Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article

Zooplankton-phytoplankton biomass and diversity relationships in the Great Lakes
Roles Conceptualization, Formal analysis, Resources, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Natural Resources Research Institute, University of Minnesota, Duluth, MN, United States of America
Roles Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing
Roles Conceptualization, Data curation, Formal analysis, Methodology, Resources, Validation, Writing – review & editing
Affiliation Department of Natural Resources and Cornell Biological Field Station, Cornell University, Ithaca, NY, United States of America
Roles Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing
Roles Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing
Roles Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – review & editing
Affiliation U.S. EPA Great Lakes National Program Office, Chicago, IL, United States of America
Roles Conceptualization, Methodology, Writing – review & editing
- Katya E. Kovalenko,
- Euan D. Reavie,
- Stephanie Figary,
- Lars G. Rudstam,
- James M. Watkins,
- Anne Scofield,
- Christopher T. Filstrup

- Published: October 26, 2023
- https://doi.org/10.1371/journal.pone.0292988
- Peer Review
- Reader Comments
Quantifying the relationship between phytoplankton and zooplankton may offer insight into zooplankton sensitivity to shifting phytoplankton assemblages and the potential impacts of producer-consumer decoupling on the rest of the food web. We analyzed 18 years (2001–2018) of paired phytoplankton and zooplankton samples collected as part of the United States Environmental Protection Agency (U.S. EPA) Great Lakes Biology Monitoring Program to examine both the long-term and seasonal relationships between zooplankton and phytoplankton across all five Laurentian Great Lakes. We also analyzed effects of phytoplankton diversity on zooplankton biomass, diversity, and predator-prey (zooplanktivore/grazer) ratios. Across the Great Lakes, there was a weak positive correlation between total algal biovolume and zooplankton biomass in both spring and summer. The relationship was weaker and not consistently positive within individual lakes. These trends were consistent over time, providing no evidence of increasing decoupling over the study period. Zooplankton biomass was weakly negatively correlated with algal diversity across lakes, whereas zooplankton diversity was unaffected. These relationships did not change when we considered only the edible phytoplankton fraction, possibly due to the high correlation between total and edible phytoplankton biovolume in most of these lakes. Lack of strong coupling between these producer and consumer assemblages may be related to lagging responses by the consumers, top-down effects from higher-level consumers, or other confounding factors. These results underscore the difficulty in predicting higher trophic level responses, including zooplankton, from changes in phytoplankton assemblages.
Citation: Kovalenko KE, Reavie ED, Figary S, Rudstam LG, Watkins JM, Scofield A, et al. (2023) Zooplankton-phytoplankton biomass and diversity relationships in the Great Lakes. PLoS ONE 18(10): e0292988. https://doi.org/10.1371/journal.pone.0292988
Editor: Hans G. Dam, University of Connecticut, UNITED STATES
Received: February 2, 2023; Accepted: October 3, 2023; Published: October 26, 2023
This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Data Availability: All relevant data are available within the manuscript and its Supporting Information files.
Funding: These data were collected as part of the U.S. Environmental Protection Agency’s (EPA’s) Great Lakes Biology Monitoring Program. Thus, the study design for sample collection and taxonomic analysis to evaluate phytoplankton and zooplankton communities was determined by the EPA, and followed methods specified by the standard operating procedures associated with this program. The funder did not determine the data analysis method, decision to publish, or assist with preparation of the manuscript beyond the scope of the contributing author affiliated with EPA.
Competing interests: The authors have declared that no competing interests exist.
Introduction
With a few rare exceptions, aquatic ecosystems in the Anthropocene have experienced changes in temperature and nutrient concentrations, which can lead to shifts in phytoplankton assemblages [ 1 – 3 ]. In many cases, these compositional changes can alter the seasonal timing and amplitude of primary productivity [ 4 , 5 ] and functional attributes of phytoplankton [ 6 , 7 ]. Changes in phytoplankton assemblage composition and dynamics can lead to decoupling of primary producers and consumers, which may destabilize planktonic food webs with cascading effects on tertiary consumers [ 8 – 10 ].
Theory predicts and observational studies have shown that greater phytoplankton diversity is linked to increased phytoplankton resource use efficiency (horizontal diversity effects within trophic levels, [ 11 ]) and to increased zooplankton growth rate, diversity, and abundance (vertical diversity effects across trophic levels [ 12 ]). Phytoplankton diversity can also directly influence consumers via biochemical diversity in food resources, which should increase zooplankton diversity [ 13 ], and these diversity effects may produce direct and indirect feedbacks to buffer primary consumer populations and entire food webs from abrupt shifts in their resource base. Because phytoplankton diversity can decrease variability in zooplankton productivity [ 12 ], greater algal diversity may support more zooplankton predators and therefore greater predator-prey ratios within the zooplankton community. However, diversity effects are not consistent across systems [ 14 ] and different measures of phytoplankton diversity can have opposing influences on horizontal and vertical diversity effects [ 15 ]. For example, communities dominated by cyanobacteria may have larger proportions of inedible taxa [ 16 ], which might limit zooplankton biomass [ 17 , 18 ] or have no impact [ 19 ]. Predator-prey biomass ratios can respond to environmental stressors when predators take longer to recover from perturbations, e.g., in isolated environments [ 20 ]; however, other studies show remarkable consistency in predator-prey ratios across a wide range of taxa and systems [ 21 , 22 ].
The structure of large lake food webs is less understood than that of smaller lakes [ 23 , 24 ], and previous vertical diversity studies have largely focused on smaller ecosystems. In the Laurentian Great Lakes, several attributes of phytoplankton assemblages, including total biovolume, cell densities, average cell sizes, and species composition, have fluctuated considerably in the last few decades, with likely causes being changes in nutrient availability, invasive species, and climate change [ 5 , 25 – 27 ]. Decreasing algal cell sizes in particular [ 27 ] could have repercussions for the entire aquatic food web, consistent with a climate change signal linked to decreasing organism sizes at community, species, and population levels across a range of ecosystems [ 28 ]. In the Great Lakes, zooplankton shifted to greater dominance by calanoid copepods, particularly Limnocalanus macrurus [ 29 ], abundances of the predatory invasive cladoceran Bythotrephes increased in some lakes [ 30 ], causing declines in some species [ 31 – 33 ] and changes vertical distribution in others due to migration to greater depths as an anti-predatory response to Bythotrephes [ 31 ]. With a wealth of long-term historical data, there have been multiple detailed analyses of trends in specific assemblages [ 25 , 34 , 35 ] and concurrent trends [ 36 , 37 ]; however, the degree of zooplankton and phytoplankton coupling, vertical diversity effects, and detailed associations between specific groups of taxa are less well understood.
Ideally, investigations of the relationships between primary producers and consumers should use high-resolution productivity data and information on feeding selectivity [ 38 ]. However, long-term high-resolution in situ productivity data are relatively sparse and often limited to smaller geographic areas (e.g., [ 39 ]), and landscape-scale analyses often rely on standing biomass. Controlled studies of feeding selectivity, usually conducted in laboratory settings, are similarly difficult to extrapolate to diverse and dynamic natural settings. We used nearly 20 years of paired zooplankton and phytoplankton data from the U.S. EPA Great Lakes Biology Monitoring Program to examine ecological associations, long-term and seasonal dynamics of zooplankton-phytoplankton coupling, and effects of phytoplankton diversity on zooplankton biomass and diversity. We predicted that there would be a positive correlation between algal biovolume and zooplankton biomass, and that the slope of this relationship would decrease over time because of increasing decoupling of the two trophic levels associated with changes in phytoplankton assemblages. We also tested relationships between algal diversity and total zooplankton biomass, zooplankton diversity, and zooplanktivore-grazer ratios, and explored group-level associations between the major types of zooplankton and algae.
Materials and methods
We used data collected as part of the U.S. Environmental Protection Agency (EPA) Great Lakes Biology and Water Quality Monitoring Programs in the pelagic Laurentian Great Lakes of North America, focusing on years which had matching phytoplankton and zooplankton data (2001–2018). Samples are collected twice per year in the spring (usually April) and summer (usually August) from 72 sites across the five Great Lakes: Lakes Erie, Ontario, Huron, Michigan, and Superior ( S1 Table ). For phytoplankton, equal volumes of water were collected by a rosette sampler from multiple depths (0, 5, 10, 20 m) at each station representing the upper 20 m of the isothermal water column in the spring or the epilimnion in the summer [ 25 ]. Four spring samples from individual depths were composited to form an integrated sample; in summer, a minimum of two and maximum of four depths (typically 0, 5, 10 m, and lower epilimnion, but fewer taken when the mixed layer is shallow) were composited to form a representative sample from the epilimnion [ 40 ]. Samples were preserved with Lugol’s iodine solution and analyzed as described in U.S. EPA Great Lakes National Program Office (GLNPO) standard operating procedure [ 41 ]. Briefly, we used the Utermöhl method [ 42 ] for soft-bodied algal identification. Subsamples were processed for detailed diatom assessment by acid digestion, slide-mounting and high-resolution microscopy. Algal specimens were also measured to allow for biovolume calculations [ 43 ].
Phytoplankton taxa were characterized as edible or inedible based on a combination of entity shape and nutritional quality. Characterization of edibility in freshwater phytoplankton has been considered previously [ 44 ], and we followed similar methods. We assumed that cyanobacteria are less desirable food organisms due to their poor nutritional quality [ 45 ]. Further, we considered a prevailing size and shape of entities (as single cells, filaments, globular colonies) greater than 50 μm to be inedible. Therefore, algae such as filamentous diatoms are considered problematic as food for zooplankton despite their high nutritional value. We acknowledge that previously published assumptions around edibility are overly simplistic, and that edibility of a given phytoplankton taxon is likely grazer-specific. For instance, some larger zooplankton taxa may be equipped to disaggregate large, filamentous diatoms into edible sizes, as noted in a limited set of species-specific studies from marine systems (e.g., [ 46 ]). Such nuances should be considered in the future, but we treat our analyses as a first attempt to evaluate this phenomenon in the Great Lakes. Using these edibility criteria, we filtered out all phytoplankton taxa with low nutritional and low shape edibility ( S2 Table ), and recalculated biovolume of remaining phytoplankton at each site.
Crustacean zooplankton and rotifers were collected by vertical tows taken across the same depth range, at the same time and stations as the phytoplankton data. All samples were collected according to U.S. EPA GLNPO standard operating procedure LG402 [ 47 ] and analyzed following LG403 [ 48 ]. Samples used here were collected using a 63 μm mesh net towed from 20 m or 1 m above the bottom, whichever was shallower, to the surface, at a rate of 0.5 m/s. As with phytoplankton, zooplankton sample collection for this program occurs 24 hours a day, and some stations are sampled during the day and some at night. Zooplankton samples from 20 m were not available for 2007 (both seasons) and for the spring season 2008–2011, and fewer stations had matching data for the two assemblages earlier in the time series. Plankton were narcotized with soda water and preserved with sucrose formalin. Separate counts with different subsampling approaches were done for crustaceans and microzooplankton (rotifers, nauplii) and data combined to densities (numbers/ m 3 ). A minimum of 400 individuals for each of the two counts were identified to the smallest practical taxonomic unit (mostly species) and up to 20 individuals in each taxonomic unit were measured for length in mm using a computerized drawing tablet [ 48 ]. Dreissenid veligers were not included in the total biomass calculations because they have not been measured consistently across the years (sensitivity analysis demonstrates that < 2% of the site-years are affected by this bias). Dry weight individual biomass (μg) was calculated from taxa-specific length-weight regressions available in the standard operating procedures [ 29 , 48 ]. Some rotifer equations used width measurements.
Statistical analyses
We used simple linear models to test for correlations between phytoplankton biovolume and zooplankton biomass, and correlations between phytoplankton and zooplankton diversity (Shannon H). All biovolume analyses were repeated with total and edible phytoplankton biovolume. In addition, we tested the relationships between zooplankton excluding predatory cladoceran ( Bythotrephes , Cercopagis , Leptodora and Polyphemus ) and Limnocalanus biomass, and edible algal biovolume and diversity, although Limnocalanus varies in its degree of zooplanktivory across the Great Lakes [ 49 ]. Data distribution was checked using qqnorm function in R and log 10 -transformation was applied to reduce skewness when warranted (biovolume and biomass data). Additionally, Spearman cross-correlation analyses were used to visualize the relationships between key groups of phytoplankton and zooplankton ( S2 Table ). Zooplankton predator ratios were calculated using the sum of predatory cladocerans ( Bythotrephes , Cercopagis , Leptodora and Polyphemus ) and Limnocalanus biomass relative to other zooplankton. Generalized additive models were fitted to visualize zooplankton predator-prey relationships with algal community metrics; model parameters were set to default as passed on to geom_smooth function in ggplot2. Time of day analyses were used to understand the relative importance of sampling time on zooplankton biomass-edible algal biovolume correlations within lakes. All analyses were done in R [ 50 ].
Across the 20 years of data and all of the lakes, there was a weak positive correlation between total algal biovolume and zooplankton biomass (P < 0.0001, R 2 = 0.19). This Great Lakes-wide correlation was season-dependent, with the overall trend driven primarily by the summer (P < 0.0001, R 2 = 0.15, vs. spring R 2 = 0.06, Fig 1 ). The relationship was also scale-dependent and varied across individual lakes, with a positive relationship in Lake Erie in both seasons and in Lake Huron in the spring, but a lack of significant correlations in lakes Ontario, Superior and Michigan in either season ( Fig 1 ). The slopes of biomass-biovolume relationship (evidence of decoupling) did not change uniformly with time (P > 0.05, Fig 2 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Data are presented for all lakes and for individual Laurentian Great Lakes by season.
https://doi.org/10.1371/journal.pone.0292988.g001
Fewer stations had matching data for the two assemblages earlier in the time series and spring data was unavailable for zooplankton between 2008–2011 (see S1 Table for complete summary of stations sampled by year).
https://doi.org/10.1371/journal.pone.0292988.g002
Total zooplankton biomass was very weakly negatively correlated with phytoplankton Shannon diversity (P = 0.001, R 2 < 0.01) and this relationship was similarly weak in both spring and summer across all lakes ( Fig 3 ). This weak negative effect was driven largely by Lake Erie, which spanned the longest gradient of both biomass and diversity, and was less pronounced in other lakes. Zooplankton diversity was likewise very weakly correlated with phytoplankton diversity (R 2 < 0.02, S1 Fig ). Most of the biomass of different zooplankton groups was unrelated or weakly negatively related to overall algal richness and diversity, with the exception of a stronger positive relationship for Limnocalanus (R 2 = 0.15, Fig 4 ). The majority of zooplankton groups had closer associations with other zooplankton groups (e.g., predatory cladoceran and rotifers), followed by biovolumes of Cyanophyta, Chlorophyta, and total algal biovolume ( Fig 4 ). Some variation in zooplankton predator-prey ratios was explained by algal diversity (P < 0.0001), whereas algal richness and biovolume did not have a strong linear effect ( S2 Fig ).
https://doi.org/10.1371/journal.pone.0292988.g003
Spearman correlation coefficients color-coded by shade intensity; all biovolume and biomass metrics have been log 10 -transformed. Relationships with visible R have P < 0.0001, whereas relationships with R < 0.10 are displayed as white text on light background.
https://doi.org/10.1371/journal.pone.0292988.g004
Edible algal biovolume was closely correlated with the overall algal biovolume (across lakes R 2 = 0.78, P < 0.0001 in each lake), with the largest discrepancy observed for Lake Erie ( Fig 5 ), where cyanobacteria are abundant in the summer. Our edibility criteria excluded algae with low nutritional value as well as those with difficult to manipulate shapes; we did not consider the two types of edibility filters separately, because even in the extreme scenario, there was a close relationship with total algal biovolume. Because of this relatively high correlation, most of the zooplankton-phytoplankton relationships were not greatly affected when considering only edible phytoplankton biovolume ( S3 and S4 Figs). Results of analyses excluding predatory cladocerans and Limnocalanus detected similarly weak trends to those for total zooplankton biomass ( S5 and S6 Figs). Examining zooplankton-phytoplankton relationship by the time of sampling demonstrated relatively minor effects of time of day on the shape of the biovolume-biomass relationship in individual lakes ( S7 Fig ). The relationship between total and edible biovolume did not exhibit directional changes over time ( S8 Fig ).
White line indicates the 1:1 ratio, the degree of departure from this line illustrates decreasing relative biovolume of edible algal taxa.
https://doi.org/10.1371/journal.pone.0292988.g005
There was a statistically significant but weak correlation between phytoplankton biovolume and zooplankton biomass across this long-term, large-scale dataset; however, it only held across the entire basin, and not individual lakes, and only in the summer. The weak correlation between phytoplankton biovolume and zooplankton biomass on a lake by lake basis could result from a lag in the response of zooplankton consumers to algal changes or variable top-down forcing on zooplankton across the lakes. If a lag in consumer response is present, we would expect the relationship to be stronger in the summer, which was generally the case, even though the correlation was still very poor in terms of predictive power and not statistically significant for most individual lakes. It is not surprising that the large trophic gradient of these lakes, from oligotrophic to meso-eutrophic, was also reflected by the gradient in zooplankton biomass and phytoplankton biovolume across the entire basin. Similarly, in other lakes the coupling between phytoplankton biomass and zooplankton biomass was limited beyond a certain productivity level [ 51 , 52 for Lakes Balaton and Lake Constance].
The slope and strength of the relationship between phytoplankton and zooplankton did not vary significantly with time, despite considerable shifts in algal and zooplankton community composition and productivity [ 5 , 25 , 29 ], providing little additional evidence for a disruption in coupling of producers and consumers. The match/mismatch hypothesis focuses on the consequences of inter-specific differences in response to climate change leading to potentially non-linear responses in the patterns of synchrony [ 9 ]. Such decoupling has already been observed in other systems as a result of a mismatch between trophic levels responding primarily to photoperiod vs. those responding to temperature [ 53 ]. In temperate lakes, the timing of thermal stratification affects the spring diatom blooms which are increasingly mismatched with keystone consumer dynamics [ 54 ]. In the Great Lakes, decreasing diatom cell sizes due to accelerated loss of larger individuals during summer stratification [ 27 ], for example, could make consumers rely on less energetically optimal smaller-sized algae. Longer ice-free periods in Lake Superior have resulted in longer stratification and increased primary production [ 5 ] and could lead to a timing mismatch between the peak of the spring bloom and zooplankton reproduction. The relationships of zooplankton biomass and diversity with edible phytoplankton were similar to those with total phytoplankton biovolume, likely because edible and total phytoplankton biovolume were closely correlated in all lakes with exception of Lake Erie, the most productive lake with a greater incidence of harmful algal blooms. Although other studies have shown that the proportion of inedible phytoplankton, particularly Cyanobacteria, increases in higher productivity lakes [ 16 , 55 , 56 ], cyanobacteria can also be abundant in oligotrophic systems [ 57 ] and can constitute a considerable part of the total biomass across large total phosphorus gradients [ 58 ]. Increasing biomass of less-edible phytoplankton, such as Cyanobacteria, has been observed to limit zooplankton resource use efficiency and the structure of trophic interactions [ 16 ]. However, the relationship between cyanobacterial blooms and zooplankton is variable, and previous studies have observed positive correlations between cyanobacteria concentrations and several groups of zooplankton [ 19 ].
Bottom-up forcing was demonstrated to be important in Lakes Michigan and Huron [ 59 ], where declines in zooplankton biomass and particularly herbivorous cladocerans were associated with simultaneous declines in spring chlorophyll indicating potential grazer limitation [ 36 , 59 , 60 ]. In other cases, changes in zooplankton are better explained by top-down forcing through increased invertebrate or fish predation [ 30 , 33 , 39 ], including changes in vertical distribution [ 61 ]. It is likely that the relative importance of these forces varies across the large spatial and trophic gradient and with season, contributing to the overall uncertainty in the zooplankton-phytoplankton relationship.
Zooplankton biomass was weakly negatively correlated with algal diversity, and it is possible that counteractive effects of algal diversity can be manifested through improved chances of balanced nutrition vs. dilution of the most nutritious taxa [ 13 ]. This effect sign was the opposite of the one we expected based on prior studies [ 12 , 13 ] possibly because pelagic Great Lakes do not include highly eutrophic waters, where extreme cyanobacterial dominance (and therefore decreased overall algal diversity) is more likely to reduce availability and diversity of preferred algal resources to the extent detrimental to consumers. Zooplankton and phytoplankton Shannon diversity were not significantly correlated in our study, providing additional evidence for inconsistent vertical diversity effects across aquatic ecosystems. Positive vertical diversity effects have been observed between bacterial and nanoflaggelate assemblages [ 62 ]; however, zooplankton diversity was not predicted by phytoplankton diversity across a wide range of marine systems [ 63 ], tropical streams [ 64 ], or temperate lakes [ 65 ].
We observed stronger correlations between the different zooplankton groups (with a particularly high correlation between predatory cladocerans and rotifers) than between zooplankton and phytoplankton. This may indirectly suggest a lack of strong feeding selectivity for zooplankton feeding on phytoplankton, at least at the division level, as well as a lack of general avoidance by zooplankton of Cyanobacteria [ 45 ], ability to adapt [ 66 ], or masking of feeding selectivity by other confounding factors. One of those factors could be availability of picoplankton, which could make an important contribution to the diets of smaller zooplankton. The predator-prey ratio of the zooplankton assemblage was weakly positively predicted by algal diversity, providing marginal support for our hypothesis that more diverse algal assemblages may support greater predator densities, which may not be surprising in the light of the overall weak links between zooplankton and phytoplankton in this system.
It is important to note that over these time scales, our dataset has temporal sampling limitations (only 2 sampling events/station/year) and lower number of stations sampled in the earlier years. Integrated samples are collected from the isothermal upper layer of the water column to favor even sampling of the phytoplankton assemblage. Although we did not see time of sampling explaining additional variation, other studies have shown that many zooplankton species have pronounced vertical migration [ 67 – 69 ] which could further contribute to the observed uncertainty about zooplankton-phytoplankton relationships. All of these factors may limit our ability to draw conclusions about the strength of temporal trends across the entire study period.
Understanding the relationships between phytoplankton and zooplankton is important for predicting the effects of climate change and nutrient loading on food web structure and higher trophic level [ 54 , 59 ]. A close correspondence between primary producer and consumer assemblages, indicative of bottom-up regulation, can make consumer populations more vulnerable to changing algal phenology and decreased overall lake productivity. However, we did not observe a close correspondence in the Great Lakes, making it more difficult to predict how the higher trophic levels would be affected by the continued changes in phytoplankton assemblages.
Supporting information
S1 fig. vertical diversity effects, or correlations between phytoplankton and zooplankton diversity..
https://doi.org/10.1371/journal.pone.0292988.s001
S2 Fig. Zooplankton predator-prey (i.e., zooplanktivore-grazer) ratios as a function of attributes of phytoplankton assemblage.
Blue line indicates a Generalized Additive Model (GAM) fit. Algal biovolume is in μm 3 /L, log 10 transformed; other metrics are diversity and richness.
https://doi.org/10.1371/journal.pone.0292988.s002
S3 Fig. Edible phytoplankton biovolume and zooplankton biomass correlations by season.
https://doi.org/10.1371/journal.pone.0292988.s003
S4 Fig. Total zooplankton biomass as a function of only edible phytoplankton diversity.
https://doi.org/10.1371/journal.pone.0292988.s004
S5 Fig. Herbivorous zooplankton biomass as a function of only edible phytoplankton biovolume.
https://doi.org/10.1371/journal.pone.0292988.s005
S6 Fig. Herbivorous zooplankton biomass as a function of only edible phytoplankton Shannon diversity.
https://doi.org/10.1371/journal.pone.0292988.s006
S7 Fig. Effects of sampling time on zooplankton biomass-edible algal biovolume correlations within lakes.
https://doi.org/10.1371/journal.pone.0292988.s007
S8 Fig. Temporal dynamics of the edible algal biovolume as a function of total algal biovolume.
Data are presented across all Great Lakes.
https://doi.org/10.1371/journal.pone.0292988.s008
S1 Table. Total number of stations sampled by year, lake and season.
Lakes: ER–Erie, HU–Huron, MI–Michigan, ON–Ontario, SU–Superior and seasons: Spr–spring, Sum–summer.
https://doi.org/10.1371/journal.pone.0292988.s009
S2 Table. Summary of phytoplankton data with edibility rankings by shape and nutritional content.
SPECCODE–standard species code; maxRelBiov–maximum relative biovolume within a sample (indicator of relative importance combined with frequency), frequency–number of samples in which the taxon was detected; DIV–division; SPECIES–species name, nutrition edibility and shape edibility–categorical rankings.
https://doi.org/10.1371/journal.pone.0292988.s010
https://doi.org/10.1371/journal.pone.0292988.s011
- View Article
- Google Scholar
- PubMed/NCBI
- 38. Wetzel RG (2001) Limnology: Lake and River Ecosystems. 3rd ed. Academic Press, San Diego. 1024 pp.
- 40. U.S. EPA (2022) Standard Operating Procedure. Great Lakes National Program Office, U.S. Environmental Protection Agency, Chicago, IL.
- 41. U.S. EPA (2010) SOP LG401, Standard Operating Procedure for Phytoplankton Analysis. Revision 05, February 2010. Great Lakes National Program Office, U.S. Environmental Protection Agency, Chicago, IL.
- 47. U.S. EPA (2017). SOP LG402. Standard Operating Procedure for Zooplankton Sample Collection and Preservation and Secchi Depth Measurement Field Procedures. Revision 12, February 2017. Great Lakes National Program Office, U.S. Environmental Protection Agency, Chicago, IL.
- 48. U.S. EPA (2017) SOP LG403, Standard Operating Procedure for Zooplankton Analysis. Revision 08, February 2017. Great Lakes National Program Office, U.S. Environmental Protection Agency, Chicago, IL.
- 50. R Core Team (2022) R: A language and environment for statistical computing. R Foundation for statistical Computing, Vienna, Austria. https://www.R-project.org/ .
- Search Menu
- Sign in through your institution
- Advance articles
- Virtual issues
- Author Guidelines
- Submission Site
- Open Access
- About Journal of Plankton Research
- Editorial Board
- Editorial History
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Books for Review
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, traits and trade-offs, trait associations and corresponding ecological strategies: emerging patterns, traits characterizing interactions across trophic levels, candidate traits for ecosystem models, future directions, acknowledgments.
- < Previous
Trait-based approaches to zooplankton communities
Corresponding editor: Roger Harris
- Article contents
- Figures & tables
- Supplementary Data
Elena Litchman, Mark D. Ohman, Thomas Kiørboe, Trait-based approaches to zooplankton communities, Journal of Plankton Research , Volume 35, Issue 3, May/June 2013, Pages 473–484, https://doi.org/10.1093/plankt/fbt019
- Permissions Icon Permissions
Zooplankton are major primary consumers and predators in most aquatic ecosystems. They exhibit tremendous diversity of traits, ecological strategies and, consequently, impacts on other trophic levels and the cycling of materials and energy. An adequate representation of this diversity in community and ecosystem models is necessary to generate realistic predictions on the functioning of aquatic ecosystems but remains extremely challenging. We propose that the use of trait-based approaches is a promising way to reduce complexity while retaining realism in developing novel descriptions of zooplankton in ecosystem models. Characterizing zooplankton traits and trade-offs will also be helpful in understanding the selection pressures and diversity patterns that emerge in different ecosystems along major environmental gradients. Zooplankton traits can be characterized according to their function and type. Some traits, such as body size and motility, transcend several functions and are major determinants of zooplankton ecological strategies. Future developments of trait-based approaches to zooplankton should assemble a comprehensive matrix of key traits for diverse groups and explore it for general patterns; develop novel predictive models that explicitly incorporate traits and associated trade-offs; and utilize these traits to explain and predict zooplankton community structure and dynamics under different environmental conditions, including global change scenarios.
Understanding and predicting the structure and function of plankton communities under different environmental conditions, including a changing climate, is an important challenge for aquatic ecologists, oceanographers and limnologists. Zooplankton are among the most abundant aquatic organisms and they occupy key trophic positions in most marine and freshwater environments ( Kiørboe, 2008a ). Knowledge of the structure and functioning of zooplankton communities is, therefore, a key component of our general understanding of aquatic ecosystems. Zooplankton in marine and freshwater environments exhibit significant diversity of ecological strategies, dominance patterns and effects on ecosystems. Adequately representing this diversity in conceptual and mathematical models is challenging and only just beginning.
Parallel challenges of representing ecological diversity exist for phytoplankton. The most common approach is to explicitly model key functional groups and their impacts on ecosystems. Such models, however, can lead to a large number of equations representing functional groups, rapidly increasing the complexity of the models ( Litchman and Klausmeier, 2008 ; Follows and Dutkiewicz, 2011 ). Another shortcoming of this approach is that setting up functional groups a priori limits model flexibility and precludes the possible rise of new functional groups under novel conditions. A more promising approach that is gaining interest is to focus on key traits rather than functional groups and to consider a continuum of traits interrelated through trade-offs ( Bruggeman and Kooijman, 2007 ; Follows et al. , 2007 ; Litchman and Klausmeier, 2008 ; Merico et al. , 2009 ). This approach permits the reduction of model complexity while maintaining an adequate representation of diversity and, moreover, it allows the emergence of species and groups with novel combinations of traits that may arise under changing environmental conditions.
Here we propose that such a trait-based approach can also be useful for describing and modeling zooplankton communities and pelagic ecosystems. We discuss possible zooplankton traits that can be included, propose a general trait classification framework and outline future research directions and main challenges to this approach. Parts of zooplankton ecology are mature fields with a wealth of studies on different aspects of zooplankton behavior, physiology and biogeography. Using existing studies for a trait-based synthesis is a productive way to gain new insights and to increase our mechanistic understanding of the structure and functioning of zooplankton communities and aquatic ecosystems in general ( Barnett et al. , 2007 ).
In all cases, fitness is a function of the feeding, growth, survival and the reproductive rates of the organism. These three fundamental activities, in turn, depend on the details of the biology of an organism, and may be expressed differently for different life forms. A combination of morphological, physiological, behavioral and life history traits is involved in these functions. Consequently, we propose classifying traits according to their type and the function in which they are involved (Fig. 1 ), similar to a recent trait classification for phytoplankton ( Litchman and Klausmeier, 2008 ). Obviously, this classification scheme is not the only possible or an exhaustive one, but we hope that it stimulates a search for general patterns and further trait categorizations.

Zooplankton trait classification according to function and type. Key traits that transcend several functions and influence many other traits are indicated in bold. Dotted lines indicate traits that may have a secondary importance for other functions.
Natural selection tends to maximize individual fitness by optimizing the net result of feeding, survival, growth and reproduction. However, there are potential conflicts—trade-offs—between these activities, and all cannot be maximized simultaneously (Fig. 2 ). For example, a non-motile ambush feeder will never encounter a mate unless it swims and, hence, sacrifices feeding ( Kiørboe, 2008b ); searching for a mate or for food increases encounter rates with predators and generates fluid disturbances that may be perceived by rheotactic predators, and thus reduces survival ( Tiselius et al. , 1997 ; Kiørboe et al. , 2010 ; Lasley-Rasher and Yen, 2012 ) and migrating to deep water during daytime to avoid visual predators ( Aksnes and Giske, 1990 ; Fiksen, 1997 ) or during nighttime to avoid non-visual predators ( Ohman, 1990 ) implies lost feeding opportunities. Thus, an organism cannot maximize performance with respect to all fundamental activities simultaneously. Energy from food must similarly be allocated to different competing functions, i.e. maintenance, growth and reproductive products (Fig. 2 ). Investment in maintenance, storage and repair enhances longevity but leaves less energy for growth and reproduction. Investment in growth allows the organism to achieve a large size and a high-reproductive potential in the future, while investment in gonads contributes directly to current reproduction.

The fundamental Darwinian missions of an organism are to feed, survive and reproduce. These activities may interfere with one another, and the energy obtained from feeding may be allocated to different competing functions: growth, reproduction and longevity/survival (investment in maintenance, storage and repair). The optimum behavior and energy allocation pattern is that which maximizes the fitness of the individual in a particular environment.
Quantifying the risks and trade-offs associated with key traits may allow us to predict the behavior, physiology and morphology that optimize the fitness of an organism in any particular environment ( Gilliam and Fraser, 1987 ) and to predict the distribution of traits along environmental gradients. We, therefore, argue that zooplankton traits and the associated trade-offs should be considered in light of their effects on fitness, as the same fitness can be achieved through optimizing different components, e.g. traits affecting feeding, survival, growth and reproduction. An explicit consideration of the trait relationships to fitness will help not only to systematize traits and determine relationships between them but would allow ecological and evolutionary perspectives to be connected in a trait-based framework.
Below we briefly describe traits of different types within each of the three fundamental functions (Fig. 1 ) and provide some examples of associated trade-offs.
Feeding traits
Feeding includes a diverse range of behavioral, morphological, physiological and life history traits (Fig. 1 ). Behavioral feeding modes include ( Kiørboe, 2011 ): (i) ambush feeding, where prey encounter depends on the motility of the prey; prey encounter may be passive and by direct interception (most protozoans), or it may depend on remote detection of prey and active prey capture (dinoflagellates, most copepods); (ii) feeding current feeding, where the feeding current is either a scanning current from which remotely detected prey are captured (copepods), or the current is passed through a filter that screens prey particles (tunicates, choanoflagellates) or over other structures that intercept the prey (many cnidarians, ctenophores) without the possibility for remote prey detection; (iii) cruise feeding, where prey are encountered via remote detection (not direct interception) and captured. The feeding mode has implications for prey selection: ambush feeders target only motile prey; feeding current feeders may be less efficient towards those motile prey that can perceive and escape feeding currents (which applies to many protists, e.g. Jakobsen, 2001 ); feeding current feeders with a scanning current may select prey based on their chemical characteristics (e.g. avoidance of toxic algae, selection of nutritious species), whereas prey retained on a filter are generally selected only by their size and shape. Each feeding mode has associated costs and benefits. For example, ambush feeding allows low-energy expenditure and a low predator encounter rate but results in relatively low feeding efficiency and a low mate encounter rate ( Kiørboe et al. , 2010 ). Employing mixotrophy as a mode of nutrition results in a trade-off between feeding efficiency and, consequently, the maximum growth rate and ability to survive low-food conditions. Some trade-offs extend beyond pairwise relationships and need to be considered in higher dimensions.
The food size spectrum consumed by the organism is another important feeding trait. The average size ratio between prey and predator in zooplankton has sometimes been assumed to be around 1:10, but there is substantial variation between taxa, with the gelatinous salps and appendicularians generally feeding on relatively small prey, while flagellates may feed on relatively large prey ( Hansen et al. , 1994 ; Lombard et al. , 2011 ). Dinoflagellates, for example, can ingest prey that are several times their cell length ( Calbet, 2008 ; Jeong et al. , 2010 ). Different groups may differ in their trophic niche breadth, at least in the size range of prey consumed: salps may consume a wide range of prey ( Vargas and Madin, 2004 ). These differences in prey size ranges and types of food have profound effects on the structure of the food webs and energy and material cycling in ecosystems.
The absolute size of zooplankton prey may be equally important because there are significant trade-offs related to absolute prey size, namely prey availability and prey selection. The biomass of small picophytoplankton fluctuates much less than the biomass of micro-phytoplankton, both seasonally and spatially ( Chisholm, 1992 ; Kiørboe, 2008b ). Large diatoms typically bloom in spring (in temperate waters) and during periods of upwelling and at spatio-temporal discontinuities in the water column (e.g. Taylor et al. , 2011 ). The biomass of picophytoplankton is constantly low, but they are the dominant phytoplankton in oligotrophic regions, i.e. in most of the ocean.
Small phytoplankton produce chemical and hydromechanical signals that are too small to allow remote detection because the signals attenuate almost instantaneously due to diffusion or viscosity. Hence, pico-sized prey cells must be collected by some automatic process by their grazers (filter feeding, diffusional deposition), which has mainly been developed by the large gelatinous forms (appendicularians, salps, doliolids) and by small flagellates. Signals from larger prey cells (nano- and microplankton) are strong enough to allow remote detection. This leads to other feeding strategies (scanning current, ambush feeding, cruise feeding) and allows for active prey selection.
Stoichiometric and nutritional requirements
Zooplankton in general have more constant nutrient ratios, such as C:N:P (carbon:nitrogen:phosphorus) than phytoplankton ( Sterner and Elser, 2002 ). A much smaller variability in elemental ratios occurs because zooplankton generally do not store or deplete such large percentages of elements in their bodies as phototrophs ( Sterner and Elser, 2002 ). Zooplankton may also have a higher content of certain essential fatty acids than their phytoplankton prey, despite their inability to synthesize them. Such trophic upgrading may be achieved by selective feeding (see above). Dinoflagellates and copepods can select prey cells based on their nutrient content (e.g. Cowles et al. , 1988 ; Meunier et al. , 2012 ). Different groups and species of zooplankton differ significantly in their average nutrient ratios and requirements ( Andersen and Hessen, 1991 ). Among freshwater zooplankton, for example, the cladoceran Daphnia has high P requirements compared with other cladocerans (e.g. Bosmina ) and, hence, low C:P and N:P ratios ( Andersen and Hessen, 1991 ). When fed on low P phytoplankton, Daphnia have slower growth and reproduction rates, demonstrating that not only food quantity but also food quality affects zooplankton growth ( Main et al. , 1997 ). According to the growth rate hypothesis, fast growing organisms contain high concentration of P-rich ribosomes and, therefore, have high P content and low C:P and N:P ratios ( Sterner and Elser, 2002 ). Freshwater zooplankton may often be P-limited ( Sterner et al. , 1993 ), while marine zooplankton may more often be limited by N or Fe ( Checkley, 1980 ; Jones et al. , 2002 ; Chen et al. , 2011 ).
Despite being more homeostatic than in phytoplankton, zooplankton stoichiometric ratios do exhibit seasonal, latitudinal and developmental variability ( Sterner and Elser, 2002 ). Potential trade-offs may include lower nutrient (e.g. phosphorus) requirements allowing survival in low-nutrient environments but leading to lower maximum growth rates according to the growth rate hypothesis ( Sterner and Elser, 2002 ).
Survival traits
Zooplankton may enhance their longevity by reducing predation risk and by adapting to periods of food shortage (e.g. over winter). Traits to minimize predation risk can occur at several successive steps in a prey–predator interaction. They include prey morphological (e.g. transparency) and behavioral traits that reduce initial encounter rates with predators, behaviors that promote successful escape once encounter has occurred, or morphological and chemical defenses that reduce the probability of successful ingestion once captured ( Ohman, 1988 ). Reduction of encounter rates with predators can be accomplished via diel vertical migration ( Aksnes and Giske, 1990 , Ohman, 1990 ), the use of other temporal or spatial refugia, or hydrodynamically cryptic swimming behavior that minimizes detectability by predators ( Ohman, 1988 ). Tissue transparency (e.g. cnidarians, chaetognaths, ctenophores, pelagic tunicates) also reduces encounter rates with visual predators. Body pigmentation in small zooplankton taxa is positively correlated with diel vertical migration ( Hays et al. , 1994 ). Sensory detection of predators ( Jakobsen, 2001 ) followed by prey escape responses ( Lenz and Hartline, 1999 ) permits motile zooplankton to evade capture. Bioluminescence may also function as a predator evasion strategy though the “burglar alarm” mechanism, i.e. by attracting the predators of the predator ( Burkenroad, 1943 ; Abrahams and Townsend, 1993 ). Although many zooplankton taxa have morphologically fixed spines and other structures that help defend against ingestion by predators, inducible mechanical defenses are probably best documented for freshwater cladocerans that develop spiny helmets in predator-rich environments ( Tollrian and Dodson, 1999 ). Dinoflagellates may be chemically defended against predators (e.g. Sykes and Huntley, 1987 ). There are obvious trade-offs associated with all these traits in terms of investment in structures, sensory apparatus, escape muscles and lost feeding opportunities, but it remains challenging to quantify these costs and, hence, to predict optimal behaviors through fitness optimization.
Strategies to survive harsh periods, typically winters (or between upwelling events in the upwelling systems), include starvation tolerance, dormancy and the production of resting stages. The trade-off is survival during harsh periods vs. reduced and/or delayed reproduction. Starvation tolerance has been studied in a range of zooplankton groups, including protozoans ( Menden-Deuer et al. , 2005 ), copepods ( Borchers and Hutchings, 1986 ), jellyfish ( Costello, 1998 ) and pteropods (Böer et al ., 2007). In copepods, starvation tolerance is typically studied in the context of winter dormancy. Preparation for dormancy in copepods is characterized by the accumulation of lipid reserves (typically wax esters) and reduced metabolism ( Ohman et al. , 1998 ) that allow survival at depth during long winters. Wax ester accumulation may be considered a proxy for “dormancy potential” and shows a characteristic latitudinal pattern in copepods, with increasing accumulation at high latitudes ( Kattner and Hagen, 2009 ).
The formation of resting stages is an alternative to dormancy: many protozoans form resting cysts ( Corliss and Esser, 1974 ), and some copepods (some species from the genera Acartia , Eurytemora and Centropages ) and many cladocerans (genera Alona , Daphnia , Ceriodaphnia and many others) produce resting eggs ( Marcus, 1996 ; Vandekerkhove et al. , 2005 ). In addition to the different physiology of resting stages, this strategy also differs from the dormancy strategy in that every individual typically produces many survival vehicles (eggs) and each egg can survive in the sediment for many years (in the extreme, up to 300 years or more for copepods, Hairston et al. , 1999 ). One would expect the formation of resting eggs to be restricted to the forms living in lakes and shallow areas of the ocean because an egg sedimented several kilometers to the deep ocean floor has a minute chance of returning to the upper ocean. Currently, little is known how these traits are related to each other and what the relevant trade-offs may be.
Reproductive traits
Zooplankton display a considerable diversity in their reproduction modes and associated traits, from asexual reproduction during at least part of the life history (some protozoans, cladocerans, tunicates and jellyfish), hermaphroditism (chaetognaths, all gelatinous forms) to sex change (some decapods and copepods) and fixed dioecious reproduction, and from internal to external fertilization. The key issue is encounter rates between either gametes or sexes, as this occurs in a 3D world where the distance to the nearest mate may be substantial. The behavior and ecology of zooplankton must to a very large extent be dictated by this ultimate Darwinian mission, but its significance is underappreciated.
Asexual reproduction and hermaphroditism with self-fertilization solve the encounter issue, but result in lower genetic diversity that potentially can impede adaptation to changing environmental conditions. For zooplankton with sexual reproduction one important distinction is whether gametes (eggs and sperm) are spawned freely into the water where fertilization takes place externally (broadcast spawning), or whether adult males and females have to meet and mate. Broadcast spawning requires the production of many gametes, mainly of sperm, and hence may limit the initial minimum size of the animals. The gelatinous plankton (cnidarians, ctenophores, tunicates) are generally broadcast spawners, while all other taxa with sexual reproduction appear to have mating. Mate finding may be facilitated by the utilization of hydrodynamic and pheromone signaling, and is rather well understood for zooplankton with mating encounters, but even broadcast spawners may need behavioral adaptations to enhance gamete encounter rates. Spawning aggregations (e.g. some appendicularians, Alldredge, 1982 ), colony formation (salps), spawning synchronization (some ctenophores, Purcell and Madin, 1991 ), self-fertilization (ctenophores, Martindale, 1987 ) may all help ensure sufficient gamete encounter rates. Sexual reproduction is wasteful (superfluous gamete production) or involves investment in sensory equipment to enhance mate encounter rates, and mate finding as well as mating itself implies elevated predation risks but allows for a higher genetic diversity, deletion of bad mutations and the promotion of good genes through sexual selection. These trade-offs are difficult to quantify.
Energy allocation and life history strategies
The energy gained from feeding must be allocated among growth, reproduction and maintenance and defines important aspects of the life history of an organism. Maintenance here includes the inescapable minimum metabolic cost to maintain body tissues, feed and locomote, but also energy invested in predator evasion and in tissue repair. The latter is rarely considered in zooplankton studies, but has implications for the rate of senescence and longevity of an organism and may vary widely among species (e.g. Ceballos and Kiørboe, 2011 ; Sichlau and Kiørboe, 2011 ). Some life histories appear to be fixed (and hence a real trait), but strategies may also be malleable in response to local conditions (known mainly for cladocerans and rotifers in freshwater). As an example, the investment in reproduction vs. growth determines the age and size at maturity. The trade-offs are relatively clear: investment in reproduction now is at the cost of reduced growth—and, hence, potential for future reproduction and reduced maintenance (and, hence, survival). Fitness optimization predicts relatively low investment in maintenance (and, hence, longevity) and early maturation at a small size when mortality is high. Copepods appear to have rather fixed life history strategies, with age and size at maturation depending solely on temperature and availability of food ( Checkley, 1980 ; Huntley and Lopez, 1992 ). Perhaps predation risk and simple optimization models predict development times in copepods well ( Kiørboe and Hirst, 2008 ). Life histories in rotifers, in contrast, are plastic, and these organisms allocate more energy to reproduction and less to maintenance in the presence of predator cues, as predicted ( Garcia et al. , 2007 ).
The maximum growth rate of zooplankton is also a result of energy allocation. While specific growth rates typically scale with the body mass to a power of about −1/4 within zooplankton groups (e.g. Hansen et al. , 1997 ), the magnitude of the maximum growth rate may vary significantly between groups ( Hirst et al ., 2003 ), suggesting different energy allocation optima. For example, pelagic tunicates typically grow much faster than, for example, copepods of comparable body mass (carbon) and at similar temperatures (by a factor of ∼5; see Hirst et al ., 2003 ). It may be hypothesized that copepods allocate more energy into predator avoidance and defense, in the form of a very well-developed sensory apparatus and powerful musculature that allows for rapid escape jumps, with a consequent lower growth rate and mortality rate than tunicates. Such relations are poorly examined but may represent fertile future research avenues.
Finally, the trade-off in the ‘progeny size’, where a large number of progeny is associated with a decreased size of individual offspring and, as a result, a decreased individual survival, determines contrasting reproductive strategies. The trade-off between high- or low-reproductive investment in individual progeny also extends to trade-offs in embryonic care. For taxa that carry their eggs, in contrast to broadcast spawners, embryonic size may be greater and the number of offspring lower, which can be compensated by the higher survivorship of protected eggs relative to those that drift freely in the plankton ( Hirst and Kiørboe, 2002 ). Hatching time of protected eggs is also greater (>3-fold) than of unprotected eggs ( Hirst and Lopez-Urrutia, 2006 ).
Traits transcending functions
The relative importance of individual traits varies. Some traits have a disproportionate influence on the overall ecology and physiology of a zooplankter, transcending multiple functions (Fig. 1 ). Adult body size and carbon density are among such traits. Maximum body sizes relates to energy allocation and size at maturity (see above). A large number of properties and vital rates scale with size, e.g. feeding rate, prey size, growth rate, metabolism, mortality and vital rates, typically increase with body mass to a power of <1 within taxonomic groups. The maximum size and size at age may therefore be used as a proxy for many traits. Another trait related to size and life form is the biomass to body volume ratio. Zooplankton separate into two main life forms related to their body carbon density: the “typical” zooplankters with carbon densities on order 10 2 mg C cm –3 body volume, and those that have inflated volumes and body carbon densities ∼2 orders of magnitude lower. The latter group includes the taxonomically diverse group of gelatinous zooplankton (tunicates, ctenophores, cnidarians, chaetognaths) but also some protists, such as Noctiluca . An immediate advantage of an inflated body volume is the increase in prey capture area and potential feeding rate, which applies across the very different feeding modes of the gelatinous taxa ( Alldredge and Madin, 1982 ; Acuna et al. , 2011 ; Kiørboe, 2011 ), but mortality rates may also be smaller for an inflated organism, because size per se can lead to lower predation mortality, the nutritional quality of a watery zooplankter is low, and high water content is often associated with tissue transparency and lower visibility to predators. This pattern is contrary to the typical trade-offs associated with feeding behaviors, where a higher feeding rate typically implies elevated predation risk, cf. above. The inflated size strategy is also found among planktonic osmotrophs and was termed the “Winnie-the-Pooh” strategy by Thingstad et al . ( Thingstad et al. , 2005 ) exactly for this reason (because Winnie, when asked whether he wanted honey or milk, answered “both”). There must be costs associated with an inflated body volume, otherwise this life form would dominate the zooplankton, but it remains a challenge to identify and quantify them. However, quantifying the trade-offs of the gelatinous vs. non-gelatinous life forms may allow us to predict the environmental conditions that select for one or the other and may be especially relevant, given the purported rise in the dominance of gelatinous forms (but see Condon et al ., 2012 ).
Motility and body shape are composite traits that affect not only feeding strategy but also influence survival (predator avoidance) and reproduction (mate encounter) and are, therefore, under complex selection pressures ( Visser, 2007 ). Both speed and patterns (e.g. pathways) vary considerably across and within species. Moving from small to large organisms, Reynolds number (Re) increases and so does the optimal shape for locomotion (from near spherical at low Re to more streamlined with increasing Re; see, e.g. Dusenberry, 2009 ): this transition is seen from nearly spherical flagellates and copepod nauplii to streamlined copepodites, etc. Among the non-gelatinous zooplankton, the dominant shape is that of fusiform copepods; even non-copepods tend to have a hydrodynamically shaped muscular body, adapted for high-escape velocities ( Verity and Smetacek, 1996 ). While most non-gelatinous plankton are propelled by appendages, flagella or cilia, the gelatinous plankton have different propulsion mechanisms: jet propulsion (salps and some jellyfish) or rowing (some medusae). A major trade-off associated with motility is that it increases encounters with both prey and predators ( Gerritsen and Strickler, 1977 ; Visser, 2007 ).
Most traits in zooplankton are not independent of one another but are correlated. These trait correlations may represent fundamental physiological constraints resulting in trade-offs that can lead to different ecological strategies that transcend taxonomic groups. Identifying such trade-offs will help define trait associations and reduce the number of traits needed to adequately describe zooplankton communities. For example, one general pattern that may emerge, and serve as a working hypothesis, is that of the two main life history types among zooplankton that feed mainly on pico- vs. microplankton:
(i) Grazers on picoplankton often have high volume-specific clearance rates. They collect prey by automatic processes (filter feeding, diffusional deposition) and have no capability to select prey on the basis of their nutritional value. They have high potential population growth rates, often accomplished through asexual reproduction (at least during the part of the life cycle), minimum investment in defense mechanisms and sensory systems, and minimum investment in overwintering strategies. Their food source is relatively stable in time and space, partly due to the controlling role of the grazers themselves, since the grazers and the prey have growth rates of similar order allowing for rapid numerical responses. The relative constancy of the food source permits minimum investment in survival during meager times. The group includes some protozooplankton (mainly heterotrophic nanoflagellates), the tunicates (at least appendicularians, salps and doliolids) and some cladocerans. The two first groups are often the main grazers of phytoplankton in the ocean and those of which we know the least.
(ii) Grazers on nano- and microplankton typically have relatively lower clearance rates, but they may be able to select prey on the basis of their nutritional content. They are generally organisms with lower potential growth rates and have mandatory sexual reproduction, high investment in defense (behaviorally or morphological) and well-developed sensory systems that allow efficient mate finding, prey selection and predator perception. They can afford low clearance and potential growth rates due to higher investment in escape behavior or defenses and, consequently, lower mortality rates. Due to the low growth rate and lagged numerical response they cannot control their prey populations, which consequently are very variable in time and space. This necessitates investment in mechanisms to survive periods of food shortage in the form of dormancy or production of resting stages (cysts, eggs). The group includes copepods, euphausiids and some protozoans, most notably the heterotrophic dinoflagellates; these groups are the dominant mesozooplankton groups in the ocean that were claimed to account only for a relatively small fraction of phytoplankton grazing in the ocean ( Calbet and Landry, 2004 ), but this view has important exceptions ( Landry et al ., 2009 ).
There are exceptions to these patterns. For example, freshwater cladocerans invest in resting stages as an adaptation to ephemeral freshwater systems, as well as in morphological defenses, and some heterotrophic nanoflagellates have been reported to be able to select prey based on their chemical content (e.g. Landry et al ., 1991 ; Jurgens and DeMott, 1995 ), although this evidence for active prey selection has later been questioned ( Boenigk et al. , 2001 ; Langlois et al. , 2009 ).
While the relative significance of zooplankters with these two alternative trait combinations to a large extent will be governed by the size structure of the phytoplankton, it is much less clear what determines whether the zooplankton communities will be dominated by heterotrophic nanoflagellates or by pelagic tunicates, for example, or, similarly, by copepods or heterotrophic dinoflagellates. Chance may of course play a role, since any enrichment mechanisms, including upwelling events, will stimulate production of whatever is there and what is seeded from deeper waters (resting stages, cysts), but differences in life history traits and associated trade-offs are likely to play a role in so far unknown ways.
Carnivorous zooplankton (e.g. ctenophores, cnidarians, predatory copepods and amphipods, chaetognaths, heteropods, fish larvae) are diverse in terms of phylogeny, morphology and behavior. For these taxa as well, trait-based organization may prove a useful means of simplifying this diversity. A first-order division among these predatory taxa is between those that search for prey visually (fish larvae and heteropods) and those that use non-visual means to locate prey (most others, e.g. Eiane et al. , 1999 ).
Given the eco-physiological and evolutionary constraints, certain traits or values of quantitative traits can only occur with a limited range of correlated traits: for example, a small-bodied zooplankter is unlikely to employ rapid swimming as an escape strategy from highly motile predators. Consequently, there are contrasting trait value associations that define different ecological strategies. Major taxonomic groups of zooplankton differ in their ecological strategies and trait associations. There is a good correspondence of taxonomic affiliation and certain trait combinations and, thus, ecological strategies, but it is not a perfect agreement and likely depends on the level of taxonomic aggregation. It may be a worthy exercise to map ecological strategies in the multi-trait space and, thus, quantitatively determine how similar or different major zooplankton groups may be.
It is well known that different zooplankton taxonomic groups are strongly associated with certain hydrographic and other physical and chemical conditions, as well as with phytoplankton composition ( Calbet, 2008 ). This likely translates into certain traits or values of quantitative traits more or less robustly associated with specific physico-chemical conditions and phytoplankton composition. Trait-based models using fitness maximization approaches may be able to predict what strategies are selected for under given environmental conditions.
A particular challenge to trait-based approaches is to define traits that describe food web interactions, the relationships between different trophic levels in particular. How might the complexity of all possible pairwise interactions (e.g. between a phytoplankton cell and a zooplankter) be reduced into a meaningful trait or a small number of traits? Some of such traits are likely to be related to cell or body size. For example, characterizing the size spectra of food particles (feeding kernels) for different size zooplankton as a function-value trait (i.e. not a single value but a function), the frequency distribution of food particles ingested, could help describe and compare diets and the effects of different groups of zooplankton on phytoplankton. Models that include frequency distributions of particle sizes ingested by different groups of grazers are starting to be implemented and provide a more realistic description of food web interactions ( Armstrong, 1999 ; Banas, 2011 ). Explicitly including stoichiometeric requirements and content of different trophic levels (e.g. consumers and their prey) may also help to represent adequately the interactions between different trophic levels ( Sterner and Elser, 2002 ; Grover, 2003 ).
The selection of traits to consider will inevitably depend on the questions asked. For many ecosystem models that focus on nutrient cycling, there are a few zooplankton traits that will likely be particularly useful for characterizing zooplankton-related processes. Such traits could be the maximum growth rates, stoichiometric requirements, grazing rates and trophic niche breadths (size distributions of food particles). To reduce the complexity of the representation of these traits, scaling relationships may be introduced ( Armstrong, 1999 ; Poulin and Franks, 2010 ), as many of these traits scale allometrically with body size ( Vidal and Whitledge, 1982 ; Hirst and Lampitt, 1998 ; Saiz and Calbet, 2007 ). The scaling relationships may be obtained empirically by compiling relevant data or derived theoretically based on scaling rules. Using these and other traits often requires a proper conversion of units (e.g. from individual-based to mass-based units). Models built to investigate the role of climate change and rising temperatures in particular will need to include the temperature dependence of many traits ( Forster et al. , 2011 ). Compiling diverse traits from empirical studies into accessible databases will allow better parameterizations of marine ecosystem models.
A promising approach to increasing the mechanistic understanding of the structure and function of zooplankton communities is to look systematically at zooplankton trait distributions along various environmental gradients, such as latitudinal gradients (associated with temperature and other physical parameters), primary productivity or nutrient concentrations. There are already such studies ( Roman et al. , 2002 ) and they can offer insights into latitudinal trait distributions. The associations of certain trait values or suites of traits and corresponding strategies with particular environmental parameters, such as hydrographic conditions, should help understand how environmental factors structure zooplankton communities and affect their functioning. This knowledge can then be used to predict potential zooplankton community reorganizations under changing environmental conditions. For example, looking at latitudinal gradients in body size or reproductive strategies can provide insights into how changing climate may affect zooplankton communities, e.g. how warming temperatures might alter the dominant body size or reproductive strategies and, consequently, lead to changes in community structure and ecosystem functioning.
There is a significant body of literature reporting various traits of different species and groups of zooplankton in marine and freshwater environments. Assembling a comprehensive zooplankton trait matrix and synthesizing the trait value distributions can be a high-payoff undertaking that will also be helpful for parameterizing zooplankton in various ecosystem models. Several such meta-analytical studies have been published and they provide excellent syntheses on the distribution and scaling of such traits as growth rates, reproduction, feeding and mortality, mostly in marine copepods ( Hirst et al. , 2003 ; Bunker and Hirst, 2004 ) but also in freshwater crustacean zooplankton ( Barnett et al. , 2007 ).
Comparing zooplankton trait distributions between marine and freshwater environments will likely provide valuable insights into the mechanisms that structure zooplankton communities in each environment. An intriguing difference in taxonomic diversity of zooplankton between the marine and freshwater realm is that marine zooplankton are much more taxonomically diverse, covering a wide range of taxa that are absent in freshwater zooplankton (salps, appendicularians, cephalopods, pteropods, etc.). This difference begs the question whether the zooplankton grazing and ecosystem effects are qualitatively and quantitatively different between the two environments. Another unanswered question is a comparison of the importance of microzooplankton in freshwater vs. marine environments. Numerous studies in marine ecosystems demonstrated that microzooplankton often are the dominant grazers, especially in oligotrophic systems ( Calbet, 2008 ). The estimates of the importance of microzooplankton in lakes are much more scarce but it is likely that the freshwater microzooplankton contribution to total grazing can be substantial as well, even in eutrophic systems ( Hambright et al. , 2007 ). It would be of interest to compare the types of marine and freshwater ecosystems that have a greater importance of micrograzers.
Allometric approaches are powerful ways to generalize the relationships among various traits. However, sometimes these relationships differ across major taxonomic groups: allometric exponents can be taxon specific. A simultaneous consideration of allometric and taxonomic constraints may improve the trait-based description of food webs ( Rall et al. , 2011 ).
Trait-based approaches to zooplankton may in the future be integrated into a general trait-based framework for modeling not only planktonic communities (bacterioplankton, phytoplankton and zooplankton) but the whole aquatic ecosystem as well, including end-to-end models encompassing multiple trophic levels and organismal groups, from bacteria, to plankton to fish and to mammals and birds.
EL acknowledges funding from the US National Science Foundation (DEB-0845932, OCE-0928819 and DEB-1136710) and MDO from NSF (OCE-1026607) via the California Current Ecosystem LTER site.
We thank Maurizio Ribera d'Alcalá, Daniele Iudicone and other staff of the Stazione Zoologica Anton Dohrn Napoli for organizing and hosting a EUROCEANS workshop on “Constraining, understanding and modeling biocomplexity in plankton communities” in Naples, Italy in 2008, where the ideas of this paper were first synthesized. We thank anonymous reviewers for helpful comments and suggestions.
Google Scholar
Google Preview
Author notes
| Month: | Total Views: |
|---|---|
| November 2016 | 2 |
| December 2016 | 6 |
| January 2017 | 14 |
| February 2017 | 35 |
| March 2017 | 59 |
| April 2017 | 78 |
| May 2017 | 146 |
| June 2017 | 85 |
| July 2017 | 48 |
| August 2017 | 61 |
| September 2017 | 115 |
| October 2017 | 150 |
| November 2017 | 82 |
| December 2017 | 125 |
| January 2018 | 174 |
| February 2018 | 202 |
| March 2018 | 218 |
| April 2018 | 262 |
| May 2018 | 170 |
| June 2018 | 61 |
| July 2018 | 60 |
| August 2018 | 67 |
| September 2018 | 68 |
| October 2018 | 184 |
| November 2018 | 85 |
| December 2018 | 70 |
| January 2019 | 88 |
| February 2019 | 124 |
| March 2019 | 145 |
| April 2019 | 134 |
| May 2019 | 194 |
| June 2019 | 90 |
| July 2019 | 83 |
| August 2019 | 71 |
| September 2019 | 90 |
| October 2019 | 113 |
| November 2019 | 117 |
| December 2019 | 74 |
| January 2020 | 78 |
| February 2020 | 99 |
| March 2020 | 96 |
| April 2020 | 89 |
| May 2020 | 61 |
| June 2020 | 72 |
| July 2020 | 108 |
| August 2020 | 105 |
| September 2020 | 97 |
| October 2020 | 102 |
| November 2020 | 112 |
| December 2020 | 113 |
| January 2021 | 105 |
| February 2021 | 121 |
| March 2021 | 165 |
| April 2021 | 126 |
| May 2021 | 164 |
| June 2021 | 86 |
| July 2021 | 97 |
| August 2021 | 106 |
| September 2021 | 124 |
| October 2021 | 143 |
| November 2021 | 141 |
| December 2021 | 106 |
| January 2022 | 86 |
| February 2022 | 106 |
| March 2022 | 147 |
| April 2022 | 206 |
| May 2022 | 158 |
| June 2022 | 87 |
| July 2022 | 114 |
| August 2022 | 116 |
| September 2022 | 138 |
| October 2022 | 137 |
| November 2022 | 147 |
| December 2022 | 123 |
| January 2023 | 118 |
| February 2023 | 135 |
| March 2023 | 210 |
| April 2023 | 168 |
| May 2023 | 173 |
| June 2023 | 105 |
| July 2023 | 137 |
| August 2023 | 110 |
| September 2023 | 189 |
| October 2023 | 210 |
| November 2023 | 190 |
| December 2023 | 94 |
| January 2024 | 179 |
| February 2024 | 188 |
| March 2024 | 215 |
| April 2024 | 204 |
| May 2024 | 156 |
| June 2024 | 107 |
| July 2024 | 126 |
| August 2024 | 142 |
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1464-3774
- Print ISSN 0142-7873
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 10 September 2021
Future phytoplankton diversity in a changing climate
- Stephanie A. Henson ORCID: orcid.org/0000-0002-3875-6802 1 ,
- B. B. Cael 1 ,
- Stephanie R. Allen 1 , 2 nAff5 &
- Stephanie Dutkiewicz ORCID: orcid.org/0000-0002-0380-9679 3 , 4
Nature Communications volume 12 , Article number: 5372 ( 2021 ) Cite this article
28k Accesses
104 Citations
106 Altmetric
Metrics details
- Biooceanography
- Marine biology
The future response of marine ecosystem diversity to continued anthropogenic forcing is poorly constrained. Phytoplankton are a diverse set of organisms that form the base of the marine ecosystem. Currently, ocean biogeochemistry and ecosystem models used for climate change projections typically include only 2−3 phytoplankton types and are, therefore, too simple to adequately assess the potential for changes in plankton community structure. Here, we analyse a complex ecosystem model with 35 phytoplankton types to evaluate the changes in phytoplankton community composition, turnover and size structure over the 21st century. We find that the rate of turnover in the phytoplankton community becomes faster during this century, that is, the community structure becomes increasingly unstable in response to climate change. Combined with alterations to phytoplankton diversity, our results imply a loss of ecological resilience with likely knock-on effects on the productivity and functioning of the marine environment.
Similar content being viewed by others

Major restructuring of marine plankton assemblages under global warming

Climate change is associated with higher phytoplankton biomass and longer blooms in the West Antarctic Peninsula
Widespread changes in Southern Ocean phytoplankton blooms linked to climate drivers
Introduction.
The socio-economic services provided by marine ecosystems are critical to human wellbeing. For example, fisheries provide almost half of Earth’s population with at least 20% of their animal protein intake 1 . Marine ecosystems also regulate Earth’s climate by absorbing and sequestering atmospheric CO 2 . Therefore, maintaining biodiversity is critical to providing resilience against future climate change and extremes 2 . At a global scale, biodiversity loss is being driven by human activities 3 , 4 , although clear trends of biodiversity decline in local ecosystems have proven difficult to identify 5 , 6 , 7 . Rather, the dominant species appear to be rapidly turned over, resulting in widespread reorganisation of ecosystems. These changes are potentially even more pronounced in the oceans than in the terrestrial realm 8 .
In addition to human pressures on habitat, anthropogenic climate change is likely to drive biodiversity loss and hence decrease ecosystem stability 2 , 9 , thus affecting both the functioning and structure of marine ecosystems 10 , 11 , 12 . Ocean warming and alterations to nutrient supply via changing circulation or stratification, combined with additional stressors such as ocean acidification and deoxygenation, are likely to force community reorganisation. Predicting future changes to marine ecosystems is challenging, partly due to the relative paucity of consistent, repeated sampling, the inherent variability over daily to interannual scales in community composition 13 , 14 , and the lack of knowledge of how future climate change and other anthropogenic stressors may combine to alter biodiversity 15 . However, with future oceans predicted to be ~ 2−4 °C warmer, more acidic, and reduced in oxygen concentration 16 , species must adapt, migrate to regions of analogous conditions, or face extinction 17 , 18 , 19 . The expected resulting changes to biodiversity are likely to affect fundamental ecosystem functioning and processes, such as biomass production and maintaining water quality 20 , 21 , 22 , as well as the entire marine ecosystem structure, with consequences for the ocean’s capacity for food production and climate regulation 23 .
As the base of the marine food web, phytoplankton play a fundamental role in setting the productivity of the entire marine ecosystem. Specific phytoplankton groups also play key roles in the biogeochemical functioning of the ocean; for example, by fixing atmospheric nitrogen (diazotrophs) or silica cycling (diatoms). Additionally, the size structure of the community affects trophic interactions, food web productivity, and carbon sequestration potential 24 , 25 , 26 . Here, we explore how phytoplankton diversity responds to a high emissions climate change scenario, similar to RCP8.5 27 , 28 , using a marine ecosystem model with 35 phytoplankton types and 16 zooplankton size classes 29 , 30 , 31 , which are able to reorganise in response to changing oceanic conditions (see “Methods”). This model thus provides a more mechanistic representation of phytoplankton community structure than correlative or niche modelling approaches 32 , 33 , 34 , and greater realism than Earth System Models (ESMs) used for IPCC projections 35 , 36 .
Niche models and correlative approaches, by necessity, assume that the contemporary relationships between environmental conditions and phytoplankton abundance or diversity will remain the same in the future. These approaches do not have a mechanistic basis, and so changes in phytoplankton diversity driven by factors other than those included in the analysis (such as temperature, latitude, etc.), or conditions outside the bounds of variability in the contemporary ocean, cannot be reliably deduced. ESMs typically employ a very simplified ecosystem model, usually incorporating only 2 or 3 phytoplankton types. These models thus capture only a very limited diversity of phytoplankton communities. ESM results have focused on the response of phytoplankton to changing nutrient supply via changing stratification and circulation, which favours small species with high nutrient affinity 37 , 38 . However, in reality, phytoplankton respond to other factors which may result in changes to their relative competitiveness, or ultimately niche loss.
Here, we use a complex ecosystem model with multiple functional groups of phytoplankton and several size classes of both phytoplankton and zooplankton types. Diversity in the model is set by several different mechanisms: the ratio of the supply rate of different limiting nutrients, the supply rate of limiting nutrients, grazing pressure, and transport/mixing 39 . Previous analysis of the modelled diversity has demonstrated that the combination of limiting nutrient supply and grazing controls the number of size classes that co-exist, and the ratio of supply rates of limiting resources contributes to setting the number of co-existing functional groups 39 . Transport and mixing tend to increase local diversity 31 . Although this model incorporates considerably more complexity than climate models, nevertheless it can only capture a fraction of the huge diversity of phytoplankton in the real ocean. Specifically, we capture diversity within biogeochemical functional groups (for example, diatoms, diazotrophs, etc.) and size classes (Extended Data Fig. 1 ). However, we do not capture the diversity that arises due to other traits, such as thermal norms, morphology, or colony formation 39 . Thus, in this study, the terms ‘richness’ and ‘diversity’ reflect functional richness and diversity, and should be understood in the context of these two important trait axes within the many different axes that set biodiversity in the real ocean.
In this study, we quantify the response of marine phytoplankton diversity to climate change, focusing on future projections of community composition and turnover. We apply a high emissions climate scenario to a complex marine ecosystem model to explore the global and regional changes in phytoplankton community composition.
Phytoplankton biomass is projected to decrease over much of the tropical and subtropical ocean due to lower nutrient supply rate (Extended Data Fig. 2 ), consistent with previous studies 35 , 38 , 40 , 41 . Increases in phytoplankton biomass occur in high latitude regions due to the retreat of sea ice, longer growing seasons, and increased growth rates at higher temperatures, again consistent with previous work 36 , 37 , 40 , 41 (Fig. 1a ). However, the increased ecological complexity of our model allows us to look beyond changes in biomass alone to uncover the community structure alterations that underlie the climate change response. Projected changes in biomass are in general reflected in phytoplankton richness, which declines by 2100 in large parts of the northern hemisphere subtropical and temperate regions (64% of area 23−55° N declines), and increases in polar and some equatorial regions (69% of area poleward of 55° or within 23° of the equator increases; Fig. 1b ). In some tropical regions, up to 30% of modelled phytoplankton types become locally extinct, whereas in polar regions colonisation exceeds extinction, and richness increases by up to 30%.
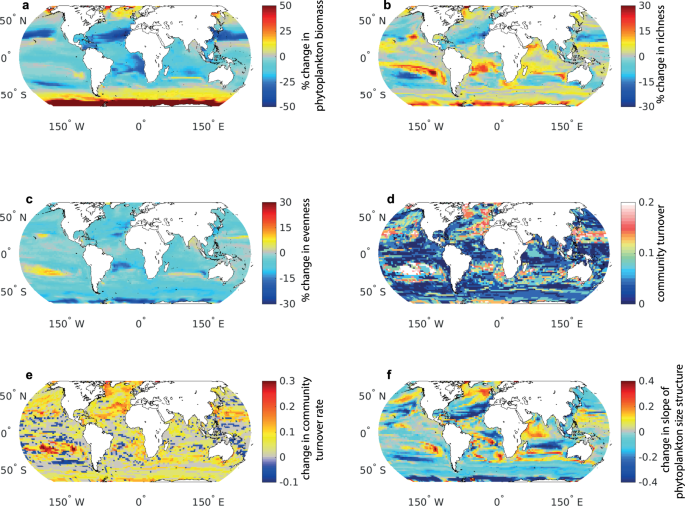
For subplots a – d , f the change between the baseline period (mean of 2005−2024) and end of the century (mean of 2081−2100) is shown. a Percent change in total phytoplankton biomass; b percent change in richness; c percent change in evenness; d community turnover; e change in community turnover rate (turnover between the mean of 2061−2080 and 2081−2100 minus mean of 2011−2030 and 2031−2040); f change in slope of the phytoplankton community size structure, where negative values indicate a greater abundance of small phytoplankton types.
The spatial patterns of phytoplankton functional group extinction and colonisation by the end of the century are illustrated in Fig. 2 . Declining nutrient supply rates (Extended Data Fig. 2 ) drive the disappearance of less competitive and larger phytoplankton types 39 (indicated by the shallowing of the slope of the size spectrum; Fig. 1f ), resulting in decreased richness in many northern hemisphere (sub)tropical regions (Fig. 2 and Extended Data Fig. 3 ). Lower nitrate relative to iron supply rate favours diazotrophs 42 , and their range thus expands polewards, particularly in the northern hemisphere. In the same regions, diatom richness decreases with the reduction in silicic acid relative to nitrate 39 (Extended Data Fig. 2 ), resulting in the extinction of up to 30% of diatom types. Reduced nutrient supply and the subsequent loss of some larger zooplankton (Extended Data Fig. 4 ) results in fewer co-existing size classes 39 . Some mixotrophic dinoflagellate types become extinct by 2100 along subtropical gyre boundaries, but intermediate nutrient supply rate (Extended Data Fig. 2 ), and an increase in smaller phytoplankton (i.e., prey; Extended Data Fig. 4 ) allow them to become more competitive in, and ultimately colonise much of, the Southern Ocean. In contrast, the distribution of picoplankton, which are better adapted to low nutrient conditions, barely changes by 2100.
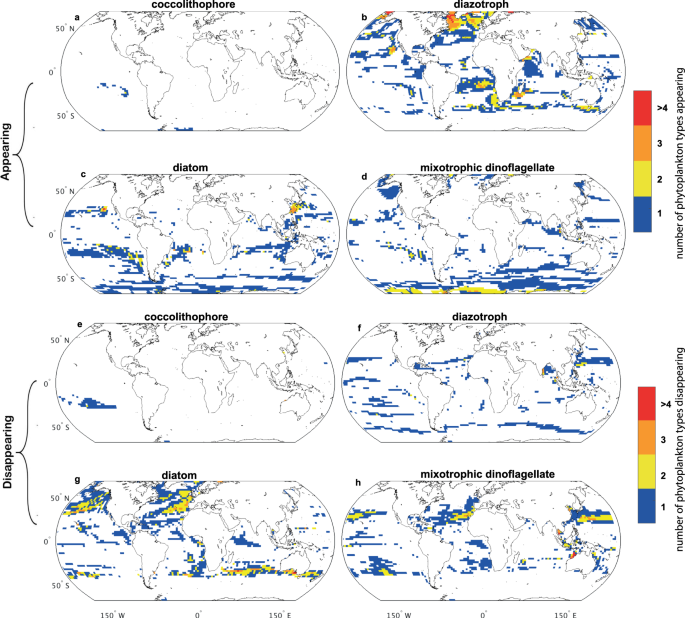
The number of phytoplankton types appearing ( a – d ) and disappearing ( e – h ) between the baseline period (mean of 2005−2024) and end of century period (mean of 2081−2100) in each of six groups: coccolithophores ( a , e ), of which there are five types; diazotrophs ( b , f ), of which there are five types; diatoms ( c , g ), of which there are 11 types; mixotrophic dinoflagellates ( d , h ), of which there are 10 types. Prokaryotes and picoeukaryotes (of which there are two types of each) do not show any significant changes. Appearance (disappearance) is defined as a type contributing >0.1% (<0.1%) to total biomass.
The Shannon diversity index, which incorporates both richness and evenness in biomass of co-existing types, declines almost globally (92% of ocean area; Extended Data Fig. 5f ), driven primarily by an almost universal decrease in evenness (93% of ocean area; Fig. 1c ). The declining evenness indicates that biomass becomes concentrated in fewer phytoplankton types by 2100 (Fig. 2 ; Extended Data Fig. 3 ).
Comparing the phytoplankton composition in the last decades of the century with the contemporary period (Fig. 1d ) demonstrates that community turnover (i.e., the proportion of types that differ between two timepoints) is highest in parts of the temperate northern hemisphere and the South Pacific subtropical gyre, with ~20% of types being exchanged. Elsewhere, turnover is lower with <10% of phytoplankton types changing at the end of the century with respect to 2005−2024. The rate of turnover however increases in the majority of the ocean by the end of the century (63% of the area; Fig. 1e ), indicating that phytoplankton community composition becomes increasingly variable (i.e., decreasingly stable) over time, both in regions of increasing and decreasing richness.
The slope of the phytoplankton size spectrum decreases by the end of the century in most sub-tropical regions (69% of the area) and in the Southern Ocean (90% of the area), i.e., the phytoplankton community shifts to dominance by smaller phytoplankton types (Fig. 1f ). In the subtropics, the decrease in size spectrum is driven by a loss of relatively more large types than smaller types. On the other hand, the decrease in overall size of the community in the Southern Ocean is driven by a larger increase in smaller types than larger types (Extended Data Fig. 4 ). In some regions, there is an increase in overall size (33% of ocean area). In the case of the North Atlantic, this is driven by an influx of larger dinoflagellates and a general loss of diatoms (Fig. 2 ).
The results presented above should be interpreted within the limitations of the ecosystem model used which, although more complex than other global models, nevertheless only includes traits for functional group and size, but not for thermal norms. The modelled geographic shifts in plankton types are therefore not a direct response to warming temperatures (i.e., due to their thermal niches 40 , 43 ), but instead are an indirect response occurring via changes to nutrient availability and relative competitiveness. However, the model metabolic processes (such as phytoplankton growth) do increase with warming waters, following an Arrhenius function 44 . Differences in temperature responses between types are likely to lead to alterations in their relative competitiveness, but such parameterisations are outside the scope of this study. The model also does not explicitly represent coastal regions (as the spatial resolution is too coarse), sea-ice communities are not explicitly modelled, and no anthropogenic impacts other than climate are simulated (e.g., runoff, pollution, habitat reduction). The modelled plankton also do not evolve or adapt to changing conditions; if plankton are able to do so on timescales comparable to those of climate change, then our results may represent a “worst-case scenario”.
The complex ecological model used here provides insights into future changes in phytoplankton diversity and community turnover. As the model is mechanistic, rather than statistical (e.g., 33 , 34 ), and represents significantly more complexity than typical ESMs (e.g., 36 , 38 ), niche loss and changes in phytoplankton types’ relative competitiveness can be assessed. Previous studies with variants of our model have examined the responses of different traits in historical runs, and knock-out and sensitivity experiments 39 , 40 , 43 . Here we present the analysis of a future climate change run, which focuses on changing phytoplankton diversity within the trait space of functional groups and size.
In the future ocean, our model predicts that biomass declines in the tropics and temperate regions (and increases at high latitudes), alongside significant shifts in phytoplankton community composition. In this model, changes in the supply of the limiting nutrient drive reduced biomass in lower latitudes 39 , while increased growth rates with warmer water lead to higher biomass in eutrophic high latitude regions. However multiple factors lead to the shifts in community structure. The reduction in macronutrient supply leads to declines in grazer abundance and trophic interactions; the combination can lead to a reduction in size classes (both lower richness and shallower slope of the size spectrum). Additionally, the ratio of supply of resources (nutrients and prey) affects the co-existence of functional groups 39 . For instance, previous analysis of this model has demonstrated that changes in the supply of nitrate relative to iron alters the distribution of diazotrophs, silica versus nitrate (or iron) supply alters diatom biogeography, and prey versus nutrient availability alters the mixotrophic dinoflagellate patterns 39 . Additionally increased stratification (and hence reduced mixing) contributes to altering diversity in the future ocean 39 , 45 . Together these mechanisms can lead to both positive and negative changes in richness (Fig. 1b ).
Other studies have explored different aspects of phytoplankton diversity and response to future change using correlative approaches. The findings of a general decrease in diversity in the tropics and increase at high latitudes (particularly in the Southern Ocean), driven by colonisation exceeding extinction 46 , 47 , broadly agree with our study (Figs. 1 b, 2 ). The ‘tropicalisation’ of diversity in temperate and polar latitudes 34 is also captured here as a shift towards smaller phytoplankton structure (Fig. 1f ) and an increase in diazotrophs and mixotrophic dinoflagellates (Fig. 2 ). Discrepancies between the projections arise because the correlative approaches must assume that the modern-day relationship between phytoplankton species distribution and environmental conditions remains the same into the future, whereas we use a mechanistic model, which permits a dynamic response of phytoplankton diversity to changing environmental forcing.
Overall, we find a decline in Shannon diversity almost globally by the end of the century (Extended Data Fig. 5 ). However, this result masks an interesting interplay between richness and evenness (“Methods”). At temperate latitudes in the North Atlantic and North Pacific, the decline in Shannon index is driven by a decline in richness, implying that existing niches close in future conditions so that extinctions exceed colonisations (Fig. 1b , Extended Data Fig. 5 ). However, in the Southern Ocean, and off-equator in the Indian Ocean and South Atlantic, the declining Shannon index is associated with decreased evenness as richness increases (Fig. 1b, c ). This indicates that, although colonisation exceeds extinction, the community becomes dominated by a few phytoplankton types, rather than its current more ‘balanced’ state.
In regions with high turnover and a decrease in richness (e.g., northwest North Atlantic, North Pacific gyre boundary; Fig. 1b, d ), extinctions exceed colonisations, suggesting that the effect of climatic change is to reduce the number of potential niches 48 . Large scale changes in species composition occur due to environmental conditions exceeding the tolerances of phytoplankton types currently extant, so that they are outcompeted under future conditions by types adapted to lower nutrient supply rates, or whose co-existence relies on specific resource supply ratios. At the polar edges of our domain, an increase in richness coincides with high turnover, implying that expanding environmental niches lead to conditions favouring colonisation without excluding extant species 7 . This suggests that these regions may be refugia for phytoplankton types pushed beyond their tolerances at lower latitudes. The greater niche redundancy of some phytoplankton types, e.g., those with similar size replacements such as mixotrophs, may also make them less vulnerable to extinction.
Phytoplankton cell size has been called a “master trait” in ocean systems, as cell size ranges over 9 orders of magnitude 49 . In our study, the projected changes in phytoplankton size structure (Fig. 1f ) imply an increasing dominance of smaller phytoplankton types. A trend toward smaller phytoplankton would have implications for both the oceans’ ecological and biogeochemical function, as regions dominated by small phytoplankton typically support less productive food webs 50 , 51 , 52 and sequester less organic carbon in the deep ocean 26 , 53 than those dominated by larger size classes.
The striking increase in turnover rate by the end of the century in this high emission scenario (Fig. 1e ) implies a reduction in niche diversity, resulting in an increased occurrence of ephemeral phytoplankton species, and fewer persistently dominant species. Higher trophic levels reliant on consistent availability of a few dominant phytoplankton types will need to adapt to a rapidly varying diet, which may be less palatable or nutritious. Increasing variability in community composition also implies a loss of ecological resilience 54 , 55 , i.e., a reduced ability to maintain ecosystem function and structure under changing conditions 56 . Although turnover in contemporary phytoplankton communities can be high on a daily to seasonal timescale 57 , 58 , 59 , species richness remains relatively stable 5 , 8 . Similarly, during environmental upheavals associated with glacial/inter-glacial periods, the diatom community structure largely recovered to its pre-perturbation structure on a ~1 million year time scale 60 . The return to an ‘equilibrium ecosystem’ state was associated with the ability of a seed population of phytoplankton to retain resistance to environmental change, suggesting that low resistance to environmental change does not necessarily equate to community fragility. Whether the phytoplankton community could recover from the perturbation associated with anthropogenic climate change (which is uni-directional on multi-decadal time scales) remains an open question. We find here that under continuing climate change in a high emissions scenario, turnover increases with time, and functional richness changes become pronounced. This implies that phytoplankton community resilience evident in contemporary populations on interannual timescales 60 , 61 , 62 may be impaired by the end of the century, resulting in an increasingly unstable marine ecosystem.
Our results reveal the potential for significant future disruption to marine phytoplankton communities in response to climate change, particularly under continued high greenhouse gas emissions. The projections highlight the potential vulnerability of phytoplankton community structure to climate change by integrating the exposure to stressors and the community’s sensitivity to those stressors. However, our model does not account for adaptation, which is likely to increase the ecological resilience of the phytoplankton community as tolerances shift to account for changing environmental conditions. Indeed, laboratory manipulation experiments have demonstrated that phytoplankton can adapt to new environmental conditions, such as warmer or more acidic waters, within a few hundred generations (i.e., 2−3 years 63 , 64 ). However, the multiple mechanisms driving the changes discussed here (nutrient supply, nutrient supply ratios, grazer control, advection) are likely to be more difficult for phytoplankton to adapt to than modest changes in temperature 65 . If organisms cannot adapt sufficiently rapidly to the development of novel climatic conditions, community reorganisation, population collapse, or other abrupt ecological shifts, may occur , 66 , 67 . However, the lack of adaptation in our model suggests that the results presented above may well be a ‘worst case scenario’.
The potential for organisms to migrate in order to remain within analogous environmental conditions has been posited 68 , 69 . However, here we find that relocation of communities (Fig. 2 ), in terms of their size classes and functional groups, does not necessarily prevent extinction by 2100 (particularly at low latitudes), and of diatoms and larger phytoplankton globally. This implies that higher trophic levels may not only need to migrate to remain in analogous climatic conditions (e.g., by tracking isotherms 70 ), but also to remain in regions of analogous diets. Although zooplankton migration speeds may be sufficiently rapid to keep pace with the northward movement of isotherms 71 , 72 , some zooplankton groups are dependent on fatty acids from specific phytoplankton species to avoid starvation, complete their life cycle, and/or survive environmental stressors 73 , 74 . If zooplankton are either unable to acquire the necessary prey items, or regions of analogous climate and analogous diet do not overlap in future, significant changes to marine food webs are likely to occur.
Long-standing ecological theory posits that diversity loss results in ecosystem instability 9 , 75 , 76 . Here we demonstrate that climate change is likely to drive altered phytoplankton diversity, and in particular a reduction in evenness, resulting in wholesale reorganisation of phytoplankton communities, and increasing instability in community structure, which will present profound challenges to the productivity of the entire marine food web. Indeed, trophic amplification may result in greater changes at higher trophic levels of the marine food web than for phytoplankton 77 . Nations dependent on fishing for their main protein source, principally low to middle-income countries, are concentrated at low latitudes 78 , where we predict substantial changes in phytoplankton diversity and biomass by the end of the century.
The ecosystem model used here has been previously described 29 , 30 and is used in the configuration detailed in ref. 31 . The ecosystem model includes 35 phytoplankton and 16 zooplankton types in seven biogeochemical functional groups covering a size distribution from 0.6 to 2425 µm equivalent spherical diameter (Extended Data Fig. 1 ). The cycling of carbon, phosphorus, nitrogen, silica, iron, and oxygen is incorporated in the model. The plankton groups consist of 2 prokaryote, 2 pico-prokaryote, 5 coccolithophore, 5 diazotroph, 11 diatom, 10 mixotrophic dinoflagellate, and 16 zooplankton types. Note that mixotrophy is only considered to occur in the dinoflagellate group, and that limited observational data restricts the representation of mixotrophy in this (and all) ecological models. All groups are modelled with constant C:N:P:Fe stoichiometry using Monod kinetics. Parameters influencing phytoplankton growth, grazing, and sinking are size-related and differ between functional groups 39 . The maximum growth rates and grazing are also determined by phytoplankton cell size 39 ; prokaryotes and picoeukaryotes (the smallest group) have the lowest nutrient affinity, while the fastest-growing types are in the 3 μm cell size range 79 . Zooplankton grazing uses a Holling III function; zooplankton will graze on plankton 5−15 times smaller than themselves, but prefer organisms 10 times smaller. Zooplankton are differentiated only by size and no differences in functionality are parameterised; we thus limit our analysis to diversity in the phytoplankton. This ecosystem model was chosen due to its high level of phytoplankton diversity, especially in terms of functionality. The model captures both size and biogeochemical differences between phytoplankton types, which impact both biogeochemical and foodweb dynamics. This ecosystem model has previously been shown to reproduce satellite and in situ observations of both size classes and functional types 30 , 31 .
The physical framework is the MIT Integrated Global System Model (IGSM 80 , 81 , 82 ). The ocean component has a 2° × 2.5° resolution in the horizontal, and 22 layers in the vertical, ranging from 10 m at the surface to 500 m thick at depth. The simulation is run from 1860 to 1990 with observed emissions of greenhouse gases, and from 1990 to 2100 with a high emissions scenario, similar to the Representative Concentration Pathway 8.5 (RCP8.5) used in the Coupled Model Intercomparison Project 5 83 . In this study, we focus on the period 2006−2100. The plankton distributions compare well with observations of both functional types and size distribution 84 , 85 , as demonstrated in previous model validation work 29 , 30 , 39 . All analyses are performed on biomass integrated over the full ocean depth (to capture any deep biomass maxima). All analysis was performed in Matlab 2019a.
Any species that contribute less than 0.1% to total biomass at that location and timestep are excluded from further analysis. Functional richness is then defined as the number of phytoplankton types (biogeochemical functional types and size classes) that coexist at a particular location and timestep. The functional Shannon index is defined as:
where s is the total number of phytoplankton types in a sample (i.e., richness), i is the total biomass of individuals in one type and p i is the proportion of biomass in type i relative to the total biomass across all types. The Shannon index decreases as both the richness and the evenness of the community decrease, where evenness is defined as: Shannon/ln(richness).
Turnover, which is the proportion of phytoplankton types that differ between two timepoints, is calculated as:
where N G is the total number of phytoplankton types gained, N L is the total number of types lost, and N T is the total number of types observed in both timepoints. This metric captures the gross change in species composition and varies between 0 (all species persist) and 1 (all species change). Here we calculate the community turnover between the mean of the baseline period (2005−2024) and the mean of the end of the century (2081−2100), shown in Fig. 1d . The increase in community turnover rate is calculated as the turnover between the mean of 2061−2080 and 2081−2100 minus the mean of 2011−2030 and 2031−2040, shown in Fig. 1e .
The slope of the phytoplankton type size spectrum was calculated by summing the biomass within each of the 16 phytoplankton size classes. The slope of log(biomass) against log(equivalent spherical diameter) was then estimated using a robust linear regression technique, the Theil−Sen estimator. The slope of the phytoplankton type size spectrum is then the slope of the regression plus 3, assuming purely spherical phytoplankton.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The model output is available from https://dataverse.harvard.edu/dataverse/gud-igsm , specifically, the depth-integrated biomass output used in this study is available from https://doi.org/10.7910/DVN/LWHQNS (ref. 86 ).
Code availability
The numerical model code is available from https://dataverse.harvard.edu/dataverse/gud-igsm , specifically at https://doi.org/10.7910/DVN/UA8VNU (ref. 87 ).
Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture http://www.fao.org/3/i2727e/i2727e00.htm (2012).
Isbell, F. et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526 , 574–577 (2015).
Article ADS CAS PubMed Google Scholar
IPBES. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services https://doi.org/10.5281/zenodo.3553579 (2019).
Tittensor, D. P. et al. A mid-term analysis of progress toward international biodiversity targets. Science 346 , 241–244 (2014).
Dornelas, M. et al. Assemblage time series reveal biodiversity change but not systematic loss. Science 344 , 296–299 (2014).
Gonzalez, A. et al. Estimating local biodiversity change: a critique of papers claiming no net loss of local diversity. Ecology 97 , 1949–1960 (2016).
Article PubMed Google Scholar
Elahi, R. et al. Recent trends in local-scale marine biodiversity reflect community structure and human impacts. Curr. Biol. 25 , 1938–1943 (2015).
Article CAS PubMed Google Scholar
Blowes, S. A. et al. The geography of biodiversity change in marine and terrestrial assemblages. Science 366 , 339–345 (2019).
McCann, K. S. The diversity–stability debate. Nature 405 , 228–233 (2000).
Loreau, M. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294 , 804–808 (2001).
Covich, A. P. et al. The role of biodiversity in the functioning of freshwater and marine benthic ecosystems. Bioscience 54 , 767–775 (2004).
Article Google Scholar
Hooper, D. U. et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75 , 3–35 (2005).
Widdicombe, C. E., Eloire, D., Harbour, D., Harris, R. P. & Somerfield, P. J. Long-term phytoplankton community dynamics in the Western English Channel. J. Plankton Res. 32 , 643–655 (2010).
Eloire, D. et al. Temporal variability and community composition of zooplankton at station L4 in the Western Channel: 20 years of sampling. J. Plankton Res. 32 , 657–679 (2010).
Hillebrand, H. et al. In Handbook on Marine Environment Protection (eds Salomon, M. & Markus, T.) 21 (Springer, 2018).
Bindoff, N. L. et al. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate (eds H.-O. Pörtner, D. C. Roberts, V. Masson-Delmotte, P. Zhai, M. Tignor, E. Poloczanska, K. Mintenbeck, A. Alegría, M. Nicolai, A. Okem, J. Petzold, B. Rama, N. M. W) Cambridge University Press (2019).
Barton, A. D., Irwin, A. J., Finkel, Z. V. & Stock, C. A. Anthropogenic climate change drives shift and shuffle in North Atlantic phytoplankton communities. Proc. Natl Acad. Sci. USA 113 , 2964–2969 (2016).
Article ADS CAS PubMed PubMed Central Google Scholar
Pecuchet, L. et al. Spatio‐temporal dynamics of multi‐trophic communities reveal ecosystem‐wide functional reorganization. Ecography 43 , 197–208 (2020).
Poloczanska, E. S. et al. Responses of marine organisms to climate change across oceans. Front. Mar. Sci . 3 , 62 (2016).
Pennekamp, F. et al. Biodiversity increases and decreases ecosystem stability. Nature 563 , 109–112 (2018).
Duffy, J. E., Godwin, C. M. & Cardinale, B. J. Biodiversity effects in the wild are common and as strong as key drivers of productivity. Nature 549 , 261–264 (2017).
Worm, B. et al. Impacts of biodiversity loss on ocean ecosystem services. Science 314 , 787–790 (2006).
Blois, J. L., Zarnetske, P. L., Fitzpatrick, M. C. & Finnegan, S. Climate change and the past, present, and future of biotic interactions. Science 341 , 499–504 (2013).
Dossena, M. et al. Warming alters community size structure and ecosystem functioning. Proc. R. Soc. B Biol. Sci. 279 , 3011–3019 (2012).
Brander, K. & Kiørboe, T. Decreasing phytoplankton size adversely affects ocean food chains. Glob. Chang. Biol . https://doi.org/10.1111/gcb.15216 (2020).
Mouw, C. B., Barnett, A., McKinley, G. A., Gloege, L. & Pilcher, D. Phytoplankton size impact on export flux in the global ocean. Glob. Biogeochem. Cycles 30 , 1542–1562 (2016).
Article ADS CAS Google Scholar
Riahi, K. et al. RCP 8.5—a scenario of comparatively high greenhouse gas emissions. Clim. Change 109 , 33–57 (2011).
Magnan, A. K. et al. Implications of the Paris agreement for the ocean. Nat. Clim. Chang. 6 , 732–735 (2016).
Article ADS Google Scholar
Kuhn, A. M. et al. Temporal and spatial scales of correlation in marine phytoplankton communities. J. Geophys. Res. Ocean. 124 , 9417–9438 (2019).
Sonnewald, M., Dutkiewicz, S., Hill, C. & Forget, G. Elucidating ecological complexity: unsupervised learning determines global marine eco-provinces. Sci. Adv. 6 , eaay4740 (2020).
Article ADS PubMed PubMed Central Google Scholar
Dutkiewicz, S., Boyd, P. W. & Riebesell, U. Exploring biogeochemical and ecological redundancy in phytoplankton communities in the global ocean. Glob. Chang. Biol. 27 , 1196–1213 (2021).
Flombaum, P., Wang, W.-L., Primeau, F. W. & Martiny, A. C. Global picophytoplankton niche partitioning predicts overall positive response to ocean warming. Nat. Geosci. 13 , 116–120 (2020).
Righetti, D., Vogt, M., Gruber, N., Psomas, A. & Zimmermann, N. E. Global pattern of phytoplankton diversity driven by temperature and environmental variability. Sci. Adv. 5 , eaau6253 (2019).
Ibarbalz, F. M. et al. Global trends in marine plankton diversity across kingdoms of life. Cell 179 , 1084–1097.e21 (2019).
Article CAS PubMed PubMed Central Google Scholar
Bopp, L. et al. Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10 , 6225–6245 (2013).
Kwiatkowski, L. et al. Twenty-first century ocean warming, acidification, deoxygenation, and upper-ocean nutrient and primary production decline from CMIP6 model projections. Biogeosciences 17 , 3439–3470 (2020).
Cabré, A., Marinov, I. & Leung, S. Consistent global responses of marine ecosystems to future climate change across the IPCC AR5 earth system models. Clim. Dyn. 45 , 1253–1280 (2015).
Bopp, L., Aumont, O., Cadule, P., Alvain, S. & Gehlen, M. Response of diatoms distribution to global warming and potential implications: a global model study. Geophys. Res. Lett. 32 , n/a−n/a (2005).
Article CAS Google Scholar
Dutkiewicz, S. et al. Dimensions of marine phytoplankton diversity. Biogeosciences 17 , 609–634 (2020).
Dutkiewicz, S., Scott, J. R. & Follows, M. J. Winners and losers: ecological and biogeochemical changes in a warming ocean. Glob. Biogeochem. Cycles 27 , 463–477 (2013).
Marinov, I., Doney, S. C. & Lima, I. D. Response of ocean phytoplankton community structure to climate change over the 21st century: partitioning the effects of nutrients, temperature, and light. Biogeosciences 7 , 3941–3959 (2010).
Dutkiewicz, S., Ward, B. A., Scott, J. R. & Follows, M. J. Understanding predicted shifts in diazotroph biogeography using resource competition theory. Biogeosciences 11 , 5445–5461 (2014).
Dutkiewicz, S. et al. Impact of ocean acidification on the structure of future phytoplankton communities. Nat. Clim. Chang. 5 , 1002–1006 (2015).
Kooijman, S. A. L. M. & Troost, T. A. Quantitative steps in the evolution of metabolic organisation as specified by the dynamic energy budget theory. Biol. Rev. 82 , 113–142 (2007).
Lévy, M., Jahn, O., Dutkiewicz, S., Follows, M. J. & D’Ovidio, F. The dynamical landscape of marine phytoplankton diversity. J. R. Soc. Interface 12 , 20150481 (2015).
Article PubMed PubMed Central Google Scholar
Beaugrand, G., Edwards, M., Raybaud, V., Goberville, E. & Kirby, R. R. Future vulnerability of marine biodiversity compared with contemporary and past changes. Nat. Clim. Chang. 5 , 695–701 (2015).
Thomas, M. K., Kremer, C. T., Klausmeier, C. A. & Litchman, E. A global pattern of thermal adaptation in marine phytoplankton. Science 338 , 1085–1088 (2012).
Hillebrand, H. et al. Biodiversity change is uncoupled from species richness trends: consequences for conservation and monitoring. J. Appl. Ecol. 55 , 169–184 (2018).
Litchman, E. & Klausmeier, C. A. Trait-based community ecology of phytoplankton. Annu. Rev. Ecol. Evol. Syst. 39 , 615–639 (2008).
Lindeman, R. L. The trophic-dynamic aspect of ecology. Ecology 23 , 399–417 (1942).
Stock, C. A. et al. Reconciling fisheries catch and ocean productivity. Proc. Natl Acad. Sci. USA 114 , E1441–E1449 (2017).
Armengol, L., Calbet, A., Franchy, G., Rodríguez-Santos, A. & Hernández-León, S. Planktonic food web structure and trophic transfer efficiency along a productivity gradient in the tropical and subtropical Atlantic Ocean. Sci. Rep. 9 , 2044 (2019).
Article ADS PubMed PubMed Central CAS Google Scholar
Cram, J. A. et al. The role of particle size, ballast, temperature, and oxygen in the sinking flux to the deep sea. Glob. Biogeochem. Cycles 32 , 858–876 (2018).
Kéfi, S., Dakos, V., Scheffer, M., Van Nes, E. H. & Rietkerk, M. Early warning signals also precede non-catastrophic transitions. Oikos 122 , 641–648 (2013).
Doncaster, C. P. et al. Early warning of critical transitions in biodiversity from compositional disorder. Ecology 97 , 3079–3090 (2016).
Gunderson, L. H. Ecological resilience—in theory and application. Annu. Rev. Ecol. Syst. 31 , 425–439 (2000).
Benedetti, F. et al. The seasonal and inter-annual fluctuations of plankton abundance and community structure in a North Atlantic Marine Protected Area. Front. Mar. Sci . 6 , 214 (2019).
Pannard, A., Bormans, M. & Lagadeuc, Y. Short-term variability in physical forcing in temperate reservoirs: effects on phytoplankton dynamics and sedimentary fluxes. Freshw. Biol. 52 , 12–27 (2007).
Vidal, T., Calado, A. J., Moita, M. T. & Cunha, M. R. Phytoplankton dynamics in relation to seasonal variability and upwelling and relaxation patterns at the mouth of Ria de Aveiro (West Iberian Margin) over a four-year period. PLoS One 12 , e0177237 (2017).
Article PubMed PubMed Central CAS Google Scholar
Cermeño, P., de Vargas, C., Abrantes, F. & Falkowski, P. G. Phytoplankton biogeography and community stability in the ocean. PLoS One 5 , e10037 (2010).
Allen, S. et al. Interannual stability of phytoplankton community composition in the North-East Atlantic. Mar. Ecol. Prog. Ser. 655 , 43–57 (2020).
Barton, A. D., Lozier, M. S. & Williams, R. G. Physical controls of variability in North Atlantic phytoplankton communities. Limnol. Oceanogr. 60 , 181–197 (2015).
Collins, S., Rost, B. & Rynearson, T. A. Evolutionary potential of marine phytoplankton under ocean acidification. Evol. Appl. 7 , 140–155 (2014).
Lohbeck, K. T., Riebesell, U. & Reusch, T. B. H. Adaptive evolution of a key phytoplankton species to ocean acidification. Nat. Geosci. 5 , 346–351 (2012).
Irwin, A. J., Finkel, Z. V., Müller-Karger, F. E. & Troccoli Ghinaglia, L. Phytoplankton adapt to changing ocean environments. Proc. Natl Acad. Sci. USA 112 , 5762–5766 (2015).
Cael, B. B. et al. Marine ecosystem changepoints spread under ocean warming in an Earth System Model. Geophys. Res. Lett.
Cael, B. B., Dutkiewicz, S. & Henson, S. A. Abrupt shifts in 21st-century plankton communities. Sci. Adv .
Parmesan, C. & Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421 , 37–42 (2003).
Burrows, M. T. et al. The pace of shifting climate in marine and terrestrial ecosystems. Science 334 , 652–655 (2011).
Chivers, W. J., Walne, A. W. & Hays, G. C. Mismatch between marine plankton range movements and the velocity of climate change. Nat. Commun. 8 , 14434 (2017).
Poloczanska, E. S. et al. Global imprint of climate change on marine life. Nat. Clim. Chang. 3 , 919–925 (2013).
Jonkers, L., Hillebrand, H. & Kucera, M. Global change drives modern plankton communities away from the pre-industrial state. Nature 570 , 372–375 (2019).
Pond, D. W., Tarling, G. A. & Mayor, D. J. Hydrostatic pressure and temperature effects on the membranes of a seasonally migrating marine copepod. PLoS One 9 , e111043 (2014).
Mayor, D. J., Sommer, U., Cook, K. B. & Viant, M. R. The metabolic response of marine copepods to environmental warming and ocean acidification in the absence of food. Sci. Rep. 5 , 13690 (2015).
Richardson, D. M. & Pyšek, P. Elton, C.S. 1958: The ecology of invasions by animals and plants. London: Methuen. Prog. Phys. Geogr. Earth Environ. 31 , 659–666 (2007).
May, R. M. Qualitative stability in model ecosystems. Ecology 54 , 638–641 (1973).
Lotze, H. K. et al. Global ensemble projections reveal trophic amplification of ocean biomass declines with climate change. Proc. Natl Acad. Sci. USA 116 , 12907–12912 (2019).
Barange, M. et al. Impacts of climate change on marine ecosystem production in societies dependent on fisheries. Nat. Clim. Chang. 4 , 211–216 (2014).
Marañón, E. et al. Unimodal size scaling of phytoplankton growth and the size dependence of nutrient uptake and use. Ecol. Lett. 16 , 371–379 (2013).
Sokolov, A. P. et al. The MIT Integrated Global System Model (IGSM) Version 2: Model Description and Baseline Evaluation Joint Program Report Series , pp. 40 https://globalchange.mit.edu/publication/14579 (2005).
Dutkiewicz, S. et al. Ocean colour signature of climate change. Nat. Commun. 10 , 578 (2019).
Monier, E., Scott, J. R., Sokolov, A. P., Forest, C. E. & Schlosser, C. A. An integrated assessment modeling framework for uncertainty studies in global and regional climate change: the MIT IGSM-CAM (version 1.0). Geosci. Model Dev. 6 , 2063–2085 (2013).
Moss, R. H. et al. The next generation of scenarios for climate change research and assessment. Nature 463 , 747–756 (2010).
Buitenhuis, E. T. et al. MAREDAT: towards a world atlas of MARine Ecosystem DATa. Earth Syst. Sci. Data 5 , 227–239 (2013).
Ward, B. A. Temperature-correlated changes in phytoplankton community structure are restricted to polar waters. PLoS One 10 , e0135581 (2015).
Dutkiewicz, S. GUD IGSM depth integrated biomass https://doi.org/10.7910/DVN/LWHQNS (2021).
Dutkiewicz, S. & Jahn, O. GUD IGSM numerical code and inputs https://doi.org/10.7910/DVN/UA8VNU (2021).
Download references
Acknowledgements
Support for S.A.H. and B.B.C. was received from NERC National Capability programme CLASS (Climate Linked Atlantic Sector Science), grant number NE/R015953/1, and funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 820989 (COMFORT). Support for S.R.A. was received from a NERC PhD studentship, NE/1498876. S.D. acknowledges support from NASA grants NNX16AR47G and 80NSSC17K0561.
Author information
Stephanie R. Allen
Present address: Plymouth Marine Laboratory, Prospect Place, Plymouth, UK
Authors and Affiliations
National Oceanography Centre, European Way, Southampton, UK
Stephanie A. Henson, B. B. Cael & Stephanie R. Allen
School of Ocean and Earth Sciences, University of Southampton, Waterfront Campus, European Way, Southampton, UK
Center for Global Change Science, Massachusetts Institute of Technology, Cambridge, MA, USA
Stephanie Dutkiewicz
Earth, Atmospheric and Planetary Sciences, Massachusetts Institute of Technology, Cambridge, MA, USA
You can also search for this author in PubMed Google Scholar
Contributions
S.A.H. and S.R.A. conceived the study, S.A.H. and B.B.C. undertook the analyses, S.D. generated the model output, all authors contributed to writing the paper.
Corresponding author
Correspondence to Stephanie A. Henson .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Robert Ptacnik and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Henson, S.A., Cael, B.B., Allen, S.R. et al. Future phytoplankton diversity in a changing climate. Nat Commun 12 , 5372 (2021). https://doi.org/10.1038/s41467-021-25699-w
Download citation
Received : 12 November 2020
Accepted : 24 August 2021
Published : 10 September 2021
DOI : https://doi.org/10.1038/s41467-021-25699-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Twenty-six years of phytoplankton pigments reveal a circumpolar class divide around the southern ocean.
- Alexander Hayward
- Matthew H. Pinkerton
- Cliff S. Law
Communications Earth & Environment (2024)
Long-term adjustment of phytoplankton structure to environmental traits at timescales during lifetime development and over generations
- Martin T. Dokulil
- Katrin Teubner
Hydrobiologia (2024)
Assessing the impact of climate change on dissolved oxygen using a flow field ecosystem model that takes into account the anaerobic and aerobic environment of bottom sediments
- Jinichi Koue
Acta Geochimica (2024)

Water mass influence on spatial and seasonal distributions of diatoms, dinoflagellates and coccolithophores in the western Barents Sea
- Qingshan Luan
- Elaine Mitchell
- Keith Davidson
Polar Biology (2024)
Climate-driven zooplankton shifts cause large-scale declines in food quality for fish
- Ryan F. Heneghan
- Jason D. Everett
- Anthony J. Richardson
Nature Climate Change (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
A comparative analyzing of zooplankton community diversity in surface layer water of reservoir via edna metabarcoding and microscopy.

Graphical Abstract
1. Introduction
2. materials and methods, 2.1. study area, sampling, and microscope-assisted identification, 2.2. edna extraction and analytical procedures, 3.1. environmental variables of sampling sites, 3.2. comparative community analysis with edna metabarcoding and msi, 3.3. characterization of zooplankton communities and their relationships with the sampling methods, 4. discussion, 4.1. limitations and effectiveness of edna metabarcoding, 4.2. comparison of zooplankton community structure according to sampling methods, 5. conclusions, author contributions, institutional review board statement, data availability statement, conflicts of interest.
Click here to enlarge figure
| Gene | 12s rRNA | 16s rRNA | 18s rRNA | COI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Genus | Species | Total | Genus | Species | Total | Genus | Species | Total | Genus | Species | ||
| Number of NCBI registrations | 281,812 | 18,982 | 51,602 | 544,387 | 36,070 | 108,443 | 1,290,909 | 46,397 | 162,665 | 3,530,058 | 47,139 | 172,650 | |
| Number of NCBI registrations of Korean organism | 4404 | 1547 | 401 | 7600 | 2330 | 630 | 8966 | 3045 | 816 | 9411 | 2298 | 513 | |
| No. | Phytoplankton | 13 | 13 | 12 | 251 | 152 | 88 | 651 | 274 | 128 | 278 | 121 | 67 |
| Zooplankton | 143 | 90 | 43 | 338 | 207 | 79 | 869 | 467 | 139 | 456 | 235 | 65 | |
| Macroinvertebrate | 976 | 433 | 116 | 1717 | 653 | 144 | 1636 | 689 | 159 | 1940 | 698 | 150 | |
| Fish | 712 | 222 | 45 | 688 | 221 | 45 | 245 | 132 | 34 | 692 | 221 | 45 | |
| % | Phytoplankton | 1.2 | 3.6 | 8.3 | 22.7 | 42.6 | 61.1 | 64.5 | 84.6 | 92.8 | 25.1 | 33.9 | 46.5 |
| Zooplankton | 9.3 | 14.8 | 27.7 | 22 | 34.1 | 51 | 56.6 | 76.9 | 89.7 | 29.7 | 38.7 | 41.9 | |
| Macroinvertebrate | 33.5 | 49.1 | 65.9 | 58.9 | 74 | 81.8 | 56.1 | 78.1 | 90.3 | 66.6 | 79.1 | 85.2 | |
| Fish | 94.9 | 98.7 | 95.7 | 91.7 | 98.2 | 95.7 | 32.7 | 58.7 | 72.3 | 92.3 | 98.2 | 95.7 | |
- Oh, H.-J.; Chae, Y.-J.; Choi, Y.; Ku, D.; Heo, Y.-J.; Kwak, I.-S.; Jo, H.; Park, Y.-S.; Chang, K.-H.; Kim, H.-W. Review and Suggestions for Applying DNA Sequencing to Zooplankton Researches: From Taxonomic Approaches to Biological Interaction Analysis. Korean J. Ecol. Environ. 2021 , 54 , 156–169. [ Google Scholar ] [ CrossRef ]
- Schminke, H.K. Entomology for the copepodologist. J. Plankton Res. 2007 , 29 , i149–i162. [ Google Scholar ] [ CrossRef ]
- Bucklin, A.; Lindeque, P.K.; Rodriguez-Ezpeleta, N.; Albaina, A.; Lehtiniemi, M. Metabarcoding of marine zooplankton: Prospects, progress and pitfalls. J. Plankton Res. 2016 , 38 , 393–400. [ Google Scholar ] [ CrossRef ]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Glob. Ecol. Conserv. 2019 , 17 , e00547. [ Google Scholar ] [ CrossRef ]
- Rourke, M.L.; Fowler, A.M.; Hughes, J.M.; Broadhurst, M.K.; DiBattista, J.D.; Fielder, S.; Walburn, J.W.; Furlan, E.M. Environmental DNA (eDNA) as a tool for assessing fish biomass: A review of approaches and future considerations for resource surveys. Environ. DNA 2022 , 4 , 9–33. [ Google Scholar ] [ CrossRef ]
- Thomsen, P.F.; Willerslev, E. Environmental DNA—An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015 , 183 , 4–18. [ Google Scholar ] [ CrossRef ]
- Lodge, D.M.; Turner, C.R.; Jerde, C.L.; Barnes, M.A.; Chadderton, L.; Egan, S.P.; Feder, J.L.; Mahon, A.R.; Pfrender, M.E. Conservation in a cup of water: Estimating biodiversity and population abundance from environmental DNA. Mol. Ecol. 2012 , 21 , 2555–2558. [ Google Scholar ] [ CrossRef ]
- Rees, H.C.; Maddison, B.C.; Middleditch, D.J.; Patmore, J.R.; Gough, K.C. Review: The detection of aquatic animal species using environmental DNA—a review of eDNA as a survey tool in ecology. J. Appl. Ecol. 2014 , 51 , 1450–1459. [ Google Scholar ] [ CrossRef ]
- Xie, R.; Zhao, G.; Yang, J.; Wang, Z.; Xu, Y.; Zhang, X.; Wang, Z. eDNA metabarcoding revealed differential structures of aquatic communities in a dynamic freshwater ecosystem shaped by habitat heterogeneity. Environ. Res. 2021 , 201 , 111602. [ Google Scholar ] [ CrossRef ]
- Kim, D.K.; Park, K.; Jo, H.; Kwak, I.S. Comparison of Water Sampling between Environmental DNA Metabarcoding and Conventional Microscopic Identification: A Case Study in Gwangyang Bay, South Korea. Appl. Sci. 2019 , 9 , 3272. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Mächler, E.; Little, C.J.; Wüthrich, R.; Alther, R.; Fronhofer, E.A.; Gounand, I.; Harvey, E.; Hürlemann, S.; Walser, J.C.; Altermatt, F. Assessing different components of diversity across a river network using eDNA. Environ. DNA 2019 , 1 , 290–301. [ Google Scholar ] [ CrossRef ]
- Heine, P.; Hausen, J.; Ottermanns, R.; Ross-Nickoll, M. Comparing eDNA metabarcoding with morphological analyses: Fungal species richness and community composition of differently managed stages along a forest conversion of Norway spruce towards European beech in Germany. For. Ecol. Manag. 2021 , 496 , 119429. [ Google Scholar ] [ CrossRef ]
- Valentini, A.; Taberlet, P.; Miaud, C.; Civade, R.; Herder, J.; Thomsen, P.F.; Bellemain, E.; Besnard, A.; Coissac, E.; Boyer, F.; et al. Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Mol. Ecol. 2016 , 25 , 929–942. [ Google Scholar ] [ CrossRef ]
- Fujii, K.; Doi, H.; Matsuoka, S.; Nagano, M.; Sato, H.; Yamanaka, H. Environmental DNA metabarcoding for fish community analysis in backwater lakes: A comparison of capture methods. PLoS ONE 2019 , 14 , e0210357. [ Google Scholar ] [ CrossRef ]
- Djurhuus, A.; Pitz, K.; Sawaya, N.A.; Rojas-Márquez, J.; Michaud, B.; Montes, E.; Muller-Karger, F.; Breitbart, M. Evaluation of marine zooplankton community structure through environmental DNA metabarcoding. Limnol. Oceanogr. Methods 2018 , 16 , 209–221. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bourque, D. Time Series Experiments Reveal that Environmental DNA Tracks Zooplankton Population Dynamics in Large Mesocosms. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2019. [ Google Scholar ]
- Keck, F.; Blackman, R.C.; Bossart, R.; Brantschen, J.; Couton, M.; Hürlemann, S.; Kirschner, D.; Locher, N.; Zhang, H.; Altermatt, F. Meta-analysis shows both congruence and complementarity of DNA and eDNA metabarcoding to traditional methods for biological community assessment. Mol. Ecol. 2022 , 31 , 1820–1835. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lopez, M.L.D.; Lin, Y.; Sato, M.; Hsieh, C.; Shiah, F.; Machida, R.J. Using metatranscriptomics to estimate the diversity and composition of zooplankton communities. Mol. Ecol. Resour. 2022 , 22 , 638–652. [ Google Scholar ] [ CrossRef ]
- Harvey, J.B.; Johnson, S.B.; Fisher, J.L.; Peterson, W.T.; Vrijenhoek, R.C. Comparison of morphological and next generation DNA sequencing methods for assessing zooplankton assemblages. J. Exp. Mar. Biol. Ecol. 2017 , 487 , 113–126. [ Google Scholar ] [ CrossRef ]
- Jo, H.; Kim, D.-K.; Park, K.; Kwak, I.-S. Discrimination of Spatial Distribution of Aquatic Organisms in a Coastal Ecosystem Using eDNA. Appl. Sci. 2019 , 9 , 3450. [ Google Scholar ] [ CrossRef ]
- Tang, C.Q.; Leasi, F.; Obertegger, U.; Kieneke, A.; Barraclough, T.G.; Fontaneto, D. The widely used small subunit 18S rDNA molecule greatly underestimates true diversity in biodiversity surveys of the meiofauna. Proc. Natl. Acad. Sci. USA 2012 , 109 , 16208–16212. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Yang, J.; Zhang, X. eDNA metabarcoding in zooplankton improves the ecological status assessment of aquatic ecosystems. Environ. Int. 2020 , 134 , 105230. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Einsle, U. Crustacea: Copepoda: Calanoida und Cyclopoida (Süsswasserfauna von Mitteleuropa) ; Schworbel, J., Zwick, P., Eds.; Fischer: Stuttgart, Germany, 1993. [ Google Scholar ]
- Mizuno, T.; Takahashi, E. An Illustrated Guide to Freshwater Zooplankton in Japan ; Tokai University Press: Hiratsuka, Japan, 1991. [ Google Scholar ]
- Addiscott, T.; Thomas, D. Tillage, mineralization and leaching: Phosphate. Soil Tillage Res. 2000 , 53 , 255–273. [ Google Scholar ] [ CrossRef ]
- Djurhuus, A.; Port, J.; Closek, C.J.; Yamahara, K.M.; Romero-Maraccini, O.; Walz, K.R.; Goldsmith, D.B.; Michisaki, R.; Breitbart, M.; Boehm, A.B.; et al. Evaluation of Filtration and DNA Extraction Methods for Environmental DNA Biodiversity Assessments across Multiple Trophic Levels. Front. Mar. Sci. 2017 , 4 , 314. [ Google Scholar ] [ CrossRef ]
- Amaral-Zettler, L.A.; McCliment, E.A.; Ducklow, H.W.; Huse, S.M. A Method for Studying Protistan Diversity Using Massively Parallel Sequencing of V9 Hypervariable Regions of Small-Subunit Ribosomal RNA Genes. PLoS ONE 2009 , 4 , e6372. [ Google Scholar ] [ CrossRef ]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science using QIIME 2. Nat. Biotechnol. 2019 , 37 , 852–857. [ Google Scholar ] [ CrossRef ]
- Shannon, C.E. Prediction and Entropy of Printed English. Bell Syst. Tech. J. 1951 , 30 , 50–64. [ Google Scholar ] [ CrossRef ]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963 , 58 , 236–244. [ Google Scholar ] [ CrossRef ]
- Beals, E.W. Bray-Curtis Ordination: An Effective Strategy for Analysis of Multivariate Ecological Data. Adv. Ecol. Res. 1984 , 14 , 1–55. [ Google Scholar ] [ CrossRef ]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979 , 86 , 420–428. [ Google Scholar ] [ CrossRef ]
- Fleiss, J.L. Measuring nominal scale agreement among many raters. Psychol. Bull. 1971 , 76 , 378–382. [ Google Scholar ] [ CrossRef ]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. (R Package Version 2.5–6). 2019. Available online: https://www.r-project.org/index.html (accessed on 1 May 2022).
- Wolak, M.; Wolak, M.M. Package ‘ICC’. Facilitating Estimation of the Intraclass Correlation Coefficient 2015. Available online: https://cran.r-project.org/web/packages/ICC/index.html (accessed on 1 May 2022).
- R Core Team. R: A Language and Environment for Statistical Computing ; R Foundation for Statistical Computing: Vienna, Austria, 2021. [ Google Scholar ]
- National Institute of Biological Resources; Biodiversity on the Korean Peninsula. Available online: https://species.nibr.go.kr/ (accessed on 1 December 2021).
- Weigand, H.; Beermann, A.J.; Čiampor, F.; Costa, F.O.; Csabai, Z.; Duarte, S.; Geigerg, M.F.; Grabowski, M.; Rimet, F.; Rulik, B.; et al. DNA barcode reference libraries for the monitoring of aquatic biota in Europe: Gap-analysis and recommendations for future work. Sci. Total Environ. 2019 , 678 , 499–524. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rivera, S.F.; Vasselon, V.; Ballorain, K.; Carpentier, A.; Wetzel, C.E.; Ector, L.; Bouchez, A.; Rimet, F. DNA metabarcoding and microscopic analyses of sea turtles biofilms: Complementary to understand turtle behavior. PLoS ONE 2018 , 13 , e0195770. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vasselon, V.; Bouchez, A.; Rimet, F.; Jacquet, S.; Trobajo, R.; Corniquel, M.; Tapolczai, K.; Domaizon, I. Avoiding quantification bias in metabarcoding: Application of a cell biovolume correction factor in diatom molecular biomonitoring. Methods Ecol. Evol. 2018 , 9 , 1060–1069. [ Google Scholar ] [ CrossRef ]
- Keck, F.; Vasselon, V.; Tapolczai, K.; Rimet, F.; Bouchez, A. Freshwater biomonitoring in the Information Age. Front. Ecol. Environ. 2017 , 15 , 266–274. [ Google Scholar ] [ CrossRef ]
- Fleiss, J.L.; Levin, B.; Paik, M.C. Statistical Methods for Rates and Proportions ; John Wiley & Sons: Hoboken, NJ, USA, 2013. [ Google Scholar ]
- Saint-Jean, L. The zooplankton. In Lake Chad ; Springer: Boston, MA, USA, 1983; pp. 199–232. [ Google Scholar ]
- Bista, I.; Carvalho, G.R.; Walsh, K.; Seymour, M.; Hajibabaei, M.; Lallias, D.; Christmas, M.; Creer, S. Annual time-series analysis of aqueous eDNA reveals ecologically relevant dynamics of lake ecosystem biodiversity. Nat. Commun. 2017 , 8 , 14087. [ Google Scholar ] [ CrossRef ] [ Green Version ]
| Month | Location | Temp. (°C) | DO (mg/L) | pH | Cond. (μS/cm) | Chl-a (μg/L) | TOC (mg/L) | TN (mg/L) | TP (mg/L) | SS (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | Inflow | 15.2 | 13.6 | 8.8 | 686 | 35.7 | 3.9 | 4.4 | 0.06 | 5.2 |
| Reservoir | 12.7 | 14.0 | 8.0 | 796 | 14.3 | 3.6 | 6.6 | 0.03 | 2.2 | |
| Outflow | 13.4 | 14.5 | 7.4 | 1,430 | 14.4 | 4.7 | 7.3 | 0.48 | 14.7 | |
| 4 | Inflow | 14.9 | 13.5 | 8.4 | 670 | 14.3 | 3.6 | 3.8 | 0.05 | 5.5 |
| Reservoir | 14.7 | 11.4 | 7.6 | 745 | 8.0 | 4.3 | 5.6 | 0.03 | 0.8 | |
| Outflow | 20.3 | 14.4 | 9.5 | 769 | 14.9 | 4.4 | 5.0 | 0.09 | 1.4 | |
| 5 | Inflow | 22.0 | 8.5 | 8.5 | 625 | 22.5 | 4.2 | 3.7 | 0.06 | 5.8 |
| Reservoir | 20.3 | 9.5 | 7.6 | 708 | 10.4 | 4.6 | 6.2 | 0.02 | 2.4 | |
| Outflow | 21.2 | 8.2 | 8.0 | 1,130 | 50.6 | 6.9 | 6.4 | 0.38 | 5.7 | |
| 6 | Inflow | 24.5 | 11.1 | 8.4 | 655 | 13.5 | 4.2 | 4.3 | 0.07 | 9.9 |
| Reservoir | 25.4 | 10.5 | 9.1 | 632 | 19.5 | 4.7 | 4.6 | 0.04 | 1.2 | |
| Outflow | 25.6 | 6.7 | 8.6 | 596 | 17.1 | 4.4 | 4.3 | 0.04 | 2.7 | |
| 7 | Inflow | 25.6 | 9.1 | 8.7 | 607 | 11.4 | 3.8 | 3.9 | 0.04 | 1.8 |
| Reservoir | 28.8 | 13.6 | 8.2 | 520 | 22.2 | 4.2 | 4.4 | 0.04 | 8.6 | |
| Outflow | 25.4 | 9.0 | 8.6 | 485 | 23.3 | 4.6 | 4.2 | 0.04 | 4.6 | |
| 8 | Inflow | 26.2 | 8.7 | 8.5 | 424 | 13.3 | 6.3 | 2.8 | 0.11 | 11.1 |
| Reservoir | 29.6 | 8.7 | 8.3 | 652 | 43.6 | 5.2 | 5.9 | 0.04 | 15.3 | |
| Outflow | 26.8 | 7.2 | 8.3 | 1,050 | 7.0 | 7.7 | 7.4 | 1.11 | 26.4 | |
| 9 | Inflow | 25.9 | 11.6 | 8.4 | 607 | 20.2 | 4.7 | 3.9 | 0.05 | 11.1 |
| Reservoir | 26.0 | 10.7 | 8.4 | 546 | 16.5 | 4.3 | 3.6 | 0.02 | 7.7 | |
| Outflow | 25.0 | 7.2 | 8.1 | 545 | 17.8 | 4.4 | 3.6 | 0.02 | 9.7 |
| Order | Family | Genus | Relative Frequency (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| eDNA | MSI | Both | eDNA | MSI | Both | eDNA | MSI | Both | eDNA | MSI | |
| Copepoda | 3 | 2 | 2 | 4 | 2 | 2 | 9 | 2 | 0 | 60.8 | 30.7 |
| Cladocera | 1 | 2 | 1 | 2 | 4 | 2 | 6 | 6 | 3 | 0.9 | 18.8 |
| Rotifera | 4 | 3 | 3 | 20 | 15 | 13 | 27 | 19 | 16 | 38.3 | 50.5 |
| MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Share and Cite
Ji, C.W.; Oh, H.-J.; Chang, K.-H.; Park, Y.-S.; Kwak, I.-S. A Comparative Analyzing of Zooplankton Community Diversity in Surface Layer Water of Reservoir Via eDNA Metabarcoding and Microscopy. Diversity 2022 , 14 , 797. https://doi.org/10.3390/d14100797
Ji CW, Oh H-J, Chang K-H, Park Y-S, Kwak I-S. A Comparative Analyzing of Zooplankton Community Diversity in Surface Layer Water of Reservoir Via eDNA Metabarcoding and Microscopy. Diversity . 2022; 14(10):797. https://doi.org/10.3390/d14100797
Ji, Chang Woo, Hye-Ji Oh, Kwang-Hyeon Chang, Young-Seuk Park, and Ihn-Sil Kwak. 2022. "A Comparative Analyzing of Zooplankton Community Diversity in Surface Layer Water of Reservoir Via eDNA Metabarcoding and Microscopy" Diversity 14, no. 10: 797. https://doi.org/10.3390/d14100797
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
ORIGINAL RESEARCH article
Phytoplankton diversity effect on ecosystem functioning in a coastal upwelling system.

- 1 Instituto de Investigaciones Marinas (IIM-CSIC), Vigo, Spain
- 2 Centro Oceanográfico de A Coruña, Instituto Español de Oceanografía, A Coruña, Spain
Species composition plays a key role in ecosystem functioning. Theoretical, experimental and field studies show positive effects of biodiversity on ecosystem processes. However, this link can differ between taxonomic and functional diversity components and also across trophic levels. These relationships have been hardly studied in planktonic communities of coastal upwelling systems. Using a 28-year time series of phytoplankton and zooplankton assemblages, we examined the effects of phytoplankton diversity on resource use efficiency (RUE, ratio of biomass to limiting resource) at the two trophic levels in the Galician upwelling system (NW Iberian peninsula). By fitting generalized least square models, we show that phytoplankton diversity was the best predictor for RUE across planktonic trophic levels. This link varied depending on the biodiversity component considered: while the effect of phytoplankton richness on RUE was positive for phytoplankton RUE and negative for zooplankton RUE, phytoplankton evenness effect was negative for phytoplankton RUE and positive for zooplankton RUE. Overall, taxonomic diversity had higher explanatory power than functional diversity, and variability in phytoplankton and zooplankton RUE decreased with increasing phytoplankton taxonomic diversity. Phytoplankton used resources more efficiently in warmer waters and at greater upwelling intensity, although these effects were not as strong as those for biodiversity. These results suggest that phytoplankton species numbers in highly dynamic upwelling systems are important for maintaining the planktonic biomass production leading us to hypothesize the relevance of complementarity effects. However, we further postulate that a selection effect may operate also because assemblages with low evenness were dominated by diatoms with specific functional traits increasing their ability to exploit resources more efficiently.
Introduction
Marine phytoplankton is responsible for roughly half of the global primary production (50 Pg C year −1 ; Chavez et al., 2011 ), contributes to nutrient cycling and regulation of climate dynamics, affects the fate of adjacent trophic levels ( Richardson and Schoeman, 2004 ), and, ultimately, constrains fishery catches ( Chassot et al., 2010 ). Marine phytoplankton is an extremely diverse group of organisms ( De Vargas et al., 2015 ), and this diversity, encompassing a large variety of life histories, is an essential factor that affects the whole structure of marine ecosystems ( Naeem, 2012 ). It is indeed the diversity of functional traits that differs among and within species and taxonomic groups, the key component determining the fitness of planktonic communities along environmental gradients and influencing the functioning of pelagic ecosystems ( Irwin and Finkel, 2018 ). Therefore, there is a need to better understand the relationship between the variability in phytoplankton diversity and its effects on ecosystem processes.
In recent years, various reviews have synthesized the effects of alterations of biodiversity (B) on ecosystem functioning (EF) concluding that biodiversity has a major role in sustaining the productivity of ecosystems and their stability ( Cardinale et al., 2012 ; Tilman et al., 2014 ). Most of this evidence comes from controlled experiments; however, field studies are also consistent with theory and experiments demonstrating the strong effect of biodiversity on ecosystem production even after accounting for abiotic forcing ( Duffy et al., 2017 ). In the marine realm, several studies have been devoted also to understand the effects that marine biodiversity has on production, biomass, or on the resilience to disturbances or invasions ( Stachowicz et al., 2007 ; van der Plas, 2019 ). Yet the majority of this experimental and field research has examined relationships in the benthos ( O'Connor and Byrnes, 2014 ; Duffy et al., 2017 ). Regarding the pelagos, recent studies have addressed BEF relationships within natural assemblages of plankton either in fresh or marine waters. A BEF relationship in the plankton was first described by Ptacnik et al. (2008) , who showed that resource-use efficiency (RUE), an ecological index that measures the proportion of supplied resources turned into new biomass ( Hodapp et al., 2019 ), scaled positively with phytoplankton taxonomic richness in multiple Fennoscandian lakes and in the Baltic Sea. Korhonen et al. (2011) also used data from boreal lakes to connect, in this case, productivity to diversity of various plankton groups showing linear, unimodal, or nonsignificant relationships between richness and biomass production depending on the spatial scale. Furthermore, Olli et al. (2014) , using long-term phytoplankton sampling, concluded that increased diversity enhanced RUE for primary producers across the brackish Baltic Sea. However, it is unclear if these BEF relationships apply to highly dynamic Eastern Boundary Upwelling Ecosystems (EBUEs). For instance, in the California Current System, an ecosystem model coupled to a circulation model showed a hump-shaped relationship between diversity and productivity with portions of the diversity-productivity scatter being dependent on geographic regions ( Goebel et al., 2013 ). Other authors did not find a relationship between phytoplankton species richness and ecosystem productivity (e.g., Cermeño et al., 2013 ).
Typically, the majority of BEF studies have used richness as the metric of biodiversity because it is easy to manipulate in experiments and to measure in the field. However, there is also evidence of the relevance of other components of biodiversity to understand pelagic processes. For instance, some authors have studied the effect(s) of evenness on planktonic ecosystem properties showing a strong negative effect on RUE in the phytoplankton of the Wadden Sea ( Hodapp et al., 2015 ) or on biomass and resource use in the phytoplankton of the Baltic Sea ( Lehtinen et al., 2017 ) highlighting the importance of the identity of dominant species. Besides taxonomic diversity, biodiversity can be assessed in terms of functional diversity, that is, accounting for the expression of multiple functional traits in the community, often concluding that functional diversity can be a better predictor of ecosystem properties than taxonomic diversity as shown for phytoplankton communities in Fennoscandian lakes ( Abonyi et al., 2018 ). Whether taxonomic or functional diversity of competing species affects ecosystem properties, it is also fundamental to incorporate trophic complexity in order to understand the effects of biodiversity across trophic levels ( Duffy et al., 2007 ). In experimental planktonic systems, Striebel et al. (2012) showed that phytoplankton diversity increased zooplankton productivity, while Filstrup et al. (2014) showed that the effect of phytoplankton evenness on RUE switched from negative at the producer level (phytoplankton) to positive at the consumer level (zooplankton) in US lakes.
Apart from average effects on ecosystem functioning, theory, experiments, and field studies predict that increasing diversity can reduce the variability of community biomass or other ecosystem properties in time and space through several mechanisms ( Loreau and de Mazancourt, 2013 ). This would occur because more diverse assemblages containing interacting species, which respond differently to the environment are more likely to buffer the effects of perturbations conferring stability to the community and maintaining the ecosystem properties in a dynamic environment ( Ives and Carpenter, 2007 ). Stability, however, is a complex and multifaceted concept with multiple components, such as variability, resistance, or resilience, which might be unrelated implying that the overall stability of an ecosystem might not be simply explained by one particular component ( Hillebrand et al., 2018 ), thus leading to different biodiversity-stability relationships ( Craven et al., 2018 ). Most analyses on biodiversity-stability relationships have been performed through experiments in terrestrial systems ( Tilman et al., 2014 ), whereas fewer studies dealt with natural ecosystems, and the majority have focused on plants (e.g., García-Palacios et al., 2018 ; but see Cusson et al., 2015 ). In the case of natural plankton assemblages, Ptacnik et al. (2008) showed that higher levels of phytoplankton taxonomic richness implied less variability of both resource use and community composition, and Shurin et al. (2007) documented a positive relationship between zooplankton diversity and community stability in temperate lakes. Despite all these research efforts, the importance of phytoplankton diversity on stability in EBUEs, so as the shape of BEF relationships between trophic levels, and the performance of functional diversity vs. taxonomic diversity have been yet poorly addressed (but see Cermeño et al., 2013 ; Goebel et al., 2013 ; Vallina et al., 2017 ).
Planktonic communities are highly dynamic with assemblages changing rapidly in response to circulation and fertilization patterns and other physical and environmental forcing. This is even more evident in coastal upwelling systems where planktonic assemblages might fluctuate at short-time scales with different assemblages characterizing the various phases of an upwelling cycle ( Marañón, 2015 ). At larger spatial and temporal scales, the structure of phytoplankton and zooplankton assemblages is affected by seasonal changes, with species' abundance responding to light conditions, temperature, nutrient inputs, or the presence of particular producers and consumers ( Wiltshire et al., 2015 ). Therefore, planktonic biodiversity is affected by the physical environment, water hydrography, and biotic variables ( Sarker et al., 2018 ). However, BEF studies have focused less on the abiotic and biotic context that might exert comparable effects to changes in species richness in mediating ecosystem properties ( Godbold, 2012 ; van der Plas, 2019 ). Thus, accounting for the environmental context in BEF relationships under natural (or experimental) conditions is crucial to understand and interpret the effects that changing biodiversity has on ecosystem properties ( García et al., 2018 ), stability ( García-Palacios et al., 2018 ), or community performance ( Schabhüttl et al., 2013 ).
In this study, we analyzed whether the phytoplankton diversity effects on ecosystem function follows theoretical and experimental expectations in real communities occurring in EBUEs for which knowledge is limited. In doing so, we used long-term time series of phytoplankton and zooplankton community data in conjunction with meteorological and hydrographic data to examine BEF relationships in a highly dynamic coastal upwelling ecosystem. In particular, we (i) evaluated the effect of two components of phytoplankton taxonomic diversity (richness and evenness) on phytoplankton and zooplankton rates of productivity to the amount of available resources (i.e., RUE), (ii) tested whether biodiversity influences planktonic RUE variability, (iii) quantified the importance of biodiversity relative to environmental conditions in driving planktonic RUE dynamics, and (iv) evaluated the explanatory power of phytoplankton taxonomic diversity vs. functional diversity in explaining planktonic RUE.
Materials and Methods
Study area and plankton sampling.
Galicia is at the northern boundary of the Iberia/Canary current EBUE ( Figure 1 ). Coastal winds at these latitudes (42° to 44° N) are seasonal; northerly winds prevail from March–April to September–October, promoting coastal upwelling, and downwelling-favorable southerly winds predominate the rest of the year. However, more than 70% of the variability in coastal winds occurs in periods of <1 month, so that the upwelling season appears as a succession of wind-stress events separated by wind-calm episodes, with a wide variety of frequencies ranging from 3 to 15 days ( Álvarez-Salgado et al., 2002 ).
Figure 1 . Map of study area showing the location of the plankton and hydrographic sampling station (black dot), and the location of the grid cell where the upwelling index was calculated (black triangle).
Phytoplankton identification and count data were obtained from the time series project RADIALES conducted by the Instituto Español de Oceanografía off A Coruña (NW Spain, Figure 1 ) ( Bode et al., 2009 ). Specifically, water samples were collected monthly with 5 L of Niskin bottles or a rosette sampler from 0-, 5-, 10-, 20-, 30-, 40-, and 70-m depths at station E2CO (water depth, 80 m; 43°25′30″N, 08°26′20″W; Figure 1 ) from January 1989 to December 2016 ( n = 313 days sampled), though sampling at 7 m ended in 1993, and from 2010 onward, samples were taken just from 3 depths between the surface and 40 m including the depth of the surface chlorophyll maximum. For each depth, samples were collected for the determination of phytoplankton abundance and inorganic nutrients and chlorophyll a concentration following the methods described in Casas et al. (1997) . Phytoplankton samples of volume 50–100 ml were preserved in Lugol's solution and kept in the dark until analysis. Depending on phytoplankton concentration, 10–25 ml of samples was allowed to settle in the Utermöhl chamber for up to 24 h. Samples were counted following the technique described by Utermöhl ( Lund et al., 1958 ) using a Nikon Diaphot TMD microscope until May 1997 and a Nikon Eclipse TE300 microscope until the end of the time series. A magnification of 100× was used for large forms, 250× for intermediate forms, and 400× for small forms. The entire slide was examined at 100× to account for large species, while only transects or smaller areas were examined at higher magnification. At least 250 cells were counted for each sample. Whenever possible, organisms were classified at the species or genus level. Species nomenclature followed the World Register of Marine Species ( http://www.marinespecies.org ). The samples were identified by two experts (M. Varela until 2010, and J. Lorenzo from 2010 to 2016). The individual biomass (in pg C) for each identified taxa was estimated from cell biovolume (in μm 3 ) after measuring the dimensions of 30–100 cells in samples distributed over all seasons and applying conversion equations from the literature (see details in Huete-Ortega et al., 2010 ). Biomass (in pg C L −1 ) of a given species in a given day and sampled depth was then calculated as the abundance (in cells L −1 ) times the cell biomass. For the purposes of the present work, we used the taxa that were systematically identified at the species level in at least 10 samples along the time series. This resulted in a set of 73 taxa, 18 of which started to be identified in 2008 ( Supplementary Table 1 ). The total biomass (obtained as the sum of the biomass of all counted species in each day and sampled depth) was significantly correlated with chlorophyll a concentration ( Supplementary Figure 1 ), and the biomass was dominated by diatoms ( Supplementary Figure 2 ). The original dataset is available at PANGAEA ( https://doi.org/10.1594/PANGAEA.908815 ) ( Bode, 2019 ). Additionally, we collated a series of morphological (cell size and ability to form chains or colonies), physiological (silica requirement, trophic strategy, and pigment composition), and behavioral (ability to swim) traits for each phytoplankton species ( Supplementary Table 1 ) using our own compilation and data from the literature ( Klais et al., 2017 ). This group of traits is of relevance for reproduction, resource acquisition, and survival ( Litchman and Klausmeier, 2008 ). Together, they affect phytoplankton fitness and are involved in several functions such as light use, nutrient uptake, or predator avoidance. Cell size can be further considered as a key trait that affects metabolism, growth rate, and community structure among others ( Marañón, 2015 ).
Zooplankton was sampled at the same station and dates as phytoplankton by means of double oblique tows from the surface to 5 m of the bottom using a 50-cm diameter Juda—Bogorov plankton net with 250-μm (until 1997) or 200-μm (from 1997 onward) mesh size. The net was equipped with a General Oceanic Flowmeter for the calculation of water filtered and a depth recorder. Samples were preserved in 2–4% sodium borate-buffered formaldehyde. Subsamples were taken to estimate total zooplankton abundances (in ind × m −3 ) by direct examination using a stereo microscope, and biomass (in μg DW × L −1 ) by weighting dried aliquots (50°C, 48 h). Further details can be found in Bode et al. (2012) . The original dataset is available at PANGAEA ( https://doi.org/10.1594/PANGAEA.908815 ) ( Bode, 2019 ).
Hydrographic Sampling and Analysis
Concurrently with the plankton samples, vertical profiles of temperature and salinity were measured with a CTD probe (Seabird SBE-25). The salinity of the CTD was checked against the salinity of bottom water samples measured with an induction salinometer Autosal 8400A calibrated with Standard Seawater ( Casas et al., 1997 ). Salinity was expressed in the practical salinity scale ( UNESCO, 1986 ). Nitrate concentration (NO 3 in μmol L −1 ) was determined by segmented flow analysis according to the standard procedures of Grasshoff et al. (1983) using a Technicon AA-II (1989–2006), a Bran–Luebbe AA3 (2007–2012), and a Seal Analytics QuAAttro 39 (2013–2016). Chlorophyll a concentration (Chl a in mg m −3 ) was measured by fluorimetric analysis of acetone extracts of phytoplankton collected on 0.8-μm pore-size membrane filters (until 1992) or GF/F filters (from 1993 onward). Specific calibrations were performed to ensure the continuity of the chlorophyll series when changing from the filter fluorometer method ( Parsons et al., 1984 ) to the spectrofluorimetric technique ( Neveux and Panouse, 1987 ) after 2001. Nutrient and chlorophyll data series are available at: https://doi.org/10.1594/PANGAEA.885413 ( Bode et al., 2018 ).
Physical Forcing
Daily upwelling index (UI in m 3 s −1 km −1 ) data, a rough estimate of the volume of water upwelled per km of coastline for the period 1989 to 2016 were downloaded from: http://www.indicedeafloramiento.ieo.es/index_UI_en.html . UI was estimated from geostrophic winds calculated from the surface atmospheric pressure fields supplied every 6 h by the US Navy Operational Global Atmospheric Prediction System (NOGAPS) model maintained by the Fleet Numerical Meteorological and Oceanography Center ( http://www.usno.navy.mil/FNMOC/ ) in a 1° × 1° grid centered at 44°N 9°W ( Figure 1 ). This cell is representative for the physical forcing that determines the impact of coastal upwelling in the region ( Bode et al., 2015 ). We used the meridional wind component, thus the UI represents the volume of water upwelled along the West–East direction with positive values of UI indicating upwelling-favorable conditions. Conversely, negative values indicate downwelling-favorable conditions. Further numerical details can be found in González-Nuevo et al. (2014) . In this study, values of UI were averaged over 15 days prior to each sampling date.
Data Analyses
Resource-use efficiency and biodiversity estimations.
Phytoplankton resource-use efficiency (RUE pp ) was calculated sensu Ptacnik et al. (2008) in terms of phytoplankton carbon biomass (pg C L −1 ) per unit of nitrate concentration (μmol L −1 ), i.e., RUE pp = phytoplankton biomass/ NO 3 - concentration. Other components such as ammonia are important for the dissolved inorganic nitrogen (DIN) pool in this region; however, this variable was not measured with the same periodicity. Nonetheless, nitrate is the main limiting nutrient for the primary production in this region ( Álvarez-Salgado et al., 1997 ). Zooplankton resource-use efficiency (RUE zp ) was calculated sensu Filstrup et al. (2014) in terms of zooplankton biomass (μg L −1 ) per unit phytoplankton carbon biomass (pg C L −1 ), i.e., RUE zp = zooplankton biomass/phytoplankton biomass. Both ratios were natural log-transformed for later modeling.
Taxonomic diversity (TD) was expressed as species richness (S) and as evenness (J) ( Pielou, 1966 ) using phytoplankton biomass as follows:
J = H H m a x where H max = ln S and H = - ∑ i = 1 S B i B t o t × l n B i B t o t where B i is the biomass of a species i , and B tot is the total biomass. Additionally, we calculated various uncorrelated multitrait-based functional diversity (FD) metrics, which capture the various aspects of functional diversity ( Mouchet et al., 2010 ). In particular, FD was expressed as functional group richness (FGR) and functional dispersion (FDis) following Laliberté and Legendre (2010) , and functional evenness (FEve) following Villéger et al. (2008) . To calculate the FD metrics, the abundance matrix was based on species biomass, and the functional trait matrix contained a quantitative variable (cell biomass expressed in log units) and other six binary variables describing other morphological, physiological, and behavioral traits (see Supplementary Table 1 ). FGR is a dendrogram-based indicator of functional groups computed from an a posteriori classification of species based on their functional traits for which the Ward method was used to create the dendrogram of the species that was cut at nine functional groups. FDis is a distance-based metric that measures the distance to the centroid of the assemblage in the trait space, and FEve is a metric that combines the evenness of species spacing in trait space and the evenness of species relative abundances. FDis and FEve metrics were weighted by the biomass of the species, and FEve was not defined for samples with fewer than three taxa ( n = 6 cases). Finally, single-trait-based indices, that is, community-weighted mean (CWM) traits were also calculated to examine phytoplankton single-trait contributions to RUE. CWMs were calculated for each trait weighted by species biomass using methods implemented by Laliberté and Legendre (2010) .
Statistical Analyses
The values of RUE pp calculated for a day i and depth j were modeled using generalized least square (GLS) models that were formulated as follows:
where α is an intercept, and ns n is a natural cubic spline describing the effect of the day of the year (DoY), i.e., the seasonality, the time trend, i.e., the interannual long-term pattern (days, i.e., consecutive days from 1989 to 2016), the depth (D), the water temperature (WT), the coastal upwelling index (UI), the phytoplankton richness (S), and the phytoplankton evenness (J). All splines had 2 degrees of freedom (df) with the exception of DoY that used 4 df. Finally, ϵ i,j is a vector of errors assumed to have mean 0 and variance σ 2 . To evaluate the variability in the response variable, i.e., a nonconstant variance, the variance in RUE pp (σ 2 ) was modeled as an exponential function of the environmental conditions (i.e., WT or UI) or the taxonomic diversity metrics (i.e., S or J). For instance, for WT: Var(ϵ i,j ) = σ 2 × exp (2 × δ × WT i,j ), where δ is an unknown parameter to be estimated that describes the estimated change in variance with water temperature. Model fitting improvement and comparison between the possible variance covariates were evaluated using the Akaike information criterion (AIC) and likelihood ratio tests ( Pinheiro and Bates, 2000 ). Covariability among predictors was evaluated using variance inflation factors (VIFs).
The values of RUE zp calculated for a day i were also modeled using GLS models that were formulated as follows:
In this case, to match the zooplankton oblique tows, WT was averaged over the water column, and phytoplankton carbon biomass (necessary to calculate RUE zp , see above), S and J were estimated based on the species biomass averaged over the water column. The rest of the parameters and variance model are as described for Equation (1).
To enable comparison of the phytoplankton and zooplankton BEF relationships using taxonomic and functional diversity, RUE pp and RUE zp calculated for a day i were related to phytoplankton S, J, and FD metrics estimated also for a day i , therefore, based on the species biomasses averaged over the water column as explained above for the RUE zp model. To quantify the bivariate relationships, we used reduced major axis (RMA) regression, and we did not include other covariates in these models because biodiversity was the best predictor as observed in the more detailed models (see Results below).
Finally, complementary analyses were further performed. In particular, we confronted the patterns found using a measure of standing stock to quantify biomass production with other alternative forms of calculating RUE pp , that is, calculated sensu Ptacnik et al. (2008) in terms of chlorophyll a (mg m −3 ) per unit of nitrate (μmol L −1 ), and sensu Lehtinen et al. (2017) in terms of primary production (mg C m −3 h −1 ) per unit of nitrate (μmol L −1 ). Primary production was obtained from Bode et al. (2019) . These models were fitted to data covering the whole period, though restricting the phytoplankton species to the 55 taxa that were consistently identified along all three decades ( Supplementary Table 1 ).
All treatment of data and analyses were performed with the software R (version 4.0.2, R Core Team, 2020 ) and using the packages “nlme 3.1-149” ( Pinheiro and Bates, 2000 ), “relaimpo 2.2-3” ( Grömping, 2006 ), “FD 1.0-12” ( Laliberté and Legendre, 2010 ), and “lmodel2 1.7-3” ( Legendre and Legendre, 1998 ).
Phytoplankton Resource-Use Efficiency
The model fitted to phytoplankton resource-use efficiency data (Equation 1, Supplementary Table 2 ) revealed that RUE pp showed a seasonal cycle peaking in late March to early April ( Figure 2A ) and a nonlinear long-term trend over the study period ( Figure 2B ). RUE pp decreased linearly with sampling depth ( Figure 2C ) and was positively related to water temperature ( Figure 2D ) and upwelling index ( Figure 2E ). Furthermore, RUE pp was related to taxonomic diversity scaling positively with phytoplankton richness ( Figure 2F ) and negatively with phytoplankton evenness ( Figure 2G ). Studying the importance of predictors for RUE pp based on the proportional marginal variance decomposition, taxonomic diversity, and richness (61.5%) in particular, was the best predictor in explaining RUE pp dynamics, whereas the environmental factors (WT and UI) played a secondary role ( Supplementary Table 2 ). Including a variance model in the GLS as an exponential function of covariates resulted in better fittings ( Supplementary Table 3 ). More specifically, the spread of RUE pp decreased with evenness that was the most optimal variance covariate. In particular, an increase in 0.2 units of phytoplankton evenness reduced RUE pp variability by 21.4% ( Supplementary Table 3 ). Finally, the RUE pp model did not show any relevant remaining patterns in the residuals ( Supplementary Figure 3 ).
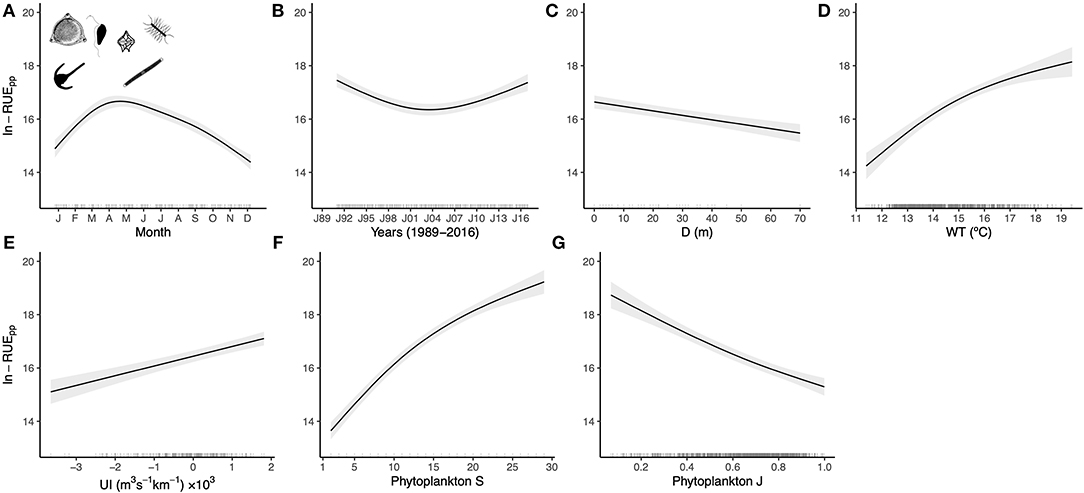
Figure 2 . Partial-effects plots from the RUE pp GLS model depicted in Equation (1). Shown are the seasonal (A) and long-term (B) changes in RUE pp , the trend with depth (C) , and the relationships with water temperature (D) , upwelling index (E) , phytoplankton richness (F) , and phytoplankton evenness (G) . Bands indicate 95% confidence intervals and the rugs along the x-axes display the distribution of the data. See ANOVA table in Supplementary Table 2 and model selection of variance covariates in Supplementary Table 3 . Silhouettes were obtained from http://www.phylopic.org .
Zooplankton Resource-Use Efficiency
The model fitted to zooplankton resource-use efficiency data (Equation 2, Supplementary Table 4 ) revealed that RUE zp showed a seasonal cycle peaking in late March to early April ( Figure 3A ) and a decreasing trend from 2003 onward ( Figure 3B ). RUE zp was positively related to water temperature ( Figure 3 C) and had a nonlinear relationship with the upwelling index ( Figure 3D ). Furthermore, RUE zp was related to phytoplankton taxonomic diversity scaling negatively with richness ( Figure 3E ) and positively with evenness ( Figure 3F ). As for the case of RUE pp , when studying the importance of predictors for RUE zp , taxonomic diversity, and richness (66.6%) in particular, was the best predictor, whereas the environmental factors played a secondary role ( Supplementary Table 4 ). Including a variance model in the GLS as an exponential function of covariates resulted in better fittings ( Supplementary Table 5 ). More specifically, the spread of RUE zp decreased with richness that was the most optimal variance covariate. In particular, an increase in 10 units of phytoplankton richness reduced RUE zp variability by 18.8% ( Supplementary Table 5 ). Finally, the RUE zp model did not show any relevant remaining patterns in the residuals ( Supplementary Figure 4 ).
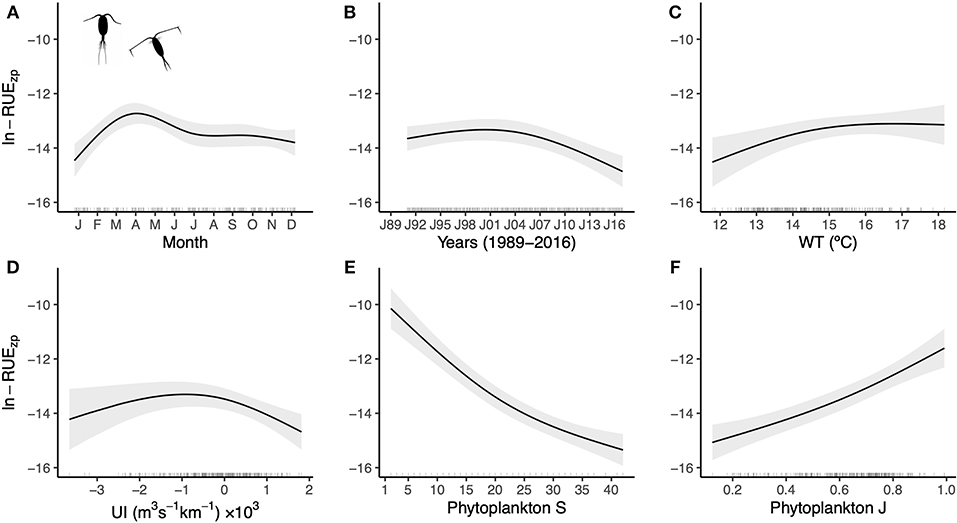
Figure 3 . Partial-effects plots from the RUE zp GLS model depicted in Equation (2). Shown are the seasonal (A) and long-term (B) changes in RUE zp , and the relationships with water temperature (C) , upwelling index (D) , phytoplankton richness (E) , and phytoplankton evenness (F) . Bands indicate 95% confidence intervals, and the rugs along the x-axes display the distribution of the data. See ANOVA table in Supplementary Table 4 , and model selection of variance covariates in Supplementary Table 5 . Silhouettes were obtained from http://www.phylopic.org .
Taxonomic and Functional Diversity Effects on Resource-Use Efficiency
Multitrait-based functional diversity metrics were unrelated, though they showed a certain degree of association with taxonomic diversity, especially between FDis and J, and FGR and S ( Supplementary Figure 5 ). Figures 4 , 5 compare the effects of taxonomic and functional diversity of phytoplankton on RUE pp and RUE zp , respectively. First, both taxonomic ( Figure 4A ) and functional ( Figure 4C ) richness had positive effects on RUE pp , while the effects were negative on RUE zp ( Figures 5A,C ). Second, both taxonomic ( Figure 4B ) and functional ( Figure 4D ) evenness had negative effects on RUE pp , while the effects were positive on RUE zp ( Figures 5B,D ). Finally, the relationship with FDis showed the same trend as for FEve for phytoplankton RUE, where more functionally similar phytoplankton assemblages had higher RUE ( Figure 4E ), and for zooplankton RUE, where zooplankton preying upon more functionally dissimilar phytoplankton assemblages had higher RUE ( Figure 5E ). Regarding the explanatory power, TD metrics had higher explanatory power (i.e., greater R 2 ) compared to FD metrics. Within TD, richness was a better predictor (48 and 47% for RUE pp and RUE zp , respectively), whereas within FD, FEve was a better predictor (29 and 20% for RUE pp and RUE zp , respectively). All slopes were statistically significant ( p < 0.0001), and in all cases, elevated RUE pp values occurred when diatom biomass was higher, while,when RUE zp values were higher, phytoplankton biomass was less dominated by diatoms.
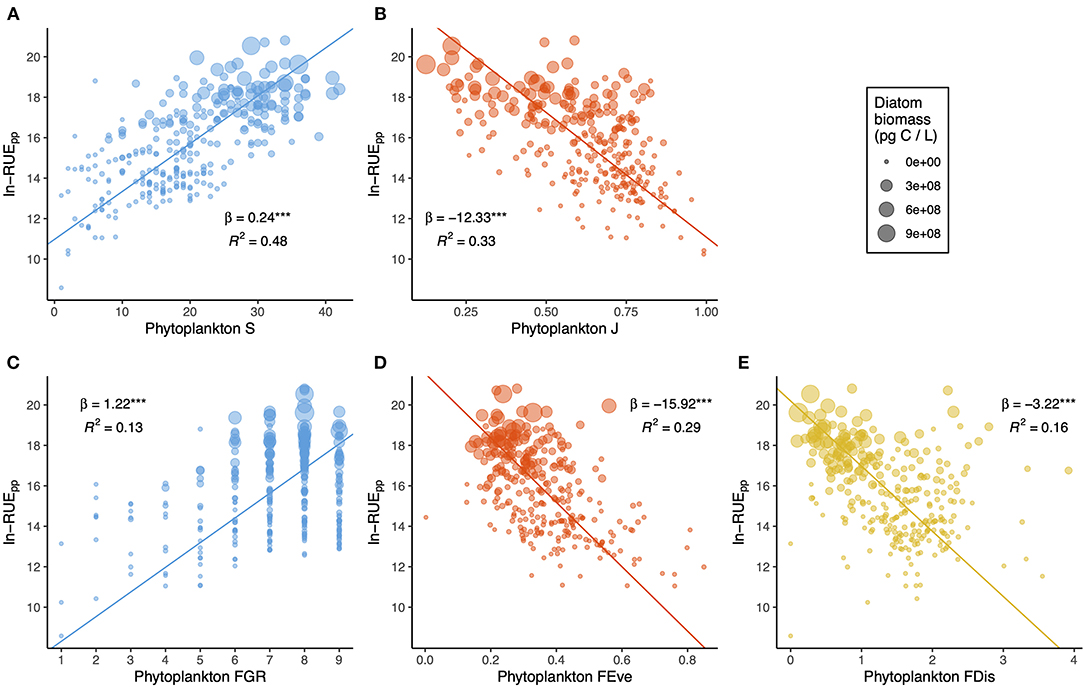
Figure 4 . Bivariate plots showing the effects of phytoplankton taxonomic (A,B) and functional (C–E) diversity on RUE pp . Dot size is scaled to diatom biomass, and lines show reduced major axis (RMA) fits. For each relationship, the slope (and statistical significance with parametric p -value < 0.0001***) and the explanatory power are shown.
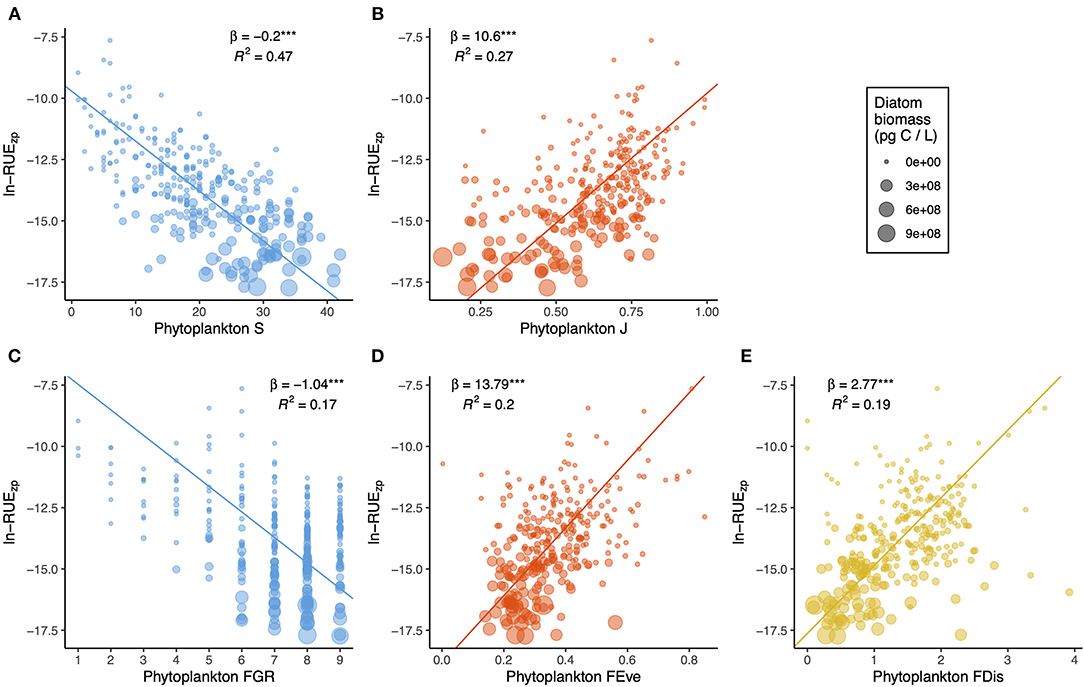
Figure 5 . Bivariate plots showing the effects of phytoplankton taxonomic (A,B) and functional (C–E) diversity on RUE zp . Dot size is scaled to diatom biomass, and lines show RMA fits. For each relationship, the slope (and statistical significance with parametric p -value < 0.0001***) and the explanatory power are shown.
Finally, regarding the phytoplankton single-trait-based indices, three CWMs had important effects on plankton RUE: the ability to use biogenic silica, the ability to swim, and the ability to form chains or colonies ( Figure 6 , Supplementary Table 6 ). For RUE pp , the largest contributor was CWM chain ( Figure 6C ), while CWM motility ( Figure 6B ) and CWM silica ( Figure 6A ) contributed less to explain phytoplankton resource use. For RUE zp , all three CWMs contributed almost equally to zooplankton resource use ( Figures 6D–F ), though the contribution of CWM motility was slightly higher ( Supplementary Table 6 ). RUE pp increased with CWM silica and CWM chain , and decreased with CWM motility . However, the direction of the effects was the opposite for the case of RUE zp .
Figure 6 . Scatterplots showing the relationships between RUE pp (A–C) and RUE zp (D–F) with CWM silica (A,D) , CWM motility (B,E) , and CWM chain (C,F) . Lines show RMA fits, and corresponding numerical data are provided in Supplementary Table 6 .
Complementary Analyses
Models using alternative calculations of RUE pp , namely, with chlorophyll a ( Supplementary Figure 6 ) and primary production ( Supplementary Figure 7 ) and fitted to data covering the whole period though restricting the phytoplankton species to the 55 taxa that were consistently identified along all three decades, resulted roughly in the same patterns as described above using a measure of standing stock to quantify the phytoplankton biomass production.
Phytoplankton and Zooplankton Resource-Use Efficiency
The model for RUE pp showed that more phytoplankton species lead to higher biomass per unit resource. This positive relationship between phytoplankton RUE and richness concurs with earlier observations made in aquatic systems both in the field ( Ptacnik et al., 2008 ) and in experiments ( Striebel et al., 2009a ). At the same time, the model revealed also a negative relationship between evenness and RUE pp , which again agrees well with previous field observations in differing aquatic systems such as is the Wadden Sea, where phytoplankton evenness was the most important driver of productivity and RUE ( Hodapp et al., 2015 ), or in Midwestern US lakes, where phytoplankton RUE was inversely related to phytoplankton evenness ( Filstrup et al., 2014 ). On the other hand, the model for RUE zp showed that zooplankton communities produced the least biomass per unit of phytoplankton biomass when feeding upon species-rich phytoplankton communities but dominated by few species or group of species. Again, this result concurs with previous findings documenting a negative relationship between phytoplankton evenness and the production of zooplankton biomass per unit of phytoplankton biomass in lakes ( Filstrup et al., 2014 ). However, it differs from Filstrup et al. (2019) who found a nonsignificant role of phytoplankton richness in driving zooplankton resource use efficiency in lakes.
In general, two nonexclusive mechanisms have been identified to explain why biodiversity enhances ecosystem function: complementarity, by which more diverse communities use limiting resources more efficiently through niche partitioning or facilitation, and the selection effect, by which more diverse communities are more likely to include a few dominant species with specific traits that drive the ecosystem functioning ( O'Connor and Byrnes, 2014 ). Separating and quantifying these processes can be addressed experimentally ( Loreau and Hector, 2001 ); however, it is rather difficult to differentiate between these two mechanisms in the field. Nevertheless, the positive effect of richness found in our study can be likely explained by the niche complementarity mechanism. This interpretation would be based on the premise that a more diverse community may include more diverse traits such as light and nutrient utilization traits reflecting a better niche differentiation in wavelength utilization and resource use ( Striebel et al., 2009a , b ; Behl et al., 2011 ). This would be important during periods of nutrient enrichment (e.g., upwelling pulses) when more and more variable resources would facilitate the coexistence of a larger number of species. On the other hand, the negative effect of evenness would indicate that certain dominant species, or group of species, would exhibit specific traits that would give them certain physiological advantages allowing for a more efficient exploitation of resources, that is, a selection effect ( Filstrup et al., 2019 ).
The simultaneous action of complementarity and selection effects would probably be associated to the heterogeneous environment that typically characterizes EBUEs. Coastal upwelling systems are highly productive regions where most of the biomass is usually composed of chain-forming diatoms especially during blooms ( Sarthou et al., 2005 ). This fact has been shown also in our study zone where diatoms are the dominant group and assemblages change rapidly between upwelling/relaxation/downwelling phases ( Casas et al., 1997 ). Diatoms are fast growing species capable of maintaining high nutrient uptake rates for longer periods, and exploit typical upwelling-intermittent nutrient pulses more effectively than other taxa of the same size ( Marañón, 2015 ). Dominance primarily reflects the distribution of traits within a community and the identity of the dominant traits, thus this fact has been recognized as an important effect to explain the fate of ecosystem processes because evenness often responds more rapidly to altered environmental constraints than species richness leading to rapid responses in ecosystem functioning ( Hillebrand et al., 2008 ). Given these premises, we suggest that the mechanistic basis for the shift in the effect of phytoplankton richness and evenness would be dependent on the diatom biomass and, more specifically, on the functional traits that diatoms have. This is evidenced by the relationships found with the community-weighted mean traits highlighting that the predominance of traits that characterizes diatom species, specially the ability to form chains and colonies, increases phytoplankton resource use. Furthermore, diatoms show high growth rates relative to their cell volume. However, nutrient traits tend to be similar among taxa for a typical cell size. This indicates that diatoms appear to be adapted to high nutrient conditions as those found within upwelling regions ( Edwards et al., 2012 ). Indeed, phytoplankton species with greater growth rates and, in particular diatoms sampled in our region tend to respond more strongly to increased upwelling ( Otero et al., 2018 ). Concurring with our results, a population growth model fitted to phytoplankton species over an annual cycle pointed out the fundamental importance of the selection effect in driving marine primary productivity ( Cermeño et al., 2016 ).
This effect of diatom dominance would translate up in the food web. For the consumer level, we found lower RUE zp when phytoplankton biomass was dominated by diatoms, which could be likely explained by several factors including the lower impact that mesozooplankton compared to microzooplankton has on phytoplankton (e.g., Fileman and Burkill, 2001 ), the inhibition that diatom exudates can exert on zooplankton grazing (e.g., Malej and Harris, 1993 ), or the reduced impact that copepod grazing has on phytoplankton biomass and production during production peaks compared to upwelling transition periods when phytoplankton biomass is lower, and dinoflagellates are more abundant (e.g., Bode et al., 2003 ). Indeed, when there was a predominance of traits in the community that characterize dinoflagellate species (e.g., ability to swim), zooplankton resource use was higher. Likewise, in lakes, when zooplankton preys upon phytoplankton communities dominated by cyanobacteria, RUE zp decreases because cyanobacteria is a poor food source for zooplankton ( Filstrup et al., 2014 ).
A simultaneous detection of a positive and negative effect of richness and evenness, respectively, on ecosystem function as the one observed here, was shown also by others either in fresh ( Filstrup et al., 2019 ) or marine ( Napoleón et al., 2014 ) waters. However, there are contrasting results on the relative contribution of the two effects, that is, the differences between the strength of the relationships. Overall, our models indicated that taxonomic richness consistently had much stronger effects on both phytoplankton and zooplankton RUE. This result contrasts with what has been observed in lakes, where evenness explained more variance in phytoplankton and zooplankton RUE than richness leading the authors to suggest that it might be the distribution of taxa rather than their number, the main driver of ecosystem function in lakes ( Filstrup et al., 2019 ). Coastal upwelling ecosystems are highly heterogeneous and contain an enormous species richness rapidly changing with the environmental conditions. Such high number of species is probably necessary to maintain the overall productivity of the system and ensures the occurrence of highly productive species. This scenario would lead to both complementarity and selection effects operating simultaneously, with complementarity being stronger when functionally different species coexist (e.g., during the spring bloom) and take advantage of a heterogeneous resource supply, whereas selection effects would be important when a broad trait space coincides with a more homogeneous landscape ( Hodapp et al., 2016 ). Nonetheless, taken altogether, our results suggest that the opposing effects of primary producer biodiversity on phytoplankton and zooplankton resource use efficiency would be of similar nature in the ocean and freshwater environment. However, there appear to be differences on the sensitivity of ecosystem functioning to richness and evenness that could be ascribed to intrinsic differences among systems, such as changes in hydrographic and biogeochemical characteristics, or the identity of the dominant taxa, or to other underlying mechanisms demanding further research.
Environmental Effects on Resource-Use Efficiency
Apart from the importance of the environmental conditions in determining the overall composition of the community outlined above, we detected direct abiotic effects on RUE and time-varying patterns. In particular, our statistical models showed an annual peak located in April for both phytoplankton and zooplankton RUE coinciding with the spring bloom ( Casas et al., 1997 ). Moreover, there were somehow an interannual inverse pattern between RUE pp and RUE zp that would point to changes in the plankton community along years. In this regard, the studied system displayed phase shifts in plankton community structure around the turn of the 21st century following changes in climate and local hydrography ( Bode et al., 2020 ). The models also revealed the influence of water temperature and upwelling intensity on RUE, albeit less pronounced than the effects of biodiversity. These effects were weak for RUE zp and especially apparent for RUE pp . Resource use efficiency had greater values when upwelling intensity was high and waters were warmer. This combination of high upwelling during the 15 days previous to the sampling date and high water temperature during the sampling date indicates the typical succession of intense upwelling followed by wind relaxation. The positive effect of upwelling contributes to fertilize the system, which promotes a proliferation of phytoplankton biomass and a more efficient use of resources during the subsequent upwelling relaxation (e.g., Huete-Ortega et al., 2010 ). The effect of temperature could also be explained because this variable favors cell growth. Besides that, RUE pp decreased with depth likely associated to the decrease in phytoplankton biomass in deeper and less illuminated waters. Therefore, the abiotic environment was found to play a role in explaining part of the variability of RUE; however, its effect was minor compared to those of biodiversity. Recent meta-analyses showed that the effects of abiotic and biotic drivers in mediating ecosystem properties can overlap or be even stronger than the effects of species loss in controlled experiments ( Godbold, 2012 ). However, Ptacnik et al. (2008) showed in the field that the relative importance of abiotic drivers vs. that of richness was minor in explaining phytoplankton RUE dynamics, thus agreeing with our results. Moreover, Hodapp et al. (2015) identified also a weaker effect of temperature and light compared to evenness on phytoplankton productivity.
Biodiversity Effects on Resource-Use Efficiency Variability
Our statistical models also showed that phytoplankton and zooplankton RUE were both less variable at higher levels of diversity, evenness in the case of RUE pp , and richness in the case of RUE zp . These results indicate that RUE pp and RUE zp would be stabilized when phytoplankton biomass is more evenly distributed, and when zooplankton preys upon richer phytoplankton communities, respectively. In general, theoretical, experimental, and field studies conclude that temporal stability of ecosystem processes would be greater at higher diversity, usually in the form of species numbers, and that those processes would be simultaneously enhanced also at higher levels of diversity ( Tilman et al., 2014 ). Our variance models do not explicitly account for the temporal scale, but we can interpret the fact that biodiversity increases RUE stability as a consequence of that; first, more diverse assemblages are more likely to include different species maintaining the ecosystem function under different environmental conditions (i.e., the insurance hypothesis), and second, more diverse assemblages may show increased asynchrony in species' abundances (i.e., the portfolio effect) favoring the stability of RUE ( Thibaut and Connolly, 2013 ). Furthermore, contrary to expectations, our results showed that the highest levels of RUE were not associated with the lowest levels of variability. This would be in line with recent synthesis of multiple experiments, which concluded that, while biodiversity of primary producers simultaneously increases both the production and stability of biomass in terrestrial and aquatic ecosystems, these effects are independent, suggesting that an ecosystem process and its variability would not necessarily reach the highest levels simultaneously with biodiversity ( Cardinale et al., 2013 ).
Taxonomic vs. Functional Diversity Effects on Resource-Use Efficiency
Our analyses included the evaluation of the performance of three FD metrics resulting in the same patterns as their TD counterparts. This is in line with other works that pointed out the validity and potential of using phytoplankton functional traits to explain community assembly and ecosystem processes both in fresh (e.g., Leruste et al., 2018 ) and marine (e.g., Breton et al., 2017 ) waters. However, our results showed that FD metrics were slightly poorer predictors of RUE than TD metrics. This observation differs from what has been previously shown by other comparative studies undertaken in fresh ( Abonyi et al., 2018 ) and marine ( Ye et al., 2019 ) waters where FD usually outperforms TD in predicting ecosystem functioning. This discrepancy could be explained in part because the set of traits included in our analysis is insufficient and does not fully capture the fine variability along the species-specific ecological niches. For instance, quantitative light utilization traits and nutrient utilization traits are determinant for species-specific metabolism; thus, both functions, efficient use of light and nitrogen acquisition, might not be well represented reducing the percentage of variance of RUE explained by FD. Functional traits are typically sourced from published laboratory experiments, usually not available for all studied species (as would be the case here) but for the most common species, thus preventing a thorough examination of FD effects on RUE for this particular upwelling ecosystem. Apart from the comparison in explanatory power between TD and FD, it is worth mentioning that, in contrast to TD, FD evenness outperformed FD richness, which was a poor predictor, suggesting that FEve captures better the distribution of species across the functional trait space and its effect on RUE. Furthermore, the observed effects of FDis highlight that an increase in functional similarity of phytoplankton increases phytoplankton production per unit of nitrate, whereas at the same time, zooplankton biomass per phytoplankton biomass was higher when phytoplankton assemblages were more functionally dissimilar. This would reinforce the hypotheses that a selection effect would occur when trait dissimilarity was low in diatom-dominated assemblages inducing greater phytoplankton RUE and lower zooplankton RUE. In line with this, Cadotte (2017) using experimental plant assemblages found opposing relationships between selection (negative) and complementarity (positive) effects with functional dispersion.
Study Limitations
Our study has some limitations. On the one hand, we have not accounted for potential cell size changes through time that might bias biomass calculations as size is expected to decrease when temperature increases ( Morán et al., 2010 ). On the other hand, and more importantly, we acknowledge that we have worked with an incomplete number of species. It is known that conventional sampling methods may underestimate phytoplankton diversity ( Cermeño et al., 2014 ); however, we used a very specific assemblage that made the dataset more homogeneous along the time series. This assemblage was indeed representative as shown by the relationship between chlorophyll a and phytoplankton carbon, and covered the most abundant species occurring in this region in each recognized upwelling/relaxation/downwelling phase ( Casas et al., 1997 ). Furthermore, this assemblage was able to explain the variability in phytoplankton and zooplankton RUE calculated in terms of chlorophyll a . Additionally, our definition of RUE was based on the quantification of biomass production using a measure of standing stock thus not accounting for gross production; nonetheless, using a direct measure of primary production (secondary production was not available), we also found comparable patterns in the dynamics of RUE pp . These complementary analyses suggest that the patterns found are robust. Therefore, we believe that, given the consistency of the sampling methods along the three decades, and the comparable results obtained using different RUE calculations, the facts outlined above could have some influence on the effect size of our models, for instance, by minimizing the effect of rare species, though not on the direction of the effects. Finally, we assumed here a single resource limitation (nitrate); thus, further investigations are needed to explore the importance of colimitation or multiple resource limitation ( Hodapp et al., 2019 ) following the dynamics of upwelling.
Conclusions
We found that taxonomic and functional diversity enhance planktonic resource use efficiency in a coastal upwelling system. The sign of this relationship differed with the components of diversity considered (richness vs. evenness) and had opposite patterns across trophic levels. These effects can be likely explained through a simultaneous complementarity effect, by which more diverse assemblages with more diverse functional traits use resources more efficiently, and a selection effect through the dominance of diatoms growing rapidly and exploiting typical upwelling-intermittent nutrient pulses more effectively than other taxa of similar cell size. Additionally, resource use efficiency was less variable at higher levels of diversity; however, greater stability was not associated with the highest levels of RUE. Our results contribute to the understanding of phytoplankton diversity effects on ecosystem processes underpinning the differing effects of two contrasting biodiversity metrics and how these relationships vary across adjacent trophic levels in a coastal upwelling area. Incorporation of this approach to the observation, experimentation, and modeling of plankton ecology in EBUEs will allow gaining understanding on the important processes occurring in these productive coastal ecosystems and how these processes are mediated by biodiversity.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.1594/PANGAEA.908815 ; https://doi.org/10.1594/PANGAEA.885413 .
Author Contributions
JO designed the project. AB provided the data. JO analyzed the data with assists from AB and XÁ-S. JO wrote the manuscript with comments from co-authors. All authors contributed to the article and approved the submitted version.
This work is part of the time series project RADIALES conducted and funded by the Instituto Español de Oceanografía ( http://www.seriestemporales-ieo.net ) with additional support from project MarRisk (Interreg POCTEP Spain-Portugal) grant number 0262 MARRISK 1 E, from grants Contrato-Programa GAIN-IEO, and grant number IN607A2018/2 of the Axencia Galega de Innovación (GAIN, Xunta de Galicia, Spain). JO was supported by a Junta para la Ampliación de Estudios Fellowship (JAE-Doc programme 2011) from the CSIC and ESF.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This article is dedicated to the memory of Dr. Manuel Varela, respected friend and colleague, who passed away on October 24, 2019. We would like to acknowledge the dedication of a large number of technicians, crew members, and scientists who contributed to the observational time series project RADIALES. We are particularly indebted to M. Varela (phytoplankton) and M. T. Álvarez-Ossorio (zooplankton) for maintaining the plankton series for more than 20 years. J. Lorenzo continued the phytoplankton counts since 2011, and E. Rey and M. A. Louro ensured the continuity of the zooplankton series. Nutrient data were provided by N. González and R. Carballo (1989–2012) and by M. Álvarez and M. Castaño (2013–2016).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.592255/full#supplementary-material
Abonyi, A., Horváth, Z., and Ptacnik, R. (2018). Functional richness outperforms taxonomic richness in predicting ecosystem functioning in natural phytoplankton communities. Fresh. Biol. 63, 178–186. doi: 10.1111/fwb.13051
CrossRef Full Text | Google Scholar
Álvarez-Salgado, X. A., Beloso, S., Joint, I., Nogueira, E., Chou, L., Pérez, F., et al. (2002). New production of the NW Iberian shelf during the upwelling season over the period 1982–1999. Deep Sea Res. I 49, 1725–1739. doi: 10.1016/S0967-0637(02)00094-8
Álvarez-Salgado, X. A., Castro, C. G., Pérez, F. F., and Fraga, F. (1997). Nutrient mineralization patterns in shelf waters of the Western Iberian upwelling. Cont. Shelf Res . 17, 1247–1270. doi: 10.1016/S0278-4343(97)00014-9
Behl, S., Donval, A., and Stibor, H. (2011). The relative importance of species diversity and functional group diversity on carbon uptake in phytoplankton communities. Limnol. Oceanogr. 56, 683–694. doi: 10.4319/lo.2011.56.2.0683
Bode, A. (2019). Monthly Series of Phytoplankton and Zooplankton Abundance as Well as Biomass Collected Between 1989 and 2016 at A Coruña (NW Spain) . PANGEA. Available online at: https://doi.pangaea.de/10.1594/PANGAEA.908815
Bode, A., Álvarez, M., García-García, L. M., Louro, M. A., Nieto-Cid, M., Ruíz-Villarreal, M., et al. (2020). Climate and local hydrography underlie recent regime shifts in plankton communities off Galicia (NW Spain). Oceans 1, 181–197. doi: 10.3390/oceans1040014
Bode, A., Álvarez, M., Ruíz-Villarreal, M., and Varela, M. (2018). Time Series of Hydrographic, Chemical and Primary Production Variables for a Shelf Station off A Coruña (NW Spain): 1989–2016 . PANGEA. Available online at: https://doi.pangaea.de/10.1594/PANGAEA.885413
Bode, A., Álvarez, M., Ruíz-Villarreal, M., and Varela, M. (2019). Changes in phytoplankton production and upwelling intensity off A Coruña (NW Spain) for the last 28 years. Ocean Dynam. 69, 861–873. doi: 10.1007/s10236-019-01278-y
Bode, A., Álvarez-Ossorio, M. T., Barquero, S., Lorenzo, J., Louro, A., and Varela, M. (2003). Seasonal variations in upwelling and in the grazing impact of copepods on phytoplankton off A Coruña (Galicia, NW Spain). J. Exp. Mar. Biol. Ecol. 297, 85–105. doi: 10.1016/S0022-0981(03)00370-8
Bode, A., Álvarez-Ossorio, M. T., Cabanas, J. M., Miranda, A., and Varela, M. (2009). Recent trends in plankton and upwelling intensity off Galicia (NW Spain). Prog. Oceanogr. 83, 342–350. doi: 10.1016/j.pocean.2009.07.025
Bode, A., Álvarez-Ossorio, M. T., Miranda, A., López-Urrutia, A., and Valdés, L. (2012). Comparing copepod time-series in the north of Spain: spatial autocorrelation of community composition. Prog. Oceanogr. 97/100, 108–119. doi: 10.1016/j.pocean.2011.11.013
Bode, A., Estévez, M. G., Varela, M., and Vilar, J. A. (2015). Annual trend patterns of phytoplankton species abundance belie homogeneous taxonomical group responses to climate in the NE Atlantic upwelling. Mar. Env. Res. 110, 81–91. doi: 10.1016/j.marenvres.2015.07.017
PubMed Abstract | CrossRef Full Text | Google Scholar
Breton, E., Christaki, U., Bonato, S., Didry, M., and Artigas, L. F. (2017). Functional trait variation and nitrogen use efficiency in temperate coastal phytoplankton. Mar. Ecol. Prog. Ser. 563, 35–49. doi: 10.3354/meps11974
Cadotte, M. W. (2017). Functional traits explain ecosystem function through opposing mechanisms. Ecol. Lett . 20, 989–996. doi: 10.1111/ele.12796
Cardinale, B. J., Duffy, J. E., Gonzalez, A., Hooper, D. U., Perrings, C., and Venail, P. (2012). Biodiversity loss and its impacts on humanity. Nature 486, 59–67. doi: 10.1038/nature11148
Cardinale, B. J., Gross, K., Fritschie, K., Flombaum, P., Fox, J. W., Rixen, C., et al. (2013). Biodiversity simultaneously enhances the production and stability of community biomass, but the effects are independent. Ecology 94, 1697–1707. doi: 10.1890/12-1334.1
Casas, B., Varela, M., Canle, M., González, N., and Bode, A. (1997). Seasonal variations of nutrients, seston and phytoplankton, and upwelling intensity off La Coruña (NW Spain). Estuar. Coast. Shelf Sci. 44, 767–778. doi: 10.1006/ecss.1996.0155
Cermeño, P., Chouciño, P., Fernández-Castro, B., Figueiras, F. G., Marañón, E., Marrasé, C., et al. (2016). Marine primary productivity is driven by a selection effect. Front. Mar. Sci. 3:173. doi: 10.3389/fmars.2016.00173
Cermeño, P., Rodríguez-Ramos, T., Dornelas, M., Figueiras, F. G., Marañón, E., Teixeira, I. G., et al. (2013). Species richness in marine phytoplankton communities is not correlated to ecosystem productivity. Mar. Ecol. Prog. Ser. 488, 1–9. doi: 10.3354/meps10443
Cermeño, P., Teixeira, I. G., Branco, M., Figueiras, F. G., and Marañón, E. (2014). Sampling the limits of species richness in marine phytoplankton communities. J. Plank. Res. 36, 1135–1139. doi: 10.1093/plankt/fbu033
Chassot, E., Bonhommeau, S., Dulvy, N. K., Mélin, F., Watson, R., Gascuel, D., et al. (2010). Global marine primary production constrains fisheries catches. Ecol. Lett. 13, 495–505. doi: 10.1111/j.1461-0248.2010.01443.x
Chavez, F. P., Messié, M., and Pennington, J. T. (2011). Marine primary production in relation to climate variability and change. Annu. Rev. Mar. Sci. 3, 227–260. doi: 10.1146/annurev.marine.010908.163917
PubMed Abstract | CrossRef Full Text
Craven, D., Eisenhauer, N., Pearse, W. D., Hautier, Y., Roscher, C., Boenisch, A. H., et al. (2018). Multiple facets of biodiversity drive the diversity-stability relationship. Nat. Ecol. Evol. 2, 1579–1587. doi: 10.1038/s41559-018-0647-7
Cusson, M., Crowe, T. P., Araújo, R., Arenas, F., Aspden, R., Bulleri, F., et al. (2015). Relationships between biodiversity and the stability of marine ecosystems: comparisons at a European scale using meta-analysis. J. Sea Res. 98, 5–14. doi: 10.1016/j.seares.2014.08.004
De Vargas, C., Audic, S., Henry, N., Decelle, J., Mahé, F., Logares, R., et al. (2015). Eukaryotic plankton diversity in the sunlit ocean. Science 348:1261605. doi: 10.1126/science.1261605
Duffy, J. E., Cardinale, B. J., France, K. E., McIntyre, P. B., Thébault, E., and Loreau, M. (2007). The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol. Lett. 10, 522–538. doi: 10.1111/j.1461-0248.2007.01037.x
Duffy, J. E., Godwin, C. M., and Cardinale, B. J. (2017). Biodiversity effects in the wild are common and as strong as key drivers of productivity. Nature 549, 261–264. doi: 10.1038/nature23886
Edwards, K. F., Thomas, M. K., Klausmeier, C. A., and Litchman, E. (2012). Allometric scaling and taxonomic variation in nutrient utilization traits and maximum growth rate of phytoplankton. Limnol. Oceanogr. 57, 554–566. doi: 10.4319/lo.2012.57.2.0554
Fileman, E., and Burkill, P. (2001). The herbivorous impact of microzooplankton during two short-term Lagrangian experiments off the NW coast of Galicia in summer 1998. Prog. Oceanog. 51, 361–383. doi: 10.1016/S0079-6611(01)00075-1
Filstrup, C. T., Hillebrand, H., Heathcote, A. J., Harpole, W. S., and Downing, J. A. (2014). Cyanobacteria dominance influences resource use efficiency and community turnover in phytoplankton and zooplankton communities. Ecol. Lett. 17, 464–474. doi: 10.1111/ele.12246
Filstrup, C. T., King, K. B. S., and McCullough, I. M. (2019). Evenness effects mask richness effects on ecosystem functioning at macro-scales in lakes. Ecol. Lett. 22, 2120–2129. doi: 10.1111/ele.13407
García, F. C., Bestion, E., Warfield, R., and Yvon-Durocher, G. (2018). Changes in temperature alter the relationship between biodiversity and ecosystem functioning. Proc. Natl. Acad. Sci. U.S.A. 115, 10989–10994. doi: 10.1073/pnas.1805518115
García-Palacios, P., Gross, N., Gaitán, J., and Maestre, F. T. (2018). Climate mediates the biodiversity-ecosystem stability relationship globally. Proc. Natl. Acad. Sci. U.S.A. 115, 8400–8405. doi: 10.1073/pnas.1800425115
Godbold, J. A. (2012). “Effects of biodiversity-environment conditions on the interpretation of biodiversity-function relations,” in Marine Biodiversity and Ecosystem Functioning: Frameworks, Methodologies, and Integration , eds. M. Solan, R. J. Aspden, and D. M. Paterson (Oxford: Oxford University Press), 101–114.
Google Scholar
Goebel, N. L., Edwards, C. A., Zehr, J. P., Follows, M. J., and Morgan, S. G. (2013). Modeled phytoplankton diversity and productivity in the California Current System. Ecol. Model. 264, 37–47. doi: 10.1016/j.ecolmodel.2012.11.008
González-Nuevo, G., Gago, J., and Cabanas, J. M. (2014). Upwelling index: a powerful tool for marine research in the NW Iberian upwelling system. J. Operat. Oceanogr. 7, 45–55. doi: 10.1080/1755876X.2014.11020152
Grasshoff, K., Erhardt, M., and Kremling, K. (1983). Methods of Seawater Analysis, 2nd Edn . Weinheim: Verlag Chemie.
Grömping, U. (2006). Relative importance for linear regression in R: The package relaimpo. J. Stat. Soft. 17:1. doi: 10.18637/jss.v017.i01
Hillebrand, H., Bennett, D. M., and Cadotte, M. W. (2008). Consequences of dominance: a review of evenness effects on local and regional ecosystem processes. Ecology 89, 1510–1520. doi: 10.1890/07-1053.1
Hillebrand, H., Langenheder, S., Lebret, K., Lindström, E., Östman, Ö., and Striebel, M. (2018). Decomposing multiple dimensions of stability in global change experiments. Ecol. Lett. 21, 21–30. doi: 10.1111/ele.12867
Hodapp, D., Hillebrand, H., Blasius, B., and Ryabov, A. B. (2016). Environmental and trait variability constrain community structure and the biodiversity-productivity relationship. Ecology 97, 1463–1474. doi: 10.1890/15-0730.1
Hodapp, D., Hillebrand, H., and Striebel, M. (2019). “Unifying” the concept of resource use efficiency in ecology. Front. Ecol. Evol. 6:233. doi: 10.3389/fevo.2018.00233
Hodapp, D., Meier, S., Muijsers, F., Badewien, T. H., and Hillebrand, H. (2015). Structural equation modeling approach to the diversity–productivity relationship of Wadden Sea phytoplankton. Mar. Ecol. Prog. Ser. 523, 31–40. doi: 10.3354/meps11153
Huete-Ortega, M., Marañón, E., Varela, M., and Bode, A. (2010). General patterns in the size scaling of phytoplankton abundance in coastal waters during a 10-year time series. J. Plank. Res. 32, 1–14. doi: 10.1093/plankt/fbp104
Irwin, A. J., and Finkel, Z. V. (2018). “Phytoplankton functional types: a trait perspective,” in Microbial Ecology of the Oceans , eds J. M. Gasol and D. L. Kirchman (Hoboken, NJ: John Wiley & Sons), 435–465.
Ives, A. R., and Carpenter, S. R. (2007). Stability and diversity of ecosystems. Science 317, 58–62. doi: 10.1126/science.1133258
Klais, R., Norros, V., Lehtinen, S., Tamminen, T., and Olli, K. (2017). Community assembly and drivers of phytoplankton functional structure. Funct. Ecol. 31, 760–767. doi: 10.1111/1365-2435.12784
Korhonen, J. J., Wang, J., and Soininen, J. (2011). Productivity-diversity relationship in lake plankton communities. PLoS ONE 6:e22041. doi: 10.1371/journal.pone.0022041
Laliberté, E., and Legendre, P. (2010). A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305. doi: 10.1890/08-2244.1
Legendre, P., and Legendre, L. (1998). Numerical Ecology, 2nd Edn . Amsterdam: Elsevier Science.
Lehtinen, S., Tamminen, T., Ptacnik, R., and Andersen, T. (2017). Phytoplankton species richness, evenness, and production in relation to nutrient availability and imbalance. Limnol. Oceanogr. 62, 1393–1408. doi: 10.1002/lno.10506
Leruste, A., Villéger, S., Malet, N., De Wit, R., and Bec, B. (2018). Complementarity of the multidimensional functional and the taxonomic approaches to study phytoplankton communities in three Mediterranean coastal lagoons of different trophic status. Hydrobiologia 815, 207–227. doi: 10.1007/s10750-018-3565-4
Litchman, E., and Klausmeier, C. A. (2008). Trait-based community ecology of phytoplankton. Annu. Rev. Ecol. Evol. Syst. 39, 615–639. doi: 10.1146/annurev.ecolsys.39.110707.173549
Loreau, M., and de Mazancourt, C. (2013). Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol. Lett . 16, 106–115. doi: 10.1111/ele.12073
Loreau, M., and Hector, A. (2001). Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76. doi: 10.1038/35083573
Lund, J. W. G., Kipling, C., and Le Cren, E. D. (1958). The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 11, 143–170. doi: 10.1007/BF00007865
Malej, A., and Harris, R. P. (1993). Inhibition of copepod grazing by diatom exudates: a factor in the development of mucus aggregates? Mar. Ecol. Prog. Ser. 96, 33–42. doi: 10.3354/meps096033
Marañón, E. (2015). Cell size as a key determinant of phytoplankton metabolism and community structure. Annu. Rev. Mar. Sci. 7, 241–264. doi: 10.1146/annurev-marine-010814-015955
Morán, X. A., López-Urrutia, Á., Calvo-Díaz, A., and Li, W. K. W. (2010). Increasing importance of small phytoplankton in a warmer ocean. Glob. Chan. Biol. 16, 1137–1144. doi: 10.1111/j.1365-2486.2009.01960.x
Mouchet, M. A., Villéger, S., Mason, N. W. H., and Mouillot, D. (2010). Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol . 24, 867–876. doi: 10.1111/j.1365-2435.2010.01695.x
Naeem, S. (2012). “Ecological consequences of declining biodiversity: a biodiversity-ecosystem function (BEF) framework for marine systems,” in Marine Biodiversity and Ecosystem Functioning: Frameworks, Methodologies, and Integration , eds M. Solan, R. J. Aspden, and D. M. Paterson (Oxford: Oxford University Press), 34–51.
Napoleón, C., Fiant, L., Raimbault, V., Riou, P., and Claquin, P. (2014). Dynamics of phytoplankton diversity structure and primary productivity in the English channel. Mar. Ecol. Prog. Ser. 505, 49–64. doi: 10.3354/meps10772
Neveux, J., and Panouse, M. (1987). Spectrofluorimetric determination of chlorophylls and pheophytins. Arch. Hydrobiol. 109, 567–581.
O'Connor, M. I., and Byrnes, J. E. K. (2014). “Biodiversity and ecosystem function: Does pattern influence process?,” in Marine Community Ecology and Conservation , eds M. D. Bertness, J. F. Bruno, B. R. Silliman, and J. J. Stachowicz (Sunderland, MA: Sinauer Associates, Inc.), 107–130.
Olli, K., Ptacnik, R., Andersen, T., Trikk, O., Klais, R., Lehtinen, S., et al. (2014). Against the tide: recent diversity increase enhances resource use in a coastal ecosystem. Limnol. Oceanogr. 59, 267–274. doi: 10.4319/lo.2014.59.1.0267
Otero, J., Bode, A., Álvarez-Salgado, X. A., and Varela, M. (2018). Role of functional trait variability in the response of individual phytoplankton species to changing environmental conditions in a coastal upwelling zone. Mar. Ecol. Prog. Ser. 596, 33–47. doi: 10.3354/meps12542
Parsons, T. R., Maita, Y., and Lalli, C. M. (1984). A Manual of Chemical and Biological Methods for Seawater Analysis . Oxford: Pergamon Press.
Pielou, E. C. (1966). The measurement of diversity in different types of biological collections. J. Theor. Biol. 13, 131–144. doi: 10.1016/0022-5193(66)90013-0
Pinheiro, J. C., and Bates, D. M. (2000). Mixed-Effects Models in S and S-Plus . New York, NY: Springer.
Ptacnik, R., Solimini, A. G., Andersen, T., Tamminen, T., Brettum, P., Lepistö, L., et al. (2008). Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proc. Natl. Acad. Sci. U.S.A. 105, 5134–5138. doi: 10.1073/pnas.0708328105
R Core Team (2020). “R: A language and environment for statistical computing,” in R Foundation for Statistical Computing (Vienna). Available online at: http://R-project.org (accessed October 30, 2020).
Richardson, A. J., and Schoeman, D. S. (2004). Climate impact on plankton ecosystems in the Northeast Atlantic. Science 305, 1609–1612. doi: 10.1126/science.1100958
Sarker, S., Lemke, P., and Wiltshire, K. H. (2018). Does ecosystem variability explain phytoplankton diversity? Solving an ecological puzzle with long-term data sets. J. Sea Res. 135, 11–17. doi: 10.1016/j.seares.2018.02.002
Sarthou, G., Timmermans, K. R., Blain, S., and Tréguer, P. (2005). Growth physiology and fate of diatoms in the ocean: a review. J. Sea Res. 53, 25–42. doi: 10.1016/j.seares.2004.01.007
Schabhüttl, S., Hingsamer, P., Weigelhofer, G., Hein, T., Weigert, A., and Striebel, M. (2013). Temperature and species richness effects in phytoplankton communities. Oecologia 171, 527–536. doi: 10.1007/s00442-012-2419-4
Shurin, J. B., Arnott, S. E., Hillebrand, H., Longmuir, A., Pinel-Alloul, B., Winder, M., et al. (2007). Diversity-stability relationship varies with latitude in zooplankton. Ecol. Lett. 10, 127–134. doi: 10.1111/j.1461-0248.2006.01009.x
Stachowicz, J. J., Bruno, J. F., and Duffy, J. E. (2007). Understanding the effects of marine biodiversity on communities and ecosystems. Annu. Rev. Ecol. Evol. Syst. 38, 739–766. doi: 10.1146/annurev.ecolsys.38.091206.095659
Striebel, M., Behl, S., Diehl, S., and Stibor, H. (2009b). Spectral niche complementarity and carbon dynamics in pelagic ecosystems. Am. Nat . 174, 141–147. doi: 10.1086/599294
Striebel, M., Behl, S., and Stibor, H. (2009a). The coupling of biodiversity and productivity in phytoplankton communities: consequences for biomass stoichiometry. Ecology 90, 2025–2031. doi: 10.1890/08-1409.1
Striebel, M., Singer, G., Stibor, H., and Andersen, T. (2012). “Trophic overyielding”: Phytoplankton diversity promotes zooplankton productivity. Ecology 93, 2719–2727. doi: 10.1890/12-0003.1
Thibaut, L., and Connolly, S. R. (2013). Understanding diversity-stability relationships: towards a unified model of portfolio effects. Ecol. Lett. 16, 140–150. doi: 10.1111/ele.12019
Tilman, D., Isbell, F., and Cowles, J. M. (2014). Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471–493. doi: 10.1146/annurev-ecolsys-120213-091917
UNESCO (1986). “Progress in oceanographic tables and standards 1983–1986, work and recommendations of the UNESCO/SCOR/ICES/IAPSO joint panel,” in UNESCO Technical Papers in Marine Science 50 (Paris: Mar Inf Cent, Div Mar Sci, UNESCO).
Vallina, S. M., Cermeño, P., Dutkiewicz, S., Loreau, M., and Montoya, J. M. (2017). Phytoplankton functional diversity increases ecosystem productivity and stability. Ecol. Model . 361, 184–196. doi: 10.1016/j.ecolmodel.2017.06.020
van der Plas, F. (2019). Biodiversity and ecosystem functioning in naturally assembled communities. Biol. Rev. 94, 1220–1245. doi: 10.1111/brv.12499
Villéger, S., Mason, N. W. H., and Mouillot, D. (2008). New multidimensional functional diversity indices for multifaceted framework in functional ecology. Ecology 89, 2290–2301. doi: 10.1890/07-1206.1
Wiltshire, K. H., Boersma, M., Carstens, K., Kraberg, A. C., Peters, S., and Scharfe, M. (2015). Control of phytoplankton in a shelf sea: determination of the main drivers based on the Helgoland Roads Time Series. J. Sea Res. 105, 42–52. doi: 10.1016/j.seares.2015.06.022
Ye, L., Chang, C. W., Matsuzaki, S. I. S., Takamura, N., Widdicombe, C. E., and Hsieh, C. H. (2019). Functional diversity promotes phytoplankton resource use efficiency. J. Ecol. 107, 2353–2363. doi: 10.1111/1365-2745.13192
Keywords: coastal upwelling, stability, functional diversity, taxonomic diversity, zooplankton, phytoplankton, nutrients, resource use efficiency
Citation: Otero J, Álvarez-Salgado XA and Bode A (2020) Phytoplankton Diversity Effect on Ecosystem Functioning in a Coastal Upwelling System. Front. Mar. Sci. 7:592255. doi: 10.3389/fmars.2020.592255
Received: 06 August 2020; Accepted: 20 October 2020; Published: 26 November 2020.
Reviewed by:
Copyright © 2020 Otero, Álvarez-Salgado and Bode. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaime Otero, jotero@iim.csic.es
† Present address: Jaime Otero, Centro Oceanográfico de Vigo, Instituto Español de Oceanografía, Vigo, Spain
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Zooplankton Diversity and Their Spatiotemporal Distribution: An Ecological Assessment from a Brackish Coastal Lagoon, Chilika, Odisha
- First Online: 04 December 2021
Cite this chapter

- Suchismita Srichandan 5 nAff6 &
- Gurdeep Rastogi 5
Part of the book series: Coastal Research Library ((COASTALRL,volume 38))
720 Accesses
Zooplankton constitutes a pivotal component in the pelagic food webs and serves as the major source of fish diet, thereby determining the productivity of coastal fisheries. Therefore, understanding zooplankton diversity and their ecology in coastal lagoon settings is a high priority research area. We examined the spatiotemporal distribution of zooplankton diversity (size >120 μm) in relation to environmental variables in Chilika lagoon. The sampling was conducted on the monthly frequency from July 2012 to June 2016 from 13 locations and identified a total of 186 zooplankton taxa which included 131 as first record from the Chilika lagoon. To date, a total inventory of 263 species of holoplankton represented by 16 diverse categories of organisms, namely, Ciliophora (51), Foraminifera (13), Tubulinea (5), Rotifera (42), Hydrozoa (1), Ctenophora (1), Nematoda (1), Polychaeta (3), Gastropoda (12), Bivalvia (5), Cladocera (13), Copepoda (95), Ostracoda (4), Malacostraca (13), Chaetognatha (2), Chordata (2), and 23 types of meroplankton were identified. Chilika lagoon exhibited a significant variation in salinity (0–35.5) at spatiotemporal scale and consisted of marine, brackish, and freshwater zooplankton along the estuarine salinity gradient. Copepods emerged as one of the most dominant and diverse zooplankton group in terms of species richness, abundance, and widespread distribution. Among the four orders of Copepoda (i.e., Calanoida, Cyclopoida, Harpacticoida, and Poecilostomatoida), Calanoida was the most abundant one. An important component of total zooplankton pool, i.e., microzooplankton (20–200 μm), was also examined in relation to environmental variables. Ciliophora dominated the microzooplankton community followed by copepod nauplii and Rotifera, except in the freshwater zone of the lagoon. Foraminifera, cirripede nauplii, gastropod veliger, and bivalve veliger were minor contributors in microzooplankton. Salinity and phytoplankton abundances were the major factors influencing microzooplankton community composition. The present study highlighted the necessity of a long-term systematic monitoring of zooplankton diversity and composition in Chilika lagoon.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

Zooplankton Community in the Boka Kotorska Bay

Zooplankton in Montenegrin Adriatic Offshore Waters

Zooplankton in the Huangyan Atoll, South China Sea: A comparison of community structure between the lagoon and seaward reef slope
Abdel-Aziz NE, Aboul Ezz SM, Abou Zaid MM, Abo-Taleb HA (2011) Temporal and spatial dynamics of rotifers in the Rosetta Estuary, Egypt. Egypt J Aquat Res 37(1):59–70
Google Scholar
Achuthankutty CT, Shrivastava Y, Mahambre GG, Goswami SC, Madhupratap M (2000) Parthenogenetic reproduction of Diaphanosoma celebensis (Crustacea: Cladocera): influence of salinity on feeding, survival, growth and neonate production. Mar Biol 137(1):19–22
Article Google Scholar
Altaff K (2004) A manual of zooplankton. University Grants Commission, New Delhi, pp 1–155
Al-Yamani FY, Skryabin V, Gubanova A, Khvorov S, Prusova I (2011) Marine zooplankton practical guide for the Northwestern Arabian Gulf. Kuwait Institute for Scientific Research, Kuwait, p 399. ISBN: 978-99966-95-07-0
Anjusha A, Jyothibabu R, Jagadeesan L, Arunpandi N (2018) Role of rotifers in microzooplankton community in a large monsoonal estuary (Cochin backwaters) along the west coast of India. Environ Monit Assess 190(5):295
Article CAS Google Scholar
Antony S, Ajayan A, Dev VV, Mahadevan H, Kaliraj S, Krishnan KA (2020) Environmental influences on zooplankton diversity in the Kavaratti lagoon and offshore, Lakshadweep Archipelago, India. Reg Stud Mar Sci 37:101330
Arreola-Lizarraga JA, Padilla-Arredondo G, Medina-Galvan J, Méndez-Rodriguez L, Mendoza-Salgado R, Cordoba-Matson MV (2016) Analysis of hydrobiological responses to anthropogenic and natural influences in a lagoon system in the Gulf of California. Oceanol Hydrobiol Stud 45(1):111–120
Azemar F, Maris T, Mialet B, Segers H, Van Damme S, Meire P, Tackx M (2010) Rotifers in the Schelde estuary (Belgium): a test of taxonomic relevance. J Plankton Res 32(7):981–997
Barik SS, Singh RK, Jena PS, Tripathy S, Sharma K, Prusty P (2019) Spatio-temporal variations in ecosystem and CO 2 sequestration in coastal lagoon: a foraminiferal perspective. Mar Micropaleontol 147:43–56
Basuri CK, Pazhaniyappan E, Munnooru K, Chandrasekaran M, Vinjamuri RR, Karri R, Mallavarapu RV (2020) Composition and distribution of planktonic ciliates with indications to water quality in a shallow hypersaline lagoon (Pulicat Lake, India). Environ Sci Pollut Res 27:1–14
Battish SK (1992) Freshwater zooplankton of India. Oxford & IBH Publishing Company, New Delhi
Bhattacharya BD, Hwang JS, Sarkar SK, Rakhsit D, Murugan K, Tseng LC (2015) Community structure of mesozooplankton in coastal waters of Sundarban mangrove wetland, India: a multivariate approach. J Mar Syst 141:112–121
Biswas SN, Godhantaraman N, Rakshit D, Sarkar SK (2013) Community composition, abundance, biomass and production rates of Tintinnids (Ciliata: Protozoa) in the coastal regions of Sundarban Mangrove wetland, India. Indian J Geomarine Sci 42(2):163–173
CAS Google Scholar
Bron JE, Frisch D, Goetze E, Johnson SC, Lee CE, Wyngaard GA (2011) Observing copepods through a genomic lens. Front Zool 8:1–15
Buikema AL Jr, Miller JD, Yongue WH Jr (1978) Effects of algae and protozoans on the dynamics of Polyarthra vulgaris. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 20(4):2395–2399
Carter JL, Schindler DE, Francis TB (2017) Effects of climate change on zooplankton community interactions in an Alaskan lake. Clim Change Responses 4(1):3
Casanova JP (1999) Chaetognatha. In: Boltovskoy D (ed) South Atlantic zooplankton. Backhuys Publishers, Leiden, pp 1353–1374
Conway DV, White RG, Hugues-Dit-Ciles J, Gallienne CP, Robins DB (2003) Guide to the coastal and surface zooplankton of the south-western Indian Ocean. Occasional Publication of the Marine Biological Association of the United Kingdom, Plymouth, p 15
Corliss JO (2002) Biodiversity and biocomplexity of the protists and an overview of their significant roles in maintenance of our biosphere. Acta Protozool 41(3):199–220
Dalal SG, Goswami SC (2001) Temporal and ephemeral variations in copepod community in the estuaries of Mandovi and Zuari—west coast of India. J Plankton Res 23(1):19–26
Dam HG (2013) Evolutionary adaptation of marine zooplankton to global change. Annu Rev Mar Sci 5:349–370
David V, Sautour B, Chardy P, Leconte M (2005) Long-term changes of the zooplankton variability in a turbid environment: the Gironde estuary (France). Estuar Coast Shelf Sci 64:171–184
Devasundaram MP, Roy JC (1954) A preliminary study of the plankton of the Chilka Lake for the years 1950 & 1951. In: Symposium on marine and fresh-water plankton in the Indo-Pacific, Indo-Pacific Fish. Com., pp 48–54
Dolan JR, Gallegos CC (1992) Trophic role of planktonic rotifers in the Rhode River Estuary, spring-summer 1991. Mar Ecol Prog Ser (Oldendorf) 85(1):187–199
Edmondson WT, Litt AH (1982) Daphnia in lake Washington 1. Limnol Oceanogr 27(2):272–293
Egloff DA, Fofonoff PW, Onbe T (1997) Reproductive biology of marine cladocerans. In: Advances in marine biology, vol 31. Academic Press, London, pp 79–167
Etile RND, Kouassi AM, Aka MNG, Pagano M, N’douba V (2009) Spatio-temporal variations of the zooplankton abundance and composition in a West African tropical coastal lagoon (Grand-Lahou, Cote d’Ivoire). Hydrobiologia 624(1):171–189
Ezz SMA, Aziz NEA, Abou Zaid MM, El Raey M, Abo-Taleb HA (2014) Environmental assessment of El-Mex Bay, Southeastern Mediterranean by using Rotifera as a plankton bio-indicator. Egypt J Aquat Res 40(1):43–57
Felipe Machado Velho L, Amodeo Lansac-Toha F, Costa Bonecker C, Zimmermann-Callegari MC (2000) On the occurrence of testate amoebae (Protozoa, Rhizopoda) in Brazilian inland waters. II. Families Centropyxidae, Trigonopyxidae and Plagiopyxidae. Acta Scientiarum Biol Sci 22:365–374
Frontalini F, Buosi C, Da Pelo S, Coccioni R, Cherchi A, Bucci C (2009) Benthic foraminifera as bio-indicators of trace element pollution in the heavily contaminated Santa Gilla lagoon (Cagliari, Italy). Mar Pollut Bull 58(6):858–877
Ganguly D, Patra S, Muduli PR, Vardhan KV, Abhilash KR, Robin RS, Subramanian BR (2015) Influence of nutrient input on the trophic state of a tropical brackish water lagoon. J Earth Syst Sci 124(5):1005–1017
Gao X, Song J, Li X (2011) Zooplankton spatial and diurnal variations in the Changjiang River estuary before operation of the Three Gorges Dam. Chin J Oceanol Limnol 29(3):591–602
Gao F, Warren A, Zhang Q, Gong J, Miao M, Sun P, Xu D, Huang J, Yi Z, Song W (2016) The all-data-based evolutionary hypothesis of ciliated protists with a revised classification of the phylum Ciliophora (Eukaryota, Alveolata). Sci Rep 6:24874
Garcia MD, Bonel N (2014) Environmental modulation of the plankton community composition and size-structure along the eutrophic intertidal coast of the Rio de la Plata estuary, Argentina. J Limnol 73(3):562–573
Gauns M, Mochemadkar S, Patil S, Pratihary A, Naqvi SWA, Madhupratap M (2015) Seasonal variations in abundance, biomass and grazing rates of microzooplankton in a tropical monsoonal estuary. J Oceanogr 71(4):345–359
Godhantaraman N (2001) Seasonal variations in taxonomic composition, abundance and food web relationship of microzooplankton in estuarine and mangrove waters, Parangipettai region, southeast coast of India. Indian J Mar Sci 30:151–160
Godhantaraman N (2002) Seasonal variations in species composition, abundance, biomass and estimated production rates of tintinnids at tropical estuarine and mangrove waters, Parangipettai, southeast coast of India. J Mar Syst 36:161–171
Godhantaraman N, Uye S (2003) Geographical and seasonal variations in taxonomic composition, abundance and biomass of microzooplankton across a brackish-water lagoonal system of Japan. J Plankton Res 25(5):465–482
Gopakumar G, Jayaprakash V (2003) Community structure and succession of brackish water rotifers in relation to ecological parameters. J Mar Biol Assoc India 45(1):20–30
Gopko M, Telesh IV (2013) Estuarine trophic state assessment: new plankton index based on morphology of Keratella rotifers. Estuar Coast Shelf Sci 130:222–230
Grahame J, Branch GM (1985) Reproductive patterns of marine invertebrates. Oceanogr Mar Biol 23:373–398
Gupta VK, Sen A, Pattnaik AK, Rastogi G, Bhadury P (2019) Long-term monitoring of benthic foraminiferal assemblages from Asia’s largest tropical coastal lagoon, Chilika, India. J Mar Biol Assoc U K 99(2):311–330
Gutierrez MF, Tavsanoglu UN, Vidal N, Yu J, Teixeira-de Mello F, Cakiroglu AI, He H, Liu Z, Jeppesen E (2018) Salinity shapes zooplankton communities and functional diversity and has complex effects on size structure in lakes. Hydrobiologia 813(1):237–255
Haridevan G, Jyothibabu R, Arunpandi N, Jagadeesan L, Biju A (2015) Influence of salinity on the life table demography of a rare Cladocera Latonopsis australis . Environ Monit Assess 187(10):643
Herzig A (1983) Comparative studies on the relationship between temperature and duration of embryonic development of rotifers. In: Biology of rotifers. Springer, Dordrecht, pp 237–246
Chapter Google Scholar
Isari S, Psarra S, Pitta P, Mara P, Tomprou MO, Ramfos A, Somarakis S, Tselepides A, Koutsikopoulos C, Fragopoulu N (2007) Differential patterns of mesozooplankters’ distribution in relation to physical and biological variables of the northeastern Aegean Sea (eastern Mediterranean). Mar Biol 151(3):1035–1050
Jones RH, Flynn KJ (2005) Nutritional status and diet composition affect the value of diatoms as copepod prey. Science 307(5714):1457–1459
Jyothibabu R, Madhu NV, Jayalakshmi KV, Balachandran KK, Shiyas CA, Martin GD, Nair KKC (2006) Impact of freshwater influx on microzooplankton mediated food web in a tropical estuary (Cochin backwaters–India). Estuar Coast Shelf Sci 69(3–4):505–518
Ka S, Mendoza-Vera JM, Bouvy M, Champalbert G, N’Gom-Ka R, Pagano M (2012) Can tropical freshwater zooplankton graze efficiently on cyanobacteria? Hydrobiologia 679(1):119–138
Kasturirangan LR (1963) A key for the identification of the more common planktonic Copepoda: of Indian coastal waters (no. 2). Council of Scientific & Industrial Research
Kawecka B, Eloranta PV (1994) The outline of algae ecology in freshwater and terrestrial environments. Wyd. Nauk. PWN, Warszawa, p 147
Kleppel GS (1993) On the diets of calanoid copepods. Mar Ecol Prog Ser 99:183–195
Kofoid CA, Campbell AS (1929) A conspectus of the marine and fresh-water Ciliata belonging to the suborder Tintinnoinea, with description of the suborder Tintinnoinea, with description of new species principally from Agassiz expedition to the eastern tropical Pacific, 1904–1905. Univ Calif Publ Zool 34:1–403
Krinsic F (1987) On the ecology of tintinnines (Ciliata—Oligotrichida, Tintinnina) in the open waters of the south Adriatic. Mar Biol 68:83–90
Kumar A, Mishra DR, Equeenuddin SM, Cho HJ, Rastogi G (2016) Differential impact of anniversary-severe cyclones on the water quality of a tropical coastal lagoon. Estuar Coasts 40(2):317–342
Kumari KB, Anbalagan R, Sivakami R (2017) Seasonal protozoan diversity in Agniar Estuary, Tamil Nadu, India. Int J Pure Appl Biosci 5(2):634–637
Liu H, Chen M, Suzuki K, Wong CK, Chen B (2010) Mesozooplankton selective feeding in subtropical coastal waters as revealed by HPLC pigment analysis. Mar Ecol Prog Ser 407:111–123
Lord AR, Boomer I, Brouwers E, Whittaker JE (2012) Ostracod taxa as palaeoclimate indicators in the quaternary. In: Developments in quaternary sciences, vol 17. Elsevier, Amsterdam, pp 37–45
Madhu NV, Jyothibabu R, Balachandran KK, Honey UK, Martin GD, Vijay JG, Shiyas CA, Gupta GVM, Achuthankutty CT (2007) Monsoonal impact on planktonic standing stock and abundance in a tropical estuary (Cochin backwaters–India). Estuar Coast Shelf Sci 73(1–2):54–64
Madhupratap M (1987) Status and strategy zooplankton of tropical Indian estuaries: a review. Bull Plankton Soc Jpn 34:65–81
Maeda M (1986) An illustrated guide to the species of the family Halteridae and Strombilidae (Oligotrichida, Ciliopora), free swimming protozoa common in aquatic environments. Bull Ocean Res Inst Univ Tokyo 19:67
Maksimenkov VV (1982) Correlation of abundance of food (for herring larvae) zooplankton and water temperature in the Korfo-Karaginsk region of Bering Sea [USSR, Clupea pallasi]. Soviet J Mar Biol 8:132–135
Mianzan HW (1999) Ctenophora. In: South Atlantic zooplankton. Backhuys Publishers, Leiden, pp 51–573
Mileikovsky SA (1971) Types of larval development in marine bottom invertebrates, their distribution and ecological significance: a re-evaluation. Mar Biol 10(3):193–213
Miron MIBD, Castellanos-Paez ME, Garza-Mourino G, Ferrara-Guerrero MJ, Pagano M (2014) Spatiotemporal variations of zooplankton community in a shallow tropical brackish lagoon (Sontecomapan, Veracruz, Mexico). Zool Stud 53(1):59
Mishra S, Panigrahy RC (1999) The tintinnids (Protozoa: Ciliata) of the Bahuda estuary, east coast of India. Indian J Mar Sci 28:219–221
Mitsch WJ, Gosselink JG (1993) Wetlands, 2nd edn. Van Nostrand Reinhold Press, New York
Molinero JC, Ibanez F, Nival P, Buecher E, Souissi S (2005) North Atlantic climate and northwestern Mediterranean plankton variability. Limnol Oceanogr 50(4):1213–1220
Muduli PR, Pattnaik AK (2020) Spatio-temporal variation in physicochemical parameters of water in the Chilika lagoon. In: Ecology, conservation, and restoration of Chilika lagoon, India. Springer, Cham, pp 203–229
Muduli PR, Kanuri VV, Robin RS, Kumar BC, Patra S, Raman AV, Rao GN, Subramanian BR (2013) Distribution of dissolved inorganic carbon and net ecosystem production in a tropical brackish water lagoon, India. Cont Shelf Res 64:75–87
Mukherjee M, Banik SK, Suresh VR, Manna RK, Panda D, Sharma AP (2014) Rotifers, their distribution, abundance and seasonal variation in Chilika lagoon. J Inland Fish Soc India 46(1):29–37
Mukherjee M, Banik SK, Pradhan SK, Sharma AP, Suresh VR, Manna RK, Panda D, Roshith CM, Mandal S (2015) Diversity and distribution of Tintinnids in Chilika lagoon with description of new records. Indian J Fish 62(1):25–32
Mukherjee M, Suresh VR, Manna RK (2018) Microplankton dynamics of a coastal lagoon, Chilika: interactive effect of environmental parameters on microplankton groups. Environ Monit Assess 190(11):689
Murray JW (2006) Ecology and applications of benthic foraminifera. Cambridge University Press, Cambridge, 426pp
Book Google Scholar
Naik S, Panigrahy RC, Mohapatra A (2008) Spatio-temporal distribution of zooplankton in Chilka lake—a Ramsar site on the Indian east coast. Indian J Sci Technol 1(3):1–5
Nandan SB, Azis PKA (1994) Zooplankton of the retting zones in the Kadinam-Kulam Estuary, Kerala. Mahasagar 27(1):59–65
Nche-Fambo FA, Tirok K, Scharler UM (2016) Hypersaline conditions cause distinct ciliate community structure in a South African estuarine lake system. J Plankton Res 38(4):878–887
Pang K, Martindale MQ (2008) Comb jellies (Ctenophora): a model for basal metazoan evolution and development. Cold Spring Harbor Protocols 2008(11):pdb-emo106
Pangle KL, Peacor SD (2009) Light-dependent predation by the invertebrate planktivore Bythotrephes longimanus . Can J Fish Aquat Sci 66(10):1748–1757
Park GS, Marshall HG (2000) The trophic contributions of rotifers in tidal freshwater and estuarine habitats. Estuar Coast Shelf Sci 51(6):729–742
Patnaik S (1973) Observation on the seasonal fluctuating of plankton in the Chilika lake. Indian J Fish 20:43–45
Pattanaik PK, Sarma ALN (1997) Qualitative and quantitative composition of zooplankton communities of Rambha Bay (Chilika Lake, Bay of Bengal). J Mar Biol Assoc India 39(1–2):49–58
Pennington JT, Rumrill SS, Chia FS (1986) Stage-specific predation upon embryos and larvae of the Pacific sand dollar, Dendraster excentricus, by 11 species of common zooplanktonic predators. Bull Mar Sci 39(2):234–240
Perez-Ruzafa A, Marcos C, Perez-Ruzafa IM, Pérez-Marcos M (2011) Coastal lagoons: “transitional ecosystems” between transitional and coastal waters. J Coast Conserv 15(3):369–392
Qin Y, Fournier B, Lara E, Gu Y, Wang H, Cui Y, Zhang X, Mitchell EAD (2013) Relationships between testate amoeba communities and water quality in Lake Donghu, a large alkaline lake in Wuhan, China. Front Earth Sci 7(2):182–190
Rakhesh M, Madhavirani KSVKS, Kumar BC, Raman AV, Kalavati C, Rao YP, Rosamma S, Rao VR, Gupta GVM, Subramanian BR (2015) Trophic–salinity gradients and environmental redundancy resolve mesozooplankton dynamics in a large tropical coastal lagoon. Reg Stud Mar Sci 1:72–84
Rakshit D, Sarkar SK (2016) Diversity, distribution and polymorphism of loricate ciliate tintinnids along Hooghly estuary, India. J Mar Biol Assoc India 58(2):61–68
Rakshit D, Biswas SN, Sarkar SK, Bhattacharya BD, Godhantaraman N, Satpathy KK (2014) Seasonal variations in species composition, abundance, biomass and production rate of tintinnids (Ciliata: Protozoa) along the Hooghly (Ganges) River Estuary, India: a multivariate approach. Environ Monit Assess 186(5):3063–3078
Rakshit D, Murugan K, Biswas JK, Satpathy KK, Ganesh PS, Sarkar SK (2017) Environmental impact on diversity and distribution of tintinnid (Ciliata: Protozoa) along Hooghly Estuary, India: a multivariate approach. Reg Stud Mar Sci 12:1–10
Ramaiah N, Nair VR (1997) Distribution and abundance of copepods in the pollution gradient zones of Bombay Harbour-Thana creek-Bassein creek, west coast of India. Indian J Mar Sci 26:20–25
Reynolds CS (1997) Vegetation processes in the pelagic: a model for ecosystem theory, vol 9. Ecology Institute, Oldendorf
Rice E, Dam HG, Stewart G (2015) Impact of climate change on estuarine zooplankton: surface water warming in Long Island Sound is associated with changes in copepod size and community structure. Estuar Coasts 38(1):13–23
Rivier IK (1998) The predatory Cladocera (Onychopoda: Podonidae, Polyphemidae, Cercopagidae) and Leptodorida of the world. Guides to the identification of the micro-invertebrates of the continental waters of the world
Sahu BK, Srichandan S, Panigrahy RC (2016) A preliminary study on the microzooplankton of Chilika Lake, a brackish water lagoon on the east coast of India. Environ Monit Assess 188(1):69
Sanders RW (1987) Tintinnids and other microzooplankton—seasonal distributions and relationships to resources and hydrography in a Maine estuary. J Plankton Res 9(1):65–77
Santangelo JM, Rocha ADM, Bozelli RL, Carneiro LS, Esteves FDA (2007) Zooplankton responses to sandbar opening in a tropical eutrophic coastal lagoon. Estuar Coast Shelf Sci 71(3–4):657–668
Saraswathi R, Sumithra P (2016) An assessment of protozoan assemblage in Kottaipattinam Seasonal estuary, Pudukkottai District, Tamil Nadu. Int J Curr Microbiol App Sci 5(2):490–494
Sen A, Bhadury P (2016) Exploring the seasonal dynamics within the benthic foraminiferal biocoenosis in a tropical monsoon-influenced coastal lagoon. Aquat Biol 25:121–138
Sharma BK, Naik LP (1996) Results on planktonic rotifers in the Narmada river (Madhya Pradesh, Central India). In: Perspectives in tropical limnology. SPB Academic Publishing, Amsterdam, pp 189–198
Shin K, Jang MC, Jang PK, Ju SJ, Lee TK, Chang M (2003) Influence of food quality on egg production and viability of the marine planktonic copepod Acartia omorii . Prog Oceanogr 57(3–4):265–277
Sladecek V (1983) Rotifers as indicators of water quality. Hydrobiologia 100(1):169–201
Sooria PM, Jyothibabu R, Anjusha A, Vineetha G, Vinita J, Lallu KR, Paul M, Jagadeesan L (2015) Plankton food web and its seasonal dynamics in a large monsoonal estuary (Cochin backwaters, India)-significance of mesohaline region. Environ Monit Assess 187(7):427
Souza LCE, Branco CWC, Domingos P, Bonecker SLC (2011) Zooplankton of an urban coastal lagoon: composition and association with environmental factors and summer fish kill. Zoologia (Curitiba) 28(3):357–364
Srichandan S, Rastogi G (2020) Spatiotemporal assessment of phytoplankton communities in the Chilika lagoon. In: Ecology, conservation, and restoration of Chilika lagoon, India. Springer, Cham, pp 251–294
Srichandan S, Kim JY, Bhadury P, Barik SK, Muduli PR, Samal RN, Pattnaik AK, Rastogi G (2015a) Spatiotemporal distribution and composition of phytoplankton assemblages in a coastal tropical lagoon: Chilika, India. Environ Monit Assess 187:47
Srichandan S, Kim JY, Kumar A, Mishra DR, Bhadury P, Muduli PR, Pattnaik AK, Rastogi G (2015b) Interannual and cyclone-driven variability in phytoplankton communities of a tropical coastal lagoon. Mar Pollut Bull 101:39–52
Steinberg DK, Landry MR (2017) Zooplankton and the ocean carbon cycle. Annu Rev Mar Sci 9:413–444
Sushchik NN, Gladyshev MI, Makhutova ON, Kalachova GS, Kravchuk ES, Ivanova EA (2004) Associating particulate essential fatty acids of the ω3 family with phytoplankton species composition in a Siberian reservoir. Freshw Biol 49(9):1206–1219
Tackx ML, De Pauw N, Van Mieghem R, Azemar F, Hannouti A, Van Damme S, Fiers F, Daro N, Meire P (2004) Zooplankton in the Schelde estuary, Belgium and the Netherlands. Spatial and temporal patterns. J Plankton Res 26(2):133–141
Thorp JH, Mantovani S (2005) Zooplankton of turbid and hydrologically dynamic prairie rivers. Freshw Biol 50(9):1474–1491
Thorson G (1946) Reproduction and larval development of Danish marine bottom invertebrates with special reference to the planktonic larvae in the sound (Oresund). Meddr Kommn Danm Fisk og Havun ders Ser Plankton 4(1):1–523
Tonno I, Agasild H, Koiv T, Freiberg R, Noges P, Noges T (2016) Algal diet of small-bodied crustacean zooplankton in a cyanobacteria-dominated eutrophic lake. PLoS One 11(4):e0154526
Varghese M, Krishnan L (2011) Ecology of rotifers in Cochin backwaters, Kerala, India. Indian J Fish 58(3):109–115
Varghese M, Jasmine S, Sreenath KR, Ranjith L, Thomas VJ (2018) Plankton productivity in lagoons of Agatti and Bangaram atolls of Lakshadweep Archipelago, India. Fish Technol 55:149–151
Venkataramana V, Sarma VVSS, Reddy AM (2017) River discharge as a major driving force on spatial and temporal variations in zooplankton biomass and community structure in the Godavari estuary India. Environ Monit Assess 189(9):474
Vineetha G, Madhu NV, Kusum KK, Sooria PM (2015) Seasonal dynamics of the copepod community in a tropical monsoonal estuary and the role of sex ratio in their abundance pattern. Zool Stud 54(1):54
Wei N, Xu RL (2014) Distinct difference of littoral rotifer community structure in two mangrove wetlands of Qi’ao Island, Pearl River estuary, China. Zool Stud 53(1):30
Xu H, Zhang W, Jiang Y, Yang EJ (2014) Use of biofilm-dwelling ciliate communities to determine environmental quality status of coastal waters. Sci Total Environ 470:511–518
Ziadi B, Dhib A, Turki S, Aleya L (2015) Factors driving the seasonal distribution of zooplankton in a eutrophicated Mediterranean Lagoon. Mar Pollut Bull 97(1–2):224–233
Download references
Acknowledgments
The study was supported from the funding of World Bank (Credit No. 4765-IN) awarded to Chilika Development Authority (Government of Odisha) under the Integrated Coastal Zone Management Project (ICZMP). Mr. Bibhuti Bhusan Dora, Research Fellow, GIS Cell of CDA, is acknowledged for the preparation of sampling map. The staff of the CDA is acknowledged for their generous help in sampling.
Author information
Suchismita Srichandan
Present address: Centurion University of Technology and Management, Bhubaneswar, Odisha, India
Authors and Affiliations
Wetland Research and Training Centre, Chilika Development Authority, Balugaon, Odisha, India
Suchismita Srichandan & Gurdeep Rastogi
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Shaheed Bhagat Singh College, University of Delhi, Delhi, India
Sughosh Madhav
Department of Civil Engineering, Jamia Millia Islamia, New Delhi, Delhi, India
Sadaf Nazneen
PGDAV College, University of Delhi, New Delhi, Delhi, India
Pardeep Singh
Rights and permissions
Reprints and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Srichandan, S., Rastogi, G. (2022). Zooplankton Diversity and Their Spatiotemporal Distribution: An Ecological Assessment from a Brackish Coastal Lagoon, Chilika, Odisha. In: Madhav, S., Nazneen, S., Singh, P. (eds) Coastal Ecosystems. Coastal Research Library, vol 38. Springer, Cham. https://doi.org/10.1007/978-3-030-84255-0_9
Download citation
DOI : https://doi.org/10.1007/978-3-030-84255-0_9
Published : 04 December 2021
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-84254-3
Online ISBN : 978-3-030-84255-0
eBook Packages : Earth and Environmental Science Earth and Environmental Science (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

IMAGES
VIDEO
COMMENTS
Zooplankton play a key role in marine biodiversity and thus have critical impacts on marine ecosystem processes 1,2,3,4.These animals are integral to the functioning of aquatic food webs because ...
Current research suggests that some zooplankton are capable of evolving a degree of tolerance to environmental contaminants, ... Zooplankton diversity and community structure vary among water bodies, due to among-water body differences in: a. The physical environment (e.g., climate, flushing rates, water body morphometry) ...
It should be noted that despite DNA metabarcoding method has been widely used in biodiversity research and monitoring [68, 79, 80], many technical issues have ... Xiong & Zhan [34] tested different clustering strategies in studying zooplankton diversity, including newly developed non-clustering (DADA2 and UNOISE 3) and clustering with different ...
Phytoplankton is a polyphyletic group with utmost. variation in size, shape, colour, type of metabolism, and life history traits. Due to the emerging knowledge. in nutritional capabilities of ...
Quantifying the relationship between phytoplankton and zooplankton may offer insight into zooplankton sensitivity to shifting phytoplankton assemblages and the potential impacts of producer-consumer decoupling on the rest of the food web. We analyzed 18 years (2001-2018) of paired phytoplankton and zooplankton samples collected as part of the United States Environmental Protection Agency (U ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... "Unraveling Zooplankton ...
We carried out a literature review to investigate the taxonomic and functional β diversity of zooplankton and its species replacement (βrepl) and richness difference (βrich) components in Brazilian rivers. In addition, the taxonomic (LCBD-t) and functional ecological uniqueness (LCBD-f) were also measured. We tested the following hypotheses: (i) The βrepl component is the main driver of ...
The evaluation of biodiversity is a primary concern for natural sciences and society, including marine ecosystems. Zooplankton plays a crucial role in marine ecosystems, transferring energy between trophic levels and participating in carbon and nutrient cycling. Zooplankton communities respond to changes in environmental conditions in complex ways and are highly susceptible to anthropogenic ...
Journal of Plankton Research, Volume 35, Issue 3, May/June 2013, Pages 473-484, ... An intriguing difference in taxonomic diversity of zooplankton between the marine and freshwater realm is that marine zooplankton are much more taxonomically diverse, covering a wide range of taxa that are absent in freshwater zooplankton (salps ...
The results showed that zooplankton samples were classified into 128 species, and Rotifera was the most common taxa. Significant seasonal differences were found among the abundance and diversity of zooplankton. Similarly, we also found significant seasonal differences among the biomass of zooplankton functional groups.
As an intermediary connection between primary producers and higher trophic levels, zooplankton are an important component of the aquatic food chain, contributing significantly to aquatic biological productivity. This study describes the zooplankton diversity and community structure, as well as their relationships with ecological factors, in homestead ponds of a coastal district along the ...
This Special Issue aims to collate papers that address zooplankton diversity in the less studied lake habitats and subhabitats. We particularly welcome papers that employ a macroecological approach to study zooplankton diversity, shedding light on the molecules, populations, species, communities, and ecosystems of zooplankton. Dr. Maciej Karpowicz
Our understanding on phytoplankton diversity has largely been progressing since the publication of Hutchinson on the paradox of the plankton. In this paper, we summarise some major steps in phytoplankton ecology in the context of mechanisms underlying phytoplankton diversity. Here, we provide a framework for phytoplankton community assembly and an overview of measures on taxonomic and ...
Zooplankton diversity. A total of 53 taxa of zooplankton were recorded, distributed among Rotifera (36), Cladocera (13), and Copepoda (4) (Table S2). The rotifers were represented by 10 families, of which Brachionidae and Lecanidae were the most representative, with 13 and 11 species, respectively.
A rigorous zooplankton study was conducted from May 2016 to February 2017, in the seagrass habitat of Lawas, Sarawak, Malaysia, to examine their temporal composition and diversity, together with ...
Abstract. The future response of marine ecosystem diversity to continued anthropogenic forcing is poorly constrained. Phytoplankton are a diverse set of organisms that form the base of the marine ...
We compared two sampling methods, eDNA metabarcoding and microscope identification (MSI), for the analysis of zooplankton diversity in reservoirs with its inflow and outflow streams. The dynamic patterns of Cladocera and Rotifera at different time points were similar between the two sampling methods, but there was a slight difference in the Copepoda. Specifically, the members of the Copepoda ...
In experimental planktonic systems, Striebel et al. (2012) showed that phytoplankton diversity increased zooplankton productivity, while Filstrup et al. (2014) showed that the effect of phytoplankton evenness on RUE switched from negative at the producer level (phytoplankton) to positive at the consumer level (zooplankton) in US lakes.
Abstract. Global human population growth rate increasing rapidly and has significant impact on natural resources. It reduces the natural water quality. Assessment of zooplankton gives valuable ...
Zooplankton constitutes a pivotal component in the pelagic food webs and serves as the major source of fish diet, thereby determining the productivity of coastal fisheries. Therefore, understanding zooplankton diversity and their ecology in coastal lagoon settings is a high priority research area.
Zooplankton data was obtained for 205 outdoor ponds and experimental tanks, from 39 different aquaculture facilities, across 13 countries. Non-indigenous taxa were recorded from 17.9% of ...
Occasional paper no 290: 1-307. ... Zooplankton diversity and physico-chemical parameters of an oxbow lake, Madhura anua was studied for a period of one year from September 2012 to August 2013 ...
2 Defence Institute of Bio-Energy Research (DIBER), HQ, Haldwani, Uttarakhand- 263139, India. *Correspondence E-mai l : [email protected]. Abstract. Zooplankton ar e cosmop olitan in ...