
- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction

How static electricity forms
Examples of static electricity, uses of static electricity.

- What was Michael Faraday’s childhood like?
- Where did Michael Faraday study?
- What did Michael Faraday discover?

static electricity
Our editors will review what you’ve submitted and determine whether to revise the article.
- Online Consortium of Oklahoma - Physical Science - Overview of Static Electricity
- BCcampus Open Publishing - Static Electricity and Charge: Conservation of Charge
- Workforce LibreTexts - Static Electricity
- LiveScience - What is Static Electricity?
- University of Birmingham - Intranet - Static Electricity - shocks and how to avoid them
- Peekskill City School District - Static Electricity
- Table Of Contents

static electricity , form of electricity resulting from the imbalance between positive and negative charges within a material that occurs when electrons (the negatively charged particles in an atom ) move from one material to another. If the electron-receiving material is either isolated or not an electrical conductor , it tends to hold on to the electrons, resulting in a buildup of electric charge . Since this charge is not moving, it is referred to as static electricity. When conditions allow the built-up charge to flow, the surplus of static electricity is discharged, and it becomes current electricity.
When different atoms make contact with one another, electrons can transfer between them. The material shedding electrons also loses negative charge, and it becomes positively charged when there are more protons (positively charged particles) than electrons. Conversely, a material that gains electrons becomes negatively charged. As more electrons move from one material to the other—due to repeated contact between them—additional negative charge will build up in a process that is referred to as the triboelectric effect.

If two objects are rubbed together—especially if the objects are insulators and the surrounding air is dry, such as when a person’s feet move across a carpet—they acquire equal and opposite charges, and an attractive force develops between them. In this example, the atoms in the person’s body strip away electrons from the carpet and leave behind a positive charge on the carpet’s fibres . The electrons become isolated in the atoms of the person’s body. However, when the person gets close to a conducting material, such as when reaching out a hand to touch a doorknob, a path to ground the built-up charge emerges, and electrons jump suddenly to the conducting material. In addition, as the person reaches for the doorknob, some strands of hair may stand on end. Because like charges repel each other, the transferred electrons that build up in the body travel to the extremities—such as individual strands of hair. (This phenomenon can be demonstrated by using a Van de Graaff generator , which uses a moving belt to gather electric charge on a metallic sphere.)
Another example of the effects of static electricity can be observed in a lightning strike, which occurs when a region of a cloud accumulates a surplus of electrical charge. Small hail particles form in a cloud when moisture in the air freezes, and these particles transfer charge as they grow, move within the cloud, and collide with one another. As additional charged hail particles form, a charge separation occurs, with air acting as an insulator between the charged particles in the cloud as well as between the cloud and the ground. When the surplus of charge is great enough, it overcomes the insulating ability of the air, and electricity is discharged between clouds or between the ground and the cloud itself.
Some of the best-known uses of static electricity occur in air filters and dust-removal devices, which take advantage of the charge differences between materials to remove airborne particles. As electrostatically charged air particles pass through the filter system, the layers of the filter, which have an opposite charge, pick them up and trap them. The buildup of static charge is not always beneficial , however. It can cause damage to important electrical components in computer chips and other components in circuits. Additionally, the friction that occurs while pumping liquids through hoses or pipelines can cause a static charge to accumulate, which can be hazardous if these liquids or the gases they produce are flammable. When this static charge comes into contact with a grounded object, it can create a spark that could ignite these materials.

Six Fun Static Electricity Experiments for Science Students
Krystal DeVille
December 18, 2023
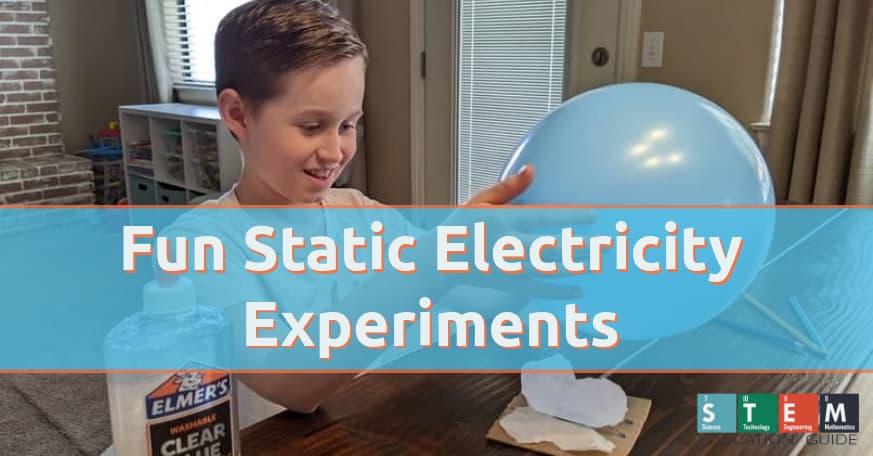
Electricity is a large part of our daily lives. Without it, we would be able to engage in any activities.
We often don’t know exactly how precious it is until we experience a power outage. Electricity doesn’t just involve currents- it also allows individuals to move, think and feel. We explore several different static electricity experiments that illustrate what this natural phenomenon can do.
Table of Contents
Experiments Using Static Electricity
Experiments using static are fun. When most people consider experiments using static electricity, then envision the one involving hair and a balloon. However, many additional experiments will amaze children of any age and can effectively also illustrate how physics and chemistry are used in creating illusions.
*Warning: These experiments may debunk some well-known magic tricks!
Before we get into all the static electricity experiments, be sure to grab my free eBook with over 25 STEM experiments for kids. For all my free downloads, check out this page here.

1. Static Electricity Butterfly

- Age: Elementary school
- Time: 15 minutes
- Difficulty Level: Easy
This experiment demonstrates how static electricity can move the wings on a tissue paper butterfly.
- Googly eyes
- Cardstock paper
- Tissue paper

Instructions:
- Begin by cutting a square piece of cardboard into a 7″x 7″ square.
- Draw butterfly wings on a piece of tissue with a pencil. Make sure that it is smaller than your square. Cut the butterfly out and place them on the cardboard piece without gluing it.
- Cut the body of the butterfly using cardstock. Once finished, glue it to the middle of the butterfly. Make sure it overlaps the cardboard to prevent the wings from flying off. The wings need to be loose to demonstrate the effects of static electricity.
- Glue googly eyes on the butterfly. You can use pipe cleaners for antennae if you would like.
- Blow up the balloon.
- Rub the balloon on your hair to provide a static (electrical charge). Hold the balloon to the top of the butterfly. It should be close to it but shouldn’t touch the butterfly. You should see the wings lower and raise as the balloon is moved closer and further in distance.

The Science Behind the Experiment
When a balloon is rubbed on hair, electrons form. Electrons go from the hair and are given to the balloon generating static. When a negatively charged balloon comes into close contact with positively charged tissue, they generate an attraction. The pull of the charged attraction enables the paper to move towards the balloon.
Static electricity experiments are fun to do. It incorporates both the principles of physics and chemistry into something very simple. It is the perfect way to engage any child in STEM education while teaching them that learning can indeed be fun.
2. Flying Bag Experiment

- Age: Any age
- Time: A few minutes
No, this isn’t done by using an updraft of air but with static electricity. This is what levitates the bag into the air.
- Light plastic bag
- Piece of material
- Plastic Rod
- Use the piece of cloth to run the plastic rod’s surface for 40 seconds.
- Flatten a plastic bag. Then rub the piece of fabric against the bag’s surface for 40 seconds.
- Release the bag and watch while it levitates in the air while the rod is waved below.
The fabric and rod become negatively charged after rubbing them together. Like charges are known to repel each other, so the bag appears to repel when the wand is waved.
My kids loved all the microscope activities we did hands-on in this article. It’s a great way of opening the world to what they can’t see!

3. Hovering Plates
- Age: Elementary School
Using magnets is not the only way that items can repel each other. Hovering plates illustrates this concept very well.
- 2 Styrofoam plates
- Piece of fabric, or your shirt
- Using the piece of fabric, rub the base of one plate.
- Put the plate (base up) on any flat surface.
- Attempt to place the other plate (base down) on top of the other plate. The two plates will repel each other.
This experiment works based on the principle of static electricity. This occurs when two things are rubbed together. The one plate receives electrons directly from the fabric and then becomes negatively charged. In turn, the electrons generated repel the other plate.
4. Bending Water Using Static Electricity

In nature, water can bend due to the moon exerting tidal forces. The same phenomenon can be accomplished by using static electricity.
- Running water
- Piece of cloth
- Plastic Rod or thick straw. My son used a smoothie straw like this because it’s thicker and works a little better.
- Use the fabric to rub the surface of the rod for 40 seconds.
- Create a stream of water by turning the tap on.
- Place the rod close to the water and watch with amazement as the stream bends.
This experiment can also be done using a comb. It would be best if you rubbed the comb against your hair for it to work. Then, you can use it to bend water.
Rubbing the material on the rod generates negatively charged ions. This repels the electrons found in the water. The water closest to the positioning of the rod receives positive charges from it. The attraction between positive and negative charges creates a force on the water, allowing it to appear as if it were bending.

5. Separating Pepper and Salt

Have you accidentally spilled both the salt and pepper? Here is a convenient way to separate them and sort through this lovely mess!
- Fabric or your shirt
- Thick straw
- Mix one teaspoon of pepper and salt thoroughly.
- Rub the straw on the fabric for 40 seconds.
- Place the straw over the mixture. The pepper should jump and adhere to the straw (if it’s held over the correct places).
The granules of pepper and salt are positively charged. As a result of gaining electrons directly from rubbing the cloth, the straw attracts these positive charges located in the mix. Since pepper is lighter, it will jump with greater ease to the straw.
6. Bubble Moving Balloon

This trick is fun and easy. It will delight young children and amaze older ones.
- Smooth sheet plastic, glass, or a kitchen plate like we used
- Dishwashing soap or bubble solution
- Charged balloon
- Spread the bubble solution on a sheet of glass or plastic. Blow larger bubbles on the sheet with the straw.
- Charge an object like a balloon.
- Place the object near the bubble and watch as they follow the charged object.
- Watch it move around the glass/plastic top.

Soapy water is drawn (attracted) to any object that is charged. When there is a large bubble, you can watch it move around.
Wrapping Up
I love static electricity experiments because they can be done with stuff you have lying around your house, not especially tools required. Our favorite experiment was the butterfly one. My three-year-old pretty much thought it was magic.
Another easy item to pick up is pop rocks. My kids had a great time with all the science experiments involving the cracking, popping treat in this article. Please check out our article, Fun Pop Rock Experiments Exploring Viscosity .

5 thoughts on “Six Fun Static Electricity Experiments for Science Students”
Great ideas here with materials that are readily available.
Thank you for ideas and a wonderful resource.
Kind Regards, Jan (Grandmother – Australia)
The salt and pepper experiment mentions a spoon in the directions, but it seems like it should be a straw according to the rest if the directions.
You’re right Kathryn, and thank you for pointing that out to me! The article has been updated 🙂
In the fourth one about bending water, one of the materials say ‘piece of clot’ not cloth. Just a minor error.
Thank you, good catch! I updated it.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
most recent

Activities and Games , Toy Gift Guides
Best stem subscription boxes for kids: hands-on reviews.

Teach Kids to Code , Activities and Games , Technology , Toy Gift Guides
11 best coding robots to teach kids to code (for all ages).

Activities and Games , Math
20 stem projects that are great for middle school.

Engineering
6 projects for learning about simple machines.

Amplify Your Outdoor Living Space with Garden Décor

35 Must-Know Stats & Facts About Natural Latex Mattresses

Easy Gardening Shortcuts for Beginners
STEM Education Guide
[email protected] STEM Education Guide 9125 SVL BOX Victorville, CA 92395
Your Compass for STEM Discovery
© 2024 STEM Education Guide
- Random article
- Teaching guide
- Privacy & cookies

Simple introductions
- What You Learned About Static Electricity Is Wrong by John Timmer. Wired, June 25, 2011.
- A Shocking New Understanding of Static Electricity by Douglas Main. Popular Mechanics, June 29, 2011.
More complex articles
- The Mosaic of Surface Charge in Contact Electrification by H. T. Baytekin, A. Z. Patashinski, M. Branicki, B. Baytekin, S. Soh, and B. A. Grzybowski. Science, July 15, 2011, Vol. 333, Issue 6040, pp.308–312.
- Antioxidants dispel static electricity by Richard Van Noorden. Nature, September 19, 2013.
- What Creates Static Electricity? by Meurig W. Williams. American Scientist, Volume 100, July/August 2012, pp.316–323.
What use is static electricity? Now static electricity is all very interesting, but what possible use is it? You can't make toast from a lightning bolt and you can't charge your cellphone simply by rubbing its case on your pullover. You might think static is one of those fascinating but ultimately quite useless bits of science that has no practical applications—but you'd be wrong: static electricity is used in all kinds of everyday technology! Laser printers and photocopiers use static electricity to build up ink on a drum and transfer it to paper . Crop spraying also relies on static electricity to help herbicides stick to the foliage of plants and distribute themselves evenly over the leaves. Factory paint-spraying robots use a similar trick to ensure that paint droplets are attracted to metal car bodies and not the machinery around them. In many power plants and chemical factories, static electricity is used in smokestacks to scrub away pollution (read more in our article on electrostatic smoke precipitators ). Photo: How can you stop air pollution spewing out from smokestacks? One way is to give a static electric charge to smoke, then funnel it through a grid of metal plates with an opposite charge, so the dirty soot particles are removed. That's how "scrubbers" (electrostatic smoke precipitators) work, such as the ones installed in these smokestacks at the McNeil biomass power plant in Burlington, VT. Photo by Warren Gretz courtesy of US DOE National Renewable Energy Laboratory (NREL) . Of course, static electricity has its drawbacks too. It can cause sparks and explosions in fuel depots and stray static is a real nuisance if you're working with electronic components. That's why engineers and chemists have developed all kinds of anti-static technologies (from simple wires to ingenious, slightly conducting paints and coatings) that prevent static buildup in sensitive places. While you're reading these words, you can be sure that someone, somewhere is trying to find a new way to harness static electricity or a better way to stop it causing problems. Static electricity may be stationary, but it's never standing still! --> --> Sponsored links (adsbygoogle = window.adsbygoogle || []).push({}); If you liked this article...
Don't want to read our articles try listening instead, find out more, on this site.
- Electrostatic smoke precipitators
On other sites
- Why static electricity is really triboelectricity : An excellent video introduction to triboelectricity from Steven Dufresne.
For younger readers
- Eyewitness: Electricity by Steve Parker. New York: Dorling Kindersley, 2005. A good solid introduction to electricity from a dependable children's science writer.
- Cool Science: Experiments with Electricity and Magnetism by Chris Woodford. New York: Gareth Stevens, 2010: Another of my books, this one is a short and simple hands-on guide to electricity and magnetism.
- Routes of Science: Electricity by Chris Woodford. New York: Rosen, 2012: One of my own books, this volume takes us through the complete history of electricity, from the ancient Greeks to modern times.
For older readers
- Hazards of Electricity and Static Electricity by BP Safety Group. Institution of Chemical Engineers, 2006. What are the dangers of static electricity and what can we do to minimize them?
- Fluttering Flag Generates Power From Wind by Prachi Patel. IEEE Spectrum, January 13, 2016. A radical new "wind turbine" uses the principle of triboelectricity to make power.
- Clean like Harry Potter with this dust-busting magic wand by Charlie Sorrel. Wired, August 5, 2011. If static electricity helps to make things dusty, can it help to clean them as well?
For teachers
- Science 101: Q: What is "Static Electricity," and How Can I See Its Effects? by Matt Bobrowsky. Science and Children, Vol 56 No 3, October 2018. A basic review of static concepts children need to know—and a few simple experiments that demonstrate them.
- Measuring static electricity: A classroom investigation to understand the triboelectric series by Carrie Perry et al, Science Scope, Vol 39, No 7, March 2016. Putting together a lesson plan that will help students map the triboelectric series for common materials.
- Static electricity by A. Henry Swann, Science and Children, Vol. 6, No. 2, October 1968, pp.28–30. Some classic classroom demonstrations and the concepts they demonstrate.
- Faraday on Static Electricity , Scientific American, May 14, 1853. A short account of one of Michael Faraday's lectures on static.
Text copyright © Chris Woodford 2012, 2018. All rights reserved. Full copyright notice and terms of use .
Rate this page
Tell your friends, cite this page, more to explore on our website....
- Get the book
- Send feedback
- Demonstrations
- Home Experiments
Balloons and Static Electricity
- by Joe Crowley
- in Home Experiments
- on January 4, 2021
Contributed by Sabrina Brickner
Introduction
- How does charge work? Can we really see how electrons work without fancy science tools?
- Someone with hair on their head
- A working faucet
- An empty metal can
- Blow up a balloon.
- Rub it on your head.
- Watch what happens to the balloon and your hair.
- Turn on your sink and put the balloon close to the water without letting the balloon touch the water.
- Watch what happens to the stream of water.
- Try moving the balloon around a little bit (without touching the water) and see what happens.
- Get an empty metal can and lie it on a hard surface (like the floor or a kitchen counter) such that it can roll.
- Put the balloon close to the can without touching them together.
- Slowly move the balloon away from the can and see what happens.
Physics Concepts and Questions
How does this work?
Static electricity arises from an electrical charge imbalance. In this experiment, when we rub the balloon against our hair, we transfer negative charge to the balloon in the form of electrons. This means that the balloon is now negatively charged, and our hair is positively charged. When we put the balloon by our hair, they attract because they are oppositely charged. This same idea of opposites attracting applies to the water coming out of the faucet, and the empty metal can.
How can the water be positively charged if we haven’t done anything to it like we did in the case of rubbing the balloon on our hair which makes our hair positively charged?
When water comes out of the faucet it is neutral, meaning that it has positive and negative charges in it. However, when we bring the balloon close to the water, some of the negative charge is repelled away into other parts of the water (the top and bottom of the stream), leaving the middle of the stream of water (by the balloon) positively charged. Since this part of the water is positively charged, and the balloon is negatively charged, they attract.
What about the metal can? Can electrons really travel through metal like they travel through water?
Yes they can! The same thing happens here! Some of the positive charge in the can goes to the other side of the can, leaving the side facing the balloon positively charged. Thus the balloon and the can attract.
Conclusions and Further Investigations
- If you rub the balloon on your head 2 times, does it bend the water more, less, or about the same as rubbing the balloon on your head 10 times?
- Does the temperature of the water make a difference?
Recent Posts
- Separating Salt & Pepper with Balloons
- Clouds from bottles: a twist!
- Communicating with Light
- Crushing Soda Cans with Air Pressure
- Buoyancy with 2 Liquids
Recent Comments
- December 2021
- January 2021
- Entries feed
- Comments feed
- WordPress.org
© 2024 UCSB Physics Circus. Built using WordPress and the Highlight Theme
Static Electricity Experiments
- Build a Roller Coaster More
- Spin Like an Ice Skater More
- Reaction Time More
- Random Walk More
- Home Meteorology More
- Vortex in a Bottle More
- Collapse a Can More
- Smoke Rings More
- The Doppler Effect More
- Build a String Telephone More
- Make a Pinhole Camera More
- Science of Bubbles More
- Measure Speed of Light More
Electricity
- Plasma Ball More
- Static Electricity More
- Build an Electromagnet More
- Fusion Cookies More
What you need:
- Plastic Pen
- Small scraps of paper (holes from a hole puncher work well)
- Rub your hair on a balloon or wool sweater. What happens to your hair? Try to stick the balloon to the wall. Does it stick?
- Rub a plastic pen on the wool sweater and hold it near a stream of water. What do you observe?
- Rub the pen on the sweater again and try to pick up small pieces of paper.
What’s going on?
In all of these experiments, we are manually moving electrons from one material to another. Your hair stands up because it is full of electrons. The electrons don’t like each other and are trying to get as far away from each other as possible. The balloon sticks to the wall because it creates an induced charge. The positive charge of the balloon attracts electrons from the wall and the balloon sticks! The same thing happens with the pen and the water and the pen and the paper.

18.1 Static Electricity and Charge: Conservation of Charge
Learning objectives.
By the end of this section, you will be able to:
- Define electric charge, and describe how the two types of charge interact.
- Describe three common situations that generate static electricity.
- State the law of conservation of charge.
What makes plastic wrap cling? Static electricity. Not only are applications of static electricity common these days, its existence has been known since ancient times. The first record of its effects dates to ancient Greeks who noted more than 500 years B.C. that polishing amber temporarily enabled it to attract bits of straw (see Figure 18.3 ). The very word electric derives from the Greek word for amber ( electron ).
Many of the characteristics of static electricity can be explored by rubbing things together. Rubbing creates the spark you get from walking across a wool carpet, for example. Static cling generated in a clothes dryer and the attraction of straw to recently polished amber also result from rubbing. Similarly, lightning results from air movements under certain weather conditions. You can also rub a balloon on your hair, and the static electricity created can then make the balloon cling to a wall. We also have to be cautious of static electricity, especially in dry climates. When we pump gasoline, we are warned to discharge ourselves (after sliding across the seat) on a metal surface before grabbing the gas nozzle. Attendants in hospital operating rooms must wear booties with a conductive strip of aluminum foil on the bottoms to avoid creating sparks which may ignite flammable anesthesia gases combined with the oxygen being used.
Some of the most basic characteristics of static electricity include:
- The effects of static electricity are explained by a physical quantity not previously introduced, called electric charge.
- There are only two types of charge, one called positive and the other called negative.
- Like charges repel, whereas unlike charges attract.
- The force between charges decreases with distance.
How do we know there are two types of electric charge ? When various materials are rubbed together in controlled ways, certain combinations of materials always produce one type of charge on one material and the opposite type on the other. By convention, we call one type of charge “positive”, and the other type “negative.” For example, when glass is rubbed with silk, the glass becomes positively charged and the silk negatively charged. Since the glass and silk have opposite charges, they attract one another like clothes that have rubbed together in a dryer. Two glass rods rubbed with silk in this manner will repel one another, since each rod has positive charge on it. Similarly, two silk cloths so rubbed will repel, since both cloths have negative charge. Figure 18.4 shows how these simple materials can be used to explore the nature of the force between charges.
More sophisticated questions arise. Where do these charges come from? Can you create or destroy charge? Is there a smallest unit of charge? Exactly how does the force depend on the amount of charge and the distance between charges? Such questions obviously occurred to Benjamin Franklin and other early researchers, and they interest us even today.
Charge Carried by Electrons and Protons
Franklin wrote in his letters and books that he could see the effects of electric charge but did not understand what caused the phenomenon. Today we have the advantage of knowing that normal matter is made of atoms, and that atoms contain positive and negative charges, usually in equal amounts.
Figure 18.5 shows a simple model of an atom with negative electrons orbiting its positive nucleus. The nucleus is positive due to the presence of positively charged protons . Nearly all charge in nature is due to electrons and protons, which are two of the three building blocks of most matter. (The third is the neutron, which is neutral, carrying no charge.) Other charge-carrying particles are observed in cosmic rays and nuclear decay, and are created in particle accelerators. All but the electron and proton survive only a short time and are quite rare by comparison.
The charges of electrons and protons are identical in magnitude but opposite in sign. Furthermore, all charged objects in nature are integral multiples of this basic quantity of charge, meaning that all charges are made of combinations of a basic unit of charge. Usually, charges are formed by combinations of electrons and protons. The magnitude of this basic charge is
The symbol q q is commonly used for charge and the subscript e e indicates the charge of a single electron (or proton).
The SI unit of charge is the coulomb (C). The number of protons needed to make a charge of 1.00 C is
Similarly, 6 . 25 × 10 18 6 . 25 × 10 18 electrons have a combined charge of −1.00 coulomb. Just as there is a smallest bit of an element (an atom), there is a smallest bit of charge. There is no directly observed charge smaller than ∣ q e ∣ ∣ q e ∣ (see Things Great and Small: The Submicroscopic Origin of Charge ), and all observed charges are integral multiples of ∣ q e ∣ ∣ q e ∣ .
Things Great and Small: The Submicroscopic Origin of Charge
With the exception of exotic, short-lived particles, all charge in nature is carried by electrons and protons. Electrons carry the charge we have named negative. Protons carry an equal-magnitude charge that we call positive. (See Figure 18.6 .) Electron and proton charges are considered fundamental building blocks, since all other charges are integral multiples of those carried by electrons and protons. Electrons and protons are also two of the three fundamental building blocks of ordinary matter. The neutron is the third and has zero total charge.
Figure 18.6 shows a person touching a Van de Graaff generator and receiving excess positive charge. The expanded view of a hair shows the existence of both types of charges but an excess of positive. The repulsion of these positive like charges causes the strands of hair to repel other strands of hair and to stand up. The further blowup shows an artist’s conception of an electron and a proton perhaps found in an atom in a strand of hair.
The electron seems to have no substructure; in contrast, when the substructure of protons is explored by scattering extremely energetic electrons from them, it appears that there are point-like particles inside the proton. These sub-particles, named quarks, have never been directly observed, but they are believed to carry fractional charges as seen in Figure 18.7 . Charges on electrons and protons and all other directly observable particles are unitary, but these quark substructures carry charges of either − 1 3 − 1 3 or + 2 3 + 2 3 . There are continuing attempts to observe fractional charge directly and to learn of the properties of quarks, which are perhaps the ultimate substructure of matter.
Separation of Charge in Atoms
Charges in atoms and molecules can be separated—for example, by rubbing materials together. Some atoms and molecules have a greater affinity for electrons than others and will become negatively charged by close contact in rubbing, leaving the other material positively charged. (See Figure 18.8 .) Positive charge can similarly be induced by rubbing. Methods other than rubbing can also separate charges. Batteries, for example, use combinations of substances that interact in such a way as to separate charges. Chemical interactions may transfer negative charge from one substance to the other, making one battery terminal negative and leaving the first one positive.
No charge is actually created or destroyed when charges are separated as we have been discussing. Rather, existing charges are moved about. In fact, in all situations the total amount of charge is always constant. This universally obeyed law of nature is called the law of conservation of charge .
Law of Conservation of Charge
Total charge is constant in any process.
In more exotic situations, such as in particle accelerators, mass, Δ m Δ m , can be created from energy in the amount Δ m = E c 2 Δ m = E c 2 . Sometimes, the created mass is charged, such as when an electron is created. Whenever a charged particle is created, another having an opposite charge is always created along with it, so that the total charge created is zero. Usually, the two particles are “matter-antimatter” counterparts. For example, an antielectron would usually be created at the same time as an electron. The antielectron has a positive charge (it is called a positron), and so the total charge created is zero. (See Figure 18.9 .) All particles have antimatter counterparts with opposite signs. When matter and antimatter counterparts are brought together, they completely annihilate one another. By annihilate, we mean that the mass of the two particles is converted to energy E , again obeying the relationship Δ m = E c 2 Δ m = E c 2 . Since the two particles have equal and opposite charge, the total charge is zero before and after the annihilation; thus, total charge is conserved.
Making Connections: Conservation Laws
Only a limited number of physical quantities are universally conserved. Charge is one—energy, momentum, and angular momentum are others. Because they are conserved, these physical quantities are used to explain more phenomena and form more connections than other, less basic quantities. We find that conserved quantities give us great insight into the rules followed by nature and hints to the organization of nature. Discoveries of conservation laws have led to further discoveries, such as the weak nuclear force and the quark substructure of protons and other particles.
The law of conservation of charge is absolute—it has never been observed to be violated. Charge, then, is a special physical quantity, joining a very short list of other quantities in nature that are always conserved. Other conserved quantities include energy, momentum, and angular momentum.
PhET Explorations
Balloons and static electricity.
Why does a balloon stick to your sweater? Rub a balloon on a sweater, then let go of the balloon and it flies over and sticks to the sweater. View the charges in the sweater, balloons, and the wall.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/college-physics-2e/pages/1-introduction-to-science-and-the-realm-of-physics-physical-quantities-and-units
- Authors: Paul Peter Urone, Roger Hinrichs
- Publisher/website: OpenStax
- Book title: College Physics 2e
- Publication date: Jul 13, 2022
- Location: Houston, Texas
- Book URL: https://openstax.org/books/college-physics-2e/pages/1-introduction-to-science-and-the-realm-of-physics-physical-quantities-and-units
- Section URL: https://openstax.org/books/college-physics-2e/pages/18-1-static-electricity-and-charge-conservation-of-charge
© Jul 9, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
May 14, 1853
Faraday on Static Electricity
On supporting science journalism.
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The following is a short abstract of a lecture recently delivered by Faraday before the Royal Institution, London, and taken iron) the " London Expositer :"— The branch of the subject to which he directed attention in this introductory lecture, was the different means by which what is called static electricity may be excited, the term "static" being applied to distinguish that condition of electric force which is excited by friction on any insulated medium, from the electricity which is developed in a current state by voltaic action. The profe sor strenuously endeavored, in the first place, to impress on the minds of his auditors the great Importance and the extraordinory character pi the torce called into action by merely rub-tMg a glass rod with a piece of silk ; that force being sufficient, when operating on light bodies, to overcome the attraction of the earth. Several experiments were exhibited to show the excitement of electricity by the least possible friction ; among which the most curious was the divergence of the gold leaves of an electrometer by the movement ot Professor Faraday's feet on the carpet whilst lie touched the top of the instrument, I With a view torprwerttact" the bodies calletf* electrics do not derive the power of exciting electricity from similarity of their constituent particles, the two highly electrical substances, gutta percha and collodion, or gun cotton, were adduced, and by the different results of their combustion, the opposite characters of their elements were exhibited. It has been generally supposed that in the excitement of electricity by friction, it is "necessary that the rubber should be ot a different material from the electric ; but that this is not an essential condition was illustrated by the following experiment:—Two strip of dried flannel were rubbed against each other transversely the assistant holding one ot the strips tightly stretched whilst Professor Faraday rubbed the other briskly across it, and on applying the latter to the electrometer, the leaves diverged. Another experiment exhibited in a very striking manner the excitement of electricity that takes place whilst combing or brushing the hair when dry. A long lock of hair combed out with a tortoiseehell comb exhibited strong electrical indications by the hairs diverging separately from each other, and when the electricity was collected by an insulated metal plate, it served, after a few repetitions, to charge a small Leyden jar, by which gunpowder wus fired. The evolution of static electricity by evaporation was illustrated by pouring water into a small heated vessel placed on the electrometer. This mode ot excitiag electricity possesses peculiar interest from its being supposed to be the cause of the electrical phenomena of the atmosphere ; though whether this arises from mere change of state, or, as some philosophers imagine, from chemical action, remains a problem to be solved. The professor stated, however, as a circumstance lavorable to the latter hypothesis, that by no experiment yet devised has the excitement of electricity been rendered manifest by evaporation at the temperatures of the atmosphere. A small boiler was on the lecture table, for the purpose of showing the excitement of electricity during the emission of high pressure steam ; but this means of excitement, though apparently op-i posed to all others previously known, may be resolved into excitement by friction, caused by the forcible rubbing together of the particles of condensed steam as they issue from the jet. Professor Faraday did not, however, allude to the searching investigations and ingeniously contrived experiments by which he established this interesting fact ; a satisfactory evidence of which is, that when the injection pipe is heated, to prevent condensation, the excitement of electricity ceases. The last means of electrical excitement noticed was the unequal expansion of some crystalline bodies by heat ; which was illustrated by experiments with tourmelin, the substance in which this property was first observed.

It’s a wonderful world — and universe — out there.
Come explore with us!
Science News Explores
Experiment: how well do different materials create static electricity.
Investigate by making your own electroscope and testing some

When you rub a balloon against your hair, it’s liable to stick! That cling is known as static electricity. We investigate the phenomenon in closer detail in this experiment.
omar alrawi/Flickr/( CC BY-NC-SA 2.0 DEED )
Share this:
- Google Classroom
By Science Buddies
November 13, 2023 at 12:00 pm
Objective : Make an electroscope to test several objects made out of different materials to see which ones produce, or conduct, the most static electricity
Areas of science : Electricity & electronics
Difficulty : Easy intermediate
Time required : ≤ 1 day
Prerequisites : None
Material availability : Readily available
Cost : Very low (under $20)
Safety : When working with electricity, take precautions and beware of electric shock
Credits : Sara Agee, Ph.D. and Teisha Rowland, Ph.D., Science Buddies; this idea was adapted from a project on how to build an electroscope on the ZOOM science activities website hosted by PBS Kids
Static electricity is the build-up of electrical charge in an object. Sometimes static electricity can suddenly discharge, like when a bolt of lightning flashes through the sky. Other times, static electricity can cause objects to cling to each other, like socks fresh out of the dryer. The static cling is an attraction between two objects with different electrical charges, positive (+) and negative (-). You can read more about electricity in the Science Buddies Electricity, Magnetism & Electromagnetism Tutorial .

You can create static electricity by rubbing one object against another object. This is because the rubbing releases negative charges, called electrons . The electrons can build up to produce a static charge. For example, when you shuffle your feet across a carpet, you are creating many surface contacts between your feet and the carpet, allowing electrons to transfer to you, building up a static charge on your skin.
You can suddenly discharge the static charge as a shock when you touch a friend or some objects. Similarly, when you rub a balloon on your head, it causes opposite static charges to build up in your hair and in the balloon. When you pull the balloon slowly away from your head, as shown in Figure 1, you can see these two static charges attracting each other. Your hair stands on end and tries to stick to the balloon!
How can static electricity be measured? One way is to use an electroscope . An electroscope is a scientific instrument that detects if there is an electrical charge, and it can show how big the electrical charge is. A drawing of one type of electroscope is shown in Figure 2, below. How does it work?
Figure 2: This is a drawing of a simple electroscope. When the electroscope receives an electrical charge (from the green rod at the top), the two gold pieces (in yellow) push apart from each other. The bigger the charge the electroscope receives, the farther apart the gold pieces are pushed. If the gold pieces have no electrical charge, they will hang straight down, touching each other.

An electrical charge is transferred to the electroscope (by touching it, as shown with the dark green rod), and the electrical charge goes into two separate metal pieces on the electroscope. In the drawing above, these two pieces are in yellow and represent two thin pieces of gold. The electrical charge makes both of these pieces have the same charge . While objects that have opposite charges are attracted to each other (like the balloon and your hair), objects that have the same charge (such as in the electroscope) are actually repelled by, or pushed away from, each other.
In the electroscope drawing, the two pieces of gold have become charged with the same charge (they are either both negatively charged, or both positively charged), so they are pushed apart from each other. The bigger the charge, the farther apart the two pieces are pushed. If the gold pieces have no charge (in other words, they are neutral ), or they have opposite charges, then they will hang straight down, touching each other.
In this science project, you will build a homemade electroscope to test several objects made out of different materials to see which ones produce, or conduct , the most static electricity. Then you will put your results together to formulate a triboelectric series , which is an ordered list that describes the type of charge an object has as a result of static electricity. The results may shock you!
Terms and Concepts
- Static electricity
- Electrical charge
- Electroscope
- Triboelectric series
- How can static electricity be measured?
- How do different materials react to static electricity?
- Which materials are neutral and which ones are charged?
- How does an electroscope work?
Materials and Equipment
- Styrofoam™ or paper cup
- Sharp pencil or skewer
- Drinking straw
- Aluminum pie pan
- Optional: clay
- Aluminum foil
- Styrofoam plate, or the Styrofoam lid from a take-out food container
- A desk or table that is not metal. For example, a wooden, plastic, or glass desk or table would work. This is because these materials do not conduct electricity as well as metal does.
- Wooden ruler, metric (a plastic ruler is more likely to build up its own static electricity and affect your measurements, so a wooden ruler is recommended)
- Plastic wrap
- Plastic, such as a flat, plastic comb
- Tissue paper
- Lab notebook
Experimental Procedure
- Make two holes near the bottom of a Styrofoam or paper cup on opposite sides. A good way to do this is by pushing a sharp pencil or skewer through the cup.
- Push a straw through both of the holes in the cup so that your setup now looks like Figure 3.
- Either securely tape the cup’s opening to the aluminum pan, as shown in Figure 4, or use clay to hold the cup to the pan. If you are using clay, stick four little balls of clay (each about 2 centimeters, or 0.8 inch, in diameter) to the rim of the cup, then turn the cup upside down and stick it to the bottom of the aluminum pie pan using the clay.
- Carefully adjust the straw’s position so that one end of the straw is right above the edge of the pan, as shown in Figure 4.
- Cut a piece of thread with a length that is about two or three times the distance between the straw and the pan’s edge. Tie a few knots in one end of the thread.
- Cut a square of aluminum foil that is about 3 centimeters (1.2 inches) on each side. Use it to make a ball around the knots in the thread, as shown in Figure 5. The ball should be about the size of a marble or a little smaller. It should be just tight enough so it does not fall off the thread.
- Tape the thread to the tip of the straw so that the ball of foil hangs straight down from the straw, just touching the edge of the pan, as shown in Figure 6. Adjust the straw’s position if needed. If the end of the thread without the ball is dangling down and touching the pan, cut the dangling part off so it does not touch the pan.
- If the straw seems loose at all, tape the straw to the cup (or wedge in some clay) so the straw does not move around when you use the electroscope.

- When you rub the balloon on the Styrofoam plate, the plate gets an electrical charge, which means there is a buildup of electrons (on either object, the balloon or the plate). Even though the plate is charged, the electrons stay where they are because Styrofoam does not conduct electricity.

- What is happening? When an object, such as the Styrofoam plate, gets an electrical charge, it can be either positive or negative. (If an object has a lot of electrons, it can have a negative charge, but if it does not have many electrons, it can have a positive charge. Whether an object tends to gain or loses electrons depends on the type of material it is made out of.) When a charged object (like the charged plate) touches the aluminum pan, the charge (or electrons) easily moves through the metal pan. Since the aluminum ball is touching the pan, the ball gets the same charge as the pan. This means that both the ball and pan have the same charge (they are either both positively or negatively charged). Because objects that have the same charge are repelled by each other, the ball is pushed away from the pan.
| Styrofoam plate | Styrofoam | 1 | |||
| 2 | |||||
| 3 | |||||
| Wool hat | Wool | 1 | |||
| 2 | |||||
| 3 | |||||
| Tissue paper | Tissue | 1 | |||
| 2 | |||||
| 3 | |||||
| Piece of cotton fabric | Cotton | 1 |
- Use a wooden ruler to measure the distance between the foil ball and the pan. The more charge there is, the more distance there will be. Be careful not to touch the ball or the edge of the plate with the ruler (or your body) when you measure this distance. In your lab notebook, make a data table like Table 1, and record your results in it. (This will be Trial 1 using Styrofoam.) In your data table, only list the objects you actually test.
- Now touch the ball with your finger. What happens? Record any observations in the data table in your lab notebook.
- Discharge your electroscope by touching the pan with your finger.
- Rub the object you want to test about 20 times with the balloon.
- Once you have charged the object, quickly lift up the electroscope (holding it by its Styrofoam or paper cup) and place the object on top of the Styrofoam plate so that the object is laying flat on the plate. Make sure the object is not touching the table. Then place the electroscope on top of the object, as shown in Figure 8. (Note: Putting the object on top of the Styrofoam plate will help prevent the electric charge from leaving the object before it can go into the electroscope.)
- Use the wooden ruler to measure the distance between the foil ball and the pan, making sure not to touch the pan or ball with your ruler (or your body). Record your results in your data table. Then touch the ball with your finger and record your observations.
- Repeat steps 6.i. to 6.iv. two more times for the same object so that you have done three trials using the same material.
- Repeat steps 6.i. to 6.v. for each object you want to test. Be sure to do two more trials using the Styrofoam plate (as you did in steps 2 to 5) so that you have done three trials with it. When you are done, you should have done a total of three trials with each object/material.
- For example, if when testing the Styrofoam plate you measured the distance to be about 0.5 centimeter (cm) in trial 1, 0.75 cm in trial 2, and 0.5 cm in trial 3, the average distance would be about 0.6 cm (since 0.5 cm + 0.75 cm + 0.5 cm equals 1.75 cm, and when divided by three this equals about 0.6 cm).
- On the x-axis (the horizontal axis) put the material that was tested, and on the y-axis (the vertical axis) put the average distance between the ball and the pan. Make a bar for each material you tested.
- You can make a graph by hand or by using a computer program such as Create a Graph .
- Which materials were the most electrically charged (had the largest distance between the ball and plate) and which were the least charged?
- Arrange the materials from most charged to least charged. This is a triboelectric series and can be written as an ordered list or chart. How do common objects rank in the series? You can do some additional research on triboelectric series and see how your results compare to other established series. What are some similarities, and what are some differences?
- Some objects become negatively charged and other objects become positively charged with static electricity. Does this kind of electroscope detect both types? How can you tell the difference between the two? Try to discover a way to sort the charged items into positively and negatively charged items.
- Static electricity is formed when many surface contacts are made between two objects. Conduct an experiment to test if the amount of static electricity formed is related to the amount of rubbing that two objects experience. See the related project, Rubbing Up Against Static Electricity .
- Static electricity is not good when it gets in your clothes! How do dryer sheets work? Try an experiment rubbing an object with a dryer sheet (like Bounce) after rubbing against the balloon. What happens to the electroscope reading after rubbing a charged object against the dryer sheet? Can you detect a difference before and after contact with the dryer sheet? If so, you can compare the results from different products. How do they compare? Which brands are most effective?
- For a more advanced experiment, try investigating static electricity in different conditions, temperatures and humidity .
- In this science project you made a simple homemade electroscope, but there are other methods of making electroscopes. Do some research on how other electroscopes are made and try to test a different electroscope design, or come up with your own!
This activity is brought to you in partnership with Science Buddies . Find the original activity on the Science Buddies website.

More Stories from Science News Explores on Physics

Scientists Say: Cosmic rays

The periodic table might soon have a new element

Lasers help put the cork on spilled oil

Scientists Say: Goldene

Scientists Say: Thought experiment

Laser-based tech can identify illegal elephant ivory

Scientists Say: Superconductor
Forget moon walking, lunar visitors. try horizontal running.
Want a daily email of lesson plans that span all subjects and age groups?
The science of static electricity - anuradha bhagwat.
2,927,978 Views
32,837 Questions Answered
Let’s Begin…
We’ve all had the experience: you’re walking across a soft carpet, you reach for the doorknob and … ZAP. But what causes this trademark jolt of static electricity? Anuradha Bhagwat sheds light on the phenomenon by examining the nature of matter.
About TED-Ed Animations
TED-Ed Animations feature the words and ideas of educators brought to life by professional animators. Are you an educator or animator interested in creating a TED-Ed Animation? Nominate yourself here »
Meet The Creators
- Educator Anuradha Bhagwat
- Director Outis
- Sound Designer Cem Misirlioglu
- Script Editor Alex Gendler
- Narrator Pen-Pen Chen
More from Awesome Nature

The weirdest (and coolest) tongues in the animal kingdom
Lesson duration 05:17
179,667 Views

Why fish are better at breathing than you are
Lesson duration 05:21
386,187 Views

These animals can hear everything
Lesson duration 05:26
196,507 Views

Scientists are obsessed with this lake
Lesson duration 05:38
789,254 Views
Choose your language
- English (US)
- English (UK)

Teach kids about static electricity
Here’s a fun way to teach your kids about static electricity. Yes, rubbing a balloon on your bonce is a big part of it, but our tutorial also features a negatively-charged jellyfish (plastic bag) and a neutrally-charged sheep (aluminium can). It’s super simple to do at home and the characters will help kids remember the science. We’re (ahem) positive you’ll love it.
Gather your equipment

Your lab equipment
Careful with the empty can. You can protect little fingers from sharp can lids by covering the opening with some tape.
Want the science-y intro? Here it is…
All things are made of atoms. Inside each atom you’ll find protons (positively charged, don’t move around), neutrons (neutral, don’t move around), and electrons (negatively charged, can move around between objects). Different materials tend to be negatively, positively or neutrally charged by default, depending on their composition.
Let’s have some fun investigating…
Step 1: Make your Neutral Sheep
Aluminium cans have a neutral charge. That means they have an equal number of protons (positively charged) and electrons (negatively charged). Decorate your aluminium can with a little face and positive/negative eyes to show he’s neutral. You can wrap your can with paper like we did, or just draw right onto the surface. Baaah.
Step 2: Make your Negative Jellyfish
Plastic bags are often negatively charged, because they easily pick up electrons from being handled. To help kids remember they are negative, cut your bag to look like a moody jellyfish and draw a face with minus-sign eyes. Don’t be fooled by the smiley face on ours: she just enjoys being negative.

Step 3: Charge up your Bossy Balloon
Balloons are neutrally charged, but when you rub them on your head they gain electrons and become negatively charged. So you can mark your balloon with a minus sign. Then, give it a good rub on your head (or clothing).
Step 4:. Make your jellyfish as negative as possible
Although the plastic has probably naturally gained some electrons from being handled, if you give it a good head-rub it will pick up extra electrons. Now she’s really, really negative. Bag humbug!
Step 5: Take Neutral Sheep for a walk
Put charged-up Bossy Balloon near Neutral Sheep and pull it away slowly. The sheep will follow the balloon faithfully. Aluminium is neutrally charged, the balloon is negatively charged. This imbalance makes them attract. How about making more balloons/sheep and challenging your little shepherds to coax them into a pen?
Step 6: Chase Negative Jellyfish away
Get your little helper to charge both balloon and jellyfish with more rubbing. Then move Bossy around underneath Negative Jellyfish—she should swim away. (Both are negatively charged, and objects with the same charge repel each other.) Ta da!
What did we learn?
All objects are made of atoms. They have different charges depending on how many (negative) electrons they have. Differently-charged objects attract (neutral sheep followed negative balloon) and similarly charged objects repel (negative jellyfish couldn’t get away from negative balloon fast enough!) Oh, and science is way more fun with characters.
Who made this?
We’re Wonderbly. We make learning fun for little minds, with children’s books such as our beautifully illustrated My Little Monster Name Book .
Loved these? Why not dream up a few Fan-static Experiments and characters of your own and share them with us on our Facebook page.
Explore Children’s books
Privacy Overview
.png)
Static Electricity Bats

Just in time to try this fun Halloween inspired activity that uses science to make bats dance. With a few simple items, kids and their parents can get batty while incorporating science, technology, engineering and math (STEM).
Items Needed:
- Tissue Paper
- Sweater or hair on your head
- Start by cutting out a bat shape from the tissue paper.
- Color in the bat shape with markers. Get creative and design it any way you like!
- Tape the bottom of the bat to a table or counter to keep it stable for the rest of the activity.
- Rub the balloon on a sweater or on your hair, then move it over to your bat shape.
- Hold the balloon above the shape and watch what happens. Move the balloon back and forth to make the bat “dance.”
- To expand on the experiment, try making the bat dance without taping it to the table. What happens? Try other materials to see what’s attracted to the balloon and not.
Shake it up! What happened?
The bat is attracted to the balloon because of static electricity – the thing that makes you go “Zap” when you walk on carpet in your socks. Everything is made up of atoms and atoms have three parts – electrons, neutrons and protons. Electrons circle the nucleus and are the easiest part of the atom to “lose” because the nucleus, which is made up of protons and neutrons, has a weaker pull on them. When two objects rub together, the electrons mix.
The balloon picks up extra electrons from the sweater or your hair and becomes negatively charged. Well, with electrons, opposites attract. So, when you put this over the bat, the bat is attracted to the balloon, because it has a positive charge and therefore, it “dances.” What happens if you remove the tape?
Source: http://inspirationlaboratories.com/challenge-discover-halloween-science/
More Experiments

Northern Virginia Science Center Foundation is a 501(c)(3) non-profit organization that operates the Children's Science Center Lab at Fair Oaks Mall and STEM programs traveling to schools and other community venues across the region. The Foundation is also developing the Northern Virginia Science Center in Dulles, VA, a world-class, interactive regional science center for families, students and learners of all ages made possible through a pivotal public-private partnership. Learn more about our mission today at childsci.org and our vision for the future at novasci.org.
What is the hypothesis for electricity?
We are long past the hypothesis stage, with regard to electricity, which is a very well understood phenomenon at this point. If you would like to know about the history of scientific investigation of electricity, there were a number of hypotheses, including the hypothesis that lightning is a form of electricity (which is true) and that nerve impulses are also a form of electricity (also true) and that electricity can be generated by some types of chemical reactions (also true) and that electricity can be used to make the Frankenstein monster come to life (not true).
Isaac Miera ∙
The scientific understanding of the nature of electricity has moved well beyond the stage of hypothesis. We have a solid theory of electricity. In general terms, electricity is a movement of electrons within a conductor.
Does hair contain electricity? (Since the hair goes up when there's static)
Add your answer:
A possible explanation or answer to a question is a?
A hypothesis
What is a verbal hypothesis?
ANSWER: A verbal hypothesis is when you say a hypothesis orallly.
What is a testable explanation of a situation?
A hypothesis.
What is m-m hypothesis?
Hypothesis? Hypothesis is a proposal intended to explain certain facts or observations
Why is hypothesis is discarded as a valid hypothesis?
when results from the experiments repeatedly fail to support the hypothesis.
Top Categories


IMAGES
VIDEO
COMMENTS
static electricity, form of electricity resulting from the imbalance between positive and negative charges within a material that occurs when electrons (the negatively charged particles in an atom) move from one material to another.If the electron-receiving material is either isolated or not an electrical conductor, it tends to hold on to the electrons, resulting in a buildup of electric charge.
Specifically, static cling is an attraction between two objects with opposite electrical charges, one positive and one negative. Static electricity can be created by rubbing one object against ...
Instructions: Spread the bubble solution on a sheet of glass or plastic. Blow larger bubbles on the sheet with the straw. Charge an object like a balloon. Place the object near the bubble and watch as they follow the charged object. Watch it move around the glass/plastic top. The Science Behind the Experiment.
Photo: Classic static: When you rub a balloon on your pullover, you create static electricity that makes it stick. The rubbing shifts electrons from your pullover (which becomes positively charged) to the latex rubber in the balloon (which becomes negatively charged). The opposite charges make the two things stick.
Figure 7.2.3 7.2. 3 shows a simple model of an atom with negative electrons orbiting its positive nucleus. The nucleus is positive due to the presence of positively charged protons. Nearly all charge in nature is due to electrons and protons, which are two of the three building blocks of most matter.
ity is tricky. Here are some tips:1. Humidity- moisture i. the air can make the air conductive. This means that the charged object will lose its ch. rge to moisture in the air over time. Dry air is less conductive so. objets will hold a charge for longer. This is also why we notice static electricity more in the winte.
Static electricity arises from an electrical charge imbalance. In this experiment, when we rub the balloon against our hair, we transfer negative charge to the balloon in the form of electrons. This means that the balloon is now negatively charged, and our hair is positively charged. When we put the balloon by our hair, they attract because ...
The balloon sticks to the wall because it creates an induced charge. The positive charge of the balloon attracts electrons from the wall and the balloon sticks! The same thing happens with the pen and the water and the pen and the paper. What you need: Balloon Plastic Pen Small scraps of paper (holes from a hole puncher work well) Try This: Rub ...
Describe three common situations that generate static electricity. State the law of conservation of charge. Figure 18.3 Borneo amber was mined in Sabah, Malaysia, from shale-sandstone-mudstone veins. When a piece of amber is rubbed with a piece of silk, the amber gains more electrons, giving it a net negative charge. At the same time, the silk ...
The last means of electrical excitement noticed was the unequal expansion of some crystalline bodies by heat ; which was illustrated by experiments with tourmelin, the substance in which this ...
Static electricity is formed when many surface contacts are made between two objects. Conduct an experiment to test if the amount of static electricity formed is related to the amount of rubbing two objects experience. See the related project, Rubbing Up Against Static Electricity. Static electricity is not good when it gets in your clothes!
Hypothesis: Procedure: Observations: Test Movement of Objects 1 2 3 Results: What did you observe about the effect of charges on the movement of objects? Conclusion: 2 S c i e n c e n o r th . c a / te a c h e r s Science North is an agency of the Government of Ontario and a registered charity #10796 2979 RR0001.
have an electric current, or, simply, electricity. Only th. negative charges (electrons) move through a wire. But you can have a buildup of either negative charges or positive charges in an ob. ect, and then that object is electrically charged. If those charges aren't moving anywhere (yet), we say th. t there is a static.
Figure 1: Static electricity makes your hair stand up! Jose Luis Pelaez Inc/DigitalVision/Getty Images. You can create static electricity by rubbing one object against another object. This is because the rubbing releases negative charges, called electrons. The electrons can build up to produce a static charge.
Socks fresh out of the dryer that cling together are a good example of this attraction in action. Specifically, static cling is an attraction between two objects with opposite electrical charges, one positive and one negative. Static electricity can be created by friction such as rubbing one object against another object.
Static electricity can be a nuisance or even a danger. The energy that makes your hair to stand on end can also damage electronics and cause explosions. However, properly controlled and ...
Educator Anuradha Bhagwat. Director Outis. Sound Designer Cem Misirlioglu. Script Editor Alex Gendler. Narrator Pen-Pen Chen. We've all had the experience: you're walking across a soft carpet, you reach for the doorknob and …. ZAP.
Experiment with Electrostatics Science Projects. (5 results) Learn how static electricity doesn't just happen by chance. Experiment with increasing static electricity, make a tool to measure it, or even save and store the charge in a homemade jar.
So you can mark your balloon with a minus sign. Then, give it a good rub on your head (or clothing). Step 4:. Make your jellyfish as negative as possible. Although the plastic has probably naturally gained some electrons from being handled, if you give it a good head-rub it will pick up extra electrons. Now she's really, really negative.
The bat is attracted to the balloon because of static electricity - the thing that makes you go "Zap" when you walk on carpet in your socks. Everything is made up of atoms and atoms have three parts - electrons, neutrons and protons. Electrons circle the nucleus and are the easiest part of the atom to "lose" because the nucleus ...
The static cling is an attraction between two objects with different charges, positive (+) and negative (-). You can create static electricity by rubbing one object against another object. This is because the rubbing releases negative charges, called electrons, that build up to produce a static charge. For example, when you shuffle your feet ...
The hypothesis for static electricity could be that when certain materials rub against each other, electrons transfer between the materials, causing one to become positively charged and one to ...
Hypothesis: Out of both objects, I think the wool socks would have more of a reaction to the static because I think the wool has more of a positive charge then the metal chair does . ... that is a good sign that a lot of static is there . 9. Reach out and touch your target. Keep in mind of how far the victim is, the closer the better ! 10 ...
Revise static electricity for your physics GCSE foundation and higher triple science exams with Bitesize interactive practice quizzes covering feedback and common errors.