
- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search

IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)
Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture
Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
- eResearch IRB NextGen Project
- Class Assignments & IRB Approval
- Operations Manual (OM)
- Authorization Agreement Process
- ORCR Policies and Procedures
- Self-Assessment Tools
- Resources and Web Links
- Single IRB-of-Record (sIRB) Process
- Certificate of Confidentiality Process
- HRPP Education Resources
- How to Register a Clinical Trial
- Maintaining and Updating ClinicalTrial.gov Records
- How to Report Clinical Trial Results
- Research Study Participation - FAQ
- International Research
- Coordinated Services & Practices (CSP)
- Collaborative Research: IRB-HSBS sIRB Process
- Data Security Guidelines
- Research Incentive Guidelines
- Routine fMRI Study Guidelines
- IRB-HSBS Website Directory and Guidance
- Waivers of Informed Consent Guidelines
- IRB Review Process
- IRB Amendment Process
- Continuing Review Process
- Incident Reporting (AE/ORIO)
- IRB Repository Application
- IRB-HSBS Education
- Newsletter Archive
You are here
- Human Subjects
- IRB Health Sciences and Behavioral Sciences (HSBS)
Informed Consent Guidelines & Templates
U-m hrpp informed consent information.
See the HRPP Operations Manual, Part 3, Section III, 6 e .
The human subjects in your project must participate willingly , having been adequately informed about the research.
- If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required.
- If the human subjects are children , in most cases you must first obtain the permission of parents in addition to the consent of the children.
Contact the IRB Office for more information .
See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent or informed consent documentation.

See the updated Basic Informed Consent Elements document for a list of 2018 Common Rule basic and additional elements.
Informed Consent Process
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent) and the presentation of that information to prospective participants.
In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document (i.e., to document the consent to participate) unless the IRB has waived the consent requirement or documentation (signature) requirement .
- Projects which collect biospecimens for genetic analysis must obtain documented (signed) informed consent.
- It is an ethical best practice to include an informed consent process for most exempt research . IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself. A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, Related Information (top right).
Informed consent documents
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations ( 45 CFR 46.116 ) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process. New with the revised 2018 Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of key information that will help potential participants understand why they might or might not want to be a part of a research study.
Key Information Elements
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information.
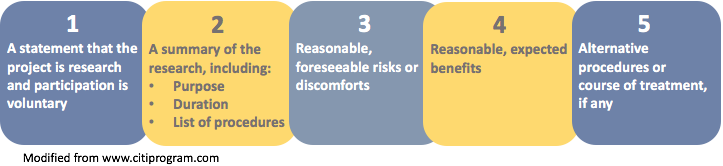
Note: Element number 5 (alternative procedures) applies primarily to clinical research.
General Information & Tips for Preparing a Consent Document
Reading level.
Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level . A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with the IRB application. Always:
For guidance on using plain language, examples, and more, visit: http://www.plainlanguage.gov/
- Tailor the document to the subject population.
- Avoid technical jargon or overly complex terms.
- Use straightforward language that is understandable.
Writing tips
The informed consent document should succinctly describe the research as it has been presented in the IRB application.
- Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I).
- Include a statement of agreement at the conclusion of the informed consent document.
- The consent doucment must be consistent with what is described in the IRB application.
Document Formating for Uploading into eResearch
- Remove "track changes" or inserted comments from the consent documentation prior to uploading the document into the IRB application (Section 10-1) for review.
- Use a consistent, clearly identified file naming convention for multiple consent/assent documents.
Informed Consent Templates
IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements (per 45 CFR 46.116 ), as well as other required regulatory and institutional language. The templates listed below include the new consent elements outlined in the 2018 Common Rule.
References and Resources
PDF. Lists the basic and additional elements required for inclusion or to be included, as appropriate to the research, in the informed consent documentation, along with the citiation number [e.g., _0116(b)(1)] within the revised Common Rule. New elements associated with the 2018 Common Rule are indicated in bold text.
Strongly recommended for studies that involve the collection of biospecimens and/or genetic or genomic analysis, particularly federally sponsored clinical trials that are required to post a consent document on a public website. Last updated: 04/10/2024.
Informed Consent documents are not reviewed by the IRB for Exempt projects. However, researchers are ethically bound to conduct a consent process with subjects. This template is suggested for use with Exempt projects. Last updated 4/17/24
(Word) Blank template with 2018 revised Common Rule key information and other required informed consent elements represented as section headers; includes instructions and recommended language. It is strongly advised that you modify this template to draft a project-specific informed consent document for your study for IRB review and approval. Last updated: 04/10/2024
(Word) General outline to create and post a flyer seeking participation in a human subjects study. Includes instructions.
(Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
For use by U-M Dearborn faculty, staff, and students conducting non-exempt human subjects research using subject pools. Last updated 4/10/24
For use by U-M Dearborn faculty, staff, and students conducting exempt human subjects research using subject pools
Researchers who will conduct data collection that is subject to the General Data Protection Regulation (GDPR) must use this template in tandem with a general consent for participation template/document.
- Child assent ages 3-6
- Child assent 7-11
- Parent permission
- Brief protocol for exempt research including data management and security questionnaire
- Child assent 12-14
- Introductory psychology subject pool general consent template
- Introductory psychology subject pool exempt consent template
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS)
Phone: (734) 936-0933 Fax: (734) 936-1852 [email protected]
- Human Subjects Protections
Sample consent and permission forms
General consent form to participate in research (DOC)
Two stage project consent form (DOC)
Parent permission form for research with child (DOC)
Child assent form (DOC)
Multiple consent form including audio-recording and quotations (DOC)
Photo and video consent form (DOC)
Video-recording consent form (DOC)
Re-contact agreement form (DOC)
Post-debriefing consent form (DOC)

Sample Consent Forms
Consent form templates.
These consent form templates have been posted for your reference. When completing and IRB submission in IRBIS, please fill in the application and use the consent form builder specific to your project. For more information, please find instructions here .
Summary of Changes to the Regulations for Informed Consent: Revised Common Rule Changes to Informed Consent and Waiver Requirements
Summary of Changes to Consent Documents:
- Informed Consent Documents – Version 2.0 Summary of Changes
- Informed Consent Documents – Version 2.1 Summary of Changes
- Informed Consent Documents – 10/26/2020 Summary of Changes
- Informed Consent Documents – 4/10/2023 Summary of Changes
| 2023-07-14 | |
| 2020-01-17 | |
| 2020-01-17 | |
| 2020-01-17 | |
| 2023-04-10 | |
| 2023-06-27 | |
| 2023-04-10 | |
| The following documents are samples. IRBIS does NOT generate these documents with application-specific information. | |
| 2017-10-30 | |
| 2024-08-09 | |
| 2017-04-17 | |
| 2018-04-19 | |
Concise Summary examples can be found here .
Guidance on the use of plain language in consent forms:
- Clinical Research Glossary
- Webinar: The Promise of Plain Language: Launching a Glossary to Support Participant Understanding of Clinical Research – Recording & Slides
There are a few additional forms that are not provided online and may be accessed below. As needed, these should be completed and uploaded to your IRB application.
Foreign Language Consent Forms
COVID-19 Related Forms:
- Spanish-IRB-COVID Information Sheet
- Spanish COVID Consent Letter v2
- Spanish COVID Informational Sheet Translation Certificate
Informed Consent Short Form (for a single subject who may be illiterate, or otherwise unable to read the consent form — used when full consent form has to be read or translated for subject).
- Informed Consent Short Form Guidance
- Simplified Chinese
HIPAA Templates
- Sample HIPAA Authorization Template
- Sample HIPAA Authorization Template in Spanish ( Certification )
- Privacy Policy

Home » Informed Consent in Research – Types, Templates and Examples
Informed Consent in Research – Types, Templates and Examples
Table of Contents

Informed Consent in Research
Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of participants.
Types of Informed Consent in Research
There are different types of informed consent in research , which may vary depending on the nature of the study, the type of participants, and the context. Some of the common types of informed consent in research include:
Written Consent
This is the most common type of informed consent, where participants are provided with a written document that explains the study and its requirements. The document typically includes information about the purpose of the study, procedures involved, risks and benefits, confidentiality, and participant rights. Participants are asked to sign the document as an indication of their willingness to participate.
Oral Consent
In some cases, oral consent may be used when a written document is not practical or feasible. Oral consent involves explaining the study and its requirements to participants verbally and obtaining their consent. This method may be used for studies with illiterate or visually impaired participants or when conducting research remotely.
Implied Consent
Implied consent is used in studies where participants’ actions are taken as an indication of their willingness to participate. For example, a participant may be considered to have given implied consent if they show up for a scheduled appointment for the study.
Opt-out Consent
This method is used when participants are given the opportunity to decline participation in a study. Participants are provided with information about the study and are given the option to opt-out if they do not wish to participate. This method is commonly used in population-based studies or surveys.
Assent is used in studies involving minors or participants who are unable to provide informed consent due to cognitive impairment or disability. Assent involves obtaining the agreement of the participant to participate in the study, along with the consent of a legally authorized representative.
Informed Consent Format in Research
Here’s a basic format for informed consent that can be customized for specific research studies:
- Introduction : Begin by introducing yourself and the purpose of the study. Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
- Study Overview : Provide a brief overview of the study, including its purpose, methods, and expected outcomes.
- Procedures : Describe the procedures involved in the study in clear, concise language. Include information about the types of data that will be collected, how they will be collected, and how long the study will take.
- Risks and Benefits : Outline the potential risks and benefits of participating in the study. Be honest and upfront about any discomfort, inconvenience, or potential harm that may be involved, as well as any potential benefits.
- Confidentiality and Privacy : Explain how participant data will be collected, stored, and used, and what measures will be taken to ensure confidentiality and privacy.
- Voluntary Participation: Emphasize that participation is voluntary and that participants can withdraw at any time without penalty. Explain how to withdraw from the study and who to contact if participants have questions or concerns.
- Compensation and Incentives: If applicable, explain any compensation or incentives that will be offered to participants for their participation.
- Contact Information: Provide contact information for the researcher or a representative from the research team who can answer questions and address concerns.
- Signature : Ask participants to sign and date the consent form to indicate their voluntary agreement to participate in the study.
Informed Consent Templates in Research
Here is an example of an informed consent template that can be used in research studies:
Introduction
You are being invited to participate in a research study. Before you decide whether or not to participate, it is important for you to understand why the research is being done, what your participation will involve, and what risks and benefits may be associated with your participation.
Purpose of the Study
The purpose of this study is [insert purpose of study].
If you agree to participate, you will be asked to [insert procedures involved in the study].
Risks and Benefits
There are several potential risks and benefits associated with participation in this study. Some of the risks include [insert potential risks of participation]. Some of the benefits include [insert potential benefits of participation].
Confidentiality
Your participation in this study will be kept confidential to the extent allowed by law. All data collected during the study will be stored in a secure location and only accessed by authorized personnel. Your name and other identifying information will not be included in any reports or publications resulting from this study.
Voluntary Participation
Your participation in this study is completely voluntary. You have the right to withdraw from the study at any time without penalty. If you choose not to participate or if you withdraw from the study, there will be no negative consequences.
Contact Information
If you have any questions or concerns about the study, you can contact the investigator(s) at [insert contact information]. If you have questions about your rights as a research participant, you may contact [insert name of institutional review board and contact information].
Statement of Consent
By signing below, you acknowledge that you have read and understood the information provided in this consent form and that you freely and voluntarily consent to participate in this study.
Participant Signature: _____________________________________ Date: _____________
Investigator Signature: ____________________________________ Date: _____________
Examples of Informed Consent in Research
Here’s an example of informed consent in research:
Title : The Effects of Yoga on Stress and anxiety levels in college students
Introduction :
We are conducting a research study to investigate the effects of yoga on stress and anxiety levels in college students. We are inviting you to participate in this study.
If you agree to participate, you will be asked to attend four yoga classes per week for six weeks. Before and after the six-week period, you will be asked to complete surveys about your stress and anxiety levels. Additionally, we will measure your heart rate variability at the beginning and end of the six-week period.
Risks and Benefits:
There are no known risks associated with participating in this study. However, the benefits of practicing yoga may include decreased stress and anxiety levels, increased flexibility and strength, and improved overall well-being.
Confidentiality:
All information collected during this study will be kept strictly confidential. Your name will not be used in any reports or publications resulting from this study.
Voluntary Participation:
Participation in this study is completely voluntary. You are free to withdraw from the study at any time without penalty.
Contact Information:
If you have any questions or concerns about this study, you may contact the principal investigator at (phone number/email address).
By signing this form, I acknowledge that I have read and understood the above information and agree to participate in this study.
Participant Signature: ___________________________
Date: ___________________________
Researcher Signature: ___________________________
Importance of Informed Consent in Research
Here are some reasons why informed consent is important in research:
- Protection of participants’ rights : Informed consent ensures that participants understand the nature and purpose of the research, the risks and benefits of participating, and their rights as participants. It empowers them to make an informed decision about whether to participate or not.
- Ethical responsibility : Researchers have an ethical responsibility to respect the autonomy of participants and to protect them from harm. Informed consent is a crucial way to uphold these principles.
- Legality : Informed consent is a legal requirement in most countries. It is necessary to protect researchers from legal liability and to ensure that research is conducted in accordance with ethical standards.
- Trust : Informed consent helps build trust between researchers and participants. When participants understand the research process and their role in it, they are more likely to trust the researchers and the study.
- Quality of research : Informed consent ensures that participants are fully informed about the research and its purpose, which can lead to more accurate and reliable data. This, in turn, can improve the quality of research outcomes.
Purpose of Informed Consent in Research
Informed consent is a critical component of research ethics, and it serves several important purposes, including:
- Respect for autonomy: Informed consent respects an individual’s right to make decisions about their own health and well-being. It recognizes that individuals have the right to choose whether or not to participate in research, based on their own values, beliefs, and preferences.
- Protection of participants : Informed consent helps protect research participants from potential harm or risks that may arise from their involvement in a study. By providing participants with information about the study, its risks and benefits, and their rights, they are able to make an informed decision about whether to participate.
- Transparency: Informed consent promotes transparency in the research process. It ensures that participants are fully informed about the research, including its purpose, methods, and potential outcomes, which helps to build trust between researchers and participants.
- Legal and ethical requirements: Informed consent is a legal and ethical requirement in most research studies. It ensures that researchers obtain voluntary and informed agreement from participants to participate in the study, which helps to protect the rights and welfare of research participants.
Advantages of Informed Consent in Research
The advantages of informed consent in research are numerous, and some of the most significant benefits include:
- Protecting participants’ autonomy: Informed consent allows participants to exercise their right to self-determination and make decisions about whether to participate in a study or not. It also ensures that participants are fully informed about the risks, benefits, and implications of participating in the study.
- Promoting transparency and trust: Informed consent helps build trust between researchers and participants by providing clear and accurate information about the study’s purpose, procedures, and potential outcomes. This transparency promotes open communication and a positive research experience for all parties involved.
- Reducing the risk of harm: Informed consent ensures that participants are fully aware of any potential risks or side effects associated with the study. This knowledge enables them to make informed decisions about their participation and reduces the likelihood of harm or negative consequences.
- Ensuring ethical standards are met : Informed consent is a fundamental ethical requirement for conducting research involving human participants. By obtaining informed consent, researchers demonstrate their commitment to upholding ethical principles and standards in their research practices.
- Facilitating future research : Informed consent enables researchers to collect high-quality data that can be used for future research purposes. It also allows participants to make an informed decision about whether they are willing to participate in future studies.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer

You may also like

What is a Hypothesis – Types, Examples and...

Research Paper – Structure, Examples and Writing...

Research Design – Types, Methods and Examples

Research Paper Abstract – Writing Guide and...

Thesis Outline – Example, Template and Writing...

Research Summary – Structure, Examples and...

- Proposal & Award Process
- Pre-Award Development
- Post-Award Management
- Policies & Procedures
- Newsletters
- Events & Announcements
- Export Control
- Intellectual Property
- Institutional Biosafety
- Internal Funding
- Funding Opportunities
- List of Centers
- How to Start a Center
- List of Committees
- Committee for International Research
- Committee for Undergraduate Research
- Graduate Research
- Student Travel Funds
- Student Research Symposium
- Student Poster Showcase
- Institutional Review Board
Consent & Assent Forms
Informed consent and assent process and forms.
“Informed consent” is the voluntary agreement of an individual, or his or her authorized representative, who has the legal capacity to give consent, and who exercises free power of choice without any form of constraint or coercion to participate in research. Consenting is a process where the researcher clearly communicates the risks and benefits of the study, the voluntary nature of participation in the study, and the expectations from the subject if they agree to participate in the study. Informed consent is a conversation between the researcher and potential research participants, and the consent form is a record of this conversation.
“Assent” is a term used to express willingness to participate in research by persons who are by definition too young to give informed consent but are old enough to understand the proposed research in general, its expected risks and possible benefits, and the activities expected of them as subjects. Assent by itself is not sufficient, however. If assent is given, informed consent must still be obtained from the subject’s parents or guardian. A person 18 years and older are considred adult and therefore one who can provide consent without parental permission.
Informed consent is not a single event or just a form to be signed – rather, it is an educational process that takes place between the investigator and the prospective subject. The informed consent and assent process is usually documented with a consent form and an assent form signed by the research participant and/or read to the research participant. In certain cases, documentation of consent may be waived and an information sheet used instead.
Once the researcher’s IRB submission is approved, the consent forms submitted by the researcher will be stamped by the IRB Office. All researchers must use IRB stamped consent forms with their research participants.
Consent and Assent Form Templates:
We recommend using the following templates when putting together the consent and assent forms for your research project. Text in red is instructions where you can insert your information. Keep the text in black as is.
- Adult Consent Form *NEW as of Fall 2023*
- Broad Consent Form
- Parent Consent Form
- Parent Information Sheet-Opt Out
- Child Assent Form (Ages 7-10)
- Child Assent Form (Ages 11-14)
- Child Assent Form (Ages 15-17)
- Information Sheet Template
- CSUSM Informed Consent Instructions
- Consent Form Requirements for Languages Other than English
- Informed consent frequently asked questions
- CSUSM Letterhead
- Regulations pertaining to Waiver of Consent
How to write the perfect consent form for research

Consent forms play a vital role in protecting both researchers and participants.
In fact, they’re an essential aspect of any study. They make sure everyone involved understands the purpose of the research, their rights, and what’ll be done with the data you collect. Consent forms also protect participants by flagging any content that might cause offence or distress, ensuring they’re informed and have the option to not take part.
Read on to discover why consent forms for research matter, what to include in one, and some handy tips and tricks for ensuring yours covers every base.
Why research consent forms matter
A thoughtful, thorough research consent form can really add to the participant’s experience – while keeping you compliant, and them safe. Here’s how.
The only way is ethics
Consent forms are a key part of ethical research. They provide a clear and concise understanding of the study's objectives, methods, and potential risks.
Asking for informed consent shows respect to your participants. It ensures they're voluntarily taking part in the study without feeling coerced or misled.
Consent forms are mandatory
All academic institutions have an ethics board (also known as the institutional review board in the US). This group must approve any research being done by researchers who are connected to the university.
Consent forms are an important part of this process. The ethics board requires them to ensure that participants understand what they’re agreeing to and that their rights are being protected.
Compliance is key
Research consent forms also serve as a legal safeguard for both researchers and participants. They outline the rights and responsibilities of each party, which helps you stay on the right side of data protection rules like the General Data Protection Regulation (GDPR).
They can also keep you out of court. They allow you to uphold participants’ rights, and your own rights, if a dispute occurs and avoid potential legal complications.
Trust matters
A well-crafted research consent form establishes trust between you and your participants.
By being transparent about the study's purpose and procedures, people will feel more comfortable engaging in your research.
Creating the perfect consent form for research
Your consent form will no doubt be unique, depending on the nature of your research and the specifics of your study.
But here are eight key components you won’t want to miss out.
1. A study description and purpose
Kick off your consent form by outlining your study's purpose and objectives. Briefly explain why the study’s being conducted.
This section should be clear, concise, and easy to understand, as it sets the stage for the rest of the form.
2. What’s expected
Describe the tasks participants will be asked to complete and any equipment they’ll need, along with an overview of the time commitment required.
With this information, participants can make an informed decision as to whether they want to take part or not.
3. Voluntary participation
Make it clear that taking part in your study is entirely voluntary. Explain that the participants can withdraw from the research at any time without penalty or negative consequences.
This a legal requirement – but it also helps you promote trust and allow participants to feel in control of their involvement.
4. Risks and rewards
Address any potential risks or discomforts that participants may experience during the study. Be open about any physical, psychological, or social risks – even if they’re small.
Don’t forget to shout about the benefits, too! If taking your study means gaining insights, learning new skills, or contributing to scientific knowledge that’ll make the world a better place, highlight it here.
5. The privacy bit
This one’s crucial. Explain what data will be collected during your study – including any sensitive info, like racial or ethnic origin, religious or political beliefs, or health status – and how this data will be used and protected.
You’ll also want to mention how the data will be stored, and for how long, as well as how participants can withdraw their consent and data.
Assure participants that their personal information will be kept confidential, and outline the measures you have in place to protect the anonymity of their responses.
If you plan on making their anonymised data available to other researchers online at some point, be really clear about this, too.
6. Compensation and incentives
Give participants full details of the compensation they’ll receive for their time. Be open about the amount, form of payment, and any conditions required to receive it.
This information helps participants understand the value of their efforts.
7. Your contact info
Offer participants a point of contact for questions, concerns, or feedback related to the study.
8. A consent statement and tick box
End your research consent form with a clear statement of consent. This should explicitly state that the participant has read and understood the information provided and agrees to participate in the study.
Then, add a button or check box participants must click to record that they’ve given their consent. This should be timestamped and, if you’re using the Prolific platform, stored alongside the participant’s Prolific ID. If a participant selects ‘Does Not Consent’, ask them to return their submission on Prolific by clicking ‘Stop Without Completing’. They shouldn’t be penalised for this.
Our top tips for writing consent forms
So, you know why you need a consent form for research and how to write a comprehensive one.
Now, make it even better with these tips and tricks.
Use clear and accessible language
Make sure your consent form is written in clear and accessible language that participants can easily understand. Steer clear of technical jargon and keep acronyms to a minimum.
Keep it simple
Try to keep your form concise and focused. Reams of text and check boxes can be overwhelming and may discourage potential participants. Rather than getting the participant to consent to several different things, try and stick to one check box that confirms whether they do or don’t consent to the study.
Be transparent
Be open and honest about the study's purpose, methods, and potential risks. If participants feel informed and at ease when taking your study, you're more likely to get better responses from them.
Test and revise
Before distributing your research consent form, have a couple of people you trust review it for clarity and comprehensibility. Use their feedback to revise it before you put it in front of participants.
Create consent forms with confidence by using our downloadable template
To make getting consent simple as possible, we’ve created a comprehensive consent form template that you can use for your research.
This template includes all the key fields required to ensure you and your participants are protected. Download the template now .
You might also like

High-quality human data to deliver world-leading research and AIs.

Follow us on
All Rights Reserved Prolific 2024
Human Subjects Division
- [email protected]
- 206.543.0098
Consent Examples
About this page.
To assist UW researchers with designing subject-focused consent, the UW IRB provides example consent forms. Many of these examples are actual UW IRB approved consent forms designed by UW researchers. Some of the examples were created using one of our consent templates . The use of our template is not required and some of the examples deviate significantly from our templates.
We encourages researchers to use the Designing the Consent Process guidance and the examples below to create consent forms and processes that: (1) are written from the perspective of the subject population being enrolled, emphasizing the Key Information that is mostly likely to assist those subjects with deciding whether to enroll; and (2) are designed and presented in a way that facilitates comprehension and understanding.
- Exempt Research Example Consents
- Expedited and Full Board Research Example Consents
- Key Information Examples
University of Washington Office of Research
Or support offices.
- Human Subjects Division (HSD)
- Office of Animal Welfare (OAW)
- Office of Research (OR)
- Office of Research Information Services (ORIS)
- Office of Sponsored Programs (OSP)
OR Research Units
- Applied Physics Laboratory (APL-UW)
- WA National Primate Research Center (WaNPRC)
Research Partner Offices
- Corporate and Foundation Relations (CFR)
- Enivronmental Health and Safety (EH&S)
- Grant and Contract Accounting (GCA)
- Institute of Translational Health Sciences (ITHS)
- Management Accounting and Analysis (MAA)
- Post Award Fiscal Compliance (PAFC)
Collaboration
- Centers and Institutes
- Collaborative Proposal Development Resources
- Research Fact Sheet
- Research Annual Report
- Stats and Rankings
- Honors and Awards
- Office of Research
© 2024 University of Washington | Seattle, WA
Office of Strategic Research Development

Working together to work wonders by providing proposal development support to enhance UTMB's research enterprise.
Request our services, announcements, new research consent form templates.
June 3, 2024 • 2:09 p.m.
The Institutional Review Board has posted updated research consent form templates and new detailed drafting guidance.
Designed with updated instructions and a newly designed key information section, these templates ensure clarity and compliance for both researchers and participants. Enhance transparency with our user-friendly and informative forms.
The new documents can be found at on the IRB Forms Page.
Right-Side Nav

- Health Care
- UTMB Support Areas

COMMENTS
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. General Consent Form Templates Social and Behavioral Research Projects (last updated 03/16/2023)
se this template if your research is NOT. derally-sponsore. A. D participants are adults.Avoid Common Problems with Consent Forms. Read these tips!1. ustomize this template to reflect the specifics of your study and participan. population.Text in [brackets] represents study-specific information that must be added.A ba.
Research Ethics Review Committee (ERC) The Research Ethics Review Committee (ERC) is a 27-member committee established and appointed by the Director-General. Its mandate is to ensure WHO only supports research of the highest ethical standards. The ERC reviews all research projects, involving human participants supported either financially or ...
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent ...
Contact. Sample consent and permission forms. General consent form to participate in research (DOC) Two stage project consent form (DOC) Parent permission form for research with child (DOC) Child assent form (DOC) Multiple consent form including audio-recording and quotations (DOC) Photo and video consent form (DOC)
The forms should be provided to participants in addition to the main study consent form. The language in these forms can also be adapted and added to consent forms for studies in which COVID-19 screening and testing procedures are being done for study purposes, i.e., the results of the screening and/or testing will be used as study data. To do ...
Assent Form Ages 7-14. 2023-06-27. Information or Fact Sheet. 2023-04-10. The following documents are samples. IRBIS does NOT generate these documents with application-specific information. Exempt Research Information Sheet. 2017-10-30. Addendum to provide additional information to subject after original consent.
Informed Consent in Research. Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of ...
Whenever you are proposing research with human participants you must provide a form, known as an Informed Consent Form (ICF), with each proposal to indicate that the research participant has decided to take part in the research of her/his own free will. If the research involves more than one group of individuals, for example healthcare users ...
How to Write. Step 1 - Download in PDF, MS Word, or OpenDocument. Step 2 - The title of the research study being conducted must be included at the top of the consent form. Step 3 - Enter the following information related to the primary researcher in the fields provided: Step 4 - The purpose of the study, the procedures, the risks, and ...
The UW IRB provides the UW research community with a variety of consent templates that align with regulatory and policy requirements and best practices as described in our main Consent guidance and guidance on Designing the Consent Process. The first two templates, marked with an asterisk, are the templates most non-exempt studies will choose from.
Informed consents should include theBelow is an example of an. ev. ew Board Hofstra University Office of. Research and Sponsored Programs 516-463-50541. Introduction and Purpose of the StudyInclude a brief overview. of the study on a level of understanding for the person who will be signing the form. Remember.
The Research Ethics Board must approve any changes to the consent form before the research begins. Changes to an approved study and its documents are done via an Amendment. Your application will be sent back, and approval delayed, if a complete consent form or consent document is not submitted with your application.
The informed consent and assent process is usually documented with a consent form and an assent form signed by the research participant and/or read to the research participant. In certain cases, documentation of consent may be waived and an information sheet used instead. Once the researcher's IRB submission is approved, the consent forms ...
The recommended or required language for each section of the consent form is listed below. If specific language is not required, you are provided with some suggested stems to begin each sentence of the section. The standard consent form MUST INCLUDE all REQUIRED sections and may include OPTIONAL sections if appropriate.
The consent signature requirements from the mother and father are summarised in table 3. Once the informed consent is obtained, the pregnant women will be included into any phase of the study unless the research project will be compromised or the patient's health (mother and/or fetus) will be in danger.
Online: A template for research projects conducted online. Video/audio Recording: A template for studies that involve recording participants. Box Gmail Blackboard Exchange Bannerweb UR Talent Web Directory Calendar Maps Library. 410 Westhampton Way. University of Richmond, VA 23173. (804) 289-8000 (800) 700-1662. Legal Policies.
Creating the perfect consent form for research. Your consent form will no doubt be unique, depending on the nature of your research and the specifics of your study. But here are eight key components you won't want to miss out. 1. A study description and purpose. Kick off your consent form by outlining your study's purpose and objectives.
Many of these examples are actual UW IRB approved consent forms designed by UW researchers. Some of the examples were created using one of our consent templates. The use of our template is not required and some of the examples deviate significantly from our templates. We encourages researchers to use the Designing the Consent Process guidance ...
the research process. The researcher should retain one copy of the consent form signed by both themse. ves and the participant. The participant should also be given a copy of the consent form as a record of wha. they have signed up to.Even if a person has signed a consent form consent should still be re-established at the poin.
The Institutional Review Board has posted updated research consent form templates and new detailed drafting guidance. Designed with updated instructions and a newly designed key information section, these templates ensure clarity and compliance for both researchers and participants. Enhance transparency with our user-friendly and informative forms.
This is a template to assist researchers in the design of their informed consent form. You must adapt this template to the requirements of your particular study, using the notes and suggestions provided. Before using this template, check whether your organisation provides a template consent form and if so, incorporate their requirements into ...
As part of the informed consent process, the consent document is designed to provide information to potential subjects about a research study so they can make an informed decision about their participation. The use of a form to document the consent process is required unless specifically waived by the IRB. One of the most common reasons for ...
The standard consent process has two separate stages: Stage 1: In this stage, information is given, and the participant is given time to reflect upon that information. They are under no obligation to respond or agree to anything. Stage 2: This is the stage where consent is obtained. The terms of the research project are gone over individually ...
INFORMED CONSENT TO PARTICIPATE IN A RESEARCH PROJECT: " Title of Study " INTRODUCTION . This form asks for your agreement to participate in a research project on topic. Your participation involves concise summary of what the subjects will be asked to do. It is expected that your participation will take approximately amount of time their ...
Working together to work wonders by providing proposal development support to enhance UTMB's research enterprise. OSRD. PROPOSAL DEVELOPMENT & MANAGEMENT. FIND A COLLABORATOR ... The Institutional Review Board has posted updated research consent form templates and new detailed drafting guidance. InfoEd Resources OnCore Resources Research ...