An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Membranes (Basel)
- PMC10384872


Gas Separation Membrane Module Modeling: A Comprehensive Review
Marcos da conceicao.
1 Department of Chemical Engineering, University of Toledo, Toledo, OH 43606, USA
Joanna Rivero
2 Department of Mechanical Engineering and Materials Science, University of Pittsburgh, Pittsburgh, PA 15213, USA
Katherine Hornbostel
Glenn lipscomb, associated data.
Not applicable.
Membrane gas separation processes have been developed for diverse gas separation applications that include nitrogen production from air and CO 2 capture from point sources. Membrane process design requires the development of stable and robust mathematical models that can accurately quantify the performance of the membrane modules used in the process. The literature related to modeling membrane gas separation modules and model use in membrane gas separation process simulators is reviewed in this paper. A membrane-module-modeling checklist is proposed to guide modeling efforts for the research and development of new gas separation membranes.
1. Introduction
This review summarizes the literature related to the development and use of membrane gas separation module models in process simulation. The manuscript is divided into the following sections:
- Introduction to membrane gas separations;
- Membrane module designs;
- Module flow patterns;
- Membrane modeling review;
- Modeling non-idealities in membrane gas separation modules;
- Gas separation process modeling applications and challenges.
A final section provides a modeling checklist for developing membrane module models that incorporate the physics and module design detail required to accurately predict performance. The overall goal is to provide the reader with the broad background needed to assess the use of membrane module models in gas separation process simulations.
2. Introduction to Membrane Gas Separations
Separation processes are a critical part of chemical and purified product production. Distillation is the main separation technology in industrial plants and is responsible for 10–15% of the world’s energy usage [ 1 ]. The energy-intensive nature of distillation has led both industry and academia to seek more efficient alternatives that can limit the rise of atmospheric carbon dioxide (CO 2 ) concentration while still meeting the desired separation targets. The use of non-thermal separation technologies such as membranes has attracted interest in several industrial sectors due to their unique advantages, such as the following [ 2 , 3 , 4 ]:
- Simple operation and installation;
- No chemical usage;
- Low energy consumption;
- Facile scale-up due to process compactness and modularity;
- Flexible integration with other process units to form hybrid processes with reduced energy consumption.
Membrane-based gas separation has become a relevant process unit that can compete with conventional processes, such as distillation, absorption, and adsorption [ 4 ]. Binary and multicomponent gas separations are achievable with membranes, making them an ideal candidate for myriad applications including but not limited to nitrogen production from air, natural gas sweetening, hydrogen recovery, biogas upgrading, gas dehydration/drying, volatile organic compound (VOC) recovery, and CO 2 capture from flue gas streams [ 5 , 6 ].
Membrane materials can be made from polymeric materials, carbon, inorganic metals and ceramics, or a combination (mixed matrix or hybrid materials) [ 5 , 7 ]. Polymeric membranes are used predominantly in industrial gas separations because they are inexpensive, easy to manufacture, and robust [ 5 ]. Polymeric membrane materials are subdivided into rubbery and glassy polymers. Glassy polymers typically possess lower permeabilities and higher selectivities than rubbery polymers due to reduced molecular motion that restricts fluctuations in free volume. This increases selectivity through enhanced molecular sieving, but it decreases gas diffusion coefficients [ 8 , 9 ].
For membranes that do not possess permanent porosity (i.e., a dense polymeric material), the most widely accepted model for transport is the solution–diffusion model. Transport is envisioned as occurring in three steps: (1) gas dissolution or sorption into the membrane on the high-pressure side of the material, (2) sorbed gas diffusion through the membrane, and (3) desorption from the membrane on the low-pressure side [ 10 ]. The driving force for transport is controlled by the chemical potential difference between the high and low pressure contacting as phases. The driving force is created by gas compression or vacuum. For ideal gas contacting phases, gas flux across the membrane is given by the following calculation:
where J i is the flux across the membrane of species i (mol/m 2 /s); Q i is the membrane permeability for species i (mol·m/m 2 /s/Pa); δ is the effective membrane thickness (m); P r and P p are the feed/retentate (high pressure) and permeate (low pressure) pressures (Pa), respectively; and x i and y i are the high- and low-pressure gas phase mole fractions (mol/mol), respectively. The permeability is equal to the product of gas solubility and diffusivity in the membrane. The ratio of permeability to membrane thickness in Equation (1) is defined as the gas permeance: q ≡ Q i δ (mol/m 2 /s/Pa). Commonly, permeance is expressed in gas permeation units (GPU) where 1 GPU = 3.35 × 10 −10 (mol/m 2 /s/Pa).
High-performance polymeric membranes typically consist of a thick, dense submicron layer on top of a porous support, as shown in Figure 1 . Ideally, the support provides mechanical support to permit the imposition of a pressure difference across the membrane without damaging it but does not pose significant resistance to permeation. Membrane permeation rates are controlled by the material comprising the dense layer, and the value of d in Equation (1) is equal to the effective thickness of this layer. Direct measurement of the layer thickness is often difficult. However, the value can be estimated from permeation rates measured for both the supported membrane and thicker samples of a dense unsupported membrane.

Representation of gas separation membrane, which typically consists of a thicker, porous support layer coated with a thin, dense selective layer.
The ability of a membrane to separate two gases is given by its selectivity, which is defined for a pair of gases, i and j , as the ratio of gas permeabilities or permeances:
where component i is the gas with the higher permeability, resulting in a selectivity greater than one. Selectivity and permeability determine the economics of a gas separation process [ 5 ]. Selectivity controls the energy (operating) cost, while permeability controls the capital (membrane area) cost. Increasing selectivity reduces the amount of gas that must permeate from the high feed pressure to the low permeate pressure to achieve product purity targets; this reduces the compression energy lost due to permeation. Increasing permeability or permeance, the gas permeation flux per unit driving force, reduces the membrane area and associated capital cost required to achieve a target feed or product flow rate.
A trade-off exists between permeability and selectivity for a given gas pair, as illustrated in Figure 2 . The permeability–selectivity combination of polymeric membrane materials reported in the literature lies below a line commonly referred to as the Robeson upper bound [ 11 ]. This observation suggests that a limit exists on the ability to increase selectivity and permeability simultaneously through changes in polymer architecture. Recent developments in membrane materials such as facilitated transport, mixed matrix, and molecular sieve membranes indicate that the upper bound can be surpassed through the addition of non-polymeric components and introduction of different transport mechanisms [ 3 , 7 ].

Generic upper bound observed for trade-off between selectivity (ratio of the permeability for the faster permeating species to that for the slower species) and permeability for a gas pair. This “Robeson plot” [ 11 ] implies that attempts to increase permeability, selectivity, or both (as suggested by the arrows) for a family of materials will always lie below the upper bound.
3. Membrane Module Designs
Large membrane areas are required for industrial gas separations to process desired gas flow rates. Membranes are packaged into modular units to form compact, high-surface-area-per-unit-volume contactors. These modules are typically combined in parallel or series inside a pressure vessel or case for use in a separation process. Three primary module designs have been developed: plate and frame, spiral wound, and shell and tube. Flat-sheet membranes are used in the plate-and-frame and spiral-wound designs, while hollow cylindrical membrane fibers are used in the shell-and-tube design [ 5 ].
3.1. Plate-and-Frame Module
Plate-and-frame modules consist of stacks of membrane sheets. Flow channels are created between adjacent membranes with a spacer. The spacers also maintain the flow channel when a pressure difference is imposed across the membrane to drive gas permeation and can mix the fluid in the flow channel to reduce concentration polarization. The spacers for the feed and permeate channels commonly are different since a finer mesh is needed to support the membrane and prevent rupture in the lower pressure gas permeate channel because the membrane is pressed into the spacer by the applied pressure difference.
Membranes are glued together along the edges such that, when the module is placed in a case, gas streams can be introduced into and removed from alternating channels through external connections, as illustrated in Figure 3 . This leads to crossflow contacting where the permeate flows in the normal direction to the feed. Restricting where the gas stream enters along the edges can introduce partial countercurrent contacting (not illustrated). Such modules are one of the oldest membrane systems and are often used in dead-end filtration. Capital costs are usually higher for plate-and-frame modules, but operational costs are typically lower due to lower pressure drops [ 5 , 12 ].

Graphic of a plate-and-frame membrane module with crossflow. Left-hand side illustrates the membrane–spacer stack and flows into and out of the stack. Right-hand side illustrates the repeating unit in the stack.
3.2. Spiral-Wound Module
Flat-sheet membranes also can be used to fabricate spiral-wound modules. Like plate-and-frame modules, membrane sheets are separated by feed and permeate spacers, and adjacent membranes are glued to allow gas introduction and removal from separate flow channels. In contrast to plate-and-frame modules, long membrane sheets are used. One long membrane is glued along the long edges of the sheet, and one short edge with a permeate spacer and central permeate collection tube on top to create a “leaf”, as illustrated in Figure 4 . The permeate collection tube is rolled to wrap the leaf around the tube and form the module. Multiple leafs can be placed in a single module to increase the total membrane area, while reducing leaf length to minimize the permeate pressure drop inside each leaf. The module is placed into a case with external connections that allow for the introduction and removal of the feed and reject along the leaf exterior and separate permeate collection from the leaf interior from a central tube.

Stepwise process for construction of a spiral-wound membrane module [ 13 ]: ( a ) a permeate spacer is placed on top of the membrane; ( b ) glue is applied along the sides of the membrane, as illustrated, and the permeate collection tube is placed along the middle of the membrane sheet; ( c ) the membrane is folded over the permeate collection tube to form a leaf or envelope; ( d , e ) a feed spacer is placed on top of the leaf, and the permeate collection tube is rolled in the direction indicated by the black arrow to wrap the spiral-wound module; ( f ) the spiral-wound module is placed inside a case to permit fluid introduction and removal. The feed and permeate flow in a crossflow configuration with all permeate being collected by the central tube.
The leaf glue lines and connection to the permeate collection tube create crossflow contacting in the module. The feed flows parallel to the permeate collection tube from one face of the module to the other, while the permeate flows perpendicular to the feed in a spiral fashion through the leaf to the permeate collection tube. Compared to plate-and-frame modules, spiral-wound modules can offer greater area per unit volume and more facile manufacture and module handling [ 5 , 12 ].
3.3. Shell-and-Tube Module
Membranes in the form of hollow fibers or tubes are commonly formed into modules like the one shown in Figure 5 . The ends of the bundle are enclosed in a tube-sheet material that seals the fiber together. The tube sheet is machined such that the fiber lumens (interior) are open, and the tube sheet can be sealed to the interior of an external cylindrical case. External ports on the end of the case allow fluid to be introduced to and removed from the fiber lumens, while ports on the circumference allow for separate fluid introduction to and removal from the shell (the space outside the fibers). Such a configuration is the mass transfer equivalent of a shell-and-tube heat exchanger and provides nominal countercurrent contacting with the potential to use a sweep stream that can dilute the permeate and thereby enhance permeation rates [ 5 , 12 ]. Larger hollow-fiber membranes, fibers with an outer diameter greater than ~0.5 cm, often are referred to as tubular or capillary membranes, but the module design is the same as for finer hollow fibers.

Graphic depiction of a hollow-fiber membrane module. In this configuration, the feed stream (blue) is introduced into the shell side and runs countercurrent to the sweep stream (green) introduced into the tube side (fiber lumens). The gas of interest for the separation (e.g., CO 2 or O 2 ) permeates from the feed side through the hollow-fiber membrane to the permeate side.
4. Module Flow Patterns
Figure 6 illustrates the three primary contacting configurations considered in module design and performance calculations [ 5 , 12 ]:
- Co-current: feed and permeate flow parallel to each other in the same direction.
- Countercurrent: feed and permeate flow parallel to each other in opposite directions.
- Crossflow: feed and permeate flow perpendicular to each other.

Membrane module flow configurations (with sweep): ( a ) co-current, ( b ) countercurrent, and ( c ) crossflow.
All three of these flow configurations can be achieved nominally in either a flat-sheet or hollow-fiber module. Additionally, all three can use a sweep stream on the permeate side of the membrane to increase the partial pressure driving force for permeation.
For ideal contacting conditions (i.e., in the absence of significant pressure drop and other module inefficiencies arising from non-uniform membrane properties and fluid distribution, as discussed later), countercurrent contacting maximizes partial pressure differences within the module and yields the best performance, as measured by module productivity (product flow rate per unit membrane area) and gas recovery (fraction of feed recovered as the desired product). However, the differences between the configurations may be small depending on the gas separation and the module stage cut (i.e., the fraction of the feed that permeates). As the stage cut decreases, concentration changes along the feed and permeate channels decrease, and performance differences between the configurations become smaller.
Membrane and module selection is a critical task when developing a new membrane gas separation process. The choice of flat-sheet or hollow-fiber membrane occurs first and dictates what module options exist. If either flat-sheet or hollow-fiber membrane modules offered inherently superior economic performance, manufacturers would opt to produce that type of module. However, since all three contacting configurations are nominally possible with both flat-sheet and hollow-fiber membranes, the choice is based primarily on membrane- and module-manufacturing expertise. The production of high-performance gas separation membranes requires creating an effective dense separation layer less than ~0.1 micron thick on a porous support that provides the requisite strength to withstand the pressure difference across the membrane but does not pose a resistance to gas permeation. The art and science of membrane formation are highly specific to the membrane type and closely guarded by manufacturers, so the development of new membranes typically relies on adapting an existing process.
While similar contacting configurations are available in flat-sheet and hollow-fiber modules, differences between the module types exist and are summarized in Table 1 [ 5 , 12 ]. Cost estimates of module manufacture vary widely and depend strongly on the degree of automation. The patent literature describes automated processes for the formation of large fiber bundles [ 14 ], but the automated manufacture of large flat-sheet modules has lagged.
Comparison of membrane module types (adapted from [ 5 , 12 ]).
| Module Type | |||
|---|---|---|---|
| Metric | Hollow Fiber | Spiral Wound | Plate and Frame |
| Ease of Countercurrent Operation | High | Low | Low |
| Ease of Permeate Sweep | High | Low | Low |
| Pressure Drop | High (lumen side) | Moderate | Low |
| Concentration Polarization | High (lumen side) | Moderate | Low |
| Degree of Manufacturing Automation | Moderate | Low | Low |
The ease of countercurrent contacting is greater in hollow-fiber modules than flat-sheet modules, due to differences in module geometry, while concentration polarization is more difficult to mitigate in hollow-fiber modules, especially in the fiber lumens which lack spacers to promote fluid mixing. For many current commercial gas separations, concentration polarization is not significant, and this would favor the shell-and-tube design. The pressure drop in both module types can be reduced by increasing the flow channel dimensions. Increasing the fiber size or spacer thickness will lead to lower pressure drops but will also reduce the membrane area per unit module volume. With shell-and-tube designs, pressure drops in the shell are lower than in the lumen which may dictate where the feed is introduced. Additionally, shell feed is preferred for a higher-pressure operation since hollow fibers are stronger when externally pressurized.
5. Membrane Modeling Review
A simulation of membrane-based gas separation systems requires the development of stable and robust models that can efficiently predict the separation performance for different flow configurations and module geometries. Mathematical modeling of gas membrane modules was first introduced by Weller and Steiner [ 15 ] in 1950, and their work has served as the basis for future model development. Shindo et al. [ 16 ] developed a calculation methodology for all five flow configurations for multicomponent gas separations. Pan [ 17 , 18 ] proposed solving module performance models with numerical integration of the governing differential equation mass balances, using split boundary conditions for the retentate and permeate side. However, this approach can be highly sensitive to the initial guess and computationally expensive when refining the solution to reduce numerical approximation errors. Khalipour et al. [ 19 ] extended Pan’s model into a system of backward finite differential equations that are solved using a Gauss–Seidel algorithm for an isothermal co-current and countercurrent module. Chowdhury et al. [ 20 ] reformulated Pan’s model as an initial value problem in which either an Adams–Moulton’s or Gear’s backward differentiation method is used for solving the nonlinear differential equations. Kovvali et al. [ 21 ] simplified Pan’s model as a set of nonlinear equations by assuming a linear relationship between the permeate and inlet stream compositions; this approach can reduce the computational effort while maintaining an acceptable accuracy. Sengupta and Sirkar [ 22 ] provide an excellent review of this early work and the numerical methods used to obtain numerical approximations to the governing equations.
Coker et al. [ 23 , 24 ] developed a stage-based approach to convert the differential mass balance equations into a set of nonlinear algebraic equations by assuming that the module consists of a series of well-mixed stages. The resulting equations are solved iteratively using a direct substitution algorithm that requires solution of a set of linear equations each iteration. The coefficients of the linear equations form a block tridiagonal matrix that allows for an efficient simultaneous solution using the Thomas Algorithm. The principal drawback of this approach is the sensitivity of the convergence process on the initial solution guess; the use of the solution for an equivalent crossflow module is recommended. This methodology is readily implemented in different computational environments and allows for a facile addition of non-idealities within the membrane module, such as pressure drop or temperature variation effects that arise due to the expansion-driven process.
The incorporation of the membrane numerical solving algorithm into a commercial process simulator such as Aspen Plus allows the user to understand how the model will behave under a range of operating conditions when connected to other process units. Commercial process simulators often do not have a membrane separation unit as part of their unit operations library; therefore, the implementation of a membrane module in a simulator such as Aspen Plus can help industry and academia further the research and development of membrane gas separation processes.
The inclusion of robust mathematical membrane models into commercial process simulators is well documented in the literature. However, some of these models are found to be simplistic due to the assumptions that are made with respect to the physical, geometrical and transport properties of the process. The baseline approach found in academic literature for membrane modeling consists of assuming the following conditions [ 15 , 16 ]:
- Steady state;
- Ideal gas behavior;
- Isothermal conditions;
- Constant permeance;
- Constant pressure in feed/retentate and permeate channels;
- No axial mixing;
- Uniform flow channel size;
- Negligible concentration polarization effects;
- Laminar flow.
Under these assumptions, the model for an “ideal” module consists of a set of differential mass balances that describe the concentration profiles of each component along the length of the module. Following Coker et al. [ 23 ], these equations can be solved by dividing the module length into a series of N well-mixed, as illustrated in Figure 7 .

Tank in series modeling schematic for countercurrent configuration. Numbers indicate the stage number with the feed introduced in the first stage.
The overall mass balances for an individual stage, as shown in Figure 8 , are given by Equations (3a)–(3c) for the countercurrent, crossflow, and co-current flow, respectively:
where R f and P f are the retentate and permeate flows, respectively; and the subscript indicates the stage the flow comes from. The component flow rates can be calculated similarly by using Equations (4a)–(4c) for the countercurrent, crossflow, and co-current flow, respectively:
where x r and y p are the mole fractions of component i in the retentate and permeate flows, respectively; and the subscripts j − 1, j , and j + 1 indicate the stage that the flow comes from.

Countercurrent membrane cell. The downward arrow indicates gas permeation from the retentate to the permeate.
Permeation across the membrane is calculated from Equation (1), using Equations (5a)–(5c) for countercurrent, crossflow, and co-current flow, respectively:
where P p ( j ) and P r ( j ) are the permeate and retentate pressures, respectively, in stage j . The retentate and permeate mole fractions must sum to unity:
where n c represents the number of components in the gas permeation process.
In the case of different module geometries, the active membrane area ( A ) for which permeation occurs is given by Equation (8) for hollow-fiber membranes based on the outer fiber diameter, and by Equation (9) for flat-sheet and spiral-wound membranes:
where L is the active permeating length of the membrane (excluding regions included in either tubesheets or glue lines), O D is the fiber outside diameter, N f is the number of fibers within the module, W is the width, N s h e e t s is the number of membrane sheets, and N is the number of discretization stages. N must be increased until the performance predictions become independent of N .
6. Modeling Non-Idealities in Membrane Gas Separation Modules
Various non-ideal effects are observed during the operation of gas separation membrane modules. These non-ideal effects can have adverse effects on gas separation performance, and, thus, these effects should be included in models to improve accuracy if possible. Table 2 summarizes models reported in the literature that have accounted for the following non-ideal effects:
- Real gas behavior;
- Pressure drop in both permeate and retentate channels;
- Non-isothermal behavior;
- Concentration polarization;
- Variable permeance;
- Non-uniform membrane properties.
Summary of available models in the literature that model the following non-ideal behaviors: (1) real gas behavior, (2) channel pressure drop, (3) non-isothermal behavior, (4) concentration polarization, (5) variable permeance, and (6) non-uniform membrane properties. Commercial software packages are also listed for models that have been integrated into process simulation software.
| References | Non-Ideal Conditions Modeled | Commercial Software Integration | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Pan [ ] | - | Lumen | - | - | - | - | - |
| Khalipour et al. [ ] | - | Lumen | - | - | - | - | - |
| Chowdhury et al. [ ] | - | Lumen and shell | - | - | - | - | Aspen Plus |
| Kovvali et al. 1994 [ ] | - | Lumen and shell | - | - | - | - | - |
| Coker et al. [ ] | - | Lumen and shell | + | - | + | - | - |
| Feng et al. [ ] | - | Lumen | - | + | - | - | - |
| Rautenbach et al. [ ] | - | Lumen | - | - | - | + | - |
| Scholz et al. [ ] | + | Lumen and shell | + | + | +++ | - | ACM |
| Miandoab et al. [ , ] | + | Lumen and shell | + | + | +++ | - | ACM |
| Che Mat et al. [ ] | + | Lumen and shell | + | - | + | - | Aspen Plus |
| Bounaceour et al. [ ] | - | - | - | - | +++ | - | Aspen Plus |
| Sonalkar et al. [ ] | - | Lumen | - | - | - | + | - |
| Ahmad et al. [ ] | - | Lumen | + | - | ++ | - | Aspen HYSIS |
| Chu et al. [ ] | - | Lumen and shell | - | - | - | - | - |
| Brinkmann et al. [ ] | + | Both channels | + | + | +++ | - | ACM |
| Mourgues et al. [ ] | - | - | - | + | - | - | - |
| Hensen [ ] | - | Lumen and shell | - | - | - | - | ACM and gPROMS |
| Marriott et al. [ ] | - | Lumen and shell | + | - | - | - | gPROMS |
| Tessendorf et al. [ ] | + | Lumen and shell | - | - | - | - | OPTISIM |
| DeJaco et al. [ ] | + | Both channels | - | - | - | - | - |
| Qi et al. [ ] | - | Permeate channel | - | - | - | - | - |
| Aiman et al. [ ] | - | Permeate channel | - | - | - | - | - |
| Rivero et al. [ ] | - | Both channels | - | - | - | - | - |
‘-’ indicates that the model did not account for that non-ideal effect, ‘+’ indicates that the model did account for that non-ideal effect. For column ‘5′, ‘+’ indicates that permeance was modeled as a function of temperature only; ‘++’ indicates that permeance was modeled as a function of temperature and pressure; and ‘+++’ indicates that permeance was modeled as a function of temperature, pressure, and composition.
The impacts of each non-ideality on improving performance predictions and module design are discussed. In all cases, including the non-ideality either led to an improvement in predictions or highlighted a key variable for consideration in future module designs. While most studies have been limited to countercurrent modules, the effect of each non-ideality would be included for crossflow and co-current flow following the approach for countercurrent flows.
6.1. Real Gas Behavior
Membrane gas separations can be carried out at high feed pressures (>10 bar), and therefore ideal gas behavior may not be accurate. This can affect the driving force for gas permeation and requires the researcher to replace the partial pressure driving force for permeation with a fugacity driving force. The Soave–Redlich–Kwong equation of state (EOS) has proven to provide a good description of the pressure–volume–temperature (PVT) properties for many industrially relevant gas mixtures [ 28 , 30 ]. A study conducted by Scholz et al. [ 27 ] for biogas separation concluded that real gas effects should be accounted for when operating at pressures exceeding 10 bar. The fugacities were calculated from the EOS and used to calculate the gas permeation rates. The availability of this equation of state is common in well-known process simulators such as Aspen Plus [ 37 ].
6.2. Friction Losses
Pressure drops are expected to occur in all module geometries in both the retentate and permeate channels, reducing the driving force for separation. A pressure drop also increases the energy requirement for the separation process. This is especially important in CO 2 -capture processes where large volumes of gas must be pushed through membranes, leading to large operating costs if pressure drops are high [ 44 ]. Pressure drops through each module will be different due to their inherent geometries. Therefore, module selection is an important aspect during process design.
Pressure drop in hollow-fiber modules is described by the Hagen–Poiseuille equation for laminar flow in both the lumen and shell side (assuming an equivalent hydraulic diameter for the shell domain). The Hagen–Poiseuille equation has been studied extensively in hollow-fiber membrane modules for different gas separations and provides a good description of experimental data [ 45 ]. For a hollow-fiber membrane module, pressure drops per stage are given by the following calculations [ 24 , 28 ]:
where μ is the retentate ( r ) or permeate ( p ) viscosity; ρ is the retentate or permeate density; ∆ P is the pressure drop of the lumen ( l ) or shell ( s ) side; I D is the internal diameter of the fiber; N f is the number of fibers; L is the fiber length; d m is the module diameter; O D is the fiber outside diameter; N is the number of stages; and L f and S f are the lumen and shell flow rate, respectively.
Lumen-side pressure drops can be detrimental to performance when designing a module with small fiber diameters, whereas shell-side pressure drops can be significant at high packing densities. Normally, pressure drops through the shell side are much smaller than those on the lumen side due to limitations on the module packing density required to introduce and remove gas through external ports on the case from the shell. Chu et al. [ 34 ] demonstrated that, for natural gas separation, pressure drops on the lumen side can lead to significant methane-retentate recovery loss, thereby increasing the pressure requirement needed for separation.
Pressure drop in flat-sheet and spiral-wound membrane modules can be approximated by the Darcy–Weisbach law expression [ 35 , 46 ]:
where λ is the friction factor, ρ is gas density, d h is the hydraulic diameter, L c is the channel length, and v is the bulk velocity.
When contrasting spiral-wound and flat-sheet sweep pressure drops for a 20 TPD CO 2 capture multistage membrane system, the flat-sheet membrane displayed a four-times-lower pressure drop (<1 psia) than the spiral-wound modules [ 47 ]. However, this difference comes at the expense of a lower packing density and, hence, larger module footprint.
6.3. Non-Isothermal Behavior
Gas permeation through a membrane is analogous to the isenthalpic expansion of a gas through a throttling valve. Therefore, as the gas permeates, changes in temperature along the length of the membrane can be expected according to the Joule–Thomson coefficients for each component. Temperature changes due to the Joule–Thomson effect impact the permeance and selectivity of the membrane due to the following Arrhenius equation for the temperature dependence of gas permeability:
where E a , i is the activation energy for component i , R is the gas constant (8.314 J/mol/K), T r is the retentate temperature at each stage j , T 0 is the reference temperature, Q i is the temperature independent pre-exponential factor, and Q ( T ) ( j , i ) is the temperature dependent permeability of component i at stage j . Note that the resistance to heat transfer across the membrane is small, so the temperature differences between permeate and retentate are often small.
Coker et al. [ 23 ] studied non-isothermal behavior for binary and multicomponent gas mixtures in a hollow-fiber membrane module. The proposed model was validated with experimental data and demonstrated that, for multicomponent natural gas separation, the temperature can decrease up to 40 °C at the 50% stage cut, and increasing the CO 2 feed stream composition led to greater temperature changes due to the high Joule–Thomson coefficient for CO 2 . Ahmad et al. [ 33 ] also demonstrated that the permeate gas temperature decreases as the CO 2 feed content increases for higher stage cuts in a non-isothermal natural gas separation experiment. However, heating of the retentate stream can occur when separating a CO 2 /H 2 stream from pre-combustion power plants, as shown by Miandoab et al. [ 28 ], due to the negative Joule–Thomson coefficient for hydrogen. Temperature-change effects are most critical to model for gas separations with gases that possess large Joule–Thomson coefficients, especially at high stage cuts and high pressure ratios [ 28 , 31 , 33 , 35 ].
6.4. Concentration Polarization
Concentration polarization influences the mass transfer process by slowing the flow of the most permeable component, while increasing the flow of the least permeable gas. Therefore, the concentration of the components changes from the boundary layer surface to inside the porous support and membrane surface. Polarization effects have been observed only for high-flux membranes and negatively affect module performance by decreasing product purity most prominently at high stage cuts [ 25 ].
Concentration polarization can be modeled by introducing a mass transfer coefficient to describe the boundary layers on either side of the membrane and the resistance of the support [ 28 , 29 ]. The dependence of the mass transfer coefficient on the module geometry and operating conditions is complex and not well understood.
Pan [ 17 ] noted the potential impact of concentration polarization in the support of a high-flux membrane and demonstrated that it can lead to effective crossflow contacting even if the module is operated nominally in countercurrent mode. Sidhoum et al. [ 48 ] showed that if the support resistance and contacting-gas-phase resistances are sufficiently low, the performance of asymmetric hollow-fiber membrane modules, with the membrane discriminating layer on the fiber outer surface, does not depend on whether the module is lumen or shell fed. Thus, proper design of the support is critical to minimize internal concentration polarization and maximize module performance.
Alpers et al. [ 49 ] documented the importance of external gas-phase concentration polarization for organic vapor separations with high-flux membranes. The apparent membrane selectivity increased dramatically with the flow rate, and the changes could be described quantitatively with an appropriate mass transfer coefficient correlation for the contacting gas phases.
Miandoab et al. [ 28 ] studied the impact of concentration polarization in a hollow-fiber membrane for pre-combustion CO 2 capture. Concentration polarization had less of an impact on CO 2 and H 2 selective membranes than other non-ideal effects. Such a conclusion concurs with the work of Mourgues et al. [ 36 ] in which concentration polarization was prominent only when component permeance exceeded 1000 GPU and selectivity was greater than 100. Scholz et al. [ 27 ] performed a similar study for assessing the impact of concentration polarization for biogas upgrading and concluded that concentration polarization can be neglected for low-flux membranes. Furthermore, Feng et al. [ 25 ] included the influence of concentration polarization for air separation in lumen-fed hollow-fiber modules and found that concentration polarization may become significant at high stage cuts.
6.5. Non-Uniform Membrane and Module Properties
Membrane module defects that arise from manufacturing practices can have detrimental effects in terms of the separation performance. In hollow-fiber membrane modules, inherent variations in fiber properties, such as permeance and internal diameter, can induce flow maldistribution effects that can dramatically affect module performance and even limit the product purity that can be achieved [ 26 ]. Similar effects can occur in flat-sheet and spiral-wound modules, where variations in permeate channel height and membrane permeance can diminish the recovery for a given retentate product and thereby increase the membrane area required for separation [ 26 , 32 ].
Sonalkar et al. [ 32 ] provided a detailed framework for studying the impact of fiber variability on module performance. The model assumes a Gaussian distribution for fiber internal diameter, selectivity, and gas permeance. A lumen-fed hollow-fiber membrane module was considered with perfect permeate mixing and no permeate mixing, and in the former, the permeate from all fibers was well mixed along the length of the module, whereas in the latter, no mixing between the fiber permeate took place. Simulation results, which were validated against experimental data from an air separation module, show that variation in inner diameter (ID) has the greatest effect on module performance as compared to varying the permeance or selectivity of the fibers.
As ID variation increases, recovery decreases. This inverse relationship is due to the dependence of flowrate on the fourth power of the inner diameter, which is given by the Hagen–Poiseuille equation. For instance, a 20% inner diameter variation can decrease the retentate recovery by as much as 30% of the original value in the case of binary air separation. Variations in fiber permeance have a moderate impact on performance compared to ID variation; a permeance variation of 30% is analogous to a 10% ID variation.
An additional study, following similar methodology, investigated the impact of channel height variation on flat-sheet membrane and plate-and-frame module performance [ 43 ]. The model shows that, as channel variability increases, recovery decreases, as the flowrate is dependent on the channel height, but to the third power. Since the dependency is smaller than a hollow-fiber membrane, the effect of flow channel variability is slightly less significant for plate-and-frame modules compared to hollow-fiber modules. However, the study demonstrates that both membrane configurations see performance decline with increasing variability, allowing for further investigations into other non-uniform conditions.
6.6. Competitive Sorption, Penetrant Blocking, and Plasticization Effects
The presence of highly condensable gases in glassy polymeric membranes can induce plasticization effects that can negatively impact the physical properties of the membrane, leading to a reduction in the gas permeance and selectivity [ 50 ]. Plasticization effects lead to enhanced diffusion of the components due to the increased segmental motion stemming from the presence of highly sorbent condensable gases such as CO 2 and CH 4 . Furthermore, competitive sorption effects and penetrant blocking can occur due to the excess free volume present in glassy polymers; these three phenomena can be mathematically described by the dual sorption/partial immobilization model [ 51 , 52 ]. Visser et al. [ 50 ] conducted mixed gas permeation experiments for a CO 2 /N 2 mixture and showed that plasticization effects dominate at high pressures and low concentrations of the inert gas, whereas competitive sorption effects become stronger at higher inert gas concentrations. A subtle balance exists between plasticization and competitive sorption, as the latter can counterbalance the effects induced by plasticization at a high concentration of the inert gas.
Commonly, permeance is assumed to be constant or temperature independent in most models; therefore, the inclusion of a pressure-, temperature-, and composition-dependent permeance is necessary to fully characterize membrane permeation. Miandoab et al. [ 29 ] presented a comprehensive permeance model for investigating these permeance dependencies based on a fugacity-dependent permeance for glassy polymers that includes membrane plasticization, penetrant blocking, and competitive sorption effects [ 51 , 53 , 54 ]. The proposed model was validated with experimental permeance measurements and used for a sensitivity analysis in biogas upgrading. The results showed that competitive sorption and plasticization by CO 2 - and H 2 O-induced free volume blocking can have a significant effect. Additionally, a humidified gas feed showed a larger decrease in performance as compared to a dry gas stream due to the induced free volume blocking by water vapor and competitive sorption effects. Scholz et al. [ 27 ] performed a similar study of biogas upgrading by using a second-order polynomial to express the pressure- and composition-dependent permeance. Although the model predicted more pronounced effects when considering competitive sorption and plasticization effects, the method employed was not an adequate description of permeation physics. Bounaceur and Ahmad et al. [ 31 , 33 ] performed similar studies; however, the former did not consider the effect of composition, whereas the latter assumed constant gas diffusivity. The influence of non-ideal effects due to membrane permeance combined with those of module operation and module fabrication defects should be taken into consideration to accurately quantify the overall module performance.
7. Modeling Applications and Challenges
7.1. dimensional modeling.
One-dimensional membrane module modeling based on an ideal contacting configuration is common in the literature. Axial variations in composition, pressure, and temperature are predicted by assuming plug flow behavior. In the case of hollow-fiber membrane modules with counter and co-current flow configurations, the one-dimensional assumption often allows researchers to make accurate predictions of experimental data since the flow is parallel to the membrane [ 17 , 24 , 25 , 39 ]. However, in the case of crossflow hollow-fiber membrane modules, variations in both the axial and radial direction can be expected due to concentration changes and pressure buildup inside the module; this behavior is analogous to that of spiral-wound and flat-sheet membrane modules in which the permeate flows perpendicular to the feed stream. DeJaco et al. [ 40 ] proposed a 1D and 2D model for simulating air separation from spiral-wound membrane modules. His results showed that the 1D model provided a good approximation to the results of the more detailed 2D model over a range of stage cuts and oxygen permeate concentrations that were fit with experimental data. Additionally, the 1D spatial distribution of momentum variables (pressure drop and velocity) was in good agreement with the one-dimensional averaged variables from the 2D model.
Brinkmann et al. [ 35 ] developed a 1D model for envelope-type and flat-sheet modules and a 2D model for spiral-wound membrane modules in which non-ideal effects were considered for all modules studied. The simulation results from the modules investigated were in good agreement with the pilot plant experiments conducted by the author. Similarly, Dias et al. [ 55 ] presented a 1D and 2D model for simulating the performance of a spiral-wound membrane module under crossflow operation. The 2D profiles obtained were in better agreement with the literature data as compared to the 1D model.
A 3D computational fluid dynamics model is expected to provide enhanced understanding of the flow and mass transfer behavior inside membrane modules, but implementing this level of modeling into a commercial process simulator becomes unpractical due to higher computational times. Moreover, Haddadi et al. [ 56 ] performed a study comparing a 1D model versus a 3D model for a hollow-fiber membrane module and concluded that both models showed good agreement with the experimental data, with less than 2% error. These results suggest that 1D models may provide good performance predictions for initial process design, but higher dimensional modules will be required to improve predictions and agreement with experimental data.
7.2. Software Implementation
Software selection for membrane module modeling is a key step for model development. The decision to model a membrane module depends on the spatial dimension used and the level of complexity of the model equations. Several works in the literature [ 27 , 28 , 29 , 37 ] have used Aspen Custom Modeler (ACM), which is an equation-oriented modeling software that has built-in numerical solvers that are capable of solving linear, nonlinear, ordinary, and partial differential equations. The customized unit operation model can be interfaced with the Aspen properties package and easily exported to Aspen Plus and HYSYS for performing flowsheet simulations. ACM offers a unique advantage over other software tools, such as MATLAB, Excel VBA, and others, because there is no need for coding the numerical method or algorithm for solving these equations, and the interfacing process with a commercial process simulator is simpler.
Additional software tools such as gPROMS have been used by Hensen [ 37 ] and Marriott et al. [ 38 ] to model hollow-fiber membrane modules under different operating conditions; the model can also be exported to Aspen Plus through CAPE-OPEN (Computer-Aided Process Engineering), which allows for the interoperability between a process modeling environment and a custom process modeling component. The incorporation of customized models in process simulators also can be performed through user-defined subroutines that are available in the program (Aspen Plus, HYSYS). However, this requires the user to code the membrane in FORTRAN, Visual Basic, C, or C++ programming languages and interface the routines with the process simulator.
If a more sophisticated modeling approach is desired for analyzing in detail the mass, momentum, and heat-transfer effects inside the membrane module, 3D software tools such as COMSOL Multiphysics, ANSYS, and OpenFoam [ 56 , 57 , 58 ] can be used to account for actual module geometry and associated fluid contacting. However, 3D models can be computationally expensive, and therefore simulation times must be balanced with the level of accuracy wanted as the meshing requirements required for the solution to be mesh independent will lead to longer run times.
7.3. Comparisons with Pilot-Scale Experiments
A great majority of the developed models for membrane gas separations are validated against experimental data from small-scale applications. There is a limited amount of work performed with model validation against pilot-plant-scale experiments. Brinkman et al. [ 35 , 59 ] developed carbon capture membrane models using the Aspen Custom Modeler (ACM) and compared them against experimental data from several field trials completed at three different locations in Germany. The operating conditions and design variables differ from each test conducted, with the purpose of evaluating the performance of the membrane module under a wide range of flows, feed compositions, and other parameters. The pilot-plant experiments were completed in a steady-state fashion over a period of two months for the first two trials and 400 h for the third one. The results from the simulation models were in good agreement with the pilot-plant data; however, the prediction was slightly poorer when concentration polarization effects became prominent at low feed-flow rates. Furthermore, high feed pressures increase the model discrepancies due to the complex behavior of the membrane permeance that was not captured by the model. A decrease in performance was not observed for the period of operation, and thus, the experiments conducted could be predicted without accounting for membrane aging.
Choi et al. [ 60 ] completed a pilot-scale membrane plant study for the separation of CO 2 from liquefied natural fired flue gas. A hollow-fiber simulation was employed to establish comparisons against the experimental data. The pilot plant in this study consists of a multistage membrane separation process with feed gas and vacuum compression. The process was operated under different operating scenarios, and a good agreement between the numerical simulation and the field data was found. However, when atmospheric pressure was applied on the permeate side, the simulations provided poorer predictions of the required membrane area.
Fixed-site carrier membranes developed by Norwegian University of Science and Technology (NTNU) in Norway were pilot tested under different environments and module configurations for CO 2 capture. Sandru et al. [ 61 ] developed plate-and-frame modules (0.25 m 2 –1.5 m 2 ) that were tested for more than six months in a coal-fired power plant in Portugal. The separation performance was consistent over the testing period and showed a good dynamic response to process upsets. However, the flat-sheet membranes were not efficient and were difficult to scale up. Therefore, Hagg et al. [ 62 ] designed a hollow-fiber membrane module (4 m 2 ) for testing in a cement plant. The module performed well and consistently under large testing periods and harsh conditions. He et al. [ 63 ] scaled up the hollow-fiber membranes (4.0 m 2 –10 m 2 ) in order to find the best operating conditions and improve the module’s performance. The membranes were subjected to several experimental runs in which multiple process parameters were varied to assess the membrane’s performance and behavior. The results from this testing campaign indicate that a bore-side-fed module provided better gas distribution within the module and, thus, improved the module’s efficiency compared to a shell-fed module. NTNU demonstrated the compactness and robustness of their membrane modules at a pilot-plant level, but they did not use uncertainty quantification within a process model to account for measurement error and bias while conducting model validation.
Facilitated transport membranes comprising polyvinylamine as a fixed carrier and an amino acid salt as a mobile carrier developed by The Ohio State University [ 64 , 65 , 66 ] also offer attractive performance for CO 2 capture. Transport models were developed and validated for the membrane with data from test coupons, and the model was used to perform technoeconomic analyses. Notably, spiral-wound modules were tested at the National Carbon Capture Center, but detailed comparisons to module simulations were not provided.
An uncertainty quantification approach was taken by DeJaco et al. [ 40 ] to help validate experimental data from an air-separation spiral-wound membrane module with simulation results. Uncertainties in seven input model parameters were evaluated in the simulation and propagated to the output variables: stage cut and permeate mole fraction. The results indicate that the uncertainty for both calculated and experimental stage cuts is predominant at low feed flows due to measurement limitations. Uncertainty in the permeate purity was found to increase when operating the module at high feed pressures; therefore, more precise measurements of component permeances are needed to accurately determine the change in permeate purity.
There has been a large quantity of bench-scale/pilot-plan-scale tests reported in the literature for different gas separations with several module types, as shown in Table 3 . However, only a few of these experimental tests were validated with membrane models, and only DeJaco et al. [ 40 ] accounted for rigorous parametric uncertainty of the process model.
Summary of several lab-scale and pilot-plant trials for multiple membrane module types. Module types listed are (1) hollow fiber (HF), (2) spiral wound (SW), and (3) flat sheet (FS).
| Reference | Gas | Module Type | Pilot Scale | Process Model Validation | UQ of Model |
|---|---|---|---|---|---|
| Feng et al. [ ] | O /N | HF | 0.046 m | + | - |
| DeJaco et al. [ ] | O /N | SW | 0.3 m | + | + |
| Brinkmann et al. [ ] | CO /N /O | FS | 12.6 m | + | - |
| Choi et al. [ ] | CO /N /O | HF | - | + | - |
| Sandru et al. [ ] | CO /N /O | FS | 1.5 m | - | - |
| Hagg et al. [ ] | CO /N /O | HF | 18 m | - | - |
| He et al. [ ] | CO /N /O | HF | 4.2–10 m | - | - |
| Salim et al. [ ] | CO /N /O /H O | SW | 1.4 m | - | - |
| White et al. [ ] | CO /N /O | SW | 1 TPD CO | - | - |
| Lin et al. [ ] | CO /CO/H /N /CH | SW | 1–40 m | - | - |
| Scholes et al. [ ] | CO /N /O | HF and SW | 5 and 7.5 m | - | - |
| Stern et al. [ ] | CO /CH | HF | 0.93 m | - | - |
| Pohlmann et al. [ ] | CO /N /O | FS | 12.5 m | - | - |
| Dai et al. [ ] | CO /N /O | HF | 0.02 m | - | - |
| Wolff et al. [ ] | CO /N /O | FS | 11–40 m | + | - |
The process models for membrane-based separation processes are predominantly deterministic in nature—rigorous parametric uncertainty quantification has not been accounted for. Sensitivity studies must be performed to assess the impact of errors in process variable measurements and help identify input parameters that have the largest contribution to the output variable of interest.
Additionally, optimization studies have been completed to identify regions where the membrane process can meet desired product purity and recovery targets. Propagating the uncertainty through the process model can allow for the estimation of uncertainty in meeting these targets. It has been proven that when taking parameter uncertainty into consideration, the model is able to accurately predict large-scale pilot-plant data for CO 2 capture in solvent systems [ 74 ]. Membrane-based CO 2 -capture technologies can clearly benefit from the implementation of uncertainty quantification into process models that can help replicate the pilot-plant data while maximizing the knowledge in the stochastic modeling and sequential design of experiments’ methodology.
8. Membrane Module Modeling Checklist
Deciding which non-ideal effects to include in module performance models during the early stages of module development is a difficult task. The most comprehensive model will include all the non-ideal effects identified in the preceding section. However, the information required to perform the calculations (e.g., dependence of permeance on temperature and pressure and variability in membrane properties) may not be immediately available.
Model complexity can be reduced by initially neglecting all non-ideal effects and assuming an ideal contacting pattern based on the module geometry, e.g., assuming countercurrent contacting for a hollow-fiber module. Module performance predictions for this ideal module model provide an upper limit on performance in terms of product recovery from the feed and membrane productivity or product flow rate per unit membrane area.
To help evaluate the impact of non-idealities, a checklist was developed, as summarized in Table 4 . Based on the available literature, this checklist provides guidelines for estimating the potential effects of non-idealities and how to modify the ideal model to account for them.
Checklist for inclusion of module non-idealities.
| Source of Nonideality | Check on Importance | Simulation Modification |
|---|---|---|
| Deviation from nominal countercurrent flow | Compare predictions for countercurrent and crossflow contacting | 2D or 3D transport simulations may be required |
| Fiber size or channel height variation | Experimental standard deviation of size variation is >10% of average | Include fiber size or channel height variation |
| Membrane permeance/selectivity variation | Experimental standard deviation of variation is >30% of average | Include permeance/selectivity |
| Pressure drops in flow channel | Evaluate pressure drops in absence of permeation in flow channels | Include momentum balance |
| Joule–Thomson effects | Evaluate temperature change upon expanding feed gas to permeate pressure | Include non-isothermal permeances and energy balance |
| Concentration polarization | Gas permeance > 1000 GPU and selectivity > 100 | Include external and internal mass transfer resistances in permeation rate calculation |
| Concentration/pressure dependent permeances | Experimental measurement of permeances over relevant range of pressures and compositions | Include appropriate expression of dependence of permeance on process variables |
| Real gas behavior | Experimental pressure > 10 bar and non-unity fugacity coefficients | Use fugacity driving force in permeation expression |
The potential need to use higher dimensional (i.e., 2D or 3D) models for nominal countercurrent modules can be evaluated by comparing performance predictions for countercurrent contacting with predictions for crossflow contacting. If the differences are significant over the anticipated operating range, higher dimensional models may be needed to capture more subtle effects associated with gas distribution into and from the module. Note that the observation of differences does not necessarily imply that higher dimensionality modeling is needed. It only suggests that if differences between simulation and experiment exist, they may be associated with non-ideal fluid contacting, but this does not rule out other non-ideality sources.
Module performance may depend on the variation in membrane and flow channel properties (i.e., ID, channel thickness, permeance, and selectivity) that occurs in membrane and module manufacture. Ideally, manufacturing quality controls reduce these variations to acceptable levels: <10% channel size variation and <30% permeance variation. However, if actual variability is higher, the resulting flow maldistribution effects can be detrimental to performance and should be included in module simulations.
An upper bound on the pressure drop within a module is provided by calculating pressure drops in the absence of permeation, i.e., for a constant flow. If significant, pressure drops must be calculated through the inclusion of a momentum balance, as described previously. Note that pressure drops can be minimized through changes in the module design and operating conditions selected for the desired separation [ 63 , 68 ].
The potential impact of Joule–Thomson effects can be determined by calculating changes in temperature associated with expansion of the feed gas to the permeate pressure, using analytical expressions or a process simulator. If significant temperature changes occur, the energy balance must be included in the simulation. Additionally, the temperature dependence of gas permeance is required. Higher temperature drops along the length of the membrane are expected at low feed temperatures and high feed pressures [ 23 , 28 , 33 ]. The extent of temperature change along the length of the membrane is also governed by the gas composition.
Concentration polarization effects may be important for gas permeances that are higher than 1000 GPU and selectivities that are larger than 100 [ 36 ]. If significant, mass transfer resistances in the contacting gas phases and the membrane porous support should be included in gas permeation rate calculations.
The potential impact of concentration- and pressure-dependent permeances is best determined from experimental permeance measurements over the anticipated range of operating conditions. Theoretical predictions of such effects may be possible using the Flory–Huggins model for rubbery polymers [ 31 , 35 ] or either the dual mode sorption/partial immobilization, ENSIC (ENgaged Species Induced Clustering), or NELF (Non-Equilibrium Lattice Fluid) models for glassy polymers [ 35 , 75 , 76 , 77 ]. These models also provide a theoretical basis for developing correlations of permeance with pressure and composition for use in module models.
The real gas behavior assumption may not be valid when operating at high pressures >10 bar [ 27 ]. An additional check on validity is provided by evaluating fugacity coefficients to determine the deviation from ideal gas behavior. If significant, a fugacity driving force is required to calculate gas permeation rates.
9. Conclusions
Mathematical modeling of membranes for gas separations is an important step for quantifying module performance. Simplified membrane models are likely to overpredict performance and lead to erroneous results when compared to experimental data. The development of robust mathematical membrane models for several gas separation applications that take into consideration non-ideal effects related to module manufacturing, operating conditions, and membrane properties are necessary for better process performance quantification and agreement with real data. Uncertainty quantification of membrane process models can help quantify the best estimates of uncertain design parameters through a stochastic modeling approach. However, uncertainty quantification in membrane models is still a novel process that needs more research that can lead to better model refinement strategies which can provide a better fit with the experimental data. The effective development of membrane models is essential for demonstrating the commercial competitiveness that membranes offer when comparing it to other separation alternatives through a techno-economic analysis.
A checklist for developing future simulations of modules that either possess improved membrane properties or are used in emerging separation areas is provided. As membrane permeances increase, the detrimental effect of concentration polarization within the support and in the external gas phases will have to be considered. This is the case for state-of-the-art membranes developed for CO 2 capture and light hydrocarbon separations. Additionally, module pressure drops and internal flow distribution will be of concern when seeking module designs to minimize the energy input required to create the chemical potential driving force for permeation. These concerns are especially important in CO 2 capture.
Abbreviations
| 1D | one dimensional |
| 2D | two dimensional |
| ACM | Aspen Custom Modeler |
| CAPE | Computer-Aided Process Engineering |
| CCS | Carbon Capture and Storage |
| DOE | Department of Energy |
| ENSIC | ENgaged Species Induced Clustering |
| FV | fiber variability |
| gPROMS | general Process Modeling System |
| GPU | Gas Permeating Unit |
| ID | internal diameter |
| JT | Joule–Thomson |
| MEA | Monoethanolamine |
| NELF | Non-Equilibrium Lattice Fluid Model |
| NTNU | Norwegian University of Science and Technology |
| OD | outside diameter |
| PVT | pressure–volume–temperature |
| VBA | Visual Basic for Applications |
| VOC | volatile organic compound |
Funding Statement
This work was performed with the support of the US Department of Energy’s Transformational Carbon Capture program. Funding was provided through contract P010267273 from the US Department of Energy.
Author Contributions
Conceptualization: M.D.C., L.N., J.R., K.H. and G.L.; writing: M.D.C., L.N., J.R., K.H. and G.L.; visualization: J.R., K.H. and G.L.; supervision: K.H. and G.L.; funding acquisition: K.H. and G.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest. The funders had no role in the conceptualization of the review and writing of the manuscript.
This project was funded by the United States Department of Energy, National Energy Technology Laboratory, in part, through a site support contract. Neither the United States Government nor any agency thereof, nor any of their employees, nor the support contractor, nor any of their employees makes any warranty, whether expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
PERSPECTIVE article
The future of membrane separation processes: a prospective analysis.

- LRGP-CNRS Université de Lorraine, Nancy, France
Membrane processes are today one of the key technologies for industrial separations and are expected to play an important role in future sustainable production systems. The combination of materials science and process engineering has historically always been an essential condition to the development of new applications for membranes. The recent development of high performance nanostructured materials, together with new production technologies (such as 3D printing) and high performance computing possibilities is expected to open new horizons to membrane processes. The different challenges and prospects to be addressed to achieve this purpose are discussed, with an emphasis on the future of process industries in terms of feedstocks, energy sources, and environmental impact.
1 Introduction: Membrane Separations Today
Membranes are usually considered as the third wave of separation processes, thermal separations (e.g., distillation, evaporation) and auxiliary phase processes (e.g. absorption, liquid extraction, adsorption) being the first and second respectively ( King, 1980 ; Koros, 2001 ). The industrial development of membrane indeed demanded advanced thin layer materials to be produced at a large scale; early attempts of microporous membranes preparation (with pore sizes below µm range) can be dated back 1920, while thin film dense membranes could not be obtained before 1960 ( Hwang and Kammermeyer, 1975 ).
The key production challenges of membranes, which are still valid today, have been soon identified: first, a highly permeable material simultaneously showing a high enough selectivity is logically absolutely necessary. The antagonism between these two performances generates a so called trade-off curve, an empirical limit based on experimental data, that is obtained for the separation of gas and liquid mixtures ( Robeson, 2008 ). The possibility to overpass the corresponding upper bond between permeance and selectivity is one of the key challenges of membrane science ( Park et al., 2017 ).
In a second step, large scale production processes of membrane and module have to be developed with zero default standards.
Finally, the membrane process has to be implemented at the best place in the industrial process, with efficient pretreatment operations, in order to ensure the longest membrane material lifetime.
The development of membrane processes thus requires a combination of 1) high performance materials (chemistry being the key discipline), 2) robust and liable module production technologies and 3) process engineering and design tools ( Prasad et al., 1994 ; Baker, 2004 ; Favre et al., 2017 ).
These three key steps (material, module, system) are sketched in Figure 1 as a science push contribution, while the application framework and constraints correspond to an industry pull action. Taking into account the tremendous developments recently achieved in the different directions shown on Figure 1 , it can be expected that the place and role of membrane processes in a large range of industrial applications will significantly expand in the near future. This prospective statement, together with the associated challenges, is detailed hereafter.
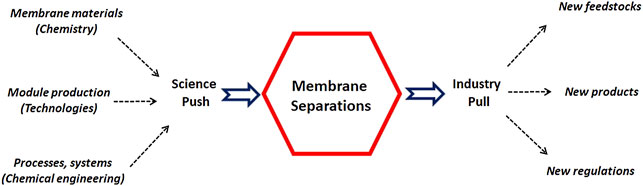
FIGURE 1 . Synopsis of the industrial development framework of membrane processes: scientific advances (innovative materials, new production technologies, and process engineering methodologies) can synergistically contribute to current and future industrial needs.
2 The Future of Membrane Processes: Challenges and Prospects
2.1 membrane materials.
To a large extent, polymers represent today the dominant material family of membrane separation processes ( Baker and Low, 2014 ). This statement applies for porous (microfiltration, ultrafiltration, dialysis) or dense (reverse osmosis, gas separation, and pervaporation) industrial membranes. Polymers effectively offer unique possibilities in terms of thin separation layer production, through cheap, scalable, liable processing technologies (phase inversion, extrusion, hollow fiber spinning, and coating) ( Nunes et al., 2020 ). Globally speaking, the permeability/selectivity trade-off is achieved based on statistical porous structures: pore size distribution in the nm to µm range for porous membranes, statistical free volume distribution in the subnanometer range for dense polymeric membranes ( Figure 2 ). The same statement holds for inorganic membranes (i.e. alumina, carbon, metal oxides, silica…), which are produced by sintering/extrusion and are mostly used for microfiltration and ultrafiltration operations.
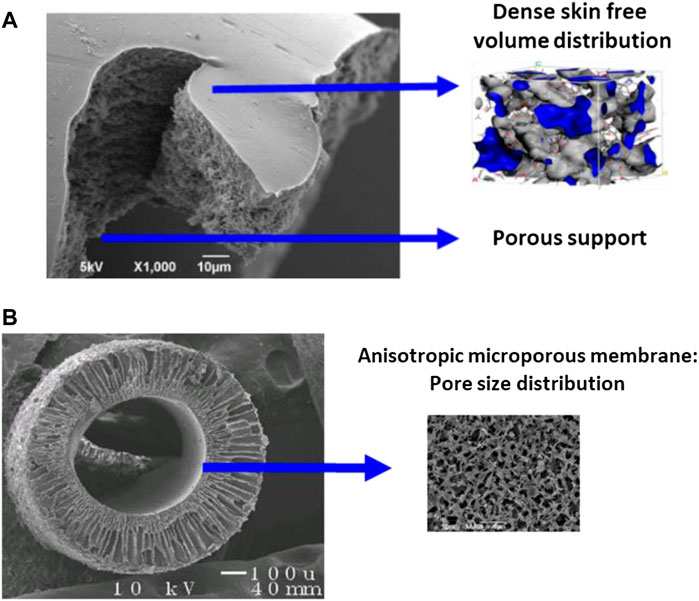
FIGURE 2 . Examples of current industrial membrane materials. (A) Dense skin asymmetric polymeric membrane (reverse osmosis, gas separations). Productivity constraints require a very thin dense layer supported on a porous structure. The separation performances result from species solubility and diffusion into a subnanometer free volume distribution matrix. (B) Porous membrane (ultrafiltration, microfiltration, dialysis, membrane contactors, and transmembrane distillation). The porous separating layer, shows a pore size distribution in the nanometer to micrometer range, depending on the liquid mixture to be treated and species to be treated be separated. This type of structure can be based on polymeric or inorganic materials.
With the advent of the nanostructured materials revolution, breakthrough performances are achievable today, mostly at lab scale for membrane materials ( Koros and Zhang, 2017 ). For instance, the classical permeability/selectivity trade-off limit of polymers for gas and, more recently, liquid separations, can be completely overpassed with materials showing a quasi-perfect monodisperse pore size such as zeolites ( Young et al., 2017 ), carbon nanotubes ( Skoulidas et al., 2002 ), Carbon Molecular Sieves (CMS) ( Koh et al., 2016 ), graphenes ( Geim, 2009 ), Metal Oxide Frameworks (MOF) ( Gascon and Kapteijn, 2010 ), among others.
The combination of ultrathin structure (down to the atom level for graphene films), together with perfect lattice structure opens the way to very high separation performances. Moreover, most of the inorganic nanostructured materials mentioned above show high temperature resistance and compatibility with a very broad range of chemicals. The limitations of polymers, with upper operating temperature usually around 100 C and sensitivity to chemicals (e.g., chlorine for ultrafiltration and reverse osmosis in biotechnology and water treatment, heavy hydrocarbons for gas separations, solvents for organic solvent nanofiltration) effectively limit today the selection of membrane processes for industrial use. More specifically, the possibility to operate membrane modules under high temperature conditions could unlock novel hybrid processes such as membrane reactors. The association of catalysis and separation function in a single unit is indeed known to often offer improved performances ( Agrawal, 2001 ; Van Kampen et al., 2021 ). Numerous studies have addressed this type of process for decades, for instance for hydrogen production with high temperature separation membranes based on palladium or inorganic membranes. The success of membrane bioreactors ( Shannon et al., 2008 ), which has been achievable with polymeric materials given the low temperature operation level (ca 30 C), could then possibly apply to a new set of chemical reactors.
2.2 Production Technologies
Besides new material developments, major changes are also expected to occur for membrane module production. The development of a new tailor made module for a new membrane material is known to be tedious, long and costly. Moreover, module/membrane industrial production most often makes use of organic solvents (i.e., for polymer dissolution) that can lead to environmental concerns. Green solvents (water, supercritical CO 2 .) have been proposed in order to limit these problems, but their use is far to be applicable to any type of polymer. Nevertheless, the large efforts and significant progress recently achieved in producing more sustainable membranes, employing green solvents and bio-based materials through the replacement of traditional toxic and harmful compounds should be stressed ( Nunes et al., 2020 ). Simultaneously, solvent resistant membranes, such as fluorinated polymers and thin film polymers showing impressive mechanical resistance have been recently developed, opening new perspectives for polymeric membranes ( Karan et al., 2015 ). Besides solvent use, potting and casing materials can also be an issue, with difficulties in terms of materials compatibility and defect free adhesion operation of resin potting for instance. Module production often relies on secret know-how. The challenges of module production also explain why the number of membrane equipment suppliers remains limited.
With the advent of 3D printing techniques, it might be that a completely new field of development emerges. The direct production of a membrane module through 3D printing in place of classical production techniques (e.g., hollow fiber spinning + resin potting) is not achievable yet, but it could become a reality in a near future. For instance, the production of ultrathin composite membrane samples, with dense skin layers down to 20 nm, has been recently reported, offering tremendous perspectives for development ( Chowdhury et al., 2018 ). Several studies recently reported 3D possibilities for different types of membrane materials and processes ( Bara et al., 2013 ; Bram et al., 2015 ; Nguyen et al., 2019 ). It has to be stressed that major limitations for large scale modules remain. Nevertheless, the direct 3D printing production of a membrane module based on either polymeric or inorganic materials could be a complete game changer. A rapid efficient module production could be achieved, with completely new possibilities offered in terms of structure.
The production constraints of a membrane, be it flat or a hollow fiber, necessarily translates into 1D type module structures. With 3D printing, complex geometries (such as fractal or constructal), possibly including in situ turbulence promoters (in place of spacers), anisotropic membrane or module structures could be possible. It is important to stress that living systems make use of membranes for numerous applications (e.g., lung, kidney…) based on complex structures, far away from the constant cross Section 1D fluid flow. This is certainly not fortuitous, but it may reflect improved performances (energy efficiency, intensification) that are largely unexplored today in membrane science with synthetic polymers.
2.3 Process Design Methods
The synergy between materials and process studies has always been a key requirement of membrane applications. Similarly to any chemical engineering target, Process Systems Engineering (PSE) tools are very efficient for membrane process design purposes today, with different software environments ( Biegler et al., 1997 ). The selection of the most efficient membrane material, together with the best place, best design and optimal operating conditions has been achieved for a great number of industrial applications ( Bozorg et al., 2019 ). Nevertheless, complex processes such as multistage or hybrid systems still address some important and partly unsolved optimization issues. Significant progress has been recently achieved in this direction, but an important paradigm shift is currently under progress.
2.4 Membrane Processes in a New Industrial Environment
With the forecasted decrease of fossil fuel use, a completely new industrial landscape is on the way to become reality in a near future. In terms of feedstocks, renewables are expected to replace fossil hydrocarbons ( Agrawal and Mallapragada, 2010 ; Favre, 2020 ). This will strongly impact the type of separation processes which are classically used in petrorefineries, with the predominant role of distillation. High performance membrane materials can drastically improve the energy efficiency of separation processes ( Sholl and Lively, 2016 ; Castel and Favre, 2018 ).
Biorefineries will require efficient separation processes, adequate to achieve operation on aqueous, diluted mixtures containing heat sensitive biomolecules. Membrane processes which offer the possibility to separate complex mixtures without heat supply and require electricity in place of thermal driving force are considered as a key technology for biorefineries.
Moreover, the use of alternative driving forces could be of interest, besides the classical pressure, power based solution. Temperature difference (such as in transmembrane distillation or thermopervaporation), sweep operation (which can replace vacuum pumping is some cases) or more exotic driving forces such as light ( Gérardin et al., 2021 ) or electrical fields ( Wilcox, 2020 ) have been mostly discarded up to now. It might be that these novel approaches are in some cases reconsidered in a sustainable industrial framework, especially in an integrated, energy efficient network.
3 Discussion
The joined rapid evolution of advanced nanostructured materials, module production technologies and process engineering tools discussed in the previous sections is expected to generate significant changes in the production, place and role of membrane processes in industry.
- The new generation of membrane materials, to a large extent based on inorganic monodisperse structures, is likely to push membrane applications, through improved separation performances, and/or new applications under high temperature or aggressive environments. A sound collaboration between materials scientists and process engineers is however absolutely necessary in order to rigorously evaluate these new perspectives.
- The possibilities offered by new materials production technologies, especially 3D printing, is likely to generate a breakthrough in membrane module production. This field of research is very large and numerous development stoppers have to be solved, but the production tool is there, with spectacular developments in material science, and technological devices.
- Besides the traditional separation function, operated by membranes for decades, new possibilities are expected to emerge, where membranes fulfill at the same time multiple tasks (filtration, catalysis, support, and heat exchange.) ( Liu et al., 2016 ). The development of biomimetic and stimuli responsive membrane materials (such as self repairing structures for instance) is also expected to lead to new applications ( di Vincenzo et al., 2021 ).
- The development of modern, artificial intelligence type tools (neural networks, surrogate models, superstructure approaches, and genetic algorithms…) in Process Systems Engineering enables today the very fast identification of the optimal membrane, process design and operating conditions ( Castel et al., 2020 ). The joined improvements of optimization algorithms and computing capacity opens the way for innovative processes, where the design of mutlimembrane, multistaged processes can be rigorously achieved.
Additionally, the shift of numerous industrial sectors from fossil to renewables feedstocks and energy offers promising perspectives for membrane applications. The rational design of downstream processes for biorefineries, which are expected to gradually replace fossil fuel based refineries, will require a smart combination of technological bricks, where membranes will for sure play a key role ( Huang et al., 2008 ; Favre and Brunetti, 2022 ).
The different aspects listed throughout this prospective analysis are tentatively summarized in a general table ( Supplementary Material ).
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material , further inquiries can be directed to the corresponding author.
Author Contributions
EF: Concept and writing.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fceng.2022.916054/full#supplementary-material
Agrawal, R., and Mallapragada, D. (2010). Chemical Engineering in a Solar Energy-Driven Sustainable Future. AIChE J. 56 (11), 2762–2768. doi:10.1002/aic.12435
CrossRef Full Text | Google Scholar
Agrawal, R. (2001). Separations: Perspective of a Process Developer/designer. AIChE J. 47 (5), 967–971. doi:10.1002/aic.690470503
Baker, R. W., and Low, B. T. (2014). Gas Separation Membrane Materials: A Perspective. Macromolecules 47, 6999–7013. doi:10.1021/ma501488s
Baker, R. W. (2004). Membrane Technology and Applications . Chichester, New York: J. Wiley .
Google Scholar
Bara, J. E., Hawkins, C. I., Neuberger, D. T., and Poppell, S. W. (2013). 3D Printing for CO2 Capture and Chemical Engineering Design. Nanomater. Energy 2 (5), 235–243. doi:10.1680/nme/13.00021
Biegler, L., Grossmann, I., and Westerberg, A. (1997). Systematic Methods for Chemical Process Design . Englewood Cliffs, NJ: Prentice-Hall .
Bozorg, M., Addis, V., Piccialli, A., Ramírez-Santos, C., Castel, I., et al. (2019). Polymeric Membrane Materials for Nitrogen Production from Air: A Process Synthesis Study. Chem. Eng. Sci. 207, 1196–1213. doi:10.1016/j.ces.2019.07.029
Bram, M., Dornseiffer, J., Hoffmann, J., Gestel, T., Meulenberg, W. A., and Stöver, D. (2015). Inkjet Printing of Microporous Silica Gas Separation Membranes. J. Am. Ceram. Soc. 98 (8), 2388–2394. doi:10.1111/jace.13657
Castel, C., Bounaceur, R., and Favre, E. (2020). Engineering of Membrane Gas Separation Processes: State of the Art and Prospects. J. Membr. Sci. Res. 6 (3), 295–303.
Castel, C., and Favre, E. (2018). Membrane Separations and Energy Efficiency. J. Membr. Sci. 548, 345–357. doi:10.1016/j.memsci.2017.11.035
Chowdhury, M. R., Steffes, J., Huey, B. D., and McCutcheon, J. R. (2018). 3D Printed Polyamide Membranes for Desalination. Science 361, 682–686. doi:10.1126/science.aar2122
PubMed Abstract | CrossRef Full Text | Google Scholar
di Vincenzo, M., Tiraferri, A., Musteata, V.-E., Chisca, S., Sougrat, R., Huang, L.-B., et al. (2021). Biomimetic Artificial Water Channel Membranes for Enhanced Desalination. Nat. Nanotechnol. 16, 190. doi:10.1038/s41565-020-00796-x
Favre, E. (2017). Polymeric Membranes for Gas Separation. Comprehensive Membrane Science and Technology . Editors E. Drioli, and L. Giorno (New York: Elsevier ), Vol. II, pp155–212.
Favre, E., and Brunetti, A. (2022). “The Impact of Membrane Engineering in the Circular Economy,” in Membrane Engineering in the Circular Economy : Renewable Sources Valorization in Energy and Downstream Processing in Agro-Food Industry . 1st edition ( Elsevier ), Vol. 1. chap 2. doi:10.1016/b978-0-323-85253-1.00013-7
Favre, E. (2020). Specialty Grand Challenges in Separation Processes. Front. Chem. Eng. 2, 1. doi:10.3389/fceng.2020.00001
Gascon, J., and Kapteijn, F. (2010). Metal-Organic Framework Membranes-High Potential, Bright Future? Angew. Chem. Int. Ed. 49, 1530–1532. doi:10.1002/anie.200906491
Geim, A. K. (2009). Graphene: Status and Prospects. Science 324, 1530–1534. doi:10.1126/science.1158877
Gérardin, F., Cloteaux, A., Simard, J., and Favre, É. (2021). A Photodriven Energy Efficient Membrane Process for Trace VOC Removal from Air: First Step to a Smart Approach. Chem. Eng. J. 419. doi:10.1016/j.cej.2021.129566
Huang, H. J., Ramaswamy, S., Tschirner, U. W., and Ramarao, B. V. (2008). A Review of Separation Technologies in Current and Future Biorefineries. Sep. Purif. Technol. 62, 1–21. doi:10.1016/j.seppur.2007.12.011
Hwang, S. T., and Kammermeyer, K. (1975). Membranes in Separations . Wiley .
Karan, S., Jiang, Z., and Livingston, A. W. (2015). Sub–10 Nm Polyamide Nanofilms with Ultrafast Solvent Transport for Molecular Separation. Science 348, 6241–1347. doi:10.1126/science.aaa5058
King, C. J. (1980). Separation Processes . 2nd ed. New York: McGraw-Hill .
Koh, D.-Y., McCool, B. A., Deckman, H. W., and Lively, R. P. (2016). Reverse Osmosis Molecular Differentiation of Organic Liquids Using Carbon Molecular Sieve Membranes. Science 353, 804–807. doi:10.1126/science.aaf1343
Koros, W. J. (2001). The 'Third Wave. J. Membr. Sci. 187 (1–2), 1. doi:10.1016/s0376-7388(01)00425-2
Koros, W. J., and Zhang, C. (2017). Materials for Next-Generation Molecularly Selective Synthetic Membranes. Nat. Mater 16, 289–297. doi:10.1038/nmat4805
Liu, Z., Wang, W., Xie, R., Ju, X. J., and Chu, L. Y. (2016). Stimuli-responsive Smart Gating Membranes. Chem. Soc. Rev. 45, 460. doi:10.1039/c5cs00692a
Nguyen, D. T., Hornbostel, K., Murialdo, M. R., Ye, C., Smith, W., Baker, S., et al. (2019). 3D Printed Polymer Composites for CO2 Capture. Industrial Eng. Chem. Res. 58 (48), 22015–22020. doi:10.1021/acs.iecr.9b04375
Nunes, S. P., Culfaz-Emecen, P. Z., Ramon, G. Z., Visser, T., Koops, G. H., Jin, W., et al. (2020). Thinking the Future of Membranes: Perspectives for Advanced and New Membrane Materials and Manufacturing Processes. J. Membr. Sci. 598, 117761. doi:10.1016/j.memsci.2019.117761
Park, H. B., Kamcev, J., Robeson, L. M., Elimelech, M., and Freeman, B. D. (2017). Maximizing the Right Stuff: The Trade-Off between Membrane Permeability and Selectivity. Science 356, 1137. doi:10.1126/science.aab0530
Prasad, R., Shaner, R. L., and Doshi, K. J. (1994). “Comparison of Membranes with Other Gas Separation Technologies,” in Polymeric Gas Separation Membranes . Editors D.R. Paul, and Y.P. Yampol׳skii (Boca Raton, FL: CRC Press ), 531–614.
Robeson, L. M. (2008). The Upper Bound Revisited. J. Membr. Sci. 320, 390.
Shannon, M. A., Bohn, P. W., Elimelech, M., Georgiadis, J. G., Mariñas, B. J., and Mayes, A. M. (2008). Science and Technology for Water Purification in the Coming Decades. Nature 452, 301–310. doi:10.1038/nature06599
Sholl, D. S., and Lively, R. P. (2016). Seven Chemical Separations to Change the World. Nature 532, 435–437. doi:10.1038/532435a
Skoulidas, A. I., Ackerman, D. M., Johnson, J. K., and Sholl, D. S. (2002). Rapid Transport of Gases in Carbon Nanotubes. Phys. Rev. Lett. 89, 185901. doi:10.1103/physrevlett.89.185901
Van Kampen, J., Boon, J., and van Sint Annaland, M. (2021). Separation Enhanced Methanol and Dimethyl Ether Synthesis. J. Mater. Chem. A 9, 14627–14629. doi:10.1039/D1TA03405G
Wilcox, J. (2020). An Electro-Swing Approach. Nat. Energy 5, 121–122. doi:10.1038/s41560-020-0554-4
Young, J. M., Kim, D., Kumar, P., Lee, P. S., Rangnekar, N., Bai, P., et al. (2017). Ultra-selective High-Flux Membranes from Directly Synthesized Zeolite Nanosheets. Nature 543, 690.
PubMed Abstract | Google Scholar
Keywords: membrane, separations, materials, engineering, sustainable, industry
Citation: Favre E (2022) The Future of Membrane Separation Processes: A Prospective Analysis. Front. Chem. Eng. 4:916054. doi: 10.3389/fceng.2022.916054
Received: 08 April 2022; Accepted: 28 April 2022; Published: 17 May 2022.
Reviewed by:
Copyright © 2022 Favre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric Favre, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
St Andrews Research Repository

- St Andrews Research Repository
- Chemistry (School of)
- Chemistry Theses
- Register / Login
MOF-based membranes for challenging gas separations
Collections, description of related resources, related resources.
Items in the St Andrews Research Repository are protected by copyright, with all rights reserved, unless otherwise indicated.
Advertisement
Review of Membrane Separation Models and Technologies: Processing Complex Food-Based Biomolecular Fractions
- Published: 02 January 2021
- Volume 14 , pages 415–428, ( 2021 )
Cite this article

- Subin R. C. K. Rajendran 1 , 2 ,
- Beth Mason 2 &
- Alan A. Doucette ORCID: orcid.org/0000-0002-9467-1002 1
2920 Accesses
25 Citations
Explore all metrics
There is growing interest in the food industry to develop approaches for large-scale production of bioactive molecules through continuous downstream processing, especially from sustainable sources. Membrane-based separation technologies have the potential to reduce production costs while incorporating versatile multiproduct processing capabilities. This review describes advances in membrane technologies that may facilitate versatile and effective isolation of bioactive compounds. The benefits and drawbacks of pressure-driven membrane cascades, functionalized membranes and electromembrane separation technologies are highlighted, in the context of their applications in the food industry. Examples illustrate the separation of functional macromolecules (peptides, proteins, oligo/polysaccharides, plant secondary metabolites) from complex food-based streams. Theoretical and mechanistic models of membrane flux and fouling are also summarized. Overcoming existing challenges of these technologies will provide the food industry with several attractive options for bioprocessing operations.
Graphical Abstract
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
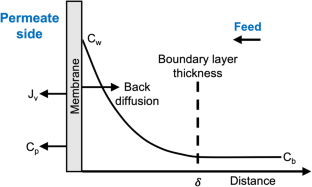
Similar content being viewed by others

Recovery Technologies for Water-Soluble Bioactives: Advances in Membrane-Based Processes

Introduction to Membrane Separation of Bioactive Compounds; Challenges and Opportunities
Food bioactive ingredients processing using membrane distillation, abbreviations.
bovine serum albumin
electrodialysis reversal
electrodialysis with ultrafiltration membranes
limiting current density
nanofiltration
polyethersulfone
ultrafiltration
Bargeman, G., Koops, G.-H., Houwing, J., Breebaart, I., van der Horst, H. C., & Wessling, M. (2002). The development of electro-membrane filtration for the isolation of bioactive peptides: The effect of membrane selection and operating parameters on the transport rate. Desalination, 149 (1), 369–374. https://doi.org/10.1016/S0011-9164(02)00824-X .
Article CAS Google Scholar
Bdiri, M., Dammak, L., Chaabane, L., Larchet, C., Hellal, F., Nikonenko, V., & Pismenskaya, N. D. (2018). Cleaning of cation-exchange membranes used in electrodialysis for food industry by chemical solutions. Separation and Purification Technology, 199 , 114–123. https://doi.org/10.1016/j.seppur.2018.01.056 .
Bdiri, M., Dammak, L., Larchet, C., Hellal, F., Porozhnyy, M., Nevakshenova, E., Pismenskaya, N., & Nikonenko, V. (2019). Characterization and cleaning of anion-exchange membranes used in electrodialysis of polyphenol-containing food industry solutions; comparison with cation-exchange membranes. Separation and Purification Technology, 210 , 636–650. https://doi.org/10.1016/j.seppur.2018.08.044 .
Bildyukevich, A. V., Plisko, T. V., Lipnizki, F., & Pratsenko, S. A. (2020). Correlation between membrane surface properties, polymer nature and fouling in skim milk ultrafiltration. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 605 , 125387. https://doi.org/10.1016/j.colsurfa.2020.125387 .
Binabaji, E., Ma, J., Rao, S., & Zydney, A. L. (2015). Theoretical analysis of the ultrafiltration behavior of highly concentrated protein solutions. Journal of Membrane Science, 494 , 216–223. https://doi.org/10.1016/j.memsci.2015.07.068 .
Binahmed, S., Hasane, A., Wang, Z., Mansurov, A., & Romero-Vargas Castrillón, S. (2018). Bacterial adhesion to ultrafiltration membranes: Role of hydrophilicity, natural organic matter, and cell-surface macromolecules. Environmental Science and Technology, 52 (1), 162–172. https://doi.org/10.1021/acs.est.7b03682 .
Article CAS PubMed Google Scholar
Brisson, G., Britten, M., & Pouliot, Y. (2007). Electrically-enhanced crossflow microfiltration for separation of lactoferrin from whey protein mixtures. Journal of Membrane Science, 297 (1–2), 206–216. https://doi.org/10.1016/J.MEMSCI.2007.03.046 .
Caus, A., Braeken, L., Boussu, K., & Van der Bruggen, B. (2009). The use of integrated countercurrent nanofiltration cascades for advanced separations. Journal of Chemical Technology & Biotechnology, 84 (3), 391–398. https://doi.org/10.1002/jctb.2052 .
Chamberland, J., Beaulieu-Carbonneau, G., Lessard, M.-H., Labrie, S., Bazinet, L., Doyen, A., & Pouliot, Y. (2017). Effect of membrane material chemistry and properties on biofouling susceptibility during milk and cheese whey ultrafiltration. Journal of Membrane Science, 542 , 208–216. https://doi.org/10.1016/J.MEMSCI.2017.08.012 .
Chuang, C.-J., Wu, C.-Y., & Wu, C.-C. (2008). Combination of crossflow and electric field for microfiltration of protein/microbial cell suspensions. Desalination, 233 (1–3), 295–302. https://doi.org/10.1016/J.DESAL.2007.09.054 .
Cifuentes-Araya, N., Pourcelly, G., & Bazinet, L. (2012). Multistep mineral fouling growth on a cation-exchange membrane ruled by gradual sieving effects of magnesium and carbonate ions and its delay by pulsed modes of electrodialysis. Journal of Colloid and Interface Science, 372 (1), 217–230. https://doi.org/10.1016/J.JCIS.2011.12.067 .
Cissé, M., Vaillant, F., Pallet, D., & Dornier, M. (2011). Selecting ultrafiltration and nanofiltration membranes to concentrate anthocyanins from roselle extract (Hibiscus sabdariffa L.). Food Research International, 44 (9), 2607–2614. https://doi.org/10.1016/j.foodres.2011.04.046 .
Córdova, A., Astudillo, C., Santibañez, L., Cassano, A., Ruby-Figueroa, R., & Illanes, A. (2017). Purification of galacto-oligosaccharides (GOS) by three-stage serial nanofiltration units under critical transmembrane pressure conditions. Chemical Engineering Research and Design, 117 , 488–499. https://doi.org/10.1016/j.cherd.2016.11.006 .
Doyen, A., Beaulieu, L., Saucier, L., Pouliot, Y., & Bazinet, L. (2011). Impact of ultrafiltration membrane material on peptide separation from a snow crab byproduct hydrolysate by electrodialysis with ultrafiltration membranes. Journal of Agricultural and Food Chemistry, 59 (5), 1784–1792. https://doi.org/10.1021/jf103739m .
Doyen, A., Husson, E., & Bazinet, L. (2013a). Use of an electrodialytic reactor for the simultaneous β-lactoglobulin enzymatic hydrolysis and fractionation of generated bioactive peptides. Food Chemistry, 136 (3–4), 1193–1202. https://doi.org/10.1016/J.FOODCHEM.2012.09.018 .
Doyen, A., Roblet, C., Beaulieu, L., Saucier, L., Pouliot, Y., & Bazinet, L. (2013b). Impact of water splitting phenomenon during electrodialysis with ultrafiltration membranes on peptide selectivity and migration. Journal of Membrane Science, 428 , 349–356. https://doi.org/10.1016/J.MEMSCI.2012.10.036 .
Durand, R., Fraboulet, E., Marette, A., & Bazinet, L. (2019). Simultaneous double cationic and anionic molecule separation from herring milt hydrolysate and impact on resulting fraction bioactivities. Separation and Purification Technology, 210 , 431–441. https://doi.org/10.1016/j.seppur.2018.08.017 .
Etzel, M. R., & Arunkumar, A. (2009). Charged ultrafiltration and microfiltration membranes in antibody purification. In Process scale purification of antibodies (pp. 325–347). Hoboken: Wiley. https://doi.org/10.1002/9780470444894.ch16 .
Chapter Google Scholar
Firdaous, L., Dhulster, P., Amiot, J., Gaudreau, A., Lecouturier, D., Kapel, R., Lutin, F., Vézina, L. P., & Bazinet, L. (2009). Concentration and selective separation of bioactive peptides from an alfalfa white protein hydrolysate by electrodialysis with ultrafiltration membranes. Journal of Membrane Science, 329 (1–2), 60–67. https://doi.org/10.1016/J.MEMSCI.2008.12.012 .
Galanakis, C. M. (2015). Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends in Food Science & Technology, 42 (1), 44–63. https://doi.org/10.1016/J.TIFS.2014.11.005 .
Gunderson, S. S., Brower, W. S., O’Dell, J. L., & Lightfoot, E. N. (2007). Design of membrane cascades. Separation Science and Technology, 42 (10), 2121–2142. https://doi.org/10.1080/01496390701444121 .
Habibi, S., Rabiller-Baudry, M., Lopes, F., Bellet, F., Goyeau, B., Rakib, M., & Couallier, E. (2020). New insights into the structure of membrane fouling by biomolecules using comparison with isotherms and ATR-FTIR local quantification. Environmental Technology , 1–18. https://doi.org/10.1080/09593330.2020.1783370 .
Hahn, T., Huuk, T., Osberghaus, A., Doninger, K., Nath, S., Hepbildikler, S., Heuveline, V., & Hubbuch, J. (2016). Calibration-free inverse modeling of ion-exchange chromatography in industrial antibody purification. Engineering in Life Sciences, 16 (2), 107–113. https://doi.org/10.1002/elsc.201400248 .
Hanspal, N. S., Waghode, A. N., Nassehi, V., & Wakeman, R. J. (2009). Development of a predictive mathematical model for coupled Stokes/Darcy flows in cross-flow membrane filtration. Chemical Engineering Journal, 149 (1–3), 132–142. https://doi.org/10.1016/J.CEJ.2008.10.012 .
Henaux, L., Thibodeau, J., Pilon, G., Gill, T., Marette, A., & Bazinet, L. (2019). How charge and triple size-selective membrane separation of peptides from Salmon protein hydrolysate orientate their biological response on glucose uptake. International Journal of Molecular Sciences, 20 (8). https://doi.org/10.3390/ijms20081939 .
Houldsworth, D. W. (1980). Demineralization of whey by means of ion exchange and electrodialysis. International Journal of Dairy Technology, 33 (2), 45–51. https://doi.org/10.1111/j.1471-0307.1980.tb01470.x .
Hung, J. J., Borwankar, A. U., Dear, B. J., Truskett, T. M., & Johnston, K. P. (2016). High concentration tangential flow ultrafiltration of stable monoclonal antibody solutions with low viscosities. Journal of Membrane Science, 508 , 113–126. https://doi.org/10.1016/j.memsci.2016.02.031 .
Husson, E., Araya-Farias, M., Gagné, A., & Bazinet, L. (2013). Selective anthocyanins enrichment of cranberry juice by electrodialysis with filtration membrane: Influence of membranes characteristics. Journal of Membrane Science, 448 , 114–124. https://doi.org/10.1016/J.MEMSCI.2013.06.061 .
Jungbauer, A., & Hahn, R. (2008). Polymethacrylate monoliths for preparative and industrial separation of biomolecular assemblies. Journal of Chromatography A. Elsevier., 1184 (1-2), 62–79. https://doi.org/10.1016/j.chroma.2007.12.087 .
Kasemset, S., Wang, L., He, Z., Miller, D. J., Kirschner, A., Freeman, B. D., & Sharma, M. M. (2017). Influence of polydopamine deposition conditions on hydraulic permeability, sieving coefficients, pore size and pore size distribution for a polysulfone ultrafiltration membrane. Journal of Membrane Science, 522 , 100–115. https://doi.org/10.1016/j.memsci.2016.07.016 .
Katz, W. E. (1979). The electrodialysis reversal (EDR) process. Desalination, 28 (1), 31–40. https://doi.org/10.1016/S0011-9164(00)88124-2 .
Kravtsov, V. A., Kulikova, I. K., Bessonov, A. S., & Evdokimov, I. A. (2019). Feasibility of using electrodialysis with bipolar membranes to deacidify acid whey. International Journal of Dairy Technology, 73 (1), 261–269. https://doi.org/10.1111/1471-0307.12637 .
Ladewig, B., & Al-Shaeli, M. N. Z. (2017). Surface modification of polyethersulfone membranes. In Fundamentals of membrane bioreactors (1st ed., pp. 87–129). Springer Nature Singapore Pvt. Ltd.. https://doi.org/10.1007/978-981-10-2014-8_4 .
Lightfoot, E. N. (2005). Can membrane cascades replace chromatography? Adapting binary ideal cascade theory of systems of two solutes in a single solvent. Separation Science and Technology, 40 (4), 739–756. https://doi.org/10.1081/SS-200047994 .
Lightfoot, E. N., Root, T. W., & O’Dell, J. L. (2008). Emergence of ideal membrane cascades for downstream processing. Biotechnology Progress, 24 (3), 599–605. https://doi.org/10.1021/bp070335l .
Liu Y, Huang H, Huo P, Gu L (2017) Exploration of zwitterionic cellulose acetate antifouling ultrafiltration membrane for bovine serum albumin (BSA) separation. Carbohydrate Polymers 165:266–275
Ma, J., Qin, L., Zhang, X., & Huang, H. (2016). Temporal evolution of the selectivity-permeability relationship during porous membrane filtration of protein solutions. Journal of Membrane Science, 514 , 385–397. https://doi.org/10.1016/j.memsci.2016.05.022 .
MacKe, S., Jerz, G., Empl, M. T., Steinberg, P., & Winterhalter, P. (2012). Activity-guided isolation of resveratrol oligomers from a grapevine-shoot extract using countercurrent chromatography. Journal of Agricultural and Food Chemistry, 60 (48), 11919–11927. https://doi.org/10.1021/jf3030584 .
Malczewska, B., & Żak, A. (2019). Structural changes and operational deterioration of the Uf polyethersulfone (Pes) membrane due to chemical cleaning. Scientific Reports, 9 (1), 1–14. https://doi.org/10.1038/s41598-018-36697-2 .
Mayani, M., Filipe, C. D. M., & Ghosh, R. (2010). Cascade ultrafiltration systems—Integrated processes for purification and concentration of lysozyme. Journal of Membrane Science, 347 (1–2), 150–158. https://doi.org/10.1016/J.MEMSCI.2009.10.016 .
Mehta, A., & Zydney, A. L. (2005). Permeability and selectivity analysis for ultrafiltration membranes. Journal of Membrane Science, 249 (1-2), 245–249. https://doi.org/10.1016/j.memsci.2004.09.040 .
Mohan, A., Rajendran, S. R. C. K., Thibodeau, J., Bazinet, L., & Udenigwe, C. C. (2018). Liposome encapsulation of anionic and cationic whey peptides: Influence of peptide net charge on properties of the nanovesicles. LWT, 87 , 40–46. https://doi.org/10.1016/J.LWT.2017.08.072 .
Nassehi, V. (1998). Modelling of combined Navier–Stokes and Darcy flows in crossflow membrane filtration. Chemical Engineering Science, 53 (6), 1253–1265. https://doi.org/10.1016/S0009-2509(97)00443-0 .
Ndiaye, N., Pouliot, Y., Saucier, L., Beaulieu, L., & Bazinet, L. (2010). Electroseparation of bovine lactoferrin from model and whey solutions. Separation and Purification Technology, 74 (1), 93–99. https://doi.org/10.1016/j.seppur.2010.05.011 .
Nevakshenova, E. E., Sarapulova, V. V., Nikonenko, V. V., & Pismenskaya, N. D. (2019). Application of sodium chloride solutions to regeneration of anion-exchange membranes used for improving grape juices and wines. Membranes and Membrane Technologies, 1 (1), 14–22. https://doi.org/10.1134/s2517751619010062 .
Ng, K. S. Y., Dunstan, D. E., & Martin, G. J. O. (2018). Influence of processing temperature on flux decline during skim milk ultrafiltration. Separation and Purification Technology, 195 , 322–331. https://doi.org/10.1016/j.seppur.2017.12.029 .
Nor, M. Z. M., Ramchandran, L., Duke, M., & Vasiljevic, T. (2016). Separation of bromelain from crude pineapple waste mixture by a two-stage ceramic ultrafiltration process. Food and Bioproducts Processing, 98 , 142–150. https://doi.org/10.1016/J.FBP.2016.01.001 .
Oussedik, S., Belhocine, D., Grib, H., Lounici, H., Piron, D. L., & Mameri, N. (2000). Enhanced ultrafiltration of bovine serum albumin with pulsed electric field and fluidized activated alumina. Desalination, 127 (1), 59–68. https://doi.org/10.1016/S0011-9164(99)00192-7 .
Patil, N. V., Janssen, A. E. M., & Boom, R. M. (2014). The potential impact of membrane cascading on downstream processing of oligosaccharides. Chemical Engineering Science, 106 , 86–98. https://doi.org/10.1016/J.CES.2013.11.007 .
Persico, M., & Bazinet, L. (2018). Fouling prevention of peptides from a tryptic whey hydrolysate during electromembrane processes by use of monovalent ion permselective membranes. Journal of Membrane Science, 549 , 486–494. https://doi.org/10.1016/J.MEMSCI.2017.12.021 .
Persico, M., Daigle, G., Kadel, S., Perreault, V., Pellerin, G., Thibodeau, J., & Bazinet, L. (2020). Predictive models for determination of peptide fouling based on the physicochemical characteristics of filtration membranes. Separation and Purification Technology, 240 , 116602. https://doi.org/10.1016/j.seppur.2020.116602 .
Picot, L., Ravallec, R., Fouchereau-Péron, M., Vandanjon, L., Jaouen, P., Chaplain-Derouiniot, M., Guérard, F., Chabeaud, A., LeGal, Y., Alvarez, O. M., Bergé, J. P., Piot, J. M., Batista, I., Pires, C., Thorkelsson, G., Delannoy, C., Jakobsen, G., Johansson, I., & Bourseau, P. (2010). Impact of ultrafiltration and nanofiltration of an industrial fish protein hydrolysate on its bioactive properties. Journal of the Science of Food and Agriculture, 90 (11), 1819–1826. https://doi.org/10.1002/jsfa.4020 .
Poulin, J.-F., Amiot, J., & Bazinet, L. (2006a). Simultaneous separation of acid and basic bioactive peptides by electrodialysis with ultrafiltration membrane. Journal of Biotechnology, 123 (3), 314–328. https://doi.org/10.1016/j.jbiotec.2005.11.016 .
Poulin, J.-F., Araya-Farias, M., Amiot, J., & Bazinet, L. (2006b). Separation of bioactive peptides by electrodialysis with ultrafiltration membrane. Desalination, 200 (1–3), 620. https://doi.org/10.1016/J.DESAL.2006.03.485 .
Pouliot, Y. (2008). Membrane processes in dairy technology—From a simple idea to worldwide panacea. International Dairy Journal, 18 (7), 735–740. https://doi.org/10.1016/j.idairyj.2008.03.005 .
Pruksasri, S., Nguyen, T.-H., Haltrich, D., & Novalin, S. (2015). Fractionation of a galacto-oligosaccharides solution at low and high temperature using nanofiltration. Separation and Purification Technology, 151 , 124–130. https://doi.org/10.1016/j.seppur.2015.07.015 .
Article CAS PubMed PubMed Central Google Scholar
Rektor, A., & Vatai, G. (2004). Membrane filtration of mozzarella whey. Desalination, 162 , 279–286. https://doi.org/10.1016/S0011-9164(04)00052-9 .
Rios, G. M., Rakotoarisoa, H., & Tarodo de la Fuente, B. (1988). Basic transport mechanisms of ultrafiltration in the presence of an electric field. Journal of Membrane Science, 38 (2), 147–159. https://doi.org/10.1016/S0376-7388(00)80876-5 .
Rizki, Z., Janssen, A. E. M., Boom, R. M., & van der Padt, A. (2019). Oligosaccharides fractionation cascades with 3 outlet streams. Separation and Purification Technology, 221 , 183–194. https://doi.org/10.1016/j.seppur.2019.03.086 .
Rizki, Z., Janssen, A. E. M., Claassen, G. D. H., Boom, R. M., & van der Padt, A. (2020b). Multi-criteria design of membrane cascades: Selection of configurations and process parameters. Separation and Purification Technology, 237 , 116349. https://doi.org/10.1016/j.seppur.2019.116349 .
Rizki, Z., Suryawirawan, E., Janssen, A. E. M., van der Padt, A., & Boom, R. M. (2020a). Modelling temperature effects in a membrane cascade system for oligosaccharides. Journal of Membrane Science, 610 , 118292. https://doi.org/10.1016/j.memsci.2020.118292 .
Robinson, C. W., Siegel, M. H., Condemine, A., Fee, C., Fahidy, T. Z., & Glick, B. R. (1993). Pulsed-electric-field crossflow ultrafiltration of bovine serum albumin. Journal of Membrane Science, 80 (1), 209–220. https://doi.org/10.1016/0376-7388(93)85145-M .
Robinson, S., Abdullah, S. Z., Bérubé, P., & Le-Clech, P. (2016). Ageing of membranes for water treatment: Linking changes to performance. Journal of Membrane Science, 503 , 177–187. https://doi.org/10.1016/J.MEMSCI.2015.12.033 .
Roblet, C., Amiot, J., Lavigne, C., Marette, A., Lessard, M., Jean, J., Ramassamy, C., Moresoli, C., & Bazinet, L. (2012). Screening of in vitro bioactivities of a soy protein hydrolysate separated by hollow fiber and spiral-wound ultrafiltration membranes. Food Research International, 46 (1), 237–249. https://doi.org/10.1016/j.foodres.2011.11.014 .
Roblet, C., Doyen, A., Amiot, J., & Bazinet, L. (2013). Impact of pH on ultrafiltration membrane selectivity during electrodialysis with ultrafiltration membrane (EDUF) purification of soy peptides from a complex matrix. Journal of Membrane Science, 435 , 207–217. https://doi.org/10.1016/J.MEMSCI.2013.01.045 .
Rohani, M. M., & Zydney, A. L. (2010). Role of electrostatic interactions during protein ultrafiltration. Advances in Colloid and Interface Science, 160 (1–2), 40–48 http://www.ncbi.nlm.nih.gov/pubmed/20688310 . Accessed 20 March 2018.
Rosenberg, E., Hepbildikler, S., Kuhne, W., & Winter, G. (2009). Ultrafiltration concentration of monoclonal antibody solutions: Development of an optimized method minimizing aggregation. Journal of Membrane Science, 342 (1–2), 50–59. https://doi.org/10.1016/J.MEMSCI.2009.06.028 .
Rossignol, N., Vandanjon, L., Jaouen, P., & Quéméneur, F. (1999). Membrane technology for the continuous separation microalgae/culture medium: Compared performances of cross-flow microfiltration and ultrafiltration. Aquacultural Engineering, 20 (3), 191–208. https://doi.org/10.1016/S0144-8609(99)00018-7 .
Article Google Scholar
Sarapulova, V., Nevakshenova, E., Nebavskaya, X., Kozmai, A., Aleshkina, D., Pourcelly, G., Nikonenko, V., & Pismenskaya, N. (2018). Characterization of bulk and surface properties of anion-exchange membranes in initial stages of fouling by red wine. Journal of Membrane Science, 559 , 170–182. https://doi.org/10.1016/j.memsci.2018.04.047 .
Shethji, J. K., & Ritchie, S. M. C. (2015). Microfiltration membranes functionalized with multiple styrenic homopolymer and block copolymer grafts. Journal of Applied Polymer Science, 132 (36), 42501. https://doi.org/10.1002/app.42501 .
Shevate, R., Kumar, M., Karunakaran, M., Canlas, C., & Peinemann, K. V. (2018). Surprising transformation of a block copolymer into a high performance polystyrene ultrafiltration membrane with a hierarchically organized pore structure. Journal of Materials Chemistry A, 6 (10), 4337–4345. https://doi.org/10.1039/c7ta09777h .
Song, L. (1998). Flux decline in crossflow microfiltration and ultrafiltration: Mechanisms and modeling of membrane fouling. Journal of Membrane Science, 139 (2), 183–200. https://doi.org/10.1016/S0376-7388(97)00263-9 .
Sun, H., Qi, D., Xu, J., Juan, S., & Zhe, C. (2011). Fractionation of polysaccharides from rapeseed by ultrafiltration: Effect of molecular pore size and operation conditions on the membrane performance. Separation and Purification Technology, 80 (3), 670–676. https://doi.org/10.1016/J.SEPPUR.2011.06.038 .
Suwal, S., Doyen, A., & Bazinet, L. (2015). Characterization of protein, peptide and amino acid fouling on ion-exchange and filtration membranes: Review of current and recently developed methods. Journal of Membrane Science. Elsevier., 496 , 267–283. https://doi.org/10.1016/j.memsci.2015.08.056 .
Suwal S, Li J, Engelberth AS, Huang J-Y (2018) Application of electro-membrane separation for recovery of acetic acid in lignocellulosic bioethanol production. Food and Bioproducts Processing 109:41–51
Suwal, S., Roblet, C., Amiot, J., Doyen, A., Beaulieu, L., Legault, J., & Bazinet, L. (2014). Recovery of valuable peptides from marine protein hydrolysate by electrodialysis with ultrafiltration membrane: Impact of ionic strength. Food Research International, 65 , 407–415. https://doi.org/10.1016/J.FOODRES.2014.06.031 .
Suwarno, S. R., Huang, W., Chew, Y. M. J., Tan, S. H. H., Trisno, A. E., & Zhou, Y. (2018). On-line biofilm strength detection in cross-flow membrane filtration systems. Biofouling, 34 (2), 123–131. https://doi.org/10.1080/08927014.2017.1409892 .
Article PubMed Google Scholar
Tadimeti, J. G. D., Kurian, V., Chandra, A., & Chattopadhyay, S. (2016). Corrugated membrane surfaces for effective ion transport in electrodialysis. Journal of Membrane Science, 499 , 418–428. https://doi.org/10.1016/J.MEMSCI.2015.11.001 .
Teng, M.-Y., Lin, S.-H., Wu, C.-Y., & Juang, R.-S. (2006). Factors affecting selective rejection of proteins within a binary mixture during cross-flow ultrafiltration. Journal of Membrane Science, 281 (1–2), 103–110. https://doi.org/10.1016/J.MEMSCI.2006.03.019 .
Valiño, V., San Román, M. F., Ibañez, R., & Ortiz, I. (2014). Improved separation of bovine serum albumin and lactoferrin mixtures using charged ultrafiltration membranes. Separation and Purification Technology, 125 , 163–169. https://doi.org/10.1016/J.SEPPUR.2014.01.023 .
Vrouwenvelder, J. S., Hinrichs, C., van der Meer, W. G. J., van Loosdrecht, M. C. M., & Kruithof, J. C. (2009). Pressure drop increase by biofilm accumulation in spiral wound RO and NF membrane systems: Role of substrate concentration, flow velocity, substrate load and flow direction. Biofouling, 25 (6), 543–555. https://doi.org/10.1080/08927010902972225 .
Wang, F., He, M., Gao, K., Su, Y., Zhang, R., Liu, Y., Shen, J., Jiang, Z., & Kasher, R. (2019). Constructing membrane surface with synergistic passive antifouling and active antibacterial strategies through organic-inorganic composite modifier. Journal of Membrane Science, 576 , 150–160. https://doi.org/10.1016/j.memsci.2019.01.047 .
Wang, M., Yuan, J., Huang, X., Cai, X., Li, L., & Shen, J. (2013). Grafting of carboxybetaine brush onto cellulose membranes via surface-initiated ARGET-ATRP for improving blood compatibility. Colloids and Surfaces B: Biointerfaces, 103 , 52–58. https://doi.org/10.1016/J.COLSURFB.2012.10.025 .
Wijmans, J. G., Nakao, S., & Smolders, C. A. (1984). Flux limitation in ultrafiltration: Osmotic pressure model and gel layer model. Journal of Membrane Science, 20 (2), 115–124. https://doi.org/10.1016/S0376-7388(00)81327-7 .
Xie, J.-H., Shen, M.-Y., Nie, S.-P., Zhao, Q., Li, C., & Xie, M.-Y. (2014). Separation of water-soluble polysaccharides from Cyclocarya paliurus by ultrafiltration process. Carbohydrate Polymers, 101 , 479–483. https://doi.org/10.1016/J.CARBPOL.2013.09.075 .
Yazdanshenas, M., Tabatabaee-Nezhad, S. A. R., Soltanieh, M., Roostaazad, R., & Khoshfetrat, A. B. (2010). Contribution of fouling and gel polarization during ultrafiltration of raw apple juice at industrial scale. Desalination, 258 (1–3), 194–200. https://doi.org/10.1016/j.desal.2010.03.014 .
Yogarathinam, L. T., Gangasalam, A., Ismail, A. F., Arumugam, S., & Narayanan, A. (2018). Concentration of whey protein from cheese whey effluent using ultrafiltration by combination of hydrophilic metal oxides and hydrophobic polymer. Journal of Chemical Technology and Biotechnology, 93 (9), 2576–2591. https://doi.org/10.1002/jctb.5611 .
Zydney, A. L. (1997). Stagnant film model for concentration polarization in membrane systems. Journal of Membrane Science, 130 (1–2), 275–281. https://doi.org/10.1016/S0376-7388(97)00006-9 .
Zydney, A. L. (2016). Continuous downstream processing for high value biological products: A review. Biotechnology and Bioengineering, 113 (3), 465–475. https://doi.org/10.1002/bit.25695 .
Download references
Acknowledgements
The authors thank Jessica Nickerson (Department of Chemistry, Dalhousie University) for creating the artwork presented in the graphical abstract and for providing helpful editorial suggestions.
SRCKR received funding through the Izaak Walton Killam Predoctoral Scholarship. This work was supported by the National Research Council of Canada (Grant Number 05145, 2017).

Author information
Authors and affiliations.
Department of Chemistry, Dalhousie University, 6274 Coburg Road, Halifax, Nova Scotia, B3H 4R2, Canada
Subin R. C. K. Rajendran & Alan A. Doucette
Verschuren Centre for Sustainability in Energy and the Environment, 1250 Grand Lake Road, Sydney, Nova Scotia, B1P 6L2, Canada
Subin R. C. K. Rajendran & Beth Mason
You can also search for this author in PubMed Google Scholar
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Correspondence to Alan A. Doucette .
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Rajendran, S.R.C.K., Mason, B. & Doucette, A.A. Review of Membrane Separation Models and Technologies: Processing Complex Food-Based Biomolecular Fractions. Food Bioprocess Technol 14 , 415–428 (2021). https://doi.org/10.1007/s11947-020-02559-x
Download citation
Received : 21 May 2020
Accepted : 17 November 2020
Published : 02 January 2021
Issue Date : March 2021
DOI : https://doi.org/10.1007/s11947-020-02559-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Membrane filtration
- Downstream processing
- Electrodialysis
- Ultrafiltration
- Bioactive molecules
- Find a journal
- Publish with us
- Track your research

Improving Advanced Polymer-Based Mixed Matrix Membrane Performance for Gas Separation
Campus location, principal supervisor, additional supervisor 1, additional supervisor 2, year of award, department, school or centre, additional institution or organisation, degree type, usage metrics.

- Separation technologies
- Home
- Research Collections
- Dissertations and Theses (Ph.D. and Master's)
Smart Methodologies for Efficient Separation of Liquid Mixtures.
Collections
Remediation of harmful language.
The University of Michigan Library aims to describe library materials in a way that respects the people and communities who create, use, and are represented in our collections. Report harmful or offensive language in catalog records, finding aids, or elsewhere in our collections anonymously through our metadata feedback form . More information at Remediation of Harmful Language .
Accessibility
If you are unable to use this file in its current format, please select the Contact Us link and we can modify it to make it more accessible to you.
- Architecture and Design
- Asian and Pacific Studies
- Business and Economics
- Classical and Ancient Near Eastern Studies
- Computer Sciences
- Cultural Studies
- Engineering
- General Interest
- Geosciences
- Industrial Chemistry
- Islamic and Middle Eastern Studies
- Jewish Studies
- Library and Information Science, Book Studies
- Life Sciences
- Linguistics and Semiotics
- Literary Studies
- Materials Sciences
- Mathematics
- Social Sciences
- Sports and Recreation
- Theology and Religion
- Publish your article
- The role of authors
- Promoting your article
- Abstracting & indexing
- Publishing Ethics
- Why publish with De Gruyter
- How to publish with De Gruyter
- Our book series
- Our subject areas
- Your digital product at De Gruyter
- Contribute to our reference works
- Product information
- Tools & resources
- Product Information
- Promotional Materials
- Orders and Inquiries
- FAQ for Library Suppliers and Book Sellers
- Repository Policy
- Free access policy
- Open Access agreements
- Database portals
- For Authors
- Customer service
- People + Culture
- Journal Management
- How to join us
- Working at De Gruyter
- Mission & Vision
- De Gruyter Foundation
- De Gruyter Ebound
- Our Responsibility
- Partner publishers

Your purchase has been completed. Your documents are now available to view.
Recent progress and challenges in membrane-based O 2 /N 2 separation
Nurul F. Himma received her bachelor’s degree in chemical engineering from Institut Teknologi Sepuluh Nopember, Indonesia, in 2013. In 2015, she completed her master’s degree from Institut Teknologi Bandung, Indonesia, under the supervision of Professor Wenten, and then worked as a research asisstant in Prof. Wenten’s laboratory. She is currently a lecturer in the Department of Chemical Engineering, Universitas Brawijaya, Indonesia. Her research interests include wastewater treatment and membrane development for environmental protection.
Anita K. Wardani graduated with bachelor’s and master’s degrees in chemical engineering from Institut Teknologi Bandung in 2013 and 2016, respectively. She is currently a PhD student in chemical engineering, Institut Teknologi Bandung, under supervision of Prof. I Gede Wenten. Her research interests focus on modification of polypropylene for air separation, water treatment, and other filtration processes.
Nicholaus Prasetya completed his bachelor’s degree in chemical engineering at Institut Teknologi Bandung (ITB). After working as a research assistant (at Research Center for Nanosciences and Nanotechnology, ITB) under Prof. Wenten’s supervision, he continued his master’s degree at Imperial College London as an awardee of Indonesia Endowment Fund for Education. He is currently a PhD student under the supervision of Dr. Bradley Ladewig at Imperial College London. His research is focused on development of light-responsive metal-organic framework (MOF) and membrane for gas separation.
Putu T.P. Aryanti received her PhD in chemical engineering from Institut Teknologi Bandung (Indonesia) in 2016 under the supervision of Prof. Wenten. Her master’s degree was received in 2002 from Institut Teknologi Sepuluh Nopember (ITS), Indonesia, and her bachelor’s degree from Institut Teknologi Nasional Malang (ITN Malang), Indonesia, in 1997. She joined the Chemical Engineering Department at Universitas Jenderal Achmad Yani (UNJANI), Indonesia, in 2016, and her current research interests include membrane preparation and modification.
I Gede Wenten received his bachelor in chemical engineering from Institut Teknologi Bandung (ITB), Indonesia, and MSc and PhD degrees from DTU Denmark. He is a professor of chemical engineering and a member of Research Center for Nanosciences and Nanotechnology, ITB. I G. Wenten has long time experience in membrane technology at both industrial and academic fields with a career spanning more than 20 years. He received several awards such as Suttle Award (1994), Toray Science and Technology Award (1996), ASEAN Outstanding Engineering Award (2010), and other national awards from academic and professional foundations. He is a founder of GDP Filter Indonesia – a membrane manufacturing company and also a founder of ASEAN Association on Membrane (Membrane Science and Technology conference).
Compared with current conventional technologies, oxygen/nitrogen (O 2 /N 2 ) separation using membrane offers numerous advantages, especially in terms of energy consumption, footprint, and capital cost. However, low product purity still becomes the major challenge for commercialization of membrane-based technologies. Therefore, numerous studies on membrane development have been conducted to improve both membrane properties and separation performance. Various materials have been developed to obtain membranes with high O 2 permeability and high O 2 /N 2 selectivity, including polymer, inorganic, and polymer-inorganic composite materials. The results showed that most of the polymer membranes are suitable for production of low to moderate purity O 2 and for production of high-purity N 2 . Meanwhile, perovskite membrane can be used to produce a high-purity oxygen. Furthermore, the developments of O 2 /N 2 separation using membrane broaden the applications of oxygen enrichment for oxy-combustion, gasification, desulfurization, and intensification of air oxidation reactions, while nitrogen enrichment is also important for manufacturing pressure-sensitive adhesive and storing and handling free-radical polymerization monomers.
About the authors

Abraham B, Asbury J, Lynch E, Teotia A. Coal-oxygen process provides CO 2 /for enhanced recovery. Oil Gas J 1982; 80: 11. Search in Google Scholar
Acharya M. Engineering design and theoretical analysis of nanoporous carbon membranes for gas separation. PhD Thesis, Univ. of Delaware, Newark, 1999. Search in Google Scholar
Adams R, Carson C, Ward J, Tannenbaum R, Koros W. Metal organic framework mixed matrix membranes for gas separations. Microporous Mesoporous Mater 2010; 131: 13–20. 10.1016/j.micromeso.2009.11.035 Search in Google Scholar
Ahamparam S, Harrison S. Oxygen enrichment in desulphurisation. Pet Technol Q 2012; 17: 51–53. Search in Google Scholar
Air Products. Air separation technology – ion transport membrane (ITM). Allentown, PA: Air Products and Chemicals, Inc., 2008. Search in Google Scholar
Air Products. Advanced oxygen for energy intensive markets: ion transport membrane (ITM) oxyge. Allentown, PA: Air Products and Chemicals, Inc., 2013. Search in Google Scholar
Alsayed MFM. Oxygen enriched combustion of high emission fuels. ME thesis, An-Najah National University, 2008. Search in Google Scholar
Ariono D, Purwasasmita M, Wenten IG. Brine effluents: characteristics, environmental impacts, and their handling. J Eng Technol Sci 2016; 48: 367–387. 10.5614/j.eng.technol.sci.2016.48.4.1 Search in Google Scholar
Ariono D, Khoiruddin K, Subagjo S, Wenten IG. Heterogeneous structure and its effect on properties and electrochemical behavior of ion-exchange membrane. Mater Res Express 2017; 4: 024006. 10.1088/2053-1591/aa5cd4 Search in Google Scholar
Arnold M, Xu Q, Tichelaar FD, Feldhoff A. Local charge disproportion in a high-performance perovskite. Chem Mater 2009; 21: 635–640. 10.1021/cm802779f Search in Google Scholar
Aroon MA, Ismail AF, Matsuura T, Montazer-Rahmati MM. Performance studies of mixed matrix membranes for gas separation: a review. Sep Purif Technol 2010; 75: 229–242. 10.1016/j.seppur.2010.08.023 Search in Google Scholar
Aryanti P, Yustiana R, Purnama R, Wenten IG. Performance and characterization of PEG400 modified PVC ultrafiltration membrane. Membr Water Treat 2015; 6: 379–392. 10.12989/mwt.2015.6.5.379 Search in Google Scholar
Aryanti P, Joscarita SR, Wardani AK, Subagjo S, Ariono D, Wenten IG. The influence of PEG400 and acetone on polysulfone membrane morphology and fouling behaviour. J Eng Technol Sci 2016; 48: 135–149. 10.5614/j.eng.technol.sci.2016.48.2.1 Search in Google Scholar
Aryanti P, Sianipar M, Zunita M, Wenten IG. Modified membrane with antibacterial properties. Membr Water Treat 2017; 8: 463–481. Search in Google Scholar
Athayde DD, Souza DF, Silva AM, Vasconcelos D, Nunes EH, da Costa JCD, Vasconcelos WL. Review of perovskite ceramic synthesis and membrane preparation methods. Ceram Int 2016; 42: 6555–6571. 10.1016/j.ceramint.2016.01.130 Search in Google Scholar
Baker RW. Future directions of membrane gas separation technology. Ind Eng Chem Res 2002; 41: 1393–1411. 10.1021/ie0108088 Search in Google Scholar
Baker RW. Membrane technology and applications, West Sussex, England: John Wiley & Sons Ltd, 2004. 10.1002/0470020393 Search in Google Scholar
Baker RW, Lokhandwala K. Natural gas processing with membranes: an overview. Ind Eng Chem Res 2008; 47: 2109–2121. 10.1021/ie071083w Search in Google Scholar
Baker RW, Low BT. Gas separation membrane materials: a perspective. Macromolecules 2014; 47: 6999–7013. 10.1021/ma501488s Search in Google Scholar
Barbosa-Coutinho E, Salim VM, Borges CP. Preparation of carbon hollow fiber membranes by pyrolysis of polyetherimide. Carbon 2003; 41: 1707–1714. 10.1016/S0008-6223(03)00129-5 Search in Google Scholar
Belaissaoui B, Le Moullec Y, Hagi H, Favre E. Energy efficiency of oxygen enriched air production technologies: cryogeny vs membranes. Sep Purif Technol 2014; 125: 142–150. 10.1016/j.seppur.2014.01.043 Search in Google Scholar
Bernardo P, Clarizia G. 30 years of membrane technology for gas separation. Chem Eng Trans 2013; 32: 1999–2004. Search in Google Scholar
Bingue JP, Saveliev AV, Kennedy LA. Optimization of hydrogen production by filtration combustion of methane by oxygen enrichment and depletion. Int J Hydrogen Energy 2004; 29: 1365–1370. 10.1016/j.ijhydene.2004.01.002 Search in Google Scholar
Borisov S, Khotimsky VS, Rebrov AI, Rykov SV, Slovetsky DI, Pashunin YM. Plasma fluorination of organosilicon polymeric films for gas separation applications. J Membr Sci 1997; 125: 319–329. 10.1016/S0376-7388(96)00254-2 Search in Google Scholar
Brisotto M, Cernuschi F, Drago F, Lenardi C, Rosa P, Meneghini C, Merlini M, Rinaldi C. High temperature stability of Ba 0.5 Sr 0.5 Co 0.8 Fe 0.2 O 3–δ and La 0.6 Sr 0.4 Co 1−y Fe y O 3–δ oxygen separation perovskite membranes. J Eur Ceram Soc 2016; 36: 1679–1690. 10.1016/j.jeurceramsoc.2016.01.029 Search in Google Scholar
Budd PM, Msayib KJ, Tattershall CE, Ghanem BS, Reynolds KJ, McKeown NB, Fritsch D. Gas separation membranes from polymers of intrinsic microporosity. J Membr Sci 2005; 251: 263–269. 10.1016/j.memsci.2005.01.009 Search in Google Scholar
Budd PM, McKeown NB, Ghanem BS, Msayib KJ, Fritsch D, Starannikova L, Belov N, Sanfirova O, Yampolskii Y, Shantarovich V. Gas permeation parameters and other physicochemical properties of a polymer of intrinsic microporosity: polybenzodioxane PIM-1. J Membr Sci 2008; 325: 851–860. 10.1016/j.memsci.2008.09.010 Search in Google Scholar
Buhre BJP, Elliott LK, Sheng CD, Gupta RP, Wall TF. Oxy-fuel combustion technology for coal-fired power generation. Prog Energy Combust Sci 2005; 31: 283–307. 10.1016/j.pecs.2005.07.001 Search in Google Scholar
Buonomenna MG, Yave W, Golemme G. Some approaches for high performance polymer based membranes for gas separation: block copolymers, carbon molecular sieves and mixed matrix membranes. RSC Advances 2012; 2: 10745–10773. 10.1039/c2ra20748f Search in Google Scholar
Bushell AF, Attfield MP, Mason CR, Budd PM, Yampolskii Y, Starannikova L, Rebrov A, Bazzarelli F, Bernardo P, Jansen JC. Gas permeation parameters of mixed matrix membranes based on the polymer of intrinsic microporosity PIM-1 and the zeolitic imidazolate framework ZIF-8. J Membr Sci 2013; 427: 48–62. 10.1016/j.memsci.2012.09.035 Search in Google Scholar
Camacho-Zuñiga C, Ruiz-Treviño FA, Hernández-López S, Zolotukhin MG, Maurer FHJ, González-Montiel A. Aromatic polysulfone copolymers for gas separation membrane applications. J Membr Sci 2009; 340: 221–226. 10.1016/j.memsci.2009.05.033 Search in Google Scholar
Centeno TA, Fuertes AB. Carbon molecular sieve gas separation membranes based on poly (vinylidene chloride-co-vinyl chloride). Carbon 2000; 38: 1067–1073. 10.1016/S0008-6223(99)00214-6 Search in Google Scholar
Chauhan R, Pandey P. Preparation of gas separation membranes and their evaluation. Def Sci J 2004; 54: 335. 10.14429/dsj.54.2047 Search in Google Scholar
Chen S-H, Chuang W-H, Wang AA, Ruaan R-C, Lai J-Y. Oxygen/nitrogen separation by plasma chlorinated polybutadiene/polycarbonate composite membrane. J Membr Sci 1997; 124: 273–281. 10.1016/S0376-7388(96)00246-3 Search in Google Scholar
Chen S-H, Yu K-C, Houng S-L, Lai J-Y. Gas transport properties of HTPB based polyurethane/cosalen membrane. J Membr Sci 2000; 173: 99–106. 10.1016/S0376-7388(00)00357-4 Search in Google Scholar
Chen J-T, Shih C-C, Fu Y-J, Huang S-H, Hu C-C, Lee K-R, Lai J-Y. Zeolite-filled porous mixed matrix membranes for air separation. Ind Eng Chem Res 2014; 53: 2781–2789. 10.1021/ie403833u Search in Google Scholar
Chong K, Lai S, Thiam H, Teoh H, Heng S. Recent progress of oxygen/nitrogen separation using membrane technology. J Eng Sci Technol 2016; 11: 1016–1030. Search in Google Scholar
Chung T-S, Kafchinski ER, Foley P. Development of asymmetric hollow fibers from polyimides for air separation. J Membr Sci 1992; 75: 181–195. 10.1016/0376-7388(92)80016-D Search in Google Scholar
Chung T-S, Kafchinski ER, Vora R. Development of a defect-free 6FDA-durene asymmetric hollow fiber and its composite hollow fibers. J Membr Sci 1994; 88: 21–36. 10.1016/0376-7388(93)E0163-E Search in Google Scholar
Chung TS, Teoh SK, Hu X. Formation of ultrathin high-performance polyethersulfone hollow-fiber membranes. J Membr Sci 1997; 133: 161–175. 10.1016/S0376-7388(97)00101-4 Search in Google Scholar
Clark GA, Rowan M. A feasibility study of using oxygen enrichment for fuel cell air independent propulsion. Melbourne: DSTO Aeronautical and Maritime Research Laboratory, 1996. Search in Google Scholar
Clausi DT, Koros WJ. Formation of defect-free polyimide hollow fiber membranes for gas separations. J Membr Sci 2000; 167: 79–89. 10.1016/S0376-7388(99)00276-8 Search in Google Scholar
Comesaña-Gándara B, Hernández A, de la Campa JG, de Abajo J, Lozano AE, Lee YM. Thermally rearranged polybenzoxazoles and poly(benzoxazole-co-imide)s from ortho-hydroxyamine monomers for high performance gas separation membranes. J Membr Sci 2015; 493: 329–339. 10.1016/j.memsci.2015.05.061 Search in Google Scholar
Crider LB, O’Mara MM, Bowles RL. A model for the diffusion of vinyl chloride monomer from PVC under various conditions of storage. J Vinyl Technol 1979; 1: 168–171. 10.1002/vnl.730010314 Search in Google Scholar
Czech Z. Development of solvent-free pressure-sensitive adhesive acrylics. Int J Adhes Adhes 2004; 24: 119–125. 10.1016/j.ijadhadh.2003.07.001 Search in Google Scholar
Czech Z, Wesolowska M. Development of solvent-free acrylic pressure-sensitive adhesives. Eur Polym J 2007; 43: 3604–3612. 10.1016/j.eurpolymj.2007.05.003 Search in Google Scholar
David L, Ismail A. Influence of the thermastabilization process and soak time during pyrolysis process on the polyacrylonitrile carbon membranes for O 2 /N 2 separation. J Membr Sci 2003; 213: 285–291. 10.1016/S0376-7388(02)00513-6 Search in Google Scholar
de Larramendi IR, Ortiz-Vitoriano N, Dzul-Bautista IB, Rojo T. Designing perovskite oxides for solid oxide fuel cells. In: Pan L, editor. Perovskite materials-synthesis, characterisation, properties, and applications. Rijeka: InTech, 2016: 589–617. Search in Google Scholar
Ding X, Cao Y, Zhao H, Wang L, Yuan Q. Fabrication of high performance Matrimid/polysulfone dual-layer hollow fiber membranes for O 2 /N 2 separation. J Membr Sci 2008; 323: 352–361. 10.1016/j.memsci.2008.06.042 Search in Google Scholar
Domínguez-Domínguez S, Berenguer-Murcia A, Morallón E, Linares-Solano A, Cazorla-Amorós D. Zeolite LTA/carbon membranes for air separation. Microporous Mesoporous Mater 2008; 115: 51–60. 10.1016/j.micromeso.2007.11.049 Search in Google Scholar
Dong G, Li H, Chen V. Challenges and opportunities for mixed-matrix membranes for gas separation. J Mater Chem A 2013; 1: 4610–4630. 10.1039/c3ta00927k Search in Google Scholar
Dong G, Woo KT, Kim J, Kim JS, Lee YM. Simulation and feasibility study of using thermally rearranged polymeric hollow fiber membranes for various industrial gas separation applications. J Membr Sci 2015; 496: 229–241. 10.1016/j.memsci.2015.08.059 Search in Google Scholar
Drioli E, Curcio E. Membrane engineering for process intensification: a perspective. J Chem Technol Biotechnol 2007; 82: 223–227. 10.1002/jctb.1650 Search in Google Scholar
Drioli E, Stankiewicz AI, Macedonio F. Membrane engineering in process intensification – an overview. J Membr Sci 2011; 380: 1–8. 10.1016/j.memsci.2011.06.043 Search in Google Scholar
Du N, Robertson GP, Song J, Pinnau I, Thomas S, Guiver MD. Polymers of intrinsic microporosity containing trifluoromethyl and phenylsulfone groups as materials for membrane gas separation. Macromolecules 2008; 41: 9656–9662. 10.1021/ma801858d Search in Google Scholar
Duan C, Jie X, Liu D, Cao Y, Yuan Q. Post-treatment effect on gas separation property of mixed matrix membranes containing metal organic frameworks. J Membr Sci 2014; 466: 92–102. 10.1016/j.memsci.2014.04.024 Search in Google Scholar
Efimov K, Halfer T, Kuhn A, Heitjans P, Caro J, Feldhoff A. Novel cobalt-free oxygen-permeable perovskite-type membrane. Chem Mater 2010; 22: 1540–1544. 10.1021/cm902882s Search in Google Scholar
Ettouney HM, El-Dessouky HT, Abou Waar W. Separation characteristics of air by polysulfone hollow fiber membranes in series. J Membr Sci 1998; 148: 105–117. 10.1016/S0376-7388(98)00144-6 Search in Google Scholar
Fang W, Liang F, Cao Z, Steinbach F, Feldhoff A. A mixed ionic and electronic conducting dual-phase membrane with high oxygen permeability. Angew Chem Int Ed 2015a; 54: 4847–4850. 10.1002/anie.201411963 Search in Google Scholar PubMed
Fang W, Steinbach F, Chen C, Feldhoff A. An approach to enhance the CO 2 tolerance of fluorite-perovskite dual-phase oxygen-transporting membrane. Chem Mater 2015b; 27: 7820–7826. 10.1021/acs.chemmater.5b03823 Search in Google Scholar
Fatemi SM, Sepehrian H, Arabieh M. Simulation studies of the separation of Kr-85 radionuclide gas from nitrogen and oxygen across nanoporous graphene membranes in different pore configurations. The European Physical Journal Plus 2016; 131: 131. 10.1140/epjp/i2016-16131-6 Search in Google Scholar
Fatyeyeva K, Dahi A, Chappey C, Langevin D, Valleton J-M, Poncin-Epaillard F, Marais S. Effect of cold plasma treatment on surface properties and gas permeability of polyimide films. RSC Advances 2014; 4: 31036–31046. 10.1039/C4RA03741C Search in Google Scholar
Favre E, Bounaceur R, Roizard D. A hybrid process combining oxygen enriched air combustion and membrane separation for post-combustion carbon dioxide capture. Sep Purif Technol 2009; 68: 30–36. 10.1016/j.seppur.2009.04.003 Search in Google Scholar
Feng X, Ivory J, Rajan VSV. Air separation by integrally asymmetric hollow-fiber membranes. AICHE J 1999; 45: 2142–2152. 10.1002/aic.690451013 Search in Google Scholar
Fernández-Barquín A, Casado-Coterillo C, Valencia S, Irabien A. Mixed matrix membranes for O 2 /N 2 separation: the influence of temperature. Membranes 2016; 6: 28. 10.3390/membranes6020028 Search in Google Scholar
Freeman BD. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules 1999; 32: 375–380. 10.1021/ma9814548 Search in Google Scholar
Freeman BD, Pinnau I. Polymeric materials for gas separations. In: Freeman BD, Pinnau I, editors. Polymer membranes for gas and vapor separation. Washington, DC: American Chemical Society, 1999: 1–27. 10.1021/bk-1999-0733 Search in Google Scholar
Fritzsche AK, Murphy MK, Cruse CA, Malon RF, Kesting RE. Characterization of asymmetric hollow fibre membranes with graded-density skins. Gas Sep Purif 1989; 3: 106–116. 10.1016/0950-4214(89)80020-9 Search in Google Scholar
Fritzsche AK, Armbruster BL, Fraundorf PB, Pellegrin CJ. The structure of the effective separating layer of asymmetric hollow fiber membranes with graded density skins. J Appl Polym Sci 1990a; 39: 1915–1932. 10.1002/app.1990.070390908 Search in Google Scholar
Fritzsche AK, Cruse CA, Murphy MK, Kesting RE. Polyethersulfone and polyphenylsulfone hollow fiber trilayer membranes spun from Lewis acid:base complexes – structure determination by SEM, DSC, and oxygen plasma ablation. J Membr Sci 1990b; 54: 29–50. 10.1016/S0376-7388(00)82068-2 Search in Google Scholar
Fu Y-J, Qui H-z, Liao K-S, Lue SJ, Hu C-C, Lee K-R, Lai J-Y. Effect of UV-ozone treatment on poly(dimethylsiloxane) membranes: surface characterization and gas separation performance. Langmuir 2010; 26: 4392–4399. 10.1021/la903445x Search in Google Scholar PubMed
Fu S, Wenz GB, Sanders ES, Kulkarni SS, Qiu W, Ma C, Koros WJ. Effects of pyrolysis conditions on gas separation properties of 6FDA/DETDA:DABA(3:2) derived carbon molecular sieve membranes. J Membr Sci 2016; 520: 699–711. 10.1016/j.memsci.2016.08.013 Search in Google Scholar
Fuertes AB. Adsorption-selective carbon membrane for gas separation. J Membr Sci 2000; 177: 9–16. 10.1016/S0376-7388(00)00458-0 Search in Google Scholar
Fuertes A, Centeno T. Carbon molecular sieve membranes from polyetherimide. Microporous Mesoporous Mater 1998; 26: 23–26. 10.1016/S1387-1811(98)00204-2 Search in Google Scholar
Gao D, Zhao J, Zhou W, Ran R, Shao Z. Influence of high-energy ball milling of the starting powder on the sintering; microstructure and oxygen permeability of Ba 0.5 Sr 0.5 Co 0.5 Fe 0.5 O 3−δ membranes. J Membr Sci 2011; 366: 203–211. 10.1016/j.memsci.2010.10.001 Search in Google Scholar
Geffroy PM, Fouletier J, Richet N, Chartier T. Rational selection of MIEC materials in energy production processes. Chem Eng Sci 2013; 87: 408–433. 10.1016/j.ces.2012.10.027 Search in Google Scholar
George SC, Ninan KN, Thomas S. Permeation of nitrogen and oxygen gases through styrene-butadiene rubber, natural rubber and styrene-butadiene rubber/natural rubber blend membranes. Eur Polym J 2001; 37: 183–191. 10.1016/S0014-3057(00)00083-5 Search in Google Scholar
Ghanem BS, McKeown NB, Budd PM, Selbie JD, Fritsch D. High-performance membranes from polyimides with intrinsic microporosity. Adv Mater 2008; 20: 2766–2771. 10.1002/adma.200702400 Search in Google Scholar
Ghosal K, Freeman BD. Gas separation using polymer membranes: an overview. Polym Adv Technol 1994; 5: 673–697. 10.1002/pat.1994.220051102 Search in Google Scholar
Gibson P, Schreuder-Gibson H, Rivin D. Transport properties of porous membranes based on electrospun nanofibers. Colloids Surf Physicochem Eng Aspects 2001; 187–188: 469–481. 10.1016/S0927-7757(01)00616-1 Search in Google Scholar
Goh PS, Ismail AF, Sanip SM, Ng BC, Aziz M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep Purif Technol 2011; 81: 243–264. 10.1016/j.seppur.2011.07.042 Search in Google Scholar
Gupta Y, Hellgardt K, Wakeman RJ. Enhanced permeability of polyaniline based nano-membranes for gas separation. J Membr Sci 2006; 282: 60–70. 10.1016/j.memsci.2006.05.014 Search in Google Scholar
Han D, Tan X, Yan Z, Li Q, Liu S. New morphological Ba 0.5 Sr 0.5 Co 0.8 Fe 0.2 O 3−α hollow fibre membranes with high oxygen permeation fluxes. Ceram Int 2013; 39: 431–437. 10.1016/j.ceramint.2012.06.044 Search in Google Scholar
Hasbullah H, Kumbharkar S, Ismail AF, Li K. Asymmetric hollow fibre membranes based on ring-substituted polyaniline and investigation towards its gas transport properties. J Membr Sci 2012; 397–398: 38–50. 10.1016/j.memsci.2012.01.007 Search in Google Scholar
Hashim SM, Mohamed AR, Bhatia S. Current status of ceramic-based membranes for oxygen separation from air. Adv Colloid Interface Sci 2010; 160: 88–100. 10.1016/j.cis.2010.07.007 Search in Google Scholar
Hassan MH, Douglas Way J, Thoen PM, Dillon AC. Single component and mixed gas transport in a silica hollow fiber membrane. J Membr Sci 1995; 104: 27–42. 10.1016/0376-7388(95)00009-2 Search in Google Scholar
He W, Liu J-j, Chen C-S, Ni M. Oxygen permeation modeling for Zr 0.84 Y 0.16 O 1.92 –La 0.8 Sr 0.2 Cr 0.5 Fe 0.5 O 3−δ asymmetric membrane made by phase-inversion. J Membr Sci 2015; 491: 90–98. 10.1016/j.memsci.2015.05.026 Search in Google Scholar
Himma NF, Wenten IG. Surface engineering of polymer membrane for air separation. AIP Conf Proc, AIP Publishing, 2016; 1840: 090005. Search in Google Scholar
Himma NF, Anisah S, Prasetya N, Wenten IG. Advances in preparation, modification, and application of polypropylene membrane. J Polym Eng 2016; 36: 329–362. 10.1515/polyeng-2015-0112 Search in Google Scholar
Himma NF, Wardani AK, Wenten IG. The effects of non-solvent on surface morphology and hydrophobicity of dip-coated polypropylene membrane. Mater Res Express 2017a; 4: 054001. 10.1088/2053-1591/aa6ee0 Search in Google Scholar
Himma NF, Wardani AK, Wenten IG. Preparation of superhydrophobic polypropylene membrane using dip-coating method: the effects of solution and process parameters. Polym Plast Technol Eng 2017b; 56: 184–194. 10.1080/03602559.2016.1185666 Search in Google Scholar
Hopkins J, Badyal JPS. CF4 plasma treatment of asymmetric polysulfone membranes. Langmuir 1996; 12: 3666–3670. 10.1021/la960038i Search in Google Scholar
Hosseini SS, Omidkhah MR, Zarringhalam Moghaddam A, Pirouzfar V, Krantz WB, Tan NR. Enhancing the properties and gas separation performance of PBI-polyimides blend carbon molecular sieve membranes via optimization of the pyrolysis process. Sep Purif Technol 2014; 122: 278–289. 10.1016/j.seppur.2013.11.021 Search in Google Scholar
Hosseini SS, Najari S, Kundu PK, Tan NR, Roodashti SM. Simulation and sensitivity analysis of transport in asymmetric hollow fiber membrane permeators for air separation. RSC Advances 2015; 5: 86359–86370. 10.1039/C5RA13943K Search in Google Scholar
Houston KS, Weinkauf DH, Stewart FF. Gas transport characteristics of plasma treated poly(dimethylsiloxane) and polyphosphazene membrane materials. J Membr Sci 2002; 205: 103–112. 10.1016/S0376-7388(02)00068-6 Search in Google Scholar
Hsu KK, Nataraj S, Thorogood RM, Puri PS. O 2 /N 2 Selectivity improvement for polytrimethylsilypropyne membranes by UV-irradiation and further enhancement by subambient temperature operation. J Membr Sci 1993; 79: 1–10. 10.1016/0376-7388(93)85013-M Search in Google Scholar
Hu C-C, Liu T-C, Lee K-R, Ruaan R-C, Lai J-Y. International Congress on Membranes and Membrane Processes. Zeolite-filled PMMA composite membranes: influence of coupling agent addition on gas separation properties. Desalination 2006; 193: 14–24. 10.1016/j.desal.2005.04.137 Search in Google Scholar
Hu J, Cai H, Ren H, Wei Y, Xu Z, Liu H, Hu Y. Mixed-matrix membrane hollow fibers of Cu 3 (BTC) 2 MOF and polyimide for gas separation and adsorption. Ind Eng Chem Res 2010; 49: 12605–12612. 10.1021/ie1014958 Search in Google Scholar
Huang A, Wang N, Caro J. Synthesis of multi-layer zeolite LTA membranes with enhanced gas separation performance by using 3-aminopropyltriethoxysilane as interlayer. Microporous Mesoporous Mater 2012; 164: 294–301. 10.1016/j.micromeso.2012.06.018 Search in Google Scholar
Huber F, Springer J, Muhler M. Plasma polymer membranes from hexafluoroethane/hydrogen mixtures for separation of oxygen and nitrogen. J Appl Polym Sci 1997; 63: 1517–1526. 10.1002/(SICI)1097-4628(19970321)63:12<1517::AID-APP2>3.0.CO;2-R Search in Google Scholar
Ilconich JB, Xu X, Coleman M, Simpson PJ. Impact of ion beam irradiation on microstructure and gas permeance of polysulfone asymmetric membranes. J Membr Sci 2003; 214: 143–156. 10.1016/S0376-7388(02)00543-4 Search in Google Scholar
Illing G, Hellgardt K, Schonert M, Wakeman RJ, Jungbauer A. Towards ultrathin polyaniline films for gas separation. J Membr Sci 2005; 253: 199–208. 10.1016/j.memsci.2004.12.031 Search in Google Scholar
Inagaki N, Ohkubo J. Plasma polymerization of hexafluoropropene/methane mixtures and composite membranes for gas separations. J Membr Sci 1986; 27: 63–75. 10.1016/S0376-7388(00)81382-4 Search in Google Scholar
Isfahani AP, Sadeghi M, Dehaghani AHS, Aravand MA. Enhancement of the gas separation properties of polyurethane membrane by epoxy nanoparticles. J Ind Eng Chem 2016; 44: 67–72. 10.1016/j.jiec.2016.08.012 Search in Google Scholar
Islam Q, Raja M, Basu R. La x Sr 1−x Co 0.35 Bi 0.2 Fe 0.45 O 3−δ (x=0.5 to 0.8): a new series of oxygen separation membrane. Int J Hydrogen Energy 2016; 41: 4682–4689. 10.1016/j.ijhydene.2016.01.021 Search in Google Scholar
Ismail AF, David LIB. A review on the latest development of carbon membranes for gas separation. J Membr Sci 2001; 193: 1–18. 10.1016/S0376-7388(01)00510-5 Search in Google Scholar
Ismail AF, Lai PY. Effects of phase inversion and rheological factors on formation of defect-free and ultrathin-skinned asymmetric polysulfone membranes for gas separation. Sep Purif Technol 2003; 33: 127–143. 10.1016/S1383-5866(02)00201-0 Search in Google Scholar
Ismail AF, Lai PY. Development of defect-free asymmetric polysulfone membranes for gas separation using response surface methodology. Sep Purif Technol 2004; 40: 191–207. 10.1016/j.seppur.2004.02.011 Search in Google Scholar
Ismail AF, Nasri NS. Effects of dope extrusion rate on the morphology and gas separation performance of asymmetric polysulfone hollow fiber membranes for 024 separation. Songklanakarin J Sci Technol 2002; 24: 833–842. Search in Google Scholar
Ismail AF, Yean LP. Review on the development of defect-free and ultrathin-skinned asymmetric membranes for gas separation through manipulation of phase inversion and rheological factors. J Appl Polym Sci 2003; 88: 442–451. 10.1002/app.11744 Search in Google Scholar
Ismail AF, Dunkin IR, Gallivan SL, Shilton SJ. Production of super selective polysulfone hollow fiber membranes for gas separation. Polymer 1999; 40: 6499–6506. 10.1016/S0032-3861(98)00862-3 Search in Google Scholar
Ismail AF, Ridzuan N, Rahman SA. Latest development on the membrane formation for gas separation. Songklanakarin J Sci Technol 2002; 24: 1025–1043. Search in Google Scholar
Ismail AF, Khulbe KC, Matsuura T. Gas separation membrane materials and structures. In: Gas separation membranes. Switzerland: Springer, Cham, 2015: 37–192. 10.1007/978-3-319-01095-3_3 Search in Google Scholar
Ivanova S, Lewis R. Producing nitrogen via pressure swing adsorption. Chem Eng Prog 2012; 108: 38–42. Search in Google Scholar
Iwa T, Kumazawa H, Bae SY. Gas permeabilities of NH 3 -plasma-treated polyethersulfone membranes. J Appl Polym Sci 2004; 94: 758–762. 10.1002/app.20961 Search in Google Scholar
Iyer P, Iyer G, Coleman M. Gas transport properties of polyimide-POSS nanocomposites. J Membr Sci 2010; 358: 26–32. 10.1016/j.memsci.2010.04.023 Search in Google Scholar
Jain IP, Agarwal G. Ion beam induced surface and interface engineering. Surf Sci Rep 2011; 66: 77–172. 10.1016/j.surfrep.2010.11.001 Search in Google Scholar
Jami’an WNR, Hasbullah H, Mohamed F, Wan Salleh WN, Ibrahim N, Ali RR. Biodegradable gas separation membrane preparation by manipulation of casting parameters. Chem Eng Tran 2015; 43: 1105–1110. Search in Google Scholar
Jansen J, Buonomenna M, Figoli A, Drioli E. Ultra-thin asymmetric gas separation membranes of modified PEEK prepared by the dry-wet phase inversion technique. Desalination 2006; 193: 58–65. 10.1016/j.desal.2005.09.024 Search in Google Scholar
Jeazet H, Staudt C, Janiak C. A method for increasing permeability in O 2 /N 2 separation with mixed-matrix membranes made of water-stable MIL-101 and polysulfone. Chem Commun (Camb) 2012; 48: 2140–2142. 10.1039/c2cc16628c Search in Google Scholar
Jiang X, Li S, Shao L. Pushing CO 2 -philic membrane performance to the limit by designing semi-interpenetrating networks (SIPN) for sustainable CO 2 separations. Energy Environ Sci 2017; 10: 1339–1344. 10.1039/C6EE03566C Search in Google Scholar
Jones CW, Koros WJ. Carbon molecular sieve gas separation membranes-I. Preparation and characterization based on polyimide precursors. Carbon 1994; 32: 1419–1425. 10.1016/0008-6223(94)90135-X Search in Google Scholar
Jones C, Gordeyev S, Shilton S. Poly (vinyl chloride)(PVC) hollow fibre membranes for gas separation. Polymer 2011; 52: 901–903. 10.1016/j.polymer.2011.01.013 Search in Google Scholar
Kawakami M, Yamashita Y, Iwamoto M, Kagawa S. Modification of gas permeabilities of polymer membranes by plasma coating. J Membr Sci 1984; 19: 249–258. 10.1016/S0376-7388(00)80228-8 Search in Google Scholar
Kesting RE, Fritzsche AK, Murphy MK, Cruse CA, Handermann AC, Malon RF, Moore MD. The second-generation polysulfone gas-separation membrane. I. The use of Lewis acid:base complexes as transient templates to increase free volume. J Appl Polym Sci 1990; 40: 1557–1574. 10.1002/app.1990.070400913 Search in Google Scholar
Kharton V, Kovalevsky A, Viskup A, Jurado J, Figueiredo F, Naumovich E, Frade J. Transport properties and thermal expansion of Sr 0.97 Ti 1−x Fe x O 3−δ (x=0.2–0.8). J Solid State Chem 2001; 156: 437–444. 10.1006/jssc.2000.9019 Search in Google Scholar
Kharton VV, Tsipis EV, Naumovich EN, Thursfield A, Patrakeev MV, Kolotygin VA, Waerenborgh JC, Metcalfe IS. Mixed conductivity, oxygen permeability and redox behavior of K 2 NiF 4 -type La 2 Ni 0.9 Fe 0.1 O 4+δ . J Solid State Chem 2008; 181: 1425–1433. 10.1016/j.jssc.2008.03.019 Search in Google Scholar
Khoiruddin K, Wenten IG. Investigation of electrochemical and morphological properties of mixed matrix polysulfone- silica anion exchange membrane. J Eng Technol Sci 2016; 48: 1–11. 10.5614/j.eng.technol.sci.2016.48.1.1 Search in Google Scholar
Khoiruddin K, Widiasa IN, Wenten IG. Removal of inorganic contaminants in sugar refining process using electrodeionization. J Food Eng 2014; 133: 40–45. 10.1016/j.jfoodeng.2014.02.015 Search in Google Scholar
Khoiruddin K, Ariono D, Subagjo, Wenten IG. Surface modification of ion-exchange membranes: methods, characteristics, and performance. J Appl Polym Sci 2017; 134: 45540. 10.1002/app.45540 Search in Google Scholar
Kim S, Lee YM. Rigid and microporous polymers for gas separation membranes. Prog Polym Sci 2015; 43: 1–32. 10.1016/j.progpolymsci.2014.10.005 Search in Google Scholar
Kim S, Chen L, Johnson JK, Marand E. Polysulfone and functionalized carbon nanotube mixed matrix membranes for gas separation: theory and experiment. J Membr Sci 2007; 294: 147–158. 10.1016/j.memsci.2007.02.028 Search in Google Scholar
Kim D-H, Im H-S, Kim M-S, Lee B-S, Lee B-S, Yoon S-W, Kim B-S, Park Y-I, Cheong S-I, Rhim J-W. Study on the gas permeation behaviors of surface fluorinated polysulfone membranes. Polymer Korea 2009; 33: 537–543. Search in Google Scholar
Koenig SP, Wang L, Pellegrino J, Bunch JS. Selective molecular sieving through porous graphene. Nat Nanotechnol 2012; 7: 728–732. 10.1038/nnano.2012.162 Search in Google Scholar
Komatsuka T, Kusakabe A, Nagai K. Characterization and gas transport properties of poly(lactic acid) blend membranes. Desalination 2008; 234: 212–220. 10.1016/j.desal.2007.09.088 Search in Google Scholar
Kong C, Wang J, Yang J, Lu J, Wang A, Zhao Q. Thin carbon-zeolite composite membrane prepared on ceramic tube filter by vacuum slip casting for oxygen/nitrogen separation. Carbon 2007; 45: 2848–2850. 10.1016/j.carbon.2007.08.041 Search in Google Scholar
Konruang S, Sirijarukul S, Wanichapichart P, Yu L, Chittrakarn T. Ultraviolet-ray treatment of polysulfone membranes on the O 2 /N 2 and CO 2 /CH 4 separation performance. J Appl Polym Sci 2015; 132: 42074. 10.1002/app.42074 Search in Google Scholar
Koros WJ, Fleming GK. Membrane-based gas separation. J Membr Sci 1993; 83: 1–80. 10.1016/0376-7388(93)80013-N Search in Google Scholar
Kottke T, Stalke D. Crystal handling at low temperatures. J Appl Crystallogr 1993; 26: 615–619. 10.1107/S0021889893002018 Search in Google Scholar
Krasowska M, Rybak A, Dudek G, Strzelewicz A, Pawełek K, Grzywna ZJ. Structure morphology problems in the air separation by polymer membranes with magnetic particles. J Membr Sci 2012; 415–416: 864–870. 10.1016/j.memsci.2012.06.005 Search in Google Scholar
Lamlong C, Taweepreda W. Coating of porous PVC-PEG memebrane with crosslinkable XSBR for O 2 /N 2 and CO 2 /N 2 separation. Polymer 2016; 96: 205–212. 10.1016/j.polymer.2016.04.069 Search in Google Scholar
Le Roux JD, Paul DR, Arendt M, Yuan Y, Cabasso I. Modification of asymmetric polysulfone membranes by mild surface fluorination Part II. Characterization of the fluorinated surface. J Membr Sci 1994a; 94: 143–162. 10.1016/0376-7388(93)E0154-C Search in Google Scholar
Le Roux JD, Paul DR, Kampa J, Lagow RJ. Modification of asymmetric polysulfone membranes by mild surface fluorination Part I. Transport properties. J Membr Sci 1994b; 94: 121–141. 10.1016/0376-7388(93)E0153-B Search in Google Scholar
Lee EH. Ion-beam modification of polymeric materials – fundamental principles and applications. Nucl Instrum Methods Phys Res, Sect B 1999; 151: 29–41. 10.1016/S0168-583X(99)00129-9 Search in Google Scholar
Lee YM, Ha SY, Lee YK, Suh DH, Hong SY. Gas separation through conductive polymer membranes. 2. Polyaniline membranes with high oxygen selectivity. Ind Eng Chem Res 1999; 38: 1917–1924. 10.1021/ie980259e Search in Google Scholar
Lee W-J, Kim D-S, Kim J-H. Preparation and gas separation properties of asymmetric polysulfone membranes by a dual bath method. Korean J Chem Eng 2000; 17: 143–148. 10.1007/BF02707135 Search in Google Scholar
Leo A, Smart S, Liu S, Diniz da Costa JC. High performance perovskite hollow fibres for oxygen separation. J Membr Sci 2011; 368: 64–68. 10.1016/j.memsci.2010.11.002 Search in Google Scholar
Li L, Qi H. Gas separation using sol-gel derived microporous zirconia membranes with high hydrothermal stability. Chin J Chem Eng 2015; 23: 1300–1306. 10.1016/j.cjche.2015.05.005 Search in Google Scholar
Li X-G, Kresse I, Springer J, Nissen J, Yang Y-L. Morphology and gas permselectivity of blend membranes of polyvinylpyridine with ethylcellulose. Polymer 2001; 42: 6859–6869. 10.1016/S0032-3861(01)00057-X Search in Google Scholar
Li Y, Cao C, Chung T-S, Pramoda KP. Fabrication of dual-layer polyethersulfone (PES) hollow fiber membranes with an ultrathin dense-selective layer for gas separation. J Membr Sci 2004; 245: 53–60. 10.1016/j.memsci.2004.08.002 Search in Google Scholar
Li F, Li Y, Chung T-S, Kawi S. Facilitated transport by hybrid POSS ® -Matrimid ® -Zn 2+ nanocomposite membranes for the separation of natural gas. J Membr Sci 2010; 356: 14–21. 10.1016/j.memsci.2010.03.021 Search in Google Scholar
Li S, Jo HJ, Han SH, Park CH, Kim S, Budd PM, Lee YM. Mechanically robust thermally rearranged (TR) polymer membranes with spirobisindane for gas separation. J Membr Sci 2013a; 434: 137–147. 10.1016/j.memsci.2013.01.011 Search in Google Scholar
Li X, Kerstiens T, Markus T. Oxygen permeability and phase stability of Ba 0.5 Sr 0.5 Co 0.8 Fe 0.2 O 3−δ perovskite at intermediate temperatures. J Membr Sci 2013b; 438: 83–89. 10.1016/j.memsci.2013.03.017 Search in Google Scholar
Li S, Jiang X, Yang Q, Shao L. Effects of amino functionalized polyhedral oligomeric silsesquioxanes on cross-linked poly(ethylene oxide) membranes for highly-efficient CO 2 separation. Chem Eng Res Des 2017; 122: 280–288. 10.1016/j.cherd.2017.04.025 Search in Google Scholar
Lin YS. Microporous and dense inorganic membranes: current status and prospective. Sep Purif Technol 2001; 25: 39–55. 10.1016/S1383-5866(01)00089-2 Search in Google Scholar
Lin H, Freeman BD. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J Membr Sci 2004; 239: 105–117. 10.1016/j.memsci.2003.08.031 Search in Google Scholar
Lin X, Xiao J, Yu Y, Chen J, Zheng G, Xu J. Gas permeabilities of poly(trimethylsilylpropyne) membranes surface modified with CF4 plasma. J Appl Polym Sci 1993; 48: 231–236. 10.1002/app.1993.070480207 Search in Google Scholar
Lin X, Qiu X, Zheng G, Xu J. Gas permeabilities of poly(trimethylsilylpropyne) membranes surface modified with CCI4 plasma. J Appl Polym Sci 1995; 58: 2137–2139. 10.1002/app.1995.070581127 Search in Google Scholar
Liu Y, Pan C, Ding M, Xu J. Gas permeability and permselectivity of photochemically crosslinked copolyimides. J Appl Polym Sci 1999; 73: 521–526. 10.1002/(SICI)1097-4628(19990725)73:4<521::AID-APP8>3.0.CO;2-P Search in Google Scholar
Liu Y, Wang R, Chung T-S. Chemical cross-linking modification of polyimide membranes for gas separation. J Membr Sci 2001; 189: 231–239. 10.1016/S0376-7388(01)00415-X Search in Google Scholar
Liu Q, Wang T, Liang C, Zhang B, Liu S, Cao Y, Qiu J. Zeolite married to carbon: a new family of membrane materials with excellent gas separation performance. Chem Mater 2006a; 18: 6283–6288. 10.1021/cm061807e Search in Google Scholar
Liu Q, Wang T, Qiu J, Cao Y. A novel carbon/ZSM-5 nanocomposite membrane with high performance for oxygen/nitrogen separation. Chem Commun 2006b: 1230–1232. 10.1039/b516519a Search in Google Scholar PubMed
Liu Q, Wang T, Guo H, Liang C, Liu S, Zhang Z, Cao Y, Su DS, Qiu J. Controlled synthesis of high performance carbon/zeolite T composite membrane materials for gas separation. Microporous Mesoporous Mater 2009; 120: 460–466. 10.1016/j.micromeso.2008.12.029 Search in Google Scholar
Liu SL, Shao L, Chua ML, Lau CH, Wang H, Quan S. Recent progress in the design of advanced PEO-containing membranes for CO 2 removal. Prog Polym Sci 2013; 38: 1089–1120. 10.1016/j.progpolymsci.2013.02.002 Search in Google Scholar
Lockwood T. Developments in oxyfuel combustion of coal. London, UK: IEA Clean Coal Centre, Report No CCC/240, August 2014. Search in Google Scholar
Lokaj J, Brožová L, Holler P, Pientka Z. Synthesis and gas permeability of block copolymers composed of poly(styrene-co-acrylonitrile) and polystyrene blocks+. Collect Czech Chem Commun 2002; 67: 267–278. 10.1135/cccc20020267 Search in Google Scholar
Lumeij M, Gilleßen M, Bouwmeester H, Markus T, Barthel J, Roitsch S, Mayer J, Dronskowski R. Influence of the Ba 2+ /Sr 2+ content and oxygen vacancies on the stability of cubic Ba x Sr 1−x Co 0.75 Fe 0.25 O 3−δ . Phys Chem Chem Phys 2014; 16: 1333–1338. 10.1039/C3CP53958J Search in Google Scholar
Luo H, Jiang H, Klande T, Cao Z, Liang F, Wang H, Caro Jr. Novel cobalt-free, noble metal-free oxygen-permeable 40Pr 0. 6 Sr 0.4 FeO 3−δ−60 Ce 0. 9 Pr 0.1 O 2−δ dual-phase membrane. Chem Mater 2012; 24: 2148–2154. 10.1021/cm300710p Search in Google Scholar
Madaeni SS, Moradi P. Preparation and characterization of asymmetric polysulfone membrane for separation of oxygen and nitrogen gases. J Appl Polym Sci 2011; 121: 2157–2167. 10.1002/app.33804 Search in Google Scholar
Madaeni SS, Enayati E, Vatanpour V. Separation of nitrogen and oxygen gases by polymeric membrane embedded with magnetic nano-particle. Polym Adv Technol 2011; 22: 2556–2563. 10.1002/pat.1800 Search in Google Scholar
Magueijo VM, Anderson LG, Fletcher AJ, Shilton SJ. Polysulfone mixed matrix gas separation hollow fibre membranes filled with polymer and carbon xerogels. Chem Eng Sci 2013; 92: 13–20. 10.1016/j.ces.2013.01.043 Search in Google Scholar
Meier IK, Langsam M, Klotz HC. Selectivity enhancement via photooxidative surface modification of polyimide air separation membranes. J Membr Sci 1994; 94: 195–212. 10.1016/0376-7388(93)E0174-I Search in Google Scholar
Ming X, Borgnakke DS, Campos MA, Olszewski P, Atreya A, Borgnakke C. Possibility of combustion furnace operation with oxygen-enriched gas from nitrogen generator. University of Michigan, ACEEE Summer Study on Energy Efficiency in Industry 2003. Search in Google Scholar
Mohamed F, Hasbullah H, Jamian WNR, Rani ARA, Saman MFK, Salleh WNH, Ali RR. Gas permeation performance of poly(lactic acid) asymmetric membrane for O 2 /N 2 separation. In: Hashim AM, editor. ICGSCE 2014: Proceedings of the International Conference on Global Sustainability and Chemical Engineering. Singapore: Springer Singapore, 2015: 149–156. 10.1007/978-981-287-505-1_18 Search in Google Scholar
Mozaffari V, Sadeghi M, Fakhar A, Khanbabaei G, Ismail A. Gas separation properties of polyurethane/poly (ether-block-amide)(PU/PEBA) blend membranes. Sep Purif Technol 2017; 185: 202–214. 10.1016/j.seppur.2017.05.028 Search in Google Scholar
Mulder M. Basic principles of membrane technology, Netherlands: Kluwer Academic Publisher, 1996. 10.1007/978-94-009-1766-8 Search in Google Scholar
Murali RS, Sankarshana T, Sridhar S. Air separation by polymer-based membrane technology. Sep Purif Rev 2013; 42: 130–186. 10.1080/15422119.2012.686000 Search in Google Scholar
Nady N, Franssen MCR, Zuilhof H, Eldin MSM, Boom R, Schroën K. Modification methods for poly(arylsulfone) membranes: a mini-review focusing on surface modification. Desalination 2011; 275: 1–9. 10.1016/j.desal.2011.03.010 Search in Google Scholar
Nagendra N, Bandopadhyay S. Room and elevated temperature strength of perovskite membrane tubes. J Eur Ceram Soc 2003; 23: 1361–1368. 10.1016/S0955-2219(02)00367-9 Search in Google Scholar
Nakata M, Kumazawa H. Gas permeability and permselectivity of plasma-treated polyethylene membranes. J Appl Polym Sci 2006; 101: 383–387. 10.1002/app.23850 Search in Google Scholar
Nishiyama N, Yamaguchi M, Katayama T, Hirota Y, Miyamoto M, Egashira Y, Ueyama K, Nakanishi K, Ohta T, Mizusawa A, Satoh T. Hydrogen-permeable membranes composed of zeolite nano-blocks. J Membr Sci 2007; 306: 349–354. 10.1016/j.memsci.2007.09.011 Search in Google Scholar
Niwa M, Kawakami H, Nagaoka S, Kanamori T, Shinbo T. Fabrication of an asymmetric polyimide hollow fiber with a defect-free surface skin layer. J Membr Sci 2000; 171: 253–261. 10.1016/S0376-7388(00)00306-9 Search in Google Scholar
Niwa M, Kawakami H, Kanamori T, Shinbo T, Kaito A, Nagaoka S. Gas separation of asymmetric 6FDA polyimide membrane with oriented surface skin layer. Macromolecules 2001; 34: 9039–9044. 10.1021/ma0113778 Search in Google Scholar
Nomura H, Kramer PW, Yasuda H. Preparation of gas separation membranes by plasma polymerization with fluoro compounds. Thin Solid Films 1984; 118: 187–195. 10.1016/0040-6090(84)90071-3 Search in Google Scholar
Ochs TL, Summers CA, Oryshchyn DB, Gross D, Patrick B, Gross A, Dogan C, Simmons W, Schoenfeld M. Oxy-fuel combustion systems for pollution free coal fired power generation. The 29th International Technical Conference on Coal Utilization and Fuel Systems, Clearwater, Florida, 2004. Search in Google Scholar
Ordonez MJC, Balkus KJ, Ferraris JP, Musselman IH. Molecular sieving realized with ZIF-8/Matrimid ® mixed-matrix membranes. J Membr Sci 2010; 361: 28–37. 10.1016/j.memsci.2010.06.017 Search in Google Scholar
Pandey P, Chauhan RS. Membranes for gas separation. Prog Polym Sci 2001; 26: 853–893. 10.1016/S0079-6700(01)00009-0 Search in Google Scholar
Pellegrino J, Radebaugh R, Mattes BR. Gas sorption in polyaniline. 1. Emeraldine base. Macromolecules 1996; 29: 4985–4991. 10.1021/ma951333x Search in Google Scholar
Peng N, Chung TS, Li KY. The role of additives on dope rheology and membrane formation of defect-free Torlon ® hollow fibers for gas separation. J Membr Sci 2009; 343: 62–72. 10.1016/j.memsci.2009.07.010 Search in Google Scholar
Pesek SC, Koros WJ. Aqueous quenched asymmetric polysulfone membranes prepared by dry/wet phase separation. J Membr Sci 1993; 81: 71–88. 10.1016/0376-7388(93)85032-R Search in Google Scholar
Pinnau I, Koros WJ. Relationship between substructure resistance and gas separation properties of defect-free integrally skinned asymmetric membranes. Ind Eng Chem Res 1991a; 30: 1837–1840. 10.1021/ie00056a024 Search in Google Scholar
Pinnau I, Koros WJ. Structures and gas separation properties of asymmetric polysulfone membranes made by dry, wet, and dry/wet phase inversion. J Appl Polym Sci 1991b; 43: 1491–1502. 10.1002/app.1991.070430811 Search in Google Scholar
Prasad R, Notaro F, Thompson DR. Evolution of membranes in commercial air separation. J Membr Sci 1994; 94: 225–248. 10.1016/0376-7388(93)E0193-N Search in Google Scholar
Purwasasmita M, Kurnia D, Mandias FC, Khoiruddin K, Wenten IG. Beer dealcoholization using non-porous membrane distillation. Food Bioprod Process 2015a; 94: 180–186. 10.1016/j.fbp.2015.03.001 Search in Google Scholar
Purwasasmita M, Nabu EBP, Khoiruddin K, Wenten IG. Non dispersive chemical deacidification of crude palm oil in hollow fiber membrane contactor. J Eng Technol Sci 2015b; 47: 426–446. 10.5614/j.eng.technol.sci.2015.47.4.6 Search in Google Scholar
Rana D, Matsuura T. Surface modifications for antifouling membranes. Chem Rev 2010; 110: 2448–2471. 10.1021/cr800208y Search in Google Scholar
Rao P, Muller M. Industrial oxygen: its generation and use. ACEEE Summer Study on Energy Efficiency in Industry, 2007. Search in Google Scholar
Rao H-X, Liu F-N, Zhang Z-Y. Preparation and oxygen/nitrogen permeability of PDMS crosslinked membrane and PDMS/tetraethoxysilicone hybrid membrane. J Membr Sci 2007; 303: 132–139. 10.1016/j.memsci.2007.07.002 Search in Google Scholar
Rautenbach R, Struck A, Melin T, Roks MFM. Impact of operating pressure on the permeance of hollow fiber gas separation membranes. J Membr Sci 1998; 146: 217–223. 10.1016/S0376-7388(98)00119-7 Search in Google Scholar
Readman JE, Olafsen A, Larring Y, Blom R. La 0.8 Sr 0.2 Co 0.2 Fe 0.8 O 3−δ as a potential oxygen carrier in a chemical looping type reactor, an in-situ powder X-ray diffraction study. J Mater Chem 2005; 15: 1931–1937. 10.1039/b416526h Search in Google Scholar
Reid BD, Ruiz-Trevino FA, Musselman IH, Balkus KJ, Ferraris JP. Gas permeability properties of polysulfone membranes containing the mesoporous molecular sieve MCM-41. Chem Mater 2001; 13: 2366–2373. 10.1021/cm000931+ Search in Google Scholar
Reinhardt H-J, Obermeyer H-D, Schreiner B, Wolf S. Oxygen enrichment for intensification of air oxidation reactions. Pullach, Germany: The Linde Group, 2015. Search in Google Scholar
Robeson LM. Correlation of separation factor versus permeability for polymeric membranes. J Membr Sci 1991; 62: 165–185. 10.1016/0376-7388(91)80060-J Search in Google Scholar
Robeson LM. Polymer membranes for gas separation. Curr Opin Solid State Mater Sci 1999; 4: 549–552. 10.1016/S1359-0286(00)00014-0 Search in Google Scholar
Robeson LM. The upper bound revisited. J Membr Sci 2008; 320: 390–400. 10.1016/j.memsci.2008.04.030 Search in Google Scholar
Rowe B, Freeman B, Paul D. Physical aging of membranes for gas separations. In: Drioli E, Barbieri G, editor. Membrane engineering for the treatment of gases volume 1: gas-separation problems with membranes. Cambridge, UK: Royal Society of Chemistry, 2011: 58–83. 10.1039/9781849733472-00058 Search in Google Scholar
Ruaan RC, Chen SH, Lai JY. Oxygen/nitrogen separation by polycarbonate/Co(SalPr) complex membranes. J Membr Sci 1997; 135: 9–18. 10.1016/S0376-7388(97)00129-4 Search in Google Scholar
Ruaan R-C, Wu T-H, Chen S-H, Lai J-Y. Oxygen/nitrogen separation by polybutadiene/polycarbonate composite membranes modified by ethylenediamine plasma. J Membr Sci 1998; 138: 213–220. 10.1016/S0376-7388(97)00237-8 Search in Google Scholar
Rybak A, Kaszuwara W. Magnetic properties of the magnetic hybrid membranes based on various polymer matrices and inorganic fillers. J Alloys Compd 2015; 648: 205–214. 10.1016/j.jallcom.2015.06.197 Search in Google Scholar
Rybak A, Grzywna ZJ, Kaszuwara W. On the air enrichment by polymer magnetic membranes. J Membr Sci 2009; 336: 79–85. 10.1016/j.memsci.2009.03.027 Search in Google Scholar
Rybak A, Grzywna ZJ, Sysel P. Mixed matrix membranes composed of various polymer matrices and magnetic powder for air separation. Sep Purif Technol 2013; 118: 424–431. 10.1016/j.seppur.2013.07.026 Search in Google Scholar
Sadeghi M, Semsarzadeh MA, Barikani M, Chenar MP. Gas separation properties of polyether-based polyurethane-silica nanocomposite membranes. J Membr Sci 2011; 376: 188–195. 10.1016/j.memsci.2011.04.021 Search in Google Scholar
Salleh W, Ismail AF. Carbon membranes for gas separation processes: recent progress and future perspective. Journal of Membrane Science and Research 2015; 1: 2–15. Search in Google Scholar
Sánchez-Soto PJ, Avilés M, del Rıo J, Ginés J, Pascual J, Pérez-Rodrıguez J. Thermal study of the effect of several solvents on polymerization of acrylonitrile and their subsequent pyrolysis. J Anal Appl Pyrolysis 2001; 58: 155–172. 10.1016/S0165-2370(00)00203-5 Search in Google Scholar
Saufi SM, Ismail AF. Fabrication of carbon membranes for gas separation – a review. Carbon 2004; 42: 241–259. 10.1016/j.carbon.2003.10.022 Search in Google Scholar
Scheffknecht G, Al-Makhadmeh L, Schnell U, Maier J. Oxy-fuel coal combustion – a review of the current state-of-the-art. Int J Greenhouse Gas Control 2011; 5, Supplement 1: S16–S35. 10.1016/j.ijggc.2011.05.020 Search in Google Scholar
Schiestel T, Kilgus M, Peter S, Caspary K, Wang H, Caro J. Hollow fibre perovskite membranes for oxygen separation. J Membr Sci 2005; 258: 1–4. 10.1016/j.memsci.2005.03.035 Search in Google Scholar
Sedigh MG, Xu L, Tsotsis TT, Sahimi M. Transport and morphological characteristics of polyetherimide-based carbon molecular sieve membranes. Ind Eng Chem Res 1999; 38: 3367–3380. 10.1021/ie9806592 Search in Google Scholar
Semsarzadeh MA, Ghalei B. Characterization and gas permeability of polyurethane and polyvinyl acetate blend membranes with polyethylene oxide-polypropylene oxide block copolymer. J Membr Sci 2012; 401–402: 97–108. 10.1016/j.memsci.2012.01.035 Search in Google Scholar
Shaddix C, Molina A. Ignition, flame stability, and char combustion in oxy-fuel combustion. In: Zheng L, editor. Oxy-fuel combustion for power generation and carbon dioxide (CO 2 ) capture. Cambridge, UK: Woodhead Publishing, 2011: 101–124. 10.1533/9780857090980.2.101 Search in Google Scholar
Shao L, Liu L, Cheng S-X, Huang Y-D, Ma J. Comparison of diamino cross-linking in different polyimide solutions and membranes by precipitation observation and gas transport. J Membr Sci 2008; 312: 174–185. 10.1016/j.memsci.2007.12.060 Search in Google Scholar
Sharpe ID, Ismail AF, Shilton SJ. A study of extrusion shear and forced convection residence time in the spinning of polysulfone hollow fiber membranes for gas separation. Sep Purif Technol 1999; 17: 101–109. 10.1016/S1383-5866(99)00024-6 Search in Google Scholar
Sianipar M, Kim SH, Khoiruddin K, Iskandar F, Wenten IG. Functionalized carbon nanotube (CNT) membrane: progress and challenges. RSC Advances 2017; 7: 51175–51198. 10.1039/C7RA08570B Search in Google Scholar
Skeen SA, Yablonsky G, Axelbaum RL. Characteristics of non-premixed oxygen-enhanced combustion: I. The presence of appreciable oxygen at the location of maximum temperature. Combust Flame 2009; 156: 2145–2152. 10.1016/j.combustflame.2009.07.009 Search in Google Scholar
Smith AR, Klosek J. A review of air separation technologies and their integration with energy conversion processes. Fuel Process Technol 2001; 70: 115–134. 10.1016/S0378-3820(01)00131-X Search in Google Scholar
Song Q, Nataraj S, Roussenova MV, Tan JC, Hughes DJ, Li W, Bourgoin P, Alam MA, Cheetham AK, Al-Muhtaseb SA. Zeolitic imidazolate framework (ZIF-8) based polymer nanocomposite membranes for gas separation. Energy Environ Sci 2012; 5: 8359–8369. 10.1039/c2ee21996d Search in Google Scholar
Staiger CL, Pas SJ, Hill AJ, Cornelius CJ. Gas separation, free volume distribution, and physical aging of a highly microporous spirobisindane polymer. Chem Mater 2008; 20: 2606–2608. 10.1021/cm071722t Search in Google Scholar
Strathmann H, Kock K, Amar P, Baker RW. The formation mechanism of asymmetric membranes. Desalination 1975; 16: 179–203. 10.1016/S0011-9164(00)82092-5 Search in Google Scholar
Strzelewicz A, Grzywna ZJ. Studies on the air membrane separation in the presence of a magnetic field. J Membr Sci 2007; 294: 60–67. 10.1016/j.memsci.2007.02.008 Search in Google Scholar
Sunarso J, Baumann S, Serra JM, Meulenberg WA, Liu S, Lin YS, Diniz da Costa JC. Mixed ionic-electronic conducting (MIEC) ceramic-based membranes for oxygen separation. J Membr Sci 2008; 320: 13–41. 10.1016/j.memsci.2008.03.074 Search in Google Scholar
Tan Y, Croiset E, Douglas MA, Thambimuthu KV. Combustion characteristics of coal in a mixture of oxygen and recycled flue gas. Fuel 2006; 85: 507–512. 10.1016/j.fuel.2005.08.010 Search in Google Scholar
Tan X, Wang Z, Meng B, Meng X, Li K. Pilot-scale production of oxygen from air using perovskite hollow fibre membranes. J Membr Sci 2010; 352: 189–196. 10.1016/j.memsci.2010.02.015 Search in Google Scholar
Tan X, Liu N, Meng B, Liu S. Morphology control of the perovskite hollow fibre membranes for oxygen separation using different bore fluids. J Membr Sci 2011; 378: 308–318. 10.1016/j.memsci.2011.05.012 Search in Google Scholar
Tanasescu S, Yáng Z, Martynczuk J, Varazashvili V, Maxim F, Teodorescu F, Botea A, Totir N, Gauckler LJ. Effects of A-site composition and oxygen nonstoichiometry on the thermodynamic stability of compounds in the Ba-Sr-Co-Fe-O system. J Solid State Chem 2013; 200: 354–362. 10.1016/j.jssc.2013.01.030 Search in Google Scholar
Tang X, Zhang X, Luo W, Wu C, Zhang Y, Ding W, Sun C. Iron-doping effects on the CO 2 tolerance of a perovskite oxygen permeable membrane. J Mater Sci 2016; 51: 3971–3978. 10.1007/s10853-015-9715-4 Search in Google Scholar
Tanihara N, Shimazaki H, Hirayama Y, Nakanishi S, Yoshinaga T, Kusuki Y. Gas permeation properties of asymmetric carbon hollow fiber membranes prepared from asymmetric polyimide hollow fiber. J Membr Sci 1999; 160: 179–186. 10.1016/S0376-7388(99)00082-4 Search in Google Scholar
Teramae T, Kumazawa H. Gas permeability and permselectivity of plasma-treated polypropylene membranes. J Appl Polym Sci 2007; 104: 3236–3239. 10.1002/app.25951 Search in Google Scholar
Teraoka Y, Zhang H-M, Furukawa S, Yamazoe N. Oxygen permeation through perovskite-type oxides. Chem Lett 1985; 14: 1743–1746. 10.1246/cl.1985.1743 Search in Google Scholar
Teraoka Y, Shimokawa H, Kang CY, Kusaba H, Sasaki K. Fe-based perovskite-type oxides as excellent oxygen-permeable and reduction-tolerant materials. Solid State Ionics 2006; 177: 2245–2248. 10.1016/j.ssi.2006.05.037 Search in Google Scholar
The Linde Group. Oxygen for the chemical industry: process intensification of air oxidations by O 2 enrichment. Murray Hill: Linde North America, Inc., 2012. Search in Google Scholar
Tin PS, Chung TS, Liu Y, Wang R, Liu SL, Pramoda KP. Effects of cross-linking modification on gas separation performance of Matrimid membranes. J Membr Sci 2003; 225: 77–90. 10.1016/j.memsci.2003.08.005 Search in Google Scholar
Torres-Trueba A, Ruiz-Treviño FA, Luna-Bárcenas G, Ortiz-Estrada CH. Formation of integrally skinned asymmetric polysulfone gas separation membranes by supercritical CO 2 . J Membr Sci 2008; 320: 431–435. 10.1016/j.memsci.2008.04.024 Search in Google Scholar
Tul Muntha S, Kausar A, Siddiq M. Progress in applications of polymer-based membranes in gas separation technology. Polym Plast Technol Eng 2016; 55: 1282–1298. 10.1080/03602559.2016.1163592 Search in Google Scholar
U.S. Department of Energy. Oxygen-Enriched Combustion. Wahington DC: Energy Efficiency and Renewable Energy, U.S. Department of Energy, 2005. Search in Google Scholar
Van Huynh C, Kong S-C. Performance characteristics of a pilot-scale biomass gasifier using oxygen-enriched air and steam. Fuel 2013; 103: 987–996. 10.1016/j.fuel.2012.09.033 Search in Google Scholar
Velianti, Park SB, Heo PW. The enhancement of oxygen separation from the air and water using poly(vinylidene fluoride) membrane modified with superparamagnetic particles. J Membr Sci 2014; 466: 274–280. 10.1016/j.memsci.2014.04.043 Search in Google Scholar
Villaluenga JPG, Seoane B, Hradil J, Sysel P. Gas permeation characteristics of heterogeneous ODPA-BIS P polyimide membranes at different temperatures. J Membr Sci 2007; 305: 160–168. 10.1016/j.memsci.2007.08.002 Search in Google Scholar
Vu DQ, Koros WJ, Miller SJ. Mixed matrix membranes using carbon molecular sieves: I. Preparation and experimental results. J Membr Sci 2003; 211: 311–334. 10.1016/S0376-7388(02)00429-5 Search in Google Scholar
Wahab MFA, Ismail AF, Shilton SJ. Studies on gas permeation performance of asymmetric polysulfone hollow fiber mixed matrix membranes using nanosized fumed silica as fillers. Sep Purif Technol 2012; 86: 41–48. 10.1016/j.seppur.2011.10.018 Search in Google Scholar
Wang D, Li K, Teo WK. Polyethersulfone hollow fiber gas separation membranes prepared from NMP/alcohol solvent systems. J Membr Sci 1996; 115: 85–108. 10.1016/0376-7388(95)00312-6 Search in Google Scholar
Wang D, Li K, Teo WK. Preparation and characterization of polyetherimide asymmetric hollow fiber membranes for gas separation. J Membr Sci 1998; 138: 193–201. 10.1016/S0376-7388(97)00229-9 Search in Google Scholar
Wang D, Li K, Teo WK. Highly permeable polyethersulfone hollow fiber gas separation membranes prepared using water as non-solvent additive. J Membr Sci 2000; 176: 147–158. 10.1016/S0376-7388(00)00419-1 Search in Google Scholar
Wang H, Huang L, Holmberg BA, Yan Y. Nanostructured zeolite 4A molecular sieving air separation membranes. Chem Commun 2002a: 1708–1709. 10.1039/b204854j Search in Google Scholar
Wang D, Teo WK, Li K. Preparation and characterization of high-flux polysulfone hollow fibre gas separation membranes. J Membr Sci 2002b; 204: 247–256. 10.1016/S0376-7388(02)00047-9 Search in Google Scholar
Wang H, Werth S, Schiestel T, Caro J. Perovskite hollow-fiber membranes for the production of oxygen-enriched air. Angew Chem Int Ed 2005; 44: 6906–6909. 10.1002/anie.200501914 Search in Google Scholar PubMed
Wang J, Xu Z, Xu Y. Preparation of poly(4-methyl-1-pentene) asymmetric or microporous hollow-fiber membranes by melt-spun and cold-stretch method. J Appl Polym Sci 2006a; 100: 2131–2141. 10.1002/app.23597 Search in Google Scholar
Wang B, Yi J, Winnubst L, Chen C. Stability and oxygen permeation behavior of Ce 0.8 Sm 0.2 O 2−δ –La 0.8 Sr 0.2 CrO 3−δ composite membrane under large oxygen partial pressure gradients. J Membr Sci 2006b; 286: 22–25. 10.1016/j.memsci.2006.06.009 Search in Google Scholar
Wang R, Meng B, Meng X, Tan X, Sunarso J, Liu L, Liu S. Highly stable La 0.6 Sr 0.4 Co 0.2 Fe 0.8 O 3−δ hollow fibre membrane for air separation swept by steam or steam mixture. J Membr Sci 2015; 479: 232–239. 10.1016/j.memsci.2015.01.006 Search in Google Scholar
Wang B, Song J, Tan X, Meng B, Liu J, Liu S. Reinforced perovskite hollow fiber membranes with stainless steel as the reactive sintering aid for oxygen separation. J Membr Sci 2016; 502: 151–157. 10.1016/j.memsci.2015.12.034 Search in Google Scholar
Wardani AK, Hakim AN, Wenten IG. Combined ultrafiltration-electrodeionization technique for production of high purity water. Water Sci Technol 2017; 75: 2891–2899. 10.2166/wst.2017.173 Search in Google Scholar
Weibel D, Vilani C, Habert A, Achete C. Surface modification of polyurethane membranes using RF-plasma treatment with polymerizable and non-polymerizable gases. Surf Coat Technol 2006; 201: 4190–4194. 10.1016/j.surfcoat.2006.08.050 Search in Google Scholar
Wenten IG, Khoiruddin K. Recent developments in heterogeneous ion-exchange membrane: preparation, modification, characterization and performance evaluation. J Eng Sci Technol 2016; 11: 916–934. Search in Google Scholar
Wenten IG, Dharmawijaya P, Aryanti P, Mukti R. LTA zeolite membranes: current progress and challenges in pervaporation. RSC Adv 2017a; 7: 29520–29539. 10.1039/C7RA03341A Search in Google Scholar
Wenten IG, Khoiruddin K, Hakim A, Himma N. The bubble gas transport method. In: Hilal N, Ismail AF, Matsuura T, Oatley-Radcliffe D, editors. Membrane Characterization. Amsterdam, Netherlands: Elsevier, 2017b: 199–218. Search in Google Scholar
Wenten IG, Khoiruddin K, Himma NF. Membrane-based carbon capture technology: challenges and opportunities in Indonesia. Adv Sci Lett 2017c; 23: 5768–5771. 10.1166/asl.2017.8827 Search in Google Scholar
Wenten IG, Aryanti PTP, Khoiruddin K, Hakim AN, Himma NF. Advances in polysulfone-based membranes for hemodialysis. J Memb Sci Res 2016; 2: 78–89. Search in Google Scholar
Wu K-K, Chang Y-C, Chen C-H, Chen Y-D. High-efficiency combustion of natural gas with 21–30% oxygen-enriched air. Fuel 2010; 89: 2455–2462. 10.1016/j.fuel.2010.02.002 Search in Google Scholar
Wu C, Gai Y, Zhou J, Tang X, Zhang Y, Ding W, Sun C. Structural stability and oxygen permeability of BaCo 1−x Nb x O 3−δ ceramic membranes for air separation. J Alloys Compd 2015; 638: 38–43. 10.1016/j.jallcom.2015.03.056 Search in Google Scholar
Xu X, Coleman MR. Preliminary investigation of gas transport mechanism in a H + irradiated polyimide-ceramic composite membrane. Nucl Instrum Methods Phys Res, Sect B 1999; 152: 325–334. 10.1016/S0168-583X(99)00067-1 Search in Google Scholar
Xu D, Dong F, Chen Y, Zhao B, Liu S, Tade MO, Shao Z. Cobalt-free niobium-doped barium ferrite as potential materials of dense ceramic membranes for oxygen separation. J Membr Sci 2014; 455: 75–82. 10.1016/j.memsci.2013.12.030 Search in Google Scholar
Yamasaki A, Tyagi RK, Fouda AE, Matsuura T, Jonasson K. Effect of solvent evaporation conditions on gas separation performance for asymmetric polysulfone membranes. J Appl Polym Sci 1999; 71: 1367–1374. 10.1002/(SICI)1097-4628(19990228)71:9<1367::AID-APP2>3.0.CO;2-H Search in Google Scholar
Yang N-T, Kathiraser Y, Kawi S. New asymmetric SrCo 0.8 Fe 0.1 Ga 0.1 O₃ −δ perovskite hollow fiber membrane for stable oxygen permeability under reducing condition. J Membr Sci 2013; 428: 78–85. 10.1016/j.memsci.2012.11.005 Search in Google Scholar
Yang F, Zhao H, Yang J, Fang M, Lu Y, Du Z, Świerczek K, Zheng K. Structure and oxygen permeability of BaCo 0.7 Fe 0.3−x In x O 3−δ ceramic membranes. J Membr Sci 2015; 492: 559–567. 10.1016/j.memsci.2015.06.012 Search in Google Scholar
Yao W, Cheng H, Zhao H, Lu X, Zou X, Li S, Li C. Synthesis, oxygen permeability, and structural stability of BaCo 0.7 Fe 0.3−x Zr x O 3−δ ceramic membranes. J Membr Sci 2016; 504: 251–262. 10.1016/j.memsci.2016.01.005 Search in Google Scholar
Ye P, Sjöberg E, Hedlund J. Air separation at cryogenic temperature using MFI membranes. Microporous Mesoporous Mater 2014; 192: 14–17. 10.1016/j.micromeso.2013.09.016 Search in Google Scholar
Ye P, Korelskiy D, Grahn M, Hedlund J. Cryogenic air separation at low pressure using MFI membranes. J Membr Sci 2015; 487: 135–140. 10.1016/j.memsci.2015.03.063 Search in Google Scholar
Yi J, Schroeder M, Martin M. CO 2 -tolerant and cobalt-free SrFe 0.8 Nb 0.2 O 3−δ perovskite membrane for oxygen separation. Chem Mater 2013; 25: 815–817. 10.1021/cm303666v Search in Google Scholar
Yi J, Schroeder M, Weirich T, Mayer J. Behavior of Ba(Co, Fe, Nb)O 3−δ perovskite in CO 2 -containing atmospheres: degradation mechanism and materials design. Chem Mater 2010; 22: 6246–6253. 10.1021/cm101665r Search in Google Scholar
Yin X, Wang J, Chu N, Yang J, Lu J, Zhang Y, Yin D. Zeolite L/carbon nanocomposite membranes on the porous alumina tubes and their gas separation properties. J Membr Sci 2010; 348: 181–189. 10.1016/j.memsci.2009.10.055 Search in Google Scholar
Yong WF, Li FY, Xiao YC, Chung TS, Tong YW. High performance PIM-1/Matrimid hollow fiber membranes for CO 2 /CH 4 , O 2 /N 2 and CO 2 /N 2 separation. J Membr Sci 2013; 443: 156–169. 10.1016/j.memsci.2013.04.037 Search in Google Scholar
Yu Y, Liu S, Wang Y, Zhang H, Li X, Jiang Z, Liu B. Asymmetric membranes prepared with trifluoromethylphenylated poly(ether ether ketone) for gas separation. High Perform Polym 2014; 27: 10–18. 10.1177/0954008314537099 Search in Google Scholar
Zeng C, Zhang L, Cheng X, Wang H, Xu N. Preparation and gas permeation of nano-sized zeolite NaA-filled carbon membranes. Sep Purif Technol 2008; 63: 628–633. 10.1016/j.seppur.2008.07.009 Search in Google Scholar
Zhang Y, Musselman IH, Ferraris JP, Balkus KJ. Gas permeability properties of Matrimid ® membranes containing the metal-organic framework Cu-BPY-HFS. J Membr Sci 2008; 313: 170–181. 10.1016/j.memsci.2008.01.005 Search in Google Scholar
Zhang K, Sunarso J, Shao Z, Zhou W, Sun C, Wang S, Liu S. Research progress and materials selection guidelines on mixed conducting perovskite-type ceramic membranes for oxygen production. RSC advances 2011; 1: 1661–1676. 10.1039/c1ra00419k Search in Google Scholar
Zhang B, Shi Y, Wu Y, Wang T, Qiu J. Preparation and characterization of supported ordered nanoporous carbon membranes for gas separation. J Appl Polym Sci 2014; 131: 39925. 10.1002/app.39925 Search in Google Scholar
Zhang C, Sunarso J, Liu S. Designing CO 2 -resistant oxygen-selective mixed ionic-electronic conducting membranes: guidelines, recent advances, and forward directions. Chem Soc Rev 2017; 46: 2941–3005. 10.1039/C6CS00841K Search in Google Scholar
Zhao H, Xu N, Cheng Y, Wei W, Chen N, Ding W, Lu X, Li F. Investigation of mixed conductor BaCo 0.7 Fe 0.3−x Y x O 3−δ with high oxygen permeability. J Phys Chem C 2010; 114: 17975–17981. 10.1021/jp106220z Search in Google Scholar
Zhu H, Jie X, Wang L, Kang G, Liu D, Cao Y. Effect of MIL-53 on phase inversion and gas separation performance of mixed matrix hollow fiber membranes. RSC Advances 2016; 6: 69124–69134. 10.1039/C6RA14823A Search in Google Scholar
Zimmerman CM, Koros WJ. Polypyrrolones for membrane gas separations. I. Structural comparison of gas transport and sorption properties. J Polym Sci, Part B: Polym Phys 1999; 37: 1235–1249. 10.1002/(SICI)1099-0488(19990615)37:12<1235::AID-POLB5>3.0.CO;2-J Search in Google Scholar
Zornoza B, Seoane B, Zamaro JM, Téllez C, Coronas J. Combination of MOFs and zeolites for mixed-matrix membranes. ChemPhysChem 2011; 12: 2781–2785. 10.1002/cphc.201100583 Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
- X / Twitter
Supplementary Materials
Please login or register with De Gruyter to order this product.
Journal and Issue
Articles in the same issue.
Login to your account
Change password, your password must have 8 characters or more and contain 3 of the following:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create a new account
Can't sign in? Forgot your password?
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Request Username
Can't sign in? Forgot your username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- Institutional Access
Cookies Notification
Our site uses javascript to enchance its usability. you can disable your ad blocker or whitelist our website www.worldscientific.com to view the full content., select your blocker:, adblock plus instructions.
- Click the AdBlock Plus icon in the extension bar
- Click the blue power button
- Click refresh
Adblock Instructions
- Click the AdBlock icon
- Click "Don't run on pages on this site"
uBlock Origin Instructions
- Click on the uBlock Origin icon in the extension bar
- Click on the big, blue power button
- Refresh the web page
uBlock Instructions
- Click on the uBlock icon in the extension bar
Adguard Instructions
- Click on the Adguard icon in the extension bar
- Click on the toggle next to the "Protection on this website" text
Brave Instructions
- Click on the orange lion icon to the right of the address bar
- Click the toggle on the top right, shifting from "Up" to "Down
Adremover Instructions
- Click on the AdRemover icon in the extension bar
- Click the "Don’t run on pages on this domain" button
- Click "Exclude"
Adblock Genesis Instructions
- Click on the Adblock Genesis icon in the extension bar
- Click on the button that says "Whitelist Website"
Super Adblocker Instructions
- Click on the Super Adblocker icon in the extension bar
- Click on the "Don’t run on pages on this domain" button
- Click the "Exclude" button on the pop-up
Ultrablock Instructions
- Click on the UltraBlock icon in the extension bar
- Click on the "Disable UltraBlock for ‘domain name here’" button
Ad Aware Instructions
- Click on the AdAware icon in the extension bar
- Click on the large orange power button
Ghostery Instructions
- Click on the Ghostery icon in the extension bar
- Click on the "Trust Site" button
Firefox Tracking Protection Instructions
- Click on the shield icon on the left side of the address bar
- Click on the toggle that says "Enhanced Tracking protection is ON for this site"
Duck Duck Go Instructions
- Click on the DuckDuckGo icon in the extension bar
- Click on the toggle next to the words "Site Privacy Protection"
Privacy Badger Instructions
- Click on the Privacy Badger icon in the extension bar
- Click on the button that says "Disable Privacy Badger for this site"
Disconnect Instructions
- Click on the Disconnect icon in the extension bar
- Click the button that says "Whitelist Site"
Opera Instructions
- Click on the blue shield icon on the right side of the address bar
- Click the toggle next to "Ads are blocked on this site"
System Upgrade on Tue, May 28th, 2024 at 2am (EDT)
Chapter 4: membrane processes for n 2 –ch 4 separation.
- Shiguang Li ,
- Zhaowang Zong ,
- Miao Yu , and
- Moises A. Carreon
Gas Technology Institute, 1700 S. Mount Prospect Road, Des Plaines, IL 60018, USA
Search for more papers by this author
Chemical and Biological Engineering Department, Colorado School of Mines, Golden, CO 80401, USA
Department of Chemical Engineering, University of South Carolina, Columbia, SC 29208, USA
An increasing demand for clean energy has led to a search for alternative energies with less environmental impact, of which natural gas is an important option. The large consumption of natural gas requires an effective technology to improve the purity of natural gas, in which inert gases such as nitrogen play a large part. However, current technologies cannot provide economic solutions to remove nitrogen from natural gas, which leads to a decrease in the heat value of the natural gas. Membrane separation by zeolites represents a viable energy-saving method that can potentially offer an effective way to remove nitrogen from natural gas. In particular, SAPO-34 zeolite membranes have been extensively studied for carbon dioxide separation because of their exceptional molecular-sieving effects, higher thermal and chemical resistances. These membranes can also be effective for N 2 –CH 4 separation due to their molecular-sieving effects and adsorption properties. In this mini review, we discuss the different technologies employed for nitrogen rejection from natural gas, emphasizing zeolite membranes as an effective and promising technology to economically separate N 2 from CH 4 .
- The influence of cellulose acetate butyrate membrane structure on the improvement of CO 2 /N 2 separation Jia Qiang Ngo, Shin Tien Lee, Zeinab Abbas Jawad, Abdul Latif Ahmad and Ren Jie Lee et al. 29 October 2019 | Chemical Engineering Communications, Vol. 207, No. 12
Recommended

Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Impact of pclnpg nanopolymeric additive on the surface and structural properties of ppsu ultrafiltration membranes for enhanced protein rejection.

1. Introduction
2. experimental work, 2.1. materials, 2.2. pclnpg synthesis, 2.3. membrane preparation, 2.4. membrane characterization, 2.5. performance tests, 3. results and discussion, 3.1. membrane morphology, 3.2. ftir analysis, 3.3. hydrophilicity of membranes, 3.4. thickness, porosity, and pore size of membranes, 3.5. membrane performance, 3.6. antifouling analysis, 4. conclusions, author contributions, data availability statement, conflicts of interest.
- Qadir, D.; Mukhtar, H.; Keong, L.K. Mixed Matrix Membranes for Water Purification Applications. Sep. Purif. Rev. 2017 , 46 , 62–80. [ Google Scholar ] [ CrossRef ]
- Wee, T.; Kim, C.; Hanif, M. Review on Wastewater Treatment Technologies. Int. J. Appl. Environ. Sci. 2016 , 11 , 111–126. [ Google Scholar ]
- Al Aani, S.; Mustafa, T.N.; Hilal, N. Ultrafiltration membranes for wastewater and water process engineering: A comprehensive statistical review over the past decade. J. Water Process. Eng. 2020 , 35 , 101241. [ Google Scholar ] [ CrossRef ]
- Li, Q.; Xu, Z.; Pinnau, I. Fouling of reverse osmosis membranes by biopolymers in wastewater secondary effluent: Role of membrane surface properties and initial permeate flux. J. Membr. Sci. 2007 , 290 , 173–181. [ Google Scholar ] [ CrossRef ]
- Hilal, N.; Ismail, A.F.; Wright, C. Membrane Fabrication ; CRC Press: Boca Raton, FL, USA, 2015. [ Google Scholar ]
- Al Aani, S.; Gomez, V.; Wright, C.J.; Hilal, N. Fabrication of antibacterial mixed matrix nanocomposite membranes using hybrid nanostructure of silver coated multi-walled carbon nanotubes. Chem. Eng. J. 2017 , 326 , 721–736. [ Google Scholar ] [ CrossRef ]
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004 , 167 , 55–63. [ Google Scholar ] [ CrossRef ]
- Zhao, S.; Wang, Z.; Wei, X.; Tian, X.; Wang, J.; Yang, S.; Wang, S. Comparison study of the effect of PVP and PANI nanofibers additives on membrane formation mechanism, structure and performance. J. Membr. Sci. 2011 , 385–386 , 110–122. [ Google Scholar ] [ CrossRef ]
- Abdullah, R.R.; Shabeeb, K.M.; Alzubaydi, A.B.; Figoli, A.; Criscuoli, A.; Drioli, E.; Alsalhy, Q. Characterization of the Efficiency of Photo-Catalytic Ultrafiltation PES Membrane Modified with Tungsten Oxide in the Removal of Tinzaparin Sodium. Eng. Technol. J. 2022 , 40 , 1–10. [ Google Scholar ] [ CrossRef ]
- Al-Araji, D.D.; Al-Ani, F.H.; Alsalhy, Q.F. The permeation and Separation Characteristics of Polymeric Membranes Incorporated with Nanoparticles for Dye Removal and Interaction Mechanisms between Polymer and Nanoparticles: A Mini Review. Eng. Technol. J. 2022 , 40 , 1399–1411. [ Google Scholar ] [ CrossRef ]
- Zhao, J.; Yang, Y.; Li, C.; Hou, L.-A. Fabrication of GO modified PVDF membrane for dissolved organic matter removal: Removal mechanism and antifouling property. Sep. Purif. Technol. 2019 , 209 , 482–490. [ Google Scholar ] [ CrossRef ]
- Dipheko, T.D.; Matabola, K.P.; Kotlhao, K.; Moutloali, R.M.; Klink, M. Fabrication and Assessment of ZnO Modified Polyethersulfone Membranes for Fouling Reduction of Bovine Serum Albumin. Int. J. Polym. Sci. 2017 , 2017 , 3587019. [ Google Scholar ] [ CrossRef ]
- Pakizeh, M.; Azinfar, F.; Safarnia, M.; Raji, F. The effects of TiO2 nanoparticles and polydopamine on the structure, separation, and antifouling properties of PPSU membrane. Sep. Sci. Technol. 2022 , 57 , 1788–1799. [ Google Scholar ] [ CrossRef ]
- Rashid, H.-O.; Ralph, S.F. Carbon nanotube membranes: Synthesis, properties, and future filtration applications. Nanomaterials 2017 , 7 , 99. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Plisko, T.; Karslyan, Y.; Bildyukevich, A. Effect of polyphenylsulfone and polysulfone incompatibility on the structure and performance of blend membranes for ultrafiltration. Materials 2021 , 14 , 5740. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ghadhban, M.Y.; Majdi, H.S.; Rashid, K.T.; Alsalhy, Q.F.; Lakshmi, D.S.; Salih, I.K.; Figoli, A. Removal of dye from a leather tanning factory by flat-sheet blend ultrafiltration (UF) membrane. Membranes 2020 , 10 , 47. [ Google Scholar ] [ CrossRef ]
- Hussein, S.S.; Ibrahim, S.S.; Toma, M.A.; Alsalhy, Q.F.; Drioli, E. Novel chemical modification of polyvinyl chloride membrane by free radical graft copolymerization for direct contact membrane distillation (DCMD) application. J. Membr. Sci. 2020 , 611 , 118266. [ Google Scholar ] [ CrossRef ]
- Mansouri, J.; Harrisson, S.; Chen, V. Strategies for controlling biofouling in membrane filtration systems: Challenges and opportunities. J. Mater. Chem. 2010 , 20 , 4567–4586. [ Google Scholar ] [ CrossRef ]
- Fang, X.; Li, J.; Li, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. Polyethyleneimine, an effective additive for polyethersulfone ultrafiltration membrane with enhanced permeability and selectivity. J. Membr. Sci. 2014 , 476 , 216–223. [ Google Scholar ] [ CrossRef ]
- Shabeeb, K.M.; Noori, W.A.; Abdulridha, A.A.; Majdi, H.S.; Al-Baiati, M.N.; Yahya, A.A.; Rashid, K.T.; Németh, Z.; Hernadi, K.; Alsalhy, Q.F. Novel partially cross-linked nanoparticles graft co-polymer as pore former for polyethersulfone membranes for dyes removal. Heliyon 2023 , 9 , e21958. [ Google Scholar ] [ CrossRef ]
- Garcia-Ivars, J.; Iborra-Clar, M.-I.; Alcaina-Miranda, M.-I.; Van der Bruggen, B. Comparison between hydrophilic and hydrophobic metal nanoparticles on the phase separation phenomena during formation of asymmetric polyethersulphone membranes. J. Membr. Sci. 2015 , 493 , 709–722. [ Google Scholar ] [ CrossRef ]
- Kang, Y.; Obaid, M.; Jang, J.; Ham, M.-H.; Kim, I.S. Novel sulfonated graphene oxide incorporated polysulfone nanocomposite membranes for enhanced-performance in ultrafiltration process. Chemosphere 2018 , 207 , 581–589. [ Google Scholar ] [ CrossRef ]
- Xiao, S.; Yu, S.; Yan, L.; Liu, Y.; Tan, X. Preparation and properties of PPSU/GO mixed matrix membrane. Chin. J. Chem. Eng. 2017 , 25 , 408–414. [ Google Scholar ] [ CrossRef ]
- Gebru, K.A.; Das, C. Removal of bovine serum albumin from wastewater using fouling resistant ultrafiltration membranes based on the blends of cellulose acetate, and PVP-TiO 2 nanoparticles. J. Environ. Manag. 2017 , 200 , 283–294. [ Google Scholar ] [ CrossRef ]
- Liu, J.; Zhong, Z.; Ma, R.; Zhang, W.; Li, J. Development of high-antifouling PPSU ultrafiltration membrane by using compound additives: Preparation, morphologies, and filtration resistant properties. Membranes 2016 , 6 , 35. [ Google Scholar ] [ CrossRef ]
- Arumugham, T.; Kaleekkal, N.J.; Doraiswamy, M. Development of new hybrid ultrafiltration membranes by entanglement of macromolecular PPSU-SO3H chains: Preparation, morphologies, mechanical strength, and fouling resistant properties. J. Appl. Polym. Sci. 2015 , 132. [ Google Scholar ] [ CrossRef ]
- Alsalhy, Q.F.; Al-Ani, F.H.; Al-Najar, A.E.; Jabuk, S.I. A study of the effect of embedding ZnO-NPs on PVC membrane performance use in actual hospital wastewater treatment by membrane bioreactor. Chem. Eng. Process. Process. Intensif. 2018 , 130 , 262–274. [ Google Scholar ] [ CrossRef ]
- Zwane, S.; Kuvarega, A.T.; Mhlanga, S.D.; Dlamini, D.S. Hydrophilic polysulfone/Lantana camara mixed matrix membranes for the removal of dyes from water. Surf. Interfaces 2018 , 13 , 216–223. [ Google Scholar ] [ CrossRef ]
- Saljoughi, E.; Amirilargani, M.; Mohammadi, T. Effect of PEG additive and coagulation bath temperature on the morphology, permeability and thermal/chemical stability of asymmetric CA membranes. Desalination 2010 , 262 , 72–78. [ Google Scholar ] [ CrossRef ]
- Farjami, M.; Vatanpour, V.; Moghadassi, A. Effect of nanoboehmite/poly(ethylene glycol) on the performance and physiochemical attributes EPVC nano-composite membranes in protein separation. Chem. Eng. Res. Des. 2020 , 156 , 371–383. [ Google Scholar ] [ CrossRef ]
- Al-Ani, D.M.; Al-Ani, F.H.; Alsalhy, Q.F.; Ibrahim, S. Preparation and characterization of ultrafiltration membranes from PPSU-PES polymer blend for dye removal. Chem. Eng. Commun. 2021 , 208 , 41–59. [ Google Scholar ] [ CrossRef ]
- Kadhim, R.J.; Al-Ani, F.H.; Al-Shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of dyes using graphene oxide (Go) mixed matrix membranes. Membranes 2020 , 10 , 366. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hosseini, S.S.; Torbati, S.F.; Shahmirzadi, M.A.A.; Tavangar, T. Fabrication, characterization, and performance evaluation of polyethersulfone/TiO2 nanocomposite ultrafiltration membranes for produced water treatment. Polym. Adv. Technol. 2018 , 29 , 2619–2631. [ Google Scholar ] [ CrossRef ]
- Mushtaq, A.; Bin Mukhtar, H.; Shariff, A.M. FTIR study of enhanced polymeric blend membrane with amines. Res. J. Appl. Sci. Eng. Technol. 2014 , 7 , 1811–1820. [ Google Scholar ] [ CrossRef ]
- Ali, A.M.; Rashid, K.T.; Yahya, A.A.; Majdi, H.S.; Salih, I.K.; Yusoh, K.; Alsalhy, Q.F.; AbdulRazak, A.A.; Figoli, A. Fabrication of gum arabic-graphene (Gga) modified polyphenylsulfone (ppsu) Mixed Matrix Membranes: A Systematic Evaluation Study for Ultrafiltration (UF) Applications. Membranes 2021 , 11 , 542. [ Google Scholar ] [ CrossRef ]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Effect of TiO2nanoparticles on the performance of polyphenylsulfone biomaterial for orthopaedic implants. J. Mater. Chem. B 2014 , 2 , 7502–7514. [ Google Scholar ] [ CrossRef ]
- Haghighat, N.; Vatanpour, V.; Sheydaei, M.; Nikjavan, Z. Preparation of a novel polyvinyl chloride (PVC) ultrafiltration membrane modified with Ag/TiO2 nanoparticle with enhanced hydrophilicity and antibacterial activities. Sep. Purif. Technol. 2020 , 237 , 116374. [ Google Scholar ] [ CrossRef ]
- Al-Ani, F.H.; Alsalhy, Q.F.; Raheem, R.S.; Rashid, K.T.; Figoli, A. Experimental investigation of the effect of implanting tio2-nps on pvc for long-term uf membrane performance to treat refinery wastewater. Membranes 2020 , 10 , 77. [ Google Scholar ] [ CrossRef ]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016 , 393 , 65–78. [ Google Scholar ] [ CrossRef ]
- Hong, J.; He, Y. Polyvinylidene fluoride ultrafiltration membrane blended with nano-ZnO particle for photo-catalysis self-cleaning. Desalination 2014 , 332 , 67–75. [ Google Scholar ] [ CrossRef ]
- Maximous, N.; Nakhla, G.; Wan, W.; Wong, K. Performance of a novel ZrO2/PES membrane for wastewater filtration. J. Membr. Sci. 2010 , 352 , 222–230. [ Google Scholar ] [ CrossRef ]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011 , 375 , 284–294. [ Google Scholar ] [ CrossRef ]
- Aljanabi, A.A.A.; Mousa, N.E.; Aljumaily, M.M.; Majdi, H.S.; Yahya, A.A.; Al-Baiati, M.N.; Hashim, N.; Rashid, K.T.; Al-Saadi, S.; Alsalhy, Q.F. Modification of Polyethersulfone Ultrafiltration Membrane Using Poly(terephthalic acid-co-glycerol-g-maleic anhydride) as Novel Pore Former. Polymers 2022 , 14 , 3408. [ Google Scholar ] [ CrossRef ]
- Balta, S.; Sotto, A.; Luis, P.; Benea, L.; Van der Bruggen, B.; Kim, J. A new outlook on membrane enhancement with nanoparticles: The alternative of ZnO. J. Membr. Sci. 2012 , 389 , 155–161. [ Google Scholar ] [ CrossRef ]
- Vatanpour, V.; Madaeni, S.S.; Khataee, A.R.; Salehi, E.; Zinadini, S.; Monfared, H.A. TiO 2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2012 , 292 , 19–29. [ Google Scholar ] [ CrossRef ]
- Arthanareeswaran, G.; Mohan, D.; Raajenthiren, M. Preparation and performance of polysulfone-sulfonated poly(ether ether ketone) blend ultrafiltration membranes. Part I. Appl. Surf. Sci. 2007 , 253 , 8705–8712. [ Google Scholar ] [ CrossRef ]
- Abdel-Karim, A.; Leaper, S.; Alberto, M.; Vijayaraghavan, A.; Fan, X.; Holmes, S.M.; Souaya, E.R.; Badawy, M.I.; Gorgojo, P. High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. Chem. Eng. J. 2018 , 334 , 789–799. [ Google Scholar ] [ CrossRef ]
- Zhou, D.; Rong, G.; Huang, S.; Pang, J. Preparation of a novel sulfonated polyphenlene sulfone with flexible side chain for ultrafiltration membrane application. Sep. Purif. Technol. 2019 , 210 , 817–823. [ Google Scholar ] [ CrossRef ]
- Falsanisi, D.; Liberti, L.; Notarnicola, M. Ultrafiltration (UF) pilot plant for municipal wastewater reuse in agriculture: Impact of the operation mode on process performance. Water 2010 , 2 , 872–885. [ Google Scholar ] [ CrossRef ]
- Cosenza, A.; Gulhan, H.; Maida, C.M.; Mannina, G. Nutrient recovery from wastewater treatment by ultrafiltration membrane for water reuse in view of a circular economy perspective. Bioresour. Technol. 2022 , 363 , 127929. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Membrane Code | PPSU wt.% | NMP wt.% | PCLNPG wt.% |
|---|---|---|---|
| M1 | 16 | 84 | 0 |
| M2 | 16 | 83.75 | 0.25 |
| M3 | 16 | 83.5 | 0.5 |
| M4 | 16 | 83.25 | 0.75 |
| M5 | 16 | 83 | 1.0 |
| M6 | 16 | 82.75 | 1.25 |
| Membrane | Pore Size (nm) | Porosity (%) | Contact Angle (°) | Thickness (µm) |
|---|---|---|---|---|
| M1 | 25.22 | 60.16 | 81.7 | 64.33 |
| M2 | 36.68 | 72.61 | 80.0 | 110.1 |
| M3 | 40.92 | 76.02 | 61.8 | 114.8 |
| M4 | 45.32 | 79.49 | 52.2 | 111.53 |
| M5 | 42.22 | 74.81 | 61.0 | 110.2 |
| M6 | 42.42 | 69.48 | 63.1 | 130.3 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Taha, Y.R.; Zrelli, A.; Hajji, N.; Al-Juboori, R.A.; Alsalhy, Q. Impact of PCLNPG Nanopolymeric Additive on the Surface and Structural Properties of PPSU Ultrafiltration Membranes for Enhanced Protein Rejection. Processes 2024 , 12 , 1930. https://doi.org/10.3390/pr12091930
Taha YR, Zrelli A, Hajji N, Al-Juboori RA, Alsalhy Q. Impact of PCLNPG Nanopolymeric Additive on the Surface and Structural Properties of PPSU Ultrafiltration Membranes for Enhanced Protein Rejection. Processes . 2024; 12(9):1930. https://doi.org/10.3390/pr12091930
Taha, Younus Rashid, Adel Zrelli, Nejib Hajji, Raed A. Al-Juboori, and Qusay Alsalhy. 2024. "Impact of PCLNPG Nanopolymeric Additive on the Surface and Structural Properties of PPSU Ultrafiltration Membranes for Enhanced Protein Rejection" Processes 12, no. 9: 1930. https://doi.org/10.3390/pr12091930
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

Share ×
Scan the QR code and open PeakVisor on your phone
❤ Wishlist ×
See all region register, peakvisor app, khanty-mansiysk autonomous okrug – ugra.
Welcome to the land of sheer silent whiteness. Its vast expanses are filled with fresh Arctic air, howling winds, and the spirit of true adventure. Come with us to the lands of the ancient Khanty and Mansi tribes that survived in this harsh climate of the Nether-Polar Urals . See the mountains that defy any logical or geological reason for their existence. Experience the wonders of this sparsely populated land where you can hardly see a human trace. Welcome to Yugra!
Flora & Fauna
Water resources, landmarks and tourism, major mountains, mount narodnaya, mount zaschita, mount neroyka, the pyramid mountain, samarovskaya mountain, ski and sports facilities, protected sites, reserves, national and natural parks, rivers and lakes, major cities, khanty-mansiysk.
The Khanty-Mansiysk Autonomous Area – Yugra (KhMAO) is located in the central part of the West Siberian Plain, stretching from west to east from the Ural Range to the Ob-Yenisei Watershed. The vast areas of this plain, as well as the Lower Priob region, are considered one of the most recently inhabited areas.

The Khanty-Mansiysk Autonomous Area (KhMAO) was established in 1930. Its name comes from two main northern indigenous peoples – the Khanty and the Mansi. From 1944 it was legally part of the Tyumen Region , but in 1993 the Area received autonomy and became a full-fledged territorial entity of the Russian Federation. It is a part of the Urals Federal District. The administrative centre is the city of Khanty-Mansiysk , whereas the largest city is Surgut. The word Yugra was introduced to the name of the Khanty-Mansiysk Autonomous Area in 2003 to pay tribute to the old name used by the locals to call the territories lying beyond the North Urals.
The KhMAO borders the Komi Republic in the north-west, the Yamalo-Nenets Autonomous District in the north, the Krasnoyarsk Area and the Tomsk Region in the east and south-east, the Tyumen Region in the south and the Sverdlovsk Region in the south-west.
The area of the territory is 534,801 sq.km, the length from north to south is 800 km, from west to east is 1400 km. The population of this huge territory is 1,674,676 people as of 2020, which is the same amount as people living in Barcelona or Munich.

The main part of the territory is a huge, poorly dissected plain where absolute elevation marks rarely exceed 200 meters above sea level. The western part of the KhMAO territory is characterized by low and middle mountainous terrains with some Alpine relief featured in the Subpolar Urals. Here are ridges and spurs of the mountain system of the North Urals and the Subpolar Urals. The maximum absolute elevations are on the border with the Komi Republic . Mount Narodnaya (1,895m) is the highest peak.
More than 800 species of higher plants grow in the Khanty-Mansi Autonomous Area . Almost the entire territory is covered by taiga forests that occupy about 52% of the area. Spruce, fir, pine, cedar, larch, birch, alder grow here. In the northern parts of the area, the composition of the vegetation is greatly influenced by perennial permafrost. Light lichen grasslands which are used as deer pastures are widespread there. Tundra dominates in the mountainous and hilly areas. River floodplains and lowlands are characterized by meadow vegetation, the so-called water meadows. High floodplains of large rivers are mainly covered with woods that mainly feature willows, birches and aspens. Forests and swamps are rich in berries and various valuable plants, most of which are used in traditional indigenous medicine.

The animal world is typical for the Russian taiga zone. There are 369 species of vertebrates. Mammals are represented by 60 species (28 of them are commercial species). The most common and valuable of them are wild reindeer, elk, fox, sable, fox, squirrel, marten, ermine, Siberian weasel, polecat, mink, weasel, otter, hare and others. Wolverine and West Siberian river beaver are included in the Red Book of Russia.
There are 256 bird species in the region, including 206 sedentary and nesting species. Some rare bird species are listed in the Red Book. There are 42 species of fish in rivers and lakes. Of these, 19 species are commercial, among them are starlet sturgeon, lelema, muksun (whitefish), pelyad, chir, lake herring, wader, tugun, freshwater cod, pike, ide, roach, bream, fir, perch, ruff, golden and silver crucian carp, carp (carp is grown in the cooling ponds of the Surgutskaya and Nizhnevartovskaya hydroelectric plants). Sturgeon is listed in the Red Book. There is an abundance of mosquitoes and gnats in the area, the greatest activity of which is in the second half of summer.

Yugra can boast of over 2 thousand large and small rivers, the total length of which is 172,000 km. The main rivers are the Ob (3,650 km), the Irtysh (3,580 km). These are some of the largest rivers in Russia. Other significant rivers include the tributaries of the Ob (the Vakh, Agan, Tromyogan, Bolshoy Yugan, Lyamin, Pim, Bolshoy Salym, Nazym, Severnaya Sosva, Kazym rivers), the tributary of the Irtysh (the Konda River) and the Sogom River. Ten rivers are over 500 km long. All the Yugra rivers with the exception of the rivers in the Ural part of the region are characterized by rather slow currents, gentle slopes, some surge wave phenomena, spring and summer floods. The Ob River basin extends over a distance of 700-200 km from the mouths of its tributaries. Such abundance of water facilitates the appearance of floodplain swamps and seasonal lakes.
The region's swamps are predominantly of the upper and transitional type. Those water basins occupy about a third of the region. About 290,000 lakes with the area of more than 1 ha are surrounded by swamps and forests. The largest lakes are Tursuntsky Tuman, Levushinsky Tuman, Vandemtor and Trmemtor. The deepest lakes are Kintus (48 m) and Syrky Sor (42 m). However, most of the lakes (about 90%) are modest and quite small and have no surface runoff.
The area is rich in resources of fresh, mineral and thermal underground waters, which are still insignificantly used.

The climate is moderately continental. Winters are harsh, snowy and long, and summers are short and relatively warm. The territory is protected from the west by the Ural Mountains but its openness from the north has a significant impact on the climate formation because cold air masses from the Arctic freely penetrate the area. The flat character of the terrain with a large number of rivers, lakes and swamps also has its impact. Most of the precipitation falls during the warm seasons. But even with a small amount of precipitation, their evaporation is very low, which as a result contributes to the formation of the zone of excessive moisture throughout the Yugra. The snow cover is stable from late October to early May, its height varies from 50 to 80 cm. The region is characterized by a rapid change of weather conditions, especially in transitional seasons (autumn and spring), as well as during the day. Late spring and early autumn frosts are rather frequent and can happen even until mid-June. Average January temperatures range from -18ºC to -24ºC (0 F to -11 F) and can reach -60ºC to -62ºC (-76 F to -80 F) when the northern cold air masses break through. The average temperature in July, the warmest month of the year, ranges from +15ºC to +20ºC (+59 F to +68 F) and on very rare days can reach a maximum temperature of +36ºC (+97 F). The prevailing wind direction is north in summer and south in winter.
The weather in the mountains is quite changeable and cool even in summer. The best time to visit the region's mountains is between July and mid-August.
The Yugra of the Khanty-Mansi Autonomous Area has a huge natural resource potential. These are oil and gas deposits, forests, gold and iron ore deposits, as well as bauxites, copper, zinc, lead, niobium, tantalum, brown and hard coal deposits, rock crystal, quartz and piezo quartz, peat deposits, etc. The region has plenty of natural resources. In terms of natural gas reserves, the Yugra ranks second in the Russian Federation after the Yamalo-Nenets Autonomous District .
The industry is dominated by oil and gas production, power generation and processing industries, including woodworking except for pulp and paper production.

The Khanty-Mansi area has very developed tourism of all kinds. There is a modern infrastructure for cultural exploration as well as for active recreation.
Fans of sports and eco-friendly tourism will be able to conquer majestic mountains and raft down picturesque rivers, enjoy the beauty of nature in nature reserves and natural parks. The hills and mountains of this area open up endless opportunities for skiing and snowboarding.
The mountainous part of the Subpolar Urals located on the territory of the Khanty-Mansi Autonomous Area is very beautiful. The highest peaks of the Ural Mountains are situated here.
Being the highest point of the whole Urals, Mount Narodnaya (1,895 m), also known as Naroda and Poenurr and translated as People's Mountain is territorially situated in the Subpolar Urals, on the border of the Yugra Area and the Komi Republic . It is the highest point in European Russia outside the Caucasus. This leads to its large topographic prominence of 1,772 metres (5,814 ft).

The top of the mountain is half a kilometre from the border towards Yugra. As for the name of the mountain, scientists could not come to a common opinion for a long time, so there are two versions. According to one version, in the Soviet years, an expedition of pioneers gave the mountain a name in honour of the Soviet people - Narodnaya (the stress is on the second syllable). According to the other version, even before the arrival of the first Soviet tourists, the peak was named after the River Naroda (the stress is on the first syllable) flowing at the foot of the mountain. The Nenets peoples called the River Naroda Naro, which means a thicket or a dense forest, and the Mansi peoples called it Poengurr or Poen-urr, which translates as the top, or head. The maps used to refer to it as Mount Naroda or Mount Naroda-Iz. Nowadays, it appears everywhere as Narodnaya.
In the 1980s, someone set a bust of Lenin on the top of the mountain. Its remains can be found there to this day. There is one more symbolic relic there – some Orthodox believers erected a worship cross on top of Mount Narodnaya after a Procession of the Cross.
The slopes of the mountain are steeper in the north-east and south-west and there are many steep rocks on them. The south-eastern and northern parts of the mountain are more gentle but they are also covered with scree. Be vigilant and careful when climbing! On the slopes of the mountain, there are many not only boulders but also caverns filled with clear water as well as ice. There are glaciers and snowfields. From the north-eastern part of the mountain, you can observe Lake Blue near which tourists and travellers like to make bivouacs.

Mesmerizing with its beauty and inaccessibility, it attracts many tourists and fans of active recreation. This majestic mountain is quite remote from the settlements, so getting to it is not an easy task. The mountain is located in the Yugyd Va National Park , so it is necessary to register in advance and get a visit permit from the park administration. How to get to the park administration and get a permit, read the article on the Yugyd Va National Park .
Mountain Zaschita (1,808 m) is the second-highest peak in the Ural Mountains, after Mount Narodnaya . Mysteriously, the name of the mountain, which roughly translates as Defense or Protection Mount, does not correlate in any way with the Mansi names of the nearby mountains and rivers. The origin of the name is unknown. There are some speculations but we will consider just one of them. On the map of the Northern Urals which was made by the Hungarian researcher Reguli the closest peak to Mount Narodnaya was called gnetying olu. Its location coincides with that of the present-day Mount Zaschita . The name gnetying olu in the Mansi can be deciphered as a mountain on which there is some help from ice. The mountain is believed to protect deer grazing on glaciers from mosquitoes. So, early topographers called the mountain more briefly – Mount Defense. Indeed, the slopes of this mountain are covered with a lot of snow and glaciers (the Yugra, Naroda, Kosyu, Hobyu glaciers and others). And it is here that the Mansi shepherds bring their deer which can rest on glaciers and snow. Summarizing all the above, we can say that Zaschita Mount is to some extent protection for deer from mosquitoes. The very name Zaschita appeared on maps with the beginning of hiking tours in the Subpolar Urals.
Mount Neroyka (1,645 m) is 100 km from Neroyka village, the closest tourist base to this peak. In the 1950s, people who were engaged in quartz mining near the mountain worked and lived in this base. Later, a gravel road was built from the village of Saranpaul to the mountain for large-scale development of the quartz deposit. In recent years, the road has not been much used and is practically not cleaned from snow in winter. There has been a plant built 20 km down from the mountain for primary processing of quartz with the use of nanotechnologies. There is an annual big camping event near the mountain. It is organized by the Tourism Department of the Khanty-Mansi Autonomous Area. You can have a 1-hour helicopter ride to the mountain from the village of Saranpaul. Should you wish to fly from the city of Khanty-Mansiysk , be prepared to fly over the taiga for 2.5-3 hours.

Quite inquisitive tourists happened to discover, by a lucky chance, a Pyramid similar to that of Cheops but four times bigger. It is located on the territory of the Narodo-Ityinsky Ridge. The closest to the pyramid is the village of Saranpaul. The sizes of the found pyramid are as follows: the height is 774 m, in comparison to the Egyptian pyramid which is 147 m; the length of a lateral edge is 230 m whereas the Egyptian pyramid is 1 km. The pyramid is located precisely according to the cardinal directions, there is not a single degree deviation at that. The origin of the pyramid is unknown, scientists are still making assumptions. No traces of human activity were found near the pyramid. The only way to get here at this time is by helicopter.
Samarovskaya Mountain is another wonder that is baffling many people. It is dividing the city of Khanty-Mansiysk into northern and southern parts. Few now living residents know that in the old days the highest part of the modern city used to bear a plural name of the Samarovsky Mountains among which there were Mount Palenina, Komissarskaya, Miroslavskaya, Filinova, and Romanova. Originally, there was a village called Samarovo amidst these mountains. Until now, many issues bewilder both residents and scientists. How could a mountain form in the middle of the West Siberian Plain? What is inside it? Won't the weight of the buildings erected on the top of the mountain affect its height? The uniqueness of Samarovskaya Mountain is that it consists of numerous large stones, boulders, rocks that are absolutely foreign to this area. Scientists have not yet come to a consensus on the mountain’s origin.

The Yugra is very famous for its ski resorts, the main of which are:
- The Cedar Ravine ski resort (Surgut city, Naberezhny Ave. 39/1)
- Three Mountains (Trekhgorie) ski resort (30 km from Nizhnevartovsk, Ermakovsky settlement)
- Stone Cape (Kamenniy Mys) ski resort (near the city of Surgut)
- Pine Urman ski resort ( Khanty-Mansiysk , Sportivnaya Str., 24)
The far-away lands of the Yugra are the blessed sanctuaries for many animals as the area is rather hostile to a human There are reserves, natural parks, wildlife sanctuaries here that aim to protect the national treasures of the lands. Having visited these regions once, you would crave for coming back again and again to feel that unique sense of unity with nature, to forget about the urban fuss and and hustles whatsoever. The harsh but beautiful nature of this extraordinary area leaves an indelible trace in the soul of every person.

On the territory of the district there are 25 specially protected natural areas, the most famous of them are:
- The reserves are two: the Malaya Sosva Reserve and the Yugan Reserve, the latter was established in 1982 as the largest reserve of taiga landscapes. The purpose of the reserves was to study unobtrusively and carefully preserve the endemic flora and fauna without disturbing natural processes. Hunting and economic activities are prohibited here, which is important for the preservation of natural ecosystems.
- The natural parks are the Samarovsky Chugas Nature Park, the Siberian Sloping Hills (Uvaly), the Numto (also called Lake Numto), and the Kondinskie Lakes.
These reserves and natural parks offer tourists their own excursion programs to make visiting their territory much more enjoyable and educational.
The Samarovsky Chugas Nature Park is located in the center of Khanty-Mansiysk , on a small hill between the Ob and Irtysh rivers.
The territory of the Siberian Sloping Hills (Uvaly) natural park is 350 km away from the city of Khanty-Mansiysk . You can get there by helicopter or by plane. The office of the park is located at 7a Pionerskaya Street, Nizhnevartovsk.
The Kondinskie Lakes Natural Park is located 380 km from Khanty-Mansiysk . Half of the park is covered with swamps, but there is also a recreational area. There you can rest, swim, do some amateur fishing, picking berries (cowberries, cranberries) and mushrooms is permitted. There is only one independent walking route here, it runs for 3 km in the deep forest. It is a cool place for kids since the park is equipped with sports grounds, a pool and a small zoo where the kids can interact with brown bear cubs. What else, try the TaiPark, it is a rope course running at the height of 2.5 meters, having 15 stages, the full length is 125 meters. There is an opportunity to order water walking tours in the town of Sovetsky, which can be reached by train from Khanty-Mansiysk .

The Numto Nature Park is located almost in the center of the West Siberian Plain, in the Beloyarsk district of the Khanty-Mansi Autonomous Area, 300 km from the city of Surgut and 200 km from the town of Beloyarsk. It is located on the border of Yugra and Yamalo-Nenets Autonomous Area. The administration of the park is located at 2, Beloyarsky micro-district, 4a. The territory of the natural park is a treasure trove of archaeological and ethnocultural monuments. As of today, there have been discovered 20 architectural monuments, including fortified and not fortified settlements, places of worship abandoned by the peoples who lived here from the Stone Age to almost the present day. Researchers have also found 65 monuments of ethnic value, the main of which are worship objects, sacred places and cemeteries.
The Malaya Sosva Reserve includes several subordinated territories and sanctuaries, including Lake Ranghe-Tour. The reserve offers a 4-km walking guided route that gets the visitors introduced to the typical features and characteristics of flora and fauna of the region. The route is called Bear Trail and you can spot bears there (don’t come close though, we’ve already written how to behave if you meet a bear in the wild). Also, you will see the River Malaya Sosva, some marshes, ancient cultural monuments and other nice sights. Permission to visit the reserve can be obtained from the administration of the reserve at Lenina Str. 46, town Sovetskiy.
As to the Yugan Nature Reserve , it is inaccessible to common hikers who are afraid of flying since there are no roads to it. The only way to get there is taking a helicopter ride. You also must obtain a permit in the administration of the reserve, go accompanied by employees of the reserve, and only on special transport of the reserve (motorboat, snowmobile). The central manor of the Reserve and the administration are located in the village of Ugut. To get to this village, you should first go to the town of Surgut, then go to the town of Pyt-Yakh, and from it there is a road to the village of Ugut. It is about 100 km from Ugut to the southern border of the reserve i, and another 25 km to the nearest cordon. The administration works from Monday to Friday. You can request a permit via mail at [email protected] , order a guided tour at [email protected]

The Yugra lands are heaven for water sports aficionados. They can have some awesome fishing or go rafting along such rivers as: the river Naroda, the Deep Sabun, etc.
The Naroda River is 140 km long. It is the left tributary of the Manya River located in the Ob River basin. The river has its origin on the south-western slope of Mount Narodnaya . It is a mountain-taiga river with rapids, swifts, numerous rolls, which attracts interest among water tourists. However, it is usually not rafted very often.
The Deep Sabun River flows through the territory of the Siberian Sloping Hills Nature Park. The park has developed multi-day water routes. It is possible to raft along the river in summer and to go skiing along it in winter.
The Kondinskie Lakes are a system of lakes along the left bank of the Konda River. The largest lake is the Arantur, with pine forests on the northern side and sandy beaches well equipped for a nice relaxing me-time. The water heats up well in summer. The small river Okunevaya and the river Maly Akh flow into the lake. The Maly Akh comes in on the west side and connects lake Arantur with Lake Pon-Tour. This lake is the richest in fish, and there is also a parking lot for fishermen here. The streams connect Pon-Tour with small lakes Krugloe and Lopukhovoye. When you look at Lopukhovoe lake, you feel as if you have found yourself in a fabulous place: more than half of its surface is covered with white lilies, as well as yellow flowers of the water-beans. Then the river Big Akh, which flows into the river Konda, connects all the lakes into a single system. Along the river there are many archeological monuments such as forts and settlements which have paths to them. The southernmost lake of the park is Ranghe-Tour.

Yugra is not the easiest destination and not the most accessible, but the effort is well worth it. You should first get to the capital of Khanty-Mansiysk Autonomous Area – the city of Khanty-Mansiysk either by air or by train.
Khanty-Mansiysk is based on the premises of the former village Samarovo founded in 1582. It used to be the territory of the Khanty people and a pit stop for coachmen who rode their wagons across the country. The village was founded by Russian Count Samara, thus the name Samarovo. The modern city actually began to develop in 1930 because amidst the Siberian taiga there finally started to appear stone houses on the high bank of the Irtysh River. In 1940, the village was renamed into Khanty-Mansiysk by the name of the peoples living on this territory – the Khanty and the Mansi, and in 1950 it received the status of a town.

The city has several attractions. Mount Samarovskaya is probably the biggest natural and scientific wonder. It divides the city in two parts and causes many concerns for urban developers who always wonder whether this mountain can move making the buildings slide or even sink in.
Another beauty is the century-old cedar grove that is within the city limits. The grove is a part of the natural park Samarovsky Chugas. The word chugas in the language of the Khanty means a lonely hill in the low river floodplain.

The park is one of the main attractions of the city, it hosts an open-air ethnographic museum called the Torum Maa, a cultural and tourist complex called Archaeopark, a biathlon center. Kids and adults, nature lovers and fans of culture love this place dearly.
A memorial sign to Yugra's discoverers is installed on top of the Samarovsky Chugas. It is a tall stele pyramid divided into three portions. On the lower level, there is a restaurant, on the second level is a small museum, and on the third level there is an observation deck, 40 m above the ground, with a magnificent view of the Irtysh River and the river port. The pyramid is decorated by the bas-relief depicting the discoverers of the region, from the 16th-century Count Samara to the geologists of the 20th century.
Another trademark of Khanty-Mansiysk is the State Museum of Nature and Man. The museum hosts a gallery and a workshop of a famous artist G. Rayshev.
The city has a lot of small monuments generously spread around the city. There is the Khanty family resting on a camp, this monument is near the airport building. You can take a pic at the Golden Tambourine located at the intersection of Gagarin Street and Mira Street. Connoisseurs of culture should also visit the Sun – the Theatre of Ob-Ugrian Peoples, it is the world's first professional theatre of Khanty and Mansi peoples. And if you are travelling with kids, the Khanty-Mansiysk Puppet Theatre is a must-visit. In the period from May to October, you can take a boat ride to the confluence of two rivers – the Ob and the Irtysh. Yugra Service Co. operates such cruises, you can find more information locally at their address Tobolsk Trakt street 4, Khanty-Mansiysk .

Explore Khanty-Mansiysk Autonomous Okrug – Ugra with the PeakVisor 3D Map and identify its summits .

PeakVisor Hiking Maps
Be a superhero of outdoor navigation with state-of-the-art 3D maps and mountain identification in the palm of your hand!


IMAGES
VIDEO
COMMENTS
A membrane reactor is a combination of chemical conversion and membrane separation process. The membrane reactor acts as a contactor and separates the reaction medium in one chamber from a second chamber containing a catalyst, enzymes, or a cell culture. The simulation of membrane reactors by various configurations is an emerging area.
Mathematical models of hollow-fibre and spiral-wound membrane modules are presented in this thesis. The models are developed from rigorous mass, momentum and energy balances and can be used to describe a generic membrane separation. This is in contrast to most existing models which are typically process specific and are only valid within
Simulation, Design and Optimization of Membrane Gas ...
In this paper, a two dimensional cross flow mathematical model for membrane separation has been incorporated with Aspen HYSYS as a user defined unit operation in order to optimize and design the membrane system for CO 2 capture from natural gas. Parameter sensitivities, along with process economics, have been studied for different design ...
2. Introduction to Membrane Gas Separations. Separation processes are a critical part of chemical and purified product production. Distillation is the main separation technology in industrial plants and is responsible for 10-15% of the world's energy usage [].The energy-intensive nature of distillation has led both industry and academia to seek more efficient alternatives that can limit ...
Porous membranes play a crucial role in many commercial applications: the desalination of seawater and brackish water via reverse osmosis and nanofiltration; the clean-up of industrial effluents by micro- and ultra-filtration or electrodialysis; and the separation of multi-component gas mixtures, to name only a few.
2 The Future of Membrane Processes: Challenges and Prospects 2.1 Membrane Materials. To a large extent, polymers represent today the dominant material family of membrane separation processes (Baker and Low, 2014).This statement applies for porous (microfiltration, ultrafiltration, dialysis) or dense (reverse osmosis, gas separation, and pervaporation) industrial membranes.
Membrane technology has undergone a long, historical development in laboratory research and achieved its first major industrial application in the 1960s [].Membrane is a kind of material with a selective separation function, and can transfer one component and restrict others because of the special properties of the components [].The main membrane technologies include microfiltration ...
Membrane Separation Process Design and Intensification
In according to current research, various MOF materials with distinct properties have been applied for membrane-based gas separation in this PhD study, and their performance is discussed in Chapters 4 to 7. In Chapter 4, two types of pure MOF membranes, crystalline and glassy, are introduced, however, they were difficult to work with and their ...
Gas separation. Gas separation membranes have played a significant role in the fields of air purification and energy recovery [96], [97]. In particular, to achieve carbon neutrality, the development of gas separation membrane technology is more and more critical as it can effectively reduce CO 2 emissions by capturing CO 2 in flue gas [98], [99].
Mathematical modelling of membrane separation Vinther, Frank Publication date: 2015 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Vinther, F. (2015). Mathematical modelling of membrane separation. ... Furthermore, the thesis consist of three separate mathematical models, each
There is growing interest in the food industry to develop approaches for large-scale production of bioactive molecules through continuous downstream processing, especially from sustainable sources. Membrane-based separation technologies have the potential to reduce production costs while incorporating versatile multiproduct processing capabilities. This review describes advances in membrane ...
The focus of this thesis is the design and testing of membranes for separation of water-in-oil (w/o) emulsions. A polycarbonate membrane treated with octadecyltrichlorosilane (OTS) is used to filter a 3 wt% w/o emulsion. The permeate is characterized to have no measurable water content by microscopy, dynamic light scattering (DLS) and differential
The work of this thesis focuses on membrane technology for gas separation due to the merit of high energy efficiency relative to conventional techniques. Advanced polymers with high fractional free volume and highly porous nanoparticles with full organic framework are selected to fabricate mixed matrix membranes (MMMs). Efforts to further improve MMMs gas separation performance include solvent ...
This research explores the systematic design of membranes, as well as, the development of smart methodologies that enable separation of a wide variety of both immiscible and miscible liquid mixtures. The first part of my thesis describes membranes that can separate oil-water mixtures, solely under gravity.
Compared with current conventional technologies, oxygen/nitrogen (O 2 /N 2 ) separation using membrane offers numerous advantages, especially in terms of energy consumption, footprint, and capital cost. However, low product purity still becomes the major challenge for commercialization of membrane-based technologies. Therefore, numerous studies on membrane development have been conducted to ...
These membranes can also be effective for N 2 -CH 4 separation due to their molecular-sieving effects and adsorption properties. In this mini review, we discuss the different technologies employed for nitrogen rejection from natural gas, emphasizing zeolite membranes as an effective and promising technology to economically separate N 2 from CH 4.
ly, while pres. ure swing adsorption only applies to verylimited cases. Membrane separation. is expected to offer a promising a. native process fornitrogen removal from natural gas. In this research, a series of P. separate nitrogen. from methane under different pressuresand temperatures. These rubbery p.
Polymeric membranes with high permselective performance are desirable for energy-saving bioalcohol separations. However, it remains challenging to design membrane microstructures with low-resistance channels and a thin thickness for fast alcohol transport. Herein, we demonstrate highly crystalline covalent organic framework (COF) membranes with ordered nanochannels as tunable transport layers ...
This research explored the use of a partially cross-linked graft copolymer (PCLNPG) as an innovative nanopolymer pore-forming agent to enhance polyphenylsulfone (PPSU) membranes for protein separation applications. The study systematically examined the impact of incorporating PCLNPG at varying concentrations on the morphological and surface properties of PPSU membranes.
Khanty-Mansi Autonomous Okrug
Khanty-Mansiysk Autonomous Okrug - Ugra in Siberia has recently started to play a major role in the Russian economy because key oil and gas extraction sites are located in this region.
The Khanty-Mansiysk Autonomous Area (KhMAO) was established in 1930. Its name comes from two main northern indigenous peoples - the Khanty and the Mansi. From 1944 it was legally part of the Tyumen Region, but in 1993 the Area received autonomy and became a full-fledged territorial entity of the Russian Federation.
The article presents the results of a sociological study conducted on the territory of the Khanty- Mansiysk Autonomous Okrug - Ugra, whose goal was to study the opinion of the population, migrants ...