Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 20 April 2021

Habitual coffee drinkers display a distinct pattern of brain functional connectivity
- Ricardo Magalhães 1 , 2 , 3 , 4 na1 ,
- Maria Picó-Pérez ORCID: orcid.org/0000-0002-1573-2445 1 , 2 , 3 na1 ,
- Madalena Esteves 1 , 2 , 3 na1 ,
- Rita Vieira ORCID: orcid.org/0000-0001-6762-406X 1 , 2 , 3 ,
- Teresa C. Castanho 1 , 2 , 3 ,
- Liliana Amorim 1 , 2 , 3 ,
- Mafalda Sousa 1 , 2 , 3 ,
- Ana Coelho 1 , 2 , 3 ,
- Henrique M. Fernandes 5 ,
- Joana Cabral ORCID: orcid.org/0000-0002-6715-0826 1 , 2 , 3 , 5 ,
- Pedro S. Moreira 1 , 2 , 3 , 6 &
- Nuno Sousa ORCID: orcid.org/0000-0002-8755-5126 1 , 2 , 3 , 7
Molecular Psychiatry volume 26 , pages 6589–6598 ( 2021 ) Cite this article
145k Accesses
33 Citations
489 Altmetric
Metrics details
- Neuroscience
Coffee is the most widely consumed source of caffeine worldwide, partly due to the psychoactive effects of this methylxanthine. Interestingly, the effects of its chronic consumption on the brain’s intrinsic functional networks are still largely unknown. This study provides the first extended characterization of the effects of chronic coffee consumption on human brain networks. Subjects were recruited and divided into two groups: habitual coffee drinkers (CD) and non-coffee drinkers (NCD). Resting-state functional magnetic resonance imaging (fMRI) was acquired in these volunteers who were also assessed regarding stress, anxiety, and depression scores. In the neuroimaging evaluation, the CD group showed decreased functional connectivity in the somatosensory and limbic networks during resting state as assessed with independent component analysis. The CD group also showed decreased functional connectivity in a network comprising subcortical and posterior brain regions associated with somatosensory, motor, and emotional processing as assessed with network-based statistics; moreover, CD displayed longer lifetime of a functional network involving subcortical regions, the visual network and the cerebellum. Importantly, all these differences were dependent on the frequency of caffeine consumption, and were reproduced after NCD drank coffee. CD showed higher stress levels than NCD, and although no other group effects were observed in this psychological assessment, increased frequency of caffeine consumption was also associated with increased anxiety in males. In conclusion, higher consumption of coffee and caffeinated products has an impact in brain functional connectivity at rest with implications in emotionality, alertness, and readiness to action.
Similar content being viewed by others
Drinking coffee enhances neurocognitive function by reorganizing brain functional connectivity
Resting-state functional connectivity and structural differences between smokers and healthy non-smokers
Brain activity during a working memory task after daily caffeine intake and caffeine withdrawal: a randomized double-blind placebo-controlled trial
Introduction.
Coffee is the most widely consumed beverage, with particular interest for human health in view of its short-term effects on attention, sleep, and memory and its long-term impact on the appearance of different diseases and on healthy span of ageing [ 1 , 2 ]. Coffee has several constituents able to impact on human health, amongst which stems caffeine, which is the most widely consumed psychostimulant in the world [ 3 ]. Despite its widespread use it is surprising to note that a thorough characterization of the chronic effects of coffee upon the human brain is still lacking. In the present work we aim to begin addressing that issue.
In the brain, caffeine acts as an antagonist of adenosine A1 and A2A receptors, leading to hyperexcitability of the central nervous system [ 3 , 4 ]. This induces acute effects in diverse domains, such as physical endurance [ 1 , 5 ], vigilance, dexterity [ 6 ], mood [ 7 , 8 ], memory [ 9 ], and cognitive function [ 1 , 8 , 10 ]. There is also evidence that coffee/caffeine intake can normalize anxiety [ 11 ], although higher doses of caffeine may be anxiogenic [ 1 , 12 ] by disrupting the HPA axis [ 13 ]. On the other hand, epidemiological and animal studies converge in concluding that coffee, caffeine and adenosine receptor antagonists attenuate the burden of neurodegenerative disorders such as Alzheimer’s [ 14 ], or psychiatric disorders such as depression [ 15 ]. Indeed, chronic antagonism of either A1 or A2 receptors seems to induce an upregulation of the former, but not the latter. The resulting altered receptor ratio may explain the shift from the acute psychomotor effects (e.g., attention, vigilance) to the longer-term actions of coffee (e.g., stress resistance, neuroprotection) effects [ 4 , 16 ].
Functional magnetic resonance imaging (fMRI) allows studying, in a noninvasive way, the function of the human brain during execution of different tasks or at rest [ 17 ]. So far, most studies using fMRI were focused on measuring the acute effects of caffeine intake in the brain. Briefly, they have reported caffeine-related increases in blood oxygenation-dependent-level (BOLD) signal in different cortical and subcortical areas during a visuomotor task [ 18 ]; an impact in working memory and perfusion in elderly subjects [ 19 , 20 ]; an increase in BOLD activation in the frontopolar and cingulate cortex during a 2-back verbal working memory task [ 21 , 22 ]; and a global caffeine-induced increase in brain entropy, possibly representing an increased processing capacity [ 23 ]. Very few studies, however, were performed to study the acute effects of caffeine in functional connectivity (FC) at rest [ 24 , 25 ]. Those few studies reveal a general trend for a caffeine-induced reduction in FC, associated with neuro-electric power fluctuations as measured through magnetoencephalography and exacerbated anticorrelations. Despite this existing literature, many aspects of the characterization of the impact of caffeine on the brain remain unexplored. Critical amongst these is the characterization of the chronic effects of habitual coffee and caffeine consumption upon the functional architecture of the brain. We are only aware of a single study that touched on this subject [ 26 ]. That work revealed an association between different habits of coffee consumption and the magnitude of BOLD signals in the visual cortex; however, it did not address possible effects on the functional connectome or resting state networks. Pursuit of the latter can present significant challenges in finding and recruiting participants with sufficient variation in consumption habits and who are willing to undergo necessary, even if short, abstinence procedures.
To tackle this gap, herein we will use whole brain approaches [ 27 , 28 , 29 ], as well as the study of brain functional dynamics [ 30 ] to compare FC and its dynamics between habitual and non-habitual coffee consumers. In addition, and because of the potential anxiogenic and HPA-disrupting role of caffeine, measures of psychological state (depression, anxiety, and stress) will also be acquired, in order to explore the potential association of habitual coffee consumption with these variables.
Subject recruitment and assessment
Participants were recruited through advertisement on the Institute’s social media, institutional e-mail, and press releases distributed among Portuguese local and national newspapers. Exclusion criteria included the presence of neurological or psychiatric disorders, habitual consumption of mind-altering substances, and the inability to undergo MRI. Two experimental groups were created according to participants’ coffee consumption habits: coffee drinkers (CD), who drank a minimum of one cup of caffeinated coffee per day; and non-coffee drinkers (NCD), who had no habits of regular consumption of coffee (less than one cup per week). Consumption of coffee as well as other caffeinated products was confirmed in a structured interview. Participants were instructed to abstain from caffeinated products for 3 h before the assessment, in order to avoid acute influence of caffeine. Fifty-six subjects were recruited (32 CD and 24 NCD). One participant from the CD group was excluded due to imaging artifacts, rendering a final sample of 31 CD and 24 NCD. Characterization of subjects was done in two (CD) or three (NCD) parts within a 3 h time-period: participants were first interviewed by a certified psychologist. This was followed by an MRI scanning session, and, in the case of the NCD, the first scanning session was followed by ingestion of coffee (Nespresso ® Ristretto, ~50 cc) before a rs-fMRI scan ~30 min thereafter. During the interview, the following data were gathered: demographic data; habits of coffee and other caffeinated products consumption; and assessment of depression, anxiety, and stress scores through the Depression, Anxiety and Stress Scales (DASS-21, [ 31 , 32 ]).
Demographic and psychological data analysis
CD and NCD groups were compared in terms of sociodemographic variables, frequency of consumption of caffeinated products, and psychological variables. Since the variables did not follow a normal distribution, nonparametric tests were applied (Wilcoxon test). Moreover, multiple regression analyses were performed, aiming to determine the association between daily consumption of caffeinated products such as coffee, tea, chocolate, etc. (0 = <1/day; 1 = 1/day; 2 = 2/day; 3 = 3 or more/day) and the psychological data measured with the DASS-21 questionnaire (controlled for sex, age, and education), independently of the groups. These analyses were performed on Matlab2020a software (The Mathworks, Inc.) and p < 0.05 was considered the threshold for statistical significance. Linear regression representations were generated in Prism 7 software (GraphPad Software, Inc.).
MRI brain imaging
Magnetic resonance imaging scans were conducted using a Siemens Verio 3T (Siemens, Erlangen, Germany) located in Hospital de Braga (Braga, Portugal) using a 32-channel head antenna. The scanning session included as an anatomical acquisition a T1-weighted sagittal magnetization-prepared rapid acquisition with gradient echo (TE/TR = 2420/4.12 ms, FA = 9°, 1 mm 3 isometric voxel size, Field-of-View = 176 × 256 × 256 mm 3 ). The resting-state fMRI (rs-fMRI) acquisition used a multi-band echo planar imaging sequence, CMRR EPI 2D (R2016A, Center for Magnetic Resonance Research, University of Minnesota, Minnesota, USA [ 33 , 34 , 35 ]) sensitive to fluctuations in the BOLD contrast (TR/TE = 1000/27 ms, FA = 62°, 2 mm 3 isometric voxel size, 64 axial slices over an in plane matrix of 100 × 100). The rs-fMRI acquisition had a duration of 7.5 min, during which subjects were instructed to remain with their eyes closed, relaxed, and let their minds wander freely.
Preprocessing of MRI data
MRI results included in this manuscript were preprocessed using fMRIPrep 1.4.1 ([ 36 ]; RRID:SCR_016216), which is based on Nipype 1.2.0 ([ 37 , 38 ]; RRID:SCR_002502). A full description of the preprocessing pipeline can be found in the Supplementary material.
Resting-state analysis
Independent component analysis.
Resting-state network (RSN) maps were analyzed voxel-wise through a probabilistic independent component analysis (ICA) as implemented in Multivariate Exploratory Linear Optimized Decomposition into Independent Components, distributed with FSL [ 39 ]. For further details check the Supplementary material.
The RSNs FC was compared between CD and NCD groups, using a nonparametric permutation procedure implemented in the randomize tool from FSL [ 40 ]. Threshold-free cluster enhancement (TFCE) was used to detect widespread significant differences and control the family-wise error rate (FWE-R) at α = 0.05. In total, 5000 permutations were performed.
Static functional connectomics analysis
To assess differences between the two groups in the functional connectome, the mean time series of the 268 regions of the Shen Atlas [ 41 ] were extracted. The Pearson correlation between time series, followed by Fisher r-to-Z transformation, were calculated to obtain matrices of FC for each subject. To overcome the issue of multiple comparisons induced by the large number of connections in the network, we applied the network-based statistics (NBS) approach [ 42 ]. A total of 5000 permutations were used, together with a FWE corrected network significance of 0.05. For more details check the Supplementary material.
Dynamic functional analysis
We applied the leading eigenvector dynamics analysis (LEiDA, [ 30 ]) approach to study the changes in the functional dynamics associated with habitual caffeine consumption. Instantaneous FC was calculated for each subject at each time point for all 268 regions of interest of the Shen atlas, using the time series extracted for the static analysis. To help visually identify phase locked (PL) states, the overlap between each anatomical region of each state to the 7 Yeo RSN’s [ 43 ], plus two other labels for the cerebellum and subcortical units, was calculated and anatomical units color coded in accordance to the best match. For more details check the reference paper or the Supplementary material.
Effects of acute coffee consumption and frequency of caffeine consumption
The significant findings obtained with ICA, NBS, and LEiDA were further explored, aiming to assess the effects of acute coffee consumption in NCD and of frequency of consumption of caffeinated products in both groups. The first were assessed by comparing NCD after coffee consumption with data from CD (independent sample t -test) and NCD (before coffee consumption; paired sample t -test). The second were evaluated by performing multiple regression analyses following the same approach described for the DASS-21 questionnaire.
Demographic analysis
CD and NCD groups did not differ in terms of age (range 19–57; p = 0.28; Z = 1.09; r = 0.15) or number of formal years of education (range 12–25; p = 0.07; Z = 1.84; r = 0.25). Frequency of consumption of caffeinated products was, as expected, higher in the CD group ( p < 0.001; Z = 6.17; r = 0.83). Sex distribution was not significantly different between groups ( χ 2 (1, N = 55) = 0.52, p = 0.42), despite the CD group presenting a slightly higher proportion of males (41.94%) in comparison with the NCD group (33.33%). Descriptive statistics can be found in Table 1 .
Effect of habitual caffeine consumption on rs-fMRI data
Independent components analysis.
Thirty components were obtained from the probabilistic ICA of CD and NCD (before consuming coffee). Fifteen of these components were found to be representative of the most typical RSNs. A tendency toward lower FC patterns in the CD group can be seen in most of these networks (see Supplementary Fig. 1 ). Despite this, we only found significant FWE-R TFCE corrected between-group differences in two of them, namely, in the somatosensory network and the limbic network (Fig. 1 ). Regarding the somatosensory network, NCD presented a pattern of higher connectivity with the right precuneus (MNI coordinates = 30, −72, 38; 7 voxels; peak t value = 4.4). Moreover, for the limbic network, NCD had higher FC in the right insula compared to CD (MNI coordinates = 42, −12, 2; 4 voxels; peak t value = 5.09). Of note, these effects were also linearly associated with the caffeinated products’ frequency of consumption. Negative correlations were found for both right precuneus ( p = 0.003; β = −1.433; adjusted R 2 = 0.162; Fig. 1B ) and right insula ( p < 0.001; β = −2.384; adjusted R 2 = 0.267; Fig. 1B ). Detailed statistics can be found in Supplementary Table 1 .
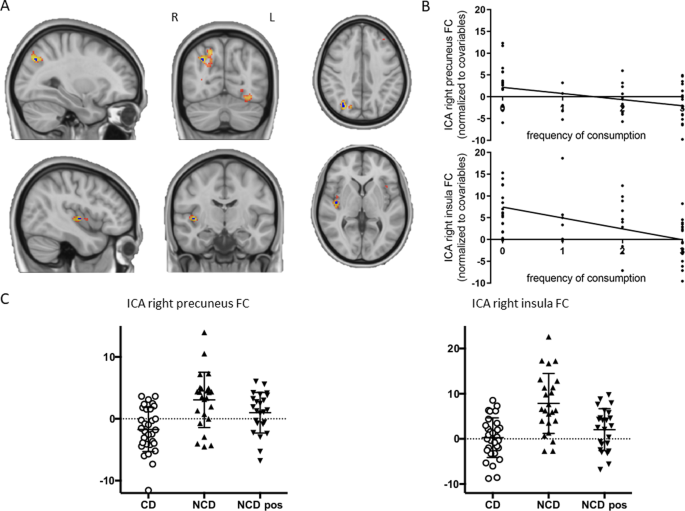
A Sagittal, coronal, and axial view of the clusters showing significant between-group differences in the connectivity between the somatosensory network and the right precuneus (top) and the limbic network and the right insula (bottom). The FWE-R TFCE corrected clusters are shown in dark blue overlaid over a more extended non-significant after multiple comparison correction cluster in hot color scale scheme, for visualization purposes. B Associations of frequency of consumption of caffeinated beverages with the mean FC of the right precuneus and the right insula. C Scatter plots showing the mean FC of the right precuneus and the right insula for the NCD before drinking coffee (NCD), the NCD after drinking coffee (NCD pos), and the CD.
Importantly, the group differences described were reduced after NCD drank coffee (see Fig. 1C ; somatosensory network: pre vs post NCD t value = 1.86, p = 0.075, post NCD vs CD t value = −2.89, p = 0.006; limbic network: pre vs post NCD t value = 3.88, p < 0.001, post NCD vs CD t value = −1.46, p = 0.15). This points to a potential causality link between coffee drinking and the above-described changes in lower connectivity in the somatosensory and in the limbic networks.
Connectomics analysis
From the connectomics analysis done using NBS, a single network of significantly higher connectivity was found in the NCD group (pre-coffee) between the thresholds of 0.005 and 0.0005 (for statistics of all thresholds see Supplementary Table 2 ). For ease of visualization, we present only the results found at the highest significant threshold of p = 0.0005 ( t (threshold) = 3.71, df = 54, p (network) = 0.043, Hedge’s g = 1.08 (large effect size), 24 nodes, 46 edges; Fig. 2A ). The full list of nodes with significant different edges between groups across all thresholds can be found in Supplementary Table 3 . Of these we highlight the Thalamus (nodes #262 and #126), the Cerebellum (left anterior Culmen #245 and bilateral Tonsils #238 and #119), the right Postcentral Gyrus (#33), the left Middle Temporal Gyrus (#197), the left Precentral Gyrus (#160), and the bilateral Caudate (#260 and #122) and Putamen (#124 and #261) as having the most strongly affected connections within the network.
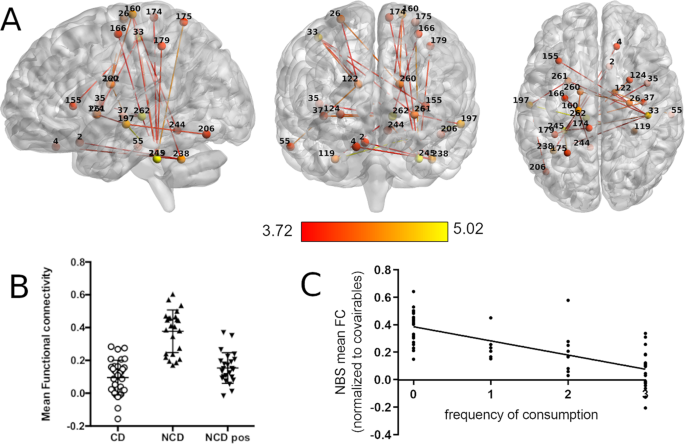
A Sagittal, coronal, and axial view of the network with nodes and edges colored in red–yellow color scheme representing the statistical t value of the difference between groups. B Scatter plot of the mean FC within the significant network for each experimental group. C Associations of frequency of consumption of caffeinated beverages with the mean FC of the network found in NBS.
When observing the average network connectivity from this network, NCD post-coffee drink displayed a significant reduction in connectivity (Fig. 2B ), leading to a profile more similar to CD ( p = 0.037, t = 2.13, df = 54) than to NCD pre-coffee drink ( p = 1.3 × 10 −7 , t = 7.4, df = 23). NBS mean FC was negatively associated with frequency of caffeine consumption ( p < 0.001; β = −0.101; adjusted R 2 = 0.506; Fig. 2C ). Detailed statistics can be found in Supplementary Table 1 .
From the dynamic FC analysis, one functional subsystem (Fig. 3A , PL state 4) was found to last significantly longer in CD (Fig. 3B , 17.95 ± 18.32 s) compared to pre-coffee NCD (8.95 ± 6.13 s) surviving correction for multiple comparisons with a corrected p = 0.038 and a medium effect size with Hedge’s g = 0.62. No BOLD phase-locking state was found to significantly differ in terms of probability of occurrence (see Supplementary Table 4 for all p values for all partition models).
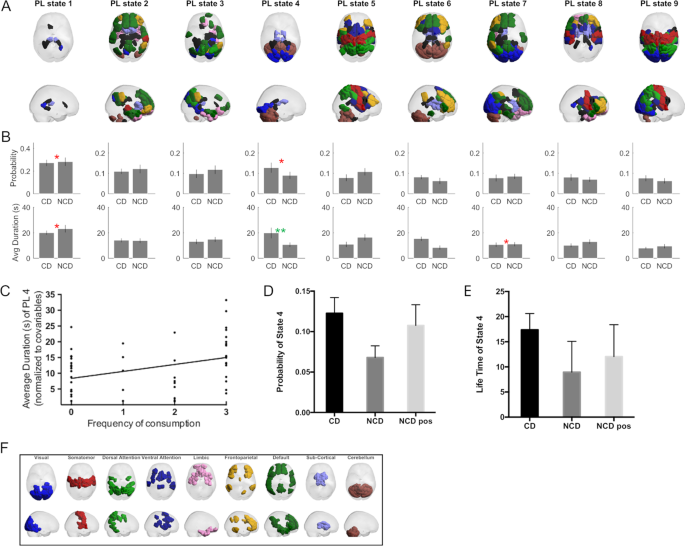
A sagittal and axial views representing the state anatomical areas of each phase locked (PL) state. B Bar plot representing the group differences between coffee and non-coffee drinkers. Differences of p < 0.05 are indicated in red, while multiple comparison surviving effects are indicated in green. C Associations of frequency of consumption of caffeinated beverages with the average duration (in seconds) of PL state 4. D Bar plot of the probability of state 4 for the CD, NCD, and NCD post caffeine consumption groups. E Life time of state 4 for the CD, NCD, and NCD post caffeine consumption groups. F Colored labels used to match each anatomical area of the PL states to different resting state networks.
This BOLD phase-locking state, corresponding to the fourth most probable state when partitioning the data into nine states, comprises a large number of nodes in the cerebellum, visual network as well as several subcortical nodes such as the bilateral thalamus and parahippocampal gyrus (mapped and color coded through the reference shown in Fig. 3F ). While this was the only result that survived correction for multiple comparisons, it is relevant to note that the equivalent LEiDA state for k = 10 is just below the threshold ( p = 0.051, Supplementary Table 4 and Supplementary Figs. 2 and 3 ). Furthermore, LEiDA lifetime results were positively correlated with frequency of caffeine consumption ( p = 0.012; β = 2.176; adjusted R 2 = 0.083; Fig. 3C ).
After drinking coffee, both the lifetime and the probability of this state in NCD became closer to the values observed in CD, with the probability not being significantly different from CD ( p = 0.5, t = 0.67, df = 54), while being significantly higher than NCD pre-coffee ( p = 0.037, t = 2.31, df = 23, Fig. 3D ). For the life time of state 4, post-coffee drink NCD were not significantly different from CD ( p = 0.177, t = 1.37, df = 54) nor the pre-drink NCD ( p = 0.107, t = 1.68, df = 23, Fig. 3E ). All results across the different k’ s can be found in Supplementary Figs. 2 and 3 and Supplementary Table 4 .
Effect of habitual caffeine consumption on psychological data
The association between coffee consumption and stress, anxiety, and depression (DASS-21) was assessed. When comparing CD and NCD groups, only stress was significantly different between groups (stress— p = 0.025; Z = 2.237; r = 0.307; anxiety— p = 0.851; Z = −0.188; r = −0.026; depression— p = 0.085; Z = 1.724; r = 0.237), with CD showing higher levels of stress than NCD (median (Med) = 6.0; interquartile range (IQR) = 6.0 vs Med = 4.0; IQR = 4.0, respectively). Of notice, particular items of the DASS-21 Stress subscale that can be related to arousal were increased in CD. Items #1 and #12, which measure difficulty to relax, presented statistically significant differences ( p = 0.007, Mann–Whitney test), while item #8, that relates to nervous arousal, presented a trend in the same direction ( p = 0.083). Interestingly, item #7 (Anxiety subscale), that is associated with skeletal musculature, despite not achieving a statistically significant difference between groups, tended to be lower in CD ( p = 0.113), suggesting a segregation between the motor and arousal loops.
When assessing the effects of frequency of caffeine consumption in self-reported variables (controlling for sex, age, and education), the positive correlation with stress was maintained ( p = 0.004; β = 1.292; adjusted R 2 = 0.135; Fig. 4A ). Moreover, a sex by anxiety interaction was found ( p = 0.023; β = 0.683; adjusted R 2 = 0.085; Fig. 4B ), which seems to be driven by a positive correlation in males. No significant effects were found for the depression subscale ( p = 0.128; β = 0.450; adjusted R 2 = 0.108; Fig. 4C ). Detailed statistics can be found in Supplementary Table 1 .
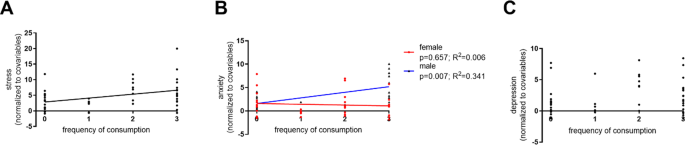
Associations of frequency of consumption of caffeinated products with the DASS-21 subscales of stress ( A ) and anxiety ( B ), and non-significant association with the depression subscale ( C ).
Herein we describe for the first time the effects of habitual coffee consumption on the human brain networks. We show that habitual CD have different patterns of FC in comparison with NCD. Our rs-fMRI analysis revealed decreased FC of the somatosensory and limbic networks in CD that correlated with the frequency of consumption of caffeinated products. Such changes were replicated in NCD after a single coffee, suggesting possible causality between coffee intake and altered patterns of brain network connectivity. Previous studies have described a reduction of similar RSN connectivity after acute caffeine ingestion [ 25 , 44 ].
Decreased FC in somatosensory and related networks in CD likely represents a more efficient and beneficial pattern of connections with respect to motor control and alertness; importantly this fits our findings of trends of increased scores in CD in the specific items of the DASS-21 scale that measure these dimensions. The other network impacted by coffee intake was the limbic network, which is involved in processing the sensory input from the external and internal environment which, by modulating memory and motivation, determine emotional, autonomic, motor, and cognitive responses [ 45 ]. A previous resting-state PET study showed reduced metabolic activity in components of this network after caffeine ingestion [ 18 ] and a study using a hedonic fMRI task showed decreased activation in neuronal areas associated with memory and reward [ 46 ] in caffeine consumers compared to non-consumers; the present FC data are consistent with those reports.
Analysis of the global functional connectome using NBS revealed a network impacted by the habitual consumption of caffeine. This widespread network of reduced FC comprised cerebellar, subcortical (striatal and thalamic), and motor cortex regions, partially matching previously reported effects of acute caffeine ingestion [ 24 , 25 ]. Interestingly, there is a clear bilateral involvement of striatal nodes and of the thalamus which, respectively, have the highest densities of A2A and A1 receptors in the brain [ 47 , 48 ]. The action of caffeine in these regions has an influence on cortico-striatal-thalamic and cerebellar-thalamocortical loops that are relevant for a variety of neuronal processes. Thus, the observed decrease in FC at rest in this network in regular caffeine-ingesting individuals reveals greater segregation of these areas with less inter-regional dependencies, favoring greater efficiency within these loops. It is relevant to note here that, even though A1 and A2A receptors are thought to mediate differential actions [ 49 ], similar effects were observed in both loops. This likely reflects the fact that fMRI provides proxy aggregate measurements of functional connections among a network of brain areas.
A previous study reported that caffeine increases brain entropy, indicating higher information processing capacity across the cerebral cortex [ 23 ]. Our LEiDA analysis revealed a dynamic state involving several cerebellar and subcortical areas, with a longer average lifetime in habitual CD. This network comprises several nodes, including the cerebellum, thalamus, and parahipocampal, lingual, and inferior occipital gyri which are relevant in the context of caffeine consumption—caffeine is known to decrease mind wandering [ 50 ] and to increase attention, alertness, and arousal [ 51 ]. In fact, the nodes implicated in this network are linked by visual processing; among these, the thalamus is critical for distributing cognitive control [ 52 ]. The lingual and inferior occipital gyrus are also implicated in visual processing, while the parahippocampus is involved in memory encoding and retrieval [ 20 , 21 ]; the latter may explain why caffeine reportedly facilitates memory processes [ 9 ]. Lastly, evidence of strong rsFC between the cerebellum, known to be also implicated in sensory processing [ 53 ] and a number of sensorial cortices [ 54 ], explains the observed increased visual alertness/attention and readiness to sensorial perception among CD individuals. While similar findings have been previously reported [ 6 ], only one other study assessed habitual CD using MRI, and did not characterize changes in FC [ 26 ]. Importantly, similarly to the other neuroimaging findings, a common pattern of connectivity dynamics was found in CD individuals and NCD subjects who drank a single coffee before scanning.
In order to provide a link with other neuropsychologic dimensions, we also assessed our subjects in the DASS-21. Interestingly, we observed habitual CD to display increased levels of stress; there was a clear positive association between the indices of stress and the amount of consumption of caffeinated drinks. Interestingly, items of the DASS-21 sub-score that showed greater variance between CD vs NCD were those related with difficulty to relax (items #1 and #12), and those related to nervous arousal (item #8), consistent with the common attribution of alertness and arousal to coffee intake. It also deserves to be mentioned that, despite the display of a higher anxiety among CD (particularly in males), there was a decrease in DASS-21 item (#7) which matches the effects on the skeletal muscles in CD; this, in turn, fits the findings of better segregation of the above-described loops. The present results extend previous studies that described an association between coffee/caffeine consumption and stress and anxiety [ 1 , 13 , 16 , 55 ] and sex [ 13 , 16 ]. It is important to note, however, that causality cannot be inferred from our study design. Our results are open to two interpretations: higher coffee/caffeine consumption leads to increased stress and anxiety; or, alternatively, higher stress and anxiety induce higher coffee/caffeine consumption. Moreover, given that resting-state studies using stress and anxiety samples have shown both decreases and increases in FC [ 56 , 57 , 58 ], the possibility that coffee/caffeine consumption elicits decreases in FC or compensates for FC beyond a certain threshold, must also be considered. While the first possibility is in line with studies showing increased anxiety upon both acute caffeine administration in humans [ 1 , 12 ] and prolonged ingestion in rodents [ 59 ] reports that greater caffeine consumption under periods of stress may help maintain synaptic homeostasis [ 60 ] as well as prevent mood disorders warrant further study in future.
The methodologies applied in the present study do not allow us to draw precise relationships between the psychological and neuroimaging results and the dosage and metabolism of caffeine among individual subjects. To study the individual responses to the acute and chronic effects of caffeinated product intake would be a complex undertaking, requiring subjects to adapt their daily habits and strict abstinence regimens. Based on our experience, recruitment of subjects for a properly balanced study is also difficult since NCD subjects are insufficiently motivated to engage in studies on the actions of caffeine. Nevertheless, we are currently developing alternative strategies that would allow us to deliver calibrated doses of caffeine during fMRI scanning sessions to better discriminate its effects from other factors (e.g., stress). Our future work will also examine inter-individual differences in response to caffeine consumption, the subjective experience of coffee consumption, as well as the influence of additional factors as the consumption of alcohol and tobacco. Despite such gaps, the data presented here represent a contribution to the knowledge of the “caffeinated brain” and how these changes underlie the behavioral effects triggered by coffee intake, with implications for physiological and pathological conditions.
Code availability
In-house scripts used in the NBS analysis are fully available online at open science framework website ( https://osf.io/qepc8/ ) and LEiDA scripts at github ( https://github.com/juanitacabral/LEiDA ).
McLellan TM, Caldwell JA, Lieberman HR. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev. 2016;71:294–312.
Article CAS PubMed Google Scholar
O’Keefe JH, DiNicolantonio JJ, Lavie CJ. Coffee for cardioprotection and longevity. Prog Cardiovascular Dis. 2018;61:38–42.
Article Google Scholar
Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133.
CAS PubMed Google Scholar
Ribeiro JA, Sebastião AM. Caffeine and Adenosine. J Alzheimer’s Dis. 2010;20:S3–15.
Article CAS Google Scholar
Southward K, Rutherfurd-Markwick KJ, Ali A. The effect of acute caffeine ingestion on endurance performance: a systematic review and meta–analysis. Sports Med. 2018;48:1913–28.
Article PubMed Google Scholar
Killgore WDS, Kamimori GH. Multiple caffeine doses maintain vigilance, attention, complex motor sequence expression, and manual dexterity during 77 h of total sleep deprivation. Neurobiol Sleep Circadian Rhythms. 2020;9:100051.
Article PubMed PubMed Central Google Scholar
Sane RM, Jadhav PR, Subhedar SN. The acute effects of decaffeinated versus caffeinated coffee on reaction time, mood and skeletal muscle strength. J Basic Clin Physiol Pharmacol. 2019;30.
Smit HJ, Rogers PJ. Effects of low doses of caffeine on cognitive performance, mood and thirst in low and higher caffeine consumers. Psychopharmacology (Berl). 2000;152:167–73.
Borota D, Murray E, Keceli G, Chang A, Watabe JM, Ly M, et al. Post-study caffeine administration enhances memory consolidation in humans. Nat Neurosci. 2014;17:201–3.
Article CAS PubMed PubMed Central Google Scholar
Franceschini S, Lulli M, Bertoni S, Gori S, Angrilli A, Mancarella M, et al. Caffeine improves text reading and global perception. J Psychopharmacol. 2020;34:315–25.
Harris A, Ursin H, Murison R, Eriksen HR. Coffee, stress and cortisol in nursing staff. Psychoneuroendocrinology 2007;32:322–30.
Bruce M. Anxiogenic effects of caffeine in patients with anxiety disorders. Arch Gen Psychiatry. 1992;49:867.
O’Neill CE, Newsom RJ, Stafford J, Scott T, Archuleta S, Levis SC, et al. Adolescent caffeine consumption increases adulthood anxiety-related behavior and modifies neuroendocrine signaling. Psychoneuroendocrinology. 2016;67:40–50.
Eskelinen MH, Kivipelto M. Caffeine as a protective factor in dementia and Alzheimer’s disease. J Alzheimer’s Dis. 2010;20:S167–74.
Wang L, Shen X, Wu Y, Zhang D. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust NZ J Psychiatry. 2016;50:228–42.
Kaster MP, Machado NJ, Silva HB, Nunes A, Ardais AP, Santana M, et al. Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci USA. 2015;112:7833–8.
Soares JM, Magalhães R, Moreira PS, Sousa A, Ganz E, Sampaio A, et al. A Hitchhiker’s guide to functional magnetic resonance imaging. Front Neurosci. 2016;10:515.
Park CA, Kang CK, Son YD, Choi EJ, Kim SH, Oh ST, et al. The effects of caffeine ingestion on cortical areas: functional imaging study. Magn Reson Imaging. 2014;32:366–71.
Haller S, Rodriguez C, Moser D, Toma S, Hofmeister J, Sinanaj I, et al. Acute caffeine administration impact on working memory-related brain activation and functional connectivity in the elderly: a BOLD and perfusion MRI study. Neuroscience. 2013;250:364–71.
Klaassen EB, de Groot RHM, Evers EAT, Snel J, Veerman ECI, Ligtenberg AJM, et al. The effect of caffeine on working memory load-related brain activation in middle-aged males. Neuropharmacology. 2013;64:160–7.
Haller S, Montandon ML, Rodriguez C, Moser D, Toma S, Hofmeister J, et al. Caffeine impact on working memory-related network activation patterns in early stages of cognitive decline. Neuroradiology. 2017;59:387–95.
Koppelstaetter F, Poeppel TD, Siedentopf CM, Ischebeck A, Verius M, Haala I, et al. Does caffeine modulate verbal working memory processes? An fMRI study. NeuroImage. 2008;39:492–9.
Chang D, Song D, Zhang J, Shang Y, Ge Q, Wang Z. Caffeine caused a widespread increase of resting brain entropy. Sci Rep. 2018;8:2700.
Tal O, Diwakar M, Wong CW, Olafsson V, Lee R, Huang MX, et al. Caffeine-induced global reductions in resting-state BOLD connectivity reflect widespread decreases in MEG connectivity. Front Hum Neurosci. 2013;7:63.
Wong CW, Olafsson V, Tal O, Liu TT. Anti-correlated networks, global signal regression, and the effects of caffeine in resting-state functional MRI. NeuroImage. 2012;63:356–64.
Laurienti PJ, Field AS, Burdette JH, Maldjian JA, Yen Y-F, Moody DM. Dietary caffeine consumption modulates fMRI measures. NeuroImage. 2002;17:751–7.
Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98.
Deco G, Kringelbach ML. Great expectations: using whole-brain computational connectomics for understanding neuropsychiatric disorders. Neuron. 2014;84:892–905.
Sporns O, Chialvo D, Kaiser M, Hilgetag C. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8:418–25.
Cabral J, Vidaurre D, Marques P, Magalhães R, Silva Moreira P, Miguel, et al. Cognitive performance in healthy older adults relates to spontaneous switching between states of functional connectivity during rest. Sci Rep. 2017;7:5135.
Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the beck depression and anxiety inventories. Behav Res Ther. 1995;33:335–43.
Pais-Ribeiro JL, Honrado A, Leal I. Contribuição para o Estudo da Adaptação Portuguesa das Escalas de Ansiedade, Depressão e Stress (EADS) de 21 itens de Lovibond e Lovibond. Psic Saúde Doenç. 2004;5:229–39.
Google Scholar
Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS ONE. 2010;5:e15710.
Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63:1144–53.
Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, et al. Evaluation of slice accelerations using multiband echo planar imaging at 3T. NeuroImage. 2013;83:991–1001.
Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111–6.
Gorgolewski KJ, Esteban O, Ellis DG, Notter MP, Ziegler E, Johnson H, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in Python. 0.13.1. Zenodo. 2017.
Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform. 2011;5:13.
Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–52.
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. NeuroImage. 2014;92:381–97.
Shen X, Tokoglu F, Papademetris X, Constable RT. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. NeuroImage. 2013;82:403–15.
Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53:1197–207.
Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65.
Article PubMed Central Google Scholar
Rack-Gomer AL, Liu TT. Caffeine increases the temporal variability of resting-state BOLD connectivity in the motor cortex. NeuroImage. 2012;59:2994–3002.
McLachlan RS. A brief review of the anatomy and physiology of the limbic system. Can J Neurological Sci. 2009;36:S84–87.
Gramling L, Kapoulea E, Murphy C. Taste perception and caffeine consumption: an fMRI study. Nutrients. 2018;11:34.
Svenningsson P. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–96.
Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn-Schmiedeberg’s Arch Pharmacol. 2000;362:364–74.
Adenosine FBB. Adenosine receptors and the actions of caffeine. Pharmacol Toxicol. 1995;76:93–101.
Mishina M, Ishiwata K. Adenosine receptor PET imaging in human brain. Int Rev Neurobiol. 2014;119:51–69.
Kahathuduwa CN, Dhanasekara CS, Chin SH, Davis T, Weerasinghe VS, Dassanayake TL, et al. l-Theanine and caffeine improve target-specific attention to visual stimuli by decreasing mind wandering: a human functional magnetic resonance imaging study. Nutr Res. 2018;49:67–78.
Brunyé TT, Mahoney CR, Lieberman HR, Taylor HA. Caffeine modulates attention network function. Brain Cogn. 2010;72:181–8.
Halassa MM, Kastner S. Thalamic functions in distributed cognitive control. Nat Neurosci. 2017;20:1669–79.
Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C, Ivry RB, et al. Consensus Paper: the role of the cerebellum in perceptual processes. Cerebellum. 2015;14:197–220.
Richards G, Smith A. Caffeine consumption and self-assessed stress, anxiety, and depression in secondary school children. J Psychopharmacol. 2015;29:1236–47.
Kolesar TA, Bilevicius E, Wilson AD, Kornelsen J. Systematic review and meta-analyses of neural structural and functional differences in generalized anxiety disorder and healthy controls using magnetic resonance imaging. NeuroImage Clin. 2019;24:102016.
Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review: theoretical review: brain activity in PTSD during rest. Depress Anxiety. 2016;33:592–605.
Xu J, Van Dam NT, Feng C, Luo Y, Ai H, Gu R, et al. Anxious brain networks: a coordinate-based activation likelihood estimation meta-analysis of resting-state functional connectivity studies in anxiety. Neurosci Biobehav Rev. 2019;96:21–30.
Yacoubi ME, Ledent C, Parmentier M, Costentin J, Vaugeois JM. The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A2A adenosine receptor antagonists. Psychopharmacology. 2000;148:153–63.
Yin Y-Q, Zhang C, Wang J-X, Hou J, Yang X, Qin J. Chronic caffeine treatment enhances the resilience to social defeat stress in mice. Food Funct. 2015;6:479–91.
Download references
This study was funded by the Institute for the Scientific Information on Coffee (ISIC) (ISIC_2017_NS); ISIC did not influence the experimental design or data analysis/interpretation. The laboratory was also supported by the project NORTE‐01‐0145‐FEDER000013 through the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER). RM, MP-P, and ME were supported by post-doctoral grants from the project ISIC_2017_NS. PSM was supported by a fellowship grant from the Fundação para a Ciência e a Tecnologia (FCT; grant number PDE/BDE/113601/2015) from the PhD-iHES program. RV was supported by a research fellowship of the project funded by FCT (UMINHO/BI/340/2018). AC was supported by a scholarship from the project NORTE-08-5639-FSE-000041 (NORTE 2020; UMINHO/BD/51/2017).
Author information
These authors contributed equally: Ricardo Magalhães, Maria Picó-Pérez, Madalena Esteves
Authors and Affiliations
Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Campus de Gualtar, Braga, Portugal
Ricardo Magalhães, Maria Picó-Pérez, Madalena Esteves, Rita Vieira, Teresa C. Castanho, Liliana Amorim, Mafalda Sousa, Ana Coelho, Joana Cabral, Pedro S. Moreira & Nuno Sousa
ICVS/3B’s, PT Government Associate Laboratory, Braga/Guimarães, Portugal
Clinical Academic Center - Braga, Braga, Portugal
NeuroSpin, CEA, CNRS, Paris-Saclay University, Gif-sur-Yvette, France
Ricardo Magalhães
Center for Music in the Brain, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark
Henrique M. Fernandes & Joana Cabral
Psychological Neuroscience Lab, CIPsi, School of Psychology, University of Minho, Braga, Portugal
Pedro S. Moreira
P5 Medical Center, Braga, Portugal
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Nuno Sousa .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Ethical approval
The present study was conducted in accordance with the principles expressed in the Declaration of Helsinki (59th amendment) and was approved by the ethics committee of Hospital de Braga. All participants gave informed written consent after the study aims were explained and were informed of the option to withdraw from the study at any time.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental materials, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Magalhães, R., Picó-Pérez, M., Esteves, M. et al. Habitual coffee drinkers display a distinct pattern of brain functional connectivity. Mol Psychiatry 26 , 6589–6598 (2021). https://doi.org/10.1038/s41380-021-01075-4
Download citation
Received : 19 December 2020
Accepted : 19 March 2021
Published : 20 April 2021
Issue Date : November 2021
DOI : https://doi.org/10.1038/s41380-021-01075-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Genome-wide association studies of coffee intake in uk/us participants of european ancestry uncover cohort-specific genetic associations.
- Hayley H. A. Thorpe
- Pierre Fontanillas
- Sandra Sanchez-Roige
Neuropsychopharmacology (2024)
Current coffee consumption is associated with decreased striatal dopamine transporter availability in Parkinson’s disease patients and healthy controls
- Minming Zhang
BMC Medicine (2023)
Perceived stress modulates the activity between the amygdala and the cortex
- Inês Caetano
- Sónia Ferreira
Molecular Psychiatry (2022)
- Sung Hoon Kang
- Jung Bin Kim
Scientific Reports (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Masks Strongly Recommended but Not Required in Maryland
Respiratory viruses continue to circulate in Maryland, so masking remains strongly recommended when you visit Johns Hopkins Medicine clinical locations in Maryland. To protect your loved one, please do not visit if you are sick or have a COVID-19 positive test result. Get more resources on masking and COVID-19 precautions .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
News & Publications
New insight into caffeine use disorder.
Johns Hopkins researchers recently conducted the most thorough evaluation to date of the prevalence and clinical significance of caffeine use disorder, as well as the correlates of meeting proposed criteria for the condition.
A new study finds potential for caffeine to cause anxiety, insomnia and other symptoms that interfered with subjects’ lives.
Mary “Maggie” Sweeney wants to make one thing clear: She has no intention of convincing people to give up their coffee or favorite caffeinated beverage. That said, the psychiatry researcher at Johns Hopkins Bayview Medical Center’s Behavioral Pharmacology Research Unit feels compelled to raise awareness about caffeine’s potential to cause distress.
Building on a long-running grant project in collaboration with Roland Griffiths , psychiatry researcher, a recent study on caffeine use disorder revealed responses to questions about caffeine use that Sweeney says were eye-opening and complementary to clinical trials conducted at Johns Hopkins — one in 2016 and one in 2019 . The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) recognized caffeine use disorder as “a condition for further study.”
Caffeine use disorder is a problematic pattern of caffeine consumption characterized by a persistent desire to cut down or control use of the substance along with unsuccessful efforts to do so despite problems caused or worsened by caffeine. Significant withdrawal symptoms or use of the drug to relieve or avoid withdrawal are also characteristics of the condition.
Sweeney, Griffiths and colleagues conducted the online research survey with 1,006 caffeine-consuming adults from across the U.S. Data were collected by an online survey panel aggregator used in other peer-reviewed research studies. The goal was to better understand caffeine use disorder’s prevalence and clinical significance in the general population.
Milligrams of caffeine per serving were calculated using typical milligrams per ounce for brewed/drip coffee (200 mg/12 oz.); brewed tea (40 mg/6 oz.); and soft drinks (40 mg/12 oz.). Total caffeine intake in a typical week from all sources was summed and divided by seven to estimate daily caffeine consumption. To qualify for the study, participants needed to report consuming some caffeine-containing beverage or supplement in a typical week.
The researchers found that 8% of the sample fulfilled DSM- proposed criteria for caffeine use disorder when the structured caffeine use disorder interview questions were adapted to the online survey format.
“What I find fascinating,” says Sweeney, “is how little people think about coffee or other caffeinated drinks as stimulants. Although for many people consumption of caffeine is benign, we learned from our study that there is a small but important subset of caffeine consumers who report that caffeine has interfered with their lives in clinically meaningful ways.”
People who met criteria for caffeine use disorder reported problems such as insomnia, gastrointestinal troubles and anxiety, which were caused by or exacerbated by caffeine. The study also found that participants who met criteria for caffeine use disorder tended to consume more caffeine, and were younger and more likely to be cigarette smokers. A larger sample or sample with greater substance use history may be necessary to detect the association between caffeine use disorder and other substance use.
About 90% of adults in the United States use caffeine regularly, says Griffiths, and their average consumption exceeds 200 milligrams of caffeine per day — more caffeine than is contained in two 6-ounce cups of coffee, or five 12-ounce cans of soft drinks.
This latest research study, notes Sweeney, is the most thorough evaluation to date of the prevalence and clinical significance of caffeine use disorder. These data complement results from their recent clinical trial, which showed that people seeking treatment for caffeine reduction were able to reduce their caffeine consumption and decrease their symptoms following the study intervention.
“In our clinical trial , our hypothesis was that people who have had trouble cutting back on caffeine on their own may be able to reduce their caffeine consumption with our guidelines to cut back over several weeks,” says Sweeney. “We also thought this could help people reduce their caffeine-related distress, such as withdrawal symptoms or consuming more caffeine than they intended.”
In both the online survey study and clinical trial, it was common for participants who met criteria for caffeine use disorder to report withdrawal symptoms from caffeine that reduced their function. Caffeine withdrawal symptoms can include headache, fatigue and irritability, which tend to peak at 24 to 48 hours after stopping caffeine, but can last for as long as 10 days in some individuals.
Prior research has also revealed that caffeine can result in withdrawal symptoms following cessation of much lower doses than previously thought. A 6-ounce cup of regular coffee delivers 100 milligrams of caffeine. Even this small amount of caffeine can cause withdrawal symptoms in some people when they stop using it regularly. Other studies have shown that caffeine doses as low as 10–20 milligrams are psychoactive.
The researchers acknowledge that caffeine can have positive health effects, such as reducing the risk of type 2 diabetes and boosting some aspects of cognition. “I want to be clear that caffeine isn’t all good or bad,” says Sweeney. “We’re not arguing that everyone needs to cut back on their consumption. A moderate amount of caffeine — up to 400 milligrams/day (about two 12-ounce cups of coffee) — is not generally associated with negative health effects. But, caffeine reduction is a good goal if caffeine causes significant impairment through withdrawal symptoms or by worsening an underlying problem, such as insomnia or anxiety.”
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Caffeine addiction: Need for awareness and research and regulatory measures
Affiliations.
- 1 Department of Psychiatry, Institute of Medical Sciences (IMS), Banaras Hindu University (BHU), Varanasi 221005, India. Electronic address: [email protected].
- 2 Department of Psychiatry, Institute of Medical Sciences (IMS), Banaras Hindu University (BHU), Varanasi 221005, India. Electronic address: [email protected].
- 3 Department of Psychiatry, Institute of Medical Sciences (IMS), Banaras Hindu University (BHU), Varanasi 221005, India. Electronic address: [email protected].
- 4 Department of Psychiatry, Institute of Medical Sciences (IMS), Banaras Hindu University (BHU), Varanasi 221005, India. Electronic address: [email protected].
- PMID: 28174076
- DOI: 10.1016/j.ajp.2017.01.008
Caffeine consumption has been constantly growing in India especially among children and youngsters. Addictive potential of caffeine has long been reported, still there is lack of awareness about caffeine abuse in India. There is an intense need for appropriate public health regulatory measures and awareness about addictive potential & harms related to caffeine. To the best of our knowledge this is first case from India highlighting several important issues with progressive caffeine abuse resulting in dependence leading to physical, psychological, academic and social consequences; psychotic symptoms during intoxication; predisposing factors as impulsivity and novelty seeking traits in pre-morbid personality; psychosis in family; poor awareness of health hazards even among medical professionals. Widely variable caffeine containing products are available but caffeine content or its safety limit is not mentioned on caffeine products in India. Due to harmful consequences, legal availability to children, growing consumption of caffeine products, it is utmost essential to recognize caffeine as addictive substance and impose regulatory measures on sale, advertisement, maximum caffeine content, health consequences and safety limits of caffeine containing products. Further school teachers, parents and medical practitioners need to be made aware of health hazards of caffeine. Caffeine use shall always be enquired from patients presenting with psychiatric complaints. Further research and survey are required on caffeine use and related problems.
Keywords: Caffeine intoxication; Caffeine related disorders; Caffeine use disorder; Stimulant.
Copyright © 2017 Elsevier B.V. All rights reserved.
PubMed Disclaimer
Similar articles
- [Caffeine--common ingredient in a diet and its influence on human health]. Wierzejska R. Wierzejska R. Rocz Panstw Zakl Hig. 2012;63(2):141-7. Rocz Panstw Zakl Hig. 2012. PMID: 22928360 Review. Polish.
- Caffeine-induced psychiatric manifestations: a review. Wang HR, Woo YS, Bahk WM. Wang HR, et al. Int Clin Psychopharmacol. 2015 Jul;30(4):179-82. doi: 10.1097/YIC.0000000000000076. Int Clin Psychopharmacol. 2015. PMID: 25856116 Review.
- Caffeine dependence in combination with a family history of alcoholism as a predictor of continued use of caffeine during pregnancy. Svikis DS, Berger N, Haug NA, Griffiths RR. Svikis DS, et al. Am J Psychiatry. 2005 Dec;162(12):2344-51. doi: 10.1176/appi.ajp.162.12.2344. Am J Psychiatry. 2005. PMID: 16330600
- Clinical importance of caffeine dependence and abuse. Ogawa N, Ueki H. Ogawa N, et al. Psychiatry Clin Neurosci. 2007 Jun;61(3):263-8. doi: 10.1111/j.1440-1819.2007.01652.x. Psychiatry Clin Neurosci. 2007. PMID: 17472594
- [Is caffeine addictive? The most widely used psychoactive substance in the world affects same parts of the brain as cocaine]. Daly JW, Holmén J, Fredholm BB. Daly JW, et al. Lakartidningen. 1998 Dec 16;95(51-52):5878-83. Lakartidningen. 1998. PMID: 9889511 Review. Swedish.
- Effects of front-of-package caffeine and sweetener disclaimers in Mexico: cross-sectional results from the 2020 International Food Policy Study. Arellano-Gómez LP, Jáuregui A, Nieto C, Contreras-Manzano A, Quevedo KL, White CM, Thrasher JF, Davis RE, Hammond D, Barquera S. Arellano-Gómez LP, et al. Public Health Nutr. 2023 Dec;26(12):3278-3290. doi: 10.1017/S1368980023002100. Epub 2023 Oct 2. Public Health Nutr. 2023. PMID: 37781769 Free PMC article.
- New Potentiometric Screen-Printed Platforms Modified with Reduced Graphene Oxide and Based on Man-Made Imprinted Receptors for Caffeine Assessment. Abd-Rabboh HSM, E Amr AE, Almehizia AA, Naglah AM, H Kamel A. Abd-Rabboh HSM, et al. Polymers (Basel). 2022 May 10;14(10):1942. doi: 10.3390/polym14101942. Polymers (Basel). 2022. PMID: 35631825 Free PMC article.
- Chronic voluntary caffeine intake in male Wistar rats reveals individual differences in addiction-like behavior. Lee CH, George O, Kimbrough A. Lee CH, et al. Pharmacol Biochem Behav. 2020 Apr;191:172880. doi: 10.1016/j.pbb.2020.172880. Epub 2020 Feb 24. Pharmacol Biochem Behav. 2020. PMID: 32105663 Free PMC article.
- Long-term Treatment with Low-Dose Caffeine Worsens BPSD-Like Profile in 3xTg-AD Mice Model of Alzheimer's Disease and Affects Mice with Normal Aging. Baeta-Corral R, Johansson B, Giménez-Llort L. Baeta-Corral R, et al. Front Pharmacol. 2018 Feb 15;9:79. doi: 10.3389/fphar.2018.00079. eCollection 2018. Front Pharmacol. 2018. PMID: 29497377 Free PMC article.
Publication types
- Search in MeSH
Related information
- PubChem Compound (MeSH Keyword)
LinkOut - more resources
Full text sources.
- Elsevier Science
Other Literature Sources
- scite Smart Citations
- MedlinePlus Consumer Health Information
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- DOI: 10.1016/j.ajp.2017.01.008
- Corpus ID: 205377025
Caffeine addiction: Need for awareness and research and regulatory measures.
- Shobhita Jain , A. S. Srivastava , +1 author Gaurav Maggu
- Published in Asian Journal of Psychiatry 4 February 2017
- Medicine, Psychology
15 Citations
Caffeine intake and its association with mental health status among pharmacy students at uitm puncak alam, chronic voluntary caffeine intake in male wistar rats reveals individual differences in addiction-like behavior, a survey on caffeine consumption and risky behaviors among a sample of secondary school students in nigeria, long-term treatment with low-dose caffeine worsens bpsd-like profile in 3xtg-ad mice model of alzheimer’s disease and affects mice with normal aging, place attachment in coffee shops: a customer perspective study in north cyprus, new potentiometric screen-printed platforms modified with reduced graphene oxide and based on man-made imprinted receptors for caffeine assessment, maternal and fetal toxicity of carisoprodol, immobilization and retention of caffeine in soil amended with ulva reticulata biochar., antagonistic interaction between caffeine and ketamine in zebrafish: implications for aquatic toxicity, risk prediction of opioid dependency using machine learning based on electronic health record, 11 references, clinical importance of caffeine dependence and abuse, caffeine awareness in children: insights from a pilot study., caffeine intake among adolescents in delhi, personality traits associated with caffeine intake and smoking, the frequency of caffeine withdrawal in a population‐based survey and in a controlled, blinded pilot experiment, caffeine and suicide: a systematic review., a review of energy drinks and mental health, with a focus on stress, anxiety, and depression, psychotropic effects of caffeine., beverage caffeine intakes in the u.s., caffeine increases striatal dopamine d2/d3 receptor availability in the human brain, related papers.
Showing 1 through 3 of 0 Related Papers
Advertisement
Caffeine Use Disorder: A Review of the Evidence and Future Implications
- Addictive Disorders in DSM-5 (JE Grant, Section Editor)
- Published: 28 May 2014
- Volume 1 , pages 186–192, ( 2014 )
Cite this article

- Merideth A. Addicott 1
16k Accesses
31 Citations
141 Altmetric
17 Mentions
Explore all metrics
The latest edition of the Diagnostic and Statistical Manual of Mental Disorders (5th edition; DSM-5) has introduced new provisions for caffeine-related disorders. Caffeine withdrawal is now an officially recognized diagnosis, and criteria for caffeine use disorder have been proposed for additional study. Caffeine use disorder is intended to be characterized by cognitive, behavioral, and physiological symptoms indicative of caffeine use despite significant caffeine-related problems, similar to other substance use disorders. However, since non-problematic caffeine use is so common and widespread, it may be difficult for some health professionals to accept that caffeine use can result in the same types of pathological behaviors caused by alcohol, cocaine, opiates, or other drugs of abuse. Yet there is evidence that some individuals are psychologically and physiologically dependent on caffeine, although the prevalence and severity of these problems is unknown. This article reviews the recent changes to the DSM, the concerns regarding these changes, and some potential impacts these changes could have on caffeine consumers.
Similar content being viewed by others

Evaluating the Validity of Caffeine Use Disorder

Craving espresso: the dialetics in classifying caffeine as an abuse drug

Estimate the prevalence of daily caffeine consumption, caffeine use disorder, caffeine withdrawal and perceived harm in Iran: a cross-sectional study
Avoid common mistakes on your manuscript.
Introduction
After centuries of cultivation and consumption, our relationship with caffeine has just undergone a major change. The latest version of the Diagnostic and Statistical Manual of Mental Disorders (5th edition; DSM-5) now includes caffeine withdrawal disorder and proposes a set of criteria for caffeine use disorder (CUD) [ 1 ]. What effect will this have on us and America’s most popular psychostimulant?
Caffeine is generally considered a functional or beneficial drug because it can improve mood and alertness at low doses. At high doses, caffeine produces aversive intoxicating effects. For this reason, caffeine consumption is typically self-limiting and compatible with a social and productive life [ 2 ]. Caffeine is thought to have little to no abuse liability, but perhaps its modest reinforcing effects enhance the desirability of beverages that already have pleasant flavors and aromas, such as coffee, tea, and soft drinks. For many of us who sit behind computer screens all day, these caffeinated beverages help us focus our attention and provide a welcome excuse to get up from our chairs once in a while. Although the question of whether we are all collectively dependent on caffeine has been raised [ 3 ], coffee drinking is thought to be “more a dedicated habit than a compulsive addiction” [ 4 ].
The majority of people who use caffeine safely every day may find it difficult to understand how caffeine use could become disordered or problematic. Of course, many coffee drinkers probably have had a personal experience with withdrawal symptoms if they skipped their morning coffee, but the remedy for that is simply a cup of coffee. But what if someone were convinced he could not function without caffeine? What if he took increasingly greater amounts of caffeine to improve his ability to function, until he began to experience the effects of caffeine intoxication or withdrawal more days than not? What if he were told that his caffeine use was physically harming his body, but he could not reduce his use? At what point does caffeine use become disordered?
A few studies have suggested that some individuals meet the criteria for substance dependence regarding their caffeine use. However, many questions remain regarding the prevalence, development, and severity of disordered caffeine use. To help answer these questions and guide future research on this topic, the DSM-5 proposes a set of criteria for CUD. This article reviews the caffeine-related changes to the DSM and the recent research and evidence for disordered caffeine use.
Diagnostic and Statistical Manual of Mental Disorders , 4th Edition (DSM-IV) Caffeine-Related Diagnoses
Caffeine withdrawal.
The fourth edition of the DSM (DSM-IV) recognized four caffeine-related diagnoses: caffeine intoxication, caffeine-induced anxiety disorder, caffeine-induced sleep disorder, and caffeine-related disorder not otherwise specified (NOS) [ 5 ]. The criteria for caffeine intoxication included recent caffeine use, usually in excess of 250 mg, and five or more symptoms that develop shortly thereafter, such as restlessness, nervousness, insomnia, gastrointestinal disturbance, and tachycardia. Of the caffeine-related diagnoses included in the DSM-IV, caffeine withdrawal is notably absent, although a proposed set of criteria was included to encourage future research. The DSM-IV recognized that “some individuals who drink large amounts of coffee display some aspects of dependence on caffeine and exhibit tolerance and perhaps withdrawal. However, the data are insufficient at this time to determine whether these symptoms are associated with clinically significant impairment that meets the criteria for Substance Dependence or Substance Abuse” (page 212) [ 5 ].
Over the past 30 years, there have been a large number of studies characterizing caffeine withdrawal symptoms (for reviews, see Griffiths and Woodson [ 6 ] and Juliano and Griffiths [ 7 ]). As a result, the DSM-5 includes diagnostic criteria for caffeine withdrawal, which consists of prolonged daily use of caffeine and three or more withdrawal symptoms occurring within 24 h of abrupt cessation or reduction of caffeine use. These symptoms include headache, marked fatigue or drowsiness, dysphoric mood/depressed mood/irritability, difficulty concentrating, and flu-like symptoms [ 1 ].
Caffeine Dependence
Concurrent with research on caffeine withdrawal, investigators have also been studying caffeine’s abuse potential. Although the DSM-IV included a substance dependence diagnosis for every other recognized substance, there were no criteria or proposed criteria for caffeine dependence. Therefore, investigators adapted the DSM-IV substance dependence criteria for caffeine use to use in their research. The criteria for substance dependence consisted of a maladaptive pattern of substance use with clinically significant impairment manifested by three or more symptoms within a 12-month period. These symptoms included (1) tolerance; (2) withdrawal; (3) substance used in larger amounts or over a longer period than intended; (4) a persistent desire or unsuccessful effort to control use; (5) a great deal of time spent obtaining, using, or recovering from the substance; (6) forgoing important activities because of the substance; and (7) substance use continued despite knowledge of having a persistent or recurrent physical or psychological problem likely to be caused or exacerbated by the substance (i.e., ‘use despite harm’) [ 5 ].
There have been four notable studies that have investigated caffeine dependence. Strain et al. recruited participants who believed they were psychologically or physiologically dependent on caffeine. The authors reported that 16 out of 27 subjects met three of four criteria for caffeine dependence, including tolerance, withdrawal, persistent desire/unsuccessful efforts to control use, and ‘use despite harm’ (e.g., using caffeine against medical advice) [ 8 ]. A similar study by Juliano et al. reported that 93 % of 94 subjects met three of seven criteria for caffeine dependence, and 55 % of subjects met five of seven criteria. Most of the interviewees reported at least one serious attempt to quit or reduce caffeine without success and 43 % were advised by a health professional to reduce caffeine use for health reasons (including cardiovascular problems, fibrocystic breast disease, pregnancy, anxiety, headaches, sleep difficulties, or to reduce caloric intake from caffeinated soft drinks) [ 9 ]. Another study by Striley et al. recruited subjects who were expected to have high rates of drug use/abuse. The authors reported that 35 % of 167 subjects endorsed three of seven caffeine dependence criteria [ 10 ]. Lastly, Hughes et al. conducted random phone surveys of Vermont residents. They reported that 30 % of 162 subjects endorsed three or more criteria, with the highest percentage of people endorsing a desire to control caffeine use, followed by spending a great deal of time with the drug, and using more caffeine than intended [ 11 ].
In summary, these studies suggest that caffeine use has the features of substance dependence for some individuals. Furthermore, they suggest that not all caffeine users can simply quit on their own, which is an attitude probably held by some health professionals [ 9 ]. However, these studies have several limitations. Three of the studies used targeted subject samples [ 8 – 10 ], and so the prevalence of caffeine dependence in the general population cannot be estimated. Additionally, in two of the studies, interviews were not conducted by psychiatric clinicians, so issues of severity and harm related to caffeine dependence may not have been adequately addressed [ 10 , 11 ]. The studies on caffeine dependence reviewed here were conducted prior to the publication of the DSM-5 in 2013. Although caffeine dependence did not become an officially recognized diagnosis in this edition, these and other studies elicited interest in the psychiatric community to learn more about disordered caffeine use.
DSM, 5th Edition (DSM-5) Caffeine-Related Diagnoses
The DSM-5 includes caffeine intoxication, caffeine withdrawal, other caffeine-induced disorders (e.g., anxiety and sleep disorders), and unspecified caffeine-related disorder. In this edition, substance abuse and substance dependence are now represented by substance use disorder (SUD), which is applied to all classes of substances except for caffeine. For this diagnosis, individuals must endorse at least two of the following criteria: (1) substance used in larger amounts or over longer period than intended; (2) a persistent desire or unsuccessful effort to control use; (3) a great deal of time spent obtaining, using, or recovering from the substance; (4) craving the substance; (5) substance use interfering with ability to fulfill major obligations; (6) substance use despite social problems related to use; (7) important occupational or social activities given up because of substance use; (8) recurrent use in situations when it is physically hazardous; (9) ‘use despite harm’; (10) tolerance; and (11) withdrawal [ 1 ].
The DSM-5 does not include a diagnosis of CUD because, according to the American Psychiatric Association (APA), it is not yet clear to what extent it is a clinically significant disorder. However, CUD is included in Section III (“Emerging Measures and Models”) of the DSM-5 to encourage further research on the impact of this condition [ 12 ]. The proposed CUD criteria are the same as other SUDs; however, the CUD diagnosis is designed to be more conservative. For a CUD diagnosis, all three of the following criteria need to be endorsed: (1) a persistent desire or unsuccessful effort to control use; (2) ‘use despite harm’; and (3) withdrawal. This higher threshold is intended to prevent over-diagnosis of CUD given the prevalence of non-problematic caffeine use in the general population [ 1 ]. These proposed criteria are intended to encourage more research on the reliability, validity, and prevalence of CUD, as well as its functional consequences on the lives of those affected by it.
Current Literature on Caffeine Use Disorder
There are three notable articles that summarize the current attitudes and information regarding caffeine withdrawal and CUD. First, a roundtable discussion with Drs. Hughes, Griffiths, Juliano, and Budney provides an excellent overview of the caffeine-related changes to the DSM-5 and explains some of the decision-making process behind the revisions, as well as the concerns about caffeine withdrawal and CUD over-diagnosis [ 13 ••]. The discussants explain how the more conservative criteria for CUD than other SUDs should help prevent over-diagnosis, but, at the same time, diagnoses included in the DSM should not be exceedingly rare. Thus, more information is needed on the prevalence of CUD before deciding whether it belongs in the DSM. Another issue raised by the panel is that there is a common perception of caffeine being a functional drug; in fact, there has been a substantial amount of research on its benefits (for review, see Glade [ 14 ]). However, once caffeine (or any other substance) has been determined to be an addictive drug, then prejudices against discussing any potentially beneficial effects often develop in the psychiatric community [ 13 ••]. This conflict of interest could interfere with future caffeine research.
A second article complements some of the issues raised by the roundtable concerning attitudes among the psychiatric community, including both researchers and clinicians. Budney et al. (2013) investigated popular opinions about caffeine dependence/CUD among members of professional societies relevant to addiction. An overwhelming majority (95 %) of those surveyed believed that caffeine cessation can produce withdrawal and 73 % thought withdrawal could have clinical importance, but fewer than half thought caffeine withdrawal should be in the DSM. A small majority (58 %) of respondents thought that some individuals could develop CUD, and 44 % believed CUD should be a DSM diagnosis [ 15 ]. These attitudes will be influenced by research published over the next few years and could affect what caffeine-related diagnoses are included in the next edition of the DSM.
Lastly, Meredith et al. provides a comprehensive review of studies on caffeine use/abuse/dependence and summarizes the existing evidence in support of the three primary CUD criteria. The authors also present a number of research directions needed to further support and understand CUD. As the authors note, the prevalence of CUD is difficult to estimate from existing studies since DSM-IV criteria for caffeine dependence were used previously and the current criteria for CUD are slightly different [ 16 ••].
Use Despite Harm
Before CUD can become an official diagnosis, more research is needed on the severity of symptoms of the three primary criteria: (1) a persistent desire or unsuccessful effort to control use; (2) substance use continued despite knowledge of having a persistent or recurrent physical or psychological problem likely to be caused or exacerbated by the substance (i.e., ‘use despite harm’); and (3) withdrawal [ 1 ]. In this author’s opinion, criterion 2 is the most contentious issue and in need of clarification. Some authors appear to accept that caffeine consumption is associated with negative health effects (e.g., Juliano et al. [ 9 ] and Striley et al.[ 10 ]), while others believe that it is not (e.g., Morelli and Simola [ 2 ], Hughes et al. [ 11 ], and Lara [ 17 ]). These opinions can influence research directions and hypotheses; therefore, closer examination of this criterion is needed to promote consensus on what health problems can define ‘use despite harm’ for CUD.
Evidence for Physical Problems Caused or Exacerbated by Caffeine
Large, acute doses of caffeine are known to cause caffeine intoxication, which can cause a significant threat to one’s health and require medical attention. McCarthy et al. reviewed caffeine-related calls to a state poison control center. Out of 254 reported cases of caffeine abuse, 106 patients were managed in an emergency department and 34 were hospitalized and/or admitted to an intensive care unit [ 18 •]. In addition, Ogawa and Ueki presented two case reports of individuals whose daily caffeine use escalated until symptoms of caffeine intoxication made medical intervention necessary [ 19 •]. Clearly, caffeine intoxication is a medically significant health problem. However, could an otherwise healthy individual meet the criterion for ‘use despite harm’ by consuming a low to moderate daily dose of caffeine? A review by Nawrot et al. on caffeine and health recommended that doses up to 400 mg/day are safe [ 20 ]; however, it is difficult to determine the health effects of low to moderate daily doses of caffeine because the effects of caffeine cannot be easily separated from the effects of caffeinated beverages, usually coffee, tea, soft drinks, or energy drinks. The antioxidant effects of polyphenols in tea and coffee are thought to have health benefits, while the excess sugars in soft drinks and energy drinks can be detrimental. Despite these confounds, there has actually been a great deal of research on the health effects of caffeine. However, the data are inconsistent.
A review of the literature on caffeine and health is outside the scope of this article, but a brief example may be informative: caffeine causes a small, temporary increase in blood pressure in normotensive adults. Tolerance to these effects may develop in some people, but caffeine could pose a threat to patients with, or at risk for, hypertension. Some studies have suggested an increased risk of sustained hypertension following coffee consumption (e.g., Palatini et al. [ 21 ]), while others have not found a significant relationship (e.g., Klag et al. [ 22 ]). Even two recent meta-analyses on caffeine and hypertension arrived at different conclusions: one found no evidence of a relationship [ 23 ] and the other found an elevated risk of hypertension associated with 1–3 cups of coffee per day, but not with 3 or more cups per day [ 24 ].
Clinicians make recommendations to their patients based on their knowledge of the literature, but the literature on caffeine and health is enormous and complicated. In at least two of the studies on caffeine dependence, subjects met the criteria for ‘use despite harm’ if they admitted using caffeine against medical advice [ 8 , 9 ]. If a clinician who read about an association between caffeine and hypertension (e.g., Palatini et al. [ 21 ]) recommended to her hypertensive patient to stop drinking coffee and he did not, should that patient meet the criteria for ‘use despite harm’ even though another physician who read a different article (e.g., Klag et al. [ 22 ]) would not have made the same recommendation?
To this author’s knowledge, low to moderate daily caffeine intake has not been proven to cause significant and irreversible health problems that would warrant medical intervention. That is not to say that caffeine does not or cannot have negative health effects, but researchers and clinicians need to agree on what physical health problems can be caused by chronic low to moderate caffeine intake. Whether or not low to moderate daily doses of caffeine can cause physical harm is an important issue to resolve since medical professionals recommend limiting/eliminating caffeine intake to some of their patients, and health concerns are a common reason for individuals to want to modify their caffeine use [ 9 ]. Furthermore, the fate of CUD in the next edition of the DSM may depend on the definition of ‘use despite harm,’ since the other two primary criteria for CUD (i.e., a persistent desire or unsuccessful effort to control use and withdrawal) could potentially be endorsed at any daily dose, even while consuming as little as 100 mg/day [ 25 ].
Evidence for Psychological Problems Caused or Exacerbated by Caffeine
The DSM-5 recognizes that some features of CUD may be positively associated with other psychiatric diagnoses [ 1 ], and there have been studies investigating whether caffeine use or withdrawal can exacerbate existing psychiatric symptoms. In particular, the anxiogenic effects of high caffeine doses can aggravate symptoms of anxiety, panic disorder, and insomnia (for reviews, see Lara [ 17 ] and Winston et al. [ 26 ]). In fact, a review of eight studies that administered a caffeine challenge to patients with panic disorder found that caffeine aggravated symptoms of anxiety and panic disorder in every study [ 27 ]. While this review provides strong evidence that caffeine can exacerbate anxiety and panic disorder, these studies were caffeine challenges and not representative of the patients’ normal caffeine intake. Patterns of actual caffeine consumption among psychiatric patients have been shown to be similar to matched controls; however, maximum lifetime intake was higher among patients [ 28 ]. In addition, the prevalence of caffeine dependence and intoxication was reportedly higher in patients, who endorsed consuming more caffeine than intended, having a desire to cut down, and using caffeine despite harm more often than controls [ 28 ]. However, even among psychiatric patients, caffeine can act as a functional drug. Low to moderate daily doses of caffeine can reduce anxiety and elevate mood, and may even improve symptoms of attention–deficit hyperactivity disorder, although large-scale clinical trials have not been conducted [ 17 ]. To date, the evidence suggests that caffeine use is associated with, but does not cause, psychiatric and substance use disorders [ 29 ]. The research on caffeine use and psychiatric disorders raises the possibility of increased risk for CUD or caffeine intoxication due to disordered use among certain patient populations and more studies are needed on the prevalence of caffeine use among individuals with psychiatric problems.
Co-Use with Other Substances
Another potential contribution to disordered caffeine use is co-use with other substances. Caffeine may facilitate the effects of other drugs of abuse [ 2 ]. In particular, combining caffeinated energy drinks with alcoholic beverages has become a popular phenomenon because high doses of caffeine may offset the subjective intoxicating effects of alcohol; this is problematic because the objective intoxicating effects of alcohol are not affected [ 30 ]. Furthermore, the co-use of caffeine and sugary soft drinks may cause cross-sensitization, especially among children, and this could lead to poor dietary habits across the lifespan [ 31 ••]. Too many caffeinated soft drinks in one’s diet could increase the risk of obesity [ 32 ] and dental caries [ 33 ] in children and adolescents.
In conclusion, future research on CUD must demonstrate that enough people, but not too many, meet the criteria for disordered caffeine use, and that the severity and frequency of problems resulting from this use significantly interfere with their well-being and daily function. In addition, tests of the reliability and validity of CUD criteria are needed, as well as clinical treatment options and their efficacy. If the same standard of harm can be met for caffeine as for other drugs of abuse, then perhaps in another 20 years CUD will be an official diagnosis in the sixth edition of the DSM (DSM-6). Official recognition of CUD could significantly impact popular opinions towards caffeinated beverages and affect their legal regulation. After all, 20 years ago, caffeine withdrawal was not an officially recognized diagnosis in the DSM-IV [ 5 ], but now there is sufficient evidence of caffeine withdrawal to warrant inclusion in the DSM-5. Many people are now aware that chronic caffeine use can result in physical dependence and there has been pressure on manufacturers of caffeinated beverages to disclose their products’ caffeine content. Some researchers have even recommended warning labels on caffeinated beverages [ 34 ].
The roundtable discussion raised an important issue: if caffeine use were proven harmful in some capacity, then a bias may develop among researchers against discussing any of its potential benefits on health, cognition, or arousal [ 13 ••]. In this event, could there also be public backlash against caffeine consumption? If so, there may be legislative pressure to limit access to caffeine, or to apply age restrictions on who can purchase and consume caffeine, in order to reduce the likelihood of caffeine-related problems among the general population. However, considering the amount of trade and commerce surrounding caffeinated beverages, caffeine use is not only a public health concern, but a major economic concern as well. It would not be surprising if coffee, tea, soft drink, and energy drink industries took an active role in dissuading official recognition of CUD in the DSM, especially if that recognition meant increased regulation of caffeinated products.
The recent research on caffeine has important considerations for health professionals and consumers. It appears that not all consumers are aware they are dependent on caffeine, or realize that their fatigue, headache, nausea, or other symptoms are related to caffeine withdrawal, instead of an illness [ 13 ••]. Several authors recommend increasing awareness among both clinicians and patients about the relationship between caffeine use, health, and psychiatric disorders [ 19 •, 28 , 34 ], and also recommend including caffeine use assessments during psychiatric evaluations [ 26 ]. Importantly, the last survey of caffeine use in America was published in 2005 [ 35 ] and this information needs updating. Lastly, a survey of what clinicians are recommending to their patients regarding caffeine use would be valuable information for researchers and health professionals. In addition to these recommendations, there are many more potential avenues for future caffeine research. On the other hand, since caffeine is the most widely used psychoactive drug in the world and there are upwards of 20,000 research articles on caffeine, there may be little left to learn about this substance and our relationship with it.
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, Fifth Edition, DSM-5. Arlington: American Psychiatric Association. 2013.
Morelli M, Simola N. Methylxanthines and drug dependence: a focus on interactions with substances of abuse. In: Fredholm BB, editor. Handbook of experimental pharmacology. Berlin Heidelberg: Springer-Verlag; 2011. p. 483–507.
Google Scholar
Nehlig A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci Biobehav Rev. 1999;23:563–76.
Article CAS PubMed Google Scholar
Satel S. Is caffeine addictive? A review of the literature. Am J Drug Alcohol Abuse. 2006;32:493–502.
Article PubMed Google Scholar
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, Fourth Edition, DSM-IV. Washington, D.C.: American Psychiatric Association. 1994.
Griffiths RR, Woodson PP. Caffeine physical-dependence - a review of human and laboratory-animal studies. Psychopharmacology. 1988;94:437–51.
Juliano LM, Griffiths RR. A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology. 2004;176:1–29.
Strain EC, Mumford GK, Silverman K, Griffiths RR. Caffeine dependence syndrome - evidence from case-histories and experimental evaluations. JAMA. 1994;272:1043–8.
Juliano LM, Evatt DP, Richards BD, Griffiths RR. Characterization of individuals seeking treatment for caffeine dependence. Psychol Addict Behav. 2012;26:948–54.
Article PubMed Central PubMed Google Scholar
Striley CW, Griffiths RR, Cottler LB. Evaluating dependence criteria for caffeine. J Caffeine Res. 2011;1:219–25.
Article CAS PubMed Central PubMed Google Scholar
Hughes JR, Oliveto AH, Liguori A, Carpenter J, Howard T. Endorsement of DSM-IV dependence criteria among caffeine users. Drug Alcohol Depend. 1998;52:99–107.
Association AP. Substance-related and addictive disorders. DSM-5 Development Fact Sheets 2013. http://www.dsm5.org/Documents/Substance%20Use%20Disorder%20Fact%20Sheet.pdf . Accessed 2 April 2014.
Striley CW, Hughes JR, Griffiths RR, Juliano LM, Budney AJ. A critical examination of the caffeine provisions in the Diagnostic and Statistical Manual, 5th Edition (DSM-5). J Caffeine Res. 2013;3:101–7. Striley moderated a roundtable discussion examining the caffeine provisions in the DSM-5. Participants were Drs. Hughes, Griffiths, Juliano, and Budney. The discussants provide an overview of the recent changes to the DSM and address the issues and concerns raised by the psychiatric community regarding those changes. Some of these concerns were that a diagnosis of caffeine withdrawal or CUD could trivialize other drug use disorders, and that disordered caffeine use could be over-diagnosed since caffeine is so widely used. The discussants emphasize that more conservative criteria are used to define caffeine withdrawal and CUD than other substance use diagnoses—to help prevent over-diagnosis—and the DSM substance use disorder diagnoses are intended for individuals who experience significant functional impairment as a consequence of their use .
Article Google Scholar
Glade MJ. Caffeine-not just a stimulant. Nutrition. 2010;26:932–8.
Budney AJ, Brown PC, Griffiths RR, Hughes JR, Juliano LM. Caffeine withdrawal and dependence: a convenience survey among addiction professionals. J Caffeine Res. 2013;3:67–71.
Meredith SE, Juliano LM, Hughes JR, Griffiths RR. Caffeine use disorder: a comprehensive review and research agenda. J Caffeine Res. 2013;3:114–30. Meredith et al. present a thorough review of the evidence in support of caffeine dependence and describe an agenda for future research on clinical, epidemiological, and genetic investigations of caffeine dependence and CUD. Of note, evidence is described in support of the three primary criteria for CUD, and three case reports of individuals seeking treatment for caffeine dependence are presented. The authors recommend more research on the prevalence of CUD in the general population and the severity of functional impairment associated with the disorder, as well as efficacious treatment options for affected individuals .
Lara DR. Caffeine, mental health, and psychiatric disorders. J Alzheimers Dis. 2010;20:S239–48.
CAS PubMed Google Scholar
McCarthy DM, Mycyk MB, DesLauriers CA. Hospitalization for caffeine abuse is associated with abuse of other pharmaceutical products. Am J Emerg Med. 2008;26:799–802. McCarthy et al. performed a retrospective review of all caffeine-related reports to the Illinois Poison Center between 2002 and 2004. Instances of caffeine abuse were included for analysis. The authors reported that caffeine was most often abused in the form of a non-dietary medication, followed by caffeine-enhanced beverages, then dietary supplements. Forty-two percent of cases were managed in an emergency department and 13 % of patients were hospitalized. The need for hospitalization was associated with concomitant abuse of other pharmaceuticals .
Ogawa N, Ueki H. Clinical importance of caffeine dependence and abuse. Psychiatry Clin Neurosci. 2007;61:263–8. The authors present two case reports of individuals whose caffeine use escalated to a point when they needed medical intervention for symptoms of caffeine intoxication. These case reports illustrate how caffeine use can become a clinically relevant disorder in some individuals. The authors also review evidence that caffeine abuse/dependence can manifest itself similar to other drugs of abuse. The authors recommend improved labeling of caffeinated products for consumer awareness .
Nawrot P, Jordan S, Eastwood J, et al. Effects of caffeine on human health. Food Addit Contam. 2003;20:1–30.
Palatini P, Dorigatti F, Santonastaso M, et al. Association between coffee consumption and risk of hypertension. Ann Med. 2007;39:545–53.
Klag MJ, Wang NY, Meoni LA, et al. Coffee intake and risk of hypertension - The Johns Hopkins precursors study. Arch Intern Med. 2002;162:657–62.
Mesas AE, Leon-Munoz LM, Rodriguez-Artalejo F, Lopez-Garcia E. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: a systematic review and meta-analysis. Am J Clin Nutr. 2011;94:1113–26.
Zhang ZZ, Hu G, Caballero B, Appel L, Chen LW. Habitual coffee consumption and risk of hypertension: a systematic review and meta-analysis of prospective observational studies. Am J Clin Nutr. 2011;93:1212–9.
Griffiths RR, Evans SM, Heishman SJ, et al. Low-dose caffeine physical-dependence in humans. J Pharmacol Exp Ther. 1990;255:1123–32.
Winston AP, Hardwick E, Jaberi N. Neuropsychiatric effects of caffeine. Adv Psychiatr Treat. 2005;11:432–9.
Vilarim MM, Araujo DMR, Nardi AE. Caffeine challenge test and panic disorder: a systematic literature review. Expert Rev Neurother. 2011;11:1185–95.
Ciapparelli A, Paggini R, Carmassi C, et al. Patterns of caffeine consumption in psychiatric patients. An Italian study. Eur Psychiatry. 2010;25:230–5.
Kendler KS, Myers J, Gardner CO. Caffeine intake, toxicity and dependence and lifetime risk for psychiatric and substance use disorders: an epidemiologic and co-twin control analysis. Psychol Med. 2006;36:1717–25.
Ferreira SE, de Mello MT, Pompeia S, de Souza-Formigoni MLO. Effects of energy drink ingestion on alcohol intoxication. Alcohol Clin Exp Res. 2006;30:598–605.
Temple JL. Caffeine use in children: what we know, what we have left to learn, and why we should worry. Neurosci Biobehav Rev. 2009;33:793–806. Review of caffeine literature, including sources of caffeine and consumption patterns; physiological mechanisms; evidence for addiction, tolerance, and sensitization; and caffeine conditioning and reinforcement value. A special emphasis is given to caffeine use among children and adolescents. In particular, cross-sensitization between caffeine and sugary beverages could set up poor dietary habits across the lifespan. Also, children and adolescents may experience pressure in the classroom and from coaches to drink caffeine for its putative performance-enhancing effects. The author concludes that research is lacking on caffeine use in children and adolescents, but the long-term effects of caffeine exposure during a critical period of development may be a cause for concern .
Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–8.
Majewski RF. Dental caries in adolescents associated with caffeinated carbonated beverages. Pediatr Dent. 2001;23:198–203.
Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks–a growing problem. Drug Alcohol Depend. 2009;99:1–10.
Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105:110–3.
Download references
Acknowledgments
This work was supported by National Institutes of Health grants K01 DA033347 (National Institute on Drug Abuse).
Compliance with Ethics Guidelines
Conflict of interest.
Merideth A. Addicott declares she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Author information
Authors and affiliations.
Department of Psychiatry and Behavioral Sciences, Duke-UNC Brain Imaging and Analysis Center, Duke University Medical Center, 2608 Erwin Rd, Box 3527, Lakeview Pavilion E, suite 300, Durham, NC, 27705, USA
Merideth A. Addicott
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Merideth A. Addicott .
Rights and permissions
Reprints and permissions
About this article
Addicott, M.A. Caffeine Use Disorder: A Review of the Evidence and Future Implications. Curr Addict Rep 1 , 186–192 (2014). https://doi.org/10.1007/s40429-014-0024-9
Download citation
Published : 28 May 2014
Issue Date : September 2014
DOI : https://doi.org/10.1007/s40429-014-0024-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Caffeine-related disorders
- Diagnostic and Statistical Manual
- Find a journal
- Publish with us
- Track your research

Caffeine Use Disorder: A Comprehensive Review and Research Agenda
Jul 19, 2018

Adapted from and full credit to Steven E. Meredith, Laura M. Juliano, John R. Hughes, and Roland R. Griffiths and Journal of Caffeine Research . This is just a summary. See full article .
Caffeine is the most widely used drug in the world. In the United States, more than 90% of adults use it regularly, and, among them, average consumption is more than 200 mg of caffeine per day – more caffeine than is contained in two 6-ounce cups of coffee or five 12-ounce cans of soft drinks. Although consumption of low to moderate doses of caffeine is generally safe, consumption of higher doses by vulnerable individuals can lead to increased risk for negative health consequences, including cardiovascular problems and perinatal complications. Moreover, a number of recent studies show that some caffeine users become addicted to or dependent on caffeine. Many of these individuals are unable to reduce consumption despite knowledge of recurrent health problems associated with continued caffeine use.
Thus, the World Health Organization and some health care professionals recognize caffeine dependence as a clinical disorder.
The diagnostic criterion that may be of most concern to health care professionals is continued caffeine use despite harm, or “Continued caffeine use despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by caffeine”. Caffeine consumption has been associated with a number of negative health consequences, including anxiety, insomnia, hypertension, myocardial infarction, bladder instability, gastroesophageal reflux, spontaneous abortion, and reduced fetal growth.
In the general-population survey conducted in the United States, 14% of caffeine users endorsed use despite harm. Many participants from this study (13%) reported that a physician or counselor had advised them to stop or reduce caffeine consumption within the last year. Medical and psychological problems that participants attributed to caffeine included heart, stomach, and urinary problems, and complaints of anxiety, depression, insomnia, irritability, and difficulty thinking. In addition, two-thirds of those surveyed endorsed at least one symptom associated with Caffeine Intoxication, a clinical disorder recognized by the ICD-10 (i.e., Caffeine Acute Intoxication) and the DSM-5 (see Table 4). For example, 39% of participants from this general population study endorsed insomnia, 30% endorsed nervousness, 24% endorsed heart pounding, 18% endorsed stomachache, and 10% endorsed muscle twitching. Seven percent of participants reported that these symptoms interfered with their performance at work, home, or school.
Although the World Health Organization already recognizes a diagnosis of Caffeine Dependence Syndrome in the ICD-10, the American Psychiatric Association has indicated that more research is needed to determine the clinical significance of Caffeine Use Disorder before the diagnosis may be recognized in the DSM as a clinical disorder.
The inclusion of Caffeine Use Disorder in the DSM-5 should help stimulate more research on caffeine dependence. More studies are needed to determine the prevalence of Caffeine Use Disorder and the severity of functional impairment associated with the disorder. In addition, research is needed to evaluate the reliability and validity of the Caffeine Use Disorder diagnostic schema and the relationship between Caffeine Use Disorder and other behavioral and mental disorders. Most importantly, however, more research is needed to determine which methods work best to treat individuals who are currently distressed by this clinically important health problem.
Adapted from and full credit to: Steven E. Meredith, Laura M. Juliano, John R. Hughes, and Roland R. Griffiths.
See full article ; this is just a summary.
- Open access
- Published: 15 June 2021

Caffeine consumption, intoxication, and stress among female university students: a cross-sectional study
- Deemah A. AlAteeq ORCID: orcid.org/0000-0003-2852-5370 1 ,
- Razan Alotaibi 1 ,
- Raneem Al Saqer 1 ,
- Njoud Alharbi 1 ,
- Maram Alotaibi 1 ,
- Reema Musllet 1 &
- Rana Alraqibah 1
Middle East Current Psychiatry volume 28 , Article number: 30 ( 2021 ) Cite this article
18k Accesses
8 Citations
17 Altmetric
Metrics details
University students use caffeine to cope with stress in spite of its adverse effects. The purpose of this study is to explore caffeine consumption among university students in Saudi Arabia, as well as its correlation with stress and caffeine intoxication. This cross-sectional study examined a convenience sample of 547 students at Princess Nourah Bint Abdulrahman University (PNU). A self-administrated questionnaire was used to assess caffeine consumption in milligrams per day, stress was assessed by the perceived stress scale (PSS), and caffeine intoxication was assessed using the DSM-5 criteria.
The mean total caffeine consumption was 424.69 ± 385.31 mg/day. High levels of caffeine consumption were found among students of non-health colleges and students who were undiagnosed with psychiatric disorders ( p values <0.040 and 0.027, respectively). A significant positive correlation was found between caffeine consumption and perceived stress ( p <0.045). Only 13.26% of all participants fulfilled the DSM-5 criteria for caffeine use disorder. The majority of participants showed moderate and high stress levels (69.9% and 18.7%).
This study revealed high caffeine consumption and perceived stress levels among female undergraduate students with a significant positive association between them. The results emphasize the importance of educational campaigns about caffeine consumption and intoxication. They also encourage the development of stress management programs. Longitudinal studies need to be designed for evidence-based intervention.
Caffeine is a stimulant of the central nervous system and metabolism that is used for recreational and for medical reasons, such as decreasing physical exhaustion and increasing mental alertness [ 1 ]. Caffeine intake has positive and negative effects. The positive effects are enhanced mood and readiness, improved ability to stay conscious and alert, and strengthened exercise performance [ 2 ]. On the other hand, negative effects may occur when caffeine intake exceeds 250 mg, it can result in a condition called caffeine intoxication. Symptoms include fidgeting, excitement, insomnia, increased urination, gastrointestinal disturbance, muscle twitching, irregular or rapid heartbeat, and psychomotor agitation according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) [ 3 ].
Students experience stressful times in college due to classes, homework, exams, projects, and extracurricular activities [ 4 ]. Studies done on college students in Puerto Rico, Saudi Arabia, and Turkey showed that 49%, 49.5%, and 58.99% of them use caffeine as a coping mechanism, respectively [ 2 , 5 , 6 ]. In order to deal with this stress and fulfill academic requirements, college students may consume caffeine in the belief that it can aid their academic performance [ 4 ].
In Bahrain, the mean of daily caffeine consumption was assessed among college students. Females were consuming less than males (246 and 306 mg/day respectively) [ 7 ]. Another study assessed caffeine consumption and sleep habits among a sample of 228 students at Princess Nourah Bint Abdulrahman University (PNU) and found that most of them had high caffeine consumption, and a need for future studies concerning caffeine intoxication was suggested [ 8 ]. In this study, we aimed to estimate the level of caffeine consumption among students at health colleges and non-health colleges in PNU and to explore the correlation of caffeine consumption (including all types of caffeinated beverages) with caffeine intoxication and perceived stress.
Study design and population
This cross-sectional study examined a convenience sample of students who were Arabic speakers at the age of 18 years or above at health and non-health colleges at PNU, Riyadh, Saudi Arabia. PNU is the first women’s university in the Middle East and also the largest one. It accommodates 33,825 students and includes 18 colleges.
A self-administrated questionnaire was distributed conveniently in October 2019 to 547 students from different colleges. Equal numbers of questionnaires were distributed to health and non-health colleges (humanities, community, and science). The comparison was chosen to be between health and non-health colleges based on the significant differences in the studying years, academic system, training requirements, and health-related knowledge. Health college students have an average of 6 years of studying and non-health college students have an average of 4 years. These factors may affect the level of their perceived stress and caffeine consumption.
The consenting process started by going to the students on the campus and explaining to them the study and asks them if they are willing to participate, mentioning that their identity is confidential as it does not require a name or an ID. If they agree to participate, a copy of the questionnaire will be given to them, and they will be encouraged to read the front page thoroughly which has a statement saying “Filling this questionnaire means you agree to be part of this study.” It also has the information and purpose of the study and the contact information.
Previous literatures showed that the average daily caffeine consumptions vary from 10 to 11.2 mg/day, and the standard deviation was around 5 mg/day. Gpower software was used to calculate the minimal size required for this study, considering alpha =0.05 (level of significance of 95%) and beta to be 0.20 (power of the study is 80%), the minimal sample size required is 548.
Data collection tool
The questionnaire included four main divisions. The first one covered demographic characteristics, academic-related characteristics, and personal clinical history. The second and third divisions covered caffeine consumption and caffeine intoxication. The fourth division assessed perceived stress level by PSS-10. The first three division of the survey were first written in Arabic then translated to English by a professional translator, then reviewed and translated into Arabic by bilingual speakers. Pilot test was done for the Arabic version, and then face validation was done by sending the survey to four mental health professionals. Furthermore, the fourth division involved the Arabic version of PSS-10, which has been validated previously by Chaaya et al. The PSS-10 was translated from English into Arabic, and then it was reviewed by a bilingual psychiatrist. After that, it was back translated into English by the psychiatrist and a comparison with the original one was done [ 9 ].
Demographic characteristics, academic-related characteristics, and personal clinical history
Demographic characteristics included age, nationality, marital status, number of children, and income, which was estimated among students as having enough income, enough income with saving, not enough income, or debt. Academic-related characteristics included college, academic level (first and second-years were counted as “juniors,” whereas third, fourth, and fifth years were accounted as “seniors”), GPA, and academic satisfaction. Personal clinical history included questions about their history of diagnosed chronic diseases or psychiatric disorders, received psychiatric help, and smoking.
- Caffeine consumption
Caffeine intake per day was measured in milligrams. A table was included in the questionnaire (Table 1 ). The common caffeinated drinks had been enlisted in the table. All common caffeinated drinks were investigated, including drinks such as coffee, decaffeinated coffee, tea, cola, citrus, and energy drinks. The table included the size and the number of cup/cans per day for each drink. A reference image was attached to illustrate the size, and the amount of fluid in ounces. The participants were asked to fill out only the size and number of the drinks they regularly consume. The amount of caffeine in each drink size was calculated (Table 2 ), which were later multiplied by the number of cups/cans consumed daily. Then, the total numbers of caffeine milligrams per day were summed. Caffeine intake was examined as “low” and “high.” Low intake was considered less than 250 mg per day, while high intake was considered more than 250 mg per day. The validity and reliability of measurements have been confirmed in similar studies [ 10 , 11 ].
Caffeine intoxication
Intoxication was assessed using the criteria of DSM-5, which includes 12 symptoms. Participants were asked if they developed symptoms during or shortly after caffeine consumption. Any participant with five or more symptoms was diagnosed with caffeine intoxication according to the criteria.
Perceived stress level
Stress level was measured using the Arabic version of the PSS, which is a 10-question tool that is used to measure perception of stress over the past 30 days. The scale was developed in 1983 [ 12 ] and was modified in 1988 by Cohen [ 13 ]. It is a validated stress questionnaire with established acceptable psychometric properties [ 14 , 15 ]. A Likert-type scale was used to capture responses to the PSS (“never,” “almost never,” “sometimes,” “fairly often,” and “very often”). A score of 0-13 is considered as low stress, 14-26 is considered moderate, and 27-40 is considered high perceived stress.
Statistical analysis
Data were analyzed using SPSS 23. We described the variables as means ± the standard deviation (SD) or percentages as appropriate. A t test was used to determine the difference between quantitative variables, while the chi-squared test was used to determine the association between qualitative variables.
The total number of participants in the study was 547, and the average age was 20.30 ± 1.91 years. The majority were Saudi (98.40%) and single (96%). More than half of the participants (59.20%) reported that their income was enough, and 29.20% reported that their income was enough with saving.
Almost half of the participants were from health colleges (50.10%), whereas the other half were from non-health colleges (49.90%). More than half of the participants were junior students (61.80%). The GPA 4.50-5.00 for 42.50% of the participants, and only 9.40% of them had a GPA less than 3.50. More than half of them were either satisfied or very satisfied with their academic achievement (46.60% and 22.80%, respectively).
A minority of the participants reported that they were diagnosed with chronic diseases and psychiatric disorders (6.80% and 10.10%, respectively). Half of those who had been diagnosed with a psychiatric disorder received psychiatric help (56.36%). In addition, the majority of participants were non-smokers (93.90; Table 3 ).
The mean total caffeine consumption per day was 424.69±385.31 mg. Specialty coffee was the most consumed caffeine source with a mean of 93.06±126.99 mg, followed by regular brewed coffee, capsule coffee, and black tea with means of 62.74±114.30 mg, 55.39±114.62 mg, and 51.60±83.98 mg, respectively (Table 4 ). The mean of low caffeine consumption group (< 250 mg/day) was 126.6±68.01 mg/day, while it was 628.28±381.6 mg/day for the high caffeine consumption group (> 250 mg/day).
A high level of caffeine consumption was significantly more evident among students of non-health colleges than health college students (53.50% versus 46.50%, respectively; p <0.040). In addition, a high level of caffeine consumption was significantly more evident among students undiagnosed with psychiatric disorders than diagnosed students (87.60% versus 12.40%, respectively; p <0.027). Moreover, a high level of caffeine consumption was significantly more evident among students who experienced caffeine intoxication symptoms than asymptomatic students (75% versus 57.1%; p < 0.005). Finally, students who had a high level of caffeine consumption had significantly higher mean scores of perceived stress than students with a low level of caffeine consumption (21.40±6.38 and 20.27±6.31, respectively; p <0.045; Table 5 ).
Symptoms of caffeine intoxication
The reported caffeine intoxication symptoms in descending order were diuresis, insomnia, tachycardia or arrhythmia, gastrointestinal disturbance, restlessness, nervousness, rambling flow of thought and speech, muscle twitching, periods of inexhaustibility, psychomotor agitation, excitement, and flushed face (43.70%, 43.50%, 38.90%, 25.80%, 16.80%, 15.90%, 13.90%, 11.70%, 11%, 9.50%, 7.50%, and 5.10%, respectively). However, more than three quarters of them had no clinically significant distress or impairment of function (78%). Only 13.26% of all participants fulfilled DSM-5 criteria for caffeine use disorder.
Perceived stress
More than two-thirds of the participants showed moderate stress levels (69.9%), whereas 18.7% reported high stress levels (Table 6 ). There were significant associations between the level of perceived stress and academic satisfaction. A high level of stress was also more evident among students who were academically very unsatisfied or not satisfied than those who were satisfied or very satisfied (25.87±6.57 and 22.43±6.20 versus 20.23±5.95 and 19.33±6.17, respectively; p <0.001).
In addition, the level of perceived stress was significantly associated with students’ income; a higher level of stress was more evident among students who expressed that they were in debt or their income is not enough those who had enough income or enough income with savings (24.11±6.95 and 23.24±7.90 versus 20.99±5.75 and 20.16±6.69, respectively; p <0.008). Another significant association was found between the level of perceived stress and some personal clinical histories. A high level of stress was more evident among students who were diagnosed with psychiatric disorders than undiagnosed students (25.20±6.31 versus 20.52±6.20, respectively; p <0.001). The level of stress was also significantly higher among students diagnosed with chronic disease than undiagnosed students (23.69±6.17 versus 20.80±6.33, respectively; p <0.008). Furthermore, a high level of stress was more evident among smokers than non-smokers (24.36±7.44 versus 20.79±0.02, respectively; p <0.002). Finally, a high level of stress was more evident among students who experienced caffeine intoxication symptoms than asymptomatic students (24.12±5.92 than 20.51±6.28, respectively; p <0.001; Table 7 ).
This study represents the first Saudi university-based survey of caffeine consumption including all types of caffeinated beverages among students from both health and non-health colleges to explore the correlation with perceived stress and caffeine intoxication. The results showed that the mean total caffeine consumption was 424.69±385.31 mg/day. This could be alarming as the recommended use for healthy adult is 400 mg/day [ 16 ]. This result is comparable to that of an Egyptian study, which found that caffeine consumption was 405.47±396.43 mg/day among university students [ 17 ]. These two results are slightly higher than a Lebanese result that showed a mean total caffeine consumption of 193.32±361.81 mg/day for medical students [ 18 ]. This can be explained by the result of the current study as it showed higher caffeine consumption among non-health college student. The lower caffeine consumption level reported by students of health colleges could be due to their awareness about the side effects of caffeine. On the other hand, the total mean caffeine consumption in the current study is much higher (by at least twice) than in other studies that were conducted among another various populations, like army soldiers (285 mg/day), psychiatric patients (281±325 mg/day), office workers (205.7±34.9 mg/day), the general populations (164.5 ± 0.9 mg/day and 193 mg/day), adolescents (25.92±41.25 mg/day and 91.5 ± 4.7 mg/day), and children (76.1 ± 6.3 mg/day) [ 10 , 11 , 19 , 20 , 21 , 22 ]. This could be due to the higher level of perceived stress that was found among university students in this study as caffeine may relieve stress [ 23 ]. In addition, other numerous factors for caffeine intake among undergraduate university students were reported in the USA including improving alertness, concentration, mood, energy, and enjoying the taste [ 24 ].
Furthermore, the level of caffeine consumption was significantly lower among students diagnosed with psychiatric disorders, which could be attributed to their awareness or previous experience of the effects of excessive caffeine consumption, which increases the risk of anxiety, panic attacks, and psychotic symptoms [ 25 , 26 ]. And those who are suffering from anxiety conditions may have more caffeine sensitivity, which contribute in caffeine avoidance due to the undesirable effects [ 27 , 28 , 29 ]. It could also be attributed to their awareness or previous experience with the potential interaction of caffeine with psychotropic drugs that are used for their psychiatric conditions, which is due to the metabolism of caffeine by CYP1A2 enzyme. Caffeine can inhibit this enzyme and cause side effects that may affect their treatment plan [ 30 ]. In addition, a high level of caffeine consumption was significantly more evident among students who experienced caffeine intoxication because the more caffeine they consume, the more symptoms they experience. A related study done in the USA showed that excessive caffeine consumption can lead to caffeine intoxication [ 4 ]. And it was found that only 13% of participants experienced caffeine intoxication according to the DSM-5 criteria. This is similar to the prevalence of intoxication that was found among psychiatric patients in Italy (10.3%), which was significantly higher compared to healthy participants (2.9%). However, comparing our results with the Italian results was limited by the samples differences as the Italian study had wider age range and more severe psychiatric cases compared to our study [ 22 ].
Perceived stress was prevalent in this study. This is not surprising as similar results were found in previous studies that were conducted among university students in Saudi Arabia, Iran, and Malaysia [ 5 , 31 , 32 , 33 ]. In addition, a significant positive relationship was found between the level of caffeine consumption and the level of perceived stress. This is supported by a previous study that found a significant positive relationship between the consumption of energy drinks and stress [ 34 , 35 ]. This might be due to the beneficial effects of caffeine in maintaining cognitive function under conditions of stress and improving work performance [ 23 ].
Moreover, smoker students reported significantly higher stress levels. There are several theories on the role of stress and smoking behaviors. Smokers use cigarettes to relieve stress. However, several studies have shown that while smoking may temporarily relieve perceived stress, it actually may generate or aggravate negative emotional states and propagate negative coping strategies, leading to higher stress levels overall [ 36 ].
Perceived stress was found to be significantly higher among students who were diagnosed with psychiatric disorder or chronic disease. This is not surprising as it is evident that stress is a risk factor for various psychiatric and medical conditions [ 37 , 38 , 39 , 40 ]. Research shows that almost every system in the body can be influenced by chronic stress. When chronic stress goes unreleased, it suppresses the body’s immune system and ultimately manifests as illness. If stress continues and the body is unable to cope, there is likely to be a breakdown of bodily resources [ 41 ].
Limitations
This is the first Saudi university-based survey of caffeine consumption among students from both health and non-health colleges that included all types of caffeinated beverages. The results provided valuable information about caffeine consumption, caffeine intoxication, and stress. However, the convenience sampling and female participants limit the generalizability of the study. Although all common caffeinated drinks were investigated in this study, other possible sources of caffeine such as caffeine pills and chocolate were not included. In addition, even if intoxication symptoms listed in the survey were developed during or shortly after caffeine intake, it was difficult to differentiate between caffeine intoxication and symptoms of other medical or psychiatric conditions. Furthermore, a cross-sectional study cannot identify causality relationships.
Caffeine is highly consumed by female undergraduate students, mostly specially coffee, and the level is significantly higher among students of non-health colleges. In addition, caffeine consumption levels are positively and significantly correlated with perceived stress levels, which were prevalent among the students. However, only 13.26% of all participants fulfilled DSM-5 criteria for caffeine use disorder which was associated with high level of stress. This emphasizes the importance of educational campaigns about caffeine consumption and intoxication. Furthermore, this study could be useful for future university education and stress management planning. It could also be used as a primary resource for future investigations. However, longitudinal studies need to be designed for evidence-based intervention. Further studies also need to involve both sexes and postgraduate students.
Availability of data and materials
All data and material of this study are available upon request from the corresponding author.
Abbreviations
Perceived stress scale
The Diagnostic and Statistical Manual of Mental Disorders, fifth edition
Uddin MS, Abu Sufian M, Hossain MF, Kabir MT, Islam MT, Rahman MM et al (2017) Neuropsychological effects of caffeine: is caffeine addictive? J Psychol Psychother 07(02):1–12
Richards G, Smith A (2015) Caffeine consumption and self-assessed stress, anxiety, and depression in secondary school children. J Psychopharmacol. 29(12):1236–1247. https://doi.org/10.1177/0269881115612404
Article CAS PubMed PubMed Central Google Scholar
American Psychiatric Association (2012) DSM-IV and DSM-5 criteria for the personality disorders. Am Psychiatr Assoc. p. 503-4 in Section II (Substance-Related and Addictive Disorders)
Simpson E, Stephenson T, Brewer D, Schwartz A, Bastin S (2016) Perceived stress, caffeine consumption, and GPA of undergraduate students at a large public university. J Nutr Educ Behav. 48(7):S102. https://doi.org/10.1016/j.jneb.2016.04.269
Article Google Scholar
Aftab MT, Naqvi AA, Al-karasneh AF, Ghori SA (2018) Impact of religiosity on subjective life satisfaction and perceived academic stress in undergraduate pharmacy students. J Pharm Bioallied Sci 10(4):192–198
Karagoz I (2019) Cmbebih 2019. IFMBE Proc - C. 73:159–163
Jahrami H, Al-Mutarid M, Penson PE, Al-Islam Faris M, Saif Z, Hammad L (2020) Intake of caffeine and its association with physical and mental health status among university students in Bahrain. Foods (Basel, Switzerland) 9(4):473
CAS Google Scholar
Alfajahan OAA (2018) Sleep habits and caffeine consumption in undergraduate female students in Saudi Arabia
Google Scholar
Chaaya M, Osman H, Naassan G, Mahfoud Z (2010) Validation of the Arabic version of the Cohen perceived stress scale (PSS-10) among pregnant and postpartum women. BMC Psychiatry 10(111):1–7
Jin MJ, Yoon CH, Ko HJ, Kim HM, Kim AS, Moon HN, Jung SP (2016) The relationship of caffeine intake with depression, anxiety, stress, and sleep in Korean adolescents. Korean J Fam Med. 37(2):111–116. https://doi.org/10.4082/kjfm.2016.37.2.111
Article PubMed PubMed Central Google Scholar
Mitchell DC, Knight CA, Hockenberry J, Teplansky R, Hartman TJ (2014) Beverage caffeine intakes in the U.S. Food Chem Toxicol 63:136–142
Article CAS Google Scholar
Cohen S, Kamarck T, Mermelstein R (1983) A global measure of perceived stress. J Health Soc Behav. 24(4):385–396. https://doi.org/10.2307/2136404
Article CAS PubMed Google Scholar
Cohen S (1988) Perceived stress in a probability sample of the United States. In: The social psychology of health. Sage Publications, Inc, Thousand Oaks, pp 31–67 (The Claremont Symposium on Applied Social Psychology.)
Lee EH (2012) Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res (Korean Soc Nurs Sci). 6(4):121–127. https://doi.org/10.1016/j.anr.2012.08.004
Article PubMed Google Scholar
Taylor JM (2015) Psychometric analysis of the ten-item perceived stress scale. Psychol Assess. 27(1):90–101. https://doi.org/10.1037/a0038100
Knight CA, Knight I, Mitchell DC, Zepp JE (2004) Beverage caffeine intake in US consumers and subpopulations of interest: estimates from the Share of Intake Panel survey. Food Chem Toxicol. 42(12):1923–1930. https://doi.org/10.1016/j.fct.2004.05.002 Available from: https://www.sciencedirect.com/science/article/pii/S0278691504001589
El-Nimr N, Bassiouny S, Tayel D (2019) Pattern of caffeine consumption among university students. J High Inst Public Heal. 0(0):153–160. https://doi.org/10.21608/jhiph.2019.56579
Samaha A, Al Tassi A, Yahfoufi N, Gebbawi M, Rached M, Fawaz MA (2020) Data on the relationship between caffeine addiction and stress among Lebanese medical students in Lebanon. Data Br. 28:104845. https://doi.org/10.1016/j.dib.2019.104845
Misaizu A, Kokubo T, Tazumi K, Kanayama M, Miura Y (2014) The combined effect of caffeine and ornithine on the mood of healthy office workers. Prev Nutr Food Sci. 19(4):367–372. https://doi.org/10.3746/pnf.2014.19.4.367
Frary CD, Johnson RK, Wang MQ (2005) Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 105(1):110–113. https://doi.org/10.1016/j.jada.2004.10.027 Available from: https://www.sciencedirect.com/science/article/pii/S000282230401702X
Lieberman HR, Stavinoha T, McGraw S, White A, Hadden L, Marriott BP (2012) Caffeine use among active duty US army soldiers. J Acad Nutr Diet 112(6):902–912.e4 Available from: https://www.sciencedirect.com/science/article/pii/S2212267212001530
Ciapparelli A, Paggini R, Carmassi C, Taponecco C, Consoli G, Ciampa G et al (2020) Patterns of caffeine consumption in psychiatric patients. An Italian study. Eur Psychiatry 25(4):230–235 Available from: https://www.cambridge.org/core/article/patterns-of-caffeine-consumption-in-psychiatric-patients-an-italian-study/FE266821259A551A179369AFDED4584C
Lieberman HR, Tharion WJ, Shukitt-Hale B, Speckman KL, Tulley R (2002) Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Psychopharmacology (Berl). 164(3):250–261. https://doi.org/10.1007/s00213-002-1217-9
Mahoney CR, Giles GE, Marriott BP, Judelson DA, Glickman EL, Geiselman PJ, Lieberman HR (2019) Intake of caffeine from all sources and reasons for use by college students. Clin Nutr. 38(2):668–675. https://doi.org/10.1016/j.clnu.2018.04.004 Available from: https://www.sciencedirect.com/science/article/pii/S0261561418301341
Addicott MA (2014) Caffeine use disorder: a review of the evidence and future implications. Curr Addict Reports. 1(3):186–192. https://doi.org/10.1007/s40429-014-0024-9
Lara DR (2010) Caffeine, mental health, and psychiatric disorders. J Alzheimer’s Dis 20(SUPPL.1):S239–S248
Lee MA, Cameron OG, Greden JF (1985) Anxiety and caffeine consumption in people with anxiety disorders. Psychiatry Res. 15(3):211–217. https://doi.org/10.1016/0165-1781(85)90078-2 Available from: https://www.sciencedirect.com/science/article/pii/0165178185900782
Lande RG, Labbate LA (1998) Caffeine use and plasma concentrations in psychiatric outpatients. Depress Anxiety 7(3):130–133. Available from:. https://doi.org/10.1002/(SICI)1520-6394(1998)7:3%3C130::AID-DA6%3E3.0.CO
Boulenger JP, Uhde TW (1982) Caffeine consumption and anxiety: preliminary results of a survey comparing patients with anxiety disorders and normal controls. Psychopharmacol Bull. 18(4):53–57 Available from: http://europepmc.org/abstract/MED/7156298
CAS PubMed Google Scholar
Broderick PJ, Benjamin AB, Dennis LW (2005) Caffeine and psychiatric medication interactions: a review. J Okla State Med Assoc. 98(8):380–384 Available from: http://europepmc.org/abstract/MED/16206866
PubMed Google Scholar
Al-sowygh ZH (2013) Academic distress, perceived stress and coping strategies among dental students in Saudi Arabia. Saudi Dent J. 25(3):97–105. Available from:. https://doi.org/10.1016/j.sdentj.2013.05.002
Moayedi F, Bastami MM, Ashouri FP, Hamadiyan H, Rasekhi S (2016) Comparison of sources and severity of perceived stress between paramedical and medical students. Int J Med Res Heal Sci. 6:183–190
Al-Dubai SA, Barua A, Ganasegeran K, Jadoo SA, Rampal KG (2014) Concurrent validity of the Malay version of Perceived Stress Scale (PSS-10). ASEAN J Psychiatry. 15:8–13
Pettit ML, Debarr KA (2011) Perceived stress, energy drink consumption, and academic performance among college students. J Am Coll Heal. 59(5):335–341. https://doi.org/10.1080/07448481.2010.510163
Errisuriz VL, Pasch KE, Perry CL (2016) Perceived stress and dietary choices: the moderating role of stress management. Eat Behav. 22:211–216. https://doi.org/10.1016/j.eatbeh.2016.06.008
Lawless MH, Harrison KA, Grandits GA, Eberly LE, Allen SS (2015) Perceived stress and smoking-related behaviors and symptomatology in male and female smokers. Addict Behav. 51:80–83. https://doi.org/10.1016/j.addbeh.2015.07.011
Sinha R, Jastreboff AM (2013) Stress as a common risk factor for obesity and addiction. Biol Psychiatry. 73(9):827–835. https://doi.org/10.1016/j.biopsych.2013.01.032 Available from: https://www.sciencedirect.com/science/article/pii/S0006322313001340
VanItallie TB (2002) Stress: a risk factor for serious illness. Metab - Clin Exp 51(6):40–45. Available from. https://doi.org/10.1053/meta.2002.33191
Kivimäki M, Kawachi I (2015) Work stress as a risk factor for cardiovascular disease. Curr Cardiol Rep 17(9):74. Available from:. https://doi.org/10.1007/s11886-015-0630-8
Article PubMed Central Google Scholar
Wang J (2005) Work stress as a risk factor for major depressive episode (s). Psychol Med. 35(6):865–871. https://doi.org/10.1017/S0033291704003241
Salleh MR (2008) Life event, stress and illness. Malaysian J Med Sci. 15(4):9–18
Download references
Acknowledgements
The authors would like to thank Prof. Amel Fayed and Prof. Halah Elmershardi.
This research was funded by the Deanship of Scientific Research at Princess Nourah Bint Abdulrahman University through the Fast-track Research Funding Program. The funding body has no role in study design, data collection, data analysis, data interpretation, or manuscript writing.
Author information
Authors and affiliations.
Clinical Sciences Department, College of Medicine, Princess Nourah Bint Abdulrahman University, P.O. Box 93949, Riyadh, 11683, Saudi Arabia
Deemah A. AlAteeq, Razan Alotaibi, Raneem Al Saqer, Njoud Alharbi, Maram Alotaibi, Reema Musllet & Rana Alraqibah
You can also search for this author in PubMed Google Scholar
Contributions
RMA and NA were responsible of designing the study. MA and RM were in charge of collecting and entering the data. And they helped in the analysis. RMA and RIA were responsible of interpreting and analyzing of the data. DA was responsible of the general process, editing, and publication of paper. All authors took a part in writing, revising and approving the final manuscript. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Deemah A. AlAteeq .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval was obtained from the Institutional Review Board at Princess Nourah bint Abdulrahman University, Riyadh, KSA (IRB-PNU:19-0234), on 20 November 2019. Informed verbal consent was acquired from all participants before enrollment in the study. The ethics committee approved the verbal consent. Using verbal consent was recommended by the IRB in surveys if the data was taken from human subjects who cannot be identified, and their responses could not put them at risk of criminal or civil liability and could not damage their reputation or employability.
Consent for publication
Not applicable.
Competing interests
No conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
AlAteeq, D.A., Alotaibi, R., Al Saqer, R. et al. Caffeine consumption, intoxication, and stress among female university students: a cross-sectional study. Middle East Curr Psychiatry 28 , 30 (2021). https://doi.org/10.1186/s43045-021-00109-5
Download citation
Received : 28 December 2020
Accepted : 27 April 2021
Published : 15 June 2021
DOI : https://doi.org/10.1186/s43045-021-00109-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Intoxication
Recommended
Kyle richards go-to truskin just launched a new serum — and it’s powered by caffeine.

The tea on celebrities’ skincare routines? From “Real Housewives” to Disney stars, plenty of famous folks rely on TruSkin .
And on Wednesday, the label expanded its lineup with a new green tea-powered product: the Caffeine Facial Serum ($16), a formula packed with energizing ingredients like matcha.
In addition to 5% caffeine, the just-dropped product contains niacinamide (to target skin texture), hyaluronic acid (for hydration) and ginseng (to help energize and balance the complexion).
TruSkin Caffeine Facial Serum

While it’s brand-new, the serum’s already garnering great reviews on Amazon .
“I found this serum to be a medium weight, smooth but not quite silky formulation that applies easily and sinks in readily,” one satisfied shopper wrote of their first impressions, while another dubbed it a “good serum for a decent price.”

If history is any indication, the new serum just might pop up in stars’ routines soon, too.
Kyle Richards once shouted out the brand’s Vitamin C Facial Serum ( $30 $20) in an Amazon livestream, calling the popular skincare ingredient a “miracle” and saying she can “see a difference” after using it, per Prevention .
Alexandra Daddario’s also a TruSkin devotee, as she applied the same serum in a Vogue “Beauty Secrets” routine breakdown.
And “Descendants” star Kylie Cantrall is such a fan, she even partnered with the brand on her own bundle of products, in addition to singing its praises on social media.
Why Trust Page Six Style Shopping
This article was written by Hannah Southwick , Commerce Writer/Reporter for Page Six Style. Hannah spies deals on actually affordable celebrity-worn styles , puts Hollywood’s favorite labels to the test and finds the beauty products that keep stars red carpet-ready. She consults stylists and industry pros — including celebs themselves — for firsthand product recommendations, trend predictions and more. In addition to writing for Page Six since 2020, her work has been featured in USA Today and Parade.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Effects of Caffeine Consumption on Attention Deficit Hyperactivity Disorder (ADHD) Treatment: A Systematic Review of Animal Studies
Javier c. vázquez.
1 Faculty of Psychology and Educational Sciences, Cognitive NeuroLab, Universitat Oberta de Catalunya, 08018 Barcelona, Spain; ude.cou@amzeugnimodo (O.M.d.l.T.); ude.cou@raloderd (D.R.-R.)
2 Neuromodulation Unit, Institut Brain 360, 08022 Barcelona, Spain
Ona Martin de la Torre
Júdit lópez palomé.
3 Consorci d’Educació de Barcelona, Centre de Màxima Complexitat Elisenda de Montcada, Generalitat de Catalunya, 08010 Barcelona, Spain; tac.cetx@482epolj
Diego Redolar-Ripoll
Associated data.
Not applicable.
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by a persistent pattern of inattention and/or hyperactivity-impulsivity. ADHD impairments arise from irregularities primarily in dopamine (DA) and norepinephrine (NE) circuits within the prefrontal cortex. Due to ADHD medication’s controversial side effects and high rates of diagnosis, alternative/complementary pharmacological therapeutic approaches for ADHD are needed. Although the number of publications that study the potential effects of caffeine consumption on ADHD treatment have been accumulating over the last years, and caffeine has recently been used in ADHD research in the context of animal models, an updated evidence-based systematic review on the effects of caffeine on ADHD-like symptoms in animal studies is lacking. To provide insight and value at the preclinical level, a systematic review based on PRISMA guidelines was performed for all publications available up to 1 September 2021. Caffeine treatment increases attention and improves learning, memory, and olfactory discrimination without altering blood pressure and body weight. These results are supported at the neuronal/molecular level. Nonetheless, the role of caffeine in modulating ADHD-like symptoms of hyperactivity and impulsivity is contradictory, raising discrepancies that require further clarification. Our results strengthen the hypothesis that the cognitive effects of caffeine found in animal models could be translated to human ADHD, particularly during adolescence.
1. Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is the most commonly diagnosed and treated mental disorder during childhood [ 1 ] and it is increasingly diagnosed and treated in during adulthood [ 2 ]. ADHD is a neurodevelopmental disorder characterized by a pattern of inattention and/or hyperactivity-impulsivity, persisting no less than six months, that is inconsistent with developmental level and has negative impact in at least two settings (academic, occupational or social) [ 3 ]. Inattention refers to important difficulties in sustaining attention to tasks that do not deliver a high level of stimulation or regular rewards, distractibility, and difficulties with organisation. Hyperactivity refers to disproportionate motor activity and difficulties with remaining still, most manifest in structured situations that involve behavioral self-control. Finally, impulsivity is a propensity to behave in response to immediate stimuli, without consideration of the risks and consequences [ 4 ]. Specific manifestations vary across individuals, and may change over the course of development. Depending on the symptoms presented, three different types of ADHD can be diagnosed: predominantly inattentive presentation, predominantly hyperactive-impulsive presentation, or combined presentation [ 3 , 4 ]. Although ADHD onset occurs during childhood and it often persists into adulthood, there is an important knowledge gap concerning ADHD lifespan aspects [ 5 ]. Population surveys suggest that ADHD occurs in most cultures in about 5% of children and about 2.5% of adults [ 6 ] and, as of 2019, it was estimated to affect 84.7 million people worldwide [ 7 ]. ADHD management recommendations depend on the country [ 8 , 9 , 10 ] and usually include psychotherapy (essentially Cognitive Behavior Therapy, CBT), lifestyle changes and medications [ 11 ]. ADHD medication treatment, however, has been historically considered controversial [ 12 ], particularly due to its side effects [ 13 , 14 , 15 ]. In the face of these controversies and high rates of diagnosis, alternative/complementary pharmacological therapeutic approaches for ADHD are needed.
Although larger ADHD models containing supplementary pathways have been suggested [ 16 , 17 ], it is widely accepted that ADHD impairments, including selective and sustained attention, impulsivity, and motor activity, arise from abnormalities in different circuits involving the prefrontal cortex [ 18 ]: sustained attention is modulated by a cortico-striato-thalamocortical (CSTC) loop that comprises the dorsolateral prefrontal cortex (DLPFC) projecting to the striatal complex. Selective attention is modulated by a cortico-striato-thalamo-cortical (CSTC) loop ascending from the dorsal anterior cingulate cortex (dACC) and projecting to the striatal complex, followed by the thalamus, and back to the dACC. Impulsivity is related to a cortico-striato-thalamocortical (CSTC) loop that contains the orbitofrontal cortex (OFC), the striatal complex, and the thalamus. Finally, motor activity, including hyperactivity and psychomotor agitation or retardation, can be modulated by a cortico-striato-thalamo-cortical (CSTC) loop arising from the prefrontal motor cortex to the lateral striatum to the thalamus and back to the prefrontal motor cortex. ADHD patients cannot activate prefrontal cortex areas in an appropriate manner when responding to cognitive tasks requiring attention and executive control, and show a dysfunction in reward and motivation, hindering cognitive control of behaviour [ 19 , 20 ]. Children diagnosed with ADHD, in this regard, need stronger incentives to adapt their behaviour [ 21 ], showing impaired responses to partial schedules of reinforcement and difficulties in delaying gratification [ 22 , 23 ].
In ADHD, inefficient information processing and arousal-related behaviours are hypothetically caused by imbalances mainly in the dopamine (DA) and norepinephrine (NE) circuits [ 24 , 25 ] and the serotonin (5-HT), glutamate (GLU), and acetylcholine (ACh) pathways within these areas of the brain [ 26 , 27 , 28 ].
Different genes are associated with the disorder, including the serotonin transporter (SERT), the synaptosomal-associated protein (SNAP-25), and the brain-derived neurotrophic factor (BDNF) [ 29 , 30 ], while some genes directly affect DA neurotransmission, including the DA transporter (DAT) or the DA receptor 4 (DRD4) [ 31 , 32 ]. In this respect, the ventral tegmental area (VTA) and locus coeruleus (LC) neurons have different targets, although their efferent fibers converge into the PFC: DA is released into the nucleus accumbens (NAcc), facilitating reward; NE is released in different posterior cortical areas, optimizing the organism reaction to significant stimuli; and both organic compounds are released into the PFC, enhancing working memory and attention in the face of significant stimuli [ 33 ].
Animal studies have provided insights into the pathological and neurochemical basis of ADHD through different types of animal model (see Figure 1 ) [ 34 ]. Among these, the spontaneously hypertensive rat (SHR) is considered an excellent and validated hyperactive model to study ADHD. Concerning its behavioral profile, SHR presents anomalies in DA neurotransmission [ 35 ] and, importantly, in adenosine neurotransmission [ 36 ].

Animal models of Attention Deficit Hyperactivity Disorder. Key for abbreviations used: SHR: spontaneously hypertensive rat, low-density lipoprotein receptor, SI: social isolated, 6-OHDA: 6-hydroxy-dopamine, ADHD: Attention Deficit Hyperactivity Disorder.
Caffeine, in this respect, is an adenosine A 1 and A 2A receptor antagonist controlling synaptic plasticity [ 37 ]. These receptors are functionally paired with certain postsynaptic DA receptors, such as D2 receptors, where DA binds and has a stimulatory effect. When adenosine binds to its receptors, this causes reduced sensitivity of D2 receptors. Antagonism of adenosine receptors by caffeine prevents adenosine from binding, enhancing dopaminergic actions [ 18 , 24 ]. In addition to these dopaminergic effects, it has been shown that caffeine also produces secondary effects on ACh and NE [ 37 , 38 , 39 ]. Moreover, caffeine’s effects on the non-selective antagonism of adenosine receptors also generate vasoconstriction in the nervous system. In this respect, it has been shown that caffeine modifies the blood perfusion signal, measured by fMRI, due to its neural and vascular effects, depending on the cerebral distribution of its receptors [ 40 ]. Similarly, the effect that caffeine may have at the cognitive level could depend on its regional effects on vascular response [ 41 ].
Nevertheless, the potential of caffeine consumption as a treatment for ADHD remains largely controversial, with studies showing efficacy in relieving ADHD-related symptoms [ 42 ], and studies failing to find superior effects when compared to first-line ADHD medication [ 43 ]. Beyond ADHD, there is an existing correlation between the daily consumption of moderate doses of caffeine and related benefits in different psychiatric disorders linked with adenosine A 2A receptor blockade controlling synaptic plasticity [ 44 ], mainly at the glutamatergic synapses [ 45 ]. Moreover, regular coffee consumption improves children’s performance in comparison to decaffeinated coffee or placebo [ 46 ]. However, some studies have reported that caffeine consumption improvement is not significantly superior to placebo [ 47 ] or methylphenidate (MPD) [ 48 ], while hyperactivity has been strongly associated with higher coffee consumption among adolescents [ 49 ].
The number of publications that study the potential effects of caffeine consumption on ADHD treatment has accumulated since 1975 (see Figure 2 ) and, over the last few years, caffeine has been used in ADHD research in the context of animal models. Surprisingly, an updated evidence-based systematic review on the effects of caffeine on ADHD-like symptoms in animal studies is lacking.

Caffeine/Attention Deficit Hyperactivity Disorder-related articles since 1975 (Source: MEDLINE).
Consequently, to provide insight and value at the preclinical level, we sought to produce a comprehensive compilation and systematically review all the relevant scientific publications that make reference to the underlying effects of caffeine intake on treating ADHD-like symptoms in animal studies.
2. Materials and Methods
We conducted a systematic review of ADHD research in the context of animal models to assess the association between caffeine and ADHD-dependent variables including attention, locomotor activity, impulsive behavior, learning, and memory.
2.1. Search Strategy
Figure 3 depicts the search strategy. We followed the guidelines and recommendations contained in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [ 50 ], in order to reliably structure the gathered information in this systematic review. Academic articles were located using two electronic databases: MEDLINE and Web of Science. Only the results from these two databases were reported, since results from other sources (Scopus, Google Scholar) did not provide any relevant new results. No restrictions regarding publication date were applied. The literature search was conducted on 5 September 2021.

Flow diagram of study’s selection based on PRISMA guidelines [ 50 ].
According to our proposal, the MEDLINE search strategy was established on the following key search terms: “caffeine” [Mesh] AND “Attention Deficit Disorder with Hyperactivity” [Mesh]. MeSH (Medical Subject Headings) terms were therefore used in the development of this search. The Web of Science search strategy was based on the following key search terms: (“attention” OR “hyperactivity” OR “ADHD”) AND “caffeine”.
2.2. Study Selection Criteria
The search was limited to preclinical and original experiments on non-human animals. The inclusion criteria were: (1) English-written, indexed studies; (2) non-human animal preclinical/experimental studies; (3) the mention of the relationship between caffeine treatment and ADHD-like symptoms; and (4) controlled studies with separately treated groups. The exclusion criteria were: (1) clinical/experimental/qualitative studies on humans; (2) not mentioning caffeine treatment and ADHD-like symptoms at all; (3) reviews, posters, conference abstracts, oral speeches, commentaries, theoretical papers, unpublished relevant studies, and other studies relevant to the topic but not published in peer-reviewed journals; and (4) case and cross-over studies.
2.3. Study Selection
Duplicates of all the databases were removed. Titles and abstracts were independently screened by two authors (J.C.V. and O.M.d.l.T.) according to the inclusion and exclusion criteria. Articles interpreted as compatible were selected for a full-text analysis to determine whether they were or were not within the inclusion criteria. Furthermore, the references of selected studies were screened in search of additional articles that met the inclusion criteria. Whenever a divergence of opinions emerged, a third author (D.R.-R.) was consulted to discuss and reach an agreement between the authors.
2.4. Data Extraction and Analyses
Following selection of the studies, data were extracted and prearranged into a table. The subsequent information was collected: (1) author/s and year; (2) species: strain, sex, and sample ( n ); (3) animal model; (4) age; (5) independent variables; (6) caffeine treatment; (7) behavioral tests/types of stress; (8) dependent variables; and (9) main results.
Due to the large number of results acquired by the search terms, strict inclusion/exclusion criteria were applied to limit the final selection of studies. Figure 2 shows the studies included in quantitative synthesis.
3.1. Study Selection
A total of 121 unique citations was initially retrieved through the combined search, after which 108 citations were excluded after full-text screening because they did not meet the inclusion criteria. Therefore, 13 studies (Pandolfo et al., 2013 [ 51 ]; Ouichi et al., 2013 [ 52 ]; Caballero et al., 2011 [ 53 ]; Ruiz-Oliveira et al., 2019 [ 54 ]; Higgins et al., 2007 [ 55 ]; França et al., 2020 [ 56 ]; Nunes et al., 2018 [ 57 ]; Szczepanik et al., 2016 [ 58 ]; Pires et al., 2010 [ 59 ]; Prediger et al., 2005 [ 60 ]; Leffa et al., 2019 [ 61 ]; Pires et al., 2009 [ 62 ]; Alves et al., 2020 [ 63 ]) on animal models were finally considered. Based on their methodology, the studies in this review could be classified as experimental ( n = 10; 76.9%), randomly assigning the subjects sample to the experimental groups, and quasi-experimental ( n = 3; 23.1%), where the groups were usually constructed according to the subject’s characteristics. The first studies relevant to the topic were from 2005, while the most recent studies included in this review were published in 2020. Table 1 describes each article individually.
Summary of included studies.
| Author/s & Year | Species, Strain, Sex & Sample ( ) | Animal Model | Age | Independent Variables | Caffeine Treatment | Behavioral Tests/ Type of Stress | Dependent Variables | Main Results |
|---|---|---|---|---|---|---|---|---|
| Szczepanik et al., 2016 | Mice C57Bl/6 wild-type (8) LDLr (8) Female | Genetic (LDLr) | 3 months 8 months | Treatment (caffeine or vehicle) Strain (C57Bl/6 wild type or LDLr) | 10 mg/kg oral route Chronic treatment (21 days) | Open-field arena | Spontaneous locomotor activity (total distance travelled) Anxiety (time in the center) Exploratory behavior (visual inspection of the occupation plot) | - LDLr mice travelled greater distances than the C57BI/6 wild type mice during the 5 min period of analysis. - Caffeine treatment induced a renormalization effect in 8 month-old mouse locomotion. - Caffeine treatment was unable to modify the hyperlocomotion observed in 3 month-old LDLr mice. - All animal groups spent a similar amount of time in the center of an open field. - Similar exploratory behavior between groups. |
| Higgins et al., 2007 | Rats LE (15–16) CD (12–16) Male | Not used | Not specified | Treatment (caffeine, SCH412348, KW-6002, DPCPX, CGS-21680, amphetamine) Strain (LE or CD) | 1 mL/kg i.p. route One dose, prior testing | Five-choice serial reaction time task Locomotor activity test | Selective attention (Correct/incorrect trials, omissions, premature and perseverative responses, choice accuracy, correct/incorrect, and magazine latency) Hypolocomotion (distance travelled) | - Caffeine, SCH 412348 and KW-6002 augmented time reaction in LE and CD, without effect on accuracy. - Effects of SCH 412348 were at doses that were not overtly psychostimulatory. - CGS-21680 reduced speed reaction and augmented omissions. A CGS-21680 lower dose reduced the increased premature response caused by amphetamine. - Caffeine’s attentional-enhancing effects were facilitated through A receptor blockade. Selective A receptor antagonists could be included as a potential therapy for ADHD. |
| Ruiz-Oliveira et al., 2019 | Zebrafish wild-type (40) Male Female | Not used | 4 months | Treatment (caffeine or vehicle) | 10 mg/L 50 mg/L drinking water Chronic treatment (14 days) | Discrimination task | Conditioned learning ability (average swimming speed, intergroup freezing, maximum speed, time spent in each area, latency to enter each area) | - 0 and 10 mg/L caffeine groups spent most of the time close to the target. - 10 mg caffeine group had the shortest latency to reach the target. - 0 and 10 mg/L caffeine groups increased the average speed and distance travelled. - Caffeine exposure at low doses seems to enhance visual cue discrimination and zebrafish performance. |
| Prediger et al., 2005 | Rats WKY (7–8) SHR (7–8) Female | Genetic (SHR) | 3 months | Treatment (caffeine or vehicle) Strain (WKY or SHR) | 1.3 mg/kg 10 mg/kg i.p. route One dose, prior testing | Water maze task | Spatial learning (escape latency, distance travelled, swimming speed) Mean arterial pressure | - SHR needed a larger amount of trials during the training session to learn the spatial information, although a similar profile to that of WKY rats during the test session, showing a selective spatial learning deficit. - Caffeine’s pre-training administration enhanced SHRs’ spatial learning deficit. - Caffeine’s post-training administration did not enhance SHRs’ test performance, although it improved WKY rats’ memory retention. - Mean blood pressure was not altered by caffeine. |
| Pires et al., 2009 | Rats WKY (15) SHR (18) Male Female | Genetic (SHR) | 3 months | Treatment (MPD, DPCPX, caffeine, ZM241385 or vehicle) Strain (WKY or SHR) | 1 mg/kg 3 mg/kg 10 mg/kg i.p. route One dose, prior testing | Object recognition task | Object recognition (investigation time, discrimination time) Mean arterial pressure | - SHR only discriminated between the most structurally distinct pairs of objects. - Pre-training administration of MPD, caffeine, the selective adenosine receptor antagonists DPCPX and ZM241385, or the association of ineffective doses of DPCPX and ZM241385, improved the performance of SHR in the object-recognition task. - The administration of the same doses of MPD and caffeine did not significantly alter the mean arterial pressure of either WKYs or SHRs. |
| Pires et al., 2010 | Rats WKY (37) SHR (38) Female | Genetic (SHR) | 25/38 days | Treatment (caffeine, MPD or vehicle) Strain (WKY or SHR) | 3 mg/kg i.p. Route. Chronic treatment (14 days) | Object recognition task | Object recognition (investigation time, discrimination time) Spontaneous locomotor activity Mean arterial pressure Body weight | - WKY rats distinguished all the items. SHRs were unable to distinguish pairs of items with slight structural alterations. - Caffeine or MPD chronic treatment enhanced SHR item-recognition deficits. The same treatments impaired the adult WKY rats’ short-term object recognition ability. - Effects were independent of variations in locomotion, arterial blood pressure, and body weight. |
| Caballero et al., 2011 | Rats 6-OHDA lesioned (9) Saline- lesioned (9) Male Female | Physical trauma (6-OHDA lesioned) | 25 days | Treatment (caffeine or vehicle) | 1 mg/mL drinking water Chronic treatment (14 days) | Olton maze behavioral assay | Motor behavior (number of arms crossed) Attention behavior (total number of arms walked, and total number of arms walked until one was repeated) | - Caffeine treatment significantly improved 6-OHDA lesioned rats’ attention deficit. - After caffeine consumption, no changes were found in motor activity. |
| Pandolfo et al., 2013 | Rats WKY (16) SHR (16) Male | Genetic (SHR) | 24 days | Treatment (caffeine or vehicle (saline)) Strain (WKY or SHR) | 2 mg/kg i.p. route Chronic treatment (twice daily for 21 days) | Attentional-set shifting; anxiety-related behavior; Y maze; locomotion -related behavior | Attention (regressive and never- reinforced errors, perseverative errors, total number of trials required before reaching 10 correct consecutive choices) Locomotion and anxiety (number of peripheral squares crossed, number of central squares crossed, percentage of central locomotion) Spatial recognition (number of entries; time spent per arm; random exploration) | - SHRs were hyperactive and showed poorer performance in the attentional set-shifting and Y-maze paradigms, displayed increased dopamine transporter density, and increased dopamine uptake in frontocortical and striatal terminals. - Chronic caffeine treatment improved memory and attention deficits, and normalized dopaminergic function in SHR. - First indication of adenosine A receptors (A R) in nerve terminals in frontal cortex. - First evidence that A R density is improved in SHR. |
| Ouichi et al., 2013 | Mice ICR (9) Male Female | Physical trauma (SI) | 4 weeks | Treatment (MPD and caffeine) SI | 0.5–1 mg/kg i.p. route One dose, prior testing | Water-finding test; aggression; modified Y-maze test; novel object recognition test; fear-conditioning test | Spatial attention (entering & drinking latency) Aggression (duration of wrestling) Spatial recognition (time spent in the new arm; total time exploring objects) Fear conditioning (freezing behavior) | - SI rats showed deficits in spatial attention on the water-finding test. Re-socialized did not reduce deficit in spatial attention. SI effect on spatial attention revealed no difference in gender or correlation with aggressive behaviour. - SI impaired conditional and contextual fear memory. - MPD and caffeine enhanced deficits in SI-induced latent learning in a manner that was reversible with cholinergic but not dopaminergic antagonists. |
| Nunes et al., 2018 | Rats WKY (5–15) SHR (5–15) Male Female | Genetic (SHR) | 15 days 28 days 50 days | Treatment (caffeine/water, caffeine/caffeine or water) Strain (WKY or SHR) | 0.3 g/L drinking water Until PND 28 | Open-field test; Novel object recognition; Y maze task | Open field test (travel distance periphery) Habituation (total travelled distance in the open field) Spatial recognition- Y maze and object recognition (exploration, discrimination ratio, number of entries, time spent in novel arm, total number of entries in three arms) | - Adolescent SHR from both sexes displayed hyperlocomotion, recognition, and spatial memory disturbances. Females displayed a lack of habituation and deteriorated spatial memory. - Caffeine was effective at improving recognition memory damage in both sexes. - Spatial memory was improved only in female SHRs. - Female SHRs displayed impaired hyperlocomotion following caffeine treatment. - SHRs of both sexes presented increases in BDNF, truncated and phospho-TrkB receptors, and phospho-CREB levels in the hippocampus. - Caffeine normalized BDNF in males and truncated TrkB receptor in both sexes. |
| Leffa et al., 2019 | Rats WKY (7–9) SHR (7–9) Male | Genetic (SHR) | 60/65 days 24 days | Treatment (WIN, AM251, caffeine or vehicle) Strain (WKY or SHR) | 2 or 5 mg/kg i.p. route Acute pretreatment, one dose Chronic treatment (21 days) | Tolerance to delay of reward; T maze | Impulsive behavior (tolerance to delay of reward) | - WIN treatment decreased large reward choices and AM251 treatment increased large reward choices in SHR. - Acute caffeine pretreatment blocked WIN effects. - Chronic caffeine treatment increased the impulsive phenotype and potentiated the WIN effects. - Cannabinoid and adenosine receptors modulate impulsive behavior in SHR. |
| Alves et al., 2020 | Rats-pregnant SHR (40–70) WKY (40–70) Female | Genetic (SHR) | In vitro | Treatment (caffeine, DMSO, LY294002, adenosine selective agonist and antagonists) Strain (WKY or SHR) | Caffeine incubation (30 µM) One dose | No behavioral task | Morphological alterations (singling, neurite branching) | - SHR neurons displayed less neurite branching, shorter maximal neurite length and decreased axonal outgrowth. - Caffeine recovered neurite branching and elongation from SHR neurons via PKA and PI3K signaling, - A R agonist (CGS 21680) promoted more neurite branching via PKA signaling. - The selective A R antagonist (SCH 58261) was efficient at recovering axonal outgrowth from SHR neurons through PI3K and not PKA signaling. |
| França et al., 2020 | Rats WKY (9) SHR (11) Male | Genetic (SHR) | 30 days 4–5 months | Treatment (caffeine or water) Physical exercise Strain (WKY or SHR) | 0.3 mg/mL, drinking water One dose | Olfactory discrimination; Open field; Object recognition; Water maze | Olfactory discrimination (time spent in compartments, numbers of crossings) Locomotor activity (total distance, time spent in the central zone) Short-term memory (total time spent exploring the objects, discrimination index) Working and procedural memories (escape latency) | - SHR showed olfactory and short-term recognition memory deficiencies from adolescence to adulthood, accompanied by lower prefrontal cortex and hippocampus SNAP-25 levels. - Caffeine and physical exercise during adolescence or adulthood repaired the olfactory discrimination ability and enhanced short-term recognition memory in SHRs. - Caffeine consumption and physical exercise during adolescence augmented hippocampus and prefrontal cortex SNAP-25, syntaxin, and serotonin levels, as well as SHRs’ striatal dopamine levels. |
Key for abbreviations used: LDLr: low-density lipoprotein receptor, LE rat: Long–Evans rat, CD rat: Cesarean-derived rat, i.p.: intraperitoneally, WKY rat: wistar Kyoto rat, SHR: spontaneously hypertensive rat, MPD: methylphenidate, 6-OHDA: 6-hydroxy-dopamine, A 2A R: Adenosine A 2A receptors, ICR mice: Institute of Cancer Research mice, SI: social isolation, PND: postnatal day, BDNF: brain-derived neurotrophic factor, SNAP-25: synaptosomal-associated protein 25.
3.1.1. Species, Animal Model, Sex, and Treatment
Most of the animal studies were performed on rodents. Ten studies were conducted with rats and two with mice. Only one of the studies used zebrafish as an animal model. Four studies used only males, five used both males and females, and four used only females. Different caffeine treatments and routes of administration were used, along with different durations ( Table 1 ). Chronic treatments were mainly performed by dilating caffeine powder in the system water, whereas acute treatments were mainly administered intraperitoneally (i.p.).
3.1.2. Animal Models of ADHD
Overall, nine studies used genetic animal models of ADHD: eight studies used SHR, and one study used the low-density lipoprotein receptor (LDLr) mouse. Finally, two studies used physical trauma to provide an epigenetic animal model of ADHD: one study caused 6-hydroxy-dopamine (6-OHDA) lesions in rats, while one study used social isolation (SI) as an intensely stressful environment in mice ( Table 1 ).
3.1.3. Behavioral Tests
Five studies used the object recognition task; four studies used the Y maze test; three studies used the open field test; two studies used the water maze test; and two studies used the novel object recognition test. In addition, other tests were performed, including the water-finding test, the five-choice serial reaction time task (5-CSRTT), the locomotor activity test, the discrimination task, the Olton maze behavioral assay, the attention set-shifting task, the fear-conditioning test, the tolerance to delay of reward task, and the olfactory discrimination test. Finally, one study induced the animals to a certain type of stress by means of social isolation and aggressivity ( Table 1 ).
3.2. Study Outcomes
The results are summarized in Table 1 . Considering the amount of data provided in the reviewed articles, we decided to categorize all the information based on caffeine’s effects on each relevant ADHD-like evaluated parameter, as follows.
3.2.1. Attention
Attention and behavioral flexibility.
Pandolfo et al. [ 51 ] examined the impact of chronic caffeine treatment during adolescence on SHR and Wistar Kyoto (WKY) rats’ performance in an attention set-shifting task, placing emphasis on response to conflict. The task was divided into different phases: familiarization, response discrimination, and visual cue discrimination. During the response discrimination phase, statistical analysis showed that vehicle-treated SHR needed a superior amount of trials to reach the benchmark of 10 consecutive correct choices, compared with WKY rats. Importantly, treatment with caffeine (2 mg/kg, i.p.) improved SHR discriminative learning in a selective manner, as indicated by a reduction in the number of trials needed to reach the benchmark, while treatment with caffeine had no effect on WKY rats. During the visual cue discrimination phase, SHR required more trials to master the task, compared with WKY rats. Once more, caffeine treatment (2 mg/kg, i.p.) diminished the number of trials needed to reach the benchmark. Finally, statistical analysis showed that vehicle-treated SHR made significantly more regressive and never-reinforced errors than vehicle-treated WKY rats. Remarkably, while treatment with caffeine (2 mg/kg, i.p.) diminished the number of these errors in SHR, it had no effect on WKY rats.
Spatial Attention
Ouchi et al. [ 52 ] tested the effect of SI on latent learning using the water-finding test, measuring entering latency and drinking latency. The authors eventually discussed the utility of SI as an ADHD epigenetic model. They socially isolated male or female Institute of Cancer Research (ICR) mice for one week or more. Subsequently, the animals displayed spatial attention deficit during the water-finding task. Five weeks of resocialization following one week of SI failed to improve this deficit. Drinking latency depended on how much attention the animal paid to environmental factors, including the location of a tap water nozzle, which they were exposed to in the training trial. Therefore, a decrease in drinking latency correlated with the animal remembering the position/location of the nozzle. Caffeine (0.3–1 mg/kg, i.p.) induced changes in drinking latency on the water-finding test, in this sense, significantly ameliorating SI-induced latent learning deficits in a dose-dependent manner, independently of gender or age.
Caballero et al. [ 53 ] examined caffeine’s therapeutic use in neonatal 6-OHDA lesioned rats, which constitute another existing ADHD animal model. At postnatal day (PND) 7, the rats were lesioned at the left striatum with 6-OHDA. At PND 25, spatial attention was measured with an eight arm radial maze, the Olton maze. The animals were then placed in the maze. The total number of arms the animals walked before completing six out of eight, or until they repeated one of them, was measured. After 14 days of treatment with caffeine, administered ad libitum into the drinking water, the authors assessed caffeine’s effects on the attention deficit of the animals, using the same task. Interestingly, the 6-OHDA lesioned rats significantly improved their attention deficit after caffeine treatment. Consequently, the authors highlighted the properties through which caffeine managed the attentional deficits occurring during the prepubertal period of ADHD.
Discrimination
Ruiz-Oliveira et al. [ 54 ] evaluated the effect of caffeine on zebrafish performance in a task requiring focus and attention, the discrimination task. The task took place in three phases: tank acclimation, training, and test. The authors used visual cues during the training trials and the test trials. Distractors, objects resembling the target, were used to confuse the fish and impair conditioning. The fish were exposed to different caffeine concentrations for 14 days: 0 mg/L (control), 10 mg/L (low), and 50 mg/L (high). Notably, low caffeine doses improved the fishes’ ability to discriminate the cues and reach the target; the fish spent most of the time close to the target where the reward was offered, and showing the shortest latency to reaching the target. The higher dose impaired the fishes’ ability to find the target; the fish demonstrated increased anxiety, a possible side effect of the substance.
Selective Attention
Higgins et al. [ 55 ] evaluated the effect of caffeine on Long–Evans (LE) and Cesarean-derived (CD) rat performance in a selective attention task, the 5-CSRTT. The effects of caffeine were compared to the selective A 2A antagonists, SCH 412348 and KW-6002, and the A 1 antagonist, DPCPX. Caffeine (3–10 mg/kg, i.p.) increased reaction time in both LE and CD rats, with no effect on accuracy, an effect replicated by SCH 412348 (0.1–1 mg/kg PO) and KW-6002 (1–3 mg/kg PO), but not DPCPX (3–30 mg/kg PO). The faster response speed was observed in both the CD and LE rat strains at 3 mg/kg, although increased premature responses were confined to the LE strain at the 10 mg/kg dose. These results suggest that the attention-enhancing effects of caffeine were mediated through A 2A receptor blockade. Selective A 2A receptor antagonists may therefore have potential as therapies for attention-related disorders, such as ADHD.
3.2.2. Hyperactivity and Impulsivity
Locomotor activity.
França et al. [ 56 ] tested the effect of caffeine on the hyperlocomotion characteristic of ADHD by examining locomotor activity. Caffeine consumption (0.3 mg/mL in drinking water) and physical exercise in running wheels for 6 weeks, either during adolescence (30 days old) or adulthood (4–5 months old), did not relate to changes in spontaneous locomotion in SHR, in an open field, during the 5 min habituation phase of the object recognition test. Ruiz-Oliveira et al. [ 54 ] studied the effects of caffeine on zebrafish (4 months old, wild type, both sexes). Low concentrations of caffeine (10 mg/L) affected locomotor parameters, increasing average speed and decreasing freezing behavior. Interestingly, the levels of freezing and locomotor behavior were the same for the 50 mg/L caffeine group and the control group. Nunes et al. [ 57 ] evaluated locomotor activity during the late childhood and the end of adolescence of male and female SHR, using an open field arena and measuring the total distance travelled in meters along the periphery during 5 min. Although caffeine (0.3 g/L) did not impact hyperlocomotion during late childhood (PND 28) in either sex, continuous treatment aggravated adolescent female SHR hyperactivity (PND 50), suggesting that the consumption of caffeine during childhood may aggravate hyperactivity in females, but only if the administration persists up to adolescence. Szczepanik et al. [ 58 ] demonstrated that young (3 months old) and middle-aged (8 months old) LDLr mice display different responses to chronic caffeine treatment in terms of motor activity. Although caffeine was unable to modify the hyperlocomotion observed in 3 months old LDLr mice, caffeine attenuated the increased locomotor activity observed in 8 months old LDLr mice. Pandolfo et al. [ 51 ] tested whether chronic treatment with caffeine was able to counteract the hyperlocomotion characteristic of ADHD in SH, during the open field test. Chronic treatment with caffeine did not alter central and total locomotion in SHR. Similarly, Pires et al. [ 59 ] showed that chronic treatment with caffeine did not produce changes in SHR locomotion during the object recognition task sample phase. Interestingly, Caballero et al. [ 53 ] showed that neonatal 6-OHDA lesioned rats, a different ADHD animal model, demonstrated a non-significant tendency to decrease their motor activity after ad libitum caffeine consumption throughout the prepubertal period during an Olton maze behavioral assay. Higgins et al. [ 55 ] conducted two separate types of locomotor activity study. In a CGS-21680-induced hypolocomotion assay, pretreatment with caffeine (3–30 mg/kg, i.p.) produced a significant attenuation of the CGS-21680 hypolocomotion at different doses, of 10 mg/kg and 30 mg/kg, in CD rats. In a second experiment, caffeine (1–30 mg/kg, i.p.) produced a dose-related increase in locomotion in the animals habituated to the test chambers. Finally, Prediger et al. [ 60 ] did not find a direct increase in locomotor performance in SHR after the administration of acute doses of caffeine (1–10 mg/kg i.p.) when using a spatial version of the Morris water maze. No alteration was observed in the swimming speed in this regard.
Impulsive Behavior
Leffa et al. [ 61 ] focused on impulsive behavior to clarify the neurobiology of ADHD. They treated SHRs with caffeine, a non-selective adenosine receptor antagonist, to assess the modulating effects of the adenosine systems on tolerance to the delay of a reward. The animals had to choose between a small, but immediate, or a large, but delayed, reward. An acute pretreatment with caffeine (2 mg/kg or 5 mg/kg) increased number of large-reward choices. Conversely, chronic treatment with caffeine (2 mg/kg, for 21 days) augmented the impulsive phenotype and decreased the number of large-reward choices.
3.2.3. Learning and Memory
Non-associative learning.
Habituation is a form of non-associative learning in which the animal’s innate response to a stimulus decreases after prolonged or repeated presentations of this stimulus. Nunes et al. [ 57 ] analyzed habituation during late childhood and the end of adolescence in male and female SHRs. The authors observed a sex and age difference in habituation, with female SHRs showing lack of habituation from childhood onwards, and male SHRs showing a lack of habituation in adolescence. These difficulties observed in female habituation, however, were overturned by treatment with caffeine (0.3 g/L) during childhood.
Working Memory
The object recognition task is recognized as a working-memory task, relies on the animal’s natural tendency for novelty, and tests the ability to discriminate between familiar and unfamiliar objects. França et al. [ 56 ] assessed working memory using an adapted version of the object recognition task, conducted in an open field during three different phases: habituation, sample and discrimination. Although the study results indicated that the disruption of the short-term recognition memory persisted into adulthood, the association of caffeine (0.3 mg/mL) and exercise during adulthood and adolescence improved short-term recognition memory in the SHR strain. Nunes et al. [ 57 ] also carried out the novel object recognition test and observed similar recognition memory disturbances in adolescent SHRs of both sexes. Nonetheless, caffeine intake (0.3 g/L) restricted to childhood restored recognition memory in adolescent SHRs of both sexes. To evaluate the potential of caffeine in ADHD therapy, Pires et al. [ 59 ] treated female WKY rats and SHR with caffeine (3 mg/kg, i.p.) for 14 consecutive days during the prepubertal period. The animals were tested during the object recognition test in the course of adulthood. While WKY rats discriminated between all the used objects, the SHRs were unable to differentiate between pairs of objects with subtle structural differences. Nonetheless, caffeine or MPD chronic treatment improved the deficits in object recognition in SHR. Pires et al. [ 62 ] showed, for the first time, the significant impairment of SHRs’ short-term object-recognition ability in comparison with WKY rats. They further investigated the effects of caffeine (1, 3 or 10 mg/kg), 30 min before the sample phase, on the performance of WKY rats and SHR of both sexes in the object recognition task. The injection of caffeine (1, 3 or 10 mg/kg, i.p.) improved the discrimination index of female SHRs, while the highest tested dose of caffeine (10 mg/kg, i.p.) increased the discrimination index of male SHRs.
Spatial Learning
The water maze task is a behavioral procedure widely used with rodents to study spatial learning or spatial memory. Prediger et al. [ 60 ] used a circular swimming pool to assess the effect of caffeine administration on spatial learning deficit in SHRs. Adult female WKY rats and SHRs were treated with caffeine (1–10 mg/kg i.p.) before or immediately after training, or before the test session. Spatial learning deficit in SHR was improved through the pre-training administration of caffeine (1–10 mg/kg i.p.). SHR test performance was not altered by the post-training administration of caffeine (3 mg/kg i.p.), although WKY rats’ memory retention was increased. Although França et al. [ 25 ] observed procedural memory impairment in adolescent SHRs during a cued version of the water maze, these normalized in adulthood.
Spatial Short-Term Memory
Given the willingness of rodents to explore new environments, the Y-Maze Test is widely used for testing the conditions affecting memory and learning. Pandolfo et al. [ 51 ] assessed SHRs’ spatial short-term memory, using a Y-maze paradigm. When compared with WKY rats, the control group SHRs displayed a spatial learning deficit. Importantly, treatment with caffeine (2 mg/kg, i.p.) during adolescence improved SHR memory impairment. Nunes et al. [ 57 ] evaluated spatial memory in male and female SHRs using the Y-maze task at PND 53. Female SHRs showed worsened spatial memory. Although caffeine (0.3 g/L) showed effectiveness against recognition memory deficiency in males and females, only female SHRs increased the number of entries in the novel arm following caffeine treatment, from PND 15 to 55, and showed spatial memory recovery.
3.2.4. Olfactory Discrimination
França et al. [ 56 ] assessed the effects of caffeine consumption (0.3 mg/mL) and physical exercise on running on wheels over 6 weeks, during either adolescence (30 days old) or adulthood (4–5 months old), by means of SHR during the olfactory discrimination test. Besides providing the first evidence of deficits in olfactory discrimination in both adolescent and adult SHRs, the authors showed how caffeine, together with physical exercise, was able to restore olfactory discrimination ability in these animals during adolescence or adulthood.
3.2.5. Blood Pressure
França et al. [ 56 ] measured systolic blood pressure using the tail-cuff method in a non-invasive manner. For animals treated during adolescence, the systolic arterial pressure was measured before (basal values) and 14, 28, and 42 days after beginning the treatment, before the behavioral tests. For the rats subjected to caffeine treatment and physical exercise during adult life, two measurements were taken, one before the protocols (basal values) and the other after the last behavioral task. Notably, the hypertensive phenotype was not significantly altered by caffeine (0.3 mg/mL) or exercise. When applied from adolescence, caffeine and exercise had no effect on the development of hypertension and at 42 days of treatment (72 days of age), all the SHRs were hypertensive. For adult animals that were already hypertensive at the beginning of the treatment, no further significant differences between groups were observed. To investigate whether the SHRs’ cognitive deficits could be directly associated with hypertension, Pires et al. [ 59 ] measured the effects of chronic caffeine administration (3 mg/kg, i.p.) during the prepubertal period on the arterial blood pressure of adult female WKY rats and female SHRs. The SHRs were hypertensive in comparison to the WKY control rats. The chronic administration of caffeine during the prepubertal period, at the same doses that reversed the cognitive deficits of adult SHR (3 mg/kg, i.p.), did not cause significant changes in blood pressure values in adulthood SHR and WKY rats. Again, Pires et al. [ 62 ] measured blood pressure after caffeine treatment to investigate whether the cognitive deficits of SHR could be directly related with hypertension. Accordingly, the arterial blood pressure (mmHg) of female WKY rats and female SHRs were measured 30 min after treatment with caffeine (1, 3, or 10 mg/kg, i.p.). As expected, the SHRs were hypertensive in comparison with the WKY control rats. However, the administration of the same doses of caffeine, which was able to improve the object discrimination deficits of the SHRs, did not significantly alter the mean arterial pressure of either the WKY rats or the SHRs. In a similar vein, Prediger et al. [ 60 ] measured the arterial blood pressure (mm Hg) of adult female WKY rats and SHRs 30 min after the injection of caffeine (1, 3 or 10 mg/kg, i.p.). Although the SHRs presented a significantly higher mean arterial pressure compared to the WKY control rats, treatment with caffeine did not significantly alter the mean arterial pressure of either the WKY or SHR groups. Caffeine was consequently able to improve the spatial learning deficits of the SHRs without varying their hypertensive state, showing that cognitive impairment in SHR might not be entirely explained by hypertension.
3.2.6. Body Weight
Pires et al. [ 59 ] measured the effects of chronic caffeine treatment during the prepubertal period on the body weight of juvenile and adult female WKY rats and female SHR. The body weights of the WKY rats and SHRs was were accordingly measured every 2 days during the treatment (14 days) with caffeine (1, 3, or 10 mg/kg, i.p.). The body weight of the adult rats also recorded during the performance of the object recognition task. Statistical comparisons indicated that juvenile rats from the SHR strain presented significantly lower mean body weight than the juvenile WKY rats. Notably, chronic treatment with caffeine did not alter the body weight of the evaluated rat strains. During adulthood, similar results for the body weight of the animals were found. Although significant strain differences were observed, chronic treatment with caffeine throughout the prepubertal period did not alter the final body weight of the animals in adulthood (regardless of strain). Likewise, Pandolfo et al. (2013) [ 51 ] found no weight differences among groups following caffeine treatment (2 mg/kg, i.p.).
3.2.7. Neurobiology
Brain levels of synaptosomal-associated protein-25.
França et al. [ 56 ] evaluated the effects of caffeine consumption (0.3 mg/mL in drinking water) and physical exercise on running wheels by measuring the brain levels of monoamine, using high-performance liquid chromatography, for 6 weeks. Regarding prefrontal cortex SNAP-25 levels, a statistical analysis revealed a significant increase in SNAP-25 levels in the prefrontal cortex in the group submitted to the combination of caffeine consumption with physical exercise. Regarding hippocampus SNAP-25 levels, the statistical analysis indicated a significant increase in hippocampal SNAP-25 levels selectively in animals submitted to the combination of caffeine consumption with physical exercise.
Brain Levels of Syntaxin
SNAP-25 is a component of the soluble N-ethylmaleimidesensitive factor attachment protein receptor (SNARE) complex, which is critical in regulating synaptic vesicle fusion and neurotransmitter release, along with syntaxin 1. Regarding prefrontal cortex syntaxin levels, a statistical analysis performed by França et al. (2020) [ 56 ] revealed a significant increase in syntaxin levels in the prefrontal cortex selectively in the group submitted to the combination of caffeine consumption and physical exercise. Regarding hippocampus syntaxin levels, the statistical analysis indicated a main effect of treatment with a marginal effect for treatment versus exercise interaction.
Brain Levels of Serotonin
França et al. [ 56 ] measured the effects of caffeine consumption and physical exercise throughout adolescence on serotonin (5-hydroxytryptamine, 5-HT) through high-performance liquid chromatography (HPLC). A statistical analysis performed by the authors showed that the combination of caffeine consumption and physical exercise during adolescence increased 5-HT levels in the prefrontal cortex of SHRs. Concerning hippocampal 5-HT levels, statistical comparisons showed that caffeine consumption and physical exercise, alone or in combination, significantly augmented hippocampal 5-HT levels.
Brain Levels of Dopamine
França et al. [ 56 ] evaluated dopamine levels in the prefrontal cortex, hippocampus, and striatum by using HPLC. Dopamine levels were not detectable in the hippocampus. Although a significant effect of treatment was observed in the prefrontal cortex, no significant effects were observed for exercise or their interaction. Statistical comparisons indicated no significant differences between groups in the levels of dopamine in the prefrontal cortex. Notably, the statistical analysis revealed significant effects of treatment, exercise, and their interaction on striatal dopamine levels. Subsequent statistical comparisons showed that caffeine intake and physical exercise, alone or in combination, significantly augmented striatal dopamine levels.
Dopamine Transporter Density
Pandolfo et al. [ 51 ] examined if the cognitive and attentional deficits of SHR and their attenuation by caffeine treatment were associated with alterations in the density of DAT in frontocortical and striatal terminals. The number of animals analyzed was four in the WKY control group, four in the WKY caffeine-treated group, three in the SHR control group, and four in the SHR caffeine-treated group. Statistical analysis showed a significant effect of the interaction between strain and treatment in the density of DAT in striatal and frontocortical synaptosomes. Consequently, DAT density was increased in both SHR brain areas of SHR and, significantly, caffeine treatment (2 mg/kg) during adolescence attenuated this enhanced DAT density in both brain areas of the SHRs, while caffeine treatment had no effect on the WKY rats.
Dopamine Uptake
Pandolfo et al. [ 51 ] tested whether a higher frontocortical density of DAT in SHR was complemented by an augmented uptake of dopamine. The authors directly measured dopamine uptake by synaptosomes. The number of animals was four per group. Both frontocortical and striatal synaptosomes from the SHRs took up almost the double amount of ( 3 H) dopamine during the 3 min incubation period than the synaptosomes from the WYK rats. Remarkably, chronic treatment with caffeine (2 mg/kg, i.p.) significantly reduced the dopamine uptake by synaptosomes from both brain areas in the SHRs when compared to vehicle-treated SHRs, while caffeine had no effect on the WKY rats.
AdenosineA 2A Receptor Density
The effects of chronic caffeine intake are generally attributed to the antagonism of A 2AR . Consequently, Pandolfo et al. [ 51 ] compared the density of A 2AR in striatal and frontocortical terminals from SHR or WKY rats treated with caffeine or saline. The number of animals analyzed was four in the WKY control group, three in the WKY caffeine-treated group, four in the SHR control group, and four in the SHR caffeine-treated group. Statistical analysis indicated a significant effect of the interaction between strain and treatment on A 2AR density both in the striatum and in the frontal cortex. Notably, fronto-cortical nerve terminals in the SHRs displayed more colocalization between A 2AR and synaptophysin immunoreactivities than in the WKY rats. This provided the first direct demonstration of the presence of A 2AR in fronto-cortical nerve terminals, and the first indication that A 2AR density is improved in SHRs.
Colocalization of Dopamine Transporter and Adenosine A 2A Receptors
Chronic treatment with caffeine is proposed to operate through A 2AR and was shown to affect DAT density and function. Pandolfo et al. [ 51 ] proved the colocalization of A 2AR and DAT in striatal and frontocortical nerve terminals. The number of animals analyzed was three in the WKY control group, four in the WKY caffeine-treated group, three in the SHR control group and three in the SHR caffeine-treated group. In the striatum, statistical analysis revealed a significant effect of strain on the colocalization of A 2AR and DAT immunoreactivities, and a subsequent comparison exhibited that nerve terminals from vehicle-treated SHR displayed a significantly lower colocalization of A 2AR and DAT in comparison with vehicle-treated WKY. In the frontal cortex, a statistical analysis revealed no significant effect of strain or treatment on the colocalization between A 2AR and DAT.
Brain-Derived Neurotrophic Factor
Nunes et al. [ 57 ] examined the effects of caffeine (0.3 g/L) administered from childhood onwards in the BDNF and its related proteins in both sexes of SHR rats. BDNF and its related proteins were therefore evaluated in the hippocampus of WKYs and SHRs of both sexes at PND 55. A statistical analysis revealed a significant effect of strain on BDNF levels, while the precursor form (proBDNF) remained unaltered. The TrkB receptor full length (TrkB-FL), phospho-TrkB, and truncated-form TrkB receptors were immunodetected in the hippocampuses of the WKYs and SHRs of both sexes. A statistical analysis revealed a significant effect of strain on the truncated form and also on phospho-TrkB. Furthermore, the transcription factor CREB was not altered either by strain or sex, although its phosphorylated form (phospho-CREB) was increased in the SHR hippocampus from both sexes. Finally, Nunes et al. [ 57 ] evaluated the impact of caffeine only on the BDNF levels and TrkB receptors (TrkB-FL, phospho-TrkB, and TrkB-T). Caffeine administered from PND 15 up to PND 55 (caff/caff) reduced the BDNF levels in the hippocampuses of SHR male rats, whereas the BDNF levels were unaltered in the SHR female rats in both schedules of treatment. In the male rats, caffeine in both schedules of treatment did not change either TrkB-FL or TrkB-T levels, whereas female SHRs showed reduced TrkB-FL and TrkB-T forms as a consequence of caffeine treatment. Neither the increased phospho-TrkB nor the CREB were modified in the hippocampuses of the SHRs following caffeine treatment.
Neuronal Development In Vitro
Alves et al. [ 63 ] investigated caffeine’s in vitro effects at the neuronal level. At first, SHR and WKY rats’ cultured frontal cortical neurons were immunostained for MAP-2 during in vitro development. Later on, somatodendritic analyses were performed, measuring branch point number, root number, and maximal and total neurite length. Neurons from the SHRs displayed fewer differentiation patterns, including neurite branching, shorter maximal neurite length, and decreased axonal outgrowth. Following a 24 h period of caffeine incubation (30 μM), the SHR neurons showed an inferior percentage of zero branch points, and a superior percentage of two branch points. A trend toward a superior percentage of one-branch-point-neurons was observed for SHR neurons following treatment with caffeine. Caffeine also promoted a rise in the total and maximal neurite length in neurons from both strains. PKA or PI3K inhibitor were subsequently used to study whether one of the transducing systems activated by adenosine receptors, and in the neuronal differentiation, are responsible for the effects produced by caffeine. PKA inhibitor KT5720 (5 μM) did not change caffeine’s ability to augment the percentage of SHR neurons with more branch points. Caffeine’s effect on the recovery of the total neurite length of the SHR neurons was obstructed by PKA inhibitor. Comparable results were seen for maximal neurite length, in which PKA inhibitor completely decreased caffeine’s effects. Finally, LY294002 (50 μM) was used as an inhibitor of PI3K and its presence blocked caffeine’s effect on the increase in the number of branch points in SHR neurons. Furthermore, caffeine’s effect on the prevention of reductions in the total neurite length were eliminated in the presence of PI3K inhibitor. Similar results were found for the maximal neurite length. The number of roots was also reduced by PI3K inhibitor in SHR neurons.
4. Discussion
ADHD is characterized by symptoms including attention deficits, impulsivity, and hyperactivity [ 3 , 4 ] that frequently persist throughout life [ 1 , 2 , 6 ]. Prefrontal cortex function modulation and attentional/behavioral regulation depends on the optimal release of signalling molecules such as NE, DA [ 24 , 25 ], as well as 5-HT, GLU, or ACh [ 26 , 27 , 28 ]. In this respect, genes, including the DAT or the DRD4 [ 31 , 32 ] or the SERT, the SNAP-25, and the BDNF [ 29 , 30 ], might play a role in causing ADHD. Therefore, agents that can lead to the optimal balance of these organic compounds are hypothetically beneficial in patients with ADHD by mainly returning prefrontal activity to adequate functional levels [ 18 , 33 ]. In this sense, it has long been discussed whether caffeine could become an effective pharmacological compound for the management of symptoms of ADHD [ 64 , 65 ].
This systematic review analyzed 13 animal studies that investigated the effects of caffeine on the modulation of ADHD-like symptoms. Overall, the reviewed results show that caffeine treatment increases attention and improves learning, memory, and olfactory discrimination without altering blood pressure and body weight.
Regarding attention, caffeine treatment improved the attentional and behavioral flexibility of SHRs [ 51 ], the spatial attention of 6-OHDA lesioned rats [ 53 ], and SI in ICR mice [ 52 ] during adolescence. Caffeine treatment improved the reaction time of LE and CD rats [ 55 ] and focus and attention in zebrafish [ 54 ] during adulthood.
Regarding learning and memory, caffeine treatment plus physical exercise during adulthood and adolescence improved working memory in SHRs [ 56 ]. In the same vein, caffeine treatment alone restored non-associative learning in female SHRs [ 57 ], improved working memory in SHRs [ 59 ], female SHRs [ 62 ], and adolescent SHRs [ 57 ]. The administration of caffeine improved spatial learning deficit in SHRs, increased memory retention in WKY rats [ 60 ], and improved spatial short-term memory in SHRs [ 51 ] and female SHRs [ 57 ].
Concerning olfactory discrimination, caffeine treatment, together with physical exercise, was able to restore olfactory discrimination in SHRs during adolescence or adulthood [ 56 ]. Concerning blood pressure, caffeine treatment did not alter the hypertensive phenotype in SHR [ 60 , 62 ] during adolescence or adult life [ 56 ], nor during the adult female SHR prepubertal period [ 59 ]. Finally, caffeine treatment did not alter body weight in SHRs [ 51 , 59 ].
If we are ever to acquire a truly in-depth understanding of ADHD pharmacotherapy, we need to face the following question: Does caffeine deserve a place in the battery of pharmacological agents for ADHD treatment, particularly during adolescence? Although previous meta-analyses [ 64 ] and reviews [ 65 ] were unable to provide any recommendations for adolescents diagnosed with ADHD, due to a lack of data, our reviewed results provide updated preclinical evidence and support the therapeutic potential of caffeine to improve attention, learning, memory, or olfactory discrimination in ADHD, especially during adolescence.
Beyond its clear effects on improving performance in tasks requiring attention, learning, memory and olfactory discrimination, without altering blood pressure and body weight, the implication of caffeine in modulating ADHD-like hyperactivity symptoms remains controversial. Indeed, caffeine treatment plus physical exercise did not affect locomotor activity in SHRs [ 56 ]. In a similar manner, caffeine treatment alone did not alter locomotion in SHR [ 51 , 59 , 60 ], preadolescent SHR [ 57 ], or young LDLr mice [ 58 ]. Nonetheless, caffeine treatment did increase locomotor activity in adolescent female SHRs [ 57 ], zebrafish [ 54 ]. Furthermore, it produced an increase related to dose in locomotion in CD rats and a significant attenuation of CGS-21680-induced hypolocomotion in CD rats [ 55 ], and it attenuated locomotor activity in middle-aged LDLr mice [ 58 ] and 6-OHDA lesioned rats throughout the prepubertal period [ 53 ]. This apparent discrepancy may have resulted from caffeine ‘s promotion of different effects according to age and sex. In this regard, Nunes et al. [ 57 ] suggested that the intake of caffeine from the childhood period onwards may aggravate hyperactivity in females, if the consumption continues up to the adolescence period. Szczepanik et al. [ 58 ] linked the age-dependent effect induced by caffeine with the idea that the blockade of adenosine A 1 /A 2A receptors attempts to renormalize a potentially maladaptive system [ 66 ], with age an important escalating factor in mice. In a different study, Ruiz-Oliveira et al. [ 54 ] proposed that caffeine-induced bursts of locomotion may be caused by a decrease in fatigue [ 67 ] rather than by an anxiogenic response. Importantly, the attenuation of motor activity by caffeine consumption was determined as a natural effect of growth rather than an effect of caffeine intake by Caballero et al. [ 53 ].
In terms of impulsivity, although acute pretreatment with caffeine increased the number of large-reward choices made by SHRs, chronic treatment with caffeine increased the impulsive phenotype and decreased choices of large rewards by SHRs [ 61 ]. This discrepancy may be explained by previous studies performed on animal models of brain diseases, showing that while acute treatment acts mainly on A 1 receptors, chronic treatment acts mainly on A 2A receptors [ 68 ]. Leffa et al. [ 61 ], in this direction, underscored the ability of the adenosine modulation system to control behavioral inhibition.
Besides reviewing animal studies deciphering the effects of caffeine in the modulation of ADHD-like symptoms, we reviewed for the first time animal studies examining the effects of caffeine and adenosine receptors on neurons isolated from SHRs, at the neuronal level.
In this respect, treatment with caffeine and physical exercise during the adolescence period augmented the quantity of SNAP-25, syntaxin, and serotonin in the prefrontal cortex and the hippocampus, as well as striatal dopamine quantity, in SHRs [ 56 ]. In a similar manner, caffeine treatment alone during the adolescence period attenuated the improvement in DAT density in the fronto-cortical and striatal terminals of SHRs and diminished the dopamine uptake by synaptosomes from SHRs’ fronto-cortical and striatal terminals [ 51 ]. Furthermore, Pandolfo et al. [ 51 ] demonstrated that fronto-cortical nerve terminals are provided with AdenosineA 2A receptor, the target of chronic caffeine exposure, whose density was found to be increased in SHRs. Caffeine treatment normalized BDNF levels in the hippocampuses of SHR males, while the same treatment normalized TrkB receptors TrkB-FL and TrkB-T SHR in the hippocampuses of SHR females [ 57 ]. Finally, neurons from SHRs showed an inferior number of zero-branch points, and a superior number of two-branch-points-neurons following in vitro caffeine treatment consisting of 24 h of caffeine incubation. After treatment with caffeine, an increase in the total and maximal neurite length and a tendency toward a superior number of one-branch-point neurons was also observed for SHR neurons. The effect of caffeine on increasing maximal neurite length, and on recuperating the entire neurite length of neurons from SHR, was entirely blocked by PKA inhibitor. LY294002, as an inhibitor of PI3K, blocked caffeine’s effects on the increase in the amount of branch points in SHR neurons. Finally, the effect of caffeine on the prevention of reductions in the total neurite length, increasing maximal neurite length, and the number of roots was eradicated by the presence of PI3K inhibitor in SHR neurons [ 63 ].
5. Conclusions
Overall, our reviewed data suggest that caffeine is a possible adjuvant pharmacological strategy for the treatment of ADHD. The compiled preclinical data support the notion that caffeine improves ADHD-like symptoms of inattention and its related learning and memory impairments without affecting blood pressure and body weight. Our results are supported at the neuronal/molecular level, and strengthen the hypothesis that the cognitive effects of caffeine found in animal models of ADHD could be translated to humans diagnosed with the disorder, particularly during adolescence. Nonetheless, caution is needed when extrapolating potential effects identified in animal studies to human patients. In this work, studies that explored caffeine’s effects on locomotor activity and impulsivity were contradictory, raising discrepancies that require further clarification. Although we consider that the reviewed results in this manuscript can potentially impact the scientific, pre-clinical, and clinical community and expand our knowledge regarding ADHD, more studies should be performed to validate our present knowledge while offering prospective clues to support caffeine as a therapeutic approach for the treatment of ADHD.
Acknowledgments
We thank William J. Giardino from Stanford University for helpful comments on this manuscript.
Author Contributions
J.C.V. contributed significantly to the conception and design of the manuscript, the acquisition of data, and data analysis and interpretation, agreeing to be responsible for all aspects of the work in ensuring that questions related to the accuracy or veracity of any part of the work are appropriately investigated and resolved. J.C.V., J.L.P., O.M.d.l.T. and D.R.-R. drafted the manuscript. D.R.-R. provided critical revision of the manuscript and final consent of the version to be published. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no potential conflict of interest regarding the research, authorship, and/or publication of this article.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
Introduction. C affeine is the most widely used drug in the world. 1 In the United States, more than 90% of adults use it regularly, and, among them, average consumption is more than 200 mg of caffeine per day 2 —more caffeine than is contained in two 6-ounce cups of coffee or five 12-ounce cans of soft drinks. 3,4 Although consumption of low to moderate doses of caffeine is generally safe ...
Meredith et al. present a thorough review of the evidence in support of caffeine dependence and describe an agenda for future research on clinical, epidemiological, and genetic investigations of caffeine dependence and caffeine use disorder. Of note, evidence is described in support of the 3 primary criteria for caffeine use disorder, and 3 ...
Introduction. Caffeine is psychoactive constituent of various widely available products e.g. Cola, energy drinks, chocolates, tea, coffee, analgesics, etc. It is most widely used (80% population world-wide) psychoactive substance (Silva et al., 2014, Ogawa and Ueki, 2007). In US 61% of general population consumed average 210-238 mg/day (Dews ...
Habitual coffee drinkers display a distinct pattern of brain ...
Caffeine as a Factor Influencing the Functioning ...
Caffeine has both positive and negative effects on this population and further research is necessary to better understand the long-term consequences of caffeine consumption. Caffeine use, dependence, and addiction are common among government HCPs in KSA. Caffeine has both positive and negative effects on this population and further research is ...
Rationale: Although reports of caffeine withdrawal in the medical literature date back more than 170 years, the most rigorous experimental investigations of the phenomenon have been conducted only recently. Objectives: The purpose of this paper is to provide a comprehensive review and analysis of the literature regarding human caffeine withdrawal to empirically validate specific symptoms and ...
About 90% of adults in the United States use caffeine regularly, says Griffiths, and their average consumption exceeds 200 milligrams of caffeine per day — more caffeine than is contained in two 6-ounce cups of coffee, or five 12-ounce cans of soft drinks. This latest research study, notes Sweeney, is the most thorough evaluation to date of ...
Findings support shift in future research to unhealthy populations, sensitive populations and interindividual variability. ... (Nawrot et al., 2003). Since then, >10,000 papers have been published related to caffeine, including hundreds of reviews on specific human health effects; however, to date, none have compared the wide range of topics ...
Background: The DSM-5 recognizes caffeine use disorder as a condition for further study, but there is a need to better understand its prevalence and clinical significance among the general population. Methods: A survey was conducted among an online sample of 1006 caffeine-consuming adults using demographic quotas to reflect the U.S. population. Caffeine consumption, DSM-proposed criteria for ...
This review examines the effects caffeine has on cognitive and physical function, since most real-world activities require complex decision making, motor processing and movement. Caffeine exerts its effects by blocking adenosine receptors. Following low (∼40 mg or ∼0.5 mg kg −1) to moderate (∼300 mg or 4 mg kg −1) caffeine doses ...
Addictive potential of caffeine has long been reported, still there is lack of awareness about caffeine abuse in India. There is an intense need for appropriate public health regulatory measures and awareness about addictive potential & harms related to caffeine. To the best of our knowledge this is first case from India highlighting several ...
Psychiatry and clinical neurosciences. 2007. TLDR. It is proposed that companies or businesses manufacturing or marketing caffeine or products containing caffeine must meet the following guidelines: clearly indicate the caffeine content of products containing comparatively higher quantities of caffeine, warn that such products should be avoided ...
Caffeine use disorder: a comprehensive review and research agenda. J Caffeine Res. 2013;3:114-30. Meredith et al. present a thorough review of the evidence in support of caffeine dependence and describe an agenda for future research on clinical, epidemiological, and genetic investigations of caffeine dependence and CUD.
Caffeine is one of the world's most consumed drugs, and is consumed in various forms such as coffee, energy drinks, soda, or chocolate. According to the Washington Post (2015), two billion cups of coffee are consumed per day worldwide . Caffeine consumption affects the cognitive function of its consumers in a variety of different ways.
caffeine use may hav e on the physical and mental health. of children and adolescents. In the United States, nearly 75% of children under the. age of 18 consume caffeine on any giv en day [4,5 ...
Caffeine is the most widely used drug in the world. In the United States, more than 90% of adults use it regularly, and, among them, average consumption is more than 200 mg of caffeine per day - more caffeine than is contained in two 6-ounce cups of coffee or five 12-ounce cans of soft drinks. Although consumption of low to moderate doses of ...
Background University students use caffeine to cope with stress in spite of its adverse effects. The purpose of this study is to explore caffeine consumption among university students in Saudi Arabia, as well as its correlation with stress and caffeine intoxication. This cross-sectional study examined a convenience sample of 547 students at Princess Nourah Bint Abdulrahman University (PNU). A ...
1. Introduction with a brief history of caffeine consumption. Caffeine (1,3,7-trimethylxanthine) is a psychostimulant purine-like alkaloid, which is found naturally in coffee, tea, cacao beans (source for chocolate and cocoa) guarana, mate, and kola nuts, though it has been identified in more than 60 plant species [1,2].It has been consumed for thousands of years by humans with stories ...
In addition to 5% caffeine, the just-dropped product contains niacinamide (to target skin texture), hyaluronic acid (for hydration) and ginseng (to help energize and balance the complexion).
BBC tennis correspondent Russell Fuller looks at the team and the sacrifices behind "absolute tennis keeno" Jack Draper's run to the US Open semi-finals.
College students use very high doses of caffeine, an average of over 800 mg/day, which is approximately double the recommended safe dosage [3]. The short-term and long-term effects of caffeine on the human body have been studied. Research to date has primarily focused on caffeine's exacerbation of anxiety, sleep disorders, and depression in ...
กาเฟอีน - วิกิพีเดีย ... กาเฟอีน
Go to: Caffeine use is increasing worldwide. The underlying motivations are mainly concentration and memory enhancement and physical performance improvement. Coffee and caffeine-containing products affect the cardiovascular system, with their positive inotropic and chronotropic effects, and the central nervous system, with their locomotor ...
Coffee is one of the most widely consumed beverages in the world and is also a major source of caffeine for most populations [1]. This special issue of Nutrients, "The Impact of Caffeine and Coffee on Human Health" contains nine reviews and 10 original publications of timely human research investigating coffee and caffeine habits and the ...
The number of publications that study the potential effects of caffeine consumption on ADHD treatment has accumulated since 1975 (see Figure 2) and, over the last few years, caffeine has been used in ADHD research in the context of animal models. Surprisingly, an updated evidence-based systematic review on the effects of caffeine on ADHD-like ...