or bases to a neutral solution. Add a small amount of the indicator to each solution and observe the color change. Record the pH value at which the color change occurs for each indicator. This experiment can be repeated with different indicators to compare their sensitivity to changes in pH.
14 - Can the concentration of iron in a solution be determined using spectrophotometry?
Prepare a series of standard solutions with known concentrations of iron. The absorbance of each standard solution would be measured using a spectrophotometer, which would create a calibration curve. A sample of the unknown solution containing iron would then be measured for its absorbance, and the concentration of iron in the solution can be determined by comparing its absorbance to the calibration curve. This process would need to be repeated for each solution being tested.
15 - Investigating the effect of the concentration of a solution on the rate of reaction between potassium permanganate and oxalic acid .
Set up a series of experiments in which different concentrations of the potassium permanganate solution are mixed with a fixed concentration of oxalic acid. The rate of reaction could be measured by monitoring the color change of the solution over time, as the potassium permanganate is reduced by the oxalic acid. The concentration of the potassium permanganate solution that produces the fastest rate of reaction could be determined, and the effect of varying concentrations of oxalic acid could also be investigated. Control variables such as temperature and stirring rate would need to be kept constant throughout the experiments.
16 - How does the presence of a common ion affect the solubility of a salt?
Prepare solutions of the salt at different concentrations and add a known amount of the common ion to each solution. The solubility of the salt in each solution can then be determined by measuring the amount of salt that remains undissolved after stirring the solution for a set period of time. Comparing the solubility of the salt in solutions with and without the common ion would determine the effect of the common ion on the salt's solubility. This process would need to be repeated for different concentrations of the common ion to determine the concentration at which it has the greatest effect on the salt's solubility.
17 - Can the rate constant of a chemical reaction be determined using kinetics experiments?
Conduct a series of experiments in which the concentration of reactants is varied while keeping all other variables constant. The rate of the reaction can be measured by monitoring the change in concentration of the reactants or products over time. The rate constant can then be calculated using the rate equation for the reaction. This process would need to be repeated for different temperatures and concentrations to determine the effect of these variables on the rate constant.
18 - Investigating the effect of different types of acids and bases on the pH of a buffer solution.
Prepare a buffer solution with a known pH and add different types of acids and bases to it. The pH of the buffer solution would be measured using a pH meter or indicator paper before and after the addition of each acid or base. The change in pH would indicate the effect of the acid or base on the buffer solution. This process would need to be repeated for each type of acid and base being tested. The results could be compared to determine which types of acids and bases have the greatest impact on the pH of the buffer solution.
19 - How does the concentration of a solution affect the absorbance of light by a colored compound?
Prepare a series of solutions with varying concentrations of the colored compound. Use a spectrophotometer to measure the absorbance of light by each solution at a specific wavelength. Plot the absorbance values against the concentration of the colored compound to create a calibration curve. Use this curve to determine the concentration of the colored compound in an unknown solution by measuring its absorbance and comparing it to the calibration curve. This process would need to be repeated for each colored compound being tested.
20 - Can the concentration of ammonia in a solution be determined using acid-base titration?
Prepare a standardized solution of a known concentration of acid or base. A sample of the ammonia solution would be titrated with the acid or base solution until the endpoint is reached, indicating that all the ammonia has reacted with the acid or base. The concentration of ammonia in the solution can then be calculated based on the volume and concentration of the acid or base solution used in the titration. This process would need to be repeated for each ammonia solution being tested.
21 - Investigating the effect of different types of buffers on the pH of a solution.
Prepare solutions of different buffers and measure their pH using a pH meter. Then, add a small amount of acid or base to each solution and measure the change in pH. The buffer that maintains the pH closest to its original value would be the most effective. This process would need to be repeated for each type of buffer being tested. The results could be graphed to visually compare the effectiveness of each buffer.
22 - How does the concentration of a solution affect the rate of reaction between iodine and sodium thiosulfate?
Prepare solutions of different concentrations of sodium thiosulfate and iodine. The reaction between the two solutions can be timed and the rate of reaction calculated for each concentration. The results can be graphed to show the relationship between concentration and reaction rate. This experiment would need to be repeated multiple times to ensure accuracy and to account for any experimental error.
23 - Can the concentration of a metal ion in a solution be determined using complexometric titration?
Prepare a standardized solution of a chelating agent, such as EDTA, of a known concentration. A sample of the metal ion solution would be titrated with the chelating agent until the endpoint is reached, indicating that all the metal ions have reacted with the chelating agent. The concentration of the metal ion in the solution can then be calculated based on the volume and concentration of the chelating agent used in the titration. This process would need to be repeated for each metal ion being tested.
24 - Investigating the effect of the length of a chain on the rate of esterification.
Set up an experiment in which different lengths of chains are used in the esterification reaction. The reaction could be monitored by measuring the amount of product formed over time using a spectrophotometer or by analyzing the product using gas chromatography. The rate of esterification could be calculated by determining the slope of the reaction curve. Comparing the rates of esterification for the different chain lengths would determine the effect of chain length on the reaction rate.
25 - How does the pH of a solution affect the rate of reaction between sodium thiosulfate and hydrochloric acid?
Set up a series of solutions with varying pH levels using hydrochloric acid and sodium thiosulfate. The reaction between the two chemicals would be timed and the time taken for the solution to turn cloudy would be recorded. The experiment would need to be repeated multiple times for each pH level to ensure accuracy. The data collected would then be used to plot a graph of the reaction rate against pH level, allowing for the relationship between pH and reaction rate to be determined.
26 - Can the concentration of a solution be determined using gravimetric analysis?
In gravimetric analysis, a known mass of the substance being analyzed is dissolved in a solvent and then reacted with a reagent that forms a precipitate with the substance of interest. The precipitate is then filtered, dried, and weighed to determine its mass. From this, the mass of the original substance can be calculated using stoichiometry. Therefore, to determine the concentration of a solution using gravimetric analysis, a known volume of the solution would need to be evaporated to dryness, and the resulting solid would be weighed. The mass of the solid would then be used to calculate the concentration of the original solution.
27 - Investigating the effect of different types of surfactants on the emulsification of oil and water.
Create a series of oil and water emulsions using different types and concentrations of surfactants. The emulsions could be visually inspected for stability and the time it takes for the oil and water to separate could be recorded. The effectiveness of each surfactant in emulsifying the oil and water could be compared by analyzing the data collected. Additionally, the size and distribution of the droplets in the emulsion could be measured using microscopy to gain a more detailed understanding of the emulsification process.
28 - How does the concentration of a solution affect the rate of reaction between potassium permanganate and hydrogen peroxide?
Set up a series of experiments in which different concentrations of potassium permanganate and hydrogen peroxide are mixed together. The reaction rate could be measured by tracking the change in color of the solution over time, as the potassium permanganate is reduced. The concentration of the reactants could be varied by diluting them with water, and the reaction rate could be measured for each concentration. The results could then be plotted on a graph to show the relationship between concentration and reaction rate.
29 - Can the concentration of sulfate ions in a solution be determined using gravimetric analysis?
To determine the concentration of sulfate ions in a solution using gravimetric analysis, a known volume of the solution would be evaporated to dryness to obtain the sulfate ions in solid form. The mass of the solid sulfate would be measured and compared to the mass of the original sample to determine the percentage of sulfate ions present. This process would need to be repeated for multiple samples of the solution to ensure accuracy and precision in the results.
30 - Investigating the effect of different types of acids and bases on the rate of reaction between hydrochloric acid and sodium thiosulfate.
Set up an experiment in which hydrochloric acid and sodium thiosulfate are mixed with different types and concentrations of acids and bases. The reaction between the two chemicals would produce a yellow precipitate of sulfur, which would gradually become less visible as the reaction progresses. The time taken for the precipitate to disappear could be measured and used to calculate the rate of reaction. Comparing the rates of reaction for the different groups would determine the effect of the acids and bases on the reaction between hydrochloric acid and sodium thiosulfate.
31 - Investigating the effects of different types of catalysts on the rate of a chemical reaction.
Set up an experiment in which a chemical reaction is carried out with different catalysts. The reaction should be monitored using a suitable method such as spectrophotometry or gas chromatography to determine the rate of the reaction. The same reaction should be carried out with each catalyst, and the results should be compared to determine the effect of the catalyst on the rate of the reaction. Control variables such as temperature, pressure, and concentration of reactants should be kept constant to ensure accurate results.
32 - How does the concentration of a reactant affect the rate of a chemical reaction?
Conduct a series of experiments in which the concentration of the reactant is varied while keeping all other variables constant. The rate of the chemical reaction can be measured by monitoring the change in concentration of the reactant or product over time. A graph can be plotted to show the relationship between the concentration of the reactant and the rate of the reaction. This can be used to determine the rate law for the reaction, which can then be used to predict the rate of the reaction under different conditions.
33 - Investigating the properties of different types of acids and bases and their behavior in different solutions.
Conduct experiments in which different types of acids and bases are added to different solutions, such as water or other acids/bases. The behavior of the acids and bases can be observed, such as whether they dissolve or react with the solution, and the pH of the solution can be measured. The properties of the acids and bases, such as their strength and reactivity, can be compared based on their behavior in the different solutions. This could also involve testing the effect of different concentrations of the acids and bases on the pH of the solution.
34 - How does the temperature affect the solubility of a solute in a solvent?
Prepare a solution of the solute in the solvent at a known concentration and temperature. The solution would then be cooled or heated to different temperatures and the solubility of the solute in the solvent would be measured at each temperature. This could be done by adding a known amount of the solute to the solvent at each temperature and measuring the amount of solute that dissolves. The results could be plotted on a solubility curve to show the relationship between temperature and solubility.
35 - Investigating the properties of different types of polymers and their behavior in different environments.
Conduct experiments in which different types of polymers are exposed to different environmental conditions, such as temperature, humidity, and UV radiation. The behavior of the polymers could be observed and measured using techniques such as tensile testing, thermal analysis, and microscopy. Comparing the properties and behavior of the different polymers in different environments would provide insights into their suitability for various applications.
Get expert help with your IB Chemistry
The world's leading online IB Chemistry tutoring provider trusted by students, parents, and schools globally.
4.93 /5 based on 486 reviews
36 - How does the concentration of a solute affect the osmotic pressure of a solution?
Set up a series of solutions with varying concentrations of the solute and measure the osmotic pressure of each solution using an osmometer. The osmotic pressure can be calculated by measuring the change in pressure as the solution is introduced to a semi-permeable membrane. The results can then be plotted on a graph to determine the relationship between solute concentration and osmotic pressure. This experiment could be repeated with different solutes to compare their effects on osmotic pressure.
37 - Investigating the properties of different types of surfactants and their behavior in different solutions.
Conduct experiments in which different types of surfactants are added to different solutions, such as water or oil. The behavior of the surfactants can be observed, including their ability to reduce surface tension and form micelles. The properties of the surfactants can also be tested, such as their solubility in different solvents and their stability under different conditions. The results of these experiments can be used to compare the effectiveness of different surfactants in different applications, such as in cleaning products or in the production of emulsions.
38 - How does the temperature affect the conductivity of an electrolyte solution?
Conductivity measurements of an electrolyte solution would need to be taken at different temperatures using a conductivity meter. The temperature of the solution can be controlled using a water bath or other temperature control device. The conductivity readings can be plotted against temperature to determine the effect of temperature on conductivity. The experiment would need to be repeated multiple times to ensure accuracy and consistency of results.
39 - Investigating the properties of different types of metal alloys and their behavior under different conditions.
Conduct experiments on different types of metal alloys under varying conditions such as temperature, pressure, and exposure to different chemicals. The properties of the alloys such as strength, ductility, and corrosion resistance could be measured and compared to determine their behavior under different conditions. This would require specialized equipment such as a tensile testing machine and a corrosion testing apparatus. The results of these experiments could be used to optimize the use of different alloys in various applications.
40 - How does the concentration of a solution affect the boiling and freezing points of the solvent?
Conduct an experiment in which different concentrations of a solution are prepared and their boiling and freezing points are measured using a thermometer. The data collected can be used to create a graph showing the relationship between concentration and boiling/freezing point. This graph can be used to determine the effect of concentration on the boiling and freezing points of the solvent. Control variables such as pressure and volume of the solution should be kept constant throughout the experiment.
41 - Investigating the properties of different types of gas laws and their behavior under different conditions.
Conduct experiments using different gases, such as helium, nitrogen, and oxygen, under varying conditions of temperature and pressure. The behavior of the gases could be observed using equipment such as pressure gauges and thermometers. The data collected could then be analyzed to determine the properties of each gas and how they behave under different conditions. This could include measuring the volume of gas at different pressures, or the pressure of gas at different temperatures. The results could then be used to develop mathematical models of gas behavior, such as the ideal gas law.
42 - How does the concentration of a solution affect the rate of diffusion and effusion?
Set up a series of experiments in which solutions of varying concentrations are placed in separate compartments of a diffusion or effusion apparatus. The rate of diffusion or effusion could be measured by tracking the movement of a dye or gas through a semi-permeable membrane separating the compartments. The rate of diffusion or effusion could then be compared across the different concentrations to determine the effect of concentration on the rate of diffusion or effusion. Control variables such as temperature and pressure would need to be kept constant throughout the experiments.
43 - Investigating the properties of different types of nuclear reactions and their behavior under different conditions.
Conduct experiments with different types of nuclear reactions, such as fission and fusion, under varying conditions such as temperature, pressure, and reactant concentration. The behavior of the reactions can be observed and recorded, and data can be analyzed to determine the properties of each type of reaction. This could include factors such as energy release, reaction rate, and byproducts produced. The results of these experiments can be used to better understand the behavior of nuclear reactions and their potential applications.
44 - How does the temperature affect the viscosity of a liquid?
Measure the viscosity of a liquid at different temperatures using a viscometer. The temperature of the liquid can be controlled using a water bath or other heating/cooling apparatus. The viscosity can be measured by timing how long it takes for the liquid to flow through the viscometer at each temperature. The results can be plotted on a graph to show how the viscosity changes with temperature. This can help determine the optimal temperature for the liquid's intended use or provide insight into the physical properties of the liquid.
45 - Investigating the properties of different types of organic compounds and their behavior under different conditions.
Conduct a series of experiments to investigate the properties of different types of organic compounds. This could involve testing their solubility in different solvents, their reactivity with other compounds, their melting and boiling points, and their behavior under different conditions such as heat or pressure. The results of these experiments could be used to develop a better understanding of the behavior and properties of organic compounds, which could have applications in fields such as medicine, agriculture, and materials science.
46 - How does the concentration of a solution affect the pH of the solution?
Prepare solutions of varying concentrations of an acidic or basic substance, such as hydrochloric acid or sodium hydroxide. The pH of each solution would be measured using a pH meter or indicator paper. The results would be recorded and analyzed to determine the relationship between the concentration of the solution and its pH. A graph could be created to visualize this relationship.
47 - Investigating the properties of different types of electrochemical cells and their behavior under different conditions.
Set up different electrochemical cells using different electrodes and electrolytes. Measure the voltage and current produced by each cell under different conditions such as temperature, concentration of electrolyte, and electrode surface area. Analyze the data to determine the behavior of each cell and compare their properties. This could include calculating the cell potential, determining the rate of reaction, and identifying any limitations or advantages of each type of cell.
48 - How does the concentration of a solution affect the color and absorption spectrum of a chromophore?
Prepare a series of solutions with varying concentrations of the chromophore. The absorption spectra of each solution could be measured using a spectrophotometer, and the color of each solution could be observed visually. By comparing the absorption spectra and colors of the different solutions, the relationship between concentration, color, and absorption spectrum of the chromophore could be determined. This could be further analyzed using mathematical models to predict the absorption spectrum and color of solutions with different concentrations of the chromophore.
49 - Investigating the properties of different types of covalent compounds and their behavior under different conditions.
Conduct experiments on different covalent compounds under varying conditions such as temperature, pressure, and pH levels. Observe and record their behavior, including changes in state, solubility, and reactivity. Analyze the data to determine the properties of each compound and how they respond to different conditions. This could involve using techniques such as spectroscopy, chromatography, and mass spectrometry to identify and characterize the compounds. The results could be used to develop a better understanding of the behavior of covalent compounds and their potential applications in various fields.
50 - How does the temperature affect the rate of diffusion and effusion?
Set up an experiment in which a gas is released in a container at a constant rate and the time it takes for the gas to diffuse or effuse through a small opening is measured at different temperatures. The temperature can be varied by immersing the container in a water bath of different temperatures. The rate of diffusion or effusion can be calculated based on the time taken for the gas to pass through the opening, and the temperature can be varied to determine its effect on the rate of diffusion or effusion. The results can be plotted on a graph to visualize the relationship between temperature and the rate of diffusion or effusion.
51 - Investigating the properties of different types of intermolecular forces and their behavior under different conditions.
Conduct experiments using different substances with different types of intermolecular forces, such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces. The substances could be tested under different conditions, such as temperature and pressure, to observe how the intermolecular forces affect their behavior. The results could be analyzed to determine the properties of each type of intermolecular force and how they interact with each other. This could lead to a better understanding of the behavior of substances in various environments.
52 - How does the concentration of a solution affect the rate of an acid-base titration?
Prepare a standardized solution of a strong acid or base of known concentration. A sample of the solution being tested would be titrated with the acid or base solution until the endpoint is reached, indicating that all the acid or base has reacted with the solution. The concentration of the solution being tested can then be calculated based on the volume and concentration of the acid or base solution used in the titration. This process would need to be repeated for solutions of varying concentrations to determine the effect of concentration on the rate of the acid-base titration.
53 - Investigating the properties of different types of coordination compounds and their behavior under different conditions.
Conduct experiments to observe the behavior of different coordination compounds under varying conditions such as temperature, pH, and concentration. The properties of the compounds such as color, solubility, and stability could be measured and compared. The results could be analyzed to determine the effect of the different conditions on the behavior of the coordination compounds. This could provide insight into the potential applications of these compounds in various fields such as medicine or materials science.
54 - How does the concentration of a solution affect the equilibrium constant of a chemical reaction?
Conduct a series of experiments in which the concentration of a reactant or product is varied while keeping other variables constant. The equilibrium constant of the chemical reaction can then be calculated using the concentrations of the reactants and products at equilibrium. This process would need to be repeated for different initial concentrations of the reactants to determine the effect of concentration on the equilibrium constant. Graphing the data would help visualize the relationship between concentration and equilibrium constant.
55 - Investigating the properties of different types of chromatography and their behavior in different separation techniques.
Conduct a series of experiments using different types of chromatography, such as paper chromatography, thin-layer chromatography, and gas chromatography. Each experiment would involve separating a mixture of substances using the chosen chromatography technique and analyzing the results to determine the effectiveness of the technique in separating the substances. The behavior of the chromatography technique could be studied by varying the solvent used, the type of stationary phase, and other experimental conditions. The results of the experiments could be compared to determine the most effective chromatography technique for different types of separations.
56 - How does the temperature affect the activation energy of a chemical reaction?
Conduct a series of experiments in which the same chemical reaction is carried out at different temperatures. The activation energy of the reaction can be calculated by measuring the rate of the reaction at each temperature and using the Arrhenius equation to determine the activation energy. The results can be plotted on a graph to show the relationship between temperature and activation energy. This would help to determine how temperature affects the rate of chemical reactions.
57 - Investigating the properties of different types of solid-state materials and their behavior under different conditions.
Conduct experiments on different types of solid-state materials, such as metals, ceramics, and polymers, under different conditions such as temperature, pressure, and humidity. The properties that could be investigated include strength, elasticity, conductivity, and thermal expansion. The results of these experiments could be used to compare the behavior of different materials and to identify the most suitable material for a particular application. The data collected could also be used to develop models and simulations to predict the behavior of materials under different conditions.
58 - How does the concentration of a solution affect the rate of a redox reaction?
Conduct a series of experiments in which the concentration of a solution is varied while keeping all other variables constant. The redox reaction could be monitored using a colorimetric assay or by measuring the change in pH of the solution. The rate of the reaction could then be calculated based on the change in absorbance or pH over time. By comparing the rates of the reaction at different concentrations, the effect of concentration on the rate of the redox reaction could be determined.
59 - Investigating the properties of different types of nanomaterials and their behavior under different conditions.
Conduct experiments with different types of nanomaterials, varying their size, shape, and composition, and observe their behavior under different conditions such as temperature, pressure, and exposure to different chemicals. The properties of the nanomaterials, such as their conductivity, reactivity, and strength, could be measured using various techniques such as microscopy, spectroscopy, and mechanical testing. The results could be analyzed to determine the optimal conditions for each type of nanomaterial and to compare their properties to identify the most suitable material for specific applications.
60 - How does the concentration of a solution affect the rate of a precipitation reaction?
Set up multiple solutions of the same reactants with varying concentrations. The rate of precipitation can be measured by tracking the time it takes for the precipitate to form or by measuring the amount of precipitate formed over a set period of time. By comparing the rates of precipitation in the different solutions, the effect of concentration on the rate of the reaction can be determined. Control variables such as temperature and stirring rate would need to be kept constant.
Remember to come up with your own original IA topic and check it with your teacher. It should be practical to conduct and relevant to the syllabus. This is a great opportunity to develop your personal interests, while advancing your knowledge of the chemistry curriculum. Online tutors agree that this list is quite extensive and can help IB students a lot with their IB Chemistry IA.
TutorChase's IB Chemistry Study Notes , IB Past Papers and IB Chemistry Questions are the perfect resource for students who want to get a 7 in their IB Chemistry exams and also prepare for the internal assessment. They are completely free, cover all topics in depth, and are structured by topic so you can easily keep track of your progress.
How is the IA graded?
The IA is worth 20% of the final grade for the IB chemistry course, whether you are studying at Higher or at Standard Level. This applies for assessments both before and after May 2025. It is graded by the student’s teacher, who is trained and certified by the International Baccalaureate organization. The report is then sent to a moderator, who will check that the report adheres to the IB guidelines and that the grade awarded is appropriate.
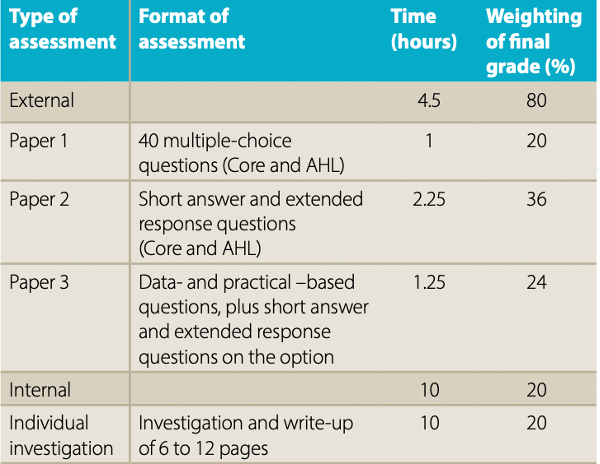
Source: IB Chemistry Subject Brief, pre-May 2025
In summary, the IA in the IB is an opportunity for students to demonstrate their understanding of the chemistry curriculum, as well as their ability to conduct independent research. It consists of a laboratory report and a reflective statement, and is worth 20% of the final grade for the course. To prepare for the assessment, students should ensure that they understand the material covered in their IB chemistry course , practice writing lab reports, review their IB Chemistry Q&A Revision Notes , and seek feedback from their teachers or tutors.
Need help from an expert?
The world’s top online tutoring provider trusted by students, parents, and schools globally.
Study and Practice for Free
Trusted by 100,000+ Students Worldwide
Achieve Top Grades in your Exams with our Free Resources.
Practice Questions, Study Notes, and Past Exam Papers for all Subjects!
Need Expert Help?
If you’re looking for assistance with IB Chemistry, get in touch with the TutorChase team and we’ll be able to provide you with an expert IB Chemistry tutor . We’ll be there every step of the way!

Professional tutor and Cambridge University researcher

Written by: Charles Whitehouse
Charles scored 45/45 on the International Baccalaureate and has six years' experience tutoring IB and IGCSE students and advising them with their university applications. He studied a double integrated Masters at Magdalen College Oxford and has worked as a research scientist and strategy consultant.
Related Posts

IB Biology IA: 60 Examples and Guidance

IB Maths IA: 60 Examples and Guidance

IB Chemistry: A Complete Guide (2024)

Hire a tutor
Please fill out the form and we'll find a tutor for you
- Select your country
- Afghanistan
- Åland Islands
- American Samoa
- Antigua and Barbuda
- Bosnia and Herzegovina
- Bouvet Island
- British Indian Ocean Territory
- Brunei Darussalam
- Burkina Faso
- Cayman Islands
- Central African Republic
- Christmas Island
- Cocos (Keeling) Islands
- Congo, The Democratic Republic of the
- Cook Islands
- Cote D'Ivoire
- Czech Republic
- Dominican Republic
- El Salvador
- Equatorial Guinea
- Falkland Islands (Malvinas)
- Faroe Islands
- French Guiana
- French Polynesia
- French Southern Territories
- Guinea-Bissau
- Heard Island and Mcdonald Islands
- Holy See (Vatican City State)
- Iran, Islamic Republic Of
- Isle of Man
- Korea, Democratic People'S Republic of
- Korea, Republic of
- Lao People'S Democratic Republic
- Libyan Arab Jamahiriya
- Liechtenstein
- Macedonia, The Former Yugoslav Republic of
- Marshall Islands
- Micronesia, Federated States of
- Moldova, Republic of
- Netherlands
- Netherlands Antilles
- New Caledonia
- New Zealand
- Norfolk Island
- Northern Mariana Islands
- Palestinian Territory, Occupied
- Papua New Guinea
- Philippines
- Puerto Rico
- Russian Federation
- Saint Helena
- Saint Kitts and Nevis
- Saint Lucia
- Saint Pierre and Miquelon
- Saint Vincent and the Grenadines
- Sao Tome and Principe
- Saudi Arabia
- Serbia and Montenegro
- Sierra Leone
- Solomon Islands
- South Africa
- South Georgia and the South Sandwich Islands
- Svalbard and Jan Mayen
- Switzerland
- Syrian Arab Republic
- Taiwan, Province of China
- Tanzania, United Republic of
- Timor-Leste
- Trinidad and Tobago
- Turkmenistan
- Turks and Caicos Islands
- United Arab Emirates
- United Kingdom
- United States
- United States Minor Outlying Islands
- Virgin Islands, British
- Virgin Islands, U.S.
- Wallis and Futuna
- Western Sahara

Alternatively contact us via WhatsApp, Phone Call, or Email

50+ IB Chemistry IA Ideas
Request free trial class, ib chemistry hl ia ideas.
IB Chemistry HL IA ideas list is available online with expert guidance and examples. The list contains 20 to 50+ IA ideas, including research questions and topic descriptions and IB Chemistry HL Internal assessment examples. Choosing the perfect IB Chemistry IA topic can be made easier by approaching it methodically and evaluating existing research before conducting further research on a topicChemistry HL IA ideas
1.) Does the time of cooking superfoods affect Vitamin C content that leaches into the water?
Experimental setup:.
To determine the amount of vitamin C content in the water after boiling the vegetables, a titration reaction will be performed. The end point of the titration is detected when the color of the solution turns blue black after all the ascorbic acid is used up (the volume of iodine solution used will be recorded)
Independent Variables:
Cooking time of superfoods, superfoods
Constant Variables:
Temperature of cooking water, Volume of water boiled, Starch indicator and amount, mass of vegetables, etc.
Dependent Variables:
Volume of iodine solution used
2.) Impact of temperature on dissolved oxygen concentration
Using the Winkler method to measure the dissolved oxygen concentrations in tap water at varying temperatures of the water samples.
Temperature of the water samples
‘Pressure and salinity of the water, Time each sample is left
DO concentration of the water sample
3.) How does amount of aspirin moving through a partially permeable membrane change with time?
To determine the concentration of aspirin passed out of the vising tubing, a titration reaction is performed. The endpoint is indicated by a permanent trace of a pink color due to the formation of a basic solution.
Number of aspirin tablets, Volume of water, Amount of indicator
Volume of Sodium Hydroxide
4.) Calculating peroxide value using iodometric titration in different cooking oils
Any friable food is put in the oil and is let to fry for about 25-30 seconds. After each cycle, some of the oil is stored aside to perform the titration. This is repeated until the same oil has undergone the frying process 5 times. The experiment is to see how the peroxide value changes for the oil when it is fried once and five times. To determine the amount of peroxide value, a titration reaction will be performed and the volume of sodium thiosulfate used in titration is recorded. The end point of the titration is detected when the color of the solution turns blue black. Using the volume of solution used in the titration procedure, the peroxide value is calculated.
Type of oil, antioxidant
Temperature of the oil, Mass of antioxidant, oil and the frying food, The concentration of Sodium Thiosulfate
The volume of sodium thiosulfate used in titration, Peroxide value
5.) Investigating the degradation of vitamin C in tablets
Samples of powdered vitamin C tablets were exposed to the atmosphere for different periods of time, and then dissolved in distilled water. 1 ml of the solution was then titrated with DCPIP (Di-chlorophenol indophenol) stock solution until the solution retained the dark blue-black color. Using the raw data obtained, the shelf life of the vitamin C tablets and the rate of degradation of Vitamin C were calculated.
The time each group of tablets are exposed to the atmosphere
Brand of the tablets, mass of Vitamin C dissolved in distilled water
Mass of Vitamin C present in the tablets
6.) How does the change in temperature of acetate buffer affect the pH of the acetate buffer when sodium hydroxide is added?
The temperature of the buffer solution will be changed by 20, 30 up to 60 degrees and the pH of the solution will be measured until the buffer reaches a pH of 12. Then, with that, the buffer capacity is calculated along with the temperature dependance of the buffer solution. Firstly, sodium acetate is prepared. Then, sodium hydroxide solution is made. Then, the acetate buffer is prepared. The burettes are then set up according to the experimental setup. Then the buffer solution is heated. Afterward, the acetate buffer solution is set up below the burette system and titration starts. The same is repeated for different temperatures.
Temperature of the acetate buffer
Initial concentration of acetate buffer and sodium hydroxide, volume of the solutions
pH of the acetate buffer
7.) Does the structure of an alcohol affect its standard enthalpy of combustion?
Set up the appropriate experiment setup. Measure water and put it into the metal can. Pour an alcohol into a spirit burner and then adjust the wick accordingly. Weigh the spirit burner with the cap. Stir the water in the metal can and record the temperature. Remove the cap of the spirit burner and light the wick. Immediately, put it under the metal can of water and continuously stir the water. When there has been a 10 degree rise in the temperature, blow out the flame and replace the cap. Reweigh the spirit burner. Repeat with other alcohols.
Alcohol used
Volume of water used, Distance from can to wick, Temperature rise, etc.
Mass of alcohol lost
8.) The effect of cyanuric acid on chlorine concentration in swimming pools with 2 hours of sun exposure. (To what extent is the concentration of free chlorin in swimming pools affected by different concentrations of cyanuric acid in water samples containing urea compared to urea free samples?)
To determine the concentration of free chlorine in the water samples, an iodometric redox titration technique is used. Three water samples with different chlorine concentration are obtained. Afterwards, Potassium iodide and starch indicator to determine if they are able to indicate these small concentrations of chlorine in the samples and change the color of the solution. Titration was performed using the water samples and the sodium thiosulfate. The amount of sodium thiosulfate used will determine the amount of free chlorine present.
Concentration of cyanuric acid
pH, temperature, initial chlorine concentration, concentration of urea, etc.
Concentration of free chlorine
9.) Investigating the effect of a transition metal catalyst on the activation energy of the reaction between Iron nitrate and Sodium thiosulfate
The reactants were placed in individual test tubes. The test tubes filled with reactant solution were placed into the test tube rack and the thermometer was put in one of the test tubes. The test tube rack was placed into the Clifton Water Bath. The test tubes were left in the water bath for 10 minutes, and the reactant solutions were made sure to have achieved the desired temperature by using the thermometer. A cross was drawn on the paper using the pen, and then placed on a surface ready for a measuring cylinder to be put above it. Five measuring cylinders were placed next to the water bath. The Iron(IlI) nitrate reactant solution was poured into the measuring cylinder placed above the cross. The sodium thiosulfate reactant solution was soon after poured into the same measuring cylinder, and then the measuring cylinder was immediately afterwards observed from above. The timer started and stopped as soon as the cross was visible to the naked eye from the top of the measuring cylinder and the time was recorded on the data table.
Temperature
Concentration and Volume of Reactant and Catalyst solutions, Reaction vessel, etc
Time taken for the reaction to reach the end point
10.) Composition of Different Shades of Lipsticks and the effect of pH on them?
Utilising chromatography, identify and analyse the various pigments within various shades of lipstick and then heating these samples and measuring the degradation of certain pigments
Lipstick shades
Degradation of certain pigments
11.) To what extent do different transition metal catalysts affect the rate of a reaction?
Procuring various transition metal catalysts and measuring their effect on the rate of any reaction by measuring the concentration of a substance with accordance to time
Transition metal catalysts
Rate of reaction
12.) Investigating the Relationship between Concentration and Optical Rotation of Sucrose Solutions using Polarimetry
In this experiment, we aim to determine the relationship between the concentration of a sucrose solution and its optical rotation. We will measure the optical rotation of sucrose solutions of varying concentrations using a polarimeter. We will prepare solutions of sucrose of concentrations ranging from 1% to 10%. Each solution will be measured in triplicate to ensure precision in the readings. The optical rotation will be recorded as degrees of rotation, and a graph of the optical rotation versus concentration will be plotted.
Concentration of the sucrose solution
The temperature of the solution, the length of the polarimeter tube, and the wavelength of the light used
Optical rotation of the sucrose solution
13.) Investigating the effect of pH on enzyme activity
Enzymes are biological catalysts that speed up chemical reactions. The activity of enzymes is affected by the pH of the solution they are in. In this experiment, you could investigate how the activity of an enzyme (e.g. catalase) changes as you vary the pH of the solution. You could measure the rate of an enzyme-catalyzed reaction (e.g. decomposition of hydrogen peroxide) at different pH values.
pH of the solution
Concentration of the enzyme, Concentration of the substrate, and the temperature
Rate of the reaction (measured as the amount of gas produced over time)

14.) Testing the Accuracy of the Winkler Method for Measuring Dissolved Oxygen in Water Samples
To do this experiment, we will collect several water samples from different sources and measure their dissolved oxygen concentration using both the Winkler method and the dissolved oxygen meter. We will then compare the results and calculate the percentage error for each method. We will also investigate the effect of temperature and pH on the accuracy of the Winkler method by repeating the measurements at different temperature and pH levels.
Water sample source, temperature, and pH
The method of measurement (Winkler method and dissolved oxygen meter)
Dissolved oxygen concentration
15.) Investigate the effect of temperature on the equilibrium constant of a chemical reaction between iodine and thiosulfate ions
To conduct this experiment, we would first prepare a solution of sodium thiosulfate and potassium iodide in a conical flask. We would then add a known amount of iodine solution to the flask and allow the reaction to reach equilibrium. This can be detected by adding starch solution, which forms a blue-black color in the presence of iodine. We would then titrate the remaining iodine with sodium thiosulfate solution to determine the concentration of iodine that was consumed in the reaction. We would then repeat the experiment at different temperatures, using a water bath or a thermostatically controlled heating mantle to maintain the temperature. We would measure the equilibrium constant at each temperature by calculating the concentration of iodine that was consumed in the reaction and using an equation.
Initial concentrations of the reactants, the volume of the reaction mixture, and the titration procedure used to determine the concentration of iodine
Equilibrium Constant
16.)Investigating the Effect of Storage Temperature on the Sulfite Concentration in Wine
The aim of this experiment is to determine how storage temperature affects the sulfite concentration in wine. Different samples of wine will be stored at various temperatures (e.g. 5°C, 15°C, and 25°C) for a set period of time. The sulfite concentration in each sample will then be measured using a spectrophotometer. The results will be analyzed to identify any correlations between storage temperature and sulfite concentration.
Storage temperature (5°C, 15°C, and 25°C)
Type of wine, initial sulfite concentration, duration of storage, volume of wine sampled, method of sulfite measurement
Sulfite concentration in wine
17.) Investigating the effect of temperature on the strength of ferromagnets
A sample of a ferromagnetic material such as iron or nickel will be subjected to different temperatures ranging from -10°C to 100°C. A magnetometer will be used to measure the strength of the material’s magnetic field. The experiment will be repeated three times for each temperature, and the average magnetic field strength will be calculated.
Type of ferromagnetic material, size and shape of the sample, the distance of the magnetometer from the sample, and the strength of the magnetometer
Magnetic field strength
18.) Determination of the Concentration of an Acid Using a Back Titration with a Base
In this experiment, you would determine the concentration of an unknown acid by back titration with a standard solution of a base. You would first react to a known amount of the acid with an excess of a known concentration of a base. The remaining base in the solution would then be titrated with a standard solution of an acid to determine the amount of base that was not consumed in the first reaction. From this, you could calculate the amount of acid that was consumed in the first reaction and therefore the concentration of the unknown acid.
the volume and concentration of the base used in the first reaction.
the volume and concentration of the unknown acid, the volume of the base added, the temperature of the solutions, the accuracy of the burette, and the pH indicator used.
the volume and concentration of the standard acid solution needed to neutralize the excess base.
19.) Investigating the effect of different ligands on the structure and stability of metal complexes
You could synthesize several metal complexes using a common transition metal (e.g. cobalt) and various organic ligands (e.g. ammonia, ethylenediamine, diethylenetriamine). You could then analyze the complexes using various techniques such as UV-Vis spectroscopy, infrared spectroscopy, and melting point determination to determine their structure and stability. You could also compare the reactivity of the complexes with different ligands by performing a substitution reaction to displace one ligand with another.
the different ligands used to synthesize the metal complexes.
the same transition metal used to synthesize all the complexes, the concentration of the metal ion in the reaction mixture, the solvent used to dissolve the metal ion and ligand, and the temperature at which the reaction is carried out.
the structural and stability differences between the metal complexes synthesized with different ligands
20.) How does the concentration of a solute affect the rate of crystal formation during the process of crystallization?
In this experiment, the effect of the concentration of a solute on the rate of crystal formation during crystallization will be investigated. Sodium chloride will be used as the solute, and distilled water will be used as the solvent. The experiment will involve dissolving varying amounts of sodium chloride in the distilled water to create solutions of different concentrations. The solutions will then be placed in a controlled environment to allow for crystallization to occur. The rate of crystal formation will be measured by observing and recording the time taken for crystals to form in each solution.
The concentration of the solute (sodium chloride)
Temperature, volume of solvent, type of solute, method of mixing the solution
The rate of crystal formation
21.) How does the wavelength of light affect the accuracy and precision of a spectrometer?
In this experiment, the accuracy and precision of a spectrometer will be investigated with respect to the wavelength of light used. A spectrometer will be set up and calibrated with a known light source, and measurements will be taken at various wavelengths using a series of calibration lamps. The spectrometer readings will be compared to known values to determine accuracy, and multiple measurements will be taken at each wavelength to determine precision.
The wavelength of light used in the experiment
The same spectrometer will be used throughout the experiment, with the same calibration lamps and settings, the temperature and humidity of the room and the same operator to avoid human error
The accuracy and precision of the spectrometer readings
22.) How does the activation energy of the reaction between hydrochloric acid and sodium thiosulfate change with temperature?
The aim of this experiment is to investigate the effect of temperature on the activation energy of the reaction between hydrochloric acid and sodium thiosulfate. To do this, a series of experiments will be conducted, in which the temperature of the reaction mixture will be varied, and the rate of the reaction will be measured. The reaction will be monitored by measuring the time taken for a cross drawn on a piece of paper to disappear. The temperature of the reaction mixture will be varied by placing the reaction vessel in a water bath set to a specific temperature.
The temperature of the reaction mixture, The concentration of the reactants
The volume of the reaction mixture, and the method of mixing
The rate of the reaction
23.) How does changing the concentration of the electrolyte affect the potential difference and current generated in a voltaic cell?
The experiment aims to investigate the effect of changing the concentration of the electrolyte on the potential difference and current generated in a voltaic cell. The experiment will involve setting up a simple voltaic cell using zinc and copper electrodes and different concentrations of a common electrolyte, such as copper sulfate solution. The potential difference generated across the electrodes will be measured using a voltmeter, and the current flowing through the cell will be measured using an ammeter. The experiment will be repeated at least three times for each concentration of the electrolyte to ensure accuracy and reliability of results.
The concentration of the electrolyte, Temperature of the electrolyte
The type of electrolyte, Surface area of the electrodes, Distance between the electrodes, and type of electrodes used
The potential difference and current
24.) How does the concentration of reactants affect the rate of reaction and the mechanism of reaction between sodium thiosulphate and hydrochloric acid?
In this experiment, the effect of concentration on the rate of reaction and the mechanism of reaction between sodium thiosulphate and hydrochloric acid will be investigated. The reaction involves the formation of a yellow precipitate of sulfur when the two solutions are mixed. By measuring the time taken for the precipitate to form, the rate of reaction can be calculated. The concentration of sodium thiosulphate and hydrochloric acid will be varied independently to determine the effect of concentration on the rate of reaction. The experiment will be repeated three times for each concentration to ensure accuracy. The temperature of the solutions and the volume of the solutions will be kept constant to ensure that these variables do not affect the rate of reaction.
Concentration and temperature of sodium thiosulphate and hydrochloric acid
The volume of the solutions
The rate of reaction
25.) How does the change in entropy affect the spontaneity of the reaction?
In this experiment, we will investigate the relationship between entropy and spontaneity by measuring the Gibbs free energy change (ΔG) of a chemical reaction at different temperatures. We will use a coffee cup calorimeter to measure the heat absorbed or released during the reaction and calculate ΔG using the equation ΔG = ΔH – TΔS, where ΔH is the enthalpy change of the reaction, T is the temperature in Kelvin, and ΔS is the change in entropy. We will vary the entropy of the system by adding different amounts of a solid solute to a fixed amount of solvent and measure the temperature at which the reaction becomes spontaneous (ΔG < 0). We will repeat the experiment with different solutes to determine the effect of molecular size and structure on entropy and spontaneity.
Amount and type of solute added to the solvent, temperature of the reaction, the volume and concentration of the solvent.
The mass and purity of the solutes, the pressure and atmospheric conditions in the laboratory, the equipment used to measure temperature and heat.
Change in Gibbs free energy, temperature at which the reaction becomes spontaneous.
26.) How does the concentration of an acid affect the shape and position of the pH curve during the titration process with a strong base?
This experiment aims to investigate the effect of the concentration of an acid on the pH curve during titration with a strong base. The experiment will involve the titration of different concentrations of hydrochloric acid with sodium hydroxide. A pH meter will be used to measure the pH of the solution at different points during the titration process, and the data will be used to plot the pH curve.
Concentration of hydrochloric acid, temperature of the solution, concentration of the base
Volume of acid and base used.
pH of the solution at different points during the titration process
27.) How does the type of alcohol used in the reaction affect the rate of esterification of carboxylic acid and alcohol?
The experiment aims to investigate the effect of different types of alcohol on the rate of esterification of carboxylic acid and alcohol using sulfuric acid as a catalyst. A series of reactions will be carried out by mixing different types of alcohols such as methanol, ethanol, propanol, and butanol with acetic acid, and then adding sulfuric acid as a catalyst. The reaction mixture will be heated under reflux for a fixed amount of time, and the products will be extracted with a separating funnel and analyzed using gas chromatography. The rate of the reaction will be calculated by measuring the amount of ester formed over time.
The type of alcohol used in the reaction (methanol, ethanol, propanol, butanol), the temperature of the reaction mixture.
The concentration of the reactants, the amount of sulfuric acid used as a catalyst, the duration of the reaction, and the analytical method used for measuring the amount of ester formed.
The rate of esterification, measured by the amount of ester formed over time.
Download our Successful College Application Guide
Our Guide is written by counselors from Cambridge University for colleges like MIT and other Ivy League colleges.
To join our college counseling program, call at +918825012255

IB Chemistry SL IA Ideas
This list of IB Chemistry SL IA ideas is a helpful resource for students to showcase their understanding of chemistry concepts and theories. The IA is a research project that accounts for a significant portion of the grade, and students can choose from a wide range of topics and IB chemistry internal assessment examples , such as organic chemistry, chemical kinetics, and applications in the real world. Students can also find expert guidance and examples to improve their own work.
1.) How do different temperature levels affect the concentration of ascorbic acid in orange juice?
To determine the amount of vitamin C content in the water after heating the orange juice (different temperatures), a titration reaction will be performed. The end point of the titration is detected when the color of the solution turns blue black after all the ascorbic acid is used up (the volume of iodine solution used will be recorded).
Temperature of orange juice
Brand of Orange Juice, Volume of orange juice for titration, Amount of Starch indicator solution
Amount of potassium iodate in titration with orange juice (vitamin C)
2.) To what extent do rust removers affect the mass of rusted iron nails?
Measure the initial mass of the rusted nail and put it into a test tube. Following that, pour in the rust removers and let the rusted nail soak in it for 5 days. Once it is done, use steel wool to remove the rust and wipe with a cloth. Measure the mass of the nail now and then compare how the mass differs while using different kinds of rust removers.
Rust remover
Constant variables:
Rusted iron nail size and mass, Amount of rust approximately
Mass of iron nail without rust
3.) Investigating the effect of temperature on the temporary hardness of the water
A set amount of tap water sample is collected in a conical flask and the EBT indicator and the buffer solution is added to it. Complex metric titration is performed by titrating the solutions against EDTA. The volume of EDTA used for the solution to turn blue indicates the end point.
Temperature of tap water
Volume of Water, Concentration of EDTA, Source of tap water, Volume of indicator and buffer
Hardness of tap water
4.) Determination of Iron content in Iron tablet
2 methods can be used to determine the iron content. 1st is the redox titration: Potassium permanganate is titrated against the iron solution (iron tablet into deionized water with sulfuric acid added to make it acidic in nature) and the end point is determined when the color changes from colorless to light pink. The 2nd method is the precipitation method: Dissolve iron tables into sulfuric acid and add concentrated nitric acid to it. Add sodium hydroxide solution to neutralize the excess acid and precipitate the iron in the solution. Put a filter paper on top of the vacuum flask and filter the precipitate. Weight the filter paper in intervals until the weight is constant.
5.) How does increasing the number of carbon atoms in an alcohol affect the enthalpy change of combustion of the alcohol?
Determining and comparing the energy released in the combustion of different alcohols, and concluding with a research on which one of them is more efficient as a fuel substituent to fossil fuels. A copper calorimeter, 3 different kinds of alcohol and a spirit burner is used to conduct the experiment.
Number of carbon atoms in the molecule
Volume of water, initial mass of alcohol
Enthalpy change of combustion of the alcohol
6.) Investigating the amount of Iodine content in Iodised Salt
By redox titration, one can determine the amount of sodium thiosulphate required to reduce iodine to Iodide ions. To begin with, a sodium thiosulfate solution is prepared. After, a 0.5% starch indicator solution is also made. Then, distilled water is added to the iodised salt for it to get dissolved. Then, hydrochloric acid and potassium iodide solution is added to the dissolved salt. The solution will turn a yellow/brown color as iodine is produced. The solution is later titrated with the sodium thiosulfate solution until the yellow/brown color of iodine becomes very pale. Titration is repeated until concordant results come.
Iodised salt
Hydrochloric acid, Potassium iodide,
Sodium thiosulfate solution
7.) To what extent does electrolyte concentration affect electroplating efficiency?
Providing different concentrations of an electrolyte(copper sulphate) in the electroplating process for an iron sheet and measuring the amount of copper electroplated within a time duration.
Various concentrations of copper sulphate solution
Mass of copper electroplated
8.) To what extent does temperature affect the calcium concentration of different types of milk?
Heating different types of milk(such as oat, almond, cow etc.) at different temperatures for a certain duration of time and measuring the calcium concentration of the sample before and after utilising complexometric titration
Varieties of Milk
Calcium concentration
9.) Investigation of the clock reaction
The experiment aims to investigate the clock reaction, which is a reaction that changes color after a certain time interval. The experiment will use the reaction between potassium iodide (KI) and sodium thiosulfate (Na2S2O3) in the presence of starch as an indicator. The experiment will vary the concentration of the reactants to determine how it affects the time it takes for the reaction to occur. By measuring the time it takes for the reaction to occur at different concentrations of reactants, we can determine the rate of reaction and calculate the activation energy.
Concentration of KI and Na2S2O3
Amount of starch used, the temperature of the reaction, and the volume of the reactants
Time it takes to change color (Reaction time)
10.) Investigating the Effect of Electrolyte Concentration on the Rate of Galvanic Cell Reactions
In this experiment, students will set up galvanic cells using zinc and copper electrodes immersed in various electrolyte solutions of different concentrations. The rate of the galvanic cell reaction will be measured by monitoring the potential difference (voltage) between the electrodes over time. The electrolyte solutions tested will include solutions of different concentrations of copper sulfate, zinc sulfate, and potassium chloride. The aim of the experiment is to determine how the concentration of the electrolyte affects the rate of the galvanic cell reaction, which can be used to predict the feasibility and efficiency of electrochemical processes.
Concentration of electrolyte solution
Type of electrode, surface area of electrode, temperature, and time of reaction.
Rate of galvanic cell reaction (measured as voltage change over time)
11.) Investigating the Effect of Varying the Amount of Acid Catalyst on the Yield of Esterification Reaction
In this experiment, the effect of varying the amount of acid catalyst on the yield of the esterification reaction will be investigated. A mixture of carboxylic acid and alcohol will be heated in the presence of sulfuric acid catalyst, and the yield of the ester product will be determined by the amount of soap produced. The experiment will be carried out by preparing several reaction mixtures with different amounts of sulfuric acid catalyst and the same amount of reactants. The reaction progress will be monitored by measuring the pH and the temperature of the reaction mixture. After the reaction is complete, the yield of soap will be determined by titration with a standardized sodium hydroxide solution.
Amount of sulfuric acid catalyst (0.1 M, 0.2 M, 0.3 M, 0.4 M)
Amount of reactants (carboxylic acid and alcohol), reaction time and temperature, concentration and volume of sodium hydroxide solution used for titration.
Yield of soap (measured by titration with sodium hydroxide)
12.) Investigating the Stoichiometry of a Chemical Reaction using Vinegar and Baking Soda
The aim of this experiment is to determine the stoichiometry of a chemical reaction between vinegar and baking soda. This experiment involves measuring the volume of CO2 gas produced during the reaction between vinegar and baking soda. The reaction equation for this experiment is CH3COOH + NaHCO3 → CH3COONa + CO2 + H2O. The experiment will be conducted by mixing different amounts of vinegar and baking soda in a flask and measuring the volume of CO2 gas produced. The volume of CO2 gas produced will be recorded at regular intervals of time using a gas syringe. The stoichiometry of the reaction will be determined by calculating the ratio of the moles of reactants and products involved in the reaction.
Independent variable:
The amount of vinegar and baking soda used in the reaction.
Control variable:
The temperature, pressure, and time of the experiment.
Dependent variable:
The volume of CO2 gas produced during the reaction.
13.) Investigating the effect of different solvents on the fluorescence of a dye
The experiment involves preparing solutions of a fluorescent dye in various solvents, such as water, ethanol, and acetone. The solutions are then exposed to a UV light source, and the intensity of the fluorescence emitted by the dye is measured using a spectrophotometer. The experiment is repeated with different concentrations of the dye in each solvent to investigate the effect of concentration on fluorescence.
The type of solvent used.
The concentration of the dye in each solution, the volume of solvent used, the wavelength of the UV light source, and the distance between the sample and the spectrophotometer.
The intensity of fluorescence emitted by the dye.
Don't forget to check our Forum
14.) investigating energy content of fuels.
In this experiment, the energy content of two different fuels, such as ethanol and gasoline, will be compared. The fuels will be burned in a calorimeter, and the heat released during the combustion reaction will be used to heat a known amount of water. The change in temperature of the water will be used to calculate the energy content of each fuel. The experiment will be repeated three times for each fuel to ensure accuracy and reliability.
The type of fuel being burned (ethanol or gasoline)
The amount of fuel used, the amount of water used, the temperature of the water before the combustion reaction, the mass of the calorimeter, the time over which the reaction occurs, the pressure and volume of the reaction environment.
The energy released during the combustion reaction (measured in joules or calories)
15.) Comparing the effectiveness of different brands or types of tablets in neutralizing excess stomach acid
To do this experiment, we could first use a pH meter to measure the initial pH of a simulated stomach acid solution (e.g. 0.1 M HCl), and record this as the dependent variable. Next, we could add a known amount of each tablet to separate portions of the acid solution, and record the time it takes for the pH to reach a neutral value of 7.0. This would be repeated for several different types of tablets and for a control (i.e. a portion of acid without any tablet added). Finally, we could calculate the average time taken to neutralize the acid for each type of tablet, and compare these values to determine which tablet is most effective in terms of speed and efficiency of acid neutralization.
type of indigestion tablet
time taken to neutralize acid solution
starting pH of acid solution, amount of acid solution, amount of tablet added, temperature of solution, stirring rate, etc.
16.) How does the concentration of citric acid affect the rate of effervescence in Alka-Seltzer tablets?
In this experiment, the rate of effervescence in Alka-Seltzer tablets will be measured by observing the time it takes for the tablet to completely dissolve and produce bubbles. To carry out the experiment, each concentration of citric acid solution will be prepared and labeled. Then, an Alka-Seltzer tablet will be added to each solution, and the time it takes for the tablet to completely dissolve and produce bubbles will be recorded. The data collected will be used to create a graph of the time it takes for the tablet to dissolve versus the concentration of citric acid solution. The graph will be used to analyze the relationship between the two variables and draw conclusions about the effect of citric acid concentration on the rate of effervescence in Alka-Seltzer tablets.
Concentration of citric acid solution, Amount of water
The temperature (room temperature), and the brand of Alka-Seltzer tablets used
Time it takes for the tablet to dissolve completely
17.) What is the enthalpy change of combustion of magnesium and how does it compare to the theoretically calculated value using Hess’s Law?
In this experiment, the enthalpy change of combustion of magnesium will be determined by measuring the heat released from the reaction between magnesium and hydrochloric acid. The heat released will be measured using a simple calorimeter. The experimental value will then be compared to the theoretically calculated value using Hess’s Law, which states that the enthalpy change of a reaction is independent of the pathway taken, provided that the initial and final conditions are the same. The enthalpy change of combustion of magnesium can be calculated by combining the enthalpy changes of the reactions that make up the overall reaction.
The mass of magnesium used in the reaction, the concentration and volume of hydrochloric acid used in the reaction
The mass and type of calorimeter used, the initial and final temperature of the reaction mixture, and the atmospheric conditions during the experiment
The heat released from the reaction
18.) How does changing the concentration of a reducing agent affect the rate of the iodine-clock reaction?
In this experiment, the iodine-clock reaction will be used to investigate the effect of changing the concentration of a reducing agent on the rate of the reaction. The reaction involves the oxidation of iodide ions by hydrogen peroxide, which is catalyzed by iodide ions. The time taken for the blue-black color to appear will be measured using a stopwatch. The reducing agent will be varied in concentration by diluting it with water. A range of concentrations will be used to determine the effect of concentration on the rate of the reaction. The experiment will be conducted at room temperature to control the temperature as a variable.
Concentration of reducing agent (varied by dilution with water), Temperature, volume of reactants
Concentration of iodine, concentration of hydrogen peroxide, use of the same stopwatch for all trials
Time taken for the blue-black color to appear (indicating completion of the reaction)
19.) How does the concentration of electrolyte affect the voltage output of an electrochemical cell?
The experiment involves constructing a simple electrochemical cell using two different metals (e.g., copper and zinc) as electrodes and a salt bridge filled with different concentrations of electrolyte (e.g., NaCl). The voltage output of the cell will be measured using a voltmeter for each concentration of electrolyte. The experiment will be repeated with different concentrations of electrolyte to observe the effect of concentration on the voltage output of the cell.
Concentration of electrolyte
The type of metals used as electrodes, the size of the electrodes, the distance between the electrodes, the temperature, the surface area of the electrodes, the type of electrolyte used, and the concentration of the metals used.
Voltage output of the electrochemical cell
20.) How does the polarity of different solvents affect the separation of ink components in paper chromatography?
The purpose of this experiment is to investigate how the polarity of different solvents affects the separation of ink components in paper chromatography. The experiment will involve placing a small spot of ink at the bottom of chromatography paper and then dipping the paper in different solvents of varying polarities. As the solvent travels up the paper, it will carry the ink components with it, and the separation of the components will be observed. The distance traveled by each component and the Rf value will be calculated for each solvent.
The polarity of the solvents used (e.g. water, ethanol, acetone, etc.), the size of the ink spot
The type of ink used, the type and size of the chromatography paper used, the temperature and humidity of the experimental environment, the length of time the paper is left in the solvent
The distance traveled by each component of the ink and the Rf value
21.) How does the energy content of different fuels compare?
The aim of this experiment is to compare the energy content of different fuels by determining the enthalpy change of combustion. The fuels used in this experiment will be ethanol, propanol, and butanol. A calorimeter will be used to measure the heat released during the combustion of each fuel. The mass of each fuel burned will be controlled, and the temperature change of water will be recorded before and after each combustion. The enthalpy change of combustion will then be calculated using the formula ΔH = q/m where ΔH is the enthalpy change, q is the heat released, and m is the mass of fuel burned.
The independent variable in this experiment is the type of fuel used (ethanol, propanol, and butanol).
The mass of fuel burned will be controlled, and the same calorimeter will be used for each combustion. The starting temperature of the water will also be controlled, as will the room temperature during the experiment.
The dependent variable is the enthalpy change of combustion, which will be calculated based on the heat released during combustion.
22.) How does the concentration of a sodium polyacrylate solution affect its ability to absorb water?
The experiment will involve creating sodium polyacrylate solutions of varying concentrations, and then measuring their ability to absorb water. This will be done by placing a small amount of each solution in a separate container, and then adding a fixed amount of water to each container. The containers will be left undisturbed for a set amount of time to allow the sodium polyacrylate to absorb as much water as possible. After the set time, the remaining water in each container will be measured, and the amount absorbed will be calculated. The results will be analyzed to determine the relationship between the concentration of the sodium polyacrylate solution and its ability to absorb water.
Concentration of sodium polyacrylate solution
Type of sodium polyacrylate used, amount of water added, temperature, and time allowed for absorption
Amount of water absorbed by the sodium polyacrylate
23.) How does the temperature affect the solubility of potassium nitrate (KNO3) in water, and how accurate are the solubility curves predicted by the literature values?
The purpose of this experiment is to determine the solubility of KNO3 in water at different temperatures and compare the results with the literature values. The experiment will involve dissolving a set amount of KNO3 in a fixed amount of water at different temperatures, ranging from 10°C to 70°C. The solutions will then be cooled and allowed to crystallize, and the mass of KNO3 crystals formed will be measured. These measurements will be used to construct a solubility curve for KNO3 at different temperatures. The solubility curve will then be compared to the literature values to assess its accuracy.
Temperature (10°C, 20°C, 30°C, 40°C, 50°C, 60°C, 70°C) Control Variables: Mass of KNO3, Volume of water, stirring rate, cooling rate, and measurement instruments
Solubility of KNO3 in water (grams of KNO3 per 100g of water)
Our Expert Tutors!

Barbara Centis
Cat 1 – ESS and Cat 2 – Biology. Chief of the IB program. Mentored 320+ students across various curricula.

Manish Kedawat
IBDP Physics HL / SL. IGCSE Physics. A-level Physics (AQA, CIE, Edexcel, OCR, and WJEC). IGCSE Physics (AQA,CIE, OCR & Edexcel)

Jacqueline Francis
IBDP Cat 1 – Business Management, IBDP Cat 1 – TOK. Taught over 130+ students across 4+ countries.

Dr. Nikita Bhan
IBDP Cat 1 & 2 November 2019. Specializes in Global Politics. Many students scored 7s; mentors 200+ students in assessments.

Specializing in Mathematics: Analysis and Approaches (HL & SL), Mathematics: Applications and Interpretation (HL & SL), and MYP (Mathematics).


Sreevidya KG
IBDP Cat 1 – Chemistry, IBDP Cat 3 – IA Chemistry, IBDP Cat 1 – TOK. Helped 2 out of 3 students achieve a 7 in IB Chemistry.
Our Student'S Results
Score 40+ in IB Exam like they did
What our Students Have to say
A few words about us from our students…

IB Tutoring Recent Blogs
Give our blog a read for anything you need

The MYP Personal Project: A Parent’s Perspective – Nurturing Your Child’s Passion

A SMOOTH PROGRESSION FROM IBMYP-1 TO IBMYP-5

Smooth Transition to IB-MYP: 10 Tips for Students and Parents

The Importance of Community Service in the IB Middle Years Programme (MYP): Nurturing Responsible Global Citizens

CAMBRIDGE VS IB-MYP

The types of assessments that you will face in the IB-MYP

IBMYP IN INDIA: Is it the right choice for you

Nurturing Effective Communication in the MYP: A Guide for Parents and Students

Nurturing Young Writers: A Guide to Elevating Your Child’s Writing Skills in the IB Middle Years Programme (MYP)

Guiding Your MYP Student’s Future: A Parent’s Conversational Toolkit
Get access to our free ib resources.

20 IB Chemistry IA Topics
An introduction to ib chemistry ia’s.
IB Chemistry IA’s (Internal Assessments) are essays that students in the International Baccalaureate program must write in order to be successful in the program. Each essay examines a different concept in chemistry, and requires comprehensive research and investigation into the subject. While completing the essays is often daunting, they are an invaluable asset to those seeking to excel in the program, as each essay carries a weight of 25% of their overall grade.
The topics for IB Chemistry IA’s vary greatly, ranging from analyzing stoichiometric processes, to studying the effects of light on photosynthesis. Furthermore, each essay must meet the criteria set forth by the International Baccalaureate program, and must follow a specific structure and style. In the following guide, we will present 20 IB Chemistry IA topics, and provide insight and guidance for each one.
The 20 IB Chemistry IA topics we will explore in this guide are:
🎓✍️ Acing Your Internal Assessment Has Never Been Easier! ✍️🎓
Are you struggling with your Internal Assessment ? Let our experts take care of it! We’ve successfully completed hundreds of IA projects across different IB courses, and we know the IB criterium inside out.
🌟 Our writers are all human and do not use CHAT-GPT, ensuring a unique and personalized touch to your project. Plus, our service is 100% confidential and risk-free, so you can trust us with your academic success.
Don’t miss out on this opportunity to secure the grade you deserve! Get started with our IB IA Writing Service today! 💡📚🔝

- Thermochemistry
- Redox Reactions
- Equilibrium
- Acid-Base Chemistry
- Periodic Trends
- Atomic Structure
- Molecular Structure
- Energy and Bonding
- Nuclear Chemistry
- Chemical Equations
- Voltaic Cells
- Descriptive Chemistry
- Quantitative Chemistry
- Bioinorganic Chemistry
- Environmental Chemistry
- Organometallic Chemistry
- Surface Chemistry
- Analytical Chemistry
We hope that this guide will provide an informative and comprehensive look into the topics of IB Chemistry IA’s. With our help, students can gain a greater understanding of the topics and make their essays stand out amongst the rest.
What is an IB Chemistry IA?
IB Chemistry IA’s, also known as Internal Assessments, are a critical part of the International Baccalaureate Chemistry course. It is a project that requires you to undertake a practical experiment and write up your work. This will account for 20-40% of your overall grade.
The experiment is designed to be student-led and it allows you to demonstrate all the key skills you have learnt throughout the year. These include your knowledge of the subject matter, data-gathering, lab safety and technical reporting.
The aim of the assessment is to show the examiner that you can ask a scientific question, plan the apparatus and method, carry out the experiment with precision, analyze and interpret the data, and write up your conclusions.
The report form needs to be completed correctly, so it is important that you familiarize yourself with its structure. This includes a title, hypothesis, materials and methods, results, discussion/conclusion, references and bibliography.
You will be marked on a variety of criteria such as accuracy of data, depth of discussion and style of writing. If you follow the assessment criteria and take the time to plan and write your report carefully, you are sure to do well.
General Tips for Achieving Success With IB Chemistry IA Reports
Essay structure and style, carry out your own research, edit and proofread your work, seek feedback from others, topic 1: preparation of salts.
The preparation of salts is a key part of IB Chemistry IAs. Salts are compounds that are made up of cations and anions, which are oppositely charged particles. When these two particles form a bond, a salt is created. In this topic, you’ll learn about the different types of salts, how to create them in your own lab, and how to approach the topic in your IA.
Types of Salts
There are many different types of salts, some of which include aluminum, ammonium, calcium, magnesium, sodium, and potassium salts. Each salt is unique in its own way and requires different amounts of ions to form them. Understanding the different types of salts will help you accurately depict the preparation of salts in your lab.
Creating Salts in the Lab
In order to successfully create salts, you must first have the cations and anions, acid, and base. To combine all these elements and create a salt, you must use the neutralization reaction. This reaction occurs when an acid reacts with a base and creates water and the salt. To achieve this in the lab, you must measure out the correct amounts of acid and base and mix them together. Once they are mixed, the salt will form and can then be collected.
Approaching the Topic in Your IA
When writing your IA on the topic of salts, there are a few things to keep in mind. First, be sure to include the background and methods used throughout the experiment. You must also be sure to discuss any findings or observations you may have noticed during the experiment. Finally, any conclusion from the experiment must be discussed, as well as any sources of error or uncertainties. Writing the IA following these steps will ensure that your IA is successful.
The second topic is all about acid-base titrations. Titration is a method that is used to determine the concentration of an unknown acid or base solution by adding small amounts of a known standard solution until the reaction is complete. This process is often done using a burette and indicator solution.
When studying titrations in IB Chemistry, you need to make sure you understand the concept of equivalence point. This is the point at which the amount of base added to the acid is exactly equal to the amount of acid originally present. Once you understand this, you can learn to calculate the concentrations of solutions before and after the reaction. It’s also important to understand the concept of end point. This is the point at which the acid-base reaction is complete and no further addition of either solution is required to reach the equivalence point.
In order to approach this topic successfully, it’s important to brush up on your basic knowledge about acids, bases, and pH. It’s also essential to understand different types of indicators and how they are used to determine the end point of the titration. Additionally, it is important to be able to compare titrations to other experiments such as oxidation-reduction titrations. Finally, practice calculating concentrations and finding the amount of solutions needed for an accurate titration.
Topic 3: Kinetics
Kinetics is the study of the rate of chemical reactions. It is a very important topic in IB Chemistry IA’s as it requires students to understand how different variables such as temperature, pressure, reactants and catalysts, affect the speed at which a reaction occurs.
When writing your IA report on kinetics, there are certain things you should focus on. Firstly, it is important to understand the general equation for the rate of a reaction, which is: Rate of reaction = k [reactants]^n. From this equation, you can infer that the rate of the reaction is dependent on the reactant concentrations and catalysts involved.
It is also important to pay close attention to the order of the reaction. The order determines how the rate of the reaction changes depending on the concentration of the reactants. For example, if the order of the reaction is 0, then the rate of the reaction will not be affected by the concentration of reactants; however, if the order is 1, then the rate will increase as the concentration of reactants increases.
Finally, when writing an IA on kinetics, make sure to include a discussion of catalyst mechanisms and their effect on the rate of the reaction. You may also want to include experiments that demonstrate the effects of temperature and other variables on the rate of the reaction. Make sure that you explain all of your results clearly and thoroughly.
Topic 4: Investigating the Entropy Change of a Substance
The entropy change of a substance is a measure of the disorder or randomness of molecules in the reaction. Entropy can be calculated by measuring the heat exchange when the reaction occurs and calculating the pressure, volume, temperature, and total number of molecules.
When investigating this topic, it is important to pay attention to the conditions that the substances are in before and after the reaction. This will help determine the entropy change of the reaction. A change in entropy can tell us whether the reaction is an exothermic or endothermic reaction, as well as other useful information.
To approach this topic, begin by researching the basics of entropy and familiarizing yourself with the equation that must be used, S = k lnW. Then, decide what experiment you would like to do, and how you are going to measure entropy. It is also important to choose a suitable reaction to calculate the entropy change for. Consider the effects of the different compounds in the reaction on the entropy.
Once you have chosen your experiment and set it up, begin to collect data. Depending on the experiment, this can involve measuring the temperature, pressure, and volume, amongst other parameters. Record all of your data meticulously in order to understand the exact changes taking place in the reaction.
Finally, calculate the entropy change of the reaction using the equation S=klnW. This will tell you the entropy change of the reaction, as well as giving you information about whether the reaction is endothermic or exothermic.
Topic 5: Electrochemistry
Electrochemistry is a branch of chemistry which studies the chemical changes caused by electric current, or the interchange of chemical and electrical energy. It includes topics such as galvanic cells, electrolysis, redox reactions and acid-base titrations.
To write a successful IB Chemistry IA regarding electrochemistry, it can be helpful to focus on the different variables that affect the rate of a reaction. These variables generally include the concentration of the solution, temperature, surface area of the reactants, pressure, and the presence of a catalyst. By studying how these variables impact the electrochemical reaction you can gain insight into the rate of the reaction, the amount of product formed, and the energy produced.
It can also be beneficial to discuss the structure of different electrochemical cells, such as galvanic, electrolytic, and fuel cells. Additionally, an IB Chemistry IA on electrochemistry should also explore topics within electroanalytical chemistry such as voltammetry, potentiometry, and amperometry.
When approaching this topic, it is important to research all the necessary information and become familiar with the different processes involved in electrochemistry. It will also be useful to create diagrams and sketches to help explain the different concepts covered in your IA.
Topic 6: Investigating the Effect of Temperature on the Rate of Reaction
In this topic, you will be investigating how temperature affects the rate of a chemical reaction. It is important to understand that when a reaction takes place, it takes some energy for it to occur and the reactants must overcome an energy barrier called an “activation energy” before they can form products.
The higher the activation energy, the slower the reaction; therefore, the rate at which products are formed is determined by the amount of energy the reactants have available. Temperature has a direct impact on the rate at which a reaction can take place. As temperature increases, so too does the amount of energy available to the reactants, thus allowing them to overcome the activation energy and speed up the rate at which the reaction takes place.
When conducting your investigation, it is important to ensure you have a safe environment for the experiment to take place in and that you understand the necessary precautions required. You should also calculate the theoretical rate of reaction at different temperatures, which can provide useful background information for comparison against actual results.
When analyzing the data obtained from the experiment, it is important to consider factors such as validity (whether the results were influenced by any outside factors) and accuracy (how close the results were to predictions made prior to the experiment). Additionally, it can be beneficial to create a graph in order to visualize any trends or patterns observed in your data.
Overall, this is an engaging and interesting topic that allows one to explore the relationship between temperature and reaction rate. With the correct planning and analysis of results, this can be a practical and informative experience.
We have made it to the seventh topic of this guide—Topic 7! This topic is all about titration, which is a fancy word for measuring how much of a substance you have in a solution.
When you are doing titration, you need to start by making a solution of a known concentration (this is called a standard solution). Then you will use that solution to find out the concentration of the unknown solution. This is done by adding the standard solution to the unknown one, drop by drop, until an endpoint is reached—this is when a color change happens and shows how much of the unknown solution has been added.
To succeed at titration, you will need to know how to make a standard solution, measure accurately, and identify when the endpoint has been reached. All of these skills take practice and experience, so be sure to get lots of practice before tackling this section of the IA.
Here are some tips for approaching titration:
- Always use an accurate measuring device (preferably a burette) when adding the standard solution to the unknown solution.
- Make sure you pay attention to the color change that indicates the endpoint has been reached.
- Be sure to calculate and record your results, as they are the main part of the IA.
Titration can be tricky, but with practice and patience, you can do it! So take the time to practice, and you will soon become an expert in titration.
The eighth topic of your IB Chemistry IA might be about acids and bases. The goal of this topic is for you to understand how acids and bases react in different environments. You’ll need to be able to recognize indicators such as pH paper or universal indicator, as well as other measurements used to assess the acidity or basicity of a solution.
You’ll also explore the different types of salts and the effect they have on the acidic or basic nature of a solution. It’s important to conduct various tests to determine the concentration of anions and cations in a solution and to understand their properties.
When approaching this topic, it’s important to keep track of the observed data and the process that led to the observation. Good record-keeping will aid in replicating experiments and effectively demonstrating the concept or principles being studied in the lab. It’s also important to consider what factors could be contributing to any anomalies seen in the data.
Finally, it’s essential to think critically about the results and implications of your experiments. This will help to further develop your research questions and objectives and guide subsequent experiments.
You have now learned about the 20 IB Chemistry IA topics in detail. These topics can range from exploring the properties of different types of molecules, to researching biological processes or chemical reactions.
We have also looked at some tips for successfully completing your IB Chemistry IA report. This includes taking into account essay structure, style, and research techniques.
By now you should have a good idea about what is expected from an IB Chemistry IA. Remember, it’s important to stay focused, and keep track of what you have accomplished so far. Doing this will help you stay organized, and ensure that your report meets all assessment requirements.
Good luck! And don’t forget to take the time to enjoy the process, as this is an opportunity to apply your knowledge in a meaningful way.

Nick Radlinsky
Nick Radlinsky is a devoted educator, marketing specialist, and management expert with more than 15 years of experience in the education sector. After obtaining his business degree in 2016, Nick embarked on a quest to achieve his PhD, driven by his commitment to enhancing education for students worldwide. His vast experience, starting in 2008, has established him as a reputable authority in the field.
Nick's article, featured in Routledge's " Entrepreneurship in Central and Eastern Europe: Development through Internationalization ," highlights his sharp insights and unwavering dedication to advancing the educational landscape. Inspired by his personal motto, "Make education better," Nick's mission is to streamline students' lives and foster efficient learning. His inventive ideas and leadership have contributed to the transformation of numerous educational experiences, distinguishing him as a true innovator in his field.
How to Prepare for IB Oral Assessments?
Preparing for IB Oral Assessments entails more than simply understanding your content; it also requires mastering the skill of effective speaking under pressure. As an experienced IB writer, I’ve seen that students who begin their preparation early, practice frequently, and grasp the exact criteria that examiners are looking for do well on these assessments.
IB CAS Projects. The Importance of Reflection
By reflecting on your CAS projects, you learn more about your strengths and flaws. This lets you make smart choices and changes as you work on your project. This process of self-reflection ensures that your CAS experience is more than just a list of things to do; it’s a valuable path of growth.
Best Effective Time Management Strategies in IB Diploma
For the IB Diploma, where homework, projects, and tests can quickly pile up, learning how to handle your time well is essential. I believe that coming up with good ways to handle your time is not only helpful, it’s necessary.
How to Develop a Research Question for IB IA?
The most important thing for a good IB Internal Assessment (IA) is coming up with a good research question. As a former IB writer, I can promise you that a well-written research question will not only help you with your research, but it will also help you keep your analysis on track and make sense.
IB English Paper 2 Writing Guide
To do well on IB English Paper 2, you need to know not only the texts, but also how to compare and contrast them in a test-like setting. I use my many years of experience as an IB teacher to give you important tips and techniques in this complete guide.
IB Paper 1 Writing Guide
As an experienced IB writer, I’ve compiled this complete guide to help you feel strong as you take on this critical part of the IB Diploma Programme. This article details the methods and skills you need to ace Paper 1, from understanding how the test is set up and choosing the right texts.
© 2024 I Bstudenthelp.com. This website is owned and operated by Udeepi OU Harju maakond, Tallinn, Lasnamäe linnaosa, Sepapaja tn 6, 15551. Disclaimer : Services we provide are only to assist the buyer like a guideline to complete any kind of writing assignment. Privacy Policy Terms and Conditions Cookie Policy Revision Policy Refund Policy
- Find A Tutor
- Geneva Tutors
- Lausanne Tutors
- Zurich Tutors
- Basel Tutors
- Online Tutors
- Maths Tutors
- Chemistry Tutors
- Physics Tutors
- Biology Tutors
- English Tutors
- History Tutors
- Geography Tutors
- Language Tutors
- Special Educational Needs
- Residential Tutors
- Primary School
- School Entrance Exams
- Middle School
- Combined Science
- Maths AA and AI
- IB Internal Assessment
- Environmental Systems & Societies (ESS)
- Sports, Exercise & Health Science
- Computer Science
- Global Politics
- Digital Society
- Business Management
- Visual Arts
- English A/B
- English Oral (IO)
- German Oral (IO)
- French Oral (IO)
- Spanish A/B
- French Ab Initio
- German Ab Initio
- Spanish Ab Initio
- IB Extended Essay
- IB Theory of Knowledge
- University Applications
- Our Approach
- Happy Parents
- School Choice
- Become a Tutor
Ideas on How to Come up With a Good Chemistry IA Topic
By TutorsPlus

Choosing a Chemistry IA topic is the crucial first step that will affect the quality of your final IB Chemistry Internal Assessment.
It shouldn’t be too broad or too specific because you don’t want to deal with too much or, vice versa, not enough data. So, it really does pay to invest time in choosing the right question for you.
Also, you need to align your topic with the IB Chemistry curriculum. It requires you to take a closer look at one of the sections of the syllabus rather than trying to experiment in a completely new area. And this is not the only limitation. You should also consider the capacity of your school lab. It is best to avoid investigations that require equipment or materials your school may not have, or if you need more than 10 hours to conduct them. Ideally, you want a good neat experiment that interests you but that has limited capacity to become overly complicated and require extra lab-time in lunch breaks or after school, which can be challenging to organise.
The best IA Chemistry topic hits the spot between your personal interests and academic rigour. In this way you will keep your motivation high and hopefully enjoy investing the time to make your IA as good as it can possibly be.
There are several ways to achieve this balance:
- Link it to your hobbies, cultural background, or daily activities. For example, you might be interested in sustainable living practices. In this case, it is a great idea to analyse the biodegradability of different cleaning products or the chemical composition of eco-friendly alternatives.
- Focus on your favourite type of chemistry. Do you like Organic chemistry best? You might be interested in investigating the synthesis of some organic medicines. Or maybe you prefer inorganic Chemistry? Then you can research different types of pigments used in paints throughout history and analyse the impact of factors like light, humidity, and temperature on their stability.
- Explore interdisciplinary connections. Chemistry has connections with Biology, Environmental Science, Food Science, and Engineering, just to name a few. If you go this route, you can, for instance, investigate how the heating of everyday acidic foods can change the concentration of ascorbic acid.
This is just one example, now it’s over to you to brainstorm others. What we can say for sure is that our IB Examiners recommend this approach. As it often allows you to identify topics that are interesting and engaging, partly because they are chosen less frequently by students.
Our detailed guide has more tips on how to pick a suitable topic for Chemistry IA, so if you don’t find a suitable topic for you from the list below, check out the article How to pick a topic and write your Chemistry IA to perfection . Good luck!
30 great Chemistry IA Topic Ideas
If none of this has given you any inspiration, you might want to try some ideas found on the internet.
However, you need to realise that they have likely been explored extensively. You don’t want to have a topic your teacher and the examiner has seen many times before.
Still, these suggestions may be useful. You just have to breathe new life into them by adding a unique twist. For example, “Investigating the effect of temperature on the rate of a chemical reaction” is quite a generic and perhapsoverused topic. However, you can turn it into “Investigating the effect of temperature on the rate of the reaction between vitamin C and an oxidising agent, and linkit to the degradation of vitamin C in fruits and vegetables stored at different temperatures”. Now this is a more unique and relevant topic.
Having said that, let us take a look at our 30+ Chemistry IA ideas.
Kinetics & Thermodynamics
- The Temperature Factor : Explore how temperature impacts the rate of a specific reaction and calculate its activation energy.
- Concentration’s Influence : Examine the effect of reactant concentration on reaction rate, determining the overall order of the reaction.
- Catalysis in Action : Investigate the impact of a catalyst on reaction rate and propose a potential mechanism for its function.
- Enthalpy Exploration : Use calorimetry to determine the enthalpy change of a reaction (combustion, neutralization, etc.)
- Equilibrium Investigations : Study a reversible reaction to determine its equilibrium constant and explore factors that shift the equilibrium position.
Organic Chemistry
- Esterification Exploration : Synthesise an ester, optimize reaction conditions, and determine its percentage yield.
- Natural Product Analysis : Investigate the composition of a natural product (e.g., essential oil) using techniques like extraction or distillation.
- Polymer Possibilities : Synthesise a polymer and characterize its properties (e.g., tensile strength, melting point).
- Drug Analysis : Determine the concentration of an active ingredient in a pharmaceutical (e.g., aspirin tablet) using titration.
Redox & Electrochemistry
- Electrochemical Cells : Construct different electrochemical cells, measure cell potentials, and explore the electrochemical series.
- Electroplating Efficiency : Investigate factors affecting the efficiency of electroplating a metal and calculate the amount deposited.
- Battling Corrosion : Examine methods of preventing the corrosion of a specific metal and evaluate their effectiveness.
- Redox Titrations : Use redox titrations to determine the concentration of an unknown solution (e.g., vitamin C content in fruit juice).
Analytical Chemistry & Spectroscopy
- Spectrophotometry Sleuthing : Use spectrophotometry to determine the concentration of a coloured compound by creating a calibration curve.
- Chromatography Creations : Employ paper or thin-layer chromatography to separate and identify components in mixtures (e.g., plant pigments, inks).
- The Mystery of Metal Ions : Identify unknown metal ions in solution using flame tests or precipitation reactions.
- Water Quality Analysis : Measure water quality parameters (pH, hardness, dissolved oxygen) in a local sample and compare them to safe standards.
Environmental Chemistry
- Biodegradation Breakdown : Investigate the biodegradability of different plastics under controlled conditions.
- Water Purification Techniques : Compare the effectiveness of various water purification methods (e.g., filtration, adsorption) in removing contaminants.
- Analysing Air Quality : Measure and analyse specific air pollutants (e.g., nitrogen dioxide, particulate matter) in your local environment.
Materials Chemistry & Sustainability
- Bioplastics Potential : Explore the synthesis and properties of bioplastics as a sustainable alternative to conventional plastics.
- Battery Power : Investigate the performance and environmental impact of different types of batteries (e.g., lithium-ion, lead-acid).
- Dye Degradation : Study the effectiveness of different methods for degrading harmful dyes used in the textile industry.
Analytical Chemistry & Data Analysis
- Developing a Sensor : Design and test a sensor for detecting a specific chemical species using a suitable indicator (e.g., pH indicator, conductivity meter).
- Chemometrics in Action : Utilise chemometric techniques (e.g., principal component analysis) to analyse and interpret complex chemical data sets.
- Modelling and Simulations : Utilise computational modelling or simulations to predict the behaviour of molecules or chemical systems.
Miscellaneous
- The Chemistry of Cooking : Investigate how a culinary process (e.g., baking, caramelization) can be explained by chemical principles.
- Chemistry in Cosmetics : Analyse the chemical components of a cosmetic product and evaluate its claims and safety.
- Decoding Flavour : Explore the relationship between the chemical composition of food and its perceived flavour by studying specific components (e.g., sugars, acids, umami compounds) and their impact on taste perception.
- Examine Flavor Deciphering: Investigate how the chemical makeup of food influences its perceived taste, focusing on particular elements (like sugars, acids, and umami compounds) and their effects on how we experience flavor.
Chemistry IA Topic Conclusion
Choosing the right Chemistry IA topic is half the battle in ensuring that your research project is set up for success.
With the 30+ ideas we provided, there will be something to give you the inspiration you need to come up with a topic that is both unique and motivating for you to work on. Remember, even if you select a topic from our list, you need to add your own angle to make sure it is different. .
If you’re still struggling to choose the direction of your experiment or need guidance in conducting it, don’t hesitate to seek help from a tutor. At TutorsPlus, we have a team of experienced Chemistry tutors who are passionate about helping students like you. With our expert guidance, you’ll be able to create a research question that not only impresses your teacher but also inspires you to pursue your scientific interests.
Ready to choose a Chemistry IA Topic and take your project to the next level? Then contact us at 022 731 8148 and [email protected] to schedule your first session and take the first step to nail your Internal Assessment.

Sara has been an education consultant for TutorsPlus for 15 years, and is an expert on international IB education. She is also a parent of two lively children.
Find a Tutor
Popular Posts

English IO – How to Ace Your IB English Literature & Language Oral

Tips to get a Top Grade in Your IGCSE English

How to get a top score in your IB TOK Exhibition
IGCSE Maths Revision Tips To Help You Today

What is the IA, EE or TOK? Everything you need to know about the IB written assignments

Maths Anxiety – How Parents Can Help
More articles from our expert tutors.

Your guide to the International School of Geneva – Part 1

How to Pass IGCSE Maths exam: Options and Strategies

Meet Diana, our expert English and IELTS tutor in Geneva
Find a Tutor Today
" * " indicates required fields
Step 1 of 5
Find the best support for your family

25+ IB Chemistry IA Topics and How to Complete Them ⚗️

Internal Assessment is an integral part of the International Baccalaureate (IB) Diploma Programme in chemistry, comprising 20% of the final assessment. The IA requires students to conduct an independent laboratory investigation and write a report on their findings.So this where the next question comes – why is it important to select a right Chemistry IA topic.
Why choosing a good topic for your IB Chemistry IA is important?
Choosing a good IA topic is crucial as it forms the foundation of the investigation and can make or break the project. So if you do not want to risk the final mark, consider selecting a topic you will be able to cover.
First, let’s take a closer look at how you should choose a topic for your Chemistry Internal Assessment.
When choosing a topic for your IB Chemistry IA, it’s important to consider the following factors:
- Relevance to the course: The topic should align with the objectives and content of the IB Chemistry course. It should demonstrate your understanding of key concepts and theories covered in the syllabus.
- Interest and curiosity: Select a topic that genuinely interests you and arouses your curiosity. This will motivate you to conduct thorough research and engage with the project more deeply.
- Feasibility and resources: Ensure that the topic is feasible and you have access to the resources needed to conduct the investigation. This includes equipment, chemicals, and data sources.
- Originality and innovation: While it’s unnecessary to come up with a completely new topic, selecting an original and innovative angle can make your IA stand out and demonstrate your creativity and critical thinking skills.
- Clarity and specificity: Choose a specific research question that can be clearly defined and answered within the project’s scope. This will help you stay focused and avoid getting overwhelmed by the vastness of the subject.
A well-chosen topic can help you score higher grades in your IA as it provides a clear and organized framework for the investigation. A good topic also allows you to apply your knowledge and skills to a real-world problem or question, which is the ultimate goal of the IA. Therefore, take your time and carefully consider your options before finalizing your IA topic.
Here are some tips for selecting the best chemistry IA topics:
- Choose a topic that interests you: The IA is a long-term project and requires a lot of time and effort. It is important to choose a topic that you are genuinely interested in, as it will keep you motivated throughout the process.
- Make sure the topic is feasible: The IA should be realistic and achievable because you should complete them within the given time frame. Consider factors such as the availability of equipment, materials, and resources.
- Keep it simple: While it is tempting to choose a complex and ambitious topic, it is important to keep it simple and focused. A narrow and well-defined topic is easier to investigate and allows you to delve deeper into the subject.
- Consider the relevance of the topic: Choose a topic that is relevant and timely, and has real-world applications. This will make the IA more meaningful and engaging.
Here are some examples of good chemistry IA topics:
- The effect of pH on the rate of enzyme-catalyzed reactions
- The synthesis and characterization of nanoparticles
- The determination of the equilibrium constant of a chemical reaction
- The analysis of food additives using spectroscopy techniques
- The determination of the molar mass of a volatile liquid using the Ideal Gas Law
Want some complex Chemistry IA ideas?

Here are some more detailed chemistry IA topics that explore the speed of different chemical reactions using a spectrometer:
- Investigating the effect of temperature on the rate of a chemical reaction
In this IA, you could use a spectrometer to measure the absorbance of a reactant or product as a function of time at different temperatures. This will allow you to determine the rate of the reaction at each temperature and plot a rate versus temperature graph.
You could also use this data to determine the activation energy of the reaction.
- Determining the rate law of a chemical reaction
In this IA, you could use a spectrometer to measure the absorbance of a reactant or product as a function of time at different concentrations. This will allow you to determine the rate of the reaction at each concentration and plot a rate versus concentration graph.
You could then use this data to determine the rate law of the reaction and calculate the rate constant.
- Analyzing the kinetics of a catalyzed reaction
In this IA, you could use a spectrometer to measure the absorbance of a reactant or product as a function of time in the presence and absence of a catalyst. This will allow you to compare the rate of the reaction with and without the catalyst and determine the effect of the catalyst on the rate.
You could also use this data to calculate the rate constant and activation energy for the catalyzed and uncatalyzed reactions.
- Evaluating the effect of a solvent on the rate of a chemical reaction
In this IA, you could use a spectrometer to measure the absorbance of a reactant or product as a function of time in different solvents. This will allow you to determine the reaction rate in each solvent and compare the rates.
You can use this data to determine the effect of the solvent on the rate constant and activation energy of the reaction.
If you want, you can buy IB Chemistry IA at our writing service.
These topics will surely give you some ideas for your chemistry IA! But I know that you want more, so let’s continue with other ideas.
And some more Chemistry IA topics for you:
Here are five more chemistry IA topics related to the effect of temperature on activation energy:
- Investigating the effect of pH on the activation energy of an enzyme-catalyzed reaction
In this IA, you could measure the rate of an enzyme-catalyzed reaction at different pH values and use this data to calculate the activation energy.
You can use a spectrometer to measure the absorbance of a reactant or product as a function of time at each pH value to determine the rate of the reaction.
- Analyzing the effect of a solvent on the activation energy of a chemical reaction
In this IA, you could measure the rate of a chemical reaction in different solvents and use this data to calculate the activation energy.
You could also use a spectrometer to measure the absorbance of a reactant or product as a function of time in each solvent to determine the rate of the reaction.
- Evaluating the effect of a catalyst on the activation energy of a chemical reaction
In this IA, you could measure the rate of a chemical reaction in the presence and absence of a catalyst and use this data to calculate the activation energy for both cases.
Use a spectrometer to measure the absorbance of a reactant or product as a function of time with and without the catalyst to determine the rate of the reaction.
- Determining the effect of temperature on the stability of a compound
In this IA, you could measure a compound’s decomposition rate at different temperatures and use this data to calculate the activation energy.
You could also use a spectrometer to measure the absorbance of the decomposition products as a function of time at each temperature to determine the reaction rate.
- Analyzing the effect of a reactant concentration on the activation energy of a chemical reaction
In this IA, you could measure the rate of a chemical reaction at different reactant concentrations and use this data to calculate the activation energy.
And now use a spectrometer to measure the absorbance of a reactant or product as a function of time at each concentration to determine the rate of the reaction.
And five more Chemistry IA ideas you can do at home:

Here are five more chemistry IA topics related to investigating energy contents in packaged chips:
- Analyzing the nutritional value of packaged chips using proximate analysis
In this IA, you could perform proximate analysis on a variety of packaged chips to determine their protein, carbohydrate, fat, and fiber content. You could also calculate the caloric value of each chip based on the proximate analysis data.
- Evaluating the effect of frying temperature on the oil content of packaged chips
In this IA, you could fry chips at different temperatures and use a moisture analyzer to determine the oil content of each batch.
Then compare the oil content of the chips fried at different temperatures to see if the frying temperature affects the oil content.
- Determining the preservative content of packaged chips using spectrophotometry
In this IA, you could use spectrophotometry to analyze the preservative content of different brands of packaged chips.
You could compare the preservative content of each brand to see if there are significant differences.
- Investigating the effect of storage temperature on the shelf life of packaged chips
In this IA, you could store chips at different temperatures and use a moisture analyzer to determine the moisture content at regular intervals.
Then you can plot the moisture content versus time to see how the storage temperature affects the shelf life of the chips.
- Analyzing the effect of packaging material on the oxygen content of packaged chips
In this IA, you could compare the oxygen content of chips packaged in different materials using a gas chromatograph.
You could then determine the effect of the packaging material on the oxygen content and shelf life of the chips.
Want some more Chem IA topics?
Here are five more chemistry IA topics related to the effect of sodium fluoride on tooth decay in the presence of carbonated drinks:
- Investigating the effect of different pH levels on the effectiveness of sodium fluoride in preventing tooth decay
In this IA, you could expose teeth to different concentrations of sodium fluoride at different pH levels and measure the amount of tooth decay using a tooth decay model.
You can compare the effect of the pH on the effectiveness of the sodium fluoride in preventing tooth decay.
- Evaluating the effect of different sweeteners on the tooth decay prevention properties of sodium fluoride
In this IA, using a tooth decay model, you could expose teeth to different concentrations of sodium fluoride in the presence of different sweeteners and measure the amount of tooth decay.
You could then compare the effect of the sweeteners on the effectiveness of the sodium fluoride in preventing tooth decay.
- Analyzing the effect of different types of carbonated drinks on the tooth decay prevention properties of sodium fluoride
In this IA, you could expose teeth to different concentrations of sodium fluoride in the presence of different types of carbonated drinks and measure the amount of tooth decay using a tooth decay model.
Then – compare the effect of the carbonated drinks on the effectiveness of the sodium fluoride in preventing tooth decay.
- Determining the effect of different storage temperatures on the stability of sodium fluoride in a toothpaste formulation
In this IA, you could prepare a toothpaste formulation containing sodium fluoride and store it at different temperatures. You could then measure the stability of the sodium fluoride at regular intervals using a spectrophotometer.
Also, you could compare the stability of the sodium fluoride at different storage temperatures to see if the temperature affects its stability.
- Investigating the effect of different brushing agents on the tooth decay prevention properties of sodium fluoride
In this IA, you could expose teeth to different concentrations of sodium fluoride in the presence of different types of toothpaste formulations containing various types of abrasives and measure the amount of tooth decay using a tooth decay model.
Afterwards, compare the abrasives’ effect on sodium fluoride’s effectiveness in preventing tooth decay.
Don’t let the stress of choosing an IA topic
Hold you back..
Are you struggling to come up with topic suggestions for your IB Internal Assessment?
Our experienced writers can help you choose the perfect topic for your IA
Tailored to your specific subject and requirements.
Simply click:

And last but not least – topics related to the current situation worldwide:
Here are five chemistry IA topics related to modern times:
- Investigating the environmental impact of plastic pollution
In this IA, you could analyze the chemical composition of plastic waste and determine its environmental impact.
Also, compare the environmental impact of different types of plastics to see if some are more harmful than others.
- Analyzing the effect of air pollution on plant growth
In this IA, you could expose plants to different levels of air pollution and measure the effect on their growth using various parameters such as height, weight, and leaf area.
You could also use spectrophotometry to measure the concentration of pollutants in the air and compare it to the effect on plant growth.
- Evaluating the effectiveness of natural antimicrobial agents against pathogenic bacteria
In this IA, you could test the antimicrobial activity of natural compounds such as essential oils against pathogenic bacteria and compare their effectiveness to synthetic antimicrobial agents.
Use spectrophotometry to measure the concentration of the antimicrobial agents and determine the minimum inhibitory concentration (MIC).
- Determining the effect of electronic waste on soil and water quality
In this IA, you could expose soil and water samples to electronic waste and measure the effect on their quality using various parameters such as pH, conductivity, and nutrient content.
You could also use spectrophotometry to measure the concentration of heavy metals and other contaminants in the soil and water.
- Analyzing the chemical composition of alternative energy sources
In this IA, you could analyze the chemical composition of different alternative energy sources such as biofuels, solar cells, and batteries and compare their properties.
Once again, you can also use spectroscopy techniques such as infrared spectroscopy and X-ray fluorescence to determine the chemical structure and elemental composition of the energy sources.
If you are also searching for other IA topics, make sure to read other related articles:
- Biology IA topics without experiment
- Experiment based Bio Internal Assessment topics
- History IA topic ideas
- Math IA topics for HL and SL students
- IB Business IA ideas
- Topics for Physics IA
- Environmental Science IA topics
Select topic for Chemistry IA that suits YOU!
Remember, the IA is an opportunity for you to demonstrate your understanding and skills in chemistry.
So please choose a topic that challenges and engages you, and have fun with it!
If you need assistance with choosing a topic or writing your Chemistry IA, IB writing service is here to help you. Simply contact us.
Get hot offers and discounts for your IB Assignments
Our writing solutions cater to all disciplines within the IB program, and we specialize in crafting academic papers for students of all levels. We follow the IB criteria.
Adhering strictly to the rigorous standards set by the IB, we deploy a methodical approach to our writing process. This ensures that every piece of content we generate not only meets but exceeds the expectations set within the program.
Contact us:
Latest Articles:

What Is EE and TOK Matrix?

Latest Extended Essay Requirements Updates for the Years 2023/2024

Is It Legit to Buy Extended Essay Online?
Our services:.
- Buy Internal Assessment
- Buy Math IA
- Buy Extended Essay
- Buy TOK Essay
- Buy TOK Exhibition
IBWritingService.com is an independent academic writing aid with no official ties to the International Baccalaureate Organization (IBO). Our use of “IB” in the domain and title is purely for identification, and we neither claim nor imply any endorsement or partnership with the IBO. Our services aim to support students’ educational needs without violating IBO policies. Trademarks mentioned are property of their owners and do not suggest affiliations. By using our services, you acknowledge our non-affiliation with the IBO and that we’re not a substitute for IBO requirements. We deny any liability for use of our services in relation to the IBO.
ALL PAPERS WRITTEN BY OUR EXPERTS AS PART OF THIS WRITING SERVICE ARE FOR REFERENCE PURPOSES ONLY. WHEN USING CONTENT PURCHASED FROM THIS WEBSITE, IT MUST BE PROPERLY REFERENCED.
- Terms & Conditions
- Revision Policy
- Privacy Policy
- Refund Policy
- Cookie Policy
© 2024. All Rights Reserved.

- Saesha Grover
IB Chemistry Internal Assessment Solved: A Guide to Acing Your Chemistry IA
The IB Chemistry IA is often dreaded by students, as they frequently envision completing notoriously difficult experiments and spending hours at a desk trying to formulate topic ideas. Given the complexity of the Chemistry subject, with numerous topics and subtopics that demand intricate knowledge and application, securing a high grade on your Internal Assessment can be the make-or-break factor for achieving your desired score in final exams and finding solace as you enter the exam room. In this guide, I will not only break down how to secure a strong Grade 7 on your Chemistry IA, but I will also share how this can be achieved in both an experimental and data-based IA . The guiding principle: keep it simple, keep it clear.
1.0 Picking your research question:
1.1: Keeping it Simple
Selecting an appropriate research question is an important step in ensuring success in your IB Chemistry IA. I can assure you that there is no need to opt for an overly complicated research question – a principle I adhered to, resulting in a 22/24 on my Chemistry HL Internal Assessment ( keep it simple, keep it clear! ). The rationale behind this, is that your goal is to showcase a thorough understanding of your chosen topic throughout your IA, rather than inadvertently revealing gaps in your knowledge.
1.2: Check out the Academic Literature
Furthermore, I strongly advocate for choosing a topic with plenty of existing academic literature. This will provide you with several sources and reports you can draw upon in your conclusion and analysis within your Internal Assessment . This allows you to make effective comparisons between the outcomes of your experiment and established findings .
1.3: Don’t Neglect a Data-based IA
In addition, I want to emphasise the potential of doing a data-based IA. In a data-based IA, you may opt for running a virtual experiment, or analysing data on a topic to answer a selected research question. If you are doing the latter, note that there are various online sources for data for Chemistry Internal Assessments, such as ChemSpider, the CRC Handbook, and PubChem. These platforms offer a lot of varied information that can be harnessed for data-based investigations.
2.0 Personal Engagement:
In your introduction, ensure that you demonstrate your personal engagement with the topic you have selected. Your personal engagement is essentially what sparked your interest in the topic you explore in your Internal Assessment. Reveal what genuinely ignited your interest, avoiding elaborate stories . Whether it's a concept learned in class or a discovered exception (e.g., trends in melting points, electronegativity, boiling points or solubility properties of elements), keep it simple, keep it clear.
3.0 Risk Assessment and Variables:
For the risk assessment and variables section of your Internal Assessment, make sure you address safety, ethical AND environmental concerns if you are doing an experimental IA. However, if you are doing a data-based IA , you can say that these were not of concern in your investigation.
When discussing the different variables in your investigation, do not just state what they are. Discuss how these variables will be manipulated and provide clear and convincing justification for why you are manipulating the variable in this way. Ensure you state which unit you are using for your experiment, and if you are not using conventional SI units, justify why .
For instance, in a database IA, do your database sources mainly cite temperature values in degrees Celsius, and is that the reason for why you are using that unit?
4.0 Methodology:
4.1: Creating a Methodology for a Databased IA
When conducting a database-based Internal Assessment (IA), a common misconception is that this type of investigation does not require a detailed methodology section. However, this is arguably one of the most important parts of your IA . In your methodology for this type of IA, clearly outline the values you plan to extract from the databases that you find, and explain how you will ensure accuracy in your experiment while presenting your data. And of course, remember to keep it simple, keep it clear .
To further enhance your databased IA , create a section beneath your methodology that serves the purpose of justifying the selection of databases for your investigation . Here, delve into discussions about the reliability of authors of the databases, and try to ascertain whether the sourced data has been cross-checked .
4.2: An Extra Tip I have for Databased IAs
An additional recommendation I have for your database IA is to actively compare multiple sources for a specific data point value that you are researching. For instance, different sources may state different values for the melting point of an element. By doing so, you can identify discrepancies between source values. This comparative analysis will ultimately contribute significantly to the overall clarity of your IA ( keep it simple, keep it clear! ).
5.0 Data Collection:
5.1: Clarity
In your data collection, I highly recommend being wary of your unit representation and notation. Your units should be in your table headings , not inside the rest of the table as well. Small things like these can go a long way. Also, make it clear when you are referring to raw versus processed data, and include sample calculations in the processed data section of your IA .
5.2: Data Based IA
In some of the data you find for an IB Chemistry IA, you may be given a range of values in tabulated form, especially instances where you require a property of a chemical unit.

In instances like these, you might make the decision to take the midpoint of your data point values. If you do this, again, be upfront, and explain why you made this decision. If something does not go your way, you can insert phrases such as “in view of this observation” or “due to this' and this helps your examiner follow your line of thinking .
6.0 Conclusion:
I recommend reminding the examiner of the purpose of your investigation at the start of your conclusion. Refer to your figures! What did Figure 1 tell you? Is this consistent with literature? Why or why not? Was there an exception in the results that you found? Use this information to discuss some of the weaknesses or limitations of your investigation. Additionally, in your figures within your IA, try and include error bars if relevant, as this allows you to discuss things like the lack or presence of error bar overlap, as well as the size of error bars and what this means in the context of the reliability of the trends that you observe in your investigation .
7.0 Extensions:
Something that I have noticed a lot of students miss in their investigations or often do not go into adequate detail over, is the extensions in their investigation. I would not brush past this in a couple of sentences, but rather would recommend writing in extensions to your IA that can tie easily onto your research question. I would also recommend explaining why or how the extensions would develop your understanding of the investigation.
In essence, “keep it simple, keep it clear” , is a mantra you should keep in mind throughout writing up your IB Chemistry Internal Assessment. This approach ensures not only a smoother IA writing process, but also a greater likelihood of achieving success in this assessment, whether it be experimental or databased!
And that's it! Best of luck for the Chemistry Internal Assessment. You’ve got this!!!
- Internal Assessment Guides
Recent Posts
The Complete IB TOK Exhibition Guide
The Complete IB Geography Internal Assessment Guide
The Complete IB Psychology Internal Assessment Guide
IB Chemistry IA Topics That ROCK | 50+ Ideas for SL and HL

Table of contents
- Writing Metier
One of the major challenges students of International Baccalaureate is finding and selecting a topic for the essay and rightly so. It can be challenging because of the plethora of options students have. Additionally, there are different levels when it comes to doing an essay for Chemistry IA. And all we know is how hard it is when it comes to selecting IB Chemistry IA topics.
With these different levels come different subjects within the discipline. For instance, you can choose between organic Chemistry or acid and bases as the topics. While the choice for the topic is entirely up to you, it is understandable that it may be a lot to deal with. And that’s why you are here reading the article that will guide you 😉
—————
You can request a custom suggestion of a Chemistry IA topic from our IB IA experts , or you can spend several minutes of your time, read this article and find awesome solutions for your next IA.
We are aware that getting a inte s is an ideal way that can boost your overall IB grade. It is therefore understandable when students take a lot of pressure when it comes to their essay. If you have selected a Chemistry from the list of subjects in the IB , you should be ready for its specifics. Students often find themselves lost when writing a lab report and choosing the topic they need to go for. Generally, the rule of thumb for Chemistry IA is that you need to measure the changing variable and its effect on another variable. This is something that remains constant in all Chemistry IA essays, despite whatever topic you go for.
Therefore, if you find yourself struggling with selecting a topic, go for the one in which altering the variables makes sense, such as concentration, surface area, temperature and such to explore the effect they may have on a variable which is dependent, something which is easy to measure, such as temperature, gas produced and ph.
Another important thing you need to keep in mind is that the topic needs to be demanding – it needs to meet the diploma-level and not just any high-school level topic. While the topic needs to be relevant to the syllabus of Chemistry, it has to be around something that is not just easily found in textbooks. It needs to be well-researched and essentially a topic you find yourself engaged and connected with. Because an IA essay requires to be well-researched, it needs to be on a topic you are fond of, so you do not feel mundane while doing it.
Now that we have covered some important basis regarding the importance of topic selection, it is time to discuss some of the options. There is no need to feel overwhelmed by this information as in the following article we will help you with whatever you are looking for. Lastly, like with Bio IA topics , make sure you run the topic and a rough idea by the instructor before you begin your work on it.
For those of you who decided to opt for Chemistry subject, another read with a list of Chemistry EE topics can be interesting. Have a read, right after you finished with this article.
Depending on what topic you choose, you might have to conduct various experiments in a lab to make sure you have the proper analysis for the essay. On the other hand, most of these topics can be done by yourself, at home given that you do the required amount of study and research.
High scoring Chemistry IA topics
Before any more delays, let’s dig deep into the knowledge and find you the best Chemistry IA ideas for the essay:
- Explore the speed of different chemical reactions by using the spectrometer
- Determine the activation energy of a particular reaction
- Exploring the dissolved oxygen content present in the water body
- Denatured of lipase and the conditions
- Enthalpy of neutralization and enthalpy changes by using calorimetry
- Concentration of drugs in multiple/various tablets
- Content of vitamin in healthy food
- Gas volume and absolute zero
- Exploring local sources and the hardness of water
- Exploring juice brands and Vitamin C percentage
- Exploring milk brands and calcium content/percentage
- Exploring tea and coffee brands and caffeine percentage
- CaC03 amount in white eggshells and brown eggshells
- Determine the chemical equilibria – Kc for reaction
- Investigating the activation energy of iodine-clock
- Exploring the percentage of chlorine in swimming pool and bleaches
- Electrolysis and factors that affect the process
- Electroplating and factors that affect the process
- Thermal decomposition and salt type present in the compound
- Electroplate metals, optimal conditions on different external factors
- Decomposing hydrogen peroxide and activation energy
- Effect of different temperature on rust on steel
- Effect of temperature on ferromagnet strength and weakness
- Explore the effectiveness of salt (different brands) and snow removal
- Paracetamol and synthesizing Dulcin
- Using UV light, speed of denaturation on animal proteins
- EDTA content in shower cleaners
- Studying the pH of soil
- Soil pH effect on chlorophyll in plants and leaves
- PH change in oxidation of coffee and tea
- Solubility of salts and hardness of water
- Testing and preparing of buffer solutions
- Investigating Cheetos (or any other packaged chips brand) and energy contents
- The effect of ionic salts on the depression of water and its freezing point
- Studying soaps and esterification
- Studying the equivalence point of strong bases/strong and weak acids
- Studying salts and solubility
- Preparing esters
- Studying paper chromatography and the effects of solvents
- Studying catalytic abilities on different metal oxides and transitions
- Studying the factors that affect the rate between propanone and iodine
- Studying the factors that affect the colors and transition on metal compounds
- Investing charcoal and absorption of organic acids
- Studying different sacrificial metals and their effect on iron rusting
- Studying water absorption polymers and their effect on water
- Studying zinc ions as a dietary supplement and its concentration
UPD! Like with Economics IA ideas , and recent Math AA ideas , we have made another update for all of yall in the current year.
Breathe Easy! We’re Handling Your Paper
- Polished Papers : Styled right, glitch-free
- Ask Away : Direct chat with your writer
- Free Goodies : Revisions, title page, and bib
- Fair Prices : Plus a money-back guarantee
- All Human : No AI, just real experts
- Private & Secure : Your details, our secret
Bye-Bye, Burnout!
Slash 15% OFF using the coupon code: BLG15WM

Check the new IB Chem IA topics below.
Temperature, catalase enzyme
1. Effect of Temperature on Activation Energy Produced during the breakdown of H202 while using catalase enzyme and aluminum inhibitor as a catalyst.
Oxygen saturation level
2. Evaluating the impact of different age groups on the oxygen saturation level measured using an oximeter after the 2-Minute Step Test for Athletes participating in the Special Olympics.
Chlorine ions
3. How do various at-home water filter brands affect the concentration of chlorine ions dissolved in the water?
4. Effect of isomers on the enthalpy of formation in the homologous series alcohols.
Saturated fat content
5. How does the saturated fat content of sunflower oil, as measured by iodine value, vary with the number of times the oil is used for frying potato chips. ❗ (Simulation based)
Changes in temperature
6. To what extent does the changes in temperature (300 to 400 K) along with the varying concentrations of Zinc and Copper (0.001, 0.002, 0.003, 0.04 and 0.005 Ml) as an electrolyte solution affect the voltage produced in a voltaic cell? ❗ (Based on Chem Reax simulation)
Nitric acid concentrations, reaction time
7. What is the effect of varying concentration of Nitric Acid (HNO3) on the rate of reaction (moldm-3 s-1) measured as the time taken for the release of 10 dm3 of CO2 gas during reaction with Calcium Carbonate (Ca CO3) chips?
8. Determining the amount of vitamin c in an orange.
Sodium fluoride
9. Effect of different concentration of Sodium Fluoride on the tooth decay in the presence of Carbonated Drink i.e. Pepsi.
Carbonated drinks
10. Effect of Volume and pH of carbonated drinks on dental erosion or tooth decay
You can also check this video by The IB Scientist to get inspiration and find more ideas for your Chemistry IA.
After doing much research, we have formulated this list and believe that these ideally serve as high-scoring Chemistry IA topics. Again, the same as with Math IA topics , or ESS , for Chemistry IA you would have to do much research on the topic you choose and make sure that you meet all the requirements for the essay. Let’s say that, for instance, you go for Chemistry IA ideas energetics.
Need a Dope Paper Written? We've Got Your Back!
Use these Chemistry IA ideas for your IB
Now, that would essentially require a lot of homework and perhaps work at the lab as well. So when it comes to lab work, you need to be in touch with your instructor to help you with the work and be aware of what you are doing.
All the best, you got this!
Free topic suggestions
Vasy kafidoff.
Vasyl Kafidoff is a co-founder and CEO at WritingMetier. He is interested in education and how modern technology makes it more accessible. He wants to bring awareness about new learning possibilities as an educational specialist. When Vasy is not working, he’s found behind a drum kit.
Similar posts
37 ib sl math ia topic ideas that actually work.
If you are here because you are stuck with an idea for your IB Mathematics Standard Level (SL) essay topic, you have come to the right place. We understand that the IB Math is the toughest subject (no kidding, we feel you) and therefore, we are here to aid you as much as we can.
55 English and Literature IA Topic Ideas and Research Questions
Unlock the full potential of your IB English IA with 55 insightful topic ideas! Learn from an experienced IB mentor how to craft a compelling research question and excel in your Internal Assessment. Gain insider tips on analysis, argumentation, and literary exploration, and explore avenues for personalized professional assistance
70+ IB Physics IA Topics and Research Questions
In the realm of IB Physics IA, students have the golden opportunity to explore a diverse range of topics, from the wonders of quantum mechanics to the principles governing our vast universe. This guide has been crafted to assist you in pinpointing a topic that not only piques your interest but also aligns with your academic pursuits. As you sift through these carefully selected topics and research questions, we hope you find that perfect match that leads to a successful and insightful project
IB Economics IA Topics | SL and HL Ideas
When working on an International Baccalaureate Internal Assessment (IA), the most important thing is to select the right topic. You can only write well when you have a topic you are interested in. Not just that, but you should also have ample information on it to be able to work.
IB Business Management IA: 60 Topic Ideas and Tips
In this insightful guide, I draw upon my extensive experience in academic writing to offer a rich array of innovative IB Business Management IA topics, coupled with expert tips to navigate the selection and research process seamlessly. As we delve into the nuances of crafting a successful IA, I share firsthand insights and real-life examples to foster a deeper understanding and appreciation of the business world.
IB Acceptance Rates at Top American Universities
Comprehensive database of university rankings and statistics. From acceptance rates to student satisfaction percentages, we provide the data you need to make an informed decision about your education. Discover the top universities in the U.S. today
We rely on cookies to give you the best experince on our website. By browsing, you agree to it. Read more

- Customer Reviews
- Extended Essays
- IB Internal Assessment
- Theory of Knowledge
- Literature Review
- Dissertations
- Essay Writing
- Research Writing
- Assignment Help
- Capstone Projects
- College Application
- Online Class
IB Chemistry IA Guide: Format, Topics, Rubric, And Assessment
by Antony W
October 20, 2022
For most students, the IB Chemistry IA (Internal Assessment) assignment lurks on the path to academic success like a sphinx with an impossible riddle. The analogy is quite right too, given that the assignments take up a hefty 20% of your total marks in the coursework.
If you are reading this, you are likely confused by the guesswork that surrounds the entire exam. Luckily for you, we at Help for Assessments are old hands at chemistry assignments including IB internal assessments. We will guide you through the process as we debunk the 'nightmare' that is a chemistry IA to make sure you ace it.
If you would love a little more practical help than this detailed guide, our team of experts is at your disposal to carry out your chemistry IA with polished skill. As a multi-faceted team, we are experts in every form of academic writing assignments and projects. Don't be shy, check out our range of services and order your very own writing expert today.
If you still want to proceed, its time you put your apprehension away as we take a practical look into how you can pass the chemistry IA.
This guide will explore the sections and format of a chemistry IA, a look into the marking criteria used, and suggestions at every point to help show you what is expected of you.
What is a Chemistry IA
The IB chemistry IA is an investigative essay that seeks to explain the chemistry behind common real-world phenomena. It seeks to assess your grasp of chemistry up to the level achieved in your coursework through practical application of the skills gained.
Unlike regular test which many students cram their way through, the chemistry IA needs a high level of independent skill, research work, and personal effort to complete.
Coupled with all the other stressful assignments in other subjects, the chemistry IA sometimes feels like an insurmountable problem partly because of the sheer amount of guesswork.
The assignment seeks to develop independent thinking and creative application of concepts learned to the real world. If you can do this successfully, you will earn top marks in the assignment. The first task you have with the chemistry internal assignment is choosing an appropriate topic.
Choosing a Topic for Your Chemistry IA
The first consideration you should take into account when choosing a topic for the IB chemistry IA is that it should be personal. That means it should be one that interests you personally, adapted to your particular cause, and related with a personal touch throughout the text.
To achieve full marks, your topic and essay must meet the conditions set out by the descriptors on IB’s chem page .
However, in the search for authenticity and originality, many students get themselves in a rut trying to invent procedures and experiments. Notice that the paper is looking for independent thinking, creativeness, and initiative.
The best way to achieve these criteria without knocking your brains out is to adapt existing experiments, procedures, or other ideas to your investigation. What you need to showcase is authentic inspiration and highly personalized methods, which you can easily do by adapting existing ones.
Let's say, for example, that you want to investigate the calories in the food you eat. Of course, that is a washed-out idea. How about you give it a new twist and investigate how cooking Ideal Protein packs affects their calorie content? This is something you can do easily with a calorimeter in the lab.
For those who don’t know, Ideal Protein is a popular, albeit shady, weight loss program when dieters substitute daily food for prepackaged ultra-refined protein packets. If you have ever tried it, you can tie it to the investigation to show innovation and inspiration.
The same goes for other common chemistry experiments you will find online, in books, journals, magazines, and other common sources.
With a topic in hand, it's time to plan the investigation and structure it accordingly.
Chemistry IA Format
You will be carrying out the IA assignment in stages, but very little of it will be actual lab work. Most of the work is in the research involved and crafting the actual write up.
So, while we are confident you can handle the actual experimentation (your instructor will be more than glad to help), let us show you how to structure the write up.
There will be seven important parts of the write up, which for the sake of simplicity we will keep calling an essay. The image below will give you a rough idea of how each section appears, but we shall also take a more in-depth look into some of the parts.
Parts of a Chemistry IA
The following image shows a sample structure of a chemistry IA. Please note that it is strictly for guidance only; in no way is it to be taken as a must-use format. Your instructor will usually provide exhaustive instructions in this regard.

Introduction
Your introduction is meant to tell your reader why they should keep reading. Here, give in very brief detail what your experiment is about, why you chose it, and stress your personal connection to the subject matter. You first person ‘I’ or ‘We’ pronouns to achieve this.
a) Background
As part of the Introduction, the background is meant to enhance the reader’s understanding of the context of your investigation. Include a general review of the science behind the concept, backed by facts and evidence.
You can also include the equations and mathematical formulas that you will be using in the assignment.
b) Hypothesis
The hypothesis is meant to be a justification for the choice of science concepts you use to explain the claim under investigation.
More technically, it should be a specific prediction about the outcome of your experiment or how the dependent variable will change with the independent one.
For example, with our example, our hypothesis might be:
“Using calorimeters to find out whether Ideal Protein packs change in calorie content value upon cooking in various ways.”
In any other discipline, this section would probably be called ‘methodology’ or ‘procedure.’ This is the part where you tell, in complete detail, how you carry out your experiment.
a) Research Question
The design part starts with a research question in the form ‘how is x dependent on Y?’ It should be a highly specific question outlining the variance you are investigating, stated without any ambiguity.
b) Variables
Outline fully what your variables are, outlining the three types:
- Independent variables - the factor you manipulate and set parameters for.
- Dependent variable - the variable you want to determine by measuring or calculating.
- Controlled variable - other variables or factors that influence the outcome and which should either be controlled or taken into consideration in the outcome.
As part of the design, the method or procedure is a detailed explanation of how you carried out the experiment or tests required to measure the variables. No matter how strong the temptation is, do not take this section off a chemistry book or lap report.
Most students trip on this section because they forget the procedure is supposed to be specially adapted and personalized for this assessment.
If you were choosing a reagent, state why you chose it. If you are choosing a flask, show the reasoning behind your choice. For example, 'use a glass stirring rod instead of a metallic one to prevent reaction with the reagent …'
It is also a good idea to include a diagram of your setup and describe any modifications you had to improvise. A picture will also do.
a) Raw Data
Record your data as soon as you collect it. This is usually done in tables where you can compare the variables easily and fill in the gaps quickly. You need to collect both quantitative (measured, e.g. 10g of residue) and qualitative data (observed, e.g. color change, bubbles).
Report every measured value and include every field on the same table where possible. If any of the data values don't match, you can enter it in different fields.
b) Analysis
Use your measured data to calculate totals, averages, correlations, among others. Draw graphs and charts as needed. Be sure to show the entirety of your working including the formulas you used. Note any uncertainties and margins of error expected.
If you have an accepted value for that kind of experiment, compare the two and see how close they come to each other. If you have graphs, make them appealing, give them names, and name them accordingly.
The conclusion should be a rewording of your research question to give an answer based on the trend observed and proved by your results. The conclusion is a three-part section that also includes:
a) Evaluation
The evaluation is a critical review of your methods and how well the procedure you chose worked to your benefit (or not). If there are any discordant data/outlier points, discuss them here.
b) Improvements
Suggest any improvements or changes you would make if you were to repeat the experiment. What would you advise anyone else using the same procedure for the experiment? Do you have any suggestions to make it better?
Marking Criteria for Your Chemistry IA
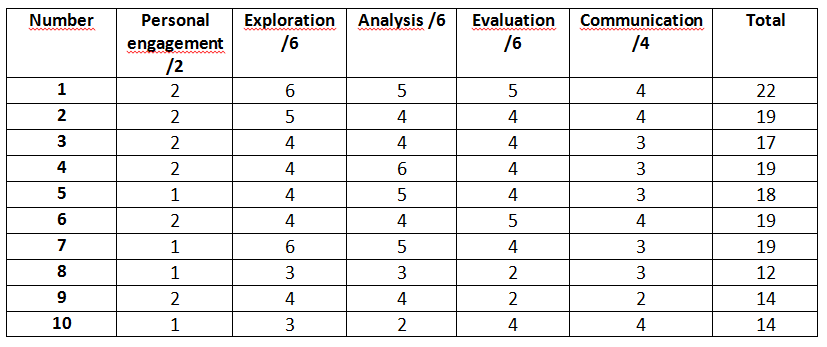
By now, you can tell that personal engagement is a pretty big deal in your chemistry IA, but it is not the only one.
Markers will rely on several other criteria to grade your work, including:
1. Exploration
Exploration is a measure of how well you establish a scientific context for the project. It measures how well focused and grounded your research question is, as well as your awareness and knowledge of safety, environmental, and ethical concerns involved, if any.
2. Methodology
This criterion measures how clearly and thoroughly you set out your procedure. You should have at least 5 variations or manipulations of the independent variable and 3 trials for each.
You should collect as many significant data points as possible, set out any safety procedures taken, and qualify each choice of equipment and supplies.
3. Analysis
This metric measures your data collection, organizational, and presentation skills. All calculations must be shown clearly, formulas displayed, and all qualitative and quantitative data recorded.
4. Evaluation
This metric contributes 6 marks to the total 20 and assesses how well the results obtained serve to prove/disprove your research question in the accepted context.
You also get points for suggesting useful improvements, noting and owning up to any weakness of the methodology, and describing the strength of the correlation between the results and the hypothesis.
5. Communication
This criterion is a measure of how clearly and effectively you presented yourself in the essay.
6. Personal Engagement
As discussed, you will need to completely own the entire project from start to finish. Choose every word with care, especially if you use textbook sources along the way. Cite each of these appropriately because, needless to say, plagiarism is a capital crime in the assessment.
Need Help With Your Chemistry IA Assignment?
Many students are tempted to rush through with the assignment to be ‘over and done with’ it. However, such IB assessments are tricky because they test not only the outcomes, but also the process, the student’s state of mind, and the overall circumstances surrounding the assessment.
If you truly want to be 'over and done with' the assignment, the only way to do it is to be diligent and do it as meticulously as you can. However, you can also hand over the entire problem to chemistry internal assessment experts at Help for Assessments to write it for you.
With years of experience and skill in the market, we will take the burden off your hands and do the entire assessment for you in record time and at the lowest prices. If you want to experience total peace of mind, order our stellar services here. We are here for you, always.
About the author
Antony W is a professional writer and coach at Help for Assessment. He spends countless hours every day researching and writing great content filled with expert advice on how to write engaging essays, research papers, and assignments.
Leave a Reply
Your email address will not be published. Required fields are marked *
Chemistry HL IA Examples
Score a home run on your Chemistry HL with our exclusive collection of Free IA Examples! Get insider tips and tricks for success.

Sell Your IA For $10 a pop!
Happy to give you $10 for every IA so we can give it away for free!
Top IA examples. Learn and excel.
Outstanding EE examples. Draft with confidence.
High-scoring IOs. Enhance your performance.
Exemplary TOK essays. Master your writing.
Top TOK exhibitions. Impress in assessments.
Top English HL essays. Write with confidence.
How Does Temperature Affect The Critical Micelle Concentration (CMC) Of The Common Ionic Surface Active Agent (Surfactant) Sodium Dodecyl Sulphate Measured By Using The Change In The Rate Of Conductivity As Concentration Is Increased?
Unlock The Chemistry Mystery - Discover How Temperature Impacts The Critical Micelle Concentration Of Sodium Dodecyl Sulphate Through Conductivity Rate Changes.
Effect Of Brewing Time On Mass Percentage Of Gallic Acid In Green Tea & Black Tea Extract
Discover How Brewing Time Influences Gallic Acid In Green Tea And Black Tea Through This Detailed IB Chemistry HL Investigation.
Effect Of Basicity Of Ammine Ligands On Thermodynamic Stability Of Ni (II) - Ammine Complexes
Discover How The Basicity Of Ammine Ligands Influences The Thermodynamic Stability Of Ni (II) - Ammine Complexes In Our Detailed IB Chemistry HL IA.
Effect Of Salinity On Kinetics Of Adsorption Of Allura Red Dye
Discover How Salinity Impacts The Adsorption Kinetics Of Allura Red Dye In Our Detailed IB Chemistry HL Sample IA.
Does The Value Of Equilibrium Constant For The Reaction Between Iron (III) And Thiocyanate In Basic Medium (Using NaOH) Depends On The Basicity Of The Medium (Expressed In Terms Of pH Ranging From 8.00 to 13.00), Determined Using Colorimetric Estimation Of Iron-III?
Unlock The Secrets Of Chemistry With Our IB Chemistry HL IA Guide On Equilibrium Constant For Iron (III) And Thiocyanate Reaction
How Does The Concentration Varied Between 1 And 5 mol dm -3 Of ZnSO 4 ⋅7H 2 O In The Aqueous State In A Cu–Zn Voltaic Cell Affect The Electrode Potential Across The Voltaic Cell And How Does This Compare To Theoretical Values Calculated By The Nernst Equation?
Discover How Varying Concentrations Of ZnSO 4 ·7H 2 O From 1 To 5 Mol Dm -3 In Cu - Zn Voltaic Cells Influence Electrode Potential Against Nernst Predictions.
How Does The Mass Of Manganese (IV) Oxide Catalyst (0.1000g, 0.2000g, 0.3000g, 0.4000g, 0.5000g) Affect The Natural Log Of The Arrhenius Constant Of The Decomposition Of Hydrogen Peroxide (H2O2) By Measuring The Rate Constant Of The Reaction With A Pressure Sensor At Different Temperatures And Calculating It Through The Arrhenius Equation
Explore How Varying Masses Of Manganese IV Oxide Catalyst Influence The Arrhenius Constant In Hydrogen Peroxide Decomposition.
Comparison Of Efficiency Of Natural & Artificial Indicators
Discover The Comparative Efficiency Of Natural Versus Artificial Indicators In Our In-Depth IB Chemistry HL IA Sample.
Correlation Of Temperature Dependence Of Free Energy Change & The Chain Length
Explore The Intriguing Correlation Between Temperature And Free Energy In Chemistry HL IA To Enhance Your Understanding And Grades.
Effect Of pH On Adsorption Of Carmoisine Red Dye By Activated Charcoal
Discover How pH Levels Influence The Adsorption Of Carmoisine Red Dye By Activated Charcoal In This Comprehensive IB Chemistry HL IA.
Investigation On The Variation In Melting Point And Boiling Point Of Hydrides Of Group 15, 16, And 17 With Respect To Their % Lonic Character
Explore The Impact Of Ionic Character On The Melting And Boiling Points Of Group 15, 16 , And 17 Hydrides In This Detailed IB Chemistry HL Investigation.
Effect Of pH On Rate Of Ketal Formation Between Propanone & Ethanol
Discover How pH Levels Influence The Ketal Formation Rate Between Propanone And Ethanol In Our Detailed IB Chemistry HL IA Analysis.
Effect Of pH On The Rate Of Acid Hydrolysis Of Keratin Protein
Discover How pH Levels Impact Acid Hydrolysis Of Keratin Protein In Our Comprehensive IB Chemistry HL Sample IA.
Effect Of Percentage Concentration Of NaCl On Adsorption Of The Food Color-Carmoisine Red Dye By Dried Bread Crumps
Discover The Impact Of NaCl Concentration On Carmoisine Red Dye Adsorption With Dried Bread Crumbs In This Detailed IB Chemistry HL IA Study.
How Does The Chemical Shift Of The Methyl Protons In 1 - H NMR Spectrum Of Ring Substituted Methyl Benzene Depends On The Type (Methyl, Hydroxy, Chloro, Carboxylic Acid & Nitro) And Position (Ortho, Meta And Para) Of The Substituent In?
Discover How Chemical Shifts Of Methyl Protons In 1-H NMR Spectra Vary By Substituent Type And Position In Our Comprehensive IB Chemistry HL Sample IA.
Correlation Of Retention Factor pH At The Iso-Electric Point And Molar Mass Of Amino Acids
Discover How Retention Factors And pH Influence Molar Mass In Our Comprehensive IB Chemistry HL Study On Amino Acids.
How Does The Mass Of Caffeine (In g) Extracted From Tetley Black Tea Leaves In Its Aqueous Extract Depends On The Temperature Of The Water In Which The Tea Leaves Are Brewed, Determined Using Solvent Extraction?
Discover How Temperature Affects Caffeine Extraction From Tetley Black Tea Through Our Detailed IB Chemistry HL Sample IA Analysis.
How Does The Exposure To UV Light (Wavelength Of 200nm) For Different Lengths Of Time (0mins, 15mins, 30mins, 45mins, 60mins) Affect The Concentration Of Vitamin C (Ascorbic Acid) Measured Through Iodine Titration
Discover How UV Light Exposure Impacts Vitamin C Levels Through Precise Iodine Titration Analysis. Explore Key Insights For IB Business Chemistry HL IA Success.
Investigation Of Trends In Spectral Data In Homologous Series Of Carboxylic Acids
Discover The Intricacies Of Carboxylic Acids Through Our Thorough IB Chemistry HL Investigation Of Spectral Data Trends.
How does the increase in temperature affect the potential of hydrogen (pH) of coffee?
Discover How Temperature Changes Influence The PH Of Coffee In Our In-Depth IB Chemistry HL Investigation.
ToK Exhibition Objects Generator
Generating ToK exhibition objects was hard. QBIX AI makes it easy.

IMAGES
VIDEO
COMMENTS
50+ Chemistry IA Ideas with Research Question Examples One of the biggest challenges facing students taking IB chemistry is coming up with a good Internal Assessment (IA) idea. It's got to be something suitably demanding for diploma-level study, it's got to be something relevant to the chemistry syllabus, it's got ...
Your IB Chemistry IA research question, in turn, can only be effectively framed once the topic you pick for your IA is inspired by past experiments and investigation ideas. Your IB Chemistry IA idea should help you aim at a specific research question and help you develop a proper methodology for your investigation.
This allows the students to explore and gain a more in depth understanding of one of the topics covered in the IB syllabus as well as further develop their laboratory, research, and scientific writing skills. The internal assessment is marked by the student's own teacher and moderated by an external marker. It is an important component of the ...
IB Chemistry IA ideas. VSEPR theory predicts the shape and bond angles of molecules based on the number of bonding and non-bonding pairs of electrons around the central atom. It does not consider the identity of the atoms or groups attached to the central atom however, and this does have an effect.
Analysis of seaweed (seaweed is a good source of Br and I). 8. Investigate the amount of CaCO3 in brown and white eggshells. 9. Compare the percentage of vitamin C in various brands of juice. 10. Investigate the hardness of water from different local sources. 11.
All Chemistry IA Examples. Starting from the May 2025 session, the Chemistry IA requirements have changed. We created a couple of exemplars to show you how the new IA should look like. It's OK to refer to the old Chemistry IA exemplars (since the new IA is quite similar) for inspiration/ideas, but make sure to follow the new requirements.
IB Chemistry IA Ideas - Investigating the Effects of Different Catalysts on Reaction Rates. The investigation delves into the intriguing realm of catalytic chemistry, exploring the profound impact that various catalysts have on the rates of chemical reactions. Catalysts play a crucial role in speeding up reactions by providing an alternative ...
Writing a Chemistry IA can be a challenging task. Luckily, some of Lanterna's top tutors have collected 25 Chemistry IA topic ideas to give you a head start! 1:1 Tutoring. How It Works ; Our Tutors; IB Tutoring; IB Retakes; iGCSE Tutoring ... You can also get in touch with us with any questions via email at [email protected] or via phone at +44 ...
Chemistry IA Ideas. Vitamin C content of superfoods: In this topic, you can explore how the time taken to cook superfoods such as kale and broccoli affects the amount of vitamin C that leaches into the water. The amount of vitamin C can be determined through an iodometric titration where the endpoint is indicated by the formation of a blue ...
Central to your IA is the research question, ... IB Chemistry IA Ideas and Research Questions - chemistrytutor.me October 19, 2018 at 2:11 pm - Reply […] Secondly, some of the projects below are just plain bad for reasons I've outlined in my post about research questions. […]
7 Pro Tips To Write A Top-Notch IB Chemistry IA; 23 IB Chemistry IA Ideas To Get You Started; Let's Wrap It Up! ... the relevance of the research question to the Chemistry syllabus prescribed by IB, and the uniqueness of the topic, to name a few. However, it isn't all bad news. We at Nail IB are devoted to providing the best guide to cracking ...
Dive into a diverse collection of innovative experiments across medicine, dentistry, engineering, and environmental science. From analyzing reaction rates to exploring pH effects on drug stability, these topics are designed to inspire your IB Chemistry IA and help you stand out.
The IA consists of a laboratory report that students must complete during their IB chemistry course. For assessments before May 2025, the report should be 6 to 12 pages in length and should include a research question, a methodology section, data analysis, and a conclusion. From May 2025, the report should be a maximum of 3,000 words.
IB Chemistry HL IA ideas list is available online with expert guidance and examples. The list contains 20 to 50+ IA ideas, including research questions and topic descriptions and IB Chemistry HL Internal assessment examples. Choosing the perfect IB Chemistry IA topic can be made easier by approaching it methodically and evaluating existing ...
Descriptive Chemistry. Quantitative Chemistry. Bioinorganic Chemistry. Environmental Chemistry. Organometallic Chemistry. Surface Chemistry. Analytical Chemistry. We hope that this guide will provide an informative and comprehensive look into the topics of IB Chemistry IA's.
30+ Chemistry IA Topic Ideas to Get You Started. The following are just but ideas to give you a clear picture of what a suitable Chemistry IA topic should look like. It's important to keep in mind that these are not by any means research questions. They're either general questions or statement, which you can modify and make as specific as ...
Choosing the right Chemistry IA topic is half the battle in ensuring that your research project is set up for success. With the 30+ ideas we provided, there will be something to give you the inspiration you need to come up with a topic that is both unique and motivating for you to work on. Remember, even if you select a topic from our list, you ...
Here are some examples of good chemistry IA topics: The effect of pH on the rate of enzyme-catalyzed reactions. The synthesis and characterization of nanoparticles. The determination of the equilibrium constant of a chemical reaction. The analysis of food additives using spectroscopy techniques.
1.1: Keeping it Simple. Selecting an appropriate research question is an important step in ensuring success in your IB Chemistry IA. I can assure you that there is no need to opt for an overly complicated research question - a principle I adhered to, resulting in a 22/24 on my Chemistry HL Internal Assessment (keep it simple, keep it clear!).
Check the new IB Chem IA topics below. One of the major challenges students of International Baccalaureate is finding and selecting a topic for the essay and rightly so. It can be challenging because of the plethora of options students have. Additionally, there are different levels when it comes to doing an essay for Chemistry IA.
October 20, 2022. For most students, the IB Chemistry IA (Internal Assessment) assignment lurks on the path to academic success like a sphinx with an impossible riddle. The analogy is quite right too, given that the assignments take up a hefty 20% of your total marks in the coursework. If you are reading this, you are likely confused by the ...
Electrochemical Reactions. Examine the factors affecting electroplating. Find how the strength of ferromagnets varies with temperature. Organic Chemistry. By using Sodium Hydroxide solutions separate the ethanol and methanol compounds. Try synthesizing a compound such as Paracetamol and aspirin. Design and test the buffer solutions. Disclaimer ...
IB Resources Question Bank IB Flashcards IA Examples EE Examples Pricing Cart Login Register (It's Free) Chemistry HL IA Examples. ... Explore Key Insights For IB Business Chemistry HL IA Success. Chemistry HL. 5/7. 144 Likes. 25 mins read. 4910 words. English. November. 2023.