Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 09 June 2020

Malaria vaccines since 2000: progress, priorities, products
- Patrick E. Duffy ORCID: orcid.org/0000-0002-4483-5005 1 &
- J. Patrick Gorres 1
npj Vaccines volume 5 , Article number: 48 ( 2020 ) Cite this article
43k Accesses
149 Citations
93 Altmetric
Metrics details
Malaria vaccine development entered a new era in 2015 when the pre-erythrocytic Plasmodium falciparum candidate RTS,S was favorably reviewed by the European Medicines Agency and subsequently introduced into national pilot implementation programs, marking the first human anti-parasite vaccine to pass regulatory scrutiny. Since the first trials published in 1997, RTS,S has been evaluated in a series of clinical trials culminating in Phase 3 testing, while testing of other pre-erythrocytic candidates (that target sporozoite- or liver-stage parasites), particularly whole sporozoite vaccines, has also increased. Interest in blood-stage candidates (that limit blood-stage parasite growth) subsided after disappointing human efficacy results, although new blood-stage targets and concepts may revive activity in this area. Over the past decade, testing of transmission-blocking vaccines (that kill mosquito/sexual-stage parasites) advanced to field trials and the first generation of placental malaria vaccines (that clear placenta-sequestering parasites) entered the clinic. Novel antigen discovery, human monoclonal antibodies, structural vaccinology, and improved platforms promise to expand on RTS,S and improve existing vaccine candidates.
Similar content being viewed by others
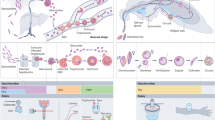
Malaria vaccines: a new era of prevention and control
Preclinical development of a Pfs230-Pfs48/45 chimeric malaria transmission-blocking vaccine

Persistent Plasmodium falciparum infections enhance transmission-reducing immunity development
Introduction.
The malaria vaccine RTS,S/AS01E (brand name Mosquirix TM ) received a favorable opinion from the European Medicines Agency (EMA) in 2015 after review of its safety and efficacy to reduce clinical Plasmodium falciparum malaria episodes in young African children. This was a milestone in vaccine development as the first human parasite vaccine passed the highest level of regulatory scrutiny (referred to as WHO-listed authority maturity level 4 (WLA ML4)) 1 . RTS,S/AS01E pilot implementation programs requested by WHO were launched in 2019 to assess safety and benefits during delivery through standard public health mechanisms. Meanwhile, novel malaria vaccine candidate clinical development has continued apace. Some new vaccine candidates seek to improve on the efficacy of RTS,S/AS01E to prevent clinical malaria in African children, while other candidates in the clinic will pursue different indications such as to protect pregnant women from malaria, or to interrupt the parasite’s cycle of transmission and thereby contribute to regional elimination of malaria by blocking P. falciparum infection or transmission to mosquitoes.
Over the past 20 years, the rate of new malaria vaccine trials registered at ClinicalTrials.gov, a major venue to register clinical trials that launched in 2000 ( https://clinicaltrials.gov/ct2/about-site/history ), has remained steady at ~10 trials each year (Table 1 ). However, trial registrations reflect shifting priorities over time: RTS,S studies maintained a consistent pace throughout albeit with larger sample sizes, while trials that assess whole sporozoite vaccines (WSV) for their safety and efficacy to reduce P. falciparum infection episodes increased in frequency in the last decade, as have transmission-blocking vaccines (TBV) that target parasite sexual stages to prevent parasite transmission to mosquitoes. Further, the first vaccine candidates to protect women from placental malaria entered the clinic in the past 5 years, and trials of blood-stage vaccines (BSV) (which target blood-stage merozoites, with the potential to control blood-stage multiplication, or abort infection during the blood stage) decreased in frequency from 2001–2010 to 2011–2020. Interest has increased in the use of vaccines for malaria elimination, or a so-called vaccine to interrupt malaria transmission (VIMT), that could include antigens expressed during pre-erythrocytic, blood-stage and/or mosquito-sexual stage development in order to reduce or halt the spread of parasites in the community 2 . P. vivax vaccine trials were registered sporadically, reflecting the dearth of resources dedicated to this neglected disease that afflicts millions each year. Notably, some promising P. vivax candidates induced functional activity in Phase 1 trials.
In this Perspective, we examine the background and rationale for different malaria vaccine concepts that target pre-erythrocytic, blood, or mosquito stages of the parasite life cycle (Fig. 1 ), we highlight the progress and limitations of several of the most prominent malaria vaccines in or nearing clinical trials since the year 2000 (Table 2 ), and we describe approaches being used to improve on the existing candidates.
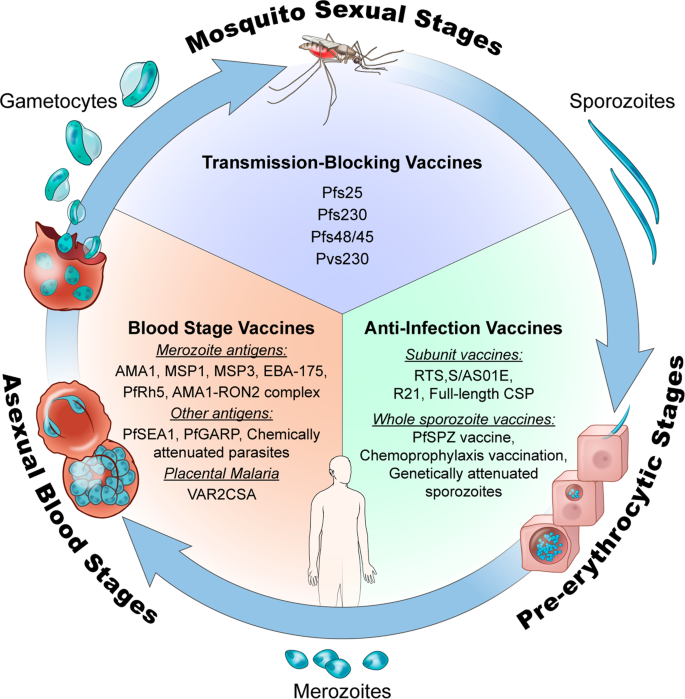
This figure was adapted from a previously published illustration 105 that has been updated to include more recent malaria vaccine candidates. Illustration by Alan Hoofring, Medical Arts Design Section, NIH.
Pre-erythrocytic vaccines
Pre-erythrocytic vaccines (PEV) target antigens from Plasmodium sporozoite and liver stages, the clinically silent forms that initiate human infection after a mosquito inoculates sporozoites into skin. PEV are designed to induce (1) antibodies against surface antigens that clear sporozoites from skin or bloodstream or block their invasion of hepatocytes, or (2) T cell responses that attack infected hepatocytes. Protective efficacy of PEV was first demonstrated in a human in the 1970s using radiation-attenuated WSV delivered through hundreds of mosquito bites; the vaccinee was protected from subsequent challenge with homologous (i.e., identical strain) 3 and heterologous 4 P. falciparum sporozoites (PfSPZ) but not from challenge with homologous blood-stage parasites 3 . PEV with high activity can completely clear pre-erythrocytic parasites before release into the bloodstream, and these have also been referred to as anti-infection vaccines (AIV).
RTS, S and CSP-based vaccines
The demonstration that WSV induce sterilizing immunity in humans coincided with the development of genetic engineering tools. The first malaria gene to be cloned encodes the major surface antigen of sporozoites called circumsporozoite protein or CSP 5 , which continues to be a major focus of vaccine development. RTS,S, the most advanced PEV, incorporates a P. falciparum CSP fragment comprising central repeat (hence “R”) and C-terminal regions (containing T cell epitopes, hence “T”) fused to hepatitis B surface antigen (“S”), or altogether “RTS”. RTS is expressed in yeast that also carry hepatitis B “S” expression cassettes, and thus synthesize S and RTS polypeptides that spontaneously co-assemble into mixed lipoprotein particles (or “RTS,S”) with the CSP fragment on their surface 6 .
RTS,S formulated in GSK’s proprietary AS01 adjuvant completed trials in adults, children, and young infants in sub-Saharan Africa 7 . The phase III trial enrolled 15,459 children at 11 centers in seven African countries, and delivered 3 doses at 1-month intervals to coincide with the Extended Program for Immunization schedule, with a booster dose 18 months after the third dose. Clinical malaria episodes (the primary efficacy endpoint) were reduced by ~36% in young children and ~26% among infants who received four vaccine doses (at 0, 1, 2, and 20 months), with statistically significant efficacy against severe malaria in young children but not infants. Efficacy waned over time, with 68% reduction in the incidence of clinical malaria in the first 6 months 8 . The vaccine prevented an estimated 1774 (95% CI 1387–2186; range across sites 205–6565) clinical malaria episodes per 1000 children that received four vaccine doses and 1363 (95% CI 995–1797) per 1000 children that received 3 doses 8 , 9 . Although vaccine efficacy tended to have a higher point estimate in lower transmission settings, this difference was not significant, and the highest numbers of cases averted were noted in areas of high malaria incidence.
In 2015, the Committee for Medicinal Products for Human Use (CHMP) of the EMA adopted a positive scientific opinion for use of RTS,S outside the European Union “in areas where malaria is regularly found, for the active immunisation of children aged 6 weeks to 17 months against malaria caused by the Plasmodium falciparum parasite, and against hepatitis B” 10 . In 2019, a pilot implementation program of RTS,S was launched in Malawi, Ghana, and Kenya to assess protective benefits and safety during routine use in real-life settings. The program will enroll more than a million children over a period of 3 years in selected areas of medium-to-high malaria burden 11 . Children will be randomized to unvaccinated or vaccinated clusters that are offered 4 doses of RTS,S. While WHO views the program as a pilot introduction into national childhood immunization schedules, the program is registered as a study (clinicaltrials.gov ID NCT03806465), prompting ethical concerns over the absence of informed consent, which was considered by WHO to be “implied” as part of the children’s routine vaccination schedule 12 .
Progress with RTS,S represents a historic milestone, but its partial efficacy leaves room for improvement. RTS,S is administered to children according to their age, in order to coincide with other routine EPI vaccinations. The period of peak vaccine-induced antibody levels (and presumed maximum protection) often does not coincide with the malaria parasite transmission season. An ongoing trial in West Africa is using RTS,S for seasonal vaccination with annual booster doses scheduled to maximize antibody responses during the peak malaria parasite transmission season (ClinicalTrials.gov ID NCT03143218). Other field trials are examining fractional dosing regimens that deliver 1/5th of the full dosage for last vaccine administration, a strategy that has increased efficacy against homologous CHMI as well as antibody somatic hypermutation and avidity in malaria-naïve adult subjects 13 .
Another approach is to improve immunogenicity of CSP-based vaccines. At Oxford’s Jenner Institute, a “next-generation RTS,S-like vaccine” called R21 dispenses with the unfused “S” and generates particles solely comprised of CSP-HBsAg fusion protein 14 . In mice, R21 was immunogenic at low doses using human-use adjuvants, and unlike RTS,S, induced minimal antibody responses to the HBsAg fusion partner. Vaccinated mice displayed sterile protection against challenge with transgenic sporozoites expressing homologous PfCSP, and R21 has advanced to Phase 1/2 testing in Africa (ClinicalTrials.gov IDs NCT02925403; NCT03580824; NCT02925403). Viral-vectored PEV candidates (including those that target CSP) have also been assessed in human trials, particularly as part of heterologous prime-boost approaches. These have induced strong CD8 T cell proliferation among other responses, but thus far have failed to exceed the protective efficacy against sporozoite challenge seen with RTS,S (reviewed in ref. 15 ).
As with many P. falciparum antigens, CSP displays high sequence diversity including in the C-terminal region targeted by RTS,S vaccine. In sieving analysis of the Phase 3 trial, RTS,S showed greater protection against parasites that matched its C-terminal sequence 16 , indicating that parasite variation can limit efficacy and that escape variants could spread. Speculatively, CSP-based vaccines that eliminate or reduce responses to variant epitopes could improve overall efficacy. Further, the N terminal region is not included in RTS,S, but is critical to hepatocyte attachment and invasion by sporozoites 17 , and naturally acquired antibodies to an N-terminal peptide are associated with protection from disease in Tanzanian children 18 . Full-length CSP candidates have been developed 19 , 20 and some have recently entered the clinic [ClinicalTrials.gov ID NCT03589794].
Structural vaccinology approaches are being applied to design improved CSP-based vaccines by defining epitopes of functional human monoclonal antibodies. Monoclonal antibodies to CSP have been prepared from humans after PEV administration or malaria parasite exposure 21 , 22 , 23 , 24 , 25 , 26 , 27 . Most anti-CSP human mAbs react to the repeat region, and a subset of these recognize or cross-react to an epitope at the junction of N-terminal and repeat regions. Antibodies to the C-terminal region have been infrequent: only 4 of 215 monoclonal antibodies derived from PfCSP-specific B cells after whole sporozoite vaccination bound the C terminal region specifically 25 . Although the sieving analysis of the RTS,S trial indicated differential efficacy against parasites with C terminal regions that did or did not match the vaccine, the few C-terminal-specific human mAbs tested failed to show functional activity in vitro or in vivo in mice after passive transfer against parasites carrying the homologous CSP sequence 25 . Functional assessment of additional C-terminal-reactive human mAbs may be useful to understand the differential efficacy of RTS,S.
Whole sporozoite vaccines
Despite evidence since the 1970s that WSV confer sterilizing immunity against sporozoite challenge of humans, WSV were not pursued as a product owing to the perception that manufacture of irradiated sporozoites was impractical for a vaccine 28 . In 2010, the company Sanaria introduced a platform technology that entails harvesting PfSPZ from the salivary glands of aseptic mosquitoes infected by cultured laboratory parasites, followed by purification, vialing, and cryopreservation in liquid nitrogen vapor phase 29 . PfSPZ are attenuated by different approaches to prepare the vaccine candidate product: radiation attenuation (called PfSPZ Vaccine), chemoattenuation achieved in vivo by concomitant administration of antimalarial drugs (called PfSPZ-CVac for chemoprophylaxis vaccination), or genetic attenuation by deletion of genes required to complete liver-stage development 30 (called PfSPZ-GA1 for the first genetically attenuated PfSPZ candidate (NCT03163121)) 31 . PfSPZ Vaccine has required direct venous inoculation to confer sterile immunity against challenge with sporozoites 32 . The logistical and potential cost challenges to implementing WSV will include (1) liquid nitrogen cold chain, (2) intravenous inoculation, (3) scale-up of manufacture.
The efficacy of WSV has been demonstrated in humans although importantly this efficacy is dose-dependent 32 , 33 , 34 . In malaria-naive adults, the level and duration of protection from homologous or heterologous sporozoite challenge depend on dose and regimen with either PfSPZ Vaccine or PfSPZ-CVac, and these have achieved high levels of sterile homologous immunity 32 , 33 , 35 , 36 , 37 . Protection against heterologous CHMI and protection beyond a few months have not yet been studied systematically. In an area of intense malaria transmission in Mali, five administrations of PfSPZ Vaccine (2.7 × 10 5 PfSPZ dosage) to adult residents reduced the risk of new P. falciparum infection by 52% in time-to-event analysis over the 24 weeks after last dose, and reduced the proportion infected across the transmission season by 29% 34 . The time-to-event efficacy achieved appears greater than that reported for RTS,S in adults using AS02 or AS01 adjuvants 38 , 39 . Additional field efficacy trials of PfSPZ Vaccine with 3-dose regimens have been completed in adults and infants (Supplementary Data Set 1 ) and await publication. In particular, an efficacy trial in Kenyan infants was completed in August 2018 (clinicaltrials.gov ID NCT02687373), and the results of that trial will allow a comparison to efficacy of RTS,S/AS01 in this key demographic group.
In malaria-naive individuals, PfSPZ-CVac using chloroquine conferred high levels of sterile immunity against homologous sporozoite challenge 40 that lasted for up to 2 years 41 , but induced sterile heterologous immunity in only a minority of vaccinees 42 . Field trials of PfSPZ-CVac have been completed or are ongoing [Supplementary Data Set 1 ] but results have not yet been published. PfSPZ-CVac approaches are a valuable translational research model to study human sterile immunity. Development as a viable vaccination strategy will require safe and reliable delivery, such as by coformulation of non-attenuated highly sensitive sporozoites and long-lived chemoprophylactic agents to ensure full chemoattenuation in vivo. GAP vaccines are being tested in malaria-naïve individuals for safety, immunogenicity and protective efficacy (clinicaltrials.gov ID NCT03168854; NCT03163121).
Improved field efficacy of WSV will require new regimens or approaches, and future studies will likely incorporate different P. falciparum strains in PfSPZ products to broaden efficacy against heterogeneous parasites that naturally circulate. Immunological analysis of WSV trials may guide approaches to improve field efficacy of PfSPZ and other PEV candidate products. WSV express thousands of malaria antigens and induce a broad immune response including CD4 T cells, CD8 T cells, γδ T cells and antibodies. Among these, the Vδ2 subset of γδ T cells and antibodies to the CSP protein have been associated with protection in human trials 37 , 43 . In SPZ-vaccinated mice, a subset of γδ T cells are required for the induction of protective CD8+ T cells that mediate killing of intrahepatocytic parasites; however, γδ T cells do not directly mediate protection against sporozoite challenge 43 . These findings are consistent with longstanding evidence in mice and in monkeys that CD8+ T cells play a key role in SPZ-induced sterile immunity 44 .
Blood-stage vaccines
BSV target the asexual parasite forms that undergo repeated multiplicative cycles in erythrocytes and cause disease and death. Cycle duration varies between malaria parasite species and determines the period between fevers, or periodicity: 1 day for P. knowlesi , 2 days for P. falciparum, P. vivax and P. ovale , and 3 days for P. malariae . At the completion of each cycle, the brood of ~1–2 dozen progeny (called merozoites) egress from host erythrocytes and within seconds each merozoite has invaded a new erythrocyte to initiate another round of multiplication (and a subset of invasive merozoites commit to generate the sexual forms that will infect mosquitoes).
Blood-stage parasites are an attractive target because this is the disease-causing stage of development, and also because passive transfer of IgG purified from semi-immune African adults was shown to clear parasitemia from African children 6 decades ago 45 , 46 and later in Thai adults 47 . Of note, the studies in Africa included children with malaria who did not receive antimalarial chemotherapy as the standard of care 45 , 46 . and hence would not now pass ethical scrutiny. In subsequent studies, immunization with whole parasite preparations rich in merozoites protected monkeys from P. falciparum infection 48 , focusing attention of vaccine developers on merozoite invasion over the ensuing years.
The challenges to developing anti-merozoite vaccines include (1) the brief time (seconds) when merozoites pass between erythrocytes and are accessible to antibodies, (2) antigenic polymorphism, (3) redundant invasion pathways, and (4) the large number of parasites that need to be targeted compared with the numerical bottlenecks attacked by PEVs and TBVs. Between 2000-2015, over 30 BSV trials registered in ClinicalTrials.gov were completed (Table 1 ), with the large majority targeting the antigens MSP1 and AMA1 and a handful targeting other antigens like EBA-175 and MSP3. In general, these trials sought to elicit high titer antibody against merozoite surface antigens that would impair parasite invasion, or in the case of MSP3, would mediate antibody-dependent cellular inhibition 49 . Ultimately, the results showed scant evidence of protection against controlled human infection or against naturally occurring infection. In particular, AMA-1 candidates induced high titer antibody that was functional by in vitro assays in two trials but failed to show efficacy against controlled infection with the homologous parasite 50 , 51 . Among all BSV candidates, only GMZ2 (consisting of conserved domains of GLURP and MSP3) showed statistically significant albeit low (14%) efficacy in a pre-specified analysis against naturally acquired infection 52 .
After these disappointments, attention turned to identifying novel BSV antigens or refining the approach to existing targets. Two vaccine candidates seek to address the issue of redundant invasion pathways: PfRH5 and the AMA1-RON2 complex.
P. falciparum reticulocyte-binding protein homolog 5 (PfRH5) binds the essential red cell receptor basigin and shows limited polymorphism 53 , and entered clinical trials using a viral-vectored prime-boost immunogen 54 . PfRH5 is the first highly conserved merozoite antigen shown to induce broadly neutralizing antibody in preclinical studies 55 . In monkeys, different combinations of PfRH5 viral-vectored and/or adjuvanted protein immunogens conferred protective immunity that controlled parasitemia after challenge with virulent heterologous parasites 56 .
Notably, natural infections induce modest or no antibody against PfRH5 55 , 57 , 58 and PfRH5 studies in monkeys showed good protection against virulent blood-stage parasite challenge but modest or no boosting of vaccine-induced antibody by infection 56 . This may limit the duration of protection conferred by a vaccine. In addition, protection in monkeys required an estimated 200 µg/mL of anti-PfRH5 IgG 56 , a high level to achieve and sustain by vaccination. Efforts to improve RH5 vaccine candidates include presentation in virus-like particles (VLP) and production of a protein vaccine in Drosophila (Schneider 2) cells 59 , 60 .
In addition, scientists at Jenner Institute have generated human mAbs from PfRH5 vaccinees and used these in structural studies to identify epitopes targeted by neutralizing, non-neutralizing and potentiating antibodies (the latter slow merozoite invasion and enhance activity of neutralizing antibodies) 61 . This knowledge will inform the design of improved Rh5 immunogens that focus the antibody response on neutralizing and potentiating epitopes.
Despite its poor efficacy in previous trials, AMA1 is an essential protein for blood-stage parasite growth. The recognition that AMA1 binds to the rhoptry neck protein RON2 at the merozoite-erythrocyte interface to initiate invasion has revived interest in AMA1 as an immunogen in complex with RON2. When complexed with RON2 peptide, AMA1 antigenicity is altered to generate more potent anti-invasion antibodies than monomeric AMA1 antigen 62 . In monkeys, AMA1-RON2 showed significantly greater protection against heterologous blood-stage challenge versus AMA1 alone, and conferred sterile protection in half the animals 62 . As with Rh5, AMA1 vaccines may be improved by structural studies of antigen-antibody complexes to determine epitopes to include or exclude in re-designed immunogens. Unlike Rh5, AMA1 displays extensive sequence variation, and therefore future studies will need to assess the number of alleles or chimeric sequences that will be required for AMA1-RON2 to confer broadly effective immunity.
Novel BSV antigens
The search for novel BSV antigens has also moved beyond merozoite targets. Parasite antigens are exported to the surface of infected erythrocytes where they are accessible to antibody for hours. Among these, the variant surface antigen family PfEMP1 is immunodominant, mediates parasite sequestration and hence virulence of P. falciparum , and is a target of naturally acquired protective antibody 63 . However its highly polymorphic sequence, large size, and cysteine-rich conformational structure have impeded vaccine development and no trials of PfEMP1-based vaccines have been reported. An exception to this is VAR2CSA, a distinctly structured PfEMP1 family member used by the parasite to sequester in the placenta, as discussed in the next section on placental malaria vaccines (PMV).
Interestingly, a non-PfEMP1-infected erythrocyte surface protein called PfGARP has just been described as the target of protective antibodies 64 . Antibodies to PfGARP induced programmed cell death of intraeythrocytic trophozoites in vitro and naturally acquired PfGARP antibodies were related to control of P. falciparum parasitemia and protection from severe malaria. In monkey studies, PfGARP vaccines conferred partial protection against P. falciparum challenge.
Parasite egress from erythrocytes has also been identified as a target of protective antibody. A differential screen of sera from children that did or did not control parasite density during infection associated protection to antibody against P. falciparum Schizont Egress Antigen 1 (PfSEA-1) 65 . Antibodies bind to intraeythrocyic PfSEA-1 and arrest P. falciparum schizont rupture in vitro, and vaccination of mice with recombinant P. berghei SEA-1 reduced parasitemia and delayed mortality after challenge with lethal P. berghei .
Given the disappointing record of subunit BSV in human trials, scientists at Griffith University in Australia are exploring whole blood-stage parasite vaccines attenuated by incubation with a DNA-binding drug (e.g., Tafuramycin-A). Unlike the PfSPZ-CVac approach (described above) that chemoattenuates parasites in vivo, chemically attenuated blood-stage parasites (CAP) are prepared in vitro before administration. In mice, CAP (but not lysed parasites) induced homologous and heterologous immunity; protection was CD4+ T cell-dependent 66 , 67 , 68 and persisted after CD8+ T cell depletion 67 . In Aotus nancymaae monkeys, a single CAP dose did not delay patent parasitemia after blood-stage parasite challenge but may have delayed drug treatment and induced CD8 T cell responses 69 . In humans, CAP were well-tolerated in malaria-naïve volunteers and induced T cell but not antibody responses 70 . A human trial of a 3-dose CAP regimen has been registered to assess efficacy against challenge with homologous blood-stage parasites [ACTRN12618001314213]. As with PfSPZ-CVac, CAP will need to be convincingly shown to be safe and implementable to be viewed as a viable vaccination strategy.
Placental malaria vaccines
PMV target chondroitin sulfate A (CSA)-binding parasites that uniquely sequester in the placenta; hence PMV represent a distinct BSV approach. While vaccines such as PEV and BSV candidates that protect the general population may also benefit pregnant women, naturally acquired protection against placental malaria offers a focused vaccine approach. Natural antibodies to CSA-binding parasites are associated with protection from placental malaria and are acquired over successive pregnancies as women in endemic areas become resistant to placental malaria 71 . Placental parasites uniformly express the distinctive PfEMP1 family member VAR2CSA that binds CSA 72 ; recombinant VAR2CSA induces antibodies that block parasite binding to CSA (reviewed in ref. 73 ). VAR2CSA is a complex target that has a large (>300 kD) extracellular domain with six DBL domains and additional interdomain regions, and a recent report identified atypical VAR2CSA with seven or eight DBL domains in some field isolates that can be functional 74 .
The first trials of VAR2CSA-based vaccines have been conducted over the past 5 years. Owing to its large size, VAR2CSA vaccine development has focused on individual domains or domain combinations. Two candidates based on N-terminal VAR2CSA fragments that have high binding affinity for CSA have completed first-in-human trials. The Drosophila cell-expressed PAMVAC was tested in different human adjuvants and proved to be safe, well-tolerated, and induced functionally active antibodies against homologous parasites 75 . PAMVAC will be tested in malaria-experienced nulligravidae next. A second subunit VAR2CSA candidate, PRIMVAC, has completed a first-in-human trial in France and Burkina Faso, which showed the vaccine was safe, immunogenic, and induced functional antibodies against the homologous VAR2CSA variant expressed by NF54-CSA infected erythrocytes. However, cross-reactivity against heterologous VAR2CSA variants was limited and only observed in the higher dose group 76 . Researchers hypothesized that an alternate schedule of immunization, antigen dose, and combinations with other VAR2CSA-based vaccines could improve the cross-reactivity against heterologous VAR2CSA variants.
Transmission-blocking vaccines
TBV incorporate surface antigens of mosquito/sexual-stages (gametes and zygotes) in order to induce antibodies that kill parasites in the mosquito bloodmeal and interrupt parasite transmission through the vector 77 , 78 . Target antigens were identified with monoclonal antibodies that were raised in rodents against gamete/zygote preparations and blocked infection of mosquitoes. The four leading candidates have been grouped as gamete surface proteins first expressed by gametocytes in human blood 79 such as Pfs230 and Pfs48/45 of P. falciparum , and zygote surface proteins expressed only post-fertilization in the mosquito host 80 , 81 such as Pfs25 and Pfs28. These antigens are cysteine-rich with multiple 6-cys or epidermal growth factor (EGF)-like domains that have been challenging to prepare as properly folded recombinant protein. Pfs25 was the first TBV candidate prepared as a recombinant protein 82 . In animal studies, Pfs25 candidates have induced equal or greater serum transmission-blocking activity as other antigens or antigen combinations 83 , 84 and hence Pfs25 has been the focus of clinical trials published to date. Ongoing trials are now examining the activity of Pfs230 vaccine candidates (ClinicalTrials.gov IDs NCT02942277; NCT03917654). Pfs230 antibodies raised in animals show lytic activity against P. falciparum gametes in the presence of complement 85 , which might similarly enhance activity of human Pfs230 antisera.
Both Pfs25 and Pfs230 recombinant antigens have shown poor immunogenicity as monomers. To enhance immunogenicity, our group prepares protein-protein conjugate vaccines by chemically coupling Pichia- expressed Pfs25 to carriers such as ExoProtein (EPA) to generate nanoparticles, and formulate these in adjuvants 86 . While several previous trials of Pfs25 candidates failed to induce adequate antibody responses or were overly reactogenic in human vaccinees, Pfs25-EPA conjugate formulated with Alhydrogel® was reported in 2016 to be well-tolerated and to induce functional antibodies in humans that block transmission of P. falciparum to mosquitoes in membrane feeding assays 87 , and this activity correlated with titers. However, functional activity in most vaccinees required 4 doses and antibody titers and activity waned rapidly.
Ongoing studies (ClinicalTrials.gov ID NCT02334462) are comparing and combining Pfs25 and Pfs230 vaccine antigens using Pichia- expressed Pfs230 domain 1 88 . These studies are also assessing the benefits of alternative adjuvants, including the GSK adjuvant AS01 used in the RTS,S vaccine (ClinicalTrials.gov ID NCT02942277; NCT03917654).
Additional TBV candidates will enter the clinic in the coming years and can be compared or combined with the current candidates. Gamete surface antigen Pfs48/45 is likely to be the next target tested in humans. Like Pfs230, Pfs48/45 expression occurs during the later stages of gametocyte development in the human red cell. Once ingested by mosquitoes, gametocytes egress from red cells as gametes. Pfs48/45 appears as GPI-anchored antigen on both male and female gametes 89 , where it forms a complex with Pfs230 90 . Pfs48/45 and Pfs230 play a role in male gamete fertility 91 . Pfs48/45 comprises three 6-cys domains, of which the C-terminal domain contains a conformational epitope targeted by potent transmission-blocking mAbs 92 .
Pfs48/45 vaccine development has been hindered by difficulty in recombinant expression of properly folded protein. Progress has recently been reported for the C-terminal 6-Cys domain as the downstream partner in a fusion with the R0 region of asexual stage Glutamate Rich Protein by expression in Lactococcus lactis 93 . The resulting antigen, called R0.6 C, reacts to conformation-dependent functional monoclonal antibodies and induces transmission-blocking antibodies in animals. Further, a chimeric protein comprising the pro-domain of Pfs230 (upstream of domain 1) and the C-terminal domain of Pfs48/45 induced significantly higher serum functional activity than did R0.6C, suggesting an additive effect of antibody to Pfs230 and Pfs48/45 94 .
Current challenges of TBV development include achieving sufficient adaptive responses that maintain high levels of antibodies over time, as well as widespread coverage to accomplish herd immunity. Furthermore, TBVs must have an exceptional safety profile since they do not confer direct benefit to the individual. TBVs could be implemented in combination with a PEV to prevent both infection in humans and transmission to mosquitoes, and could similarly be combined with BSV that reduce transmission to assess additive or synergistic activity.
Vivax vaccines
P. vivax causes an estimated 14.3 million malaria episodes each year and is the leading cause of malaria in Asia and Latin America 95 . Although it has been historically designated as benign tertian malaria, P. vivax is increasingly recognized as a public health threat causing severe morbidity and mortality 96 . Further, sterile heterologous immunity against P. vivax has been demonstrated 4 , 97 . Despite this, P. vivax research suffers from a dearth of resources since the funds dedicated to malaria research—which are not commensurate to the scope of the problem in any case—are predominantly allocated to P. falciparum research. This inadequate investment is particularly short-sighted, since vaccines may disproportionately benefit P. vivax control: dormant liver forms called hypnozoites produced by P. vivax (but not by P. falciparum ) allow the parasite to relapse repeatedly over months or years and thwart efforts to control or eliminate this species, hence the benefit of durable immunological protection conferred by vaccines.
P. vivax vaccine development has generally followed P. falciparum efforts. Vivax vaccines tested in humans include orthologues (PvCSP and Pvs25, respectively) of the PEV (PfCSP) and TBV (Pfs25) candidates that have commanded greatest attention for P. falciparum . However, PvCSP vaccine prepared as a monomer formulated in GSK’s AS01 adjuvant failed to induce sterile protection against challenge with P. vivax sporozoites 98 , and Pvs25 expressed in S. cerevisiae formulated in Montanide ISA 51 caused systemic reactogenicity that prompted termination of the clinical trial 99 . Notably, when formulated in Alhydrogel®, Pvs25 was well-tolerated, and the antibody responses, though modest, showed functional transmission-blocking activity in mosquito feeding assays that correlated to antibody concentration 100 . Based on clinical progress with the P. falciparum candidate Pfs230D1-EPA, the P. vivax candidate Pvs230D1-EPA is currently being manufactured in anticipation of trials that may launch in 2021.
P. vivax BSV trials have focused on Duffy-Binding Protein (PvDBP) which binds the Duffy Antigen Receptor for Chemokines (DARC) on erythrocytes and is required for merozoite invasion. Two DBP candidates have completed Phase 1 trials, including a viral-vectored 101 and a recombinant protein candidate 102 . Both candidates induced strain-transcending functional antibodies measured in vitro. Using human mAbs generated through vaccination or natural vivax exposure, structural studies have identified functional and non-functional epitopes that will provide a rational basis to improve the design of PvDBP immunogens 103 , 104 .
Conclusions
The landscape for malaria vaccines in 2020 is very different from that in the year 2000. The pre-erythrocytic vaccine (PEV) product RTS,S/AS01E has proven to be safe and efficacious for reducing clinical malaria in African children. Upon completion of ongoing implementation programs in 2022 in three African countries, the results will be reviewed by international bodies including WHO and RTS,S/AS01E will be considered by national policy decision-makers for broader use in Africa.
While RTS,S reduces clinical malaria risk in African children, newer PEV candidates such as R21/Matrix M, PfSPZ whole sporozoite vaccines, and full-length CSP immunogens seek to improve on its efficacy. In parallel, TBV have advanced to Phase 2 clinical trials over the past decade. Efficacious TBV can be combined with the most effective pre-erythrocytic vaccines to pursue malaria elimination programs in combination with other malaria control tools. The substantial progress made with P. falciparum vaccine candidates that have demonstrated efficacy or activity in human trials justifies increased investment in P. vivax vaccines to pursue similar goals.
BSV that target merozoite invasion proteins have delivered disappointing efficacy results in clinical trials over the past 20 years. Novel or improved immunogens that target non-redundant merozoite invasion pathways may improve on these dismal results. Meanwhile, vaccines against other BSV targets such as infected red cell surface proteins, schizont egress antigens, or intact infected erythrocytes that have been attenuated, are progressing in preclinical and clinical studies.
PMV represent a distinct type of BSV by targeting the surface antigens of CSA-binding infected erythrocytes that sequester in intervillous spaces and cause placental malaria. Two vaccine candidates that target VAR2CSA, the immunodominant surface antigen of CSA-binding infected erythrocytes, have completed first-in-human trials. An initial report suggests that these vaccines can induce functional activity against homologous parasites. Future studies will determine whether they can induce heterologous activity that is boosted during naturally occurring pregnancy malaria infections to confer durable protection over successive pregnancies.
Malaria vaccine candidates are progressing in clinical trials and RTS,S has advanced to implementation. The question remains how well can malaria vaccines work, and how can we best deploy them to the advantage of the communities devastated by malaria. Scientists are pursuing antigen discovery, structural vaccinology studies, and improved platforms to expand on or improve our existing portfolio of candidates. As the portfolio advances in development, adequate resources are needed to develop promising candidates; as more candidates transition to products, we must ensure these valuable new interventions are optimally deployed to maximize their benefits in the fight against this ancient scourge.
Data availability
All relevant data are included in the submitted manuscript.
WHO. (World Health Organization, Geneva, Switzerland, 2017).
The mal, E. R. A. C. G. O. V. A research agenda for malaria eradication: vaccines. PLOS Med. 8 , e1000398 (2011).
Google Scholar
Clyde, D. F., Most, H., McCarthy, V. C. & Vanderberg, J. P. Immunization of man against sporozite-induced falciparum malaria. Am. J. Med. Sci. 266 , 169–177 (1973).
CAS PubMed Google Scholar
Clyde, D. F. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am. J. Trop. Med. Hyg. 24 , 397–401 (1975).
Dame, J. B. et al. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum . Science 225 , 593–599 (1984).
Cohen, J. Vaccine composition against malaria. USA patent (1996).
Rts, S. C. T. P. et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med . 367 , 2284–2295 (2012).
Rts, S. C. T. P. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 11 , e1001685 (2014).
Rts, S. C. T. P. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386 , 31–45 (2015).
EMA. First malaria vaccine receives positive scientific opinion from EMA , < https://www.ema.europa.eu/en/news/first-malaria-vaccine-receives-positive-scientific-opinion-ema > (2015).
WHO. MVIP countries: Ghana, Kenya, and Malawi , < https://www.who.int/immunization/diseases/malaria/malaria_vaccine_implementation_programme/pilot_countries_ghana_kenya_malawi/en/ > (2019).
Doshi, P. WHO’s malaria vaccine study represents a “serious breach of international ethical standards”. BMJ 368 , m734 (2020).
PubMed Google Scholar
Regules, J. A. et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J. Infect. Dis. 214 , 762–771 (2016).
Collins, K. A., Snaith, R., Cottingham, M. G., Gilbert, S. C. & Hill, A. V. S. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci. Rep. 7 , 46621 (2017).
CAS PubMed PubMed Central Google Scholar
Hoffman, S. L., Vekemans, J., Richie, T. L. & Duffy, P. E. The march toward malaria vaccines. Vaccine 33 (Suppl 4), D13–D23 (2015).
Neafsey, D. E. et al. Genetic diversity and protective efficacy of the RTS,S/AS01 malaria vaccine. N. Engl. J. Med . 373 , 2025–2037 (2015).
Rathore, D., Sacci, J. B., de la Vega, P. & McCutchan, T. F. Binding and invasion of liver cells by Plasmodium falciparum sporozoites. Essential involvement of the amino terminus of circumsporozoite protein. J. Biol. Chem. 277 , 7092–7098 (2002).
Bongfen, S. E. et al. The N-terminal domain of Plasmodium falciparum circumsporozoite protein represents a target of protective immunity. Vaccine 27 , 328–335 (2009).
Cawlfield, A. et al. Safety, toxicity and immunogenicity of a malaria vaccine based on the circumsporozoite protein (FMP013) with the adjuvant army liposome formulation containing QS21 (ALFQ). Vaccine 37 , 3793–3803 (2019).
Herrera, R. et al. Reversible conformational change in the Plasmodium falciparum circumsporozoite protein masks its adhesion domains. Infect. Immun. 83 , 3771–3780 (2015).
Imkeller, K. et al. Antihomotypic affinity maturation improves human B cell responses against a repetitive epitope. Science 360 , 1358–1362 (2018).
Kisalu, N. K. et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat. Med . 24 , 408–416 (2018).
Oyen, D. et al. Cryo-EM structure of P. falciparum circumsporozoite protein with a vaccine-elicited antibody is stabilized by somatically mutated inter-Fab contacts. Sci. Adv. 4 , eaau8529 (2018).
Pholcharee, T. et al. Diverse antibody responses to conserved structural motifs in Plasmodium falciparum circumsporozoite protein. J. Mol. Biol. 432 , 1048–1063 (2020).
Scally, S. W. et al. Rare PfCSP C-terminal antibodies induced by live sporozoite vaccination are ineffective against malaria infection. J. Exp. Med. 215 , 63–75 (2018).
Tan, J. et al. A public antibody lineage that potently inhibits malaria infection through dual binding to the circumsporozoite protein. Nat. Med. 24 , 401–407 (2018).
Triller, G. et al. Natural parasite exposure induces protective human anti-malarial antibodies. Immunity 47 , 1197–1209.e1110 (2017).
SmithklineBeechamBiologicalsWilde, M. D. Hybrid protein between CS from Plasmodium and HBsAG. (1991).
Hoffman, S. L. et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum. Vaccin 6 , 97–106 (2010).
Mueller, A. K., Labaied, M., Kappe, S. H. & Matuschewski, K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433 , 164–167 (2005).
Richie, T. L. et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine 33 , 7452–7461 (2015).
Seder, R. A. et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341 , 1359–1365 (2013).
Mordmuller, B. et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 542 , 445–449 (2017).
Sissoko, M. S. et al. Safety and efficacy of PfSPZ vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect. Dis. 17 , 498–509 (2017).
Epstein, J. E. et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight 2 , e89154 (2017).
PubMed PubMed Central Google Scholar
Epstein, J. E. et al. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science 334 , 475–480 (2011).
Ishizuka, A. S. et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat. Med. 22 , 614–623 (2016).
Bojang, K. A. et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 358 , 1927–1934 (2001).
Polhemus, M. E. et al. Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area. PLoS ONE 4 , e6465 (2009).
Roestenberg, M. et al. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 361 , 468–477 (2009).
Roestenberg, M. et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377 , 1770–1776 (2011).
Walk, J. et al. Modest heterologous protection after Plasmodium falciparum sporozoite immunization: a double-blind randomized controlled clinical trial. BMC Med . 15 , 168 (2017).
Zaidi, I. et al. gammadelta T cells are required for the induction of sterile immunity during irradiated sporozoite vaccinations. J. Immunol. 199 , 3781–3788 (2017).
Weiss, W. R. & Jiang, C. G. Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLoS ONE 7 , e31247 (2012).
Cohen, S., Mc, G. I. & Carrington, S. Gamma-globulin and acquired immunity to human malaria. Nature 192 , 733–737 (1961).
Edozien, J. C., Gilles, H. M. & Udeozo, I. O. K. Adult and cord-blood gamma-globulin and immunity to malaria in Nigerians. Lancet 280 , 951–955 (1962).
Sabchareon, A. et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45 , 297–308 (1991).
Siddiqui, W. A. An effective immunization of experimental monkeys against a human malaria parasite, Plasmodium falciparum . Science 197 , 388–389 (1977).
Sirima, S. B., Cousens, S. & Druilhe, P. Protection against malaria by MSP3 candidate vaccine. N. Engl. J. Med. 365 , 1062–1064 (2011).
Payne, R. O. et al. Demonstration of the blood-stage Plasmodium falciparum controlled human malaria infection model to assess efficacy of the P. falciparum apical membrane antigen 1 vaccine, FMP2.1/AS01. J. Infect. Dis. 213 , 1743–1751 (2016).
Spring, M. D. et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS ONE 4 , e5254 (2009).
Sirima, S. B. et al. A phase 2b randomized, controlled trial of the efficacy of the GMZ2 malaria vaccine in African children. Vaccine 34 , 4536–4542 (2016).
Crosnier, C. et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum . Nature 480 , 534–537 (2011).
Payne, R. O. et al. Human vaccination against RH5 induces neutralizing antimalarial antibodies that inhibit RH5 invasion complex interactions. JCI Insight https://doi.org/10.1172/jci.insight.96381 (2017).
Douglas, A. D. et al . The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat. Commun 2 , 601 (2011).
Douglas, A. D. et al. A PfRH5-based vaccine is efficacious against heterologous strain blood-stage Plasmodium falciparum infection in aotus monkeys. Cell Host Microbe 17 , 130–139 (2015).
Tran, T. M. et al. Naturally acquired antibodies specific for Plasmodium falciparum reticulocyte-binding protein homologue 5 inhibit parasite growth and predict protection from malaria. J. Infect. Dis. 209 , 789–798 (2014).
Villasis, E. et al. Anti- Plasmodium falciparum invasion ligand antibodies in a low malaria transmission region, Loreto, Peru. Malar. J. 11 , 361, https://doi.org/10.1186/1475-2875-11-361 (2012).
Article CAS PubMed PubMed Central Google Scholar
Hjerrild, K. A. et al. Production of full-length soluble Plasmodium falciparum RH5 protein vaccine using a Drosophila melanogaste r Schneider 2 stable cell line system. Sci. Rep. 6 , 30357 (2016).
Jin, J. et al. Production, quality control, stability, and potency of cGMP-produced Plasmodium falciparum RH5.1 protein vaccine expressed in Drosophila S2 cells. NPJ Vaccines 3 , 32 (2018).
Alanine, D. G. W. et al. Human antibodies that slow erythrocyte invasion potentiate malaria-neutralizing antibodies. Cell 178 , 216–228.e221 (2019).
Srinivasan, P. et al. A malaria vaccine protects Aotus monkeys against virulent Plasmodium falciparum infection. NPJ Vaccines https://doi.org/10.1038/s41541-017-0015-7 (2017).
Tessema, S. K. et al. Protective immunity against severe malaria in children is associated with a limited repertoire of antibodies to conserved PfEMP1 variants. Cell Host Microbe 26 , 579–590 e575, https://doi.org/10.1016/j.chom.2019.10.012 (2019).
Article CAS PubMed Google Scholar
Raj, D. K. et al. Anti-PfGARP activates programmed cell death of parasites and reduces severe malaria. Nature , https://doi.org/10.1038/s41586-020-2220-1 (2020).
Raj, D. K. et al. Antibodies to PfSEA-1 block parasite egress from RBCs and protect against malaria infection. Science 344 , 871–877 (2014).
Good, M. F. et al. Cross-species malaria immunity induced by chemically attenuated parasites. J. Clin. Invest. https://doi.org/10.1172/JCI66634 (2013).
Raja, A. I. et al. Chemically attenuated blood-stage Plasmodium yoelii parasites induce long-lived and strain-transcending protection. Infect. Immun. 84 , 2274–2288 (2016).
Raja, A. I., Stanisic, D. I. & Good, M. F. Chemical attenuation in the development of a whole-organism malaria vaccine. Infect. Immun. https://doi.org/10.1128/IAI.00062-17 (2017).
De, S. L. et al. Persistence and immunogenicity of chemically attenuated blood stage Plasmodium falciparum in Aotus monkeys. Int J. Parasitol. 46 , 581–591 (2016).
Stanisic, D. I. et al. Vaccination with chemically attenuated Plasmodium falciparum asexual blood-stage parasites induces parasite-specific cellular immune responses in malaria-naive volunteers: a pilot study. BMC Med . 16 , 184 (2018).
Fried, M., Nosten, F., Brockman, A., Brabin, B. J. & Duffy, P. E. Maternal antibodies block malaria. Nature 395 , 851–852 (1998).
Salanti, A. et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol . 49 , 179–191 (2003).
Fried, M. & Duffy, P. E. Designing a VAR2CSA-based vaccine to prevent placental malaria. Vaccine 33 , 7483–7488 (2015).
Doritchamou, J. Y. A. et al. Placental malaria vaccine candidate antigen VAR2CSA displays atypical domain architecture in some Plasmodium falciparum strains. Commun. Biol. 2 , 457 (2019).
Mordmuller, B. et al. First-in-human, randomized, double-blind clinical trial of differentially adjuvanted PAMVAC, a vaccine candidate to prevent pregnancy-associated malaria. Clin. Infect. Dis. 69 , 1509–1516 (2019).
Sirima, S. B. et al. PRIMVAC vaccine adjuvanted with Alhydrogel or GLA-SE to prevent placental malaria: a first-in-human, randomised, double-blind, placebo-controlled study. Lancet Infect. Dis . https://doi.org/10.1016/S1473-3099(19)30739-X (2020).
Carter, R. & Chen, D. H. Malaria transmission blocked by immunisation with gametes of the malaria parasite. Nature 263 , 57–60 (1976).
Gwadz, R. W. Successful immunization against the sexual stages of Plasmodium gallinaceum . Science 193 , 1150–1151 (1976).
Carter, R. & Kaushal, D. C. Characterization of antigens on mosquito midgut stages of Plasmodium gallinaceum . III. Comparison of surface antigens of male and female gametes and zygotes. Mol. Biochem.Parasitol. 13 , 235–241 (1984).
Duffy, P. E., Pimenta, P. & Kaslow, D. C. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J. Exp. Med . 177 , 505–510 (1993).
Grotendorst, C. A., Kumar, N., Carter, R. & Kaushal, D. C. A surface protein expressed during the transformation of zygotes of Plasmodium gallinaceum is a target of transmission-blocking antibodies. Infect. Immun. 45 , 775–777 (1984).
Barr, P. J. et al. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J. Exp. Med . 174 , 1203–1208 (1991).
Kapulu, M. C. et al. Comparative assessment of transmission-blocking vaccine candidates against Plasmodium falciparum . Sci. Rep. 5 , 11193 (2015).
Menon, V. et al. Assessment of antibodies induced by multivalent transmission-blocking malaria vaccines. Front. Immunol. 8 , 1998 (2017).
Read, D. et al. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol. 16 , 511–519 (1994).
Radtke, A. J. et al. Adjuvant and carrier protein-dependent T-cell priming promotes a robust antibody response against the Plasmodium falciparum Pfs25 vaccine candidate. Sci. Rep. 7 , 40312 (2017).
Talaat, K. R. et al. Safety and Immunogenicity of Pfs25-EPA/Alhydrogel(R), a Transmission Blocking Vaccine against Plasmodium falciparum : An Open Label Study in Malaria Naive Adults. PLoS ONE 11 , e0163144 (2016).
MacDonald, N. J. et al. Structural and immunological characterization of recombinant 6-cysteine domains of the Plasmodium falciparum sexual stage protein Pfs230. J. Biol. Chem. 291 , 19913–19922 (2016).
Kocken, C. H. et al. Cloning and expression of the gene coding for the transmission blocking target antigen Pfs48/45 of Plasmodium falciparum . Mol. Biochem. Parasitol. 61 , 59–68 (1993).
Kumar, N. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol. 9 , 321–335 (1987).
van Dijk, M. R. et al. A central role for P48/45 in malaria parasite male gamete fertility. Cell 104 , 153–164 (2001).
Roeffen, W. et al. Plasmodium falciparum : production and characterization of rat monoclonal antibodies specific for the sexual-stage Pfs48/45 antigen. Exp. Parasitol. 97 , 45–49 (2001).
Singh, S. K. et al. Construct design, production, and characterization of Plasmodium falciparum 48/45 R0.6C subunit protein produced in Lactococcus lactis as candidate vaccine. Micro. Cell Fact. 16 , 97 (2017).
Singh, S. K. et al. Pfs230 and Pfs48/45 fusion proteins elicit strong transmission-blocking antibody responses against Plasmodium falciparum . Front Immunol. 10 , 1256 (2019).
Battle, K. E. et al. Mapping the global endemicity and clinical burden of Plasmodium vivax , 2000-17: a spatial and temporal modelling study. Lancet 394 , 332–343 (2019).
Baird, J. K. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin. Microbiol. Rev. 26 , 36–57 (2013).
Arevalo-Herrera, M. et al. Protective Efficacy of Plasmodium vivax Radiation-Attenuated Sporozoites in Colombian Volunteers: A Randomized Controlled Trial. PLoS Negl. Trop. Dis. 10 , e0005070 (2016).
Yadava, A. et al. Protective efficacy of a Plasmodium vivax circumsporozoite protein-based vaccine in Aotus nancymaae is associated with antibodies to the repeat region. PLoS Negl. Trop. Dis. 8 , e3268 (2014).
Wu, Y. et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE 3 , e2636 (2008).
Malkin, E. M. et al. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine 23 , 3131–3138 (2005).
Payne, R. O. et al. Human vaccination against Plasmodium vivax Duffy-binding protein induces strain-transcending antibodies. JCI Insight https://doi.org/10.1172/jci.insight.93683 (2017).
Singh, K. et al. Malaria vaccine candidate based on Duffy-binding protein elicits strain transcending functional antibodies in a Phase I trial. NPJ Vaccines 3 , 48 (2018).
Rawlinson, T. A. et al. Structural basis for inhibition of Plasmodium vivax invasion by a broadly neutralizing vaccine-induced human antibody. Nat. Microbiol . 4 , 1497–1507 (2019).
Urusova, D. et al. Structural basis for neutralization of Plasmodium vivax by naturally acquired human antibodies that target DBP. Nat. Microbiol. 4 , 1486–1496 (2019).
Doumbo, O. K., Niare, K., Healy, S. A., Sagara, I. & Duffy, P. E. in Towards Malaria Elimination - A Leap Forward (eds, Manguin, S. & Dev, V.) (InTechOpen, 2018).
Coelho, C.H., Doritchamou, J.Y.A., Zaidi, I. & Duffy, P.E. Advances in malaria vaccine development: report from the 2017 malaria vaccine symposium. NPJ Vaccines 2 , 34 (2017).
Download references
Acknowledgements
The authors are supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank David L. Narum and Sara A. Healy for their critical review of the manuscript, and Alan Hoofring from the NIH Medical Arts Design Section for assistance in designing the illustration in Fig. 1
Author information
Authors and affiliations.
Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA
Patrick E. Duffy & J. Patrick Gorres
You can also search for this author in PubMed Google Scholar
Contributions
P.E.D. drafted and J.P.G. edited the manuscript. J.P.G. prepared the tables and figures with input from P.E.D.
Corresponding author
Correspondence to Patrick E. Duffy .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary-materials, supplementary data set 1, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Duffy, P.E., Patrick Gorres, J. Malaria vaccines since 2000: progress, priorities, products. npj Vaccines 5 , 48 (2020). https://doi.org/10.1038/s41541-020-0196-3
Download citation
Received : 27 January 2020
Accepted : 14 May 2020
Published : 09 June 2020
DOI : https://doi.org/10.1038/s41541-020-0196-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Genetic polymorphism and evidence of signatures of selection in the plasmodium falciparum circumsporozoite protein gene in tanzanian regions with different malaria endemicity.
- Beatus M. Lyimo
- Catherine Bakari
- Deus S. Ishengoma
Malaria Journal (2024)
Genetic diversity and natural selection analysis of VAR2CSA and vir genes: implication for vaccine development
- Joseph Hawadak
- Vineeta Singh
Genomics & Informatics (2024)
- Patrick E. Duffy
- J. Patrick Gorres
- Michal Fried
Nature Reviews Microbiology (2024)
Optimized Refolding Buffers Oriented Humoral Immune Responses Versus PfGCS1 Self-Assembled Peptide Nanoparticle
- Leila Nourani
- Anita Lotfi
- Akram Abouie Mehrizi
Molecular Biotechnology (2024)
An immunoinformatics approach for design and validation of multi-subunit vaccine against Plasmodium falciparum from essential hypothetical proteins
- Prajna Ritaparna
- Rajani Kanta Mahapatra
Journal of Parasitic Diseases (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.

At last, a malaria vaccine has passed important clinical trials
Promising early results suggest we may have a new tool in the battle against the pernicious mosquito-borne parasite.
Every second, seven people somewhere on Earth encounter one of humankind’s most prolific killers: a shape-shifting parasite carried in the saliva of female mosquitoes that can evade our immune systems and live in our livers and blood cells. Every two minutes, the parasite claims another victim under the age of five years old—and brings another round of heartbreak and loss. This grim cycle plays out every hour, every day, every week, every year.
For more than a decade, Halidou Tinto has squared off against this killer. Tinto, an epidemiologist, expert on malaria, and a regional director of Burkina Faso’s Institute of Research in Health Sciences, serves the district of Nanoro, some 50 miles northwest of the capital Ouagadougou. With the arrival of the African monsoon each summer, malaria cases spike in Nanoro and communities across the country. Burkina Faso, a country of 20 million, records about 11 million malaria cases a year—as well as 4,000 deaths.
But after months of speaking with local families about participating in a new malaria vaccine trial, years of experience with running medical trials in the area, and decades of global research behind him, Tinto’s site in Nanoro is home to something else: hope.
In a study published in The Lancet on Wednesday , an international team has shared promising new data on a potential vaccine. The phase two trial, based on 450 children in Nanoro, evaluated the R21 malaria vaccine candidate, which has been under development in the United Kingdom for more than a decade. Researchers found that after children received three shots in an eight-week period and a booster 12 months later, the R21 vaccine was 77 percent effective at stopping malaria, when compared against a control rabies vaccine, rather than a standard placebo.
R21 is the first vaccine candidate for malaria to cross the 75 percent threshold, a goal the World Health Organization (WHO) first set in 2013. If borne out in bigger trials, R21 could add another powerful tool to the world’s malaria-fighting toolkit.
“We are enthusiastic, but we still need phase three trials to confirm the efficacy and the safety of the vaccine before we move on,” says Tinto, one of the study’s senior authors.
A complex parasite
The stakes are high. In 2019, the world saw an estimated 229 million cases of malaria , which killed some 409,000 people—two thirds of whom were young children.
In the past two decades, the world has made enormous progress toward curbing malaria, thanks to widespread use of bed nets, rapid diagnosis, and the seasonal use of preventive antimalarial drugs. Between 2000 and 2015, with all of these interventions, the incidence of malaria cases among at-risk populations fell by 27 percent . But in recent years, progress has stalled. But between 2015 and 2020 cases declined by less than two percent.
To make meaningful progress once again, the WHO is eager to introduce a malaria vaccine into the mix. More than 140 different malaria vaccine candidates are in development. For now, none are formally approved.
Making a malaria vaccine is extremely difficult, in part because this disease is complex. Most cases of malaria are caused by the parasite Plasmodium falciparum , whose genome contains more than 5,000 genes—far more than the mere 12 rattling around inside the coronavirus that causes COVID-19. “There’s a lot of interest and a lot of excitement around vaccines at the moment, because of COVID-19 … but obviously, we’re targeting something quite different,” says lead study author Mehreen Datoo , a physician and doctoral candidate at Oxford’s Jenner Institute who is helping lead R21’s clinical development.
Unlike bacteria and viruses, parasites such as Plasmodium go through several life stages in the human body, which makes designing vaccines for them even harder. As a female mosquito sticks its proboscis into a person’s skin for a blood meal, Plasmodium parasites in the mosquito’s saliva can be transferred into the person’s bloodstream. Within half an hour, these parasites leave the bloodstream and set up shop in the liver, where they multiply by the thousands.
Next, the parasites return to the bloodstream, where they multiply rapidly in a vicious cycle: entering a red blood cell, replicating inside it, and then bursting the infected cell. Some of these parasites mature further, and once inside a mosquito that happens to drink the infected person’s blood, these Plasmodium work their way through the wall of the bug’s gut and enter its salivary glands—beginning the cycle anew.
At each point in the human body, Plasmodium multiplies, which means that the best way to cut off an infection is to stop it early, preferably before it starts infecting red blood cells. But how?
Engineering the new vaccine
For decades, researchers have focused on the Plasmodium life stage that first enters the human bloodstream, which is called a sporozoite. In 1983, researchers found that sporozoites are covered in a protein that provokes a strong response from the immune system. In 1987, researchers at the U.S. pharmaceutical company GlaxoSmithKline developed a test malaria vaccine based on this protein, which is called circumsporozoite protein, or CSP.
GlaxoSmithKline’s idea was to engineer carrier proteins that would contain bits of CSP and self-assemble into microscopic spherical blobs—technically called “virus-like particles”—that could then be injected into the human body, where they would trigger an immune response. If pathogens coated in the same protein later appeared, the immune system would show up ready to rumble. This technique is already used to make vaccines today. If you’ve been vaccinated for human papillomavirus (HPV) or hepatitis B, you’ve received a vaccine based on a virus-like particle.
You May Also Like

Are we entering a new era of mosquito control?

Is malaria making a comeback in the U.S.?

This brain-eating amoeba is on the rise
In malaria’s case, researchers attached a snippet of CSP onto a protein plucked from the surface of the hepatitis B virus, which researchers already knew clumped together into spherical particles. When these proteins are made en masse in engineered yeast, they glom together into particles studded with bits of Plasmodium protein that encourage the body to make antibodies against CSP.
This vaccine, called RTS,S, is the single most tested vaccine candidate for malaria . (It’s produced commercially by GlaxoSmithKline under the name Mosquirix.) For the better part of three decades, researchers, philanthropies including the Gates Foundation, and GlaxoSmithKline have tried to get RTS,S off the ground. Trials have shown it to be safe, and in 2015, the European Medicines Agency gave it a positive recommendation, but not approval (primarily because it’s not being marketed in the EU). Since 2019, RTS,S has been given to more than 650,000 children in Ghana, Kenya, and Malawi, through pilot programs supported by the WHO.
Trials of RTS,S showed that in high-transmission areas where children can come down with malaria upwards of six times a year, the vaccine prevented some 4,500 cases of malaria for every 1,000 children vaccinated. Models suggest that for every 200 children given RTS,S, one child’s life will be saved.
“ To put this in perspective, [RTS,S has] about the same efficacy as the efficacy of a bed net—and we’ve seen the dramatic decline in malaria morbidity and mortality with bed nets,” says WHO epidemiologist Mary Hamel, who manages the organization’s Malaria Vaccine Implementation Program. “This is something you could add on top.”
But relative to other vaccines—such as the astoundingly effective COVID-19 vaccines—RTS,S is a modest performer. Trials found that in the first year after vaccination, for every nine unvaccinated people who got malaria, four vaccinated people did, translating to an efficacy of roughly 55 percent. Four years post-vaccination, efficacy dropped to roughly 36 percent.
The WHO recognized that a more effective vaccine could save more lives, so it set an audacious goal in 2013. By 2030, the health agency proclaimed, it wanted to see a 75 percent effective malaria vaccine.
Enter R21, the vaccine candidate in the Burkina Faso trial. R21 works similarly to RTS,S: attach a bit of Plasmodium protein to a hepatitis B protein, and make a spherical particle that stimulates the immune system.
But thanks to improvements in vaccine manufacturing techniques, R21’s particle is more efficient. As it turns out, there’s less Plasmodium protein on the outside of the RTS,S particle than there theoretically could be. For every hepatitis B protein that has a snippet of Plasmodium CSP, four do not. In R21, however, every protein has a Plasmodium snippet—giving the surface of its virus-like particle many more sites for antibodies to recognize and bind.
Lab studies of R21 began at Oxford from 2010 to 2012, and early “challenge” trials of the vaccine began several years later, with healthy volunteers in Oxford, London, and Southampton, U.K., who agreed to be infected with malaria to test the vaccine’s safety. These early results were promising enough to get the Serum Institute of India, one of the world’s biggest vaccine manufacturers, involved. In 2018, the institute licensed the vaccine from Oxford, agreeing to produce 200 to 300 million doses of R21 per year if it was formally registered.
Two years later, in May 2019, the bigger 450-person phase two trial in Burkina Faso began, in a health district centered on Nanoro. Tinto and his colleagues were extremely well-prepared: They had administered one of the trial sites for the RTS,S vaccine.
Fighting a neglected disease
Hamel, the WHO epidemiologist, lauded the R21 results. But like the study’s coauthors, she urged caution until after the 4,800-person phase three trials, which are starting in five sites in Burkina Faso, Kenya, Mali, and Tanzania. According to Tinto, results are likely in late 2023 or early 2024. Datoo adds that the R21 team could start the approvals process as soon as late 2022, if African legislators consider giving the vaccine emergency authorizations like those issued for COVID-19 vaccines.
One key question is how well the R21 vaccine protects against malaria under different transmission settings. In Burkina Faso, malaria cases spike in the country’s wet season, which lasts from June to November. In other parts of Africa, transmission persists year-round. In the R21 trial, researchers intentionally timed the three doses—which are each administered four weeks apart—to come right before the upswing of Burkina Faso’s wet season, to synchronize the high antibody levels triggered by the vaccine with the peak of malaria season.
For Hamel, the past two years—even with all the challenges of COVID-19—have shown just how effective vaccines might be against malaria. The WHO-backed pilot programs for the RTS,S vaccine are still on track, despite the pandemic’s disruptions to local health care systems. What’s more, broader studies of childhood vaccination programs in Africa have shown that among households where children don’t regularly sleep under bed nets, some 70 percent of children are vaccinated. If a malaria vaccine were deployed at scale and given alongside other childhood vaccinations, large numbers of children who currently can’t access other malaria interventions would at least have a malaria vaccine’s protection.
COVID-19 has also underscored just how much progress can be made when the global community acts with urgency to address a medical crisis. Hamel wishes that sense of urgency—and the resulting funds and logistical support—were there for malaria, too. “I think the biggest roadblock is complacency,” she says. “If this year was the first year that there were 265,000 deaths of children under five from malaria, we’d say it’s an emergency, and we’d get on top of it. But we’ve become accustomed to it.”
Related Topics
- PUBLIC HEALTH

Some antibiotics are no longer as effective. That's as concerning as it sounds.

This parasite uses a clone army to suck out the guts of its enemies

We got rid of BPA in some products—but are the substitutes any safer?

Melanoma is overdiagnosed at ‘alarming’ rates. Here’s what to know.
Fungi could be the key to major cancer research breakthroughs
- Environment
- Paid Content
History & Culture
- History & Culture
- Destination Guide
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
Clinical trial confirms efficacy, safety of low-cost malaria vaccine
UK Aid / Flickr cc
A phase 3 randomized controlled trial confirms that the R21/Matrix-M malaria vaccine is 67% to 75% effective against the mosquito-borne illness in young children in four African countries.
Study data, published yesterday in The Lancet , also confirm a good safety profile for the vaccine, which costs only $2 to $4 per dose, per the World Health Organization (WHO).
A University of Oxford–led research team randomly assigned 4,800 children aged 5 months to 3 years in Burkina Faso, Kenya, Mali, and Tanzania to receive either the malaria vaccine or a rabies control vaccine from April 2021 to January 2022. Follow-up was 1 year.
A total of 3,103 vaccine recipients and 1,541 controls were included in a modified per-protocol analysis; 51.9% were boys, and 48.1% were girls. The vaccine was given in three doses 4 weeks apart, followed by a booster 12 months after the third dose. Half of the children were enrolled at two sites with seasonal malaria transmission, and half were recruited from standard sites with perennial malaria spread.
Highest VE in kids aged 5 to 17 months
Vaccine effectiveness (VE) at 1 year was 75% (95% confidence interval [CI], 71% to 79%) at seasonal sites and 68% (95% CI, 61% to 74%) at standard sites for time to first malaria infection. VE against multiple infections was 75% (95% CI, 71% to 78%) at seasonal sites and 67% (95% CI, 59% to 73%) at standard sites.
Mild waning occurred over 1 year at similar rates at both site types. The investigators noted a rate reduction of 868 (95% CI, 762 to 974) cases per 1,000 children-years at seasonal sites and 296 (95% CI, 231 to 362) at standard sites.
Children aged 5 to 17 months had the highest VE regarding time to first clinical malaria episode at seasonal (79% [95% CI, 73% to 84%) and standard (75% [95% CI, 65% to 83%) sites. This age-group also had significantly greater immune responses than those aged 18 to 36 months.
The vaccine was well tolerated, with 18.6% of 1,615 participants experiencing injection-site pain and 46.7% of 1,615 experiencing fever. The number of adverse events of special interest and serious adverse events didn't differ significantly between the two vaccine groups. No vaccine-related deaths were reported.
The WHO recommended the vaccine in October 2023.
Low cost, fast scale-up
"This low-cost, high-efficacy vaccine is already licensed by several African countries, and recently received a WHO policy recommendation and prequalification, offering large-scale supply to help reduce the great burden of malaria in sub-Saharan Africa," the study authors wrote.
The vaccine was developed by the University of Oxford and vaccine maker Serum Institute of India, which is ready to roll out the first 25 million doses in the next 3 or 4 months, according to a university news release . Up to 200 million doses are predicted to be available in the coming years.
"The continued high efficacy of this new vaccine in field trials is very encouraging, and consistent with the high efficacy and excellent durability observed in a smaller four-year phase IIb trial," chief investigator Adrian Hill, MD, PhD, of the University of Oxford, said in the release.
This low-cost, high-efficacy vaccine is already licensed by several African countries, and recently received a WHO policy recommendation and prequalification, offering large-scale supply to help reduce the great burden of malaria in sub-Saharan Africa.
In a related commentary , Vasee Moorthy, BMBCh, PhD, of the WHO, and colleagues said that R21/Matrix-M will augment the 18 million doses of the RTS,S/AS01 malaria vaccine expected to be available this year and next (annual demand is expected to be 80 million to 100 million doses).
"Two key advantages of R21/Matrix-M are the price, which is expected to be substantially lower than the current RTS,S/AS01 price, and the much higher near-term supply capability," they wrote.
Moorthy and colleagues urged the integration of malaria vaccines into WHO-recommended high-impact measures for infection control in areas of moderate to high transmission. "Effective malaria control depends on the strategic deployment of a mix of partially effective interventions, including vaccines," they concluded. "Importantly, financing for malaria vaccines must be additive to and not replacing existing WHO-recommended malaria control measures if the unacceptable burden of malaria mortality is to be curtailed."
Related news
Monoclonal antibody offers strong malaria protection in children.
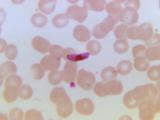
Study highlights heavy global burden of infectious diseases

Climate change, other pressures erode malaria progress, WHO says

Arkansas reports locally acquired malaria case

WHO recommends second malaria vaccine
CDC details locally acquired malaria cases as PAHO calls for better surveillance
Trial of malaria vaccine plus drugs confirms two-thirds reduction in infections, deaths

Maryland reports first local malaria case in 4 decades
This week's top reads
3 studies uncover flu, covid vaccine reluctance, impact of mental illness on uptake.
The findings come on the cusp of the Northern Hemisphere flu and respiratory syncytial virus seasons and rising US COVID-19 cases.
New report: COVID more severe, longer-lasting than other respiratory diseases
COVID-19 participants most often reported fatigue, muscle aches, headache, and loss of taste and/or smell, consistent with the more extensive systemic COVID-19 effects.

Symptomatic contacts reported in probe into Missouri H5N1 flu case
Also, sequencing confirmed N1 and identified two HA mutations, one of which could affect vaccine development.

Officials await testing clues from Missouri H5 avian flu case as Michigan reports more affected cows
CDC lab experts are working on sequencing to identify the neuraminidase and detail the genome, which could yield clues about the source.
No clear exposure source in Missouri H5 avian flu case
While sequencing shows similarity to the virus in cattle, the epi investigation might not be able to pinpoint a source.
US COVID activity remains elevated, though some markers decline
Wastewater levels are still high, but some indicators such as hospitalizations trended downward.
CDC warns of Salmonella outbreak linked to eggs
Of the 65 people sickened, 24 have required hospitalization.
Long-COVID rate among disabled people double that of able-bodied
People with disabilities are also more likely to have severe COVID-19, face treatment refusals, and be hospitalized, the researchers say.

H5N1 avian flu virus detected in wastewater from 10 Texas cities
Before March 2024, H5N1 had not been detected in 1,337 wastewater samples analyzed by the team.
California officials confirm local dengue case
The case follows two local cases reported in 2023, one in Pasadena and the other in Long Beach.

Our underwriters
Unrestricted financial support provided by.

- Antimicrobial Resistance
- Chronic Wasting Disease
- All Topics A-Z
- Resilient Drug Supply
- Influenza Vaccines Roadmap
- CIDRAP Leadership Forum
- Roadmap Development
- Coronavirus Vaccines Roadmap
- Antimicrobial Stewardship
- Osterholm Update
- Newsletters
- About CIDRAP
- CIDRAP in the News
- Our Director
- Osterholm in the Press
- Shop Merchandise
Main navigation
- Our Articles
- Dr. Joe's Books
- Media and Press
- Our History
- Public Lectures
- Past Newsletters
- Photo Gallery: The McGill OSS Separates 25 Years of Separating Sense from Nonsense
Subscribe to the OSS Weekly Newsletter!
Register for the trottier 2024 symposium, the malaria vaccine’s success story hides legitimate concerns.

- Add to calendar
- Tweet Widget
It has been a good year for vaccines. Remarkably effective inoculations were engineered, tested, and rolled out to rein in the COVID-19 pandemic which has killed over 4.8 million people, and on October 6 the World Health Organization (WHO) officially recommended a “groundbreaking malaria vaccine for children at risk.” It’s hard to wrap our heads around the numbers that make up the fabric of mass infectious illnesses like malaria. In 2019, the number of cases of malaria all over the world was estimated at 229 million . That’s a little over the total population of Pakistan, the fifth most populous country on Earth. For that same year, deaths from malaria added up to 409,000. Two-thirds of those deaths were in children under the age of five.
The announcement by the WHO that a vaccine against malaria, more than thirty years in the making, could finally be recommended was greeted with joy in the media. But our vaccine efficacy expectations, raised aloft by the COVID-19 vaccines’ stunning results, need to be tampered down in this case. And while anti-vaccination activists claim, wrongly, that the approved RNA vaccines are “experimental” and are administered to people without their informed consent, the way in which the malaria vaccine’s implementation was pilot-tested in three African countries has raised the ethical questions of what constitutes research and whether or not proper consent was indeed secured in those children.
A protean adversary
If the crash course the public received in 2020 about how a respiratory virus infects the body was a beginner’s class in infectious diseases, malaria is the advanced lesson. A coronavirus spitting out its genetic guts inside our cells so that their replication machinery will make more copies of it is plain sailing compared to the complex, shape-shifting life cycle of malaria, a disease that wrangles at least three different organisms. First, there’s the infected human. Then, there’s the female mosquito, which could be any one of 41 species under the larger umbrella known as Anopheles . Finally, and most interesting of all, there’s the vector, the go-between, the shapeshifter itself. It’s a microscopic, single-celled parasite called Plasmodium whose adaptable existence has forced exotic nomenclature from the pens of scientists, words to describe its life stages like “merozoites” and “schizont.”
Put simply, the mosquito bites you and its saliva delivers the Plasmodium parasite inside your body. For the first week or two, the malaria parasite enters the liver stage of its existence: it replicates itself asexually inside the human liver. Its numbers grow. Eventually, it infects new red blood cells, and thus begins the blood stage and with it the symptoms of malaria. For the uncomplicated form of the disease, these include the non-specific symptoms we associate with the flu, like fever, headache, chills, and body aches. For the severe form of malaria, this tiny parasite can cause acute injury of the lungs and kidneys, coma, and birth complications with long-term consequences. The parasite continues to make copies of itself asexually inside the red blood cells until the cells burst and the parasites look for new cells to infect. Some of the Plasmodium parasites turn into the equivalent of immature sperm and eggs, sexual cells known scientifically as gametes. When you get bitten by another mosquito, these immature gametes get scooped up and the parasite’s life cycle continues inside its new mosquito host. The “sperm” and “egg” mature, meet-cute, and lead to new infectious versions of the parasite ready to travel from the mosquito’s long proboscis and into a fresh human host.
To complicate matters, Plasmodium is not just one thing. It includes six species of parasites that are known to give humans malaria, the most famous of which is Plasmodium falciparum , responsible for the vast majority of malaria deaths. But other species—specifically P. vivax and P. ovale —have the superpower to lay dormant in the form of hypnozoites, which can awaken and cause a relapse of the infection months or years later.
This ability the malaria parasite has to change over the course of its life cycle—a good word for that being “protean,” after the mythological Greek god Proteus who would assume different shapes—has really vexed researchers. Developing a vaccine against a coronavirus is relatively easy, but against this? Which form of the parasite should even be displayed as the crown jewel of a vaccine? The asexual form that couch-surfs in our liver? The one that spreads in the blood? The immature gametes? The mosquito stage? Should we use the full parasite or a simple protein from it? Given that different forms of the parasite can express different proteins, selecting one such protein for the vaccine also spoils us for choice, unless we should choose two or three proteins together to cover more bases. Different strategies were used by scientists over decades, and while a small number of vaccines managed to make it to a phase II trial in humans, their efficacy was judged “modest” and the immunity they granted was not sustained.
Finally, a large clinical trial in thousands of children and infants yielded encouraging results in 2015 for a specific malaria vaccine that targeted the liver stage of the parasite’s life cycle. The vaccine is commonly called RTS,S (an initialism for a shockingly long descriptor), with Mosquirix as its trade name. Whether or not it will be a safe and effective “game-changer” requires a closer look at the results of its testing and how the testing itself was conducted.
“A serious breach of international ethics standards”
Mosquirix prevents 4 in 10 cases of malaria . In terms of efficacy, we have certainly seen better, with two doses of the MMR vaccine being 97% effective against measles and 88% effective against mumps. Even the COVID-19 vaccines had higher efficacies in their clinical trials. When compared to the WHO’s goal of having licensed malaria vaccines with efficacies of at least 75% by the year 2030, Mosquirix clearly doesn’t check the box.
But given the enormity of the problem, preventing 4 in 10 cases of malaria is still an impressive achievement, especially given that Mosquirix is the only vaccine of a crop of 25 to successfully make it through all three phases of human testing. This vaccine could save the lives of tens of thousands of children each year . However, as the results of the Mosquirix clinical trials came to light, a number of key questions lingered. Would a four-dose regimen of the vaccine be feasible in the real world of sub-Saharan Africa, where most people are affected by malaria? Would vaccine recipients assume they were fully protected and dismiss other protective measures, like insecticide-treated bed nets? Given the complexities of malaria, the potential for reinfection, and the relatively short length of the phase III trial, would the vaccine really prevent deaths in the long run? A seven-year follow-up in children who participated in the phase II trial of Mosquirix revealed that the efficacy of the vaccine had gone down over time. There was a rebound effect later on in areas particularly prone to malaria. And more disturbingly, in the biggest trial of the vaccine, three safety signals were picked up: there was a ten times higher rate of meningitis, a higher chance of cerebral malaria, and a doubling of deaths from all causes in girls who had received the vaccine and not the placebo. Were these effects real or chance artefacts?
To answer these questions, the WHO launched a pilot evaluation of the vaccine roll-out in Malawi, Ghana, and Kenya, and this is where we find what has been described as “a serious breach of international ethics standards.” This pilot was registered with ClinicalTrials.gov . Its master protocol clearly calls it a “study” and it contains many sections dedicated to “research questions” and “research methods.” Indeed, areas within these countries were randomized to either receive the vaccine or not. This is why there was outrage in the scientific community when it was revealed that the WHO had not used informed consent during this pilot study.
Informed consent is when a patient is properly informed about the potential risks, benefits, and alternatives of an intervention and, being of sound mind, can decide to go forward with it or not. Ever since the Nazis’ sickening experiments, informed consent has become enshrined in medical ethics. But in the case of the Mosquirix pilot study, the WHO denied that it was a research activity and stated it had used “implied consent,” meaning that the children who received the vaccine and their parents or guardians were not informed that they were taking part in a study. What emerged out of an investigation by the British Medical Journal is that the WHO said it had sent training material to country partners about the potential risks, although the association with an increased risk of deaths among girls seen in the clinical trial was not mentioned in the training material. The vaccine deployment was handled by the countries as part of routine vaccinations. It is this protean roll-out—appearing clearly as a risk assessment research project to some people and as a routine vaccination campaign to others—that the WHO used to recently endorse the wider use of Mosquirix, to much media acclaim.
When interviewed by German radio station Deutschlandfunk , Professor Charles Weijer, who co-wrote the ethical rules on the kind of randomized design the WHO used for their pilot study and which were adapted in collaboration with the WHO, declared that the WHO was violating the very rules it had co-authored . This waiver of informed consent looks to Weijer like there is one standard for research in wealthier countries and a different standard for research done in poorer countries. “It looks like colonial science to me,” he told the interviewer.
But what about the potential risks of the Mosquirix vaccine detected in the clinical trial? Were they seen in the pilot roll-out? According to the WHO, it is now clear that there is no link between the vaccine and these original concerns, but the lack of follow-up at an individual level, the low vaccine coverage, and the short duration of this pilot study (which, to be fair, is still on-going) mean that the actual effect of the vaccine on female mortality, real or not, may have been missed, according to a 2020 analysis by Dr. Christine Benn of the University of Southern Denmark and colleagues. These safety signals, for meningitis, cerebral malaria, and deaths from all causes in girls, have to be sufficiently addressed. A petition , which now has close to 35,000 signatures, is calling for the WHO to be more transparent about its pilot evaluation and to answer the ethical questions that have been raised.
What a mess. The fight against malaria has been plagued by difficulties. Plasmodium is a slippery beast to successfully sketch out for the “Wanted” poster that is a vaccine. It owns many fake moustaches. Mosquirix began testing in 1987, the year that saw the release of Good Morning, Vietnam and the first Lethal Weapon movie. It has reportedly cost over USD 750 million , a substantial bill that was mostly settled by GlaxoSmithKline and the Gates Foundation. Its efficacy is not great but it can’t be dismissed, especially considering the magnitude of the problem. Other interventions exist to prevent or treat malaria, but none are perfect. Bed nets are affordable, but in 2016, only a little over half of people at risk for malaria in sub-Saharan Africa were sleeping under one. Not everyone wants to spend the whole night under a net and, as a WHO spokesperson explained , the nets don’t stop mosquitos by day. Treatment with the drug artemisinin, which led to the 2015 Nobel Prize in Physiology or Medicine, has saved millions of lives, but the emergence of drug resistance in Southeast Asia is sounding the alarm. A vaccine, even if it does not prevent every case of malaria, would be a useful part of the armamentarium.
But doubts remain, in my mind, about Mosquirix’s safety. It is worth pointing out that travellers to Africa will, by and large, not be eligible for Mosquirix, as the WHO has recommended its use only in children at the moment. This vaccine is meant for the children who live in countries where malaria is widespread. There are, of course, other vaccines in the works, like R21/MM which was recently tested in a phase II clinical trial with 450 children and found to have an efficacy of up to 77%. We will have to see if it and others can clear the hurdle of making it through a phase III trial.
It is high time Africa got a safe and effective vaccine against malaria, but ethical standards and transparency cannot be sacrificed. A vaccine’s protection does not simply come from its building blocks. It also comes from trust.
Note: To any reader genuinely curious as to what Mosquirix’s generic name, RTS,S/AS01E, stands for, this article unfurls it as “the central repeat region (R) and T-cell epitopes (T) of P. falciparum circumsporozoite protein carried by the hepatitis B surface antigen (HBsAg, S), and co-expressed within Saccharomyces cerevisiae with unfused copies of HBsAg(S)/adjuvant system 01E.” Worth infinite points in Scrabble.
Take-home message: -Malaria is a life-threatening infection caused by a parasite, Plasmodium, which humans can get from the bite of a female mosquito from the Anopheles family -The first vaccine against malaria to be recommended by the WHO, Mosquirix, has a moderate efficacy against the disease which affects hundreds of millions of people, predominantly in Africa -In further testing the vaccine in Africa prior to its recommendation, the WHO has come under fire for not properly informing the people who received the vaccine of its potential risks and for claiming that this testing was not actually “research” -In the largest clinical trial of Mosquirix, certain risks were increased in the group that received the vaccine, and some scientists have argued that the further testing done since may not have been able to detect if these risks were indeed caused by the vaccine or if they were due to chance
@CrackedScience
What to read next
Another bullet fired at highly processed food 11 sep 2024.

Should You Be Worried About Eating Burned Toast? 6 Sep 2024

No Tolerance for IgG Food Intolerance Tests 29 Aug 2024

Waxing Lyrical About Fruit Wax 21 Aug 2024

Doc of Detox Tries to Rewrite All of Medicine 9 Aug 2024

Coffee for the Brain 7 Aug 2024


Department and University Information
Office for science and society.

Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Malaria Vaccine Candidate Could Prevent Infection During Pregnancy
An experimental malaria vaccine could offer protection during pregnancy for up to 2 years without a booster dose, according to a randomized clinical trial conducted in Mali. The 3-dose vaccine, known as Plasmodium falciparum sporozoite (PfSPZ) vaccine, had previously been tested successfully in adults in other parts of Africa.
This was the first clinical trial to test the vaccine’s safety and efficacy among people who were planning to become pregnant after immunization, according to the researchers. Approximately 300 healthy adults in Mali aged 18 to 38 years received a treatment to remove malaria parasites, then 3 injections of either a placebo or the PfSPZ vaccine, at higher or lower doses.
In the 55 people who became pregnant after the final vaccine dose, the vaccine was 65% effective at the lower dose and 86% effective at the higher dose. Among the 155 women who became pregnant over the course of the 2-year study, vaccine efficacy was 57% at the lower dose and 49% at the higher dose. Most adverse reactions were mild.
“Existing measures are not protecting women from malaria in pregnancy…and our results indicate that PfSPZ vaccine might be a suitable candidate,” the researchers wrote in The Lancet .
Published Online: September 13, 2024. doi:10.1001/jama.2024.18197
See More About
Anderer S. Malaria Vaccine Candidate Could Prevent Infection During Pregnancy. JAMA. Published online September 13, 2024. doi:10.1001/jama.2024.18197
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Seasonal use case for the RTS,S/AS01 malaria vaccine: a mathematical modelling study
Affiliations.
- 1 MRC Centre for Global Infectious Disease Analysis, Imperial College London, London UK.
- 2 MRC Centre for Global Infectious Disease Analysis, Imperial College London, London UK; School of Population Health, University of New South Wales, Sydney, NSW, Australia.
- 3 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso.
- 4 Malaria Research and Training Center, University of Sciences, Technologies, and Techniques of Bamako, Bamako, Mali.
- 5 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso; Institut National de Santé Publique - Centre Muraz, Bobo-Dioulasso, Burkina Faso.
- 6 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso; Institut Sciences et Techniques, Bobo-Dioulasso, Burkina Faso.
- 7 Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, UK.
- 8 International Statistics and Epidemiology Group, London School of Hygiene and Tropical Medicine, London, UK.
- 9 MRC Centre for Global Infectious Disease Analysis, Imperial College London, London UK. Electronic address: [email protected].
- PMID: 36400084
- DOI: 10.1016/S2214-109X(22)00416-8
Background: A 2021 clinical trial of seasonal RTS,S/AS01 E (RTS,S) vaccination showed that vaccination was non-inferior to seasonal malaria chemoprevention (SMC) in preventing clinical malaria. The combination of these two interventions provided significant additional protection against clinical and severe malaria outcomes. Projections of the effect of this novel approach to RTS,S vaccination in seasonal transmission settings for extended timeframes and across a range of epidemiological settings are needed to inform policy recommendations.
Methods: We used a mathematical, individual-based model of malaria transmission that was fitted to data on the relationship between entomological inoculation rate and parasite prevalence, clinical disease, severe disease, and deaths from multiple sites across Africa. The model was validated with results from a phase 3b trial assessing the effect of SV-RTS,S in Mali and Burkina Faso. We developed three intervention efficacy models with varying degrees and durations of protection for our population-level modelling analysis to assess the potential effect of an RTS,S vaccination schedule based on age (doses were delivered to children aged 6 months, 7·5 months, and 9 months for the first three doses, and at 27 months of age for the fourth dose) or season (children aged 5-17 months at the time of first vaccination received the first three doses in the 3 months preceding the transmission season, with any subsequent doses up to five doses delivered annually) in seasonal transmission settings both in the absence and presence of SMC with sulfadoxine-pyrimethamine plus amodiaquine. This is modelled as a full therapeutic course delivered every month for four or five months of the peak in transmission season. Estimates of cases and deaths averted in a population of 100 000 children aged 0-5 years were calculated over a 15-year time period for a range of levels of malaria transmission intensity (Plasmodium falciparum parasite prevalence in children aged 2-10 years between 10% and 65%) and over two west Africa seasonality archetypes.
Findings: Seasonally targeting RTS,S resulted in greater absolute reductions in malaria cases and deaths compared with an age-based strategy, averting an additional 14 000-47 000 cases per 100 000 children aged 5 years and younger over 15 years, dependent on seasonality and transmission intensity. We predicted that adding seasonally targeted RTS,S to SMC would reduce clinical incidence by up to an additional 42 000-67 000 cases per 100 000 children aged 5 years and younger over 15 years compared with SMC alone. Transmission season duration was a key determinant of intervention effect, with the advantage of adding RTS,S to SMC predicted to be smaller with shorter transmission seasons.
Interpretation: RTS,S vaccination in seasonal settings could be a valuable additional tool to existing interventions, with seasonal delivery maximising the effect relative to an age-based approach. Decisions surrounding deployment strategies of RTS,S in such settings will need to consider the local and regional variations in seasonality, current rates of other interventions, and potential achievable RTS,S coverage.
Funding: UK Medical Research Council, UK Foreign Commonwealth & Development Office, The Wellcome Trust, and The Royal society.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
PubMed Disclaimer
Conflict of interest statement
Declaration of interests ABH has received personal consultancy fees from WHO outside the submitted work. ABH was previously engaged by Pfizer to advise on modelling respiratory syncytial virus vaccination strategies for which she received no financial compensation. ACG has a data-transfer agreement with GSK Vaccines to cover ongoing analysis of trial data related to the RTS,S/AS01 vaccine. ACG does not receive any funding from GSK for this work. All other authors declare no competing interests.
- Seasonal targeting of the RTS,S/AS01 malaria vaccine: a complementary tool but sustained funding is required. Silal SP. Silal SP. Lancet Glob Health. 2022 Dec;10(12):e1693-e1694. doi: 10.1016/S2214-109X(22)00477-6. Lancet Glob Health. 2022. PMID: 36400073 No abstract available.
Publication types
- Search in MeSH
Grants and funding
- G98669/MRC_/Medical Research Council/United Kingdom
- 220658/Z/20/Z/WT_/Wellcome Trust/United Kingdom
- MR/R015600/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full text sources.
- ClinicalKey
- Elsevier Science
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Methodology
- Open access
- Published: 25 April 2022
Assessing the safety, impact and effectiveness of RTS,S/AS01 E malaria vaccine following its introduction in three sub-Saharan African countries: methodological approaches and study set-up
- Nicolas Praet 1 nAff16 ,
- Kwaku Poku Asante 2 , 3 ,
- Marie-Cecile Bozonnat 4 ,
- Elaine Jacqueline Akité 1 ,
- Patrick Odum Ansah 5 ,
- Laurence Baril 6 ,
- Owusu Boahen 2 ,
- Yolanda Guerra Mendoza 1 ,
- Valerie Haine 1 ,
- Simon Kariuki 7 ,
- Mathieu Lamy 8 ,
- Kenneth Maleta 9 ,
- Randy Mungwira 9 ,
- Latif Ndeketa 10 ,
- Abraham Oduro 5 ,
- Bernhards Ogutu 11 , 12 ,
- Fredrick Olewe 11 ,
- Martina Oneko 7 ,
- Mattéa Orsini 4 ,
- Francois Roman 1 ,
- Edith Roset Bahmanyar 13 ,
- Dominique Rosillon 1 nAff17 ,
- Lode Schuerman 1 ,
- Valentine Sing’oei 14 ,
- Dianne J. Terlouw 10 , 15 ,
- Stéphanie Wéry 1 ,
- Walter Otieno 14 &
- Jean-Yves Pirçon 1
Malaria Journal volume 21 , Article number: 132 ( 2022 ) Cite this article
6244 Accesses
15 Citations
14 Altmetric
Metrics details
This article has been updated
Following a 30-year development process, RTS,S/AS01 E (GSK, Belgium) is the first malaria vaccine to reach Phase IV assessments. The World Health Organization-commissioned Malaria Vaccine Implementation Programme (MVIP) is coordinating the delivery of RTS,S/AS01 E through routine national immunization programmes in areas of 3 countries in sub-Saharan Africa. The first doses were given in the participating MVIP areas in Malawi on 23 April, Ghana on 30 April, and Kenya on 13 September 2019. The countries participating in the MVIP have little or no baseline incidence data on rare diseases, some of which may be associated with immunization, a deficit that could compromise the interpretation of possible adverse events reported following the introduction of a new vaccine in the paediatric population. Further, effects of vaccination on malaria transmission, existing malaria control strategies, and possible vaccine-mediated selective pressure on Plasmodium falciparum variants, could also impact long-term malaria control. To address this data gap and as part of its post-approval commitments, GSK has developed a post-approval plan comprising of 4 complementary Phase IV studies that will evaluate safety, effectiveness and impact of RTS,S/AS01 E through active participant follow-up in the context of its real-life implementation.
EPI-MAL-002 (NCT02374450) is a pre-implementation safety surveillance study that is establishing the background incidence rates of protocol-defined adverse events of special interest. EPI-MAL-003 (NCT03855995) is an identically designed post-implementation safety and vaccine impact study. EPI-MAL-005 (NCT02251704) is a cross-sectional pre- and post-implementation study to measure malaria transmission intensity and monitor the use of other malaria control interventions in the study areas, and EPI-MAL-010 (EUPAS42948) will evaluate the P. falciparum genetic diversity in the periods before and after vaccine implementation.
GSK’s post-approval plan has been designed to address important knowledge gaps in RTS,S/AS01 E vaccine safety, effectiveness and impact. The studies are currently being conducted in the MVIP areas. Their implementation has provided opportunities and posed challenges linked to conducting large studies in regions where healthcare infrastructure is limited. The results from these studies will support ongoing evaluation of RTS,S/AS01 E ’s benefit-risk and inform decision-making for its potential wider implementation across sub-Saharan Africa.
Graphic abstract
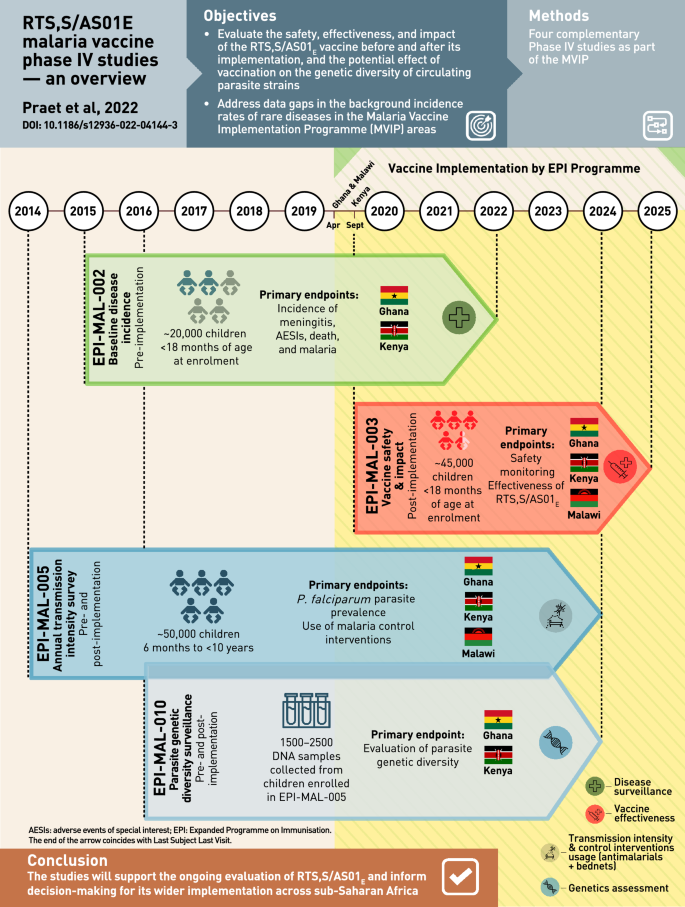
RTS,S/AS01 E (GlaxoSmithKline (GSK), Belgium) is a pre-erythrocytic Plasmodium falciparum malaria vaccine developed for routine immunization of young children living in malaria-endemic countries. In the pivotal Phase III trial, 4 doses of RTS,S/AS01 E administered to children aged 5 months or older reduced clinical malaria by 39% and severe malaria by 29% over 4 years of follow-up [ 1 , 2 ]. In addition, vaccination with RTS,S/AS01 E was associated with a reduction in overall hospitalizations, and hospitalizations due to malaria, severe anaemia and the need for blood transfusion [ 1 , 3 , 4 ]. RTS,S/AS01 E was generally well tolerated and although more reactogenic than control vaccines, local and systemic symptoms were generally transient and mild-to-moderate in intensity [ 1 , 4 ]. There was a higher incidence of febrile convulsions in RTS,S/AS01 E recipients versus controls after vaccination in children aged 5 months or older, with the risk mainly during the first 3 days after vaccination [ 3 ]. Three safety signals were identified during the study. In the 5–17 months age group, higher incidences of meningitis (any cause) and cerebral malaria cases were observed in RTS,S/AS01 E -vaccinated children than in children vaccinated with control vaccines [ 3 ]. In addition, there was a gender-specific imbalance in mortality, with higher mortality rates in girls vaccinated with RTS,S/AS01 E compared to girls vaccinated with control vaccines, without differences in risk factors, time to death or causes of death that could explain the results. No such difference was observed in boys. Detailed case evaluation indicated that these imbalances were likely to be chance findings due to unexpectedly low rates of meningitis in the control group (1 case in approximately 3000 children followed for almost 4 years), or a low mortality rate in girls in the control group, or the lack of biological plausibility to explain the causal relationship to RTS,S/AS01 E vaccination [ 3 ].
In 2015, RTS,S/AS01 E received a positive scientific opinion from the European Medicines Agency [ 5 ]. In a 2016 position paper, the World Health Organization (WHO) acknowledged that several uncertainties related to programmatic feasibility, RTS,S/AS01 E impact and safety remained. The WHO, therefore, adopted the recommendations of the Strategic Advisory Group of Experts on Immunization and the Malaria Policy Advisory Committee who jointly endorsed pilot implementation of the vaccine in 3–5 settings in sub-Saharan Africa [ 6 ]. Under the programme, 3 vaccine doses are being administered to children 5–9 months of age in areas of moderate-to-high transmission of malaria, with a fourth dose 15–18 months later [ 6 ].
In April 2017, the WHO announced that RTS,S/AS01 E would be first introduced in selected areas in Ghana, Kenya and Malawi by the respective routine national immunization programmes in the framework of the Malaria Vaccine Implementation Programme (MVIP). Authorization for use of RTS,S/AS01 E in this context was granted on 24 April 2018 by the Ghana Food and Drug Board, 11 May 2018 by the Kenya Pharmacy and Poisons Board, and 16 May 2018 by the Malawi Pharmacy and Medicines Regulatory Authority. Vaccination started on 23 April 2019 in Malawi, 30 April 2019 in Ghana and 13 September 2019 in Kenya [ 7 ]. RTS,S/AS01 E is the first vaccine to be implemented as a complementary tool to existing interventions under the Global Technical Strategy for Malaria, 2016–2030 [ 8 ]. In 2022 the WHO recommended that RTS,S/AS01 E should be used for the prevention of P. falciparum malaria in children living in regions with moderate to high malaria transmission as part of a comprehensive malaria control strategy [ 9 ].
The MVIP is a collaboration between selected countries and international private and public partners established by the WHO to coordinate, support and evaluate the introduction of RTS,S/AS01 E. Key aspects of the MVIP have been summarized by the WHO in an on-line series of question and answers, in a summary of key milestones in the journey to vaccine implementation, and in the 2021 SAGE report of the RTS,S/AS01 E vaccine [ 10 , 11 , 12 ]. GSK is donating the RTS,S/AS01 E vaccine doses necessary to the MVIP (up to 10 million doses) [ 7 ]. In addition to RTS,S/AS01 E introduction, the MVIP evaluates the vaccine safety, impact, and effectiveness in order to generate information necessary to inform potential future policy for the deployment of RTS,S/AS01 E on a broader scale. A first step is the WHO-commissioned Malaria Vaccine Pilot Evaluation (MVPE). This consists of household surveys, and sentinel hospital and community mortality surveillance, building on routine systems. The MVPE will measure the programmatic feasibility of delivering a 4-dose vaccine schedule, vaccine safety in routine use, and the impact of the malaria vaccine on severe malaria and all-cause mortality. This evaluation is largely based on passive follow-up and comparison of the occurrence of vaccine safety and impact study endpoints between vaccine implementation areas (exposed clusters) and areas where the vaccine is not yet implemented (unexposed clusters) [ 13 ]. Second, as a part of the MVIP, GSK has designed a comprehensive post-approval plan that includes 4 observational studies to assess RTS,S/AS01 E vaccine safety, effectiveness, impact, and the potential effect of vaccination on the genetic diversity of circulating parasite strains. This paper presents an overview of GSK’s post-approval plan currently being conducted in the MVIP areas [ 11 ]. The challenges associated with conducting large observational studies in regions with limited healthcare infrastructures are discussed, as well as the opportunities to leverage existing collaborations, research infrastructure and global expertise. Over the coming years, these studies will contribute to the ongoing assessment of the RTS,S/AS01 E benefit-risk profile, and to informing decisions for its potential wider implementation across malaria endemic areas of Africa.
Overview of GSK’s RTS,S/AS01 E post-approval plan
Many low and lower-middle income countries where malaria is endemic have little or no data on background incidence rates of rare diseases such as those that may be reported as adverse events following immunization. This may prevent robust post-authorization vaccine safety and effectiveness monitoring, and can lead to delays in detecting safety signals, potentially contributing to vaccine hesitancy related to the new vaccine, or to other vaccines introduced in the future. In these settings, disease surveillance studies conducted prior to vaccine introduction can be used to determine reliable background rates of specific diseases/events that can be compared with post-introduction observations. In order to further monitor the benefit-risk profile of RTS,S/AS01 E , GSK’s post-approval plan is designed to assess vaccine safety, impact, and effectiveness in a real-life setting. It comprises 4 GSK-sponsored Phase IV studies (Fig. 1 ) including a before-after comparison in which data collected in the pre-RTS,S/AS01 E vaccine introduction study, EPI-MAL-002 (NCT02374450), and the post-vaccine introduction study, EPI-MAL-003 (NCT03855995), are compared. For operational reasons, the before-after comparison is being conducted in Ghana and Kenya only. In addition, because the Ministries of Health of the implementing countries are not introducing RTS,S/AS01 E into all national areas, vaccine safety and impact will also be assessed using a contemporaneous comparison between exposed and unexposed areas of the study endpoints. Moreover, since annual and/or geographical variations in malaria incidence may occur as a result of changes in malaria transmission intensity or in malaria control intervention coverage, these potential confounders are monitored in the EPI-MAL-005 study (NCT02251704).
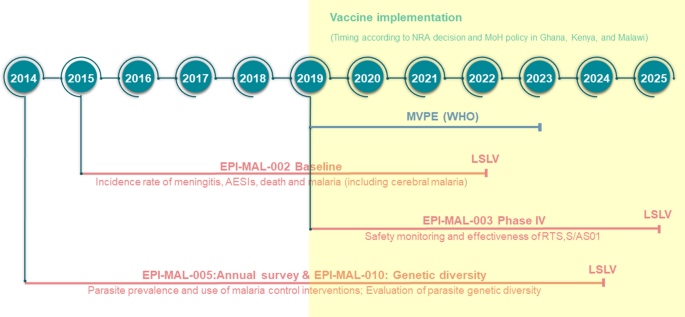
Overview GSK’s RTS,S/AS01 E vaccine post-approval plan embedded within the Malaria Vaccine Implementation Programme. AESIs adverse events of special interest, LSLV last subject last visit, MoH Ministry of Health, MVPE Malaria Vaccine Programme Evaluation, WHO World Health Organization, NRA National Regulatory Authority
EPI-MAL-005 is an observational cross-sectional study assessing P. falciparum parasite prevalence (as a proxy for transmission intensity) and malaria control intervention coverage over 10 annual surveys in the EPI-MAL-002 and EPI-MAL-003 study areas. Results from EPI-MAL-005 will be used to adjust the temporal and contemporaneous comparison analyses for potential year-to-year variation during the conduct of EPI-MAL-002 and EPI-MAL-003. Finally, because P. falciparum has evolved multiple mechanisms to vary cell surface antigens and evade the host’s immune response, an ancillary study to EPI-MAL-005, EPI-MAL-010 (EUPAS42948), has been designed to monitor parasite genetic diversity before and after vaccine implementation.
Assessment of vaccine safety, effectiveness and impact (EPI-MAL-002 and EPI-MAL-003)
Study EPI-MAL-002 is designed to collect incidence data of pre-defined health outcomes, i.e., adverse events of special interest (AESI). These include rare events potentially associated with vaccination (Table 1 ), meningitis, malaria (including severe malaria and cerebral malaria), death and other health outcomes leading to hospitalization, before RTS,S/AS01 E vaccine introduction. To assess vaccine safety, effectiveness and impact, these baseline incidence rates will be compared with rates documented in the post-implementation study EPI-MAL-003 which commenced when RTS,S/AS01 E vaccination was introduced by the Ministries of Health (Fig. 2 ). RTS,S/AS01 E vaccine implementation follows a phased introduction in which RTS,S/AS01 E is introduced into some areas (exposed clusters) but not others (unexposed clusters). Thus, in addition to the before-after comparison, EPI-MAL-003 also includes a contemporaneous comparison of endpoints of interest between vaccinated and unvaccinated study participants.
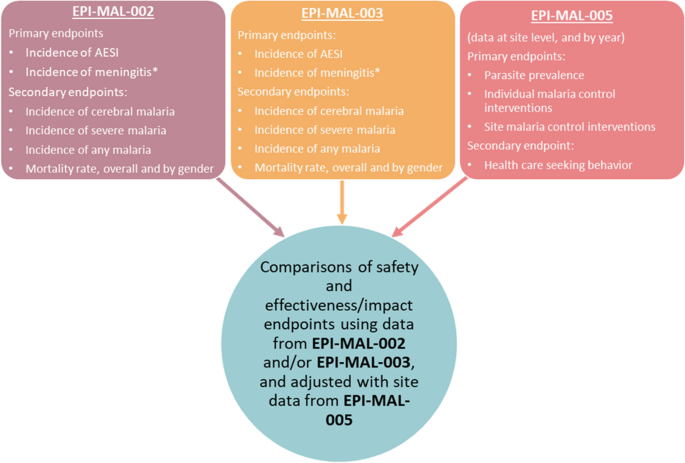
Evaluation of safety, effectiveness and impact using data collected as part of the RTS,S/AS01 E post-approval plan. AESI adverse events of special interest. Asterisk indicates the potential risk of meningitis will be monitored on ongoing basis using the maximized sequential probability ratio test (MaxSPRT) method
Study design and population of EPI-MAL-002 and EPI-MAL-003
GSK’s Phase IV study package is fully embedded in the MVIP. Therefore, selection of participating clusters depended on the cluster identification process led by the Ministries of Health according to the WHO guidance [ 14 ]. EPI-MAL-002 is being conducted in Ghana and Kenya and EPI-MAL-003 in Ghana, Kenya and Malawi. There are 4 study sites (corresponding to 4 clusters of the MVIP) in each country, of which 2 became exposed clusters and 2 became unexposed clusters in EPI-MAL-003. The EPI-MAL-002 and EPI-MAL-003 studies will enroll prospective cohorts of approximately 20,000 and 45,000 children, respectively. The areas participating in EPI-MAL-002 became exposed clusters in EPI-MAL-003 once vaccination commenced, to allow the before-after comparison. EPI-MAL-003 added additional unexposed clusters to allow the contemporaneous comparison. Additionally, both exposed and unexposed clusters were added in Malawi that was not included in study EPI-MAL-002.
To allow direct comparisons of endpoints, EPI-MAL-002 and EPI-MAL-003 are strictly identical in terms of design and conduct. Both are multi-country observational studies with prospective cohort event monitoring. Children < 18 months of age are enrolled during routine immunization with the pentavalent diphtheria-tetanus-pertussis-hepatitis B and Haemophilus influenzae type b vaccine, through direct invitation or when hospitalized before routine immunization. Similarly, EPI-MAL-003 is enrolling children presenting for routine immunization, regardless of their future vaccination status with RTS,S/AS01 E .
In both studies, follow-up until approximately 5 years of age (corresponding to around 2 years after the fourth dose of RTS,S/AS01 E in children in the exposed clusters in EPI-MAL-003) consists of active surveillance for outpatient and inpatient visits by each enrolled participant and of 10 home visits conducted according to a specific time frame (prospective cohort). In addition, hospital-based disease surveillance is organized across the entire study area for infants and children who are not enrolled in the prospective cohort. In other words, throughout the whole study period, all hospitalized children under the age of 5 years who are not already enrolled in the prospective cohort and who live within the study areas are eligible for enrolment in the hospital-based disease surveillance.
Objectives and endpoints
The study objectives are to estimate the incidence of protocol-defined events, including AESI, meningitis, other adverse events leading to hospitalization, death, and malaria (including severe malaria and cerebral malaria). AESI (Table 1 ) are events that have historically been associated with other vaccines, that may be hypothetically associated with RTS,S/AS01 E given that this vaccine has relatively new components compared to other widely used vaccines, or that were identified from the results of the Phase III efficacy study (meningitis, severe malaria and cerebral malaria, gender-specific mortality) [ 1 , 3 ]. Estimated incidences will be used to monitor (1) vaccine safety and (2) vaccine effects (direct, indirect, total and overall effects) on the incidence of any malaria, severe malaria, cerebral malaria, all-cause hospitalizations, malaria-attributable hospitalizations, the prevalence of anaemia in hospitalized children, and the mortality rate. The direct effect (effectiveness) of RTS,S/AS01 E will compare malaria-related events in vaccinated and unvaccinated children from exposed clusters enrolled in active surveillance. Vaccine impact (indirect, total and overall effects) will be investigated by comparing the incidence of malaria-related events in unvaccinated children (either from EPI-MAL-002 or from EPI- MAL-003 unexposed clusters) with the incidence of events in children from EPI-MAL-003 exposed clusters (either unvaccinated children [indirect effect], vaccinated children [total effect] or both [overall effect]).
Methods and analysis
The studies are conducted in the setting of routine medical practice and local laboratory testing (first-line testing), and include a strong support component comprised of study-specific trainings, telemedicine support, second-line laboratory testing, and consultation with an external expert panel for final case classification. These tools are likely to enhance case detection and ascertainment rates. Both EPI-MAL-002 and EPI-MAL-003 use identical surveillance in order to allow a robust comparison of study outcomes before and after the RTS,S/AS01 E vaccine introduction.
Freely given and written or witnessed and thumb-printed informed consent is obtained from each study participant’s parent/legal representative prior to study participation. Protocol-specified procedures include the collection of demographic data, vaccination records, medical history, medical care episodes, adverse events, use of malaria control measures, development delays or death, and a physical examination when contacts between study staff and subjects occur. The protocol requires collection of a sample of 5 ml of blood for testing at an external laboratory (second-line laboratory) in all suspected cases of AESI and meningitis. All study participants are treated according to good medical and routine practices [ 15 ]. In cases where a lumbar puncture is performed according to routine medical practice, when possible an aliquot of cerebro-spinal fluid (CSF) is required to be sent to the second-line laboratory for testing. An independent panel of external medical experts perform blinded reviews of all suspected cases of meningitis and cerebral malaria, as well as any case of any other endpoint for which the diagnosis is equivocal. The cause of death is determined from medical records when available, or through verbal autopsy for children who die in the community.
Incidence rates of safety endpoints will be calculated and compared based on both before-after and between cluster comparisons using a univariable and multivariable Poisson regression model adjusted for specific covariates, including if applicable, those identified in EPI-MAL-005. The vaccination status of participants will be confirmed using data from individual vaccination cards, vaccination registers and health and demographic surveillance system (HDSS) or equivalent surveillance system. Incidence rates will be computed using person-time denominators for group of interest. Each child will contribute person-time until study end or 5 years of age, whichever occurs first.
In addition, the potential risk of meningitis is being monitored in near real time using the maximized sequential probability ratio test (MaxSPRT) method [ 16 ]. MaxSPRT is a continuous sequential test that allows an early detection of safety event signals. Among vaccinated subjects, the maximum likelihood and the log-likelihood ratios are estimated each month if new meningitis cases are detected. The upper limit is estimated based on the results (meningitis incidence estimate) of EPI-MAL-002. If the log-likelihood ratio reaches a critical value, the comparison between vaccinated and unvaccinated study participants will be done. If the signal is confirmed, further investigation will be performed with additional focus on the subset of children diagnosed with meningitis.
Vaccine effectiveness (direct effect) and impact (overall, indirect, and total effects in the same population) of RTS,S/AS01 E on the incidence of malaria will be estimated [ 17 ]. Cases of any malaria identified during outpatient visits and hospitalizations at all healthcare facilities will be expressed per person-year of observation for contributing enrolled children.
EPI-MAL-002 started in January 2016 and an interim analysis has been conducted to provide preliminary results from 14,329 children who participated in the prospective cohort monitoring prior to the introduction of RTS,S/AS01 E vaccination [ 18 ]. EPI-MAL-003 started in March 2019 and is currently ongoing.
Estimating malaria transmission intensity and the use of malaria control interventions (EPI-MAL-005)
It is expected that the use of RTS,S/AS01 E will lead to a reduction in the incidence of malaria disease in vaccinated subjects in EPI-MAL-003 compared to baseline rates recorded in EPI-MAL-002. However, annual fluctuations in malaria incidence occur due to changes in transmission intensity influenced by rainfall patterns or changes in how malaria control interventions are used. By monitoring malaria transmission intensity and the coverage of malaria control interventions in the EPI-MAL-002 and EPI-MAL-003 study sites during the conduct of these studies, EPI-MAL-005 will allow a more accurate estimate of the true impact of RTS,S/AS01 E vaccination. This study will contribute to the analysis of vaccine effectiveness and vaccine impact by identifying variables to be used as covariates to adjust the before-after and between cluster comparisons.
Study design and population
EPI-MAL-005 has a cross-sectional design with yearly surveys coinciding with the recruitment and follow-up periods of the EPI-MAL-002 and EPI-MAL-003 studies. At each survey, 600 participants aged 6 months to < 10 years are randomly selected from the sites participating in the survey in that year, stratified by age group. The selection process will be repeated every year meaning that the subjects will be different in each cross-sectional survey except if they are re-selected in a subsequent survey by chance. The surveys occur during the period of peak malaria transmission that varies from end September to mid-December in western African sites, from end April to mid-August in eastern and southern African sites [ 19 , 20 ].
The co-primary study objectives are estimation of P. falciparum parasite prevalence (in order to characterize malaria transmission intensity), and of the use of malaria control interventions (insecticide-treated nets, long-lasting insecticidal nets, indoor residual spraying, seasonal malaria chemoprevention, intermittent preventative treatment in infants, and artemisinin-based combination therapy).
At each survey, demographic information, medical and vaccination history, information on healthcare-seeking behaviors in the previous 14 days, fever in the last 24 h, and use of malaria control measures (bednets for the night before the visit, coils/repellents over the previous 7 days, anti-malaria medication over the previous 14 days), are recorded for all participants. During the survey visit, axillary body temperature is measured, and a capillary blood sample is collected for malaria testing by microscopy and nucleic acid amplification tests (both asexual and sexual parasitaemia). In the event of fever at the time of the visit, or fever or other symptoms/signs of clinical malaria reported in the previous 24 h, a rapid diagnostic test is conducted, and the participant treated if the test is positive. Participants identified as being parasite-positive following microscopy are treated according to national guidelines.
Parasite prevalence and use of malaria control measures are computed by study site, age group, RTS,S/AS01 E vaccination status and gender. Annual fluctuations in parasite prevalence are estimated using the Cochran-Armitage trend test. The agreement between parasitaemia as measured by microscopy versus nucleic acid amplification tests is assessed using the Cohen’s Kappa coefficient and the Landis and Koch scale. A risk factor analysis for malaria infection is conducted using a multivariable logistic regression analysis.
The study started in October 2014 and the first two surveys have been published [ 21 ]. At the time of the study completion, data from approximately 50,000 participants will be available, providing a comprehensive picture on malaria prevalence variations across the study sites.
Monitoring P. falciparum genetic diversity (EPI-MAL-010)
Although the central NANP amino acid repeat sequence of the circumsporozoite protein used as a major component of the RTS,S vaccine antigen is normally well conserved across parasite strains, P. falciparum is a pathogen with high variability and a high number of different circulating haplotypes. The parasite uses numerous mechanisms to vary cell surface antigens and evade the host immune response. For this reason, there is a potential concern that widespread implementation of RTS,S/AS01 E could drive the selection of specific parasite variants or alter the number of parasite haplotypes over time by exerting selective pressure. EPI-MAL-010 is an ancillary study to EPI-MAL-005 that will monitor the genetic diversity of circumsporozoite protein sequences in the P. falciparum parasite population before and after vaccine implementation.
EPI-MAL-010 has a longitudinal, cross-sectional study design and uses capillary blood samples collected from participants enrolled in EPI-MAL-005 over 7 survey years. Samples are from participants aged 6 months to < 5 years with P. falciparum parasitaemia confirmed by microscopy and/or nucleic acid amplification tests, and collected before and after RTS,S/AS01 E implementation in two study sites: Kintampo (Ghana) in Western Africa and Kombewa (Kenya) in Eastern Africa.
This study is estimating P. falciparum haplotype prevalence (i.e., the proportion of participants infected with a specific haplotype) and frequency (i.e., the proportion of a specific haplotype among all detected malaria clones) in participants aged 6 months to < 5 years vaccinated or not with RTS,S/AS01 E .
Amplicon sequencing is conducted on samples tested positive for P. falciparum by microscopy and/or nucleic acid amplification tests. Trends in the prevalence of specific P. falciparum haplotypes with a frequency of at least 5% will be assessed by a logistic regression model. Multinomial logistic regression will be used to describe the annual fluctuations in haplotype frequency using the 3D7 haplotype as the reference group.
Study set-up opportunities and challenges
Considering the specificities of the study setting, GSK together with its local scientific partners, conducted comprehensive study feasibility assessments in which both scientific and operational aspects were carefully balanced to allow generation of robust data. During the planning phase, global experts provided advice on study design and execution. Evolving circumstances, external constraints and the involvement of multiple stakeholders required adaptability and flexibility, balancing an optimal study design with real-world constraints. Designing, setting-up and conducting a complex post-approval plan in sub-Saharan Africa brings some important setting-specific considerations: (1) data collection needs to be performed using prospective follow-up because existing data collection systems and databases may be sub-optimal or absent; (2) vaccine safety, effectiveness and impact data have to be collected in a healthcare environment where case detection and ascertainment may be challenging because of limited healthcare infrastructure and diagnostic capability; (3) there may be limited access to health care in remote settings; (4) background incidence rates of many diseases including study-specific endpoints are limited or not available; (5) standard laboratory procedures may not exist across all study sites; and (6) sample storage and transportation from remote locations can be challenging.
More information on key parameters that were considered in the framework of the study feasibility assessment is provided below. Study site selection was based on specific criteria: (1) the existence of pre-existing scientific research infra-structure capable of expanding beyond routine data collection. Several of the finally selected study sites have research experience in conducting clinical trials with RTS,S/AS01 E , and have developed capability in terms of training, experience and quality of healthcare that resulted from previous study participation. However, for some sites without such past experience, additional investment was required to set up baseline structures and procedures; (2) the existence of a HDSS, part of the International Network for the Demographic Evaluation of Populations and Their Health (INDEPTH), or of an equivalent surveillance system. HDSS sites have a demographic database in place that updates, on a regular basis, the number of births, deaths, immigrations and emigrations, and potentially vaccinations and population health outcomes. The number of HDSS sites in Ghana, Kenya and Malawi being limited, a population census had to be fully established or partially enhanced in approximately half of the study sites.
The EPI-MAL-002 and EPI-MAL-003 studies are mainly based on data collection in the framework of routine medical practice, which may hamper the ascertainment of diseases requiring more advanced diagnostic tools. A full understanding of the structure and capacity of the healthcare system in each country where the studies were planned was required, including the assessment of the capacities for case detection and the ascertainment of study endpoints in each study site. As an outcome of this assessment, specific tools were put in place during the study preparation and conduct to enhance case detection and diagnostic capabilities. These include regular and ongoing medical and pharmacovigilance trainings, telemedicine support (Réseau en Afrique Francophone pour la Télémédecine, Switzerland; Agence de Médecine Préventive, Ivory Coast), the distribution of job aids, the support of local laboratories and the set-up of a central reference laboratory (Clinical Laboratory Services, South Africa) for blood and CSF testing. These enhancement tools should increase the likelihood of reaching the highest possible level of diagnostic certainty. For the key endpoint of meningitis, medical and non-medical staffs have received training on meningitis case detection and ascertainment, which includes information on the national guidelines for case management (including lumbar puncture and testing of CSF) with secondary testing to be performed at a central laboratory when sample volume permits.
This multi-country initiative involves key local and global public health partners. The scientific and operational constraints and the complexity of the study set-up provide opportunities for collaborations and alignment in healthcare approaches between countries to promote best practice. However, turnover of trained personal in the study areas is likely to be an ongoing challenge.
Within countries, the MVIP, and more specifically the study design, set-up and conduct, promote collaboration between different authorities, such as routine healthcare system, diagnostic services, national immunization services, epidemiological research, and National Malaria Control Programmes.
Despite best laid plans, the coronavirus disease 2019 (COVID-19) pandemic in Africa continues to unfold and its impact is evolving. In this environment, the MVIP is continuing and measures are being taken to protect the welfare and safety of participants and study staff, and to ensure data integrity [ 22 ]. No major change in the rate of RTS,S/AS01 E vaccination has been observed during the pandemic thus far, although it might be expected that hospitalization practices may change in order to limit admissions to children with serious or critical conditions and avoid hospital crowding. However, the potential impact of COVID-19 on the evaluation of AESI as determined in pre-vaccination pre-COVID-19 period requires continuous monitoring of the situation. Sensitivity analyses may be conducted considering COVID-19 pandemic periods for both studies.
Conclusions and perspectives
The implementation and the safety, effectiveness and impact evaluation of RTS,S/AS01 E in a real-life setting is a unique and complex undertaking that requires the establishment of large-scale and strong partnerships. Assessing the benefit-risk profile of a 3-dose primary schedule vaccine with a 4 th dose booster administered beyond the usual Expanded Programme on Immunization schedule in low and lower-middle income countries requires the establishment of ad-hoc tools and quality control methods to allow collection of robust and reliable data. Whilst technical and operational expertise is key to achieve this goal, human and financial resource needs should not be underestimated. The effort has been based on the WHO-led MVIP, existing research platforms and expertise in the implementing countries, and significant commitment by GSK. The experience with RTS,S/AS01 E in sub-Saharan Africa highlights important aspects to be considered when planning and implementing a vaccine post-approval plan in low and lower-middle income countries. The RTS,S/AS01 E experience will pave the way for the development and implementation of new generation malaria vaccines, and of other vaccines for the developing world.
Availability of data and materials
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com . To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website.
Change history
23 may 2022.
Following the original publication of this article, the graphic abstract has been updated with the DOI information 10.1186/s12936-022-04144-3, which had erreonously been omitted from the originally published version.
Abbreviations
Adverse events of special interest
Coronavirus disease 2019
Cerebrospinal fluid
GlaxoSmithKline Biologicals SA
Health and demographic surveillance system
International Network for the Demographic Evaluation of Populations and Their Health
Maximized sequential probability ratio test
World Health Organization
Malaria Vaccine Programme Evaluation
Malaria Vaccine Implementation Programme
RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45.
European Medicines Agency. Mosquirix H-W-2300. Product information. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/document_listing/document_listing_000395.jsp&mid= . Accessed 22 May 2020.
Guerra Mendoza Y, Garric E, Leach A, Lievens M, Ofori-Anyinam O, Pircon JY, et al. Safety profile of the RTS, S/AS01 malaria vaccine in infants and children: additional data from a phase III randomized controlled trial in sub-Saharan Africa. Hum Vaccin Immunother. 2019;15:2386–98.
Article Google Scholar
RTS,S Clinical Trials Partnership. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Eng J Med. 2012;367:2284–95.
European Medicines Agency. Mosquirix H-W-2300. European Public Assessment Report. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/document_listing/document_listing_000395.jsp&mid= . Accessed 19 March 2020.
Malaria vaccine: WHO position paper-January 2016. Wkly Epidemiol Rec. 2016;91:33–51.
WHO. Q&A on the malaria vaccine implementation programme (MVIP). Geneva, World Health Organization. https://www.who.int/malaria/media/malaria-vaccine-implementation-qa/en/ . Accessed 22 January 2020.
WHO. Global Technical Strategy for Malaria 2016–2030. Geneva, World Health Organization, 2015. https://www.who.int/malaria/areas/global_technical_strategy/en/ . Accessed 19 March 2020. .
Malaria vaccine: WHO position paper – March 2022. Wkly Epidemiol Rec. 2022;97 61–80.
WHO. Malaria: The malaria vaccine implementation programme (MVIP). Geneva, World Health Organization, 2020. Q&A. https://www.who.int/news-room/questions-and-answers/item/malaria-vaccine-implementation-programme . Accessed 08 March 2022.
WHO. Full evidence report on the RTS,S/AS01 malaria vaccine. SAGE Yellow Book for October 2021. 5.1 malaria. https://cdn.who.int/media/docs/default-source/immunization/mvip/full-evidence-report-on-the-rtss-as01-malaria-vaccine-for-sage-mpag-(sept2021).pdf?sfvrsn=c9737be_5 Accessed 08 March 2022.
WHO. Key milestones in the development of the Malaria Vaccine Implementation Programme (MVIP): from pilot recommendation to vaccine introduction. Geneva, World Health Organization. https://www.who.int/docs/default-source/immunization/mvip/mvip-milestones-to-programme-development-final.pdf?sfvrsn=14768db0_4 . Accessed 16 March 2022.
ClinicalTrials.gov. Malaria vaccine evaluation programme (MVPE). 2019. https://clinicaltrials.gov/ct2/show/NCT03806465?term=vaccine&cond=Malaria&cntry=GH&age=0&draw=2&rank=7 . Accessed 26 May 2021.
WHO. Ghana, Kenya and Malawi to take part in WHO malaria vaccine pilot programme. Geneva, World Health Organization, 2017. https://www.afro.who.int/news/ghana-kenya-and-malawi-take-part-who-malaria-vaccine-pilot-programme . Accessed 22 January 2020.
WHO. Guidelines for the treatment of malaria. 3rd Edn. Geneva, World Health Organization, 2015. http://www.who.int/malaria/publications/atoz/9789241549127/en/ . Accessed 26 February 2019.
Kulldorff M, Davis R, Kolczak M, Lewis E, Lieu T, Platt R. A maximized sequential probability ratio test for drug and vaccine safety surveillance. Seq Anal. 2011;30:58–78.
Halloran ME, Haber M, Longini IM Jr, Struchiner CJ. Direct and indirect effects in vaccine efficacy and effectiveness. Am J Epidemiol. 1991;133:323–31.
Article CAS Google Scholar
RTS,S Epidemiology EPI-MAL-002 Study Group. Baseline incidence of meningitis, malaria, mortality and other health outcomes in infants and young sub-Saharan African children prior to the introduction of the RTS,S/AS01(E) malaria vaccine. Malar J. 2021;20:197.
Mzilahowa T, Hastings IM, Molyneux ME, McCall PJ. Entomological indices of malaria transmission in Chikhwawa district, Southern Malawi. Malar J. 2012;11:380.
Roca-Feltrer A, Schellenberg JR, Smith L, Carneiro I. A simple method for defining malaria seasonality. Malar J. 2009;8:276.
RTS,S Epidemiology EPI-MAL-005 Study Group. Estimating annual fluctuations in malaria transmission intensity and in the use of malaria control interventions in five sub-Saharan African countries. Am J Trop Med Hyg. 2020;103:1883–92.
WHO. Malaria vaccine implementation programme: one year on. Geneva, World Health Organization, 2020. https://www.who.int/immunization/diseases/malaria/malaria_vaccine_implementation_programme/en/ . Accessed 22 May 2020.
Download references
Acknowledgements
The authors thank the children, their families and their communities who participated or will participate in this study. The authors acknowledge the study team members of all research sites: Kintampo Health Research Centre, Navrongo Health Research Centre, University of Malawi College of Medicine, Malawi Liverpool Wellcome Trust Clinical Research Programme, Centre for Research in therapeutic Sciences (CREATES), Kenya Medical Research Institute and KEMRI-Walter Reed Project. The authors also acknowledge the healthcare system institutions for their help and expertise. The authors thank the following individuals or groups for their invaluable contribution to the study: Effua Usuf and PATH: Cheryl Keech. The authors also thank the Modis platform on behalf of GSK for writing assistance provided by Joanne Wolter and manuscript coordination provided by Pauline De Berdt; and the TVF platform on behalf of GSK for designing the infographic provided by Martin Todd.
Elaine Jacqueline Akité, Patrick Odum Ansah, Laurence Baril, Owusu Boahen, Yolanda Guerra Mendoza, Valerie Haine, Simon Kariuki, Mathieu Lamy, Kenneth Maleta, Randy Mungwira, Latif Ndeketa, Abraham Oduro, Bernhards Ogutu, Fredrick Olewe, Martina Oneko, Mattéa Orsini, Francois Roman, Edith Roset Bahmanyar, Dominique Rosillon, Lode Schuerman, Valentine Sing’oei, Dianne J. Terlouw, Stéphanie Wéry—Authors listed in alphabetical order
This work was supported and funded by GlaxoSmithKline Biologicals SA and PATH. The studies EPI-MAL-002 (NCT02374450), EPI-MAL-003 (NCT03855995), EPI-MAL-005 (NCT02251704) and EPI-MAL-010 (GSK study identifier: 205071) are sponsored by the GSK group of companies. GlaxoSmithKline Biologicals SA funded all costs associated with the development and the publishing of the present manuscript.
Author information
Nicolas Praet
Present address: Janssen Pharmaceutica NV, Beerse, Belgium
Dominique Rosillon
Present address: DESiRE-Consulting, Sorée, Belgium
Authors and Affiliations
GSK, Wavre, Belgium
Nicolas Praet, Elaine Jacqueline Akité, Yolanda Guerra Mendoza, Valerie Haine, Francois Roman, Dominique Rosillon, Lode Schuerman, Stéphanie Wéry & Jean-Yves Pirçon
Kintampo Health Research Centre, Research and Development Division, Ghana Health Service, Kintampo North Municipality, Kintampo, Ghana
Kwaku Poku Asante & Owusu Boahen
London School of Hygiene and Tropical Medicine, London, UK
Kwaku Poku Asante
Clinics C/O GSK, Wavre, Belgium
Marie-Cecile Bozonnat & Mattéa Orsini
Navrongo Health Research Centre, Research and Development Division, Ghana Health Service, Navrongo, Ghana
Patrick Odum Ansah & Abraham Oduro
Institut Pasteur, Phnom Penh, Cambodia
Laurence Baril
Kenya Medical Research Institute, Centre for Global Health Research, Kisumu, Kenya
Simon Kariuki & Martina Oneko
Aixial C/O GSK, Wavre, Belgium
Mathieu Lamy
University of Malawi College of Medicine, Mangochi, Malawi
Kenneth Maleta & Randy Mungwira
Malawi Liverpool Wellcome Trust Clinical Research Programme, Kamuzu University of Health Sciences, Blantyre, Malawi
Latif Ndeketa & Dianne J. Terlouw
Centre for Research in Therapeutic Sciences (CREATES), Strathmore University, Nairobi, Kenya
Bernhards Ogutu & Fredrick Olewe
Kenya Medical Research Institute, Centre for Clinical Research, Nairobi, Kenya
Bernhards Ogutu
HQ Global Clinical, Organon International GmbH, Luzern, Switzerland
Edith Roset Bahmanyar
KEMRI-Walter Reed Project, US Army Medical Research Directorate-Kenya, Kombewa, Kenya
Valentine Sing’oei & Walter Otieno
Liverpool School of Tropical Medicine, Liverpool, UK
Dianne J. Terlouw
You can also search for this author in PubMed Google Scholar
Contributions
NP, KPA, MCB, POA, LB, OB, VH, ML, KM, AO, BO, FO, MOr, FR, ERB, DR, LS, WO and JYP designed the studies. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Nicolas Praet .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
EJA, YGM, VH, FR, LS, SW and JYP are employees of the GSK group of companies. At the time of the study NP was an employee of the GSK group of companies and is now employed by Janssen Pharmaceutica NV. At the time of the study DR was an employee of the GSK group of companies and is now a freelance consultant at DESiRE-Consulting. NP, YGM, ML, FR, LS, DR and JYP hold shares in the GSK group of companies. ML is employee of Aixial on behalf of the GSK group of companies. MOr and MCB are employees of 4Clinics on behalf of the GSK group of companies. KPA, POA, OB, SK, KM, RM, LN, AO, BO, FO, MOn, VS, DJT and WO via their institutions, received grants from the GSK group of companies for the conduct of this study/work. KPA, OB, SK, WO, VS, KM and DJT via their institutions, received grants from the GSK group of companies for the conduct of studies outside the submitted work.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Praet, N., Asante, K.P., Bozonnat, MC. et al. Assessing the safety, impact and effectiveness of RTS,S/AS01 E malaria vaccine following its introduction in three sub-Saharan African countries: methodological approaches and study set-up. Malar J 21 , 132 (2022). https://doi.org/10.1186/s12936-022-04144-3
Download citation
Received : 04 June 2021
Accepted : 29 March 2022
Published : 25 April 2022
DOI : https://doi.org/10.1186/s12936-022-04144-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- RTS,S/AS01 E
- Plasmodium falciparum
- Effectiveness
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]

Candidate Malaria Vaccine Provides Lasting Protection
Two NIH-supported trials of an experimental malaria vaccine in healthy Malian adults found that all three tested regimens were safe.
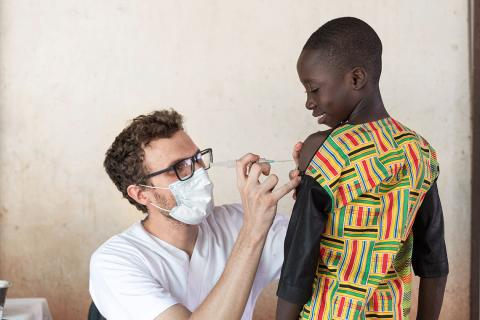
Photo: Riccardo Mayer/Shutterstock
One of the trials enrolled 300 healthy women ages 18 to 38 who anticipated becoming pregnant soon after immunization. That trial began with drug treatment to remove malaria parasites, followed by three injections spaced over a month of either placebo or the investigational vaccine at one of two dosages. Both dosages of the vaccine candidate conferred a significant degree of protection from parasite infection and clinical malaria that was sustained over two years without a booster dose—a first for any malaria vaccine.
In an exploratory analysis of women who conceived during the study, the vaccine offered significant protection from malaria in pregnancy. If confirmed through additional clinical trials, the approach modeled in this study could open improved ways to prevent malaria in pregnancy.
Malaria parasites such as the species Plasmodium falciparum (Pf) can cause illness in people of any age, but pregnant women, infants and very young children are especially vulnerable. Malarial parasitemia in pregnancy causes an estimated 50,000 maternal deaths and 200,000 stillbirths in Africa each year.
The trials were co-led by investigators from NIAID and the University of Sciences, Techniques and Technologies, Bamako (USTTB), Mali. The investigational vaccine used in both trials was PfSPZ Vaccine.
In the first year of the current trial, 55 women became pregnant following the vaccination regimen. Vaccine efficacy against parasitemia (before or during pregnancy) was 65% in the low dose group and 86% in the high dose group. Among 155 women who became pregnant across both study years, the lower and higher dose vaccines were 57 and 49% effective, respectively.
“Preconception immunization is a new strategy to reduce [malaria] mortality for women in pregnancy,” the researchers note. They plan to investigate the safety of PfSPZ administered during pregnancy, then examine its efficacy given preconception or during pregnancy in larger clinical trials.
The NIH Record
The NIH Record , founded in 1949, is the biweekly newsletter for employees of the National Institutes of Health.
Published 25 times each year, it comes out on payday Fridays.
Associate Editor: Dana Talesnik [email protected] (link sends e-mail)
Assistant Editor: Eric Bock [email protected] (link sends e-mail)
Staff Writer: Amber Snyder [email protected] (link sends e-mail)
- NIH Record Home
- Current Issue
- Past Issues
- Submission Deadlines
- Privacy Policy
- Disclaimers
- Accessibility
- Freedom of Information Act
- No Fear Act
- HHS Vulnerability Disclosure
- Office of Inspector General
- '' Subscribe
NIH…Turning Discovery Into Health ®
National Institutes of Health 9000 Rockville Pike
U.S. Department of Health and Human Services (link is external)
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- WHO’s malaria vaccine...
WHO’s malaria vaccine study represents a “serious breach of international ethical standards”
Linked analysis.
WHO’s rollout of malaria vaccine in Africa: can safety questions be answered after only 24 months?
- Related content
- Peer review
- Peter Doshi , associate editor
- pdoshi{at}bmj.com
Experts are troubled by the apparent lack of informed consent in a large, cluster randomised study of the malaria vaccine. Peter Doshi reports
A large scale malaria vaccine study led by the World Health Organization has been criticised by a leading bioethicist for committing a “serious breach” of international ethical standards. The cluster randomised study in Africa is already under way in Malawi, Ghana, and Kenya, where 720 000 children will receive the RTS,S vaccine, known as Mosquirix, over the next two years. 1 2 3
Mosquirix, the world’s first licensed malaria vaccine, was positively reviewed by the European Medicines Agency, but its use is being limited to pilot implementation, in part to evaluate outstanding safety concerns that emerged from previous clinical trials. 3 These were a rate of meningitis in those receiving Mosquirix 10 times that of those who did not, increased cerebral malaria cases, and a doubling in the risk of death (from any cause) in girls. 2
Charles Weijer, a bioethicist at Western University in Canada, told The BMJ that the failure to obtain informed consent from parents whose children are taking part in the study violates the Ottawa Statement, a consensus statement on the ethics of cluster randomised trials, of which Weijer is the lead author, and the Council for International Organizations of Medical Sciences’ International Ethical Guidelines. “The failure to require informed consent is a serious breach of international ethical standards,” he said.
Implied consent
WHO contends that the study is a “pilot introduction” and not a “research activity.” It says that those children living in areas randomised to receive the new vaccine will do so as part of each country’s routine vaccination schedule and that consent is “implied.”
“An implied consent process is one in which parents are informed of imminent vaccination through social mobilisation and communication, sometimes including letters directly addressed to parents. Subsequently, the physical presence of the child or adolescent, with or without an accompanying parent at the vaccination session, is considered to imply consent,” said a WHO spokesperson.
Christine Stabell Benn of the University of Southern Denmark, professor in global health and a vaccine expert who recently published concerns about WHO’s study in The BMJ , 4 added her concerns: “I think parents should be made aware of this doubled female mortality. Imagine that this mortality was a true finding (and remember that it comes on top of five other non-live vaccines being associated with increased female mortality 5 6 7 8 9 ). If true, then how will this be perceived by the participants—that their children were unknowingly involved in a huge experiment by the authorities? This could be a disaster for public trust in vaccines and health authorities.”
In the study, areas are being randomly assigned to either receive malaria vaccine or not. After two years, WHO intends to analyse the data between the two groups to make a decision about whether to recommend wider rollout of the vaccine to other countries. 4
Recipients of the malaria vaccine are not being informed that they are in a study. And the extent to which parents are being given information about the known safety concerns before vaccination is unclear. “Information on vaccination is provided to the community and to parents through health talks and community outreach—among other methods, and parents who present for vaccination do so with the option to vaccinate their children or not,” WHO says.
Weijer says that so called implied consent is “no substitute for informed consent. Indeed, implied consent is no consent at all. We have no assurance that parents in fact received information about the study let alone that they understood it.”
Safety signals
What information parents are provided with in practice is hard to judge. WHO sent The BMJ some training information that it says it has shared with country partners about Mosquirix’s potential risks. The material lists the increased rates of meningitis and cerebral malaria observed in trials and states that they will be monitored. But the potential for increased risk of death among girls is not mentioned.
In a post hoc analysis of the GlaxoSmithKline phase III trial, WHO reported that the all cause mortality rate was “about twofold higher in females” given malaria vaccine versus those in the control arm. 10 A more detailed analysis of the data showed that at study end, 67 of 2967 female children (2.3%) in the malaria vaccine group and 17 of 1503 female children (1.1%) in the control group had died (relative risk 2.00 (95% confidence interval 1.18 to 3.39); risk difference 1.1%, (0.4% to 1.9%); P=0.009). 4 11
When asked why the female mortality signal was not included, WHO cited “insufficient evidence to classify gender specific mortality as a known or potential risk.”
Anders Björkman, a malaria expert at the Karolinska Institute who coauthored the recent analysis published by The BMJ , 4 rejected WHO’s characterisation of gender specific mortality as not even rising to the level of a “potential risk.” “Whether the evidence to call it a known risk is sufficient or not,” he said, “it remains a potential risk, so that is a wrong statement according to me.”
Questionable ethics
It is unclear whether any ethical bodies specifically reviewed and signed off on the “implied consent” process already under way. The BMJ asked WHO whether the agency’s Research Ethics Review Committee, which approved the study protocol in February 2018, waived the requirement for individual informed consent.
WHO did not answer the question directly, instead referring to the process as one used by the ministries of health in Ghana, Kenya, and Malawi. “The vaccine deployment is led by the countries and it is done in the context of routine vaccinations, where there is no requirement for written individual consent.” It said that “care givers are free to decline if they do not wish their child to receive the vaccine.”
McGill bioethicist Jonathan Kimmelman commented, “If an activity is classified as research, then all sorts of rules and oversight mechanisms are activated. For example, the activity must receive prospective ethical review. Unless certain conditions are met, human subjects must provide informed consent.” He added, “The fact that the activity has been registered in clinicaltrials.gov [ NCT03806465 ] amounts to an open declaration that this is research.”
Weijer doubted a research ethics committee would have ever given permission for waiving the need for informed consent. “It is difficult to see how a research ethics committee could have approved a waiver of consent for the WHO malaria vaccine pilot cluster randomized trial,” pointing out that neither the Ottawa Statement 12 nor the CIOMS international ethical guidelines (that WHO says it follows 13 ) support the use of waivers of consent in cluster trials of drugs or vaccines.
He also noted that the human rights provisions of the Malawi constitution include a specific provision prohibiting the use of a waiver under any circumstances: “No person shall be subjected to medical or scientific experimentation without his or her consent.”
Competing interests: See https://www.bmj.com/about-bmj/editorial-staff/peter-doshi
Provenance and peer review: Commissioned; not externally peer reviewed.
- ↵ World Health Organization. Proposed framework for policy decision on RTS,S/AS01 malaria vaccine. 2019 Apr 10. https://www.who.int/malaria/mpac/proposed-framework-for-policy-decision-on-rtss-as01-malaria-vaccine.pdf
- ↵ Framework for Policy Decision on RTS,S/AS01 Working Group, WHO Secretariat. Malaria vaccine implementation programme (MVIP): proposed framework for policy decision on RTS,S/AS01 malaria vaccine. 2019 Mar. https://www.who.int/immunization/sage/meetings/2019/april/1_Session_7_Framework_for_Policy_Decision_on_RTSS-AS01_-_MALARIA_VACCINE_(for_print).pdf
- Fisker AB ,
- Björkman A ,
- Garly M-L ,
- Martins CL ,
- Nielsen J ,
- Rodrigues A ,
- Biering-Sørensen S ,
- Andersen A ,
- ↵ Malaria vaccine: WHO position paper-January 2016 . Wkly Epidemiol Rec 2016 ; 91 : 33 - 51 . pmid: 26829826 OpenUrl PubMed
- Grimshaw JM ,
- Eccles MP ,
- Ottawa Ethics of Cluster Randomized Trials Consensus Group
- ↵ World Health Organization. Research ethics review committee. 2020 https://www.who.int/ethics/review-committee/en/
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Assessing the safety, impact and effectiveness of RTS,S/AS01 E malaria vaccine following its introduction in three sub-Saharan African countries: methodological approaches and study set-up
Nicolas praet.
1 GSK, Wavre, Belgium
16 Present Address: Janssen Pharmaceutica NV, Beerse, Belgium
Kwaku Poku Asante
2 Kintampo Health Research Centre, Research and Development Division, Ghana Health Service, Kintampo North Municipality, Kintampo, Ghana
3 London School of Hygiene and Tropical Medicine, London, UK
Marie-Cecile Bozonnat
4 Clinics C/O GSK, Wavre, Belgium
Elaine Jacqueline Akité
Patrick odum ansah.
5 Navrongo Health Research Centre, Research and Development Division, Ghana Health Service, Navrongo, Ghana
Laurence Baril
6 Institut Pasteur, Phnom Penh, Cambodia
Owusu Boahen
Yolanda guerra mendoza, valerie haine, simon kariuki.
7 Kenya Medical Research Institute, Centre for Global Health Research, Kisumu, Kenya
Mathieu Lamy
8 Aixial C/O GSK, Wavre, Belgium
Kenneth Maleta
9 University of Malawi College of Medicine, Mangochi, Malawi
Randy Mungwira
Latif ndeketa.
10 Malawi Liverpool Wellcome Trust Clinical Research Programme, Kamuzu University of Health Sciences, Blantyre, Malawi
Abraham Oduro
Bernhards ogutu.
11 Centre for Research in Therapeutic Sciences (CREATES), Strathmore University, Nairobi, Kenya
12 Kenya Medical Research Institute, Centre for Clinical Research, Nairobi, Kenya
Fredrick Olewe
Martina oneko, mattéa orsini, francois roman, edith roset bahmanyar.
13 HQ Global Clinical, Organon International GmbH, Luzern, Switzerland
Dominique Rosillon
17 Present Address: DESiRE-Consulting, Sorée, Belgium
Lode Schuerman
Valentine sing’oei.
14 KEMRI-Walter Reed Project, US Army Medical Research Directorate-Kenya, Kombewa, Kenya
Dianne J. Terlouw
15 Liverpool School of Tropical Medicine, Liverpool, UK
Stéphanie Wéry
Walter otieno, jean-yves pirçon, associated data.
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com . To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website.
Following a 30-year development process, RTS,S/AS01 E (GSK, Belgium) is the first malaria vaccine to reach Phase IV assessments. The World Health Organization-commissioned Malaria Vaccine Implementation Programme (MVIP) is coordinating the delivery of RTS,S/AS01 E through routine national immunization programmes in areas of 3 countries in sub-Saharan Africa. The first doses were given in the participating MVIP areas in Malawi on 23 April, Ghana on 30 April, and Kenya on 13 September 2019. The countries participating in the MVIP have little or no baseline incidence data on rare diseases, some of which may be associated with immunization, a deficit that could compromise the interpretation of possible adverse events reported following the introduction of a new vaccine in the paediatric population. Further, effects of vaccination on malaria transmission, existing malaria control strategies, and possible vaccine-mediated selective pressure on Plasmodium falciparum variants, could also impact long-term malaria control. To address this data gap and as part of its post-approval commitments, GSK has developed a post-approval plan comprising of 4 complementary Phase IV studies that will evaluate safety, effectiveness and impact of RTS,S/AS01 E through active participant follow-up in the context of its real-life implementation.
EPI-MAL-002 (NCT02374450) is a pre-implementation safety surveillance study that is establishing the background incidence rates of protocol-defined adverse events of special interest. EPI-MAL-003 (NCT03855995) is an identically designed post-implementation safety and vaccine impact study. EPI-MAL-005 (NCT02251704) is a cross-sectional pre- and post-implementation study to measure malaria transmission intensity and monitor the use of other malaria control interventions in the study areas, and EPI-MAL-010 (EUPAS42948) will evaluate the P. falciparum genetic diversity in the periods before and after vaccine implementation.
GSK’s post-approval plan has been designed to address important knowledge gaps in RTS,S/AS01 E vaccine safety, effectiveness and impact. The studies are currently being conducted in the MVIP areas. Their implementation has provided opportunities and posed challenges linked to conducting large studies in regions where healthcare infrastructure is limited. The results from these studies will support ongoing evaluation of RTS,S/AS01 E ’s benefit-risk and inform decision-making for its potential wider implementation across sub-Saharan Africa.
Graphic abstract

RTS,S/AS01 E (GlaxoSmithKline (GSK), Belgium) is a pre-erythrocytic Plasmodium falciparum malaria vaccine developed for routine immunization of young children living in malaria-endemic countries. In the pivotal Phase III trial, 4 doses of RTS,S/AS01 E administered to children aged 5 months or older reduced clinical malaria by 39% and severe malaria by 29% over 4 years of follow-up [ 1 , 2 ]. In addition, vaccination with RTS,S/AS01 E was associated with a reduction in overall hospitalizations, and hospitalizations due to malaria, severe anaemia and the need for blood transfusion [ 1 , 3 , 4 ]. RTS,S/AS01 E was generally well tolerated and although more reactogenic than control vaccines, local and systemic symptoms were generally transient and mild-to-moderate in intensity [ 1 , 4 ]. There was a higher incidence of febrile convulsions in RTS,S/AS01 E recipients versus controls after vaccination in children aged 5 months or older, with the risk mainly during the first 3 days after vaccination [ 3 ]. Three safety signals were identified during the study. In the 5–17 months age group, higher incidences of meningitis (any cause) and cerebral malaria cases were observed in RTS,S/AS01 E -vaccinated children than in children vaccinated with control vaccines [ 3 ]. In addition, there was a gender-specific imbalance in mortality, with higher mortality rates in girls vaccinated with RTS,S/AS01 E compared to girls vaccinated with control vaccines, without differences in risk factors, time to death or causes of death that could explain the results. No such difference was observed in boys. Detailed case evaluation indicated that these imbalances were likely to be chance findings due to unexpectedly low rates of meningitis in the control group (1 case in approximately 3000 children followed for almost 4 years), or a low mortality rate in girls in the control group, or the lack of biological plausibility to explain the causal relationship to RTS,S/AS01 E vaccination [ 3 ].
In 2015, RTS,S/AS01 E received a positive scientific opinion from the European Medicines Agency [ 5 ]. In a 2016 position paper, the World Health Organization (WHO) acknowledged that several uncertainties related to programmatic feasibility, RTS,S/AS01 E impact and safety remained. The WHO, therefore, adopted the recommendations of the Strategic Advisory Group of Experts on Immunization and the Malaria Policy Advisory Committee who jointly endorsed pilot implementation of the vaccine in 3–5 settings in sub-Saharan Africa [ 6 ]. Under the programme, 3 vaccine doses are being administered to children 5–9 months of age in areas of moderate-to-high transmission of malaria, with a fourth dose 15–18 months later [ 6 ].
In April 2017, the WHO announced that RTS,S/AS01 E would be first introduced in selected areas in Ghana, Kenya and Malawi by the respective routine national immunization programmes in the framework of the Malaria Vaccine Implementation Programme (MVIP). Authorization for use of RTS,S/AS01 E in this context was granted on 24 April 2018 by the Ghana Food and Drug Board, 11 May 2018 by the Kenya Pharmacy and Poisons Board, and 16 May 2018 by the Malawi Pharmacy and Medicines Regulatory Authority. Vaccination started on 23 April 2019 in Malawi, 30 April 2019 in Ghana and 13 September 2019 in Kenya [ 7 ]. RTS,S/AS01 E is the first vaccine to be implemented as a complementary tool to existing interventions under the Global Technical Strategy for Malaria, 2016–2030 [ 8 ]. In 2022 the WHO recommended that RTS,S/AS01 E should be used for the prevention of P. falciparum malaria in children living in regions with moderate to high malaria transmission as part of a comprehensive malaria control strategy [ 9 ].
The MVIP is a collaboration between selected countries and international private and public partners established by the WHO to coordinate, support and evaluate the introduction of RTS,S/AS01 E. Key aspects of the MVIP have been summarized by the WHO in an on-line series of question and answers, in a summary of key milestones in the journey to vaccine implementation, and in the 2021 SAGE report of the RTS,S/AS01 E vaccine [ 10 – 12 ]. GSK is donating the RTS,S/AS01 E vaccine doses necessary to the MVIP (up to 10 million doses) [ 7 ]. In addition to RTS,S/AS01 E introduction, the MVIP evaluates the vaccine safety, impact, and effectiveness in order to generate information necessary to inform potential future policy for the deployment of RTS,S/AS01 E on a broader scale. A first step is the WHO-commissioned Malaria Vaccine Pilot Evaluation (MVPE). This consists of household surveys, and sentinel hospital and community mortality surveillance, building on routine systems. The MVPE will measure the programmatic feasibility of delivering a 4-dose vaccine schedule, vaccine safety in routine use, and the impact of the malaria vaccine on severe malaria and all-cause mortality. This evaluation is largely based on passive follow-up and comparison of the occurrence of vaccine safety and impact study endpoints between vaccine implementation areas (exposed clusters) and areas where the vaccine is not yet implemented (unexposed clusters) [ 13 ]. Second, as a part of the MVIP, GSK has designed a comprehensive post-approval plan that includes 4 observational studies to assess RTS,S/AS01 E vaccine safety, effectiveness, impact, and the potential effect of vaccination on the genetic diversity of circulating parasite strains. This paper presents an overview of GSK’s post-approval plan currently being conducted in the MVIP areas [ 11 ]. The challenges associated with conducting large observational studies in regions with limited healthcare infrastructures are discussed, as well as the opportunities to leverage existing collaborations, research infrastructure and global expertise. Over the coming years, these studies will contribute to the ongoing assessment of the RTS,S/AS01 E benefit-risk profile, and to informing decisions for its potential wider implementation across malaria endemic areas of Africa.
Overview of GSK’s RTS,S/AS01 E post-approval plan
Many low and lower-middle income countries where malaria is endemic have little or no data on background incidence rates of rare diseases such as those that may be reported as adverse events following immunization. This may prevent robust post-authorization vaccine safety and effectiveness monitoring, and can lead to delays in detecting safety signals, potentially contributing to vaccine hesitancy related to the new vaccine, or to other vaccines introduced in the future. In these settings, disease surveillance studies conducted prior to vaccine introduction can be used to determine reliable background rates of specific diseases/events that can be compared with post-introduction observations. In order to further monitor the benefit-risk profile of RTS,S/AS01 E , GSK’s post-approval plan is designed to assess vaccine safety, impact, and effectiveness in a real-life setting. It comprises 4 GSK-sponsored Phase IV studies (Fig. 1 ) including a before-after comparison in which data collected in the pre-RTS,S/AS01 E vaccine introduction study, EPI-MAL-002 (NCT02374450), and the post-vaccine introduction study, EPI-MAL-003 (NCT03855995), are compared. For operational reasons, the before-after comparison is being conducted in Ghana and Kenya only. In addition, because the Ministries of Health of the implementing countries are not introducing RTS,S/AS01 E into all national areas, vaccine safety and impact will also be assessed using a contemporaneous comparison between exposed and unexposed areas of the study endpoints. Moreover, since annual and/or geographical variations in malaria incidence may occur as a result of changes in malaria transmission intensity or in malaria control intervention coverage, these potential confounders are monitored in the EPI-MAL-005 study (NCT02251704).

Overview GSK’s RTS,S/AS01 E vaccine post-approval plan embedded within the Malaria Vaccine Implementation Programme. AESIs adverse events of special interest, LSLV last subject last visit, MoH Ministry of Health, MVPE Malaria Vaccine Programme Evaluation, WHO World Health Organization, NRA National Regulatory Authority
EPI-MAL-005 is an observational cross-sectional study assessing P. falciparum parasite prevalence (as a proxy for transmission intensity) and malaria control intervention coverage over 10 annual surveys in the EPI-MAL-002 and EPI-MAL-003 study areas. Results from EPI-MAL-005 will be used to adjust the temporal and contemporaneous comparison analyses for potential year-to-year variation during the conduct of EPI-MAL-002 and EPI-MAL-003. Finally, because P. falciparum has evolved multiple mechanisms to vary cell surface antigens and evade the host’s immune response, an ancillary study to EPI-MAL-005, EPI-MAL-010 (EUPAS42948), has been designed to monitor parasite genetic diversity before and after vaccine implementation.
Assessment of vaccine safety, effectiveness and impact (EPI-MAL-002 and EPI-MAL-003)
Study EPI-MAL-002 is designed to collect incidence data of pre-defined health outcomes, i.e., adverse events of special interest (AESI). These include rare events potentially associated with vaccination (Table (Table1), 1 ), meningitis, malaria (including severe malaria and cerebral malaria), death and other health outcomes leading to hospitalization, before RTS,S/AS01 E vaccine introduction. To assess vaccine safety, effectiveness and impact, these baseline incidence rates will be compared with rates documented in the post-implementation study EPI-MAL-003 which commenced when RTS,S/AS01 E vaccination was introduced by the Ministries of Health (Fig. 2 ). RTS,S/AS01 E vaccine implementation follows a phased introduction in which RTS,S/AS01 E is introduced into some areas (exposed clusters) but not others (unexposed clusters). Thus, in addition to the before-after comparison, EPI-MAL-003 also includes a contemporaneous comparison of endpoints of interest between vaccinated and unvaccinated study participants.
Summary of safety endpoints for evaluation in studies EPI-MAL-002 and EPI-MAL-003
| Study endpoints | Event |
|---|---|
| Adverse events of special interest* | Acute disseminated encephalomyelitis, encephalitis, Guillain-Barré syndrome, generalized convulsive seizure, hypotonic hypo-responsive episode, intussusception, hepatic insufficiency, renal insufficiency, juvenile chronic arthritis, Stevens Johnson syndrome and toxic epidermal necrolysis, Henoch Schonlein purpura, Kawasaki disease, diabetes mellitus type 1, thrombocytopenia and anaphylaxis |
| Meningitis* | Etiology confirmed meningitis, etiology confirmed, probable and clinically suspected meningitis, clinically suspected meningitis |
| Malaria | Any malaria, severe malaria, cerebral malaria |
| Deaths | Deaths all causes, death all causes: female/male |
| Other adverse events leading to hospitalization | Anemia, gastroenteritis, lower respiratory tract infection, sepsis, upper respiratory tract infection, skin infection, malnutrition, conjunctivitis, helminthic infection, urinary tract infection, bacterial infection, burn |
*Co-primary endpoints

Evaluation of safety, effectiveness and impact using data collected as part of the RTS,S/AS01 E post-approval plan. AESI adverse events of special interest. Asterisk indicates the potential risk of meningitis will be monitored on ongoing basis using the maximized sequential probability ratio test (MaxSPRT) method
Study design and population of EPI-MAL-002 and EPI-MAL-003
GSK’s Phase IV study package is fully embedded in the MVIP. Therefore, selection of participating clusters depended on the cluster identification process led by the Ministries of Health according to the WHO guidance [ 14 ]. EPI-MAL-002 is being conducted in Ghana and Kenya and EPI-MAL-003 in Ghana, Kenya and Malawi. There are 4 study sites (corresponding to 4 clusters of the MVIP) in each country, of which 2 became exposed clusters and 2 became unexposed clusters in EPI-MAL-003. The EPI-MAL-002 and EPI-MAL-003 studies will enroll prospective cohorts of approximately 20,000 and 45,000 children, respectively. The areas participating in EPI-MAL-002 became exposed clusters in EPI-MAL-003 once vaccination commenced, to allow the before-after comparison. EPI-MAL-003 added additional unexposed clusters to allow the contemporaneous comparison. Additionally, both exposed and unexposed clusters were added in Malawi that was not included in study EPI-MAL-002.
To allow direct comparisons of endpoints, EPI-MAL-002 and EPI-MAL-003 are strictly identical in terms of design and conduct. Both are multi-country observational studies with prospective cohort event monitoring. Children < 18 months of age are enrolled during routine immunization with the pentavalent diphtheria-tetanus-pertussis-hepatitis B and Haemophilus influenzae type b vaccine, through direct invitation or when hospitalized before routine immunization. Similarly, EPI-MAL-003 is enrolling children presenting for routine immunization, regardless of their future vaccination status with RTS,S/AS01 E .
In both studies, follow-up until approximately 5 years of age (corresponding to around 2 years after the fourth dose of RTS,S/AS01 E in children in the exposed clusters in EPI-MAL-003) consists of active surveillance for outpatient and inpatient visits by each enrolled participant and of 10 home visits conducted according to a specific time frame (prospective cohort). In addition, hospital-based disease surveillance is organized across the entire study area for infants and children who are not enrolled in the prospective cohort. In other words, throughout the whole study period, all hospitalized children under the age of 5 years who are not already enrolled in the prospective cohort and who live within the study areas are eligible for enrolment in the hospital-based disease surveillance.
Objectives and endpoints
The study objectives are to estimate the incidence of protocol-defined events, including AESI, meningitis, other adverse events leading to hospitalization, death, and malaria (including severe malaria and cerebral malaria). AESI (Table (Table1) 1 ) are events that have historically been associated with other vaccines, that may be hypothetically associated with RTS,S/AS01 E given that this vaccine has relatively new components compared to other widely used vaccines, or that were identified from the results of the Phase III efficacy study (meningitis, severe malaria and cerebral malaria, gender-specific mortality) [ 1 , 3 ]. Estimated incidences will be used to monitor (1) vaccine safety and (2) vaccine effects (direct, indirect, total and overall effects) on the incidence of any malaria, severe malaria, cerebral malaria, all-cause hospitalizations, malaria-attributable hospitalizations, the prevalence of anaemia in hospitalized children, and the mortality rate. The direct effect (effectiveness) of RTS,S/AS01 E will compare malaria-related events in vaccinated and unvaccinated children from exposed clusters enrolled in active surveillance. Vaccine impact (indirect, total and overall effects) will be investigated by comparing the incidence of malaria-related events in unvaccinated children (either from EPI-MAL-002 or from EPI- MAL-003 unexposed clusters) with the incidence of events in children from EPI-MAL-003 exposed clusters (either unvaccinated children [indirect effect], vaccinated children [total effect] or both [overall effect]).
Methods and analysis
The studies are conducted in the setting of routine medical practice and local laboratory testing (first-line testing), and include a strong support component comprised of study-specific trainings, telemedicine support, second-line laboratory testing, and consultation with an external expert panel for final case classification. These tools are likely to enhance case detection and ascertainment rates. Both EPI-MAL-002 and EPI-MAL-003 use identical surveillance in order to allow a robust comparison of study outcomes before and after the RTS,S/AS01 E vaccine introduction.
Freely given and written or witnessed and thumb-printed informed consent is obtained from each study participant’s parent/legal representative prior to study participation. Protocol-specified procedures include the collection of demographic data, vaccination records, medical history, medical care episodes, adverse events, use of malaria control measures, development delays or death, and a physical examination when contacts between study staff and subjects occur. The protocol requires collection of a sample of 5 ml of blood for testing at an external laboratory (second-line laboratory) in all suspected cases of AESI and meningitis. All study participants are treated according to good medical and routine practices [ 15 ]. In cases where a lumbar puncture is performed according to routine medical practice, when possible an aliquot of cerebro-spinal fluid (CSF) is required to be sent to the second-line laboratory for testing. An independent panel of external medical experts perform blinded reviews of all suspected cases of meningitis and cerebral malaria, as well as any case of any other endpoint for which the diagnosis is equivocal. The cause of death is determined from medical records when available, or through verbal autopsy for children who die in the community.
Incidence rates of safety endpoints will be calculated and compared based on both before-after and between cluster comparisons using a univariable and multivariable Poisson regression model adjusted for specific covariates, including if applicable, those identified in EPI-MAL-005. The vaccination status of participants will be confirmed using data from individual vaccination cards, vaccination registers and health and demographic surveillance system (HDSS) or equivalent surveillance system. Incidence rates will be computed using person-time denominators for group of interest. Each child will contribute person-time until study end or 5 years of age, whichever occurs first.
In addition, the potential risk of meningitis is being monitored in near real time using the maximized sequential probability ratio test (MaxSPRT) method [ 16 ]. MaxSPRT is a continuous sequential test that allows an early detection of safety event signals. Among vaccinated subjects, the maximum likelihood and the log-likelihood ratios are estimated each month if new meningitis cases are detected. The upper limit is estimated based on the results (meningitis incidence estimate) of EPI-MAL-002. If the log-likelihood ratio reaches a critical value, the comparison between vaccinated and unvaccinated study participants will be done. If the signal is confirmed, further investigation will be performed with additional focus on the subset of children diagnosed with meningitis.
Vaccine effectiveness (direct effect) and impact (overall, indirect, and total effects in the same population) of RTS,S/AS01 E on the incidence of malaria will be estimated [ 17 ]. Cases of any malaria identified during outpatient visits and hospitalizations at all healthcare facilities will be expressed per person-year of observation for contributing enrolled children.
EPI-MAL-002 started in January 2016 and an interim analysis has been conducted to provide preliminary results from 14,329 children who participated in the prospective cohort monitoring prior to the introduction of RTS,S/AS01 E vaccination [ 18 ]. EPI-MAL-003 started in March 2019 and is currently ongoing.
Estimating malaria transmission intensity and the use of malaria control interventions (EPI-MAL-005)
It is expected that the use of RTS,S/AS01 E will lead to a reduction in the incidence of malaria disease in vaccinated subjects in EPI-MAL-003 compared to baseline rates recorded in EPI-MAL-002. However, annual fluctuations in malaria incidence occur due to changes in transmission intensity influenced by rainfall patterns or changes in how malaria control interventions are used. By monitoring malaria transmission intensity and the coverage of malaria control interventions in the EPI-MAL-002 and EPI-MAL-003 study sites during the conduct of these studies, EPI-MAL-005 will allow a more accurate estimate of the true impact of RTS,S/AS01 E vaccination. This study will contribute to the analysis of vaccine effectiveness and vaccine impact by identifying variables to be used as covariates to adjust the before-after and between cluster comparisons.
Study design and population
EPI-MAL-005 has a cross-sectional design with yearly surveys coinciding with the recruitment and follow-up periods of the EPI-MAL-002 and EPI-MAL-003 studies. At each survey, 600 participants aged 6 months to < 10 years are randomly selected from the sites participating in the survey in that year, stratified by age group. The selection process will be repeated every year meaning that the subjects will be different in each cross-sectional survey except if they are re-selected in a subsequent survey by chance. The surveys occur during the period of peak malaria transmission that varies from end September to mid-December in western African sites, from end April to mid-August in eastern and southern African sites [ 19 , 20 ].
The co-primary study objectives are estimation of P. falciparum parasite prevalence (in order to characterize malaria transmission intensity), and of the use of malaria control interventions (insecticide-treated nets, long-lasting insecticidal nets, indoor residual spraying, seasonal malaria chemoprevention, intermittent preventative treatment in infants, and artemisinin-based combination therapy).
At each survey, demographic information, medical and vaccination history, information on healthcare-seeking behaviors in the previous 14 days, fever in the last 24 h, and use of malaria control measures (bednets for the night before the visit, coils/repellents over the previous 7 days, anti-malaria medication over the previous 14 days), are recorded for all participants. During the survey visit, axillary body temperature is measured, and a capillary blood sample is collected for malaria testing by microscopy and nucleic acid amplification tests (both asexual and sexual parasitaemia). In the event of fever at the time of the visit, or fever or other symptoms/signs of clinical malaria reported in the previous 24 h, a rapid diagnostic test is conducted, and the participant treated if the test is positive. Participants identified as being parasite-positive following microscopy are treated according to national guidelines.
Parasite prevalence and use of malaria control measures are computed by study site, age group, RTS,S/AS01 E vaccination status and gender. Annual fluctuations in parasite prevalence are estimated using the Cochran-Armitage trend test. The agreement between parasitaemia as measured by microscopy versus nucleic acid amplification tests is assessed using the Cohen’s Kappa coefficient and the Landis and Koch scale. A risk factor analysis for malaria infection is conducted using a multivariable logistic regression analysis.
The study started in October 2014 and the first two surveys have been published [ 21 ]. At the time of the study completion, data from approximately 50,000 participants will be available, providing a comprehensive picture on malaria prevalence variations across the study sites.
Monitoring P. falciparum genetic diversity (EPI-MAL-010)
Although the central NANP amino acid repeat sequence of the circumsporozoite protein used as a major component of the RTS,S vaccine antigen is normally well conserved across parasite strains, P. falciparum is a pathogen with high variability and a high number of different circulating haplotypes. The parasite uses numerous mechanisms to vary cell surface antigens and evade the host immune response. For this reason, there is a potential concern that widespread implementation of RTS,S/AS01 E could drive the selection of specific parasite variants or alter the number of parasite haplotypes over time by exerting selective pressure. EPI-MAL-010 is an ancillary study to EPI-MAL-005 that will monitor the genetic diversity of circumsporozoite protein sequences in the P. falciparum parasite population before and after vaccine implementation.
EPI-MAL-010 has a longitudinal, cross-sectional study design and uses capillary blood samples collected from participants enrolled in EPI-MAL-005 over 7 survey years. Samples are from participants aged 6 months to < 5 years with P. falciparum parasitaemia confirmed by microscopy and/or nucleic acid amplification tests, and collected before and after RTS,S/AS01 E implementation in two study sites: Kintampo (Ghana) in Western Africa and Kombewa (Kenya) in Eastern Africa.
This study is estimating P. falciparum haplotype prevalence (i.e., the proportion of participants infected with a specific haplotype) and frequency (i.e., the proportion of a specific haplotype among all detected malaria clones) in participants aged 6 months to < 5 years vaccinated or not with RTS,S/AS01 E .
Amplicon sequencing is conducted on samples tested positive for P. falciparum by microscopy and/or nucleic acid amplification tests. Trends in the prevalence of specific P. falciparum haplotypes with a frequency of at least 5% will be assessed by a logistic regression model. Multinomial logistic regression will be used to describe the annual fluctuations in haplotype frequency using the 3D7 haplotype as the reference group.
Study set-up opportunities and challenges
Considering the specificities of the study setting, GSK together with its local scientific partners, conducted comprehensive study feasibility assessments in which both scientific and operational aspects were carefully balanced to allow generation of robust data. During the planning phase, global experts provided advice on study design and execution. Evolving circumstances, external constraints and the involvement of multiple stakeholders required adaptability and flexibility, balancing an optimal study design with real-world constraints. Designing, setting-up and conducting a complex post-approval plan in sub-Saharan Africa brings some important setting-specific considerations: (1) data collection needs to be performed using prospective follow-up because existing data collection systems and databases may be sub-optimal or absent; (2) vaccine safety, effectiveness and impact data have to be collected in a healthcare environment where case detection and ascertainment may be challenging because of limited healthcare infrastructure and diagnostic capability; (3) there may be limited access to health care in remote settings; (4) background incidence rates of many diseases including study-specific endpoints are limited or not available; (5) standard laboratory procedures may not exist across all study sites; and (6) sample storage and transportation from remote locations can be challenging.
More information on key parameters that were considered in the framework of the study feasibility assessment is provided below. Study site selection was based on specific criteria: (1) the existence of pre-existing scientific research infra-structure capable of expanding beyond routine data collection. Several of the finally selected study sites have research experience in conducting clinical trials with RTS,S/AS01 E , and have developed capability in terms of training, experience and quality of healthcare that resulted from previous study participation. However, for some sites without such past experience, additional investment was required to set up baseline structures and procedures; (2) the existence of a HDSS, part of the International Network for the Demographic Evaluation of Populations and Their Health (INDEPTH), or of an equivalent surveillance system. HDSS sites have a demographic database in place that updates, on a regular basis, the number of births, deaths, immigrations and emigrations, and potentially vaccinations and population health outcomes. The number of HDSS sites in Ghana, Kenya and Malawi being limited, a population census had to be fully established or partially enhanced in approximately half of the study sites.
The EPI-MAL-002 and EPI-MAL-003 studies are mainly based on data collection in the framework of routine medical practice, which may hamper the ascertainment of diseases requiring more advanced diagnostic tools. A full understanding of the structure and capacity of the healthcare system in each country where the studies were planned was required, including the assessment of the capacities for case detection and the ascertainment of study endpoints in each study site. As an outcome of this assessment, specific tools were put in place during the study preparation and conduct to enhance case detection and diagnostic capabilities. These include regular and ongoing medical and pharmacovigilance trainings, telemedicine support (Réseau en Afrique Francophone pour la Télémédecine, Switzerland; Agence de Médecine Préventive, Ivory Coast), the distribution of job aids, the support of local laboratories and the set-up of a central reference laboratory (Clinical Laboratory Services, South Africa) for blood and CSF testing. These enhancement tools should increase the likelihood of reaching the highest possible level of diagnostic certainty. For the key endpoint of meningitis, medical and non-medical staffs have received training on meningitis case detection and ascertainment, which includes information on the national guidelines for case management (including lumbar puncture and testing of CSF) with secondary testing to be performed at a central laboratory when sample volume permits.
This multi-country initiative involves key local and global public health partners. The scientific and operational constraints and the complexity of the study set-up provide opportunities for collaborations and alignment in healthcare approaches between countries to promote best practice. However, turnover of trained personal in the study areas is likely to be an ongoing challenge.
Within countries, the MVIP, and more specifically the study design, set-up and conduct, promote collaboration between different authorities, such as routine healthcare system, diagnostic services, national immunization services, epidemiological research, and National Malaria Control Programmes.
Despite best laid plans, the coronavirus disease 2019 (COVID-19) pandemic in Africa continues to unfold and its impact is evolving. In this environment, the MVIP is continuing and measures are being taken to protect the welfare and safety of participants and study staff, and to ensure data integrity [ 22 ]. No major change in the rate of RTS,S/AS01 E vaccination has been observed during the pandemic thus far, although it might be expected that hospitalization practices may change in order to limit admissions to children with serious or critical conditions and avoid hospital crowding. However, the potential impact of COVID-19 on the evaluation of AESI as determined in pre-vaccination pre-COVID-19 period requires continuous monitoring of the situation. Sensitivity analyses may be conducted considering COVID-19 pandemic periods for both studies.
Conclusions and perspectives
The implementation and the safety, effectiveness and impact evaluation of RTS,S/AS01 E in a real-life setting is a unique and complex undertaking that requires the establishment of large-scale and strong partnerships. Assessing the benefit-risk profile of a 3-dose primary schedule vaccine with a 4 th dose booster administered beyond the usual Expanded Programme on Immunization schedule in low and lower-middle income countries requires the establishment of ad-hoc tools and quality control methods to allow collection of robust and reliable data. Whilst technical and operational expertise is key to achieve this goal, human and financial resource needs should not be underestimated. The effort has been based on the WHO-led MVIP, existing research platforms and expertise in the implementing countries, and significant commitment by GSK. The experience with RTS,S/AS01 E in sub-Saharan Africa highlights important aspects to be considered when planning and implementing a vaccine post-approval plan in low and lower-middle income countries. The RTS,S/AS01 E experience will pave the way for the development and implementation of new generation malaria vaccines, and of other vaccines for the developing world.
Acknowledgements
The authors thank the children, their families and their communities who participated or will participate in this study. The authors acknowledge the study team members of all research sites: Kintampo Health Research Centre, Navrongo Health Research Centre, University of Malawi College of Medicine, Malawi Liverpool Wellcome Trust Clinical Research Programme, Centre for Research in therapeutic Sciences (CREATES), Kenya Medical Research Institute and KEMRI-Walter Reed Project. The authors also acknowledge the healthcare system institutions for their help and expertise. The authors thank the following individuals or groups for their invaluable contribution to the study: Effua Usuf and PATH: Cheryl Keech. The authors also thank the Modis platform on behalf of GSK for writing assistance provided by Joanne Wolter and manuscript coordination provided by Pauline De Berdt; and the TVF platform on behalf of GSK for designing the infographic provided by Martin Todd.
Elaine Jacqueline Akité, Patrick Odum Ansah, Laurence Baril, Owusu Boahen, Yolanda Guerra Mendoza, Valerie Haine, Simon Kariuki, Mathieu Lamy, Kenneth Maleta, Randy Mungwira, Latif Ndeketa, Abraham Oduro, Bernhards Ogutu, Fredrick Olewe, Martina Oneko, Mattéa Orsini, Francois Roman, Edith Roset Bahmanyar, Dominique Rosillon, Lode Schuerman, Valentine Sing’oei, Dianne J. Terlouw, Stéphanie Wéry—Authors listed in alphabetical order
Abbreviations
| AESI | Adverse events of special interest |
| COVID-19 | Coronavirus disease 2019 |
| CSF | Cerebrospinal fluid |
| GSK | GlaxoSmithKline Biologicals SA |
| HDSS | Health and demographic surveillance system |
| INDEPTH | International Network for the Demographic Evaluation of Populations and Their Health |
| MaxSPRT | Maximized sequential probability ratio test |
| WHO | World Health Organization |
| MVPE | Malaria Vaccine Programme Evaluation |
| MVIP | Malaria Vaccine Implementation Programme |
Author contributions
NP, KPA, MCB, POA, LB, OB, VH, ML, KM, AO, BO, FO, MOr, FR, ERB, DR, LS, WO and JYP designed the studies. All authors read and approved the final manuscript.
This work was supported and funded by GlaxoSmithKline Biologicals SA and PATH. The studies EPI-MAL-002 (NCT02374450), EPI-MAL-003 (NCT03855995), EPI-MAL-005 (NCT02251704) and EPI-MAL-010 (GSK study identifier: 205071) are sponsored by the GSK group of companies. GlaxoSmithKline Biologicals SA funded all costs associated with the development and the publishing of the present manuscript.
Availability of data and materials
Declarations.
Not applicable.
EJA, YGM, VH, FR, LS, SW and JYP are employees of the GSK group of companies. At the time of the study NP was an employee of the GSK group of companies and is now employed by Janssen Pharmaceutica NV. At the time of the study DR was an employee of the GSK group of companies and is now a freelance consultant at DESiRE-Consulting. NP, YGM, ML, FR, LS, DR and JYP hold shares in the GSK group of companies. ML is employee of Aixial on behalf of the GSK group of companies. MOr and MCB are employees of 4Clinics on behalf of the GSK group of companies. KPA, POA, OB, SK, KM, RM, LN, AO, BO, FO, MOn, VS, DJT and WO via their institutions, received grants from the GSK group of companies for the conduct of this study/work. KPA, OB, SK, WO, VS, KM and DJT via their institutions, received grants from the GSK group of companies for the conduct of studies outside the submitted work.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
For the 5 μg R21/50 μg Matrix-M regimen, vaccine efficacy against multiple clinical malaria episodes was 77% (95% CI 70-82) over 2 years of follow-up. 8 Phase 3 trial results from five sites in east and west Africa of a four-dose regimen demonstrated 72% vaccine efficacy (68-75) in the two sites in which the vaccine was delivered under ...
The Malaria Vaccine Implementation Programme showed that RTS,S/AS01 has a favourable safety profile and was associated with a 30% reduction in cases of severe malaria. 3 This followed an earlier phase 3 study, where, with a median follow-up of 48 months, vaccine efficacy against clinical malaria was 36% in infants aged 5-17 months and 26% in ...
Here, we discuss recent clinical advances in vaccine development and highlight ongoing challenges for the future. Malaria is one of the most devastating infectious diseases in humans, responsible ...
In a multicountry, phase 3 trial involving young children, 4 the malaria vaccine RTS,S/AS01 E, a viruslike particle expressing the Plasmodium falciparum circumsporozoite protein and hepatitis B ...
There was a minimum 2-week interval between administration of the study vaccine and any Expanded Programme on Immunisation vaccine. ... and 18 months (seasonal sites). Severe malaria case definitions were similar to the clinical malaria case definitions, with the addition of specified criteria of disease severity (definitions are detailed in ...
A recent trial showed that seasonal malaria chemoprevention plus an annual booster of RTS,S/AS01 through 5 years of age (after the initial three-dose vaccine series is started at 5 to 17 months of ...
Malaria vaccines are urgently needed in the fight against this devastating disease that is responsible for almost half a million deaths each year. Here, we discuss recent clinical advances in vaccine development and highlight ongoing challenges for the future. Subject terms: Vaccines, Malaria.
Introduction. The malaria vaccine RTS,S/AS01E (brand name Mosquirix TM) received a favorable opinion from the European Medicines Agency (EMA) in 2015 after review of its safety and efficacy to ...
2. Expectations and realities of malaria vaccines to date. A malaria vaccine has long been seen as a potential game changer in the fight against malaria, although vaccine development began at a time when the emphasis in international health was shifting from immediate goal of world-wide malaria eradication to control, with the Alma-Ata agreement focusing on primary health care (World Health ...
The malaria vaccination landscape has seen significant advancements with the recent endorsement of RTS,S/AS01 and R21/Matrix-M vaccines, which target the pre-erythrocytic stages of Plasmodium falciparum (Pf) infection. However, several challenges remain to be addressed, including the incomplete protection afforded by these vaccines, their dependence on a single Pf antigen, and the fact that ...
Modern malaria vaccine development stemmed from studies in the 1960s that immunized mice with irradiated sporozoites. There was continual progress on malaria vaccine candidates. The first-ever malaria vaccine (and also the first parasite vaccine), RTS,S AS01 was approved for widespread use on 6 October 2021.
In a study published in The Lancet on Wednesday, an international team has shared promising new data on a potential vaccine.The phase two trial, based on 450 children in Nanoro, evaluated the R21 ...
A phase 3 randomized controlled trial confirms that the R21/Matrix-M malaria vaccine is 67% to 75% effective against the mosquito-borne illness in young children in four African countries.. Study data, published yesterday in The Lancet, also confirm a good safety profile for the vaccine, which costs only $2 to $4 per dose, per the World Health Organization (WHO).
Here we present a case study of a phase III malaria vaccine clinical trial. Through qualitative interviews with researchers and caregivers of pediatric participants we elucidate themes that shape the clinical trial system. These themes can be a useful complementary planning tool to existing research guidelines for clinical trial researchers.
Plasmodium falciparum reticulocyte-binding protein homolog 5 (RH5) is a unique asexual blood-stage malaria vaccine candidate because of its high conservation and essential biological function of binding to basigin on the erythrocyte surface. Recent studies by Barrett et al., Wang et al., and King et al., have brought RH5-based vaccine development a step forward based on a rational antigen ...
The study population were in mostly moderate and low transmission intensity sites. 75% efficacy (95% CI 71-78) was reported against all episodes of malaria during 12 months of follow-up when the vaccine was given in three primary doses just before the malaria season in areas where malaria is highly seasonal, with a booster dose sustaining ...
It has been a good year for vaccines. Remarkably effective inoculations were engineered, tested, and rolled out to rein in the COVID-19 pandemic which has killed over 4.8 million people, and on October 6 the World Health Organization (WHO) officially recommended a "groundbreaking malaria vaccine for children at risk." It's hard to wrap our heads around the numbers that make up the fabric ...
An experimental malaria vaccine could offer protection during pregnancy for up to 2 years without a booster dose, according to a randomized clinical trial conducted in Mali. The 3-dose vaccine, known as Plasmodium falciparum sporozoite (PfSPZ) vaccine, had previously been tested successfully in adults in other parts of Africa.. This was the first clinical trial to test the vaccine's safety ...
Abstract. Background: A 2021 clinical trial of seasonal RTS,S/AS01 E (RTS,S) vaccination showed that vaccination was non-inferior to seasonal malaria chemoprevention (SMC) in preventing clinical malaria. The combination of these two interventions provided significant additional protection against clinical and severe malaria outcomes.
The trial is designed to evaluate vaccine efficacy, safety, and immunogenicity for 32 months after the first dose of study vaccine in children 6 to 12 weeks of age or 5 to 17 months of age at ...
EPI-MAL-005 (NCT02251704) is a cross-sectional pre- and post-implementation study to measure malaria transmission intensity and monitor the use of other malaria control interventions in the study areas, and EPI-MAL-010 (EUPAS42948) will evaluate the P. falciparum genetic diversity in the periods before and after vaccine implementation.
Mathematical models of malaria dynamics have been useful for estimating the public health effect of malaria vaccination beyond the estimates obtained from clinical trials. 11 In this study, we combine data from the phase 3b trial 9 and an individual-based transmission model of Plasmodium falciparum transmission to understand the case for ...
Vaccine efficacy against parasitemia (before or during pregnancy) was 65% in the low dose group and 86% in the high dose group. Among 155 women who became pregnant across both study years, the lower and higher dose vaccines were 57 and 49% effective, respectively.
Experts are troubled by the apparent lack of informed consent in a large, cluster randomised study of the malaria vaccine. Peter Doshi reports A large scale malaria vaccine study led by the World Health Organization has been criticised by a leading bioethicist for committing a "serious breach" of international ethical standards. The cluster randomised study in Africa is already under way ...
EPI-MAL-005 (NCT02251704) is a cross-sectional pre- and post-implementation study to measure malaria transmission intensity and monitor the use of other malaria control interventions in the study areas, and EPI-MAL-010 (EUPAS42948) will evaluate the P. falciparum genetic diversity in the periods before and after vaccine implementation.