An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Bioethics Resources for Investigators & Research Ethics Committees
A collection of resources for scientific investigators conducting research in the developing world, and ethics committees reviewing it.
- Guidelines (non-regulatory)
Regulations
Practical aids, research ethics committees, guidelines and regulations.
Guidelines (non-regulatory) Human Subjects Research in General These are the key non-regulatory documents that are likely to be cited by ethicists and research ethics committees. The Nuremberg Code The World Medical Association Declaration of Helsinki Council for International Organizations of Medical Sciences (CIOMS), International Ethical Guidelines for Biomedical Research Involving Human Subjects . The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research (USA) PREVENT Project: Pregnant Women & Vaccines Against Emerging Epidemic Threats: Ethics Guidance for Preparedness, Research, and Response , September 2018 HIV/AIDS UNAIDS 2012. Ethical considerations in biomedical HIV prevention trials National Institutes of Health, USA. NIH Guidance for Addressing the Provision of Antiretroviral Treatment for Trial Participants Following their Completion of NIH-Funded HIV Antiretroviral Treatment Trials in Developing Countries Research in Developing Countries The Nuffield Council. The Ethics of Research Related to Healthcare in Developing Countries . 2002. The Nuffield Council is a UK-based independent body that researches and reports on topical issues in bioethics. They published a follow-up discussion paper on this same topic in 2005. National Bioethics Advisory Commission, USA. Ethical and Policy Issues in International Research: Clinical Trials in Developing Countries . Research in Humanitarian Crises Post-Research Ethics Analysis (PREA) - A research project investigating ethical issues in health research in humanitarian crises. PREA tool - Provides researchers and other stakeholders with a pragmatic tool that assists learning lessons about the actual ethical challenges that develop during health research in humanitarian crises. R2HC Research Ethics Tool Elrha, September 2017 Disasters: Core Concepts and Ethical Theories [Open access] Springer Nature, supported by Disaster Bioethics/COST Action, 2018 Genetics and Genomics Human Genome Organisation (HUGO) - HUGO Ethics Committee . Statements on several issues, including genetics research and benefit sharing. UNESCO. Universal Declaration on the Human Genome and Human Rights . The Office for Human Research Protections (OHRP) in the US has guidance on the application of US regulations to genetic research and research on biological samples . See "Coded Private Information," "Biological Specimens," and "Tissue Storage/Repositories." The US National Institutes of Health also have guidance documents relating to Genome-Wide Association Studies (GWAS). National Bioethics Advisory Commission, USA. Research Involving Human Biological Materials: Ethical Issues and Policy Guidance .
US Regulations Code of Federal Regulations Title 45 Part 46 . Food and Drug Administration (FDA). Title 21 Part 50 Protection Of Human Subjects . A helpful FDA and HHS comparison chart showing the similarities and differences between FDA and HHS regulations. Compilations of National Regulations ClinRegs from the National Institute of Allergy and Infectious Diseases (NIAID). A central resource for exploring and comparing international clinical research regulations. Global Research Ethics Map . Harvard School of Public Health. As of March 2009, provides guides to the human subjects protections in 11 countries: Botswana, Ghana, Kenya, Kuwait, Malawi, Nepal, Pakistan, Philippines, Senegal, South Africa, Tanzania. From the Office for Human Research Protections (OHRP) at HHS: International Compilation of Human Research Protections - Lists the approximately 1,100 laws, regulations, and guidelines that govern human subjects research in over 100 countries, as well as standards from a number of international and regional organizations. Compilation of European GDPR guidances as of July 24, 2018 UNESCO Global Ethics Observatory includes a database containing ethics related legislation and guidelines from 34 countries.
- Research Ethics in Epidemics and Pandemics: A Casebook published by PAHO in 2024, free through Springer Open Access.
- WHO's Special Programme for Research and Training in Tropical Diseases (TDR). Standards and Operational Guidance for Ethics Review of Health-Related Research with Human Participants . Provides guidance for setting up and developing standard operating procedures for research ethics committees and ethical review systems.
- Foreign Grants Information . NIH Office of Extramural Research (OER) has a website for foreign applicants and grantees. It goes through the grants process and includes a section on human subjects protections.
- OER also have a page providing HHS and NIH requirements and resources for all members of the extramural community involved in human subjects research
- The Elements of a Successful Informed Consent [Video] created by the Human Subjects Protection Team of the National Institute of Mental Health (NIMH) Office of the Clinical Director. This video is intended for use by novice clinical investigators and clinical research staff.
- Global Health Reviewers A "resource for all those involved in the ethical and regulatory review of research, particularly in resource-limited settings. A variety of materials and forums are provided to assist committee members from Ethics Review Committees, Institutional Review Boards, and Food and Drug Administration regulatory committees."
- Iberoamerican Bioethics Network The network, with headquarters at FLACSO-Argentina, has subcoordinator centers in Brazil, Mexico and Spain. The Iberoamerican Bioethics Network is a dependent of the IAB (International Association of Bioethics).
- Inventory of research ethics committees in Africa and for Latin America and the Caribbean From Health Research Web, the inventory includes contact information for each REC.
- IRB Primer: Incidental and Secondary Findings [Archive] From the from the archive of the U.S. Presidential Commission for the Study of Bioethical Issues , the IRB Primer is designed to help institutional review boards (IRBs) understand and implement the Bioethics Commission's recommendations regarding how to manage incidental and secondary findings ethically in the research setting.
The PAHO Regional Program on Bioethics strengthens the development of bioethics. The program builds capacity in bioethics in the region and support PAHO and Member States in the integration of bioethics in all health-related activities.
- Research Ethics in Africa , by Mariana Kruger, Paul Ndebele, Lyn Horn This free e-book for research ethics committee members focuses on research ethics issues in Africa.
- Strategic Initiative for Developing Capacity in Ethical Review (SIDCER) A network of independently established regional fora for ethical review committees, health researchers and invited partner organizations with an interest in the development of ethical review. The regional fora are composed of Asia and Western Pacific (FERCAP), former Russian states (FECCIS), Latin America (FLACEIS), Africa (PABIN) and North America (FOCUS).
- Africa - The Pan-African Bioethics Initiative (PABIN) aims to strengthen ethical awareness and discussion across the African continent. They are currently surveying research ethics committees in Africa.
- Asia - The Forum for Ethical Review Committees in Asia and the Western Pacific Region (FERCAP) is a regional organization of researchers and members of ethics review committees.
- Latin America - The Latin American Forum of Research Ethics Committees in Health (FLACEIS) is located at FLACSO-Argentina. From its creation in 2002 there have been three or four lectures each year about issues relevant for research ethics committees.
To view Adobe PDF files, download current, free accessible plug-ins from the Adobe's website .
Updated April 29, 2024

Country selection
Regulatory Authority
Scope of assessment, regulatory fees, ethics committee, scope of review, ethics committee fees, oversight of ethics committees.
Clinical Trial Lifecycle
Submission Process
Submission content, timeline of review, initiation, agreements & registration, safety reporting, progress reporting.
Sponsorship
Definition of Sponsor
Site/investigator selection, insurance & compensation, risk & quality management, data & records management, personal data protection.
Informed Consent
Documentation Requirements
Required elements, participant rights, emergencies, vulnerable populations, children/minors, pregnant women, fetuses & neonates, mentally impaired.
Investigational Products
Definition of Investigational Product
Manufacturing & import, quality requirements, product management, definition of specimen, specimen import & export, consent for specimen, requirements, additional resources.
Information Not Yet Incorporated Into Country Profile:
New FDA Final Rule Issued for Informed Consent Exceptions for Minimal Risk Clinical Investigations The Food and Drug Administration (FDA) issued a final rule , which went into effect on January 22, 2024. The final rule allows an institutional review board (IRB) to waive or alter certain informed consent elements, or waive the informed consent requirement for certain FDA-regulated minimal risk clinical investigations.
Clinical Trials Registries
- ClinicalTrials.gov listing of studies in United States
- International Clinical Trials Registry Platform (ICTRP) consolidated listing of studies in United States
Ethics Committees
- Database of institutional review boards/ethics committees registered with the United States Department of Health and Human Services (HHS) Office for Human Research Protections (OHRP)
Funding & Institutions
- World RePORT database of funding organizations, research organizations, and research programs in United States
- HHS OHRP database of institutions with approved Federalwide Assurances (FWAs) for the protection of human subjects
US Profile Updated
Us profile updated in clinregs, united states: ohrp issues guidance for research impacted by covid-19, united states: fda and nih issue guidance for clinical trials impacted by covid-19.
Other Regulatory Databases
- United States Department of Health and Human Services (HHS) Office for Human Research Protections (OHRP) International Compilation of Human Research Standards for United States
- Health Research Web - United States
This profile covers the role of the Department of Health & Human Services (HHS) ’s Food & Drug Administration (FDA) in reviewing and authorizing investigational new drug applications (INDs) to conduct clinical trials using investigational drug or biological products in humans in accordance with the FDCAct , 21CFR50 , and 21CFR312 . Regulatory requirements for federally funded or sponsored human subjects research, known as the Common Rule ( Pre2018-ComRule and RevComRule ), which the HHS and its Office for Human Research Protections (OHRP) implements in subpart A of 45CFR46, are also examined. Lastly, additional HHS requirements included in subparts B through E of 45CFR46 are described in this profile, where applicable, using the acronym 45CFR46-B-E . (Please note: ClinRegs does not provide information on state level requirements pertaining to clinical trials.)
Food & Drug Administration
As per the FDCAct , 21CFR50 , and 21CFR312 , the FDA is the regulatory authority that regulates clinical investigations of medical products in the United States (US). According to USA-92 , the FDA is responsible for protecting public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices.
An overview of the FDA structure is available in USA-33 . Several centers are responsible for pharmaceutical and biological product regulation, including the Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER) . Additionally, per USA-88 , the Office of Clinical Policy (OCLiP) develops good clinical practice and human subject protection policies, regulation, and guidance.
See USA-47 for a list of FDA clinical trials related guidance documents.
Office for Human Research Protections and Common Rule Agencies
Per USA-93 , the OHRP provides leadership in the protection of the rights, welfare, and well-being of human research subjects for studies conducted or supported by the HHS. The OHRP helps ensure this by providing clarification and guidance, developing educational programs and materials, maintaining regulatory oversight, and providing advice on ethical and regulatory issues in biomedical and social-behavioral research.
USA-65 states that the Common Rule ( Pre2018-ComRule and RevComRule ) outlines the basic provisions for institutional ethics committees (ECs) (referred to as institutional review boards (IRBs) in the US), informed consent, and Assurances of Compliance. See USA-65 for a list of US departments and agencies that follow the Common Rule, which are referred to as Common Rule departments/agencies throughout the profile.
The RevComRule applies to all human subjects research that is federally funded or sponsored by a Common Rule department/agency (as identified in USA-65 ), and: 1) was initially approved by an EC on or after January 21, 2019; 2) had EC review waived on or after January 21, 2019; or 3) was determined to be exempt on or after January 21, 2019. (Per USA-55 and USA-74 , the RevComRule is also known as the “2018 Requirements.”) For 2018 Requirements decision charts consistent with the RevComRule , including how to determine if research is exempt, see USA-74 . For more information about the RevComRule , see USA-66 .
Per the RevComRule , the Pre2018-ComRule requirements apply to research funded by a Common Rule department/agency (as identified in USA-65 ) that, prior to January 21, 2019, was either approved by an EC, had EC review waived, or was determined to be exempt from the Pre2018-ComRule . Institutions conducting research approved prior to January 21, 2019 may choose to transition to the RevComRule requirements. The institution or EC must document and date the institution's determination to transition a study on the date the determination to transition was made. The research must comply with the RevComRule beginning on that date. For pre-2018 Requirements decision charts consistent with the Pre2018-ComRule , including how to determine if research is exempt, see USA-74 .
See USA-54 for additional information regarding compliance with the Pre2018-ComRule and the RevComRule .
USA-65 indicates that the FDA, despite being a part of the HHS, is not a Common Rule agency. Rather, the FDA is governed by its own regulations, including the FDCAct and 21CFR50 . However, the FDA is required to harmonize with the Pre2018-ComRule and the RevComRule whenever permitted by law.
If a study is funded or sponsored by HHS, and involves an FDA-regulated product, then both sets of regulations will apply. See G-RevComRule-FDA for additional information.
Other Considerations
Per USA-16 , the US is a founding regulatory member of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The US has adopted several ICH guidance documents, including the E11(R1) Addendum: Clinical Investigation of Medicinal Products in the Pediatric Population ( US-ICH-E11 ), E17 General Principles for Planning and Design of Multiregional Clinical Trials ( US-ICH-E17 ), and E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1) ( US-ICH-GCPs ), which are cited throughout this profile.
Contact Information
As per USA-81 , USA-91 , and USA-90 , the contact information for the FDA is as follows:
Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 Telephone (general inquiries): (888) 463-6332
CDER Telephone (drug information): (301) 796-3400 CDER Email: [email protected]
CBER Telephone: (800) 835-4709 or (240) 402-8010 CBER Email (manufacturers assistance): [email protected] CBER Email (imports): [email protected] CBER Email (exports): [email protected]
Office for Human Research Protections
Per USA-82 , the contact information for the OHRP is as follows:
Office for Human Research Protections 1101 Wootton Parkway, Suite 200 Rockville, MD 20852 Telephone: (866) 447-4777 or (240) 453-6900 Email (general inquiries): [email protected]
Department of Health & Human Services
According to USA-83 , the contact information for the HHS is as follows:
US Department of Health & Human Services Hubert H. Humphrey Building 200 Independence Avenue, S.W. Washington, D.C. 20201 Call Center: (877) 696-6775
In accordance with the FDCAct , 21CFR50 , and 21CFR312 , the Food & Drug Administration (FDA) has authority over clinical investigations for drug and biological products regulated by the agency. 21CFR312 specifies that the scope of the FDA’s assessment for investigational new drug applications (INDs) includes all clinical trials (Phases 1-4). Based on 21CFR56 and 21CFR312 , institutional ethics committee (EC) review of the proposed clinical investigation may be conducted in parallel with the FDA review of the IND. However, EC approval must be obtained prior to the sponsor being permitted to initiate the clinical trial. (Note: Institutional ECs are referred to as institutional review boards (IRBs) in the United States (US)).
As delineated in 21CFR312 and USA-42 , sponsors are required to submit an IND to the FDA to obtain an agency exemption to ship investigational drug(s) across state lines to conduct drug or biologic clinical trial(s). An IND specifically exempts an investigational drug or biologic from FDA premarketing approval requirements that would otherwise be applicable. 21CFR312 states that “‘IND’ is synonymous with ‘Notice of Claimed Investigational Exemption for a New Drug.’"
According to USA-42 , the FDA categorizes INDs as either commercial or non-commercial (research) and classifies them into the following types:
- Investigator INDs - Submitted by physicians who both initiate and conduct the investigation, and who are directly responsible for administering or dispensing the investigational drug.
- Emergency Use INDs - Enable the FDA to authorize experimental drugs in an emergency situation where normal IND submission timelines cannot be met. Also used for patients who do not meet the criteria of an existing study protocol, or if an approved study protocol does not exist.
- Treatment INDs - Submitted for experimental drugs showing potential to address serious or immediately life-threatening conditions while the final clinical work is conducted and the FDA review takes place.
Per the G-PharmeCTD , non-commercial products refer to products not intended to be distributed commercially and include the above listed IND types.
As indicated in the G-IND-Determination , in general, human research studies must be conducted under an IND if all of the following research conditions apply:
- A drug is involved as defined in the FDCAct
- A clinical investigation is being conducted as defined in 21CFR312
- The clinical investigation is not otherwise exempt from 21CFR312
The G-IND-Determination states that biological products may also be considered drugs within the meaning of the FDCAct .
Further, per 21CFR312 and the G-IND-Determination , whether an IND is required to conduct an investigation of a marketed drug primarily depends on the intent of the investigation and the degree of risk associated with the use of the drug in the investigation. See 21CFR312 and the G-IND-Determination for detailed exemption conditions for marketed drugs.
Clinical Trial Review Process
As delineated in 21CFR312 , the FDA's primary objectives in reviewing an IND are to ensure human participant safety and rights in all phases of the investigation. Phase 1 submission reviews focus on assessing investigation safety, and Phase 2 and 3 submission reviews also include an assessment of the investigation’s scientific quality and ability to yield data capable of meeting marketing approval statutory requirements. An IND may be submitted for one (1) or more phases of an investigation.
As per USA-41 and USA-94 , the FDA’s Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER) receive IND submissions for drugs, therapeutic biological products, and other biologicals. Per the FDCAct and 21CFR312 , an IND automatically goes into effect 30 calendar days from receipt, unless the FDA notifies the sponsor that the IND is subject to a clinical hold, or the FDA has notified the sponsor earlier that the trial may begin. A clinical hold is an order the FDA issues to delay or suspend a clinical investigation. If the FDA determines there may be grounds for imposing a clinical hold, an attempt will be made to discuss and resolve any issues with the sponsor prior to issuing the clinical hold order. See 21CFR312 for more information on clinical holds.
According to USA-41 , with respect to sponsor-investigators, once the FDA receives the IND, an IND number will be assigned and the application will be forwarded to the appropriate reviewing division. A letter will be sent to the sponsor-investigator providing notification of the assigned IND number, date of receipt of the original application, address where future submissions to the IND should be sent, and the name and telephone number of the FDA person to whom questions about the application should be directed.
As indicated in 21CFR312 , the FDA may at any time during the course of the investigation communicate with the sponsor orally or in writing about deficiencies in the IND or about the FDA's need for more data or information. Furthermore, on the sponsor's request, the FDA will provide advice on specific matters relating to an IND.
21CFR312 indicates that once an IND is in effect, a sponsor must submit a protocol amendment if intending to conduct a study that is not covered by a protocol already contained in the IND, there is any change to the protocol that significantly affects the safety of subjects, or a new investigator is added to carry out a previously submitted protocol. A sponsor must submit a protocol amendment for a new protocol or a change in protocol before its implementation, while protocol amendments to add a new investigator or to provide additional information about investigators may be grouped and submitted at 30-day intervals. See 21CFR312 for more information on protocol amendments.
As per 21CFR312 , if no subjects are entered into a clinical study two (2) years or more under an IND, or if all investigations under an IND remain on clinical hold for one (1) year or more, the IND may be placed by the FDA on inactive status. An IND that remains on inactive status for five (5) years or more may be terminated. See 21CFR312 for more information on inactive status.
21CFR312 indicates that the FDA may propose to terminate an IND based on deficiencies in the IND or in the conduct of an investigation under an IND. If the FDA proposes to terminate an IND, the agency will notify the sponsor in writing, and invite correction or explanation within a period of 30 days. If at any time the FDA concludes that continuation of the investigation presents an immediate and substantial danger to the health of individuals, the FDA will immediately, by written notice to the sponsor, terminate the IND. See 21CFR312 for more information on FDA termination.
For more information on CDER and CBER internal policies and procedures for accepting and reviewing applications, see USA-96 and USA-95 , respectively.
Expedited Processes
USA-84 further indicates that the FDA has several approaches to making drugs available as rapidly as possible:
- Breakthrough Therapy – expedites the development and review of drugs which may demonstrate substantial improvement over available therapy
- Accelerated Approval – allow drugs for serious conditions that fill an unmet medical need to be approved based on a surrogate endpoint
- Priority Review – a process by which the FDA’s goal is to take action on an application within six (6) months
- Fast Track – facilitates the development and expedites the review of drugs to treat serious conditions and fill an unmet medical need
See USA-84 and USA-85 for more information on each process. Additionally, see the FDCAct , as amended by the FDORA , for changes to the accelerated approval process.
The G-RWDRWE-Reg , issued as part of the FDA’s Real-World Evidence (RWE) Program (see USA-17 ), discusses the applicability of the 21CFR312 IND regulations to various clinical study designs that utilize real-world data (RWD). See the G-RWDRWE-Reg for more information.
For information on the appropriate use of adaptive designs for clinical trials and additional information to provide the FDA to support its review, see G-AdaptiveTrials .
For research involving cellular and gene therapy, see the guidance documents at USA-80 .
The Food & Drug Administration (FDA) does not levy a fee to review investigational new drug submissions.
However, per the FDCAct , FDARA , and USA-45 , the FDA has the authority to assess and collect user fees from companies that produce certain human drug and biological products as part of the New Drug Application (NDA). Per USA-43 , the NDA is the vehicle through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing in the United States. The data gathered during the animal studies and human clinical trials of an investigational new drug become part of the NDA.
As indicated in 21CFR50 , 21CFR56 , and 21CFR312 , the United States (US) has a decentralized process for the ethics review of clinical investigations. The sponsor must obtain institutional level ethics committee (EC) approval for each study. (Note: Institutional ECs are referred to as institutional review boards (IRBs) in the US.)
As set forth in 21CFR50 , 21CFR56 , and 21CFR312 , all clinical investigations for drug and biological products regulated by the Food & Drug Administration (FDA) require institutional EC approval.
The Pre2018-ComRule and the RevComRule also require that human subjects research receive institutional EC approval. However, note that these regulations’ definition of “human subject” does not include the use of non-identifiable biospecimens. Therefore, the use of non-identifiable biospecimens in research does not, on its own, mandate the application of the Pre2018-ComRule to such research. However, the RevComRule does require federal departments or agencies implementing the policy to work with data experts to reexamine the meaning of “identifiable private information” and “identifiable specimen” within one (1) year of the effective date and at least every four (4) years thereafter. In particular, these agencies will collaboratively assess whether there are analytic technologies or techniques that could be used to generate identifiable private information or identifiable specimens.
(See USA-65 for a list of Common Rule departments/agencies, and the Regulatory Authority section for more information on when the Pre2018-ComRule and the RevComRule apply to research.)
Per the RevComRule , for non-exempt research (or exempt research that requires limited EC review) reviewed by an EC not operated by the institution doing the research, the institution and the EC must document the institution's reliance on the EC for research oversight and the responsibilities that each entity will undertake to ensure compliance with the RevComRule . Compliance can be achieved in a variety of ways, such as a written agreement between the institution and a specific EC, through the research protocol, or by implementing an institution-wide policy directive that allocates responsibilities between the institution and all ECs not operated by the institution. Such documentation must be part of the EC’s records. The G-HHS-Inst-Engagemt can help an institution to determine if a research study can be classified as non-exempt.
Ethics Committee Composition
As stated in 21CFR56 , the Pre2018-ComRule , and the RevComRule , an EC must be composed of at least five (5) members with varying backgrounds to promote complete and adequate research proposal review. The EC must be sufficiently qualified through member experience, expertise, and diversity, in terms of race, gender, cultural backgrounds, and sensitivity to issues such as community attitudes, to promote respect for its advice and counsel in safeguarding human participants’ rights and welfare. EC members must possess the professional competence to review research activities and be able to ascertain the acceptability of proposed research based on institutional commitments and regulations, applicable laws, and standards. In addition, if an EC regularly reviews research involving vulnerable populations, the committee must consider including one (1) or more individuals knowledgeable about and experienced in working with those participants. See the Vulnerable Populations section for details on vulnerable populations.
At a minimum, each EC must also include the following members:
- One (1) primarily focused on scientific issues
- One (1) focused on nonscientific issues
- One (1) unaffiliated with the institution, and not part of the immediate family of a person affiliated with the institution
No EC member may participate in the initial or continuing review of any project in which the member has a conflicting interest, except to provide EC requested information.
Terms of Reference, Review Procedures, and Meeting Schedule
As delineated in 21CFR56 , ECs must follow written procedures for the following:
- Conducting initial and continuing reviews, and reporting findings and actions
- Determining which projects require review more often than annually, and which projects need verification from sources other than the investigator that no material changes have occurred since the previous EC review
- Ensuring that changes in approved research are not initiated without EC review and approval except where necessary to eliminate apparent immediate hazards to participants
- Ensuring prompt reporting to the EC, institution, and FDA of changes in research activity; unanticipated problems involving risks to participants or others; any instance of serious or continuing noncompliance with these regulations or EC requirements or determinations; or EC approval suspension/termination
Per the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs , ECs must establish and follow written procedures for the following:
- Conducting initial and continuing reviews, and reporting findings and actions to the investigator and the institution
- Ensuring prompt reporting to the EC of proposed changes in research and ensuring that investigators conduct the research in accordance with the terms of the EC approval until any proposed changes have received EC review and approval, except where necessary to eliminate apparent immediate hazards to participants
- Ensuring prompt reporting to the EC, the institution, the FDA, and the Department of Health & Human Services (HHS) ’ Office for Human Research Protections (OHRP) of any unanticipated problems involving risks to participants or others; any instance of serious or continuing noncompliance with these regulations or EC requirements or determinations; or EC approval suspension/termination.
21CFR56 , the Pre2018-ComRule , and the RevComRule further require that an institution, or where appropriate an EC, prepare and maintain adequate documentation of EC activities, including copies of all research proposals reviewed. The applicable records must be retained for at least three (3) years after completion of the research. For more details on the EC records included in this requirement, see the Pre2018-ComRule , the RevComRule , and 21CFR56 .
See G-IRBProcs for detailed FDA guidance on EC written procedures to enhance human participant protection and reduce regulatory burden. The guidance includes a Written Procedures Checklist that incorporates regulatory requirements as well as recommendations on operational details to support the requirements.
Per 21CFR56 , the Pre2018-ComRule , and the RevComRule , proposed research must be reviewed during convened meetings at which a majority of the EC members are present, including at least one (1) member whose primary concerns are nonscientific, except when an expedited review procedure is used. Research is only considered approved if it receives the majority approval of attending members.
Refer to the Pre2018-ComRule , the RevComRule , 21CFR56 , the G-IRBProcs , and the G-IRBFAQs for detailed EC procedural requirements.
In addition, per the Pre2018-ComRule , the RevComRule , and the G-HHS-Inst-Engagemt , any institution engaged in non-exempt human subjects research conducted or supported by a Common Rule department/agency (as identified in USA-65 ) must also submit a written assurance of compliance to OHRP. According to USA-59 , the Federalwide Assurance (FWA) is the only type of assurance of compliance accepted and approved by OHRP for HHS-funded research. See USA-57 for more information on FWAs.
21CFR56 , 21CFR312 , the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs state that the primary scope of information assessed by the institutional ethics committee (EC) (referred to as an institutional review board (IRB) in the United States (US)) relates to maintaining and protecting the dignity and rights of research participants and ensuring their safety throughout their participation in a clinical trial. As delineated in 21CFR56 , the Pre2018-ComRule , and the RevComRule , the EC must also pay special attention to reviewing informed consent and to protecting the welfare of certain classes of participants deemed to be vulnerable. (See the Vulnerable Populations; Children/Minors; Pregnant Women, Fetuses, & Neonates; Prisoners; and Mentally Impaired sections for additional information about these populations). The EC is also responsible for ensuring a competent review of the research protocol, evaluating the possible risks and expected benefits to participants, and verifying the adequacy of confidentiality safeguards.
See USA-65 for a list of Common Rule departments/agencies, and the Regulatory Authority section for more information on when the Pre2018-ComRule and the RevComRule apply to research.
Role in Clinical Trial Approval Process
In accordance with 21CFR56 and 21CFR312 , the Food & Drug Administration (FDA) must review an investigational new drug application (IND) and an EC must review and approve the proposed study prior to a sponsor initiating a clinical trial. The institutional EC review of the clinical investigation may be conducted in parallel with the FDA review of the IND. However, EC approval must be obtained prior to the sponsor being permitted to initiate the clinical trial. According to 21CFR56 , the Pre2018-ComRule , and the RevComRule , the EC may approve, require modifications in (to secure approval), or disapprove the research.
Refer to the G-RevComRule-FDA for information on the impact of the RevComRule on studies conducted or supported by the Department of Health & Human Services (HHS) that must also comply with FDA regulations.
Per 21CFR56 , the Pre2018-ComRule , the RevComRule , and the G-IRBContRev , an EC has the authority to suspend or terminate approval of research that is not being conducted in accordance with the EC’s requirements or that has been associated with unexpected serious harm to participants. Any suspension or termination of approval will include a statement of the reasons for the EC’s action and will be reported promptly to the investigator, appropriate institutional officials, and the department or agency head (e.g., the FDA). See the G-IRBContRev for additional information and FDA recommendations on suspension or termination of EC approval.
Expedited Review
21CFR56 , the Pre2018-ComRule , and the RevComRule indicate that the FDA and HHS maintain a list of research categories that may be reviewed by an EC through an expedited review procedure (see the G-IRBExpdtdRev for the list). An EC may use the expedited review procedure to review the following:
- Some or all of the research appearing on the list and found by the reviewer(s) to involve no more than minimal risk
- Minor changes in previously approved research during the period (of one (1) year or less) for which approval is authorized
- Under the RevComRule , research for which limited EC review is a condition of exemption
21CFR56 , the Pre2018-ComRule , and the RevComRule specify that under an expedited review procedure, the review may be carried out by the EC chairperson or by one (1) or more experienced reviewers designated by the chairperson from among the EC’s members. In reviewing the research, the reviewers may exercise all of the authorities of the EC except that the reviewers may not disapprove the research. A research activity may be disapproved only after review in accordance with the EC’s non-expedited review procedure.
Continuing Review and Re-approval
21CFR56 and the G-IRBContRev state that any clinical investigation must not be initiated unless the reviewed and approved study remains subject to continuing review at intervals appropriate to the degree of risk, but not less than once a year. The G-IRBContRev notes that when continuing review of the research does not occur prior to the end of the approval period specified by the EC, EC approval expires automatically. A lapse in EC approval of research occurs whenever an investigator has failed to provide continuing review information to the EC, or the EC has not conducted continuing review and re-approved the research by the expiration date of the EC approval. In such circumstances, all research activities involving human participants must stop. Enrollment of new participants cannot occur after the expiration of EC approval.
In addition, per the G-IRBContRev , research that qualified for expedited review at the time of initial review will generally continue to qualify for expedited continuing review. For additional information and FDA recommendations regarding continuing review, see the G-IRBContRev .
The Pre2018-ComRule similarly indicates that the EC must conduct reviews at intervals appropriate to the degree of risk, but not less than once per year. However, the RevComRule provides the following exceptions to the continuing review requirement, unless an EC determines otherwise:
- Research eligible for expedited review
- Research reviewed by the EC in accordance with the limited EC review described in Section 46.104 of the RevComRule
- Research that has progressed to the point that it involves data analysis and/or accessing follow-up clinical data from procedures that are part of clinical care
Exemptions under the Revised Common Rule
Per the RevComRule , certain categories of research are exempt from EC review, and some “exempt” activities require limited EC review or broad consent. Users should refer to Section 46.104 of the RevComRule for detailed information on research categories specifically exempt from EC review, or exempt activities requiring limited EC review or broad consent.
Per USA-54 , for secondary research that does not qualify for an exemption under the RevComRule , the applicant must either apply for a waiver of the informed consent requirement from the EC, obtain study-specific informed consent, or obtain broad consent.
Further, the RevComRule modifies what constitutes research to specifically exclude the following types of research:
- Scholarly and journalistic activities
- Public health surveillance activities authorized by a public health authority to assess onsets of disease outbreaks or conditions of public health importance
- Collection and analysis of information, biospecimens, or records by or for a criminal justice agency for criminal investigative activities
- Authorized operational activities in support of intelligence, homeland security, defense, or other national security missions
See the G-IRBFAQs , the G-OHRP-IRBApprvl , and USA-54 for frequently asked questions regarding EC procedures, approval with conditions, example research, expedited review, limited review, and continuing review.
Per the FDA’s G-IRBReview , an EC may review studies that are not performed on-site. When an institution has a local EC, the written procedures of that EC or of the institution should define the scope of studies subject to review by that EC. A non-local EC may not become the EC of record for studies within that defined scope unless the local EC or the administration of the institution agree. Any agreement to allow review by a non-local EC should be in writing. For more information, see G-IRBReview .
Cooperative Research Studies
In the event of multicenter clinical studies, also known as cooperative research studies, taking place at US institutions that are subject to the RevComRule , the institutions must rely on a single EC to review that study for the portion of the study conducted in the US. The reviewing EC will be identified by the Common Rule department/agency (as identified in USA-65 ) supporting or conducting the research or proposed by the lead institution subject to the acceptance of the department/agency. The exceptions to this requirement include: when multicenter review is required by law (including tribal law) or for research where any federal department or agency supporting or conducting the research determines that the use of a single EC is not appropriate.
Designed to complement the RevComRule , per the NIHNotice16-094 and the NIHNotice17-076 , the National Institutes of Health (NIH) issued a final policy requiring all institute-funded multicenter clinical trials conducted in the US to be overseen by a single EC, unless prohibited by any federal, tribal, or state law, regulation, or policy.
For more information on multicenter research, see the FDA’s G-CoopRes . For more information on how new sites added to ongoing cooperative research can follow the same version of the Common Rule, see the HHS Office for Human Research Protections (OHRP) ’s G-ComRuleCnsstncy .
Many institutional ethics committees (ECs) (referred to as institutional review boards (IRBs) in the United States (US)) charge fees to review research proposals submitted by industry-sponsored research or other for-profit entities. However, this varies widely by institution. Neither the Department of Health & Human Services (HHS) nor the Food & Drug Administration (FDA) regulate institutional EC review fees. Because each EC has its own requirements, individual ECs should be contacted to confirm their specific fees.
As delineated in 21CFR56 and 45CFR46-B-E , the Department of Health & Human Services (HHS) and the HHS’ Food & Drug Administration (FDA) have mandatory registration programs for institutional ethics committee (ECs), referred to as institutional review boards (IRBs) in the United States (US). A single electronic registration system ( USA-28 ) for both agencies is maintained by HHS’ Office for Human Research Protections (OHRP) .
Registration, Auditing, and Accreditation
In accordance with the G-IRBReg-FAQs and USA-61 , EC registration with the HHS OHRP system ( USA-28 ) is not a form of accreditation or certification by either the FDA that the EC is in full compliance with 21CFR56 , or by the HHS that the EC is in full compliance with 45CFR46-B-E . Neither EC competence nor expertise is assessed during the registration review process by either agency.
According to 21CFR56 and the G-IRBReg-FAQs , the FDA requires each EC in the US, that either reviews clinical investigations regulated by the agency under the FDCAct or reviews investigations intended to support research or marketing permits for agency-regulated products, to register electronically in the HHS OHRP system ( USA-28 ). Only individuals authorized to act on the EC’s behalf are permitted to submit registration information. Non-US ECs may register voluntarily. The G-IRBReg-FAQs also indicates that while registration of non-US ECs is voluntary, the information the FDA receives from them is very helpful.
As stated in 21CFR56 and the G-IRBReg-FAQs , any EC not already registered in the HHS OHRP system ( USA-28 ) must submit an initial registration prior to reviewing a clinical investigation in support of an investigational new drug application (IND). The HHS OHRP system ( USA-28 ) provides instructions to assist users, depending on whether the EC is subject to regulation by only the OHRP, only the FDA, or both the OHRP and the FDA.
21CFR56 and the G-IRBReg-FAQs indicate that FDA EC registration must be renewed every three (3) years. EC registration becomes effective after review and acceptance by the HHS.
See 21CFR56 and the G-IRBReg-FAQs for detailed EC registration submission requirements. See the G-IRBInspect for FDA inspection procedures of ECs.
Per the Pre2018-ComRule and RevComRule , institutions engaging in research conducted or supported by a Common Rule department/agency (as identified in USA-65 ) must obtain an approved assurance that it will comply with the Pre2018-ComRule or RevComRule requirements and certify to the department/agency heads that the research has been reviewed and approved by an EC provided for in the assurance.
Per USA-59 , a Federalwide Assurance (FWA) of compliance is a document submitted by an institution (not an EC) engaged in non-exempt human subjects research conducted or supported by HHS that commits the institution to complying with Pre2018-ComRule or RevComRule requirements. FWAs also are approved by the OHRP for federalwide use, which means that other federal departments and agencies that have adopted the Federal Policy for the Protection of Human Subjects ( Pre2018-ComRule or RevComRule ) may rely on the FWA for the research that they conduct or support. Institutions engaging in research conducted or supported by non-HHS federal departments or agencies should consult with the sponsoring department or agency for guidance regarding whether the FWA is appropriate for the research in question.
Per USA-54 , institutions do not need to change an existing FWA because of the RevComRule . See USA-57 for more information on FWAs.
Per 45CFR46-B-E and USA-61 , all ECs that review human subjects research conducted or supported by HHS and are to be designated under an OHRP FWA must register electronically with the HHS OHRP system ( USA-28 ). An individual authorized to act on behalf of the institution operating the EC must submit the registration information. EC registration becomes effective for three (3) years when reviewed and approved by OHRP.
Per USA-59 , an institution must either register its own EC (an “internal” EC) or designate an already registered EC operated by another organization (“external” EC) after establishing a written agreement with that other organization. Additionally, each FWA must designate at least one (1) EC registered with the OHRP. The FWA is the only type of assurance of compliance accepted and approved by the OHRP.
See 45CFR46-B-E , USA-58 , and USA-61 for detailed registration requirements and instructions.
As delineated in 21CFR312 , USA-42 , and USA-52 , the United States (US) requires the sponsor to submit an investigational new drug application (IND) for the Food & Drug Administration (FDA) 's review and authorization to obtain an exemption to ship investigational drug or biological products across state lines and to administer these investigational products in humans. Per 21CFR312 and the G-IND-Determination , whether an IND is required to conduct an investigation of a drug to be marketed (this includes biological products under the FDCAct ) primarily depends on the intent of the investigation, and the degree of risk associated with the use of the drug in the investigation. See the Scope of Assessment section for more information.
In addition, per 21CFR56 and 21CFR312 , institutional ethics committee (EC) (institutional review board (IRB) in the US) review of the clinical investigation may be conducted in parallel with the FDA review of the IND. However, EC approval must be obtained prior to the sponsor being permitted to initiate the clinical trial.
Regulatory Submission
According to 21CFR312 , meetings between a sponsor and the FDA may be useful in resolving questions and issues raised during the course of a clinical investigation. The FDA encourages such meetings to the extent that they aid in the evaluation of the drug and in the solution of scientific problems concerning the drug, to the degree the FDA's resources permit. See 21CFR312 for more information on meetings with the FDA.
A sponsor who is conducting a clinical trial to support a future marketing application may ask to meet with the FDA for a special protocol assessment (SPA) to help ensure the clinical trial can support the application. For more information, see G-SPA .
Additionally, the G-FDAComm describes the FDA’s philosophy regarding timely interactive communication with IND sponsors, the scope of appropriate interactions between review teams and sponsors, the types of advice appropriate for sponsors to seek from the FDA in pursuing their drug development programs, and general expectations for the timing of FDA response to sponsor inquiries. See the G-FDAComm for more information.
According to the G-PharmeCTD , which implements FDCAct requirements, and as described in USA-34 and USA-53 , commercial IND submissions must be submitted in the Electronic Common Technical Document (eCTD) format. Noncommercial INDs are exempt from this eCTD format submission requirement. “Noncommercial products” refer to products not intended to be distributed commercially, including investigator-sponsored INDs and expanded access INDs (e.g., emergency use and treatment INDs). However, the G-AltrntElecSubs indicates that sponsors and applicants who receive an exemption or a waiver from filing in eCTD format should still provide those exempted or waived submissions electronically, in an alternate format.
The G-AltrntElecSubs and USA-35 indicate that for both eCTD and alternate electronic formats, submissions should include only FDA fillable forms and electronic signatures. Scanned images of FDA fillable forms should not be submitted. In addition, before making an electronic submission, a pre-assigned application number should be obtained by contacting the FDA’s Center for Drug Evaluation and Research (CDER) or Center for Biologics Evaluation and Research (CBER) . See USA-35 for more information on requesting an application number.
For more information and detailed requirements on eCTD submissions, see the G-PharmeCTD , the G-eCTDTech , USA-35 , and USA-36 . Additionally, the G-CBER-ElecINDs provides instructions on how to submit an IND using an electronic folder structure on a CD-ROM.
According to the G-eCTDspecs and USA-7 , eCTD submissions sized 10 GB and under for most applications must be submitted via the FDA Electronic Submissions Gateway (ESG) ( USA-44 ). However, the G-eCTDspecs adds that the FDA also recommends the use of USA-44 for submissions greater than 10 GB when possible. See USA-8 for information on how to create an account.
As indicated in the G-eCTDspecs , physical media greater than 10 GB should be submitted using a USB drive. For specific instructions on how to submit physical media, email CDER at [email protected] or CBER at [email protected] . See the G-eCTDspecs for additional physical media information.
The IND must be submitted in English. As indicated in 21CFR312 , the sponsor must submit an accurate and complete English translation of each part of the IND that is not in English. The sponsor must also submit a copy of each original literature publication for which an English translation is submitted.
According to USA-41 and USA-94 , paper submissions of INDs should be sent to CDER or CBER at the following locations, as appropriate:
Drugs (submitted by Sponsor-Investigators):
Food and Drug Administration Center for Drug Evaluation and Research (CDER) Central Document Room 5901-B Ammendale Rd. Beltsville, MD 20705-1266
Therapeutic Biological Product (submitted by Sponsor-Investigators) :
Food and Drug Administration Center for Drug Evaluation and Research (CDER) Therapeutic Biological Products Document Room 5901-B Ammendale Rd. Beltsville, MD 20705-1266
Center for Biologics Evaluation and Research-Regulated Products:
Food and Drug Administration Center for Biologics Evaluation and Research (CBER) Document Control Center 10903 New Hampshire Avenue WO71, G112 Silver Spring, MD 20993-0002
(Note: Per USA-94 , CBER also accepts electronic media via mail, but electronic or email submission is preferred.)
Based on information provided in 21CFR312 , for paper IND submissions, the sponsor must submit an original and two (2) copies, including the original submission and all amendments and reports.
Ethics Review Submission
Each EC maintains its own procedures and processes for review. Consequently, there is no stated regulatory requirement for clinical trial submission processes.
Regulatory Authority Requirements
As specified in 21CFR312 , an investigational new drug application (IND) to the Food & Drug Administration (FDA) must include the following documents, in the order provided below:
- Cover sheet (Form FDA 1571 ( USA-76 )) (including, but not limited to: sponsor contact information, investigational product (IP) name, application date, phase(s) of clinical investigation to be conducted, and commitment that the institutional ethics committee (EC) (institutional review board (IRB) in the United States (US)) will conduct initial and continuing review and approval of each study proposed in the investigation)
- Table of contents
- Introductory statement and general investigational plan
- Investigator’s brochure (IB)
- Chemistry, manufacturing, and control data
- Pharmacology and toxicology data
- Previous human experience with the IP
- Additional information (e.g., drug dependence and abuse potential, radioactive drugs, pediatric studies)
- Relevant information (e.g., foreign language materials and number of copies - see Submission Process section for details)
For detailed application requirements, see 21CFR312 . In addition, see USA-40 for other IND forms and instructions.
Furthermore, for information on the appropriate use of adaptive designs for clinical trials and additional information to provide to the FDA to support its review, see G-AdaptiveTrials .
The G-RWDRWE-Doc states that to facilitate the FDA’s internal tracking of submissions that include real-world data (RWD) and real-world evidence (RWE), sponsors and applicants are encouraged to identify in their submission cover letters certain uses of RWD/RWE. For more information, see the G-RWDRWE-Doc .
The FDCAct , as amended by the FDORA , requires sponsors to submit diversity action plans for certain clinical trials, such as a clinical investigation of a new drug that is a phase 3 study. See the FDORA for more details. (Note: The FDA’s guidance on diversity action plans is currently in draft. The ClinRegs team will continue to monitor this requirement and incorporate any updates as appropriate).
According to the G-PedStudyPlans , a sponsor who is planning to submit to the FDA a marketing application (or supplement to an application) for a new active ingredient, new indication, new dosage form, new dosing regimen, or new route of administration is required to submit an initial pediatric study plan (iPSP), if required by the Pediatric Research Equity Act (PREA) . An exception to this is if the drug is for an indication granted an orphan designation. For additional details and recommendations to sponsors regarding the submission of an iPSP, see the G-PedStudyPlans .
Ethics Committee Requirements
Each EC has its own application form and clearance requirements, which can differ significantly regarding application content requirements. However, the requirements listed below comply with 21CFR56 as well as the US-ICH-GCPs and are basically consistent across all US ECs.
As per 21CFR56 , the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs , the EC should obtain the following documents and must ensure the listed requirements are met prior to approving the study (Note: The regulations provide overlapping and unique elements so each of the items listed below will not necessarily be in each source):
- Clinical protocol
- Informed consent forms (ICFs) and participant information (the RevComRule also requires information regarding whether informed consent was appropriately sought and documented, or waived)
- Participant recruitment procedures
- Safety information
- Participant payments and compensation
- Investigator(s) current Curriculum Vitaes (CVs)
- Additional required EC documentation
- Risks to participants are minimized and are reasonable in relation to anticipated benefits
- Participant selection is equitable
- Adequate provisions are made to protect participant privacy and maintain confidentiality of data, where appropriate; the Department of Health & Human Services (HHS) will issue guidance to assist ECs in assessing what provisions are adequate to protect participant privacy and maintain the confidentiality of data
Per the RevComRule , where limited EC review applies, the EC does not need to make the determinations outlined above. Rather, limited EC review includes determinations that broad consent will be/was obtained properly, that adequate protections are in place for safeguarding the privacy and confidentiality of participants, and (for secondary studies) that individual research results will not be returned to participants. See USA-65 for a list of Common Rule departments/agencies, and the Regulatory Authority section for more information on when the Pre2018-ComRule and the RevComRule apply to research.
See 21CFR56 , the Pre2018-ComRule , the RevComRule , and section 3 of the US-ICH-GCPs for additional EC submission requirements.
Clinical Protocol
According to the US-ICH-GCPs , the clinical protocol should contain the following elements:
- General information
- Background information
- Trial objectives and purpose
- Trial design
- Participant selection/withdrawal
- Participant treatment
- Efficacy assessment
- Safety assessment
- Direct access to source data/documents
- Quality control/quality assurance
- Data handling/recordkeeping
- Financing/insurance
- Publication policy
- For complete protocol requirements, see section 6 of the US-ICH-GCPs .
Per the NIHNotice17-064 , and provided in USA-29 and USA-27 , the National Institutes of Health (NIH) and the FDA developed a clinical trial protocol template with instructional and example text for NIH-funded investigators to use when writing protocols for phase 2 and 3 clinical trials that require IND applications.
As delineated in 21CFR56 and 21CFR312 , institutional ethics committee (EC) (institutional review board (IRB) in the United States (US)) review of the clinical investigation may be conducted in parallel with the Food & Drug Administration (FDA) 's review of the investigational new drug application (IND). However, EC approval must be obtained prior to the sponsor being permitted to initiate the clinical trial.
Regulatory Authority Approval
Per the FDCAct and 21CFR312 , initial INDs submitted to the FDA’s Center for Drug Evaluation and Research (CDER) or Center for Biologics Evaluation and Research (CBER) automatically go into effect in 30 calendar days, unless the FDA notifies the sponsor that the IND is subject to a clinical hold, or the FDA has notified the sponsor earlier that the trial may begin. As indicated in 21CFR312 , the FDA will provide the sponsor with a written explanation of the basis for the hold as soon as possible, and no more than 30 days after the imposition of the clinical hold. See 21CFR312 for more information on clinical hold timelines. For more information on CDER and CBER internal policies and procedures for reviewing applications, see USA-96 and USA-95 , respectively.
According to USA-41 and USA-42 , clinical studies must not be initiated until 30 days after the FDA receives the IND, unless the FDA provides earlier notification that studies may begin.
Ethics Committee Approval
Each EC maintains its own procedures and processes for review. Consequently, there is no stated regulatory requirement for a standard timeline of review and approval of the clinical trial. However, according to the US-ICH-GCPs , the institutional EC should review a proposed clinical trial within a reasonable time.
In accordance with 21CFR312 , USA-41 , and USA-42 , a clinical trial can only commence after the investigational new drug application (IND) is reviewed by the Food & Drug Administration (FDA) , which will provide a written determination within 30 days of receiving the IND. No waiting period is required following the 30-day FDA review period, unless the agency imposes a clinical hold on the IND or sends an earlier notification that studies may begin. Per 21CFR312 and 21CFR56 , ethics approval from an institutional ethics committee (EC) (known as institutional review board (IRB) in the United States (US)) is also required before a clinical trial can commence.
As per 21CFR312 , once an IND has been submitted and following the 30-day review period, the sponsor is permitted to import an investigational product (IP). (See the Manufacturing & Import section for additional information).
See the G-CTDiversity for FDA recommendations to sponsors on increasing enrollment of underrepresented populations in their clinical trials.
Clinical Trial Agreement
Prior to the trial’s commencement, as addressed in the 21CFR312 and the G-1572FAQs , the sponsor must obtain from the investigator(s) a signed Statement of Investigator, Form FDA 1572 ( USA-77 ). This form serves as the investigator’s agreement to provide certain information to the sponsor and to ensure compliance with the FDA’s clinical investigation regulations. Refer to the 21CFR312 , the G-1572FAQs , and USA-40 for further information.
The US-ICH-GCPs indicates that the sponsor must obtain the investigator’s/institution’s agreement:
- To conduct the trial in compliance with good clinical practice (GCP), with the applicable regulatory requirement(s), and with the protocol agreed to by the sponsor and given approval/favorable opinion by the EC;
- To comply with procedures for data recording/reporting;
- To permit monitoring, auditing, and inspection; and
- To retain the trial-related essential documents until the sponsor informs the investigator/institution these documents are no longer needed.
The sponsor and the investigator/institution must sign the protocol, or an alternative document, to confirm this agreement.
Clinical Trial Registration
The FDAMA , the FDAAA , and 42CFR11 require the responsible party, either the sponsor or the principal investigator (PI) designated by the sponsor, to register electronically with the ClinicalTrials.gov databank ( USA-78 ). Per the FDAAA and 42CFR11 , the sponsor/PI must register no later than 21 calendar days after the first human participant is enrolled in a trial.
42CFR11 expands the legal requirements for submitting clinical trial registration information and results for investigational products that are approved, licensed, or cleared by the FDA.
The National Institutes of Health (NIH) issued NIHTrialInfo to complement 42CFR11 requirements. This policy requires all NIH-funded awardees and investigators conducting clinical trials, funded in whole or in part by the NIH, regardless of study phase, type of intervention, or whether they are subject to the regulation, to ensure that they register and submit trial results to ClinicalTrials.gov ( USA-78 ).
See 42CFR11 , the NIHTrialInfo , and USA-49 for detailed information on ClinicalTrials.gov ( USA-78 ). See also the FDA’s G-DataBankPnlty for clarification on the types of civil money penalties that may be issued for failing to register a clinical trial.
Safety Reporting Definitions
In accordance with 21CFR312 , the G-IND-Safety , 42CFR11 , and USA-38 , the following definitions provide a basis for a common understanding of safety reporting requirements in the United States (US):
- Adverse Event – Any untoward medical occurrence associated with the use of a drug in humans, whether or not considered drug related
- Suspected Adverse Reaction – Any adverse event where there is a reasonable possibility that the drug caused the adverse event
- Adverse Reaction – Any adverse event caused by a drug. Adverse reactions are a subset of all suspected adverse reactions where there is reason to conclude that the drug caused the event
- Serious Adverse Event/Serious Suspected Adverse Reaction – An adverse event/suspected adverse reaction that results in death, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, causes persistent or significant disability/incapacity, results in a congenital anomaly/birth defect, or leads to a substantial disruption of the participant’s ability to conduct normal life functions
- Unexpected Adverse Event/Unexpected Suspected Adverse Reaction – An adverse event/suspected adverse reaction that is not listed in the investigator’s brochure (IB), or is not listed at the specificity or severity that has been observed; or if an IB is not required or available, is not consistent with the risk information described in the general investigational plan or elsewhere in the application
- Life-threatening Adverse Event/Life-threatening Suspected Adverse Reaction – An adverse event/suspected adverse reaction is considered “life-threatening” if its occurrence places the participant at immediate risk of death. It does not include an adverse event/suspected adverse reaction that, had it occurred in a more severe form, might have caused death
According to the G-HHS-AEReqs , the Department of Health & Human Services (HHS) ’s 45CFR46 regulations (the Pre2018-ComRule , the RevComRule , and 45CFR46-B-E ) do not define the terms “adverse event” or “unanticipated problems.” However, the Pre2018-ComRule and the RevComRule do contain requirements relevant to reviewing and reporting these incidents. See the G-HHS-AEReqs , the G-IRBRpting , the Pre2018-ComRule , and the RevComRule for further information.
Safety Reporting Requirements
Investigator Responsibilities
As delineated in 21CFR312 and the G-IND-Safety , the investigator must comply with the following reporting requirements:
- Serious adverse events, whether or not considered drug related, must be reported immediately to the sponsor
- Study endpoints that are serious adverse events must be reported in accordance with the protocol unless there is evidence suggesting a causal relationship between the drug and the event. In that case, the investigator must immediately report the event to the sponsor
- Non-serious adverse events must be recorded and reported to the sponsor according to the protocol specified timetable
- Report promptly to the ethics committee (EC) all unanticipated problems involving risk to human participants or others where adverse events should be considered unanticipated problems
Sponsor Responsibilities
As delineated in 21CFR312 , the G-IND-Safety , and USA-38 , the sponsor must report any suspected adverse reaction or adverse reaction that is both serious and unexpected. An adverse event is only required to be reported as a suspected adverse reaction if there is evidence to suggest a causal relationship between the drug and the adverse event .
The sponsor is required to notify the Food & Drug Administration (FDA) and all participating investigators in a written safety report of potential serious risks, from clinical trials or any other source, as soon as possible, but no later than 15 calendar days after the sponsor determines the information qualifies for reporting. Additionally, the sponsor must notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction as soon as possible, but no later than seven (7) calendar days following receipt of the information. The sponsor is required to submit a follow-up safety report to provide additional information obtained pertaining to a previously submitted safety report. This report should be submitted without delay, as soon as the information is available, but no later than 15 calendar days after the sponsor initially receives the information.
Per 21CFR312 and the G-IND-Safety , the sponsor must also report the following:
- Any findings from epidemiological studies, pooled analyses of multiple studies, or clinical studies (other than those reported in the safety report), whether or not conducted under an investigational new drug application (IND), and whether or not conducted by the sponsor, that suggest a significant risk in humans exposed to the drug
- Any findings from animal or in vitro testing, whether or not conducted by the sponsor, that suggest a significant risk in humans exposed to the drug
- Any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or IB
In each safety report, the sponsor must identify all safety reports previously submitted to the FDA concerning a similar suspected adverse reaction and must analyze the significance of the suspected adverse reaction in light of previous, similar reports, or any other relevant information. Refer to 21CFR312 and the G-IND-Safety for more details on these safety reporting requirements.
As part of the clinical trial results information submitted to ClinicalTrials.gov ( USA-78 ), 42CFR11 requires the responsible party, either the sponsor or the principal investigator (PI) designated by the sponsor, to submit three (3) tables of adverse event information. The tables should consist of the following summarized data:
- All serious adverse events
- All adverse events, other than serious adverse events, that exceed a frequency of five (5) percent in any arm of the trial
- All-cause mortalities
Per 42CFR11 and USA-70 , this information must be submitted no later than one (1) year after the primary completion date of the clinical trial. Submission of trial results may be delayed as long as two (2) years if the sponsor or PI submits a certification to ClinicalTrials.gov ( USA-78 ) that either: 1) the FDA has not yet approved, licensed, or cleared for marketing the investigational product (IP) being studied; or 2) the manufacturer is the sponsor and has sought or will seek approval within one (1) year.
See 42CFR11 for detailed adverse event reporting requirements.
Form Completion & Delivery Requirements
As per 21CFR312 , the G-IND-Safety , and USA-38 , the sponsor must submit each safety report in a narrative format on Form FDA 3500A ( USA-75 ), or in an electronic format that the FDA can process, review, and archive, and be accompanied by Form FDA 1571 ( USA-76 ) (cover sheet).
As per the G-IND-Safety and USA-38 , the submission must be identified as follows:
- “IND safety report” for 15-day reports
- “7-day IND safety report” for unexpected fatal or life-threatening suspected adverse reaction reports
- “Follow-up IND safety report” for follow-up information
The report must be submitted to the appropriate review division (i.e., Center for Drug Evaluation and Research (CDER) or Center for Biologics Evaluation and Research (CBER) ). Per USA-38 , the FDA recommends that sponsors submit safety reports electronically. Other means of rapid communication to the respective review division’s Regulatory Project Manager (e.g., telephone, facsimile transmission, email) may also be used. Per USA-90 , fatality reports to CBER should be sent to [email protected] .
Additionally, 21CFR312 and the G-IND-Safety indicate that the FDA will accept foreign suspected adverse reaction reports on CIOMS Form I (See USA-13 and USA-3 ) instead of Form FDA 3500A ( USA-75 ). See USA-38 and USA-48 for additional information.
Interim and Annual Progress Reports
As per the US-ICH-GCPs , the investigator should promptly provide written reports to the sponsor and the institutional ethics committee (EC) (institutional review board (IRB) in the United States (US)) on any changes significantly affecting the conduct of the trial, and/or increasing the risk to participants.
As specified in 21CFR312 , the investigator must furnish all reports to the sponsor who is responsible for collecting and evaluating the results obtained. In addition, per 21CFR56 and the US-ICH-GCPs the investigator should submit written summaries of the trial status to the institutional EC annually, or more frequently, if requested by the institutional EC.
21CFR312 states that the sponsor must submit a brief annual progress report on the investigation to the Food & Drug Administration (FDA) within 60 days of the anniversary date that the investigational new drug went into effect. The report must contain the following information for each study:
- Title, purpose, and description of patient population, and current status
- Summary of the participants screened (e.g., failed screenings; participants enrolled, withdrawn, or lost to follow-up; and other challenges)
- Summary information - including information obtained during the previous year’s clinical and nonclinical investigations
- Description of the general investigational plan for the coming year
- Updated investigator’s brochure, if revised
- Description of any significant Phase 1 protocol modifications not previously reported in a protocol amendment
- Brief summary of significant foreign marketing developments with the drug
- A log of any outstanding business for which the sponsor requests a reply, comment, or meeting
As indicated in 42CFR11 , trial updates must be submitted to ClinicalTrials.gov ( USA-78 ) according to the following guidelines:
- Not less than once every 12 months for updated general trial registration information
- Not later than 30 calendar days for any changes in overall recruitment status
- Not later than 30 calendar days after the trial reaches its actual primary completion date, the date the final participant was examined or received an intervention for the purposes of final collection data for the primary outcome
Final Report
As indicated in 21CFR312 , an investigator must provide the sponsor with an adequate report shortly after completion of the investigator’s participation in the investigation. There is no specific timeframe stipulated for when the report should be completed.
The US-ICH-GCPs also states that upon the trial’s completion, the investigator should inform the institution and the investigator/institution should provide the EC with a summary of the trial’s outcome, and supply the FDA with any additional report(s) required of the investigator/institution.
Additionally, per 42CFR11 and USA-70 , the sponsor or the principal investigator (PI) designated by the sponsor must submit results for applicable investigational product (IP) clinical trials to USA-78 no later than one (1) year following the study’s completion date. Submission of trial results may be delayed as long as two (2) years if the sponsor or PI submits a certification to USA-78 that indicates either: 1) the FDA has not yet approved, licensed, or cleared the IP being studied for marketing; or 2) the manufacturer is the sponsor and has sought or will seek approval within one (1) year. The results information must include data on the following:
- Participant flow
- Demographic and baseline characteristics
- Outcomes and statistical analysis
- Adverse events
- The protocol and statistical analysis plan
- Administrative information
See USA-49 for more information and 42CFR11 for more detailed requirements. See NIHTrialInfo for specific information on dissemination of NIH-funded clinical trial data.
As per 21CFR312 , 21CFR50 , and the US-ICH-GCPs , a sponsor is defined as a person who takes responsibility for and initiates a clinical investigation. The sponsor may be an individual or pharmaceutical company, governmental agency, academic institution, private organization, or other organization. The sponsor does not actually conduct the investigation unless the sponsor is a sponsor-investigator. 21CFR312 , 21CFR50 , and the US-ICH-GCPs define a sponsor-investigator as an individual who both initiates and conducts an investigation, and under whose immediate direction the investigational product is administered or dispensed.
In addition, 21CFR312 and the US-ICH-GCPs state that a sponsor may transfer responsibility for any or all obligations to a contract research organization (CRO).
Any trial-related responsibilities transferred to and assumed by a CRO should be specified in writing, and those obligations not covered by the written description will be deemed not to have been transferred. Further, a CRO that assumes any sponsor obligations must comply with the specific regulations delineated in 21CFR312 and will be subject to the same regulatory action as the sponsor for failure to comply with any obligation assumed under these regulations. However, per the US-ICH-GCPs , although a sponsor may transfer all trial-related duties and functions to a CRO, the sponsor is ultimately responsible for the study data’s quality and integrity.
As indicated in 21CFR312 , a sponsor may be either domestic or foreign.
As set forth in 21CFR312 and the US-ICH-GCPs , the sponsor is responsible for selecting the investigator(s) and the institution(s) for the clinical trial and for ensuring that the investigator(s) are qualified by training and experience. Prior to permitting an investigator(s) to conduct a study, the sponsor must obtain the following:
- Signed investigator’s statement (Form FDA 1572 ( USA-77 ))
- Curriculum vitae
- Financial disclosure information
As addressed in the G-1572FAQs , Form FDA 1572 ( USA-77 ) serves as the investigator’s agreement to provide certain information to the sponsor and to assure compliance with the Food & Drug Administration (FDA) 's clinical investigation regulations. Refer to the G-1572FAQs and USA-40 for further information.
In addition, prior to the start of the study, the sponsor must provide the investigator(s) with the protocol and the investigator’s brochure.
See G-InvstgtrResp for more information on investigator responsibilities.
As per the G-InvstgtrAdmin , the FDA may disqualify a clinical investigator from receiving investigational drugs (including biologics) if the FDA determines that the investigator has repeatedly or deliberately violated the agency’s regulations, or submitted false information to the sponsor or FDA in any required report. See the G-InvstgtrAdmin for more details.
Foreign Sponsor Responsibilities
No information is currently available.
Data and Safety Monitoring Board
As per 21CFR50 and the G-DMCs , Data and Safety Monitoring Boards (DSMBs), (also known as a Data Monitoring Committees (DMCs)), are not required by FDA regulations, except in the case of research conducted in emergency settings in which fulfilling the informed consent requirement is unfeasible. In this case, as stated in 21CFR50 , the FDA requires the establishment of an independent data monitoring committee to exercise oversight of the clinical investigation. See the G-DMCs for FDA recommendations on DSMB/DMC establishment.
Additionally, the Pre2018-ComRule and the RevComRule indicate that for all human subjects research funded and/or sponsored by a Common Rule department/agency (as identified in USA-65 ), the institutional ethics committee (EC) (institutional review board (IRB) in the United States (US)) must ensure that, when appropriate, the research plan makes adequate provisions for monitoring the data collected during the study to ensure participant safety. Moreover, per the NIHDataSftyMntrng and USA-72 , all National Institutes of Health (NIH) -funded clinical trials require a Data and Safety Monitoring Plan and monitoring should be commensurate with risk. DSMBs are also required for multi-site clinical trials with interventions that involve potential participant risk. See the NIHDataSftyMntrng and USA-72 for detailed Department of Health & Human Services (HHS) /NIH requirements.
Although not specified as a sponsor requirement, the US-ICH-GCPs states that a DSMB may be established to assess the progress of a clinical trial, including the safety data and the critical efficacy endpoints at intervals, and to recommend to the sponsor whether to continue, modify, or stop a trial.
Multicenter Studies
For all human subjects research funded and/or sponsored by a Common Rule department/agency, institutions that are located in the US and engaged in multicenter research/cooperative research studies must use a single EC to review the research. See the Scope of Review section , the RevComRule , and G-CoopRes for additional information.
The US-ICH-GCPs indicates that in the event of a multicenter clinical trial, the sponsor must ensure that:
- All investigators conduct the trial in strict compliance with the protocol agreed to by the sponsor, and given EC approval
- The case report forms (CRFs) are designed to capture the required data at all multicenter trial sites
- Investigator responsibilities are documented prior to the start of the trial
- All investigators are given instructions on following the protocol, complying with a uniform set of standards to assess clinical and laboratory findings, and completing the CRFs
- Communication among investigators is facilitated
See US-ICH-E17 for additional FDA guidance related to multi-regional clinical trials.
The United States (US) regulations do not require insurance.
Compensation
The G-IRBFAQs state that institutional policy, not Food & Drug Administration (FDA) regulation, determines whether compensation and medical treatment(s) will be offered and the conditions that might be placed on participant eligibility for compensation or treatment(s).
Injury or Death
According to the US-ICH-GCPs , the sponsor's policies and procedures should address the costs of treatment of trial subjects in the event of trial-related injuries in accordance with the applicable regulatory requirement(s).
As specified in 21CFR50 , the Pre2018-ComRule , the RevComRule , and US-ICH-GCPs , for research involving more than minimal risk, participants must be informed as to whether any compensation or medical treatments are available in the event of trial-related injuries. See the Required Elements section for additional information.
Trial Participation
As per the FDA’s G-SbjctPayment , compensation for participation is considered a recruitment incentive and not a benefit, and is often offered when the participant’s health benefits are remote or non-existent. Payment amounts and schedules should be presented to the institutional ethics committee (EC) (institutional review board (IRB) in the US) at the time of the initial review. The EC should ensure the payment amount and the proposed method and timing of disbursement are not coercive or present undue influence and are also included in the informed consent document. Payment to participants who withdraw may be made at the time that they would have completed the study. While the entire payment should not be contingent upon completion of the entire study, a small payment provided as an incentive for completion is acceptable to the FDA. Further, the FDA does not consider reimbursement for travel expenses to and from the clinical trial site and associated costs such as airfare, parking, and lodging to raise issues regarding undue influence.
Quality Assurance/Quality Control
Per the US-ICH-GCPs , the sponsor should implement a system to manage quality throughout all stages of the trial process, focusing on trial activities essential to ensuring participant protection and the reliability of trial results. The quality management system should use a risk-based approach that includes:
- During protocol development, identify processes and data that are critical to ensure participant protection and the reliability of trial results
- Identify risks to critical trial processes and data
- Evaluate the identified risks, against existing risk controls
- Decide which risks to reduce and/or which risks to accept
- Document quality management activities and communicate to those involved in or affected by these activities
- Periodically review risk control measures to ascertain whether the implemented quality management activities are effective and relevant
- In the clinical study report, describe the quality management approach implemented in the trial and summarize important deviations from the predefined quality tolerance limits and remedial actions taken
As stated in the US-ICH-GCPs , the sponsor is responsible for implementing and maintaining quality assurance (QA) and quality control (QC) systems with written standard operating procedures (SOPs) to ensure that trials are conducted and data generated, recorded, and reported in compliance with the protocol, the US-ICH-GCPs , and the applicable regulatory requirements. The sponsor is responsible for obtaining agreement from all involved parties to ensure direct access to all trial related sites, source data/documents, reports for monitoring and auditing purposes, and inspection by domestic and foreign regulatory authorities. QC should be applied to each stage of data handling to ensure that all data are reliable and have been correctly processed. A written agreement must be signed by both the sponsor and the investigator or any other parties involved with the clinical trial, verifying that all parties agree to the trial protocol, the monitoring and auditing practices, the SOPs, and their respective duties.
Per the G-ICH-E19 , the Food & Drug Administration (FDA) has adopted the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH)’s E19 guidance, A Selective Approach to Safety Data Collection in Specific Late-Stage Pre-Approval or Post-Approval Clinical Trials. The document describes circumstances in which it may be appropriate to reduce the collection of safety data in late-stage pre-approval and post-approval clinical trials, e.g., long-term outcome trials, when appropriate and with agreement from regulatory authorities. See the G-ICH-E19 for more information.
Furthermore, the FDA’s G-CTEmrgncy provides general considerations to assist sponsors, institutional ethics committees (ECs) (institutional review boards (IRBs) in the United States (US)), and clinical investigators in assuring the safety of trial participants, maintaining compliance with good clinical practice (GCP), and minimizing risks to trial integrity during disasters and public health emergencies that may lead to a major disruption of clinical trial conduct and operations. See the G-CTEmrgncy for more information.
See the G-eHealthRecords for the FDA’s guidance related to the use of electronic health records in clinical research.
Additionally, the G-CovariatesCT provides the FDA’s recommendations for the use of covariates in the analysis of randomized, parallel group clinical trials that are applicable to both superiority trials and noninferiority trials. See the G-CovariatesCT for more information.
Additionally, see USA-47 for a list of FDA clinical trials related guidance documents.
See USA-6 for information on the National Institutes of Health (NIH) ’s data management and sharing policy, the NIHDataMngmnt , which applies to all research that is funded or conducted in whole or in part by the NIH, and results in the generation of scientific data.
Monitoring Requirements
As part of its QA system, the US-ICH-GCPs notes that the sponsor should ensure the trial is monitored and audited. The purpose of the audit should be to evaluate trial conduct and compliance with the protocol, SOPs, the US-ICH-GCPs , and other applicable regulatory requirements. The sponsor should appoint auditors to review the clinical trial. The sponsor should ensure that the auditors are qualified by training and experience, and the auditor’s qualifications should be documented. The sponsor must also ensure that the audit is conducted in accordance with the sponsor’s own SOPs and the auditor observations are documented. The sponsor should develop a systematic, prioritized, risk-based approach to monitoring clinical trials. The extent and nature of monitoring is flexible and permits varied approaches that improve effectiveness and efficiency. The sponsor may choose on-site monitoring, a combination of on-site and centralized monitoring, or where justified, centralized monitoring. The sponsor should document the rationale for the chosen monitoring strategy (e.g., in the monitoring plan).
The FDA’s G-RiskMntrng states that for each clinical trial, the sponsor should develop a monitoring plan that describes the monitoring methods, responsibilities, and requirements for the trial. The monitoring plan should include a brief description of the study, its objectives, and the critical data and study procedures, with particular attention to data and procedures that are unusual in relation to clinical routine. The monitoring plan should also require training of study site staff. Additionally, the plan should communicate the specific risks to be addressed by monitoring and should provide those involved in monitoring with adequate information to effectively carry out their duties. The FDA also encourages greater use of centralized monitoring practices, where appropriate, with correspondingly less emphasis on on-site monitoring. Centralized monitoring techniques should be used to the extent appropriate and feasible to:
- Supplement or reduce the frequency and extent of on-site monitoring with monitoring activities that can be done as well or better remotely or with monitoring activities that can be accomplished using centralized processes only. Examples include monitoring data quality through routine review of submitted data, as well as completing administrative and regulatory tasks.
- Target on-site monitoring by identifying higher risk clinical sites (e.g., sites with data anomalies or a higher frequency of errors, protocol violations, or dropouts relative to other sites).
For more FDA guidance on a risk-based approach to monitoring and monitoring plans, see the G-RiskMntrng and the G-RiskMntrngQA .
Premature Study Termination/Suspension
As delineated in 21CFR312 and the US-ICH-GCPs , if the sponsor determines the study presents an unreasonable and significant risk to the participants, the sponsor must discontinue the study as soon as possible, and no later than five (5) working days after making the determination. The sponsor must also notify the FDA, all ECs, and all investigators who have participated in the study about the termination. Additionally, the sponsor must ensure the disposition of all remaining drugs and provide the FDA with a full report on the sponsor’s actions.
According to the US-ICH-GCPs , if it is discovered that noncompliance significantly affects or has the potential to significantly affect participant protection or reliability of trial results, the sponsor should perform a root cause analysis and implement appropriate corrective and preventive actions. Further, the EC should also be informed promptly and provided the reason(s) for the termination or suspension by the sponsor.
The G-InfrmdCnsnt , which is the FDA’s discussion of the regulations in 21CFR50 , further states that if a study is terminated, participants should be provided with as much information as possible regarding the reason for the termination. Such a discussion provides an opportunity to address questions that participants may have about an investigational product (IP) that was administered to them (e.g., immediate safety concerns, ability to participate in another clinical trial, and appropriate waiting period to do so) and what long-term follow-up may be available or necessary.
21CFR312 indicates that if the FDA terminates an investigational new drug application (IND) based on deficiencies in the IND or in the conduct of an investigation under an IND, the sponsor must end all clinical investigations conducted under the IND and recall or otherwise provide for the disposition of all unused supplies of the drug. See 21CFR312 for more information on FDA termination.
Electronic Data Processing System
Per the US-ICH-GCPs , when using electronic trial data handling processing systems, the sponsor must ensure and document that the electronic data processing system conforms to the sponsor’s established requirements for completeness, accuracy, reliability, and consistency of intended performance. To validate such systems, the sponsor should use a risk assessment approach that takes into consideration the system’s intended use and potential to affect human subject protection and reliability of trial results. In addition, the sponsor must maintain standard operating procedures (SOPs) that cover system setup, installation, and use. The SOPs should describe system validation and functionality testing, data collection and handling, system maintenance, system security measures, change control, data backup, recovery, contingency planning, and decommissioning. With respect to the use of these computerized systems, the responsibilities of the sponsor, investigator, and other parties should be clear, and the users should receive relevant training. Refer to the US-ICH-GCPs for additional information.
Records Management
As set forth in 21CFR312 and the US-ICH-GCPs , the sponsor must retain all sponsor-specific essential documents pertaining to the trial for at least two (2) years after a marketing application (known as a new drug application (NDA)) is approved for the drug; or if a NDA is not approved, until two (2) years after shipment and delivery of the drug for investigational use is discontinued and the Food & Drug Administration (FDA) has been notified. The sponsor should also inform the investigator(s)/institution(s) in writing of the need for record retention and when the trial-related records are no longer needed. Additionally, per 21CFR312 , the sponsor must upon request from the FDA, permit an officer or employee to access, copy, and verify any records and reports relating to the clinical investigation. Upon written request by the FDA, the sponsor must also submit the records or reports (or copies of them) to the agency.
In addition, the US-ICH-GCPs states that the sponsor and investigator/institution should maintain a record of the location(s) of their respective essential documents including source documents. The storage system used during the trial and for archiving (irrespective of the type of media used) should allow for document identification, version history, search, and retrieval. The sponsor should ensure that the investigator has control of and continuous access to the data reported to the sponsor. The investigator/institution should have control of all essential documents and records generated by the investigator/institution before, during, and after the trial.
Responsible Parties
As stated in USA-86 , the HIPAA Privacy Rule establishes the conditions under which protected health information (PHI) may be used or disclosed by covered entities for research purposes (Per USA-87 , the Privacy Rule is located at 45CFR160 and Subparts A and E of 45CFR164 ; see USA-87 for more information). The Privacy Rule builds upon protections, described in Department of Health & Human Services (HHS) (the Pre2018-ComRule and the RevComRule ) and Food & Drug Administration (FDA) ( 21CFR50 and 21CFR56 ) regulations, that help ensure the privacy of participants and the confidentiality of information. (Please note: ClinRegs does not provide information on state level personal data protection requirements.)
Per the Privacy Rule, a covered entity means: a health plan; a health care clearinghouse; or a health care provider who transmits any health information in electronic form in connection with a transaction covered by the Privacy Rule.
Data Protection
According to the FDA’s G-CertCnfdntlty , a Certificate of Confidentiality (CoC) is intended to help protect the privacy of human subject research participants from whom identifiable, sensitive information is being collected or used in furtherance of the research. CoCs must be issued for federally funded human subject research that collects or uses identifiable, sensitive information (mandatory CoCs). For non-federally funded research, issuance of CoCs is not required but may be issued at the discretion of the FDA (discretionary CoCs). If an institutional ethics committee (EC) (institutional review board (IRB) in the United States) determines that data collected in a clinical trial are sufficiently sensitive to warrant requesting a CoC, then the EC may request that a CoC be obtained in order to secure EC approval. Any disagreement between an EC, sponsor, and/or investigators regarding the need to request a CoC for a study should be resolved by communications among the parties. See the G-CertCnfdntlty for more information on CoCs.
NIH Privacy Requirements
The NIHPrvcy indicates that the HHS’ National Institutes of Health (NIH) follows the PrvcyAct , which includes procedures for: 1) protecting records that can be retrieved by personal identifiers such as a name, social security number, or other identifying number or symbol, and 2) persons to access their identifiable records and to request correction(s) of these records. See the NIHPrvcy and the PrvcyAct for more information.
Consent for Processing Personal Data
Per USA-86 , the Privacy Rule defines the means by which individuals will be informed of uses and disclosures of their medical information for research purposes, and their rights to access information about themselves held by covered entities. Researchers may obtain, create, use, and/or disclose individually identifiable health information in the course of conducting research. Under the Privacy Rule, covered entities are permitted to use and disclose PHI for research with individual authorization, or without individual authorization under limited circumstances. To use or disclose PHI without authorization by the research participant, a covered entity must obtain one (1) of the following:
- Documented EC or privacy board approval
- Representations from the researcher that the use or disclosure of the PHI is solely to prepare a research protocol (or for similar purposes preparatory to research), the researcher will not remove any PHI from the covered entity, and PHI for which access is sought is necessary for the research purpose
- Research on protected health information of decedents
- Limited data sets with a data use agreement
- Research use/disclosure with individual authorization
- Accounting for research disclosures
See USA-86 for more information on these circumstances.
Obtaining Consent
In all United States (US) clinical trials, a freely given informed consent is required to be obtained from each participant in accordance with the requirements set forth in 21CFR50 for Food & Drug Administration (FDA) regulated clinical trials, and the Pre2018-ComRule or the RevComRule for federally funded or sponsored clinical trials. (See USA-65 for a list of Common Rule departments/agencies, and the Regulatory Authority section for more information on agency-specific compliance.) Department of Health & Human Services (HHS) -funded or sponsored clinical trials must also comply with 45CFR46-B-E . The FDA has also adopted the US-ICH-GCPs as guidance.
As per 21CFR50 , the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs , the informed consent form (ICF) is viewed as an essential document that must be reviewed and approved by an institutional ethics committee (EC) (institutional review board (IRB) in the US) and provided to the FDA with the investigational new drug application (IND).
Per the G-RevComRule-FDA , the informed consent requirements of the RevComRule are not inconsistent with FDA regulations. Therefore, there may not be a need for sponsors or investigators to develop, and have ECs review, two (2) separate ICFs for research that must comply with both the RevComRule and FDA regulations. (See the Required Elements section for ICF content details.) Per the RevComRule , which took effect January 21, 2019, for each clinical trial conducted or supported by a federal department or agency, one (1) EC-approved ICF used to enroll subjects must be posted by the awardee or the federal department or agency component conducting the trial on a publicly available federal website that will be established as a repository for such ICFs. According to USA-12 , two (2) federal websites have been identified to meet this requirement: ClinicalTrials.gov ( USA-78 ) and a docket folder on Regulations.gov ( USA-79 ). According to the RevComRule , if the federal department or agency supporting or conducting the clinical trial determines that certain information should not be made publicly available on a federal website (e.g., confidential, commercial information), such federal department or agency may permit or require redactions to the information posted. The ICF must be posted on the federal website after the clinical trial is closed to recruitment and no later than 60 days after the last study visit by any subject, as required by the protocol.
According to 21CFR50 , the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs , the investigator must provide detailed research study information to the participant and/or the legal representative(s) or guardian(s). ICF content should be briefly and clearly presented orally and in writing, in a manner that is easy to understand and commensurate with the comprehension level of the research participants, and without coercion or unduly influencing a potential participant to enroll in the clinical trial. The participant and/or the legal representative(s) or guardian(s), should also be given adequate time to consider whether to participate.
As indicated in 21CFR50 , the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs , none of the oral and written information concerning the research study should contain any language that causes the participant and/or the legal representative(s) or guardian(s) to waive or appear to waive legal rights, or that releases or appears to release the investigator, sponsor, institution or its agents from liability for negligence.
Additionally, per the RevComRule , participants must be provided with the information that a “reasonable person” would want to have in order to make an informed decision and an opportunity to discuss that information. Furthermore, the RevComRule requires that the informed consent, except for broad consent, must begin with a concise and focused presentation of the key information and organized to facilitate comprehension. Broad consent may be obtained in lieu of a full informed consent only with respect to the storage, maintenance, and secondary research uses of private identifiable information and identifiable biospecimens. See USA-54 and USA-60 for additional information regarding informed consent and broad consent requirements.
In addition, per 21CFR50 , the Pre2018-ComRule , and the RevComRule , the ICF may be presented as either a full length written ICF or as a short form stating the consent requirements have been presented orally. The full length written ICF may be presented orally but must then be provided to the participant and/or a legal representative(s) or guardian(s) to read before it is signed.
See the FDA’s G-ElectronicIC for recommendations on the use of electronic systems and processes that may employ multiple electronic media to obtain informed consent for both HHS-regulated human subject research and FDA-regulated clinical investigations of medical products.
See the G-InfrmdCnsnt for the FDA’s discussion of the regulations in 21CFR50 . Also, see USA-54 and USA-60 for additional information regarding informed consent.
According to 21CFR50 , the US-ICH-GCPs , and the G-IRBFAQs , the EC should determine the need to re-consent enrolled participants in the event of an ICF modification due to protocol changes or new information which may, in turn, affect the willingness of already enrolled participants to continue in the study. The communication of this information should be documented.
The G-IRBFAQs indicates that the FDA does not require re-consenting of participants who have completed their active participation in the study, or of participants who are still actively participating when the change will not affect their participation. One such case is when the change will be implemented only for subsequently enrolled participants.
Language Requirements
21CFR50 , the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs state that any information provided must be in a language understandable to the participant and/or the legal representative(s) or guardian(s).
As delineated in the FDA’s G-InfrmdCnsnt , when non-English speaking participants are enrolled in a study, ECs and investigators must ensure that the information provided to prospective participants and/or their legal representative(s) or guardian(s) is in a language that is understandable to them. The EC must review and approve all consent documents that are to be used by investigators to document the informed consent. When translation and interpretation are needed for written and oral information to be presented to participants, the FDA recommends that the EC review and approve reasonable procedures for ensuring that the translations will be prepared by a qualified individual or entity, and that interpretation assistance is available. The FDA also recommends that whenever non-English speaking participants are enrolled in a study, appropriate interpreter services be made available throughout the course of the study.
USA-63 also states that when an oral presentation of the ICF is provided, the witness present should be fluent in both English and the participant’s language, and the translator may serve as the witness. See the G-InfrmdCnsnt and USA-63 for detailed information.
Documenting Consent
As set forth in 21CFR50 , the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs , the participant and/or a legal representative(s) or guardian(s) must sign and date an EC-approved written ICF. A written copy of the form must be given to the participant and/or a legal representative(s) or guardian(s). In addition, the RevComRule explicitly allows electronic signatures for consent documentation.
Per 21CFR50 , the Pre2018-ComRule , and the RevComRule , if the consent information is only presented orally using the short form, the participant and/or the legal representative(s) or guardian(s) must sign the form, the witness must sign both the short form and a copy of the summary once consent has been provided, and the person obtaining the consent must sign a copy of the summary. A copy of both the summary and the short form must be given to the participant and/or the legal representative(s) or guardian(s). The FDA’s G-InfrmdCnsnt further states that participants who cannot write can instead indicate their consent by "making their mark" on the consent document. In these situations, a note should be included in participant case histories indicating the reason for the lack of a signature and date as required in 21CFR50 . The date consent was obtained should be recorded in this note.
According to the US-ICH-GCPs , where the participant is illiterate and/or the legal representative(s) and/or guardian(s) is illiterate, an impartial witness should be present during the entire informed consent discussion. The witness should sign and date the ICF after the following steps have occurred:
- The written ICF and any other written information to be provided to the participant is read and explained to the participant or the legal representative(s)/guardian(s)
- The participant or the legal representative(s)/guardian(s), has orally consented to the participant’s involvement in the trial, and has signed and dated the ICF, if capable of doing so
Per the US-ICH-GCPs , before participating in the study, the participant or the legal representative(s)/guardian(s) should receive a copy of the signed and dated ICF.
Waiver of Consent
Per the Pre2018-ComRule and the RevComRule , the EC may waive the requirement to obtain a signed ICF if it finds any of the following:
- The ICF would risk a breach of confidentiality by linking the participant to the study
- The research presents minimal risk and involves no procedures for which written consent is required outside of the study
The RevComRule also adds that the EC may waive the requirements to obtain a signed ICF if the participants are part of a distinct cultural group or community in which signing the form is not the norm, the research presents minimal risk, and there is an alternative approach to document informed consent.
The Pre2018-ComRule and the RevComRule further indicate that in cases where the documentation requirement is waived, the EC may require the investigator to provide the participant or the legal representative(s)/guardian(s) with a written statement regarding the research.
In addition, the Pre2018-ComRule states that for an EC to approve a general waiver or alteration of consent, the EC must find that:
- The research involves no more than minimal risk
- The research could not practicably be carried out without the requested waiver or alteration
- If the research involves using identifiable private information or identifiable biospecimens, the research could not practicably be carried out without using such information or biospecimens in an identifiable format
- The waiver or alteration will not adversely affect the rights and welfare of the participants
- Whenever appropriate, the participant will be provided with additional pertinent information after participation
In the G-MinRiskWaiver , the FDA informs sponsors, investigators, and ECs that it does not intend to object to an EC waiving or altering informed consent requirements for certain minimal-risk, clinical investigations.
Furthermore, the Pre2018-ComRule , the RevComRule , and the G-MinRiskWaiver specify that although voluntary informed consent is always a requirement for every trial, the EC may approve a waiver or alteration of consent if the study involves a public benefit and service program conducted by or subject to the approval of state or local officials and could not be carried out without the waiver or alteration.
Based on 21CFR50 , the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs , the informed consent form (ICF) must include the following statements or descriptions, as applicable (Note: The regulations provide overlapping and unique elements so each of the items listed below will not necessarily be in each source):
- The study purpose, procedures, and expected duration of the trial
- Identification of any experimental procedures
- Any expected risks or discomforts to the participant, and when applicable, to an embryo or fetus
- Any expected benefits to the participant
- Disclosure of appropriate alternative procedures that might be advantageous to the participant
- Confidentiality of records identifying the participant will be maintained and the possibility that the Food & Drug Administration (FDA) may inspect the records
- Compensation and/or treatment available for the participant in the case of trial-related injury
- Contact information for relevant individuals to contact in the event of a trial-related injury
- That participation is voluntary, that refusal to participate will involve no penalty or loss of benefits to which the participant is otherwise entitled, and that the participant can withdraw from the trial at any time without penalty or loss of otherwise entitled benefits
- Foreseeable circumstances under which the investigator may remove the participant without consent
- Any expenses the participant needs to pay to participate in the trial
- The consequences of a participant’s decision to withdraw from the study, and procedures for orderly withdrawal by the participant
- Any significant new findings developed during the study that may affect a participant’s willingness to continue participation
- Approximate number of participants in the study
As per 21CFR50 , for FDA-regulated research, the following statement must be included on the informed consent documents: “A description of this clinical trial will be available on https://www.ClinicalTrials.gov , as required by U.S. Law. This Web site will not include information that can identify you. At most, the Web site will include a summary of the results. You can search this Web site at any time.”
In the G-InfrmdCnsnt , the FDA also recommends the consent document advise participants that data collected on them up until the point of their withdrawal from a study will remain part of the study database and may not be removed. See the G-InfrmdCnsnt for additional FDA discussion of the regulations in 21CFR50 .
The RevComRule also requires the following statements to be included in the ICF:
- Whether research results will be disclosed to participants
- Whether or not the participant’s information or biospecimens will be used or distributed for future research
- That participant’s biospecimens (even if identifiers are removed) may be used for commercial profit and if the participant will share in this profit
- Whether biospecimens research may include whole genome sequencing
Compensation Disclosure
The FDA’s G-InfrmdCnsnt further states that if no compensation in the event of injury is available, the consent process should include a statement informing the participant. See the G-InfrmdCnsnt for an example statement.
In accordance with 21CFR50 , 21CFR312 , the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs , the United States’ (US) ethical standards promote respect for all human beings and safeguard the rights of research participants. A participant’s rights must also be clearly addressed in the informed consent form (ICF) and during the informed consent process.
The Right to Participate, Abstain, or Withdraw
As set forth in 21CFR50 , the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs , a potential participant and/or a legal representative(s) or guardian(s) must be informed that participation is voluntary, that the participant may withdraw from the research study at any time, and that refusal to participate will not involve any penalty or loss of benefits to which the participant is otherwise entitled.
The Right to Information
As delineated in 21CFR50 , the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs , a potential research participant and/or a legal representative(s) or guardian(s), has the right to be informed about the nature and purpose of the research study, its anticipated duration, study procedures, any potential benefits or risks, any compensation for participation or injury/treatment, and any significant new information regarding the research study.
The Right to Privacy and Confidentiality
As per 21CFR50 , the Pre2018-ComRule , and the RevComRule , participants should be given a statement describing the extent, if any, to which confidentiality of records identifying them will be maintained. Per the US-ICH-GCPs , all participants must be afforded the right to privacy and confidentiality, and the ICF must provide a statement that recognizes this right. It is the responsibility of the investigator(s) to safeguard the confidentiality of research data to protect the identity and records of research participants.
The RevComRule does allow the use of identifiable private information or biospecimens in instances where the institutional ethics committee (EC) (institutional review board (IRB) in the US) determines the research could not practicably be carried out without the information. Furthermore, it removes the requirement for the investigator to seek a waiver of informed consent to obtain information or biospecimens to screen, recruit, or determine eligibility of prospective participants. See USA-54 for additional information on identifiable private information or biospecimens, USA-65 for a list of Common Rule departments/agencies, and the Regulatory Authority section for more information on when the Pre2018-ComRule and the RevComRule apply to research.
The G-InfrmdCnsnt , which is the Food & Drug Administration (FDA) ’s discussion of the regulations in 21CFR50 , delineates how data should be handled when an enrolled participant decides to withdraw from a trial. Data collected on participants up to the time of withdrawal from clinical investigations of drugs conducted under an investigational new drug application (IND) must remain in the study database to maintain the scientific validity of the research. The FDA recommends that participants be advised in the consent document that the data collected on them up until the point of their withdrawal will remain part of the study database and may not be removed. If a participant withdraws from the interventional portion of the clinical investigation but agrees to continued follow-up not addressed in the original consent document, the investigator must obtain the participant’s informed consent for this limited participation using an EC-approved consent document. If a participant withdraws from the interventional portion of a clinical investigation and does not consent to continued follow-up of associated clinical outcome information, the investigator must not access the participant’s medical record or other confidential records that would require additional consent from the participant. However, such records may be accessed consistent with the original consent process, without additional consent, to obtain information collected prior to the participant’s withdrawal from the study. See the G-InfrmdCnsnt for additional information.
The Right of Inquiry/Appeal
21CFR50 , the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs state that the research participant and/or a legal representative(s) or guardian(s), should be provided with contact information for the sponsor and the investigator(s) to address trial-related inquiries and/or to appeal against a violation of the participant’s rights.
The Right to Safety and Welfare
The US-ICH-GCPs clearly states that a research participant’s right to safety and the protection of the participant’s health and welfare must take precedence over the interests of science and society.
21CFR50 , 21CFR56 , the US-ICH-GCPs , and the G-ICEmrgncyReqs make provisions to protect the rights of a research participant during the informed consent process when the procedure is complicated by life-threatening medical emergencies, public health emergencies, or military operations.
Medical Emergencies
As per the US-ICH-GCPs , in an emergency, if the signed informed consent form (ICF) has not been obtained from the research participant and/or a legal representative(s) or guardian(s), or if an effective treatment is lacking but the investigational product (IP) could address the participant’s emergency needs, the clinical trial may be conducted. However, the method used on the participant must be explained clearly in the trial protocol, and the institutional ethics committee (EC) (referred to as an institutional review board (IRB) in the United States (US)) must approve the protocol in advance. The participant and/or the legal representative(s) or guardian(s) should be informed about the trial as soon as possible, and consent to continue and other consent should be requested, as appropriate.
Emergency Use Situation
21CFR56 describes emergency use as the use of a test article, such as an IP, on a human participant in a life-threatening situation in which no standard acceptable treatment is available, and in which there is not sufficient time to obtain EC approval.
21CFR50 and the G-EmrgncyUse indicate that even in an emergency use situation, obtaining participant consent is required unless the investigator and a physician not participating in the trial certify in writing the following:
- The participant is confronted by a life-threatening situation
- Informed consent cannot be obtained due to an inability to communicate with the participant
- Time is insufficient to obtain consent from the participant’s legal representative(s) and/or guardian(s)
- No alternative methods of approved or generally recognized therapy are available
Per 21CFR50 and the G-EmrgncyUse , if immediate use of the IP is, in the investigator's opinion, required to preserve the participant’s life and time is not sufficient to obtain an independent physician’s determination prior to using the IP, the investigator’s determinations should be carried out. However, within five (5) working days following the use of the IP, the investigator’s decision must be reviewed and evaluated in writing by a physician not participating in the investigation. According to 21CFR50 , 21CFR56 , and the G-EmrgncyUse , the investigator must also notify the EC within five (5) working days.
21CFR56 , the G-EmrgncyUse , and the G-IRBFAQs further state that following emergency use of the IP, EC review and approval is required for any subsequent use of the IP.
Emergency Research
The G-ICEmrgncyReqs defines emergency research as a planned clinical investigation that requires prior written Food & Drug Administration (FDA) authorization to proceed, and involves participant(s) who are in a life-threatening situation for which available treatments or in vitro diagnostic tests are unproven or unsatisfactory.
21CFR50 and the G-ICEmrgncyReqs delineate that for emergency research, the EC may approve the investigation without requiring the consent of all the participants if the EC (with the concurrence of a licensed physician who is an EC member or EC consultant, and not otherwise participating in the investigation) finds and documents the following:
- The participants are in a life-threatening situation, available treatments are unproven or unsatisfactory, and the collection of valid scientific evidence is necessary to determine the safety and effectiveness of particular interventions
- Obtaining informed consent is not feasible because: (i) the participants will not be able to give their informed consent as a result of their medical condition; (ii) the intervention under investigation must be administered before consent from the participants’ legal representative(s) and/or guardian(s) is feasible; and (iii) there is no reasonable way to identify prospectively the individuals likely to become eligible for participation in the clinical investigation
- Participation in the research holds out the prospect of direct benefit to the participants
- The clinical investigation could not practicably be carried out without the waiver
- The proposed investigational plan defines the length of the potential therapeutic window based on scientific evidence, and the investigator has committed to attempting to contact a legal representative and/or guardian for each participant within that window of time and, if feasible, to asking them for consent within that window rather than proceeding without consent
- The EC has reviewed and approved informed consent procedures and an informed consent document consistent with 21CFR50
- Additional protections of the rights and welfare of the participants will be provided
See 21CFR50 and the G-ICEmrgncyReqs for more details.
USA-60 notes that in certain emergency circumstances, the Department of Health & Human Services (HHS) Secretarial waiver of informed consent under 46.101(i) of the RevComRule may be applicable. The HHS waiver applies to research that may be carried out in human participants who need emergency therapy and for whom, because of the participants’ medical condition and the unavailability of the participants’ legal representative(s) and/or guardian(s), no legally effective informed consent can be obtained. Furthermore, if the research is regulated by the FDA, the HHS waiver permits the research to be conducted under a comparable provision. See the G-HHS-Emrgncy for additional guidance, USA-65 for a list of Common Rule departments/agencies, and the Regulatory Authority section for more information on when the RevComRule applies to research.
Military Operations
21CFR50 and 10USC55 indicate that in the case of IP administration to a member of the armed forces in connection with participation in a particular military operation, the requirement for the member’s prior consent may be waived only by the US President. The US President may grant the waiver only after determining, in writing, that obtaining consent is not feasible; is contrary to the best interests of the military personnel; or is not in the interests of national security. See 21CFR50 and 10USC55 for detailed requirements.
As per 21CFR56 , the Pre2018-ComRule , the RevComRule , and the US-ICH-GCPs , in all United States (US) clinical trials, research participants selected from vulnerable populations must be provided additional protections to safeguard their health and welfare during the informed consent process. Institutional ethics committees (ECs) (institutional review boards (IRBs) in the US) must pay special attention to protecting such participants. (See USA-65 for a list of Common Rule departments/agencies, and the Regulatory Authority section for more information on when the Pre2018-ComRule and the RevComRule apply to research.)
21CFR56 and the US-ICH-GCPs require special considerations for vulnerable populations and characterize them as those whose willingness to volunteer in a trial may be unduly influenced by the expectation, whether justified or not, of benefits associated with participation, or of a retaliatory response for refusing to participate. Examples of these participants include members of a group with a hierarchical structure, such as medical, pharmacy, dental, and nursing students; subordinate hospital and laboratory personnel; pharmaceutical industry employees; members of the armed forces; and persons kept in detention. Per 21CFR56 and US-ICH-GCPs , other vulnerable subjects include children, pregnant women, physically or mentally disabled persons, patients with incurable diseases, persons in nursing homes, economically or educationally disadvantaged persons, patients in emergency situations, ethnic minority groups, homeless persons, nomads, refugees, minors, and those incapable of giving consent.
The Pre2018-ComRule describes children, prisoners, pregnant women, handicapped persons, mentally disabled persons, or economically or educationally disadvantaged persons as vulnerable populations. The RevComRule describes children, prisoners, individuals with impaired decision-making capacity, or economically or educationally disadvantaged persons as vulnerable populations.
For more guidance documents related to vulnerable populations, see USA-64 .
See the Children/Minors; Pregnant Women, Fetuses, & Neonates; Prisoners; and Mentally Impaired sections for additional information about these vulnerable populations.
As set forth in 21CFR50 and 45CFR46-B-E , children are defined as persons who have not attained the legal age for consent to treatments or procedures involved in the research, under the applicable law of the jurisdiction in which the study will be conducted. USA-25 further states that the age of majority in most states is 18 and therefore for legal purposes, children are those individuals who have not reached the age of 18. See USA-25 for a table delineating the legal age of majority by state in the United States (US).
Per the Pre2018-ComRule and the RevComRule , children require additional safeguards to be included in any research study in order to protect their rights and welfare. (See USA-65 for a list of Common Rule departments/agencies, and the Regulatory Authority section for more information on when the Pre2018-ComRule and the RevComRule apply to research.)
As delineated in the US-ICH-GCPs , when the research participant is a minor, informed consent should be obtained from a legal representative(s) or guardian(s). All pediatric participants should be fully informed about the trial and its risks and benefits in a language and in terms that they are easily able to understand. If capable, the participant should sign and date the written informed consent.
For all clinical trials that do not involve greater than minimal risk, 21CFR50 and 45CFR46-B-E state that a study may only be conducted if adequate provisions are made to obtain the child’s assent and the permission of their legal representative(s) or guardian(s).
For all clinical trials that involve greater than minimal risk but present the prospect of direct benefit to the child, 21CFR50 and 45CFR46-B-E indicate that a study may only be conducted if the following applies:
- The risk is justified by the anticipated benefit to the child
- The anticipated benefit is greater than or equal to the available alternative approaches
- Adequate provisions are made to obtain the child’s assent and the permission of their legal representative(s) or guardians
For all clinical trials involving children/minors that involve greater than minimal risk and do not present the prospect of direct benefit to the child, but will likely result in increased knowledge about the child’s disorder or condition, 21CFR50 and the 45CFR46-B-E state that a study may only be conducted if the following applies:
- The risk is slightly greater than minimal
- The trial presents experiences that are similar to those associated with the child’s actual or expected medical, dental, psychological, social, or educational situation
- Adequate provisions are made to obtain the child’s assent and the permission of their legal representative(s) or guardian(s)
For all clinical trials that present a reasonable opportunity to further understand, prevent, or alleviate a serious problem affecting the health or welfare of children/minors but is not otherwise approvable per 21CFR50 and 45CFR46-B-E , a study may only be conducted if the following applies:
- The institutional ethics committee (EC) (institutional review board (IRB) in the US) finds that the investigation presents a reasonable opportunity to further the understanding, prevention, or alleviation of a serious problem affecting the health or welfare of children, and,
- The Commissioner of Food and Drugs consults with an expert panel and has an opportunity for public review and comment to determine that the investigation satisfies the conditions of one (1) of the other earlier described research types, or the following conditions are met: the investigation will be conducted in accordance with sound ethical principles and adequate provisions are made for soliciting the assent of children and the permission of their legal representative(s) or guardian(s)
Per the RevComRule , certain exemptions may apply to observational research involving children. See the RevComRule for details.
For additional Food & Drug Administration (FDA) guidance on clinical research in children, see US-ICH-E11 and USA-60 . Additionally, see the G-InfrmdCnsnt for FDA discussion of the regulations in 21CFR50 .
Assent Requirements
Per 21CFR50 and 45CFR46-B-E , when determining whether children/minors are capable of providing assent, the EC must consider their age, maturity, and psychological state. Assent from a child/minor is not necessary for proceeding with the clinical trial if the following applies:
- The capability of some or all of the children/minors is so limited that they cannot reasonably be consulted
- The trial presents a potential direct benefit that is important to the health or well-being of the children/minors and is only available through the investigation
Further, the EC may waive assent, even if the children/minors are capable of providing assent, if it finds and documents the following:
- Trial involves no more than minimal risk
- The waiver will not negatively affect the rights and welfare of the children/minors
- The trial could not be implemented without the waiver
- The children/minors will be given additional information after participation, whenever appropriate
When legal representative or guardian permission is necessary, the EC must determine whether the permission of one (1) legal representative or guardian is sufficient, or if permission from both is required. If the EC determines assent is required, it must also determine whether and how assent must be documented. 21CFR50 and 45CFR46-B-E do specify, however, that the consent of both legal representative(s) or guardian(s) is required in the following cases:
- When there is greater than minimal risk to the child with no direct benefit to the child, but the study will likely result in increased knowledge about the child’s disorder or condition
- Research that presents an opportunity to understand, prevent, or alleviate a serious problem affecting the health or welfare of children/minors, but is not otherwise approvable
Exceptions to the two (2) legal representatives’ and/or guardians’ consent requirement are when one (1) legal representative or guardian is deceased, unknown, incompetent, or not reasonably available, or, when only one (1) legal representative or guardian has legal responsibility for the care and custody of the child.
The G-InfrmdCnsnt indicates that when obtaining legal representative or guardian permission, in the event that the legal representative(s) or guardian(s) of a child does not understand English, the permission must be obtained and documented in a language that is understandable to the legal representative(s) or guardian(s). The child who will be participating in the research should not be used as an interpreter for the legal representative(s) or guardian(s), even if the child is fluent in English and may be able to assent. Further, legal representative or guardian permission and child assent should be viewed as an ongoing process throughout the duration of a clinical investigation. If and when a child who was enrolled in a clinical investigation with legal representative or guardian permission reaches the legal age of consent, that participant no longer meets the definition of a child under 21CFR50 , and the investigator should obtain the participant’s informed consent prior to performing any further research interventions and/or procedures involving that participant. See the G-InfrmdCnsnt for additional FDA discussion of the regulations in 21CFR50 .
As per 21CFR50 and 45CFR46-B-E , for studies involving women of childbearing age or who are pregnant, a statement should be provided in the informed consent form (ICF) indicating that the treatment or procedure may involve risks to the participant, embryo, or fetus, which are currently unforeseeable. According to the US-ICH-GCPs , the ICF should include a statement on the reasonably foreseeable risks or inconveniences to the participant, and when applicable, to an embryo, fetus, or nursing infant.
Per the Pre2018-ComRule , pregnant women require additional safeguards to be included in any research study in order to protect their rights and welfare. Furthermore, according to the RevComRule , all of the available exemptions of the RevComRule for observational research may be applied to research involving pregnant women, fetuses, and neonates. See the RevComRule for details. (See USA-65 for a list of Common Rule departments/agencies, and the Regulatory Authority section for more information on when the Pre2018-ComRule and the RevComRule apply to research.)
All Department of Health & Human Services (HHS) -sponsored or -funded research involving pregnant women, human fetuses, neonates of uncertain viability, or nonviable neonates must comply with Subpart B of 45CFR46-B-E .
Pregnant Women and Fetuses
As per 45CFR46-B-E , pregnant women and fetuses may participate in research if all of the following criteria are met:
- Preclinical and clinical studies have been conducted and provide data for assessing potential risks, where scientifically appropriate
- Risk to the fetus is caused solely by procedures that provide potential direct benefit to the woman or fetus. If there is no potential direct benefit, then the risk to the fetus cannot be greater than minimal, and the intent of the study is to develop important biomedical knowledge that cannot be obtained otherwise
- Least possible risk involved for achieving the research objectives
- Consent is obtained from the woman for studies that provide potential direct benefit to the pregnant woman and/or fetus, and studies with minimal risk to the fetus conducted to develop important biomedical knowledge that cannot be obtained otherwise
- Consent is obtained from the pregnant woman and the father if the study provides potential direct benefit solely to the fetus. Paternal consent is not required if the father is unavailable, incompetent, temporarily incapacitated, or the pregnancy was a result of incest or rape
- All individuals providing consent are fully informed about the foreseeable impact on the fetus or neonate
- No inducements will be offered to terminate a pregnancy
- Participants will not be involved in determining the timing, method, or procedures for terminating a pregnancy
- Participants will not be involved in determining the viability of a neonate
45CFR46-B-E states that neonates may not be involved in research unless all of the following criteria are met:
- All individuals providing consent are fully informed about the foreseeable impact on the neonate
Neonates of uncertain viability may not be involved in research unless the institutional ethics committee (EC) (institutional review board (IRB) in the United States (US)) determines the following additional conditions are met:
- Research provides the potential for increasing the probability of survival to the point of viability, and involves the least possible risk
- The purpose is to develop important biomedical knowledge that cannot be obtained otherwise and there is no added risk resulting from the research
- Informed consent is obtained from either parent, or if neither parent is able to provide consent, then consent is obtained from the neonate’s legal representative and/or guardian. Paternal consent is not required if pregnancy was a result of incest or rape.
Nonviable neonates may not be involved in research unless the following additional conditions are met:
- Vital functions will not be maintained artificially
- Research will not terminate the heartbeat or respiration
- The purpose is to develop important biomedical knowledge that cannot be obtained otherwise, and there is no added risk resulting from the research
- Consent is obtained from both parents. If neither parent is able to provide consent, informed consent of one (1) parent will suffice. Paternal consent is not required if pregnancy was a result of incest or rape. Consent of a legal representative or guardian of either or both parents will not suffice.
Viable neonates may only be included in research to the extent permitted by and in accordance with the RevComRule and subparts B and D of 45CFR46-B-E .
21CFR56 , 45CFR46-B-E , and the US-ICH-GCPs include prisoners in their description of vulnerable populations. As set forth in 45CFR46-B-E , a prisoner is defined as any individual involuntarily confined or detained in a penal institution. Prisoners are considered vulnerable because incarceration could affect their ability to make a voluntary decision regarding participation in research.
Per the Pre2018-ComRule and the RevComRule , prisoners require additional safeguards to be included in any research study in order to protect their rights and welfare. As delineated in the RevComRule , none of its observational research exemptions may be applied to research involving prisoners, except for research aimed at involving a broader subject population that only incidentally includes prisoners. (See USA-65 for a list of Common Rule departments/agencies, and the Regulatory Authority section for more information on when the Pre2018-ComRule and the RevComRule apply to research.)
45CFR46-B-E states that prisoners may participate in biomedical or behavioral research conducted or supported by the Department of Health & Human Services (HHS) only if the following criteria are met:
- The institution conducting the research has certified to the HHS Secretary that the research has been approved by the institutional ethics committees (EC) (institutional review board (IRB) in the United States (US)); research involves minimal risk; and studies focus on the possible causes, effects, and processes of incarceration and criminal behavior, prisons as institutional structures, or prisoners as incarcerated persons
- Research should focus on conditions specifically affecting prisoners as a class, or practices that have the intent and likelihood of improving the health or well-being of participants only after the HHS Secretary has consulted the appropriate experts, and a Federal Register notice is published indicating intent to approve such research
See USA-62 for more HHS information on prisoner research.
As per 45CFR46-B-E , ECs have additional approval responsibilities when reviewing research studies involving prisoners. An EC must only approve these studies if it determines that:
- The research under review represents one (1) of the permissible categories of research delineated in Subpart C
- The prisoner’s judgement will not be impaired by any possible advantages accruing to the prisoner through participation in the research, when compared to the general living conditions, medical care, quality of food, amenities, and opportunity for earnings in the prison
- Research risks are commensurate with those that would be accepted by non-prisoner volunteers
- Procedures for participant selection within the prison are fair to all prisoners and immune from arbitrary intervention by prison authorities or prisoners
- Information is presented in a language understandable to the prisoner population
- Adequate assurance exists that parole boards will not take into account a prisoner's participation in the research in making decisions regarding parole, and each prisoner is clearly informed in advance that participation in the research will have no effect on parole
- As needed, adequate provisions have been made for follow-up examination or care of participants, taking into account the varying lengths of individual prisoners' sentences, and for informing participants of this fact
See Subpart C of 45CFR46-B-E for additional EC requirements related to prisoner research.
In accordance with 21CFR56 , the Pre2018-ComRule , and the US-ICH-GCPs , an institutional ethics committee (EC) (institutional review board (IRB) in the United States (US)) must approve the participation of research participants who are mentally incapable of giving consent. According to the G-InfrmdCnsnt , which is the Food & Drug Administration (FDA) ’s discussion of the regulations in 21CFR50 , impaired consent capacity may involve partial impairment, impairment that fluctuates over time, or complete impairment. Consent capacity can be affected by a wide range of disorders and conditions, such as dementia, stroke, traumatic brain injury, intellectual and developmental disabilities, serious mental illness, intoxication, and delirium.
Per the Pre2018-ComRule and the RevComRule , this population requires additional safeguards to be included in any research study to protect the rights and welfare of participants likely to be vulnerable to coercion or undue influence. (See USA-65 for a list of Common Rule departments/agencies, and the Regulatory Authority section for more information on when the Pre2018-ComRule and the RevComRule apply to research.)
USA-60 further indicates that while Department of Health & Human Services (HHS) regulations do not provide specific procedures, it is expected that for research involving adult participants with mental illnesses or cognitive impairments, the EC and investigator(s) must be knowledgeable about the condition and any level of impairment that is likely to be present in the participant population.
As stated in the FDA’s G-InfrmdCnsnt , ECs and investigators should carefully consider whether the inclusion in research of individuals who lack consent capacity is ethically appropriate and scientifically necessary. Considerations that may help address these challenges and provide additional safeguards include:
• Assessing consent capacity of prospective participants, for example, through use of an independent, qualified professional
• Establishing a waiting period in the decision-making process to allow additional time for decision-making
• Using methods to enhance consent capacity, for example through (1) simplification and/or repetition of information, (2) involvement of a participant advocate or trusted family member/friend to assist when sharing information about the clinical investigation, and (3) refraining from discussions during periods of heightened impairment, when possible
• Assessing a participant’s understanding after information about the clinical investigation has been imparted, for example, through use of a questionnaire
• Re-assessing consent capacity after initiation of the clinical investigation for participants with progressive disorders whose cognition may decline
• Involving a legally authorized representative and/or guardian either initially or later in the clinical investigation if consent capacity diminishes
• Assessing whether prospective participants who cannot provide legally effective consent on their own behalf may nonetheless be able to provide some form of oral agreement at the outset of the study and, as appropriate, throughout the course of the research (e.g., for participants with progressive disorders), and how such oral agreement would be documented
• Emphasizing the voluntary nature of the decision to participate and the right to withdraw at any time
• Determining whether the EC or a third party should observe the consent process
See the G-InfrmdCnsnt for additional information and FDA discussion of the regulations in 21CFR50 .
As delineated in 21CFR312 , an investigational new drug is defined as a new drug or biological drug that is used in a clinical investigation. This includes a biological product that is used in vitro for diagnostic purposes. The terms ‘investigational drug’ and ‘investigational new drug’ are deemed to be synonymous for the purposes of this part.
Additionally, the US-ICH-GCPs defines an investigational product as a pharmaceutical form of an active ingredient or placebo being tested or used as a reference in a clinical trial, including a product with a marketing authorization when used or assembled (formulated or packaged) in a way different from the approved form, or when used for an unapproved indication, or when used to gain further information about an approved use.
Manufacturing
According to 21CFR312 and USA-42 , the Food & Drug Administration (FDA) is responsible for authorizing the manufacture of investigational products (IPs) (also known as investigational new drugs in the United States (US)).
Per 21CFR312 , sponsors that use an IP not already subject to a manufacturer’s investigational new drug application (IND) or marketing application are required to provide all of the technical chemistry, manufacturing, and control (CMC) information outlined in the application content and format requirements section of 21CFR312 , unless such information may be referenced from applicable scientific literature. Sponsors using an IP already subject to a manufacturer’s application should follow the same general application format but may, if authorized by the manufacturer, refer to the manufacturer’s application to provide the technical (CMC) information supporting the proposed clinical investigation.
Moreover, as stated in 21CFR312 , a sponsor may ship an IP to the investigators named in the IND under the following conditions:
- Thirty (30) days after the FDA receives the IND, or
- FDA provides earlier authorization to ship the IP
The sponsor is responsible for complying with the principles of good manufacturing practice (GMP) as specified in 21CFR210 , the G-CGMP-Phase1 , and the G-INDPrep . The US-ICH-GCPs also states that the sponsor must ensure that the products are manufactured in accordance with GMPs.
As set forth in 21CFR312 , the FDA is also responsible for authorizing the import and export of IPs. An IP may be imported into the US if it is subject to an IND that is in effect for it and complies with one (1) of the following requirements:
- The IP consignee is the IND sponsor, or
- The consignee is a qualified investigator named in the IND, or
- The consignee is the domestic agent of a foreign sponsor, is responsible for the control and distribution of the IP, and the IND identifies the consignee and describes what, if any, actions the consignee will take with respect to the IP
Investigator's Brochure
In accordance with 21CFR312 and the US-ICH-GCPs , the sponsor is responsible for providing investigators with an Investigator’s Brochure (IB). The IB must contain all of the relevant information on the investigational new drug(s)/investigational product(s) (IPs) obtained through the earlier research phases. The sponsor must also update the IB as significant new information becomes available.
As specified in 21CFR312 and the US-ICH-GCPs , the IB must provide coverage of the following areas (Note: The regulations provide overlapping and unique elements so each of the items listed below will not necessarily be in each source):
- A brief description of the drug substance and the formulation, including the structural formula, if known
- A summary of the pharmacological and toxicological effects of the drug in animals and, to the extent known, in humans
- A summary of the pharmacokinetics and biological disposition of the drug in animals and, if known, in humans
- A summary of information relating to safety and effectiveness in humans obtained from prior clinical studies
- A description of possible risks and side effects to be anticipated on the basis of prior experience with the drug under investigation or with related drugs, and of precautions or special monitoring to be done as part of the investigational use of the drug
- Summary of data and guidance for the investigator
See 21CFR312 and the US-ICH-GCPs for detailed IB content guidelines.
For investigational new drug applications (INDs) that include clinical data provided from studies conducted outside of the United States (US), 21CFR312 states that the sponsor or applicant must submit a description of the actions taken to ensure that the research conformed to good clinical practices (GCPs). See Section 312.120 of 21CFR312 for detailed requirements.
Quality Management
According to USA-39 , submitting a copy of the Certificate of Analysis (CoA) of the clinical batch is suggested, but not required by the Food & Drug Administration (FDA) .
The US-ICH-GCPs state that the sponsor must maintain a CoA to document the identity, purity, and strength of the IP(s) to be used in the clinical trial.
Investigational new drug/investigational product (IP) labeling in the United States (US) must comply with the requirements set forth in Section 312.6 of 21CFR312 , which include the following:
- The immediate package of an IP intended for human use must bear a label with the following statement: “Caution: New Drug-Limited by Federal (or US) law to investigational use”
- The label or labeling of an IP must not bear any false or misleading statements and must not represent that the IP is safe or effective for the purposes for which it is being investigated
The appropriate Food & Drug Administration (FDA) Center Director may grant an exception or alternative to the requirements above for specific lots, batches, or other units of a human drug or biological product that is or will be included in the Strategic National Stockpile.
In addition, the US-ICH-GCPs states that the IP must be coded and labeled in a manner that protects the blinding, if applicable.
Supply, Storage, and Handling Requirements
As defined in the US-ICH-GCPs , the sponsor must supply the investigator(s)/institution(s) with the investigational new drug(s)/investigational product(s) (IP(s)), including the comparator(s) and placebo, if applicable. The IPs must also be suitably packaged in a manner that will prevent contamination and unacceptable deterioration during transport and storage.
Per 21CFR312 , the US-ICH-GCPs , the G-CGMP-Phase1 , and the G-INDPrep , the sponsor must ensure the following (Note: The regulations provide overlapping and unique elements so each of the items listed below will not necessarily be in each source):
- IP product quality and stability over the period of use
- IP manufactured according to any applicable good manufacturing practices (GMPs)
- Proper coding, packaging, and labeling of the IP(s)
- Acceptable storage temperatures, conditions, and times for the IP
- Timely delivery of the IP(s)
Refer to the US-ICH-GCPs , the G-CGMP-Phase1 , and the G-INDPrep for detailed sponsor-related IP requirements.
Record Requirements
According to 21CFR312 , the sponsor must maintain adequate records showing the receipt, shipment, or other disposition of the IP. These records are required to include, as appropriate, the name of the investigator to whom the drug is shipped, and the date, quantity, and batch or code mark of each such shipment. The sponsor is also required to maintain records showing financial interest paid to investigators. See 21CFR312 for more details.
As per 21CFR312 and the US-ICH-GCPs , the sponsor and the investigator(s) must retain the clinical investigation records and reports for two (2) years after a marketing application (known as a New Drug Application (NDA)) is approved for the IP; or, if an NDA is not approved, until two (2) years after shipment and delivery of the IP is discontinued for investigational use and the Food & Drug Administration (FDA) has been so notified.
A specimen, referred to as patient specimen in 49CFR173 , is defined as human or animal material collected directly from humans or animals and transported for research, diagnosis, investigational activities, or disease treatment or prevention. Patient specimen includes excreta, secreta, blood and its components, tissue and tissue swabs, body parts, and specimens in transport media (e.g., transwabs, culture media, and blood culture bottles).
In addition, 42CFR73 defines specimen as samples of material from humans, animals, plants, or the environment or isolates or cultures from such samples for diagnosis, verification, or proficiency testing.
The RevComRule defines an identifiable biospecimen as one for which the identity of the participant is or may readily be ascertained by the investigator or associated with the biospecimen. (See USA-65 for a list of Common Rule departments/agencies, and the Regulatory Authority section for more information on when the RevComRule applies to research.)
Import/Export
The import and export of human specimens, also known as patient/diagnostic specimens/substances or human biological materials in the United States (US), is governed by several federal agencies working cooperatively to ensure the safe transport of these materials. These agencies include, but are not limited to, the Department of Transportation (DOT) ’s Pipeline and Hazardous Materials Safety Administration (PHMSA) , the Centers for Disease Control and Prevention (CDC) ’s Import Permit Program (IPP) , the Department of Health & Human Services (HHS) , the United States Postal Service (USPS) , and the International Air Transport Association (IATA) . The IATA has also adopted all of the hazardous materials requirements set forth in the Technical Instructions for the Safe Transport of Dangerous Goods by Air ( USA-10 ) published biannually by the United Nations (UN) ’ International Civil Aviation Organization (ICAO) .
Infectious Specimens
Per 49CFR173 , 42CFR73 , 42CFR71 , USA-21 , USA-4 , USA-11 , and USA-31 , DOT’s PHMSA, IATA, USPS, and CDC’s IPP refer to an infectious specimen/substance as a Division 6.2 material (Category A or Category B), or a select agent, etiologic agent, toxin, or a vector of human disease. The CDC’s IPP is specifically responsible for the importation of infectious specimens/substances/biological agents/vectors of human disease per 42CFR71 and for regulating the possession, use, and transfer of select agents and toxins per 42CFR73 . See 42CFR71 , 42CFR73 , USA-31 , and USA-73 for further information and permit applications for these import/transfer programs.
Additionally, the Department of Commerce (DOC) ’s Bureau of Industry and Security is responsible for regulating the export of a wide range of infectious specimens that may require a DOC license. Refer to the Commerce Control List (CCL) in 15CFR774 and USA-30 to determine if a DOC export permit is required for specific specimens.
According to 49CFR173 , USA-21 , and USA-4 , certain materials and specimens are exempt from the DOT’s PHMSA, IATA, and USPS requirements for import/export of infectious specimens. These include materials that do not contain infectious substances; non-infectious biological materials from humans, animals, or plants; and specimens for which there is a low probability that the sample is infectious. Exempt human or animal specimens are not subject to regulation as hazardous materials but are subject to specific packaging procedures that must be followed when shipped. Please refer to 49CFR173 , USA-21 , USA-4 , and USA-11 for detailed DOT, IATA, and USPS shipping instructions.
NIH Specimen Requirements
The HHS’ National Institutes of Health (NIH) researchers must also comply with all applicable federal and international air and ground transport laws and regulations. Researchers must also receive prior authorization from the NIH’s Quarantine Permit Service Office to obtain permits for the import, transfer, or export of all specimens to the NIH. Detailed instructions about how to proceed are outlined in USA-71 .
Per USA-2 , the NIH also requires researchers to use an agreement (e.g., Material Transfer Agreement (MTA) or contract) to transfer materials among academic, nonprofit, and/or industrial organizations. See USA-2 for detailed MTA requirements and Appendix 4 for a sample MTA.
As delineated in the G-IC-IVDs , the Food & Drug Administration (FDA) only provides informed consent guidance with respect to its regulations governing the informed consent requirement when human specimens are used for FDA-regulated in vitro diagnostic device investigations.
Informed consent requirements guiding Department of Health & Human Services (HHS) -conducted or -supported research on human research participants is regulated by the Pre2018-ComRule and 45CFR46-B-E .
Per the Pre2018-ComRule and the G-SpecimensResrch , the HHS views research involving human subject specimens as research involving human participants and subject to informed consent requirements, if the specimens obtained may be classified as identifiable private information. Identifiable private information or identifiable specimens are those that can be linked to specific individuals by the investigator(s) either directly or indirectly through coding systems. The RevComRule further defines an identifiable biospecimen as one for which the identity of the participant is or may readily be ascertained by the investigator. See the Pre2018-ComRule , RevComRule , the G-SpecimensResrch , USA-2 , USA-9 , and USA-1 for additional information. See also the G-SpecimensResrch for exemptions to this definition.
Additionally, as defined by the HHS’ National Institutes of Health (NIH) in USA-72 , research with specimens, cells, cell lines, or data involves human subjects when:
- The specimens, cells, or data must be or must have been obtained from individuals who are alive, and must be or must have been obtained by an investigator conducting research; and
- The investigator either must be obtaining or must have obtained specimens, cells, or data through interaction or intervention with living individuals, or must be obtaining or have obtained individually identifiable private information.
See USA-72 for detailed frequently asked questions (FAQs) on this topic.
Per the Pre2018-ComRule , the RevComRule , and USA-2 , prior to collecting, storing, or using a research participant’s biological specimen(s), consent must be obtained from the participant and/or a legal representative(s). See USA-65 for a list of Common Rule departments/agencies, and the Regulatory Authority section for more information on when the Pre2018-ComRule and the RevComRule apply to research.
The RevComRule requires the informed consent form to provide one (1) of the following statements about any research that involves the collection of identifiable private information or identifiable biospecimens:
- A statement that identifiers might be removed from the identifiable private information or identifiable biospecimens and that, after such removal, the information or biospecimens could be used for future research studies or distributed to another investigator for future research studies without additional informed consent from the subject or the legally authorized representative, if this might be a possibility
- A statement that the subject's information or biospecimens collected as part of the research, even if identifiers are removed, will not be used or distributed for future research studies
- A statement that the subject's biospecimens (even if identifiers are removed) may be used for commercial profit and whether the subject will or will not share in this commercial profit
- Whether the research will (if known) or might include whole genome sequencing (i.e., sequencing of a human germline or somatic specimen with the intent to generate the genome or exome sequence of that specimen)
Furthermore, the RevComRule delineates the requirements of broad consent—an alternative consent process—for the storage, maintenance, and secondary research use of private information or identifiable biospecimens. Broad consent requires that the following information be provided to the participant and/or the legal representative(s) or guardian(s):
- Certain basic elements from the normal consent process related to risks, benefits, confidentiality, voluntary statement, commercial profit, contact information, and whole genome sequencing elements
- Types of research that may be conducted
- A description of the information or biospecimens that might be used in future research, whether sharing might occur; and the types of institutions or researchers that might conduct research
- A description of the length of time that the information or biospecimens may be stored, maintained, and used
- A statement that participants will or will not be informed of the details of any specific research studies that might be subsequently conducted
- A statement that research results either will or will not be disclosed to participants
- An explanation of whom to contact for answers to questions about the subject's rights and about storage and use of the subject's identifiable private information or identifiable biospecimens, and whom to contact in the event of a research-related harm.
The RevComRule does allow the use of identifiable information or biospecimens in instances where the institutional ethics committee (EC) (institutional review board (IRB) in the United States (US)) determines the research could not practicably be carried out without the information in that form. Furthermore, it removes the requirement for the investigator to seek a waiver of informed consent to obtain information or biospecimens to screen, recruit, or determine eligibility of prospective participants. See USA-54 for more information on broad consent and informed consent waivers.
The HHS’ G-StoredData-Tissues and USA-2 recommend that the following be included in informed consent documents for biospecimen collection:
- A clear description of the operation of the biospecimen resource including details such as whether identifiable information will be maintained by the biospecimen resource and/or whether research results will be linked to the biospecimen
- Conditions under which samples and data will be released to recipient investigators
- Procedures for protecting the privacy of human research participants and confidentiality of data
- Specific descriptions of the nature and purpose of the research
- Information about the consequences of DNA typing if human genetic research is anticipated
(See the Required Elements and Participant Rights sections for additional information on informed consent).

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- What is ethics?
- How is ethics different from morality?
- Why does ethics matter?
- Is ethics a social science?

institutional review board
Our editors will review what you’ve submitted and determine whether to revise the article.
- National Center for Biotechnology Information - PubMed Central - Institutional Review Boards
institutional review board (IRB) , in the United States , ethics committee that reviews proposed and ongoing research involving human subjects. The institutional review board (IRB) exists to protect the rights and safety of human subjects who participate in research studies. The need for an IRB became apparent in the 1960s and 1970s, largely as a result of the Tuskegee Syphilis Study , in which human subjects received substandard medical care without their consent . The IRB system subsequently was established with the passage of the National Research Act of 1974. The Office of Human Research Protections, within the U.S. Department of Health and Human Services , is responsible for the registration of IRBs and their oversight.
Initially focused on biomedical research, IRBs later were also developed for research in the social sciences and liberal arts (e.g., for research involving living history interviews). Institutions seeking federal funding must have an IRB, and the IRB must review and approve federally funded research studies. Most institutions require IRB approval for all research that involves human subjects, not only that funded by the federal government.
The IRB process begins before participants are recruited for a study. The study protocol must satisfy the three basic principles of the Belmont Report (a summary of ethical principles and guidelines for the conduct of research involving human subjects): 1) respect for persons, 2) beneficence (avoiding harm to subjects and maximizing the benefits compared with the risks of participation), and 3) justice . Once the study is approved, the IRB is charged with overseeing the research from an ethics perspective. This oversight usually is exercised through two mechanisms. First, participants are provided with a means of contacting the IRB directly if they have concerns, and, second, the IRB conducts periodic reviews of the study to monitor the research progress and address any ethical issues. The review process usually occurs annually. Although rarely used, the IRB can carry out additional reviews and actively conduct surprise inspections of research records.
IRBs for institutions receiving U.S. federal funds are required to have at least five members, though most institutions have more. The IRB must include members who represent diverse bodies of knowledge relevant to the conduct of ethical research. For example, at least one member must be from the scientific community and knowledgeable about scientific research, and there must be at least one member from outside the scientific community; this person should advocate for the nonscientific issues relevant to ethical conduct of research, such as legal issues and standards for professional conduct. In addition, at least one person must be from outside the institution; this person (who may also serve as the nonscientific member) usually is a community member and represents the community standard for assessing the ethics of a study.
When the research proposed is outside the expertise of the IRB members, the IRB can invite experts in the research area to provide additional information in the review; however, these consultants are not allowed to vote. Most IRBs use a consensus approach (i.e., votes must be unanimous) to reach a decision, although some IRBs allow a majority vote. When a majority vote is used, the community member typically still has substantial power because most IRBs will not override the perspective of the community member. The administration of an institution (e.g., president of a university or director of a hospital ) must allow the IRB to function independently, without undue influence related to funding pressures or other administration priorities.
- Department of Health and Human Services
- National Institutes of Health

We are excited to welcome Dr. Yukiko Asada, PhD and Dr. Robert Steel, PhD as the newest members of our department's faculty.

The NIH Bioethics offers several courses, training and lecture opportunities related to bioethical issues. Learn more.

Our interdisciplinary research is published in a range of high impact medical, legal, scientific, philosophical, social science and bioethics journals.

The Department of Bioethics hosts Ethics Grand Rounds four times per year. Ethics Grand Rounds is designed to provide a venue to learn about and discuss important issues in bioethics.

Christine Grady , RN, PhD
Chief, Department of Bioethics
Welcome to the NIH Department of Bioethics
Phone: 301.496.2429 Fax: 301.496.0760 Email: [email protected] Twitter: @NIHBioethics
Featured Training
Fellowship Opportunities
The NIH Department of Bioethics welcomes applications for fully funded two-year postbaccalaureate and postdoctoral research fellowships. Fellows will:
- Be centrally involved in the daily activities and intellectual life of our interdisciplinary department.
- Participate in weekly bioethics seminars, case conferences, ethics consultations, IRB deliberations, and other educational opportunities at NIH.
- Study ethical issues related to biomedical research, clinical practice, genetics, biotechnology, public health, health policy, and other important issues in bioethics.
- Conduct mentored theoretical and empirical research. For a typical fellow, this research yields multiple first-authored publications in academic journals.
We do not require or expect bioethics experience and encourage anyone with a strong interest to apply.
Multinational Capacity Building
We have several initiatives designed to build capacity in low- and middle-income countries, including:
- Mentored research program for early career bioethics scholars from low- and middle-income countries
- International workshops in health policy and research ethics for researchers, governmental officials, and IRB and Ethics Committee members
- International workshops to support a network of young scholars in bioethics from low- and middle-income countries
- Three month IRB training program that brings participants from resource poor settings to the NIH for training in ethics and IRB review
- Joint NIH-University of Bergen Ph.D. program for candidates from low- and middle-income countries. Please contact the Department or Reidar Lie for more information [ disclaimer ] about this program
- Of the fellowships that we offer , the two-year postdoctoral training program is open to both international and domestic participants. The post-baccalaureate training program is open only to domestic participants.
- Short-term appointments for visiting scholars and researchers from around the world
Featured Projects
Continuing trial responsibilities for implantable neural devices Saskia Hendriks, Christine Grady et al., Neuron Read more
Building a "We" With Deliberative Dialogue in Pursuit of Health for All Yukiko Asada et al., AJPHn Read more
Research Faculty and Head of the Bioethics Consultation Service
We invite scholars with outstanding credentials and experience to apply for the position of Research Faculty and Head of the Bioethics Consultation Service in the Department of Bioethics at the NIH Clinical Center.
The selected candidate will also lead important empirical and/or conceptual research on theoretical and applied issues in bioethics and will oversee and guide the planning, management, and implementation of the Bioethics Consultation Service. They should be an independent scholar with an established (or potential) career as a recognized leader in their respective field, with a strong record of publication.
You are now leaving the NIH Clinical Center website.
This external link is provided for your convenience to offer additional information. The NIH Clinical Center is not responsible for the availability, content or accuracy of this external site.
The NIH Clinical Center does not endorse, authorize or guarantee the sponsors, information, products or services described or offered at this external site. You will be subject to the destination site’s privacy policy if you follow this link.
More information about the NIH Clinical Center Privacy and Disclaimer policy is available at http://www.cc.nih.gov/disclaimers.html
Committees: Research Ethics Committees
- Reference work entry
- First Online: 01 January 2022
- Cite this reference work entry

- Ana Borovecki 2
238 Accesses
Research ethics committees have become a permanent fixture when it comes to ethics of research. They are essential part of quality control of research protocol, and their existence and work are thoroughly described in all important international documents dealing with research ethics issues. In this contribution history, development and different types of research ethics committees are discussed. The functions, structure, and locale of research ethics committees are also addressed. The global dimension of the work of research ethics committees is also discussed.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

Research: Human Subjects

Committees, Research Ethics Committees (See Research Ethics; Research Ethics Committees)
Borovecki, A., ten Have, H., & Oreskovic, S. (2009). Ethics committees in Croatia: Studies in bioethics . Saarbrücken: VDM Verlag Dr. Müller.
Google Scholar
Bouëssau, M. S., et al. (2009). Research ethics committees: Basic concepts for capacity building . Geneva: WHO.
Glasa, J. (Ed.). (2000). Ethics committees in central and Eastern Europe . Bratislava: Institute of Medical Ethics and Bioethics.
Huriet, C. (2009). Article 19: ethics committees. In H. A. M. J. ten Have & S. J. Michele (Eds.), The UNESCO universal declaration on bioethics and human rights background principles and application (pp. 265–270). Paris: UNESCO.
Jonsen, A. R., Veatch, R. M., & le Roy, W. (1998). Source book in bioethics. A documentary history . Washington, DC: Georgetown University Press.
Levine, R. J. (2004). Research ethics committees. In W. T. Reich (Ed.), Encyclopaedia of bioethics (Vol. IV, pp. 2311–2316). New York, NY: Macmillan Simon and Schuster.
ten Have, H. (2005). Establishing bioethics committees guide No. 1 (pp. 40–52). Paris: UNESCO.
Further Readings
Amdur, R., & Bankert, E. A. (2011). Institutional review board: Member handbook (3rd ed.). Sudbury, MA: Jones and Bartlett Publishers.
Emanuel, E. J., et al. (Eds.). (2008). The Oxford textbook of clinical research ethics (pp. 541–588). Oxford/New York: Oxford University Press.
Schrag, Z. M. (2010). Ethical imperialism. Institutional Review Boards and the Social Sciences, 1965–2009 . Baltimore: The Johns Hopkins University Press.
Download references
Author information
Authors and affiliations.
Andrija Stampar School of Public Health, School of Medicine, University of Zagreb, Zagreb, Croatia
Ana Borovecki
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ana Borovecki .
Editor information
Editors and affiliations.
Center for Healthcare Ethics, Duquesne University, Pittsburgh, PA, USA
Henk ten Have
Rights and permissions
Reprints and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this entry
Cite this entry.
Borovecki, A. (2016). Committees: Research Ethics Committees. In: ten Have, H. (eds) Encyclopedia of Global Bioethics. Springer, Cham. https://doi.org/10.1007/978-3-319-09483-0_104
Download citation
DOI : https://doi.org/10.1007/978-3-319-09483-0_104
Published : 19 January 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-319-09482-3
Online ISBN : 978-3-319-09483-0
eBook Packages : Religion and Philosophy Reference Module Humanities and Social Sciences Reference Module Humanities
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Federal Policy for the Protection of Human Subjects ('Common Rule')
The current U.S. system of protection for human research subjects is heavily influenced by the Belmont Report , written in 1979 by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report outlines the basic ethical principles in research involving human subjects. In 1981, with this report as foundational background, HHS and the Food and Drug Administration revised, and made as compatible as possible under their respective statutory authorities, their existing human subjects regulations.
The Federal Policy for the Protection of Human Subjects or the “Common Rule” was published in 1991 and codified in separate regulations by 15 Federal departments and agencies, as listed below. The HHS regulations, 45 CFR part 46 , include four subparts: subpart A, also known as the Federal Policy or the “Common Rule”; subpart B, additional protections for pregnant women, human fetuses, and neonates; subpart C, additional protections for prisoners; and subpart D, additional protections for children. Each agency includes in its chapter of the Code of Federal Regulations [CFR] section numbers and language that are identical to those of the HHS codification at 45 CFR part 46, subpart A. For all participating departments and agencies the Common Rule outlines the basic provisions for IRBs, informed consent, and Assurances of Compliance. Human subject research conducted or supported by each federal department/agency is governed by the regulations of that department/agency. The head of that department/agency retains final judgment as to whether a particular activity it conducts or supports is covered by the Common Rule. If an institution seeks guidance on implementation of the Common Rule and other applicable federal regulations, the institution should contact the department/agency conducting or supporting the research.
The list below displays the agencies and departments that have signed onto the Common Rule and their CFR numbers. Hyperlinks are to areas of a department or agency Web site that have been suggested to HHS as entry points for those interested in human subject protection activities of the department or agency.
General information:
- Of these, 15 agencies are official signatories with the rule codified in their own Code of Federal Regulations (CFR) sections
- 4 departments and agencies follow the Pre-2018 Common Rule because of executive order or statutory mandate (Department of Homeland Security, Social Security Administration, Office of the Director of National Intelligence, and Central Intelligence Agency)
- There is 1 new signatory to the revised Common Rule (Department of Labor)
- 2 agencies that followed the pre-2018 Common Rule because of executive order or statutory mandate have become official signatories to the revised Common Rule (Department of Homeland Security and Social Security Administration)
- 1 original signatory (Department of Justice) intends to become an official signatory to the revised Common Rule
- You can find more information about the FDA regulations here
Common Rule Departments and Agencies:
For additional details about the agencies and departments that have signed onto the Common Rule, including contact information and links to relevant webpages/resources, please visit https://www.hhs.gov/ohrp/education-and-outreach/revised-common-rule/common-rule-departments-agencies/index.html .
| No. | Dept. or Agency | CFR Citation (2018) | Authority | Status under Pre-2018 Requirements | Status under 2018 Requirements |
|---|---|---|---|---|---|
| Department of Homeland Security | 6 CFR Part 46 | 5 U.S.C. 301; P.L. 107-296, sec. 102, 306(c); P.L. 108-458, sec. 8306. | Follows Common Rule and all subparts per statute (Pub. L. 108-458, title VIII, section 8306) | Common Rule Signatory | |
| Department of Agriculture | 7 CFR Part 1c | 5 U.S.C. 301; 42 U.S.C. 300v-1(b). | Common Rule Signatory | Common Rule Signatory | |
| Department of Energy | 10 CFR Part 745 | 5 U.S.C. 301; 42 U.S.C. 7254; 42 U.S.C. 300v-1(b). | Common Rule Signatory | Common Rule Signatory | |
| National Aeronautics and Space Administration | 14 CFR Part 1230 | 5 U.S.C. 301; 42 U.S.C. 300v-1(b). | Common Rule Signatory | Common Rule Signatory | |
| Department of Commerce ( ) | 15 CFR Part 27 | 5 U.S.C. 301; 42 U.S.C. 300v-1(b). | Common Rule Signatory | Common Rule Signatory | |
| Social Security Administration | 20 CFR Part 431 | 5 U.S.C. 301; 42 U.S.C. 289(a). | SSA and HHS split in 1995. Pursuant to the transition rules provided in Section 106 of title 1 of Pub.L. 103-296, SSA has been required to apply the CR to its research. | Common Rule Signatory | |
| 22 CFR Part 225 | 5 U.S.C. 301; 42 U.S.C. 300v-1(b), unless otherwise noted. | Common Rule Signatory | Common Rule Signatory | ||
| Department of Housing and Urban Development | 24 CFR Part 60 | 5 U.S.C. 301; 42 U.S.C. 300v-1(b) and 3535(d). | Common Rule Signatory | Common Rule Signatory | |
| Department of Justice ( ) | 28 CFR Part 46 | Common Rule Signatory | Intends to become an official signatory | ||
| Department of Labor | 29 CFR Part 21 | 5 U.S.C. 301; 29 U.S.C. 551. | Not a Common Rule Signatory | Common Rule Signatory | |
| 32 CFR Part 219 | 5 U.S.C. 301. | Common Rule Signatory | Common Rule Signatory | ||
| 34 CFR Part 97 | 5 U.S.C. 301; 20 U.S.C. 1221e-3, 3474. | Common Rule Signatory | Common Rule Signatory | ||
| Department of Veterans Affairs ( ) ( ) | 38 CFR Part 16 | 5 U.S.C. 301; 38 U.S.C. 501, 7331, 7334; 42 U.S.C. 300v-1(b). | Common Rule Signatory | Common Rule Signatory | |
| Environmental Protection Agency ( ) | 40 CFR Part 26 | 5 U.S.C. 301; 7 U.S.C. 136a(a) and 136w(a)(1); 21 U.S.C. 346a(e)(1)(C); sec. 201, Pub. L. 109-54, 119 Stat. 531; and 42 U.S.C. 300v-1(b). | Common Rule Signatory | Common Rule Signatory | |
| 45 CFR Part 46 | Common Rule Signatory | Common Rule Signatory | |||
| 45 CFR Part 690 | 5 U.S.C. 301; 42 U.S.C. 300v-1(b). | Common Rule Signatory | Common Rule Signatory | ||
| Department of Transportation | 49 CFR Part 11 | 5 U.S.C. 301; 42 U.S.C. 300v-1(b). | Common Rule Signatory | Common Rule Signatory | |
| Office of the Director of National Intelligence | None | EO 12333 (1981), amended by EO 13284 (2003), EO 13355 (2004), and EO 13470 (2008) | Follows CR because of EO 12333, as amended. | Follows CR because of EO 12333, as amended. | |
| Central Intelligence Agency | None | EO 12333 (1981), amended by EO 13284 (2003), EO 13355 (2004), and EO 13470 (2008) | Follows CR because of EO 12333, as amended. | Follows CR because of EO 12333, as amended. | |
| Consumer Product Safety Commission | 16 CFR Part 1028 | 5 U.S.C. 301; 42 U.S.C. 300v-1(b) | Common Rule Signatory | Common Rule Signatory |
Disclaimer Policy: Links with this icon ( ) mean that you are leaving the HHS website.
- The Department of Health and Human Services (HHS) cannot guarantee the accuracy of a non-federal website.
- Linking to a non-federal website does not mean that HHS or its employees endorse the sponsors, information, or products presented on the website. HHS links outside of itself to provide you with further information.
- You will be bound by the destination website's privacy policy and/or terms of service when you follow the link.
- HHS is not responsible for Section 508 compliance (accessibility) on private websites.
For more information on HHS's web notification policies, see Website Disclaimers .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Institutional Review Boards: Purpose and Challenges
Affiliation.
- 1 Department of Bioethics, Clinical Center, National Institutes of Health, Bethesda, MD. Electronic address: [email protected].
- PMID: 26042632
- PMCID: PMC4631034
- DOI: 10.1378/chest.15-0706
Institutional review boards (IRBs) or research ethics committees provide a core protection for human research participants through advance and periodic independent review of the ethical acceptability of proposals for human research. IRBs were codified in US regulation just over three decades ago and are widely required by law or regulation in jurisdictions globally. Since the inception of IRBs, the research landscape has grown and evolved, as has the system of IRB review and oversight. Evidence of inconsistencies in IRB review and in application of federal regulations has fueled dissatisfaction with the IRB system. Some complain that IRB review is time-consuming and burdensome without clear evidence of effectiveness at protecting human subjects. Multiple proposals have been offered to reform or update the current IRB system, and many alternative models are currently being tried. Current focus on centralizing and sharing reviews requires more attention and evidence. Proposed changes to the US federal regulations may bring more changes. Data and resourcefulness are needed to further develop and test review and oversight models that provide adequate and respectful protections of participant rights and welfare and that are appropriate, efficient, and adaptable for current and future research.
PubMed Disclaimer
Timeline of regulations and…
Timeline of regulations and guidance regarding IRB review. ANPRM = Advance Notice…
Similar articles
- American Society of Clinical Oncology policy statement: oversight of clinical research. American Society of Clinical Oncology. American Society of Clinical Oncology. J Clin Oncol. 2003 Jun 15;21(12):2377-86. doi: 10.1200/JCO.2003.04.026. Epub 2003 Apr 29. J Clin Oncol. 2003. PMID: 12721281
- Operational Characteristics of Institutional Review Boards (IRBs) in the United States. Nesom GL, Petrof I, Moore TM. Nesom GL, et al. AJOB Empir Bioeth. 2019 Oct-Dec;10(4):276-286. doi: 10.1080/23294515.2019.1670276. Epub 2019 Oct 16. AJOB Empir Bioeth. 2019. PMID: 31618119 Free PMC article.
- Burdens on research imposed by institutional review boards: the state of the evidence and its implications for regulatory reform. Silberman G, Kahn KL. Silberman G, et al. Milbank Q. 2011 Dec;89(4):599-627. doi: 10.1111/j.1468-0009.2011.00644.x. Milbank Q. 2011. PMID: 22188349 Free PMC article. Review.
- Are central institutional review boards the solution? The National Heart, Lung, and Blood Institute Working Group's report on optimizing the IRB process. Mascette AM, Bernard GR, Dimichele D, Goldner JA, Harrington R, Harris PA, Leeds HS, Pearson TA, Ramsey B, Wagner TH. Mascette AM, et al. Acad Med. 2012 Dec;87(12):1710-4. doi: 10.1097/ACM.0b013e3182720859. Acad Med. 2012. PMID: 23095928 Free PMC article.
- Ethical standards for medical research in the Israeli military - review of the changes in the last decade. Hassidim A, Kayouf R, Yavnai N, Panush N, Dagan D, Bader T, Hartal M. Hassidim A, et al. Isr J Health Policy Res. 2016 Dec 1;5:53. doi: 10.1186/s13584-016-0113-4. eCollection 2016. Isr J Health Policy Res. 2016. PMID: 27980720 Free PMC article. Review.
- The Benefit of an Umbrella Protocol: Reducing Challenges in Orthopedic Oncology Research. Simister SK, Tse S, Saade A, Sweeney CA, Wise BL, Thorpe SW, Randall RL. Simister SK, et al. J Clin Med. 2024 Mar 8;13(6):1551. doi: 10.3390/jcm13061551. J Clin Med. 2024. PMID: 38541777 Free PMC article.
- Towards an understanding of global brain data governance: ethical positions that underpin global brain data governance discourse. Ochang P, Eke D, Stahl BC. Ochang P, et al. Front Big Data. 2023 Nov 9;6:1240660. doi: 10.3389/fdata.2023.1240660. eCollection 2023. Front Big Data. 2023. PMID: 38025947 Free PMC article.
- Awareness of Medical Professionals Regarding Research Ethics in a Tertiary Care Hospital in Riyadh, Saudi Arabia: A Survey to Assess Training Needs. Alahmad G, Alshahrani KM, Alduhaim RA, Alhelal R, Faden RM, Shaheen NA. Alahmad G, et al. Healthcare (Basel). 2023 Oct 12;11(20):2718. doi: 10.3390/healthcare11202718. Healthcare (Basel). 2023. PMID: 37893792 Free PMC article.
- Continuous quality improvement: reducing informed consent form signing errors. Hsu TW, Huang CH, Chuang LJ, Lee HC, Wong CS. Hsu TW, et al. BMC Med Ethics. 2023 Aug 4;24(1):59. doi: 10.1186/s12910-023-00933-w. BMC Med Ethics. 2023. PMID: 37542298 Free PMC article.
- APOE Gene Associated with Dementia-Related Traits, Depression, and Anxiety in the Hispanic Population. Xu C, Padilla V, Lozano S, Gamez D, Su BB, Wang X, Maestre G, Wang K. Xu C, et al. Genes (Basel). 2023 Jul 6;14(7):1405. doi: 10.3390/genes14071405. Genes (Basel). 2023. PMID: 37510309 Free PMC article.
- McCarthy C. The origins and policies that govern institutional review boards. In: Emanuel E, Grady C, Crouch R, Lie R, Miller F, Wendler D, eds. The Oxford Textbook of Clinical Research Ethics. New York, NY: Oxford University Press; 2008:541-550.
- US Code of Federal Regulations. Title 45CFR, part 46. http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html . Accessed January 5, 2015.
- US Code of Federal Regulations. Title 21CFR, part 56. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CF... . Accessed January 5, 2015.
- US Code of Federal Regulations. Title 45CFR.46.101 (h). - PubMed
- US Code of Federal Regulations. Title 21 CFR 312.120; guidance for industry and FDA staff FDA acceptance of foreign clinical studies not conducted under an IND frequently asked questions.US Food and Drug Administration website. http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM294729.pdf . Accessed April 12, 2015.
Publication types
- Search in MeSH
Related information
- Cited in Books
Grants and funding
- Intramural NIH HHS/United States
LinkOut - more resources
Full text sources.
- Elsevier Science
- Europe PubMed Central
- Ovid Technologies, Inc.
- PubMed Central
Other Literature Sources
- scite Smart Citations
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

U.S. Government Accountability Office
Institutional Review Boards: Actions Needed to Improve Federal Oversight and Examine Effectiveness
Institutional Review Boards (IRBs) assess the ethics and safety of research studies involving human subjects, such as behavioral studies or clinical trials for new drugs or medical devices.
Health and Human Services oversees about 2,300 U.S.-based IRBs through routine or for-cause inspections to assess if they are following federal laws when reviewing research. But few IRBs are inspected. For example, one HHS agency aims to do just 3-4 routine inspections each year. Also, HHS agencies haven't examined how many inspections are needed or if inspections could be changed to further reduce risks to human subjects.
Our recommendations address this.

What GAO Found
Institutional review boards (IRB) are groups that review ethical and safety considerations for research involving human subjects, such as clinical trials.
General Institutional Review Board (IRB) Process
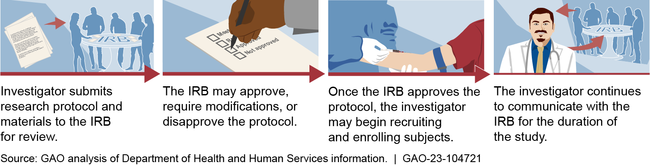
Most IRBs are based at universities, according to Department of Health and Human Services (HHS) data. University-based IRBs were also responsible for reviewing most research involving certain investigational drugs from calendar years 2012 through 2020, according to Food and Drug Administration (FDA) data. Some IRBs are independent, meaning they are not part of institutions that conduct or sponsor research. FDA data show these independent IRBs have reviewed an increasing share of investigational drug research: 25 percent of this research in 2012, and 48 percent in 2021. At the same time, the number of independent IRBs has decreased largely due to consolidation; this is, in part, related to private equity investment in IRBs.
FDA and HHS's Office for Human Research Protections (OHRP) oversee about 2,300 U.S.-based IRBs (operated by about 1,800 separate organizations, which may register and operate one or more IRB) through routine or for-cause inspections. These inspections assess whether IRBs follow federal regulations when reviewing research. FDA and OHRP consider several factors when selecting organizations for inspections, such as the volume of research reviewed. However, GAO found the agencies inspect relatively few IRBs. OHRP officials said they aim to conduct three to four routine inspections annually, while FDA conducted an average of 133 inspections annually between fiscal years 2010 and 2021. Neither agency has conducted a risk-based assessment of their IRB inspection program to help ensure they inspect enough IRBs annually and to optimize their responsibilities in protecting human subjects. Such an approach would be consistent with federal risk management principles.
While the agencies oversee IRBs to determine their adherence to regulations, OHRP and FDA have not assessed to what extent IRB reviews are effective in protecting human subjects. This is because the agencies have not determined the best approaches for doing so. Evaluating effectiveness is challenging in part due to an absence of validated measures and because IRBs are only one part of the framework of stakeholders responsible for protecting human subjects. Convening stakeholders to identify approaches for evaluating IRB effectiveness would be consistent with OHRP and FDA responsibilities and change management practices, and would help provide assurance that IRBs are successful in protecting human subjects.
Why GAO Did This Study
IRBs review research studies involving human subjects to ensure that risks to subjects are minimized and participants have sufficient information to consent to participate. In the past, IRBs were based at research institutions, such as academic centers. Over time, independent IRBs have played a more prominent role in reviewing research on human subjects. Some policymakers and others have raised questions about the increased use of independent IRBs and the effects on protecting human subjects.
GAO was asked to examine independent IRBs, processes used to protect human subjects, and standards of IRB quality, among other things. This report describes the composition of the IRB market and examines OHRP and FDA oversight of IRBs, among other objectives.
GAO reviewed federal laws and regulations and articles published between 2010 and June 2021; analyzed IRB registration, drug application, and inspection data; and interviewed FDA and OHRP officials, experts and stakeholders, and 11 IRBs selected for variation in type, size, and other factors.
Recommendations
GAO is making four recommendations, including that HHS and FDA conduct annual risk assessments to determine if the agencies are routinely inspecting an adequate number of IRBs and to optimize the use of inspections in the oversight of IRBs and protection of research participants, and examine and implement approaches for measuring IRB effectiveness. HHS concurred with the recommendations.
Recommendations for Executive Action
| Recommendation | ||
|---|---|---|
| Department of Health and Human Services | The Assistant Secretary for Health should ensure that OHRP takes steps to ensure the accuracy of protocol data collected in OHRP's IRB registry. This could include updating instructions to IRBs and examining data accuracy for a sample of IRBs. (Recommendation 1.) | |
| Department of Health and Human Services | The Assistant Secretary for Health should ensure that OHRP conducts an annual risk assessment to determine whether the agency is conducting an adequate number of routine IRB inspections and to optimize the use of IRB inspections in the oversight of IRBs and protection of research participants. (Recommendation 2) | |
| Food and Drug Administration | The Commissioner of the Food and Drug Administration should conduct an annual risk assessment to determine whether the agency is conducting an adequate number of routine IRB inspections and to optimize the use of IRB inspections in the oversight of IRBs and protection of research participants. (Recommendation 3) | |
| Department of Health and Human Services | The Secretary of Health and Human Services should ensure that OHRP and FDA convene stakeholders to examine approaches for measuring IRB effectiveness in protecting human subjects, and implement the approaches as appropriate. These could include effectiveness measures; peer audits of IRB meetings and decisions; mock protocols; surveys of IRB members, investigators, and human research participants; or other approaches. (Recommendation 4) |
Full Report
Gao contacts.
John Dicken Director [email protected] (202) 512-7114
Office of Public Affairs
Sarah Kaczmarek Acting Managing Director [email protected] (202) 512-4800
- Subscribe to our Newsletter
popular searches
The Concordat to Support Research Integrity
Upcoming Webinar Series
Publications
Annual Conference
Research Ethics Support and Review
Code of Practice for Research
Checklist for Researchers
Checklist during COVID-19
Case Study Packs
Researcher Checklist of Ethics Applications
Concordat Self-Assessment Tool
What is a Research Ethics Committee?
- Newsletter issues
Research ethics committees (RECs) are an important part of a healthy research culture. Their role is to consider the ethical implications of research. Traditionally this has focussed on the need to protect research participants (both human and animal), but in recent years their role in supporting researchers, and promoting research integrity more generally, has been increasingly recognised.
Two types of RECs
It is important to distinguish two types of research ethics committees. The first type is often set up to consider ethical issues that may be relevant to researchers working in specific areas. These might include the ethics of research into genetic modification, climate engineering, dual-use research (e.g., research with military applications), or research using potentially contentious methodologies such as “ human challenge ” trials (where participants are intentionally infected with diseases such as COVID). As these are difficult and complex areas, the main output is often in the form of guidance or position statements that can be applied by researchers, their institutions, funders, and ultimately policymakers. Consequently, these committees are convened at a fairly high level by organisations with an interest in the area of research being considered. They normally include scientific and legal experts alongside those with a specific interest in the topic under consideration (such as patient groups).
But the second, and far more common, type of research ethics committee is those set up by universities, research organisations, or health care providers (such as the NHS) to consider the ethical issues relating to individual, and often very specific, research projects. These Research Ethics Committees — abbreviated as RECs and referred to as Institutional Review Boards (IRBs) in the United States — provide a point-in-time review of a very detailed research protocol before the research is allowed to start. They aim to provide an opinion as to whether the research, if carried out in accordance with the detailed protocol, will meet accepted ethical norms. Exactly what these norms are, and how they can be addressed, is a complex question that may need to take into account guidance created by the first type of ethics committee described above. As such, although RECs still need to have suitably experienced individuals, it is more important that they are also suitably independent from the researcher (and their funder) to ensure they give an ethical opinion that is free from as many conflicts of interest as possible. Scientific or research expertise is important, but so is the voice of non-expert members. Quite often researchers will not be allowed to publish their work if they cannot prove it was reviewed by a REC before it started.
REC review supports research and researchers
REC review is criticised by researchers as being too lengthy, burdensome, or bureaucratic. This is often because it is confused with wider governance processes relating to issues such as data protection, health and safety, financial management, etc. While such issues are important, the fact that they are related to specific, often legally prescribed, arrangements means that they are governance issues that are the responsibility of the research institution (e.g., the university) to review and approve. The distinction between governance approvals , and ethics opinion , is extremely important if the aim is to create systems that provide robust, but proportionate, support to research and researchers. While in some contexts committees are expected to review both governance and ethics issues, there is an increasing recognition that governance is best handled separately by expert research officers, freeing RECs to consider the more complex ethical issues that may arise in any given research project.
Written by Dr Simon Kolstoe, UKRIO Trustee .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

National Institute of Environmental Health Sciences
Your environment. your health., research ethics timeline, david b. resnik, j.d., ph.d., bioethicist, niehs/nih florian w. hofweber.
Note: This list is the authors’ own interpretation of some important events in the history of research ethics and does not include every event that some people might regard as important. We welcome suggestions for additions, revisions, etc. Please send them to David B. Resnik .
Mughal emperor Akbar the Great performs an experiment to determine whether children who grow up in a mute environment will learn language. He ordered twelve infants to be raised by mute nurses who communicated with each other via sign language. He later came back to discover that the twelve children did not learn an audible language but instead communicated in sign. Similar experiments have been done by other monarchs, many with the purpose of discovering the “original” language.
Philosopher and scientist Francis Bacon (1561-1626) publishes The Novum Organon , , in which he argues that scientific knowledge should be based on observation and experimentation and not on tradition and authority and that it should benefit humanity.
Physicist and astronomer Galileo Galilea (1564-1642) publishes his Dialogue on Two World Systems , in which he defends a heliocentric theory of the solar system, a view that contradicted the Catholic Church’s position that the Earth does not move but that the Sun moves around it. In 1633, Galileo appeared before an inquisitor from the Catholic Church. He was ordered to recant his views and was sentenced to house arrest for the remainder of his life. The Church banned his book. In 1992, 359 years after Galileo’s arrest, Pope John Paul II formally apologized for its treatment of Galileo.
The Royal Society of London for Improving Natural Knowledge, the world’s first scientific organization, is established for the purpose of realizing Bacon’s vision of science. The Royal Society publishes the world’s first scientific journal Philosophical Transactions of the Royal Society of London , in 1665.
TThe Royal Society of London institutes peer review procedures for articles submitted to Philosophical Transactions .
English physician Edward Jenner (1749-1823), the “father” of immunology, tests a vaccine for smallpox on an 8-year-old boy, James Phipps. Jenner and others had observed that dairymaids did not get smallpox. Jenner theorized that this was because they were exposed to cowpox. Jenner tested this hypothesis by inoculating Phipps with cowpox and then exposing him to smallpox. Phipps did not get smallpox. During Jenner’s time, 10-20% of the global population died from smallpox each year.
English mathematician and inventor Charles Babbage (1791-1871) publishes Reflections on the Decline of Science in England, And Some of Its Causes , in which he argues that many of his colleagues were engaging in dishonest research practices, including fabricating, cooking, trimming, and fudging data. Babbage invented a programmable, mechanical computing machine.
Charles Darwin (1809-1882) and Lord Alfred Wallace publish The Origin of Species , which proposes a theory of evolution of living things by natural selection. The book generates a great deal of controversy because it proposes that human beings were not created by God (as most religions claimed) but descended from apes. Darwin collected most of the data for the theory while serving as the ship’s naturalist on the voyage of the HMS Beagle (1831-1836). He waited over twenty years to publish his ideas because he knew they would meet with strong opposition and he wanted to ensure that he could back up his claims with evidence and arguments. George Lyell urged Darwin to publish his theory after reading a paper by Alfred Wallace that proposed a theory similar to Darwin’s, so that Darwin could establish priority. Instead, Darwin shared credit with Wallace.
Roberts Bartholow (1831-1904) was treating a mentally disabled patient, 30-year-old Mary Rafferty, who had a two-inch hole in her skull caused by a cancerous ulcer. He inserted electrodes into the hole to study the effects of electrical stimulation on her brain. Rafferty experienced pain, distress, convulsions, and seizures, and fell into a coma a died in a few days.
The Massachusetts Supreme Court rules in McDonald v. Massachusetts General Hospital that a charitable hospital is not liable for the actions of its employees. This was an important case for human research ethics because courts that followed its reasoning shielded hospitals from legal liability from medical experiments performed by employees. Later, in Schloendorff v. Society of New York Hospital (1914), the New York Court of Appeals ruled that Mary Schloendorff could sue her doctors, but not the hospital, for unconsented surgery. This legal principle, known as the Schloendorff rule, stood until 1957, when the New York Court of Appeals overturned it in Bing v. Thunig.
French chemist and microbiologist Louis Pasteur (1822-1895) tested a rabies vaccine on a 9-year-old boy, Joseph Meister, who had been attacked by a rabid dog two days before. The vaccine worked, and by 1886 Pasteur was treating hundreds of people. The vaccine had been tested on dogs but not humans. Pasteur could have been prosecuted for vaccinating people because he was not a licensed physician.
Giuseppe Sanarelli (1864-1940) injects bacteria into five patients without their consent to test his hypothesis that the bacteria cause yellow fever. The patients all developed yellow fever symptoms and three died. However, the hypothesis later was proven false by Walter Reed. Many physicians sharply criticized Sanarelli’s experiments as being immoral.
U.S. Army physician Walter Reed (1851-1902) conducts medical experiments in Cuba in the early 1900s which show that Aedes aegypti mosquitos carry yellow fever. Yellow fever had been a major public health problem in the Caribbean and Central America that killed thousands of people each year and threatened commerce and U.S. military operations. Reed asked participants who had never had yellow fever to allow themselves to be bitten by mosquitos that had fed on patients with yellow fever, or to be injected with blood from a yellow fever patient. The participants signed informed consent forms (believed to be the first know use of this documentation) stating that they understood the risks of the experiment, including the possibility of death. The forms were translated into Spanish. Participants received $100 in gold and additional $100 and free medical care if they contracted yellow fever. Family members of participants who died also received $100. Two of Reed’s collaborators, James Carroll and Jesse Lazear, volunteered for the experiment. They both developed yellow fever and Lazear died. Reed had been planning to volunteer for the experiment, but Carroll talked him out of it due to his age (the disease was more deadly for patients over 40 years old, such as Reed). A total of 33 volunteers participated in the experiment, including 18 Americans (2 civilians and 16 soldiers) and 15 Spanish immigrants. Six people died from yellow fever. The surviving military personnel received medals and government pensions, and the Army’s Medical Center in Washington, DC was named after Reed.
Robert Millikan (1868-1953) performs oil drop experiments to determine the charge of an electron. Millikan received a Nobel Prize for this research in 1923. Historians and journalists who studied Millikan’s notebooks discovered that he did not report 33 out of 149 oil drop observations that he had marked as “fair” or “poor.” Millikan also did not name his student, Harvey Fletcher, as an author on the paper that reported the results of these experiments, even though Fletcher made important contributions to the design of these experiments, such as suggesting that Millikan use oil droplets instead of water droplets.
Museum curator Charles Dawson discovers a skull in at Piltdown gravel bed near Surrey, U.K. It was thought to be the fossilized remains of a species in between humans and apes (i.e., “a missing link”). A controversy surrounded the skull for decades and many scientists believed it to be fake. Chemical analyses performed in 1953 confirmed these suspicions by showing that the skull is a combination of a human skull and orangutan jaw, which had been treated with chemicals to make them appear old. The identity of the forger is still unknown, though most historians suspect Dawson.
The University of Wisconsin establishes the Wisconsin Alumni Foundation (WARF), an independent organization that manages intellectual property (e.g. patents) and investments owned by the university and supports scientific innovation and discovery on campus. At that time, few universities owned or managed patents. WARF helps Harry Steenbock develop his invention for fortifying fats with vitamin D.
The Tuskegee Syphilis Study, sponsored by the U.S. Department of Health, Education and Welfare, begins in 1932. The study investigated the effects of untreated syphilis in 400 African American men from the Tuskegee, Alabama area. The researchers did not tell the subjects that they were in an experiment. Most subjects who attended the Tuskegee clinic thought they were getting treatment for "bad blood." Researchers withheld treatment for the disease from participants even when penicillin, an effective form of treatment, became widely available in the 1950s. The study ended in 1972, after a news story from the Associated Press alerted the public and Congress to the ethical problems with the research. The U.S. government settled a lawsuit brought by the participants and their families. In 1997, President William Clinton issued an official apology on behalf of the U.S. government to surviving participants and their families.
Japanese scientists working at Unit 731 performed morally abominable experiments on thousands of Chinese prisoners or war, including biological and chemical weapons experiments, vaccination experiments, and wound-healing and surgical studies, including vivisections. The U.S. government agreed to not prosecute the scientists for war crimes in exchange for data from the biological and chemical weapons research. Unit 731 of the Imperial Japanese Army also conducted research on Korean prisoners/civilians (such as Dong Ju Yoon (arguably the most famous modern era Korean poet) and Chung-Chun Lee (a Korean national hero and freedom fighter)), as well as Mongolians, Manchurians (separate from Chinese), and Russians.
German scientists conducted morally abominable research on concentration camp prisoners, including experiments that exposed subjects to freezing temperatures, low air pressures, ionizing radiation and electricity, and infectious diseases; as well as wound-healing and surgical studies. The Allies prosecuted the German scientists for war crimes in the Nuremberg Trials. The Nuremberg Code provided the legal basis for prosecuting the scientists.
Two German refugee scientists, Frisch and R.E. Peierls, warn the U.S. about Germany's nuclear weapons program. Physicist Albert Einstein (1879-1955) sends a letter to President Roosevelt warning him about the threat posed by Germany. The letter, which was written by Leó Szilárd in consultation with Edward Teller and Eugene Wigner, was signed by Einstein. The letter suggested that the U.S. should develop a nuclear weapons program.
The U.S. conducts the $2 billion ($34 billion in 2023 dollars) Manhattan Project to develop an atomic bomb. General Leslie Groves directs the Project and physicist Robert Oppenheimer (1904-1967) oversees the scientific work. Other notable scientists who worked on the project included Hans Beth (1906-2005), Enrico Fermi (1901-1954), Richard Feynman (1918-1988), and Edward Teller (1908-2003). At the time, the negative health effects of radiation were poorly not well-understood and the scientists working on the project were exposed to extremely large doses of radiation through the handling of plutonium and uranium. The first atomic bomb was detonated in the Jornada del Muerto Desert in New Mexico on July 16, 1945.
The U.S. Department of Energy sponsors secret research on the effects of radiation on human beings. Subjects were not told that they were participating in the experiments. Experiments were conducted on cancer patients, pregnant women, and military personnel. These experiments included in total several hundred releases of radiation on human subjects. They were often done to test weaponry or safety equipment. These experiments were investigated decades after they happened by the Advisory Committee on Human Radiation Experiments, which was created in 1994 by President William Clinton after he declassified documents pertaining to this research. This committee also discovered that at least several hundred Uranium miners died of lung cancer, partly as a result of the government failing to properly ventilate the mines.
The U.S. drops atomic bombs on Hiroshima and Nagasaki, Japan on August 6 and 9, killing an estimated 200,000 civilians. After the bombs were dropped, Oppenheimer and other scientists led the “atoms for peace” movement.
Engineer and Head of the U.S. Office of Scientific Research and Development Vannevar Bush (1890-1974) writes the report Science: The Endless Frontier for President Franklin Roosevelt. The report argues for a major increase in government spending on science and defends the ideal of a self-governing scientific community free from significant public oversight. It advocates for investment in science and technology as a means of promoting national security and economic development.
The U.S. Congress passes a law transferring atomic energy development from military to civilian control. The law leads to the formation of the Atomic Energy Commission, which promotes peaceful uses of nuclear energy.
The Nuremberg Code, the first international code of ethics for research on human subjects, is adopted. The Code requires that research cannot take place without the subject’s consent; that research must be scientifically well-designed and socially valuable; and that research must minimize harm and suffering and should not involve a significant risk of death or disabling injury.
Norbert Wiener, the founder of cybernetics, published an article in the Atlantic Monthly titled "A Scientist Rebels" in which he refuses to conduct research for the military.
Alfred Kinsey (1894-1956) publishes Sexual Behavior in the Human Male . Five years later, he publishes Sexual Behavior in the Human Female . These books were very controversial, because they examined topics which were regarded as taboo at the time, such as masturbation, orgasm, intercourse, promiscuity, and sexual fantasies. Kinsey could not obtain public funding for the research, so he funded it privately through the Kinsey Institute.
The Soviet Union tests an atomic bomb, marking the beginning of the Cold War.
Believing that the Soviet Union had discovered a form of mind control, the CIA starts a covert program called MKUltra with the purpose of developing mind control techniques that could be used for interrogation or brainwashing. MKultra researchers subjected unwitting participants to psychological torture involving the administration of electric shocks and the psychoactive drug LSD. The program also involved hiring Nazi and Japanese doctors who had performed unethical experiments on living human subjects. The MKUltra program came to the public’s attention during Congressional Hearings held from 1975 to 1977.
James Watson (1928-) and Francis Crick (1916-2004) propose a model for the structure of DNA, for which they eventually would share the Nobel Prize with Maurice Wilkins (1916-2004) in 1962. An X-ray diffraction photo of DNA (known as Photo 51) generated by Rosalind Franklin (1920-1958) was crucial for verifying Watson and Crick’s model. Wilkins showed Watson and Crick Photo 51 without Franklin’s permission. Watson/Crick and Wilkins/Franklin published their papers in the same issue of the journal Nature in 1953. Neither Wilkins nor Franklin were named as authors on the Watson/Crick paper (and vice versa). Franklin was not awarded the Nobel Prize because she died in 1958 from ovarian cancer at age 37, and the prize is not awarded posthumously.
The U.S. Atomic Energy Commission revokes Oppenheimer’s security clearance based on its determination that, due to his associations with members of the Communist Party, he could not be trusted to be loyal to the U.S. and was a security risk.
Saul Krugman, Joan Giles and other researchers conduct hepatitis experiments on mentally disabled children at The Willowbrook State School. They intentionally infected subjects with a mild form of hepatitis for the purpose of developing a vaccine to a stronger form of the disease, which was endemic at Willowbrook. Children were infected both by injecting them with the hepatitis and making them drink chocolate milk mixed with the feces of people infected with the disease. The experiments were approved by the New York Department of Health. The Willowbrook State School itself was a center of controversy for abuse and neglect of children. In a speech to Congress in 1965, Robert F. Kennedy called the school a “snake pit.”
The Soviets launch Sputnik, the first satellite to orbit the Earth, which triggers the U.S. government to increase its investments in science and technology to avoid falling behind in the space race.
In 1957, thalidomide is marketed in West Germany as medication to treat morning sickness during pregnancy. About 10,000 infants, mostly in West Germany, are born with severe birth defects as a result of exposure to this drug. 2,000 children die from thalidomide exposure. In 1960, Frances Kathleen Oldham Kelsey (1914-2015), a drug reviewer for the FDA, refused to approve the drug. Soon, countries around the world ban the drug. Kelsey is awarded the President's Award for Distinguished Federal Civilian Service in 1962.
President John F. Kennedy commits the U.S. to the goal of putting a man on the moon by the end of the decade.
Rachel Carson (1907-1964) publishes Silent Spring , which alerts people to the harmful environmental and public health effects pesticides, especially DDT. Her book launches the environmentalist movement.
Stanley Milgram (1933-1984) conducts his "electric shock" experiments, which proved that people are willing to do things that they consider to be morally wrong when following the orders of an authority. The experiments, which had several variations, included a learner, a teacher, and a researcher. The learner was connected to electrodes. If the learner gave an incorrect response to a question, the researcher would instruct the teacher to push a button on a machine to give the learner an electric shock. Teachers were willing to do this even when the dial on the machine was turned up to “dangerous” levels and the learner were crying out in pain and asking for the experiments to stop. In reality, no shocks were given. The purpose of the experiments was to test subjects’ willingness to obey an authority figure. Since then, other researchers who have repeated these experiments have obtained similar results.
Due to fear of the long-lasting impacts of nuclear fallout, dozens of countries signed the Partial Nuclear Test Ban Treaty, which bans nuclear weapons tests, except those conducted underground.
The Public Health Service publishes its Guide to the Care and Use of Laboratory Animals , which describes standards, practices, and procedures for conducting experiments with animals and protecting their welfare. The Guide has been revised eight times, most recently in 2011.
The World Medical Association publishes Declaration at Helsinki, Ethical Principles for Research Involving Human Subjects. The Helsinki Declaration has been revised numerous times, most recently in 2013.
The U.S. Surgeon General's office issues its first of several reports on health problems related to smoking.
The Association for Assessment and Accreditation of Laboratory Animal Care (AALAC) is established as an organization that accredits institutions which perform experiments on laboratory animals. AAALAC evaluates organizations based on their compliance with standards, practices, and procedures described in the Public Health Service’s Guide to the Care and Use of Laboratory Animals .
Henry Beecher (1904-1976) publishes an article in the N ew England Journal of Medicine describing 22 unethical studies reported in the medical literature. Beecher selected these studies from 186 he had found in medical journals and said they represented a pattern of unethical behavior. Though Beecher did not include the names of researchers and institutions in his article, it is likely that Example 16 was the Willowbrook Experiment and Examples 1-3 were examples of methodologies that had been used in the Tuskegee Study.
The U.S. Congress adopts the Animal Welfare Act, which protect animals used in research, excluding rodents and birds. The Act was adopted partly due to the fear of dogs being stolen to be used in research. During the 1960s, various states adopt or revise animal cruelty laws that protect agricultural, domestic, wild, and laboratory animals.
The United States, Soviet Union, and United Kingdom sign the Treaty on the Nonproliferation of Nuclear Weapons and agree to pursue nuclear disarmament policies. Today, 190 countries have signed the treaty. Notably, India, Pakistan, Israel, and North Korea are not parties to the treaty.
The U.S. lands the first man on the moon, Neil Armstrong.
The U.S., Soviet Union, and dozens of other countries sign the Biological Weapons and Toxins Convention, which prohibits the development, production, acquisition, transfer, stockpiling and use of biological and toxin weapons but allows research on defensive countermeasures to biological weapons and toxins. However, the Soviet Union continues to conduct secret research on offensive bioweapons during the 1970s and 1980s.
After conducting hearings on unethical research involving human subjects, including the Tuskegee study, Congress passes the National Research Act in 1973, which President Nixon signs in 1974. The Act authorizes federal agencies (e.g., the NIH and FDA) to develop human research regulations. The regulations require institutions to form Institutional Review Boards (IRBs) to review and oversee research with human subjects.
Leaders of the emerging field of recombinant DNA research meet in Asilomar, CA to discuss biosafety issues and develop biosafety protocols. In 1974, Paul Berg and other top scientists call for a voluntary moratorium on recombinant DNA experiments until these risks are better understood and appropriate safety protocols are in place. In 1975, Berg and others published a paper in describing some biosafety principles and recommendations for recombinant DNA experiments.
The NIH forms the recombinant DNA advisory committee (RAC) to provide guidance for NIH-funded recombinant DNA experiments. In 1976, the RAC publishes guidelines for recombinant DNA research, which include the requirement that funded institutions establish institutional biosafety committees (IBCs) to oversee recombinant DNA experiments. Although not required by NIH policy, most IBCs also oversee other types of dangerous biological research, such as research involving the collection and storage of deadly pathogens.
William Summerlin admits to fabricating data by using a marker to make black spots on white mice at Sloan Kettering Cancer Institute. He was developing a technique for transplanting skin grafts.
Monsanto Corporation and Harvard University reach a deal for the first major corporate investment in a university.
Peter Singer publishes Animal Liberation , which provides a philosophical defense of the animal rights movement. Singer argues that all sentient beings have inherent moral value and that to think otherwise is a form of bias which he calls speciesism, i.e., the view that human beings are inherently superior to other forms of life. Singer argues that many socially accepted ways of using animals, such as for food, sport, or experimentation, are unethical.
Harvard entomologist E.O. Wilson (1929-2021) publishes Sociobiology , which reignites the centuries-old "nature vs. nurture" debate. His book proposes biological and evolutionary explanations of human behavior and culture.
The Animal Liberation Front, a radical animal rights group, is formed. This group has engaged in tactics involving raiding labs, destroying lab equipment, releasing animals into the wild, and threatening scientists. The ALF and related groups have caused millions of dollars in property damage.
Louise Brown, the world’s first baby conceived by in vitro fertilization, is born in the U.K., alive and healthy.
The National Commission for the Protection of Human Subjects in Biomedical and Behavioral Research publishes The Belmont Report: Principles of Ethical Research on Human Subjects . The Report articulates three ethical principles for research with human subjects, respects for persons, beneficence, and justice, and provides a conceptual foundation for a major revision of the U.S. federal research regulations in 1981.
Congress passes the Bayh-Dole Act, which allows researchers to patent inventions developed with government funds; the Act is amended by the Technology Transfer Act in 1986.
In Diamond v. Chakrabarty , the U.S. Supreme Court rules that a genetically modified bacterium can be patented because it is the product of human ingenuity. This sets a precedent for patents on other life forms and helps to establish solid intellectual property protection for the new biotechnology industry.
The Whitehead Institute is established at MIT, which represents a major private investment in a university. Other universities follow this example and begin forming complex partnerships with industry.
The Department of Health, Education, and Welfare (a precursor to the Department of Health and Human Services, DHHS) conducts major revisions of the federal human research regulations for human subjects research.
John Darsee, a postdoctoral fellow at Harvard, is accused of fabricating data. 17 of his papers were retracted.
William Broad and Nicholas Wade publish Betrayers of Truth . The book claims that there is more misconduct in science than researchers want to admit and suggests that famous scientists, including Isaac Newton, Gregor Mendel, and Robert Millikan were not completely honest with their data. Their book helps to launch an era of "fraud busting" in science.
Members of a religious cult led by Bhagwan Rajneesh sprayed salmonella bacteria on salad bars in ten restaurants in The Dalles, Oregon in an effort to make people too sick to vote in Wasco County elections so that the cult’s candidates would win. 751 people developed salmonella poisoning, but fortunately no one died. This is the first known bioterrorist attack on U.S. soil.
After collaborating with Robert Gallo on isolating HIV from biological samples provided by AIDS patients, Luc Montagnier accuses Gallo of misappropriating an HIV strain and accuses him of research misconduct, for which Gallo is found innocent. Gallo and Montagnier also have a dispute about who should be credited with discovering HIV and who can patent a test for the virus. The U.S. and French governments reach an agreement to settle the controversy.
The Animal Welfare Act is amended to create the Animal Welfare Information Center, the purpose of which is to improve the public’s access to animal welfare information and to develop more humane methods of animal research. The amendment also requires that government funded animal research be reviewed by Animal Care and Use Committees.
Aerospace engineer Roger Boisjoly warns NASA about possible O-ring failure during the Space Shuttel Challenger launch, due to cold weather. The O-rings, which are made of rubber, are designed to function properly at temperatures as low as 32° F (0° C), but the predicted air temperature at launch time was 26° F (−3° C). Boisjoly meets with NASA officials to discuss the problem, but they decide to go ahead with the launch, and the Challenger explodes, killing all seven crew members. A special committee investigating the accident determines that the O-rings did not seal properly due to the cold weather, which allowed rocket fuels to leak and explode. Nobel Prize-winning physicist Richard Feyman plays a key role in convincing the committee that the explosion was due to O-ring failure.
A NIMH panel concludes that Steven Breuning fabricated and falsified data in 24 paper. Breuning is convicted of defrauding the federal government in 1988.
Martin Luther King is accused of plagiarizing his Ph.D. dissertation.
Margot O'Toole, a post-doctoral student at the Whitehead Institute, has some questions about data in a paper authored by six of her colleagues and published in the journal Cell in 1986. She asks to examine Thereza-Imanishi-Kari's lab notebooks, which seem to be inconsistent with published results. She accuses Imanishi-Kari of fabricating and falsifying data. The ensuing investigation leads to inquiries by MIT and Tufts as well as the NIH and a Congressional committee chaired by Rep. John Dingell. Nobel Prize winner David Baltimore is one of the co-authors on the disputed paper. Although he was not accused of misconduct, Baltimore resigns as President of Rockefeller University. He described the investigation, which was covered by the New York Times, as a "witch hunt." An appeals board at the DHHS eventually exonerated Imanishi-Kari, who admitted only to poor record keeping.
Harvard and Dow Chemical patent a genetically engineered mouse used to study cancer.
The PHS forms two agencies, the Office of Scientific Integrity and the Office of Scientific Integrity Review to investigate scientific misconduct and provide information and support for universities. It also amends its definition of misconduct. The two agencies are reorganized in 1992 as the Office of Research Integrity (ORI).
The NIH requires that all graduate students on training grants receive education in responsible conduct of research. In the ensuing years, these requirements are expanded to include post-doctoral trainees and intramural researchers. NSF adopted RCR training requirements in 2010.
Stanley Pons and Martin Fleischmann hold a press conference at the University of Utah to announce that they have discovered a way to produce nuclear fusion at room temperatures. After dozens of labs across the world fail to reproduce their results, they are accused of fraud, sloppiness, and self-deception.
The National Academy of Science (NAS) publishes On Being A Scientist (revised in 1994 and 2009), which is a free, short book on research ethics for scientists in training.
The U.S. launches the Human Genome Project, a $20 billion effort to map and sequence the human genome.
W. French Anderson begins the first human gene therapy clinical trial on patients with ADA deficiency, a genetic disease that affects the immune system.
In Moore v. Regents of the University of California , the California Supreme Court rules that researchers have intellectual property rights in a cell line derived from Moore's tissue, but that Moore did not have any property rights in his own tissue. The Court also rules that the researchers violated Moore's right to informed consent by not disclosing their commercial interests in his tissue sample to him. Most courts have followed this ruling by holding that patients relinquish their property rights to tissues when they donate them to research or when they are leftover (“abandoned”) during surgery of medical procedures.
Congress investigates conflicts of interest (COIs) involving Pharmatec and the University of Florida and other COIs in biomedical research.
Europeans oppose the introduction of genetically manipulated foods and crops. Consumers in the U.S. are more receptive to GM plants and animals. After banning GM crops in 1998, the European Union allows the cultivation of GM crops but requires GM foods to be labeled as such. In 2022, the U.S. mandates the labelling of GM foods.
U.S. federal agencies revise their human research regulations. All U.S. government agencies now accept one basic regulatory framework, known as "the Common Rule" (45 CFR 46).
NAS publishes Responsible Science: Ensuring the Integrity of the Research Process . TThe book estimates the incidence of misconduct, discusses some of the causes of misconduct, proposes a definition of research misconduct, and recommends some strategies for preventing misconduct and promoting research integrity.
The Public Health Service (PHS), which funds NIH research, consolidates the Office of Scientific Integrity and the Office of Scientific Integrity Review into the Office of Research Integrity (ORI). ORI develops policies, procedures, policies, and regulations for preventing, reporting, and investing research misconduct; reviews and monitors misconduct investigations conducted by PHS-funded institutions and makes recommendations concerning misconduct findings and administrative; provides technical assistance to institutions that are responding to misconduct allegations; and supports research, conferences, and education on the responsible conduct of research.
In Daubert v. Merrell Dow Pharmaceuticals the U.S. Supreme Court rules that federal judges serve as the gatekeepers for admitting scientific testimony in court and that they can use a variety of criteria, including testability, reliability, peer review, and general acceptance for determining whether testimony is scientific. The intent of the ruling was to allow cutting-edge science that might not be generally accepted to be admitted as evidence in the courtroom. Prior to Daubert, federal judges followed the general acceptance standard articulated in Frye vs. United States (1923). State courts follow either the Daubert or Frye standard, depending on the jurisdiction.
Fertility researchers successfully clone human embryos.
Harvard psychologist Richard Herrnstein and Charles Murray publish The Bell Curve , a controversial book that reignites the centuries old debate about biology, race, and intelligence.
Roger Poisson admits to fabricating and falsifying patient data in NIH-funded breast cancer clinical trials in order allow his patients to qualify for enrollment and have access to experimental treatments.
The NIH applies for patents on thousands of gene fragments in order to undercut private efforts to patent gene fragments. The Patent Office rejects the NIH's applications because they did not clearly define a practical use for the fragments.
A Commission chair by Kenneth Ryan, convened by NIH, holds meetings on defining, investigating, and preventing scientific misconduct.
The Clinton Administration declassifies information about secret human radiation experiments conducted from the 1940s-1980s and issues an apology.
Two scientists who worked at Philip Morris, Victor DeNobel and Paul Mele, testify before Congress about secret research on the addictive properties of nicotine. If the research had been made public, the FDA or Congress might have taken additional steps to regulate tobacco as a drug. Many states and individuals brought litigation against tobacco companies, which led to a $206 billion settlement between tobacco companies and 46 states. The scientific community also publishes more data on the dangers of second-hand smoke.
Boots Pharmaceuticals pressures Betty Dong to withdraw a paper from publication in JAMA showing that its drug, Synthroid, is not more effective than generic equivalents at treating hypothyroidism.
Dozens of studies are published in biomedical journals which provide data on the relationship between the source of research funding and financial interests and the outcomes of research studies in the biomedical sciences, and the close relationship between academic researchers and the pharmaceutical and biotechnology industries.
The NIH and NSF revise their conflict of interest policies.
Scientists and defense analysts become concerned about the use of chemical or biological weapons by a terrorist group after Aum Shinrikyo, a Japanese doomsday cult, releases sarin gas in a Tokyo subway, killing 12 people and sending 5,500 to hospitals. The group also attempted (unsuccessfully) to spray anthrax spores over Tokyo. In 1998, terrorism experts warn about the use of biological or chemical weapons by Osama bin Laden and Saddam Hussein.
Over 200 religious leaders, led by biotechnology critic Jeremy Rifkin, protest the patenting of plants, animals, and human body parts in Washington, D.C.
Dolly, the world's first cloned sheep, is born; her birth is announced in 1997. Several European nations ban human cloning. Congress considers a bill to ban all human cloning but decides not to after scientists argue that the bill would undermine biomedical research.
Physics professor Alan Sokal submits a paper titled “Transgressing the Boundaries: Towards a Transformative Hermeneutics of Quantum Gravity” to Social Text , a cultural studies journal. Sokal filled the paper with errors of fact and reasoning and outright nonsense that any well-qualified physicist would be able to spot in order test the rigor of the journal’s peer review process. The paper proposed that quantum gravity does not exist independently of human beings and is a social and linguistic construct. After the paper was accepted for publication, Sokal revealed the hoax.
The ICMJE, representing over 400 biomedical journals, revises its authorship guidelines. The ICMJE recommends that to be an author on a scientific paper one must make a substantial contribution to research design, data collection, data analysis, or data interpretation and write the paper or critically revise and review it.
In an article published in New England Journal of Medicine ,Peter Lurie and Sidney Wolfe accuse the NIH, WHO, UN and CDC of designing and conducting unethical clinical on the prevention of mother-child transmission of HIV in developing countries, because the trials include placebo control groups even though the experimental treatment had been tested in western nations and proven effective. Representatives NIH Director Harold Varmus and CDC Director David Satcher argue that placebo control groups were needed to ensure scientific rigor because the dose of the experimental treatment being tested in the trials was much lower than the dose that had been proven effective. The dispute spurs a closer examination of international research ethics codes and guidelines.
Scientists perfect methods for growing human embryonic stem cells. Some countries ban the research; others promote it.
Craig Venter forms Celera Genomics and begins a private effort to sequence the human genome, using dozens of automated sequencing machines.
Apotex forces Nancy Olivieri, a clinical researcher at the University of Toronto, to withdraw a paper that exposes safety concerns about its drug deferiprone, which is used to treat thalassemia. The company tries to discredit Olivieri and have her fired.
Eighteen-year-old Jessie Gelsinger dies in a human gene therapy experiment at the University of Pennsylvania. The event triggers heightened scrutiny of conflicts of interest in human subjects research, including institutional conflicts of interest. Penn settles a lawsuit brought by the Gelsinger family for an undisclosed amount of money.
Human research lawsuits increase dramatically. Alan Milstein, from the law firm Sherman, Silverstein, Kohl, Rose & Podolsky, P.A., instigates 13 lawsuits against researchers, universities, pharmaceutical companies, and Institutional Review Board members.
The U.S. NIH and OHRP require all people conducting or overseeing human subjects research to have training in research ethics.
The U.S. Office of Science and Technology Policy finalizes a federal definition of misconduct as "fabrication, falsification or plagiarism" but not "honest error or differences of opinion.” Misconduct must be committed knowingly, intentionally, or recklessly.
ORI proposes mandatory training in responsible conduct of research (RCR) for all researchers on PHS grants, including junior senior investigators, students, and technicians. Several scientific associations and universities oppose the policy as an unnecessary and un-funded mandate. The Bush Administration suspends the ORI proposal in 2001 on the grounds that the agency failed to follow proper procedures for proposing new government regulations. Many research institutions voluntarily expand their RCR training programs.
Celera and the Human Genome Project both finish 99% complete drafts of the human genome and publish their results in Science and Nature .
Congress debates legislation on human cloning but does not adopt any laws.
Several journals, including Nature and JAMA , experiment with requiring authors to describe their responsibilities (e.g., designed experiments, collected data, analyzed data, wrote a first draft of the paper, etc.) when publishing research. Today, many journals follow this policy.
The Bush Administration announces that the NIH will only fund human embryonic stem cell research on approximately 64 cell lines created from leftover human embryos.
In the fall of 2001, Bruce Ivins, a biodefense researcher at the U.S. Army Medical Research Institute of Infectious Diseases in Fort Detrick, allegedly sent letters laced with anthrax spores to Senators Tom Daschle and Patrick Leahy and several members of the media. The attacks, which killed five people, sickened 17, and exposed dozens of postal workers to anthrax spores, created tremendous anxiety in a nation already reeling from the terrorist attacks of September 11, 2001. The Federal Bureau of Investigation’s lengthy and costly investigation eventually named Ivins as the suspect in 2008, but Ivins committed suicide before he could be apprehended and brought to trial.
Bell Labs determines that Jan Hendrick Schön, a rising star working in condensed matter physics and nanotechnology who published dozens of articles in a short period of time in prestigious journals, had fabricated and falsified data. 28 papers authored by Schön were retracted.
The President's Council on Bioethics recommends that the U.S. ban reproductive cloning and enact a moratorium on research cloning.
Historian Stephen Ambrose is accused of plagiarism.
The NAS publishes Integrity in Scientific Research , which recommends that universities develop programs for education in responsible conduct of research (RCR) as well as policies and procedures to deal with research ethics.
North Korea declares that it has a secret nuclear weapons program and warns that it has other "more powerful" weapons.
Scientists publish several papers in prominent journals with direct implications for bioterrorism. A paper published in the Journal of Virology described a method for genetically engineering a form of mousepox virus that is much deadlier than the naturally occurring strain. A paper published in Science showed how to make the poliovirus by obtaining supplies from a mail-order company. A paper published in PNAS develop a mathematical model for showing how many people would be killed by infecting the U.S. milk supply with botulinum toxin. In 2003, the American Society for Microbiology (ASM), the National Academy of Sciences, and the Center for Strategic and International Studies held a meeting to discuss the censorship biological research that poses security risks. Journals agree to self-censor some research.
The U.S. invades Iraq with the stated purpose of eliminating its chemical, biological, and nuclear weapons programs. The U.S. found evidence of weapons programs but no actual weapons.
The EPA suspends the CHEERS study due to criticism from advocacy groups and members of Congress, who claimed that the study was intentionally exposing children to pesticides and targeting minority groups. The EPA revised its human subjects rules in response to a Congressional mandate to strengthen protections for children and pregnant or nursing women.
Ronald Reagan, Jr. makes a presentation in support of federal funding for embryonic stem cell research to the Democratic Convention. Stem cell research (and therapeutic cloning) become hot issues in the 2004 Presidential election.
Merck withdraws its drug Vioxx from the market, due to safety and liability issues. As many as 50,000 people had a heart attack or stroke while take the drug, and thousands sued the company. As early as 2001, Merck scientists suspected that Vioxx could increase the cardiovascular risks, but researchers funded by Merck did not publish some of the data that would support these suspicions, even though they reported it to the FDA. In 2001, the FDA warned Merck that it had misrepresented Vioxx’s safety profile to the public and in 2002 it issued a black box warning for the drug. A systematic review of antidepressant medications known as selective serotonin uptake inhibitors (SSRIs) found that some of these drugs increase the risks of suicide in adolescents and children. The review included data from the U.K.’s Committee on Safety in Medicines, which had not been previously published. Patients, parents, researchers, and policymakers accused companies intentionally hiding this data from the public, and New York Attorney General Eliot Spitzer sued Glaxo for fraud. As a result of these problems related to data suppression, government agencies (including the FDA) and journals now require clinical trials to be registered on a publicly available website. Registration includes important information about the studies, including research design, interventions, and methods; research sites and personnel; contact information; and research results (but not raw data).
In response to criticism from Congress, the NIH revises its conflict of interest rules for employees. The rules place restrictions on ownership of stock in substantially affected organizations (such as pharmaceutical or biotech companies) by employees and prohibit employees with consulting with industry for pay.
Seoul University research Woo Suk Hwang admits to fabricating data in two papers published in the journal Science . In the papers, Hwang claimed that he had used nuclear transfer techniques to develop patient-specific human embryonic stem cells.
University of Vermont researcher Eric Poehlman admits to fabricating or falsifying data in 15 federal grants and 17 publications. Poehlman served a year and day in federal prison and agreed to pay the U.S. government $180,000 in fines.
In response to recommendations from a National Research Council report titled “Biotechnology in the Age of Terrorism,” the Department of Health and Human Services establishes the National Science Advisory Board for Biosecurity (NSABB) to provide advice and guidance to federal agencies, scientists, and journals concerning oversight and public of research in biotechnology or biomedicine which can be readily applied to cause significant harm to public health, agriculture, the economy, or national security (i.e. “dual use” research).
Someone hacked into the email server at the University of East Anglia Climatic Research Unit (CRU) and posted on the internet thousands of emails exchanged between climate change researchers at the CRU and researchers around the world. The emails showed that the researchers refused to share data and computer codes with climate change skeptics, who called the incident "climategate." The Intergovernmental Panel on Climate Change (IPCC), which relies heavily on data and models from CRU researchers, vowed to promote greater openness in climate research.
The Obama Administration announces it will significantly expand NIH funding of human embryonic stem cell research which had been restricted under the Bush Administration.
The National Science Foundation (NSF) announces RCR training requirements for funded investigators, students, and trainees. The NIH expands and strengthens its RCR training requirements.
While doing research on the Tuskegee Syphilis Study, Susan Reverby, Professor of Women’s Studies at Wellesley College, uncovered documents concerning unethical research experiments on human subjects conducted by the U.S. government in Guatemala from 1946 to 1948. The research involved intentionally infecting over 1300 subjects with syphilis to test the effectiveness of penicillin in preventing this disease. Only 700 subjects were given penicillin and 83 died as a result of the study. The subjects were not informed that they were participating in an experiment.
Lancet retracts a paper, published in 1998 by Andrew Wakefield and colleagues, linking the measles, mumps, and rubella vaccine to autism. Lancet retracted the paper after an investigation by journalist Brian Deer found that Wakefield had not disclosed a significant financial interest and had not obtained ethics board approval for the study. Wakefield’s research had been supported by a law firm that was suing vaccine manufacturers, and lawyer for the firm had helped Wakefield recruit patients. Wakefield did not disclose his relationship to the law firm in the 1998 paper. In 2010, the U.K.’s General Medical Council revoked Wakefield’s license to practice medicine. In 2011, Deer published an article in the British Medical Journal accusing Wakefield of fabricating and falsifying data in the 1998 paper. Deer based his findings on discrepancies between data reported in the paper and the data from patient records.
Jeffrey Beale publishes a list of what he calls “predatory journals.” Predatory journals are profit-driven journals that charge high fees for open access publication, promise rapid publication, and have poor (or nonexistent) standards for peer review. Beale later withdraws his list due to legal pressure from journals.
Ivan Oransky and Adam Marcus launch Retraction Watch, a blog that post retractions of scientific papers and articles related to research integrity.
The World Conference on Research Integrity releases the Singapore Statement on Research Integrity, a code of ethics for scientists in various disciplines.
The NIH and NSF revise their conflict of interest rules for funded research.
Journalist Rebecca Skloot publishes a widely-acclaimed book about Henrietta Lacks, an African American woman who provided the tissue for a widely-used cell line known as HeLa (an abbreviation of her name). In 1951, Lacks underwent treatment for cervical cancer at Johns Hopkins Hospital and died later that year. Researchers discovered that they were able to culture the cells from Lacks’ tumor and keep them alive, which was the first time that scientists had been able to grow a human cell line. HeLa cells have been used in thousands of laboratories around the world in biomedical experiments. Skloot was interested in finding out where the HeLa cell line came from, and she discovered that it came from Henrietta Lacks. Skloot interviewed Lacks’ family and learned that researchers had grown her tumor cells without her consent and without providing the family any compensation, which was a common practice at that time. Skloot decided to donate profits from her book to a private foundation she started whose purpose is to raise awareness about the role the human biological materials play in research and issues related to consent and ownership. In 2013, the NIH reached an agreement with Lacks’ family concerning access to genomic data from NIH-owned HeLa cell lines. The agreement gives the family control over access to the data and acknowledgment in scientific papers. In 2021, the Lacks family sued Thermo Fisher Scientific, a company that commercialized the the cell line. The lawsuit claims that the company unjustly profited from Lacks' tissue without her consent.
Several authors publish papers documenting a dramatic increase in the number of retracted papers since 2001 and that the majority of the retractions are due to research misconduct.
Two papers embroiled in controversy were published in Science and Nature after months of debate about their implications for bioterrorism. The papers reported the results of NIH-sponsored research conducted by a team working in the Netherlands, led by Ron Fouchier, and a team working at the University of Wisconsin, led by Yoshihiro Kawaoka. The researchers were able to genetically modify an H5N1 avian flu virus so that it can be transmitted by air between mammals. Currently, avian flu can only be contracted through direct contact with birds. The virus is highly lethal, with a case fatality rate of over 50%. Over 300 people have died from the virus since 1997. The National Science Advisory Board for Biosecurity (NSABB) initially recommend that the papers be published in redacted form, with key details removed and only made available to responsible scientists, so the terrorists or others could not use the information to make deadly bioweapons. However, the NSABB changed its mind and recommended full publication of both papers after learning more about the value of the research for public health (e.g., monitoring of bird populations, vaccine development), biosafety measures for containing the virus, how difficult it would be for terrorists to replicate the work, and legal problems with redacted publication.
The NIH launches the reproducibility initiative in response to published reports of problems with the reproducibility of biomedical and social/behavioral research. The initiative includes guidelines and educational materials for promoting rigor, transparency, openness, and reproducibility in research and reducing bias and error. Scientific journals also develop policies designed to promote reproducibility.
In Association for Molecular Pathology et al. v. Myriad Genetics , the U.S. Supreme Court rules that isolated and purified DNA cannot be patented. Only DNA that has been modified by human beings can be patented. The ruling invalidates Myriad’s patents on BRCA1 and BRCA2 genes and creates uncertainty concerning the legal validity of other types of patents on isolated and purified chemicals, such as chemicals derived from medicinal plants.
Haruko Obokata, a biochemist at the RIKEN Center for Developmental Biology in Kobe, Japan, and coauthors published two high-profile papers in Nature describing a method for converting adult spleen cells in mice into pluripotent stem cells by means of chemical stimulation and physical stress. Several weeks after the papers were published, researchers at the RIKEN Center were unable to reproduce the results and they accused Obokata, who was the lead author on the papers, of misconduct. The journal retracted both papers in July after an investigation by the RIKEN center found that Obokata had fabricated and falsified data. Later that year, Obokata’s advisor, Yoshiki Sasai, committed suicide by hanging himself.
The Department of Health and Human Services (DHHS) announces a pause on new funding of gain of function (GOF) genetic manipulation experiments involving SARS, MERS, and other potential pandemic pathogens (PPPs) and asks the NSABB to review the issues and make policy recommendations. GOF research is research that attempts to genetically manipulate a pathogen to enable it to acquire a new function, such as increased transmissibility or virulence. The NSABB recommended that the DHHS should lift the funding pause after implementing an oversight framework that minimizes and manages the risks of GOF research with PPPs, which DHHS did in 2017.
A research team led by Ralph Baric at the University of North Carolina publishes a paper in Nature Medicine reporting the results of an experiment in which they used a mouse-adapted SARS-CoV backbone to create a chimeric virus that expresses the spike protein of SHC014. The virus was able to use angiotensin-converting enzyme 2 (ACE2) receptors to infect and replicate in human airway cells and caused pathogenesis in mouse cells. The authors hypothesized that coronavirus pools found in horseshoe bats maintain spike proteins that give them the capability of infecting humans and stated that there is “a potential risk of SARS-CoV re-emergence from viruses currently circulating in bat populations.” Baric’s research was funded by the NIH. Shi Zengli, the second to last author on the paper, provided the genomic sequence for the spike protein. Shi conducted similar experiments at the Wuhan Institute of Virology. Baric’s lab was Biosafety Level 3, but Shi’s was only Biosafety Level 2. The paper became embroiled in controversy in 2021 when U.S. Senator Rand Paul alleged that it violated DHHS’s funding pause on GOF research.
The NIH places a temporary moratorium on funding for experiments involving human-animal chimeras while it revises existing policies that govern this research. The NIH lifted the moratorium the following year.
17 federal agencies publish the Final Rule for revisions to the Common Rule, which becomes effective in 2019. The revisions clarify and expand categories are research that are exempted from the Common Rule, enhance informed consent requirements, relax the need for continuing review once a study has stop recruiting participants, and requires a single IRB be responsible for reviewing multisite research.
Drawing inspiration from the Sokal hoax of 1996, in 2017-2018, philosopher Peter Boghossian, mathematician James Lindsay, and social commentator Helen Pluckrose submit bogus papers to race, gender, queer, fat, and sexuality studies journals to test their peer review standards. Several of the papers were published. The authors revealed their hoax in 2019. Boghossian and Pluckrose authored a paper titled “The Conceptual Penis as a Social Construct,” which was published in Cogent Social Sciences . In the paper, they argued that the human penis is not an anatomical organ but a social construct whose purpose is to perpetuate toxic masculinity.
In October, He Jiankui, a scientist of the Southern University of Science and Technology in Shenzhen, China, announces the birth of the world’s first gene edited babies, both girls. He claims that he used CRISPR-Cas 9 technology to modify the CCR5 gene to give the girls immunity to HIV. The announcement generates outrage around the world and many scientists and policymakers call for a ban on creating gene edited babies.
During the COVID-19 pandemic, which killed millions of people around the world, scientists worked feverishly to conduct research that could help combat this public health crisis. To make data and results available as quickly as possible, many journals expedited their peer review process for COVID-19 submissions and scientists published articles on pre-print servers before undergoing peer review. Unfortunately, the rush to publish led to the dissemination of invalid, irreproducible, and falsified results in some cases. Dozens of COVID-19 papers had to be retracted or withdrawn. Two prominent biomedical journals, the New England Journal of Medicine and the Lancet , retracted papers after the editors learned that the healthcare analytics company Surgisphere, which provided the data used in the studies, was not making the raw data available to independent scientists who found inconsistencies in the data and wanted to do an audit. The journals also learned that Surgisphere did not make all the data available to the scientists who authored the papers.
In early 2020, at the beginning of the COVID-19 pandemic, US scientists claimed that the SARS-CoV-2 virus that causes the disease probably moved from horseshoe bats into the human population via an undetermined intermediate host species. Others believed that SARS-CoV-2 was a genetically engineered pathogen that had escaped from a laboratory at the Wuhan Institute of Virology (WIV) in China. The World Health Organization attempted to investigate the origins of the virus by interviewing scientists at WIV and inspecting facilities, but they were unable to obtain all the information they needed. During 2020, there was a scientific consensus that SARS-CoV-2 probably had a natural origin. Opinions began to change in 2021, however, when highly respected science writer Nicholas Wade published several articles critically examining the two competing hypotheses: natural origin vs. laboratory escape. Wade argued that there was substantial evidence supporting the laboratory escape hypothesis, because an intermediate host species had not been found despite intense searching and a key part the protein that the virus uses to infect cells, known as the furin cleavage site, does not occur in related coronaviruses. After Wade’s articles were published, many scientists started publishing papers examining evidence concerning the origins of SARS-CoV-2. However, the origins of SARS-CoV-2 remain a mystery.
Neuroscientist Marc Tessier-Lavigne resigns his position as President of Stanford University after a special committee appointed by the Board of Trustees found that he failed to retract or correct 12 papers with serious flaws in a timely fashion. Tessier-Lavigne was a principal author on five of the papers, four of which included fabricated or falsified data or images. The Committee did not find that Tessier-Lavigne engaged in research misconduct. Commentators on the PubPeer website, which provides post-peer review of scientific papers, had alleged in November 2022 that digital images in the papers had been duplicated or otherwise manipulated.

Contact Science Directorate
Committee on human research.

The charge of the Committee on Human Research is:
To facilitate the conduct of and training in scientifically and ethically responsible research involving humans, and establish and maintain cooperative relations with organizations sharing common interests.
To examine issues related to the ethics of and regulatory requirements for research involving humans and disseminate accurate information about such research.
To develop and disseminate guidelines for protecting the rights and welfare of humans involved in research, and consult on the implementation of these guidelines.
The committee shall consist of six members elected by the Board of Scientific Affairs. Each year, two members will be elected for a term of three years. At least one member of the committee shall be an early career psychologist. The committee shall report to Council through the Board of Scientific Affairs.
Staff liaison
Kimberly Boller , PhD
2023 Chair : Albert “Buddy” Poje, PhD (2022–24) University of Kansas Medical Center
2023 Co-chair : Monica Biernat, PhD (2022–24) University of Kansas
Rona Carter, PhD (2023–25) University of Michigan
Donelson Forsyth, PhD (2023–26) University of Richmond
Anjali Thapar, PhD (2023–26) Bryn Mawr University
CHR statements and commentaries
Learning from Cases of Research Misconduct (APA Monitor on Psychology)
Guidelines for Ethical Conduct of Behavioral Projects Involving Human Participants by High School Students
Annual reports
2022 (PDF, 110KB)
2021 (PDF, 115KB)
2020 (PDF, 86KB)
2019 (PDF, 108KB)
2018 (PDF, 148KB)
2017 (PDF, 128KB)
- Johns Hopkins Berman Institute of Bioethics
Research Ethics Committee Assessment Toolkit (RECAT)
The Research Ethics Committee Assessment Toolkit (RECAT) is designed to facilitate evaluation of the operational needs of Research Ethics Committees (RECs) globally to inform local quality assurance and quality improvement efforts. The toolkit is published open-access for non-commercial use.
Click the Toolkit below to download .

The RECAT was developed by the African Bioethics Consortium (ABC) whose members include the Johns Hopkins University-Fogarty African Bioethics Training Program, the University of Zambia School of Medicine, the University of Botswana Office of Research & Development, and the Makerere University College of Health Sciences.
Financial support to develop the RECAT was provided to Johns Hopkins University Bloomberg School of Public Health and Berman Institute of Bioethics though a US National Institutes of Health, Fogarty International Center and National Institute of Allergy and Infectious Diseases supplemental grant under Award No. R25TW001604. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
While we make this freely available, we ask that you please reference the following citation when using or adapting the RECAT:
African Bioethics Consortium. (2017) Research Ethics Committee Assessment Toolkit (RECAT). Johns Hopkins University. Version 1.0. Baltimore Maryland USA.
We also kindly request that you share your experiences with using the RECAT with us so we can better understand its potential application and continue to make future improvements as necessary.
Feedback can be sent via email to: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Korean Med Sci
- v.38(25); 2023 Jun 26
- PMC10293659

Ethics Committees: Structure, Roles, and Issues
Pankti mehta.
1 Department of Clinical Immunology and Rheumatology, King George’s Medical University, Lucknow, India.
Olena Zimba
2 Department of Clinical Rheumatology and Immunology, University Hospital in Krakow, Krakow, Poland.
3 National Institute of Geriatrics, Rheumatology and Rehabilitation, Warsaw, Poland.
4 Department of Internal Medicine N2, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine.
Armen Yuri Gasparyan
5 Departments of Rheumatology and Research and Development, Dudley Group NHS Foundation Trust (Teaching Trust of the University of Birmingham, UK), Russells Hall Hospital, Dudley, UK.
Birzhan Seiil
6 Department of Biology and Biochemistry, South Kazakhstan Medical Academy, Shymkent, Kazakhstan.
Marlen Yessirkepov
An Ethics Committee (EC) is an independent body composed of members with expertise in both scientific and nonscientific arenas which functions to ensure the protection of human rights and the well-being of research subjects based on six basic principles of autonomy, justice, beneficence, nonmaleficence, confidentiality, and honesty. MEDLINE, Scopus, and Directory of Open Access Journals were searched for studies relevant to this topic. This review is focused on the types of research articles that need EC approval, the submission process, and exemptions. It further highlights the constitution of ECs, their duties, the review process, and the assessment of the risk-benefit of the proposed research including privacy issues. It’s pertinent for academicians and researchers to abide by the rules and regulations put forth by ECs for upholding of human rights and protecting research subjects primarily, as well as avoiding other issues like retraction of publications. Despite various issues of cost, backlogs, lack of expertise, lesser representation of laypersons, need for multiple approvals for multisite projects, conflicts of interest, and monitoring of ongoing research for the continued safety of participants, the ECs form the central force in regulating research and participant safety. Data safety and monitoring boards complement the ECs for carrying out continuous monitoring for better protection of research subjects. The establishment of ECs has ensured safe study designs, the safety of human subjects along with the protection of researchers from before the initiation until the completion of a study.
Graphical Abstract

INTRODUCTION
The journey of the role of ethics in biomedical research began with “The Doctor’s Trial” post-World War II in which 23 doctors and administrators were tried for war crimes, crimes against humanity, and conducting research without informed consent. This judgment, known as the “Nuremberg Code” was one of the first international ethical standards which gave a ten-point rule with respect to the protection of human research participants. The core principle was the requirement of voluntary consent of human subjects and respecting human autonomy. 1 , 2
However, some researchers continued to ignore the code and violations like the Willow Brook Hepatitis Study (1956), Jewish Chronic Disease Study (1963), and 22 others were highlighted by Beecher in 1966. 3 , 4 This led to the composition of the Declaration of Helsinki by the World Medical Association in Finland in 1964 with revisions at regular intervals. 5 This affirmed the principles highlighted in the Nuremberg Code stating that research should be conducted upholding the interests and rights of the human subjects. It proposed for the first time, the submission of a research protocol to an ethics committee (EC) before the initiation of the study ( Fig. 1 ). 6

EC refers to “Committees established by professional societies, health facilities, or other institutions to consider decisions that have bioethical implications. The role of these committees may include consultation, education, mediation, and/or review of policies and practices.” Committees that consider the ethical dimensions of patient care are Clinical ECs whereas committees established to protect the welfare of research subjects are Research ECs. 7 In this review, we will be using the terms ECs and Research ECs interchangeably.
It was in the 1960s that most nations developed guidelines regarding the formation of ECs with the main task of protection of human subjects. 8 EC is an independent body composed of members with expertise in both scientific and nonscientific arenas which functions to ensure the protection of human rights and the well-being of research subjects. ECs can be of two types—Institutional Review Boards (IRBs) or Institutional ECs (IECs) (referred to IRB or IEC by different countries) that are formally constituted by an institution to review research projects for that institute. An independent EC is an autonomous EC that is not part of any institute and performs the same functions independently. It is helpful for institutes that don’t have an IRB.
Despite these regulations, the unethical standards of the Tuskegee Syphilis study emerged in 1972 in which treatment was denied to the participants in order to study the natural course of the disease. This was followed by the conversion of the National Research Act in the USA into law (1974) and the setting up of the national commission of ‘International Ethical Guidelines for Biomedical Research Involving Human Subjects’ that submitted the Belmont report in 1979. The Belmont report described the role of assessment of risk-benefit of research involving human subjects, appropriate guidelines for selection of human subjects, and definition of informed consent. It was based on the three pillars of ethics- respect, beneficence, and justice. 9 , 10 It stressed the need for the approval of studies by an EC in accordance with the 1975 revision of the World Medical Association in Tokyo. Subsequently, countries like China, India, and South Korea adopted and legalized the need for submission of protocols to ECs from the 1980s onwards. 11 , 12 , 13 , 14
ECs function on six basic principles 15 :
- 1. Autonomy: respect the patient’s right to act on his/her own value and choice.
- 2. Justice: fair treatment of the research subjects.
- 3. Beneficence: work for the benefit of the patient.
- 4. Nonmaleficence: primum non-nocere or first do no harm to the patient.
- 5. Confidentiality: privacy protection.
- 6. Honesty: truthfulness in terms of the study.
Ethics approval is required for most research studies to uphold the above-mentioned principles, and protect the participants as well as the researcher. 16
In this narrative review, we aim to study the structure and function of ECs or IRBs with a focus on the composition, role, violations, and development perspectives of ECs.
Searches through MEDLINE (PubMed) and Scopus were performed in line with previously published recommendations. 17
Articles published till March 15, 2023 were reviewed using the following keywords: ("Ethics Committees, Clinical/classification"[Mesh] OR "Ethics Committees, Clinical/economics"[Mesh] OR "Ethics Committees, Clinical/ethics"[Mesh] OR "Ethics Committees, Clinical/history"[Mesh] OR "Ethics Committees, Clinical/legislation and jurisprudence"[Mesh] OR "Ethics Committees, Clinical/organization AND administration"[Mesh] OR "Ethics Committees, Clinical/standards"[Mesh] OR "Ethics Committees, Clinical/statistics and numerical data"[Mesh] OR "Ethics Committees, Clinical/trends"[Mesh]). Additional searches about subtopics were also carried out (“Data Safety Monitoring Boards” OR “Independent Data Review Committees”, “Institutional Review Boards” OR “Ethics Committees” and “Problems” OR “Issues”).
Articles in languages other than English, and reviews, conference proceedings, and editorials were excluded. Relevant articles searchable at the Directory of Open Access Journals and references of included articles were also processed for eligibility and inclusion for this narrative review. 18 , 19 , 20 , 21
RESEARCH, SUBMISSION PROCESS, AND EXEMPTIONS
IRB approval is required for most research to protect human rights and assess the scientific soundness of the research. For this, we first need to understand what research is. Research is defined as “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge” ( Table 1 ). 8
| Type of studies | Details about the need for approvals | |
|---|---|---|
| Studies involving interaction or intervention | ||
| 1. Clinical Trials- pilot, all phases and designs | A pilot study with an intention to contribute to general knowledge (fits the criteria for research) | |
| All phases of clinical trials require separate approvals. | ||
| 2. All interventional studies | Even those using standard of care or non-pharmacologic interventions need approval. | |
| 3. Diagnostic tests and devices | Analysis of data and biologic specimens need approvals. | |
| 4. Medical records review | Those identifying private identifying information need approvals. | |
| 5. Case reports | 1–3 cases don’t require IRB approvals, preferably patient consent should be taken for it nevertheless. | |
| Case series need approvals as they are hypothesis testing. | ||
| 6. Quality improvement and cost benefit analysis | Whenever it fits the definition of research, i.e., intent to generalize knowledge present. | |
| 7. Product evaluations | Whenever it fits the definition of research, i.e., intent to generalize knowledge present. | |
| 8. Public health surveillance | Those mandated by law, e.g., reporting of communicable diseases are exempt from IRB review. | |
| Studies not involving direct interaction or intervention | ||
| 1. Use of preexisting medical records or stored specimens | IRB review required if identifying information is recorded. | |
| 2. Databases, registries, biobanks of biomedical specimens | IRB review required for confidentiality purposes. | |
| Surveys/Interviews | Collection of identifying or sensitive information requires IRB approval. | |
IRB = Institutional Review Board.
An EC approval is required for studies with more than minimal risk to the subjects where the intention is to publish findings or contribute to the scientific knowledge, studies involving the compilation or analysis of data containing patient identifying information, studies with any risk of physical or mental discomfort to participants or their families, and studies on vulnerable groups. 22 Minimal risk refers to the probability of discomfort posed by the research is not greater than that ordinarily encountered in routine daily life activities of an average healthy individual. 5 , 8 , 22
Thus, even surveys and archived data that contain patient identifying information (name, age, address) and sensitive information (illicit drug use, comorbidities, communicable diseases, e.g., HIV AIDS) need ethical approval to uphold the privacy and anonymity of the participants as well as protection the possibility of psychological discomfort to them. 10 , 23 , 24 , 25
Some studies may be exempted from ethical approval including most educational research, case reports on one to three patients (without any hypothesis testing), those that pose no risk to the participants, involve information freely available in the open domain for the community, analysis of open-source datasets or anonymized datasets obtained from other researchers with due informed consent taken at the time of primary data collection, research evaluating the public health programs or government public schemes. 26 , 27 However, a formal exemption is to be decided by the IRB and not the investigator. 8 , 28
For projects requiring an EC approval, the type of reviews includes expedited and a full board review. Expedited review is for research involving no more than minimal risk to the subjects, minor revisions of an already approved study, and is usually conducted by an experienced person or the chair of the IRB. A full board review on the other hand is for research with greater than minimal risk to the subjects or those involving vulnerable populations. This is reviewed extensively by a full IRB meeting.
The documents usually required for a full ethics review include the name of the applicant with designation, approval of the head of the department, research/trial protocol, ethical issues if any, and plans to address them, written informed consent form (and assent forms) in the language the participant understands, data collection tools, patient information sheet, regulatory clearances (e.g., Drug Controller General of India in India for drug trials), finance and funding details, Insurance, statement of conflicts of interest, information about payment or compensation to the subjects, scientific or departmental review board permission, Curriculum Vitae of the investigators, declaration of interests and any other relevant information. 29 , 30 Waivers of consent may be provided for no more than minimal risk to the subjects when the waiver will not endanger the rights and welfare of the subjects like retrospective studies, secondary analysis of data wherein consent had been taken previously, use of open access databases with anonymized data, and emergency research as seen fit by the EC. 31 , 32 In emergencies like the coronavirus disease 2019 pandemic, waivers may be provided if the patient is incapacitated or in life-threatening situations where there is no time for informed consent. Pandemics like these may even call for common documents for risk disclosure and audio/video/electronic consent. 33 , 34
EC PERSONNEL, THEIR EXPERIENCE, AND DUTIES
ECs have the primary responsibility of reviewing research and its alignment with the Good Clinical Practice (GCP) guidelines. 35 The research design must be scientifically sound and conducted in an ethical way to include human subjects with voluntary informed consent.
The composition of ECs varies depending on the country, center, volume, and nature of the research reviewed. However, there are some basic recommendations laid down by national authorities and GCP. 30
- a) Most countries in Europe, the USA, and South Korea have a requirement of at least five members whereas recommendations in China and India need a minimum of seven members and a maximum of 12–15 members. 14 , 36 , 37 , 38 , 39
- b) At least one member who is autonomous, independent of the institution or trial site. It is mandatory that the chairperson of the EC is not part of the institution where the research is to be conducted.

Others include a member secretary from within the institution and members from the scientific field. The composition should have an adequate gender and age representation with a blend of basic scientists, clinician scientists, one legal expert, one social scientist, one philosopher, and one layperson. Review of research involving vulnerable populations like children, pregnant women, handicapped, prisoners, etc., must involve one member with expertise in dealing with that population. 40 , 41 It is also desirable to have a member or expert advisor for special areas of research who has proficiency in that field.
Responsibilities
The chairperson has the primary responsibility of independent and smooth functioning of the EC, ensuring the participation of all members, seeking Conflict of Interests from all members, and handling complaints against the researchers and EC members. 39 It’s the responsibility of the member secretary to schedule EC meetings, handle documentation, organize an effective review of proposals, define and maintain adherence to standard operating procedures (SOPs), train EC members, and assess the need for expedited reviews/exemption from review. 39 The members of the scientific community have the primary responsibility of reviewing the research protocols and their scientific soundness. The non-scientist member is crucial to safeguard the human subjects and practical issues of the research. 40 , 41 However, studies have shown lesser participation by laypersons as compared to scientific members. A study conducted across 10 academic centers across the USA with 20 IRB meetings recorded noted that 29 community members were present in 17 of those meetings. They were primary reviewers in only two of the 93 submitted protocols due to refusal on grounds of lack of knowledge regarding medical research. Even as secondary and tertiary reviewers, they were less active and were more likely to focus on issues related to confidentiality. However, they played a greater role when they were not designated reviewers. 42
The EC or IRBs function to review and approve research protocols, monitor ongoing research involving human subjects with the aims of continual protection of human volunteers, advancement of research, and protecting the institute from litigation. Its main role is the protection of the human rights, autonomy, confidentiality, and welfare of the research subjects especially vulnerable populations. The GCP recommends the following for duties of the IRB ( Table 2 , Fig. 3 ) 35 :
| Review process | Details | |
|---|---|---|
| Ensuring that risks to the subject are minimized | ||
| 1. Eligibility criteria | Drug X if metabolized through the kidney, will patients with reduced renal function be excluded? | |
| If not, what is the rationale for inclusion? | ||
| 2. Subject monitoring | If the drug has possible hematologic side effects, how and at what interval is the monitoring planned? | |
| 3. Are the proposed procedures well justified? | If a renal biopsy is planned at the end of the study to look for histopathologic remission, is it justified? Is it part of routine clinical care? | |
| 4. Comparison of risks with SOC | Additional risks conferred by the drug X as compared to SOC like MMF or CYC. | |
| 5. Are the research personnel qualified? | Is training in medical ethics and GCP done? | |
| Are trained personnel (rheumatologist/nephrologists in this case) managing the research participants? | ||
| 6. Are the SOPs in place for the exclusion of subjects? | What is the level of cytopenia or severity of infection at which drug X will be stopped? | |
| 7. Appropriate location | Rheumatology and Nephrology set-up, availability of ICU backup | |
| Risk benefit assessment | ||
| 1. Risks different from SOC | Is the risk of cytopenia or infections the same as SOC? If yes, easier for IRB to approve. | |
| 2. Frequency, severity and reversibility of side effects. Potential delayed effects | Short-lasting adverse events like reversible cytopenia is acceptable as long as the benefits outweigh the risk. Any chance of delayed renal dysfunction would be difficult for the IRB to assess. | |
| 3. Vulnerable populations | If the researcher plans to include vulnerable subjects like children, elderly, pregnant women, it is difficult for the IRB to approve. | |
| 4. Perception of risks amongst subjects | Risk of death with the drug is not acceptable. | |
| 5. Preclinical and clinical studies | Results of animal and phase 1/phase 2 data about drug X to assess safety and efficacy in favor of the drug X will help the approval process. | |
| 6. Continuation of SOC/Drug wash out | Will SOC be continued along with drug X? | |
| Will a wash out period be given for SOC before initiation of the study? | ||
| 7. Type of study design | RCT preferred over open label studies to assess safety and efficacy in an unbiased manner. | |
| 8. Potential for direct benefit or indirect benefit to science or society | In this case, there is a potential benefit to the subject and society. | |
| Equitable selection of subjects | ||
| 1. Target population | Ethnic representation as different severity of lupus nephritis across Caucasians, African Americans, Asians. | |
| 2. Inclusion and exclusion | Inclusion or exclusion of children, pregnant women, chronic kidney disease, etc., need to be confirmed. | |
| Informed consent | ||
| 1. Description of the informed consent process | Information, nature, time given to the subject for providing the information, etc., need to be assessed. | |
| 2. Appropriateness of the process | Assent in case of children, appropriateness of the language. | |
| 3. Components | Patient information about lupus nephritis and drugs, potential risks, potential benefits, confidentiality, compensation, voluntary process need to be assessed. | |
| Monitoring data | ||
| 1. Data safety monitoring plan as to how adverse events will be monitored and action taken | Frequency and method need to be assessed. | |
| Protection of subject privacy and confidentiality | ||
| 1. Location of data collection | Adequate space for interviewing, minimal personnel with access to patient data. | |
| 2. Sensitive data | Any data involving stigmatizing medical conditions, behaviors, and genetic information. | |
| 3. Data storage | Method and plans for data protection. | |
| 4. Data recording | Anonymous/coded. | |
| 5. Accessing patient records | Personnel with valid access. | |
| 6. Information in the consent forms about the measures planned and risks of breach in confidentiality | ||
| Vulnerable populations | Children, prisoners, mentally disabled people, or economically or educationally disadvantaged people, should explicitly describe what measures are in place to ensure that the subjects’ rights and welfare are adequately protected. | |
e.g., An immunosuppressive drug “X” being evaluated for patients with Lupus Nephritis.
IRB = Institutional Review Board, SOC = standard of care, GCP = Good Clinical Practice, MMF = mycophenolate mofetil, CYC = cyclophosphamide, ICU = intensive care unit, SOP = standard operating procedure, RCT = randomized controlled trial.

- • The IRB should obtain and review all the necessary documents for the research/trial within a reasonable time and document its views following standardized operating procedures with clear identification of the dates for approval, modifications, disapproval, or termination of an ongoing trial that was initially approved in writing.
- • Qualification of the investigators should be considered for the proposed research.
- • Reviewing of ongoing research as appropriate to the risks involved (at least once a year).
- • Protocols indicating exemption of prior consent of the subject or their legally acceptable representative (e.g., emergency situations) should be assessed in detail for all the regulatory needs.
- • Review the sum and method of compensatory payment to subjects if required.
- • Functions should be performed as per written SOPs which should comply with the GCP guideline.
Most IRBs conduct meetings regularly (one–two per month depending on the number of protocols) and SOPs are followed as per the national governing authority.
An EC review is a continuous process and is needed before the initiation of research, before the extension of the approval period, prior to modifications to an already approved study, for monitoring of any adverse events, and until all the data collection and analysis is complete. 8 An oversight to the monitoring of trials (usually single center, early phase, less risky) is provided by the IRBs through annual reviews, adverse event monitoring, and reporting of undue events by the principal investigator (PI). However, complex clinical trials and/or multicenter, randomized controlled trials, interventional studies with pre-existing concerns about safety, or study participants who might need additional protection through an additional committee referred to as the Data Safety Monitoring Board (DSMB). 43 , 44
RISKS, BENEFITS, CONFIDENTIALITY, AND PRIVACY ISSUES IN RESEARCH PROTOCOLS
The role of the EC is not only to provide direct protection to human subjects from physical or mental harm but also to weigh the risks and benefits involved in the research. It must be assessed if the study is designed to add to the current scientific knowledge base and help society. 8
The research protocol is the document that includes the research question, aims and objectives, a critical literature review, methodology, and statistical plan. It is pertinent that the IRB reviews the protocol with respect to the clarity and focus of the research question; and whether the study design is suitable to answer the same. This is decided by the chair or a special departmental committee ( Table 2 , Fig. 3 ).
Privacy and confidentiality are a part and parcel of every physician-patient relationship. Needless to say, this must be maintained in a researcher-human subject relationship as well. It helps build trust, curbs participant anxiety, maintains their dignity, and above all their autonomy. 10 The International Committee of Medical Journal Editors recommends that authors must ensure that nonessential information like names, initials hospital record numbers, etc., are omitted during data collection, storage, and publication whenever possible. 45 However, there’s an extent to which this confidentiality can be maintained. Information required for scientific purposes (e.g., clinical photographs) or those with mandated legal reporting may breach participant privacy. This needs to be explained to the participant and recorded in written informed consent ( Table 2 ).
The role of the IRB with respect to privacy and confidentiality is to:
- • Review the consent document and assess the sensitivity of the information, the duration for which it will be held, the usefulness of the information, and the ability to protect it.
- • For multicenter projects, review the measures taken by the research team to maintain the privacy of the research subjects including the number of personnel with access to the information, data storage, and transfer.
- • An ongoing review of the research must include monitoring of confidentiality issues to check for maintenance of the same and the need for a revised privacy protection plan.
- • Educate researchers and IRB members regarding the data privacy and protection process. 46
Review of informed consent by IRBs is especially important in low-middle-income countries. There are various issues related to the lack of understanding of the information provided, maintaining privacy due to interference by family members, and the inability to assess risk and benefit by the research participant. IRBs have an additional responsibility to ensure that studies have minimal/no risk to the participant, the consent forms are clear and simple to understand and ensure the proper process of obtaining informed consent is being followed without undue pressure or coercion to participate in the study. 47
VIOLATIONS OF ETHICS APPROVAL RULES AND REGULATIONS
Violations of IRB approval rules like lack of approval, lack of approval of modifications to the protocol, and lack of informed consent can result in dire aftermaths for the authors. It can result in the withdrawal of the article if it’s still in press, retraction if it’s already published, and even removal if it has legal consequences. The number of papers retracted as searched on the retraction database 48 is steadily increasing by the decade from 474 in the 1990s to 6120 in the 2010s. The most common reason for retraction is plagiarism whereas violation of IRB rules accounts for 4–5% of all retractions. 49 , 50 When consultations for ethical inquiries to the Korean Association of Medical Journal Editor were analyzed, the most common reason was duplicate publications (12 of 80) with issues with IRB approval (5 of 80) and informed consent (6 of 80). 51 Some of the examples of types of studies and their reasons for retractions have been summarized in Table 3 .
| No. | Health science (750) | Title | Type | Reasons | Date of publication | Date of retraction |
|---|---|---|---|---|---|---|
| 1 | Alternative medicine (15) | Retraction note: Improved treatment of asthma by using natural sources of antioxidants | Clinical Trial | • Lack of IRB approval | 06/26/2013 | 09/25/2014 |
| • Informed consent: none | ||||||
| 2 | Anesthesia (183) | Retraction note: Efficacy of dexmedetomidine as an adjunct to ropivacaine in bilateral dual-transversus abdominis plane blocks in patients with ovarian cancer who underwent cytoreductive surgery | Clinical Trial | • Lack of IRB approval for modifications | 08/18/2021 | 08/11/2022 |
| 3 | Cardiology/Cardiothoracic surgery (77) | Virtual reality-guided aortic valve leaflet reconstruction for type 0 bicuspid aortic stenosis | Case Report | • Lack of IRB approval for using a device not approved by the national authority | 06/01/2022 | 11/08/2022 |
| - Lack of patient consent | ||||||
| 4 | Dermatology (13) | Influence of maternal diet during lactation and use of formula feeds on development of atopic eczema in high risk infants | Clinical Trial | • Concerns/issues about authorship | 07/22/1989 | 10/28/2015 |
| • Ethical violations by author | ||||||
| • Falsification/fabrication of data | ||||||
| • Investigation by company/institution | ||||||
| • Misconduct: official investigation/finding | ||||||
| • Misconduct by author | ||||||
| • Upgrade/update of prior notice | ||||||
| 5 | Endocrinology (21) | Relationship between chronic kidney disease staging and vitamin D deficiency: a retrospective study | Medical Records Review | + Concerns/issues about authorship | 01/13/2022 | 03/17/2022 |
| + Concerns/issues about data | ||||||
| + False/forged authorship | ||||||
| + Investigation by journal/publisher | ||||||
| + Lack of IRB/IACUC approval | ||||||
| 6 | Gastroenterology (77) | The profile of the key pro-inflammatory cytokines in the serum of patients with CD and their association with the disease severity and activity | Prospective Observational Study | + Concerns/issues about authorship | 11/21/22 | 03/02/2023 |
| + Lack of IRB/IACUC approval | ||||||
| + Paper mill | ||||||
| + Unreliable results | ||||||
| 7 | Immunology (55) | Immunotherapy of HIV-infected patients with Gc protein-derived macrophage activating factor (GcMAF) | Clinical Study | • Lack of IRB approval | 11/28/2008 | 08/11/2014 |
| 8 | Neurology (76) | The association of interleukin-16 gene polymorphisms with IL-16 serum levels and risk of multiple sclerosis | Observational Study | + Concerns/issues about data | 02/02/17 | 01/06/21 |
| + Lack of IRB/IACUC approval | ||||||
| 9 | Obstetrics and gynecology (64) | Retraction note: Is early intervention using Mansoura-VV uterine compression sutures an effective procedure in the management of primary atonic postpartum hemorrhage?: a prospective study | Prospective Observational Study | • Lack of IRB approval | 05/31/17 | 02/04/2023 |
| 10 | Pediatrics (57) | Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis | Prospective Observational Study | • Lack of IRB approval | 09/02/2019 | 05/11/2018 |
Violations can be assessed before the studies are published for those with IRB approval. It is the responsibility of the IRBs to monitor whether ongoing studies are abiding by the ethical regulations and whether the approved protocol is being followed. A study conducted in India by an IRB at a tertiary care hospital in Mumbai monitored 12 clinical trials from 2011–2017. The most common violations were related to informed consent, followed by a lack of understanding of protocol and protocol deviations. This was corrected by re-taking of the informed consent and retraining in GCP by the IRB. 52 A similar study in Uganda done from 2007–2010 with monitoring of 40 research projects also found a similar frequency and reasons for violations. 53
Journal editors routinely check if a statement mentioning whether ethics approval was sought has been mentioned in the manuscript. Depending on the journal and type of article, further details of the EC approval can be sought by the journal editorial board. 54
ISSUES AND ONGOING DEVELOPMENTS
ECs were developed to provide ethical oversight to clinical research. But here are various issues associated with the functioning of IRBs.
- • Composition: Most studies indicate a skewed gender representation in the structure IRBs. Further, the participation of laypersons on the board is minimal. 14 , 42 , 55 , 56 , 57
- • Overburdened IRBs, delays, and operational costs: The IRB reviews have been associated with delays from over 4 to 7 months on average from surveys conducted across the USA. 58 , 59 A delay in biomedical research can translate into more than monetary loss as biomedical research saves lives and a delay in the approvals can result in greater loss of life. 60 An older survey conducted across 63 institutions (with 20 being low volume, 24 intermediate volume, and 19 being high volume centers) in the USA in 2005 reported the median amount spent by academic medical centers on IRB was $750,000/year with an average of $559 per review. The main costs are divided across staff salary, board salary, space, outsourcing of the reviews, travel, supplies, and equipment. 61 Over the years, there is a definite increase in the number of ongoing research projects thus increasing these costs further. Furthermore, documentation of Food and Drug Administration (FDA) warning letters to IRBs was predominantly related to paperwork stressing on documentation of reviews and meetings rather than ethical issues. 62 Increasing paperwork further results in delays and added costs. These deficiencies are more marked in developing nations like India and China dealing with issues like lack of regulation, informal ethics reviews, lack of supervision, and insufficient ethics review capacity. 63 , 64
- • Multi-site projects: With multicenter projects on the rise, a single protocol is often reviewed by multiple IRBs. In a review of 17 articles reported from UK, USA and Europe, which underwent multiple IRB reviews of the same protocol there were discrepancies in the judgment. Five of 26 reported rejection at some and acceptance by some IRBs. However, there were great differences in the protocol revisions, consent, patient information sheets, risk-benefit assessment, and compensation arrangements. 65 Keeping these issues in mind, the Common Rule in the USA was revised in 2017 with IRB approval required only from one center for multisite projects. 66 This may be extrapolated to other nations or consideration of an expedited review at other sites when fully reviewed at one IRB can be considered.
- • Independent EC and IEC: Independent ECs have inherent tissues of limitation of knowledge about the local community and use of these may promote IRB shopping. Whereas, local IRBs can have conflicts of interest as colleagues of investigators may be on the review board. Thus, a central IRB can alleviate some of these concerns by avoiding repetitive reviews, minimizing conflicts, and establishing a centralized adverse event reporting system. 67 , 68 , 69 A central IRB can be formed by experts on a particular subject or by a group of institutes like the National Cancer Institute’s Central IRB and the Biomedical Research Alliance of New York respectively. 70 , 71
- • Scientific expertise of the IRB reviewers: The IRB reviewers may lack the scientific expertise to review sophisticated research projects that may affect the quality of the research. 11 , 14 , 57 , 72 Regular training in research ethics and GCP along with adequate consultations with external experts is needed. This can be done at a national, regional, and international level. First, by identifying core issues and then solutions for them by focused training. 73 Training of EC members is conducted across Central Asia and Eastern Europe under the framework of Forum for Ethics Committees in the Confederation of Independent States and Strategic Initiative for Developing Capacity in Ethical Review program that train members regarding GCP, bioethics, the establishment of an EC, review processes and SOPs, choosing independent consultants, and confidentiality agreements. 74
- • Review of studies involving complementary and integrative medicine (CIM) is a challenge due to the lack of quality evidence to support the basis for their use. Moreover, most international regulatory bodies and research regulations do not address CIM, thus leaving the review process and decision-making to the IRBs. However, it is to be emphasized here studies irrespective of the type (modern or CIM) must be reviewed using the same principles of respect, beneficence, and justice. Well-designed studies on CIM are essential to ascertain the health and safety of patients. 75
DSMB is defined by the FDA, USA as “a group of individuals with pertinent scientific expertise that review research data of an ongoing trial on a regular basis, advises the sponsor/or researcher regarding the continuing safety of research subjects and those yet to be recruited into the research trial, and advises as to the continuing validity and scientific merit of the trial.” 76 It’s an autonomous entity independent of the researchers, sponsors, and the IRB so as to control data sharing and protect the authenticity of the clinical trial from unfavorable impact. 35 It was first developed in the USA in the 1960s as the NIH began sponsoring multicenter trials, the first trial was the Coronary Drug Project which used a DSMB for monitoring. 77 Over time, it became a common practice for the sponsors to have experienced scientific personnel serving on these committees. Although the FDA does not mandate DSMB for all trials, DSMBs are generally recommended for large, multi-site studies evaluating treatments that intend to reduce mortality and morbidity.
DSMBs are usually constituted for:
- • The study outcome is such that a highly encouraging or detrimental result is a possibility in an interim analysis that may require an early termination of the study on ethical grounds.
- • When the safety concerns are high, e.g., invasive therapy is administered.
- • Previous data suggesting serious toxicity with the study treatment.
- • Studies involving vulnerable populations.
- • Studies including subjects at an increased risk of death or serious outcomes.
- • Large, multisite, long-duration studies.
In India, it is recommended by the Indian GCP guidelines that the sponsor may establish a DSMB to assess the progress of the trial, and in 2006 Indian Council of Medical Research (ICMR) mandated a DSMB to review data emerging from research on interventions in the emergency setting. 39 These were updated in 2012 by the ICMR to include all stem cell research involving human subjects. The SOPs for the constitution and responsibilities of the DSMB are laid down by the World Health Organization and are similar across USA, Europe, and South Korea. 78 , 79
DSMBs are constituted by scientific members and are appointed by the funding agency, before the recruitment of the first subject in the trial. It can consist of as few as three members and is typically constituted of clinicians and at least one biostatistician. Others that may be included are medical ethicists, other scientists, etc. The most important requisite is that the members should be independent of the sponsors, investigators IRBs, regulatory authorities, and site or study staff. They should have no conflicts of interest with the sponsors, researchers, or study staff.
The functions of the DSMB are:
- • To uphold participant safety.
- • Ensure credibility and integrity of the trial for future subjects.
- • Ensure the timely conclusion of the study so that the results can be disseminated.
- • Identify protocol violations if any.
- • Identify unexpectedly high dropouts and evaluate for the same.
- • Ensure the validity of the results.
The above functions are carried out by an initial organizational meeting to understand the protocol and safety monitoring plan followed by an early safety review meeting to review early safety information. Continuing periodic reviews to assess safety, efficacy, and the progress of the trial are then carried out with reporting of serious adverse events. 44 A final meeting is to be held at the termination of a study. DSMBs function independently of the IRBS but the PIs must submit DSMB reports or minutes to the IRB.
Dramatic instances in which trials have been stopped prematurely on the recommendation of the DSMB include the withdrawal of rofecoxib and celecoxib in two trials on the prevention of colonic polyps due to increased cardiovascular events. 80 , 81
We have come a long way from the horrific ethical compromises in clinical studies in history to establishing adequate safety for the human subjects participating in clinical research today. The establishment of the IRB or EC has ensured safe study designs and the safety of human subjects right from before the study initiation until its completion. This is further supplanted by additional boards like DSMBs. However, we still need studies assessing the outcomes of the ECs on a global basis and addressing various issues that are still pertinent to the working of the ECs. 82
Disclosures: The authors have no potential conflicts of interest to disclose.
Author Contributions:
- Conceptualization: Mehta P, Zimba O, Gasparyan AY, Seiil B, Yessirkepov M.
- Data curation: Mehta P.
- Writing - original draft: Mehta P.
- Writing - review & editing: Mehta P, Zimba O, Gasparyan AY, Seiil B, Yessirkepov M.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- FDA Organization
- Center for Drug Evaluation and Research (CDER)
- CDER Offices and Divisions
Institutional Review Boards (IRBs) and Protection of Human Subjects in Clinical Trials
Under FDA regulations, an Institutional Review Board is group that has been formally designated to review and monitor biomedical research involving human subjects. In accordance with FDA regulations, an IRB has the authority to approve, require modifications in (to secure approval), or disapprove research. This group review serves an important role in the protection of the rights and welfare of human research subjects.
The purpose of IRB review is to assure, both in advance and by periodic review, that appropriate steps are taken to protect the rights and welfare of humans participating as subjects in the research. To accomplish this purpose, IRBs use a group process to review research protocols and related materials (e.g., informed consent documents and investigator brochures) to ensure protection of the rights and welfare of human subjects of research.
Regulations: Good Clinical Practice and Clinical Trials . Comprehensive list of regulations governing human subject protection and the conduct of clinical trials. Guidance for Institutional Review Boards and Clinical Investigators . A series of Information Sheets providing the Agency's current guidance on the protection of people who are subjects of research. Topics include Institutional Review Boards and Sponsor-Investigator-IRB interrelationships, FDA clinical investigator inspections and sanctions, clinical trials protocols, informed consent, and documents necessary for the conduct of clinical trials.
Information for Health Professionals . Additional links to information on subject protection from FDA and other government agencies.
Clinical Safety Data Management (PDF - 49KB) . Provides the definitions and terminology associated with clinical safety experience and the standards for expedited reporting of adverse drug reactions that occur during clinical trials.
FDA Compliance Program 7348.809 - BIMO for Institutional Review Boards (PDF - 1050MB).
FDA Compliance Program 7348.811 - Bioresearch Monitoring: Clinical Investigators.
Institutional Review Board Questions: Contact the Office of Good Clinical Practice, 301-796-8340, or [email protected]
Main Navigation
- Contact NeurIPS
- Code of Ethics
- Code of Conduct
- Create Profile
- Journal To Conference Track
- Diversity & Inclusion
- Proceedings
- Future Meetings
- Exhibitor Information
- Privacy Policy
NeurIPS 2024, the Thirty-eighth Annual Conference on Neural Information Processing Systems, will be held at the Vancouver Convention Center
Monday Dec 9 through Sunday Dec 15. Monday is an industry expo.

Registration
Pricing » Registration 2024 Registration Cancellation Policy »
Conference Hotels NeurIPS has contracted Hotel guest rooms for the Conference at group pricing, requiring reservations only through this page. Please do not make room reservations through any other channel, as it only impedes us from putting on the best Conference for you. We thank you for your assistance in helping us protect the NeurIPS conference.
Announcements
- See the Visa Information page for changes to the visa process for 2024.
- The call for High School Projects has been released
- The Call For Papers has been released
- The accepted competitions have been released.
Latest NeurIPS Blog Entries [ All Entries ]
| Aug 02, 2024 | |
| Jun 19, 2024 | |
| Jun 04, 2024 | |
| May 17, 2024 | |
| May 07, 2024 | |
| Apr 17, 2024 | |
| Apr 15, 2024 | |
| Mar 03, 2024 | |
| Dec 11, 2023 | |
| Dec 10, 2023 |
Important Dates
| Mar 15 '24 11:46 AM PDT * | ||
| Apr 05 '24 (Anywhere on Earth) | ||
| Apr 21 '24 (Anywhere on Earth) | ||
| Main Conference Paper Submission Deadline | May 22 '24 01:00 PM PDT * | |
| May 22 '24 01:00 PM PDT * | ||
| Jun 14 '24 (Anywhere on Earth) | ||
| Sep 05 '24 (Anywhere on Earth) | ||
| Main Conference Author Notification | Sep 25 '24 06:00 PM PDT * | |
| Datasets and Benchmarks - Author Notification | Sep 26 '24 (Anywhere on Earth) | |
| Workshop Accept/Reject Notification Date | Oct 09 '24 (Anywhere on Earth) | |
| Oct 23 '24 (Anywhere on Earth) | ||
| Oct 30 '24 (Anywhere on Earth) | ||
| Nov 15 '24 11:00 PM PST * | ||
Timezone: |
If you have questions about supporting the conference, please contact us .
View NeurIPS 2024 exhibitors » Become an 2024 Exhibitor Exhibitor Info »
Organizing Committee
General chair, program chair, workshop chair, workshop chair assistant, tutorial chair, competition chair, data and benchmark chair, diversity, inclusion and accessibility chair, affinity chair, ethics review chair, communication chair, social chair, journal chair, creative ai chair, workflow manager, logistics and it, mission statement.
The Neural Information Processing Systems Foundation is a non-profit corporation whose purpose is to foster the exchange of research advances in Artificial Intelligence and Machine Learning, principally by hosting an annual interdisciplinary academic conference with the highest ethical standards for a diverse and inclusive community.
About the Conference
The conference was founded in 1987 and is now a multi-track interdisciplinary annual meeting that includes invited talks, demonstrations, symposia, and oral and poster presentations of refereed papers. Along with the conference is a professional exposition focusing on machine learning in practice, a series of tutorials, and topical workshops that provide a less formal setting for the exchange of ideas.
More about the Neural Information Processing Systems foundation »
| NeurIPS uses cookies to remember that you are logged in. By using our websites, you agree to the placement of cookies. |
What is George Santos accused of? Looking back at allegations after guilty plea
Santos, who lied about being jewish, pleaded guilty to two of the 23 charges against him after reaching an agreement with prosecutors that could see him spending up to eight years in prison.
Moments after former Rep. George Santos pleaded guilty to wire fraud and identity theft , he told reporters that he was doing the right thing by recognizing his lies.
"Pleading guilty is a step I never imagined I’d take, but it is a necessary one because it is the right thing to do," the New York Republican said on Monday. "It’s not only a recognition of my misrepresentation to others, but more profoundly, it is my own recognition of the lies I told myself over these past years."
The plea comes nearly a year after he was indicted on 23 counts , including laundering campaign funds to pay for personal expenses, charging donor' credit cards and accepting unemployment benefits while he was working. He would have gone to trial in September had he not reached a plea agreement with prosecutors.
"Today, for what may seem like the first time since he started his campaign for Congress, Mr. Santos told the truth about his criminal schemes," U.S. Attorney Breon Peace said in a news release. "His flagrant and disgraceful conduct has been exposed and will be punished."
U.S. District Judge Joanna Seybert said that Santos could face up to eight years in prison at his sentencing on Feb. 7. At minimum, he faces two years in prison and agreed to pay nearly $374,000 under terms of the plea agreement.
Here's a look at all the allegations against the former politician in the short time since he joined Congress in January 2023, including lying about being Jewish and the grandson of a Holocaust survivor.
Why was George Santos expelled from Congress?
The House of Representative's voted 311-114 to expel Santos from office in December after he was indicted on the charges .
Before Santos' ousting, a House Ethics Committee report found substantial evidence that he committed federal crimes and misused campaign funds for personal expenses, like purchasing Botox and luxury apparel brands.
The report included more than 170,000 pages of documents, testimony from witnesses and financial statements alleging that Santos attempted to "exploit" his House campaign, deceive donors and lied about his campaign finances.
Both Democrats and Republicans called for Santos' resignation before the ousting. Lawmakers who voted against expelling him voiced concerns that doing so was premature as he had not been convicted.
Santos heavily denied the report's findings at the time, calling the investigators "biased."
What did the ethics committee find?
The ethics committee's report found that Santos fabricated multiple parts of his past, including that he worked at Goldman Sachs investment bank.
A subcommittee formed to investigate the former congressman probe reported "a complex web of unlawful activity involving Representative Santos’ campaign, personal, and business finances."
"Representative George Santos cannot be trusted," the subcommittee report said. "At nearly every opportunity, he placed his desire for private gain above his duty to uphold the Constitution, federal law, and ethical principles."
Santos lied about being Jewish and grandson of Holocaust survivors
Other lies by Santos include claiming to be the grandson of Holocaust survivors and that he is Jewish.
He claimed his maternal grandfather was forced to flee from Ukraine to Brazil to escape Nazism, CNN reported.
In December 2022, the New York Times reported that Santos lied about where he went to college, his bank job and falsifying records relating to his financial status.
Weeks later, Santos told the New York Post that he did fabricate his job experience and college education, adding that "my sins are embellishing my resume." He also responded to reports that he lied about being Jewish, saying he had claimed he was "Jew-ish."
Former aide accused Santos of sexual harassment
In February 2023, a former aide filed a complaint to the House Ethics Committee accusing him of sexual harassment.
The aide claimed Santos inappropriately touched him in the New York lawmaker's private office. Santos denied the allegation from the aide, who is the editor-in-chief of the Ohio news outlet the Scioto Valley Guardian.
"It's comical," Santos told CNN at the time. "Of course, I deny that claim."
Contributing: Bart Jansen , Ken Tran , Rachel Looker
MEMBERSHIP PROGRAMS
- Law.com Pro
- Law.com Pro Mid-Market
- Global Leaders In Law
- Global Leaders In Law Advisers
- Private Client Global Elite
MEDIA BRANDS
- Law.com Radar
- American Lawyer
- Corporate Counsel
- National Law Journal
- Legal Tech News
- New York Law Journal
- The Legal Intelligencer
- The Recorder
- Connecticut Law Tribune
- Daily Business Review
- Daily Report
- Delaware Business Court Insider
- Delaware Law Weekly
- New Jersey Law Journal
- Texas Lawyer
- Supreme Court Brief
- Litigation Daily
- Deals & Transactions
- Law Firm Management
- Legal Practice Management
- Legal Technology
- Intellectual Property
- Cybersecurity
- Law Journal Newsletters
- Analyst Reports
- Diversity Scorecard
- Kirkland & Ellis
- Latham & Watkins
- Baker McKenzie
- Verdict Search
- Law.com Compass
- China Law & Practice
- Insurance Coverage Law Center
- Law Journal Press
- Lean Adviser Legal
- Legal Dictionary
- Law Catalog
- Expert Witness Search
- Recruiters Directory
- Editorial Calendar
Legal Newswire
- Lawyer Pages
- Law Schools
- Women in Influence (WIPL)
- GC Profiles
- How I Made It
- Instant Insights
- Special Reports
- Resource Center
- LMA Member Benefits
- Legal Leaders
- Trailblazers
- Expert Perspectives
- Lawjobs.com
- Book Center
- Professional Announcements
- Asset & Logo Licensing

Content Source
Content Type

About Us | Contact Us | Site Map
Advertise | Customer Service | Terms of Service
FAQ | Privacy Policy
Copyright © 2021 ALM Global, LLC.
All Rights Reserved.

- Law Topics Litigation Transactional Law Law Firm Management Law Practice Management Legal Technology Intellectual Property Cybersecurity Browse All ›
- LegalTech Event (current)
- LegalWeek Event Perspectives (current)
- All Sections Events Cybersecurity & Privacy Legal Operations Products & Software Cases & Legislation Updates From the Experts Vendor Updates Expert Witness Search Lawjobs.com CLE Center Book Center Law.com Radar Public Notices Sitemap

Generative AI: A Legal Ethics Roadmap and Reference Guide
The American Bar Association's Standing Committee on Ethics and Professional Responsibility released ABA Formal Opinion 512: Generative Artificial Intelligence Tools on July 29. Here’s a roadmap of how we got here, and a reference guide to where we are with select case law and legal ethics rules in the era of GenAI.
August 11, 2024 at 05:58 PM
16 minute read
Artificial Intelligence
Share with Email
Thank you for sharing.
Monday marks the first full day of ILTACON , the annual celebration of law, technology, and the professionals practicing in this intersection, presented by the International Legal Technology Association (ILTA). As legal technology teams gather in Nashville this week, one wouldn’t be surprised if the 2024 edition were renamed “AICON.”
At least 55 of ILTACON 2024’s sessions have some reference to artificial intelligence, and for almost every attendee in Nashville this week, legal ethics should be an important issue—especially when it comes to generative AI (abbreviated often as “GenAI” or, in the case of the American Bar Association, “GAI”).
Want to continue reading? Become an ALM Digital Reader for Free!
Benefits of a digital membership.
- Free access to 1 article* every 30 days
- Access to the entire ALM network of websites
- Unlimited access to the ALM suite of newsletters
- Build custom alerts on any search topic of your choosing
- Search by a wide range of topics
Register Now
Already have an account? Sign In Now
*May exclude premium content
You Might Like

The Next Frontier: AI Agents for Law Firms
By Jay Madheswaran, Eve

Summer’s in the aiR: Recent Updates From Relativity
By Stephanie Wilkins

Coinbase Slams SEC's Proposal to Amend Definition of Term 'Exchange'
By Michael A. Mora

Minnesota State Bar Takes Big Step Toward Launching Gen AI Regulatory Sandbox
By Rhys Dipshan
Trending Stories
'Big Law Killed My Husband': An Open Letter From a Sidley Partner's Widow
The American Lawyer
'Increasingly Rare': These Law Firms Still Maintain Smaller Partner Pay Spreads
The Law Firms With the Largest Partner Pay Spreads
Simpson Thacher to Face UK Tribunal Over Anti-Money Laundering Compliance Allegations
International Edition
Simpson Partner Heads to Paul Hastings, as Firm Plots Growth in Banking, Finance

- 25 Years of the Am Law 200: Is Size as a Strategy a Winning Formula?
- People, Places & Profits, Part III: Are Law Firm Financial Metrics Keeping Pace With Inflationary Growth?
- The A-List, Innovation, and Professional Development: How Market Trends Are Impacting What it Takes to Be a Well-Rounded Firm
Featured Firms
Law Offices of Gary Martin Hays & Associates P.C. 75 Ponce De Leon Ave NE Ste 101 Atlanta , GA 30308 (470) 294-1674 www.garymartinhays.com
Law Offices of Mark E. Salomone 2 Oliver St #608 Boston , MA 02109 (857) 444-6468 www.marksalomone.com
Smith & Hassler 1225 N Loop W #525 Houston , TX 77008 (713) 739-1250 www.smithandhassler.com
Presented by BigVoodoo
More From ALM
- Events & Webcasts
The New York Law Journal honors attorneys and judges who have made a remarkable difference in the legal profession in New York.
The African Legal Awards recognise exceptional achievement within Africa s legal community during a period of rapid change.
Consulting Magazine identifies the best firms to work for in the consulting profession.
SALARY/STEP INCREASES 3% Annual Across the Board Salary Increases on February 2025/2026 (Salary Increases contingent upon assessed values fo...
Rawle & Henderson s Blue Bell, Pennsylvania office seeks an Attorney with at least five years of civil defense litigation experience to ...
Company DescriptionCruser, Mitchell is a national law firm, seeking an associate for its Bergen County office. Our lawyers possess a unique ...
Professional Announcement
Full Page Announcement
Subscribe to Legaltech News
Don't miss the crucial news and insights you need to make informed legal decisions. Join Legaltech News now!
Already have an account? Sign In
Advertisement
Supported by
What to Know About the Democratic National Convention
Vice President Kamala Harris and her running mate, Gov. Tim Walz of Minnesota, will be the stars in Chicago.
- Share full article

By Maggie Astor
Follow the latest news on Kamala Harris and the Democratic National Convention.
The Democratic National Convention is almost upon us, following the Republican convention last month. Once it’s over, it will be a 75-day sprint to Election Day.
Here is what to know about the convention.
When is the Democratic National Convention?
The convention will run from Monday, Aug. 19, through Thursday, Aug. 22.
Where is it?
It will be held at two venues in Chicago: The United Center, an arena on the city’s West Side, will host evening events — that is, the prime-time programming and speeches intended for public consumption. McCormick Place, which is downtown near Lake Michigan, will host daytime events, largely official party business and meetings.
Who will be there?
Vice President Kamala Harris and her running mate, Gov. Tim Walz of Minnesota, will be the stars, and President Biden is expected to speak as well. But the full list and schedule of the speakers has not yet been released, and Ms. Harris’s campaign and the Democratic National Convention committee have declined so far to confirm any names besides Ms. Harris and Mr. Walz.
Republicans, during their convention, didn’t publicize their speaking schedule until close to the start of each night’s programming.
Typically, though, conventions feature a wide array of prominent people within the party, such as governors, members of Congress and former elected officials. Spouses of the nominees and other family members often speak or make appearances. And Americans who aren’t famous generally get some stage time as well, speaking about personal experiences that touch the themes and policies the nominee wants to emphasize.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .

IMAGES
COMMENTS
The Office for Human Research Protections (OHRP) provides leadership in the protection of the rights, welfare, and wellbeing of human subjects involved in research conducted or supported by the U.S. Department of Health and Human Services (HHS). OHRP is part of the Office of the Assistant Secretary for Health in the Office of the Secretary of ...
An institutional review board (IRB), also known as an independent ethics committee (IEC), ethical review board (ERB), or research ethics board (REB), is a committee at an institution that applies research ethics by reviewing the methods proposed for research involving human subjects, to ensure that the projects are ethical.The main goal of IRB reviews is to ensure that study participants are ...
This free e-book for research ethics committee members focuses on research ethics issues in Africa. Strategic Initiative for Developing Capacity in Ethical Review (SIDCER) A network of independently established regional fora for ethical review committees, health researchers and invited partner organizations with an interest in the development ...
Additionally, the Pre2018-ComRule and the RevComRule indicate that for all human subjects research funded and/or sponsored by a Common Rule department/agency (as identified in USA-65), the institutional ethics committee (EC) (institutional review board (IRB) in the United States (US)) must ensure that, when appropriate, the research plan makes ...
institutional review board (IRB), in the United States, ethics committee that reviews proposed and ongoing research involving human subjects. The institutional review board (IRB) exists to protect the rights and safety of human subjects who participate in research studies. The need for an IRB became apparent in the 1960s and 1970s, largely as a ...
Mentored research program for early career bioethics scholars from low- and middle-income countries; International workshops in health policy and research ethics for researchers, governmental officials, and IRB and Ethics Committee members; International workshops to support a network of young scholars in bioethics from low- and middle-income ...
Standard 7. Ethical basis for decision-making in research ethics committees. The REC bases its decisions about research that it reviews on a coherent and consistent application of the ethical principles articulated in international guidance documents and human rights instruments, as well as any national laws or policies consistent with those principles.
The research ethics committee (REC) is constituted according to a charter or other document that establishes the manner in which members and the Chair will be appointed. The appointing entity ensures that the REC has a multidisciplinary and multisectoral membership, that its composition is gender balanced, that it reflects the social and cultural diversity of the communities from which ...
Johns Hopkins Medicine Institutional Review Board (a research ethics committee in USA) provides an annual Community Day during which the public is invited to dialog with them about research ethics, research protections, and the research review process. 10 Events like these are important because research ethics committees and research ...
In the United States, research ethics committees are usually called institutional review boards or IRBs since from the beginning, they were placed at those institutions that were performing research. Since they were mainly dealing with the research on human subjects, they were also called human investigation committee by some institutions in ...
The Federal Policy for the Protection of Human Subjects or the "Common Rule" was published in 1991 and codified in separate regulations by 15 Federal departments and agencies, as listed below. The HHS regulations, 45 CFR part 46, include four subparts: subpart A, also known as the Federal Policy or the "Common Rule"; subpart B ...
When most people think of ethics (or morals), they think of rules for distinguishing between right and wrong, such as the Golden Rule ("Do unto others as you would have them do unto you"), a code of professional conduct like the Hippocratic Oath ("First of all, do no harm"), a religious creed like the Ten Commandments ("Thou Shalt not kill..."), or a wise aphorisms like the sayings of Confucius.
Institutional review boards (IRBs) or research ethics committees provide a core protection for human research participants through advance and periodic independent review of the ethical acceptability of proposals for human research. IRBs were codified in US regulation just over three decades ago and are widely required by law or regulation in ...
Institutional Review Boards (IRBs) assess the ethics and safety of research studies involving human subjects, such as behavioral studies or clinical trials for new drugs or medical devices. Health and Human Services oversees about 2,300 U.S.-based IRBs through routine or for-cause inspections to assess if they are following federal laws when ...
News. Research ethics committees (RECs) are an important part of a healthy research culture. Their role is to consider the ethical implications of research. Traditionally this has focussed on the need to protect research participants (both human and animal), but in recent years their role in supporting researchers, and promoting research ...
A research ethics timeline from 1620 to present. ... These experiments were investigated decades after they happened by the Advisory Committee on Human Radiation Experiments, which was created in 1994 by President William Clinton after he declassified documents pertaining to this research. ... The United States, Soviet Union, and United Kingdom ...
The charge of the Committee on Human Research is: To facilitate the conduct of and training in scientifically and ethically responsible research involving humans, and establish and maintain cooperative relations with organizations sharing common interests. To examine issues related to the ethics of and regulatory requirements for research ...
A Guide for the Ethics Committee Editors Johan PE Karlberg and Marjorie A Speers ... Chief Compliance Counsel, Pfizer R&D, New York, USA - contacted Johan PE Karlberg in May 2009 and proposed the project for an ethics guide. ... research nurses, research support staff, ethics committee . 6 administrators, contract and budget development ...
African Bioethics Consortium. (2017) Research Ethics Committee Assessment Toolkit (RECAT). Johns Hopkins University. Version 1.0. Baltimore Maryland USA. We also kindly request that you share your experiences with using the RECAT with us so we can better understand its potential application and continue to make future improvements as necessary.
A Medical Research Ethics Committee (MREC) in the Netherlands. [2] A Comité de Protection des Personnes (CPP) in France. In the United States, an ethics committee is usually known as an institutional review board (IRB) or research ethics board (REB) and is dedicated to overseeing the rights and well-being of research subjects participating in ...
It proposed for the first time, the submission of a research protocol to an ethics committee (EC) before the initiation of the study (Fig. 1).6. Open in a separate window. ... This was followed by the conversion of the National Research Act in the USA into law (1974) and the setting up of the national commission of 'International Ethical ...
Ethics committees are the primary mechanism for dealing with ethical issues in hospitals in the United States today [1-3]. Present in nearly every US hospital, ethics committees were virtually nonexistent in the 1960s and '70s and, as recently as the early 1980s, were present in only 1 percent of US hospitals [4].
It includes an introduction to research ethics (module 1), a module on the role and responsibilities of Research Ethics Committees (module 2.1), several modules on the national regulation in given countries from the North and the South (module 3) and a detailed module on informed consent (module 4). ... USA Type of training/training methods. On ...
To accomplish this purpose, IRBs use a group process to review research protocols and related materials (e.g., informed consent documents and investigator brochures) to ensure protection of the ...
The Neural Information Processing Systems Foundation is a non-profit corporation whose purpose is to foster the exchange of research advances in Artificial Intelligence and Machine Learning, principally by hosting an annual interdisciplinary academic conference with the highest ethical standards for a diverse and inclusive community.
Before Santos' ousting, a House Ethics Committee report found substantial evidence that he committed federal crimes and misused campaign funds for personal expenses, like purchasing Botox and ...
The American Bar Association's Standing Committee on Ethics and Professional Responsibility released ABA Formal Opinion 512: Generative Artificial Intelligence Tools on July 29. Here's a roadmap ...
Vice President Kamala Harris and her running mate, Gov. Tim Walz of Minnesota, will be the stars in Chicago. By Maggie Astor The Democratic National Convention is almost upon us, following the ...