An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(9); 2017 Sep


60 years ago, Francis Crick changed the logic of biology
Matthew cobb.
School of Biological Sciences, University of Manchester, Manchester, United Kingdom
In September 1957, Francis Crick gave a lecture in which he outlined key ideas about gene function, in particular what he called the central dogma. These ideas still frame how we understand life. This essay explores the concepts he developed in this influential lecture, including his prediction that we would study evolution by comparing sequences.
Introduction
This month marks the 60th anniversary of one of the most significant lectures in the history of biology. It was given on 19 September 1957 by Francis Crick as part of a Society for Experimental Biology symposium on the Biological Replication of Macromolecules, held at University College London. Originally entitled ‘Protein synthesis,’ the title acquired a magisterial introductory ‘On’ during writing up for publication the following year [ 1 ]. The lecture went far further than its title suggested: as Crick pointed out in the opening paragraph, he also addressed ‘the other central problems of molecular biology—those of gene action and nucleic acid synthesis.’
Crick’s talk is now often called the ‘central dogma’ lecture, for it was here that he first publicly presented this frequently misunderstood concept. While this was highly significant, the content of the lecture was even richer—it also saw Crick outline his view of the nature of life and of genetic information and the source of protein folding as well as making two bold and spectacularly accurate predictions: that there must exist a small ‘adaptor’ molecule (now known as tRNA) that could bring amino acids to the site of protein synthesis and that in the future, scientists would be able to explore rich evolutionary sources of information by comparing sequence data. In this one brief lecture, Crick profoundly influenced how we think. In The Eighth Day of Creation , journalist Horace Judson went so far as to claim that on that day 60 years ago, Crick “permanently altered the logic of biology [ 2 ].”
Crick’s presentation
Crick’s hour-long lecture was given on the third day of a leisurely 4-day meeting (at most four talks a day), with participants from France, the United States, Belgium, and Hungary as well as a solid contingent of Britons. One of the French speakers was molecular geneticist François Jacob, for whom this was his first encounter with Crick. The impression Crick made was lasting—30 years later, Jacob recalled the lecture:
“Tall, florid, with long sideburns, Crick looked like the Englishman seen in illustrations to 19th century books about Phileas Fogg or the English opium eater. He talked incessantly. With evident pleasure and volubly, as if he was afraid he would not have enough time to get everything out. Going over his demonstration again to be sure it was understood. Breaking up his sentences with loud laughter. Setting off again with renewed vigour at a speed I often had trouble keeping up with…Crick was dazzling.” [ 3 ]
There is no manuscript of Crick’s actual talk, only the 11,000-word article that was published in 1958, which Crick prepared for publication in October 1957. [ 4 ] This version would presumably have been too long for Crick to read out in his 60-minute slot, even if he did speak incredibly quickly and, as he recalled, ‘ran overtime’ [ 2 ]. According to the acknowledgement in the paper, the version with which we are all familiar was the product of many discussions with Sydney Brenner, who also played a role in “redrafting” the manuscript, presumably for publication.
Crick’s opening statement may seem unsettling to the modern reader:
“I shall…argue that the main function of the genetic material is to control (not necessarily directly) the synthesis of proteins. There is a little direct evidence to support this, but to my mind the psychological drive behind this hypothesis is at the moment independent of such evidence.”
This highlights how uncertain scientists were at the time about gene function—as Crick pointed out, at the time, not everyone accepted that nucleic acids were involved in protein synthesis [ 5 ]. In 1957, ribosomes were known only as microsomes, and their function and composition was uncertain; messenger RNA was still undreamt of—it would be properly identified only in the summer of 1960, and the discovery was not published until the following year [ 6 , 7 , 8 ].
Faced with the lack of experimental evidence as to how genes produced proteins, Crick fell back on what he excelled in: outlining general, bold concepts that drew together a wide variety of strands into a compelling whole. As he recalled: “In looking back I am struck…by the brashness which allowed us to venture powerful statements of a very general nature [ 9 ].”
Protein synthesis and the sequence hypothesis
Crick had been thinking at a very high level about the relation between DNA, RNA, and protein for several years, partly inspired by documents and letters that were exchanged between members of the 20-strong RNA Tie Club, a loose discussion group that included Brenner, Jim Watson, and a host of physicists and mathematicians, led by George Gamow [ 10 ]. In 1954, Watson wrote a series of letters to Crick as he tried to grapple with the role of RNA, which he jokingly called ‘the mysteries of life’ [ 11 ]. Watson initially thought that DNA might be chemically converted into RNA but gradually shifted his view and ended up arguing that DNA acted as a template for RNA, an answer he described as ‘not ugly’ [ 12 ].
Crick took these ideas and the experimental data that increasingly suggested that RNA was some kind of intermediate between DNA and protein (these data referred to ribosomes rather than mRNA) and developed a scheme to explain the relations between these three classes of biological molecules. In so doing, he had to get to grips with what exactly was in a gene and what took place if DNA was used as a template for RNA—not in biochemical terms, but in the most abstract way possible.
To do this, Crick had to resolve an issue that had been perplexing scientists since he and Watson introduced the concept of “genetic information” in their second, less often-read 1953 Nature article [ 13 ]. Although the idea had been rapidly and widely adopted, no one was clear what exactly genetic information might consist of. In his 1957 lecture, Crick gave a disarmingly straightforward definition—information in this context was simply ‘the determination of a sequence of units.’ This highlighted the existence of a link between the base sequences of nucleic acids and those of amino acids in a protein—they pointed to the reality of the genetic code. This in turn enabled Crick to conceptualize the link between gene and protein. He called this link “the flow of information” and added this concept to the factors that were generally accepted to describe protein synthesis and, indeed, life itself—the flow of matter and the flow of energy.
This definition of information raised a problem. Proteins are 3-dimensional (3D) structures whereas a DNA sequence is 1-dimensional (1D). Crick recognized that there might be some unknown source of information that enabled proteins to fold, but he argued that the ‘more likely hypothesis’ was that ‘folding is simply a function of the order of the amino acids.’ In other words, 3D protein structure is an emergent property of the 1D sequence. This simple ‘sequence hypothesis,’ as he termed it, remains essentially true today, despite the acknowledged role of molecular chaperones.
The central dogma
The most widely known of the powerful statements made by Crick in his lecture related to the flow of information between genes and proteins [ 14 ]. He had been musing about this for some time and in October 1956 wrote a set of notes entitled ‘Ideas on protein synthesis’ that took up 2 pages [ 15 ]. The second sentence of this document read, “The Central Dogma: ‘Once information has got into a protein it can’t get out again. Information here means the sequence of the amino acid residues, or other sequences related to it.’” This statement was repeated several times in the September 1957 lecture and also appeared in a Scientific American article on nucleic acids, which Crick published in October 1957 [ 16 ].
In Crick’s 1956 notes, this definition of the central dogma was followed by a diagram illustrating his idea, with arrows drawn in blue biro ( Fig 1 ). This figure was never published, although Crick did draw it on the blackboard when giving talks (see Fig 2 , from 1963—he may have done something similar in September 1957), and a slightly amended version was eventually published in 1970 [ 17 ].

Credit: Wellcome Library, London.

Note the drawing of the central dogma on the blackboard. Credit : Cold Spring Harbor Laboratory .
For Crick, four kinds of information transfer clearly existed: DNA → DNA (DNA replication), DNA → RNA (the first step of protein synthesis), RNA → protein (the second step of protein synthesis) and RNA → RNA (RNA viruses copying themselves). There were two steps for which there was no evidence but that Crick thought were possible (hence the dotted lines in the figure): DNA → protein (this would mean RNA was not involved in protein synthesis) and RNA → DNA (structurally possible, but at the time, there no was no perceptible biological function).
Just as striking were the three flows of information that Crick considered to be impossible due to both lack of evidence and lack of biochemical mechanism. These were protein → protein, protein → RNA, and above all, protein → DNA. This was what Crick meant when he said that once information had gone from DNA into the protein, it could not get out of the protein and go back into the genetic code. This is the central dogma.
Crick admitted that the direct evidence for this hypothesis was ‘negligible’ and that it had a ‘speculative nature,’ but he defended his approach by pointing out that cosmologists had no qualms about constructing theories without adequate experimental data. That implicit comparison with grand theories of the universe is justified, for Crick was laying out the foundations of a new way of understanding how the cell works. The simplicity of the sequence hypothesis and the central dogma, together with the focus on information, brought a clear explanatory power to the synthesis of protein molecules that could take virtually any form and could ‘do almost anything,’ as Crick put it. Once the cell’s fundamental activity was conceived of in this way, everything fell into place. Crick advised his listeners to attempt to explain protein synthesis without these two basic principles—it was ‘an instructive exercise,’ he said. ‘One generally ends in the wilderness,’ he claimed.
Students are now often mistakenly taught that the central dogma is something like ‘DNA → RNA → protein’ (as popularised by Watson in his 1965 textbook Molecular Biology of the Gene [ 18 ]) or, even less precisely, ‘DNA makes RNA makes protein’ (as first suggested by Jean Brachet in 1960 [ 19 ]). This view, which went back to André Boivin in 1949 [ 20 ] and Alexander Dounce in 1953 [ 21 ], was very different to what Crick had in mind (it also confuses students, who often fail to grasp what the arrows mean or ‘makes’ implies [ 22 ]).
In 1970, following the discovery by Howard Temin and David Baltimore of reverse transcriptase, which enables information to flow in the direction RNA → DNA, Nature published an editorial entitled ‘Central dogma reversed’ [ 23 ]. Crick wrote a slightly tetchy response, repeating what he had actually stated in 1957, and rightly insisting that he had never argued that RNA → DNA was impossible [ 17 ]. In a distinctly undogmatic approach, he emphasised that our knowledge of cell biology was remarkably limited and that surprises might be in store, pointing to the example of the disease scrapie in which a protein seemed to act as an infectious agent (Stanley Prusiner later described this as a prion). However, even in the case of scrapie and other prion diseases, infection involves a change in conformation, not de novo synthesis.
Crick’s essential argument still holds: protein synthesis relies on nucleic acids, and once the genetic information has got into the protein, it cannot alter the DNA sequence. Despite recent excitement about transgenerational epigenetic inheritance due to histone modifications, DNA methylation, or other temporary modifications of material surrounding the genetic sequence, there is no evidence in any organism that the information in a DNA sequence can be rewritten from information in a protein.
In one aspect of the central dogma, Crick was mistaken. In reality, the ‘Central Dogma’ was anything but a dogma. Crick later claimed that he had not properly understood the meaning of ‘dogma’—Jacques Monod had to explain to him exactly what it meant. An indication of the truth of this assertion can be seen in the lecture when he states that the name that he has coined emphasizes the speculative nature of the idea—a dogma is not speculative. As Crick later acknowledged, a more accurate description would have been ‘basic assumption’ [ 17 ]. This does not sound quite so sexy, but it would have removed a lot of subsequent misunderstanding. Perhaps if Crick had not used such a dramatic turn of phrase, many subsequent critics would not have become so exercised about the question.
RNA and the adaptor
Crick used his lecture to publicly air another key idea about protein synthesis that he had been developing in private. In 1955, he circulated a note to the RNA Tie Club entitled ‘On degenerate templates and the adaptor hypothesis’ [ 24 ]. In this document, he argued that it was structurally impossible for any nucleic acid to act as a template for a particular amino acid; the duo of Crick and Brenner therefore came up with what Brenner called ‘the adaptor hypothesis’—an unknown class of molecule that would act like an electric plug adaptor, taking amino acids to the ribosome for protein assembly.
Crick was understandably unable to predict the nature of these adaptor molecules, but he felt that it was likely that they would contain nucleotides, which would be able to pair with both DNA and the RNA site of protein synthesis. Even allowing for the fact that he did not yet fully grasp the role of ribosomal RNA, Crick’s vision was astonishingly clear:
“The template could consist of perhaps a single chain of RNA…Each adaptor molecule containing, say, a di- or trinucleotide would each be joined to its own amino acid by a special enzyme. These molecules would then diffuse to the microsomal particles and attach to the proper place on the basis of the RNA by base-pairing.”
Crick and Brenner’s prediction would soon be proven correct—as Crick was giving his talk, Hoagland and Zamecnik were putting the finishing touches to their paper describing the isolation of the adaptor, which was eventually called tRNA [ 25 ].
Crick and evolutionary biology
There were two aspects of Crick’s lecture that related to evolutionary thinking. The first was that the central dogma supported the neo-Darwinian view that it was impossible for any character that was acquired during an organism’s life to affect its hereditary characters. This provided support for the widespread hostility to the view that had been held by Darwin, Lamarck, and others, according to which, patterns of use and disuse could lead to changes in the frequency of characters in subsequent generations.
Although in most organisms, including bacteria, plants, and even some animals, there is no separation between the copies of DNA used for protein synthesis and those used for transmitting genetic information to the next generation, Crick could see no conceivable mechanism whereby changes acquired during life could feed back into the DNA sequence. This was later considered to be an additional argument against Lamarckian inheritance and a reinforcement of Weismann’s separation of the germ and somatic cell lines (something that applies only to most animals) [ 2 ]. However, Crick did not mention either of these ideas.
The other evolutionary aspect to Crick’s lecture came in a brief and little-noticed aside, in which he essentially predicted the development of phylogenetics. In 1957, protein sequencing was extremely primitive, while sequencing DNA was two decades in the future. Complete amino acid sequences for insulin had been described for just five species, but nevertheless, Crick could see the way things would go. In an incredibly prescient prediction, he stated:
“Biologists should realise that before long we shall have a subject which might be called ‘protein taxonomy’—the study of the amino acid sequences of the proteins of an organism and the comparison of them between species. It can be argued that these sequences are the most delicate expression possible of the phenotype of an organism and that vast amounts of evolutionary information may be hidden away within them.”
This insight appears to have had little impact on thinking about the potential power of studying sequences—the history of bioinformatics [ 26 ] is generally traced back to the work of Dick Eck [ 27 ], Margaret Dayhoff [ 28 ], and Emile Zuckerkandl and Linus Pauling [ 29 ] in the early 1960s, none of whom cited Crick’s lecture. Further exploration of the work of the early bioinformaticians may reveal currently-unknown direct connections with Crick’s ideas, but whatever the case, the clarity of this vision underlines the power of Crick’s thinking.
It took some time for Crick’s lecture to exert its influence. Despite Jacob’s vivid description of how Crick presented his ideas, there is no indication that the content immediately changed the thinking of those in the audience. Only one of the other presentations at the symposium made any reference to Crick’s novel ideas in the revised printed version, and even here, the authors appear to have thought that Crick was indeed being dogmatic in his views because he speculated rather than strictly limiting himself to the experimental evidence [ 30 ].
Since then, the renown of the lecture has grown, and it has been cited over 800 times. The pattern of citations is U-shaped, with an early peak of 28 in 1962, followed by a trough of a handful of citations per year between 1971 and 1990, rising to 52 citations in 2014. Crick was later quite harsh on his lecture, describing it as ‘a mixture of good and bad ideas, of insight and nonsense’ [ 9 ]. This seems unfair—any nonsense is primarily due to lack of experimental evidence at the time. The reason why people still return to a 60-year-old lecture is because of the power of its ideas and the clarity with which they are presented. Crick’s style and intellectual verve continue to be both influential and inspirational; everyone should read or reread this brilliant lecture by one of the 20th century’s greatest scientists, a lecture that changed how we think.
Funding Statement
Cold Spring Harbor Laboratory Sydney Brenner Research Scholarship. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Provenance: Not commissioned; externally peer reviewed.
A world in one dimension: Linus Pauling, Francis Crick and the central dogma of molecular biology
- February 2006
- History and Philosophy of the Life Sciences 28(4):491-512
- 28(4):491-512

- University of Geneva
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Mikhail Voloshin

- Eric de Bony de Lavergne
- INT J MOL SCI

- Chrysa Nikopoulou

- Peter Tessarz

- Vivian Ling
- Lijing Jiang

- F. Haurowitz
- Linus Pauling
- Lois N. Magner

- DAEDALUS-US
- A. de Vries

- F.M. Rombouts
- Joshua Lederberg
- F. H. C. Crick
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The Central Dogma revisited: Insights from protein synthesis, CRISPR, and beyond
Affiliations.
- 1 School of Graduate Studies, Rutgers University, Newark, New Jersey, USA.
- 2 Department of Medicine, Rutgers New Jersey Medical School, Newark, New Jersey, USA.
- PMID: 35199457
- DOI: 10.1002/wrna.1718
Francis Crick advanced two distinct but interrelated fundamental principles of molecular biology: (1) the Sequence Hypothesis and (2) the Central Dogma. The Sequence Hypothesis defines biological information transfer as the residue-by-residue transfer of sequence information between nucleic acids and to proteins. This is commonly summarized as DNA ➔ RNA ➔ protein and is colloquially referred to as the Central Dogma. More specifically, however, the Central Dogma expounded by Crick included a critical restriction, stipulating that "once sequential information has passed into protein it cannot get out again." Under this definition, the Central Dogma has stood the test of time despite challenges. In principle, a violation of the Central Dogma could transpire through synthetic biology or by natural occurrence. To address these possibilities, we draw insights from existing modes of information transfer in protein synthesis and from synthetic Clustered Regularly-Interspaced Short Palindromic Repeats (CRISPR) gene-editing. We introduce a three-part evaluation scheme, which we apply to the CRISPR/Cas9 system and the more recent CRISPR prime editing system. Potential mechanisms by which engineered sequence editing systems might violate the Central Dogma are considered. We conclude that although information transfer in protein synthesis and CRISPR gene-editing remain within the bounds of the Central Dogma, the underlying mechanisms point toward an avenue of synthetic biology that could directly violate the Central Dogma. Finally, we speculate on some of the theoretical and practical implications of a protein-derived information transfer system. This article is categorized under: RNA Evolution and Genomics > Ribonomics RNA Interactions with Proteins and Other Molecules > Protein-RNA Interactions: Functional Implications Translation > Mechanisms.
Keywords: CRISPR; central dogma; protein synthesis; synthetic biology.
© 2022 Wiley Periodicals LLC.
PubMed Disclaimer
Similar articles
- Gene Therapy with CRISPR/Cas9 Coming to Age for HIV Cure. Soriano V. Soriano V. AIDS Rev. 2017 Oct-Dec;19(3):167-172. AIDS Rev. 2017. PMID: 29019352
- Engineered CRISPR Systems for Next Generation Gene Therapies. Pineda M, Moghadam F, Ebrahimkhani MR, Kiani S. Pineda M, et al. ACS Synth Biol. 2017 Sep 15;6(9):1614-1626. doi: 10.1021/acssynbio.7b00011. Epub 2017 Jun 7. ACS Synth Biol. 2017. PMID: 28558198 Review.
- Development of a CRISPR/Cas9 System for Methylococcus capsulatus In Vivo Gene Editing. Tapscott T, Guarnieri MT, Henard CA. Tapscott T, et al. Appl Environ Microbiol. 2019 May 16;85(11):e00340-19. doi: 10.1128/AEM.00340-19. Print 2019 Jun 1. Appl Environ Microbiol. 2019. PMID: 30926729 Free PMC article.
- Recent advances in CRISPR/Cas9 mediated genome editing in Bacillus subtilis. Hong KQ, Liu DY, Chen T, Wang ZW. Hong KQ, et al. World J Microbiol Biotechnol. 2018 Sep 29;34(10):153. doi: 10.1007/s11274-018-2537-1. World J Microbiol Biotechnol. 2018. PMID: 30269229 Review.
- CRISPR/Cas genome editing to optimize pharmacologically active plant natural products. Dey A. Dey A. Pharmacol Res. 2021 Feb;164:105359. doi: 10.1016/j.phrs.2020.105359. Epub 2020 Dec 4. Pharmacol Res. 2021. PMID: 33285226 Review.
- Cell-type specific and differential expression of LINC-RSAS long noncoding RNA declines in the testes during ageing of the rat. Danga AK, Kour S, Kumari A, Rath PC. Danga AK, et al. Biogerontology. 2024 Jun;25(3):543-566. doi: 10.1007/s10522-023-10088-1. Epub 2024 Feb 14. Biogerontology. 2024. PMID: 38353919
- "Molecular Biology"-Pleonasm or Denotation for a Discipline of Its Own? Reflections on the Origins of Molecular Biology and Its Situation Today. Greslehner GP. Greslehner GP. Biomolecules. 2023 Oct 12;13(10):1511. doi: 10.3390/biom13101511. Biomolecules. 2023. PMID: 37892193 Free PMC article. Review.
- Osteosarcoma transcriptome data exploration reveals STC2 as a novel risk indicator in disease progression. Wang Z, Zeng Z, Gao F, Gui Z, Du J, Shen N, Shang Y, Yang Z, Shang L, Wei R, Ma W, Wang C. Wang Z, et al. BMC Med Genomics. 2023 Feb 20;16(1):30. doi: 10.1186/s12920-023-01456-4. BMC Med Genomics. 2023. PMID: 36803385 Free PMC article.
- Multiple-Gene Regulation for Enhanced Antitumor Efficacy with Branch-PCR-Assembled TP53 and MYC Gene Nanovector. Cheng L, Lu L, Chen Z, Ma D, Xi Z. Cheng L, et al. Molecules. 2022 Oct 16;27(20):6943. doi: 10.3390/molecules27206943. Molecules. 2022. PMID: 36296536 Free PMC article.
- Anzalone, A. V., Koblan, L. W., & Liu, D. R. (2020). Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nature Biotechnology, 38(7), 824-844. https://doi.org/10.1038/s41587-020-0561-9
- Anzalone, A. V., Randolph, P. B., Davis, J. R., Sousa, A. A., Koblan, L. W., Levy, J. M., Chen, P. J., Wilson, C., Newby, G. A., Raguram, A., & Liu, D. R. (2019). Search-and-replace genome editing without double-strand breaks or donor DNA. Nature, 576(7785), 149-157. https://doi.org/10.1038/s41586-019-1711-4
- Baltimore, D., Eggers, H. J., Franklin, R. M., & Tamm, I. (1963). Poliovirus-induced RNA polymerase and the effects of virus-specific inhibitors on its production. Proceedings of the National Academy of Sciences of the United States of America, 49(6), 843-849.
- Baltimore, D. (1970). Viral RNA-dependent DNA polymerase: RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature, 226(5252), 1209-1211. https://doi.org/10.1038/2261209a0
- Boothby, T. C., Tapia, H., Brozena, A. H., Piszkiewicz, S., Smith, A. E., Giovannini, I., Rebecchi, L., Pielak, G. J., Koshland, D., & Goldstein, B. (2017). Tardigrades use intrinsically disordered proteins to survive desiccation. Molecular Cell, 65(6), 975-984. https://doi.org/10.1016/j.molcel.2017.02.018
Publication types
- Search in MeSH
Related information
Grants and funding.
- P41 GM103311/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full text sources.

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Loading metrics
Open Access
Essays articulate a specific perspective on a topic of broad interest to scientists.
See all article types »
60 years ago, Francis Crick changed the logic of biology
* E-mail: [email protected]
Affiliation School of Biological Sciences, University of Manchester, Manchester, United Kingdom
- Matthew Cobb

Published: September 18, 2017
- https://doi.org/10.1371/journal.pbio.2003243
- Reader Comments
In September 1957, Francis Crick gave a lecture in which he outlined key ideas about gene function, in particular what he called the central dogma. These ideas still frame how we understand life. This essay explores the concepts he developed in this influential lecture, including his prediction that we would study evolution by comparing sequences.
Citation: Cobb M (2017) 60 years ago, Francis Crick changed the logic of biology. PLoS Biol 15(9): e2003243. https://doi.org/10.1371/journal.pbio.2003243
Copyright: © 2017 Matthew Cobb. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: Cold Spring Harbor Laboratory Sydney Brenner Research Scholarship. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Provenance: Not commissioned; externally peer reviewed.
Introduction
This month marks the 60th anniversary of one of the most significant lectures in the history of biology. It was given on 19 September 1957 by Francis Crick as part of a Society for Experimental Biology symposium on the Biological Replication of Macromolecules, held at University College London. Originally entitled ‘Protein synthesis,’ the title acquired a magisterial introductory ‘On’ during writing up for publication the following year [ 1 ]. The lecture went far further than its title suggested: as Crick pointed out in the opening paragraph, he also addressed ‘the other central problems of molecular biology—those of gene action and nucleic acid synthesis.’
Crick’s talk is now often called the ‘central dogma’ lecture, for it was here that he first publicly presented this frequently misunderstood concept. While this was highly significant, the content of the lecture was even richer—it also saw Crick outline his view of the nature of life and of genetic information and the source of protein folding as well as making two bold and spectacularly accurate predictions: that there must exist a small ‘adaptor’ molecule (now known as tRNA) that could bring amino acids to the site of protein synthesis and that in the future, scientists would be able to explore rich evolutionary sources of information by comparing sequence data. In this one brief lecture, Crick profoundly influenced how we think. In The Eighth Day of Creation , journalist Horace Judson went so far as to claim that on that day 60 years ago, Crick “permanently altered the logic of biology [ 2 ].”
Crick’s presentation
Crick’s hour-long lecture was given on the third day of a leisurely 4-day meeting (at most four talks a day), with participants from France, the United States, Belgium, and Hungary as well as a solid contingent of Britons. One of the French speakers was molecular geneticist François Jacob, for whom this was his first encounter with Crick. The impression Crick made was lasting—30 years later, Jacob recalled the lecture:
“Tall, florid, with long sideburns, Crick looked like the Englishman seen in illustrations to 19th century books about Phileas Fogg or the English opium eater. He talked incessantly. With evident pleasure and volubly, as if he was afraid he would not have enough time to get everything out. Going over his demonstration again to be sure it was understood. Breaking up his sentences with loud laughter. Setting off again with renewed vigour at a speed I often had trouble keeping up with…Crick was dazzling.” [ 3 ]
There is no manuscript of Crick’s actual talk, only the 11,000-word article that was published in 1958, which Crick prepared for publication in October 1957. [ 4 ] This version would presumably have been too long for Crick to read out in his 60-minute slot, even if he did speak incredibly quickly and, as he recalled, ‘ran overtime’ [ 2 ]. According to the acknowledgement in the paper, the version with which we are all familiar was the product of many discussions with Sydney Brenner, who also played a role in “redrafting” the manuscript, presumably for publication.
Crick’s opening statement may seem unsettling to the modern reader:
“I shall…argue that the main function of the genetic material is to control (not necessarily directly) the synthesis of proteins. There is a little direct evidence to support this, but to my mind the psychological drive behind this hypothesis is at the moment independent of such evidence.”
This highlights how uncertain scientists were at the time about gene function—as Crick pointed out, at the time, not everyone accepted that nucleic acids were involved in protein synthesis [ 5 ]. In 1957, ribosomes were known only as microsomes, and their function and composition was uncertain; messenger RNA was still undreamt of—it would be properly identified only in the summer of 1960, and the discovery was not published until the following year [ 6 , 7 , 8 ].
Faced with the lack of experimental evidence as to how genes produced proteins, Crick fell back on what he excelled in: outlining general, bold concepts that drew together a wide variety of strands into a compelling whole. As he recalled: “In looking back I am struck…by the brashness which allowed us to venture powerful statements of a very general nature [ 9 ].”
Protein synthesis and the sequence hypothesis
Crick had been thinking at a very high level about the relation between DNA, RNA, and protein for several years, partly inspired by documents and letters that were exchanged between members of the 20-strong RNA Tie Club, a loose discussion group that included Brenner, Jim Watson, and a host of physicists and mathematicians, led by George Gamow [ 10 ]. In 1954, Watson wrote a series of letters to Crick as he tried to grapple with the role of RNA, which he jokingly called ‘the mysteries of life’ [ 11 ]. Watson initially thought that DNA might be chemically converted into RNA but gradually shifted his view and ended up arguing that DNA acted as a template for RNA, an answer he described as ‘not ugly’ [ 12 ].
Crick took these ideas and the experimental data that increasingly suggested that RNA was some kind of intermediate between DNA and protein (these data referred to ribosomes rather than mRNA) and developed a scheme to explain the relations between these three classes of biological molecules. In so doing, he had to get to grips with what exactly was in a gene and what took place if DNA was used as a template for RNA—not in biochemical terms, but in the most abstract way possible.
To do this, Crick had to resolve an issue that had been perplexing scientists since he and Watson introduced the concept of “genetic information” in their second, less often-read 1953 Nature article [ 13 ]. Although the idea had been rapidly and widely adopted, no one was clear what exactly genetic information might consist of. In his 1957 lecture, Crick gave a disarmingly straightforward definition—information in this context was simply ‘the determination of a sequence of units.’ This highlighted the existence of a link between the base sequences of nucleic acids and those of amino acids in a protein—they pointed to the reality of the genetic code. This in turn enabled Crick to conceptualize the link between gene and protein. He called this link “the flow of information” and added this concept to the factors that were generally accepted to describe protein synthesis and, indeed, life itself—the flow of matter and the flow of energy.
This definition of information raised a problem. Proteins are 3-dimensional (3D) structures whereas a DNA sequence is 1-dimensional (1D). Crick recognized that there might be some unknown source of information that enabled proteins to fold, but he argued that the ‘more likely hypothesis’ was that ‘folding is simply a function of the order of the amino acids.’ In other words, 3D protein structure is an emergent property of the 1D sequence. This simple ‘sequence hypothesis,’ as he termed it, remains essentially true today, despite the acknowledged role of molecular chaperones.
The central dogma
The most widely known of the powerful statements made by Crick in his lecture related to the flow of information between genes and proteins [ 14 ]. He had been musing about this for some time and in October 1956 wrote a set of notes entitled ‘Ideas on protein synthesis’ that took up 2 pages [ 15 ]. The second sentence of this document read, “The Central Dogma: ‘Once information has got into a protein it can’t get out again. Information here means the sequence of the amino acid residues, or other sequences related to it.’” This statement was repeated several times in the September 1957 lecture and also appeared in a Scientific American article on nucleic acids, which Crick published in October 1957 [ 16 ].
In Crick’s 1956 notes, this definition of the central dogma was followed by a diagram illustrating his idea, with arrows drawn in blue biro ( Fig 1 ). This figure was never published, although Crick did draw it on the blackboard when giving talks (see Fig 2 , from 1963—he may have done something similar in September 1957), and a slightly amended version was eventually published in 1970 [ 17 ].
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Credit: Wellcome Library, London.
https://doi.org/10.1371/journal.pbio.2003243.g001
Note the drawing of the central dogma on the blackboard. Credit : Cold Spring Harbor Laboratory .
https://doi.org/10.1371/journal.pbio.2003243.g002
For Crick, four kinds of information transfer clearly existed: DNA → DNA (DNA replication), DNA → RNA (the first step of protein synthesis), RNA → protein (the second step of protein synthesis) and RNA → RNA (RNA viruses copying themselves). There were two steps for which there was no evidence but that Crick thought were possible (hence the dotted lines in the figure): DNA → protein (this would mean RNA was not involved in protein synthesis) and RNA → DNA (structurally possible, but at the time, there no was no perceptible biological function).
Just as striking were the three flows of information that Crick considered to be impossible due to both lack of evidence and lack of biochemical mechanism. These were protein → protein, protein → RNA, and above all, protein → DNA. This was what Crick meant when he said that once information had gone from DNA into the protein, it could not get out of the protein and go back into the genetic code. This is the central dogma.
Crick admitted that the direct evidence for this hypothesis was ‘negligible’ and that it had a ‘speculative nature,’ but he defended his approach by pointing out that cosmologists had no qualms about constructing theories without adequate experimental data. That implicit comparison with grand theories of the universe is justified, for Crick was laying out the foundations of a new way of understanding how the cell works. The simplicity of the sequence hypothesis and the central dogma, together with the focus on information, brought a clear explanatory power to the synthesis of protein molecules that could take virtually any form and could ‘do almost anything,’ as Crick put it. Once the cell’s fundamental activity was conceived of in this way, everything fell into place. Crick advised his listeners to attempt to explain protein synthesis without these two basic principles—it was ‘an instructive exercise,’ he said. ‘One generally ends in the wilderness,’ he claimed.
Students are now often mistakenly taught that the central dogma is something like ‘DNA → RNA → protein’ (as popularised by Watson in his 1965 textbook Molecular Biology of the Gene [ 18 ]) or, even less precisely, ‘DNA makes RNA makes protein’ (as first suggested by Jean Brachet in 1960 [ 19 ]). This view, which went back to André Boivin in 1949 [ 20 ] and Alexander Dounce in 1953 [ 21 ], was very different to what Crick had in mind (it also confuses students, who often fail to grasp what the arrows mean or ‘makes’ implies [ 22 ]).
In 1970, following the discovery by Howard Temin and David Baltimore of reverse transcriptase, which enables information to flow in the direction RNA → DNA, Nature published an editorial entitled ‘Central dogma reversed’ [ 23 ]. Crick wrote a slightly tetchy response, repeating what he had actually stated in 1957, and rightly insisting that he had never argued that RNA → DNA was impossible [ 17 ]. In a distinctly undogmatic approach, he emphasised that our knowledge of cell biology was remarkably limited and that surprises might be in store, pointing to the example of the disease scrapie in which a protein seemed to act as an infectious agent (Stanley Prusiner later described this as a prion). However, even in the case of scrapie and other prion diseases, infection involves a change in conformation, not de novo synthesis.
Crick’s essential argument still holds: protein synthesis relies on nucleic acids, and once the genetic information has got into the protein, it cannot alter the DNA sequence. Despite recent excitement about transgenerational epigenetic inheritance due to histone modifications, DNA methylation, or other temporary modifications of material surrounding the genetic sequence, there is no evidence in any organism that the information in a DNA sequence can be rewritten from information in a protein.
In one aspect of the central dogma, Crick was mistaken. In reality, the ‘Central Dogma’ was anything but a dogma. Crick later claimed that he had not properly understood the meaning of ‘dogma’—Jacques Monod had to explain to him exactly what it meant. An indication of the truth of this assertion can be seen in the lecture when he states that the name that he has coined emphasizes the speculative nature of the idea—a dogma is not speculative. As Crick later acknowledged, a more accurate description would have been ‘basic assumption’ [ 17 ]. This does not sound quite so sexy, but it would have removed a lot of subsequent misunderstanding. Perhaps if Crick had not used such a dramatic turn of phrase, many subsequent critics would not have become so exercised about the question.
RNA and the adaptor
Crick used his lecture to publicly air another key idea about protein synthesis that he had been developing in private. In 1955, he circulated a note to the RNA Tie Club entitled ‘On degenerate templates and the adaptor hypothesis’ [ 24 ]. In this document, he argued that it was structurally impossible for any nucleic acid to act as a template for a particular amino acid; the duo of Crick and Brenner therefore came up with what Brenner called ‘the adaptor hypothesis’—an unknown class of molecule that would act like an electric plug adaptor, taking amino acids to the ribosome for protein assembly.
Crick was understandably unable to predict the nature of these adaptor molecules, but he felt that it was likely that they would contain nucleotides, which would be able to pair with both DNA and the RNA site of protein synthesis. Even allowing for the fact that he did not yet fully grasp the role of ribosomal RNA, Crick’s vision was astonishingly clear:
“The template could consist of perhaps a single chain of RNA…Each adaptor molecule containing, say, a di- or trinucleotide would each be joined to its own amino acid by a special enzyme. These molecules would then diffuse to the microsomal particles and attach to the proper place on the basis of the RNA by base-pairing.”
Crick and Brenner’s prediction would soon be proven correct—as Crick was giving his talk, Hoagland and Zamecnik were putting the finishing touches to their paper describing the isolation of the adaptor, which was eventually called tRNA [ 25 ].
Crick and evolutionary biology
There were two aspects of Crick’s lecture that related to evolutionary thinking. The first was that the central dogma supported the neo-Darwinian view that it was impossible for any character that was acquired during an organism’s life to affect its hereditary characters. This provided support for the widespread hostility to the view that had been held by Darwin, Lamarck, and others, according to which, patterns of use and disuse could lead to changes in the frequency of characters in subsequent generations.
Although in most organisms, including bacteria, plants, and even some animals, there is no separation between the copies of DNA used for protein synthesis and those used for transmitting genetic information to the next generation, Crick could see no conceivable mechanism whereby changes acquired during life could feed back into the DNA sequence. This was later considered to be an additional argument against Lamarckian inheritance and a reinforcement of Weismann’s separation of the germ and somatic cell lines (something that applies only to most animals) [ 2 ]. However, Crick did not mention either of these ideas.
The other evolutionary aspect to Crick’s lecture came in a brief and little-noticed aside, in which he essentially predicted the development of phylogenetics. In 1957, protein sequencing was extremely primitive, while sequencing DNA was two decades in the future. Complete amino acid sequences for insulin had been described for just five species, but nevertheless, Crick could see the way things would go. In an incredibly prescient prediction, he stated:
“Biologists should realise that before long we shall have a subject which might be called ‘protein taxonomy’—the study of the amino acid sequences of the proteins of an organism and the comparison of them between species. It can be argued that these sequences are the most delicate expression possible of the phenotype of an organism and that vast amounts of evolutionary information may be hidden away within them.”
This insight appears to have had little impact on thinking about the potential power of studying sequences—the history of bioinformatics [ 26 ] is generally traced back to the work of Dick Eck [ 27 ], Margaret Dayhoff [ 28 ], and Emile Zuckerkandl and Linus Pauling [ 29 ] in the early 1960s, none of whom cited Crick’s lecture. Further exploration of the work of the early bioinformaticians may reveal currently-unknown direct connections with Crick’s ideas, but whatever the case, the clarity of this vision underlines the power of Crick’s thinking.
It took some time for Crick’s lecture to exert its influence. Despite Jacob’s vivid description of how Crick presented his ideas, there is no indication that the content immediately changed the thinking of those in the audience. Only one of the other presentations at the symposium made any reference to Crick’s novel ideas in the revised printed version, and even here, the authors appear to have thought that Crick was indeed being dogmatic in his views because he speculated rather than strictly limiting himself to the experimental evidence [ 30 ].
Since then, the renown of the lecture has grown, and it has been cited over 800 times. The pattern of citations is U-shaped, with an early peak of 28 in 1962, followed by a trough of a handful of citations per year between 1971 and 1990, rising to 52 citations in 2014. Crick was later quite harsh on his lecture, describing it as ‘a mixture of good and bad ideas, of insight and nonsense’ [ 9 ]. This seems unfair—any nonsense is primarily due to lack of experimental evidence at the time. The reason why people still return to a 60-year-old lecture is because of the power of its ideas and the clarity with which they are presented. Crick’s style and intellectual verve continue to be both influential and inspirational; everyone should read or reread this brilliant lecture by one of the 20th century’s greatest scientists, a lecture that changed how we think.
- View Article
- PubMed/NCBI
- Google Scholar
- 2. Judson HF. The eighth day of creation: makers of the revolution in biology. Plainview: Cold Spring Harbor Laboratory Press; 1996.
- 3. Jacob F. The statue within. London: Unwin Hyman; 1988.
- 4. Crick FHC. On protein synthesis; 1957. Manuscript. Cold Spring Harbor Laboratory Archives, SB/11/5/4. http://libgallery.cshl.edu/items/show/52220 .
- 5. Cobb M. Life’s greatest secret: the race to crack the genetic code. London: Profile; 2015.
- 9. Crick F. What mad pursuit: a personal view of scientific discovery. Cambridge, Mass: Basic; 1998.
- 10. Watson JD. Genes, girls and Gamow. Oxford: Oxford University Press; 2001.
- 11. JD Watson to FHC Crick, 13 February 1954. Wellcome Library, Box 26 Folder PP/CRI/H/1/42/3. https://profiles.nlm.nih.gov/ps/access/SCBBWY.pdf
- 12. JD Watson to FHC Crick, 15 October 1954. Wellcome Library, Box 26 Folder PP/CRI/D/2/45. https://profiles.nlm.nih.gov/ps/access/SCBBJQ.pdf
- 15. Crick FHC. Ideas on protein synthesis (Oct. 1956). Unpublished note. Wellcome Library, PPCRI/H/2/6. https://wellcomelibrary.org/item/b18174139
- 18. Watson JD. Molecular biology of the gene. New York: W. A. Benjamin; 1965.
- 19. Brachet J. The biological role of nucleic acids. New York: Elsevier; 1965.
- 24. Crick FHC. On degenerate templates and the adaptor hypothesis. Unpublished note, RNA Tie Club; 1955. Wellcome Library, PPCRI/H/1/38. https://wellcomelibrary.org/item/b18186300
- 26. Stevens H. Life out of sequence: a data-driven history of bioinformatics. Chicago: University of Chicago Press; 2013.
- Search Menu
- Sign in through your institution
- Advance Articles
- Virtual Issues
- High-Impact Research Collection
- Celebrate 40 years of MBE
- Perspectives
- Discoveries
- Cover Archive
- Brief Communications
- Submission site
- Author guidelines
- Open access
- Self-archiving policy
- Reasons to submit
- About Molecular Biology and Evolution
- About the Society for Molecular Biology and Evolution
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, conclusions, materials and methods, supplementary material, acknowledgments, data availability.
- < Previous
Diversity and Molecular Evolution of Nonvisual Opsin Genes across Environmental, Developmental, and Morphological Adaptations in Frogs
- Article contents
- Figures & tables
- Supplementary Data
John L Boyette, Rayna C Bell, Matthew K Fujita, Kate N Thomas, Jeffrey W Streicher, David J Gower, Ryan K Schott, Diversity and Molecular Evolution of Nonvisual Opsin Genes across Environmental, Developmental, and Morphological Adaptations in Frogs, Molecular Biology and Evolution , Volume 41, Issue 6, June 2024, msae090, https://doi.org/10.1093/molbev/msae090
- Permissions Icon Permissions
Nonvisual opsins are transmembrane proteins expressed in the eyes and other tissues of many animals. When paired with a light-sensitive chromophore, nonvisual opsins form photopigments involved in various nonvisual, light-detection functions including circadian rhythm regulation, light-seeking behaviors, and seasonal responses. Here, we investigate the molecular evolution of nonvisual opsin genes in anuran amphibians (frogs and toads). We test several evolutionary hypotheses including the predicted loss of nonvisual opsins due to nocturnal ancestry and potential functional differences in nonvisual opsins resulting from environmental light variation across diverse anuran ecologies. Using whole-eye transcriptomes of 81 species, combined with genomes, multitissue transcriptomes, and independently annotated genes from an additional 21 species, we identify which nonvisual opsins are present in anuran genomes and those that are also expressed in the eyes, compare selective constraint among genes, and test for potential adaptive evolution by comparing selection between discrete ecological classes. At the genomic level, we recovered all 18 ancestral vertebrate nonvisual opsins, indicating that anurans demonstrate the lowest documented amount of opsin gene loss among ancestrally nocturnal tetrapods. We consistently found expression of 14 nonvisual opsins in anuran eyes and detected positive selection in a subset of these genes. We also found shifts in selective constraint acting on nonvisual opsins in frogs with differing activity periods, habitats, distributions, life histories, and pupil shapes, which may reflect functional adaptation. Although many nonvisual opsins remain poorly understood, these findings provide insight into the diversity and evolution of these genes across anurans, filling an important gap in our understanding of vertebrate opsins and setting the stage for future research on their functional evolution across taxa.
Animals rely on light detection to accomplish many biologically critical functions. Visual photosensitivity allows animals to acquire food, locate mates, and avoid predators. In recent years, the role of nonvisual photosensitivity has received increasing attention, revealing a diverse suite of physiologically important nonvisual functions including calibration of circadian rhythms, regulation of light-seeking behaviors, and initiation of seasonal reproductive changes ( Nakane et al. 2010 , 2014 ; Fernandes et al. 2012 ; Andrabi et al. 2023 ). The basis of animal photosensitivity lies in the conversion of light stimuli into neural stimuli—a process known as phototransduction—which is initiated by photopigments embedded in the membranes of light-sensitive cells. Each photopigment is composed of a transmembrane opsin protein encapsulating a light-sensitive chromophore. Different opsins confer distinct ranges of spectral sensitivity to their respective photopigments by maximally absorbing a specific wavelength of light. Upon absorption of light, the chromophore photoisomerizes, undergoing a conformational change that initiates phototransduction, generating a neural signal that can be interpreted for either visual or nonvisual functions ( Terakita 2005 ; Hunt and Collin 2014 ). The function of opsins in initiating phototransduction and light detection is illustrated in Fig. 1 .

General overview of opsin function in vertebrates exemplified using the eye of a frog ( Boana albomarginata , pictured here). 1) Light enters the eye and is focused on the retina. 2) Light reaches a photopigment (composed of an opsin and chromophore) embedded in the membrane of a light-sensitive retinal cell. The photopigment maximally absorbs a specific wavelength of light. In this example, the photopigment maximally absorbs blue light. 3) Absorption of light stimulates photoisomerization of the chromophore encapsulated within the opsin. 4) A neural signal is generated, processed in the retina and sent to the brain to be further processed and interpreted for visual or nonvisual purposes.
Opsins are divided broadly into eight groups based on amino-acid sequence similarity, molecular function, and signaling properties. The vertebrate visual opsin group contains opsins involved in initiating the formation of visual images, including rod opsin (RH1) and the cone opsins (LWS, RH2, SWS1, and SWS2). These opsins are associated with “bleaching” photopigments, meaning that following light exposure and photoisomerization, the chromophore dissociates from the opsin and renders the photopigment unreactive until the visual pigment can be regenerated with a new chromophore ( Tsukamoto 2014 ). Opsins in the retinal photoisomerase group (RGR) and the peropsin group (RRH) function to regenerate chromophores used by bleaching photopigments ( Terakita 2005 ; Radu et al. 2008 ; Zhang 2015 ). The other opsin groups include the encephalopsin/tmt-opsin group (OPN3 and TMT1 to 3), the Gq-coupled opsin/melanopsin group (OPN4m and OPN4x), the Go-coupled opsin group, the neuropsin group (NEUR1 to 6), and the paraphyletic vertebrate nonvisual opsin group (parietopsin [PAR], parapinopsin [PARA], vertebrate ancient opsin [VAOP], and pinopsin [PIN]), which forms a clade with the visual opsins. These opsin groups are generally found in photoreceptor cells in various major organs including the eyes, brain, and skin of many vertebrates (e.g. Foster and Bellingham 2004 ; Nakane et al. 2010 ; Davies et al. 2015 ; Kelley and Davies 2016 ). These opsins function in either bleaching or “nonbleaching” photopigments. Nonbleaching photopigments, also referred to as bistable photopigments, retain their chromophore following light exposure and photoisomerization. This is relevant to nonvisual photopigments because many are expressed in extraocular tissues that lack the specialized chromophore-regeneration mechanisms of the retina ( Tsukamoto 2014 ; Steindal and Whitmore 2020 ). For the purposes of this study, we broadly refer to all opsins outside the vertebrate visual opsin group as nonvisual opsins. A summary of vertebrate opsin diversity and known spectral sensitivities, tissue expression, and functions is presented in Fig. 2 .

Summarized diversity of exemplar spectral sensitivities, tissue expression, and functions across visual and nonvisual opsins. Phylogenetic hypothesis based on Beaudry et al. (2017) and Davies et al. (2015) . Peak absorbance measurements and corresponding color are displayed beside the taxa in which each measurement was observed. Note that these values may vary across lineages. Tissue expression based on transcriptomic profiles in zebrafish ( Davies et al. 2015 ). Citations for peak absorbance and functional overview notes can be found in supplementary table S1, Supplementary Material online.
The scope of opsin diversity coupled with the breadth of tissues and taxa expressing these genes suggests that the biological relevance of light detection extends far beyond the visual system. Among vertebrates, teleost fishes demonstrate the greatest opsin diversity, with 10 visual and 32 nonvisual opsins reported in zebrafish ( Davies et al. 2015 ). This diversity likely arose through whole-genome duplication events, and the retention of these opsin genes in zebrafish is hypothesized to confer an adaptive advantage in dynamic freshwater light environments. Mammals, on the other hand, have lost multiple opsins, with 11 opsins (two visual) inferred to have been lost ancestrally, and an additional three opsins (one visual) lost in placentals ( Gemmell et al. 2020 ). This disparity of opsin diversity across vertebrates emphasizes the importance of ecology in the evolution of opsin systems. For example, the loss of opsin diversity in mammals is hypothesized to result from a “nocturnal bottleneck” in which ancestral mammals transitioned to nocturnal lifestyles and encountered a reduced need for broad spectral sensitivity ( Gerkema et al. 2013 ; Borges et al. 2018 ). Ecological transitions to low-light environments are also thought to explain the loss of opsin diversity in other tetrapod taxa, including geckos ( Pinto et al. 2019 ), crocodilians ( Emerling 2017 ), snakes ( Davies et al. 2009 ; Schott et al. 2018 ; Gower et al. 2022 ), whales ( Meredith et al. 2013 ), burrowing rodents ( Emerling and Springer 2014 ), and nocturnal primates ( Kawamura and Kubotera 2004 ). These examples highlight the impact of low-light ecological transitions on opsin diversity and evolution; however, the influence of other environmental, developmental, and morphological adaptations remains poorly studied.
Frogs and toads (Anura, hereafter collectively “frogs”) provide an opportune system in which to investigate opsin diversity and evolution because they demonstrate remarkable variation in activity period, habitat, distribution, life history, and pupil shape (e.g. Wiens et al. 2006 ; Moen et al. 2013 ; Thomas et al. 2022a ). This variation exposes frogs to diverse light environments and sensory constraints, which in turn introduce unique evolutionary challenges to the nonvisual system that we hypothesize have driven functional adaptation, and loss, of nonvisual opsins. Specifically, the nocturnal bottleneck hypothesis exemplifies a connection between the evolution of nonvisual opsins and adaptation to new activity periods and habitats in several tetrapod groups but has not yet been investigated in anurans. We also hypothesize that species distribution has influenced nonvisual opsin functional evolution, because species distributed outside tropical zones experience more predictable seasonal variation in photoperiod ( Canavero and Arim 2009 ; Borah et al. 2019 ). Variation in both seasonality and photoperiod has implications for nonvisual opsin function because these proteins are involved in responses to seasonality ( Nakane et al. 2010 ) and regulation of circadian rhythm ( Göz et al. 2008 ). Many frog species also experience a dramatic shift in light environment across development as they metamorphose from aquatic larvae to terrestrial adults. We hypothesize that this biphasic life history subjects nonvisual opsins to disparate environmental constraints and selective pressures across metamorphosis, resulting in adaptive decoupling in biphasic frog species ( Schott et al. 2022 ). Furthermore, a subset of frog species, known as direct developers, lack a free-living aquatic larval stage, which provides an opportunity to test whether species with different life history strategies exhibit differences in selection across nonvisual opsins. Finally, frogs demonstrate a strikingly diverse suite of pupil shapes that regulate the amount of light reaching the retina through pupillary constriction ( Malmström and Kröger 2006 ; Thomas et al. 2022a ). We hypothesize that this morphological diversity is associated with nonvisual opsin evolution because these proteins have been implicated in the regulation of pupillary light responses ( Keenan et al. 2016 ). Taken together, the environmental, developmental, and morphological diversity of frogs makes them an attractive study system in which to investigate nonvisual opsin diversity and evolution.
Here, we extract nonvisual opsin genes from de novo whole-eye transcriptome assemblies of 81 frog species. Sampling only eye transcriptomes may provide an incomplete picture of nonvisual opsin diversity because these genes are expressed in many extraocular tissues; therefore, we supplement our whole-eye transcriptome sampling with publicly available genomes, multitissue transcriptomes, and independently annotated genes from an additional 21 species. Together, these 102 frog species represent 34 of 56 currently recognized frog families, including a broad sampling of environmental, developmental, and morphological adaptations. We predict that this variation has influenced the diversity and molecular evolution of the nonvisual opsins ( Fig. 3 ). We aim to (i) identify which nonvisual opsin genes are expressed in the eyes of frogs and test whether a nocturnal ancestry has driven opsin gene loss in frog genomes; (ii) compare selection among nonvisual opsin genes; and (iii) test hypotheses of adaptive evolution by comparing selection among frogs with differing ecologies.

Variation in adult activity period, adult habitat, distribution, life history, and pupil shape across our species sampling. Each column represents one of seven trait partitions used to analyze shifts in selective constraint across discrete environmental, developmental, and morphological transitions in frogs. Filled (colored) bubbles in trait columns indicate the foreground partition for selection analyses (e.g. diurnal). Unfilled (gray) bubbles indicate the background partition for selection analyses (e.g. nondiurnal). Phylogenetic hypothesis based on several large-scale phylogenetic studies ( Pyron and Wiens 2011 ; Feng et al. 2017 ; Jetz and Pyron 2018 ; Streicher et al. 2018 ). Trait coding citations are available in supplementary table S4, Supplementary Materials online. Photographs by M.K.F. ( Rhinophrynus dorsalis and Xenopus tropicalis ), J.W.S. and D.J.G. ( Lepidobatrachus laevis and Cornufer guentheri ), J.L.B. ( Brachycephalus pitanga , Haddadus binotatus , Vitreorana uranoscopa , Rhinella icterica , Gastrophryne olivacea , and L. catesbeianus ), Christian Irian ( Hyperolius tuberculatus ), and John Clare ( P. adspersus ).
Fourteen Nonvisual Opsins Consistently Expressed in Frog Eyes
Our total sampling included 92 whole-eye transcriptomes from 81 species, 19 genomes from 15 additional species, and multitissue transcriptomes or independently annotated genes from six additional species. Across the frog genomes, we recovered all 18 nonvisual opsins inferred to be present in the ancestral vertebrate ( Beaudry et al. 2017 ; Gemmell et al. 2020 ), although recovery success varied across genes. OPN3 was recovered from the fewest genomes (10), while NEUR4 and PIN were recovered in all 19 genomes ( supplementary table S5, Supplementary Material online). Most cases where a gene was not recovered are likely due to incomplete genome coverage and assembly. However, there is evidence for the loss of NEUR2 in some frog lineages because we were unable to recover this gene from the multiple hylid and bufonid genomes that are presently available (five species). Furthermore, this gene was not recovered from any of the eye transcriptomes with the exception of a single partial transcript in Spea bombifrons .
In terms of expression in the eye, we found that four genes ( NEUR2 , OPN3 , PAR , and PARA ) were expressed in very few samples (0 to 11). For the four species in which our sampling included both an eye transcriptome and a genome ( Lithobates catesbeianus , Pyxicephalus adspersus , Scaphiopus couchii , and S. bombifrons ), NEUR2 , OPN3 , PAR , and PARA were mostly absent from the eye transcriptome and present in the genome (with a few exceptions noted in supplementary table S5, Supplementary Material online). These four genes were dropped from downstream analyses because their low rates of recovery success limited our ability to generate reliable phylogenies and perform selection analyses. We recovered the remaining 14 nonvisual opsins with some degree of consistency (ranging from 34.2% to 94.7% recovery of whole or partial coding sequences, detailed in supplementary table S5, Supplementary Materials online) across our total sampling.
Evidence for Positive Selection in a Subset of Frog Nonvisual Opsins
To determine the overall selective constraint acting on each nonvisual opsin, we used the PAML M0 model to estimate the average rate ratio of nonsynonymous to synonymous substitutions ( d N / d S or ω ) across all codon sites in each gene alignment. These tests revealed fairly consistent selective constraint acting on frog nonvisual opsins, with most genes demonstrating mean ω values between 0.09 and 0.18 as illustrated in Fig. 4a . Only NEUR6 fell outside of this range, with an elevated mean ω value of 0.25. Taken together, all 14 nonvisual opsins have a mean ω < 1, indicating negative purifying selection. This is expected in most functional protein-coding genes, whose proteins are made up of a high proportion of invariable amino acids (with ω near 0) due to strong functional constraints ( Yang et al. 2000 ). However, genes demonstrating overall negative selection may still contain positively selected codon sites. We tested for this using the PAML M8 model, which unlike the M0 model, allows ω to vary between sites in a gene. The M8 model is compared with the null models M7 and M8a (which allow ω to vary but constrain ω ≤ 1) to test for the presence of positively selected sites using a likelihood ratio test (LRT). Using this approach, we found statistically significant positive selection at a proportion of sites in PIN , TMT1 , TMT2 , and VAOP as illustrated in Fig. 4b . The most extreme signature of positive selection was detected in PIN , which had an ω value of 3.17 (M8 vs. M8a: LRT = 13.2, P < 0.001). For comparison, the second most elevated signature of positive selection was observed in VAOP , with an ω value of 1.90 (M8 vs. M8a: LRT = 4.91, P = 0.027). Because the PAML M8 model estimates ω as a single parameter, it is possible for ω to be overestimated in instances of synonymous rate variation across a phylogeny. We tested for this using BUSTED with synonymous rate variation and identified statistically significant evidence of episodic diversifying selection in NEUR4 , NEUR5 , OPN4m , and OPN4x , which do not overlap with the genes identified by our PAML M8 results supplementary fig. S1, Supplementary Material online. Analyses with BUSTED that instead do not allow synonymous rate variation do not have evidence for positive selection in three of the four genes identified by M8 ( TMT1 , TMT2 , and VAOP ). PIN was significant with M8 and BUSTED without synonymous rate variation, but not BUSTED with synonymous rate variation; although the estimated rates were similar, the test was not significant (LRT = 3, P = 0.107). An opposite pattern is seen in two of the four genes identified with BUSTED where not allowing synonymous rate variation results in statistical nonsignificance ( NEUR4 and NEUR5 ), whereas no difference was found for the other two genes ( OPN4m and OPN4x ). These results suggest that not accounting for synonymous rate variation is not a cause of false inferences of positive selection but does result in statistical differences. Differences between BUSTED and M8 appear primarily due to differences in model formulation (including the use of a discretized beta distribution to determine site classes in M8 vs. three site classes in BUSTED). Overall, these results provide evidence for positive selection on frog nonvisual opsins and suggest that adaptive evolution may be occurring within a subset of these genes. Complete PAML random sites models and BUSTED results are presented in supplementary tables S6 and S7, Supplementary Material online, while the number and position of positively selected sites estimated with the PAML M8 model are detailed in supplementary table S8, Supplementary Material online.

Patterns of selective constraint across nonvisual opsin genes. a) The PAML M0 analysis averages ω values across all codon sites in a gene alignment. Among nonvisual opsins, NEUR6 had the most elevated ω while RGR had the least elevated ω , suggesting variation in selective constraint among nonvisual opsin genes. b) The PAML M8 analysis tests for the presence of positively selected codon sites in a gene alignment. The ω of the positively selected site class is shown. Four nonvisual opsins demonstrated statistically significant ( P < 0.05) evidence for positively selected sites (indicated with an asterisk). Note that graphs a) and b) use different scales along the x axes.
Shifts in Selective Constraint among Nonvisual Opsins Are Associated with Variation in Adult Activity Period, Adult Habitat, Distribution, Life History, and Pupil Shape
Because analyses of gene and species phylogenetic topologies sometimes yielded different significant results, we gave greater weight to results where the same partition was significant across analyses of both topologies and reported only those results here unless otherwise noted. Full results are available in supplementary tables S9 to S11, Supplementary Material online. Significant differences in selective constraint were detected for many of our trait partitions ( Fig. 5 ). The adult activity partition was significant in four genes and was the best-fit partition for OPN4x ( ω diu = 0.35/ ω nondiu = 0.28, P = 0.006), PIN ( ω diu = 0.35/ ω nondiu = 0.28, P = 0.051), and VAOP ( ω diu = 0.26/ ω nondiu = 0.21, P = 0.056), indicating that within each gene, the difference in selective constraint between foreground (diurnal) and background (nondiurnal) groups was greater than the difference in any other partition. The adult activity partitions for PIN and VAOP were significant for analyses of the gene topologies ( P = 0.016 and 0.039, respectively) and were marginally nonsignificant ( P = 0.051 and 0.056, respectively) for analyses of the species topologies, yet the adult activity partitions were the best fits for PIN and VAOP across both sets of topologies. Among the three adult habitat partitions, the aquatic partition was significant in three genes and was the best fit for NEUR3 ( ω aqu = 0.34/ ω nonaqu = 0.26, P = 0.003) and NEUR4 ( ω aqu = 0.30/ ω nonaqu = 0.21, P = 0.001). The scansorial partition was significant in two genes and was the best fit for NEUR6 ( ω sca = 0.48/ ω nonsca = 0.32, P < 0.001). The secretive partition was significant in one gene, with no best fits. The distribution partition was significant and a best fit for TMT3 ( ω trp = 0.26/ ω nontrp = 0.33, P = 0.019). Interestingly, the life history partition was the most frequent significant partition among nonvisual opsins, with eight genes having a significant difference in selective constraint between direct-developing and biphasic species. The life history partition was also the best-fit partition for five of these genes, including NEUR1 ( ω drd = 0.38/ ω bip = 0.28, P = 0.016), OPN4m ( ω drd = 0.48/ ω bip = 0.27, P < 0.001), RRH ( ω drd = 0.35/ ω bip = 0.24, P = 0.028), TMT1 ( ω drd = 0.44/ ω bip = 0.26, P < 0.001), and TMT2 ( ω drd = 0.44/ ω bip = 0.25, P < 0.001). Finally, because OPN4m has been implicated in regulating the pupillary light response, we tested the elongated pupil partition to explore how constricted pupil shape might relate more generally to nonvisual opsin evolution. The elongated pupil partition was not significant in OPN4m across topologies, but was otherwise significant in two genes, and was the best fit for RGR ( ω elp = 0.21/ ω nonelp = 0.33, P = 0.002).

Shifts in selective pressure on nonvisual opsin genes across frog trait partitions as illustrated in Fig. 3 . The ω ( d N / d S ) values of the divergent site class using CmC analysis of nonvisual opsin species topologies are shown, highlighting the difference between the foreground (filled circle) and the background (unfilled circle) partitions for each gene. Under each trait partition, genes with statistically significant ( P < 0.05) shifts in selection across analyses of both gene and species topologies are shown. Best-fit partitions are indicated with a star. Complete PAML results are available in supplementary tables S9 to S11, Supplementary Material online.
We used RELAX to test specific hypotheses regarding nonvisual opsin evolution in genes with known functions that demonstrated a significant shift in selective constraint across a partition. These analyses revealed significant evidence for relaxed selection acting on PIN , OPN4m , OPN4x , and VAOP in diurnal species ( K = 0.81, 0.11, 0.89, and 0.77, P ≤ 0.029). Furthermore, these analyses revealed evidence for relaxed selection in six of the eight significant direct-developing partitions, including NEUR1 ( K = 0.66, P = 0.004), NEUR3 ( K = 0.47, P = 0.002), NEUR6 ( K = 0.68, P = 0.007), OPN4m ( K = 0.11, P < 0.001), RRH ( K = 0.58, P = 0.006), and TMT2 ( K = 0.52, P < 0.001). A full summary of our RELAX results is available in supplementary table S12, Supplementary Material online.
Using a combination of de novo assembled whole-eye transcriptomes and previously published genomic and transcriptomic resources, we obtained nonvisual opsin sequences from 102 frog species spanning 34 families. We consistently recovered 14 nonvisual opsin genes from frog eye transcriptomes, and positive selection was detected in a subset of these genes, most notably PIN . We also found variation in selective constraint between frog lineages partitioned by adult activity period, adult habitat, distribution, life history, and pupil shape, which may reflect functional adaptation in frog nonvisual opsin genes. Below, we discuss these findings with respect to our current understanding of nonvisual opsin diversity, expression, function, and evolution.
Unexpected Nonvisual Opsin Diversity across Frogs
The common ancestor of all vertebrates is estimated to have had a genomic complement of 18 nonvisual opsins ( Beaudry et al. 2017 ; Gemmell et al. 2020 ). However, over the course of vertebrate evolution, nonvisual opsin diversity has shifted across groups. Nonvisual opsin gene losses have been most apparent in groups with primarily nocturnal evolutionary histories, including between nine and 11 losses in mammals, nine losses in snakes, five losses in geckos, and four losses in crocodilians ( Gemmell et al. 2020 ). These losses have all been attributed to a “nocturnal bottleneck” in which ancestors of each group transitioned to nocturnal lifestyles and encountered a reduced need for broad spectral sensitivity ( Emerling and Springer 2014 ; Borges et al. 2018 ; Pinto et al. 2019 ). Adults of most frog species are primarily nocturnal, and this activity period is thought to be the ancestral condition for anurans ( Anderson and Wiens 2017 ). Given this evolutionary history, we expected frogs to have reduced nonvisual opsin diversity compared to the common ancestor of vertebrates. Instead, we recovered remarkable nonvisual opsin diversity across frogs, with 14 of 18 nonvisual opsins consistently recovered from eye tissues. Of the four genes we failed to consistently recover, two genes, PAR and PARA , are thought to be expressed exclusively in the pineal region of the brain, and thus, it is not surprising that we had limited success recovering these nonvisual opsins from our eye transcriptome data set. The inconsistent recovery of the other two genes, NEUR2 and OPN3 , is less straightforward to understand. To our knowledge, there is no published expression profile or functional study of NEUR2 in any taxa, which limits our ability to speculate on why we failed to consistently recover this gene. On the other hand, OPN3 studies in other vertebrates report expression in many tissues, including the retina, brain, and liver ( Halford et al. 2001 ). Thus, the absence of OPN3 in all of our 92 frog eye transcriptomes is surprising given reports of its expression in eye or retinal tissue across diverse taxa, including human retinas ( Halford et al. 2001 ), chicken retinas ( Rios et al. 2019 ), and zebrafish eyes ( Davies et al. 2015 ). Although the retina is reported to have the greatest opsin diversity of any tissue ( Davies et al. 2015 ), some nonvisual opsins may be expressed at very low levels ( Do 2019 ), and there remains a possibility that both NEUR2 and OPN3 are expressed at levels below our threshold of detection, especially considering that we sequenced whole-eye tissue and not isolated retinal tissue.
Comparatively, across our whole-genome data set, we had much greater success recovering PAR and PARA , with whole or partial sequences recovered from 18 of 19 genomes for both genes ( supplementary table S5, Supplementary Materials online). This suggests that both PAR and PARA are retained in frogs; however, these genes appear to be expressed primarily in extraocular tissues. We also recovered NEUR2 and OPN3 with greater consistency across the whole-genome data set, with whole or partial sequences recovered in 11 and 10 genomes, respectively ( supplementary table S5, Supplementary Materials online). Additionally, for both NEUR2 and OPN3 , we found no evidence of nonfunctionalization among our recovered sequences, although we found preliminary support for the loss of NEUR2 in hylids and bufonids, based on consistent absence in the five genomes from those taxa. Consequently, we conclude that none of the ancestral vertebrate nonvisual opsins has been lost across frogs and, instead, frogs appear to have maintained a remarkably diverse repertoire of these genes.
Adaptive Decoupling, the Nocturnal Bottleneck, and an Alternative Hypothesis of Opsin Evolution
Frogs have maintained an unexpectedly diverse complement of nonvisual opsins, especially for a group demonstrating primarily nocturnal activity patterns. This may be due, at least in part, to the biphasic life history of most frogs. Although many fully metamorphosed frogs are nocturnal, this is not necessarily true of their aquatic larvae. Instead, many species that are nocturnal as adults are active and forage in daylight as larvae ( Beiswenger 1977 ; Griffiths and Mylotte 1988 ; Ding et al. 2014 ). This biphasic life history may subject nonvisual opsins to disparate environmental constraints and selective pressures across metamorphosis, resulting in adaptive decoupling between larvae active in bright light and adults active in low light ( Ebenman 1992 ; Schott et al. 2022 ). Adaptive decoupling may complicate our ability to test expectations of the nocturnal bottleneck hypothesis, which posits low-light adaptation as the most proximate driver of opsin evolution and diversity in many taxa ( Emerling 2017 ; Borges et al. 2018 ; Pinto et al. 2019 ), because it obscures the distinction between light-adapted and dark-adapted species.
In this study, we partitioned frogs based on adult activity period. This was necessary because larval activity period is poorly characterized across frogs. Despite this potential lack of nuance, a significant difference in ω between diurnal and nondiurnal frog species was detected in four genes with clade model C (CmC), and in each case, the diurnal species demonstrated elevated ω . This elevated rate of nonsynonymous nucleotide substitution among diurnal frogs may be the product of either relaxed or adaptive selection. Considering the nocturnal bottleneck hypothesis, we predicted that the elevated ω values observed in diurnal lineages are driven by intensified (i.e. adaptive) selection on nonvisual opsins because broad spectral sensitivity is likely adaptive in diurnal species. Our RELAX analyses did not support this prediction, instead revealing significant evidence for relaxed selection in all four genes ( OPN4m , OPN4x , PIN , and VAOP ; supplementary table S12, Supplementary Material online). Considering the unexpected diversity of frog nonvisual opsins alongside these selection signatures, it appears that expectations of the nocturnal bottleneck hypothesis do not hold true for frogs, and we suggest this may be due to widespread adaptive decoupling across frogs.
Through the lens of adaptive decoupling, our findings may offer support for an alternative hypothesis of opsin evolution. Beaudry et al. (2017) critiqued the nocturnal bottleneck hypothesis’ focus on evolutionary transitions to low-light ecologies and instead focused on developmental transitions as driving opsin evolution and diversity. They pointed to opsin losses in Mammalia, Aves, and Squamata, emphasizing that these groups undergo much of their development within the dark confines of a womb or shelled egg. By contrast, most amphibians and fishes undergo free-living larval development in relatively bright environments and appear to have retained large opsin repertoires ( Davies et al. 2015 ; this paper). Opsin diversity imparts sensitivity to a broad range of light wavelengths, which is likely beneficial to larval amphibians and fishes, whose translucent or transparent bodies make them particularly susceptible to photo-oxidative stress and DNA damage caused by exposure to light ( Beaudry et al. 2017 ). However, Beaudry et al. (2017) only considered opsin losses in Squamata at a coarse taxonomic scale and it should be noted that losses in squamates are restricted to ancestrally nocturnal groups (i.e. snakes and geckos; Gemmell et al. 2020 ). While this should cast doubt on the relevance of egg-based development in driving opsin diversity and evolution, the emphasis on development is still worthwhile. For example, recent annotation of the tuatara genome revealed only one opsin loss despite nocturnal ancestry ( Gemmell et al. 2020 ). This maintenance of opsin diversity was hypothesized to result from the tuatara's unusual life history, in which juvenile tuatara often adopt diurnal and arboreal lifestyles to avoid predation by cannibalistic adults, which hunt primarily at night. Thus, tuataras and many frogs experience disparate light environments across life stages, which may explain why these groups demonstrate the lowest documented levels of opsin gene loss among ancestrally nocturnal tetrapod groups.
The importance of adaptive decoupling in driving opsin evolution may further explain why the life history partition was the most frequently significant partition among nonvisual opsins, with eight genes demonstrating a significant difference in ω between direct-developing and biphasic frog species with CmC. The life history partition was also the best fit for five of these genes, and in each case, the direct-developing species demonstrated elevated ω . Considering the adaptive decoupling hypothesis, we predicted that elevated ω values in direct-developing lineages are driven by relaxed selection on nonvisual opsins because these genes are less adaptive in species without larvae. Our RELAX analyses supported this prediction, revealing significant evidence for relaxed selection in six of the genes with significant direct-development CmC partitions ( NEUR1 , NEUR3 , NEUR6 , OPN4m , RRH , and TMT2 ; supplementary table S12, Supplementary Material online). In parallel analyses of frog visual opsins, we did not observe the same pattern of repeated significance across direct-development partitions ( Schott et al. 2024a ), suggesting that the pattern observed in nonvisual opsins is specific to these genes and not necessarily reflective of broadly elevated ω in photosensitivity genes across the genomes of direct-developing species. Collectively, these results support the hypothesis that many nonvisual opsins are especially relevant in species whose complex life histories expose them to disparate light environments across development.
OPN4, Behavioral Light Responses, and Life History
In zebrafish larvae, OPN4 plays a role in triggering nondirectional, stochastic hyperactivity in darkness, resulting in the aggregation of larvae into illuminated areas where they remain due to reduced activity ( Fernandes et al. 2012 ). This behavior, known as dark photokinesis, is understood to drive zebrafish larvae out of dark areas, allowing them to maintain a homeostatic distribution in illuminated waters. Similar behavioral light responses have been observed in frog larvae (e.g. Muntz 1963 ; Jaeger and Hailman 1976 ; Beiswenger 1977 ; Roberts 1978 ; Branch 1983 ; Fraker 2008 ; Ding et al. 2014 ). Given these observations, we hypothesized that OPN4 may play a role in regulating the behavioral light responses of frog larvae. We tested this hypothesis using the direct-development partition from our PAML clade-model analyses with the two OPN4 genes found in frogs. We found a significant difference in selective constraint acting on OPN4m between direct-developing and biphasic frog species, with the former demonstrating elevated ω . Our RELAX analysis indicated that this elevated ω is the result of relaxed selection in direct-developing species. Considering our understanding of OPN4's function in larval zebrafish, the relaxed selection observed in direct-developing frogs may be evidence of reduced functional relevance in species without aquatic larvae. Furthermore, OPN4m is differentially expressed in leopard frog eyes across metamorphosis, with significantly reduced expression in fully metamorphosed juveniles compared to larvae ( Schott et al. 2022 ). Taken together, these findings suggest that the function of OPN4m is especially relevant in larval frogs, potentially contributing to behavioral light responses. However, further functional study in a broad diversity of frogs is needed to support this hypothesis.
PIN and Low-Light Photoreception
PIN was first discovered in the chicken pineal gland in 1994, making it the first opsin to be characterized in an extraocular organ ( Okano et al. 1994 ). Because expression of PIN was initially observed only in the pineal gland, the opsin was thought to function strictly in regulating the production and secretion of melatonin ( Csernus et al. 1999 ). However, low levels of PIN expression were later reported in the outer retina of a gecko ( Taniguchi et al. 2001 ) and more recently in rod cells in the outer retina of a fish and frog ( Sato et al. 2018 ). In addition to being expressed in rod cells, PIN also exhibits a thermal isomerization rate strikingly similar to that of RH1, the rod visual opsin responsible for high-sensitivity dim-light vision. This observation suggests that, at least in the eyes of fishes and frogs, PIN may function as an RH1-like visual pigment contributing to low-light photoreception ( Sato et al. 2018 ). We found a significant difference in selective constraint acting on PIN between diurnal and nondiurnal frogs, with diurnal species having an elevated ω value. Considering PIN's hypothesized role in low-light photoreception, we predicted that this elevated ω value is the result of relaxed selective constraint in diurnal species, which are likely less dependent on low-light photoreception to sense their environments. The RELAX analysis supports this hypothesis, revealing significant evidence for relaxed selection in diurnal species and supporting PIN's hypothesized role in low-light photoreception.
Nonvisual Opsins and Seasonality
Tropically distributed species are typically exposed to less seasonal variation in photoperiod, often causing them to rely on humidity cues to synchronize physiological and behavioral changes with seasonality ( Canavero and Arim 2009 ; Borah et al. 2019 ). In amphibians, seasonal precipitation has historically received the most attention as a potential cue stimulating physiological and behavioral changes related to reproduction ( Feder and Burggren 1992 ; Duellman and Trueb 1994 ; Stebbins and Cohen 1997 ). However, growing evidence indicates that photoperiod sensitivity may be the most proximal factor determining seasonal changes in physiology and activity in amphibians ( Canavero and Arim 2009 ). Nonvisual opsins have been implicated in the seasonal sensitivity of birds (specifically NEUR1; Nakane et al. 2010 ) and may serve a similar function in frogs. To test if selection signatures support this hypothesis, the distribution partition was designed to approximate exposure to seasonal variation in tropical versus nontropical taxa. We found a significant difference in selective constraint of a single gene ( TMT3 ) between tropical and nontropical frogs, with nontropical species demonstrating elevated ω values.
As far as we are aware, nothing is known about TMT3 function in frogs, and more broadly, little is known about its function in vertebrates. We had predicted the distribution partition would be significant for NEUR1, which is reported to regulate seasonal reproductive changes in quail, including thyroid-stimulating hormone secretion and subsequent testicular growth ( Nakane et al. 2014 ). However, NEUR1 showed differences in selective constraint only for the direct-developing partition. Like birds, frogs demonstrate many conspicuous seasonal changes associated with breeding. These changes include hormone secretion and testicular growth ( Delgado et al. 1989 ), as well as nuptial pad development ( Willaert et al. 2013 ) and dynamic sexual dichromatism ( Bell et al. 2017 ). One particularly well-studied example of seasonal reproductive changes is found in subtropical Leishan mustache toads ( Leptobrachium leishanense ). During the breeding season, males of this species develop mustache-like nuptial spines on their maxillary skin. The development of these spines has been linked to seasonal steroid biosynthesis and thyroid hormone secretion ( Li et al. 2019 ), implicating similar hormonal pathways to those activated by NEUR1 in quail. It is possible that TMT3, rather than NEUR1, is contributing to seasonal sensitivity and reproductive changes in frogs. TMT2, a very closely related opsin, has been implicated in behavioral adjustment of medaka fish in response to the onset of cold temperatures ( Zekoll et al. 2021 ), creating a precedent for seasonally linked function within the tmt-opsin group. If TMT3 is playing a role in the seasonal response, we would expect that this elevated ω is the product of adaptive selection in nontropical species due to the greater seasonal variation in the photoperiod they experience. However, our RELAX analysis revealed no signature of relaxed or adaptive selection in nontropical species. This may be because partitioning species as tropical or nontropical fails to capture exactly which species rely on photoperiod to cue seasonal reproduction. A better test of TMT3's relevance to seasonal reproduction might be to partition frogs that are observed to reproduce continuously throughout the year, such as the tropical toad Duttaphrynus melanostictus ( Jørgensen et al. 1986 ) and frogs that reproduce on a strictly seasonal basis. This test would require a more extensive understanding of seasonal reproduction in frogs than is available in current literature, as well as denser taxonomic sampling of nonvisual opsins. Given our findings, TMT3 warrants further study to clarify its role in frogs, including the possibility of regulating seasonal reproductive changes and as a candidate for the basis of seasonal sensitivity in frogs.
Frogs offer a compelling system in which to study the evolution of light sensitivity across diverse ecologies, morphologies, and life history strategies. We found that frogs have retained a diverse repertoire of nonvisual opsins, with 14 genes consistently recovered from frog eye transcriptomes. At the genomic level, frogs appear to have broadly maintained all 18 ancestral vertebrate nonvisual opsins and thus demonstrate the lowest documented rate of opsin gene loss among ancestrally nocturnal tetrapod groups. Signatures of positive selection were detected in a subset of these genes. We also found variation in selective constraint between discrete ecological, life history, and morphological classes, which may reflect functional adaptation in frog nonvisual opsin genes. Our findings provide genomic support for emerging hypotheses of nonvisual opsin evolution, including the role of PIN in low-light photoreception and the adaptive importance of many nonvisual opsins in species with complex life histories.
Species Sampling
Our sampling included a total of 92 whole-eye transcriptomes from 81 species, 19 genomes from 15 additional species, and multitissue transcriptomes or independently annotated genes from 6 additional species. Of the 92 whole-eye transcriptomes, 83 of these samples were collected from adult frogs, and the remaining nine were collected from larval frogs ( supplementary table S2, Supplementary Materials online). Frogs were sampled from wild populations in Australia, Brazil, Cameroon, Ecuador, Equatorial Guinea, French Guiana, Gabon, the Seychelles, the United Kingdom, and the United States ( supplementary table S2, Supplementary Materials online). Additional species were obtained from commercial dealers or captive colonies. Most individuals were kept in complete darkness (i.e. were dark adapted) for 3+ h prior to euthanasia (via immersion in a solution of MS222) because one eye was removed for microspectrophotometry measurements as part of another study ( Schott et al. 2024a ). Whole eyes were extracted, punctured, and placed in RNAlater (Ambion) for at least 24 h at 4 °C to allow the RNAlater to saturate the cells prior to freezing and storage at −80 °C until use. Some samples were collected at remote field sites and were kept as cool as possible in RNAlater prior to freezing at −80 °C at the earliest opportunity. Voucher specimens and tissues for further genetic analysis were accessioned in natural history museums ( supplementary table S2, Supplementary Materials online). To supplement the phylogenetic and ecological diversity of species we collected, we searched GenBank for publicly available frog genomes and multitissue transcriptomes to include in our analyses. These included 19 genomes and 6 multitissue transcriptomes or independently annotated genes from adult, larval, or mixed adult/larval frog samples ( supplementary table S3, Supplementary Materials online). Our combined sampling includes 102 species, representing 34 of 56 currently recognized frog families ( Frost 2024 ; Fig. 3 ).
Transcriptome Sequencing and Assembly
Total RNA was extracted from whole eyes using the Promega Total SV RNA Extraction Kit (Promega). Tissue was homogenized in lysis buffer using the Qiagen Tissuelyzer (10 min at 20 Hz). Messenger RNA library construction was performed using the Kapa HyperPrep mRNA Stranded with Riboerase Kit (Roche). Each indexed sample was pooled in equimolar amounts and sequenced with paired-end 150-bp reads on a HiSeq4000 at the QB3 Genomics Core at the University of California, Berkeley or a NovaSeq6000 at the UT Arlington North Texas Genome Center. Prior to assembly, adapters and low-quality bases ( q < 5) were removed with TrimGalore! ( https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ ), which implements Cutadapt ( Martin 2011 ). Read pairs shorter than 36 bp after trimming were discarded, as were unpaired reads. The quality of processed reads was assessed with FastQC ( http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ ). Transcriptome assembly of each sample was performed de novo using Trinity ( Grabherr et al. 2011 ) incorporating all paired reads following the standard protocol.
Nonvisual Opsin Sequence Recovery
Eighteen nonvisual opsin coding sequences were extracted from frog genomes, consisting of six neuropsins ( NEUR1 to 6 ), encephalopsin ( OPN3 ), two melanopsins ( OPN4m and OPN4x ), PAR , PARA , PIN , retinal g-protein coupled receptor ( RGR ), RRH , three teleost multiple tissue opsins ( TMT1 to 3 ), and VAOP , using query sequences from tetrapods ( Gemmell et al. 2020 ) and nucleotide BLAST searches ( Altschul et al. 1990 ). We used the discontiguous megablast approach with an e -value cutoff of 1 × 10 −10 to identify transcript hits, which were imported into MEGA, aligned with the reference, and manually trimmed to the coding sequence ensuring the longest open-reading frame was recovered. Gene identifications were confirmed by BLAST searches against the NCBI nucleotide database and later by phylogenetic analysis. Partial transcripts of the same gene (e.g. due to incomplete transcript assembly or sequence coverage) were combined to produce as complete a coding sequence as possible. We found that fully automated approaches often produced incomplete or incorrect transcripts. Complete sequences were compiled and in turn used as queries for additional BLAST extraction from the frog eye transcriptomes and genomes and for searches against the NCBI nucleotide database. BLAST results from whole genomes were extracted using a custom parser. Recovered coding sequences for each gene were assembled and aligned using codon alignment with MACSE ( Ranwez et al. 2011 ). Alignments were manually edited to remove terminal stop codons, remove unique insertions, and trim nonhomologous regions that were due to annotation errors or transcript variants. When present, premature stop codons were converted to sequence gaps to enable their inclusion in downstream analyses ( Yohe et al. 2017 ; Janiak et al. 2018 ). Several genes showed considerable variation at the beginning or ends and these were aligned using PRANK codon alignment, which can better resolve insertions, but at the cost of increased computation time ( Löytynoja and Goldman 2005 ). A coding sequence was considered whole if it was recovered in entirety from start to stop codon, and partial if the sequence was incomplete but more than 50% of codons were recovered relative to the reference sequence. Coding sequences were excluded from analyses if less than 50% of codons were recovered.
Species Trait Classification
Frog species were partitioned into seven binary trait categories that we predicted to influence the evolution of nonvisual light detection. The adult activity period partition separated diurnal (including strictly diurnal and mixed diurnal/nocturnal species) vs. nondiurnal species. Our three adult habitat partitions separated aquatic (including semiaquatic) vs. nonaquatic, scansorial vs. nonscansorial, and secretive (defined as species with lifestyles generally dominated by low-intensity light, including fossorial, burrowing, and leaf-litter species) vs. nonsecretive. We used a distribution partition as a proxy for seasonality, separating tropical vs. nontropical species. Our life history partition separated direct developing vs. biphasic species. Finally, our pupil shape partition separated species with elongated (horizontal or vertical pupils) vs. nonelongated pupils (other symmetrical shapes), because this distinction appears to be ecologically meaningful in frogs ( Thomas et al. 2022a ). Partitions for all traits are illustrated in Fig. 3 . We used peer-reviewed literature, online natural history resources, field guides, and field observations to partition species into these trait categories ( supplementary table S4, Supplementary Materials online). These partitions largely conform to trait scoring in previous studies of frog visual biology ( Thomas et al. 2020 , 2022a , 2022b ; Mitra et al. 2022 ; Schott et al. 2024a ).
Selection Analyses
To estimate the strength and form of selection acting on anuran nonvisual opsins, each data set was analyzed using codon-based likelihood models from the codeml program of the PAML 4 software package ( Yang 2007 ). Maximum likelihood (ML) gene trees were inferred using PhyML 3 ( Guindon and Gascuel 2003 ) under the GTR + G + I nucleotide model with a BioNJ starting tree, the best of NNI and SPR tree improvement, and approximate Bayes-like branch supports (aByes; Anisimova et al. 2011 ). Because individual gene trees do not always reflect species’ evolutionary histories, it is a common approach in selection analyses to compare results from gene-tree topologies to those that reflect the current understanding of evolutionary relationships among species (species-tree topology) to ensure that results are robust to minor topological differences (e.g. Schott et al. 2018 ; Van Nynatten et al. 2021 ). To produce species-tree topologies for each gene, we generated a topology that matched expected species relationships based on several large-scale phylogenies ( Pyron and Wiens 2011 ; Feng et al. 2017 ; Jetz and Pyron 2018 ; Streicher et al. 2018 ) and trimmed these to match the taxon sampling of the individual genes ( Boyette et al 2024 ).
All analyses were performed twice; once using the ML gene-tree topology, and again using the species-tree topology. All topologies were rooted on Ascaphus truei and modified to contain the basal trichotomy required by PAML 4. PAML analyses were carried out for each alignment with the two tree topologies using the BLASTPHYME interface ( Schott et al. 2019 ). Random site models (M0, M1a, M2a, M2a_rel, M3, M7, M8a, and M8) were used to estimate the rates of nonsynonymous to synonymous nucleotide substitutions ( ω or d N / d S ), infer alignment-wide selection patterns, and test for positive selection acting on nonvisual opsin genes. For genes with evidence of positively selected sites, we used BUSTED ( Murrell et al. 2015 ), implemented on the Datamonkey webserver ( Delport et al. 2010 ), to test for evidence of synonymous rate variation that could influence our estimates of positive selection. To test if shifts in selection among nonvisual opsins corresponded to variation in adult activity period, adult habitat, distribution, life history, and pupil shape, we used PAML CmC ( Bielawski and Yang 2004 ). These clade models test for evidence of a codon site class demonstrating a shift in selection between prepartitioned “foreground” and “background” groups (e.g. diurnal frogs and nondiurnal frogs), which can be any combination of branches and clades within a phylogeny. CmC is compared to the null model M2a_rel and assumes that some sites evolve conservatively across the phylogeny (two classes of sites where 0 < ω 0 < 1 and ω 1 = 1), whereas a class of sites is free to evolve differently among two or more partitions (e.g. ω D1 > 0 and ω D1 ≠ ω D2 > 0; Weadick and Chang 2012 ). The partition schemes were tested in each nonvisual opsin, with each partition corresponding to one of the seven binary trait categories illustrated in Fig. 3 . PAML analyses were run using varying ω starting values (1, 2, and 3) to increase the likelihood of finding global optima. If models failed to converge (worse likelihood score than the null model), we increased the range and frequency of starting values (e.g. 0.5 intervals from 0.5 to 3.5). Significance and best fit among model pairs were determined using a LRT with a χ 2 distribution and a significance threshold of 5% ( α = 0.05).
In cases where PAML analyses found significantly elevated ω in a particular group of interest (e.g. elevated ω in diurnal frogs compared to nondiurnal frogs), we used RELAX ( Wertheim et al. 2015 ), implemented on the Datamonkey web server ( Delport et al. 2010 ), to determine if the elevated ω was the product of relaxed selective constraint (i.e. lack of selection against a change) or adaptive selection (i.e. selection for a change). Such a distinction is useful when attempting to interpret the biological significance of an elevated ω value. RELAX produces a selection intensity parameter, or K value, which modulates the degree to which different ω site classes diverge from neutrality ( ω = 1) in prepartitioned background and foreground groups. When K < 1, this indicates relaxed selection on foreground group branches compared to background group branches. Alternatively, when K > 1, this indicates adaptive selection on foreground group branches compared to background group branches.
Supplementary material is available at Molecular Biology and Evolution online.
We thank the following field companions who helped obtain specimens for this work: Hannah Augustijnen, Abraham G. Bamba Kaya, C. Guillherme Becker, Gabriela Bittencourt-Silva, Itzue Calviedes Solis, Patrick Campbell, Diego Cisneros-Heredia, Simon Clulow, Christian L. Cox, Mateo Davila, Paul Doughty, Juvencio Eko Mengue, TJ Firneno, Carl Franklin, Philippe Gaucher, Ivan Gomez- Mestre, Shakuntala Devi Gopal, Jon and Krittee Gower, Célio F. B. Haddad, Anthony Herrel, Sunita Janssenswillen, Jim Labisko, H. Christoph Liedtke, Simon Loader, Simon Maddock, Michael Mahony, Renato A. Martins, Matthew McElroy, Christopher Michaels, Nicki Mitchell, Justino Nguema Mituy, Diego Moura, Martin Nsue, Daniel M. Portik, Ivan Prates, Kim Roelants, Corey Roelke, Lauren Scheinberg, Bruno Simões, Ben Tapley, Elie Tobi, Rose Upton, Mark Wilkinson, and Molly Womack. We thank the Gabon Biodiversity Program and Bioko Biodiversity Protection Program for logistical support in the field; Grant Webster, Scott Keogh, and Jared Grummer for advice on where to find key species; Carolina Reyes-Puig for help with specimen numbers; and Jodi Rowley and Stephen Mahony for assistance exporting tissues for analysis. Sampling was conducted following IACUC protocols (NHMUK, NMNH 2016-012, UNESP Rio Claro CEUA-23/2017, UTA A17.005, ANU A2017/47) and with scientific research authorizations (the United States: Texas Parks and Wildlife Division SR-0814-159, North Cascades National Parks NCCO-2018-SCI-0009; Brazil: ICMBio MMA 22511-4, ICMBio SISBIO 30309-12; the United Kingdom: NE Licence WML-OR04; French Guiana: RAA: R03-2018-06-12-006; Gabon: CENAREST AR0020/17; Australia: New South Wales National Parks & Wildlife Service SL102014, Queensland Department of National Parks WITK18705517; Equatorial Guinea: INDEFOR-AP 0130/020-2019). This research was supported by grants from the Natural Environment Research Council, UK (NE/R002150/1), the National Science Foundation (DEB-1655751), and an NSERC Discovery Grant (to R.K.S.). J.L.B. was supported by the NMNH Natural History Research Experience REU program (NSF-OCE:1560088). The authors thank two anonymous reviewers and the Associate Editor for constructive feedback that substantially improved the manuscript.
The data underlying this article are available on NCBI under Bioproject PRJNA1073881 and on Zenodo ( Boyette et al. 2024 ; Schott, Fujita, Streicher, Gower, Thomas, and Bell 2024 ), as well as in the Supplementary Materials online. See supplementary table S2, Supplementary Materials online for individual BioSample and SRA accession numbers.
Altschul SF , Gish W , Miller W , Myers EW , Lipman DJ . 1990 . Basic local alignment search tool . J Mol Biol 215 ( 3 ): 403 – 410 . https://doi.org/10.1016/S0022-2836(05)80360-2 .
Google Scholar
Anderson SR , Wiens JJ . 2017 . Out of the dark: 350 million years of conservatism and evolution in diel activity patterns in vertebrates . Evolution 71 ( 8 ): 1944 – 1959 . https://doi.org/10.1111/evo.13284 .
Andrabi M , Upton BA , Lang RA , Vemaraju S . 2023 . An expanding role for nonvisual opsins in extraocular light sensing physiology . Annu Rev Vis Sci 9 ( 1 ): 245 – 267 . https://doi.org/10.1146/annurev-vision-100820-094018 .
Anisimova M , Gil M , Dufayard J-F , Dessimoz C , Gascuel O . 2011 . Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes . Syst Biol 60 ( 5 ): 685 – 699 . https://doi.org/10.1093/sysbio/syr041 .
Beaudry FEG , Iwanicki TW , Mariluz BRZ , Darnet S , Brinkmann H , Schneider P , Taylor JS . 2017 . The non-visual opsins: eighteen in the ancestor of vertebrates, astonishing increase in ray-finned fish, and loss in amniotes . J Exp Zool B: Mol Dev Evol 328 ( 7 ): 685 – 696 . https://doi.org/10.1002/jez.b.22773 .
Beiswenger RE . 1977 . Diel patterns of aggregative behavior in tadpoles of bufo americanus, in relation to light and temperature . Ecology 58 ( 1 ): 98 – 108 . https://doi.org/10.2307/1935111 .
Bell RC , Webster GN , Whiting MJ . Breeding biology and the evolution of dynamic sexual dichromatism in frogs . J Evol Biol . 2017 : 30 ( 12 ): 2104 – 2115 . https://doi.org/10.1111/jeb.13170 .
Bielawski JP , Yang Z . 2004 . A maximum likelihood method for detecting functional divergence at individual codon sites, with application to gene family evolution . J Mol Evol 59 ( 1 ): 121 – 132 . https://doi.org/10.1007/s00239-004-2597-8 .
Borah BK , Renthlei Z , Trivedi AK . 2019 . Seasonality in terai tree frog ( Polypedates teraiensis ): role of light and temperature in regulation of seasonal breeding . J Photochem Photobiol B: Biol 191 : 44 – 51 . https://doi.org/10.1016/j.jphotobiol.2018.12.005 .
Borges R , Johnson WE , O’Brien SJ , Gomes C , Heesy CP , Antunes A . 2018 . Adaptive genomic evolution of opsins reveals that early mammals flourished in nocturnal environments . BMC Genomics 19 ( 1 ): 121 . https://doi.org/10.1186/s12864-017-4417-8 .
Boyette JL , Bell RC , Fujita MK , Thomas KN , Streicher JW , Gower DJ , Schott RK . 2024 . Data from: diversity and molecular evolution of non-visual opsin genes across environmental, developmental, and morphological adaptations in frogs. [Data set] . Zenodo . https://doi.org/10.5281/zenodo.10573191 .
Branch L . 1983 . Social behavior of the tadpoles of phyllomedusa vaillanti . Copeia 1983 ( 2 ): 420 . https://doi.org/10.2307/1444385 .
Canavero A , Arim M . 2009 . Clues supporting photoperiod as the main determinant of seasonal variation in amphibian activity . J Nat History 43 ( 47-48 ): 2975 – 2984 . https://doi.org/10.1080/00222930903377539 .
Csernus V , Becher P , Mess B . 1999 . Wavelength dependency of light-induced changes in rhythmic melatonin secretion from chicken pineal gland in vitro . Neuro Endocrinol Lett 20 : 299 – 304 .
Davies WIL , Cowing JA , Bowmaker JK , Carvalho LS , Gower DJ , Hunt DM . 2009 . Shedding light on serpent sight: the visual pigments of henophidian snakes . J Neurosci. 29 ( 23 ): 7519 – 7525 . https://doi.org/10.1523/JNEUROSCI.0517-09.2009 .
Davies WIL , Tamai TK , Zheng L , Fu JK , Rihel J , Foster RG , Whitmore D , Hankins MW . 2015 . An extended family of novel vertebrate photopigments is widely expressed and displays a diversity of function . Genome Res 25 ( 11 ): 1666 – 1679 . https://doi.org/10.1101/gr.189886.115 .
Delgado MJ , Gutirrez P , Alonso-Bedate M . Seasonal cycles in testicular activity in the frog, Rana perezi . Gen Comp Endocrinol . 1989 : 73 ( 1 ): 1 – 11 . https://doi.org/10.1016/0016-64808990049-X .
Delport W , Poon AFY , Frost SDW , Kosakovsky Pond SL . 2010 . Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology . Bioinformatics 26 ( 19 ): 2455 – 2457 . https://doi.org/10.1093/bioinformatics/btq429 .
Ding G-H , Lin Z-H , Zhao L-H , Fan X-L , Wei L . Effects of light intensity on activity in four sympatric anuran tadpoles . Zool Res . 2014 : 35 : 332 . https://doi.org/10.13918/j.issn.2095-8137.2014.4.332 .
Do MTH . 2019 . Melanopsin and the intrinsically photosensitive retinal ganglion cells: biophysics to behavior . Neuron 104 ( 2 ): 205 – 226 . https://doi.org/10.1016/j.neuron.2019.07.016 .
Duellman WE , Trueb L . Biology of amphibians . Baltimore (MD) : Johns Hopkins University Press ; 1994 .
Google Preview
Ebenman B . 1992 . Evolution in organisms that change their niches during the life cycle . Am Nat 139 ( 5 ): 990 – 1021 . https://doi.org/10.1086/285370 .
Emerling CA . 2017 . Archelosaurian color vision, parietal eye loss, and the crocodylian nocturnal bottleneck . Mol Biol Evol 34 ( 3 ): 666 – 676 . https://doi.org/10.1093/molbev/msw265 .
Emerling CA , Springer MS . 2014 . Eyes underground: regression of visual protein networks in subterranean mammals . Mol Phylogenet Evol 78 : 260 – 270 . https://doi.org/10.1016/j.ympev.2014.05.016 .
Feder ME , Burggren WW . Environmental physiology of the amphibians . Chicago (IL) : University of Chicago Press ; 1992 . https://press.uchicago.edu/ucp/books/book/chicago/E/bo3636401.html .
Feng Y-J , Blackburn DC , Liang D , Hillis DM , Wake DB , Cannatella DC , Zhang P . Phylogenomics reveals rapid, simultaneous diversification of three major clades of Gondwanan frogs at the Cretaceous–Paleogene boundary . Proc Natl Acad Sci . 2017 : 114 : E5864 . https://doi.org/10.1073/pnas.1704632114 .
Fernandes AM , Fero K , Arrenberg AB , Bergeron SA , Driever W , Burgess HA . 2012 . Deep brain photoreceptors control light-seeking behavior in zebrafish larvae . Curr Biol 22 ( 21 ): 2042 – 2047 . https://doi.org/10.1016/j.cub.2012.08.016 .
Foster RG , Bellingham J . 2004 . Inner retinal photoreceptors (IRPs) in mammals and teleost fish . Photochem Photobiol Sci. 3 ( 6 ): 617 – 627 . https://doi.org/10.1039/b400092g .
Fraker ME . 2008 . The influence of the circadian rhythm of green frog ( Rana clamitans ) tadpoles on their antipredator behavior and the strength of the nonlethal effects of predators . Am Nat 171 ( 4 ): 545 – 552 . https://doi.org/10.1086/528961 .
Frost DR. 2024 . Amphibian species of the world: an online reference. Version 6.2 (January 26, 2024). Electronic Database accessible at https://amphibiansoftheworld.amnh.org . American Museum of Natural History, New York, USA [Internet]. https://amphibiansoftheworld.amnh.org/index.php
Gemmell NJ , Rutherford K , Prost S , Tollis M , Winter D , Macey JR , Adelson DL , Suh A , Bertozzi T , Grau JH , et al. 2020 . The tuatara genome reveals ancient features of amniote evolution . Nature 584 ( 7821 ): 403 – 409 . https://doi.org/10.1038/s41586-020-2561-9 .
Gerkema MP , Davies WIL , Foster RG , Menaker M , Hut RA . The nocturnal bottleneck and the evolution of activity patterns in mammals . Proc R Soc B Biol Sci . 2013 : 280 ( 1765 ): 20130508 . https://doi.org/10.1098/rspb.2013.0508 .
Gower DJ , Hauzman E , Simões BF , Schott RK . Eyes, vision and the origins and early evolution of snakes. In: Gower DJ , Zaher H , editors. The origin and early evolutionary history of snakes . Cambridge : Cambridge University Press ; 2022 . p. 316 – 348 .
Göz D , Studholme K , Lappi DA , Rollag MD , Provencio I , Morin LP . 2008 . Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms . PLoS One 3 ( 9 ): e3153 . https://doi.org/10.1371/journal.pone.0003153 .
Grabherr MG , Haas BJ , Yassour M , Levin JZ , Thompson DA , Amit I , Adiconis X , Fan L , Raychowdhury R , Zeng Q , et al. 2011 . Trinity: reconstructing a full-length transcriptome without a genome from RNA-Seq data . Nat Biotechnol 29 ( 7 ): 644 – 652 . https://doi.org/10.1038/nbt.1883 .
Griffiths RA , Mylotte JMGAVJ . Diel patterns of activity and vertical migration in tadpoles of the common toad, Bufo bufo . Herpetol J . 1988 : 1 : 223 – 226 .
Guindon S , Gascuel O . 2003 . A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood . Syst Biol 52 ( 5 ): 696 – 704 . https://doi.org/10.1080/10635150390235520 .
Halford S , Freedman Melanie S , Bellingham J , Inglis SL , Poopalasundaram S , Soni BG , Foster RG , Hunt DM . 2001 . Characterization of a novel human opsin gene with wide tissue expression and identification of embedded and flanking genes on chromosome 1q43 . Genomics 72 ( 2 ): 203 – 208 . https://doi.org/10.1006/geno.2001.6469 .
Hunt DM , Collin SP . The evolution of photoreceptors and visual photopigments in vertebrates. In: Hunt DM , Hankins MW , Collin SP , Marshall NJ , editors. Evolution of visual and non-visual pigments. Springer Series in Vision Research . Boston, MA : Springer US ; 2014 . p. 163 – 217 . https://doi.org/10.1007/978-1-4614-4355-1_6
Jaeger RG , Hailman JP . 1976 . Ontogenetic shift of spectral phototactic preferences in anuran tadpoles . J Comp Physiol Psychol 90 ( 10 ): 930 – 945 . https://doi.org/10.1037/h0077275 .
Janiak MC , Chaney ME , Tosi AJ . 2018 . Evolution of acidic mammalian chitinase genes (CHIA) is related to body mass and insectivory in primates . Mol Biol Evol 35 ( 3 ): 607 – 622 . https://doi.org/10.1093/molbev/msx312 .
Jetz W , Pyron RA . 2018 . The interplay of past diversification and evolutionary isolation with present imperilment across the amphibian tree of life . Nat Ecol Evol 2 ( 5 ): 850 – 858 . https://doi.org/10.1038/s41559-018-0515-5 .
Jørgensen CB , Shakuntala K , Vijayakumar S . Body size, reproduction and growth in a tropical toad, bufo melanostictus, with a comparison of ovarian cycles in tropical and temperate zone anurans . Oikos . 1986 : 46 ( 3 ): 379 . https://doi.org/10.2307/3565838 .
Kawamura S , Kubotera N . 2004 . Ancestral loss of short wave-sensitive cone visual pigment in lorisiform prosimians, contrasting with its strict conservation in other prosimians . J Mol Evol 58 ( 3 ): 314 – 321 . https://doi.org/10.1007/s00239-003-2553-z .
Keenan WT , Rupp AC , Ross RA , Somasundaram P , Hiriyanna S , Wu Z , Badea TC , Robinson PR , Lowell BB , Hattar SS . 2016 . A visual circuit uses complementary mechanisms to support transient and sustained pupil constriction . eLife 5 : e15392 . https://doi.org/10.7554/eLife.15392 .
Kelley JL , Davies WIL . The biological mechanisms and behavioral functions of opsin-based light detection by the skin . Front Ecol Evol . 2016 : 4 . https://doi.org/10.3389/fevo.2016.00106 .
Li J , Yu H , Wang W , Fu C , Zhang W , Han F , Wu H . Genomic and transcriptomic insights into molecular basis of sexually dimorphic nuptial spines in Leptobrachium leishanense . Nat Commun . 2019 : 10 ( 1 ). https://doi.org/10.1038/s41467-019-13531-5 .
Löytynoja A , Goldman N. 2005 . An algorithm for progressive multiple alignment of sequences with insertions . Proc Natl Acad Sci . 102 ( 30 ): 10557 – 10562 . https://doi.org/10.1073/pnas.0409137102 .
Malmström T , Kröger RHH . 2006 . Pupil shapes and lens optics in the eyes of terrestrial vertebrates . J Exp Biol 209 ( 1 ): 18 – 25 . https://doi.org/10.1242/jeb.01959 .
Martin M . 2011 . Cutadapt removes adapter sequences from high-throughput sequencing reads . EMBnet J 17 ( 1 ): 10 – 12 . https://doi.org/10.14806/ej.17.1.200 .
Meredith RW , Gatesy J , Emerling CA , York VM , Springer MS . 2013 . Rod monochromacy and the coevolution of cetacean retinal opsins . PLoS Genet 9 ( 4 ): e1003432 . https://doi.org/10.1371/journal.pgen.1003432 .
Mitra AT , Womack MC , Gower DJ , Streicher JW , Clark B , Bell RC , Schott RK , Fujita MK , Thomas KN. 2022 . Ocular lens morphology is influenced by ecology and metamorphosis in frogs and toads . Proc R Soc B: Biol Sci 289 ( 1987 ): 20220767 . https://doi.org/10.1098/rspb.2022.0767 .
Moen DS , Irschick DJ , Wiens JJ. 2013 . Evolutionary conservatism and convergence both lead to striking similarity in ecology, morphology and performance across continents in frogs . Proc R Soc B: Biol Sci 280 ( 1773 ): 20132156 . https://doi.org/10.1098/rspb.2013.2156 .
Muntz WRA . 1963 . The development of phototaxis in the frog ( Rana temporaria ) . J Exp Biol 40 ( 2 ): 371 – 379 . https://doi.org/10.1242/jeb.40.2.371 .
Murrell B , Weaver S , Smith MD , Wertheim JO , Murrell S , Aylward A , Eren K , Pollner T , Martin DP , Smith DM , et al. 2015 . Gene-wide identification of episodic selection . Mol Biol Evol 32 ( 5 ): 1365 – 1371 . https://doi.org/10.1093/molbev/msv035 .
Nakane Y , Ikegami K , Ono H , Yamamoto N , Yoshida S , Hirunagi K , Ebihara S , Kubo Y , Yoshimura T . 2010 . A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds . PNAS 107 ( 34 ): 15264 – 15268 . https://doi.org/10.1073/pnas.1006393107 .
Nakane Y , Shimmura T , Abe H , Yoshimura T . 2014 . Intrinsic photosensitivity of a deep brain photoreceptor . Curr Biol 24 ( 13 ): R596 – R597 . https://doi.org/10.1016/j.cub.2014.05.038 .
Okano T , Yoshizawa T , Fukada Y . 1994 . Pinopsin is a chicken pineal photoreceptive molecule . Nature 372 ( 6501 ): 94 – 97 . https://doi.org/10.1038/372094a0 .
Pinto BJ , Nielsen SV , Gamble T . 2019 . Transcriptomic data support a nocturnal bottleneck in the ancestor of gecko lizards . Mol Phylogenet Evol 141 : 106639 . https://doi.org/10.1016/j.ympev.2019.106639 .
Pyron AR , Wiens JJ . 2011 . A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians . Mol Phylogenet Evol 61 ( 2 ): 543 – 583 . https://doi.org/10.1016/j.ympev.2011.06.012 .
Radu RA , Hu J , Peng J , Bok D , Mata NL , Travis GH . 2008 . Retinal pigment epithelium-retinal g protein receptor-opsin mediates light-dependent translocation of all-trans-retinyl esters for synthesis of visual chromophore in retinal pigment epithelial cells . J Biol Chem 283 ( 28 ): 19730 – 19738 . https://doi.org/10.1074/jbc.M801288200 .
Ranwez V , Harispe S , Delsuc F , Douzery EJP . 2011 . MACSE: multiple alignment of coding sequences accounting for frameshifts and stop codons . PLoS One 6 ( 9 ): e22594 . https://doi.org/10.1371/journal.pone.0022594 .
Rios MN , Marchese NA , Guido ME . Expression of non-visual opsins opn3 and opn5 in the developing inner retinal cells of birds. Light-responses in müller glial cells . Front Cell Neurosci . 2019 : 13 . https://doi.org/10.3389/fncel.2019.00376 .
Roberts A . 1978 . Pineal eye and behaviour in Xenopus tadpoles . Nature 273 ( 5665 ): 774 – 775 . https://doi.org/10.1038/273774a0 .
Sato K , Yamashita T , Kojima K , Sakai K , Matsutani Y , Yanagawa M , Yamano Y , Wada A , Iwabe N , Ohuchi H , et al. 2018 . Pinopsin evolved as the ancestral dim-light visual opsin in vertebrates . Commun Biol 1 ( 1 ): 1 – 10 . https://doi.org/10.1038/s42003-018-0164-x .
Schott RK , Bell RC , Loew ER , Thomas KN , Gower DJ , Streicher JW , Fujita MK . 2022 . Transcriptomic evidence for visual adaptation during the aquatic to terrestrial metamorphosis in leopard frogs . BMC Biol 20 ( 1 ): 138 . https://doi.org/10.1186/s12915-022-01341-z .
Schott RK , Fujita MK , Streicher JW , Gower DJ , Thomas KN , Bell RC . Frog eye transcriptome assemblies [data set] . Zenodo . 2024b . https://doi.org/10.5281/zenodo.10620583 .
Schott RK , Fujita MK , Streicher JW , Gower DJ , Thomas KN , Loew ER , Bamba Kaya AG , Bittencourt-Silva GB , Guillherme Becker C , Cisneros-Heredia D , et al. Diversity and evolution of frog visual opsins: spectral tuning and adaptation to distinct light environments . Mol Biol Evol. 2024a : 41 ( 4 ): msae049 . https://doi.org/10.1093/molbev/msae049 .
Schott RK , Gow D , Chang BS. 2019 . BlastPhyMe: a toolkit for rapid generation and analysis of protein-coding sequence datasets. bioRxiv 059881. https://doi.org/10.1101/059881 , preprint: not peer reviewed.
Schott RK , Van Nynatten A , Card DC , Castoe TA , Chang BSW . 2018 . Shifts in selective pressures on snake phototransduction genes associated with photoreceptor transmutation and dim-light ancestry . Mol Biol Evol 35 ( 6 ): 1376 – 1389 . https://doi.org/10.1093/molbev/msy025 .
Stebbins RC , Cohen NW . A natural history of amphibians . Princeton (NJ) : Princeton University Press ; 1997 . https://press.princeton.edu/books/paperback/9780691102511/a-natural-history-of-amphibians .
Steindal IAF , Whitmore D . Zebrafish circadian clock entrainment and the importance of broad spectral light sensitivity . Front Physiol . 2020 : 11 . https://doi.org/10.3389/fphys.2020.01002/full .
Streicher JW , Miller EC , Guerrero PC , Correa C , Ortiz JC , Crawford AJ , Pie MR , Wiens JJ . 2018 . Evaluating methods for phylogenomic analyses, and a new phylogeny for a major frog clade (Hyloidea) based on 2214 loci . Mol Phylogenet Evol 119 : 128 – 143 . https://doi.org/10.1016/j.ympev.2017.10.013 .
Taniguchi Y , Hisatomi O , Yoshida M , Tokunaga F . 2001 . Pinopsin expressed in the retinal photoreceptors of a diurnal gecko . FEBS Lett 496 ( 2–3 ): 69 – 74 . https://doi.org/10.1016/S0014-5793(01)02395-X .
Terakita A . 2005 . The opsins . Genome Biol 6 ( 3 ): 213 . https://doi.org/10.1186/gb-2005-6-3-213 .
Thomas KN , Gower DJ , Bell RC , Fujita MK , Schott RK , Streicher JW. 2020 . Eye size and investment in frogs and toads correlate with adult habitat, activity pattern and breeding ecology . Proc R Soc B: Biol Sci 287 ( 1935 ): 20201393 . https://doi.org/10.1098/rspb.2020.1393 .
Thomas KN, Gower DJ, Streicher JW, Bell RC, Fujita MK, Schott RK, Liedtke HC, Haddad CFB, Becker CG, Cox CL, et al. Ecology drives patterns of spectral transmission in the ocular lenses of frogs and salamanders. Funct Ecol. 2022b: 36 :850–864.
Thomas KN , Rich C , Quock RC , Streicher JW , Gower DJ , Schott RK , Fujita MK , Douglas RH , Bell RC . Diversity and evolution of amphibian pupil shapes . Biol J Linn Soc . 2022a : 137 ( 3 ): 434 – 449 . https://doi.org/10.1093/biolinnean/blac095 .
Tsukamoto H . Diversity and functional properties of bistable photopigments. In: Hunt DM , Hankins MW , Collin SP , Marshall NJ , editors. Evolution of visual and non-visual pigments. Springer Series in Vision Research . Boston (MA) : Springer US ; 2014 . p. 219 – 239 . https://doi.org/10.1007/978-1-4614-4355-1_7
Van Nynatten A , Castiglione GM , Gutierrez EdeA , Lovejoy NR , Chang BSW . 2021 . Recreated ancestral opsin associated with marine to freshwater croaker invasion reveals kinetic and spectral adaptation . Mol Biol Evol 38 ( 5 ): 2076 – 2087 . doi: 10.1093/molbev/msab008 .
Weadick CJ , Chang BSW . 2012 . An improved likelihood ratio test for detecting site-specific functional divergence among clades of protein-coding genes . Mol Biol Evol 29 ( 5 ): 1297 – 1300 . https://doi.org/10.1093/molbev/msr311 .
Wertheim JO , Murrell B , Smith MD , Kosakovsky Pond SL , Scheffler K . 2015 . RELAX: detecting relaxed selection in a phylogenetic framework . Mol Biol Evol 32 ( 3 ): 820 – 832 . https://doi.org/10.1093/molbev/msu400 .
Wiens JJ , Graham CH , Moen DS , Smith SA , Reeder TW . 2006 . Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: treefrog trees unearth the roots of high tropical diversity . Am Nat 168 ( 5 ): 579 – 596 . https://doi.org/10.1086/507882 .
Willaert B , Bossuyt F , Janssenswillen S , Adriaens D , Baggerman G , Matthijs S , Pauwels E , Proost P , Raepsaet A , Schoofs L . Frog nuptial pads secrete mating season-specific proteins related to salamander pheromones . J Exp Biol . 2013 . https://doi.org/10.1242/jeb.086363 .
Yang Z . 2007 . PAML 4: phylogenetic analysis by maximum likelihood . Mol Biol Evol 24 ( 8 ): 1586 – 1591 . https://doi.org/10.1093/molbev/msm088 .
Yang Z , Nielsen R , Goldman N , Pedersen A-MK . 2000 . Codon-substitution models for heterogeneous selection pressure at amino acid sites . Genetics 155 ( 1 ): 431 – 449 . https://doi.org/10.1093/genetics/155.1.431 .
Yohe LR , Abubakar R , Giordano C , Dumont E , Sears KE , Rossiter SJ , Dávalos LM . 2017 . Trpc2 pseudogenization dynamics in bats reveal ancestral vomeronasal signaling, then pervasive loss . Evolution 71 ( 4 ): 923 – 935 . https://doi.org/10.1111/evo.13187 .
Zekoll T , Waldherr M , Tessmar-Raible K . Characterization of tmt-opsin2 in medaka fish provides insight into the interplay of light and temperature for behavioral regulation . Front Physiol . 2021 : 12 . https://doi.org/10.3389/fphys.2021.726941 .
Zhang Y . Why do we study animal toxins? Zool Res . 2015 : 36 : 183 – 222 . https://doi.org/10.13918/j.issn.2095-8137.2015.4.183 .
Supplementary data
| Month: | Total Views: |
|---|---|
| May 2024 | 274 |
| June 2024 | 673 |
| July 2024 | 438 |
| August 2024 | 75 |
Email alerts
Citing articles via.
- Author Guidelines
Affiliations
- Online ISSN 1537-1719
- Copyright © 2024 Society for Molecular Biology and Evolution
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Bioinformatics approaches for the molecular characterization and structural elucidation of a hypothetical protein of aedes albopictus †.

1. Introduction
2. materials and methods, 2.1. selection of the hypothetical protein and sequence retrieval, 2.2. physicochemical properties analysis, 2.3. identification and validation of the secondary structure, 2.4. three-dimensional structure prediction and validation, 2.5. ligand binding site or active site determination, 2.6. pathogenecity prediction, 3. results and discussion, 3.1. protein sequence retrieval, 3.2. identification of the physicochemical properties, 3.3. identification and validation of the secondary structure, 3.4. the three-dimensional protein structure anticipation and assessment, 3.5. ligand binding site or active site determination, 3.6. pathogenecity prediction, 4. conclusions, author contributions, data availability statement, conflicts of interest.
- Gloria-Soria, A.; Payne, A.F.; Bialosuknia, S.M.; Stout, J.; Mathias, N.; Eastwood, G.; Ciota, A.T.; Kramer, L.D.; Armstrong, P.M. Vector competence of Aedes albopictus populations from the Northeastern United States for chikungunya, dengue, and Zika Viruses. Am. J. Trop. Med. Hyg. 2021 , 104 , 1123. [ Google Scholar ] [ CrossRef ]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Neglected Trop. Dis. 2019 , 13 , e0007213. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Xie, L.M.; Yin, X.; Bi, J.; Luo, H.M.; Cao, X.J.; Ma, Y.W.; Liu, Y.L.; Su, J.W.; Lin, G.L.; Guo, X.G. Identification of potential biomarkers in dengue via integrated bioinformatic analysis. PLoS Neglected Trop. Dis. 2021 , 15 , e0009633. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Murray, N.E.A.; Quam, M.B.; Wilder-Smith, A. Epidemiology of dengue: Past, present and future prospects. Clin. Epidemiol. 2013 , 5 , 299–309. [ Google Scholar ] [ PubMed ]
- Al Asad, M.; Shorna, S.A.; Saikat, A.S.; Uddin, E. Structure-Based Functional Annotation of an Uncharacterized Conserved Protein of Acinetobacter baumannii: An in-silico Approach. Eng. Proc. 2023 , 37 , 25. [ Google Scholar ] [ CrossRef ]
- Zhao, J.; Cao, Y.; Zhang, L. Exploring the computational methods for protein-ligand binding site prediction. Comput. Struct. Biotechnol. J. 2020 , 18 , 417–426. [ Google Scholar ] [ CrossRef ]
- Dukka, B.K. Structure-based Methods for Computational Protein Functional Site Prediction. Comput. Struct. Biotechnol. J. 2013 , 8 , e201308005. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Martin-Martin, I.; Smith, L.B. Aedes albopictus D7 Salivary Protein Prevents Host Hemostasis and Inflammation. Biomolecules 2020 , 10 , 1372. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Saboia-Vahia, L.; Borges-Veloso, A.; Cuervo, P.; Junqueira, M.; Mesquita-Rodrigues, C.; Britto, C.; Domont, G.B.; De Jesus, J.B. Protein expression in the midgut of sugar-fed Aedes albopictus females. Parasites Vectors 2012 , 5 , 1–25. [ Google Scholar ] [ CrossRef ]
- Gong, J.; Chen, Y.; Pu, F.; Sun, P.; He, F.; Zhang, L.; Li, Y.; Ma, Z.; Wang, H. Understanding membrane protein drug targets in computational perspective. Curr. Drug Targets 2019 , 20 , 551–564. [ Google Scholar ] [ CrossRef ]
- Rahman, M.F.; Rahman, M.R.; Islam, T.; Zaman, T.; Shuvo, M.A.; Hossain, M.T.; Islam, M.R.; Karim, M.R.; Moni, M.A. A bioinformatics approach to decode core genes and molecular pathways shared by breast cancer and endometrial cancer. Inform. Med. Unlocked 2019 , 17 , 100274. [ Google Scholar ] [ CrossRef ]
- Mills, C.L.; Beuning, P.J.; Ondrechen, M.J. Biochemical functional predictions for protein structures of unknown or uncertain function. Comput. Struct. Biotechnol. J. 2015 , 13 , 182–191. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- F de Azevedo, W. Molecular dynamics simulations of protein targets identified in Mycobacterium tuberculosis. Curr. Med. Chem. 2011 , 18 , 1353–1366. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2021 , 49 , D10–D17. [ Google Scholar ] [ CrossRef ]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003 , 31 , 3784–3788. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Geourjon, C.; Deleage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics 1995 , 11 , 681–684. [ Google Scholar ] [ CrossRef ]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018 , 46 , W296–W303. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing structure coverage for over 214 million protein sequences. Nucleic Acids Res. 2024 , 52 , D368–D375. [ Google Scholar ] [ CrossRef ]
- Hollingsworth, S.A.; Karplus, P.A. A fresh look at the Ramachandran plot and the occurrence of standard structures in proteins. Biomol. Concepts 2010 , 1 , 271–283. [ Google Scholar ] [ CrossRef ]
- Jiménez, J.; Doerr, S.; Martínez-Rosell, G.; Rose, A.S.; De Fabritiis, G. DeepSite: Protein-binding site predictor using 3D-convolutional neural networks. Bioinformatics 2017 , 33 , 3036–3042. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jendele, L.; Krivak, R.; Skoda, P.; Novotny, M.; Hoksza, D. PrankWeb: A web server for ligand binding site prediction and visualization. Nucleic Acids Res. 2019 , 47 , W345–W349. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Binkowski, T.A.; Naghibzadeh, S.; Liang, J. CASTp: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2003 , 31 , 3352–3355. [ Google Scholar ] [ CrossRef ]
- Tomer, R.; Patiyal, S.; Dhall, A.; Raghava, G.P. Prediction of celiac disease associated epitopes and motifs in a protein. Front. Immunol. 2023 , 14 , 1056101. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bendl, J.; Stourac, J.; Salanda, O.; Pavelka, A.; Wieben, E.D.; Zendulka, J.; Brezovsky, J.; Damborsky, J. PredictSNP: Robust and accurate consensus classifier for prediction of disease-related mutations. PLoS Comput. Biol. 2014 , 10 , e1003440. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tavtigian, S.V.; Deffenbaugh, A.M.; Yin, L.; Judkins, T.; Scholl, T.; Samollow, P.B.; de Silva, D.; Zharkikh, A.; Thomas, A. Comprehensive statistical study of 452 BRCA1 missense substitutions with classification of eight recurrent substitutions as neutral. J. Med. Genet. 2006 , 43 , 295–305. [ Google Scholar ] [ CrossRef ]
- Sahay, A.; Piprodhe, A.; Pise, M. In silico analysis and homology modeling of strictosidine synthase involved in alkaloid biosynthesis in catharanthus roseus. J. Genet. Eng. Biotechnol. 2020 , 18 , 44. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ikai, A. Thermostability and aliphatic index of globular proteins. J. Biochem. 1980 , 88 , 1895–1898. [ Google Scholar ] [ PubMed ]
- Bhowmick, S.; Saha, A.; AlFaris, N.A.; ALTamimi, J.Z.; ALOthman, Z.A.; Aldayel, T.S.; Wabaidur, S.M.; Islam, M.A. Structure-based identification of galectin-1 selective modulators in dietary food polyphenols: A pharmacoinformatics approach. Mol. Divers. 2022 , 26 , 1697–1714. [ Google Scholar ] [ CrossRef ]
- Mathe, E.; Olivier, M.; Kato, S.; Ishioka, C.; Hainaut, P.; Tavtigian, S.V. Computational approaches for predicting the biological effect of p53 missense mutations: A comparison of three sequence analysis based methods. Nucleic Acids Res. 2006 , 34 , 1317–1325. [ Google Scholar ] [ CrossRef ]
- Montenegro, L.R.; Lerário, A.M.; Nishi, M.Y.; Jorge, A.A.; Mendonca, B.B. Performance of mutation pathogenicity prediction tools on missense variants associated with 46, XY differences of sex development. Clinics 2021 , 76 , e2052. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| Sequence | Start | End | Score | Hydrophobicity | Hydropathicity | Hydrophilicity | Charge | Mol wt. |
|---|---|---|---|---|---|---|---|---|
| MIFFMVMPIMIGGFGNWLVP | 63 | 82 | 0.34 | 0.34 | 1.65 | −1.31 | 0 | 2301.25 |
| FFMVMPIMIGGFGNWLVPLM | 65 | 84 | 0.32 | 0.33 | 1.61 | −1.31 | 0 | 2301.25 |
| MPIMIGGFGNWLVPLMLGAP | 69 | 88 | 0.32 | 0.27 | 1.2 | −1.03 | 0 | 2114.98 |
| FPRMNNMSFWMLPPSLTLLL | 92 | 111 | 0.32 | 0.05 | 0.54 | −0.89 | 1 | 2409.27 |
| MNNMSFWMLPPSLTLLLSSS | 95 | 114 | 0.32 | 0.08 | 0.58 | −0.86 | 0 | 2270.02 |
| NNMSFWMLPPSLTLLLSSSM | 96 | 115 | 0.32 | 0.08 | 0.58 | −0.86 | 0 | 2270.02 |
| NMSFWMLPPSLTLLLSSSMV | 97 | 116 | 0.4 | 0.13 | 0.97 | −0.95 | 0 | 2255.05 |
| MSFWMLPPSLTLLLSSSMVE | 98 | 117 | 0.33 | 0.14 | 0.97 | −0.81 | −1 | 2270.06 |
| SFWMLPPSLTLLLSSSMVEN | 99 | 118 | 0.36 | 0.09 | 0.7 | −0.73 | −1 | 2252.97 |
| FWMLPPSLTLLLSSSMVENG | 100 | 119 | 0.33 | 0.11 | 0.72 | −0.75 | −1 | 2222.95 |
| FIGVNLTFFPQHFLGLAGMP | 416 | 435 | 0.32 | 0.21 | 0.98 | −1.05 | 0.5 | 2206.95 |
| TPSFPMQLSSSIEWYHTLPP | 479 | 498 | 0.42 | −0.02 | −0.33 | −0.59 | −0.5 | 2318.92 |
| Mutants | GV | GD | Prediction |
|---|---|---|---|
| M63P | 0.00 | 86.59 | Class C65 |
| F65M | 0.00 | 28.53 | Class C25 |
| M69P | 0.00 | 86.59 | Class C65 |
| F92L | 0.00 | 21.82 | Class C15 |
| M95S | 0.00 | 134.86 | Class C65 |
| N96M | 0.00 | 141.15 | Class C65 |
| N97V | 0.00 | 132.88 | Class C65 |
| M98E | 0.00 | 126.08 | Class C65 |
| S99N | 0.00 | 46.24 | Class C45 |
| F100G | 0.00 | 153.13 | Class C65 |
| F416P | 0.00 | 113.73 | Class C65 |
| T479P | 0.00 | 37.56 | Class C35 |
| Mutants | predictSNP | MAPP | PhD-SNP | Polyphen-2 | SIFT | SNAP | PANTHER | Ranging |
|---|---|---|---|---|---|---|---|---|
| M63P | 86.91% | 87.71% | 85.82% | 56.23% | 79.28% | 88.52% | 76.65% | 1 |
| F65M | 86.91% | 78.34% | 81.73% | 63.43% | 79.28% | 72.04% | 76.00% | 1 |
| M69P | 86.91% | 87.71% | 81.73% | 81.14% | 79.28% | 84.85% | 65.27% | 1 |
| F92L | 60.55% | 75.11% | 73.26% | 67.64% | 79.28% | 62.21% | 61.08% | 2 |
| M95S | 86.91% | 84.18% | 77.34% | 55.08% | 79.28% | 72.04% | 69.00% | 1 |
| N96M | 86.91% | 84.18% | 60.80% | 81.14% | 79.28% | 84.85% | 69.86% | 1 |
| N97V | 86.91% | 77.12% | 67.62% | 81.14% | 79.28% | 84.85% | 66.07% | 1 |
| M98E | 86.91% | 91.38% | 87.52% | 67.52% | 79.28% | 84.85% | 68.66% | 1 |
| S99N | 86.91% | 76.54% | 60.80% | 43.12% | 79.28% | 80.51% | 71.86% | 1 |
| F100G | 86.91% | 81.93% | 60.80% | 81.14% | 79.28% | 84.85% | 78.00% | 1 |
| F416P | 86.91% | 87.71% | 85.82% | 64.97% | 79.28% | 80.51% | 74.45% | 1 |
| T479P | 74% | 68% | 51% | 87% | 46% | 83% | 63% | 3 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Asad, M.A.; Shorna, S.A.; Mizan, M.; Nath, R.D.; Saikat, A.S.M.; Uddin, M.E. Bioinformatics Approaches for the Molecular Characterization and Structural Elucidation of a Hypothetical Protein of Aedes albopictus . Eng. Proc. 2024 , 67 , 14. https://doi.org/10.3390/engproc2024067014
Asad MA, Shorna SA, Mizan M, Nath RD, Saikat ASM, Uddin ME. Bioinformatics Approaches for the Molecular Characterization and Structural Elucidation of a Hypothetical Protein of Aedes albopictus . Engineering Proceedings . 2024; 67(1):14. https://doi.org/10.3390/engproc2024067014
Asad, Mamun Al, Surya Afrin Shorna, Md. Mizan, Rajib Deb Nath, Abu Saim Mohammad Saikat, and Md. Ekhlas Uddin. 2024. "Bioinformatics Approaches for the Molecular Characterization and Structural Elucidation of a Hypothetical Protein of Aedes albopictus " Engineering Proceedings 67, no. 1: 14. https://doi.org/10.3390/engproc2024067014
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 19 August 2024

Recruitment of Cdc48 to chloroplasts by a UBX-domain protein in chloroplast-associated protein degradation
- Na Li 1 &
- R. Paul Jarvis ORCID: orcid.org/0000-0003-2127-5671 1
Nature Plants ( 2024 ) Cite this article
101 Accesses
3 Altmetric
Metrics details
- Cell biology
- Chloroplasts
- Protein trafficking in plants
- Proteolysis in plants
- Transgenic plants
The translocon at the outer chloroplast membrane (TOC) is the gateway for chloroplast protein import and so is vital for photosynthetic establishment and plant growth. Chloroplast-associated protein degradation (CHLORAD) is a ubiquitin-dependent proteolytic system that regulates TOC. In CHLORAD, cytosolic Cdc48 provides motive force for the retrotranslocation of ubiquitinated TOC proteins to the cytosol but how Cdc48 is recruited is unknown. Here, we identify plant UBX-domain protein PUX10 as a component of the CHLORAD machinery. We show that PUX10 is an integral chloroplast outer membrane protein that projects UBX and ubiquitin-associated domains into the cytosol. It interacts with Cdc48 via its UBX domain, bringing it to the chloroplast surface, and with ubiquitinated TOC proteins via its ubiquitin-associated domain. Genetic analyses in Arabidopsis revealed a requirement for PUX10 during CHLORAD-mediated regulation of TOC function and plant development. Thus, PUX10 coordinates ubiquitination and retrotranslocation activities of CHLORAD to enable efficient TOC turnover.
Similar content being viewed by others
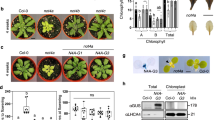
RETRACTED ARTICLE:The Arabidopsis NOT4A E3 ligase promotes PGR3 expression and regulates chloroplast translation

Chloroplast SRP43 autonomously protects chlorophyll biosynthesis proteins against heat shock

Mild proteasomal stress improves photosynthetic performance in Arabidopsis chloroplasts
Most chloroplast proteins (>90%) are synthesized in the cytosol and imported into chloroplasts post-translationally. The chloroplast protein import machinery consists of two translocons, a translocon located in the outer chloroplast membrane (TOC) and a translocon in the inner chloroplast membrane (TIC). Core components of the TOC are the β-barrel protein, TOC75, and the GTPases TOC159 and TOC33—all named in accordance with their molecular masses in kilodaltons. TOC75 forms a membrane channel for protein conductance, whereas TOC159 and TOC33 function as receptors by binding the transit peptides of precursor proteins via their cytosolic GTPase domains 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 .
Chloroplast protein import is dynamically regulated by chloroplast-associated protein degradation (CHLORAD), a ubiquitin-dependent proteolytic system that targets the TOC apparatus 9 , 10 . By reconfiguring the TOC machinery, CHLORAD action facilitates changes in the organelle’s proteome, functions and morphology. Such CHLORAD-mediated TOC regulation enables the biogenesis and operation of chloroplasts (and of other members of the plastid family of organelles) to be responsive to developmental and environmental cues, including stress 11 , 12 , 13 .
The first characterized CHLORAD component was the ubiquitin E3 ligase suppressor of ppi locus 1 (SP1). The SP1 protein is located in the chloroplast outer envelope membrane (OEM) and has a cytosol-facing RING domain and two transmembrane (TM) spans flanking an intermembrane space (IMS) domain that binds to TOC protein targets 9 . Analysis of sp1 -mutant and SP1-overexpressor Arabidopsis plants showed that SP1 expression levels correlate inversely with the abundance of TOC proteins, resulting in the suppression or enhancement of the pale-green ppi1 (TOC33 knockout) 14 mutant phenotype. Such manipulation of SP1 expression also has developmental consequences. For instance, the sp1 -mutant plants showed delayed de-etiolation and leaf senescence, whereas SP1-overexpressor plants showed acceleration of these processes 9 , 12 .
Other important components of the CHLORAD system are SP2 and Cdc48. The SP2 protein is located in the OEM and, like TOC75, it is a member of the OMP85 superfamily of β-barrel proteins with 16 predicted TM β-strands. This protein was shown to act as a translocon for the retrotranslocation of ubiquitinated TOC proteins 10 . In contrast with SP1 and SP2, Cdc48 is largely located in the cytosol. This protein provides the motive force for extracting TOC components ubiquitinated by SP1 to the cytosol, where degradation by the 26S proteasome occurs, and in this it cooperates with the SP2 membrane channel 10 .
The Cdc48 protein is a member of the ATPases associated with diverse cellular activities (AAA) family and it plays essential functions in a plethora of cellular processes 15 . Its ability to function in so many different pathways depends in part on its adaptor proteins, which control its targeting and activity 16 . For instance, the heterodimeric UFD1–NPL4 complex is a well-characterized adaptor that participates in many ubiquitin-dependent Cdc48-driven processes, including endoplasmic reticulum (ER)-associated protein degradation (ERAD) 17 . Most adaptor proteins possess conserved Cdc48-binding motifs, including ubiquitin regulatory X (UBX), UBXL, SHP, VBM, VIM and PUB 18 , 19 , 20 . Many of these adaptors are not individually essential for cell growth and survival, implying functional redundancies among them. The UBX-domain-containing proteins constitute by far the largest family of Cdc48 adaptor proteins 21 .
The UBX domain comprises approximately 80 amino acid residues and it shares substantial structural similarity with ubiquitin. Proteins possessing the domain are involved in substrate recruitment to Cdc48 and in the temporal and spatial regulation of Cdc48 activity 22 , 23 , 24 . The plant UBX-domain (PUX) proteins define a family of plant proteins that possess a conserved UBX domain for direct interaction with Cdc48. In Arabidopsis there are 16 PUX genes, most of which are largely uncharacterized 25 . That said, emerging knowledge on the structure and function of PUX proteins provides an indication of their functional links to Cdc48. Several family members appear to serve as adaptors to recruit Cdc48 to specific organelles 26 , 27 , whereas others are involved in regulating the activity of Cdc48 (refs. 28 , 29 , 30 ). Among the PUX proteins in Arabidopsis , PUX10 is the only protein with obvious membrane-anchoring hydrophobic regions. Previous studies identified PUX10 as a lipid droplet (LD)-anchored protein that mediates the degradation of ubiquitinated oleosins during seed germination 31 , 32 . Intriguingly, PUX10 was also reported to undergo relocalization to chloroplasts during seed maturation 31 .
In this study we investigated the role of PUX10 in chloroplast biogenesis in detail, providing information on the localization, topology, interactions and functions of the protein. On the basis of our results, we conclude that PUX10 is a key part of the CHLORAD machinery for TOC protein degradation. The UBX domain of PUX10 recruits cytosolic Cdc48 to the chloroplast surface, while its ubiquitin-associated (UBA) domain binds to ubiquitinated TOC proteins, thereby bringing them into close proximity with Cdc48 for retrotranslocation.
Localization and topology analysis of the PUX10 protein
With the aim of identifying Cdc48 adaptors that act in CHLORAD, we conducted a subcellular localization screening analysis of all expressed PUX proteins in Arabidopsis . In this analysis, PUX10 was the only PUX protein showing distinct association with chloroplasts (Extended Data Fig. 1 ). The PUX10 protein has two predicted TM spans, an amino-terminal (N-terminal) UBA domain, and a carboxy-terminal (C-terminal) UBX domain (Fig. 1a ). Wishing to understand the function of PUX10, we began by studying its localization in greater detail. To investigate the role of the two predicted TM domains in the localization of PUX10, a truncated PUX10 variant lacking the TM domains (ΔTM1/2) was generated. Both intact and deleted versions of PUX10 were fused with yellow fluorescent protein (YFP) and transiently expressed in protoplasts. Confocal visualization indicated that the full-length protein was localized in chloroplasts (in the chloroplast envelope) and that the truncated version was located in the cytosol (Fig. 1b ). This indicated that PUX10 shows chloroplast envelope membrane localization dependent on its TM domains. To corroborate this conclusion, stable transgenic plants expressing various PUX10 forms fused to YFP were generated and analysed. This analysis indicated that the TM domains alone contribute to the chloroplast localization of PUX10; deletion of the UBA and UBX domains did not affect its localization (Fig. 1c ). Lastly, we compared transgenic plants expressing the full-length PUX10–YFP fusion under the control of native or strong constitutive ( 35S ) promoters, again viewing the localization patterns by confocal microscopy (Extended Data Fig. 2 ). Similar results were obtained, indicating that neither the expression system nor the expression level had a major effect on the predominant subcellular localization of PUX10. Collectively, these results showed that the TM domains of PUX10 are responsible for anchoring the protein in the chloroplast envelope membrane in leaves.
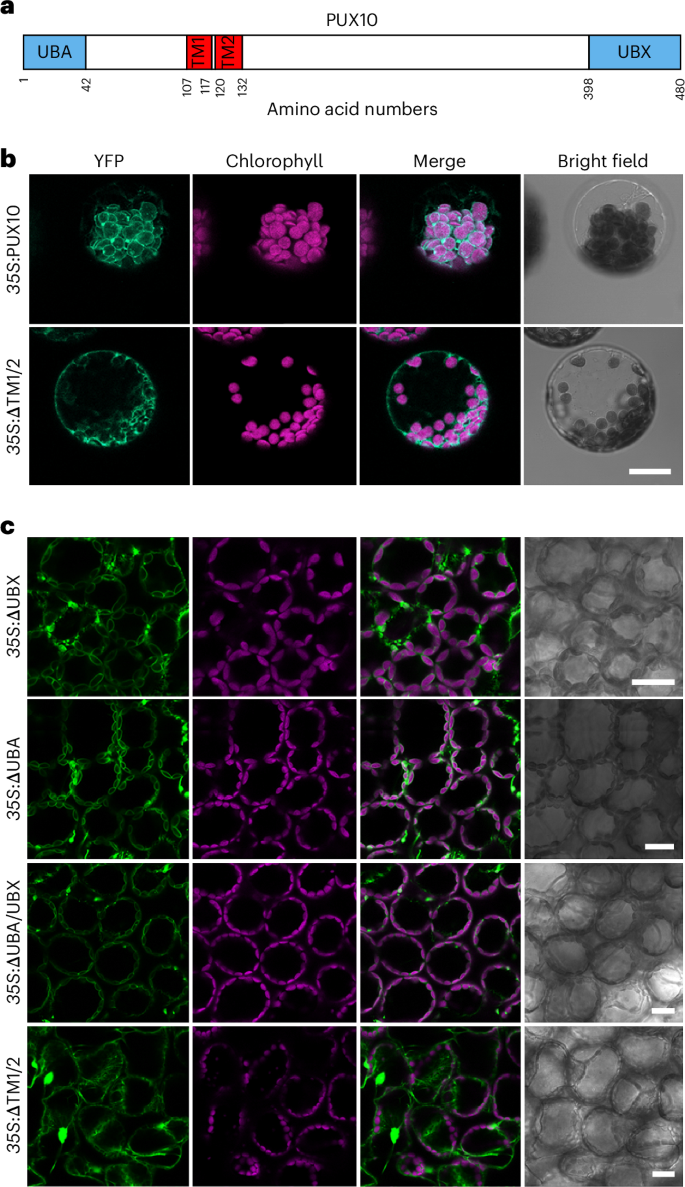
a , Protein domain map for PUX10 showing its UBA, potential TM and UBX domains. b , Analysis of PUX10 localization upon transient expression. Protoplasts transiently expressing different YFP-tagged PUX10 variants (under the control of the 35S promoter) were analysed by confocal microscopy. Representative protoplasts are shown. Exposure times and gain settings were identical in each case. Localization of PUX10–YFP to the chloroplast envelope (top) depended on the TM domains, as revealed by a double-TM deletion mutant (bottom). Scale bar, 20 µm. c , Analysis of PUX10 localization in transgenic plants. Constructs encoding different variants of PUX10 lacking the indicated domains (under the control of the 35S promoter) were used to stably transform Arabidopsis plants. Rosette leaves taken from 28-day-old T 1 plants were visualized by confocal microscopy. Representative images are shown as in b . Similar localization of the YFP signals was observed in 5–10 independent T 1 transgenic plants. Exposure times and gain settings were identical. Scale bars, 20 µm.
Next, to investigate the topology of the PUX10 protein, we generated transgenic plants expressing a PUX10–HA construct, with an HA tag fused to the C terminus of PUX10. First, alkaline extraction was applied to determine whether PUX10 is a peripheral or integral membrane protein. Chloroplasts isolated from the transgenic plants were treated with 100 mM Na 2 CO 3 (pH 11.5) to remove non-integrated proteins from the membranes. Then, membrane pellet and soluble fractions were recovered and analysed alongside a total chloroplast sample by immunoblotting. Almost all of the PUX10–HA protein was found in the membrane pellet fraction, with very little in the soluble fraction, indicating that PUX10 is indeed an integral chloroplast membrane protein (Fig. 2a ).
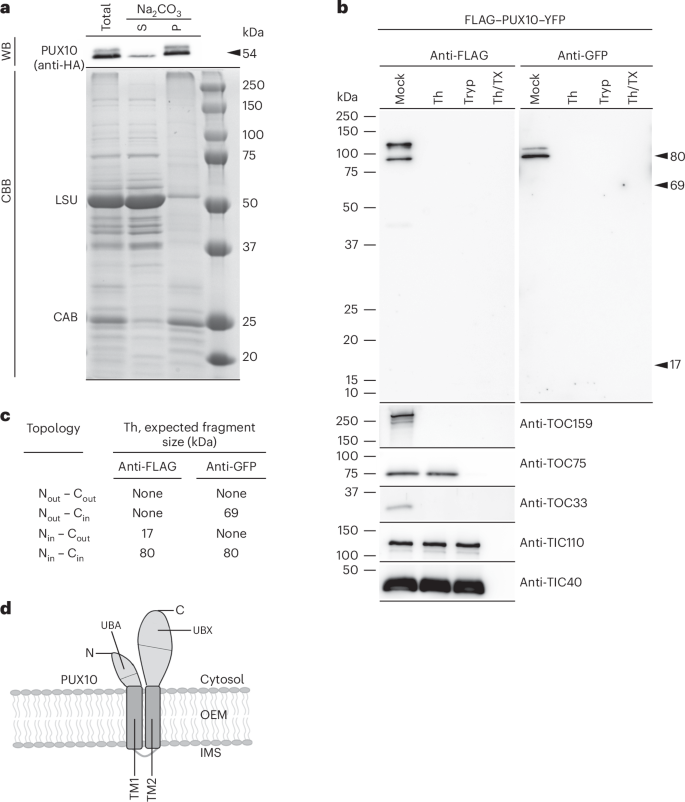
a , Alkaline extraction analysis of PUX10. Chloroplasts isolated from transgenic plants expressing PUX10–HA were fractionated into membrane pellet (P) and soluble supernatant (S) fractions after high-pH (Na 2 CO 3 ) washing. The samples were analysed by anti-HA immunoblotting to detect the PUX10–HA protein (western blotting, WB; top) and by Coomassie brilliant blue staining (CBB; bottom). Endogenous marker proteins (RuBisCo large subunit (LSU); chlorophyll a / b binding proteins (CAB)) partitioned as expected. b , c , Protease protection analysis of the PUX10 protein. b , chloroplasts isolated from transgenic plants overexpressing FLAG–PUX10–YFP were subjected to treatment using thermolysin (Th), trypsin (Tryp), thermolysin plus Triton X-100 (Th/TX) or buffer lacking protease (mock). Immunoblotting using antibodies to the FLAG and YFP tags was conducted to assess the protease accessibility of the protein termini. The arrowheads indicate the positions of protected proteolytic fragments that would be expected after thermolysin protease treatment if alternative PUX10 topologies exist. Separate immunoblot analysis of five endogenous marker proteins (TOC and TIC components), using the same samples, confirmed the efficiency of the protease treatments. c , four possible topologies of the PUX10 protein in the OEM can be envisaged. The expected sizes of protected fragments for each possible topology following thermolysin treatment are shown; the positions of these are indicated in b with arrowheads. d , Schematic drawing showing the topology of PUX10 according to the protease treatment results.
Source data
On the basis of the presence of two predicted TM spans in PUX10 and the similarity of its overall domain architecture to that of Ubx2, a yeast homologue 33 , it was hypothesized that the topology of PUX10 is such that both the UBA and UBX domains face the cytosol, enabling the protein to access both ubiquitinated substrates and Cdc48, as Ubx2 does in yeast. However, such a topological arrangement of PUX10 had not previously been assessed experimentally. To do this, we generated transgenic plants expressing a FLAG–PUX10–YFP construct, with FLAG and YFP tags fused to the N terminus and C terminus of PUX10, respectively. A protease protection assay using isolated chloroplasts and thermolysin or trypsin proteases was used (Fig. 2b ). Thermolysin cannot penetrate beyond the OEM, which means it will digest only those OEM proteins that are exposed at the organelle surface (that is, normally facing the cytosol) without causing major damage to the integrity of the chloroplast 34 . In contrast, trypsin can partially disrupt the integrity of the OEM and thus gain access to the IMS, where it may digest regions of OEM or inner envelope membrane (IEM) proteins that extend into the IMS 35 , 36 .
Thus, chloroplasts were isolated from the FLAG–PUX10–YFP transgenic plants and either mock treated or treated with thermolysin or trypsin (the former with or without Triton X-100 detergent to disrupt the chloroplast membranes, as a control). All samples were analysed by immunoblotting using anti-FLAG or anti-GFP sera, or antisera against the following control proteins: TOC159 and TOC33 (which are OEM proteins with large cytosolic domains and were sensitive to thermolysin treatment as expected), and TOC75, TIC110 and TIC40 (which are deeply embedded in the OEM or in the IEM and were resistant to thermolysin and/or trypsin treatment as expected) (Fig. 2b ). The full-length FLAG–PUX10–YFP fusion protein (~80 kDa) was detectable using both tags in the absence of protease treatment. However, both the N terminus (FLAG tagged) and the C terminus (YFP tagged) of PUX10 showed strong sensitivity to thermolysin, implying that PUX10 is located in the OEM with both termini facing the cytosol. Indeed, protected fragments that would be expected for alternative topological arrangements were absent (Fig. 2b,c ) and complete degradation of PUX10 by trypsin treatment further supported this conclusion. Altogether, these results provided direct evidence for the OEM localization of PUX10, for the existence of two TM spans in the N-terminal hydrophobic region of PUX10, and for the cytosolic orientation of both the UBA and UBX domains, ensuring their accessibility to ubiquitinated substrates and Cdc48, respectively (Fig. 2d ).
PUX10 recruits Cdc48 to the chloroplast envelope
To further understand the function of PUX10, two transfer-DNA (T-DNA) insertion mutants were obtained: pux10-1 (SAIL-1187-B06) and pux10-4 (WiscDslox424B8) (Extended Data Fig. 3a ). On the basis of reverse transcription–polymerase chain reaction (RT-PCR) analysis of PUX10 expression, both pux10-1 and pux10-4 were considered to be null mutants of PUX10 (Extended Data Fig. 3b ). However, there were no obvious phenotypic differences between the mutants and wild-type (WT) plants under standard growth conditions (Extended Data Fig. 3c ). As the two pux10 mutants are phenotypically identical (Extended Data Fig. 3d,e ) 31 , the data presented hereafter are for pux10-1 only as a representative allele.
Although the pux10 -knockout mutants appeared phenotypically normal, transgenic plants overexpressing full-length PUX10 (PUX10-OX) were severely dwarfed (Fig. 3a and Extended Data Fig. 4 ). In addition to the plants overexpressing intact PUX10, lines expressing truncated PUX10 forms were also generated and these showed different phenotypes. Transgenic lines with the UBX domain deleted (ΔUBX), both UBX and UBA domains deleted (ΔUBX/UBA) and both TM domains deleted (ΔTM1/2) all showed similar phenotypes to the WT. However, the same dwarfism as seen for full-length PUX10 overexpression was observed in the transgenic plants with the UBA domain deleted (ΔUBA; Fig. 3a ). These phenotypic differences implied that overexpression of PUX10 can have a dominantly acting negative effect on plant growth and that both the UBX and TM domains of PUX10 are essential for this effect to be mediated.
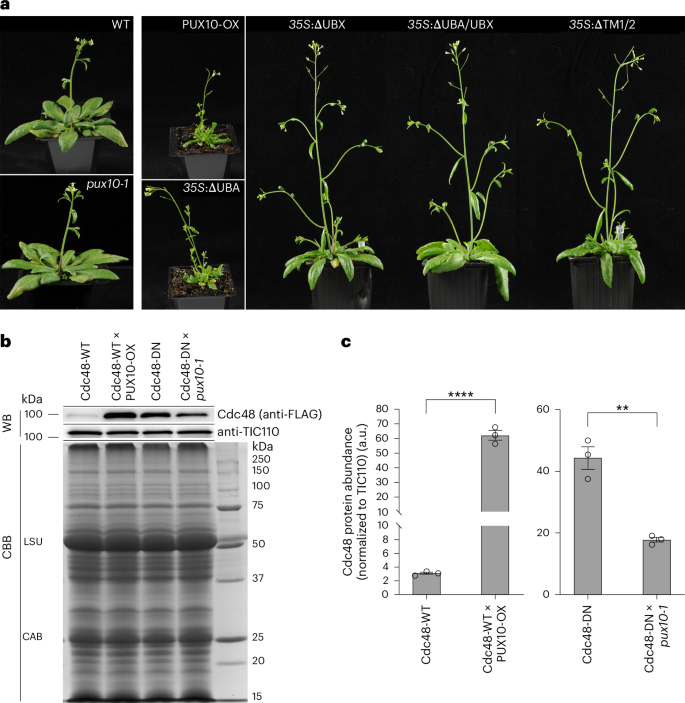
a , Phenotypes of WT, pux10-1 and transgenic plants, the latter expressing various forms of the PUX10 protein under the constitutive 35S promoter. Representative individuals are shown. Identical camera settings were used and all images are at the same magnification. Two pux10 alleles were phenotypically identical and so only pux10-1 is presented here as a representative allele. The WT and pux10-1 plants were 5 weeks old and all the overexpression or 35S lines were 6 weeks old (see Extended Data Fig. 4 for 3-week-old WT, pux10 (two alleles) and transgenic plants). b , c , The extent of Cdc48 localization to chloroplasts depends on the expression level of PUX10. b , chloroplasts isolated from plants expressing different FLAG-tagged Cdc48 variants (either WT or DN) in different genetic backgrounds (either PUX10-OX or pux10-1 ) were analysed by immunoblotting after verification that the genetic background did not influence total FLAG-tagged protein expression. Anti-FLAG antibody was used to detect Cdc48, and TIC110 was analysed as an endogenous loading control. An equivalent Coomassie-brilliant-blue-stained gel was prepared to show equal loading of the samples. Cdc48-DN showed significantly enhanced chloroplast association relative to Cdc48-WT, which showed weak chloroplast association, consistent with published data 10 . c , quantification of the changes in abundance of chloroplast-associated Cdc48 in the PUX10-OX (left) or pux10-1 (right) backgrounds was performed. Band intensities were quantified and normalized to corresponding TIC110 data; the data are presented relative to the relevant control genotype in arbitrary units. Values shown are means ± s.e.m. from three biological replicates. Asterisks indicate significance according to an unpaired two-tailed Student’s t -test. ** P = 0.0020, **** P < 0.0001.
Given that UBX is a Cdc48-binding domain and that Cdc48 has a wide spectrum of activity in various organelles and compartments, one can hypothesize that the dwarfism observed in both full-length PUX10 overexpression and ΔUBA-expressing plants was due to greatly disrupted subcellular distribution of Cdc48 due to the high-level expression of these UBX-domain proteins. It is noteworthy that no dwarfism was observed in ΔTM1/2 transgenic plants, implying that the expression of this cytosolic UBX-domain protein does not similarly disrupt the distribution of Cdc48. The selective overaccumulation of Cdc48 on chloroplasts via the UBX domain of PUX10, or its depletion from the cytosol or other compartments as a consequence, might be responsible for the developmental aberrancy seen in these transgenic plants.
To address this hypothesis, we made use of oestradiol-inducible Cdc48-WT–FLAG and Cdc48-DN–FLAG constructs 10 , which were introduced into PUX10-OX and pux10 backgrounds, respectively, via genetic crossing. The Cdc48-WT protein shows only weak association with chloroplasts, whereas the Cdc48-DN protein, which is a dominant-negative (DN) mutant with stabilized substrate binding, shows more stable association with chloroplasts. Chloroplasts were isolated from the resulting transgenic plants and analysed by immunoblotting using anti-FLAG antibody to assess the extent of chloroplast association of Cdc48 (Fig. 3b,c ). We observed that the weaker chloroplast-localized signal for Cdc48-WT–FLAG was significantly enhanced in the PUX10-OX background, whereas the strong chloroplast-localized signal for Cdc48-DN–FLAG was conversely reduced in the pux10 -knockout background. This result indicated that the expression of PUX10 has a strong influence on the accumulation of Cdc48 at chloroplasts.
PUX10 interacts with Cdc48 via its UBX domain
Proteins with a UBX domain have been shown to recruit Cdc48 to specific subcellular locales or organelles via direct interaction between the UBX domain and the N-terminal domain of Cdc48 (ref. 21 ). To investigate whether this may be the case for PUX10, we performed bimolecular fluorescence complementation (BiFC) assessments using the pSATN BiFC system 37 , in which the YFP variant EYFP (Clontech) is split between amino acid residues 174 and 175 to yield complementary N-terminal (nYFP) and C-terminal (cYFP) fragments. Full-length PUX10 and ΔUBX (PUX10 with the UBX-domain deleted) were fused with cYFP, and Cdc48 was fused with nYFP. The resulting constructs encoding complementary nYFP and cYFP fragments were co-expressed in pairs in Arabidopsis protoplasts. Meanwhile, as a control, we also transfected protoplasts with a single construct encoding Cdc48 fused to full-length YFP. Subsequent confocal microscopy analysis indicated that the Cdc48–YFP protein, expressed alone, is located predominantly in the cytosol (Fig. 4a ). In the BiFC analysis, several key observations were made (Fig. 4b,c ). First, fluorescence signals were observed when Cdc48 and full-length PUX10 fusions were co-expressed, indicating that these two proteins can interact. Second, these BiFC signals were localized to the chloroplast envelope membrane, supporting the view that PUX10 mediates the relocalization of Cdc48 to the chloroplast surface. Third, the detected interaction depended on the UBX domain of PUX10 because the BiFC signals were significantly reduced when ΔUBX was used instead of the full-length PUX10 protein.
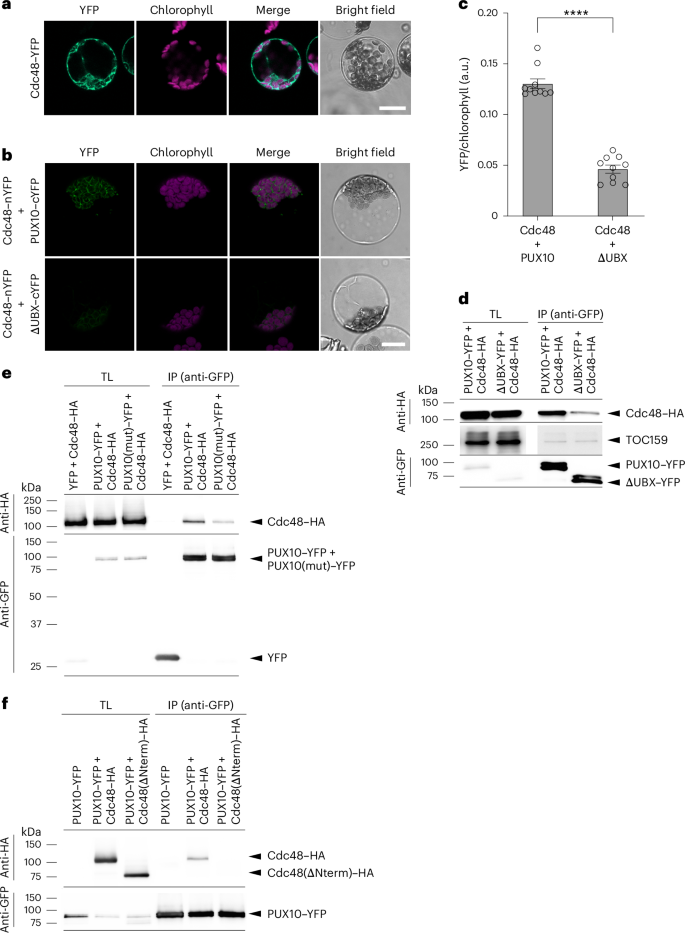
a , Localization analysis of the Cdc48 protein. Protoplasts transiently expressing a Cdc48–YFP construct were analysed by confocal microscopy. A representative protoplast is shown. Scale bar, 20 µm. b , c , BiFC analysis of the interaction between PUX10 and Cdc48. b , protoplasts co-expressing proteins fused to nYFP or cYFP fragments of YFP were visualized by confocal microscopy. Protoplasts showing typical results are shown. Exposure times and gain settings were identical. Scale bar, 20 µm. c , relative intensities of the BiFC signals were quantified and normalized with respect to chlorophyll autofluorescence. Each measurement was of a different field of view area; each area contained ~40 protoplasts. The values shown are means ± s.e.m. from ten measurements. Asterisks indicate significance according to an unpaired two-tailed Student’s t -test. *** P < 0.0001. d , Co-IP analysis of the interaction between PUX10 and Cdc48. Protoplasts transiently co-expressing the indicated proteins were solubilized and subjected to anti-GFP co-IP analysis. Anti-GFP immunoblot analysis verified the enrichment of the PUX10–YFP or ΔUBX–YFP proteins, whereas anti-HA analysis assessed co-purification of Cdc48–HA. Endogenous TOC159 protein was detected using anti-TOC159 antibody. e , Co-IP analysis of the interaction between Cdc48 and PUX10 with a UBX-domain triple-point mutation. Protoplasts transiently co-expressing the indicated proteins were solubilized and subjected to anti-GFP co-IP analysis. Anti-GFP immunoblot analysis verified the enrichment of the PUX10–YFP or PUX10(mut)–YFP proteins, whereas anti-HA analysis assessed co-purification of Cdc48–HA. f , Co-IP analysis of the interaction between PUX10 and Cdc48 lacking its N terminus. Protoplasts transiently co-expressing the indicated proteins were solubilized and subjected to anti-GFP co-IP analysis. Anti-GFP immunoblot analysis verified the enrichment of the PUX10–YFP proteins, whereas anti-HA analysis assessed co-purification of Cdc48–HA or Cdc48(∆Nterm)–HA. TL, total lysate.
To corroborate the findings of the BiFC analysis, co-immunoprecipitation (co-IP) experiments were performed. Constructs encoding either full-length PUX10 or the ΔUBX variant fused to YFP (that is, PUX10–YFP or ΔUBX–YFP) were co-expressed in protoplasts along with a previously described construct encoding HA-tagged Cdc48 (Cdc48–HA) 10 . Here, PUX10–YFP and ΔUBX–YFP were used as the bait proteins and were immunoprecipitated with anti-GFP beads after solubilization, while Cdc48–HA acted as the prey (Fig. 4d ). As expected, the results clearly indicated an interaction between full-length PUX10 and Cdc48. However, this interaction was strongly disrupted when ΔUBX was used, which is consistent with the observations from the BiFC analysis.
Next, we used AlphaFold 38 , 39 to analyse the interaction between PUX10 and Cdc48 in silico. The analysis provides two intrinsic model-accuracy estimates (predicted template modelling (pTM) and interface pTM (ipTM)) and we used a combination of these two estimates as the confidence metric. An ipTM + pTM score of 0.5 or more is considered to be indicative of a reliable interaction 39 , 40 . Although analysis of a polypeptide pair comprising full-length PUX10 and full-length Cdc48 scored slightly below 0.5, it did score noticeably higher than pairs including one or both of the proteins in truncated form (that is, PUX10 lacking the UBX domain and Cdc48 lacking the N terminus) (Extended Data Fig. 5a ). The low score associated with the analysis of the full-length proteins might have been related to a lack of similar structures in the training data, the presence of disordered or flexible regions or difficulty in modelling the interactions of large multi-domain proteins. Indeed, when polypeptide pairs including one or both of the domains of interest in isolated form were analysed, scores in excess of 0.75 were obtained (Extended Data Fig. 5a ). Inspection of the predicted three-dimensional (3D) folds of the different interaction pairs showed that the structural arrangement at the interaction interface was highly similar regardless of whether full-length proteins or isolated UBX and N domains were used (Extended Data Fig. 5b–d ). Thus, overall, these data are strongly supportive of the hypothesis that PUX10 and Cdc48 interact directly via their UBX and N domains.
The UBX domain has a β-β-α-β-β-α-β secondary structure 41 . An exposed arginine residue in strand 1 and an FPR motif in the loop connecting strands 3 and 4 form a highly conserved surface patch (R…FPR, where the ellipsis represents intervening residues) (Extended Data Fig. 6 ). This R…FPR motif was found to be the major binding site of the UBX domain and its mutation greatly reduced its Cdc48/p97 binding 42 . To address whether the R…FPR surface patch of PUX10 is important for the Cdc48–PUX10 interaction, we generated a mutant PUX10 (PUX10(mut)) with a triple-point mutation in the R…FPR motif (R409A, F450S and R452A). Constructs encoding either WT PUX10 or PUX10(mut) fused to YFP (that is, PUX10–YFP or PUX10(mut)–YFP) were co-expressed in protoplasts along with a previously described construct encoding HA-tagged Cdc48 (Cdc48–HA) 10 . In parallel, a free YFP construct was co-expressed with Cdc48–HA to serve as a negative control. Here PUX10–YFP and PUX10(mut)–YFP were used as the bait proteins and were immunoprecipitated with anti-GFP beads after solubilization, while Cdc48–HA acted as the prey (Fig. 4e ). As expected, the results clearly showed that the binding of PUX10 to Cdc48 was substantially reduced by the triple-point mutation. Therefore, the conserved R…FPR surface patch is essential for PUX10 binding to Cdc48.
It is well known that most Cdc48 adaptor proteins, including UBX proteins, bind to the N-terminal domain of Cdc48 (ref. 19 ). Indeed, our predictions by AlphaFold pointed to an interaction between the PUX10 UBX domain and the Cdc48 N terminus (Extended Data Fig. 5 ). To corroborate the prediction from AlphaFold, co-IP analysis was performed. Constructs encoding either full-length Cdc48 or Cdc48 lacking the N terminus (that is, Cdc48–HA or Cdc48(∆Nterm)–HA) were co-expressed in protoplasts along with the construct encoding PUX10–YFP. Here PUX10–YFP was used as the bait protein and was immunoprecipitated with anti-GFP beads after solubilization, while Cdc48–HA and Cdc48(∆Nterm)–HA acted as the prey (Fig. 4f ). As expected, the binding of Cdc48 to PUX10 was abolished by the truncation of the N terminus.
Taken together, these results showed that PUX10 is able to bind the N terminus of Cdc48 through its UBX domain to recruit Cdc48 to the chloroplast envelope membrane.
PUX10 interacts with the CHLORAD machinery
The Cdc48 ATPase was previously shown to form a complex with SP1 and SP2 at the surface of the chloroplast, enabling the ubiquitin-dependent degradation of TOC proteins by the cytosolic 26S proteasome in CHLORAD 10 . To investigate the possibility that PUX10 is involved in these processes, we assessed whether PUX10 also associates with SP1 and SP2.
First, BiFC assays were used to test for interactions between PUX10 and SP1. Using the BiFC system previously described, full-length PUX10 was fused with cYFP, and SP1 and the negative control proteins sensitive to freezing 2 (SFR2) and cyclin-dependent kinase A1 (CDKA1) were fused with nYFP. Construct pairs encoding complementary nYFP and cYFP fragments were transiently co-expressed in Arabidopsis protoplasts and any reconstituted YFP signals were visualized by confocal microscopy (Fig. 5a ). In this way, PUX10 was found to interact with SP1 at the chloroplast envelope membrane, whereas neither of the controls (the chloroplast membrane protein SFR2 nor the cytosolic protein CDKA1) showed appreciable interaction with PUX10.
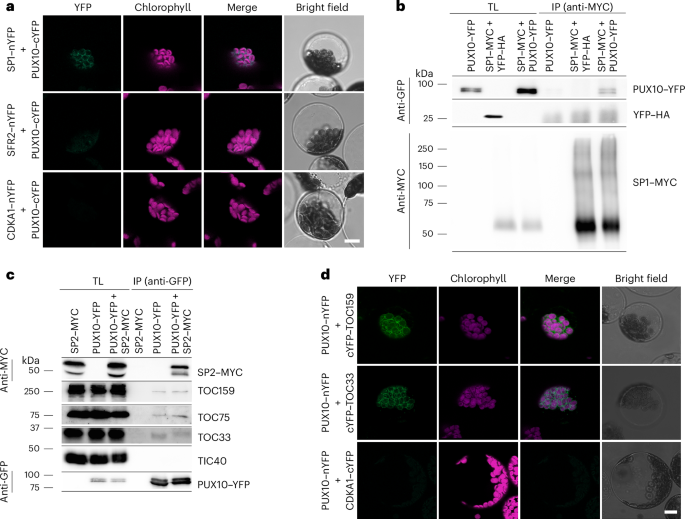
a , BiFC analysis of the interaction between PUX10 and SP1. Protoplasts transiently co-expressing the SP1 and PUX10 proteins fused to nYFP and cYFP fragments of YFP, respectively, were analysed by confocal microscopy. In parallel, SFR2 was used as a chloroplast-localized negative control and CDKA1 was used as a cytosol-localized negative control. Representative protoplasts are shown. Exposure times and gain settings were identical. Scale bar, 10 µm. b , Co-IP analysis of the interaction between PUX10 and SP1. Protoplasts expressing the indicated proteins were solubilized and subjected to anti-MYC co-IP analysis. Anti-MYC tag immunoblot analysis verified the enrichment of the SP1–MYC protein and anti-GFP analysis assessed co-purification of PUX10–YFP or YFP–HA. c , Co-IP analysis of the interaction between PUX10 and SP2 or TOC proteins. Protoplasts expressing the indicated proteins were solubilized and subjected to anti-GFP co-IP analysis. Anti-GFP tag immunoblot analysis verified the enrichment of the PUX10–YFP protein and anti-MYC analysis assessed co-purification of SP2–MYC. Further immunoblot analysis using antibodies to key TOC components was used to detect co-purification of endogenous TOC proteins; similar analysis of TIC40 provided a negative control to confirm the specificity of the detected interactions. d , BiFC analysis of the interactions between PUX10 and major TOC protein isoforms. Protoplasts transiently co-expressing PUX10 and either TOC159 or TOC33 fused to nYFP and cYFP fragments of YFP, respectively, were analysed by confocal microscopy. In parallel, CDKA1 was used as a cytosol-localized negative control instead of the TOC components. Representative protoplasts are shown as in a . Exposure times and gain settings were identical. Scale bar, 10 µm.
To corroborate the results from the BiFC experiments and validate the interaction between PUX10 and SP1, co-IP experiments were performed. The construct encoding PUX10–YFP was transiently expressed in protoplasts, either in combination with a construct encoding MYC-tagged SP1 (SP1–MYC) or alone. In parallel, a YFP–HA construct was co-expressed with SP1–MYC in protoplasts to serve as a further negative control. The SP1–MYC and YFP–HA constructs were previously described 10 . Here SP1–MYC served as the bait and was immunoprecipitated with anti-MYC beads after solubilization of the transfected cells, with PUX10–YFP and YFP–HA serving as prey (Fig. 5b ). As expected, only PUX10–YFP (not YFP–HA) was found to co-precipitate with SP1–MYC, indicating a specific interaction between PUX10 and SP1.
BiFC was judged to be an unsuitable method for analysing potential interactions with SP2 given its multi-membrane-spanning structure. Therefore, co-IP analysis was used to assess the interaction between PUX10 and SP2. Constructs encoding PUX10–YFP and MYC-tagged SP2 (SP2–MYC) were transiently co-expressed in protoplasts. In parallel, the two constructs were also singly transfected into protoplasts in control experiments. The SP2–MYC construct was previously described 10 . In this analysis, PUX10–YFP acted as the bait and was immunoprecipitated with anti-GFP beads after solubilization of the cells, with SP2–MYC acting as the prey (Fig. 5c ). The results clearly showed a strong, specific interaction between PUX10 and SP2.
PUX10 interacts with TOC proteins
On the basis of the above-described results, we hypothesized that PUX10 participates in the regulation of the TOC apparatus in a similar fashion to SP1 and SP2. Therefore, the co-IP samples generated above (Fig. 5c ) were further analysed using antibodies to TOC and TIC components in additional immunoblotting experiments. The results showed that all three TOC proteins (TOC159, TOC75 and TOC33) had co-precipitated with PUX10, whereas no association was detected for TIC40 (Fig. 5c ). This indicated specific interactions between PUX10 and the TOC complex.
To complement these results with spatial information, the interactions between PUX10 and TOC proteins were also assessed in BiFC experiments. In this case, PUX10 was fused to the nYFP fragment, and TOC159, TOC33 and the negative control protein CDKA1 were all fused to the cYFP fragment. Complementary construct pairs were transiently expressed in protoplasts and any resulting YFP signals were visualized by confocal microscopy (Fig. 5d ). As expected, PUX10 was found to interact with both TOC159 and TOC33 at the chloroplast envelope. However, no appreciable interaction was observed for the negative control protein CDKA1, indicating that the detected PUX10–TOC interactions were specific. Moreover, similar BiFC analyses indicated that TOC132 and TOC34 (which are minor isoforms of TOC159 and TOC33, respectively, in Arabidopsis ) also interact with PUX10 at the chloroplast envelope (Extended Data Fig. 7 ).
As noted earlier, PUX10 possesses an N-terminal UBA domain, which is a well-known ubiquitin-binding module. To investigate whether PUX10 indeed has the capacity to bind ubiquitin, co-IP analysis was performed. Constructs encoding either full-length PUX10 or the ΔUBA variant fused to YFP (that is, PUX10–YFP or ΔUBA–YFP) were co-expressed in protoplasts along with a previously described construct encoding FLAG-tagged ubiquitin (FLAG–Ub) 9 . Here the YFP fusions acted as bait and were immunoprecipitated using anti-GFP beads after solubilization, with FLAG–Ub acting as the prey (Fig. 6a ). The results showed a strong interaction between full-length PUX10 and ubiquitin (in fact, polyubiquitin smears), and this interaction was dependent on the UBA domain. Given that TOC proteins are ubiquitinated by SP1 before being targeted to the cytosolic 26S proteasome for degradation, it was hypothesized that the UBA domain of PUX10 is important for the interaction between PUX10 and ubiquitinated TOC proteins. Thus, the co-IP analysis above was repeated using HA-tagged TOC33 (TOC33–HA) 10 in place of FLAG-Ub (Fig. 6b ). The results clearly indicated that PUX10 is capable of interacting with polyubiquitinated TOC33 through its UBA domain. The fact that unmodified TOC33–HA was similarly precipitated most likely reflects the fact that ubiquitinated and unmodified proteins are present together in complexes. To corroborate the interaction between PUX10 and ubiquitinated TOC proteins, a reciprocal assay using the same constructs was performed. In this assay, the TOC33–HA acted as bait and was immunoprecipitated using anti-HA beads after solubilization, with PUX10–YFP and ∆UBA–YFP acting as the prey (Fig. 6c ). The results confirmed the interaction between PUX10 and (ubiquitinated) TOC33, and that this interaction is dependent on the UBA domain.
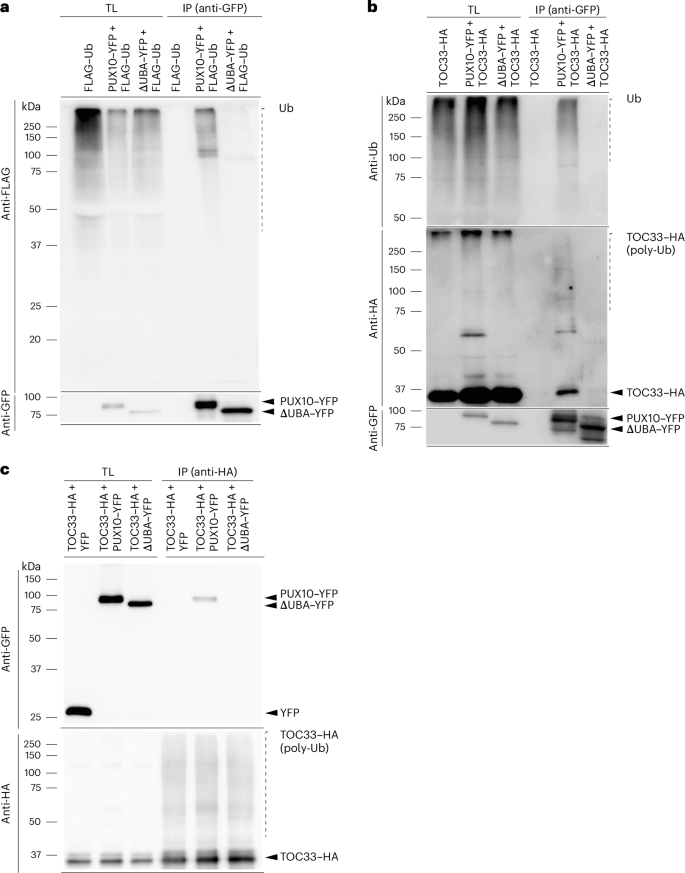
a , Analysis of the interaction of PUX10 with ubiquitin. Protoplasts transiently expressing the indicated proteins were solubilized and subjected to anti-GFP co-IP analysis. Anti-GFP immunoblot analysis verified the enrichment of the PUX10–YFP or ΔUBA–YFP proteins, and anti-FLAG analysis assessed co-purification of FLAG-tagged (poly)ubiquitin. b , Analysis of the interaction of PUX10 with ubiquitinated TOC33. Protoplasts expressing the indicated proteins were solubilized and subjected to anti-GFP co-IP analysis. Anti-GFP immunoblot analysis verified the enrichment of the PUX10–YFP or ΔUBA–YFP proteins, and anti-HA analysis assessed co-purification of TOC33–HA; both unmodified (see arrowhead) and high-molecular-weight modified forms of TOC33–HA were detected. Parallel analysis of the samples by anti-ubiquitin immunoblotting provided evidence that the high-molecular-weight species were polyubiquitinated TOC33. c , Analysis of the interaction of ubiquitinated TOC33 with WT PUX10 or PUX10 lacking its UBA domain. Protoplasts co-expressing the indicated proteins were solubilized and subjected to anti-HA co-IP analysis. Anti-HA immunoblot analysis verified the enrichment of the TOC33, and anti-GFP analysis assessed co-purification of the PUX10–YFP or ΔUBA–YFP proteins. Both unmodified (see arrowhead) and high-molecular-weight modified forms of TOC33–HA were enriched. Dashed verticle lines indicate ubiquitinated proteins. poly-Ub, polyubiquitin; Ub, ubiquitin.
Collectively, these results from different BiFC and co-IP experiments showed a clear association between PUX10 and the components and substrates of CHLORAD at the chloroplast OEM.
The pux10 mutation suppresses the ppi1 phenotype
The interaction of PUX10 with TOC proteins suggested a functional link between PUX10 and the TOC apparatus. To investigate this possibility, the pux10-1 mutation was introduced into the ppi1 single-mutant background 14 and, as a control, the tic110 /+ mutant background 43 . The resulting pux10-1 ppi1 double-mutant plants showed a moderate increase in chlorophyll content and leaf size relative to single-mutant ppi1 control plants (Fig. 7a,b ). In contrast, pux10-1 tic110 /+ double-mutant control plants showed no phenotypic differences from tic110 / + single-mutant plants, which show mild chlorosis, indicating that the effect on ppi1 was specific; note that the tic110 genotype was analysed in the heterozygous state as the homozygous state is lethal 43 .
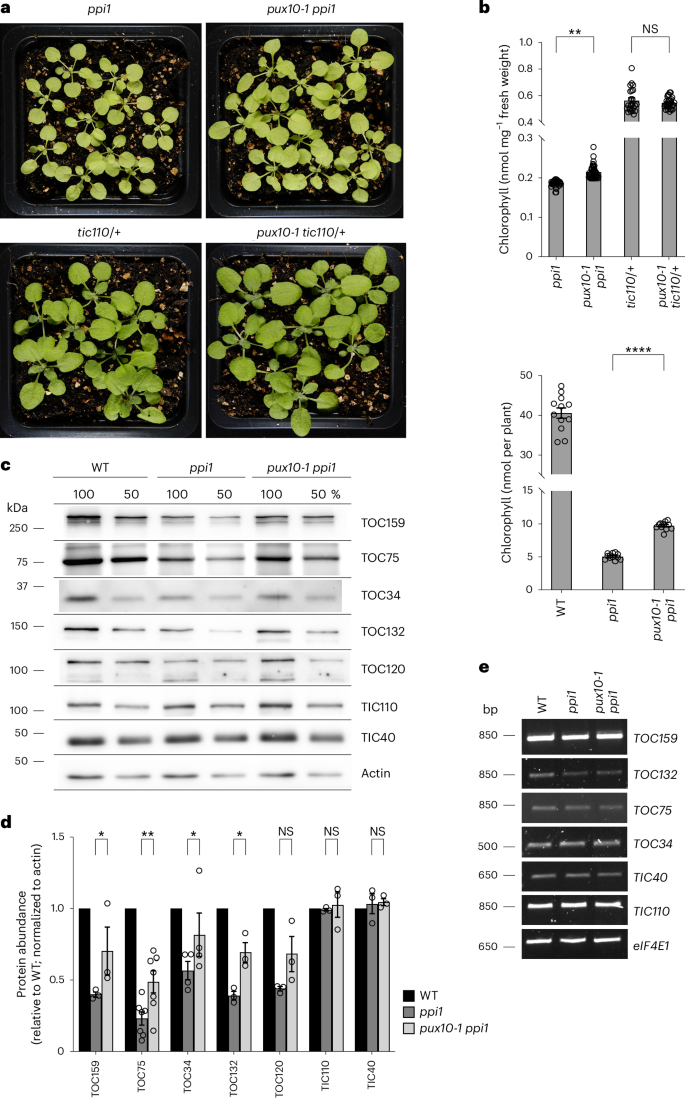
a , Phenotypes of 3-week-old pux10-1 ppi1 and control seedlings grown on soil. Controls included the pux10-1 tic110 / + double mutant; the tic110 genotype was analysed in the heterozygous state as the homozygous state is lethal 43 . Representative plant images are shown. Identical camera settings were used and all images are at the same magnification. b , Quantification of chlorophyll concentration in pux10-1 ppi1 double-mutant and control plants. Measurements were taken on the day of photography in a . First, the leaves were analysed using a Konica Minolta SPAD-502 meter (top). The values shown are means ± s.e.m. from 30 leaves per genotype. Second, chlorophyll in the aerial tissues of the plants was extracted and quantified using a spectrophotometer (bottom). The values shown are means ± s.e.m. from five plants per genotype. Asterisks indicate significance according to an unpaired two-tailed Student’s t -test. ** P < 0.005, **** P < 0.0001. c , d , Immunoblot analysis of TOC and TIC protein levels in WT, ppi1 and pux10-1 ppi1 plants. c , four-week-old plants of indicated genotypes were subjected to immunoblotting analysis. Two different loadings of each sample (100% and 50%) were analysed. Actin was used as a loading control. Representative blot images are shown. d , quantification of the immunoblot data presented in c , and of other similar datasets, was performed. Band intensities were quantified and normalized to corresponding actin data; the data are presented as ratios relative to the WT value for each protein. Data were derived from multiple technical replicates and were representative of three biological replicates. Sample size ( n ) for each protein was as follows: TOC159 (3), TOC75 (7), TOC34 (4), TOC132 (3), TOC120 (3), TIC110 (3) and TIC40 (3). The values shown are means ± s.e.m. Asterisks indicate significance according to an unpaired two-tailed Student’s t -test. * P < 0.05, ** P < 0.005. e , Analysis of mRNA expression of important translocon component genes in WT, ppi1 and pux10-1 ppi1 plants. Total RNA isolated from 20-day-old plants was analysed by RT-PCR for the indicated genes and the reference gene eIF4E1 . Amplifications used a limited number of cycles to avoid saturation. NS, not significant.
To investigate the basis for this ppi1 suppression effect, total protein extracts were prepared from WT, ppi1 and pux10-1 ppi1 plants and the abundance of TOC proteins in the samples was analysed by immunoblotting. Notably, all tested TOC proteins showed substantially recovered levels in the pux10-1 ppi1 double-mutant plants, relative to ppi1 , whereas the IEM proteins TIC40 and TIC110 were largely unaffected by the pux10 mutation (Fig. 7c,d ). These changes in TOC protein abundance were not attributable to pretranslational events because TOC transcript levels were comparable in the different genotypes (Fig. 7e ). Thus, the ppi1 suppression mediated by pux10 , much like that mediated by sp1 and sp2 as previously described 9 , 10 , is linked to partially restored TOC protein accumulation.
The pux10 mutation abrogates effects of SP1 overexpression
Having identified the interaction between PUX10 and SP1, we wished to investigate a possible functional link between the two components. It is well known that the sp1 mutation suppresses the ppi1 phenotype to produce bigger and greener plants that have increased abundance of TOC proteins and chlorophyll and improved chloroplast ultrastructural organization 9 . Conversely, SP1 overexpression (SP1-OX) greatly enhances the chlorosis of ppi1 by increasing the ubiquitination of the residual TOC proteins to prime their proteasomal degradation, leading to severe depletion of TOC proteins, reduced chlorophyll concentration, and smaller and paler plants 9 . Given that Cdc48 is important for the degradation of ubiquitinated TOC proteins 10 and that PUX10 recruits Cdc48 to the chloroplast OEM (as shown earlier), we hypothesized that PUX10 is required for SP1 function and therefore for the excessive TOC protein removal seen upon SP1 overexpression. If this is indeed the case, then the pux10 mutation should block or reduce the severe phenotypic effects of SP1-OX in ppi1 plants.
To test this hypothesis, the pux10-1 mutation was crossed into the SP1-OX ppi1 background, and triple homozygous pux10-1 SP1-OX ppi1 plants were identified via phenotyping, genotyping and RT-PCR analysis (Extended Data Fig. 8 ). As expected, SP1-OX ppi1 plants were much smaller and more chlorotic than ppi1 plants. Most interestingly, and in accordance with the above hypothesis, the pux10-1 SP1-OX ppi1 plants were substantially greener and larger than SP1-OX ppi1 plants, although they were not completely recovered to the level of ppi1 single-mutant plants (Fig. 8a ). Chlorophyll concentration was significantly increased in pux10-1 SP1-OX ppi1 plants relative to SP1-OX ppi1 plants, although still considerably less than in ppi1 (Fig. 8b ); this was consistent with the visible difference between the pux10-1 SP1-OX ppi1 and ppi1 plants.
a , Phenotypes of 4-week-old pux10-1 SP1-OX ppi1 triple-homozygous and control seedlings grown on soil. Representative plant images are shown. Identical camera settings were used and all images are at the same magnification. b , Quantification of chlorophyll concentration in the plants. Measurements were taken on the day of photography in a using a Konica Minolta SPAD-502 meter. The values shown are means ± s.e.m. from 30 leaves. Asterisks indicate significance according to an unpaired two-tailed Student’s t -test. **** P < 0.0001. c – e , Ultrastructure analysis of rosette leaf chloroplasts in pux10-1 SP1-OX ppi1 and control plants. c , samples from 4-week-old plants were analysed by TEM and typical organelles are shown. Scale bar, 1 µm. The presented electron micrographs, and other similar micrographs, were used to determine chloroplast cross-sectional area ( d ) and thylakoid development ( e ), which includes the number of lamellae per granal stack and the number of membrane interconnections per granal stack. The values shown are means ± s.e.m. from 60 chloroplasts per genotype in d and 50–80 chloroplasts per genotype in e . Asterisks indicate significance according to an unpaired two-tailed Student’s t -test. **** P < 0.0001. f , g , Immunoblot analysis of TOC and TIC protein levels in pux10-1 SP1-OX ppi1 and control plants. f , four-week-old plants of the indicated genotypes were subjected to immunoblotting analysis. Equal amounts of the different samples were loaded. Histone H3 was used as a loading control. Representative blot images are shown. g , quantification of the immunoblot data presented in f , and of other similar datasets, was performed. Band intensities were quantified and normalized to corresponding H3 data; the data are presented as ratios relative to the ppi1 value for each protein in arbitrary units. Data were derived from multiple technical replicates and were representative of three biological replicates. Sample size ( n ) for each protein was as follows: TOC75 (5), TOC159 (3), TOC132 (3), TOC34 (4), TIC110 (3) and TIC40 (5). The values shown are means ± s.e.m. Asterisks indicate significance according to an unpaired two-tailed Student’s t -test. ** P = 0.0016, *** P = 0.0006, **** P < 0.0001. NS, not significant.
To determine whether the suppression of chlorosis observed in pux10-1 SP1-OX ppi1 plants was linked to effects on chloroplast biogenesis, the organelles were analysed by transmission electron microscopy (TEM). This analysis showed that pux10-1 SP1-OX ppi1 plants possess chloroplasts of increased size and improved ultrastructure compared with SP1-OX ppi1 plants (Fig. 8c ). Quantitative analysis showed a clear increase in chloroplast cross-sectional area in pux10-1 SP1-OX ppi1 relative to SP1-OX ppi1 (Fig. 8d ), and there were increased numbers of thylakoid lamellae per granal stack and interconnections between granal stacks (Fig. 8e ). Nonetheless, the overall development of chloroplasts in pux10-1 SP1-OX ppi1 was still less than that in ppi1 , which was consistent with the visible differences between the plants.
In SP1-OX ppi1 plants, the severe phenotype is related to reduced abundance of TOC proteins 9 . To test whether the suppression effect observed in pux10-1 SP1-OX ppi1 plants is linked to restored TOC protein abundance, total protein extracts from the plants were analysed by immunoblotting. This analysis revealed substantial increases in TOC protein abundance in pux10-1 SP1-OX ppi1 plants relative to SP1-OX ppi1 plants (Fig. 8f,g ). However, in line with previous observations concerning the sp1 ppi1 mutant 9 , there were no comparable differences in the levels of TIC components, indicating that the effects of PUX10, similarly to those of SP1, are specific to TOC components.
To further explore the physiological significance of PUX10, we conducted an analysis of leaf senescence. The expression level of SP1 was previously shown to influence leaf senescence and other developmental processes in which plastids (the organelle family to which chloroplasts belong) change type 9 , 12 . High levels of SP1 activity promote the reorganization of the TOC machinery so that it is better able to bring about the organellar proteome changes that underly such transitions, and in the case of aging or dark-treated leaves, this results in accelerated leaf senescence (due to accelerated conversion of chloroplasts into gerontoplasts) 9 , 12 . To determine whether PUX10 also plays a role in such developmental processes, the pux10-1 mutation was crossed into the SP1-OX background and the resulting pux10-1 SP1-OX plants were subjected to leaf-senescence analysis, along with WT, sp1 and SP1-OX control plants (Extended Data Fig. 9 ).
In line with previously published results, the sp1 mutant showed retarded leaf senescence, as judged by reduced visible yellowing and a smaller decline in the maximal photochemical efficiency of photosystem II (variable fluorescence ( F v )/maximum fluorescence ( F m )) relative to WT, whereas SP1-OX leaves showed accelerated senescence. Interestingly, the pux10-1 SP1-OX leaves showed a significant reduction in senescence compared with SP1-OX leaves based on both visual assessment of the material and measurements of the F v / F m parameter (Extended Data Fig. 9 ). Thus, these data show the physiological importance of PUX10 during an important developmental transition and further support the conclusion that PUX10 cooperates with SP1 in the CHLORAD pathway.
Thousands of nucleus-encoded proteins are imported into chloroplasts via the TOC–TIC apparatus in the chloroplast envelope membranes 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 . Hence, protein import is vital for chloroplast biogenesis and operation and for plant growth and development. The CHLORAD system regulates the turnover of TOC proteins to manipulate protein import and thereby optimizes the organellar proteome and functions 8 . Three key components of CHLORAD have been identified (SP1, SP2 and Cdc48), which work cooperatively in the ubiquitination, retrotranslocation and degradation of TOC proteins. The Cdc48 ATPase drives the extraction of target proteins through the SP2 channel, following their ubiquitination by SP1; but how Cdc48 is recruited to the chloroplast surface to enable such action was previously unknown.
Previous studies identified PUX proteins as being involved in regulating various activities of Cdc48 in plants 26 , 27 , 28 , 29 , 30 but their potential involvement in CHLORAD was unexplored. The PUX10 protein was recently identified as an LD-localized Cdc48 adaptor protein that regulates the degradation of LD proteins during embryogenesis and seed germination 31 , 32 . Intriguingly, the subcellular localization of PUX10 swiftly changed from LDs to chloroplasts during seed maturation 31 . Given its relevance to the interpretation of our study, we further analysed the subcellular localization of PUX10. During embryogenesis, PUX10 was predominantly localized in LDs at the bent cotyledon stage; however, when embryos reached the mature green stage, it was predominantly localized in chloroplasts and a characteristic ring-shaped distribution around the chloroplast envelope was detected (Supplementary Fig. 1 ). During germination, PUX10 was found to be associated with LDs at the earliest stages (6 h of germination; although it should be noted that plastid localization at this stage could not be ruled out owing to the difficulty in detecting these organelles due to the lack of chlorophyll) but then later PUX10 was detected around chloroplasts (24 h of germination) before finally becoming clearly and predominantly located at the surface of the chloroplasts (after 48 h of germination) (Supplementary Fig. 1 ). Thus, it appears that PUX10 has multifunctional roles that are delivered in a phased manner during plant development.
In leaves, we found PUX10 to be distinctly and predominantly associated with the chloroplasts, where it was clearly localized in the envelope (Fig. 1 and Extended Data Figs. 1 and 2 ). Nonetheless, colocalization analysis indicated that a minor fraction of PUX10 is associated with the ER (Supplementary Fig. 2 ), as was also described in previous studies 31 , 32 . Interestingly, such partitioning between organelles was also observed for Ubx2 and FAS-associated factor 2 (FAF2, also known as Ubxd8), which are homologues of PUX10 in yeast and mammals, respectively 33 , 44 , 45 , 46 , 47 , 48 . The Ubx2 protein is best known for its role in recruiting Cdc48 to the ER membrane in ERAD but it was also shown to perform a similar role in mitochondria 47 . Therefore, multilocation operation may be a conserved feature of this group of proteins. It will be interesting to investigate in future work whether PUX10 indeed participates in ER-associated protein quality control processes, in addition to CHLORAD.
To elucidate the function of PUX10 in chloroplasts, a key step was to establish the topology of the protein. Our analysis showed that PUX10 is anchored in the chloroplast OEM by two TM spans such that both termini face the cytosol (Figs. 1 and 2 ). Thus, the UBA and UBX domains both have cytosolic orientation, enabling interactions of PUX10 with different sets of functional partners. Our results indicated that PUX10 interacts with Cdc48 via its UBX domain, enabling it to recruit Cdc48 to the chloroplast envelope (Figs. 3 and 4 ), and with ubiquitinated TOC proteins via its UBA domain (Figs. 5 and 6 ). Thus, PUX10 effectively acts as a bridge that brings together cytosolic Cdc48 and ubiquitinated TOC proteins in the OEM to promote the retrotranslocation step of CHLORAD. Indeed, PUX10 was also shown to interact with SP1 and SP2 (Fig. 5 ), supporting the notion that it is a key component of the CHLORAD system acting in the regulated turnover of the TOC machinery.
This view was confirmed through genetic analysis. Apart from effects on LD size during embryogenesis 31 , 32 and seed germination (Extended Data Fig. 3d,e ), Arabidopsis pux10 single mutants showed no obvious phenotypic differences from WT plants (Extended Data Figs. 3 and 4 ), much like sp1 and sp2 mutants 9 , 10 . However, the pux10 mutation did suppress the pale phenotype of ppi1 . Importantly, this suppression was linked to increased TOC protein abundance (Fig. 7 ), paralleling closely the ppi1 suppression mediated by the sp1 and sp2 mutations 9 , 10 . This provided strong evidence that PUX10 is involved in chloroplast functions in leaves and most likely in the regulation of TOC activity by CHLORAD.
As a core component of the CHLORAD system, the E3 ubiquitin ligase SP1 labels TOC proteins with ubiquitin for degradation by the cytosolic 26S proteasome. Thus, when it is overexpressed in an already TOC-compromised background ( ppi1 ), severe chlorosis results 10 . Intriguingly, when the pux10 mutation was introduced into the SP1-OX ppi1 background, the resulting plants were much larger and greener than SP1-OX ppi1 plants, and presented increased abundancies of chlorophyll and TOC proteins and improved chloroplast ultrastructural organization (Fig. 8 ). This provided a clear demonstration that PUX10 is required for SP1 function in vivo. Previous work showed that SP1 plays a crucial role in plant developmental transitions in which plastids change type, such as leaf senescence 9 , 12 . We found that the stimulating effect of SP1 overexpression on leaf senescence 9 was attenuated by the pux10 mutation (Extended Data Fig. 9 ), providing further strong evidence that PUX10 and SP1 act in the same pathway (that is, CHLORAD) and of the physiological importance of PUX10.
It is noteworthy that the phenotypic suppression of ppi1 delivered by pux10 (Fig. 8 ) was not as strong as that delivered by the sp1 or sp2 mutations 9 , 10 , and that the pux10 mutation only partially blocked the phenotypic consequences of SP1 overexpression. These observations suggest that there may be functional redundancy between PUX10 and as-yet-unknown proteins, such as other members of the PUX family 49 or other Cdc48 adaptor proteins 19 , 20 . Although our comprehensive analysis of PUX protein localization did not identify any other family members with distinct chloroplast localization (Extended Data Fig. 1 ), we cannot completely rule out the possibility that additional PUX proteins function at the chloroplast surface. It is also possible that PUX10 is only strictly required in specific situations—more so than either SP1 or SP2, which are clearly responsible for core functions of the CHLORAD system (that is, substrate ubiquitination and conductance, respectively). Regardless, it will be interesting to explore in future studies what other factors are involved in the ubiquitin-mediated degradation of TOC proteins.
Collectively, our work has unveiled PUX10 as a chloroplast-bound adaptor protein that recruits Cdc48 to the chloroplast surface, promoting its interaction with ubiquitinated TOC proteins so that they may be extracted into cytosol for degradation by the 26S proteasome. In view of its physical and functional association with the established CHLORAD machinery, we conclude that PUX10 is a bona fide component of the CHLORAD system (Extended Data Fig. 10 ).
Plant materials and growth conditions
All Arabidopsis thaliana plants used in this work were of the Columbia-0 (Col-0) ecotype. Two T-DNA mutant lines, pux10-1 (SAIL_1187_B06) and pux10-4 (WiscDsLox424B8), were obtained from the Salk Institute Genomic Analysis Laboratory (SIGnAL) 50 via the Nottingham Arabidopsis Stock Centre and confirmed by genomic PCR and RT-PCR, as previously described 51 . The pux10-1 mutant has been previously described 31 , 32 but the pux10-4 mutant has not previously been studied. Two further alleles of pux10 , namely pux10-2 and pux10-3 , have been described by other research groups 31 , 32 but were not used in this study. The ppi1, tic110/+ , sp1 and sp1 ppi1 mutants and the SP1-OX and SP1-OX ppi1 transgenic lines have been previously described 9 , 14 , 43 . The ER, mitochondria and Golgi marker lines were provided by Dr Niloufer G. Irani and the late Dr Ian Moore of Oxford University 52 , 53 , 54 .
For most experiments, plants were grown on soil: 80% (v/v) compost (modular seed; Sinclair) and 20% (v/v) vermiculite (fine particle size; Sinclair Pro). For in vitro growth, seeds were surface sterilized, sown on Murashige–Skoog (MS) agar medium in petri plates, cold treated at 4 °C and kept in a growth chamber (Percival Scientific) thereafter, as previously described 55 . All plants were grown under a long-day cycle (16 h light and 8 h dark, 100–120 µE m −2 s −1 ) at 20 °C with ~60% relative humidity. For the induction of CDC48-WT or CDC48-DN expression in the corresponding transgenic lines, 8-day-old seedlings were transferred to liquid MS medium supplemented with 4 μM oestradiol (Sigma) and incubated for an additional 2 days as previously described 10 .
For germination assays, seeds were surface sterilized, sown on MS agar medium in petri plates, cold treated at 4 °C and kept in a growth chamber (Percival Scientific) thereafter at 20 °C under continuous light.
Physiological studies
Chlorophyll measurement was performed as previously described by using a Konica Minolta SPAD-502 meter for the analysis of rosette-stage plants 56 , or following N , N ′-dimethylformamide extraction using a spectrophotometer for the analysis of seedlings 14 , 57 , 58 , 59 .
Dark treatments for the induction of leaf senescence were conducted as previously described 9 , 60 . Developmentally equivalent leaves of 28-day-old plants were wrapped in aluminium foil while still attached to the plant and then left under standard growth conditions for 5 days. F v / F m was determined by measuring chlorophyll fluorescence using a CF Imager (Technologica) as previously described 9 , 12 . Three experiments were performed and approximately ten leaves (each one from a different plant) were analysed per genotype in each experiment.
Plasmid constructs
All primers used are listed in Supplementary Table 1 . The SP1–MYC, SP2–MYC, YFP–HA, FLAG–Ub, TOC33–HA, Cdc48–HA and Cdc48–YFP constructs have all been previously described 9 , 10 . The Cdc48(∆Nterm)–HA construct was generated by using modified 5′ and 3′ primers to amplify from Col-0 cDNA a truncated Cdc48 coding sequence (CDS) (encoding a polypeptide lacking the first 190 residues), which was then cloned into the pDONR201 entry vector (Invitrogen) and subcloned into the modified p2GW7 vector 61 with a C-terminal HA tag for protoplast transfection. The new PUX10-related constructs for this study were generated as follows. The PUX10 CDS was amplified in different forms from Col-0 cDNA by using primers at the 5′ and 3′ termini of the CDS; by using a modified 5′ primer that adds an N-terminal FLAG tag; by using alternative 5′ and/or 3′ primers to generate the ΔUBX, ΔUBA and ΔUBX/UBA variants; and by using modified 5′ and 3′ primers to generate the triple UBX-domain point mutations of PUX10(mut). The PUX10 CDS lacking the coding region of the TM domains (ΔTM1/2) was generated by overlap-extension PCR. The PUX10 promoter ( pPUX10 ) was amplified from Col-0 genomic DNA by using primers that add 5′ HindIII and 3′ SpeI sites. To generate a modified pK7YWG2 binary vector 61 , the amplified PUX10 promoter sequence was cloned into pGEM-T Easy (Promega), sequenced, and then subcloned into 5′ HindIII and 3′ SpeI sites of pK7YWG2 to replace the 35S promoter. The Gateway Cloning System (Invitrogen) and vectors driven by the 35S promoter (with the exception of pPUX10 ) were used for most constructs, and all donor vectors were verified by DNA sequencing. To generate C-terminal YFP tag fusions, the PUX10 CDSs (all forms) were cloned into the pDONR201 entry vector (Invitrogen) and then subcloned either into the p2GWY7 vector 61 for protoplast transfection or into the pK7YWG2 or the modified ( pPUX10 ) pK7YWG2 binary vector 61 for stable plant transformation. The full-length PUX10 CDS (no tag) was also cloned into a previously described modified binary vector pH2GW7, which provides a C-terminal HA tag for stable plant transformation 9 , 10 . To generate BiFC constructs, selected CDSs were cloned into the pGEM-T Easy vector, sequenced and then subcloned as follows: into 5′ BglII and 3′ SalI sites of the pSAT4A-nEYFP-N1 and pSAT4A-cEYFP-N1 vectors 37 , 62 for PUX10–nYFP and PUX10–cYFP, respectively; into 5′ XholI and 3′ EcoRI sites of the pSAT4A-cEYFP-N1 vector for ΔUBX–cYFP; and into 5′ KpnI and 3′ XmaI sites of the pSAT4A-nEYFP-N1 vector for SFR2–nYFP. The Cdc48–nYFP, SP1–nYFP, CDKA–nYFP, cYFP–TOC33, cYFP–TOC159, cYFP–TOC34 and cYFP–TOC132 constructs have all been previously described 10 , 63 . Routine sequence analyses for generating plasmid constructs were conducted using DNAStar Lasergene v.7.2.
Protoplast isolation and transient assays
Protoplast isolation and transient assays were carried out as previously described 64 . When required, bortezomib (Selleckchem) (prepared as a 10 mM stock solution in DMSO) was added to the protoplast culture medium after 15 h of incubation to a final concentration of 5 μM; subsequently, the culture was incubated for a further 2–3 h before analysis. For YFP fluorescence or co-IP assays, 0.1 ml (~10 5 ) or 0.6 ml (~10 6 ) aliquots of protoplasts were transfected, respectively, with either 5 μg or 30 μg of plasmid DNA. The samples were analysed after 15–18 h.
Generation of transgenic lines
The 35 :PUX10–YFP (PUX10-OX in Fig. 3a ), 35S :ΔUBX–YFP, 35S :ΔUBA–YFP, 35S :ΔUBX/UBA–YFP, 35S :ΔTM1/2–YFP, 35S :FLAG–PUX10–YFP, 35S :PUX10–HA (PUX10-OX in Fig. 3b ) and pPUX10 :PUX10–YFP transgenic plants were generated by Agrobacterium -mediated floral dip transformation 65 . Transformants were selected by using MS medium containing either kanamycin (for the pK7YWG2 vector) or hygromycin B (for the modified pH2GW7 vector). At least ten T 2 lines for each genotype were analysed by confocal microscopy, immunoblotting or RT-PCR. The 35S :PUX10–HA transgene was introduced into the CDC48-WT background 10 (and the pux10-1 mutation was introduced into the CDC48-DN background 10 ) (Fig. 3b ) by crossing the corresponding genotypes, followed by immunoblotting or semi-quantitative RT-PCR analysis in the F 2 generation.
TEM was performed using mature rosette leaves as previously described 59 . Images were taken using an FEI Tecnai T12 TEM from three biological replicates (different leaves from different individual plants) and analysed using Fiji ImageJ-win32 (ref. 66 ). Quantitative analyses (Fig. 8d,e ) were based on at least 60 different plastids per genotype and were representative of 3 individuals per genotype. Chloroplast cross-sectional area was estimated as previously described 43 , 59 by using the equation π × 0.25 × length × width. Numbers of thylakoid lamellae per granal stack and of interconnections between granal stacks were determined as previously described 9 , 43 in a total of ~130 resolvable grana across 3 individuals per genotype.
All fluorescence microscopy and BiFC experiments were conducted at least three times with the same results and typical images are presented. For the imaging of YFP, GFP, RFP and chlorophyll fluorescence signals, in most cases (except for Fig. 1c and Extended Data Fig. 2 ), protoplasts were examined by using a Leica TCS SP5 confocal microscope equipped with a Leica HC Plan Apochromat CS2 63.0× UV water immersion lens as previously described 63 , 67 . For Fig. 1c and Extended Data Fig. 2 , small leaf tissue samples (~0.5 cm × 0.5 cm) were mounted in perfluorodecalin (PFD) before imaging as described above. PFD easily infiltrates leaf tissue to fill the intercellular air spaces of the mesophyll, enabling high-resolution confocal imaging of the mesophyll 68 . For confocal microscopy experiments, typically YFP fluorescence, chlorophyll autofluorescence, merged YFP and chlorophyll fluorescence and bright-field images are presented.
For BiFC assays, plasmid DNA for two constructs (one nYFP fusion and one cYFP fusion) was co-transfected into WT Arabidopsis protoplasts. After overnight incubation, reconstituted YFP signals were analysed by confocal imaging. All images were captured using the same settings to enable comparisons.
For the analysis of LDs, embryos and young seedlings were obtained from different siliques of plants grown on soil or from seeds germinated on MS agar medium in petri plates. Embryos and young seedlings were gently squeezed out of the seed coat using jeweller forceps (Sigma-Aldrich) on a microscope slide under a dissection microscope. They were then stained with Nile Red (dissolved to a concentration of 4 mg ml −1 in DMSO and then diluted 500-fold in water before use; Sigma-Aldrich) for 30 min on the microscope slide, before a coverslip was applied with gentle pressure to flatten the stained material. Confocal microscopy was performed using a Zeiss LSM 880 Airy Scan. Excitation (ex) and emission (em) parameters for the detection of the different fluorophores were as follows: YFP (ex/em, 514 nm/521–551 nm), Nile Red (ex/em, 561 nm/580–671 nm) and chlorophyll (ex/em, 633 nm/670–700 nm). The embryos and young seedlings, after their dissection from siliques or germinated seeds, respectively, were also imaged using a Zeiss Stemi 508 microscope equipped with a Axiocam 105 colour camera.
The diameter of LDs was measured using Fiji ImageJ-win32 by drawing the diameter using the ‘line’ tool and measuring it using the ‘measure’ function of the software 69 .
Chloroplast isolation and protein topology analysis
Chloroplasts were isolated from plants grown in vitro for 8–10 days after the induction of the CDC48-WT and CDC48-DN constructs. To induce expression of the transgenes, 8-day-old seedlings were transferred from MS agar medium to MS liquid medium supplemented with 4 μM oestradiol (Sigma) and incubated for an additional 2 days with gentle shaking under standard growth conditions. Chloroplast isolation and alkaline extraction were performed as previously described 70 , 71 . Protease treatments were performed as previously described with some minor modifications 72 : 500 µg ml −1 thermolysin (with or without 1% Triton X-100) or 500 µg ml −1 trypsin was used. After the protease treatments, chloroplast pellets were added directly to 2× sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) loading buffer (see below) and then analysed by immunoblotting.
SDS–PAGE, immunoblotting and co-IP
SDS–PAGE and immunoblotting were performed as previously described 43 , 73 with minor modifications. When necessary, gels were stained with InstantBlue Protein Stain (Expedeon).
The primary antibodies used were as follows, with dilution factors provided in parentheses. To identify TOC or TIC components, we used anti-atTOC75-III POTRA-domain 51 (1:1,000), anti-atTOC159 A-domain 74 (1:5,000), anti-atTOC132 A-domain 9 (1:1,000); anti-atTOC120 A-domain 51 (1:1,000), anti-atTOC33 peptide 75 (1:500), anti-atTOC34 (1:2,000; AS07 238; Agrisera), anti-atTIC110 stromal domain 76 , 77 (1:5,000) and anti-atTIC40 stromal domain 51 (1:100,000). To detect unrelated proteins as loading controls, we used anti-actin (1:3,000; AS132640; Agrisera) and anti-histone H3 (1:1,000; ab1791; Abcam). Other primary antibodies we used were anti-HA tag (1:1,000; H6908; Sigma), anti-c-MYC tag (1:1,000; ab9106; Abcam), anti-GFP (which detects both GFP and YFP; 1:1,000; SAB4301138; Sigma), anti-FLAG tag (1:1,000; F7425; Sigma) and anti-ubiquitin (1:2,000; 662099; Merk) 10 .
The secondary antibody was anti-rabbit IgG conjugated with horseradish peroxidase (1:5,000; 12-348; Sigma). Chemiluminescence was detected using the EZ-ECL Enhanced Chemiluminescence Detection Kit for HRP (Biological Industries, Sartorius) and an ImageQuant LAS-4000 imager (GE Healthcare). Band intensities were quantified using Aida Image Analyzer v.4.27 software (Raytest). Quantification data were obtained from the results of at least three experiments all showing a similar trend. Typical images are shown in all figures.
For co-IP using YFP-tagged proteins, total protein (~500 mg) was extracted from protoplasts in IP buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA and 1% Triton X-100) containing 0.5% plant protease inhibitor cocktail (Sigma) and centrifuged at 20,000 g for 10 min at 4 °C. The clear lysate was then incubated with 50 μl of GFP-Trap Magnetic Agarose (ChromoTek) for 2 h to overnight at 4 °C with slow rotation. After 6 washes with 500 μl of IP-washing buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA and 0.5% Triton X-100), bound proteins were eluted by boiling in 2× SDS–PAGE loading buffer (50 mM Tris-HCl, pH 6.8, 20% glycerol, 1% SDS and 0.1 M dithiothreitol) for 5 min, and analysed by SDS–PAGE and immunoblotting. A similar procedure was adopted for co-IP using MYC-tagged proteins, except that 50 μl of EZview Red Anti-c-Myc Affinity Gel (Sigma) was used instead of the GFP-Trap Magnetic Agarose.
Multiple protein sequence alignment
Protein sequences of PUX10 and UBX2 homologues were obtained from a variety of sources including Phytozome 78 and Uniprot 79 by using Arabidopsis PUX10 as a query in BLASTP. Multiple sequences were aligned by the ClustalW multiple method, using the BioEdit Alignment Editor software package v.7.2.5.
Protein complex prediction in silico
The 3D structures of complexes formed by the PUX10 + Cdc48, PUX10(∆UBX) + Cdc48, PUX10 + Cdc48(∆Nterm) and PUX10(∆UBX) + Cdc48(∆Nterm) polypeptide pairs were predicted using Alphafold-Multimer (an extension of AlphaFold2 that uses artificial intelligence to predict protein–protein complexes 39 ) as previously described 40 . This analysis was performed by Homma Scientific. The 3D structures of the PUX10(UBX) + Cdc48, PUX10 + Cdc48(Nterm) and PUX10(UBX) + Cdc48(Nterm) polypeptide pairs were predicted by AlphaFold2 using UCSF ChimeraX 80 . Both methods produced two intrinsic model accuracy estimates (ipTM and pTM) and we used a combination of these two estimates (0.8 ipTM + 0.2 pTM) as the confidence metric 39 , 40 .
Statistics and reproducibility
Statistical calculations (mean, s.e.m. and t -test) were performed using GraphPad Prism v.8.3.0 software. The statistical significance of differences between two experimental groups was assessed by using a two-tailed Student’s t -test. Differences between two datasets were considered significant at P < 0.05.
The protoplast transient expression analyses of protein localization in Figs. 1b and 4a and Extended Data Fig. 1 were repeated three times independently with similar results. The BiFC assays in Fig. 5a,d and Extended Data Fig. 7 were repeated a minimum of three times independently with similar results. The stable transformations for analysing protein localization in Fig. 1c and Extended Data Fig. 2 were conducted once, although multiple independent lines were analysed in each case. The membrane protein topology analysis in Fig. 2a,b was repeated three times independently with similar results. The co-IP assays for analysing protein–protein interactions in Figs. 4d–f , 5b,c and 6 were repeated a minimum of three times independently with similar results. The semi-quantitative RT-PCR analyses of gene expression in Fig. 7e and Extended Data Figs. 3b and 8b were repeated twice independently with similar results.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated or analysed during this study are included in this published article or its Supplementary Information . Gene sequences for the following proteins from A. thaliana were used experimentally in this study: PUX1 ( At3g27310 ), PUX2 ( At2g01650 ), PUX3 ( At4g22150 ), PUX4 ( At4g04210 ), PUX5 ( At4g15410 ), PUX6 ( At3g21660 ), PUX7 ( At1g14570 ), PUX8 ( At4g11740 ), PUX9 ( At4g00752 ), PUX10 ( At4g10790 ), PUX11 ( At2g43210 ), PUX12 ( At3g23605 ), PUX13 ( At4g23040 ), SP1 ( At1g63900 ), SP2 ( At3g44160 ), CDC48A ( At3g09840 ), TOC159 ( At4g02510 ), TOC33 ( At1g02280 ), TOC120 ( At3g16620 ), TOC132 ( At2g16640 ), TOC34 ( At5g05000 ), TOC75 ( At3g46740 ), TIC110 ( At1g06950 ), TIC40 ( At5g16620 ), CDKA1 ( At3g48750 ), SFR2 ( At3g06510 ) and ubiquitin ( At4g05320 ). Amino acid sequences of the UBX domains of the following proteins from different species were used in this study: Oryza sativa Os10g37630 ( AAP54662 ), Zea mays GRMZM2G159538 ( AQL10361 ), Marchantia polymorpha Mapoly0001s0291 ( PTQ50274 ), Chlamydomonas reinhardtii Cre03.g200100 ( A0A2K3DZI1 ), Saccharomyces cerevisiae Ubx2 ( Q04228 ) and Homo sapiens UBXD8/FAF2 ( Q96CS3 ). Sequences were obtained from the TAIR ( https:// www.arabidopsis.org/ ), Phytozome ( https://phytozome.jgi.doe.gov/pz/portal.html ), Ensembl Plants ( https://plants.ensembl.org/index.html ), Uniprot ( https://www.uniprot.org/ ) or National Center for Biotechnology Information ( https://www.ncbi.nlm.nih.gov/ ) databases. Source data are provided with this paper.
Jarvis, P. & Lopez-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 14 , 787–802 (2013).
CAS PubMed Google Scholar
Kessler, F. & Schnell, D. Chloroplast biogenesis: diversity and regulation of the protein import apparatus. Curr. Opin. Cell Biol. 21 , 494–500 (2009).
Li, H. M. & Chiu, C. C. Protein transport into chloroplasts. Annu. Rev. Plant Biol. 61 , 157–180 (2010).
Shi, L. X. & Theg, S. M. The chloroplast protein import system: from algae to trees. Biochim. Biophys. Acta 1833 , 314–331 (2013).
Paila, Y. D., Richardson, L. G. L. & Schnell, D. J. New insights into the mechanism of chloroplast protein import and its integration with protein quality control, organelle biogenesis and development. J. Mol. Biol. 427 , 1038–1060 (2015).
Sjuts, I., Soll, J. & Bolter, B. Import of soluble proteins into chloroplasts and potential regulatory mechanisms. Front. Plant Sci. 8 , 168 (2017).
PubMed PubMed Central Google Scholar
Nakai, M. New perspectives on chloroplast protein import. Plant Cell Physiol. 59 , 1111–1119 (2018).
Sun, Y. & Jarvis, R. P. Chloroplast proteostasis: import, sorting, ubiquitination, and proteolysis. Annu. Rev. Plant Biol. 74 , 259–283 (2023).
Ling, Q. H., Huang, W. H., Baldwin, A. & Jarvis, P. Chloroplast biogenesis is regulated by direct action of the ubiquitin-proteasome system. Science 338 , 655–659 (2012).
Ling, Q. H. et al. Ubiquitin-dependent chloroplast-associated protein degradation in plants. Science 363 , 836–848 (2019).
Google Scholar
Ling, Q. H. & Jarvis, P. Regulation of chloroplast protein import by the ubiquitin E3 ligase SP1 is important for stress tolerance in plants. Curr. Biol. 25 , 2527–2534 (2015).
CAS PubMed PubMed Central Google Scholar
Ling, Q. H. et al. The chloroplast-associated protein degradation pathway controls chromoplast development and fruit ripening in tomato. Nat. Plants 7 , 655–666 (2021).
Mohd Ali, S. et al. Multiple ubiquitin E3 ligase genes antagonistically regulate chloroplast-associated protein degradation. Curr. Biol. 33 , 1138–1146 (2023).
Jarvis, P. et al. An Arabidopsis mutant defective in the plastid general protein import apparatus. Science 282 , 100–103 (1998).
Begue, H., Jeandroz, S., Blanchard, C., Wendehenne, D. & Rosnoblet, C. Structure and functions of the chaperone-like p97/CDC48 in plants. Biochim. Biophys. Acta 1861 , 3053–3060 (2017).
CAS Google Scholar
Baek, G. H. et al. Cdc48: a Swiss army knife of cell biology. J. Amino Acids 2013 , 183421 (2013).
Ye, Y. H., Meyer, H. H. & Rapoport, T. A. Function of the p97-Ufd1-Npl4 complex in retrotranslocation from the ER to the cytosol: dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J. Cell Biol. 162 , 71–84 (2003).
Allen, M. D., Buchberger, A. & Bycroft, M. The PUB domain functions as a p97 binding module in human peptide N-glycanase. J. Biol. Chem. 281 , 25502–25508 (2006).
Hanzelmann, P. & Schindelin, H. The interplay of cofactor interactions and post-translational modifications in the regulation of the AAA+ ATPase p97. Front. Mol. Biosci. 4 , 21 (2017).
Li, J. L. et al. The CDC48 complex mediates ubiquitin-dependent degradation of intra-chloroplast proteins in plants. Cell Rep. 39 , 110664 (2022).
Schuberth, C. & Buchberger, A. UBX domain proteins: major regulators of the AAA ATPase Cdc48/p97. Cell. Mol. Life Sci. 65 , 2360–2371 (2008).
McNeill, H., Knebel, A., Arthur, J. S. C., Cuenda, A. & Cohen, P. A novel UBA and UBX domain protein that binds polyubiquitin and VCP and is a substrate for SAPKs. Biochem. J. 384 , 391–400 (2004).
Schuberth, C., Richly, H., Rumpf, S. & Buchberger, A. Shp1 and Ubx2 are adaptors of Cdc48 involved in ubiquitin-dependent protein degradation. EMBO Rep. 5 , 818–824 (2004).
Song, E. J., Yim, S. H., Kim, E., Kim, N. S. & Lee, K. J. Human Fas-associated factor 1, interacting with ubiquitinated proteins and valosin-containing protein, is involved in the ubiquitin-proteasome pathway. Mol. Cell. Biol. 25 , 2511–2524 (2005).
Zhang, J. R., Vancea, A. I., Hameed, U. F. S. & Arold, S. T. Versatile control of the CDC48 segregase by the plant UBX-containing (PUX) proteins. Comput. Struct. Biotechnol. J. 19 , 3125–3132 (2021).
Gallois, J. L. et al. Functional characterization of the plant ubiquitin regulatory X (UBX) domain-containing protein AtPUX7 in Arabidopsis thaliana . Gene 526 , 299–308 (2013).
Huang, A. B. et al. Proximity labeling proteomics reveals critical regulators for inner nuclear membrane protein degradation in plants. Nat. Commun. 11 , 3284 (2020).
Marshall, R. S. Hua, Z., Mali, S., McLoughlin, F. & Vierstra, R. D. ATG8-binding UIM proteins define a new class of autophagy adaptors and receptors. Cell 177 , 766–781.e24 (2019).
Park, S., Rancour, D. M. & Bednarek, S. Y. Protein domain-domain interactions and requirements for the negative regulation of Arabidopsis CDC48/p97 by the plant ubiquitin regulatory X (UBX) domain-containing protein, PUX1. J. Biol. Chem. 282 , 5217–5224 (2007).
Rancour, D. M., Park, S., Knight, S. D. & Bednarek, S. Y. Plant UBX domain-containing protein 1, PUX1, regulates the oligomeric structure and activity of Arabidopsis CDC48. J. Biol. Chem. 279 , 54264–54274 (2004).
Deruyffelaere, C. et al. PUX10 is a CDC48A adaptor protein that regulates the extraction of ubiquitinated oleosins from seed lipid droplets in Arabidopsis . Plant Cell 30 , 2116–2136 (2018).
Kretzschmar, F. K. et al. PUX10 is a lipid droplet-localized scaffold protein that interacts with CELL DIVISION CYCLE48 and is involved in the degradation of lipid droplet proteins. Plant Cell 30 , 2137–2160 (2018).
Neuber, O., Jarosch, E., Volkwein, C., Walter, J. & Sommer, T. Ubx2 links the Cdc48 complex to ER-associated protein degradation. Nat. Cell Biol. 7 , 993–998 (2005).
Cline, K., Wernerwashburne, M., Andrews, J. & Keegstra, K. Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol. 75 , 675–678 (1984).
Chou, M. L. et al. Tic40, a membrane-anchored co-chaperone homolog in the chloroplast protein translocon. EMBO J. 22 , 2970–2980 (2003).
Sveshnikova, N., Grimm, R., Soll, J. & Schleiff, E. Topology studies of the chloroplast protein import channel Toc75. Biol. Chem. 381 , 687–693 (2000).
Citovsky, V. et al. Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. J. Mol. Biol. 362 , 1120–1131 (2006).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596 , 583–589 (2021).
Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. Preprint at bioRxiv https://doi.org/10.1101/2021.10.04.463034 (2022).
Homma, F., Huang, J. & van der Hoorn, R. A. L. AlphaFold-Multimer predicts cross-kingdom interactions at the plant-pathogen interface. Nat. Commun. 14 , 6040 (2023).
Buchberger, A., Howard, M. J., Proctor, M. & Bycroft, M. The UBX domain: a widespread ubiquitin-like module. J. Mol. Biol. 307 , 17–24 (2001).
Dreveny, I. et al. Structural basis of the interaction between the AAA ATPase p97/VCP and its adaptor protein p47. EMBO J. 23 , 1030–1039 (2004).
Kovacheva, S. et al. In vivo studies on the roles of Tic110, Tic40 and Hsp93 during chloroplast protein import. Plant J. 41 , 412–428 (2005).
Schuberth, C. & Buchberger, A. Membrane-bound Ubx2 recruits Cdc48 to ubiquitin ligases and their substrates to ensure efficient ER-associated protein degradation. Nat. Cell Biol. 7 , 999–1006 (2005).
Suzuki, M. et al. Derlin-1 and UBXD8 are engaged in dislocation and degradation of lipidated ApoB-100 at lipid droplets. Mol. Biol. Cell 23 , 800–810 (2012).
Wang, C. W. & Lee, S. C. The ubiquitin-like (UBX)-domain-containing protein Ubx2/Ubxd8 regulates lipid droplet homeostasis. J. Cell Sci. 125 , 2930–2939 (2012).
Martensson, C. U. et al. Mitochondrial protein translocation-associated degradation. Nature 569 , 679–683 (2019).
Zheng, J., Cao, Y., Yang, J. & Jiang, H. UBXD8 mediates mitochondria-associated degradation to restrain apoptosis and mitophagy. EMBO Rep. 23 , e54859 (2022).
Liu, Y. D. & Li, J. M. Endoplasmic reticulum-mediated protein quality control in Arabidopsis . Front. Plant Sci. 5 , 162 (2014).
Alonso, J. M. et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana . Science 301 , 653–657 (2003).
PubMed Google Scholar
Kasmati, A. R., Topel, M., Patel, R., Murtaza, G. & Jarvis, P. Molecular and genetic analyses of Tic20 homologues in Arabidopsis thaliana chloroplasts. Plant J. 66 , 877–889 (2011).
Logan, D. C. & Leaver, C. J. Mitochondria-targeted GFP highlights the heterogeneity of mitochondrial shape, size and movement within living plant cells. J. Exp. Bot. 51 , 865–871 (2000).
Teh, O. K. & Moore, I. An ARF-GEF acting at the Golgi and in selective endocytosis in polarized plant cells. Nature 448 , 493–496 (2007).
Zheng, H. Q., Kunst, L., Hawes, C. & Moore, I. A GFP-based assay reveals a role for RHD3 in transport between the endoplasmic reticulum and Golgi apparatus. Plant J. 37 , 398–414 (2004).
Aronsson, H. & Jarvis, P. A simple method for isolating import-competent Arabidopsis chloroplasts. FEBS Lett. 529 , 215–220 (2002).
Ling, Q. H., Huang, W. H. & Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana . Photosynth. Res. 107 , 209–214 (2011).
Porra, R. J., Thompson, W. A. & Kriedemann, P. E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975 , 384–394 (1989).
Constan, D., Patel, R., Keegstra, K. & Jarvis, P. An outer envelope membrane component of the plastid protein import apparatus plays an essential role in Arabidopsis . Plant J. 38 , 93–106 (2004).
Huang, W. H., Ling, Q. H., Bedard, J., Lilley, K. & Jarvis, P. In vivo analyses of the roles of essential Omp85-related proteins in the chloroplast outer envelope membrane. Plant Physiol. 157 , 147–159 (2011).
Schelbert, S. et al. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis . Plant Cell 21 , 767–785 (2009).
Karimi, M., De Meyer, B. & Hilson, P. Modular cloning in plant cells. Trends Plant Sci. 10 , 103–105 (2005).
Tzfira, T. et al. pSAT vectors: a modular series of plasmids for autofluorescent protein tagging and expression of multiple genes in plants. Plant Mol. Biol. 57 , 503–516 (2005).
Watson, S. J. et al. Crosstalk between the chloroplast protein import and SUMO systems revealed through genetic and molecular investigation in Arabidopsis . eLife 10 , e60960 (2021).
Wu, F. H. et al. Tape- Arabidopsis sandwich—a simpler Arabidopsis protoplast isolation method. Plant Methods 5 , 16 (2009).
Zhang, X. R., Henriques, R., Lin, S. S., Niu, Q. W. & Chua, N. H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1 , 641–646 (2006).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9 , 671–675 (2012).
Kirchhelle, C. et al. The specification of geometric edges by a plant Rab GTPase is an essential cell-patterning principle during organogenesis in Arabidopsis . Dev. Cell 36 , 386–400 (2016).
Littlejohn, G. R., Gouveia, J. D., Edner, C., Smirnoff, N. & Love, J. Perfluorodecalin enhances in vivo confocal microscopy resolution of Arabidopsis thaliana mesophyll. New Phytol. 186 , 1018–1025 (2010).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9 , 676–682 (2012).
Ling, Q. H. & Jarvis, P. Analysis of protein import into chloroplasts isolated from stressed plants. J. Vis. Exp. https://doi.org/10.3791/54717 (2016).
Chu, C. C. & Li, H. M. Determining the location of an Arabidopsis chloroplast protein using in vitro import followed by fractionation and alkaline extraction. Methods Mol. Biol. 774 , 339–350 (2011).
Froehlich, J. Studying Arabidopsis envelope protein localization and topology using thermolysin and trypsin proteases. Methods Mol. Biol. 774 , 351–367 (2011).
Kovacheva, S., Bedard, J., Wardle, A., Patel, R. & Jarvis, P. Further in vivo studies on the role of the molecular chaperone, Hsp93, in plastid protein import. Plant J. 50 , 364–379 (2007).
Bauer, J. et al. The major protein import receptor of plastids is essential for chloroplast biogenesis. Nature 403 , 203–207 (2000).
Aronsson, H., Combe, J. & Jarvis, P. Unusual nucleotide-binding properties of the chloroplast protein import receptor, atToc33. FEBS Lett. 544 , 79–85 (2003).
Aronsson, H. et al. Nucleotide binding and dimerization at the chloroplast pre-protein import receptor, atToc33, are not essential in vivo but do increase import efficiency. Plant J. 63 , 297–311 (2010).
Inaba, T. et al. Arabidopsis Tic110 is essential for the assembly and function of the protein import machinery of plastids. Plant Cell 17 , 1482–1496 (2005).
Goodstein, D. M. et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40 , D1178–D1186 (2012).
Bateman, A. et al. UniProt: the Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 51 , D523–D531 (2023).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30 , 70–82 (2021).
Download references
Acknowledgements
We thank Q. Ling for helping to initiate the study and for many discussions during the course of the work. We thank E. Johnson and C. Melia for TEM conducted in the Sir William Dunn School of Pathology EM Facility, Z. Lewis for initiating the subcellular localization screening of PUX proteins, N. Buayam for technical support with LD staining and confocal imaging, N. G. Irani and I. Moore for the organelle marker lines, F. Homma for assistance with the structural analysis, and P. Bota and J. Bateman for technical assistance. This work was supported by grants from UK Research and Innovation–Biotechnology and Biological Sciences Research Council (UKRI-BBSRC; grant numbers BB/K018442/1, BB/N006372/1, BB/R016984/1, BB/R009333/1, BB/V007300/1, BB/W015021/1 and BB/X000192/1) to R.P.J. and by a PhD studentship from the Oxford Interdisciplinary Bioscience Doctoral Training Partnership (UKRI-BBSRC grant number BB/M011224/1) to N.L. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript (AAM) version arising from this submission.
Author information
Authors and affiliations.
Section of Molecular Plant Biology, Department of Biology, University of Oxford, Oxford, UK
Na Li & R. Paul Jarvis
You can also search for this author in PubMed Google Scholar
Contributions
N.L. designed and conducted the experiments, analysed the data and wrote the article. R.P.J. conceived of the study, supervised the work, analysed the data and wrote the article.
Corresponding author
Correspondence to R. Paul Jarvis .
Ethics declarations
Competing interests.
The application of CHLORAD as a technology for crop improvement is covered by a patent application (number WO2019/171091 A). The authors declare no other competing interests.
Peer review
Peer review information.
Nature Plants thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 subcellular localization analysis of arabidopsis pux proteins by confocal microscopy..
Protoplasts transiently expressing YFP-tagged PUX proteins under the constitutive 35S promoter were analysed by confocal microscopy. Representative protoplasts are presented. Exposure times and gain settings were identical. Scale bar = 20 µm. Note that PUX14 , PUX15 and PUX16 were designated as pseudogenes in a previous report, based on the presence of frameshift and nonsense mutations, and so were excluded from this analysis 26 .
Extended Data Fig. 2 Localization of PUX10 in chloroplasts in transgenic plants.
Constructs encoding PUX10-YFP driven by the native PUX10 promoter ( pPUX10 ) or by the constitutive 35S promoter ( p35S ) were used for stable plant transformation. Rosette leaves taken from 28-day-old T 1 transgenic plants were visualized by confocal microscopy. Representative images are presented. Similar localization of the YFP signals was observed in 5 to 10 independent T 1 transgenic plants. Exposure times and gain settings were identical. Scale bars = 20 µm.
Extended Data Fig. 3 Molecular and phenotypic characterization of two pux10 T-DNA insertion mutants.
a , Schematic representation of the Arabidopsis PUX10 genomic locus (At4g10790), annotated with the positions of the pux10 T-DNA insertion mutations. The positions of PCR primers used in b are also indicated, with arrows. Black boxes show exons, interconnecting white boxes show introns, and grey boxes show untranslated regions. Abbreviations: LB, left border sequences of the SAIL and Wisconsin T-DNA insertions; ATG, translation initiation codon; Stop, translation termination codon; bp, base pairs. b , Analysis of PUX10 mRNA expression in the pux10 mutants and a corresponding wild-type control. Total RNA isolated from 3-week-old seedlings was analysed by RT-PCR using the indicated primers (primer positions are marked in a, and their sequences are listed in Supplementary Table 1 ). The eIF4E1 gene was similarly analysed as a control for sample normalization. Wild-type genomic DNA (gDNA) was analysed as a positive control, and to rule out possible DNA contamination of the samples. Amplifications employed a limited number of cycles, and products were analysed by agarose gel electrophoresis. c , Phenotypes of 6-week-old wild-type, pux10-1 and pux10-4 plants grown on soil. Representative individuals are shown. Identical camera settings were employed, and all images are at the same magnification. d , e , Lipid droplet size in wild-type, pux10-1 and pux10-4 seedlings. Based on previous results 31 , we compared the size of lipid droplets (LDs) at the young seedling stage (34 h of germination) in the different plant genotypes. d , LDs were stained with Nile Red and visualized in the hypocotyl epidermis by confocal microscopy. Representative Nile Red fluorescence and brightfield images are shown. Exposure times and gain settings were identical. Scale bar = 10 µm. e , Quantification of LD size in wild-type, pux10-1 and pux10-4 plants. Images presented in d , and other similar images, were analysed. Values shown are means ± s.e.m. from 20 LDs per genotype. Asterisks indicate significance according to an unpaired two-tailed Student’s t -test (**** P < 0.0001; ns, not significant). The two pux10 mutants showed significantly and similarly reduced LD size compared to wild type, supporting the conclusion that the two pux10 alleles are equivalent.
Extended Data Fig. 4 Visible appearance of wild-type, pux10 mutant, and transgenic plants.
Plants were grown on soil under standard conditions for 3 weeks before photography, and representative individuals are shown. Identical camera settings were employed, and all images are at the same magnification.
Extended Data Fig. 5 AlphaFold analysis of the interaction between PUX10 and Cdc48.
a , Analysis of the interaction between PUX10 and Cdc48 using full-length or deleted forms of the proteins, or isolated domains, using AlphaFold. Box plots show the ipTM+pTM scores for each of five models for each of the protein pairs, and provide an indication of the likelihood of the relevant interaction. In each case, the box spans the interquartile range, the whiskers indicate the minimum and maximum scores, and the line inside the box represents the median. b , Structural model from the AlphaFold prediction of the interaction between the two full-length proteins. Yellow highlights the N terminus of Cdc48 while blue highlights the UBX domain of PUX10; the three residues of the highly-conserved R…FPR surface patch in the UBX domain are shown as green spheres. Outside of the highlighted domains, both Cdc48 and PUX10 are displayed in grey. c , Higher magnification image of the Cdc48-PUX10 interaction interface shown in b . Three residues of the highly-conserved R…FPR surface patch in the UBX domain are labelled in red. d , Structural model from the AlphaFold prediction of the interaction between the UBX domain of PUX10 and the N terminus of Cdc48 (that is, isolated domains). Orange highlights the N terminus of Cdc48 while magenta highlights the UBX domain of PUX10; the three residues of the highly conserved R…FPR surface patch in the UBX domain are shown as blue spheres and are labelled in red.
Extended Data Fig. 6 UBX domain alignment of selected PUX10 homologues.
Amino-acid sequence alignment of the UBX domains of PUX10 and PUX10/Ubx2 homologues from different species (as indicated). Black highlights identical amino acids, while grey highlights amino acids that are similar. Residues of the highly conserved R…FPR surface patch of the UBX domain are marked with red arrowheads.
Extended Data Fig. 7 BiFC analysis of the interactions between PUX10 and minor TOC protein isoforms.
Protoplasts transiently co-expressing PUX10 and either Toc132 or Toc34 fused to nYFP and cYFP fragments of YFP protein, respectively, were analysed by confocal microscopy. Representative protoplasts are presented. Exposure times and gain settings were identical. Scale bar = 10 µm.
Extended Data Fig. 8 Identification and preliminary analysis of pux10-1 SP1-OX ppi1 plants.
a , Phenotype screening for 4-week-old pux10-1 SP1-OX ppi1 plants grown on soil. The pux10-1 mutant was crossed to an SP1-OX ppi1 transgenic line, and pale F 2 individuals showing a greener phenotype than SP1-OX ppi1 controls were identified and carried forward for genotyping and further analysis. Three representative pux10-1 SP1-OX ppi1 F 3 lines and controls plants are presented. Identical camera settings were employed, and all images are at the same magnification. b , Analysis by RT-PCR of the overexpression of SP1 in the selected F 3 lines. Total RNA samples isolated from 2-week-old plants of the F 3 lines shown in a , and from appropriate controls, were analysed by RT-PCR for SP1 and the reference gene eIF4E1 . The SP1-OX ppi1 line acted as a positive control, while ppi1 and sp1 ppi1 acted as negative controls (the native SP1 mRNA was below the level of detection in ppi1 ). Amplifications employed a limited number of cycles to avoid saturation, and products were analysed by agarose gel electrophoresis.
Extended Data Fig. 9 Evaluation of the effect of PUX10 on SP1-dependent dark-induced leaf senescence.
a , Visual assessment of leaf senescence. Attached leaves of 4-week-old plants of the indicated genotypes were left uncovered (top; control) or were covered with aluminium foil (bottom; covered), and the plants were kept under standard growth conditions for 5 days before leaf detachment and photography. A representative leaf from each genotype for each condition is shown. Identical camera settings were employed, and all images are at the same magnification. b , Quantitative assessment of photosynthesis. The maximum photochemical efficiency of photosystem II ( F v / F m ) was measured for leaves similar to those presented in a using a CF Imager, to estimate the extent of senescence. The data were derived from eight leaves per genotype (for control, uncovered), and ten leaves per genotype (for senescence, covered). Values shown are means ± s.e.m. The asterisks indicate significance according to an unpaired two-tailed Student’s t -test (**** P < 0.0001; ns, not significant).
Extended Data Fig. 10 A model for the role of PUX10 in the CHLORAD pathway.
In CHLORAD, TOC proteins are marked with polyubiquitin through the action of E1, E2 and SP1 E3 ligase enzymes. The PUX10 protein is integrated in the chloroplast OEM via its transmembrane domains (TMs), and it acts to recruit Cdc48 from the cytosol to the chloroplast surface. Moreover, PUX10 acts as a link or bridge between the ubiquitinated TOC proteins and Cdc48, through its cytosol-oriented UBA and UBX domains respectively. Thus, PUX10 facilitates the retrotranslocation of ubiquitinated TOC proteins from the chloroplast OEM. Upon dislocation into the cytosol, ubiquitin is cleaved from TOC proteins by yet unknown deubiquitinase (DUB) enzymes and the TOC proteins are degraded by the 26S proteasome. Arabidopsis TOC proteins are shown and labelled by convention according to their molecular masses in kilodaltons. Putative unknown proteins (?) that share functional redundancy with PUX10 are shown. Abbreviations: CHLORAD, chloroplast-associated protein degradation; Cdc48, cell division cycle protein 48; DUBs, deubiquitinases; IMS, intermembrane space; OEM, outer envelope membrane; POTRA, polypeptide transport-associated domain; RING, really interesting new gene; SP1, suppressor of ppi1 locus 1; SP2, suppressor of ppi1 locus 2; TM, transmembrane domain; Ub, ubiquitin; UBA, ubiquitin-associated; UBX, ubiquitin regulatory X; 26SP, 26S proteasome.
Supplementary information
Supplementary information.
Supplementary Figs. 1 and 2 and Table 1.
Reporting Summary
Source data fig. 2.
Unprocessed western blots.
Source Data Fig. 3
Source data fig. 4, source data fig. 5, source data fig. 6, source data fig. 7.
Unprocessed western blots and gels.
Source Data Fig. 8
Source data extended data fig. 3.
Unprocessed gels.
Source Data Extended Data Fig. 8
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Li, N., Jarvis, R.P. Recruitment of Cdc48 to chloroplasts by a UBX-domain protein in chloroplast-associated protein degradation. Nat. Plants (2024). https://doi.org/10.1038/s41477-024-01769-x
Download citation
Received : 09 August 2023
Accepted : 20 July 2024
Published : 19 August 2024
DOI : https://doi.org/10.1038/s41477-024-01769-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
COMMENTS
In its simplest form it [the Sequence Hypothesis] assumes that the specificity of a piece of nucleic acid is expressed solely by the sequence of its bases, and that this sequence is a (simple) code for the amino acid sequence of a particular protein. This hypothesis appears to be rather widely held. ... Central dogma of molecular biology This ...
The sequence hypothesis lies at the very nexus of the genomic enterprise to improve human health, the crowning achievement of which was the sequencing of the human genome, completed in 2003. ... [10] Crick F. Central dogma of molecular biology. Nature. 1970; 227 (5258):561-563. [Google Scholar] [11] Anfinsen CB. Principles that govern the ...
The simplicity of the sequence hypothesis and the central dogma, together with the focus on information, brought a clear explanatory power to the synthesis of protein molecules that could take virtually any form and could 'do almost anything,' as Crick put it. ... (as popularised by Watson in his 1965 textbook Molecular Biology of the Gene ...
One fundamental principle of molecular biology is the Sequence Hypothesis formulated in 1958 by Sir Francis Crick [1]. In essence, it states that the genetic information as present in DNA is passed on 1:1 to RNA to protein. While still valid, a variety of exceptions to the Sequence Hypothesis has been discovered.
On 30 December 1961, a paper was published in Nature that became an instant classic of molecular biology [].It combined theory and experimentation in a striking display of the incisive thinking of the first author, Francis Crick, and of his intensely productive interactions with his friend, colleague and co-author, Sydney Brenner [].Simply using the power of genetics, Crick, Brenner and their ...
Francis Crick advanced two distinct but interrelated fundamental principles of molecular biology: (1) the Sequence Hypothesis and (2) the Central Dogma. The Sequence Hypothesis defines biological information transfer as the residue-by-residue transfer of sequence information between nucleic acids and to proteins.
Hardly a ringing endorsement of his own hypothesis for such a crucial feature of modern molecular genetics: peptide folding to protein is the process that transforms DNA sequence information into ...
Later, Crick expanded this insight in the sequence hypothesis, which stated that 'the specificity of a piece of nucleic acid is expressed solely by the sequence of its bases, and that this sequence is a (simple) code for the amino acid sequence of a particular protein' ... Despite joining the Laboratory of Molecular Biology in Cambridge, he ...
A world in one dimension: Linus Pauling, Francis Crick and the central dogma of molecular biology. ... was encoded in the one-dimensional sequence of nucleic acids. ... the 'Sequence hypothesis ...
The Sequence Hypothesis defines biological information transfer as the residue-by-residue transfer of sequence information between nucleic acids an … Francis Crick advanced two distinct but interrelated fundamental principles of molecular biology: (1) the Sequence Hypothesis and (2) the Central Dogma.
MRC Laboratory of Molecular Biology. Hills Road, Cambridge CB2 2QH. The central dogma of molecular biology deals with the detailed residue-by-residue t ransfer of sequential information. It states that such information cannot be transferred from protein to either. protein or nucleic acid. "The central dogma, enunciated by Crick in 1958 and the ...
Conversation on Central Dogma Page 2 of 11. molecule can be copied to create another molecule with the same information (sequence). In other words, one molecule ('mother') acts as a template or guide for the synthesis of another molecule ('daughter') with the same information. This property of reproduction - creation of units of ...
The 'central dogma' of molecular biology states that sequence information can be transferred among nucleic acids, and from nucleic acids to proteins, but sequence information cannot be transferred among proteins, or from proteins to nucleic acids. ... The first he simply called the sequence hypothesis, but the second seemed to require a ...
the central problem of molecular biology. He encapsulates the problem in a form that will be called (in a few pages) the Sequence Hypothesis, which states: The structure, and therefore the function, of a protein is determined solely by the order of its amino acids. In a bit that will be followed by:
The simplicity of the sequence hypothesis and the central dogma, together with the focus on information, brought a clear explanatory power to the synthesis of protein molecules that could take virtually any form and could 'do almost anything,' as Crick put it. ... (as popularised by Watson in his 1965 textbook Molecular Biology of the Gene ...
theory, is the sequence hypothesis and the digital rather than the analog or blending character (Jenkin, 1867) of inheritance as Darwin (1809-82) and his contemporaries believed (Fisher, 1930). Watson and Crick's solution of the structure of DNA and its application in biology would not have been so important if it had not been for their
A transcription unit is a linear sequence of DNA that extends from a transcription start site to a transcription stop site (Figure 4). Figure 4. The promoter, a DNA sequence that lies upstream of ...
The molecular clock hypothesis states that DNA and protein sequences evolve at a rate that is relatively constant over time and among different organisms. A direct consequence of this constancy is ...
Crick's central dogma of molecular biology. When Francis Crick first introduced the Central Dogma in 1958, ... As Graur (2019) points out, the Central Dogma—a negative thesis—is often fallaciously conflated with the Sequence Hypothesis—a positive thesis. Crick himself (1958 & 1970) maintained a distinction between these two principles. ...
[14] [15] However, Rosalind Ridley in Molecular Pathology of the Prions (2001) has written that "The prion hypothesis is not heretical to the central dogma of molecular biology—that the information necessary to manufacture proteins is encoded in the nucleotide sequence of nucleic acid—because it does not claim that proteins replicate ...
The Sequence Hypothesis, Crick wrote, `assumes that the specificity of a piece of nucleic acid is expressed solely by the sequence of its bases, and that this sequence is a (simple) code for the amino acid sequence of a particular protein' ... Molecular Biology of the Gene. On the synthesis of DNA, RNA, and proteins, Watson wrote: ...
Opsins are divided broadly into eight groups based on amino-acid sequence similarity, molecular function, and signaling properties. ... and functions across visual and nonvisual opsins. Phylogenetic hypothesis based on Beaudry et al. (2017) and Davies et al. ... Supplementary material is available at Molecular Biology and Evolution online.
The most critical issues in computational biology are characterizing and predicting uncharacterized proteins' secondary and tertiary structures from their uploaded amino acid sequences in databases. Aedes albopictus (A. albopictus), sometimes referred to as the Asian tiger mosquito or forest mosquito and the carrier of dengue-like diseases, has many proteins, many of which are still poorly ...
Crick offered the Sequence Hypothesis: the sequence of nucleotide bases in DNA or RNA directly determines A revised central dogma - the cognitive flow of information Self-organization is considered fundamental to the reiterating pattern formations and regulated energy dissipation that characterize living systems, separating living ...
This glossary of cellular and molecular biology is a list of definitions of terms and concepts commonly used in the study of cell biology, molecular biology, and related disciplines, including molecular genetics, biochemistry, and microbiology. [1] It is split across two articles: Glossary of cellular and molecular biology (0-L) lists terms beginning with numbers and those beginning with the ...
To address this hypothesis, ... Multiple sequences were aligned by the ClustalW multiple method, using the BioEdit Alignment Editor software package v.7.2.5. ... Section of Molecular Plant Biology ...